Abstract
Elevations in brain interleukin-1 beta (IL-1β) during chronic stress exposure have been implicated in behavioral and cognitive impairments associated with depression and anxiety. Two critical regulators of brain IL-1β production during times of stress are glucocorticoids and catecholamines. These hormones work in opposition to one another to inhibit (via glucocorticoid receptors) or stimulate (via beta-adrenergic receptors: β-AR) IL-1 β production. While chronic stress often heightens both corticosterone and catecholamine levels, it remains unknown as to how chronic stress may affect the “yin-yang” balance between adrenergic stimulation and glucocorticoid suppression of brain IL-1β. To investigate this further, male and female rats underwent 4 days of stress exposure or served as non-stressed controls. On day 5, animals were administered propranolol (β-AR antagonist), metyrapone (a glucocorticoid synthesis inhibitor), vehicle, or both drugs and brain IL-1β mRNA was measured by rtPCR in limbic brain areas. In males, administration of propranolol had no effect on IL-1β expression in non-stressed controls but significantly reduced IL-1β in the hippocampus and amygdala of chronically stressed animals. In females, propranolol significantly reduced IL-1β in the amygdala and hypothalamus of both control and stressed rats. In male rats, metyrapone treatment significantly increased IL-1β mRNA regardless of stress treatment in all brain areas, while in female rats metyrapone only increased IL-1β in the hypothalamus. Interestingly, propranolol treatment blocked the metyrapone-induced increase in brain IL-1β indicating the increase in brain IL-1β following metyrapone treatment was due to increase β-AR activation. Additional studies revealed that metyrapone significantly increases norepinephrine turnover in the hypothalamus and medial prefrontal cortex in male rats and that microglia appear to be the cell type contributing to the production of IL-1β. Overall, data reveal that stress exposure in male rats affects the regulation of brain IL-1β by the norepinephrine-β-AR pathway, while stress had no effect in the regulation of brain IL-1β in female rats.
Keywords: catecholamines, norepinephrine, glucocorticoids, beta-adrenergic receptor, corticosterone, cytokines
1. Introduction
Exposure to stressful life events are associated with numerous health impairments including increased risk of developing affective disorders (Kendler et al., 1999). Inflammatory cytokines are implicated in mediating some of the cognitive and behavioral impairments observed in individuals suffering from depression (Capuron et al., 2004; Miller and Raison, 2016). In rodent models, stress-induced brain interleukin-1 beta (IL-1β) appears to be a critical cytokine that mediates depression, anxiety, and cognitive impairments (Goshen et al., 2008; Maier and Watkins, 1995; Wohleb et al., 2014). Acute exposure to mild or moderate stressors typically does not elevate brain IL-1β (Deak et al., 2003; Plata-Salamán et al., 2000; Porterfield et al., 2011) while exposure to repeated stress or severe acute stress causes persistent elevations and/or sensitized brain IL-1β production (Hueston and Deak, 2014; O’Connor et al., 2003; Wohleb et al., 2011). Thus, investigating how repeated stress exposure leads to the dysregulation of brain IL-1β is particularly important for understanding the mechanisms underlying stress-induced psychopathology and for developing new therapeutics targets.
Glucocorticoids and catecholamines are two of the primary factors involved in the regulation of IL-1β during times of stress. The acute effect of glucocorticoids is to suppress inflammatory cytokine production via binding to glucocorticoid receptors that modulate transcriptional responses of inflammatory genes (Smoak and Cidlowski, 2004). Individuals with low cortisol levels or adrenalectomized animals with low corticosterone levels show greater inflammatory cytokine production (Baker et al., 2001; Kunz-Ebrecht et al., 2003; Nguyen et al., 1998; Nguyen et al., 2000). In contrast, catecholamines act via adrenergic receptors (ARs) to stimulate IL-1β production (Blandino et al., 2006; Johnson et al., 2005). Central administration of isoproterenol, a β-AR agonist, induces IL-1β within the brain (Johnson et al., 2008) while propranolol, a β-AR antagonist, blocks the induction of brain IL-1β following a multitude of stressors including footshock (Blandino et al., 2006; Johnson et al., 2005), bacteria challenge (Johnson et al., 2008), stroke (Craft and DeVries, 2006), and surgery (Ley et al., 2012). Thus, glucocorticoids and catecholamines have a “yin-yang” relationship in the regulation of brain cytokines during times of stress. In support of this hypothesis, a similar regulatory relationship between norepinephrine and glucocorticoids has been observed on prostaglandins in the cortex (García-Bueno et al., 2008) suggesting a common relationship by which stress hormones regulate important factors of the immune response.
Repeated stress exposure can lead to greater noradrenergic and glucocorticoid responses. This can be observed by increased basal release of central norepinephrine (Bhatnagar and Vining, 2003; Morilak et al., 2005) and increased circulating corticosterone (Fernandes et al., 2002) and also greater evoked responses of each to subsequent stimuli (Bhatnagar and Vining, 2003; Pardon et al., 2003; Xu et al., 2004). We previously demonstrated that four days of repeated stress exposure is sufficient to increase norepinephrine turnover in limbic brain areas (i.e. prefrontal cortex, amygdala, and hypothalamus) (Porterfield et al., 2012), elevate basal corticosterone levels (Lowrance et al., 2016), exaggerate fear conditioning (Camp et al., 2012), decrease impulse control (Camp and Johnson, 2015), decrease sucrose preference, alter β-AR expression and enhance β-AR mediated IL-1β production (Porterfield et al., 2012) in male Fischer rats. Despite the increase in norepinephrine turnover and circulating corticosterone following repeated stress exposure, basal IL-1β levels are largely unaffected indicating a balance remains between stimulatory and inhibitory regulation of brain IL-1β at this time.
We believe the heightened brain norepinephrine and circulating corticosterone observed after four days of repeated stress exposure is the “first phase” towards the dysregulation in basal levels of brain IL-1β that ultimately leads to the onset of depression, anxiety, and cognitive impairments. Here we more closely examined how repeated stress exposure affects the regulation of basal levels of brain IL-1β by manipulating the “yin-yang” balance between β-AR stimulation and glucocorticoid suppression in male and female Fischer rats that underwent 4 days of stress exposure. Twenty-four hours after the final stressor, animals were administered propranolol (a β-AR antagonist), metyrapone (a glucocorticoid synthesis inhibitor), vehicle, or both drugs. Two hours following drug administration brain IL-1β mRNA was measure by rtPCR in limbic brain areas. Since we observe increases in both norepinephrine and corticosterone following four days of repeated stress exposure, we hypothesized that blockade of β-ARs would reveal a greater suppression of brain IL-1β while blockade of glucocorticoid production would reveal a greater stimulatory drive in stressed animals compared to non-stressed animals. While female rats display greater locus coeruleus-norepinephrine activation and corticosterone production following stress exposure compared to males (Curtis et al., 2006; Lu et al., 2015), we had no a priori reason to believe that there would be sex differences in how these stress hormones regulated brain IL-1β levels. Finally, to uncover the potential cellular source of IL-1β, microglia from the hypothalamus and hippocampus were isolated from saline and metyrapone treated animals. Microglia are the innate immune cell of the brain and have been implicated in being the cellular source of brain IL-1β during times of stress (Blandino et al., 2006), thus we hypothesized that metyrapone would result in an increase in IL-1β mRNA in isolated microglia.
2. Materials and Methods
2.1. Animals
Adult male and female Fischer rats were used for all studies in this investigation. Fischer animals are known to be more stress responsive compared to most other strains, which allows us to minimize the stress necessary to induce endocrine, cytokine, and behavioral changes. For example, restraint stress is sufficient to increase hypothalamic IL-1β in Fischer rats but fails to do so in Sprague-Dawley rats unless combined with additional stressors (Deak et al., 2005) or animals are pretreated with a norepinephrine reuptake inhibitor (Porterfield et al., 2011). Animals were single-housed in Plexiglas cages and provided environmental enriched (e.g. PVC tubes, plastic whiffle balls). Food and water were available ad libitum (except when food was removed as part of the chronic stress paradigm). Animals were maintained on a 12h light-dark cycle beginning at 07:00 h. Animals were given two weeks to adjust to being singled-housed, which was done to be consistent with past studies from our laboratory and eliminate social dominance hierarchy. Animals were handled daily to habituate animals to manipulations. Care and use of the animals was in accordance with protocols approved by the Kent State University Institutional Care and Use Committee.
2.2. Chronic stress paradigm
Rats were exposed to stressors for 4 days as previously described (Camp et al., 2012). Rats received two stressors each day, one between the times of 09:00–10:00 and one at 16:00 that lasted overnight, except on day 4 when animals were only exposed to stress between the times of 09:00–10:00 to avoid acute effects of stress on manipulations the following day. On day 1, rats were exposed to 1h restraint in a decapi-cone and then food deprived overnight. On day 2, rats received two (2s, 1.5mA) foot shocks in an operant box where they remained for 30min then exposed to constant light conditions overnight. On day 3, rats were exposed to cage tilt at 45° for 3h and wet bedding overnight. On day 4, rats were re-exposed to the operant box for 30min with two (2s, 1.5mA) foot shocks. Non-stressed control animals remained in their home cage throughout the stress paradigm, but otherwise were handled the same as animals that underwent stress. Pharmacological manipulations were performed on day 5 in the absence of acute stress challenge to examine how previous stress exposure alters the neuroendocrine regulation of basal brain IL-1β.
2.3. Drug administration
All pharmacological agents were purchased from Sigma-Aldrich and dissolved in sterile, endotoxin-free saline. On Day 5, approximately 24h after the last stress exposure, animals were administered saline, propranolol, metyrapone, or both propranolol + metyrapone. Propranolol, a beta-adrenergic receptor antagonist, was administered intraperitoneally at a dose of 10mg/kg, which we previously demonstrated blocks stress-induced brain IL-1β (Johnson et al., 2005). Metyrapone, a corticosterone synthesis inhibitor that inhibits steroid 11β-hydroxylase, was administered subcutaneously at a dose of 100mg/kg. As an indicator that metyrapone reduced circulating corticosterone levels, we measured circulating adrenocorticotropic hormone (ACTH) by EIA (ALPCO Salem, NH) to demonstrate the negative feedback on the hypothalamus-pituitary-adrenal axis had been reduced (see Supplemental Materials Figure 1S).
2.4. Tissue collection
All animals were euthanized by rapid decapitation 2h after administration of the pharmacological agents and brains placed in a chilled brain matrix where consistent slices could be obtained. Medial prefrontal cortex, hypothalamus, amygdala, and hippocampus were microdissected on a chilled glass plate. After removal of the olfactory bulbs, the medial prefrontal cortex was isolated from a 2mm thick slice ranging from approximately 5mm to 3mm anterior of Bregma where approximately 1mm of tissue was dissected on each side of the midline; hypothalamus was isolated from a 2mm thick slice ranging from approximately −1.2mm to −3.2mm posterior of Bregma where the slice was further dissected in half at the top of the 3rd ventricle and approximately 2mm of tissue was dissected on each side of the midline; the amygdala was dissected out of the same anterior-posterior slice by removing cortex from the tissue section remaining just lateral to the optic tracks; the hippocampus was isolated from a 4mm thick section ranging from approximately −3.2mm to −7.2mm posterior of Bregma where the hippocampus was separated from cortex and underlying brain structures. Tissue was placed into Eppendorf Snap-Cap Microcentrifuge tubes and froze in liquid nitrogen before being stored at −80°C until tissue was processed.
2.5. Quantitative real-time PCR
Dissected brain tissue was homogenized by sonication for 10sec in lysis buffer from Qiagen (Chatsworth, CA) RNeasy Mini kit. Tubes were spun briefly to pellet the mixture. The lysate was transferred to new 1.5-ml tubes. Total RNA was extracted by using the Qiagen RNeasy Mini kit following the manufacturer’s instructions including a DNase treatment. Quality and concentration of RNA was obtained using a Nanodrop and only samples with corresponding 260/280 and 260/230 ratios greater than 1.7 were further processed. Approximately, 200ng of isolated RNA was then reversed transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Cheshire, UK). The target cDNA was amplified by polymerase chain reaction (PCR). All qPCR assays were carried out in triplicate using the Brilliant III Ultra-Fast QPCR Master Mix (Agilent Technologies; Santa Clara, CA, USA) and using TaqMan Gene Expression Probes (ThermoFisher Scientific). The assay identification of each gene was IL-1β (Rn99999009_m1) and GAPDH (Rn01775763_g1). The cDNA quantities were normalized by using the housekeeping gene GAPDH as a reference and the 2−ΔΔct method was used to analyze relative gene expression as previously described (Porterfield et al., 2011).
2.6. Norepinephrine turnover
To assess changes in norepinephrine release following drug treatment, norepinephrine turnover rates were calculated. Brain areas were collected from saline injected animals and metyrapone injected animals and placed in snap-cap Eppendorf tubes and flash frozen in liquid nitrogen. Tissue was stored at −80 ℃until Iscove’s solution containing 2% aprotinin (Sigma-Aldrich) was added in a volume between 250 and 500ul as previously described (Porterfield et al., 2011). Tissue was homogenized using a sonic dismembrator and samples centrifuged at 10,000 rpm at 4 ℃ for 10 min. The supernatant was removed and commercially available EIAs (Rocky Mountain Diagnostics, Colorado Springs, CO) were used to measure levels of norepinephrine (NE) and normetanephrine (NMN). Samples were diluted 1:10 for NMN and 1:250 for NE. Both assays were performed according to manufacturer’s instructions. Neurotransmitter levels were corrected for dissection size by dividing total protein levels measured by Bradford Assay. NE turnover was determined by calculating NMN/NE.
2.7. Microglia Isolation
Two hours after vehicle or metytrapone administration, animals were deeply anesthetized with 60mg/kg pentobarbitol and perfused with ice-cold 0.9% saline. The hippocampus and hypothalamus were dissected and microglia isolated as previously described (Frank et al., 2007). Briefly, tissue was homogenized in 0.01M PBS + 0.2% L-glucose into a single cell suspension and filtered through a 40μm strainer before centrifuging for 5min at 1,000rpm. Cells were resuspended in 1ml of 70% isotonic Percoll and 2ml of 50% Percoll layered on top following a layer of 1ml sterile PBS, and cells were centrifuged for 20min at 2,000rpm. Cells were collected at the 70%−50% interface, washed with PBS, and centrifuged for 5min at 1,000rpm. Cells were re-suspended in PBS and counted using a hemocytometer prior to being placed in lysis buffer from Applied Biosystems (Foster City, CA) Arcturus PicoPure RNA Isolatation Kit and placed in −80℃ until processed. We previously demonstrated this isolation protocol results in approximately a 98.6% CD11b/c+ cell population (Johnson et al., 2013).
2.8. Statistics
A multi-factorial ANOVA was used to compare fold changes in IL-1β mRNA levels between control and stressed animals receiving either saline, propranolol, metyrapone, or combination of both propranolol and metyrapone. Statistically significant (alpha set at 0.05) main effects and interactions were followed by post-hoc analysis to compare individual group means when necessary. A t-test was used to compare norepinephrine turnover and IL-1β mRNA production in isolated microglia between saline and metyrapone-injected animals.
2.9. Experimental Design
Experiment 1 & 2 examined the effects of chronic stress on the “yin-yang” balance between β-ARs and glucocorticoids in the regulation of basal levels of brain IL-1β in male rats. We hypothesize that blockade of β-ARs would result in a greater suppression of IL-1β mRNA in animals exposed to chronic stress due to elevated circulating corticosterone in these animals (Lowrance et al., 2016). Alternatively, blocking corticosterone production would result in a greater stimulation of IL-1β in animals exposed to chronic stress due to increased levels of norepinephrine in these animals (Porterfield et al., 2012). To test this hypothesis, male Fischer rats (n= 6–7/group) were either exposed to 4 days of chronic stress or served as home cage controls. In Experiment 1, animals received an injection of saline or propranolol 24h after the last stress exposure and 2 h later animals were euthanized for measurement of brain IL-1β mRNA. In Experiment 2, animals received an injection of either saline, metyrapone or a combination of metyrapone and propranolol 24h after the last stress exposure and 2 h later euthanized for the measurement of brain IL-1β mRNA.
Experiment 3 examined the effects of chronic stress on the “yin-yang” balance between β-ARs and glucocorticoids in the regulation of basal levels of brain IL-1β in female rats. This was the first study in our laboratory to examine if female rats show the same “yin-yang” balance of brain IL-1β regulation as male rats. Female Fischer rats (n= 6/group) were either exposed to 4 days of chronic stress or served as home cage controls. On the fifth day, animals received an injection of either saline, metyrapone, propranolol, or a combination of metyrapone and propranolol and 2 h later animals were euthanized for measurement of IL-1β mRNA.
Experiment 4 examined the effect of metyrapone on norepinephrine turnover in limbic brain regions in male rats. We hypothesized that since metyrapone treatment resulted in an increase in IL-1β mRNA expression throughout the brain and that propranolol inhibited this increase that metyrapone treatment may result in an increase in norepinephrine turnover. Male Fischer rats (n=9–10/group) were given an injection of either saline or metyrapone and 2 h later animals were euthanized for measure of norepinephrine turnover.
Experiment 5 examined the effect of metyrapone on norepinephrine turnover in limbic brain regions in female rats. Since metyrapone had no effect on brain IL-1β in female rats, we hypothesized that metyrapone treatment would have no effect on norepinephrine turnover in females. Female Fischer rats (n= 8/group) were given an injection of either saline or metyrapone and 2 h later animals were euthanized for measure of norepinephrine turnover.
Experiment 6 examined the effect of metyrapone on IL-1β mRNA in microglia. Catecholamines can stimulate the production of IL-1β in microglia (Ryan et al., 2016), thus we hypothesized that microglia may be a cellular source of the increase in IL-1β following metyrapone. To test this, male Fischer rats (n=4/group) were given an injection of either saline or metyrapone and 2 h later animals were euthanized and microglia were isolated from the hypothalamus and hippocampus for the measurement of IL-1β mRNA.
3. Results
3.1. The effect of stress on β-AR regulation of brain IL-1β mRNA in male rats
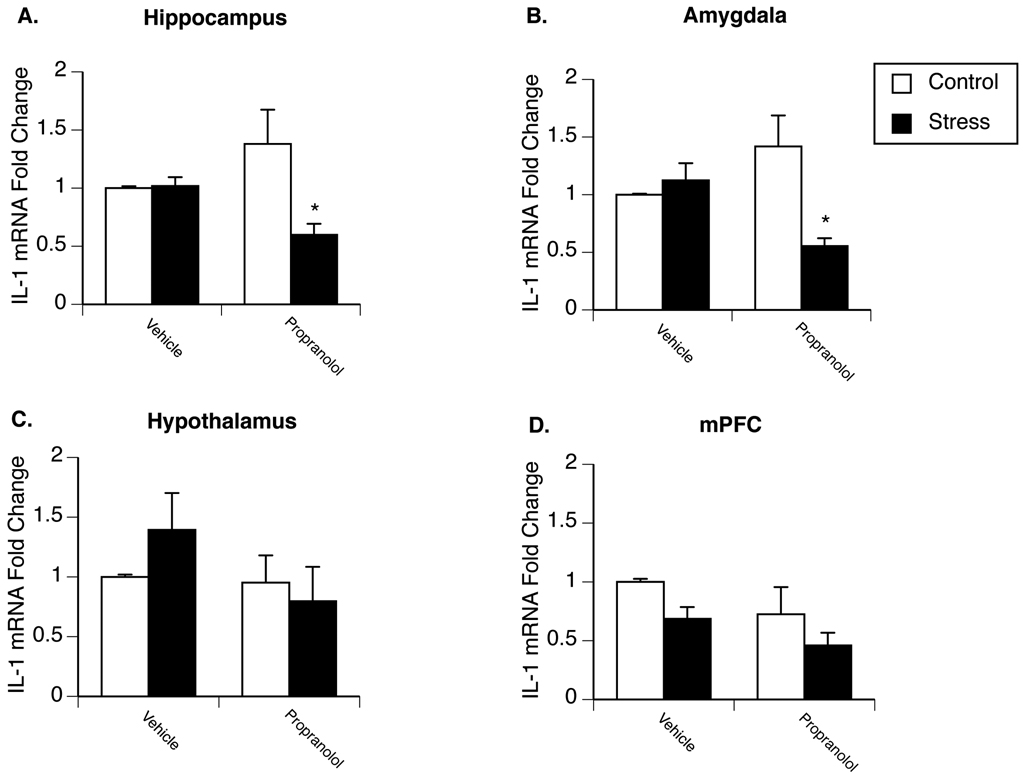
Experiment 1 examined the effect of chronic stress on β-AR regulation of basal brain IL-1β mRNA in male rats. Figure 1A–D present fold change of IL-1β mRNA in the hippocampus, amygdala, hypothalamus, and medial prefrontal cortex of non-stressed controls compared to animals exposed to 4 days of repeated stress exposure. Prior stress did not affect IL-1β mRNA expression in any brain region compared to vehicle-injected control animals. Administration of propranolol 2h prior to tissue collection had no effect on IL-1β mRNA levels in control animals; however, a significant interaction between stress and drug treatment was observed in both the hippocampus [F(1,26)=7.273; p=0.013] (Fig. 1A) and amygdala [F(1,20)=9.779; p=0.007] (Fig. 1B). Additional analyses revealed a significant decrease in IL-1β mRNA expression in chronically stressed animals following propranolol treatment in the both the amygdala and hippocampus.
Figure 1.
Effects of stress on β-adrenergic receptor regulation of brain IL-1β mRNA expression in male rats. Rats were exposed to 4 days of stress or remained in their home cage to serve as controls. Twenty-four hours after the last stressor animals were administered either saline or propranolol and two hours later euthanized for measurement of IL-1β mRNA in the hippocampus (A), amygdala (B), hypothalamus (C), and medial prefrontal cortex (D). Results revealed a significant interaction between stress and drug treatment in the hippocampus and amygdala. * represent significant (p < 0.05) decreases in IL-1β mRNA compared to vehicle-treated stress rats. Values represent mean IL-1β mRNA fold change compared to non-stressed, vehicle-treated animals. Symbols and bars represent group mean ± SEM.
3.2. The effect of stress on glucocorticoid regulation of brain IL-1β mRNA in male rats.
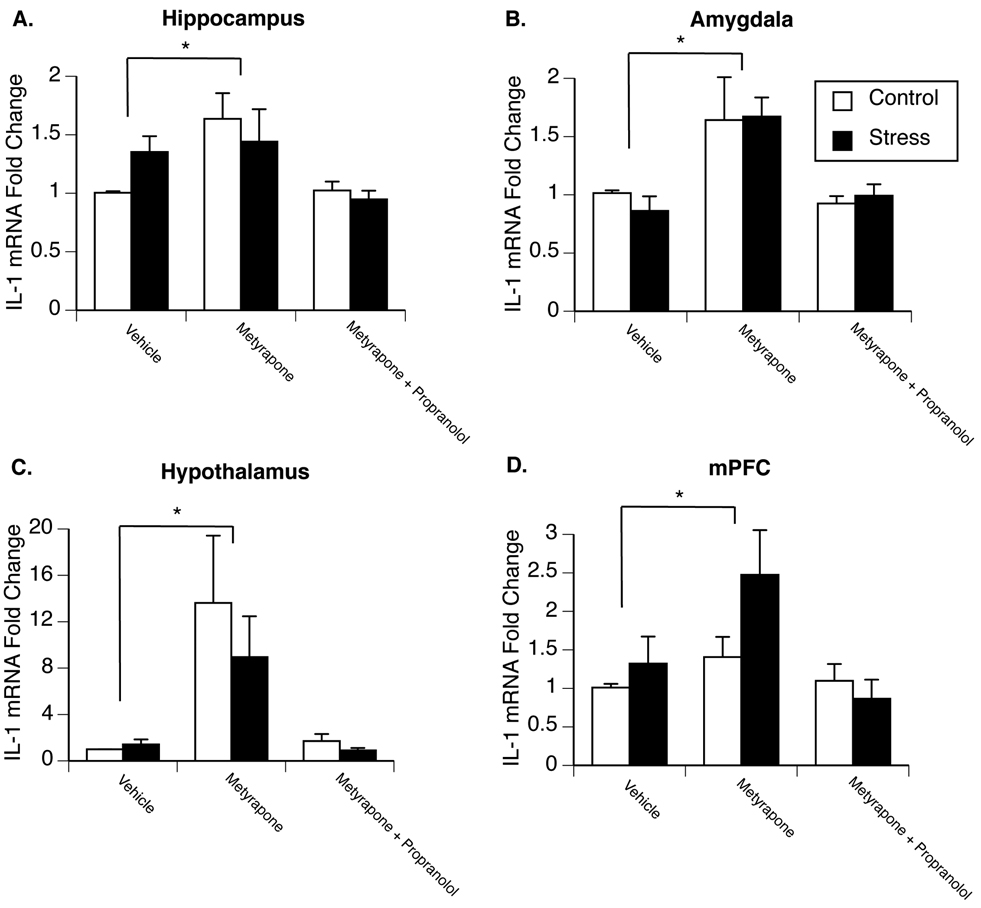
Experiment 2 examined how chronic stress exposure might alter the way glucocorticoids regulate IL-1β mRNA in male rats. Figure 2A–D present fold change of IL-1β mRNA in the hippocampus, amygdala, hypothalamus, and medial prefrontal cortex of control and stressed animals administered either vehicle, metyrapone, or both metyrapone and propranolol 2h prior to tissue collection. As previously observed, 4 days of repeated stress exposure did not significantly alter IL-1β mRNA expression in any brain region compared to saline-injected control animals. No significant interaction between metyrapone and stress was found; however, there was a significant main effect of metyrapone-treatment in all brain areas Metyrapone significantly increased IL-1β mRNA levels in the hippocampus [F(2,39)=5.665; p=0.008] (Fig. 2A), amygdala [F(2,35)=9.056; p=0.001] (Fig. 2B), hypothalamus [F(2,39)=7.631; p=0.002] (Fig 2C), and medial prefrontal cortex [F(2,35)=4.038; p=0.028] (Fig. 2D) compared to vehicle-injected animals. Interestingly, no increase in IL-1β mRNA was observed in animals that received a combination of metyrapone and propranolol. In additional animals it was observed that metyrapone also significantly increased IL-1β protein production in the hypothalamus (see supplemental Figure S2).
Figure 2.
Effects of stress on glucocorticoid regulation of brain IL-1β mRNA expression in male rats. Rats were exposed to 4 days of stress or remained in their home cage to serve as controls. Twenty-four hours after the last stressor animals were administered either saline, metyrapone, or a combination of metyrapone and propranolol and two hours later euthanized for measurement of IL-1β mRNA in the hippocampus (A), amygdala (B), hypothalamus (C), and medial prefrontal cortex (D). A significant main effect of metyrapone was observed in all brain areas. * represent significant (p < 0.05) main effect of metyrapone treatment compared to vehicle-injected animals. Values represent mean IL-1β mRNA fold change compared to non-stressed, vehicle-treated animals. Symbols and bars represent group mean ± SEM.
3.3. The effect of stress on glucocorticoid and β-AR regulation of brain IL-1β mRNA in female rats.
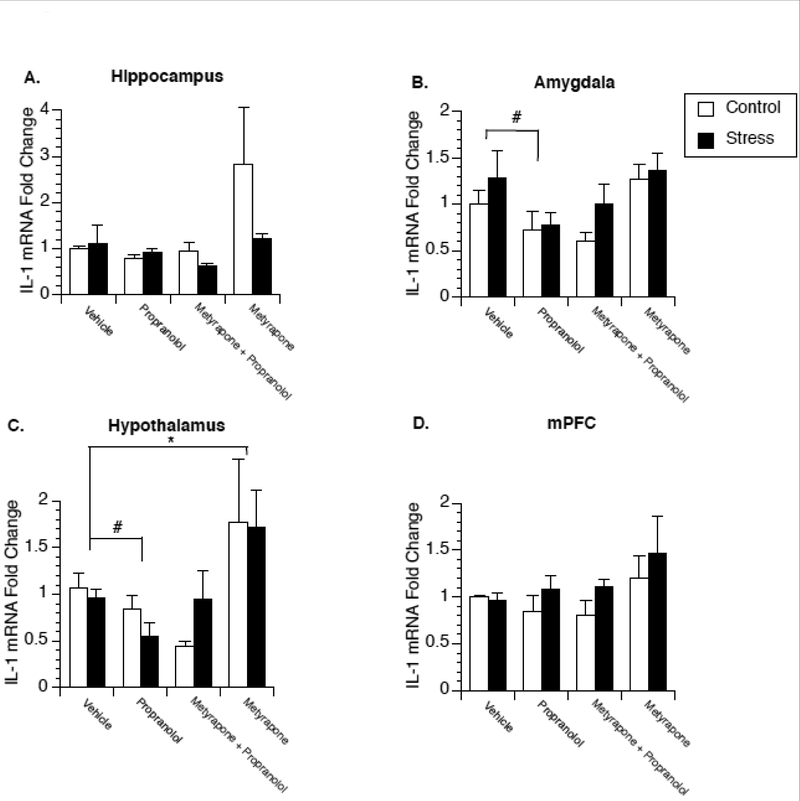
Experiment 3 examined whether similar changes in the regulation of brain IL-1β would be observed in female rats following repeated stress exposure. Figure 3A–D present fold change of IL-1β mRNA in control and stressed female rats administered saline, propranolol, metyrapone, or propranolol in combination with metyrapone. Similar to males, repeated stress exposure over a 4 day period did not significantly alter IL-1β mRNA expression in any brain region compared to saline-injected controls, and metyrapone treatment significantly increased IL-1β mRNA expression, although this was only observed in the hypothalamus [F(3,47)=5.584; p=0.003] (Fig 3C) and not in other brain areas. In contrast to males, a main effect of propranolol was observed in both the amygdala [F(3,40)=4.613; p=0.007] (Fig. 3B) and the hypothalamus [F(3,38)=4.498; p=0.009] (Fig. 3C) where propranolol-injected animals showed significantly lower IL-1β mRNA compared to saline-inject animals. No effect of drug treatment was observed in the hippocampus (Fig 3A) or medial prefrontal cortex (Fig 3D).
Figure 3.
Effect of stress on glucocorticoid and β-adrenergic receptor regulation of brain IL-1β expression in female rats. Rats were exposed to 4 days of stress or remained in their home cage to serve as controls. Twenty-four hours after the last stressor animals were administered either saline, propranolol, metyrapone, or a combination of metyrapone and propranolol and two hours later euthanized for measurement of IL-1β mRNA in the hippocampus (A), amygdala (B), hypothalamus (C), and medial prefrontal cortex (D). A significant main effect of propranolol was observed in the amygdala and hypothalamus. A significant main effect of metyrapone was observed in the hypothalamus. * represent significant (p < 0.05) main effect of metyrapone treatment compared to saline-injected animals. # represent significant (p < 0.05) main effect of propranolol treatment compared to saline-injected animals. Values represent mean IL-1β mRNA fold change compared to non-stressed, vehicle treated animals. Symbols and bars represent group mean ± SEM.
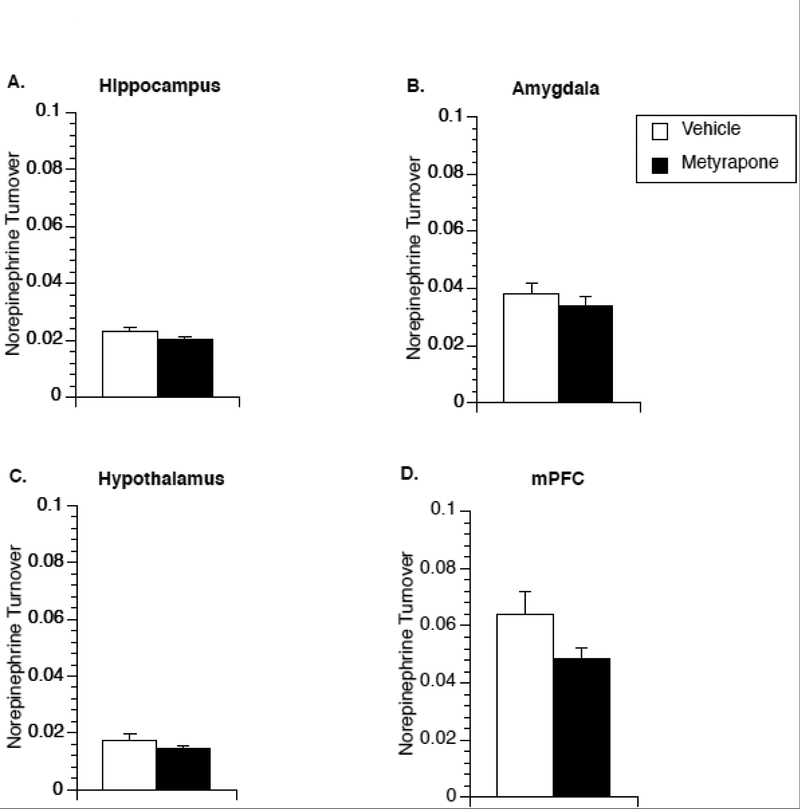
3.4. The effect of metyrapone on norepinephrine turnover in limbic brain regions in male rats.
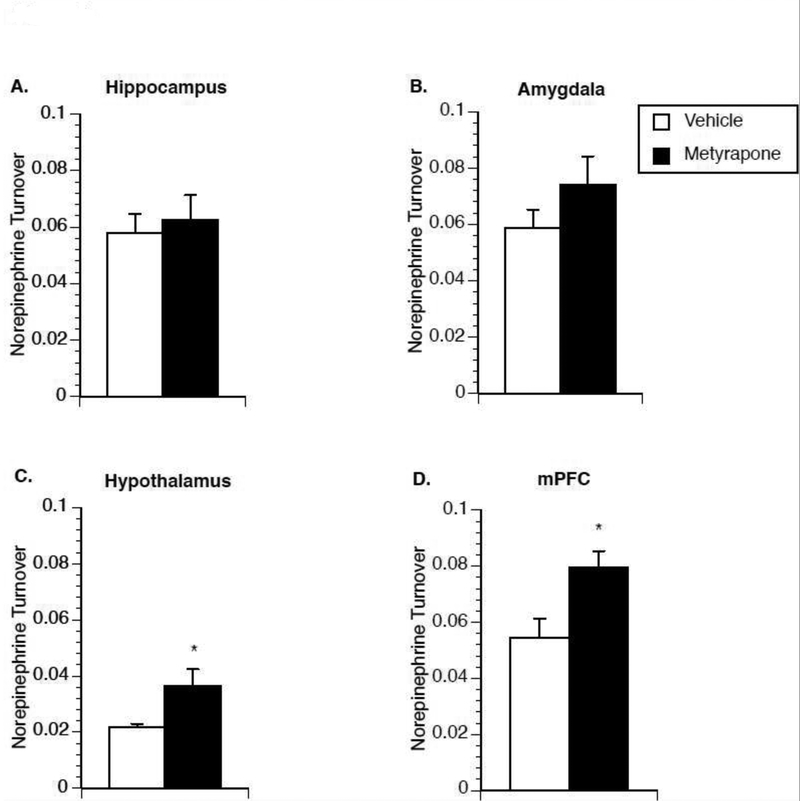
Metyrapone, in male rats, had a pronounced stimulatory effect on IL-1β mRNA expression in multiple brain regions that was blocked by co-administration of propranolol, suggesting that glucocorticoids may mediate IL-1β production via regulation of norepinephrine release. To test whether metyrapone affects norepinephrine release, Experiment 4 measured norepinephrine turnover in brain tissue of non-stressed male rats collected 2h after injection of either metyrapone or saline. Metyrapone-treated animals showed a significant increase in norepinephrine turnover in the hypothalamus [t(17)=−2.276; p=0.036] (Fig 4C) and medial prefrontal cortex [t(17)=−2.798; p=0.012] (Fig 4D).
Figure 4.
Effect of metyrapone on brain norepinephrine turnover in male rats. Two hours after saline or metyrapone administration animals were euthanized for measurement of norepinephrine turnover in the hippocampus (A), amygdala (B), hypothalamus (C), and medial prefrontal cortex (D). A significant main effect of metyrapone treatment was observed in the hypothalamus and medial prefrontal cortex. * represent significant (p < 0.05) main effect of metyrapone treatment compared to saline-injected controls. Values represent group means of norepinephrine turnover. Symbols and bars represent group mean ± SEM.
3.5. The effect of metyrapone on norepinephrine turnover in limbic brain regions in female rats.
Compared to males, metyrapone had relatively little effect on IL-1β mRNA production in females. To explore whether this difference may be due to potential sex differences in corticosterone’s regulation of norepinephrine turnover, Experiment 5 measured norepinephrine turnover in brain tissue collected 2h after injection of either metyrapone or saline in female rats. No significant differences in norepinephrine turnover were observed between saline and metyrapone injected animals in any of the brain regions collected (Fig 5A–D).
Figure 5.
Effect of metyrapone on brain norepinephrine turnover in female rats. Two hours after saline or metyrapone administration animals were euthanized for measurement of norepinephrine turnover in the hippocampus (A), amygdala (B), hypothalamus (C), and medial prefrontal cortex (D). No significant effect was observed in any brain region. Values represent group means of norepinephrine turnover. Symbols and bars represent group mean ± SEM.
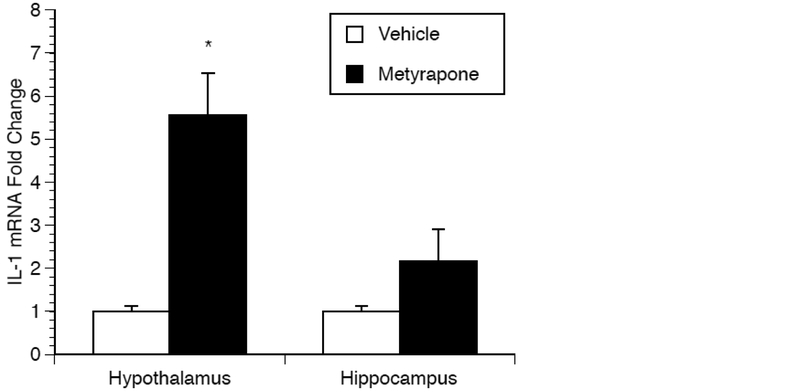
3.6. The effect of metyrapone on microglia IL-1β mRNA production.
Metyrapone significantly increased IL-1β in male rats across all brain regions. To determine whether microglia contribute to the increase in IL-1β production, microglia were isolated from the hippocampus and hypothalamus of male rats that received an injection of either saline or metyrapone. A significant main effect of drug treatment on IL-1β mRNA production was observed [F(1,15)=26.537; p=0.0001], along with a significant interaction between brain region and treatment [F(1,15)=9.268; p=0.011] (Fig. 6). Posthoc analysis revealed a significant increase in IL-1β mRNA in the hypothalamus of metyrapone treated animals (p=0.041) compared to vehicle controls. The IL-1β mRNA increase observed in the hippocampus fell short of significance.
Figure 6.
Effect of metyrapone on microglia IL-1β mRNA production. Two hours after saline or metyrapone administration animals were euthanized and microglia isolated for the measurement of IL-1β mRNA in the hippocampus and hypothalamus. A significant interaction was observed between brain region and treatment. * represents significant (P< 0.05) effect of metyrapone compared to vehicle control. Values represent mean IL-1β mRNA fold change compared to vehicle treated animals. Symbols and bars represent group mean ± SEM.
4. Discussion
Studies presented here tested the hypothesis that the “yin-yang” balance between corticosterone and catecholamines becomes heightened following repeated stress exposure (i.e. stressed animals show both greater circulating corticosterone and greater brain norepinephrine turnover) and this would result in greater fluctuation in brain IL-1β in stressed animals when these regulatory hormones were manipulated. We predicted that blocking β-ARs would reveal a greater suppression in brain IL-1β while blocking corticosterone production would reveal a greater stimulatory drive on brain IL-1β in stressed animals compared to non-stressed animals. Part of our original hypothesis was supported in male rats where the administration of propranolol decreased IL-1β mRNA in the hippocampus and amygdala of stressed animals but had no effect in non-stressed controls. This supports our previous work demonstrating propranolol does not affect baseline IL-1β levels in non-stressed male rats (Johnson et al., 2005; Johnson et al., 2008), and demonstrates that, at least in some brain areas, the increase in brain norepinephrine release in stressed animals (Porterfield et al., 2012) likely compensates for a greater inhibitory drive on IL-1β. Despite the fact that greater β-AR signaling appears to be necessary to maintain basal IL-1β levels in stressed male rats, blockade of corticosterone production following metyrapone did not result in significantly greater IL-1β mRNA production in stressed animals compared to non-stressed controls animals as originally predicted. However, we observed interesting sex differences in how corticosterone regulates brain IL-1β that might help explain this.
One of our most interesting findings was the sex difference in the way glucocorticoids regulate brain IL-1β expression. In males, inhibiting the synthesis of corticosterone by metyrapone resulted in widespread increases in IL-1β mRNA expression throughout the brain, while in females increases were restricted to the hypothalamus (and even there the increases were approximately 1/10th that observed in males). The sex differences may involve the magnitude by which glucocorticoids regulate norepinephrine release between males and females since metyrapone-treatment increased norepinephrine turnover in the hypothalamus and medial prefrontal cortex of male rats while having no effect on norepinephrine turnover in females In both males and females, propranolol blocked the increase in IL-1β mRNA induced by metyrapone-treatment indicating the increase in IL-1β expression was dependent on β-AR activation. Ryan et al. (2016) demonstrated that β-ARs regulate IL-1β production in microglia. To examine if microglia were a potential cellular source of IL-1β mRNA in our studies, we used a Percoll gradient to isolate microglia (i.e. enriched population of CD11b+ cells) from the brain of male rats injected with either vehicle or metyrapone. We observe large increases in IL-1β mRNA from cells isolated from the hypothalamus of animals treated with metyrapone. This supports previous studies that indicate microglia are the primary cellular source of IL-1β in response to catecholamine release during stress exposure (Blandino et al., 2006; Wohleb et al., 2012).
There are several limitations to our experiments that need to be considered. First is the fact that increased norepinephrine turnover was not observed in all brain areas where IL-1β expression was observed to increase, which would have been expected if norepinephrine release was driving the IL-1β production. This may be due to the limitation of using normetanephrine as the metabolite for calculating norepinephrine turnover. Normetanephrine is generated during the metabolism of norepinephrine by catechol-O-methyl-transferase (COMT). While it is useful to measure as an index of norepinephrine release when analyzing a large number of brain areas, it does not have the temporal resolution or sensitive as other measures (e.g. microdialysis or fast-scan cyclic voltammetry). Another limitation is that male and female rats were not run in the same experiments thus statistical analysis cannot directly compare quantitative differences between sexes. However, our data indicate qualitative differences in the way male and female rats respond to different pharmacological agents (i.e. propranolol, metyrapone) and therefore additional studies are warranted to investigate sex differences in the way glucocorticoids regulate norepinephrine release and brain cytokines.
Our data support previous work in male rats demonstrating corticosterone feeds back to suppress norepinephrine release (Pacák et al., 1993). Previous studies examined the suppressive effects of corticosterone on norepinephrine release during times of stress when corticosterone levels are high and at sufficient levels to bind to the low affinity glucocorticoid receptors (GR). In contrast, we administered metyrapone during the early morning (2–3h after lights-on) when corticosterone levels are at their trough, a time when corticosterone is thought to primarily bind to the high affinity mineralocorticoid receptors (MR) (De Kloet et al., 1987; De Kloet and Reul, 1987). This indicates that MR likely regulated the suppressive effects corticosterone had on norepinephrine release in our studies. Our studies were not designed to specifically examine MR/GR differences in the regulation of brain cytokines, rather we investigated the possible mechanisms by which metyrapone increased IL-1β mRNA. Additional studies are needed to elucidate how brain cytokines are regulated by glucocorticoids and how changes in MR/GR expression, which can occur following stress exposure (Lisieski et al., 2018; Zhang et al., 2017), impact brain cytokine regulation.
Sexually dimorphic responses of locus coeruleus neurons may explain why stress exposure increases β-ARs regulation of brain IL-1β mRNA in males but not females. The locus coeruleus is the primary location of noradrenergic neurons that project to forebrain areas including the prefrontal cortex, hippocampus, and other limbic brain areas. Neural excitability of locus coeruleus neurons is greater in females compared to males in response to corticotropin-releasing factor (CRF) or stress exposure (Bangasser et al., 2018; Bangasser and Wiersielis, 2018; Curtis et al., 2006). If female rats have greater norepinephrine release in response to being handled and the stress of receiving an injection compared to male rats, it is possible this may explain why propranolol was able to lower IL-1β mRNA in female control animals but not in males. Following stress exposure, locus coeruleus neural activity becomes sensitized in male rats but is not changed in female rats (Curtis et al., 2006); that is, stressed males show a leftward shift in their CRF dose response curve for stimulating locus coeruleus neurons such that a stressed male rat shows similar sensitivity to non-stressed females. Sensitization of locus coeruleus neurons in stressed male rats may be why they show similar regulation of brain IL-1β following propranolol administration compared to non-stressed female rats. An example of this is in the amygdala where propranolol administration reduced IL-1β mRNA in stressed and non-stressed female and stressed male rats but had no effect in non-stressed male rats. If brain cytokines contribute to depression and anxiety, then greater sensitivity of norepinephrine-β-AR regulation of brain IL-1β may be one factor that increases one’s susceptibility to these psychopathologies.
Given the growing evidence that stress-induced brain IL-1β contributes to the onset of depression and cognitive deficits in laboratory animals, it is important to better understand how brain IL-1β is regulated and how the regulation changes with repeated stress exposure. As demonstrated here, repeated stress exposure does not always result in greater basal levels of IL-1β throughout the brain. This is in agreement with other studies that fail to detect increases in basal brain IL-1β following chronic mild stress (Mormède et al., 2002; Porterfield et al., 2012) or repeated social defeat (Audet et al., 2011; Hueston et al., 2011). While others have reported basal increases in IL-1β following chronic mild stress (Goshen et al., 2008) or repeated social defeat (Wohleb et al., 2012; Wood et al., 2015), it is unclear whether persistent increases in brain IL-1β is necessary to mediate stress-induced behavioral changes or whether transient elevations in brain IL-1β during stress exposure or the dysregulation of IL-1β to environmental stimuli following stress exposure are critical. Data presented here reveal potential sex differences in the regulation of brain IL-1β, and demonstrate for the first time that glucocorticoids can indirectly regulate brain IL-1β expression through the modulation of norepinephrine release. Repeated stress exposure alters the regulation of brain IL-1β in male rats making the regulation more closely resemble that observed in non-stressed female rats. The behavioral and physiological consequences of these sex differences and changes in cytokine regulation need to be further explored.
Supplementary Material
Highlights.
Stress exposure affects the regulation of brain IL-1 in male but not female rats
Glucocorticoids suppress norepinephrine turnover in male but not female rats
Metyrapone increases brain IL-1 more prominently in male compared to female rats
Propranolol blocks the increase in brain IL-1 observed following metyrapone treatment
Acknowledgements:
This work was supported by the National Institutes of Health [MH099580 and MH114049]. D.F.B. and J.D.J. contributed to the conception and design of the study, D.F.B., K.M.G., A.K., A.P., and P.B.D. participated in acquisition of data, D.F.B. and J.D.J. analyzed and interpreted the data, D.F.B. drafted the article, D.F.B. and J.D.J. revised the article, and D.F.B., K.M.G., A.K., A.P., P.B.D., J.D.J. approved the final version of the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflicts of interest to declare.
Bibliography
- Audet MC, Jacobson-Pick S, Wann BP, Anisman H, 2011. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav Immun 25, 1197–1205. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, Chrousos GP, Geracioti TD, 2001. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 9, 209–217. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Eck SR, Ordoñes Sanchez E, 2018. Sex differences in stress reactivity in arousal and attention systems. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR, 2018. Sex differences in stress responses: a critical role for corticotropin-releasing factor. Hormones (Athens) 17, 5–13. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, 2003. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav 43, 158–165. [DOI] [PubMed] [Google Scholar]
- Blandino P, Barnum CJ, Deak T, 2006. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol 173, 87–95. [DOI] [PubMed] [Google Scholar]
- Camp RM, Johnson JD, 2015. Repeated stressor exposure enhances contextual fear memory in a beta-adrenergic receptor-dependent process and increases impulsivity in a non-beta receptor-dependent fashion. Physiol Behav 150, 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp RM, Remus JL, Kalburgi SN, Porterfield VM, Johnson JD, 2012. Fear conditioning can contribute to behavioral changes observed in a repeated stress model. Behav Brain Res 233, 536–544. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R, 2004. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun 18, 205–213. [DOI] [PubMed] [Google Scholar]
- Craft TK, DeVries AC, 2006. Role of IL-1 in poststroke depressive-like behavior in mice. Biol Psychiatry 60, 812–818. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ, 2006. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31, 544–554. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Ratka A, Reul JM, Sutanto W, Van Eekelen JA, 1987. Corticosteroid receptor types in brain: regulation and putative function. Ann N Y Acad Sci 512, 351–361. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Reul JM, 1987. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology 12, 83–105. [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG, 2003. Exposure to forced swim stress does not alter central production of IL-1. Brain Res 972, 53–63. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Deak MM, Tammariello SP, 2005. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull 64, 541–556. [DOI] [PubMed] [Google Scholar]
- Fernandes GA, Perks P, Cox NK, Lightman SL, Ingram CD, Shanks N, 2002. Habituation and cross-sensitization of stress-induced hypothalamic-pituitary-adrenal activity: effect of lesions in the paraventricular nucleus of the thalamus or bed nuclei of the stria terminalis. J Neuroendocrinol 14, 593–602. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF, 2007. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun 21, 47–59. [DOI] [PubMed] [Google Scholar]
- García-Bueno B, Madrigal JL, Pérez-Nievas BG, Leza JC, 2008. Stress mediators regulate brain prostaglandin synthesis and peroxisome proliferator-activated receptor-gamma activation after stress in rats. Endocrinology 149, 1969–1978. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R, 2008. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13, 717–728. [DOI] [PubMed] [Google Scholar]
- Hueston CM, Barnum CJ, Eberle JA, Ferraioli FJ, Buck HM, Deak T, 2011. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the Sprague Dawley rat. Physiol Behav 104, 187–198. [DOI] [PubMed] [Google Scholar]
- Hueston CM, Deak T, 2014. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol Behav 124, 77–91. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M, 2005. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135, 1295–1307. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Cortez V, Kennedy SL, Foley TE, Hanson H, Fleshner M, 2008. Role of central beta-adrenergic receptors in regulating proinflammatory cytokine responses to a peripheral bacterial challenge. Brain Behav Immun 22, 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Zimomra ZR, Stewart LT, 2013. Beta-adrenergic receptor activation primes microglia cytokine production. J Neuroimmunol 254, 161–164. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA, 1999. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156, 837–841. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A, 2003. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav Immun 17, 373–383. [DOI] [PubMed] [Google Scholar]
- Ley EJ, Clond MA, Bukur M, Park R, Chervonski M, Dagliyan G, Margulies DR, Lyden PD, Conti PS, Salim A, 2012. β-adrenergic receptor inhibition affects cerebral glucose metabolism, motor performance, and inflammatory response after traumatic brain injury. J Trauma Acute Care Surg 73, 33–40. [DOI] [PubMed] [Google Scholar]
- Lisieski MJ, Eagle AL, Conti AC, Liberzon I, Perrine SA, 2018. Single-Prolonged Stress: A Review of Two Decades of Progress in a Rodent Model of Post-traumatic Stress Disorder. Front Psychiatry 9, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrance SA, Ionadi A, McKay E, Douglas X, Johnson JD, 2016. Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress. Psychoneuroendocrinology 68, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wu XY, Zhu QB, Li J, Shi LG, Wu JL, Zhang QJ, Huang ML, Bao AM, 2015. Sex differences in the stress response in SD rats. Behav Brain Res 284, 231–237. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR, 1995. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res 695, 279–282. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO, 2005. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry 29, 1214–1224. [DOI] [PubMed] [Google Scholar]
- Mormède C, Castanon N, Médina C, Moze E, Lestage J, Neveu PJ, Dantzer R, 2002. Chronic mild stress in mice decreases peripheral cytokine and increases central cytokine expression independently of IL-10 regulation of the cytokine network. Neuroimmunomodulation 10, 359–366. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF, 1998. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci 18, 2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, Watkins LR, Maier SF, 2000. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res 859, 193–201. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF, 2003. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res 991, 123–132. [DOI] [PubMed] [Google Scholar]
- Pacák K, Kvetnanský R, Palkovits M, Fukuhara K, Yadid G, Kopin IJ, Goldstein DS, 1993. Adrenalectomy augments in vivo release of norepinephrine in the paraventricular nucleus during immobilization stress. Endocrinology 133, 1404–1410. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Ma S, Morilak DA, 2003. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res 971, 55–65. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Bedard T, Merali Z, Anisman H, 2000. Neither acute nor chronic exposure to a naturalistic (predator) stressor influences the interleukin-1beta system, tumor necrosis factor-alpha, transforming growth factor-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Bull 51, 187–193. [DOI] [PubMed] [Google Scholar]
- Porterfield VM, Gabella KM, Simmons MA, Johnson JD, 2012. Repeated stressor exposure regionally enhances beta-adrenergic receptor-mediated brain IL-1β production. Brain Behav Immun 26, 1249–1255. [DOI] [PubMed] [Google Scholar]
- Porterfield VM, Zimomra ZR, Caldwell EA, Camp RM, Gabella KM, Johnson JD, 2011. Rat strain differences in restraint stress-induced brain cytokines. Neuroscience 188, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KM, Griffin É, Ryan KJ, Tanveer R, Vanattou-Saifoudine N, McNamee EN, Fallon E, Heffernan S, Harkin A, Connor TJ, 2016. Clenbuterol activates the central IL-1 system via the β2-adrenoceptor without provoking inflammatory response related behaviours in rats. Brain Behav Immun 56, 114–129. [DOI] [PubMed] [Google Scholar]
- Smoak KA, Cidlowski JA, 2004. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev 125, 697–706. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP, 2012. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37, 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF, 2011. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31, 6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF, 2014. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci 34, 2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Wood CS, Lombard CM, Lee CS, Zhang XY, Finnell JE, Valentino RJ, 2015. Inflammatory Factors Mediate Vulnerability to a Social Stress-Induced Depressive-like Phenotype in Passive Coping Rats. Biol Psychiatry 78, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GP, Van Bockstaele E, Reyes B, Bethea T, Valentino RJ, 2004. Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. J Neurosci 24, 8193–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fan Y, Raza MU, Zhan Y, Du XD, Patel PD, Zhu MY, 2017. The regulation of corticosteroid receptors in response to chronic social defeat. Neurochem Int 108, 397–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.