Abstract
Introduction
Children with mild traumatic brain injury (mTBI) typically recover quickly, however approximately 15% experience persistent post-concussive symptoms (PPCS) past 3 months. The microstructural pathology associated with underlying persistent symptoms is poorly understood but is suggested to involve axonal injury to white matter tracts. Diffusion tensor imaging (DTI) can be used to visualize and characterize damage to white matter microstructure of the brain.
Objective
We aimed to investigate white matter microstructure in children with persistent concussive symptoms as compared to typically developing controls, alongside evaluating differences in white matter changes over time and how this relates to symptom recovery.
Methods
The current study is a prospective, longitudinal, controlled cohort study of children with mTBI. 104 children aged 8 to 18 years with a mTBI (72 symptomatic; 32 asymptomatic) were recruited from the Alberta Children's Hospital and compared to 20 healthy controls. Microstructural evidence of white matter injury was evaluated using DTI one month post injury and repeated 4 to 6 weeks later. Primary outcomes included fractional anisotropy and mean diffusivity of the corticospinal tracts, uncinate fasciculi, and motor fibers of the corpus callosum. Post-concussive symptoms were also measured using the Post-Concussion Symptom Inventory (PCSI) taken at both time points.
Results
Fractional anisotropy of the left uncinate fasciculi was lower in symptomatic children compared to controls (F(2,119) = 3.582, p = 0.031). No other significant differences were observed.
Conclusions
Our findings provide evidence of microstructural injury following mTBI in children with ongoing post-concussive symptoms one month post injury. The changes were persistent 4–6 weeks later. Further longitudinal studies of white matter microstructure in PPCS will be helpful to clarify whether these white matter alterations resolve over time.
Keywords: Diffusion tensor imaging, Mild traumatic brain injury, Pediatrics, Persistent post-concussive symptoms, Uncinate fasciculus
Highlights
-
•
Children with persistent post-concussive symptoms exhibit evidence of microstructural injury
-
•
Microstructural evidence of injury persists at two months post injury.
-
•
This research supports delayed return to contact sports in children with persistent symptoms.
1. Introduction
Traumatic brain injury (TBI) contributes significantly to the neurological morbidity seen in pediatric populations (McKinlay et al., 2008; Langlois et al., 2005; Thornhill et al., 2000; Maas et al., 2008). Mild TBI (mTBI) accounts for 90% of TBIs seen in the emergency department (Barlow et al., 2010). Fourteen percent of children with mTBI will experience persistent post concussive syndrome (PPCS), in which a constellation of symptoms (i.e., physical, cognitive, behavioural, and affective) continue at least three months post injury (Barlow et al., 2010). PPCS can cause significant disability in childhood (Barlow et al., 2010; Yeates et al., 2009; Centers for disease control, 2010), delaying return to school and physical activity as well as potentially impeding academic performance and social integration (Gagnon et al., 2005; Moran et al., 2011; Emanuelson et al., 2003; Ewing-Cobbs et al., 1990; Ponsford et al., 2011; Yeates and Taylor, 2005).
The neural correlates of acute mTBI have been well characterized (Cernak et al., 2010; Creed et al., 2011; Giza and Hovda, 2014; Giza et al., 2006; Gosselin et al., 2010; Henninger et al., 2007; Henry et al., 2010; Lipton et al., 2008) although the pathophysiology of persistent symptoms is less understood (Barlow et al., 2010; Yeates et al., 2009; Centers for disease control, 2010). Focal and diffuse axonal injury (DAI) due to traumatic shearing forces have been identified as major pathological contributors to poor clinical outcome following TBI (Meythaler et al., 2001). Such injury can be investigated using diffusion tensor imaging (DTI) (Levin, 2003).
DTI measures the movement of water molecules throughout the brain and produces quantitative measures of anisotropy and diffusivity, measures that are suggestive of white matter microstructure such as axonal density and myelination (Basser, 1995), as well as fiber coherence and orientation (Beaulieu, 2002). The most common diffusion measures are fractional anisotropy (FA) and mean diffusivity (MD). In children with moderate or severe TBI, lower FA in the large white matter tracts such as the corpus callosum (CC), internal capsule, inferior fronto-occipital fasciculus and uncinate fasciculus (UF) have been observed compared to healthy controls (Ewing-Cobbs et al., 2006; Wilde et al., 2006; McCauley et al., 2011). Further, reduced FA in the corpus callosum has been seen both acutely (Henry et al., 2011) and 3 years post injury in a range of TBI severity (Hulkower et al., 2013; Wozniak et al., 2007). Several cross-sectional studies have investigated white matter microstructure in the acute (<1 month post injury) (Henry et al., 2011; Smits et al., 2011) and chronic (>6 months post injury) (Wozniak et al., 2007; Yuan et al., 2007) stages of mTBI recovery. However, no studies have explored such changes over time in children who are slow to recover from mTBI (i.e., PPCS). Such longitudinal data is vital when trying to understand neural repair and recovery following mTBI and its relation to the ongoing symptomology. Studies using non-invasive techniques to investigate motor cortex neurophysiology have identified differences in the cortical excitability patterns in children with PPCS (Garvey et al., 2001; Urban et al., 2015; Seeger et al., 2017). Therefore, changes over time specifically within the corticospinal tracts and motor fibers of the corpus callosum are interesting targets for research.
The goal of this study was to use DTI to examine white matter microstructure in children with PPCS following mTBI. More specifically, we examine cross-sectional white matter differences between groups, as well as longitudinal changes in white matter over time within and between groups, and correlated changes to symptom recovery. We hypothesized that children with PPCS would show differences in white matter microstructure, specifically lower FA and higher MD within the UF, corticospinal tracts, and corpus callosum, compared to asymptomatic children who had clinically recovered and healthy controls. We also hypothesized that children with PPCS would show resolution of FA and MD changes in targeted white matter tracts over time, and that these would relate to symptom recovery.
2. Methods
2.1. Participants
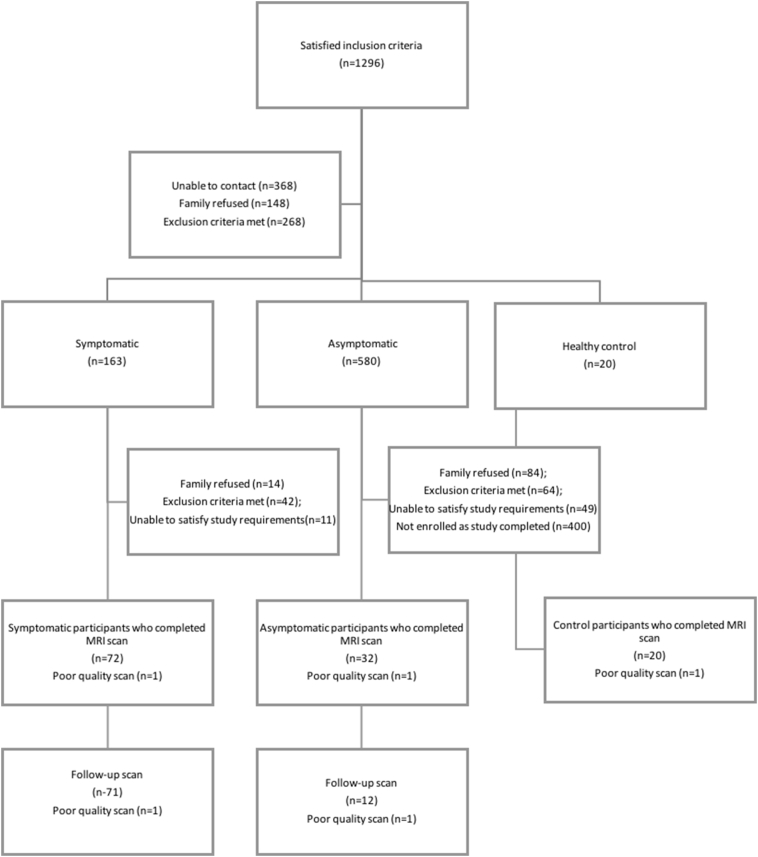
The current study included 124 children between 8 and 18 years of age (mean age 14.3 +/− 2.46 years; 54.8% female) presenting to the Emergency Department (ED) at the Alberta Children's Hospital (ACH) and were diagnosed with a mTBI or concussion by the ED physician. See Table 1 for demographic characteristics. mTBI was defined as traumatically induced alteration of consciousness where participants experienced either a loss of consciousness (LOC), had a Glasgow Coma Scale (GCS) score of 13 or 14, or had one or more acute symptoms of concussion (Moran et al., 2011; Barlow et al., 2015). Participants were enrolled one month post injury and written informed consent and assent were obtained. Exclusion criteria were: a past medical or psychiatric history requiring the use of psychotropic medications or hospitalization, previous mTBI within the past 3 months or ongoing symptoms from a previous mTBI, contraindications to MRI (Keel et al., 2001), as well as participants whose injury involved assault, suspected abuse or the use of alcohol or other substances at the time of injury. Controls of similar age and sex were identified from typically developing friends and siblings of mTBI participants using the same exclusion criteria. The recruitment process is demonstrated in Fig. 1. This study was approved by the University of Calgary Conjoint Health Research Ethics Board (REB13–0372).
Table 1.
Demographic and injury details of study participants. Post-concussive symptom inventory completed by youth (PCSI-Y). *Indicates significant differences between mTBI group and controls (p < 0.05). +Indicates significant differences between symptomatic and asymptomatic mTBI groups (p < 0.05).
| Controls | Asymptomatic | PPCS | |
|---|---|---|---|
| Age (±SD) | 14.36 (3.2) | 13.84 (2.5) | 14.09 (2.6) |
| Gender, F % | 60 | 34 | 63 |
| Handedness, R % | 100 | 91 | 90 |
| Previous concussion (%) | |||
|
100 | 56 | 63 |
|
44 | 37 | |
|
9 | 18 | |
|
6 | 11 | |
| Cause of injury (%) | |||
|
82 | 71 | |
|
12 | 7 | |
|
3 | 10 | |
|
3 | 12 | |
| Witnessed, % | 96 | 92 | |
| Loss of consciousness, % | 16 | 13 | |
| Retrograde amnesia, % | 22 | 23 | |
| Anterograde amnesia, % | 28 | 29 | |
| Time since injury at Scan 1 (days, SD) | – | 40.09 (9) | 37.58 (5.8) |
| PCSI-Y | 0 (1) | 4.13 (5.8) | 27.16 (21.3)* |
| Time since injury at Scan 2 (days, SD) | – | 84.7 (12.4) | 74.6 (7.1) |
| Change in PCSI-Y | – | −10 (13.1) | 27.17 (22.6)* |
Fig. 1.
Participant recruitment diagram. Visual flow chart of participant recruitment, screening, and data collection. Note that children who had contraindications for MRI (e.g., metal braces) were included within the population unable to commit to study requirements.
2.2. Recovery status
The Post-Concussion Symptom Inventory (PCSI) questionnaire was used to determine post-concussive symptoms in all participants. The questionnaire assessed symptoms in 4 domains: somatic, cognitive, emotional, and sleep and has been shown to be a reliable measure of PCS with good internal reliability (Barlow et al., 2010; Barlow et al., 2015). Clinically significant symptomology was defined as an increase of 10 points compared to retrospective pre-injury PCSI report collected at the same time (symptomatic group) (Barlow et al., 2014). The asymptomatic group consisted of participants who had returned to pre-injury PCSI scores or less and who reported no differences compared to their pre-injury status. Symptomatic and asymptomatic groups completed the PSCI questionnaire one month post injury, as well as at the follow-up time point. For comparison, healthy control participants completed the PCSI at the first time point only.
2.3. MRI data acquisition
Neuroimaging was performed using the Alberta Children's Hospital (ACH) General Electric 3 T MR750w MRI scanner (GE Healthcare, Waukesha, WI), with a 32-channel head coil. Participants laid supine inside the scanner with foam padding placed around the head to prevent movement. Diffusion weighted images were obtained with an axial SSE DTI 32 direction spin echo sequence with TR = 11,500 ms, TE = 69.1 ms, FOV = 22.0, 32 diffusion encoding directions (b = 750 s/mm2), 3 non-diffusion encoding directions (b = 0 s/mm2), number of slices = 62, and voxel size of 0.6 × 0.6 × 2.2 mm. Symptomatic and asymptomatic groups completed DTI one month post injury and at the follow-up time point, whereas controls underwent DTI at the first time point only.
2.4. DTI data analysis & tractography
DTI volumes were visually inspected and volumes containing motion or artifacts were removed. Participants had a minimum of 24 diffusion weighted volumes (median 32 ± 1.26) and 2 non-diffusion weighted volumes remaining (median 3 ± 0.1). DTI data was then pre-processed in ExploreDTI (Leemans et al., 2009) in which corrections for Gibbs Free Ringing, eddy currents, and EPI distortions (Leemans and Jones, 2009) were done. Following pre-processing, scans were compiled and the most representative subject was selected using Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006) via FSL (Smith et al., 2004) up to the post-registration command. The selected scan is determined as the scan that required the least warping to best fit other scans and was used as a study specific target scan for semi-automated tractography (Keihaninejad et al., 2012).
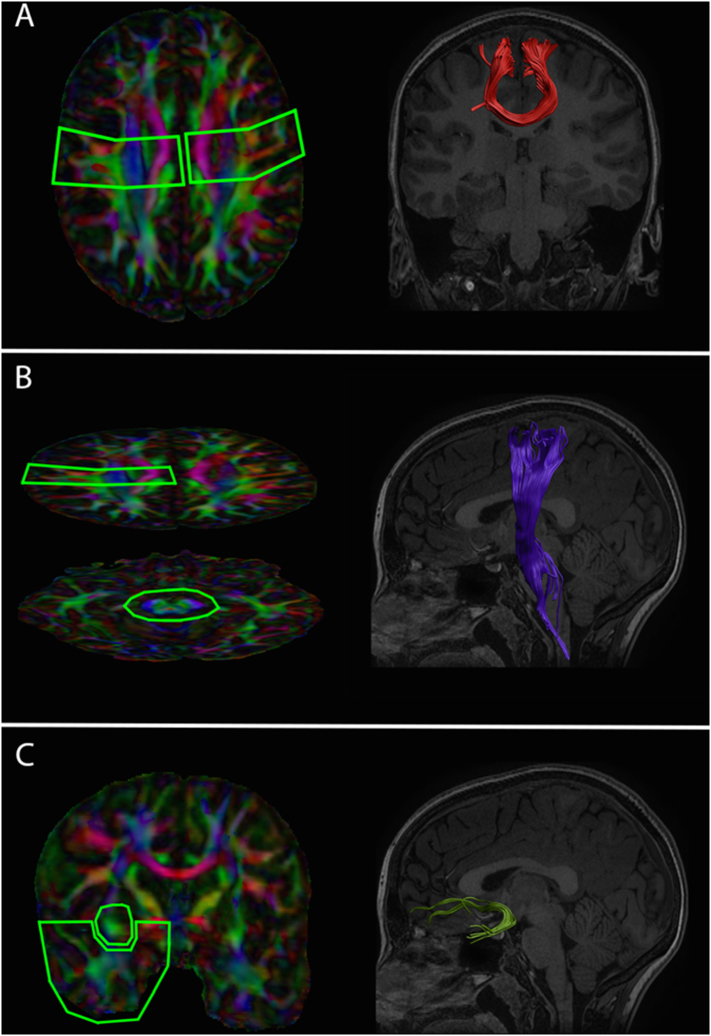
Inclusion and exclusion region of interests (ROI) were manually drawn on the selected target scan using a priori information of CST, CC, and UF tract location (Mori et al., 2005) (see Fig. 2). Deterministic streamline semi-automated tractography was performed in ExploreDTI (Leemans et al., 2009), in which the ROIs drawn on the target scan were aligned and projected onto each participant to delineate the CST, UF, and motor fibers of the CC. Inclusion ROIs used to delineate the bilateral CSTs were drawn axially at the level of the decussation of the superior cerebellar peduncle and at the posterior limb of the internal capsule (Fig. 2B). For tracking of the CC both of the ROIs drawn at the decussation of the superior cerebellar peduncle used in the isolation of the bilateral CSTs, were included to capture CC fibers projecting to the cortical motor strip. Two inclusion ROIs were used in the isolation of the bilateral UF, with the first drawn coronally anterior to the genu of the corpus callosum, and the second drawn coronally at the level of the anterior commissure (Fig. 2C). Calculations of average tract fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were calculated for each tract in each participant.
Fig. 2.
Tract Inclusion ROIs and Visual Depiction of Isolated Fibers. Inclusion ROIs used to delineate the motor fibers of the corpus callosum (A), left corticospinal fibers (B), and the left uncinate fasciculus fibers (C), and the resulting fiber tracts shown on the T1-weigthed anatomical image from the target representative scan. Right CST and UF fibers were isolated using similar inclusion ROIs to the above. Note that cortical inclusion ROIs used to isolate the left and right CST were used to delineate the corpus callosum fibers projecting to the cortical motor strip.
2.5. Along tract measurements
Along tract analyses were also performed, whereby FA, MD, AD, and RD were extracted at 56 points along the CST and CC fibers, and 40 points along the UF fibers. The number of points for each fiber was calculated by taking half of the mean fiber length for each individual tract (Leemans et al., 2009). Along tract analyses were performed on scans from each group, at the first time point only.
2.6. Statistical analysis
Statistical analyses were performed using SPSS V23.0 (SPSS Inc., Chicago, Illinois). Independent sample t-tests were run to examine group differences in age, sex, and symptom scores. One-way Analysis of Covariance (ANCOVA), controlling for age and sex, were used to investigate differences in diffusion measures between groups at one month post injury and at follow-up. For along fiber analyses, a multivariate analysis of covariance (MANCOVA), controlling for age and sex, was used to compare group differences in FA, MD, AD, and RD at each point along the fibers. Repeated measures ANCOVAs were run to investigate within group sessional changes in diffusion parameters, controlling for age and sex. In order to address multicollinearity and associated reduced power, a multivariate canonical discriminant analysis was run to determine group differences on along fiber tractography. This approach provides a set of discriminant factor scores (obtained as a linear combination of the variables) that best separate the groups. These factor scores are used to plot observations as points on a map and group membership is awarded based on nearest neighbour approach.
Kendall's correlation analyses with a Bonferroni correction were performed to analyze the relationships between the PCSI symptom domains (total score in the somatic, cognitive, emotional and sleep domains) and white matter microstructure parameters from scans at the first time point. Next, to determine if changes in white matter microstructure within the CST, UF, and motor fibers of the CC were associated with recovery from PCSI symptoms, changes in PCSI scores between sessions were compared with changes in FA and MD between sessions, in the symptomatic group, using Pearson correlations.
Multiple comparison corrections, using false discovery rate (FDR), were done for all analyses. Results are reported both corrected and uncorrected for multiple comparisons. Data may be provided upon reasonable request to the corresponding author.
3. Results
3.1. Participants
Seventy-two symptomatic participants, 32 asymptomatic participants, and 20 healthy controls were recruited at one month post injury, with initial imaging being performed at 38 (± 7) days post injury. One participant was removed from each the symptomatic and asymptomatic groups due to poor quality scans (>6 motion corrupted volumes). Two additional participants were removed from the symptomatic group due to unsuccessful tractography; leaving a final sample of 69 symptomatic scans and 31 asymptomatic scans for analyses (Fig. 1). Repeat imaging was performed in 72 symptomatic and 13 asymptomatic participants at 74.6 (±7.1) and 84.7 (±12.4) days post injury, respectively. One participant was removed from each of the symptomatic and asymptomatic groups due to poor quality scans and 2 participants were removed from the symptomatic group due to unsuccessful tractography; resulting in 68 symptomatic scans and 12 asymptomatic scans for analyses (Fig. 1).
Demographics and injury details are provided in Table 1. Groups did not significantly differ in age at any time point (p > 0.1), however the asymptomatic group had significantly fewer females (x2(2) = 7.722, p = 0.021). Summary group level data provided in Table 2.
Table 2.
Group-level data. Ages expressed as mean (SD).
| N at Scan 1 | 124 |
| N at Scan 2 | 83 |
| Age at Scan 1 | 14.29 (2.5) |
| Age at Scan 2 | 14.26 (2.4) |
| Gender (% Female) | 55 |
| Handedness (% RH) | 92 |
Table 3. Group-level data.
3.2. Post-concussive SYMPTOM inventory scores
Significantly higher symptom scores on the PCSI at one month post injury were reported in the symptomatic group (27.16 (21.3)) when compared to the asymptomatic group (4.13 (5.8)) (t(136) = 7.51, p ≤0.0001, Cohen's d = 1.983); these survived multiple comparisons. Healthy controls completed the pre-injury PCSI only (0 (1)). Higher scores on the PCSI remained at follow-up in the symptomatic group when compared to the asymptomatic group (t(104) = 1.103, p = 0.136) below significance level. Within the symptomatic group, significant changes in PCSI scores between the first and second sessions were found (t(188) = 7.075, p ≤0.0001, Cohen's d = 1.033) showing recovery of symptoms by two months post injury.
Only 7% of children with persistent symptoms in our study had a predominant symptom domain (> 20% discrepancy compared to their other domains); for instance, 3 children had marked emotional symptoms, 1 had predominant physical symptoms, and one had predominant cognitive symptoms.
3.3. Group comparisons of white matter microstructure one month post injury
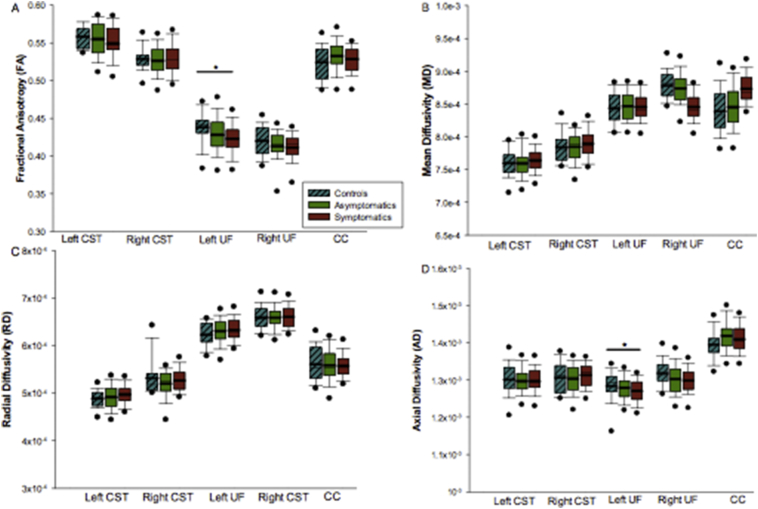
Group comparisons of DTI measures in each tract are shown in Fig. 3. No significant between group differences in FA or MD of the left or right CST (FA L CST: F(2,121) = 1.106,p = 0.334; FA R CST: F(2,120) = 0.362, p = 0.697) (MD L CST: F(2,121) = 0.697,p = 0.5; MD R CST F(2,120) = 0.733,p = 0.483) or CC (CC FA: F(2,121) = 1.013, p = 0.366) (CC MD: F(2,121) = 0.359,p = 0.699) were found.
Fig. 3.
Group comparisons of white matter microstructure one month post injury. (A) Group differences in FA; significantly lower FA of the left UF was observed in the symptomatic group. (B) Groups differences in MD values; no significant group differences were noted. (C) Group differences in RD values; no significant group differences were noted. D) Group differences in AD; significantly lower AD of the left UF in the symptomatic group. Note that box plots represent the median and interquartile range, with thick black lines indicating the group mean. Black circles indicate data points falling outside of the 95% confidence interval, while error bars indicate the range in diffusion values.
There was a significant between group effect using ANCOVA (F(2,119) = 3.582, p = 0.031), with post hoc analyses indicating that the symptomatic group had lower FA in the left UF compared to controls (t(2,119) = 2.654, p = 0.027, Cohen's d = 0.713) (Fig. 3). There was also a significant group effect (F(2,119) = 4.339, p = 0.015), with post hoc analyses indicating that the symptomatic group also had higher AD in the left UF compared to controls (t(2,119) = 2.945, p = 0.012, Cohen's d = 0.153). Significant differences between groups in the left UF did not survive correction for multiple comparisons.
Finally, no significant group differences were noted in MD of the left UF (F(2,104) = 1.260, p = 0.103), FA or MD of the right UF (FA R UF F(2,104) = 0.941, p = 0.631; MD R UF F(2,104) = 1.055, p = 0.383) or other UF, CST and CC diffusivity measures between groups.
The relationship between each of the 4 domains of PCSI and DTI parameters were explored using non-parametric analyses (Kendall's tau test), however, no significant relationships were found after Bonferroni correction (see Table 3).
Table 3.
Kendall Tau (a) correlation matrix (all groups at session 1). The 4 domains of PCSS symptoms were correlated with DTI indices, * = p < 0.05 following Bonferroni correction.
| Physical | Cognitive | Emotional | Fatigue | |
|---|---|---|---|---|
| Physical | 0.934 | – | – | – |
| Cognitive | 0.6383* | 0.884 | – | – |
| Emotional | 0.4715* | 0.4830* | 0.812 | – |
| Fatigue | 0.4834* | 0.5236* | 0.4602* | 0.853 |
| Age | −0.003 | 0.047 | 0.077 | 0.119 |
| LU_FA | −0.159 | −0.125 | −0.100 | −0.033 |
| LU_MD | 0.090 | 0.031 | 0.019 | −0.081 |
| LU_AD | −0.014 | −0.043 | −0.038 | −0.098 |
| LU_RD | 0.119 | 0.079 | 0.044 | −0.052 |
| RU_FA | −0.128 | −0.094 | −0.091 | −0.020 |
| RU_MD | 0.038 | 0.011 | −0.031 | −0.084 |
| R_AD | −0.028 | −0.037 | −0.078 | −0.074 |
| RU_RD | 0.105 | 0.074 | 0.025 | −0.043 |
| LCST_FA | −0.124 | −0.065 | −0.097 | −0.101 |
| LCST_MD | 0.100 | 0.084 | 0.031 | 0.031 |
| LCST_AD | 0.007 | 0.025 | −0.035 | −0.037 |
| LCST_RD | 0.126 | 0.078 | 0.097 | 0.082 |
| RCST_FA | −0.020 | 0.015 | −0.072 | −0.034 |
| RCST_MD | 0.055 | 0.065 | 0.078 | 0.078 |
| RCST_AD | 0.050 | 0.071 | 0.038 | 0.034 |
| RCST_RD | 0.060 | 0.038 | 0.065 | 0.080 |
| CC_FA | −0.144 | −0.056 | −0.070 | −0.022 |
| CC_MD | 0.039 | −0.023 | 0.002 | −0.094 |
| CC_AD | −0.027 | −0.046 | −0.050 | −0.121 |
| CC_RD | 0.100 | 0.007 | 0.028 | −0.052 |
3.4. Along tract analysis of white matter microstructure one month post injury
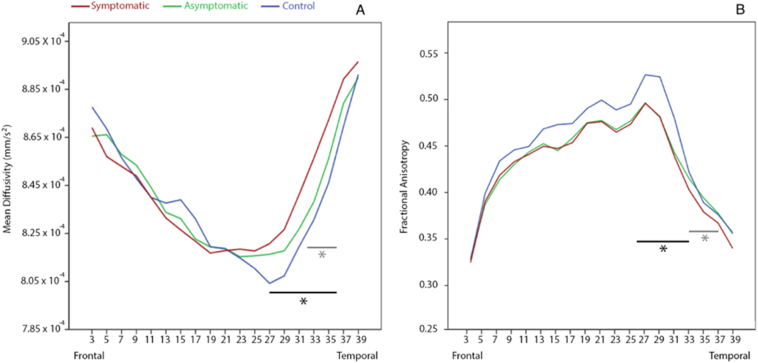
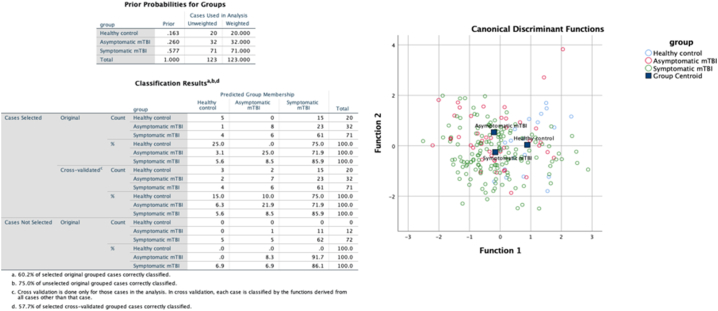
Measures of FA and MD at all points along the CC and the left or right CST did not significantly differ between either mTBI groups and controls (F(104) = 1.11, p = 0.281). Lower FA values were observed in the left UF (points 27–32) in both mTBI groups compared to controls (F(68) = 1.6, p = 0.034) (Fig. 4A-C); points 29–31 of left UF FA survived correction for multiple comparisons. Further, MD of the left UF was significantly higher in both mTBI groups compared to controls (F(68) = 1.7, p = 0.016); points 29–34 of the left UF MD survived correction for multiple comparisons. Corresponding significant points for FA and MD of the left UF were clustered around the ‘hook’ region. Canonical discriminant analysis demonstrated significant group classification based on factor scores of individual observations of FA in the left UF (see supplementary material). The CDA group classification based on factor scores of individual observations. DCA misclassified 48 of 207 observations at session one, including 15 from the healthy control group, 24 from the asymptomatic mTBI group, and 10 from the symptomatic mTBI group (159 of 207 correct classifications, p > 0.05) in the left UF. In the right UF, 54 misclassifications were made, 15 in the healthy control group, 30 in the asymptomatic group, and 9 in the symptomatic group (153 of 207 correct classifications). In the right CST, 46 misclassifications were made, including 12 from the healthy control group, 27 from the asymptomatic group, and 7 from the symptomatic group (160 of 206 correct classifications). In the left CST, 65 misclassifications were made, including 19 from the healthy control group, 40 from the asymptomatic group, and 6 from the symptomatic group (141 of 206 correct classifications).
Fig. 4.
Group comparisons of left uncinate fasciculus along fiber analysis. (A) MD of left UF for controls (blue line), asymptomatic (green line), and symptomatic (red line) groups; significantly higher MD in the mTBI groups was noted compared to controls. (B) FA of left UF for controls (blue line), asymptomatic (green line), and symptomatic (red line) groups; significantly lower FA in the mTBI groups was noted compared to controls. Note that black bars indicate significant differences between control and symptomatic groups, while grey bars indicate significant differences between control and asymptomatic groups. No significant differences were identified between symptomatic and asymptomatic groups.
3.5. Longitudinal group comparisons of white matter microstructure & correlates of PCS symptomology
No between session differences in FA, MD, AD, or RD were observed between or within mTBI groups for all white matter tracts (p > 0.1). As previously stated, higher scores on the PCSI remained at follow-up in the symptomatic group when compared to the asymptomatic group (t(104) = 1.103, p = 0.136). An exploratory analysis examining the association of diffusion changes with symptom changes was done in symptomatic children with significant improvement in symptom scores from time point one to time point two (i.e., 10 point improvement in PCSI score). The relationship between each of the 4 domains of PCSI and DTI parameters were explored using non-parametric analyses (Kendall's tau test), however, no significant relationships were found after Bonferroni correction (see Table 2).”
No significant associations were observed between symptom scores and FA or MD in the left UF. No significant associations were observed between symptom scores and FA or MD in the left UF Fig. 5.
Fig. 5.
Observations in the canonical discriminant analysis space for each group. The probabilities of group membership before analysis is shown. Classification results for original and cross-validated groups are shown. Two discriminant factors (linear combinations of variables) were used to classify group membership.
4. Discussion
Our study is the first to examine the association between white matter microstructure and persistent post-concussive symptoms in children following mTBI. We found lower FA and AD values in the left uncinate fasciculus in children with persistent symptoms compared to asymptomatic children and healthy controls. Although average MD values were not higher within the UF, higher MD values were seen in the along tract analysis.
The current study's findings of lower FA within the UF, is in keeping with previous research showing lower FA within major white matter fibers following mild TBI (Henry et al., 2011; Hulkower et al., 2013; Wozniak et al., 2007). For instance, McCauley et al., describes reduction in FA values at 3 months post injury, in both right and left UF, and correlated these with changes in prospective memory (McCauley et al., 2011). In addition to lower FA values in the left UF, we also found lower AD values in the left UF in symptomatic children compared with asymptomatic and control children. To our knowledge, this is the first study to report altered AD of the UF after pediatric mTBI. As histological studies suggest changes in AD are specific to the axon (Song et al., 2003), the lower AD values following mTBI in our study is suggestive of axonal injury or dysfunction after mTBI. Finally, while inconsistent findings have been noted for changes in MD after TBI (Henry et al., 2011; Hulkower et al., 2013), the current study's findings support a relationship between MD and microstructural injury within the UF following mTBI.
As concussion has a widespread effect on the brain with injury to various localized regions, we expected to see effects on widespread tracts in the brain, however, findings were localized to the UF. The UF is a well-characterized white matter tract that connects limbic anatomy of the temporal lobe to the orbitofrontal cortex. Clinically, abnormalities of the UF white matter microstructure have been implicated in a number of psychiatric illnesses such as anxiety and depression (Bhatia et al., 2018; Lai et al., 2013). Given that the nature of the UF is as an association tract, the cognitive or affective impairment following mTBI could be specific to functional localization of the injury, on the tract. Functional localization of UF white matter has been explored in individuals with epilepsy by visualization of white matter networks (Neal et al., 2018), however no such work has been done in mTBI populations. Indeed, children report emotional symptoms and heightened anxiety following mTBI (Emery et al., 2016), in addition to cognitive deficits. As such, perhaps the heterogeneity of symptoms may be in part related to the associative involvement of the UF tract, as well as variations in injury profile.
The absence of findings in the CST and motor collosal fibers may be related to these fibers being primarily involved in motor performance. Following mTBI motor symptomology is not commonly reported, however previous evidence does show changes in the motor tracts following mTBI (Singh et al., 2010; Kasahara et al., 2012). Mechanism or severity of injury may be a factor in motor involvement, with force or direct impact leading to motor deficits. Additionally, our developing population may be less likely to show motor deficits than impairments to regions that haven't fully matured.
In a study of 15 high school football athletes with PPCS, McCauley et al. found similar FA and MD values to controls 5.8 months (range 3 to 12 months) post-injury, although decreased FA values were seen in those athletes with cognitive complaints (Mori et al., 2005). Although these findings may reflect post-injury white matter alterations, these values could also be an indication of pre-injury differences in populations who may be more prone to trauma (Bigler et al., 1992). Demonstrating change from pre-injury status or change toward control group values over time can help elucidate this.
Contrary to our hypothesis, however, there was no evidence of change in tissue microstructure over time. As Wallerian degeneration can progress for up to 2 months post injury (Povlishock et al., 1992; Adams et al., 1989), our results are somewhat reassuring in that no further decreases in FA or increases in MD occurred. However, the lack of white matter changes in symptomatic children over time may suggest that residual white matter injury after mTBI requires more time to resolve: if at all. Animal models of Wallerian degeneration have provided evidence of peak axonal damage occurring at approximately three weeks post injury and persisting past twelve weeks (Maxwell et al., 2015). Subsequently, the time frame in our study may be too short to detect changes in tissue integrity, especially if white matter recovery lags behind clinical change. More recent mechanistic research has also implicated that damaged myelin may be cleared more slowly than other neural debris, creating a discord between imaging findings and clinical neural recovery (Armstrong et al., 2016). With this in mind, we acknowledge the possibility that imaging findings may be apparent without associated symptoms, and vice versa. For example, symptomatic athletes were similar to control athletes in cortical thickness and cerebral metabolism measured using MRS (Tremblay et al., 2014). In contrast, pediatric studies have reported changes in cerebral perfusion (Barlow et al., 2017) and cerebral blood flow (Giza and Hovda, 2014) in children with clinical recovery. Further, studies have reported increased FA following mTBI, hypothesizing that altered FA may be secondary to edema around the axons (Lipton et al., 2008). The ability to detect changes to FA or MD may also be impeded by other pathophysiological consequences of TBI such as inflammation of tissue (Giza and Hovda, 2014). Finally, the number of participants in our asymptomatic sample diminished significantly at the follow-up scan, reducing our paired sample to 12 participants. Thus, the resultant small sample size may be underpowered to properly represent white matter changes in asymptomatic brains.
While we found some evidence to support microstructural injury in children with PPCS, the significant decrease in symptoms by 4-6 weeks post injury was not reflected in white matter changes over time. The lack of changes in diffusion measures and, in turn, inability to relate white matter changes t symptom recovery is not totally unexpected due to the heterogeneity of PCS symptoms and our primary focus on motor tracts. PCS syndrome is a complex disorder with multiple underlying mechanisms including: pre-injury factors such as a propensity for headaches or dizziness, and environmental factors such as: post injury differences in symptom phenotypes and rate of recovery; and biomechanical forces involved in the insult (Cernak et al., 2010; Creed et al., 2011; Giza and Hovda, 2014; Giza et al., 2006; Gosselin et al., 2010; Henninger et al., 2007; Henry et al., 2010; Lipton et al., 2008). In light of this it may be that children exhibiting very similar symptom profiles may show more homogeneity in tract changes, such as the correlation between working memory and lower FA values in the UF (Diehl et al., 2008). Inconsistency between presence of symptoms and evidence of microstructural injury indicates further research is needed. Additionally, in order to further explore the association of DTI metrics with white matter recovery a wider longitudinal window may be required, including an immediate scan following injury or a long-term follow-up to frame the recovery more accurately.
It is important to note that there are some limitations to our study. The groups had unequal sample sizes, with a smaller asymptomatic group. Nevertheless, this is still a larger sample than the majority of other studies to date. Further, only 12 of the asymptomatic participants returned for repeat imaging, which may have affected our ability to detect differences over time between groups. A weakness in any study of symptoms following a mTBI is the subjective nature of the symptoms, the lack of a gold standard diagnostic test, and the role of psychosocial factors and environment on outcome trajectory (Tonks et al., 2011). As PPCS symptoms are present in healthy as well as chronically ill populations (Iverson et al., 2010) and there is a tendency to view the past to be more favourable over time (Brooks et al., 2014), symptoms can be under-reported following mTBI. While, all participants in our study had a detailed clinical evaluation, PPCS is a diagnosis based on clinical presentation which should be considered when interpreting results. The inclusion of a cognitive evaluation as a clinical outcome to correlate to white matter integrity may add greater clinical correlation. Finally, all of these children had a normal clinical examination (physical and history), reducing the likelihood of another medical condition contributing to symptoms or pathology. The heterogeneity of our population allows generalization of results to children who present for medical attention due to prolonged symptoms at one month post injury. However, previous studies have focused on differences due to mechanism of insult (Caeyenberghs et al., 2011; Adams et al., 1977), as different populations may have different risk factors for poor outcome such as subclinical injuries in some sports (e.g. football) suggesting diversity of mechanism may contribute to the applicability of our results to a general population.
5. Conclusions
In summary, children with PPCS at one month post injury showed differences in diffusivity measures compared to healthy controls. Our findings provide support for potential microstructural injury in the left UF following mTBI in children with ongoing post-concussive symptoms at one month post injury. Longitudinal group differences in white matter changes were not observed. Further exploration of specific symptom clusters and related tracts with baseline imaging is required to better understand the relationship between microstructural injury and presence of persistent mTBI symptoms.
Acknowledgments
Acknowledgements
We would like to acknowledge Zahra Ofoghi, Aneesh Khetani, and Dr. Jessica Reynolds for their contribution to this project.
Funding
This study was funded by the Canadian Institutes of Health Research (grant number: 293375); and the Faculty of Medicine, University of Calgary.
Declarations of interest
None
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101842.
Appendix A. Supplementary data
Supplementary material
References
- Adams H., Mitchell D.E., Graham D.I., Doyle D. Diffuse brain damage of immediate impact type. Its relationship to'primary brain-stem damage'in head injury. Brain. 1977;100(3):489–502. doi: 10.1093/brain/100.3.489. [DOI] [PubMed] [Google Scholar]
- Adams J.H., Doyle D., Ford I., Gennarelli T.A., Graham D.I., McLellan D.R. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathol. 1989;15(1):49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Armstrong R.C., Mierzwa A.J., Marion C.M., Sullivan G.M. White matter involvement after TBI: clues to axon and myelin repair capacity. Exp. Neurol. 2016;275:328–333. doi: 10.1016/j.expneurol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Barlow K.M., Crawford S., Stevenson A., Sandhu S.S., Belanger F., Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatr. 2010;126(2):e374. doi: 10.1542/peds.2009-0925. (peds-2009) [DOI] [PubMed] [Google Scholar]
- Barlow K.M., Brooks B.L., MacMaster F.P., Kirton A., Seeger T., Esser M.…Kirk V. A double-blind, placebo-controlled intervention trial of 3 and 10 mg sublingual melatonin for post-concussion syndrome in youths (PLAYGAME): study protocol for a randomized controlled trial. Trials. 2014;15(1):271. doi: 10.1186/1745-6215-15-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow K.M., Crawford S., Brooks B.L., Turley B., Mikrogianakis A. The incidence of postconcussion syndrome remains stable following mild traumatic brain injury in children. Pediatr. Neurol. 2015;53(6):491–497. doi: 10.1016/j.pediatrneurol.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Barlow K.M., Marcil L.D., Dewey D., Carlson H.L., MacMaster F.P., Brooks B.L., Lebel R.M. Cerebral perfusion changes in post-concussion syndrome: a prospective controlled cohort study. J. Neurotrauma. 2017;34(5):996–1004. doi: 10.1089/neu.2016.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8(7):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bhatia K.D., Henderson L.A., Hsu E., Yim M. Reduced integrity of the uncinate fasciculus and cingulum in depression: a stem-by-stem analysis. J. Affect. Disord. 2018;235:220–228. doi: 10.1016/j.jad.2018.04.055. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Kurth S.M., Blatter D., Abildskov T.J. Degenerative changes in traumatic brain injury: post-injury magnetic resonance identified ventricular expansion compared to pre-injury levels. Brain Res. Bull. 1992;28(4):651–653. doi: 10.1016/0361-9230(92)90119-i. [DOI] [PubMed] [Google Scholar]
- Brooks B.L., Khan S., Daya H., Mikrogianakis A., Barlow K.M. Neurocognition in the emergency department after a mild traumatic brain injury in youth. J. Neurotrauma. 2014;31(20):1744–1749. doi: 10.1089/neu.2014.3356. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., Geurts M., Linden C.V., Smits-Engelsman B.C., Sunaert S., Swinnen S.P. Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabil. Neural Repair. 2011;25(6):492–502. doi: 10.1177/1545968310394870. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2010. Heads Up: Facts for Physicians about Mild Traumatic Brain Injury (MTBI) [Google Scholar]
- Cernak I., Chang T., Ahmed F.A., Cruz M.I., Vink R., Stoica B., Faden A.I. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev. Neurosci. 2010;32(5–6):442–453. doi: 10.1159/000320085. [DOI] [PubMed] [Google Scholar]
- Creed J.A., DiLeonardi A.M., Fox D.P., Tessler A.R., Raghupathi R. Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. J. Neurotrauma. 2011;28(4):547–563. doi: 10.1089/neu.2010.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl B., Busch R.M., Duncan J.S., Piao Z., Tkach J., Lüders H.O. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49(8):1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Emanuelson I., Andersson Holmkvist E., Björklund R., Stålhammar D. Quality of life and post-concussion symptoms in adults after mild traumatic brain injury: a population-based study in western Sweden. Acta Neurol. Scand. 2003;108(5):332–338. doi: 10.1034/j.1600-0404.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- Emery C.A., Barlow K.M., Brooks B.L., Max J.E., Villavicencio-Requis A., Gnanakumar V., Yeates K.O. A systematic review of psychiatric, psychological, and behavioural outcomes following mild traumatic brain injury in children and adolescents. Can. J. Psychiatr. 2016;61(5):259–269. doi: 10.1177/0706743716643741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L., Levin H.S., Fletcher J.M., Miner M.E., Eisenberg H.M. The Children's orientation and amnesia test: relationship to severity of acute head injury and to recovery of memory. Neurosurg. 1990;27(5):683–691. [PubMed] [Google Scholar]
- Ewing-Cobbs L., Hasan K.M., Prasad M.R., Kramer L., Bachevalier J. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. Am. J. Neuroradiol. 2006;27(4):879–881. [PMC free article] [PubMed] [Google Scholar]
- Gagnon I., Swaine B., Friedman D., Forget R. Exploring children's self-efficacy related to physical activity performance after a mild traumatic brain injury. J. Head Trauma Rehabil. 2005;20(5):436–449. doi: 10.1097/00001199-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Garvey M.A., Ziemann U., Becker D.A., Barker C.A., Bartko J.J. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin. Neurophysiol. 2001;112(8):1451–1460. doi: 10.1016/s1388-2457(01)00581-8. [DOI] [PubMed] [Google Scholar]
- Giza C.C., Hovda D.A. The new neurometabolic cascade of concussion. Neurosurg. 2014;75(suppl_4):S24–S33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza C.C., Maria N.S.S., Hovda D.A. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J. Neurotrauma. 2006;23(6):950–961. doi: 10.1089/neu.2006.23.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin N., Saluja R.S., Chen J.K., Bottari C., Johnston K., Ptito A. Brain functions after sports-related concussion: insights from event-related potentials and functional MRI. Phys. Sportsmed. 2010;38(3):27–37. doi: 10.3810/psm.2010.10.1805. [DOI] [PubMed] [Google Scholar]
- Henninger N., Sicard K.M., Li Z., Kulkarni P., Dützmann S., Urbanek C., Fisher M. Differential recovery of behavioral status and brain function assessed with functional magnetic resonance imaging after mild traumatic brain injury in the rat. Crit. Care Med. 2007;35(11):2607–2614. doi: 10.1097/01.CCM.0000286395.79654.8D. [DOI] [PubMed] [Google Scholar]
- Henry L.C., Tremblay S., Boulanger Y., Ellemberg D., Lassonde M. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J. Neurotrauma. 2010;27(1):65–76. doi: 10.1089/neu.2009.0962. [DOI] [PubMed] [Google Scholar]
- Henry L.C., Tremblay J., Tremblay S., Lee A., Brun C., Lepore N., Lassonde M. Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma. 2011;28(10):2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- Hulkower M.B., Poliak D.B., Rosenbaum S.B., Zimmerman M.E., Lipton M.L. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. Am. J. Neuroradiol. 2013;34(11):2064–2074. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson G.L., Lange R.T., Brooks B.L., Lynn Ashton Rennison V. “Good old days” bias following mild traumatic brain injury. Clin. Neuropsychol. 2010;24(1):17–37. doi: 10.1080/13854040903190797. [DOI] [PubMed] [Google Scholar]
- Kasahara K., Hashimoto K., Abo M., Senoo A. Voxel-and atlas-based analysis of diffusion tensor imaging may reveal focal axonal injuries in mild traumatic brain injury—comparison with diffuse axonal injury. Magn. Reson. Imaging. 2012;30(4):496–505. doi: 10.1016/j.mri.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Keel J.C., Smith M.J., Wassermann E.M. A safety screening questionnaire for transcranial magnetic stimulation. Clin. Neurophysiol. 2001;112(4):720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Keihaninejad S., Ryan N.S., Malone I.B., Modat M., Cash D., Ridgway G.R., Ourselin S. The importance of group-wise registration in tract based spatial statistics study of neurodegeneration: a simulation study in Alzheimer's disease. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0045996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.H., Wu Y.T., Yu P.L., Yuan W. Improvements in white matter micro-structural integrity of right uncinate fasciculus and left fronto-occipital fasciculus of remitted first-episode medication-naive panic disorder patients. J. Affect. Disord. 2013;150(2):330–336. doi: 10.1016/j.jad.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Langlois J.A., Rutland-Brown W., Thomas K.E. The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 2005;20(3):229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Leemans A., Jeurissen B., Sijbers J., Jones D.K. 17th Annual Meeting of Intl Soc Mag Reson Med. vol. 209. International Society for Magnetic Resonance in Medicine; Berkeley, CA, USA: 2009. ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data; p. 3537. April. [Google Scholar]
- Levin H.S. Neuroplasticity following non-penetrating traumatic brain injury. Brain Inj. 2003;17(8):665–674. doi: 10.1080/0269905031000107151. [DOI] [PubMed] [Google Scholar]
- Lipton M.L., Gellella E., Lo C., Gold T., Ardekani B.A., Shifteh K., Branch C.A. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma. 2008;25(11):1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- Maas A.I., Stocchetti N., Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Maxwell W.L., Bartlett E., Morgan H. Wallerian degeneration in the optic nerve stretch-injury model of traumatic brain injury: a stereological analysis. J. Neurotrauma. 2015;32(11):780–790. doi: 10.1089/neu.2014.3369. [DOI] [PubMed] [Google Scholar]
- McCauley S.R., Wilde E.A., Bigler E.D., Chu Z., Yallampalli R., Oni M.B., Hunter J.V. Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. J. Neurotrauma. 2011;28(4):503–516. doi: 10.1089/neu.2010.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A., Grace R.C., Horwood L.J., Fergusson D.M., Ridder E.M., MacFarlane M.R. Prevalence of traumatic brain injury among children, adolescents and young adults: prospective evidence from a birth cohort. Brain Inj. 2008;22(2):175–181. doi: 10.1080/02699050801888824. [DOI] [PubMed] [Google Scholar]
- Meythaler J.M., Peduzzi J.D., Eleftheriou E., Novack T.A. Current concepts: diffuse axonal injury–associated traumatic brain injury. Arch. Phys. Med. Rehabil. 2001;82(10):1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- Moran M.L.M., Taylor H.G., Rusin J., Bangert B., Dietrich A., Nuss K.E., Yeates K.O. Do postconcussive symptoms discriminate injury severity in pediatric mild traumatic brain injury? J. Head Trauma Rehabil. 2011;26(5):348. doi: 10.1097/HTR.0b013e3181f8d32e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Wakana S., Van Zijl P.C., Nagae-Poetscher L.M. Elsevier; 2005. MRI Atlas of Human White Matter. [DOI] [PubMed] [Google Scholar]
- Neal E.G., Maciver S., Vale F.L. Multimodal, noninvasive seizure network mapping software: a novel tool for preoperative epilepsy evaluation. Epilepsy Behav. 2018;81:25–32. doi: 10.1016/j.yebeh.2018.01.033. [DOI] [PubMed] [Google Scholar]
- Ponsford J., Cameron P., Fitzgerald M., Grant M., Mikocka-Walus A. Long-term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J. Neurotrauma. 2011;28(6):937–946. doi: 10.1089/neu.2010.1516. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T., Erb D.E., Astruc J. Axonal response to traumatic brain injury: reactive axonal change, deafferentation, and neuroplasticity. J. Neurotrauma. 1992;9:S189–S200. [PubMed] [Google Scholar]
- Seeger T.A., Kirton A., Esser M.J., Gallagher C., Dunn J., Zewdie E.…Barlow K.M. Cortical excitability after pediatric mild traumatic brain injury. Brain Stimul. 2017;10(2):305–314. doi: 10.1016/j.brs.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Singh M., Jeong J., Hwang D., Sungkarat W., Gruen P. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn. Reson. Imaging. 2010;28(1):22–40. doi: 10.1016/j.mri.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Niazy R.K. Advances in functional and structural MR image analysis and implementation as FSL. Open Neuroimage J. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Open Neuroimage J. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smits M., Houston G.C., Dippel D.W., Wielopolski P.A., Vernooij M.W., Koudstaal P.J.…van der Lugt A. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiol. 2011;53(8):553–563. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ju W.K., Lin S.J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Open Neuroimage J. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Thornhill S., Teasdale G.M., Murray G.D., McEwen J., Roy C.W., Penny K.I. Disability in young people and adults one year after head injury: prospective cohort study. Bmj. 2000;320(7250):1631–1635. doi: 10.1136/bmj.320.7250.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks J., Huw Williams W., Yates P., Slater A. Cognitive correlates of psychosocial outcome following traumatic brain injury in early childhood: comparisons between groups of children aged under and over 10 years of age. Clin. Child. Psychol. Psychiatr. 2011;16(2):185–194. doi: 10.1177/1359104511403583. [DOI] [PubMed] [Google Scholar]
- Tremblay S., Beaulé V., Proulx S., Tremblay S., Marjańska M., Doyon J., Théoret H. Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin. Neurophysiol. 2014;125(7):1371–1379. doi: 10.1016/j.clinph.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban K.J., Barlow K.M., Jimenez J.J., Goodyear B.G., Dunn J.F. Functional near-infrared spectroscopy reveals reduced interhemispheric cortical communication after pediatric concussion. J. Neurotrauma. 2015;32(11):833–840. doi: 10.1089/neu.2014.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A., Chu Z., Bigler E.D., Hunter J.V., Fearing M.A., Hanten G., Levin H.S. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wozniak J.R., Krach L., Ward E., Mueller B.A., Muetzel R., Schnoebelen S., Lim K.O. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 2007;22(5):555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates K.O., Taylor H.G. Neurobehavioural outcomes of mild head injury in children and adolescents. Pediatr. Rehabil. 2005;8(1):5–16. doi: 10.1080/13638490400011199. [DOI] [PubMed] [Google Scholar]
- Yeates K.O., Taylor H.G., Rusin J., Bangert B., Dietrich A., Nuss K.…Jones B.L. Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatr. 2009;123(3):735–743. doi: 10.1542/peds.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Holland S.K., Schmithorst V.J., Walz N.C., Cecil K.M., Jones B.V., Wade S.L. Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. Am. J. Neuroradiol. 2007;28(10):1919–1925. doi: 10.3174/ajnr.A0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material