ABSTRACT
Chaperone-mediated autophagy (CMA) is a selective form of autophagy that allows the elimination and recycling of cytosolic proteins endowed with a KFERQ-like motif into the lysosome. During this process, the proteins to be degraded are recognized by cellular chaperones such as HSC70 and presented to the CMA receptor LAMP2A, which then translocate them into lysosomes for degradation. In this punctum, we discuss the mechanisms underlying the response and resistance to Azacitidine (Aza) in MDS/AML cell lines and bone marrow CD34+ blasts from MDS/AML patients. We show that treatment of MDS/AML cell lines and bone marrow samples from MDS/AML patients with Aza triggers loss of LAMP2 expression leading to CMA defects. LAMP2 deficiency is responsible for CMA defects, Aza resistance and hypersensitivity to lysosome and autophagy inhibitors. Low levels of LAMP2 expression in CD34+ blasts from MDS/AML patients correlate with an absence of response to Aza and are associated to a pejorative overall survival. We propose that CD34+/LAMP2Low patients at diagnosis or who become CD34+/LAMP2Low during the course of treatment with Aza could receive an autophagy inhibitor available in the clinic.
KEYWORDS: AML, azacytidine, CMA, lysosome inhibitors, MDS
MyeloDysplastic syndromes (MDS) consist of a heterogeneous spectrum of myeloid clonal hematological disorders that appear frequently in elderly patients. This disease incorporates 2 major clinical features: a differentiation defect of hematopoietic cells that results in bone marrow failure associated with peripheral blood cytopenias, and expansion of at least one abnormal hematopoietic clone which undergoes, in 30% of MDS cases, evolution to acute myeloid leukemia (AML). Treatments for AML and higher-risk MDS in younger patients have remained unchanged for the past 4 decades. These treatments include a dose-intensive chemotherapy based on a combination of anthracyclines with cytarabine (Ara-C), and, for eligible patients, bone marrow transplantation. Given the high toxicity of AML-like chemotherapy in older patients with higher-risk MDS, azacytidine (Aza) has now replaced the anthracycline-Ara-C combination. These agents can achieve a sustained remission in a majority of cases and even a cure in some patients. However, almost all patients relapse with a progressively more chemoresistant disease. The mechanisms underlying this resistance process remain a conundrum and are currently subjected to intense research, not only for fundamental biological insights but also to define appropriate therapeutic strategies to durably circumvent the relapsed disease.
LAMP2 (lysosomal-associated membrane protein 2) is a member of a family of lysosomal membrane glycoproteins that also includes LAMP1. LAMP1/2 proteins represent almost 50% of all lysosomal membrane proteins and play important roles in the protection and maintenance of the functionality of lysosomes. The LAMP2 gene gives rise to 3 alternative mRNA splicing variants encoding the LAMP2 protein isoforms called LAMP2A, LAMP2B and LAMP2C. Each isoform is involved in a specific form of autophagy: macroautophagy for LAMP2B, chaperone-mediated autophagy (CMA) for LAMP2A and RNA and DNAphagy for LAMP2C. LAMP2A is the receptor for CMA, a highly selective form of autophagy that allows the specific degradation by lysosomes of cytosolic proteins endowed with a KFERQ motif.
To decipher the mechanisms of resistance to Aza in MDS, we derived Aza-resistant cells by multiple round of Aza treatment of the human SKM1 MDS cell line [1]. Transcriptional profiling of these cells established that multiple lysosome-related enzymes are markedly downregulated in Aza-resistant cells compared to their sensitive counterpart. Among those, a lysosomal membrane protein, LAMP2, was functionally validated as an essential mediator of resistance to Aza in MDS. Importantly, the expression of all 3 LAMP2 isoforms, LAMP2A, LAMP2B, and LAMP2C are reduced in Aza-resistant cells compared to their sensitive counterpart. LAMP2A plays a central role in CMA, and we confirmed that the decreased expression of LAMP2 is associated with a blockade of CMA and an increased stabilization of known CMA substrates such as MLLT11/AF1Q and BCL2L10 that play a role in the resistance and the survival of MDS cell lines to chemotherapy, respectively (Figure 1). In addition, LAMP2 knockdown in MDS and AML cells promotes resistance to Aza, and re-expression of LAMP2 in LAMP2-deficient MDS and AML cells restores sensitivity to the drug and promotes degradation of MLLT11/AF1Q and BCL2L10 through CMA. Of note, analysis of 2 cohorts of MDS and AML patients revealed that patients with low LAMP2 expression exhibit a worse clinical outcome than those with high LAMP2 expression. Finally, blasts from Aza-resistant MDS-AML patients, which express a low level of LAMP2 are highly sensitive to autophagy inhibitors including bafilomycin A1 and hydroxychloroquine ex vivo, conversely to those of Aza-sensitive patients exhibiting a high level of LAMP2.
Figure 1.
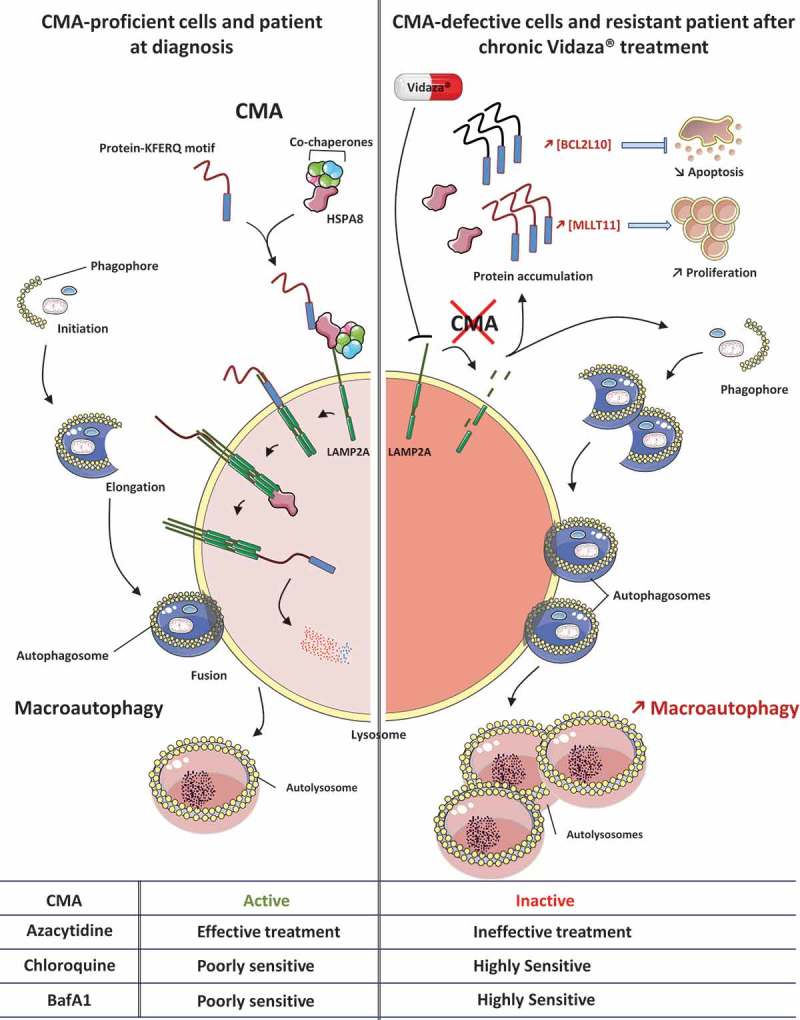
Inhibition of CMA is compensated by activation of macroautophagy in Aza-resistant MDS and AML cell lines and patients. In CMA-proficient cells and MDS-AML patients at diagnosis, CMA is fully operational and protein substrates endowed with a KFERQ-like motif are readily degraded within lysosomes. Macroautophagy also proceeds efficiently and allows the elimination of unfolded proteins and damaged organelles to ensure proper cellular homeostasis. In CMA-defective cells or MDS-AML patients chronically treated with Aza, progressive loss of LAMP2 expression through epigenetic mechanisms leads to CMA defects and accumulation of CMA substrates. Among them, BCL2L10 and MLLT11/AF1Q exert crucial roles in cell fate, limiting apoptosis on the one hand and increasing proliferation on the other hand to promote cell survival and potentially leukemogenesis. This allows the emergence of LAMP2-negative cells with higher proliferative capacities. In Aza-resistant MDS and AML cells, the defect of CMA is compensated by an increase in macroautophagy, that can be therapeutically exploited through the use of lysosomal inhibitors, and more specially FDA-approved molecules such as hydroxychloroquine. BafA1, bafilomycin A1.
Aza remains a front-line player in the therapeutic armamentarium for high-risk MDS and AML patients. However, all patients inexorably relapse, and no treatment options are available following Aza failure. We reported that LAMP2 expression progressively declines in Aza-treated patients and is associated with a poor overall survival. In addition, we demonstrated that Aza-resistant cell lines and patients respond very well to inhibitors of autophagy as attested to by the strong anti-leukemic effect of hydroxychloroquine and other lysosomal inhibitors on Aza-resistant cells from MDS-AML patients. This could be of utmost importance for Aza-resistant MDS-AML patients.
The LAMP2 gene is localized on the X chromosome, and LAMP2 deficiency is associated with Danon disease, a rare X-linked form of cardiomyopathy. The pathological hallmark of Danon disease is the accumulation of autophagic vacuoles enriched in lysosomal enzymes and autophagy-related markers. Importantly, lamp1 lamp2 double-knockout mice exhibit accumulation of autophagic vacuoles in many tissues suggesting impaired autophagosome maturation rather than impaired formation, as is the case in Aza-resistant cell lines defective for LAMP2. How is LAMP2 expression repressed in Aza-resistant MDS and AML cell lines and patients? One likely explanation is that Aza might promote demethylation of the promoter of a LAMP2 X-linked repressor, leading to inhibition of LAMP2 transcription. Following this hypothesis, MDS cells treated with Aza would progressively lose LAMP2 expression, causing striking defects in the elimination of CMA substrates such as MLLT11/AF1Q and BCL2L10. Next, selection of LAMP2-deficient cells that accumulate survival proteins would confer a survival advantage to Aza-resistant cells. What could be the nature of this repressor? A pertinent candidate is XIST, a long non-coding RNA responsible for X inactivation in females. Indeed, Aza has been shown to reactivate XIST in different cell line models. We have recently observed that it is also the case in MDS-AML cell lines. This could also partly explain why MDS is more frequent in males than in females. This loss of LAMP2 expression could represent an ‘Achille Heel’ and a therapeutic window for Aza-resistant MDS and AML patients.
In the present study, we used gain- and loss-of-function experiments to investigate the effect of LAMP2 on Aza resistance. It will be of utmost interest to determine which specific LAMP2 isoform is involved in the mechanism of resistance to nucleoside analogs. A synthetic lethal shRNA-based screen could be also used to identify autophagy and lysosomal-related genes whose targeting may restore vulnerability to chemotherapy in cells depleted for each LAMP2 isoform.
Nevertheless, we propose that LAMP2 repression, which occurs in the course of hypomethylating agent treatment in MDS and AML patients, might open a therapeutic window for the use of well-characterized autophagy inhibitors already used in the clinic.
Funding Statement
This work was supported by the Association Laurette Fugain [ALF 2016-08]; INCA [PRTK-045, 2013–2015]; Fondation ARC pour la Recherche sur la Cancer 2017–2019.
Acknowledgments
Our research is supported by Inserm, the Fondation ARC pour la Recherche contre le Cancer (Equipe Labellisée 2018-2020), the Association Laurette Fugain, the Canceropole PACA and Institut National des Canceropoles, INCA (PRTK 2012-045). The ANR
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Dubois A, Furstoss N, Calleja A, Zerhouni M, Cluzeau T, Savy C, et al. LAMP2 expression dictates azacytidine response and prognosis in MDS/AML. Leukemia 2019. 2019 Jan 3. doi: 10.1038/s41375-018-0336-1. [DOI] [PubMed] [Google Scholar]


