Abstract
Background and Aims
Depending on the species, water stress affects different growth and developmental processes, mainly due to changes in hydraulic properties and hormonal signalling. This study compared the impact of water stress on tree development and organ growth in three apple cultivars.
Methods
Trees were differentially irrigated to induce water stress or to provide well-watered conditions in their second and third years of growth. Effects of water stress were evaluated at tree scale by shoot number and proportions of the different types of shoots, and at shoot scale by metamer appearance rate, growth duration and arrest time, as well as organ size.
Key Results
Water stress promoted early growth cessation, prolonged summer arrests and decreased growth resumptions, thus modifying within-tree shoot demography in favour of short shoots. Growth cessations occurred in mild water stress conditions before any difference in stem water potential appeared. No major impact was observed on organ size. Consistently with tree ontogeny, the number of shoots that resumed growth after summer arrest decreased with years, but more in water-stressed than well-watered conditions.
Conclusions
Even though the impact of water stress differed slightly among cultivars, the reduction in neoformation and increase in summer arrest played a common role in apple tree morphological responses and led to stress avoidance by early reduction of tree leaf area.
Keywords: Drought, shoot growth dynamics, polycyclism, rhythmic growth, shoot expansion, tree architecture, Malus × domestica
INTRODUCTION
Plant development results from meristem activity through a sequence of developmental phases, referred to as ‘ontogeny’. During ontogeny, the morphological characteristics of plant entities, such as growth units or annual shoots, change over time (Nozeran, 1984). In many tree species, meristems are protected during the winter period by buds, which contain a number of preformed organs (Barthélémy and Caraglio, 2007). After bud burst, depending on the species, shoot growth may continue after the exhaustion of the preformed organs with the production of new ones, a process termed ‘neoformation’ (Gordon et al., 2006; Barthélémy and Caraglio, 2007). During tree ontogeny, a progressive decrease in shoot neoformation and length occurs with tree age (e.g. Costes et al., 2003, for the apple tree). Since neoformation also depends on the current season’s conditions and bud position within the tree, the relative extent of preformed and neoformed growth is important for understanding tree plasticity.
At the whole-plant level, architectural analysis is based on the hypothesis that plant structures are built repeatedly by constructional units, which are organized at different levels. The most basic architectural element is the metamer, which comprises a node, a leaf, an axillary bud and a subtending internode (White, 1979). The succession of metamers builds an axis, by either continuous or rhythmic growth (Barthélémy and Caraglio, 2007). In fruit trees, annual shoots can grow for a range of durations, forming different types of shoots (short, medium or long) with different length and composition in preformed/neoformed organs (Costes et al., 2006). Annual shoots can consist of a single growth unit, defined as a portion of stem that develops during an uninterrupted period of growth (Hallé and Martin, 1968). In the apple tree, when constituted of preformed organs only, the annual shoot remains short whereas when constituted of preformed and neoformed organs the shoot becomes medium or long, depending on internode elongation and growth duration. In some cases, growth may stop and resume, leading to annual shoots consisting of two successive growth units (Seleznyova et al., 2008). Such polycyclic shoots represent an intermediate stage between long monocyclic and medium shoots. During tree ontogeny, these polycyclic shoots are more frequent in the adolescent phase of apple tree life than during adult phases (Costes and Guédon, 2012).
Plant architecture affects leaf area distribution within the canopy, which further determines microclimate conditions and many processes, such as light interception, transpiration and carbon acquisition (Niinemets, 2007). Architectural analysis allows the investigation of tree plasticity, i.e. the extent to which the architecture can be modified by environmental factors (Barthélémy and Caraglio, 2007; Seleznyova et al., 2008). Moreover, the level of plasticity in organ production strongly depends on the studied species. Indeed, most single-stem species (maize, Fournier and Andrieu, 2000; sunflower, Dosio et al., 2003) have lower plasticity in organ production than other woody plants or herbaceous indeterminate species displaying high neoformation capacity (pea, Turc and Lecoeur, 1997; kiwi fruit, Seleznyova et al., 2002; grapevine, Davidson and Remphrey, 1994; Pallas and Christophe, 2015).
Numerous studies have shown, in a large range of species, that soil moisture greatly influences leaf and shoot extension rate (Belaygue et al., 1996; Palacio and Montserrat-Marti, 2005), individual leaf and shoot size (Gasque et al., 2016) or the leaf production rate by apical meristems of first- and second-order axes (Lebon et al., 2006). In the apple tree, water deficit has been shown to dramatically depress shoot extension (Irving and Drost, 1987; Ebel et al., 1995) and leaf area (Mills et al., 1996). In a range of 1-year-old apple genotypes the reduction in leaf area results from a combined reduction in leaf number and individual leaf area (Lauri et al., 2016). Under moderate stress, this reduction in plant morphogenesis was observed before any decrease in plant photosynthesis. This lack of connection between carbon supply and growth or morphogenesis was observed to be associated with a reduction in water flux to growing cells, with modifications in cell wall properties and hormonal signalling (Muller et al., 2011). In contrast, under severe water stress, when photosynthesis is severely affected, carbon starvation can also contribute to some extent to reduction in plant morphogenetic activity (Tardieu et al., 2011). Water stress also decreases the total number of growth units and accelerates tree ontogeny for the ‘Granny Smith’ cultivar grown under a Mediterranean climate (Yang et al., 2016). However, less attention has been paid to changes in shoot growth dynamics (metamer appearance rate and shoot growth duration), including the frequency of summer growth arrest. This latter phenomenon, also called ‘summer dormancy’, is a possible strategy to survive long-term environmental stresses in temperate perennials. In particular, summer drought has been shown to temporarily arrest growth in sub-shrubs and grasses under climates prone to summer water stress (Palacio and Montserrat-Marti, 2005; Volaire et al., 2009). The shoot summer arrest phenomenon has also been observed in apple trees (Foster et al., 2007; Lauri et al., 2016). Furthermore, the response to drought is cultivar-dependent and closely tied to the intensity and duration of stress as well as the developmental stage at which it occurs (Lopez et al., 2015; Lauri et al., 2016). Thus, shoot growth dynamics, including summer dormancy and its regulation at tree scale, are potential sources of tree plasticity for adaptive strategies to mitigate the impact of rapidly changing environments (Garris et al., 2009).
The present study investigated the effects of a summer water stress on young tree architecture in three cultivars observed at different scales of tree organization (organ, shoot and whole tree) in the Mediterranean region, where water restriction commonly occurs during late spring and summer and is likely to become more severe in the future (Beniston et al., 2007). We focused on metamer appearance dynamics of long shoots, final shoot and organ dimensions, and the demography of the different types of shoots within the trees, during two consecutive years. The responses of the three cultivars to water stress were compared in order to decipher the similarities and differences in their adaptive strategies. Stem water potential was measured during the experiment to quantify water stress intensity and to explore the coordination between morphogenetic and developmental processes under water stress.
MATERIALS AND METHODS
Field experiments, environmental conditions and tree management
In March 2015, 20 scions of ‘Ariane’, ‘Braeburn’ and ‘Fuji’ cultivars were grafted onto M9 rootstock and planted at the Sud-Expé experimental station (Marsillargues, 43.67°N 4.18°E) in the south of France. Trees were located in three blocks of 20 trees, with 2 × 4 m spacing and north–south orientation. Each block contained two sets of ten trees subjected to either well-watered (WW) or water-stressed (WS) conditions in summers 2016 and 2017. The final number of replicates was 9, 8 and 9 and 9, 10 and 8 for ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under WW and WS conditions, respectively, because some trees were either damaged or died during the experiment (Supplementary Data Fig. S1).
In 2016 and 2017, the experimental period lasted 5 months (from 10 May to 10 September). In 2016, the average daily mean temperature and vapour pressure deficit during the experimental period were 21.4 °C and 1.0 kPa, respectively. The total rainfall was limited and equal to 89.4 mm. In 2017, the climate was even drier, with similar average daily mean temperature (22.02 °C) and vapour pressure deficit (0.85 kPa) but lower rainfall (67.6 mm) (Supplementary Data Fig. S2).
In this experiment, all the fruits were removed at the end of May in 2015 and 2016. In 2017, fruits were thinned to maintain only one fruit per inflorescence. Trees were not pruned throughout the entire experiment to enable us to observe an unmodified architecture. Fertilizers were added to avoid any mineral deficiency.
Irrigation management and stem water potential measurements
Irrigation was applied using micro-sprayers located between the trees, with a flow rate of 27 L h−1. In 2015, trees were irrigated in order to avoid a soil water deficit, to ensure successful development during the first year after planting. The WW and WS treatments were applied according to two different irrigation schedules in the summers of 2016 and 2017 (Supplementary Data Fig. S1). In both years, two consecutive periods of increasing stress intensity were imposed. This irrigation management was used to impose progressive WS during summer and to try to reach a severe water deficit condition at the end of summer, as observed in the Mediterranean climate. Tensiometer sensors (Watermark®, Spectrum Technologies, Plainfield, IL, USA) located in the middle of every subplot were used to monitor soil water potential at 30- and 60-cm depths every 15 min throughout the experiment.
In 2015, all the trees were irrigated twice a week using an amount of water corresponding 1.93 mm d−1 from the beginning of May until harvest, ensuring well-watered conditions for all the trees in the field. In this year, soil water potential (SWP) remained to close to 0, confirming the absence of a soil water deficit. In 2016, irrigation was applied to WW trees twice a week from 1 May until 13 July at 1.93 mm d−1 and then three times a week (2.89 mm d−1) until the end of the experiment. In 2017, WW trees were irrigated three times per week (2.89 mm d−1) throughout the growing season from 7 May. During the first period of WS, from 1 and 7 May until 5 August and 24 July, in 2016 and 2017, respectively, irrigation was restricted on WS trees to half of the quantity provided to WW trees. The second period of WS began after these dates and lasted until the end of the experiments. During this period, irrigation of WS trees was stopped.
Midday water potentials were measured on one leaf per tree on four dates (13 July, 8 August, 24 August and 30 August) in 2016 and three dates (20 July, 8 August and 29 August) in 2017. These measurements were performed with a pressure chamber (Model 3005, Soilmoisture Equipment, Santa Barbara, USA) after placing leaves in an aluminium bag for around 4 h to stop transpiration (Goldhamer and Fereres, 2001).
Tree characteristics at the end of the growing season
Tree topology was observed for all the trees and coded in the form of multiscale tree graphs (Godin and Caraglio, 1998) at the end of the growth period in 2015, 2016 and 2017, using the same method as previously described by Costes et al. (2003). Each tree was decomposed into three levels of organization corresponding to the axis, growth units and metamers. Four growth unit types were defined: long (length ≥20 cm), medium (5 cm ≤ length < 20 cm), short (length <50 cm) and floral (or ‘bourse’). Since annual shoots (representing the part of each axis produced during one year) can be composed of more than one growth unit, they were also classified according to their length as described for growth units.
At the end of the growing season, the internode number, length, bottom diameter and top diameter were evaluated on long shoots and on the new part of the trunk produced in each year. Average diameters were calculated as the mean values of shoot bottom and top diameters. These measurements were not performed on short and medium shoots because most medium and short shoots stopped growing before WS application (average dates of growth arrest of medium shoots were 25 June and 10 June in 2016 and 2017, respectively). The basal diameter increment was computed as the difference in average diameter measured during two consecutive years.
Final areas of individual leaves were measured along nine long shoots per cultivar and per water treatment (three shoots per tree on three trees) at the end of September 2017. The number of measured leaves was 143 and 132 for ‘Ariane’ in WW and WS treatments, respectively, 83 and 57 for ‘Braeburn’ and 102 and 88 for ‘Fuji’. On these shoots, individual leaf areas were measured with a leaf area meter (LI 3100 Area Meter; LI-COR, Lincoln, NE, USA) on a third of the leaves. Average leaf areas were calculated and compared separately for leaves that appeared before or after the complete cessation of irrigation.
Shoot growth dynamics
In 2016, growth dynamics observations were performed on all the current-year long shoots (either in terminal or lateral positions), corresponding to 64, 117 and 74 shoots of WW trees and 51, 106 and 75 shoots of WS trees for ‘Ariane’, ‘Braeburn’ and ‘Fuji’, respectively. In 2017, the new current-year medium and long annual shoots that arose from terminal buds of the long laterals that were tagged in 2016 were observed. The number of long shoots observed in 2017 was 65, 97 and 76 shoots of WW trees and 64, 105 and 70 shoots of WS trees for ‘Ariane’, ‘Braeburn’ and ‘Fuji’, respectively. Shoot growth dynamics were monitored by placing plastic rings of different colours along the shoots, on the specific nodes bearing the newest fully expanded leaf every week in 2016 and every 2 weeks in 2017.
Metamer appearance dynamics
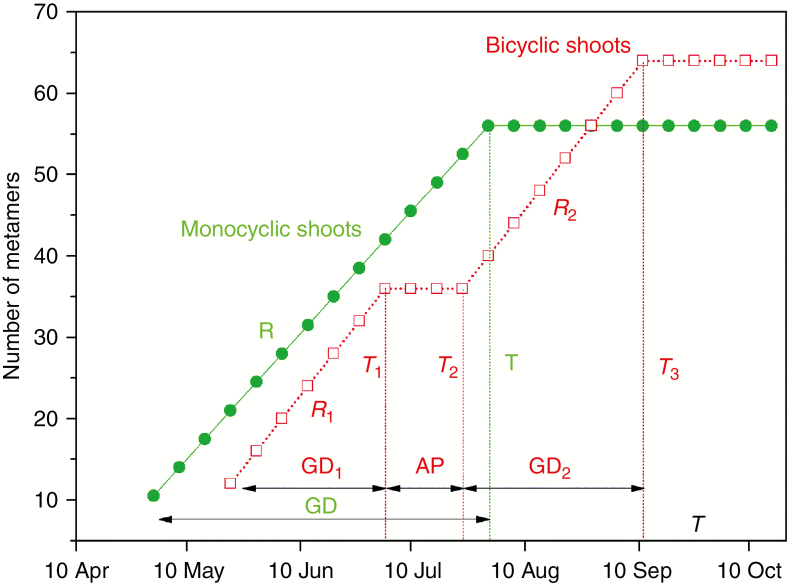
Analyses of metamer appearance dynamics were performed on long shoots only, because most of the medium shoots stopped growing before the onset of water stress. Long shoot growth dynamics were modelled using the package ‘segmented’ of R software (R Development Core Team, 2012), with piecewise linear functions with one and three break points (Fig. 1 and Appendix) to represent the absence or presence of growth arrest, respectively. The best adjustment (using one or three breakpoints) was determined based on the smallest Bayesian information criterion value (Kass and Raftery, 1995). Shoots with only one breakpoint at the end of the growing season were considered to be monocyclic shoots, whereas shoots with an intermediate growth arrest (three breakpoints) were considered to be bicyclic shoots. Monocyclic shoots were characterized by a metamer appearance rate (R) and a growth duration (GD), and bicyclic shoots by two metamer appearance rates (before and after the growth cessation, R1 and R2, respectively), two growth durations (before and after growth cessation, GD1 and GD2, respectively) and by the duration of the rest period (AP).
Fig. 1.
Schematic diagram of the growing pattern of monocyclic (green points, solid line) and bicyclic (red points, dotted line) shoots. Monocyclic shoots were characterized by a leaf appearance rate (R), a growth cessation date (T) and a growth duration (GD), and bicyclic shoots by two leaf appearance rates (before and after growth arrest, R1 and R2, respectively), two growth durations (before and after growth arrest, GD1 and GD2, respectively), an arrest date (T1), a growth resumption date (T2), a duration of the rest period (AP) and a growth cessation date (T3).
Statistical analyses
Fixed-effect models considering year and water treatment as well as their interactions were fitted against the observed variables. Depending on the variable, this analysis was performed considering each year separately or considering a year effect within the model. The effect of initial tree growth was considered in the analyses of the total shoot number in 2016 and 2017 by taking the total shoot number in 2015 as a covariate. Linear models and generalized linear models (Poisson family) were used for continuous and countable variables, respectively, and the significance of each effect was tested with an ANOVA procedure after checking the normality of residuals and equality of variance. Data were log-transformed in some cases to meet this requirement.
Post hoc multiple comparisons were carried out to determine significant differences among cultivars and between water treatments. Fisher’s LSD tests were performed when the raw data were normally distributed and had homogeneous variance. In other case, non-parametric tests (Fisher’s LSD test with ranks) were done. For the total shoot number in 2016 and 2017, post hoc analysis was performed taking the total shoot number in 2015 as a covariate. Significant differences between proportions of different types of shoots (short, medium and long, bicyclic versus monocyclic) were tested for each combination of cultivar and water treatment for each year separately. The analysis was followed by a post hoc test using the ‘chisq.post.hoc’ function of the ‘fifer’ package of R software.
RESULTS
Soil and stem water potentials
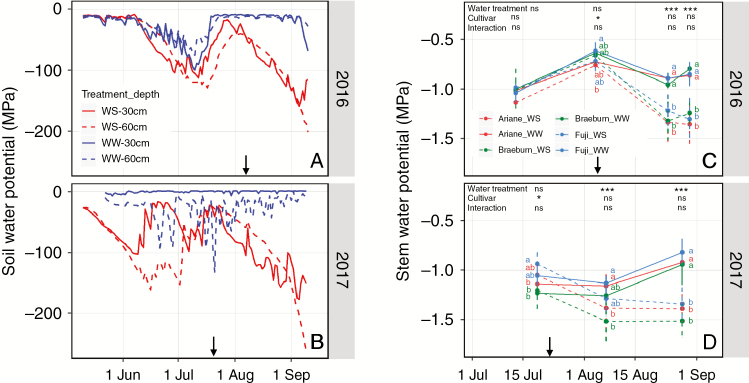
During the first period of WS, when the amount of water provided to WS trees was half that provided for WW trees, soil water potential (SWP) (Fig. 2A, B) was slightly lower in WS compared with WW conditions (minimum −0.09 and −0,12 MPa in WW and WS in 2016; −0.07 and −0.12 MPa in 2017, respectively; mean −0.03 and −0.05 MPa in WW and WS in 2016; −0.02 and −0.07 MPa in 2017, respectively). In 2016, a slight decrease in SWP was observed for WW trees from the end of June, probably due to an insufficient amount of irrigation during this period of high evaporative demand (Fig. 2A). There was a slow increase in SWP after this date, when the amount of water provided to trees was increased. This change in irrigation schedule also impacted stem water potential (Fig. 2C, D), which values were relatively lower at the end of June (around −1.0MPa), comparatively to mid-July (around −0.7MPa). In 2017, during this first period, SWP was stable on WW trees expect some short decreases between irrigation days (mean −0.006 and −0.03 MPa at 30 and 60 cm depth, respectively), whereas much lower values were observed for WS (mean −0.05 and −0.08 MPa at 30 and 60 cm depth, respectively). At the end of this first period, no significant impact of water treatment was observed on stem water potential whatever the year (Fig. 2C, D). In the second WS period, after the complete cessation of irrigation, SWP regularly decreased down to values close to −0.15 MPa at 30 cm depth and close to or lower than −0.20 MPa at 60 cm depth in both years. This strong decrease in SWP led to a significant decrease in stem water potential for WS compared with WW trees. At the end of this period, stem water potential in 2016 was −0.92 and −1.42 MPa for WW and WS trees, respectively, and −0.81 and −1.32 MPa in 2017. A slight cultivar effect was observed on stem water potential just before the complete cessation of irrigation, with a lower stem water potential for ‘Ariane’ in 2016 and for ‘Braeburn’ in 2017 (Fig. 2C, D).
Fig. 2.
Changes in soil water potential at 30 and 60 cm depths (A, B) for WW and WS trees and stem water potential (C, D) for apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under WS and WW conditions in 2016 and 2017. Measurements of stem water potential were performed on four dates in 2016 (13 July, 8 August, 24 August, 30 August) and three dates in 2017 (20 July, 8 August, 29 August). For each date, statistical analyses were performed using a linear model with water regime, cultivar effects and their interaction. ANOVA was used to assess the significance of each effect (ns, not significant, * 0.01 < P < 0.05, *** P < 0.001). For each cultivar–water treatment combination, different letters indicate significant differences (P < 0.05) in post hoc comparisons. Italic letters refer to the cultivar under water-stressed conditions. Bars represent the standard deviation. Arrows represent the date separating the two irrigation periods; in the first period WS trees were provided with a water quantity half of that provided for WW trees and in the second period irrigation was completely stopped for WS trees in both years.
Total number of shoots and within-tree shoot demography
The mean total number of shoots produced per tree during the first year after planting (2015) showed contrasting values depending on the cultivar, ‘Braeburn’ developing a higher number of shoots (more than ten) than the two other cultivars, which displayed a very low shoot number (one to three; Table 1). Although all the trees were grown under the same irrigation regime in 2015, the number of different shoot types showed some variability among trees subjected to either WW or WS conditions in the following years. At the end of 2015 and for ‘Ariane’ and ‘Braeburn’, the number of shoots was lower for the trees that were subjected to WS in 2016 and 2017 compared with those subjected to WW conditions, even though the total number of lateral shoots remained quite low in both conditions (Table 1). Therefore, the initial growth in 2015 was considered as a covariate between trees subjected to WW and WS conditions for subsequent analyses of the total shoot number per tree (Supplementary Data Figure S3).
Table 1.
Mean total number of shoots developed per tree in 2015 for apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ subjected to WW or WS conditions in 2016 and 2017. Statistical analyses were performed using a generalized linear model (Poisson family) with WS, cultivar effect and their interaction. ANOVA was used to assess the significance of each effect (ns, not significant, *0.01 < P < 0.05, ** 0.001 < P < 0.01, *** P < 0.001). For each shoot type, different letters indicate significant differences (P < 0.05) in post hoc comparisons
| Year | Cultivar | Plot | Number of shoots per tree | |||
|---|---|---|---|---|---|---|
| Short | Medium | Long | Total | |||
| 2015 | ‘Ariane’ | WW | 0.89b | 0.00b | 1.00c | 1.89b |
| WS | 0.10c | 0.00b | 1.00c | 1.10c | ||
| ‘Braeburn’ | WW | 10.00a | 1.11a | 4.44a | 15.56a | |
| WS | 6.33a | 1.00a | 3.11b | 10.44a | ||
| ‘Fuji’ | WW | 0.40bc | 0.20b | 1.10c | 1.70bc | |
| WS | 0.88bc | 0.63b | 1.38c | 2.88bc | ||
| Treatment effect | * | ns | ns | * | ||
| Cultivar effect | *** | *** | *** | *** | ||
| Interaction | ** | ns | ns | * |
As expected, the total shoot number per tree increased as trees got older (Table 2). Significant year and initial shoot number (2015) effects were observed on the total shoot number per tree. In the second and third years of growth, ‘Braeburn’ still displayed a higher number of shoots than ‘Ariane’ and ‘Fuji’ (Table 2). The ranking of ‘Fuji’ and ‘Braeburn’ for the total number of shoots per tree changed depending on the year; in 2016 ‘Fuji’ displayed a significantly lower number of shoots than ‘Ariane’, whereas the reverse was observed in 2017 (around 150 and 75 for ‘Fuji’ and ‘Ariane’, respectively). An ontogeny effect was observed through an increase in the proportion of short shoots and a decrease in the proportions of long and medium shoots in 2017 compared with 2016 (Table 3) for ‘Braeburn’ and ‘Fuji’. Nevertheless, some differences among cultivars were observed: in 2016 ‘Ariane’ and ‘Braeburn’ displayed a significantly lower proportion of long shoots than ‘Fuji’, whereas in 2017 ‘Ariane’ displayed the highest proportion of long shoots and proportionally fewer short shoots than other cultivars.
Table 2.
Mean total shoot number per tree of apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under WW and WS conditions at the end of growing season in 2016 and 2017. Statistical analyses were performed using two generalized linear models (Poisson family), the first taking into account the initial number of shoots per tree, water treatment and cultivar effects and the interaction between water treatment and cultivar (lower part of the table), and the second taking into account year, initial shoot, water treatment and cultivar effects (right side of the table). ANOVA was then used to assess the significance of each effect (ns, not significant, * 0.01 < P < 0.05, ** 0.001 < P < 0.01, *** P < 0.001). For each year, different letters indicate significant differences (P < 0.05) in post hoc comparisons, taking into account shoot number in 2015 as a covariate
| Cultivar | Water treatment | Total shoot number | Year effect | Initial shoot number effect | Water treatment effect | Cultivar effect | |
|---|---|---|---|---|---|---|---|
| 2016 | 2017 | ||||||
| ‘Ariane’ | WW | 36.00b | 76.33d | ||||
| WS | 38.00b | 73.33d | |||||
| ‘Braeburn’ | WW | 91.88a | 429.67a | *** | *** | *** | *** |
| WS | 66.00a | 231.67b | |||||
| ‘Fuji’ | WW | 24.22c | 157.33c | ||||
| WS | 25.75c | 153.33c | |||||
| Initial shoot number effect | * | ** | |||||
| Water treatment effect | ns | *** | |||||
| Cultivar effect | *** | *** | |||||
| Interaction(water treatment and cultivar) | ns | *** |
Table 3.
Proportions of different types of shoots (short, medium and long) of apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under WW and WS conditions in 2016 and 2017. Significant differences between proportions of different types of shoots were tested for each cultivar–water treatment combination, considering each year separately, using the χ2 test (***P < 0.001). For each year, this analysis was followed by a post hoc test (P < 0.05) for pairwise comparisons, and significant differences between each shoot type–cultivar combination are represented by different letters
| Year | Cultivar | Water treatment | Shoot proportion | |||
|---|---|---|---|---|---|---|
| Short | Medium | Long | ||||
| 2016 | ‘Ariane’ | WW | 0.71 | 0.06 | 0.23 | bc |
| WS | 0.79 | 0.03 | 0.18 | c | ||
| ‘Braeburn’ | WW | 0.71 | 0.07 | 0.23 | b | |
| WS | 0.75 | 0.05 | 0.20 | bc | ||
| ‘Fuji’ | WW | 0.50 | 0.05 | 0.45 | a | |
| WS | 0.55 | 0.04 | 0.41 | a | ||
| χ 2 test | *** | |||||
| 2017 | ‘Ariane’ | WW | 0.58 | 0.20 | 0.22 | ab |
| WS | 0.67 | 0.13 | 0.20 | a | ||
| ‘Braeburn’ | WW | 0.91 | 0.03 | 0.06 | d | |
| WS | 0.90 | 0.04 | 0.06 | d | ||
| ‘Fuji’ | WW | 0.71 | 0.13 | 0.16 | c | |
| WS | 0.77 | 0.09 | 0.14 | b | ||
| χ 2 test | *** |
The WS treatment decreased the total shoot number per tree only in 2017 in a three-way ANOVA with initial shoot number (2015), water treatment and cultivar effects. This significant difference was found for ‘Braeburn’ only when WW and WS trees were compared for each cultivar separately (Table 2). Trees also tended to have a higher proportion of short shoots and a relatively lower proportion of long and medium shoots under WS than WW conditions in both 2016 and 2017 (Table 3). Nevertheless, this effect was of low intensity and was significant for ‘Fuji’ in 2017 only.
Annual shoot polycyclism
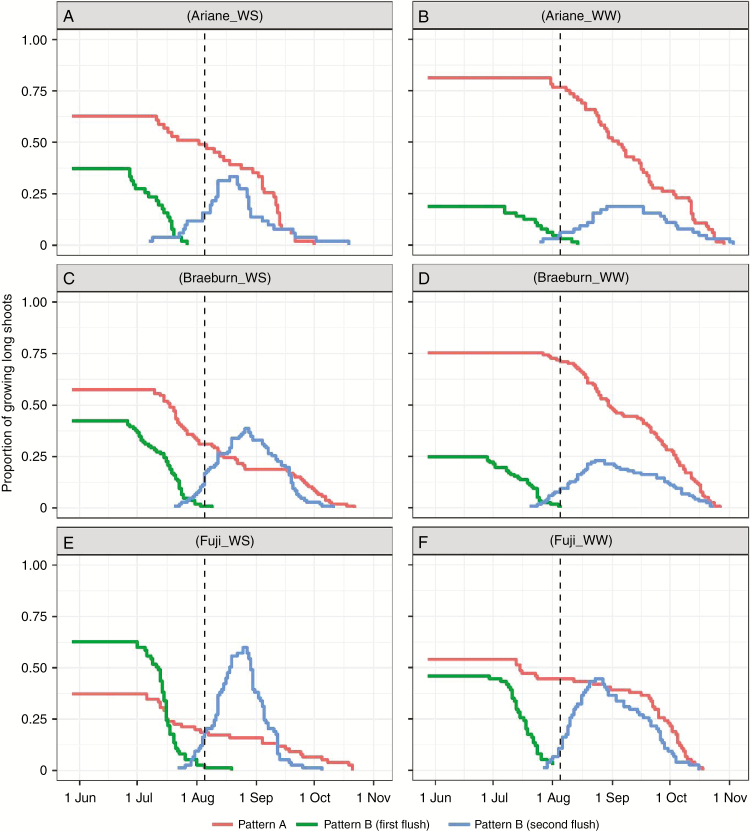
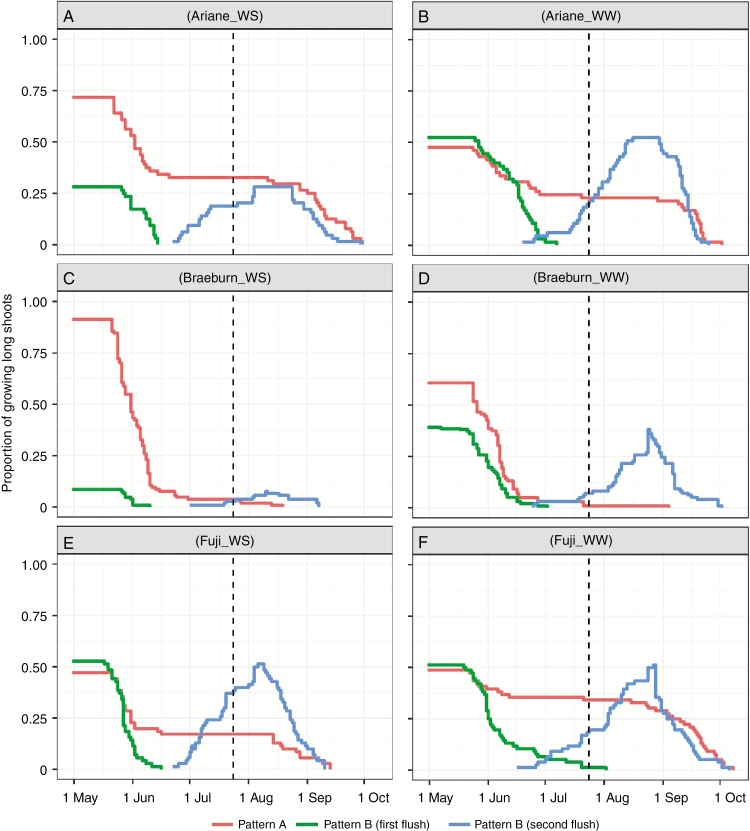
Annual shoots of long laterals were classified as either monocyclic or bicyclic depending on the presence of a summer arrest. The monocyclic shoots could also be divided into two sub-categories (Figs 3 and 4): type-A monocyclic shoots kept growing until the final cessation date of the second flush in bicyclic shoots; type-B monocyclic shoots stopped growing earlier, at a date close to the arrest date of the first flush in bicyclic shoots. Due to tree ontogeny, the proportion of type-A monocyclic shoots decreased with tree age for all cultivars and under both water treatment conditions. Concomitantly, under WW conditions the proportion of bicyclic shoots increased between the two years (‘Ariane’, +12 %, Figs 3B and 4B; ‘Braeburn’, +14 %, Figs 3D and 4D; ‘Fuji’, +5 %, Figs 3F and 4F). ‘Ariane’ and ‘Braeburn’ had a higher proportion of type-A monocyclic shoots in 2016 (about 75 % of shoots under WW conditions) and exhibited a strong decrease in 2017. In contrast, ‘Fuji’ was prone to polycyclism but with a quite constant proportion of type-A monocyclic shoots between the two years (~50 %).
Fig. 3.
Changes in the proportions of growing monocyclic and bicyclic shoots in 2016 during the annual cycle for apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under WW and WS conditions. Proportions were computed as the ratio of the number of growing shoots in each category to the total number of long shoots. The dotted line represents the date separating the two irrigation periods; in the first period WS trees were provided with half the water quantity provided to WW trees and in the second irrigation was completely stopped for WS trees in both years.
Fig. 4.
Changes in the proportions of growing monocyclic and bicyclic shoots in 2017 during the annual cycle for apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under WW and WS conditions. Proportions were computed as the ratio of the number of growing shoots in each category to the total number of long shoots. The dotted line represents the date separating the two irrigation periods; in the first period WS trees were provided with half the water quantity provided to WW trees and in the second irrigation was completely stopped for WS trees in both years
The WS treatment decreased the proportion of type-A monocyclic shoots in both years (Figs 3 and 4B, D, F), Concurrently, WS increased the proportion of bicyclic shoots significantly in the three cultivars in 2016 (+37, +42 and +63 % for ‘Ariane’, ‘Braeburn’ and ‘Fuji’, respectively). Conversely, in 2017 WS did not affect the proportion of polycyclic shoots for ‘Fuji’ (51.3 and 52.9 % under WW and WS conditions, respectively), whereas it decreased this proportion for ‘Ariane’ (52.3 and 28.1 % under WW and WS conditions) and ‘Braeburn’ (39.2 and 8.6 % under WW and WS conditions).
Shoot growth dynamics
Shoot growth dynamics were characterized by the rate of metamer emergence, the growth duration of growth units, considering both monocyclic and bicyclic shoots, and the duration of the rest period (AP parameter) between the two growth units in bicyclic shoots (Table 4). In all cultivars, the monocyclic shoots corresponded to a total growth duration of 120 d in 2016 for WW trees; this duration was strongly reduced in 2017 (Table 4). In 2016, the total growth duration of bicyclic shoots was similar to that of type-A monocyclic shoots, but was separated into two periods of 65 and 53 d in ‘Ariane’, 68 and 46 d in ‘Braeburn’ and 63 and 39 d in ‘Fuji’ for WW trees. In 2017 these periods lasted 53 and 47 d in ‘Ariane’, 54 and 29 d in ‘Braeburn’ and 55 and 39 d in ‘Fuji’. A cultivar effect was observed in 2016 on the growth duration of the second growth unit only and in 2017 on all growth units (type-A monocyclic, first and second growth units of bicyclic shoots).
Table 4.
Growth dynamic parameters of monocyclic and bicyclic long shoots of apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under WW and WS conditions. Statistical analyses were performed using a linear model considering water treatment and cultivars and their interaction for each year separately. ANOVA was then used to assess the significance of each effect (ns, not significant, * 0.01 < P < 0.05, ** 0.001 < P < 0.01, *** P < 0.001). For each year and each parameter, different letters indicate significant differences (P < 0.05) in post hoc comparisons
| Year | Cultivar | Water treatment | Monocyclic shoots | Bicyclic shoots | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | GD1 | N metamers † | R 1 | GD1† | AP | R 2 † | GD2† | N metamers | |||
| (No. d−1) | (d) | – | (No. d−1) | (d) | (d) | (No. d−1) | (d) | – | |||
| 2016 | ‘Ariane’ | WW | 0.42d | 113.50d | 50.14a | 0.42c | 64.58b | 19.58a | 0.46b | 52.91d | 46.65a |
| WS | 0.39c | 99.56bc | 36.31bcd | 0.42c | 49.67a | 24.28ac | 0.57c | 28.70bc | 36.44bc | ||
| ‘Braeburn’ | WW | 0.34ab | 120.26d | 38.26bc | 0.31a | 68.02b | 24.56ac | 0.35a | 46.038cd | 37.00b | |
| WS | 0.33a | 98.48b | 31.73d | 0.32a | 66.15b | 27.08c | 0.45b | 31.65b | 33.89c | ||
| ‘Fuji’ | WW | 0.36b | 119.56d | 40.94b | 0.36b | 62.91b | 23.44ab | 0.47b | 38.83c | 38.35b | |
| WS | 0.35ab | 99.18bc | 34.03cd | 0.35b | 62.20b | 25.57bc | 0.53c | 24.90a | 34.23bc | ||
| Water treatment effect | ** | *** | *** | ns | ns | * | *** | *** | *** | ||
| Cultivar effect | *** | ns | *** | *** | ** | ns | *** | ** | *** | ||
| Interaction | ns | ns | ns | ns | ns | ns | ns | ns | ns | ||
| 2017 | ‘Ariane’ | WW | 0.38ab | 104.28ab | 34.58a | 0.41a | 53.21ab | 41.49cd | 0.38a | 46.95a | 39.06a |
| WS | 0.34b | 105.54ab | 32.09a | 0.40ab | 59.7a | 37.22d | 0.34ab | 52.79a | 40.72a | ||
| ‘Braeburn’ | WW | 0.35ab | 55.93c | 18.32b | 0.41ab | 54.43ab | 66.27a | 0.40a | 28.53b | 29.42b | |
| WS | 0.38ab | 51.23c | 17.95b | 0.40ab | 44.22b | 63.43ab | 0.34ab | 22.93b | 23.89c | ||
| ‘Fuji’ | WW | 0.26c | 136.66a | 31.57a | 0.37b | 54.72ab | 51.19b | 0.32b | 38.61c | 29.85b | |
| WS | 0.28c | 89.4b | 21.27b | 0.38ab | 50.27b | 49.32bc | 0.34ab | 37.46c | 28.55bc | ||
| Water treatment effect | ns | ** | ** | ns | ns | ns | ns | ns | * | ||
| Cultivar effect | *** | *** | *** | ns | ns | *** | * | *** | *** | ||
| Interaction | * | * | ** | ns | ns | ns | ns | ns | ns |
R, GD and Nmetamers for monocyclic shoots are the metamer appearance rate, growth duration and final number of metamers per shoot, respectively.
R 1, GD1, AP, R2, GD2 and Nmetamers for bicyclic shoots are the metamer appearance rate in the first flush, growth duration in the first flush, arrest period, metamer appearance rate in the second flush, and growth duration in the second flush and the final number of metamers, respectively.
†Log-transformed variables were used for linear models.
The WS treatment affected the growth duration more than the rate of metamer appearance in both monocyclic and bicyclic shoots. The mean growth duration of monocyclic shoots was significantly reduced by around 20 d over the growing season in the three cultivars in 2016 and by >40 d, but for ‘Fuji’ only, in 2017 (Table 4). This decrease could reflect (1) a decrease in the growth duration of one category of monocyclic shoots only, either type-A or type-B, or of the two categories, or (2) an increase in the proportion of type-B monocyclic shoots. For bicyclic shoots, the effect of WS on growth duration was more pronounced in 2016 than in 2017 on the one hand, and on the second than on the first growth unit on the other hand (Table 4). In 2016, the growth duration of the first growth unit was reduced in ‘Ariane’ only, whereas the second growth unit was reduced in all cultivars. In this year, WS also increased the duration of the rest period (AP parameter) between the two growth units. In 2017, even though a general decrease in the growth duration and an increase in the rest period were observed, these differences were no longer significant. The rate of metamer appearance was significantly decreased by WS only, in monocyclic shoots in 2016 (Table 4). Surprisingly, WS significantly increased the rate of metamer appearance during the second flush of bicyclic shoots in 2016 (Table 4).
Final characteristics of annual shoots along trunks and long laterals
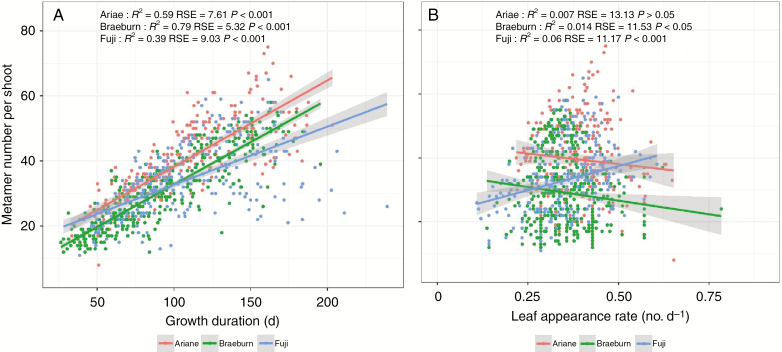
At annual shoot scale, tree ontogeny resulted in smaller values of metamer number, shoot length and diameter in 2017 compared with 2016, for shoots either along the trunks or in lateral positions (Table 5). Significant cultivar effects were observed for all the variables in both years. ‘Ariane’ had a higher number of metamers, longer annual shoot length with larger diameter on the trunk and longer lateral shoots than the other cultivars (Table 5). ‘Ariane’ also had significantly larger leaves (31.13 and 26.31 cm2 in early and late stages, respectively) than ‘Braeburn’ (20.96 and 16.31 cm2) and ‘Fuji’ (23.13 and 22.65 cm2) (Table 6). Whatever the cultivar, the final metamer number per shoot appeared correlated to shoot growth duration, whereas it was not associated with any variation in metamer appearance rate (Fig. 5). Furthermore, final shoot length appeared to be more associated with variations in metamer number (R2 = 0.58–0.88) than in individual internode length (estimated as the ratio of shoot length to metamer number; R2 = 0.06–0.25) (Supplementary Data Figure S4).
Table 5.
Characteristics of annual shoots of the trunk and annual lateral long shoots at the end of the growing season for apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under WW and WS conditions. Statistical analyses were performed using linear models considering water treatment and cultivars and their interaction for each year separately. ANOVA was then used to assess the significance of each effect (ns, not significant, * 0.01 < P < 0.05, ** 0.001 < P < 0.01, *** P < 0.001). For each year, different letters indicate significant differences (P < 0.05) in post hoc comparisons
| Year | Cultivar | Water treatment | Annual shoots of the trunk | Annual lateral long shoots | ||||
|---|---|---|---|---|---|---|---|---|
| Metamer number | Length | Average diameter | Metamer number | Length | Average diameter | |||
| (cm) | (mm) | (cm) | (mm) | |||||
| 2016 | ‘Ariane’ | WW | 66.22a | 92.22a | 11.59a | 46.83a | 69.68a | 8.48a |
| WS | 50.44b | 68.67b | 9.77b | 34.81cd | 51.30cd | 7.69b | ||
| ‘Braeburn’ | WW | 49.88c | 62.13bcd | 9.31bc | 37.51bc | 49.04de | 7.25b | |
| WS | 40.00bc | 55.44cd | 9.23bc | 32.53d | 45.98e | 7.20b | ||
| ‘Fuji’ | WW | 46.56bc | 69.56bcd | 8.34c | 38.89b | 63.19b | 7.59b | |
| WS | 38.38bc | 61.38d | 8.35c | 33.52d | 56.95c | 7.12b | ||
| Water treatment effect | *** | ns | ns | *** | *** | * | ||
| Cultivar effect | *** | *** | *** | *** | *** | *** | ||
| Interaction | ns | ns | ns | ** | ** | ns | ||
| 2017 | ‘Ariane’ | WW | 49.00a | 64.00a | 8.57a | 32.34a | 46.20a | 6.46a |
| WS | 41.00a | 52.67ab | 7.67ab | 29.43a | 43.84a | 6.27a | ||
| ‘Braeburn’ | WW | 26.33b | 43.00b | 6.91ab | 23.77b | 33.45bc | 5.51b | |
| WS | 22.33b | 40.00b | 6.00b | 18.74c | 28.77c | 4.75bc | ||
| ‘Fuji’ | WW | 25.67b | 36.33b | 6.01b | 24.34b | 37.11b | 5.27b | |
| WS | 24.33b | 38.33b | 5.89b | 18.34c | 31.39bc | 4.83bc | ||
| Water treatment effect | ns | ns | ns | *** | * | * | ||
| Cultivar effect | *** | * | * | *** | *** | *** | ||
| Interaction | ns | ns | ns | ns | ns | ns |
Table 6.
Average final area of individual leaves on lateral long shoots in different growth stages in 2017 for apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under WW and WS conditions. Statistical analyses were performed using two linear models first considering water treatment and cultivars and their interaction for each growing stage (lower part of the table) and then considering the stage effect for each cultivar–water treatment combination (right side of the table). ANOVA was then used to assess the significance of each effect (ns, not significant, * 0.01 < P < 0.05, *** P < 0.001). For each year, different letters indicate significant differences (P < 0.05) in post hoc comparisons
| Year | Cultivar | Water treatment | Average individual leaf area (cm2) | Stage effect | |
|---|---|---|---|---|---|
| Early stage: before irrigation stop | Late stage: after irrigation stop | ||||
| 2017 | ‘Ariane’ | WW | 32.16a | 31.41a | ns |
| WS | 31.13a | 26.31b | *** | ||
| ‘Braeburn’ | WW | 22.68b | 20.68cd | ns | |
| WS | 20.96b | 16.31d | * | ||
| ‘Fuji’ | WW | 23.94b | 23.04c | ns | |
| WS | 23.13b | 22.65cd | ns | ||
| Water treatment effect | ns | *** | |||
| Cultivar effect | *** | *** | |||
| Interaction | ns | ns |
Fig. 5.
Relationships between final metamer number, growth duration and leaf appearance rate of long shoots for apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’. Data for monocyclic and bicyclic shoots in both years were plotted together. Lines represent the correlation line for each cultivar. Coefficients of determination (R2) and their associated significances are shown for each cultivar. The number of shoots was 243, 418 and 292 for ‘Ariane’, ‘Braeburn’ and ‘Fuji’, respectively. RSE are the residual standard error.
Under WS, the annual shoot growth dynamics led to a significant decrease in the final metamer number (Table 4). In monocyclic shoots, the final number of metamers was reduced in 2016 for the three cultivars, and in 2017 for ‘Fuji’ only. For bicyclic shoots, the reduction in metamer number under WS was significant in 2016 in ‘Ariane’ and ‘Braeburn’ only, whereas in 2017 a significant decrease was observed for ‘Braeburn’ only (Table 4).
A WS effect was also observed when annual shoots were distinguished depending on their position, i.e. either terminal or laterals, with a stronger impact on lateral than on terminal ones (Table 5). The annual shoots of trunks were affected by WS in ‘Ariane’ only, whereas none of the variables were affected by WS in the two other cultivars in either year (Table 5); metamer number was reduced from 66.22 to 50.44, length from 92.22 to 68.67 cm and average diameter from 11.59 to 9.77 mm. However, these reductions were of a lesser extent in 2017 and not significant. The long lateral shoots were more affected by WS than trunks since significantly lower numbers of metamers per annual shoot were observed under WS than under WW conditions for all cultivars in the two years, except for ‘Ariane’ in 2017. The WS treatment also affected annual shoot length and diameter, though the effect was of lesser extent and not significant in 2017 for all the cultivars. Individual internode length was not affected by WS, except for a slight increase that was probably artefactual in ‘Braeburn’ (‘Ariane’, 1.47 and 1.49 cm under WW and WS, respectively; ‘Fuji’, 1.61 and 1.69 cm; ‘Braeburn’, 1.38 and 1.52 cm, P < 0.05). After the complete cessation of irrigation, a lower individual leaf area under WS compared with WW conditions was found for ‘Ariane’ (−16 %) and ‘Braeburn’ (−21 %). This decrease was not significant for ‘Fuji’ (−2 %) (Table 6).
DISCUSSION
Apple trees, like many other fruit trees, are characterized by shoot polymorphisms (Crabbé, 1987), such that shoots can be distinguished based on polycyclism and growth characteristics. Seleznyova et al. (2008) have proposed that annual shoots with uninterrupted extension can be distinguished from polycyclic, monocyclic extending and non-extending shoots. Such a classification corresponds to the shoot types we identified, with annual uninterrupted shoots corresponding to type-A monocyclic shoots (Figs 3 and 4), monocyclic extended shoots corresponding to type-B monocyclic shoots, polycyclic corresponding to bicyclic shoots and non-extending shoots corresponding to short shoots. Observation of these different shoot types over a period of years provided us with a framework to decipher the respective effects of ontogeny, cultivar and water treatments. An ontogenetic gradient was clearly observed in our study, as revealed by the increasing proportion of short shoots and the lower proportion of uninterrupted extending shoots between 2016 and 2017 (Table 3, Figs 3 and 4).
In this study, WS was clearly shown to interact with tree ontogeny since WS accelerated tree ontogeny, reduced neoformation by promoting earlier growth cessation and induced more frequent and longer summer arrests, leading to a reduced number of metamers per shoot. In contrast, the impact on organ dimensions, i.e. average diameter of long shoots (Table 3) and final individual leaf area of leaves that expanded after WS application (Table 6), was quite limited. This is consistent with previous studies, which have shown in plants with high neoformation capacity that the organ production process is the most reduced process under WS (Seleznyova et al., 2002; Pallas and Christophe, 2015).
Intensity and duration of WS
Water stress can be imposed in various ways, for instance by providing less water without a difference in irrigation time (Negrón et al., 2013) or using different irrigation frequencies (Silva et al., 2012). In both examples, the total amount of water supplied in WS was about 60 % of that in the WW treatment and irrigation remained the same throughout the growth period. In our study the irrigation schedule was managed in two phases. First, the amount of water supplied to control trees was half of that provided to WS trees until the end of July or early August, by reducing the irrigation duration without changing the irrigation frequency. Second, irrigation was completely stopped after these dates in order to mimic the increasing WS during summer that is observed under the Mediterranean climate. This WS management led to SWP values close to −0.2 MPa, similar to those observed in previous studies (Virlet et al., 2014; Lauri et al., 2016).
During the first period, lasting until end of July, reducing the amount of water provided to WS trees did not affect stem water potential despite a slight decrease being observed in SWP, which indicated a low intensity of WS perceived by the trees. Conversely, during the period lasting until the end of the growing season, stem water potential values decreased for WS plants to about −1.3 MPa. This value was similar to the range observed in almond trees under medium water treatment (−1.2 to −1.4 MPa) and slightly higher than the range with severe stress treatment (−1.5 to −1.8 MPa; Naor, 2010). Also, these values were higher than the range of water deficit experienced in peach trees (−1.4 to −1.8 MPa; Davidson et al., 2017). This led us to consider that a WS of medium intensity was experienced by the trees in our study.
Water stress reduces neoformation and promotes summer arrests
Water stress reduced neoformation, leading to a lower proportion of annual uninterrupted shoots and a higher proportion of short shoots in both years of the experiment (Figs 3 and 4, Tables 3 and 5). Growth cessation mainly occurred in July, under a mild water intensity that did not modify stem water potential (Fig. 2). Such a very rapid response could be mediated by hormone regulation, especially by ABA originating in roots (Sobeih et al., 2004), as it has been observed under WS of very low intensity without any direct impact of the carbon supply at the plant scale (Muller et al., 2011).
In 2016, the increase in the proportion of bicyclic shoots under the WS condition can be interpreted as a strategy of stress avoidance by inducing summer dormancy. Indeed, in this year neoformation was potentially still very active, whereas WS was set up and increasing. This can be interpreted as a strategy of perennials to survive long-term environmental stresses, e.g. high temperature and drought, by inducing a dormant stage during summer (Ofir and Kigel, 2007; Volaire et al., 2009; Li et al., 2012). In our case, the stress avoidance strategy resulted in summer arrest rather than in leaf senescence or dehydration, as possibly observed in some perennial cereals (Volaire and Norton, 2006). This summer dormancy could be compared with eco-dormancy since it is a temporary arrest during the growing season, probably mediated by environmental factors without internal control (Lang et al., 1987).
In 2017, due to ontogeny, the proportion of shoots that ceased growing in July increased, leading to monocyclic shoots with reduced growth duration. Moreover, fewer shoots were allowed to resume growth after the summer arrest than in 2016. Therefore, in 2017 WS prolonged summer arrest duration in bicyclic shoots and promoted earlier final growth cessation in all shoots. The present study reveals intertwined effects of tree ontogeny and WS, both relying on fine tuning of growth cessations and resumptions within the annual growth cycle of each shoot. This indicates that more attention should be paid to mechanisms leading to organogenesis cessation.
Water stress impacts shoot growth dynamics
The growth dynamics of long shoots was determined by intermediate variables: the metamer appearance rate and the durations of rest and growth. The analysis of the responses of these variables to soil water deficit showed a large difference in sensitivity, with a stronger impact on growth duration than on metamer appearance rate. As previously commented, this revealed a lower neoformation capacity under WS.
Here, metamer appearance rate was estimated as the slope of the regression between metamer number and date (Fig. 1), as in other plants, e.g. kiwifruit (Morgan et al., 1985), quinoa (Bertero et al., 2000), wheat (Gutiérrez-Boem and Thomas, 1998) and groundnut (Leong and Ong, 1983). In contrast to some annual plants for which leaf emergence rate has been shown to vary with time depending on temperature (Turc and Lecoeur, 1997), the leaf emergence rate when expressed in leaves per day is often considered to be constant in trees. Indeed, a relatively steady leaf appearance rate unrelated to changes in temperature, radiation and photoperiod has been found in the peach tree (Davidson et al., 2015).
For long monocyclic shoots, a decrease in metamer appearance rate was found mainly for ‘Ariane’ (Table 4). In contrast, the metamer appearance rate of bicyclic shoots displayed quite complex changes depending on soil water availability and year that could be related to the summer arrest duration. In 2016, a higher rate was observed under WS after the summer dormancy period for all three cultivars (Table 4). This higher rate was associated with the longer arrest period in WS trees than WW ones. We hypothesize that the number of preformed leaves produced in the terminal buds is proportional to the summer arrest duration, thus leading to a higher rate after growth resumption in WS. This hypothesis is consistent with the identical arrest period and the absence of any effect of WS on the growth rate during the second growth unit in 2017.
Water stress has a differential effect on shoots depending on their within-tree position
The effect of WS may depend on tree age, shoot type and soil moisture level since it has been reported that metamer number is unaffected by WS in first-order shoots of 1-year-old peach trees (Hipps et al., 1995) and in epicormic shoots of almond trees (Negrón et al., 2013). Besides, for grapevine, first-order shoots were not affected by moderate WS, whereas the number of metamers on sylleptic branches was reduced, while under water stress both types of shoots were affected (Lebon et al., 2006; Pallas and Christophe, 2015). In our study, less sensitivity of neoformation to WS was observed on the trunk than on lateral axes (Table 5), confirming different sensitivity dependent on shoot type. This could result from higher hydraulic resistance to water flux in branches than in the main stem, as suggested by Wilson (2000). Another, complementary explanation is provided by studies showing that the increase in ABA/cytokinines ratio under WS conditions may increase apical dominance, thus inhibiting the growth of lateral axes (Stoll et al., 2000).
Cultivar responses to WS: common and specific strategies
Although there was a general trend due to ontogeny that was similar for the three genotypes, some specificity could be observed in the within-tree shoot demography. Indeed, ‘Ariane’ maintained a relative high proportion of medium and long shoots and ‘Fuji’ maintained almost the same proportions of the different types of long shoots (monocyclic versus bicyclic) between 2016 and 2017. This could suggest a different progression in ontogeny depending on the cultivar, with more rapid ontogeny in ‘Braeburn’ compared with the two other cultivars.
Interestingly, even though all genotypes exhibited a reduction in long-shoot neoformation under WS conditions, the total number of shoots per tree, but not their proportion, was affected in ‘Braeburn’. This number was not affected in ‘Fuji’ and ‘Ariane’, whereas different proportions of short, medium and long shoots were observed under WW and WS conditions. This suggests that, for a given number of metamers of a parent shoot, the number of buds remaining latent and the branching pattern could be modified in a manner specific to the cultivar. Further analyses of the branching patterns (Renton et al., 2006) in the three cultivars in WS and WW conditions are needed to deepen our comprehension of the relationships between neoformation reduction and effects on total shoot number at the tree scale.
Finally, in this study, despite common responses, the three cultivars had different strategies of reaction to WS depending on their genetic growth potential. ‘Braeburn’ had many shoots (mainly short shoots), so it responded to WS partly by reducing shoot number, and also partly via changing neoformation of long shoots. However, ‘Ariane’ and ‘Fuji’ developed many fewer laterals but with a higher proportion of long shoots. They thus reacted to WS mainly by reducing neoformation. Although we have not shown this directly, we assume that the different morphological responses depending on the architectural type of each cultivar have the same result of limiting the transpiring leaf area in conditions of water scarcity. Our study thus showed a common reaction through the enhancement of tree ontogeny, the reduction of neoformation and the promotion of summer arrest, as well as the existence of responses specific to each cultivar. We argue that the balance between common and specific responses should be taken into account in seeking full comprehension of cultivar adaptation of any species to climatic constraints.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: field layout of the experiment. Figure S2: changes in daily rainfall, temperature and vapour pressure deficit during the experimental period in 2016 and 2017. Figure S3: relationships between initial total shoot number in 2015 and total shoot number per tree of apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under well-watered and water-stressed conditions in 2016 and 2017. Figure S4: relationships between shoot length and metamer number per shoot and mean metamer length for apple cultivars ‘Ariane’, ‘Braeburn’ and ‘Fuji’ under well-watered and water-stressed conditions in 2016 and 2017.
ACKNOWLEDGEMENTS
We acknowledge the staff of the Sud-Expé Experimental Station for their technical support, and the China Scholarship Council (CSC) for funding the PhD scholarship of D.C.
APPENDIX
Equations for metamer appearance dynamics
where Nleaves(d) is the number of leaves on the monocyclic shoot at date d, R is the leaf appearance rate (number d−1), T is the growth stop date, d is the date and b is the intercept.
where Nleaves(d) is the number of leaves on the bicyclic shoot at date d, R1 and R2 are the metamer appearance rate (number d−1) in the first and second flush of bicyclic shoots, respectively, T1 and T2 are the arrest start and stop dates, respectively, T3 is the growth cessation date, d is the date and b is the intercept.
LITERATURE CITED
- Barthélémy D, Caraglio Y. 2007. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany 99: 375–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaygue C, Wery J, Cowan A, Tardieu F. 1996. Contribution of leaf expansion, rate of leaf appearance, and stolon branching to growth of plant leaf area under water deficit in white clover. Crop Science 36: 1240–1246. [Google Scholar]
- Beniston M, Stephenson DB, Christensen OB. 2007. Future extreme events in European climate: an exploration of regional climate model projections. Climatic Change 81: 71–95. [Google Scholar]
- Bertero HD, King RW, Hall AJ, et al. 2000. Photoperiod and temperature effects on the rate of leaf appearance in quinoa (Chenopodium quinoa). Functional Plant Biology 27: 349–356. [Google Scholar]
- Costes E, Guédon Y. 2012. Deciphering the ontogeny of a sympodial tree. Trees 26: 865–879. [Google Scholar]
- Costes E, Sinoquet H, Kelner JJ, Godin C. 2003. Exploring within-tree architectural development of two apple tree cultivars over 6 years. Annals of Botany 91: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes E, Lauri P, Eacute, Regnard JL. 2006. Analyzing fruit tree architecture: implications for tree management and fruit production. Horticultural Reviews 32: 1–61. [Google Scholar]
- Crabbé J. 1987. Aspects particuliers de la morphogénèse caulinaire des végétaux ligneux et introduction à leur étude quantitative. Brussels: Editions IRSIA. [Google Scholar]
- Davidson A, Silva DD, Quintana B, Dejong TM. 2015. The phyllochron of Prunus persica shoots is relatively constant under controlled growth conditions but seasonally increases in the field in ways unrelated to patterns of temperature or radiation. Scientia Horticulturae 184: 106–113. [Google Scholar]
- Davidson A, Da Silva D, Dejong TM. 2017. The phyllochron of well-watered and water deficit mature peach trees varies with shoot type and vigour. AOB Plants 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson CG, Remphrey WR. 1994. Shoot neoformation in clones of Fraxinus pennsylvanica in relation to genotype, site and pruning treatments. Trees 8: 205–212. [Google Scholar]
- Dosio GA, Rey H, Lecoeur J, et al. 2003. A whole-plant analysis of the dynamics of expansion of individual leaves of two sunflower hybrids. Journal of Experimental Botany 54: 2541–2552. [DOI] [PubMed] [Google Scholar]
- Ebel RC, Proebsting EL, Evans RG. 1995. Deficit irrigation to control vegetative growth in apple and monitoring fruit growth to schedule irrigation. HortScience 30: 1229–1232. [Google Scholar]
- Foster T, Kirk C, Jones WT, et al. 2007. Characterisation of the DELLA subfamily in apple (Malus × domestica Borkh.). Tree Genetics & Genomes 3: 187–197. [Google Scholar]
- Fournier C, Andrieu B. 2000. Dynamics of the elongation of internodes in maize (Zea mays L.). Effects of shade treatment on elongation patterns. Annals of Botany 86: 1127–1134. [Google Scholar]
- Garris A, Clark L, Owens C, et al. 2009. Mapping of photoperiod-induced growth cessation in the wild grape Vitis riparia. Journal of the American Society for Horticultural Science 134: 261–272. [Google Scholar]
- Gasque M, Martí P, Granero B, González-Altozano P. 2016. Effects of long-term summer deficit irrigation on ‘Navelina’ citrus trees. Agricultural Water Management 169: 140–147. [Google Scholar]
- Godin C, Caraglio Y. 1998. A multiscale model of plant topological structures. Journal of Theoretical Biology 191: 1–46. [DOI] [PubMed] [Google Scholar]
- Goldhamer DA, Fereres E. 2001. Irrigation scheduling protocols using continuously recorded trunk diameter measurements. Irrigation Science 20: 115–125. [Google Scholar]
- Gordon D, Damiano C, Dejong TM. 2006. Preformation in vegetative buds of Prunus persica: factors influencing number of leaf primordia in overwintering buds. Tree Physiology 26: 537–544. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Boem FH, Thomas GW. 1998. Phosphorus nutrition affects wheat response to water deficit. Agronomy Journal 90: 166–171. [Google Scholar]
- Hallé F, Martin R. 1968. Etude de la croissance rythmique chez l’hevea (Hevea brasiliensis Mull.-Arg. Euphorbiacees-Crotonoidees). Adansonia 8: 475–503. [Google Scholar]
- Hipps N, Pages L, Huguet J, Serra V. 1995. Influence of controlled water supply on shoot and root development of young peach trees. Tree Physiology 15: 95–103. [DOI] [PubMed] [Google Scholar]
- Irving D, Drost J. 1987. Effects of water deficit on vegetative growth, fruit growth and fruit quality in Cox’s orange pippin apple. Journal of Horticultural Science 62: 427–432. [Google Scholar]
- Kass RE, Raftery AE. 1995. Bayes factors. Journal of the American Statistical Association 90: 773–795. [Google Scholar]
- Lang GA, Early JD, Darnell RL, Martin GC. 1987. Endo-, para-, and eco-dormancy: physiological terminology and classification for dormancy research. HortScience 22: 371–377. [Google Scholar]
- Lauri P-E, Barigah TS, Lopez G, et al. 2016. Genetic variability and phenotypic plasticity of apple morphological responses to soil water restriction in relation with leaf functions and stem xylem conductivity. Trees 30: 1893–1908. [Google Scholar]
- Lebon E, Pellegrino A, Louarn G, Lecoeur J. 2006. Branch development controls leaf area dynamics in grapevine (Vitis vinifera) growing in drying soil. Annals of Botany 98: 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong S, Ong C. 1983. The influence of temperature and soil water deficit on the development and morphology of groundnut (Arachis hypogaea L.). Journal of Experimental Botany 34: 1551–1561. [Google Scholar]
- Li XF, Shao XH, Deng XJ, et al. 2012. Necessity of high temperature for the dormancy release of Narcissus tazetta var. chinensis. Journal of Plant Physiology 169: 1340–1347. [DOI] [PubMed] [Google Scholar]
- Lopez G, Pallas B, Martinez S, et al. 2015. High-throughput phenotyping of an apple core collection: identification of genotypes with high water use efficiency. Acta Horticulturae 1150: 335–340. [Google Scholar]
- Mills T, Behboudian M, Clothier B. 1996. Water relations, growth, and the composition of ‘Braeburn’ apple fruit under deficit irrigation. Journal of the American Society for Horticultural Science 121: 286–291. [Google Scholar]
- Morgan D, Warrington I, Halligan E. 1985. Effect of temperature and photosynthetic photon flux density on vegetative growth of kiwifruit (Actinidia chinensis). New Zealand Journal of Agricultural Research 28: 109–116. [Google Scholar]
- Muller B, Pantin F, Génard M, et al. 2011. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. Journal of Experimental Botany 62: 1715–1729. [DOI] [PubMed] [Google Scholar]
- Naor A. 2010. Irrigation scheduling and evaluation of tree water status in deciduous orchards. Horticultural Reviews 32: 111–165. [Google Scholar]
- Negrón C, Contador L, Lampinen BD, et al. 2013. Differences in proleptic and epicormic shoot structures in relation to water deficit and growth rate in almond trees (Prunus dulcis). Annals of Botany 113: 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U. 2007. Photosynthesis and resource distribution through plant canopies. Plant Cell and Environment 30: 1052–1071. [DOI] [PubMed] [Google Scholar]
- Nozeran R. 1984. Integration of organismal development. In: Barlow PW, Carr DJ, eds. Positional controls in plant development. Cambridge: Cambridge University Press, 375–402. [Google Scholar]
- Ofir M, Kigel J. 2007. Regulation of summer dormancy by water deficit and ABA in Poa bulbosa ecotypes. Annals of Botany 99: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio S, Montserrat-Marti G. 2005. Bud morphology and shoot growth dynamics in two species of Mediterranean sub-shrubs co-existing in gypsum outcrops. Annals of Botany 95: 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas B, Christophe A. 2015. Relationships between biomass allocation, axis organogenesis and organ expansion under shading and water deficit conditions in grapevine. Functional Plant Biology 42: 1116–1128. [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2012. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Renton M, Guédon Y, Godin C, Costes E. 2006. Similarities and gradients in growth unit branching patterns during ontogeny in ‘Fuji’ apple trees: a stochastic approach. Journal of Experimental Botany 57: 3131–3143. [DOI] [PubMed] [Google Scholar]
- Seleznyova AN, Thorp TG, Barnett AM, Costes E. 2002. Quantitative analysis of shoot development and branching patterns in Actinidia. Annals of Botany 89: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleznyova AN, Tustin DS, Thorp TG. 2008. Apple dwarfing rootstocks and interstocks affect the type of growth units produced during the annual growth cycle: precocious transition to flowering affects the composition and vigour of annual shoots. Annals of Botany 101: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva DD, Kane ME, Beeson RC. 2012. Changes in root and shoot growth and biomass partition resulting from different irrigation intervals for Ligustrum japonicum Thunb. HortScience 47: 1634–1640. [Google Scholar]
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ. 2004. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. Journal of Experimental Botany 55: 2353–2363. [DOI] [PubMed] [Google Scholar]
- Stoll M, Loveys B, Dry P. 2000. Hormonal changes induced by partial rootzone drying of irrigated grapevine. Journal of Experimental Botany 51: 1627–1634. [DOI] [PubMed] [Google Scholar]
- Tardieu F, Granier C, Muller B. 2011. Water deficit and growth. Co-ordinating processes without an orchestrator? Current Opinion in Plant Biology 14: 283–289. [DOI] [PubMed] [Google Scholar]
- Turc O, Lecoeur J. 1997. Leaf primordium initiation and expanded leaf production are co-ordinated through similar response to air temperature in pea (Pisum sativum L.). Annals of Botany 80: 265–273. [Google Scholar]
- Virlet N, Lebourgeois N, Martinez S, Costes E, Labbé S. Regnard JL. 2014. Stress indicators based on airborne thermal imagery for field phenotyping a heterogeneous tree population for response to water constraints. Journal of Experimental Botany 65: 5429–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Norton M. 2006. Summer dormancy in perennial temperate grasses. Annals of Botany 98: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Norton M, Lelièvre F. 2009. Summer drought survival strategies and sustainability of perennial temperate forage grasses in Mediterranean areas. Crop Science 49: 2386–2392. [Google Scholar]
- White J. 1979. The plant as a metapopulation. Annual Review of Ecology & Systematics 10: 109–145. [Google Scholar]
- Wilson BF. 2000. Apical control of branch growth and angle in woody plants. American Journal of Botany 87: 601–607. [PubMed] [Google Scholar]
- Yang W, Pallas B, Durand JB, Martinez S, Han M, Costes E. 2016. The impact of long-term water stress on tree architecture and production is related to changes in transitions between vegetative and reproductive growth in the ‘Granny Smith’ apple cultivar. Tree Physiology 36: 1369–1381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.