ABSTRACT
Local immunotherapies such as the intratumoral injection of oncolytic compounds aim at reinstating and enhancing systemic anticancer immune responses. LTX-315 is a first-in-class, clinically evaluated oncolytic peptide-based local immunotherapy that meets these criteria. Here, we show that LTX-401, yet another oncolytic compound designed for local immunotherapy, depicts a similar safety profile and that sequential local inoculation of LTX-401 was able to cure immunocompetent host from subcutaneous MCA205 and TC-1 cancers. Cured animals exhibited long-term immune memory effects that rendered them resistant to rechallenge with syngeneic tumors. Nevertheless, the local treatment with LTX-401 alone had only limited abscopal effects on secondary contralateral lesions. Anticancer effects resulting from single as well as sequential injections of LTX-401 were boosted in combination with PD-1 and CTLA-4 immune checkpoint blockade (ICB), and sequential LTX-401 treatment combined with double ICB exhibited strong abscopal antineoplastic effects on contralateral tumors underlining the potency of this combination therapy.
KEYWORDS: Oncolysis, anticancer therapy, immunogenic cell death, checkpoint blockade
Introduction
The word ‘oncolysis’ usually evokes oncolytic viruses, i.e. viruses that selectively infect and kill cancer cells.1,2 However, oncolysis can also be achieved by applying physical, chemical or pharmacological agents to tumor in a way that the cancer is locally destroyed. For example, cationic ampholytic peptides or peptide mimetics can be used to target cellular membranes, causing their disruption by detergent-like effects. We have been characterizing the mode of action of several oncolytic reagents of this class such as LTX-315, LTX-401, DTT-205 and DTT-304. LTX-315 turned out to preferentially target mitochondrial membranes;3,4 while LTX-401 destroys Golgi apparatus-associated membranes,5–7 thus setting off a different cell-destroying cascade.7 DTT-205 and DTT-304, instead, have a tropism for lysosomal membranes, thus activating yet another cell death subroutine.8 Despite their diversity, these oncolytic peptide and peptide derivatives all appear to be able to stimulate anticancer immune responses, at least in specific circumstances. Thus, cancer cells treated with LTX-315 or LTX-401 in vitro can stimulate a protective antineoplastic immune response when injected into rodents.3,7,9–13 Moreover, LTX-315 treatments sensitize to subsequent immune checkpoint blockade with αCTLA-4 antibodies,11 while tumors that are successfully cured with DTT-205 or DTT-304 established a long-term immune response rendering them resistant against later rechallenge with the cancer cells that had been eliminated from them.8
Here, we compared the safety profiles of different lytic peptides to discover that LTX-401 (similar to LTX-315) was far less toxic than DTT-205 and DTT-304. We also investigated the capacity of LTX-401 to stimulate anticancer immune responses in three different experimental setups. Our results support the hypothesis that oncolysis by LTX-401 is highly immunogenic.
Results and discussion
Differential toxicity of oncolytic agents
In a retrospective study, the safety profiles of oncolytic peptides and peptidomimetics at their therapeutically effective doses were compared. The onset of manifest discomfort or death that was defined as an endpoint for in vivo experimentation was evaluated for the sequential treatment of tumors established on C57Bl/6 mice with therapeutically effective doses of LTX-315, LTX-401, DTT-205 and DTT-304 (Figure 1A). Data from in vivo experimentation utilizing oncolytic compounds in C57BL/6 mice bearing palpable subcutaneous solid tumors were evaluated by enumerating global survival (Figure 1B). Whereas both experimental compounds DTT-205 and DTT-304 led to death in a certain number of animals, LTX-401, similar to LTX-315, that is under clinical evaluation in patients with transdermally accessible tumors, did not depict any signs of adverse toxicity in vivo.
Figure 1.
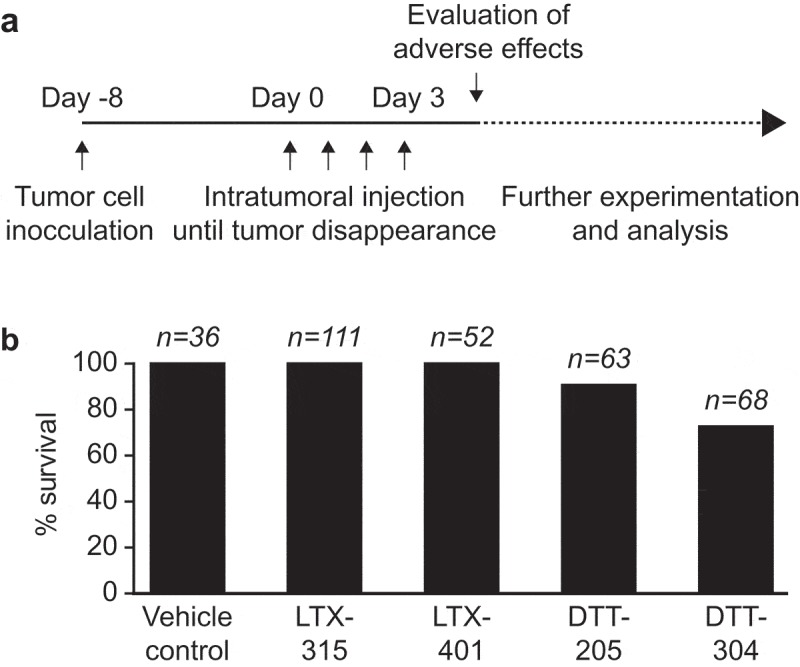
Absence of adverse toxicity in LTX-401-treated mice. In a retrospective study, the adverse toxicity of sequential intratumoral injections of oncolytic peptides and peptidomimetics at they are respective therapeutically effective dose such as LTX-315 (0.3 mg/injection), LTX-401 (0.25 mg/injection), DTT-205 and DTT-304 (both at 1.5 mg/injection) was evaluated. Note that these doses have been optimized to obtain an optimal antitumor effect. Data from in vivo experimentation utilizing oncolytic compounds in C57BL/6 mice bearing palpable subcutaneous solid tumors were evaluated and global survival was enumerated (A). The percentage of animals that survived without severe discomfort requiring their euthanasia is indicated (B).
Stimulation of anticancer immunity by LTX-401
To identify the capacity of LTX-401 to stimulate anticancer immune responses, we treated established MCA205 fibrosarcomas growing subcutaneously (s.c.) in C57BL/6 mice by daily intratumoral injection (up to 4 times) of the compound (or, as a control, its vehicle PBS) until macroscopically detectable tumors disappeared or persisted (Figure 2A). This procedure greatly reduced tumor growth (Figure 2B) and extended the longevity of the mice, yielding a survival rate of ~22% at 60 days post-diagnosis (Figure 2C). At this point, tumor-free mice were rechallenged by s.c. inoculation of the same tumor type (MCA205, injected into the opposite flank) or antigenically unrelated mammary carcinoma AT3 cells (injected into the same flank, though at a site distant from the primary MCA205 fibrosarcoma). Importantly, all mice that were cured by LTX-401 treatment from their primary MCA205 tumor became resistant to rechallenge with MCA205 (but not AT3) cancer cells (Figure 2D), supporting the idea that they had developed a long-term immunological memory. Very similar results were obtained when MCA205 fibrosarcomas were replaced by non-small cell lung adenocarcinoma TC-1 cells (that have been engineered to express the human papilloma virus antigen E6). Again, local therapy with LTX-401 injections was able to greatly reduce the growth of primary TC-1 carcinomas (Figure 2E), yielding a 60% survival rate at 60 days (Figure 2F). Again, mice that were cured became resistant to rechallenge with TC-1 (but not MCA205) cells (Figure 2G), confirming the contention that a tumor-specific memory immune response had been established.
Figure 2.
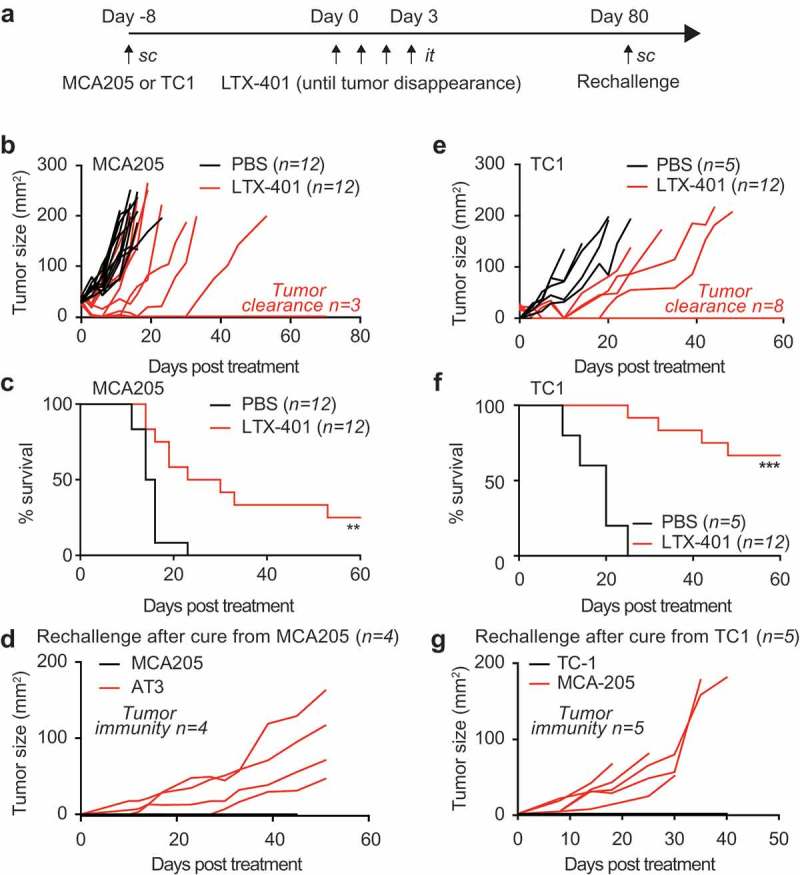
In vivo activity of LTX-401 on subcutaneous MCA205 fibrosarcoma in immunocompetent animals. Mouse fibrosarcoma MCA205 cells were injected subcutaneously in syngeneic C57BL/6 mice and palpable tumors arising thereof were treated with sequential intratumoral injections of 0.25 mg LTX-401 as indicated in (A). LTX-401 induced efficient oncolysis an effect that is reflected in reduced tumor growth (B), and increased overall survival (C) (Chi2 test, **p < 0.01, n = 12). Rechallenge of animals cured from MCA205 fibrosarcoma (pooled from several experiments) with MCA205 several weeks after the initial therapy on the contralateral flank and challenged with syngeneic mouse AT3 breast cancer cells on the ipsilateral side resulted in efficient rejection of MCA205 but aggressive tumor growth of AT3 (D). Thus, LTX-401 caused the generation of immunological memory that sufficed in rejecting isogenic tumors. Similar effects were obtained when mouse lung cancer TC-1 cells were inoculated subcutaneously in syngeneic C57BL/6 mice and tumors and were treated when palpable with repeated injections of 0.25 mg LTX-401 intratumorally as shown in (A). LTX-401 induced efficient oncolysis and tumor control in some animals reflected in reduced tumor growth and cure of some animals (E), and increased overall survival (F) (Chi2 test, ***p < 0.001). Some of the animals cured from subcutaneous TC-1 lung cancers were rechallenged with TC-1 several weeks after the initial therapy on the contralateral and challenged with syngeneic mouse MCA205 fibrosarcoma cells on the ipsilateral side. This maneuver resulted in efficient rejection of TC-1 but aggressive tumor growth of MCA205 (G). Thus, also in this model system LTX-401 caused the generation of immunological memory in cured animals that sufficed in rejecting isogenic tumors.
Limited abscopal effects of LTX-401-mediated tumor lysis
In the next step, we interrogated the experimental system for the induction of abscopal effects, i.e. a presumably immune-mediated long-distance effect of the injected LTX-401 peptide that would reduce the growth of a non-injected tumor. For this, we inoculated mice with MCA205 fibrosarcomas into the right flank to create a primary tumor (that became palpable 8 days later) and shortly later (4 days after the first injection) a similar amount of MCA205 cells into the left flank to create a secondary tumor. Only the primary tumor was injected for up to 4 days until tumor disappearance with LTX-401 (or PBS as a vehicle control) and the growth of both the primary (treated) and secondary (untreated) tumors were monitored. The local treatment had a significant tumor growth reducing effect on primary tumors (Figure 3A,B,D) yet only limited effects on secondary contralateral tumors (Figure 3C,E). Hence, LTX-401-mediated oncolysis alone is not sufficient to induce a therapeutically relevant abscopal effect.
Figure 3.
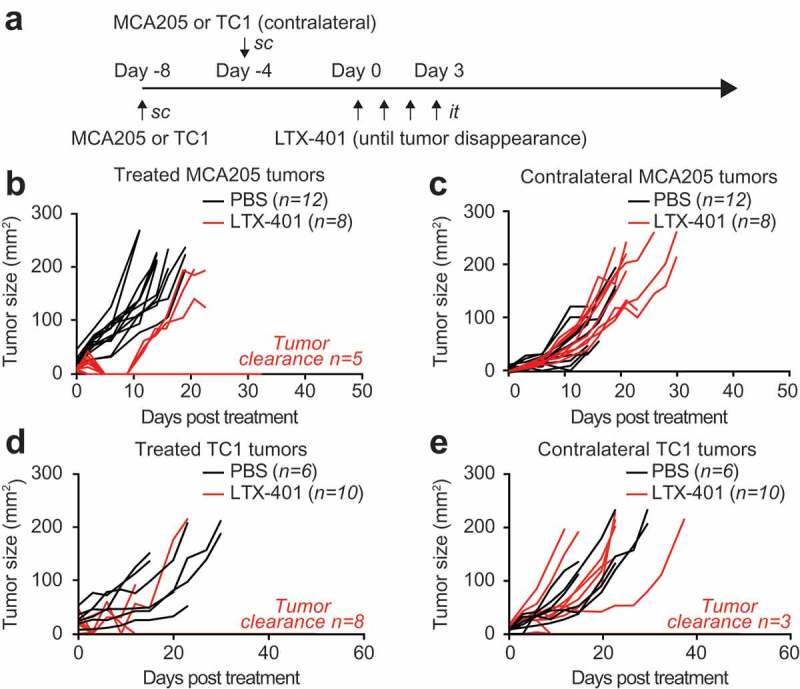
LTX-401 treatment of subcutaneous MCA205 fibrosarcoma induces limited abscopal effects. C57BL/6 mice were inoculated with murine fibrosarcoma MCA205 subcutaneously on one side and four days later on the contralateral flank. Palpable primary tumors arising from the first injection were treated with sequential intratumoral injections of 0.25 mg LTX-401 as indicated in (A). LTX-401 induced efficient oncolysis in primary tumors which is reflected in reduced tumor growth and tumor clearance in some animals (B). In parallel tumors arising from untreated secondary lesions were monitored and abscopal effects were documented as tumor growth in separate curves (C). Lung cancer TC-1 cells were injected subcutaneously into C57BL/6 mice on one flank and four days later on the contralateral side. Palpable primary tumors arising from the first injection were treated with sequential injections of 0.25 mg LTX-401 intratumorally as indicated in (A). LTX-401 induced efficient oncolysis in primary tumors which is reflected in reduced tumor growth and tumor clearance in some animals (D). In parallel tumors arising from untreated secondary lesions were monitored and abscopal effects were documented as tumor growth curves (E).
Combinatorial effects of LTX-401 and anti-CTLA4-mediated immune checkpoint blockade
Given the capacity of local LTX-401 therapy to induce memory immune responses and abscopal effects on distant lesions, we investigated the possibility that this agent would sensitize cancers to therapy with immune checkpoint blockers targeting CTLA-4 or PD-1. For this, established MCA205 fibrosarcomas were injected only once intratumorally and then subjected to immunotherapy with antibodies specific for CTLA-4, PD-1 or a combination of both (Figure 4A). Continuous tumor monitoring led to the conclusion that the most efficient therapeutic regimen consisted in a combination of all three anticancer agents (LTX-401, αCTLA-4 and αPD-1). In contrast, single-agent therapies appeared to be relatively inefficient in this setting (Figure 4B–F). When the survival of tumor-bearing animals was monitored, the combination of LTX-401 and dual checkpoint blockade (αCTLA-4 plus αPD-1) or the combination of LTX-401 and αCTLA-4 (but not αPD-1) alone were able to significantly extend life expectancy (Figure 4G–H). Again, mice that had been rendered tumor-free for more than 50 days resisted rechallenge with the same cancer cell type from which they had been cured (MCA205), yet readily developed TC-1 cancers (Figure 4I). Thus, as to be expected, mice that had been cured by a combination of local oncolysis and systemic immunotherapy had established a specific anticancer immune response. Sequential treatments of LTX-401 in combination with dual checkpoint blockade increased this effect and was able to achieve 100% clearance of treated tumors (Figure 5A,B,D). Of note in six out of nine animals the combination of LTX-401 with dual checkpoint blockade not only cleared the treated tumor but also exhibited abscopal neoplastic effects on distant tumors (Figure 5C,E).
Figure 4.
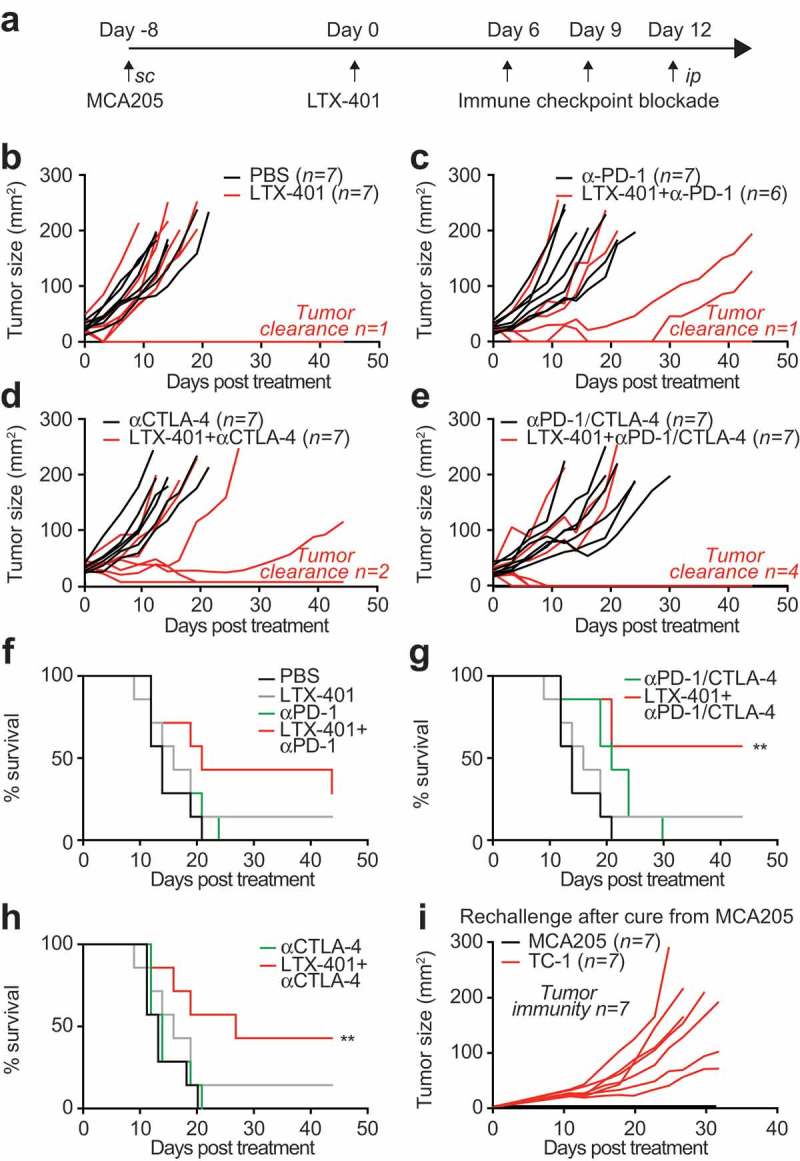
Combination of single LTX-401 treatment with immune checkpoint blockade increases its efficacy. C57BL/6 mice subcutaneously implanted with MCA205 fibrosarcoma were injected intratumorally when tumors became palpable with a single injection of 0.25 mg LTX-401 as indicated in (A). Immune checkpoint blockade was mounted by sequential injections of monoclonal antibodies targeting CTLA-4 or PD-1 alone or in combination at day 6, 9 and 12 post treatment. LTX-401 induced efficient oncolysis which is reflected in reduced tumor growth and tumor clearance in some animals. This effect was increased in combination with CTLA-4 and PD-1 cotreatment as depicted in separate tumor growth curves (B-E) and overall survival plots (Chi2 test, **p < 0.01) (F-H). Rechallenge of animals cured from MCA205 fibrosarcoma with MCA205 several weeks after the initial therapy on the contralateral and challenged with syngeneic mouse TC-1 lung cancer cells on the ipsilateral side resulted in efficient rejection of MCA205 but aggressive tumor growth of TC-1 (I). Thus, LTX-401 caused the generation of immunological memory that led to the rejection of isogenic tumors.
Figure 5.
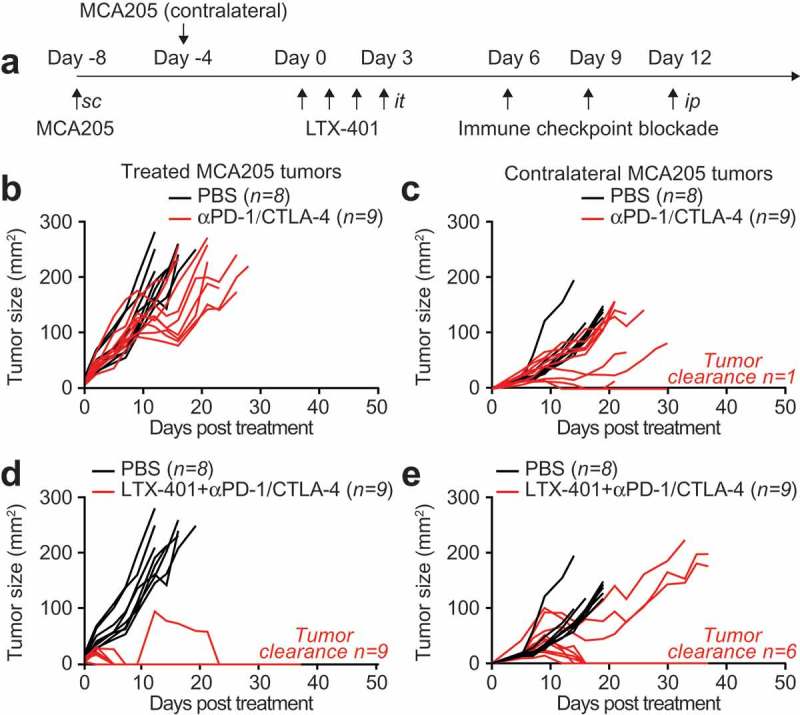
Sequential LTX-401 treatment with double immune checkpoint blockade exhibits systemic antitumor immunity C57BL/6 mice were inoculated with murine fibrosarcoma MCA205 subcutaneously on one side and four days later on the contralateral flank. Palpable primary tumors arising from the first injection were treated with sequential injections of 0.25 mg LTX-401 as indicated in (A). Immune checkpoint blockade was mounted by sequential injections of double immune checkpoint blockade with monoclonal antibodies targeting CTLA-4 or PD-1 at day 6, 9 and 12 post treatment. Immune checkpoint inhibition alone did not control tumor growth of LTX-401-treated primary (B) and distant (C) tumors. In contrast, combination of sequential LTX-401 treatment with double immune checkpoint blockade cleared 100% of LTX-401-treated primary tumors (D) and depicted abscopal effects on distant tumors (E).
Concluding remarks
The results of this study suggest that LTX-401 can provide oncolysis with a relatively favorable safety profile when the agent is injected into established tumors. Indeed, LTX-401 could be injected daily on 4 subsequent days with zero fatalities. Oncolysis by LTX-401 yielded a rapid macroscopic response and allowed most of the tumors to become undetectable following several local injections. This procedure likely does not simply cause necrotic (and passive) lysis of neoplastic and stromal cells. Rather, it triggered some immunogenic events, be it the induction of immunogenic cell death or by the liberation of tumor-associated antigens from their tumor towards the draining lymph nodes.14 Irrespective of the exact molecular mechanisms accounting for these effects, there are at least three lines of evidence pleading in favor of LTX-401-triggered cancer-specific immunogenicity. First, animals that had been cured from their established cancers by LTX-401 became resistance to rechallenge with the same cancer type. Second, one single intratumoral injection of LTX-401 (which turned out to be inefficient on its own in reducing tumor volume) sensitized the cancers to subsequent immunotherapy with antibodies blocking CTLA-4 or, more so, a combination of antibodies targeting CTLA-4 and PD-1. Third, in mice bearing two neoplastic lesions, one which was treated and another one that was left untreated, the combination of LTX-401 with double immune checkpoint blockade did not only reduce the growth of the locally injected tumor but also mediated long-distance (abscopal) effects resulting in the control of most of the non-injected cancers.
Altogether, these results convincingly demonstrate that LTX-401 mediated oncolysis may advantageously combine with established immunotherapeutic regimens.
Materials & methods
Chemicals, cell culture media and supplements
All supplements and cell culture media were purchased from Thermo Fisher Scientific (Carlsbad, CA, US). LTX-401 was provided by Lytix Biopharma (Oslo, Norway). Cell culture support and consumables were obtained from Greiner Bio-One (Monroe, CA, US). Mouse fibrosarcoma cells MCA-205 and murine lung cancer TC-1 cells were cultured in Glutamax®-containing DMEM medium supplemented with 10% fetal bovine serum (FBS), and 10 mM HEPES. Cells were maintained in a humidified incubator at 37°C with an atmosphere containing 5% CO2.
In vivo experimentation
Female wild-type C57BL/6 mice at the age of 6–8 weeks were obtained from Harlan France (Gannat, France) and kept under controlled conditions in the animal facility at the Gustave Roussy Campus Cancer in specific pathogen-free and temperature-controlled environment with 12 h day, 12 h night cycles and received food and water ad libitum. Animal experiments were conducted in compliance with the EU Directive 63/2010, and protocols 2013_094A and were approved by the Ethical Committee of the Gustave Roussy Campus Cancer (CEEA IRCIV/IGR no. 26, registered at the French Ministry of Research). MCA205 or TC-1 tumors were established in C57BL/6 hosts by subcutaneously inoculating 5 × 105 cells. When tumors became palpable, either 0.25 mg (for each injection of sequential treatment) or 0.4 mg (for single injection) of LTX-401 was injected intratumorally. Remaining tumor tissue was treated on subsequent days accordingly and animal well-being and tumor growth were monitored. Mice were sacrificed when tumor size reached end-point or signs of obvious discomfort associated with the treatment were observed always following the EU Directive 63/2010 and our Ethical Committee advice. Surviving and tumor-free animals were analyzed and kept for more than 30 days before subcutaneous rechallenge with 5 × 105 live TC-1 in one flank and 5 × 105 live MCA205 cells injected in the contralateral flank. In the case of previously injected TC-1 tumor-free animals, the location of the injected cells was inverted. Animals were monitored and tumor growth documented regularly until end-points were reached or signs of obvious discomfort were observed. Statistical analysis was performed employing two-way ANOVA analysis followed by Bonferroni’s test comparing to control conditions (* p < 0.05, ** p < 0.01 and ***p < 0.001). Abscopal effects were analyzed by inoculating C57BL/6 hosts subcutaneously with 5 × 105 MCA205 or TC-1 cells. Four days later the same number of cells was inoculated on the contralateral side to establish a second syngeneic tumor. Following exclusively primary tumors were treated with the oncolytic LTX-401 as described above and tumor sizes of both primary and secondary tumor were monitored and documented regularly until end-points were reached or signs of obvious discomfort were observed.
Retrospective study
The adverse toxicity of sequential intratumoral injections of oncolytic peptides and peptidomimetics at their respective therapeutically effective dose such as LTX-315 (0.3 mg/injection), LTX-401 (0.25 mg/injection), DTT-205 and DTT-304 (both at 1.5 mg/injection) was evaluated. Data from in vivo experimentation utilizing oncolytic compounds in C57BL/6 mice bearing palpable subcutaneous solid tumors were collected from these two studies,11,8 and unpublished data. Animals that depicted adverse toxic effects were enumerated and the percentage of mice that depicted an onset of discomfort upon treatment was plotted.
Immune checkpoint blockade
Immune checkpoint blockade was mounted by sequential intraperitoneal injections of 200 µg monoclonal antibodies targeting PD-1 (Clone 29F.1A12, BioXcell, West Lebanon, NH, USA) or CTLA-4 (Clone 9D9, BioXcell) alone or in combination at day 6, 9 and 12 post treatment. Animals were monitored and tumor growth documented regularly until end-points were reached or signs of obvious discomfort were observed. Statistical analysis was performed employing two-way ANOVA analysis followed by Bonferroni’s test comparing to control conditions (* p < 0.05, ** p < 0.01 and ***p < 0.001).
Funding Statement
WX,HZ and YW were supported by the China Scholarship Council, GK and LZ are supported by the Ligue contre le Cancer (équipes labelisées); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); the Seerave foundation; the Swiss Bridge Foundation, ISREC and the Paris Alliance of Cancer Research Institutes (PACRI). This project was supported by the Norwegian Research Council (254800, 257967).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Abbreviations
- DAMP
danger-associated molecular pattern
- ICD
immunogenic cell death
References
- 1.Fend L, Yamazaki T, Remy C, Fahrner C, Gantzer M, Nourtier V, Preville X, Quemeneur E, Kepp O, Adam J, et al. Immune checkpoint blockade, immunogenic chemotherapy or IFN-alpha blockade boost the local and abscopal effects of oncolytic virotherapy. Cancer Res. 2017;77(15):4146–4157. doi: 10.1158/0008-5472.CAN-16-2165. [DOI] [PubMed] [Google Scholar]
- 2.Bommareddy PK, Shettigar M, Kaufman HL.. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18(8):498–513. doi: 10.1038/s41577-018-0014-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H, Forveille S, Sauvat A, Yamazaki T, Senovilla L, Ma Y, Liu P, Yang H, Bezu L, Muller K, et al. The oncolytic peptide LTX-315 triggers immunogenic cell death. Cell Death Dis. 2016;7:e2134. doi: 10.1038/cddis.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, Forveille S, Sauvat A, Sica V, Izzo V, Durand S, Muller K, Liu P, Zitvogel L, Rekdal O, et al. The oncolytic peptide LTX-315 kills cancer cells through Bax/Bak-regulated mitochondrial membrane permeabilization. Oncotarget. 2015;6(29):26599–26614. doi: 10.18632/oncotarget.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes-da-Silva LC, Jimenez AJ, Sauvat A, Xie W, Souquere S, Divoux S, Storch M, Sveinbjornsson B, Rekdal O, Arnaut LG, et al. Recruitment of LC3 to damaged Golgi apparatus. Cell Death Differ. 2018. doi: 10.1038/s41418-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes-da-Silva LC, Zhao L, Bezu L, Zhou H, Sauvat A, Liu P, Durand S, Leduc M, Souquere S, Loos F, et al. Photodynamic therapy with redaporfin targets the endoplasmic reticulum and Golgi apparatus. Embo J. 2018;37(13). doi: 10.15252/embj.201798354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Sauvat A, Gomes-da-Silva LC, Durand S, Forveille S, Iribarren K, Yamazaki T, Souquere S, Bezu L, Muller K, et al. The oncolytic compound LTX-401 targets the Golgi apparatus. Cell Death Differ. 2016;23(12):2031–2041. doi: 10.1038/cdd.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, Mondragon L, Xie W, Mauseth B, Leduc M, Sauvat A, Gomes-da-Silva LC, Forveille S, Iribarren K, Souquere S, et al. Oncolysis with DTT-205 and DTT-304 generates immunological memory in cured animals. Cell Death Dis. 2018;9(11):1086. doi: 10.1038/s41419-018-1127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nestvold J, Wang MY, Camilio KA, Zinocker S, Tjelle TE, Lindberg A, Haug BE, Kvalheim G, Sveinbjornsson B, Rekdal O.. Oncolytic peptide LTX-315 induces an immune-mediated abscopal effect in a rat sarcoma model. Oncoimmunology. 2017;6(8):e1338236. doi: 10.1080/2162402X.2017.1338236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sveinbjornsson B, Camilio KA, Haug BE, Rekdal O. LTX-315: a first-in-class oncolytic peptide that reprograms the tumor microenvironment. Future Med Chem. 2017;9(12):1339–1344. doi: 10.4155/fmc-2017-0088. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki T, Pitt JM, Vetizou M, Marabelle A, Flores C, Rekdal O, Kroemer G, Zitvogel L. The oncolytic peptide LTX-315 overcomes resistance of cancers to immunotherapy with CTLA4 checkpoint blockade. Cell Death Differ. 2016;23(6):1004–1015. doi: 10.1038/cdd.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camilio KA, Rekdal O, Sveinbjornsson B. LTX-315 (Oncopore): a short synthetic anticancer peptide and novel immunotherapeutic agent. Oncoimmunology. 2014;3:e29181. doi: 10.4161/onci.29181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eike LM, Mauseth B, Camilio KA, Rekdal O, Sveinbjornsson B. The cytolytic amphipathic beta(2,2)-Amino acid LTX-401 Induces DAMP release in melanoma cells and causes complete regression of B16 Melanoma. PLoS One. 2016;11(2):e0148980. doi: 10.1371/journal.pone.0148980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]


