Abstract
Purpose:
To summarize the evidence from the 2018 Physical Activity Guidelines Advisory Committee Scientific Report, including new evidence from an updated search of the effects of physical activity on maternal health during pregnancy and postpartum.
Methods:
An initial search was undertaken to identify systematic reviews and meta-analyses published between 2006 and 2016. An updated search then identified additional systematic reviews and meta-analyses published between January 2017 through February 2018. The searches were conducted in PubMed®, CINAHL, and Cochrane Library and supplemented through hand-searches of reference lists of included articles and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results:
The original and updated searches yielded a total of 76 systematic reviews and meta-analyses. Strong evidence demonstrated that moderate-intensity physical activity reduced the risk of excessive gestational weight gain, gestational diabetes, and symptoms of postpartum depression. Limited evidence suggested an inverse relationship between physical activity and risk of preeclampsia, gestational hypertension, and antenatal anxiety and depressive symptomology. Insufficient evidence was available to determine the impact of physical activity on postpartum weight loss, postpartum anxiety, and affect during both pregnancy and postpartum. For all health outcomes, there was insufficient evidence to determine whether the relationships varied by age, race/ethnicity, socioeconomic status, or pre-pregnancy weight status.
Conclusions:
The gestational period is an opportunity to promote positive health behaviors that can have both short- and long-term benefits for the mother. Given the low prevalence of physical activity in young women in general, and the high prevalence of obesity and cardiometabolic diseases among the U.S. population, the public health importance of increasing physical activity in women of child-bearing age, before, during, and following pregnancy is substantial.
Keywords: Anxiety, Exercise, Gestational Diabetes, Depression, Preeclampsia, Weight Gain
INTRODUCTION
Pregnancy is a unique period of life for most women. The multiple hormonal, physiologic, and biomechanical changes that occur, such as increased blood volume and heart rate, weight gain, and shift in the center of mass, almost always proceed normally. For women experiencing a healthy pregnancy, regular engagement in physical activity of moderate-intensity for at least 20 to 30 minutes per day on most or all days of the week has been recommended during pregnancy and the postpartum period by the American College of Obstetricians and Gynecologists (ACOG) in 2015 (1) and reaffirmed in 2017 (2). Similarly, the 2008 Physical Activity Guidelines for Americans recommended 150 to 300 minutes per week of moderate-intensity aerobic activity during pregnancy and postpartum spread throughout the week (3). These recommendations were made in an effort to prevent a number of complications that may occur during the gestational period. Such complications include the development of diabetes, gestational hypertensive disorders, and fetal growth impairments that are associated with increased risk of adult cardiovascular disease and early mortality in the mother (4) and possibly in their offspring (5).
Despite substantial advances in scientific knowledge and development of guidelines to promote physical activity in pregnancy, most pregnant women do not achieve the current physical activity recommendations, and many continue to be inactive during and following pregnancy (6,7). In fact, only 23% to 29% of pregnant women at any gestational stage in the United States (U.S.) met the minimum physical activity guidelines, based on National Health and Nutrition Examination Survey (NHANES) data collected between 2007 and 2014 (8). Moreover, women who were active prior to pregnancy report that their physical activity level decreased once they became pregnant (9). There is also evidence that during postpartum, women may not return to their earlier physical activity levels for reasons such as lack of time, fatigue, or depressive symptoms (10).
The 2018 Physical Activity Guidelines Advisory Committee (PAGAC) Pregnancy and Postpartum Work Group recently conducted a systematic review of the evidence concerning the relationship between physical activity and various health outcomes during pregnancy and the postpartum period (defined up to 12 months following delivery). Results of this review were published in the 2018 PAGAC Scientific Report (11). This current article summarizes the evidence from the 2018 PAGAC Scientific Report, including new evidence from an updated search of the effects of physical activity on maternal health during pregnancy and postpartum.
METHODS
The PAGAC Pregnancy Work Group addressed four major questions (11):
-
1.
What is the relationship between physical activity and weight gain during pregnancy and weight loss during postpartum?
-
2.
What is the relationship between physical activity and the incidence of gestational diabetes mellitus (GDM)?
-
3.
What is the relationship between physical activity and the incidence of preeclampsia and hypertensive disorders during pregnancy?
-
4)
What is the relationship between physical activity and affect, anxiety, and depression during pregnancy and postpartum?
Questions 1 through 4 had the following subquestions: a) What dose of physical activity is associated with the reported quantitative benefit or risk?; b) Is there a dose-response relationship? If yes, what is the shape of the relationship; and c) Does the relationship vary by age, race/ethnicity, socioeconomic status, or pre-pregnancy weight status?
Literature Search Strategy and Study Selection
The Work Group first identified two high-quality existing reports: 1) the 2008 Physical Activity Guidelines Advisory Committee Report (12); and 2) the 2015 ACOG Committee Opinion on Physical Activity and Exercise During Pregnancy and the Postpartum Period (1). After reviewing these documents, the Work Group decided that they could serve as a foundation for describing the relationship between physical activity and maternal heath during pregnancy and postpartum (refer to Table F8-3 in the PAGAC Scientific Report).
To identify the most recent pertinent literature, the Work Group utilized the literature searches conducted by three of the 2018 PAGAC subcommittees that had outcomes of interest related to the pregnancy and postpartum questions. Seven searches for systematic reviews (SR) meta-analyses (MA), pooled analyses, and high quality reports conducted by other PAGAC subcommittees were considered to provide potentially pertinent information (Table 1). An initial search was undertaken in October, 2016 to include publications from 2006 to 2016. The searches were conducted in PubMed®, CINAHL, and Cochrane Library and supplemented through hand-searches of reference lists of included articles. Findings were reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (13).
Table 1.
Research questions from other subcommittees that provided evidence to answer questions related to pregnancy and postpartum (2018 PAGAC Scientific Report).
| Subcommittee, Question number | Question |
|---|---|
| Cardiometabolic Health and Weight Management, Q1 | What is the relationship between physical activity and prevention of weight gain? |
| Cardiometabolic Health and Weight Management, Q2 | In people with normal blood pressure or pre-hypertension, what is the relationship between physical activity and blood pressure? |
| Cardiometabolic Health and Weight Management, Q3 | In adults without diabetes, what is the relationship between physical activity and incident type 2 diabetes? |
| Brain Health, Q2 | What is the relationship between physical activity and quality of life? |
| Brain Health, Q3 | What is the relationship between physical activity and: (1) affect, (2) anxiety, and (3) depressed mood and depression? |
| Brain Health, Q4 | What is the relationship between physical activity and sleep? |
| Aging, Q2 | What is the relationship between physical activity and physical function? (The search for this question was not restricted to older age groups). |
All search results that included “gestation,” “postp,” “pregn,” “natal,” or “maternal” in the title or abstract were provided to the Work Group. The title, abstract, and full-text triage review process was the same as that used for other 2018 PAGAC topics (11, 14). The Work Group relied on these publications as the sources of potential evidence regarding quantifiable benefits or risks of physical activity, as well as the dose associated with specific health outcomes. The Work Group also completed one supplementary search by adding “eclampsia” and “preeclampsia” to the Cardiometabolic Health and Weight Management Subcommittee search on hypertension. In March, 2018, an updated systematic review was undertaken to identify additional systematic reviews and meta-analyses published between January 2017 through February 2018.
Quality Assessment
The evidence to inform each of the Work Group’s four questions and subquestions was graded as strong, moderate, limited, or “grade not assignable” based on several criteria, including applicability, generalizability, risk of bias/study limitations, quantity and consistency of results across studies, and magnitude and precision of effect. These criteria are described in Supplemental Table 1 (see Table, Supplemental Digital Content 1, 2018 Physical Activity Guidelines Advisory Committee Grading Criteria).
RESULTS
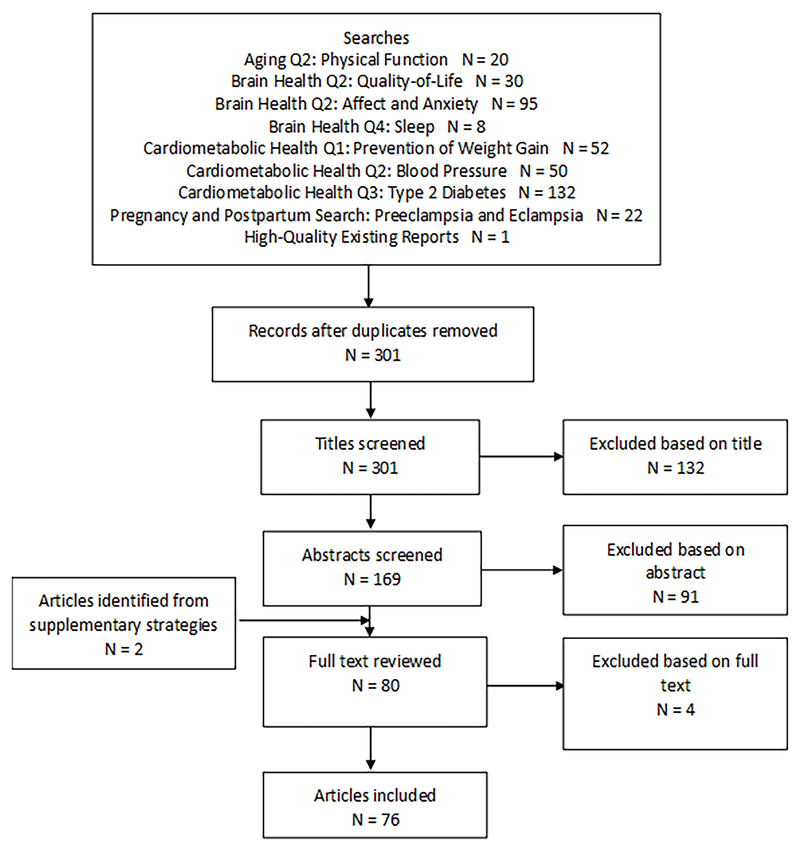
After duplicates were removed, a total of 254 articles were identified through the initial search process and the titles were reviewed by two of the three members of the Work Group. A total of 122 articles were deemed potentially relevant based on the title search (Figure 1). The abstracts of these papers then were reviewed by at least two members of the Work Group. Quality for each SR, MA, or pooled analysis was assessed using AMSTARExBP (15). Risk of bias was assessed for each study using an adapted version of the United States Department of Agriculture (USDA) Nutrition Evidence Library Bias Assessment Tool (16). Two original review articles were added to the group of articles being reviewed at full text, and thus, a total of 73 articles were determined to be potentially relevant and the full papers were retrieved and reviewed.
Figure 1. -.
Flow diagram of search strategy and study selection for both the initial 2018 PAGAC Scientific Report and the updated search. *denotes articles from the updated search.
The updated search (conducted in March 2018) identified 47 articles, of which 7 were deemed relevant for full text review. After full text review by three members of the Working Group, four papers were excluded because they failed to meet the inclusion criteria. Of the remaining three reviews from the updated search, one provided information about gestational weight gain, GDM, and hypertensive disorders (17); one about gestational hypertensive disorders (18); and one about post-partum depression (19). Therefore, the initial and updated searches yielded a total of 76 articles, 38 of which are reported on in this current review (Figure 1).
Table 2 summarizes the level of evidence for the relationship between physical activity and each health outcome during pregnancy and postpartum. Overall, there was strong evidence demonstrating an inverse relationship between physical activity during pregnancy and gestational weight gain, GDM, and postpartum depression.
Table 2 –
Summary of the level of evidence for the relationship between physical activity and each health outcome during pregnancy and postpartum.
| Overall Evidence | Dose | Dose-Response | Effect Modification by Sociodemographic Factors or Weight | |
|---|---|---|---|---|
|
Gestational Weight Gain |
Strong | Limited | Limited | Not assignable |
| Weight Loss During Postpartum | Not assignable | Not assignable | Not assignable | Not assignable |
| Gestational Diabetes | Strong | Limited | Limited | Not assignable |
|
Pre-eclampsia/Gestational Hypertension |
Limited | Limited | Limited | Not assignable |
| Antenatal Affect, Anxiety, and Depression | Not assignable | Not assignable | Not assignable | Not assignable |
| Limited | Not assignable | Not assignable | Not assignable | |
| Limited | Not assignable | Not assignable | Not assignable | |
| Postpartum Affect, Anxiety, and Depression | Not assignable | Not assignable | Not assignable | Not assignable |
| Not assignable | Not assignable | Not assignable | Not assignable | |
| Strong | Not assignable | Not assignable | Not assignable |
Gestational Weight Gain
In the 2018 PAGAC Scientific Report, 11 systematic reviews provided strong evidence that women assigned to physical activity interventions gain about 1 kilogram (kg) less weight during pregnancy than women in comparison groups. Of the nine reviews that included meta-analyses (20–28), all but one reported significantly less weight gained in the physical activity group. The other meta-analysis included only pregnant women who were overweight or obese and reported significantly attenuated weight gain among active versus inactive women who were obese but not among those who were overweight (26).
One meta-analysis (20) reviewed 30 randomized controlled trials (RCTs). Based on a meta-analysis of 18 of those RCTs, which included 1,598 women performing a structured exercise program and 1,605 receiving standard care, the standardized mean difference (SMD) in gestational weight gain was −1.11 kg (95% confidence interval (CI): −1.59 to −0.69), with women in the exercise group gaining less weight than women receiving standard care. The other meta-analyses of RCTs (21–28) reported similar standardized mean differences in gestational weight gain between exercising and control women, ranging from −0.36 kg (95% CI: −0.64 to −0.09; 5 studies) (24) to −2.22 kg (95% CI: −3.14 to −1.30; 3 studies) (21). The updated search identified a meta-analysis that analyzed participant-level data by the International Weight Management in Pregnancy (i-WIP) Collaborative Group (17) that further corroborated the finding that women who were physically active during pregnancy experience attenuated weight gain compared with women who are not (SMD=−0.73 kg; 95% CI: −1.11 to −0.34 kg; 15 studies).
Several of the systematic reviews and meta-analyses (20, 22, 23) examined the relationship between physical activity and “excess” weight gain, as defined by the Institute of Medicine Guidelines (29). In general, women who reported physical activity during pregnancy experienced a significantly lower risk of excess weight gain compared with women who did not, with pooled effect sizes ranging from 18% (20) to 23% (23). Based on this literature review, the overall evidence was strong for an inverse association between physical activity and excess gestational weight gain. Muktabhant et al. (23) also examined the relationship between exercise during pregnancy and “low” or insufficient gestational weight gain. Women from the general population having a normal (18.5-24.9 kg/m2) BMI (“low risk”) or any BMI (“mixed risk”) experienced a marginally-greater chance of “low” weight gain compared with the non-exercising control group (average RR=1.20; 95%CI: 1.00 to 1.43; 3 studies). There was no relationship between exercise and insufficient weight gain among women whose pregnancies were considered high risk and who also were overweight or obese (“high risk”)(average RR=1.03; 95%CI:0.66-1.60; 3 studies).
Dose and Dose-Response.
The dose of physical activity prescribed in the RCTs varied among the studies. Similarly, the assessment and categorization of reported leisure-time physical activity was not consistent. It appears, however, that most RCT interventions used an exercise regimen involving primarily aerobic activity of moderate-intensity (walking, swimming, aerobic exercise), occurring at least three times per week for a duration of 30 to 60 minutes per session. This dose of physical activity is similar to both ACOG Guidelines and the 2008 Physical Activity Guidelines recommendations (1, 3).
Most of the reviews did not assess whether maternal physical activity and gestational weight gain had a dose-response relationship. Indirect evidence of a dose-response relationship was suggested, however, by the observation that adherence to the prescribed exercise program was significantly higher in the “successful” interventions (22), and the observation in a meta-analysis of 28 RCTs in which the mean difference in gestational weight gain between the exercise and control groups was inversely correlated with both the duration (in weeks) of the intervention (Pearson Product Moment Correlation Coefficient (r) =−0.51; P=0.023) and the volume (hours per week) of exercise prescribed (r =−0.45; P=0.05) (28). The evidence grade for the dose and dose-response relationship between physical activity and gestational weight gain was limited.
Sociodemographic Factors and Weight Status.
None of the systematic reviews or meta-analyses from the 2018 PAGAC Scientific Report assessed whether the purported relationship between physical activity and gestational weight gain varied by age, race/ethnicity, socioeconomic status, or pre-pregnancy weight status. The i-WIP Collaborative Group meta-analysis (17), which analyzed participant-level data from 15 RCTs (N= 2915), reported that the inverse relationship between physical activity and gestational weight gain did not vary by age, race/ethnicity, or pre-pregnancy weight status.
With regard to weight status, most of the findings were reported among women of normal weight (i.e., body mass index (BMI)=18.5-24.9 kg/m2). However, four systematic reviews (22, 23, 26, 28) stratified their data by pre-pregnancy weight status [i.e., normal weight, overweight (BMI=25-29.9 kg/m2), or obese (BMI ≥30 kg/m2)]. Three of these studies observed stronger effects among pregnant women of normal weight, compared with those who were overweight or obese (22, 23, 28). One meta-analysis of women who were overweight or obese (26) reported a greater difference in gestational weight gain between the exercise and control groups among women with obesity (SMD=−0.91 kg; 95% CI: −1.66 to −0.16; 3 studies), but not in women who were overweight (SMD=−0.12; 95% CI: −0.52 to 0.26; 3 studies). In contrast, the meta-analysis from the i-WIP Collaborative Group (17) reported that the inverse relation between physical activity and gestational weight gain did not vary across different subgroups of women categorized by BMI (normal weight, overweight, obese). Thus, the evidence grade for effect modification on the relationship between physical activity and gestational weight gain was not assignable.
Weight Loss during the Postpartum Period
A total of five systematic reviews and meta-analyses (21, 30–33) that included only six original research articles and a total of 287 participants addressed the relationship between physical activity and weight loss during the postpartum period. Most of these reviews reported no significant difference in weight loss between women who performed physical activity during postpartum (alone, without dietary restriction) and the control group. Due to the insufficient number of studies linking physical activity to postpartum weight loss, an evidence grade for this relationship was not assignable.
Gestational Diabetes Mellitus
Eight of 13 meta-analyses from the 2018 PAGAC Scientific Report described higher levels of physical activity to be associated with statistically significant reductions in the risk of GDM (Table 3) (20, 34–40), four of 13 meta-analyses reported non-significant reductions (41–44), and one meta-analysis reported a non-significant increase (45). The reduced relative risk (RR) of GDM (regardless of statistical significance) ranged from 0.45 to 1.01 with a median value of RR=0.73. The updated search identified one additional meta-analysis of 10 RCTs (N=2700 women) that also reported a significantly lower risk of GDM among women participating in physical activity interventions compared with those in a control condition [odds ratio (OR)=0.67; 95% CI: 0.46 to 0.99] (17). Notably, this risk reduction in the incidence of GDM reported in many of these meta-analyses is similar to the 25-30% reduction in the risk of type 2 diabetes among the general population that is associated with 150 to 300 min/week of moderate-intensity physical activity (refer to 2018 PAGAC Scientific Report, Part F, Chapter 5 for more details).
Table 3.
Summary of Findings from 14 Meta-Analyses of the Relationship Between Pre-Pregnancy and Early Pregnancy Physical Activity and Risk of Gestational Diabetes Mellitus
| Author, year | Study Design | Effect (95% CI) |
|---|---|---|
| PRE-PREGNANCY PHYSICAL ACTIVITY | ||
| Aune, 2016 | Cohort (N=8) | sRR=0.78 (0.61-1.00) |
| Tobias, 2011 | RCT (N=7) | pOR=0.45 (0.28-0.75) |
| EARLY PREGNANCY PHYSICAL ACTIVITY | ||
| iWIP Collaborative Group, 2017* | RCT (N=10) | OR=0.67 (0.46 to 0.99) |
| Aune, 2016 | Cohort (N=5) RCT (N=12) Combined (N=17) |
sRR=0.97 (0.73-1.28) sRR=0.69 (0.50-0.96) sRR=0.80 (0.64-1.00) |
| DaSilva, 2017 | Cohort (N=6) RCT (N=10) |
sOR=0.75 (0.55-1.01) sOR=0.67 (0.49-0.92) |
| DiMascio, 2016 | RCT (N=4) | sRR=0.51 (0.31-0.82) |
| Han, 2011 | RCT (N=3) | sRR=1.10 (0.66-1.84) |
| Mudhravdata, 2015 | RCT (N=3) | pOR=0.77 (0.33-1.79) |
| Oostdam, 2011 | RCT (N=3) | risk difference= −0.05 (−0.20-0.10) |
| Russo, 2015 | RCT (N=10) | sRR=0.72 (0.58-0.91) |
| Sanabria-M, 2015 | RCT (N=8) | sRR=0.69 (0.52-0.91) |
| Song, 2016 | RCT (N=10) | sRR=0.77 (0.54-1.09) |
| Tobias, 2011 | RCT (N=5) | pOR=0.76 (0.70-0.83) |
| Yin, 2014 | RCT (N=6) | sRR=0.91 (0.57-1.44) |
| Yu, 2017 | RCT (N=5) | SMD=0.59 (0.39-0.88) |
| Zheng, 2017 | RCT (N=4) | SMD=0.62 (0.43-0.89) |
Note: Studies with statistically significant findings are in bold type.
Identified in the updated search. CI = confidence interval; OR= odds ratio; RCT = randomized controlled trial; sRR=standardized relative risk, sOR=standardized odds ratio, pOR=pooled odds ratio, and SMD=standardized mean difference.
Aune et al. (34) reviewed 23 studies of total physical activity (leisure-time, occupational, and household activity combined) and of leisure-time physical activity performed before or during early pregnancy and the incidence of GDM. Those women who reported performing highest levels of total physical activity before pregnancy experienced a significantly lower risk of GDM compared with women reporting lowest levels of total activity (RR=0.62; 95% CI: 0.41 to 0.94; 4 studies), whereas high versus low levels of total activity performed during early pregnancy did not significantly lower the risk of GDM (RR=0.66; 95% CI: 0.36 to 1.21; 3 studies). On the other hand, women performing the highest levels of moderate-intensity leisure-time physical activity either before (RR=0.78; 95% CI: 0.61 to 1.00; 8 studies) or during pregnancy (RR=0.80; 95% CI: 0.64 to 1.00; 12 studies) significantly lowered their risk of GDM by about 20% (34). Women who performed such physical activity both before and during pregnancy lowered their risk by 59% (RR=0.41; 95% CI: 0.23 to 0.73; 2 studies) compared with those reporting no physical activity during both time-periods. High versus low levels of vigorous activity performed before pregnancy significantly lowered the risk of GDM by nearly 25% (summary RR=0.76; 95% CI: 0.66 to 0.88; 3 studies), but this was not the case for vigorous activity performed during pregnancy (RR=0.95; 95% CI: 0.55 to 1.63; 2 studies). Based on this review of literature, the overall evidence was strong for an inverse association between physical activity and GDM.
Dose and Dose-Response.
The dose of physical activity prescribed in the RCTs varied among the studies. Similarly, the assessment and categorization of reported leisure-time physical activity from observational studies was not detailed nor consistent. Most RCT interventions used a physical activity regimen involving primarily aerobic activity of at least moderate-intensity (walking, cycling, swimming, aerobic dance), occurring at least three times per week for a duration of 30 to 60 minutes per session, which is similar to both ACOG Guidelines and the 2008 Physical Activity Guidelines (1, 3).
Aune et al., (34) performed a dose-response analysis and reported that each 5 hour per week increment in pre-pregnancy physical activity lowered the risk of GDM by about 30% (RR=0.70; 95 % CI: 0.49-1.01; 3 studies), with significant evidence of non-linearity (P<0.005). A similar relationship was not observed for physical activity performed during early pregnancy (RR=0.98; 95% CI: 0.87-1.09; 3 studies). Evidence from two observational studies in the meta-analysis by Tobias et al. (38) suggests that women who walked at a brisk pace before pregnancy and for a longer duration significantly lowered their risk of GDM compared with women who walked at a casual pace for shorter durations (pooled odds ratio (OR)=0.59; 95% CI: 0.30 to 0.87). The evidence grade for the dose and dose-response relationship between physical activity and GDM was limited.
Sociodemographic factors and weight status.
Almost none of the systematic reviews or meta-analyses assessed whether the relationship between physical activity and GDM varied by age, race/ethnicity, or socioeconomic status. The review by Song et al. (43) reported that physical activity during pregnancy had a significant impact on GDM risk in women ages 30 years and older, but not in women younger than age 30 years. The i-WIP Collaborative Group reported that the benefits of physical activity to the reduction in risk of GDM were similar across the different subgroups of women categorized by age, race/ethnicity, or BMI (17).
Preeclampsia and Gestational Hypertension
Hypertensive disorders during pregnancy include preeclampsia and gestational hypertension. Preeclampsia is characterized by high blood pressure, high levels of protein in the urine (proteinuria), and swelling in the hands and feet. Gestational hypertension is elevated blood pressure without concomitant signs of preeclampsia such as proteinuria. Its relationship, if any, with preeclampsia is unclear.
Nine reviews from the 2018 PAGAC Scientific Report provided only limited evidence of an inverse relationship between total volume of physical activity and risk of preeclampsia or incident gestational hypertension (20, 23, 35, 40, 46–50). One meta-analysis that included cohort and case-control studies reported a beneficial association between higher levels of physical activity and reduced risk of preeclampsia from both pre-pregnancy (RR=0.65; 95% CI: 0.47 to 0.89; 5 studies) and early pregnancy physical activity (RR=0.79; 95% CI: 0.70 to 0.91; 11 studies) (46). The meta-analysis of 10 cohort studies by Kasawara, et al. (47) reported no association between leisure-time physical activity and preeclampsia (OR=0.99; 95% CI: 0.93 to 1.05). In contrast, their meta-analysis of six case-control studies reported a significantly lower odds of preeclampsia (OR=0.77; 95% CI: 0.64 to 0.91) with physical activity performed in pre-pregnancy (summarized from only two studies) being more effective (OR=0.56; 95% CI: 0.41-0.76) than physical activity performed during pregnancy (OR=0.77; 95% CI:0.64 to 0.91). Three meta-analyses comprising RCTs and cohort studies found no association between physical activity and preeclampsia; one of the studies examined pre-pregnancy physical activity (20) while the other two studies examined early pregnancy physical activity (23, 40).
One systematic review (48) and one meta-analysis (35) examined the relationship between physical activity and hypertensive disorders during pregnancy. Di Mascio reported a RR of 0.21 (95% CI: 0.09-0.45; 3 studies) for hypertensive disorders among women performing moderate-intensity leisure activities (aerobic dance, cycling, hydrotherapy, and resistance exercises) during pregnancy, compared with women performing no activity (35). The updated search identified two additional meta-analyses (17, 18) pertaining to physical activity and hypertensive disorders during pregnancy. The i-WIP Collaborative Group (17) reported null findings (OR= 0.74; 95% CI: 0.42 to 1.33); however, Magro-Malosso, et al. (18) reported a significantly lower incidence of gestational hypertensive disorders (RR=0.39; 95% CI: 0.20 to 0.73; 7 studies) and gestational hypertension (RR=0.54; 95% CI: 0.32 to 0.91; 16 studies) and a similar incidence of preeclampsia (RR=0.37; 95% CI: 0.12 to 1.15; 6 studies) in pregnant women assigned to aerobic exercise (without dietary counseling) groups compared with women assigned to standard care control groups. Based on this review of literature, the overall evidence was limited for an inverse association between physical activity and both preeclampsia and gestational hypertension.
Dose and Dose-Response.
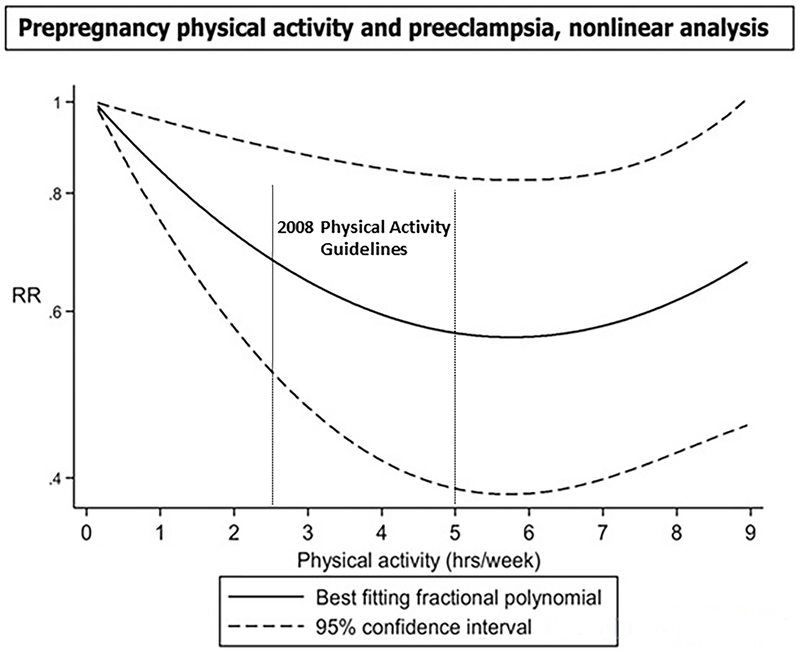
The meta-analysis by Aune, et al. (46) was the only review to report on the dose-response relation between physical activity and risk of preeclampsia. In their analysis of pre-pregnancy physical activity, the results indicated a 28% lower risk of preeclampsia for each 1 hour per day increment in physical activity (OR=0.72; 95% CI: 0.53 to 0.99; 3 studies) and a 22% lower risk for each 20 metabolic equivalent of task (MET)-hours/week increment (OR=0.78: 95% CI: 0.63 to 0.96; 2 studies). This relationship appeared non-linear, with a flattening of the curve at higher levels of physical activity. Indeed, there was a 40% reduction in risk up to 5–6 hours/week but no further reductions at higher physical activity levels (Figure 2). With regard to physical activity performed during early pregnancy, the risk of preeclampsia was reduced in a linear manner by 17% for each 1 hour/day increment in physical activity (OR=0.83; 95% CI: 0.72 to 0.95; 7 studies) and by 15% for every 20 MET-hours/week increment (OR=0.85; 95% CI: 0.68 to 1.07; 3 studies). The evidence grade for the dose and dose-response relationship between physical activity and both pre-eclampsia and gestational hypertension was limited.
Figure 2. –
The dose-response relationship between pre-pregnancy physical activity and risk of preeclampsia. The results indicate a 28% lower risk of preeclampsia for each 1 hour/day increment in activity, compared with no activity (OR=0.72; 95% CI: 0.53 to 0.99). Adapted with permission. [Aune D, Saugstad OD, Henriksen T, Tonstad S. Physical activity and the risk of preeclampsia: a systematic review and meta-analysis. Epidemiology. 2014;25(3):331-343.]
Sociodemographic factors and weight status.
There was no available evidence that evaluated whether the relationship between physical activity and preeclampsia varied by age, race/ethnicity, socioeconomic status, or weight status. Mutkabhant, et al. (23) analyzed their data according to pre-pregnancy weight status (normal weight; overweight; or obese) and observed that even among pregnant women with overweight or obesity, there was no difference in risk of preeclampsia (based on two studies) between women in the exercise groups and those in the control groups (RR=1.60; 95% CI: 0.38 to 6.73). The i-WIP Collaborative Group reported that the relationship between physical activity and hypertensive disorders during pregnancy were similar across the different age, race/ethnicity, and BMI subgroups of women (17). The evidence grade for effect modification on the relationship between physical activity and both preeclampsia and gestational hypertension was not assignable.
Physical Activity, Affect, Anxiety, and Depression during Pregnancy and Postpartum
We identified no systematic reviews or meta-analyses that examined the relationship between physical activity and affect, either during pregnancy or during the postpartum period. We found limited evidence that yoga performed during pregnancy significantly reduced anxiety symptomology (51, 52); however, no systematic reviews or meta-analyses were found that examined this relationship during the postpartum period. There was also limited evidence to suggest that higher levels of physical activity were associated with reduced symptoms of depression during pregnancy (51, 52). On the other hand, strong evidence demonstrated that there was an inverse relationship between physical activity and reduced symptoms of depression during postpartum.
With regard to antenatal anxiety and depressive symptoms, Sheffield, et al. (51) provided a systematic review of 13 studies (7 of which were RCTs) that examined the effects of practicing yoga during pregnancy on symptoms of anxiety and depression during that same time period. Of the five studies that evaluated anxiety symptomology, all of them reported statistically significant improvements in the State/Trait Anxiety Inventory scores following a yoga intervention and six of seven studies observed a statistically significant improvement in the Center for Epidemiologic Studies Depression scale score. Shivakumar, et al. (52) reported that women who were more physically active during pregnancy reported reduced symptoms of anxiety in one of three studies that examined symptoms of anxiety, while two other studies in the same review both reported reduced symptoms of depression in pregnant adolescent girls who performed physical activity compared with their sedentary counterparts.
Two meta-analyses (53, 54) and one systematic review (55) examined the relationship between physical activity and symptoms of depression during the postpartum period. The updated search identified an additional meta-analysis of 13 RCTs (19). McCurdy et al. (53) examined 16 RCTs comparing light-to-moderate intensity aerobic exercise (initiated in the first year postpartum) to standard care in postpartum women (N=1,327) with (10 RCTs) and without (6 RCTs) mild to moderate depression. In general, depressive symptoms scores (based on the Edinburgh Postnatal Depression Scale (EPDS)) were lower among those in postpartum exercise intervention groups compared with those in control groups (pooled SMD= −0.34; 95% CI: −0.50 to −0.19). Among the 10 treatment RCTs in women with postpartum depression, a moderate beneficial effect of exercise on depressive symptoms also was observed (SMD= −0.48; 95% CI: −0.73 to −0.22) relative to the control group. Moreover, in women classified with depression pre-intervention (defined as an EPDS score greater than 12), exercise increased the odds of resolving depression post-intervention by 54% (OR=0.46; 95% CI: 0.25 to 0.84; 3 trials; N=173) compared with the control group. It is not clear, however, whether these benefits were independent of medication or social support. In the six prevention trials (i.e., women without depression) a beneficial effect of postpartum exercise was observed based on the EPDS score (SMD= −0.22; 95% CI: −0.36 to −0.08) compared with standard care.
These findings are consistent with those from a smaller review and meta-analysis by Poyatos-Leon and colleagues (54), which reported improved postpartum depressive symptomology (measured by EPDS or by the Beck Depression Inventory (BDI)) among women performing physical activity during pregnancy and the postpartum period, compared with those who were not (effect size (ES)=0.41; 95% CI: 0.28 to 0.54; 12 studies). Of note, the benefits of physical activity were more pronounced in women who met criteria for postpartum depression (ES=0.67; 95% CI: 0.44-0.90; 6 studies), compared with those who did not (ES=0.29; 95% CI: 0.14 to 0.45). Most (10 of 12) of the interventions started during the postpartum period and involved a variety of activities, such as walking, aerobics, Pilates, yoga, and stretching. Similarly, Pritchett, et al. (19) performed a meta-analysis of 13 RCTs (7 trials recruited postpartum women with depression; 6 trials recruited postpartum women from the general population). In general, postpartum aerobic exercise interventions significantly reduced depressive symptoms (assessed by EPDS, BDI, or Diagnostic and Statistical Manual of Mental Disorders IV diagnosis) in women with postpartum depression (SMD= −0.32; 95% CI: −0.63 to −0.00) as well as in postpartum women without it (SMD=−0.57; 95% CI: −1.12 to −0.02). In the exercise only interventions (i.e., no co-interventions of social support or dietary counseling; N=8 RCTs), exercise had a marginal effect in reducing postpartum depressive symptoms (SMD= −0.56; 95% CI: −1.13 to 0.01).
Dose and Dose-Response.
Insufficient information was available to determine the dose of physical activity associated with improved affect and reduced anxiety and depressive symptomology. Most of the RCTs reviewed in the recently added meta-analysis by Pritchett, et al. (19) observed improvements in postpartum depressive symptoms from about 30 minutes of moderate-intensity activity, performed 3 to 5 times weekly, for 4 weeks to 6 months duration. The evidence grade for the dose and dose-response relationship between physical activity and affect, anxiety, and depression was not assignable.
Sociodemographic Factors and Weight Status.
There was no available evidence that tested whether the relationship between physical activity and affect, anxiety, or depression during pregnancy or postpartum varied by age, race/ethnicity, socioeconomic status, or pre-pregnancy weight status. The evidence grade for effect modification on the relationship between physical activity and both antenatal and postpartum affect, anxiety, and depression was not assignable.
DISCUSSION
The gestational period is an opportunity to promote positive health behaviors that can have both short- and long-term benefits for the mother. Given the low prevalence of physical activity in young women in general (56) and the high prevalence of obesity and cardiometabolic diseases among the U.S. population (57), the importance of increasing physical activity levels in women of child-bearing age, before, during, and following pregnancy is substantial. The 2008 PAGAC Scientific Report concluded that for women with a healthy pregnancy, regular physical activity probably reduces the risk of gestational diabetes, possibly reduces the risk of preeclampsia, and appears to improve mood both during and after pregnancy (12). Our findings in 2018 support those from 2008 and extend them in several ways. Strong evidence now shows that moderate-intensity physical activity commensurate with the current recommendations (150-300 min/week) reduces the risk of excessive gestational weight gain, GDM, and symptoms of postpartum depression. Unfortunately, only about 23% to 29% of pregnant women living in the U.S. meet even the minimum physical activity recommendations (8), and therefore, the majority of pregnant women receive few or none of the physical and emotional health benefits of being physically active.
We found strong evidence that physically active pregnant women (i.e., those meeting at least the minimum ACOG or 2008 Physical Activity Guidelines of 150 min/week of moderate-intensity activity) gain less weight than their non-active counterparts and are about 18% to 23% less likely to exceed the Institute of Medicine recommendations for healthy weight gain (29). Because gestational weight gain is attenuated in women who are active during pregnancy, they are also at lower risk of excessive postpartum weight retention, future obesity, and birth of an infant with macrosomia (58). Although not systematically examined by the 2018 PAGAC, active pregnant women also appear to be at lower risk of undergoing a Cesarean section (23, 27, 28, 35, 45), and appear at no greater risk of preterm delivery (23, 27, 35, 39, 40) than are inactive women. Additional information on weight gain patterns in physically active pregnant women, according to IOM recommendations and their pre-pregnancy weight status, would increase the clinical value of these findings substantially.
There was also strong evidence demonstrating that women who meet ACOG Physical Activity Guidelines during pre-pregnancy or during pregnancy are about 25% to 30% less likely to develop GDM than their inactive peers. This is significant because gestational diabetes mellitus occurs in approximately 5% to 9% of women, and those with GDM are also at increased risk of delivery by Cesarean section and having an infant with macrosomia and/or neonatal hypoglycemia (59). Gestational diabetes also is associated with a 7-fold increase in the risk of developing type 2 diabetes after pregnancy (59).
Finally, about 10% of women experience postpartum depression, with nearly 25% of them still in treatment after one year (60). This review provides strong evidence that physically active women experience significantly fewer symptoms of depression during the postpartum period compared with their inactive counterparts. In fact, the benefits of physical activity to postpartum depression are consistent with those for depressive symptoms among the general population as indicated in the 2018 PAGAC Scientific Report (see Part F. Chapter 3; Brain Health; Question 3).
The Need for Future Research
In sum, the health benefits documented in this review confirm the substantial public health importance of regular participation in moderate-intensity physical activity before, during, and after pregnancy. However, both the 2018 Scientific Report (11) and this umbrella review underscore the need for future research in several areas. For example, there is a need to investigate longitudinally the timing of the physical activity exposure (e.g., pre-pregnancy, early pregnancy, throughout pregnancy postpartum) relative to specific maternal outcomes of interest. For some pregnancy outcomes like excessive weight gain, GDM, or preeclampsia, pre- or early-pregnancy physical activity may be sufficient for reducing risk during the entire gestational period. For other issues such as postpartum weight loss or depression, however, postpartum physical activity may be more important than activity at other stages of pregnancy for promoting weight loss, mitigating depressive symptoms, and improving quality of life. The determinants and barriers to postpartum exercise also need further study.
Second, the safety and benefits of vigorous-intensity physical activity to maternal health are less well-documented than those for light- to moderate-intensity activity, and this type of activity may be discouraged by some health care providers. There are substantial numbers of women who participate regularly in vigorous physical activity (e.g., running, cycling, rowing) prior to pregnancy, who may want to continue such activity for as long as possible throughout pregnancy. Information from such studies would provide valuable information on minimal effective levels of vigorous activity, as well as on maximal threshold levels for safety concerns (e.g., insufficient gestational weight gain, hyperthermia, musculoskeletal injuries, or low birth weight) that may affect the health of mothers and their offspring .
Finally, most of the experimental research on physical activity during pregnancy relies on the 2008 Physical Activity Guidelines or the 2015 ACOG recommendations of 150 minutes per week of moderate-intensity activity. Limited evidence suggests that certain types of physical activity, such as prolonged standing or lifting heavy loads performed in an occupational setting, may have different health effects for pregnant women than when performed during leisure time (49). The validity of this claim needs to be determined, as well as whether these differential findings are caused by the nature of the activities, the setting itself, or perhaps by confounding factors such as socioeconomic status, educational attainment, or age. Also, there are limited data concerning the dose-response relationships between any type of physical activity (performed before, during, or after pregnancy) and important pregnancy outcomes such as GDM and preeclampsia. Some data suggest a non-linear relation between pre-pregnancy activity and these outcomes (34, 46), while data on early pregnancy physical activity show a more linear dose-response curve (46). Examining the impact of different types, intensities, doses, and timing of physical activity across various domains (leisure-time, occupational, household, transportation) on a range of maternal outcomes would significantly advance current knowledge and inform both clinical and public health practice.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contributions of Sarah Prowitt, MPH (HHS) for management support; Anne Brown Rodgers, HHS consultant for technical writing support; and ICF librarians, abstractors, and additional support staff.
Role of the Funder/Sponsor
HHS staff provided general administrative support to the Committee and assured that the Committee adhered to the requirements for Federal Advisory Committees. HHS also contracted with ICF, a global consulting services company, to provide technical support for the literature searches conducted by the Committee. HHS and ICF staff collaborated with the Committee in the design and conduct of the searches by assisting with the development of the analytical frameworks, inclusion/exclusion criteria, and search terms for each primary question; using those parameters, ICF performed the literature searches.
Footnotes
Conflicts of Interest and Source of Funding
The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate manipulation. The Committee’s work was supported by the U.S. Department of Health and Human Services (HHS). Committee members were reimbursed for travel and per diem expenses for the five public meetings; Committee members volunteered their time. The authors report no other potential conflicts of interest.
This paper is being published as an official pronouncement of the American College of Sports Medicine. This pronouncement was reviewed for the American College of Sports Medicine by members-at-large and the Pronouncements Committee. Disclaimer: Care has been taken to confirm the accuracy of the information present and to describe generally accepted practices. However, the authors, editors, and publisher are not responsible for errors or omissions or for any consequences from application of the information in this publication and make no warranty, expressed or implied, with respect to the currency, completeness, or accuracy of the contents of the publication. Application of this information in a particular situation remains the professional responsibility of the practitioner; the clinical treatments described and recommended may not be considered absolute and universal recommendations.
REFERENCES
- 1.American College of Obstetricians and Gynecologists. Physical activity and exercise during pregnancy and the postpartum period. Committee Opinion No. 650. Obstet Gynecol. December 2015;126:e135–e142. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists Website. Available at: https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Physical-Activity-and-Exercise-During-Pregnancy-and-the-Postpartum-Period.
- 3.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washington, DC: US: Department of Health and Human Services; 2008. [Google Scholar]
- 4.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2014;36:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaize AN, Pearson KJ, and Newcomer SC. Impact of maternal exercise during pregnancy on offspring chronic disease susceptibility. Exerc Sports Sci Rev. 2015; 43: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evenson KR, Barakat R, Brown WJ, et al. Guidelines for physical activity during pregnancy: comparisons from around the world. Am J Lifestyle Med. 2014;8(2):102–121. doi: 10.1177/1559827613498204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evenson KR, Mottola MF, Owe KM, Rousham EK, Brown WJ. Summary of international guidelines for physical activity following pregnancy. Obstet Gynecol Survey. 2014;69(7):407–414. doi: 10.1097/OGX.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesketh KR, Evenson KR. Prevalence of U.S. pregnant women meeting ACOG 2015 physical activity guidelines. Am J Prev Med. 2016;41:387–389. doi: 10.1016/j.amepre.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coll C, Domingues M, Santos I, Matijasevich A, Horta BL, Hallal PC. Changes in Leisure-Time Physical Activity From the Prepregnancy to the Postpartum Period: 2004 Pelotas (Brazil) Birth Cohort Study. J Phys Act Health. 2016. April;13(4):361–5. doi: 10.1123/jpah.2015-0324. Epub 2015 Sep 17. [DOI] [PubMed] [Google Scholar]
- 10.Borodulin K, Evenson KR, Herring AH. Physical activity patterns during pregnancy through postpartum. BMC Women’s Health 2009; 9: Available at http://www.biomedcentral.com/1472-6874/9/32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2018 Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report. Washington, D.C.: U.S. Department of Health and Human Services; 2018. Accessed May, 2018. [Google Scholar]
- 12.2008 Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report. Washington, D.C.: U.S. Department of Health and Human Services; 2008. Accessed January, 2018. [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLOS Medicine. 2009; 6(7): e1000100 10.1371/journal.pmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres A, Tennant B, Rubiero-Lucus I, Vaux-Bjerke A, Piercy K, Bloodgood B. Umbrella and Systematic Review Methodology to Support the 2018 Physical Activity Guidelines Advisory Committee. J Phys Act Health. 2018.doi 10.1123/jpah.2018-0372. PMID 30336718. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BT, MacDonald HV, Bruneau ML Jr, et al. Methodological quality of meta-analyses on the blood pressure response to exercise: a review. J Hypertens. 2014;32(4):706–723. doi: 10.1097/HJH.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Agriculture (USDA). 2015 Dieteary Guidelines Advisory Committee (DGAC) nutrition evidence library methodology. 2017. https://www.cnpp.usda.gov/sites/default/files/usda_nutrition_evidence_flbrary/2015DGAC-SR-Methods.pdf. Accessed January 16, 2018.
- 17.The International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomized trials. BMJ 2017;358:j3119 10.1136/bmj.j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magro-Malosso ER, Saccone G, Di Tommaso M, Roman A, and Berghella V. Exercise during pregnancy and risk of gestational hypertensive disorders: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2017; 96:921–931. [DOI] [PubMed] [Google Scholar]
- 19.Pritchett RV, Daley AJ and Jolly K. Does aerobic exercise reduce postpartum depressive symptoms? A systematic review and meta-analysis. Br J Gen Pract 2017; DOI: 10.3399/bjgp17X692525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva SG, Ricardo LI, Evenson KR, Hallal PC. Leisure-time physical activity in pregnancy and maternal-child health: a systematic review and meta-analysis of randomized controlled trials and cohort studies. Sports Med. 2017;47(2):295–317. doi: 10.1007/s40279-016-0565-2. [DOI] [PubMed] [Google Scholar]
- 21.Elliott-Sale KJ, Barnett CT, Sale C. Systematic review of randomised controlled trials on exercise interventions for weight management during pregnancy and up to one year postpartum among normal weight, overweight and obese women. Pregnancy Hypertens. 2014;4(3):234. doi: 10.1016/j.preghy.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 22.McDonald SM, Liu J, Wilcox S, Lau EY, Archer E. Does dose matter in reducing gestational weight gain in exercise interventions? A systematic review of literature. J Sci Med Sport. 2016;19(4):323–335. doi: 10.1016/j.jsams.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;(6):Cd007145. doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanabria-Martinez G, Garcia-Hermoso A, Poyatos-Leon R, Alvarez-Bueno C, Sanchez-Lopez M, Martinez-Vizcaino V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta-analysis. BJOG. 2015;122(9):1167–1174. doi: 10.1111/1471-0528.13429. [DOI] [PubMed] [Google Scholar]
- 25.Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, von Kries R. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG. 2011;118(3):278–284. doi: 10.1111/j.1471-0528.2010.02801.x. [DOI] [PubMed] [Google Scholar]
- 26.Sui Z, Grivell RM, Dodd JM. Antenatal exercise to improve outcomes in overweight or obese women: a systematic review. Acta Obstet Gynecol Scand. 2012;91(5):538–545. doi: 10.1111/j.1600-0412.2012.01357.x. [DOI] [PubMed] [Google Scholar]
- 27.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;(344):e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiebe HW, Boule NG, Chari R, Davenport MH. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet Gynecol. 2015;125(5):1185–1194. doi: 10.1097/AOG.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine and National Research Council. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 30.Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev. 2013;(7):CD005627. doi: 10.1002/14651858.CD005627.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger AA, Peragallo-Urrutia R, Nicholson WK. Systematic review of the effect of individual and combined nutrition and exercise interventions on weight, adiposity and metabolic outcomes after delivery: evidence for developing behavioral guidelines for post-partum weight control. BMC Pregnancy Childbirth. 2014;14:319. doi: 10.1186/1471-2393-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nascimento SL, Pudwell J, Surita FG, Adamo KB, Smith GN. The effect of physical exercise strategies on weight loss in postpartum women: a systematic review and meta-analysis. Int J Obes (Lond). 2014;38(5):626–635. doi: 10.1038/ijo.2013.183. [DOI] [PubMed] [Google Scholar]
- 33.van der Pligt P, Willcox J, Hesketh KD, et al. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obes Rev. 2013;14(10):792–805. doi: 10.1111/obr.12053. [DOI] [PubMed] [Google Scholar]
- 34.Aune D, Sen A, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis of epidemiological studies. Eur J Epidemiol. 2016;31(10):967–997. doi: 10.1007/s10654-016-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Mascio D, Magro-Malosso ER, Saccone G, Marhefka GD, Berghella V. Exercise during pregnancy in normal-weight women and risk of preterm birth: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2016;215(5):561–571. doi: 10.1016/j.ajog.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Russo LM, Nobles C, Ertel KA, Chasan-Taber L, Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(3):576–582. doi: 10.1097/AOG.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 37.Sanabria-Martinez G, Garcia-Hermoso A, Poyatos-Leon R, Alvarez-Bueno C, Sanchez-Lopez M, Martinez-Vizcaino V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta-analysis. BJOG. 2015;122(9):1167–1174. doi: 10.1111/1471-0528.13429. [DOI] [PubMed] [Google Scholar]
- 38.Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care. 2011;34(1):223–229. doi: 10.2337/dc10-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Xie R, Shen C, Shu L. Effect of exercise during pregnancy to prevent gestational diabetes mellitus: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. May 2017:1–6. doi: 10.1080/14767058.2017.1319929. [DOI] [PubMed] [Google Scholar]
- 40.Zheng J, Wang H, Ren M. Influence of exercise intervention on gestational diabetes mellitus: a systematic review and meta-analysis. J Endocrinol Invest. April 2017. doi: 10.1007/s40618-017-0673-3. [DOI] [PubMed] [Google Scholar]
- 41.Madhuvrata P, Govinden G, Bustani R, Song S, Farrell TA. Prevention of gestational diabetes in pregnant women with risk factors for gestational diabetes: a systematic review and meta-analysis of randomised trials. Obstet Med. 2015;8(2):68–85. doi: 10.1177/1753495X15576673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oostdam N, van Poppel MN, Wouters MG, van Mechelen W. Interventions for preventing gestational diabetes mellitus: a systematic review and meta-analysis. J Women’s Health (Larchmt). 2011;20(10):1551–1563. doi: 10.1089/jwh.2010.2703. [DOI] [PubMed] [Google Scholar]
- 43.Song C, Li J, Leng J, Ma RC, Yang X. Lifestyle intervention can reduce the risk of gestational diabetes: a meta-analysis of randomized controlled trials. Obes Rev. 2016;17(10):960–969. doi: 10.1111/obr.12442. [DOI] [PubMed] [Google Scholar]
- 44.Yin YN, Li XL, Tao TJ, Luo BR, Liao SJ. Physical activity during pregnancy and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2014;48(4):290–295. doi: 10.1136/bjsports-2013-092596. [DOI] [PubMed] [Google Scholar]
- 45.Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2012;(7):Cd009021. doi: 10.1002/14651858.CD009021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aune D, Saugstad OD, Henriksen T, Tonstad S. Physical activity and the risk of preeclampsia: a systematic review and meta-analysis. Epidemiology. 2014;25(3):331–343. doi: 10.1097/EDE.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 47.Kasawara KT, do Nascimento SL, Costa ML, Surita FG, e Silva JL. Exercise and physical activity in the prevention of pre-eclampsia: systematic review. Acta Obstet Gynecol Scand. 2012;91(10):1147–1157. doi: 10.1111/j.1600-0412.2012.01483.x. [DOI] [PubMed] [Google Scholar]
- 48.Fazzi C, Saunders DH, Linton K, Norman JE, Reynolds RM. Sedentary behaviours during pregnancy: a systematic review. Int J Behav Nutr Phys Act. 2017;14(1):32. doi: 10.1186/s12966-017-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonzini M, Coggon D, Palmer KT. Risk of prematurity, low birthweight and pre-eclampsia in relation to working hours and physical activities: a systematic review. Occup Environ Med. 2007;64(4):228–243. doi: 10.1136/oem.2006.026872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolf HT, Owe KM, Juhl M, Hegaard HK. Leisure time physical activity and the risk of pre-eclampsia: a systematic review. Matern Child Health J. 2014;18(4):899–910. doi: 10.1007/s10995-013-1316-8. [DOI] [PubMed] [Google Scholar]
- 51.Sheffield KM, Woods-Giscombe CL. Efficacy, feasibility, and acceptability of perinatal yoga on women’s mental health and well-being: a systematic literature review. J Holist Nurs. 2016;34(1):64–79. doi: 10.1177/0898010115577976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shivakumar G, Brandon AR, Snell PG, et al. Antenatal depression: a rationale for studying exercise. Depress Anxiety. 2011;28(3):234–242. doi: 10.1002/da.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCurdy AP, Boule NG, Sivak A, Davenport MH. Effects of exercise on mild-to-moderate depressive symptoms in the postpartum period: a meta-analysis. Obstet Gynecol 2017;129(6):1087–1097. doi: 10.1097/AOG.0000000000002053. [DOI] [PubMed] [Google Scholar]
- 54.Poyatos-León R, García-Hermoso A, Sanabria-Martínez G, Álvarez-Bueno C, Cavero-Redondo I, Martínez-Vizcaíno V. Effects of exercise-based interventions on postpartum depression: a meta-analysis of randomized controlled trials. Birth. 2017;44(3):200–208. doi: 10.1111/birt.12294. [DOI] [PubMed] [Google Scholar]
- 55.Teychenne M, York R. Physical activity, sedentary behavior, and postnatal depressive symptoms: a review. Am J Prev Med. 2013;45(2):217–227. doi: 10.1016/j.amepre.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities - United States, 2011. Morb Mort Week Rep 2013;62(17):326–330. [PMC free article] [PubMed] [Google Scholar]
- 57.Hales Craig M. Fryar Cheryl D. Carroll Margaret D. Freedman David S. Ogden Cynthia L. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–1725. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deputy NP, Sharma AJ, Kim SY. Gestational weight gain – United States, 2012 and 2013. Morb Mortal Wkly Rep. 2015;64:1215–1220. doi: 10.15585/mmwr.mm6443a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rasmussen MH, Strom M, Wohlfahrt J, Videbech P, Melbye M. Risk, treatment duration, and recurrence risk of postpartum affective disorder in women with no prior psychiatric history: a population-based cohort study. PLoS Med. 2017;14(9):e1002392. doi: 10.1371/journal.pmed.1002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.