Abstract
Inflammatory bowel disease (IBD) is characterized by chronic, recurring inflammation of the digestive tract. Current therapeutic approaches are limited and include biologics and steroids such as anti-TNFα monoclonal antibodies and corticosteroids, respectively. Significant adverse drug effects can occur for chronic usage and include increased risk of infection in some patients. GPR4, a pH-sensing G protein-coupled receptor, has recently emerged as a potential therapeutic target for intestinal inflammation. We have assessed the effects of a GPR4 antagonist, 2-(4-((2-Ethyl-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)methyl)phenyl)-5-(piperidin-4-yl)-1,3,4-oxadiazole (GPR4 antagonist 13, also known as NE-52-QQ57) in the dextran sulfate sodium (DSS)-induced acute colitis mouse model. The GPR4 antagonist 13 inhibited intestinal inflammation. The clinical parameters such as body weight loss and fecal score were reduced in the GPR4 antagonist 13 treatment group compared to vehicle control. Macroscopic disease indicators such as colon shortening, splenic expansion, and mesenteric lymph node enlargement were all reduced in severity in the GPR4 antagonist 13 treated mice. Histopathological features of active colitis were alleviated in GPR4 antagonist 13 treatment groups compared to vehicle control. Finally, inflammatory gene expression in the colon tissues and vascular adhesion molecule expression in the intestinal endothelia were attenuated by GPR4 antagonist 13. Our results indicate that GPR4 antagonist 13 provides a protective effect in the DSS-induced acute colitis mouse model, and inhibition of GPR4 can be explored as a novel anti-inflammatory approach.
Keywords: GPR4, G protein-coupled receptor, antagonist, colitis, inflammatory bowel disease
1. Introduction
Inflammatory bowel disease (IBD) is complex and characterized by loss of intestinal homeostasis resulting in dysregulated mucosal immune responses. Uncontrolled chronic gastrointestinal inflammation can result in severe complications of which include fistulas, stenosis, and abscesses (Blumberg, 2009; Brown and Mayer, 2007; Kaser et al., 2010). Furthermore, IBD can have extrainstestinal manifestations such as arthritis, autoimmune chronic active hepatitis, and uveitis (Brown and Coviello, 2015). Additionally, IBD can also increase the risk of the development of colon cancer (Mattar et al., 2011). The current therapeutic landscape for IBD is limited and is predominately focused on symptomatic management (Chandel et al., 2015; Neurath, 2017; Su et al., 2018).
IBD can take the form of ulcerative colitis (UC) and Crohn’s disease (CrD). Each disease contains overlapping, yet distinct features. One interesting feature that has been noted in IBD is the display of reduced local intra-intestinal luminal pH in IBD patients compared to healthy controls (Barkas et al., 2013; Fallingborg, 1999; Fallingborg et al., 1990; Fallingborg et al., 1993; Nugent et al., 2001). It is also well documented local tissue pH can be reduced during active inflammation, predominately owing to heightened metabolic byproducts from excessive leukocyte infiltration accompanied by impaired cellular waste removal (Justus et al., 2015; Lardner, 2001; Okajima, 2013). These studies collectively suggest the intestinal mucosa and lumen can be acidic in IBD patients. It remains poorly investigated, however, how cellular constituents involved in the maintenance of the mucosal immune response sense altered pH in the intestine and subsequently alter function.
G-protein coupled receptors (GPCRs) are implicated in normal intestinal function as well as in pathological contribution during active IBD. One such family of GPCRs are the proton-sensing GPCRs, which consist of GPR4, GPR65 (TDAG8), and GPR68 (OGR1) (Chen et al., 2011; Dong et al., 2017; Dong et al., 2013b; Ishii et al., 2005; Justus et al., 2013; Justus et al., 2017; Liu et al., 2010; Ludwig et al., 2003; Sanderlin et al., 2015; Tomura et al., 2005; Wang et al., 2004; Yang et al., 2007). These receptors are activated by protonation of extracellular histidine residues in acidic environments. Each of these receptors have recently been implicated in the modulation of intestinal inflammation (de Valliere et al., 2015; Lassen et al., 2016; Sanderlin et al., 2017; Wang et al., 2018). Several studies have shown GPR4 is responsible for acidosis-induced endothelial cell inflammation and can functionally increase leukocyte adhesion with endothelial cells (Chen et al., 2011; Dong et al., 2017; Dong et al., 2013b; Tobo et al., 2015). GPR4 genetic deletion in mice has also been shown to reduce intestinal inflammation and mucosal leukocyte infiltration in both inducible and spontaneous colitis mouse models (Sanderlin et al., 2017; Wang et al., 2018). However, no efforts have been made to investigate the effects of pharmacological modulators of proton-sensing GPCRs in intestinal inflammation.
In this study, we have assessed the function and efficacy of a GPR4 antagonist, 2-(4-((2-Ethyl-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)methyl)phenyl)-5-(piperidin-4-yl)-1,3,4-oxadiazole (GPR4 antagonist 13, also known as NE-52-QQ57), within the dextran sulfate sodium (DSS)-induced acute colitis mouse model as a potential therapeutic for the remediation of intestinal inflammation. GPR4 antagonist 13 was recently developed and characterized by Novartis and shown effective following oral administration (Velcicky et al., 2017). GPR4 antagonist 13 was capable of reducing inflammation in the rat antigen-induced arthritis model, angiogenesis in the mouse chamber implant model, and inflammation-associated nociception in the rat complete Freund’s adjuvant model (Velcicky et al., 2017). Furthermore, oral pharmacokinetics, GPR4 target selectivity, dosage and potency was thoroughly evaluated and GPR4 antagonist 13 was poised for further evaluation (Velcicky et al., 2017). Another study evaluated GPR4 antagonist 13 in mouse ventilatory responses and observed no obvious toxicities (Hosford et al., 2018). Our results demonstrated that GPR4 antagonist 13 administration reduced disease severity, histopathological features, inflammatory gene expression within the colon, and endothelial specific adhesion molecule expression within the inflamed intestinal tissue in the DSS-induced colitis mouse model.
2. Materials and methods
2.1. Dextran sulfate sodium (DSS)-induced colitis mouse model and GPR4 antagonist 13 delivery
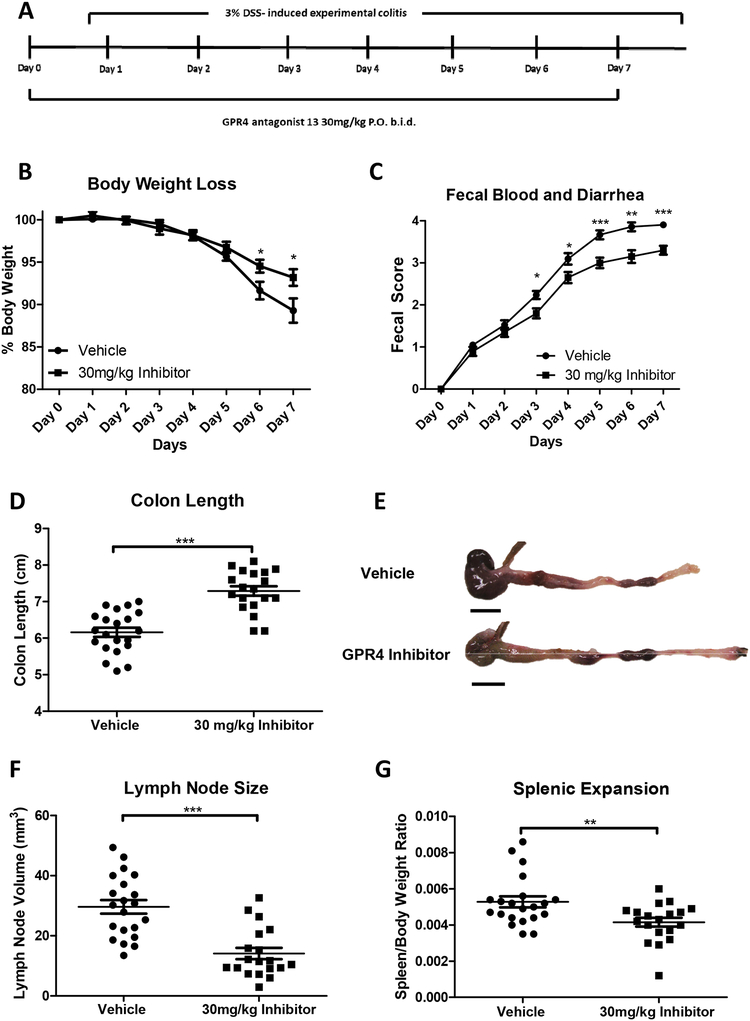
Male and female mice ages 9–10 weeks in the C57BL/6 background were used for experiments. As previously described, mice were maintained specific pathogen free and housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility (Sanderlin et al., 2017). Acute experimental colitis was induced by the addition of 3% (w/v) Dextran Sulfate Sodium Salt (DSS) ad libitum into the normal drinking water of mice (36,000–50,000 M.Wt, Lot# Q5229, MP Biomedical, Solon, OH). Mice were provided normal pelleted diet ad libitum during experimentation (ProLab 2000, Purina Mills, St. Louis, MO). Mice were treated for seven days with 3% DSS with replacement of the 3% DSS solution every two days. As previously described, during each day of the experimental course clinical parameters for disease severity were assessed (Sanderlin et al., 2017). Mice were weighed for body weight loss evaluation and feces was assessed for fecal blood and diarrhea using the Hemoccult Single Slides screening test (Beckman Coulter, Brea, CA). For GPR4 antagonist 13 administration, the small molecule (>99% purity) was suspended in 0.5% methylcellulose/ 0.5% Tween 80/ 99% water and a dosage of 30mg/kg (b.i.d.) was given to mice as previously described (Velcicky et al., 2017). This dosage was chosen based on our previous testing of increasing doses (3, 10 and 30 mg/kg) of which the best efficacy was observed with 30 mg/kg (Velcicky et al., 2017). Furthermore, the toxicity studies performed during the development of the compound showed no hepatopathy. On day one, mice were orally gavaged with either vehicle or 30mg/kg GPR4 antagonist 13 in the morning followed by addition of 3% DSS into the drinking water and another dose of GPR4 antagonist 13 or vehicle in the afternoon. On days two through six, mice were orally gavaged with vehicle or GPR4 antagonist 13 twice a day (b.i.d.). On day seven mice were euthanized for tissue collection and macroscopic disease indicator measurement (Fig. 1A). Mice were dissected as previously described and mesenteric lymph node expansion, colon shortening, and splenic expansion were measured (Sanderlin et al., 2017).
Fig. 1. GPR4 antagonist 13 reduces clinical severity and macroscopic disease indicators of intestinal inflammation in mice.
Mice were provided GPR4 antagonist 13 P.O. twice a day (b.i.d.) during experimental time course (A). Mouse body weight loss (B) and fecal blood and diarrhea scores (C) were daily measured. Mouse colon length (D-E), mesenteric lymph node expansion (F), and splenic enlargement (G) were also assessed upon tissue collection. Vehicle: N=21 (10 male/11 female) and GPR4 antagonist 13: N=19 (10male/9 female). Data are presented as mean ± S.E.M. and was analyzed for statistical significance using the t-test between vehicle and GPR4 antagonist 13 groups. (*P < 0.05, **P < 0.01, *** P < 0.001). Scale bar = 1cm.
2.2. Sample collection for histology and molecular analysis
Following the completion of the seven-day DSS treatment, mice were euthanized and tissue was collected for histology and molecular analysis. The spleen and liver were resected and weighed for assessment of expansion. The mesenteric lymph nodes were identified using a dissection microscope, measured for volume calculation, and stored in 10% buffered formalin as previously described (Sanderlin et al., 2017). The gastrointestinal tract was then removed from the mouse and the colon was separated from the cecum at the ileocecal junction. Next, the colon content was removed by PBS flushing and opened along the anti-mesenteric border. Colon tissue approximately 1mm in width was removed from the anus to proximal colon. This tissue was then cut into thirds for distal, middle, and proximal colon segments and promptly snap frozen in liquid nitrogen for molecular analysis. The remaining colon segment was fixed in 10% buffered formalin (VWR) and cut into thirds as distal, middle, and proximal colon segments for histological analysis.
2.3. Histopathological analysis
Distal, middle, proximal colon segments, and liver tissues were fixed in 10% buffered formalin, embedded in paraffin and sectioned at 5μm thickness. Slides were then stained with hematoxylin and eosin (H&E) for histopathological analysis. Colon segments were evaluated by operators blind to sample identification according to previously published criteria with minor modifications (Erben et al., 2014). Briefly, each colon segment was evaluated in four recurring locations. Each location was evaluated for leukocyte infiltration, epithelial damage, and mucosal architecture distortions. The leukocyte infiltration score included severity scores of 1= mild, 2= moderate, and 3= severe with regard to both degree and location of cellular infiltrates. The mucosal architecture score included 1= focal epithelial erosions, 2= focal ulcerations, and 3= extended ulcerations. The sum score of these parameters represents the histopathological score of colitis severity. Liver tissues were evaluated for histological indicators of drug-induced toxicity.
2.4. RNA collection and real time RT-PCR
Total RNA was collected from the distal colon segments using the IBI Scientific DNA/RNA/Protein extraction Kit (MidSci, St. Louis, MO). 500ng of RNA was reversed transcribed using SuperScript IV and diluted 1:10 in ultrapure water (Gibco, Gaithersburg, MD). TaqMan pre-designed primer-probe sets used for the evaluation of specific gene expression (Applied Biosystems, Waltham, MA). TaqMan primer-probe sets and subsequent assay ID include 18S rRNA; (Hs99999901_s1), PTGS2; (Mm00478374_m1), VCAM-1; (Mm01320970_m1), MAdCAM-1; (Mm00522088_m1), E-selectin; (Mm00441278_m1), IL1-β; (Mm00434228_m1), TNF-α; (Mm00443258_m1), IL-10; (Mm01288386_m1), and IL-6; (Mm00446190_m1). Real-time PCR was performed in duplicate with a program of 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Data was acquired using the QuantStudio 3 Real-Time PCR system and analyzed using the 2−ΔCt method.
2.5. Immunohistochemistry
Immunohistochemistry (IHC) was performed as previously described (Sanderlin et al., 2017). Briefly, serial 5μm paraffin-embedded sections of distal, middle, and proximal colon segments were deparaffinized and hydrated to water. Antigen retrieval was then performed using Tris-EDTA pH 9.0 with 0.1% Tween 20. Following endogenous peroxidase blocking and AVIDIN/BIOTIN block (Invitrogen, Carlsbad, CA), tissue sections were incubated with primary antibodies against VCAM-1 (Abcam, ab134047, 1:100, Cambridge, MA), MAdCAM-1 (Abcam, ab90680, 1:500, Cambridge, MA), TNF-α (Bioss, bs-2081R, 1:1000, Woburn, MA) or E-selectin/CD62E (Abcam, ab18981, 1:1000, Cambridge, MA) overnight at 4°C. The rat or rabbit VECTASTAIN Elite ABC HRP kit was then used according to the manufactures protocol (Vector Laboratories, Burlingame, CA). The ImmPACT DAB (Vector Laboratories, Burlingame, CA) substrate was utilized for signal detection. Slides were then dehydrated and mounted. Pictures were taken with the Zeiss AxioImager.M2 with Axiocam 503 digital color camera.
2.6. Blood vessel VCAM-1 and E-selectin intensity score and MAdCAM-1 positive vessel enumeration
After IHC was performed for VCAM-1 and E-selectin on colon tissue segments, VCAM-1 and E-selectin intensity was blindly assessed by two independent operators from distal, middle, and proximal segments. Scoring criteria included 1= none/minimal, 2= mild, 3= moderate, and 4= high signal intensity. Each tissue segment was completely evaluated using 10× and 20× objectives and subsequently scored for intensity. For MAdCAM-1 positive vessel enumeration, vessels were assessed from the distal, middle, and proximal colon segments and total MAdCAM-1 positive vessels were counted using 10× and 20× objectives. The total colon length was recorded in centimeters and results were shown as MAdCAM-1 positive vessels/centimeter.
2.7. Statistical analysis
GraphPad Prism software was utilized for all statistical analysis. Results were recorded as the mean ± standard error. When analysis was performed between two groups the unpaired t test or Mann-Whitney test was utilized. All comparisons P < 0.05 are considered statistically significant where * P < 0.05, ** P < 0.01, and *** P< 0.001.
3. Results
3.1. GPR4 antagonist 13 reduces the severity of colitis in the acute DSS-induced colitis mouse model
Wild-type C57BL/6 mice were initiated on the acute DSS colitis model and given vehicle or 30mg/kg of GPR4 antagonist 13 b.i.d. by oral gavage (Fig. 1A). During each day of the experimental course mouse body weight in conjunction with fecal blood and diarrhea scores were evaluated to provide clinical assessment of disease severity between vehicle and GPR4 antagonist 13 groups. GPR4 antagonist 13 treated mice were protected from body weight loss commencing from day five through seven (Fig. 1B). Mice given vehicle lost 11–14% body weight by day seven while mice provided GPR4 antagonist 13 lost between 5–6% body weight. Fecal blood and diarrhea scores provided further indication GPR4 antagonist 13 protects against intestinal inflammation as mouse fecal scores were reduced in GPR4 antagonist 13 treated mice compared to vehicle (Fig. 1C). Fecal blood and diarrhea could be observed in mice with progressive severity from day one through seven. Vehicle mice developed heighted fecal scores earlier than GPR4 antagonist 13 treated mice and maintained more severe scores throughout the seven-day DSS experiment. These results collectively provide evidence GPR4 antagonist 13 can blunt the clinical severity of DSS-induced intestinal inflammation.
3.2. Clinical severity and macroscopic disease indicators are reduced in mice treated with GPR4 antagonist 13
Upon completion of the final day of the acute colitis mouse model, mice were dissected for assessment of macroscopic disease indicators such as colon shortening, mesenteric lymph node expansion, and splenic expansion. We observed that mice treated with GPR4 antagonist 13 had reduced colon shortening when compared to vehicle (colon length ~7.3cm versus ~6.1cm, respectively) (Figs. 1D and 1E). Mesenteric lymph nodes (MLNs) were also collected, and the volume was measured in vehicle and GPR4 antagonist 13 treated mice. Vehicle MLN volume was expanded by more than 2-fold when compared to GPR4 antagonist 13 mice (Fig. 1F). These results indicate GPR4 antagonist 13 reduces colonic inflammation and associated expansion of MLNs when compared to vehicle. Finally, the spleen to body weight ratio was assessed in vehicle and GPR4 antagonist 13 DSS-treated mice. We observed reduced splenic expansion in mice treated with GPR4 antagonist 13 when compared to vehicle suggesting reduced disease severity due to GPR4 inhibition (Fig. 1G). Furthermore, liver weight was assessed for indicators of drug-induced liver toxicity. No differences in liver gross morphology or weight between vehicle and GPR4 antagonist 13 treated mice were observed (Supplemental Fig. 1).
3.3. GPR4 inhibition reduces histopathological features in mouse colon
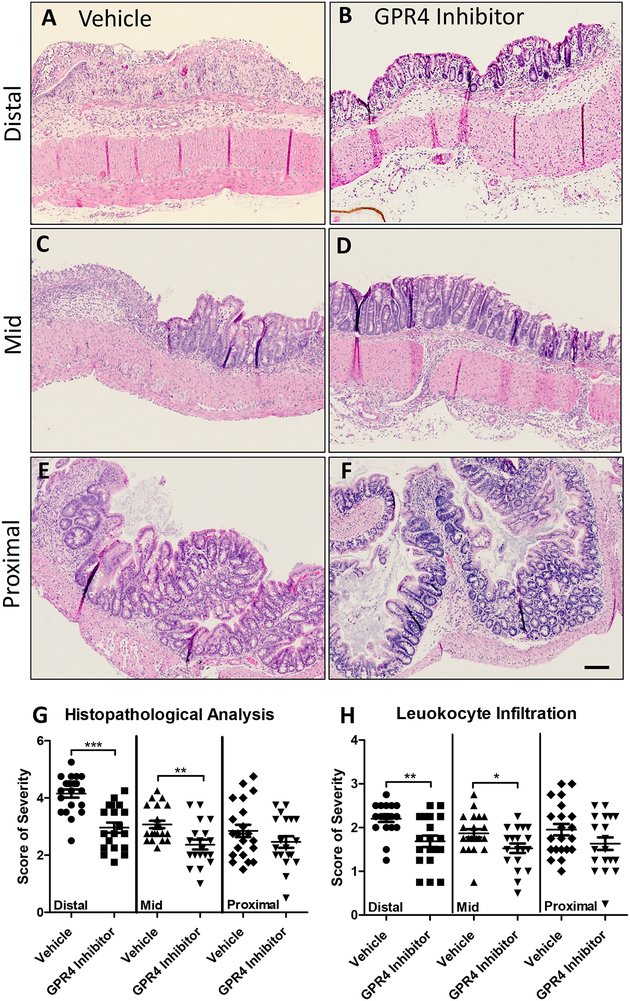
To assess the effects of GPR4 inhibition at the histological level, distinct pathological cellular features of colitis were assessed in the distal, middle, and proximal colon segments. Some such features assessed in the colon were leukocyte infiltration, epithelium erosion, crypt distortion, and mucosal ulceration. In both vehicle and GPR4 antagonist 13 DSS-treated mice, the highest degree of histopathology can be observed in the distal colon segments followed by the middle and proximal, respectively. The observation that intestinal inflammation is most severe in the distal colon within the DSS model are consistent with literature (Chassaing et al., 2014; Kim et al., 2012; Laroui et al., 2012; Perse and Cerar, 2012). When comparing vehicle and GPR4 antagonist 13 mouse groups, the degree of histopathology was significantly reduced by GPR4 antagonist 13 when compared to vehicle (Figs. 2A–2G). This GPR4 antagonist 13-mediated reduction in histopathology occurred in both the distal and middle colon segments (P < 0.01) with a trend in reduction at the proximal segment, though not statically significant. Interestingly, the degree of leukocyte infiltration was also reduced by GPR4 antagonist 13 when compared to vehicle (Fig. 2H). Liver tissues were also assessed for drug-induced liver pathologies. No overt histopathological features could be observed (Supplemental Fig. 1).
Fig. 2. GPR4 antagonist 13 reduces histopathological parameters of intestinal inflammation in the inflamed mouse colon.
Distinct histopathological features of intestinal inflammation were assessed and scored for degree of severity. Representative pictures of vehicle distal (A), Middle (C), and proximal (E) colon segments compared to GPR4 antagonist 13 distal (B), middle (D), and proximal (F) colon segments. Graphical representation of total histopathological parameters (G) and leukocyte infiltration score (H). Vehicle: N=21 (10 male/11 female) and GPR4 antagonist 13: N=19 (10male/9 female). Data are presented as mean ± S.E.M. and was analyzed for statistical significance using the t-test between vehicle and GPR4 antagonist 13 groups between each colon segment. (*P < 0.05, **P < 0.01, *** P < 0.001). 10× objective. Scale bar = 100μm.
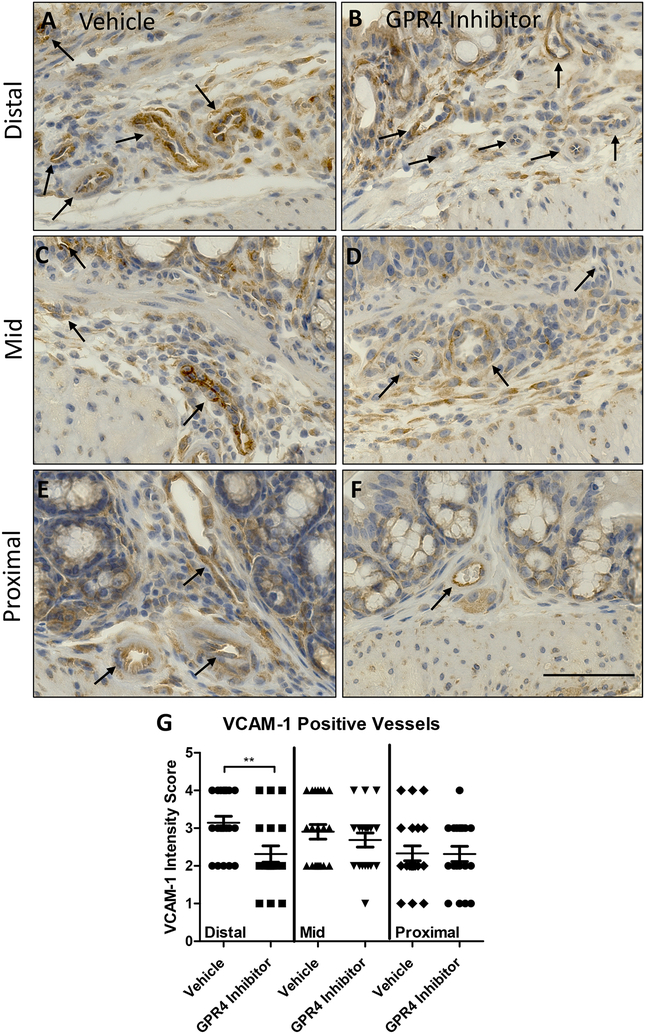
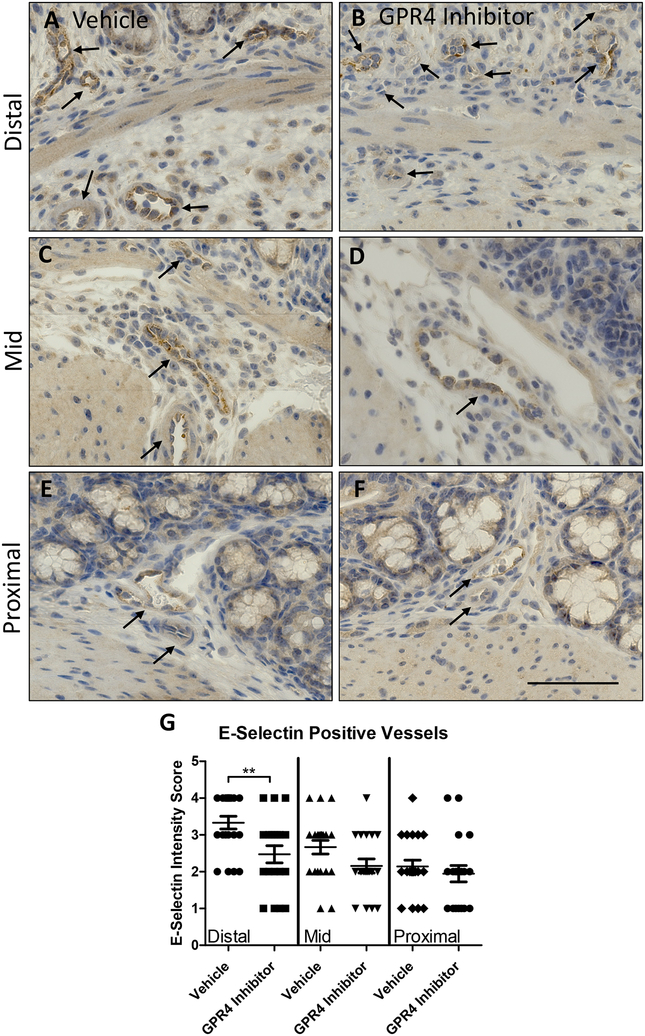
3.4. Endothelium specific VCAM-1 and E-selectin expression are reduced by GPR4 antagonist 13
Previous studies have demonstrated GPR4 activation in endothelial cells (ECs) induces vascular cell adhesion molecule-1 (VCAM-1) and E-selectin expression (Chen et al., 2011; Dong et al., 2013b; Tobo et al., 2015). Additionally, reports have shown GPR4 genetic deletion can reduce VCAM-1 and E-selectin expression in the vascular endothelium (Sanderlin et al., 2017). Here we assessed the protein expression signal intensity of VCAM-1 and E-selectin of intestinal microvascular endothelial cells in the distal, middle, and proximal colon mucosa segments of vehicle and GPR4 antagonist 13 DSS-treated mice (Figs. 3 and 4). The intensity scoring of VCAM-1 and E-selectin expression revealed highest signal within the distal intestinal endothelia with progressive intensity reduction from the middle and proximal colon, respectively. These data are consistent with the observation of reduced leukocyte infiltration in the middle and proximal colon segments compared to the distal segment (Fig. 2H). Interestingly, VCAM-1 and E-selectin signal intensity in intestinal microvascular ECs was reduced in GPR4 antagonist 13 DSS-treated mice when compared to vehicle in the distal colon and a trend of reduction in the middle colon (Figs. 3 and 4). Collectively, these data provide evidence GPR4 antagonist 13 reduces EC activation and subsequently leukocyte infiltration into the inflamed intestinal mucosa.
Fig. 3. GPR4 antagonist 13 reduces VCAM-1 protein expression in colon microvascular endothelial cells.
VCAM-1 protein expression intensity was assessed by immunohistochemistry (IHC) in colon microvascular endothelial cells. Representative pictures of vehicle distal (A), middle (C), and proximal (E) colon segments compared to GPR4 antagonist 13 distal (B), middle (D), and proximal (F) colon segments followed by graphical representation of VCAM-1 intensity score (G). Vehicle: N=21 (10 male/11 female) and GPR4 antagonist 13: N=19 (10male/9 female). Data are presented as mean ± S.E.M. and was analyzed for statistical significance using the t-test between vehicle and GPR4 antagonist 13 groups between each colon segment. (**P < 0.01). 40× objective. Scale bar = 100μm. Black arrows indicate blood vessels.
Fig. 4. GPR4 antagonist 13 reduces E-selectin protein expression in colon microvascular endothelial cells.
E-selectin protein expression intensity was assessed by IHC in colon microvascular endothelial cells. Representative pictures of vehicle distal (A), middle (C), and proximal (E) colon segments compared to GPR4 antagonist 13 distal (B), middle (D), and proximal (F) colon segments followed by graphical representation of E-selectin intensity score (G). Vehicle: N=21 (10 male/11 female) and GPR4 antagonist 13: N=19 (10male/9 female). Data are presented as mean ± S.E.M. and was analyzed for statistical significance using the t-test between vehicle and GPR4 antagonist 13 groups between each colon segment. (**P < 0.01). 40× objective. Scale bar = 100μm. Black arrows indicate blood vessels.
Immunohistochemical analysis also revealed expression of VCAM-1 and E-selectin on cell types not regulated by GPR4. VCAM-1 expression could be strongly observed in activated fibroblasts and other mononuclear cells within the intestinal mucosa (Supplemental Figs. 2A and 2B). Previous reports have observed expression of VCAM-1 in skeletal muscle, fibroblast, and some leukocyte populations (Epperly et al., 2002; Ulyanova et al., 2005). E-selectin expression could be detected within the colon epithelium (Supplemental Figs. 2C and 2D). These results are consistent with previous studies showing E-selectin can be expressed on the colon epithelium and in mononuclear cells in the intestinal mucosa during active colitis (Vainer et al., 1998). Furthermore, additional studies have shown E-selectin is expressed in T cells and can be upregulated by pro-inflammatory mediators (Bajnok et al., 2017; Harashima et al., 2001). We observed similar levels of signal intensity of VCAM-1 and E-selectin in cell types other than intestinal microvascular endothelial cells between vehicle and GPR4 antagonist 13 groups (Supplemental Fig. 2).
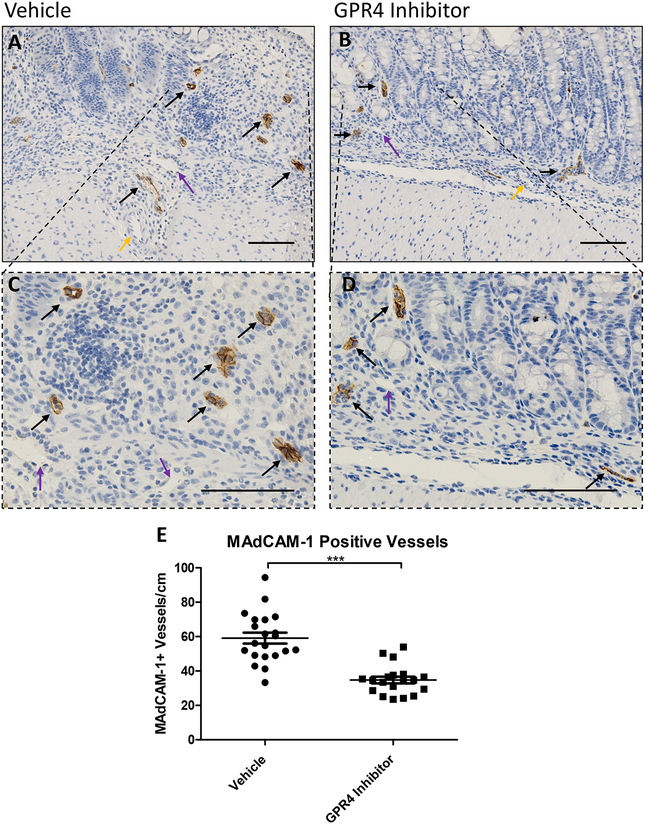
In addition to VCAM-1 and E-selectin expression analysis, we assessed the expression intensity and distribution of mucosal vascular addressin cell adhesion molecule-1 (MAdCAM-1) in colon tissues. We observed prominent MAdCAM-1 expression in intestinal microvasculature with high expression density in the lamina propria (Fig. 5). No MAdCAM-1 expression could be observed in arteries and extramural blood vessels and minimal expression could be detected in lymphatic endothelial cells. MAdCAM-1 expression signal intensity in the intestinal microvasculature were similar between vehicle and GPR4 antagonist 13 DSS-treated mouse groups (Figs. 5A and 5B). Total number of vessels positive for MAdCAM-1, however, were markedly reduced in GPR4 antagonist 13 mice compared to vehicle control mice (Figs. 5A–5D). Total MAdCAM-1 positive vessels were counted per centimeter (cm) from the entire length of the colon. Vehicle treated mice had ~60 MAdCAM-1 positive vessels/cm compared to ~35 MAdCAM-1 positive vessels/cm in GPR4 antagonist 13 treated mice (Fig. 5E).
Fig. 5. GPR4 antagonist 13 reduces MAdCAM-1 positive vessels in the mouse colon.
No difference in MAdCAM-1 protein expression intensity was observed between vehicle and GPR4 antagonist 13 colon microvascular endothelial cells; however, differences in total number of MAdCAM-1 positive vessels were observed between the two treatment groups. Representative pictures of vehicle distal (A, C) compared to GPR4 antagonist 13 (B, D). Graphical representation of MAdCAM-1+ blood vessels per colon centimeter are depicted (E). Vehicle: N=21 (10 male/11 female) and GPR4 antagonist 13: N=19 (10male/9 female). Data are presented as mean ± S.E.M. and was analyzed for statistical significance using the t-test between vehicle and GPR4 antagonist 13 groups. (*** P < 0.001). 20× and 40× objective. Black arrow indicates microvasculature, purple arrow indicates lymphatic endothelial cell, yellow arrow indicates artery. Scale bar = 100μm.
3.5. Inflammatory gene expression is reduced in the distal colon by GPR4 antagonist 13
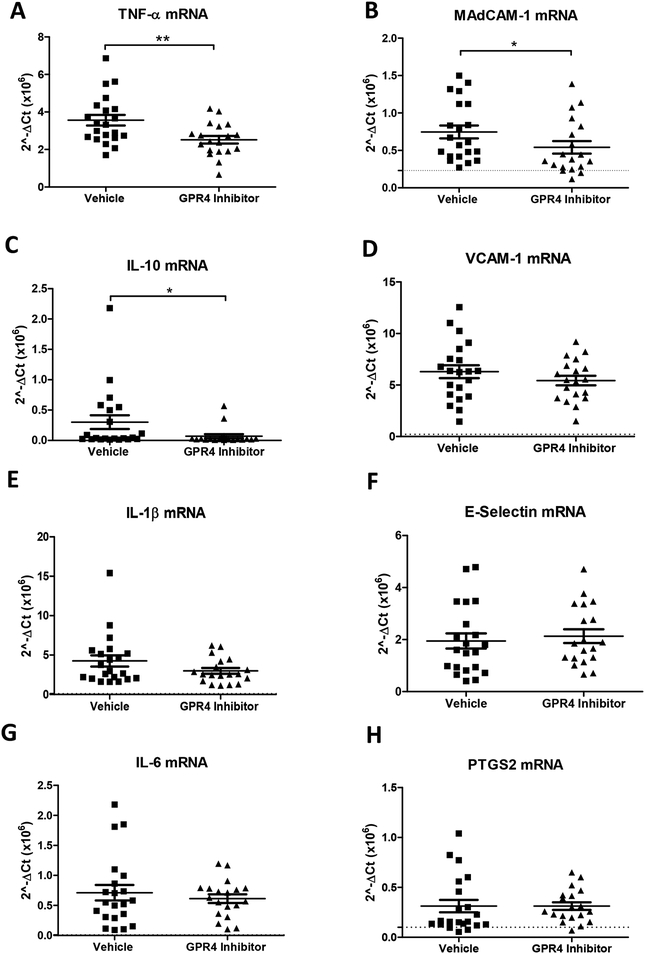
Following the assessment of histopathology and endothelial cell-specific inflammatory protein expression, we assessed inflammatory gene expression at the whole tissue level to discern the anti-inflammatory effects of GPR4 antagonist 13. Inflammatory genes were measured from the distal colon segment of vehicle and GPR4 antagonist 13 DSS-treated mice. Additionally, distal colon segments of wild-type mice not treated with DSS were collected and assessed to serve as a baseline gene expression reference. Inflammatory mediators such as TNF-α, IL-1β, IL-6, IL-10, and PTGS2 (COX-2) were measured between vehicle and GPR4 antagonist 13 groups (Fig. 6). A statistically significant reduction of TNF-α and IL-10 gene expression could be appreciated in the GPR4 antagonist 13 group compared to vehicle. Reduction of TNF-α is correlated with reduced inflammation in the mouse distal colon of the GPR4 antagonist 13 group. We also assessed TNF-α protein expression in the distal colon by immunohistochemical analysis. An appreciable reduction of TNF-α expression could be observed in immune cell infiltrates and endothelial cells within GPR4 antagonist 13 treated mice compared to vehicle control (Supplemental Fig. 3). Contrary to TNF-α, IL-10 can inhibit the function of macrophages and other inflammatory cells which are required for optimal pathogen clearance and subsequent inflammatory resolution (Couper et al., 2008; Kessler et al., 2017; Rojas et al., 2017). As such, reduced levels of IL-10 mRNA in the tissues of GPR4 antagonist 13 treated mice could potentially enhance pathogen clearance during the acute inflammatory phase. Furthermore, vehicle administered mice also had a trend in heighted expression of the inflammatory genes IL-1β and IL-6 when compared to the GPR4 antagonist 13 treated group. In addition to inflammatory cytokines, adhesion molecule gene expression including E-selectin, MAdCAM-1, and VCAM-1 were assessed. A statistically significant reduction of MAdCAM-1 expression could be discerned in the GPR4 antagonist 13 treated mice compared to vehicle (Fig. 6B). These results are in line with reduced MAdCAM-1 positive vessels in the distal colon of GPR4 antagonist 13 mice compared to vehicle (Fig. 5). VCAM-1 gene expression was modestly reduced in the GPR4 antagonist 13 group compared to vehicle and no trend in reduction could be discerned for E-selectin at the whole tissue level (Figs. 6D and 6F). As VCAM-1 and E-selectin are expressed in cells that are not regulated by GPR4 (Supplemental Fig. 2), whole tissue gene expression analysis presents complications for assessing VCAM-1 and E-selectin expression specific to GPR4 regulated vascular endothelial cells. To overcome this complication, we assessed VCAM-1 and E-selectin protein expression specific to vascular endothelial cells as described above (Figs. 3 and 4). Taken together, the results indicate that GPR4 antagonist 13 reduces inflammatory gene expression in the distal colon of DSS-treated mice.
Fig. 6. GPR4 antagonist 13 reduces inflammatory gene expression in the distal colon.
Tissue level gene expression of cytokines, adhesion molecules, and an inflammatory enzyme were assessed in the distal colon segment of DSS-treated mice given vehicle or GPR4 antagonist 13. Graphical representation of TNF-α (A), MAdCAM-1 (B), IL-10 (C), VCAM-1 (D), IL-1β (E), E-selectin (F), IL-6 (G), and PTGS2 (H) gene expression. Vehicle: N=21 (10 male / 11 female) and GPR4 antagonist 13: N=19 (10male / 9 female). Data are presented as mean ± S.E.M. and was analyzed for statistical significance using the Mann-Whitney test between vehicle and GPR4 antagonist 13 groups between each colon segment. (*P < 0.05, **P < 0.01). Dotted black line are indicative of untreated control C57Bl/6 mice (N= 2 male/2 female).
4. Discussion
The local pH of the inflammatory tissue is characteristically acidic. The contribution to reduced tissue pH during active inflammation is predominately owing to the heavy influx of immune cells to sites of inflammation and hypoxia (Lardner, 2001; Okajima, 2013). The pH within these tissue microenvironments can fluctuate within the physiological range to severely acidic. Furthermore, some groups have evaluated the pH within gastrointestinal tract of patients with active IBD (Fallingborg et al., 1993; Nugent et al., 2001). These reports suggest the intraluminal colonic pH can be altered in patients with active colitis. Molecular mechanisms for sensing extracellular pH during intestinal inflammatory responses have only recently been investigated. The pH-sensing GPCRs, including TDAG8 (GPR65), OGR1 (GPR68), and GPR4, have emerged as regulators of intestinal inflammation (de Valliere et al., 2015; Hutter et al., 2018; Lassen et al., 2016; Sanderlin et al., 2017; Wang et al., 2018).
Initial studies on the effects of acidic pH-induced activation of GPR65 have provided an anti-inflammatory role for GPR65 in macrophages, among other immune cell populations, during inflammation (Jin et al., 2014; Mogi et al., 2009). Furthermore, genetic variances within the GPR65 gene were correlated to increased risk of the development of a variety of inflammatory diseases of which include inflammatory bowel disease (Barrett et al., 2009; Beecham et al., 2013; Cortes et al., 2013; Franke et al., 2010; Hardin et al., 2014; Jostins et al., 2012; Karnes et al., 2014; Okada et al., 2014; Sawcer et al., 2011). These data implicate pH homeostasis and cellular pH sensing mechanisms in the regulation of inflammation. A recent study investigated GPR65 in a bacteria-induced colitis model and found GPR65 protects against intestinal inflammation (Lassen et al., 2016). GPR68 has also been investigated and found to potentiate intestinal inflammation through regulating macrophage inflammatory programs in response to acidic pH (de Valliere et al., 2015). Finally, GPR4 has been studied in both acute and chronic intestinal inflammation mouse models (Sanderlin et al., 2017; Wang et al., 2018). Previous reports indicate GPR4 is predominately expressed in vascular endothelial cells (ECs) (Chen et al., 2011; Dong et al., 2013b; Yang et al., 2007). GPR4 expression was evaluated within normal and inflamed mouse colon tissues and was found to be predominately expressed within intestinal ECs (Sanderlin et al., 2017). Several studies implicate GPR4 as a regulator of EC activation in response to extracellular acidosis by increasing vascular adhesion molecules such as VCAM-1, E-selectin, and ICAM-1 and functionally increasing EC-leukocyte interactions (Chen et al., 2011; Dong et al., 2013b; Tobo et al., 2015). Previous studies have implicated these mechanistic insights into mouse colitis models. We have previously demonstrated genetic deletion of GPR4 in mice reduced intestinal inflammation in an acute DSS-induced colitis mouse model (Sanderlin et al., 2017). Additionally, GPR4 knockout (KO) mice had reduced neutrophil, macrophage, and T cell infiltration into the intestinal mucosa. The reduced leukocyte infiltration was associated with GPR4-dependant reduction of vascular adhesion molecule expression in the intestinal ECs. Another group assessed the functional role of GPR4 in chronic DSS-induced inflammation and the IL-10 KO spontaneous colitis mouse model (Wang et al., 2018). Mice devoid of GPR4 had reduced intestinal inflammation and subsequent mucosal CD4+ T helper cell infiltration. These studies collectively suggest GPR4 mediates intestinal inflammation through increasing gut leukocyte recruitment and subsequent extravasation into the intestinal mucosa. Inhibition of GPR4 may be explored as a novel anti-inflammatory approach.
In this study we show that the GPR4 antagonist 13 can reduce intestinal inflammation in the DSS-induced acute experimental colitis mouse model. Upon initiation of DSS-induced colitis, mice provided 30mg/kg of the GPR4 antagonist 13 b.i.d. during the 7-day colitis insult were protected from body weight loss, fecal blood and diarrhea, colon shortening, mesenteric lymph node expansion, and splenic enlargement. Colon histopathology was also alleviated in mice given the GPR4 antagonist. Following the observation of reduced leukocyte infiltration in GPR4 antagonist-treated mice, coupled with previous reports showing that GPR4 can stimulate vascular adhesion molecule expression in vitro and in vivo, we performed immunohistochemistry to evaluate the protein expression of VCAM-1, E-selectin, and MAdCAM-1 specific to the cell type regulated by GPR4, namely, ECs within the intestinal microvasculature. A notable reduction of VCAM-1 and E-selectin expression in ECs as well as a reduced number of MAdCAM-1 positive microvessels were observed within the distal colon segments of mice given the GPR4 antagonist compared to vehicle. These observations suggest that the GPR4 antagonist 13 reduces the expression of vascular adhesion molecules on ECs and thus attenuates leukocyte-endothelium adhesion and extravasation (Fig. 7).
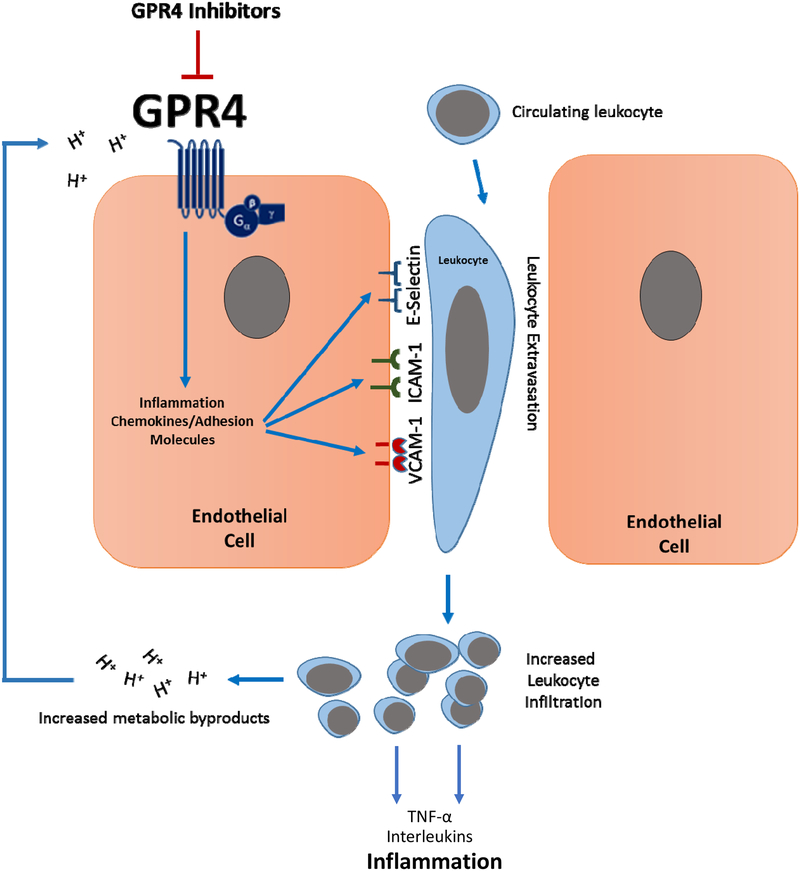
Fig. 7. Model of proposed mechanism of the anti-inflammatory action of GPR4 inhibitors.
GPR4 activation by protons in the extracellular milieu mediates the activation of vascular endothelial cells, the recruitment of immune cells and subsequent leukocyte extravasation into the inflamed tissue. Heavy immune cell infiltration into the inflammatory loci will result in further production of protons, as well as pro-inflammatory mediators, and subsequently maintain tissue inflammation and GPR4 activation. Inhibition of GPR4 activity by pharmacological intervention may present a novel approach to reduce inflammation by attenuating vascular endothelial cell activation and leukocyte infiltration into inflamed tissues.
Upon immunohistochemical evaluation of VCAM-1 and E-selectin within the colon, notable expression could also be detected in cell types not known to be regulated by GPR4. VCAM-1 could be observed on fibroblasts, macrophages, and other mononuclear cells within the colon. These observations are supported by several other studies describing basal and inflammation-associated VCAM-1 upregulation on activated fibroblasts and inflammatory cells (Epperly et al., 2002; Ulyanova et al., 2005). Studies have also observed E-selectin expression is not restricted to the vascular endothelium as E-selectin can be expressed by the colonic epithelium, mononuclear cells of which can include T cells (Bajnok et al., 2017; Harashima et al., 2001; Vainer et al., 1998). Our results were consistent with these reports as E-selectin could be detected in colonic epithelial cells and mononuclear cell populations within the inflamed mucosa (Supplemental Fig. 2). Notably, the expression of VCAM-1 and E-selectin in fibroblasts, colonic epithelial cells and mononuclear cells was at a similar level in the colon of GPR4 antagonist 13 and vehicle treated mice. These observations may explain why the mRNA expression levels of VCAM-1 and E-selectin in the whole colon tissue were either just slightly reduced or not changed in the mice treated with GPR4 antagonist 13 compared to vehicle as these cell types are not regulated by GPR4.
In addition to vascular adhesion molecules, the expression of TNF-α and IL-10 was also significantly reduced in the distal colon tissue of mice given the GPR4 antagonist when compared to vehicle. TNF-α and IL-10 mRNA reduction at the whole tissue level is most likely due to the reduction of inflammatory cells into the colon observed in the GPR4 antagonist 13 treated mice. Increased IL-10 mRNA levels are commonly observed in the acute DSS-induced colitis mouse model and in mucosal T cells of patients with active ulcerative colitis (Egger et al., 2000; Melgar et al., 2003). GPR4 antagonist 13-mediated reduction of IL-10 mRNA is in line with reduced leukocyte infiltration. Furthermore, as mentioned above, reduced IL-10 mRNA levels could aid optimal pathogen clearance and subsequent inflammatory resolution and enhance pathogen clearance during the acute inflammatory phase (Couper et al., 2008; Kessler et al., 2017; Rojas et al., 2017). A modest reduction of IL-1β and IL-6 could also be observed in mice treated with the GPR4 antagonist. Collectively, these results implicate GPR4 inhibitors as novel anti-inflammatory agents for colitis-associated intestinal inflammation.
GPR4 inhibitors have proved effective in the reduction of endothelial inflammation and subsequent tissue inflammation in vitro and in vivo, respectively (Dong et al., 2013a; Dong et al., 2017; Fukuda et al., 2016; Hosford et al., 2018; Miltz et al., 2017; Sanderlin et al., 2015; Tobo et al., 2015; Velcicky et al., 2017). A group of imidazo-pyridine and pyrazolopyrimidine derivatives were previously found to elicit inhibitory effects on GPR4-mediated EC activation (Dong et al., 2013a; Dong et al., 2017; Hosford et al., 2018; Miltz et al., 2017; Tobo et al., 2015; Velcicky et al., 2017). Further studies revealed this class of compounds could reduce mortality events in a myocardial infraction mouse model (Fukuda et al., 2016). Recently, the imidazopyridine derivatives were further characterized as an orally active GPR4 antagonist 39c (Miltz et al., 2017). This compound is commercially available and used to provide proof of concept in the pro-inflammatory role of GPR4 in endothelial inflammation (Dong et al., 2017; Dong et al., 2013b). However, due to off-target effects such as cross-reactivity with the histamine H3 receptor, GPR4 antagonist 39c was not further pursued (Miltz et al., 2017; Velcicky et al., 2017). Finally, the GPR4 antagonist 13, a pyrazolopyrimidine derivative, was developed and evaluated (Velcicky et al., 2017). Common off-targets, such as the H3 receptor and hERG channel, were evaluated for GPR4 antagonist 13 binding and the compound proved selective to the GPR4 receptor. In vivo pharmacokinetic studies proved GPR4 antagonist 13 had good profiles of oral exposure and low clearance. Additionally, an in vitro safety profile assessment for GPR4 antagonist 13 against a panel of receptors, enzymes, and ion channels indicated GPR4 antagonist 13 did not significantly inhibit these targets. Therefore, GPR4 antagonist 13 was assessed in several animal models and was found to inhibit inflammation as well as hyperplasia and angiogenesis in vivo (Velcicky et al., 2017). Taken together, the optimized GPR4 antagonist 13 displays a good pharmacological profile and has proven orally active for in vivo use. Nonetheless, there are some aspects requiring close attention for the use of GPR4 inhibitor for anti-inflammatory effects.
Previous studies have shown that a fraction of GPR4 knockout mice display vascular abnormalities associated with mouse perinatal complications (Yang et al., 2007). GPR4-null mice also exhibit decreased renal acid excretion (Sun et al., 2010). Other reports have shown GPR4 is expressed in retrotrapezoid nucleus (RTN) neurons and is involved CO2 chemosensing to regulate breathing (Kumar et al., 2015). A recent study evaluated the central nervous system (CNS) effects of GPR4 antagonist 13 and observed no effects on hemodynamics, blood oxygen levels, and cerebral blood flow (Hosford et al., 2018). However, GPR4 antagonist 13 reduced hypercapnic response to CO2 but had no effect when mice were under anesthesia. These studies suggest that current GPR4 inhibitors have a good overall pharmacological profile but might not be taken during pregnancy or if renal and respiratory complications exist.
Taken together, this study is the first to evaluate the effects of the pharmacological inhibition of GPR4 on intestinal inflammation. We have shown that GPR4 antagonization can reduce the degree of intestinal inflammation in the DSS-induced acute experimental colitis mouse model. Further studies need to be done to evaluate GPR4 inhibition in other diseases characterized by dysregulated inflammation. Additional efforts are also needed to develop and evaluate new classes of GPR4 inhibitors for further pharmacological characterization.
Supplementary Material
Liver weight assessment and histological evaluation. GPR4 antagonist 13 does not induce observable liver pathology when compared to vehicle control mice. Representative pictures of normal vehicle (A) and GPR4 antagonist 13 (B) liver histology. Weight measurements of livers between vehicle and GPR4 antagonist 13 male (C) and female (D) mice. Vehicle: N=21 (10 male/11 female) and GPR4 antagonist 13: N=19 (10 male/9 female). Data are presented as mean ± S.E.M. and was analyzed for statistical significance using the t-test between vehicle and GPR4 antagonist 13 groups. No statistically significant difference was observed. Scale bar = 100μm.
VCAM-1 and E-selectin expression was assessed in non-endothelial cells within the colon. VCAM-1 is expressed in non-endothelial mononuclear cells and fibroblasts and E-selectin is expressed in the intestinal epithelium. Representative pictures of VCAM-1 in vehicle (A) and GPR4 antagonist 13-treated mice (B) and E-selectin in vehicle (C) and GPR4 antagonist 13-treated mice (D) in the colon. Scale bar = 100μm. Red arrows indicate VCAM-1 positive cells and black arrows indicate E-selectin positive cells.
TNF-α protein expression was assessed by immunohistochemistry in immune cells and endothelial cells within the distal colon segment. TNF-α expression is reduced in colon tissues from mice treated with GPR4 antagonist 13 when compared to vehicle. Representative pictures of TNF-α in vehicle (A) and GPR4 antagonist 13-treated mice (B). 40× objective. Representative pictures of high magnification (63×) TNF-α-positive intestinal microvascular endothelial cells between vehicle (C) and GPR4 antagonist 13 (D) treatment groups. Scale bar = 100μm. Red arrows indicate endothelial cells and black arrows indicate leukocytes.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (1R15DK109484–01, to L.V.Y). We would also like to thank Tiffany Troutman and Joani Oswald for their excellent help with histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
L.V.Y. is the inventor on a U.S. patent (US 8207139 B2). J.V. and P.L. are employees of Novartis Institutes for BioMedical Research.
References
- Bajnok A, Ivanova M, Rigo J Jr., Toldi G, 2017. The Distribution of Activation Markers and Selectins on Peripheral T Lymphocytes in Preeclampsia. Mediators Inflamm 2017, 8045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkas F, Liberopoulos E, Kei A, Elisaf M, 2013. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann Gastroenterol 26, 23–28. [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS, 2009. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 41, 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, Shah TS, Spencer C, Booth D, Goris A, Oturai A, Saarela J, Fontaine B, Hemmer B, Martin C, Zipp F, D’Alfonso S, Martinelli-Boneschi F, Taylor B, Harbo HF, Kockum I, Hillert J, Olsson T, Ban M, Oksenberg JR, Hintzen R, Barcellos LF, Agliardi C, Alfredsson L, Alizadeh M, Anderson C, Andrews R, Sondergaard HB, Baker A, Band G, Baranzini SE, Barizzone N, Barrett J, Bellenguez C, Bergamaschi L, Bernardinelli L, Berthele A, Biberacher V, Binder TM, Blackburn H, Bomfim IL, Brambilla P, Broadley S, Brochet B, Brundin L, Buck D, Butzkueven H, Caillier SJ, Camu W, Carpentier W, Cavalla P, Celius EG, Coman I, Comi G, Corrado L, Cosemans L, Cournu-Rebeix I, Cree BA, Cusi D, Damotte V, Defer G, Delgado SR, Deloukas P, di Sapio A, Dilthey AT, Donnelly P, Dubois B, Duddy M, Edkins S, Elovaara I, Esposito F, Evangelou N, Fiddes B, Field J, Franke A, Freeman C, Frohlich IY, Galimberti D, Gieger C, Gourraud PA, Graetz C, Graham A, Grummel V, Guaschino C, Hadjixenofontos A, Hakonarson H, Halfpenny C, Hall G, Hall P, Hamsten A, Harley J, Harrower T, Hawkins C, Hellenthal G, Hillier C, Hobart J, Hoshi M, Hunt SE, Jagodic M, Jelcic I, Jochim A, Kendall B, Kermode A, Kilpatrick T, Koivisto K, Konidari I, Korn T, Kronsbein H, Langford C, Larsson M, Lathrop M, Lebrun-Frenay C, Lechner-Scott J, Lee MH, Leone MA, Leppa V, Liberatore G, Lie BA, Lill CM, Linden M, Link J, Luessi F, Lycke J, Macciardi F, Mannisto S, Manrique CP, Martin R, Martinelli V, Mason D, Mazibrada G, McCabe C, Mero IL, Mescheriakova J, Moutsianas L, Myhr KM, Nagels G, Nicholas R, Nilsson P, Piehl F, Pirinen M, Price SE, Quach H, Reunanen M, Robberecht W, Robertson NP, Rodegher M, Rog D, Salvetti M, Schnetz-Boutaud NC, Sellebjerg F, Selter RC, Schaefer C, Shaunak S, Shen L, Shields S, Siffrin V, Slee M, Sorensen PS, Sorosina M, Sospedra M, Spurkland A, Strange A, Sundqvist E, Thijs V, Thorpe J, Ticca A, Tienari P, van Duijn C, Visser EM, Vucic S, Westerlind H, Wiley JS, Wilkins A, Wilson JF, Winkelmann J, Zajicek J, Zindler E, Haines JL, Pericak-Vance MA, Ivinson AJ, Stewart G, Hafler D, Hauser SL, Compston A, McVean G, De Jager P, Sawcer SJ, McCauley JL, 2013. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 45, 1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg RS, 2009. Inflammation in the intestinal tract: pathogenesis and treatment. Dig Dis 27, 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SJ, Mayer L, 2007. The immune response in inflammatory bowel disease. Am J Gastroenterol 102, 2058–2069. [DOI] [PubMed] [Google Scholar]
- Brown SR, Coviello LC, 2015. Extraintestinal Manifestations Associated with Inflammatory Bowel Disease. Surg Clin North Am 95, 1245–1259. [DOI] [PubMed] [Google Scholar]
- Chandel S, Prakash A, Medhi B, 2015. Current scenario in inflammatory bowel disease: drug development prospects. Pharmacol Rep 67, 224–229. [DOI] [PubMed] [Google Scholar]
- Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M, 2014. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104, Unit 15 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Dong L, Leffler NR, Asch AS, Witte ON, Yang LV, 2011. Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway. PLoS One 6, e27586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, Leo P, Cremin K, Pryce K, Harris J, Lee S, Joo KB, Shim SC, Weisman M, Ward M, Zhou X, Garchon HJ, Chiocchia G, Nossent J, Lie BA, Forre O, Tuomilehto J, Laiho K, Jiang L, Liu Y, Wu X, Bradbury LA, Elewaut D, Burgos-Vargas R, Stebbings S, Appleton L, Farrah C, Lau J, Kenna TJ, Haroon N, Ferreira MA, Yang J, Mulero J, Fernandez-Sueiro JL, Gonzalez-Gay MA, Lopez-Larrea C, Deloukas P, Donnelly P, Bowness P, Gafney K, Gaston H, Gladman DD, Rahman P, Maksymowych WP, Xu H, Crusius JB, van der Horst-Bruinsma IE, Chou CT, Valle-Onate R, Romero-Sanchez C, Hansen IM, Pimentel-Santos FM, Inman RD, Videm V, Martin J, Breban M, Reveille JD, Evans DM, Kim TH, Wordsworth BP, Brown MA, 2013. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 45, 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper KN, Blount DG, Riley EM, 2008. IL-10: the master regulator of immunity to infection. J Immunol 180, 5771–5777. [DOI] [PubMed] [Google Scholar]
- de Valliere C, Wang Y, Eloranta JJ, Vidal S, Clay I, Spalinger MR, Tcymbarevich I, Terhalle A, Ludwig MG, Suply T, Fried M, Kullak-Ublick GA, Frey-Wagner I, Scharl M, Seuwen K, Wagner CA, Rogler G, 2015. G Protein-coupled pH-sensing Receptor OGR1 Is a Regulator of Intestinal Inflammation. Inflamm Bowel Dis 21, 1269–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Krewson E, Bliss D, Tulis DA, Yang LV, 2013a. Abstract 11587: Acidosis/GPR4 signaling regulates inflammatory and endoplasmic reticulum stress responses in vascular endothelial cells. Circulation 128, A11587. [Google Scholar]
- Dong L, Krewson EA, Yang LV, 2017. Acidosis Activates Endoplasmic Reticulum Stress Pathways through GPR4 in Human Vascular Endothelial Cells. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Li Z, Leffler NR, Asch AS, Chi JT, Yang LV, 2013b. Acidosis Activation of the Proton-Sensing GPR4 Receptor Stimulates Vascular Endothelial Cell Inflammatory Responses Revealed by Transcriptome Analysis. PLoS One 8, e61991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW, 2000. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 62, 240–248. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Bar-Sagi D, Archer H, Carlos T, Guo H, Greenberger JS, 2002. Pulmonary irradiation-induced expression of VCAM-I and ICAM-I is decreased by manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) gene therapy. Biol Blood Marrow Transplant 8, 175–187. [DOI] [PubMed] [Google Scholar]
- Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat MM, Zeitz M, Siegmund B, Kuhl AA, 2014. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol 7, 4557–4576. [PMC free article] [PubMed] [Google Scholar]
- Fallingborg J, 1999. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull 46, 183–196. [PubMed] [Google Scholar]
- Fallingborg J, Christensen LA, Ingeman-Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH, Rasmussen SN, 1990. Measurement of gastrointestinal pH and regional transit times in normal children. J Pediatr Gastroenterol Nutr 11, 211–214. [DOI] [PubMed] [Google Scholar]
- Fallingborg J, Christensen LA, Jacobsen BA, Rasmussen SN, 1993. Very low intraluminal colonic pH in patients with active ulcerative colitis. Dig Dis Sci 38, 1989–1993. [DOI] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D’Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panes J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D’Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M, 2010. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 42, 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Ito S, Watari K, Mogi C, Arisawa M, Okajima F, Kurose H, Shuto S, 2016. Identification of a Potent and Selective GPR4 Antagonist as a Drug Lead for the Treatment of Myocardial Infarction. ACS Med Chem Lett 7, 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima S, Horiuchi T, Hatta N, Morita C, Higuchi M, Sawabe T, Tsukamoto H, Tahira T, Hayashi K, Fujita S, Niho Y, 2001. Outside-to-inside signal through the membrane TNF-alpha induces E-selectin (CD62E) expression on activated human CD4+ T cells. J Immunol 166, 130–136. [DOI] [PubMed] [Google Scholar]
- Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, van Beek EJ, Make BJ, Crapo JD, Silverman EK, Hersh CP, 2014. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford PS, Mosienko V, Kishi K, Jurisic G, Seuwen K, Kinzel B, Ludwig MG, Wells JA, Christie IN, Koolen L, Abdala AP, Liu BH, Gourine AV, Teschemacher AG, Kasparov S, 2018. CNS distribution, signalling properties and central effects of G-protein coupled receptor 4. Neuropharmacology 138, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter S, van Haaften WT, Hunerwadel A, Baebler K, Herfarth N, Raselli T, Mamie C, Misselwitz B, Rogler G, Weder B, Dijkstra G, Meier CF, de Valliere C, Weber A, Imenez Silva PH, Wagner CA, Frey-Wagner I, Ruiz PA, Hausmann M, 2018. Intestinal activation of pH-sensing receptor OGR1 (GPR68) contributes to fibrogenesis. J Crohns Colitis. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kihara Y, Shimizu T, 2005. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem 280, 9083–9087. [DOI] [PubMed] [Google Scholar]
- Jin Y, Sato K, Tobo A, Mogi C, Tobo M, Murata N, Ishii S, Im DS, Okajima F, 2014. Inhibition of interleukin-1beta production by extracellular acidification through the TDAG8/cAMP pathway in mouse microglia. J Neurochem 129, 683–695. [DOI] [PubMed] [Google Scholar]
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH, 2012. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus CR, Dong L, Yang LV, 2013. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol 4, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus CR, Sanderlin EJ, Dong L, Sun T, Chi JT, Lertpiriyapong K, Yang LV, 2017. Contextual tumor suppressor function of T cell death-associated gene 8 (TDAG8) in hematological malignancies. J Transl Med 15, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus CR, Sanderlin EJ, Yang LV, 2015. Molecular Connections between Cancer Cell Metabolism and the Tumor Microenvironment. Int J Mol Sci 16, 11055–11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnes JH, Cronin RM, Rollin J, Teumer A, Pouplard C, Shaffer CM, Blanquicett C, Bowton EA, Cowan JD, Mosley JD, Van Driest SL, Weeke PE, Wells QS, Bakchoul T, Denny JC, Greinacher A, Gruel Y, Roden DM, 2014. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost 113, 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS, 2010. Inflammatory bowel disease. Annu Rev Immunol 28, 573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler B, Rinchai D, Kewcharoenwong C, Nithichanon A, Biggart R, Hawrylowicz CM, Bancroft GJ, Lertmemongkolchai G, 2017. Interleukin 10 inhibits pro-inflammatory cytokine responses and killing of Burkholderia pseudomallei. Sci Rep 7, 42791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Shajib MS, Manocha MM, Khan WI, 2012. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA, 2015. PHYSIOLOGY. Regulation of breathing by CO(2) requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardner A, 2001. The effects of extracellular pH on immune function. J Leukoc Biol 69, 522–530. [PubMed] [Google Scholar]
- Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D, 2012. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One 7, e32084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen KG, McKenzie CI, Mari M, Murano T, Begun J, Baxt LA, Goel G, Villablanca EJ, Kuo SY, Huang H, Macia L, Bhan AK, Batten M, Daly MJ, Reggiori F, Mackay CR, Xavier RJ, 2016. Genetic Coding Variant in GPR65 Alters Lysosomal pH and Links Lysosomal Dysfunction with Colitis Risk. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Nakakura T, Tomura H, Tobo M, Mogi C, Wang JQ, He XD, Takano M, Damirin A, Komachi M, Sato K, Okajima F, 2010. Each one of certain histidine residues in G-protein-coupled receptor GPR4 is critical for extracellular proton-induced stimulation of multiple G-protein-signaling pathways. Pharmacol Res 61, 499–505. [DOI] [PubMed] [Google Scholar]
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K, 2003. Proton-sensing G-protein-coupled receptors. Nature 425, 93–98. [DOI] [PubMed] [Google Scholar]
- Mattar MC, Lough D, Pishvaian MJ, Charabaty A, 2011. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res 4, 53–61. [PMC free article] [PubMed] [Google Scholar]
- Melgar S, Yeung MM, Bas A, Forsberg G, Suhr O, Oberg A, Hammarstrom S, Danielsson A, Hammarstrom ML, 2003. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol 134, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltz W, Velcicky J, Dawson J, Littlewood-Evans A, Ludwig MG, Seuwen K, Feifel R, Oberhauser B, Meyer A, Gabriel D, Nash M, Loetscher P, 2017. Design and synthesis of potent and orally active GPR4 antagonists with modulatory effects on nociception, inflammation, and angiogenesis. Bioorg Med Chem 25, 4512–4525. [DOI] [PubMed] [Google Scholar]
- Mogi C, Tobo M, Tomura H, Murata N, He XD, Sato K, Kimura T, Ishizuka T, Sasaki T, Sato T, Kihara Y, Ishii S, Harada A, Okajima F, 2009. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J Immunol 182, 3243–3251. [DOI] [PubMed] [Google Scholar]
- Neurath MF, 2017. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 14, 269–278. [DOI] [PubMed] [Google Scholar]
- Nugent SG, Kumar D, Rampton DS, Evans DF, 2001. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 48, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, Kochi Y, Ohmura K, Suzuki A, Yoshida S, Graham RR, Manoharan A, Ortmann W, Bhangale T, Denny JC, Carroll RJ, Eyler AE, Greenberg JD, Kremer JM, Pappas DA, Jiang L, Yin J, Ye L, Su DF, Yang J, Xie G, Keystone E, Westra HJ, Esko T, Metspalu A, Zhou X, Gupta N, Mirel D, Stahl EA, Diogo D, Cui J, Liao K, Guo MH, Myouzen K, Kawaguchi T, Coenen MJ, van Riel PL, van de Laar MA, Guchelaar HJ, Huizinga TW, Dieude P, Mariette X, Bridges SL Jr., Zhernakova A, Toes RE, Tak PP, Miceli-Richard C, Bang SY, Lee HS, Martin J, Gonzalez-Gay MA, Rodriguez-Rodriguez L, Rantapaa-Dahlqvist S, Arlestig L, Choi HK, Kamatani Y, Galan P, Lathrop M, Eyre S, Bowes J, Barton A, de Vries N, Moreland LW, Criswell LA, Karlson EW, Taniguchi A, Yamada R, Kubo M, Liu JS, Bae SC, Worthington J, Padyukov L, Klareskog L, Gregersen PK, Raychaudhuri S, Stranger BE, De Jager PL, Franke L, Visscher PM, Brown MA, Yamanaka H, Mimori T, Takahashi A, Xu H, Behrens TW, Siminovitch KA, Momohara S, Matsuda F, Yamamoto K, Plenge RM, 2014. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506, 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F, 2013. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal 25, 2263–2271. [DOI] [PubMed] [Google Scholar]
- Perse M, Cerar A, 2012. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol 2012, 718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JM, Avia M, Martin V, Sevilla N, 2017. IL-10: A Multifunctional Cytokine in Viral Infections. J Immunol Res 2017, 6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderlin EJ, Justus CR, Krewson EA, Yang LV, 2015. Emerging roles for the pH-sensing G protein-coupled receptors in response to acidotic stress. Cell Health Cytoskelet 7, 99–109. [Google Scholar]
- Sanderlin EJ, Leffler NR, Lertpiriyapong K, Cai Q, Hong H, Bakthavatchalu V, Fox JG, Oswald JZ, Justus CR, Krewson EA, O’Rourke D, Yang LV, 2017. GPR4 deficiency alleviates intestinal inflammation in a mouse model of acute experimental colitis. Biochim Biophys Acta 1863, 569–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, Saarela J, Bellenguez C, Fontaine B, Gillman M, Hemmer B, Gwilliam R, Zipp F, Jayakumar A, Martin R, Leslie S, Hawkins S, Giannoulatou E, D’Alfonso S, Blackburn H, Martinelli Boneschi F, Liddle J, Harbo HF, Perez ML, Spurkland A, Waller MJ, Mycko MP, Ricketts M, Comabella M, Hammond N, Kockum I, McCann OT, Ban M, Whittaker P, Kemppinen A, Weston P, Hawkins C, Widaa S, Zajicek J, Dronov S, Robertson N, Bumpstead SJ, Barcellos LF, Ravindrarajah R, Abraham R, Alfredsson L, Ardlie K, Aubin C, Baker A, Baker K, Baranzini SE, Bergamaschi L, Bergamaschi R, Bernstein A, Berthele A, Boggild M, Bradfield JP, Brassat D, Broadley SA, Buck D, Butzkueven H, Capra R, Carroll WM, Cavalla P, Celius EG, Cepok S, Chiavacci R, Clerget-Darpoux F, Clysters K, Comi G, Cossburn M, Cournu-Rebeix I, Cox MB, Cozen W, Cree BA, Cross AH, Cusi D, Daly MJ, Davis E, de Bakker PI, Debouverie M, D’Hooghe MB, Dixon K, Dobosi R, Dubois B, Ellinghaus D, Elovaara I, Esposito F, Fontenille C, Foote S, Franke A, Galimberti D, Ghezzi A, Glessner J, Gomez R, Gout O, Graham C, Grant SF, Guerini FR, Hakonarson H, Hall P, Hamsten A, Hartung HP, Heard RN, Heath S, Hobart J, Hoshi M, Infante-Duarte C, Ingram G, Ingram W, Islam T, Jagodic M, Kabesch M, Kermode AG, Kilpatrick TJ, Kim C, Klopp N, Koivisto K, Larsson M, Lathrop M, Lechner-Scott JS, Leone MA, Leppa V, Liljedahl U, Bomfim IL, Lincoln RR, Link J, Liu J, Lorentzen AR, Lupoli S, Macciardi F, Mack T, Marriott M, Martinelli V, Mason D, McCauley JL, Mentch F, Mero IL, Mihalova T, Montalban X, Mottershead J, Myhr KM, Naldi P, Ollier W, Page A, Palotie A, Pelletier J, Piccio L, Pickersgill T, Piehl F, Pobywajlo S, Quach HL, Ramsay PP, Reunanen M, Reynolds R, Rioux JD, Rodegher M, Roesner S, Rubio JP, Ruckert IM, Salvetti M, Salvi E, Santaniello A, Schaefer CA, Schreiber S, Schulze C, Scott RJ, Sellebjerg F, Selmaj KW, Sexton D, Shen L, Simms-Acuna B, Skidmore S, Sleiman PM, Smestad C, Sorensen PS, Sondergaard HB, Stankovich J, Strange RC, Sulonen AM, Sundqvist E, Syvanen AC, Taddeo F, Taylor B, Blackwell JM, Tienari P, Bramon E, Tourbah A, Brown MA, Tronczynska E, Casas JP, Tubridy N, Corvin A, Vickery J, Jankowski J, Villoslada P, Markus HS, Wang K, Mathew CG, Wason J, Palmer CN, Wichmann HE, Plomin R, Willoughby E, Rautanen A, Winkelmann J, Wittig M, Trembath RC, Yaouanq J, Viswanathan AC, Zhang H, Wood NW, Zuvich R, Deloukas P, Langford C, Duncanson A, Oksenberg JR, Pericak-Vance MA, Haines JL, Olsson T, Hillert J, Ivinson AJ, De Jager PL, Peltonen L, Stewart GJ, Hafler DA, Hauser SL, McVean G, Donnelly P, Compston A, 2011. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HJ, Chiu YT, Chiu CT, Lin YC, Wang CY, Hsieh JY, Wei SC, 2018. Inflammatory bowel disease and its treatment in 2018: Global and Taiwanese status updates. J Formos Med Assoc. [DOI] [PubMed] [Google Scholar]
- Sun X, Yang LV, Tiegs BC, Arend LJ, McGraw DW, Penn RB, Petrovic S, 2010. Deletion of the pH sensor GPR4 decreases renal acid excretion. J Am Soc Nephrol 21, 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobo A, Tobo M, Nakakura T, Ebara M, Tomura H, Mogi C, Im DS, Murata N, Kuwabara A, Ito S, Fukuda H, Arisawa M, Shuto S, Nakaya M, Kurose H, Sato K, Okajima F, 2015. Characterization of Imidazopyridine Compounds as Negative Allosteric Modulators of Proton-Sensing GPR4 in Extracellular Acidification-Induced Responses. PLoS One 10, e0129334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura H, Wang JQ, Komachi M, Damirin A, Mogi C, Tobo M, Kon J, Misawa N, Sato K, Okajima F, 2005. Prostaglandin I(2) production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J Biol Chem 280, 34458–34464. [DOI] [PubMed] [Google Scholar]
- Ulyanova T, Scott LM, Priestley GV, Jiang Y, Nakamoto B, Koni PA, Papayannopoulou T, 2005. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood 106, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainer B, Nielsen OH, Horn T, 1998. Expression of E-selectin, sialyl Lewis X, and macrophage inflammatory protein-1alpha by colonic epithelial cells in ulcerative colitis. Dig Dis Sci 43, 596–608. [DOI] [PubMed] [Google Scholar]
- Velcicky J, Miltz W, Oberhauser B, Orain D, Vaupel A, Weigand K, Dawson King J, Littlewood-Evans A, Nash M, Feifel R, Loetscher P, 2017. Development of Selective, Orally Active GPR4 Antagonists with Modulatory Effects on Nociception, Inflammation, and Angiogenesis. J Med Chem 60, 3672–3683. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Kon J, Mogi C, Tobo M, Damirin A, Sato K, Komachi M, Malchinkhuu E, Murata N, Kimura T, Kuwabara A, Wakamatsu K, Koizumi H, Uede T, Tsujimoto G, Kurose H, Sato T, Harada A, Misawa N, Tomura H, Okajima F, 2004. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J Biol Chem 279, 45626–45633. [DOI] [PubMed] [Google Scholar]
- Wang Y, de Valliere C, Imenez Silva PH, Leonardi I, Gruber S, Gerstgrasser A, Melhem H, Weber A, Leucht K, Wolfram L, Hausmann M, Krieg C, Thomasson K, Boyman O, Frey-Wagner I, Rogler G, Wagner CA, 2018. The Proton-activated Receptor GPR4 Modulates Intestinal Inflammation. J Crohns Colitis 12, 355–368. [DOI] [PubMed] [Google Scholar]
- Yang LV, Radu CG, Roy M, Lee S, McLaughlin J, Teitell MA, Iruela-Arispe ML, Witte ON, 2007. Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor. Mol Cell Biol 27, 1334–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Liver weight assessment and histological evaluation. GPR4 antagonist 13 does not induce observable liver pathology when compared to vehicle control mice. Representative pictures of normal vehicle (A) and GPR4 antagonist 13 (B) liver histology. Weight measurements of livers between vehicle and GPR4 antagonist 13 male (C) and female (D) mice. Vehicle: N=21 (10 male/11 female) and GPR4 antagonist 13: N=19 (10 male/9 female). Data are presented as mean ± S.E.M. and was analyzed for statistical significance using the t-test between vehicle and GPR4 antagonist 13 groups. No statistically significant difference was observed. Scale bar = 100μm.
VCAM-1 and E-selectin expression was assessed in non-endothelial cells within the colon. VCAM-1 is expressed in non-endothelial mononuclear cells and fibroblasts and E-selectin is expressed in the intestinal epithelium. Representative pictures of VCAM-1 in vehicle (A) and GPR4 antagonist 13-treated mice (B) and E-selectin in vehicle (C) and GPR4 antagonist 13-treated mice (D) in the colon. Scale bar = 100μm. Red arrows indicate VCAM-1 positive cells and black arrows indicate E-selectin positive cells.
TNF-α protein expression was assessed by immunohistochemistry in immune cells and endothelial cells within the distal colon segment. TNF-α expression is reduced in colon tissues from mice treated with GPR4 antagonist 13 when compared to vehicle. Representative pictures of TNF-α in vehicle (A) and GPR4 antagonist 13-treated mice (B). 40× objective. Representative pictures of high magnification (63×) TNF-α-positive intestinal microvascular endothelial cells between vehicle (C) and GPR4 antagonist 13 (D) treatment groups. Scale bar = 100μm. Red arrows indicate endothelial cells and black arrows indicate leukocytes.