Abstract
The global prevalence of hyperlipidaemia is increasing rapidly and high dietary fat intake is a major risk factor for developing hyperlipidaemia. An in-vivo biological investigation was carried out on ethanolic extract of Momordica charantia, a plant belonging to the family Cucurbitaceae for the evaluation of antihyperlipidemic activity and serum uric acid reducing potential. In our study, 25 healthy male mice were selected randomly and grouped into 5 groups (5 animals in each group). Lipid and uric acid profile were estimated after 21 days of treatment by using the enzymatic colourimetric GPO-PAP method. Results showed that ethanolic extract of M. charantia at a dose of 200 mg/kg body weight showed significant (p < 0.05) cholesterol and triglyceride level reduction profile when co-administrated with 20% fat and normal feed respectively. Atorvastatin was used as standard. Data from pathological examination showed that the average weight of the heart of the mice was normal for every group when compared with control. Gr-2 (normal and extract feed) showed significant (p ˂ 0.05) increased of liver and kidney weight rather than experimental groups; however, these values were lower than the values for the control group. Uric acid level determination revealed that the ethanolic extract of M. charantia reduced serum uric acid level both in experimental groups (Gr-2 and Gr-3). Thus a considerable correlation was found between serum uric acid reducing potentials of the present plant extract with a lipid-lowering profile. This plant can be further investigated thoroughly as a potential source of chemically interesting and biologically important drug candidates.
Keyword: Molecular biology
1. Introduction
Cardiovascular disorders are the most common causes of death, where lifestyle imparts a significant impact on the health of the people. The modernization of society brings untoward changes in dietary pattern. Nowadays food menu contains high saturated fats and refined sugars, however fibre content is low [1]. It is now established that hyperlipidaemia is responsible for the premature development of atherosclerosis and other cardiovascular complications. Hyperlipidaemia contributes to a drastic threat to the spread and expansion of atherosclerosis and coronary heart diseases (CHD) [2]. Atherosclerosis is now recognised to be an inflammatory condition and responsible for the development of cerebrovascular diseases, ischemic heart diseases (IHD), and peripheral vascular diseases, where all of these diseases account almost 29% of total deaths in the whole world in 2005 [3]. Hypertension, hypercholesterolemia, diabetics and obesity are considered as the major risk factors for most of cardiovascular disorders with significant impairment of lipid profiles [2, 4]. Among all of the cardiovascular disorders, IHD is a major risk factor in the pathogenesis of preoperative adverse cardiovascular events which leads to significant morbidity and mortality of the high-risk surgical patient population [2]. Recent studies also suggested that lipid-associated disorders are not only attributed to the total serum cholesterol but also with the distribution among different lipoproteins, where low-density lipoproteins (LDL) are the major carriers of cholesterol towards tissues with an atherogenic potentiality and the high-density lipoproteins (HDL) carry cholesterol from peripheral tissues to the liver. Thus HDL gives protection against many cardiac problems and obesity [5]. Some recent data suggest that higher level of serum uric acid (hyperuricemia) is closely related with visceral fat accumulation [6] and some other metabolic disorders such as glucose intolerance, elevated blood pressure, dyslipidaemia and atherosclerotic cardiovascular diseases, conceptualized as the metabolic syndrome [7]. Furthermore, it was reported recently that reduction in visceral fat was associated with significant falls in serum uric acid level, and that both serum adiponectin concentration and visceral fat area were significantly correlated with serum uric acid level [8]. The treatments of hyperlipidemia mainly rely on a patient's cholesterol profile. Many antihyperlipidemic agents are commercially available such as the statin, fibrates, niacin, bile acids, ezetimibe etc. All of these agents having the potentiality of reducing cholesterol level, however, they also have remarkable side effects [9]. Most common side effects of traditional hypolipidemic drugs are hyperuricemia, diarrhoea, flushing, nausea, myositis, gastric irritation, dry skin and abnormal liver function etc. [10].
In many parts of the world, plants-derived preparations have been used for the treatment of hyperlipidemia. Antihyperlipidemic agents from plant origin have become popular because of their less toxicity, easy availability and absorption in the body than currently used drugs. Nowadays Ayurveda, Unani and Chinese practitioners prescribe numerous herbal drugs which have hypoglycemic, hypolipidemic and antihypertensive properties for the treatment of cardiovascular disorders [11, 12]. Plants containing biologically active chemical substances such as saponins, tannins, essential oils, flavonoids, alkaloids and other chemical compounds are considered as the possible candidates for the herbal therapy [13, 14]. Our present interest is on M. charantia belongs to family Cucurbitaceae, also called bitter melon, balsam pear and bitter gourd. It is widely distributed in tropical regions of South East and Far East Asia to Australia and South Africa. M. charantia possesses antidiabetic, antihyperlipidemic and anticarcinogenic anti-HIV, anthelmintic, antitumor, and wound healing properties [15, 16, 17, 18, 19]. Fresh juice of bitter melon inhibited lipogenesis [20]. In addition, ingestion of M. charantia as food may regulate glucose uptake by activating a protein called AMPK and increases insulin sensitivity [21]. In this study, we want to investigate the possibility of antihyperlipidemic activity and qualitative study of plasma uric acid reducing the potential of the ethanolic extract of M. charantia seeds.
2. Materials and methods
2.1. Collection and identification of plant parts
Ripe fruits of M. charantia were brought from the local market of Noakhali district, Chittagong, Bangladesh on June 2016. The plant's material was identified and authenticated by the National Herbarium, Mirpur, Dhaka. The voucher specimen number was (Accession no# DACB 42292).
2.2. Drying, grinding and cold extraction of the seeds of M. charantia
Collected seeds were separated from undesirable plant parts washed thoroughly and air dried at room temperature for 14 days. The plant parts were ground into a coarse powder with the help of a suitable grinder. The powder was stored in an airtight container and kept in a cool, dark and dry place [22]. About 800 gm of powdered material was taken in a clean, flat-bottomed glass container and soaked in 2L of distilled ethanol. The container with its content was sealed by aluminium foil and kept for a period 15 days accompanying occasional shaking and stirring. The whole mixture then underwent a coarse filtration by fresh cotton plug and finally with a Whatman filters paper. The volume of the filtrate was then reduced by using an evaporator (Buchi Rotavapor) at low temperature and pressure.
2.3. Materials
Atorvastatin was collected form Incepta Pharmaceuticals Ltd, Bangladesh. All other reagents used in this study were analytical grades.
2.4. Preparation of extract and standard drug suspension
In order to administer the extract at doses of 200 mg/kg body weight of mice [23], the extracts were measured accurately and triturated in a unidirectional way by adding a small amount of distilled water. After proper mixing of extract, distilled water was slowly added to make the final volume of the suspension up to 5 ml. Atorvastatin at the dose of 1 mg/kg body weight [24] was prepared by breakdown the tablet by mortar pestle, accurately weighing and dissolved in a small amount of water. Water was added slowly to make the final volume of the suspension up to 5 ml. To stabilize the suspension, it was stirred well by vortex mixture.
2.5. Experimental animals
Swiss albino male mice, used for this study were collected from the animal house of Jahangirnagar University, Savar, Dhaka, Bangladesh. Feeding of mice was done by standard laboratory pellet diet and water at libitum at a temperature of 25 ± 20 °C and 55 ± 10% relative humidity. They were allowed to acclimatize for 7 days to the laboratory conditions before conducting the experiment [25]. The protocol used in this study in mice model was carried out based on the guideline of the Institutional Animal Ethics Committee. All authors hereby declare that "Principles of laboratory animal care" (NIH publication No. 85-23, revised 1985) were followed, as well as specific national laws where applicable. All experiments have been examined and approved by the ethical committee of Noakhali Science and Technology University.
2.6. Study design
During this study exactly 25 healthy male mice were randomly selected and divided into 5 groups (each group contains 5 mice).
Gr-1(Control group): All of the mice were fed normal food.
Gr-2: Mice were fed with normal diet along with 200 mg/kg body weight extract.
Gr-3(Obese group/HFD): Mice fed with high-fat diet- 20% (w/w) cow fat with a normal diet.
Gr-4: Mice fed by high-fat diet with 200 mg/kg body weight extract.
Gr-5 (Standard): Mice fed with normal food, and atorvastatin at a dose of 1 mg/kg body weight.
Mice of all groups were treated daily with their respective foods and water for a period of 11th days and each of the mice was weighed every day. At the end of the experiment, mice were anaesthetized before killing by decapitation and blood collected for analysis.
2.7. Determination of serum cholesterol, triglyceride, and uric acid level
Serum cholesterol (SC) level was calculated by enzymatic endpoint (CHOD-PAP) method [26]; whereas serum triglyceride (TG) was estimated by enzymatic colourimetric GPO-PAP method [27] respectively using a double beam spectrophotometer (Shimadzu, Japan). Serum uric acid (SUA) level was measured by using the method of Fossate et al. (enzymatic colourimetric method) with slight modification [28].
2.8. Pathological examination of organ weight
After completing the 25 days experimental period, ketamine (500 mg/kg) was administered intraperitoneally at the 26th day to anaesthetize the animal before sacrificing. After sacrificing, the specific organs of interest were separated from the carcasses and preserved in normal saline. We separated the liver, kidney and heart of each mouse. After collecting the organ, each organ of mice was weighing separately and the average weights of liver, kidney and heart of each group were compared statistically.
2.9. Statistical analysis
All the results were expressed as Mean ± SEM (Standard Error of Mean). P-value was calculated by one way ANOVA followed by Dunnet's t-test using SPSS software (version 22.0). All the groups were compared with control. P < 0.05 was considered to be statistically significant and p < 0.001 was considered to be highly significant.
3. Results
3.1. Acute toxicity test
During acute toxicity test, 2000 mg/kg body weight of extract was given and no death was observed during the experimental investigation. All of the control and treated rats survived until the end of the treatment period.
3.2. Body weight and growth
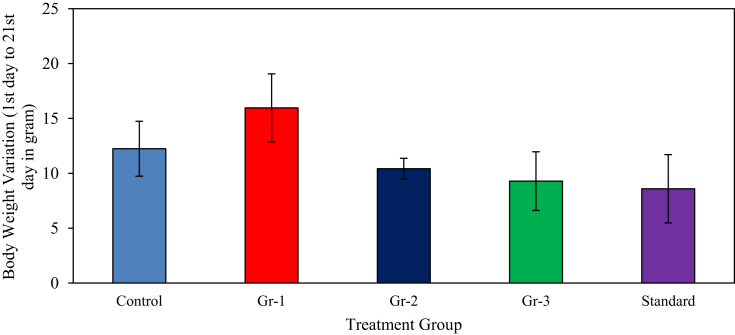
A noticeable downward tendency in body weight gaining of the mice was seen for Gr-2 when compared with mice of other groups. The average weight of the control group shows a regular and gradual rise in the graph, whereas the weight gaining trend is a parallel manner for Gr-1 mice (HFG) with respect to control group mice. This growth trend is somehow less for Gr-2 and Gr-3. Weight losing tendency was little more for Gr-3 when compared to Gr-1 and Gr-2. Standard drug lower the body gaining trend remarkably. Comparisons of body weight variation were shown in Fig. 1.
Fig. 1.
Body weight variation of mice of different groups. Here, Gr-1 = 20% fat feeding group; Gr-2 = Normal food and M. charantia extract (200 mg/kg) feeding group; Gr-3 = 20% fat and M. charantia feeding group; Control = Normal food feeding group; Standard = Atorvastatin (10 mg/kg body weight) feeding group.
3.3. Analysis of organ weight
From the data found in Table 1, we see that the average weight of liver has been decreased more significantly in both Gr-1 and Gr-2 (p < 0.01) and showed significantly lower value for Gr-3 (p < 0.05), while compared to control. Similar results were also found when comparing the weight of heart among five experimental groups, but none of this value was found to be statistically significant. Finally, the average weight of the kidney was lowered significantly (p < 0.05) for Gr-1 and Gr-2 while comparing with the control subject (Table 1).
Table 1.
Organ weight variation among the mice of different groups.
| Treatment groups | Average weight of the heart (gm) | Average weight of the liver (gm) | Average weight of the kidney (gm) |
|---|---|---|---|
| Control | 0.2 ± 0.04 | 2.04 ± 0.075 | 0.29 ± 0.005 |
| Gr-1 | 0.2 ± 0.04 | 1.38 ± 0.035** | 0.20 ± 0.015* |
| Gr-2 | 0.18 ± 0.07 | 1.56 ± 0.06* | 0.23 ± 0.015* |
| Gr-3 | 0.155 ± 0.015 | 1.36 ± 0.04** | 0.20 ± 0.026 |
| Standard | 0.131 ± 0.02 | 1.6 ± 0.148 | 0.18 ± 0.005** |
Values are represented as Mean ± SEM (n = 5); *p < 0.05, **p < 0.01.
3.4. Biochemical examination
From the biochemical examination of blood serum, we have found both the serum cholesterol (SC) and triglyceride l (TG) level were decreased in both Gr-2 and Gr-3 when compared to control group (Gr-1) who fed with fat diet showed highest SC and TG level in blood. All the experimental groups showed statistically significant (p < 0.05) result except Gr-2 when compared with control. Again in the case of SC level determination, Gr-1 and Gr-2 showed the statistically significant result (Table 2). Our final finding was focused on serum uric acid (UA) level, where Gr-1 reveal highest value and groups nursed by plant extract with normal, and fat-containing diet showed the lowest value while comparing with control subject (Table 3).
Table 2.
Analysis of serum triglyceride and cholesterol level of mice of different groups.
| Treatment group | Triglyceride level (mg/dl) | Cholesterol level (mg/dl) |
|---|---|---|
| Control | 226.66 ± 4.67* | 104.52 ± 9.08 |
| Gr-1 | 372.83 ± 0.5*** | 128.1 ± 3.65*** |
| Gr-2 | 180 ± 2.30 | 96.07 ± 1.02*** |
| Gr-3 | 339.9 ± 6.6*** | 81.05 ± 0.80 |
| Standard | 130.5 ± 7.58 | 79.50 ± 4.48 |
Values are represented as Mean ± SEM (n = 5); *p < 0.05, ***p < 0.001.
Table 3.
Analysis of serum uric acid level of mice of different groups.
| Treatment group | Plasma Uric acid level (mg/dl) |
|---|---|
| Control | 30.4 ± 6.4 |
| Gr-1 | 48 ± 8.2* |
| Gr-2 | 18.7 ± 1.2*** |
| Gr-3 | 18.4 ± 2.4** |
| Standard | 16.3 ± 4.3 |
Values are represented as Mean ± SEM (n = 5); *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
Obesity is considered as major risk factors for the development of health-related problems. It's prevalence becoming high throughout the whole world. Many studies on the human model reported that increased fat intake causes the weight gain tendency and followed by develops obesity with other metabolic complications [29]. A high-fat-diet-induced obese animal model has been commonly used for studying obesity-related changes [30]. In our study, we also found that mice fed with a high-fat diet increased the body weight significantly when compared to normal diet mice. While the treatment with the ethanolic extract of M. charantia along with normal diet or high-fat diet reducing the body weight. Although M. charantia causes a reduction in the body weight regardless of diet, this plant extract is more prominent to inhibit the weight gain in HFD induced obesity. Moreover, our seed ethanolic extract decreased the body weight of mice without showing any mortality and toxicity during the study period. This study result is in accordance with the research study of Hidaka et al., Mori et al., Astel et al., [30, 31, 32]. Data from pathological examination showed that the average weight of the heart of the mice remains normal in all groups when compared with control. However, the weight of the liver and kidney of the mice of Gr-2 and Gr-3 decreased significantly. The changes in the absolute and relative organ weights indicate incurrence of toxicity in mice [33]. During this study the weight of kidney of the mice of M. charantia decreased significantly, which is strongly supported by findings of Teoh et al., [34]. The study by Teoh et al., suggested that when extract of M. charantia has given to diabetic rates their weight of kidney decrease significantly, where all the necrotic changes observed in the proximal and distal convoluted tubules along with the deposits were found to be absent in the diabetic rats treated with the MC extract. The group that was treated with M. charantia extract showed features of healing i.e. normal glomerulus, absence of inflammatory cells, normal basement membrane and capillaries, decrease in the mucopolysaccharide and hyaline deposit, respectively. The tissue necrosis was also observed to decrease in the group treated with M. charantia extract [34]. Our study revealed that the ethanolic extract of M. charantia fruits showed the most significant serum TG lowering (p < 0.001) and insignificant SC lowering (p > 0.05) activities. Besides these seeds extract showed insignificant serum TG lowering (p > 0.05) and more significant SC lowering (p > 0.01) activity when the study was carried on mice of normal food and extract group. The result showed that there was an increase in TC and serum TG level in HFD group than the control. It was also showed that administration of the extract at 200 mg/kg body weight along with HFD lower TC, TG level compared with 20% fat group. The hypolipidemic activity of the ethanolic extract of the M. charantia seeds found to be less efficacious than that of the standard drug atorvastatin. The decrease of blood TG level may be attributed to the hepatocellular damage. According to the research studies of Breckenridge et al., Carlson et al., Connelly et al., serum TG level increases due to the defect in hepatic lipase in human beings [33, 35, 36].
Obesity is often accompanied by hyperuricemia [6, 7, 8]. Adipose tissue is responsible for abundant expressions and activities of Xanthine oxidoreductase (XOR), and obese adipose tissues have higher XOR activities than those of control mice. During the last step of purine metabolism, XOR catalyses the oxidation of xanthine and hypoxanthine into uric acid. Dysfunction of obese adipose tissue could be related to overproduction of uric acid. Xanthine oxidase (XO) inhibitors block the terminal step in uric acid biosynthesis, which can lower the serum uric acid (SUA) concentration. In folk medicine, M. charantia has been used as an important traditional herbal medicine due to its vast bioactive compounds like-kaempferol, quercetin, 5, 7-dimethoxycoumarin, alkaloids, campaign, and pseudocarpaine [37]. Any of these bioactive compounds may contribute to XO inhibitory activities. XO has previously received considerable attention because of its physiological functions such as antioxidant activity. Our research finding is endorsed by many research works to determine the antihyperlipidemic activities of different plant extract. However, here we found some new finding. Tsushima et al. revealed that the SUA level indicates a hyperlipidemic condition in mice model. Adipose tissue can secrete uric acid in the mouse [38]. Thus low SUA level reveals a lesser generation of adipocyte on mice model. Our main goal was to validate this new finding by using our experimental plant extract. We, fortunately, found that our experimental plant extract showed decreased SUA level in both Gr-2 and Gr-3 mice. Our finding endorsed that seed extract of M. charantia reduced SUA level significantly. There are possibilities that reduction of serum urate (SU) level results from the facilitation of uricase by the extract or facilitation of the excretion of serum urea. However, there is a question arise that as a human does not contain uricase, how SU level decrease if we want this plant extract for therapeutic use? The answer raises the hypothesis that there is a possibility of reduction of SU level by this extract due to the increase of urinary urate excretion through glomerular filtration or tubular secretion. There is another claim come on our mind that although human does not contain uricase; any nutraceuticals or bioactive compound in plant extract may facilitate the reduction of SU level by inhibiting its absorption in gout.
5. Conclusion
Although our present study does not represent the total lipid-lowering profile of M. charantia; by considering the cholesterol, triglyceride, and uric acid lowering index, it is clear that seeds of this plant greatly reduce the initiation of obesity. We also found a close relationship between hyperlipidemia and hyperuricemia. By considering the potential, we can conclude that there is a possibility of developing anti-obese and lipid-lowering drugs from M. charantia seeds. Further studies should be necessary to find out the bioactive metabolites and warrant this finding on the human model for potential therapeutic effects.
5.1. Limitation
Although our present study represents that M. charantia greatly reduce the initiation of obesity by considering the cholesterol, triglyceride, and uric acid lowering index, while a major limitation was failed to represent HDL and LDL index. Other major limitations were absent of histological assay, small experimental animal size, and integration of these data to human subjects.
Declarations
Author contribution statement
Md. Saddam Hussain: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nusrat Jahan: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Md. Mamun Or Rashid, Naimur Rahman: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mohammad Salim Hossain: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Umay Chen: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the authority of Bangladesh National Herbarium for identifying our experimental plants and their seeds. Authors also want to show their gratitude to the Department of Pharmacy, Jahangirnagar University for supporting us through providing experimental animals.
References
- 1.Brad B.I.C., Odebolt M.A., Algoma P.U. Effect of Persia americana leaf extract on body weight and liver lipids in rats fed with hyperlipidemic diet. Afr. J. Biotechnol. 2007;16(8):1007–1111. [Google Scholar]
- 2.Howard-Alpe G.M., Sear J.W., Foex P. Methods of detecting atherosclerosis in non-cardiac surgical patients; the role of biochemical markers. Br. J. Anaesth. 2006;97:758–769. doi: 10.1093/bja/ael303. [DOI] [PubMed] [Google Scholar]
- 3.Reddy K.S., Shah B., Varghese C., Ramadoss A. Responding to the challenge of chronic diseases in India. Lancet. 2005;366:1744–1749. doi: 10.1016/S0140-6736(05)67343-6. [DOI] [PubMed] [Google Scholar]
- 4.Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 5.Sattivel A., Rao H., Balajiaghavendran Anti peroxidative and Antihyperlipidemic nature of Ulva lactuca crude polysaccharide on D-galactose amine induced hepatitis in rats. Food Chem. Toxicol. 2000;46:3262–3267. doi: 10.1016/j.fct.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Hikita M., Ohno I., Mori Y., Ichida K., Yokose T., Hosoya T. Relationship between hyperuricemia and body fat distribution. Intern Med. 2007;46:1353–1358. doi: 10.2169/internalmedicine.46.0045. [DOI] [PubMed] [Google Scholar]
- 7.Rathmann W., Haastert B., Icks A., Giani G., Roseman J.M. Ten-year change in serum uric acid and its relation to changes in other metabolic risk factors in young black and white adults: the CARDIA study. Eur. J. Epidemiol. 2007;22:439–445. doi: 10.1007/s10654-007-9132-3. [DOI] [PubMed] [Google Scholar]
- 8.Tamba S., Nishizawa H., Funahashi T., Okauchi Y., Ogawa T., Noguchi M., Fujita K., Ryo M., Kihara S., Iwahashi H., Yamagata K., Nakamura T., Shimomura I., Matsuzawa Y. Relationship between the serum uric acid level, visceral fat accumulation and serum adiponectin concentration in Japanese men. Intern Med. 2008;47:1175–1180. doi: 10.2169/internalmedicine.47.0603. [DOI] [PubMed] [Google Scholar]
- 9.Durrington P. Dyslipidaemia. Lancet. 2003;362:717–731. doi: 10.1016/S0140-6736(03)14234-1. [DOI] [PubMed] [Google Scholar]
- 10.Speight T.M. Maclennan and Petty; Australia: 1987. Avery's Drug Treatment Principles and Practice of Clinical Pharmacology and Therapeutics. [Google Scholar]
- 11.Kumari K., Augusti K.T. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. J. Ethnopharmacol. 2007;109:367–371. doi: 10.1016/j.jep.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Son I.S., Kim J.H., Sohn H.Y., Son K.H., Kim J.S., Kwon C.S. Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.),on high-cholesterol fed rats. Biosci. Biotechnol. Biochem. 2007;71:3063–3071. doi: 10.1271/bbb.70472. [DOI] [PubMed] [Google Scholar]
- 13.Harborne J.B. first ed. Chapman and Hall; London: 1973. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 14.Sofowora A. second ed. Spectrum Books; Ibadan: 1996. Medicinal Plant and Traditional Medicine in Africa; p. 112. [Google Scholar]
- 15.Bano F. Effect of aqueous extracts of Momordica charantia on body weight of rats. J. Basic Appl. Sci. 2011;7(1):1–5. [Google Scholar]
- 16.Agarwal R., Beohar T. Chemo-preventive and Anti-carcinogenic effects of Momordica charantia extract. J. Cancer Prev. 2010;11(2):371–375. [PubMed] [Google Scholar]
- 17.Fang F. Bitter gourd (Momordica charantia) is a cornucopia of health. Curr. Mol. Med. 2011;11(5):417–436. doi: 10.2174/156652411795976583. [DOI] [PubMed] [Google Scholar]
- 18.Das P., Prasad S., Dam T. Anti-helminthic effects of Momordica charantia. J. Altern. Complement. Med. 2006;12(3):299–301. doi: 10.1089/acm.2006.12.299. [DOI] [PubMed] [Google Scholar]
- 19.Prasad V., Jain V., Girish D., Dorle A.K. Wound healing property of Momordica charrantia L. fruit powder. J. Herb. Pharmacother. 2006;6(3):105–115. doi: 10.1080/j157v06n03_05. [DOI] [PubMed] [Google Scholar]
- 20.Nerurkar P., Lee Y.K., Nerurkar V. Momordica charantia (bitter melon) inhibits primary human adipocyte differentiation by modulating adipogenic genes. BMC Complement Altern. Med. 2010;34:10. doi: 10.1186/1472-6882-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan M.J., Ye J.M., Turner N., Hohnen-Behrens C., Ke C.Q., Tang C.P., Chen T., Weiss H.C., Gesing E.R., Rowland A., James D.E., Ye Y. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem. Biol. 2008;15:263–273. doi: 10.1016/j.chembiol.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Atata R.F., Sani A., Ajewole S.M. Effect of stem bark extracts of Enantia chloranta on some clinical isolates. Niger. Soc. Exp. Biol. 2003;15(2):84–92. [Google Scholar]
- 23.Arefin S., Hossain M.S., Neshe S.A., Rashid M.M.O., Amin M.T., Hussain M.S. Tartrazine induced changes in physiological and biochemical parameters in Swiss albino mice, Mus musculus. JRP. 2017;21(3):564–569. [Google Scholar]
- 24.Hamed M.R., Hassanein N.M.A., Ali A.A., EL-Nahhas T.M.Y. An experimental study on the therapeutic efficacy of the combined administration of herbal medicines with atorvastatin against hyperlipidemia in rats. J. Appl. Sci. Res. 2010;6(11):1730–1744. [Google Scholar]
- 25.Neshe S.A., Arefin S., Hussain M.S., Das A., Karmakar P., Hossain M.S. Safety evaluation of chocolate Brown dye in Swiss albino mice. J. Nutr. Disord. Ther. 2016;6:195. [Google Scholar]
- 26.Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 27.Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973;19(5):476–482. [PubMed] [Google Scholar]
- 28.Fossati P., Prencipe L., Bari G. Use of 3, 5-dichloro-2- hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymatic assay of uric acid in serum and urine. Clin. Chem. 1980;26:227–231. [PubMed] [Google Scholar]
- 29.Moutinho I.L.D., Bertges L.C., Assis R.V.C. Prolonged use of the food dye tartrazine (FD&C yellow n° 5) and its effects on the gastric mucosa of Wistar rats. Braz. J. Biol. 2007;67(1):141–145. doi: 10.1590/s1519-69842007000100019. [DOI] [PubMed] [Google Scholar]
- 30.Hidaka S., Okamoto Y., Arita M.A. Hot water extract of Chlorella pyrenoidosa reduces body weight and serum lipids in ovariectomized rats. Phytother Res. 2004;18(2):164–168. doi: 10.1002/ptr.1178. [DOI] [PubMed] [Google Scholar]
- 31.Mori S., Satou M., Kanazawa S., Yoshizuka N., Hase T., Tokimitsu I., Takema Y., Nishizawa Y., Yada T. Body fat mass reduction and up-regulation of uncoupling protein by novel lipolysis-promoting plant extract. Int. J. Biol. Sci. 2009;5(4):311–318. doi: 10.7150/ijbs.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Astell K.J., Mathai M.L., Su X.Q. Plant extracts with appetite suppressing properties for body weight control: a systematic review of double blind randomized controlled clinical trials. Complement. Ther. Med. 2013;21(4):407–416. doi: 10.1016/j.ctim.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Imamura S., Kobayashi J., Nakajima K., Sakasegawa S., Nohara A., Noguchi T., Kawashiri M.A., Inazu A., Deeb S.S., Mabuchi H., Brunzell J.D. A novel method for measuring human lipoprotein lipase and hepatic lipase activities in postheparin plasma. JLR (J. Lipid Res.) 2008;49:1431–1437. doi: 10.1194/jlr.M700528-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teoh S.L., Latiff A.A.B.D., Das S. Histological changes in the kidneys of experimental diabetic rats fed with Momordica charantia (bitter gourd) extract. Rom. J. Morphol. Embryol. 2010;51(1):91–95. [PubMed] [Google Scholar]
- 35.Carlson L.A., Hommoquist L., Nilsson-Ehle P. Deficiency of hepatic lipase activity in post-heparin plasma in familial hyper-alpha-tryglyceridaemia. Acta Med. Scand. 1986;219:435–447. doi: 10.1111/j.0954-6820.1986.tb03337.x. [DOI] [PubMed] [Google Scholar]
- 36.Fazio S., Linton M.F. Elevated high-density lipoprotein (HDL) levels due to hepatic lipase mutations do not reduce cardiovascular disease risk: another strike against the HDL dogma. J. Clin. Endocrinol. Metab. 2009;94(4):1081–1083. doi: 10.1210/jc.2009-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madalaa N.E., Piatera L., Duberya I., Steenkampa P. Distribution patterns of flavonoids from three Momordica species by ultra-high performance liquid chromatography quadrupole time of flight mass spectrometry: a metabolomic profiling approach. Rev. Bras. Farmacogn. 2016;26(4) [Google Scholar]
- 38.Tsushima Y., Nishizawa H., Tochino Y., Nakatsuji1 H., Sekimoto R., Nagao H., Shirakura T., Kato K., Imaizumi K., Takahashi H., Tamura M., Maeda N., Funahashi T., Shimomura I. Uric acid secretion from adipose tissue and its increase in obesity. JBC Papers. 2013;113:4850–4894. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]