Abstract
Objectives:
Interacting with socially assistive robots (SAR) has been shown to influence human behaviors and emotions. This study sought to review the literature on SAR intervention for reducing pediatric distress and pain in medical settings.
Methods:
Databases (PubMed, Cochrane Library, CINAHL, PsycINFO, ERIC, Web of Science, Engineering Village, Scopus, Google Scholar, IEEE Xplore) were searched from database inception to January 2018 with the aid of a medical librarian. Included studies examined any SAR intervention for reducing pain or improving emotional well-being in children related to physical or psychiatric care, with outcomes assessed by some quantitative measure. Study quality was assessed using the modified Downs and Black checklist (max score 28). The review is registered in PROSPERO (CRD42016043018).
Results:
Eight studies met eligibility criteria and represented 206 children. Of the two studies using Wong-Baker’s FACES scale, one study claimed to be effective at reducing pain (Cohen’s d = 0.49–0.62), while the other appeared effective only when parents and child interacted with SAR together. Distress was evaluated using validated measures in four studies, three of which showed reduction in distress while one showed no difference. Satisfaction surveys from four studies showed that children were interested in using SAR again. Quality scores ranged from 8–26.
Conclusions:
There is limited evidence suggesting that SAR interventions may reduce distress and no clear evidence showing reduction in pain for children in medical settings. Engineers are conducting interventions using SAR in pediatric populations. Healthcare providers should be engaged in technology research related to children to facilitate testing and improve effectiveness of these systems.
Keywords: robots, pain, anxiety
Introduction:
The distress and pain experienced by children undergoing health care treatments has been linked to post-traumatic stress disorder, avoidance of medical care as adults, and increased sensitivity to future painful stimuli [1–4]. In order to mitigate such pain and distress, anesthetic or anxiolytic medications are often used, but administration of the drugs themselves may cause pain, distress, or side-effects [5, 6]. Non-pharmacologic techniques are gaining in popularity, and evidence shows that psychological interventions including distraction, behavioral feedback, or coping exercises can be as effective in reducing distress [7–9]. Recent advances in technology have allowed the development of highly distracting and engaging tablet games, robotics, and virtual reality that can be tools to enhance and deepen such interventions. For instance, virtual reality has shown reduced pain intensity during phlebotomy[10] and IV placement compared to control [11] and tablet distraction has reduced pain in pediatric burn patients undergoing hydrotherapy [12].
Socially Assistive Robotics (SAR) represents another promising opportunity for mitigating distress during painful medical procedures. SAR systems establish communication and create a shared relationship without touching the human, by utilizing embodied interaction that may involve expressiveness, personality, dialog, empathy, and adaptation skills. It is not yet known what mechanisms produce human physiologic and behavior change resulting from such interaction, but the human-robot relationship has been used to promote healthy outcomes. In adult health settings, SAR systems have been explored extensively for various uses with the elderly, including for improving cognitive skills in Alzheimer’s patients [13, 14], reducing feelings of loneliness and depression [15], and increasing function following stroke [16], among numerous others. Relevant to pediatrics, SAR systems have been extensively explored in the context of autism diagnosis, intervention and therapy, and have been shown to increase verbalization, socialization, and emotional expression, among other desired effects [17]. In non-autistic children, SAR systems have been used to promote child learning, provide therapy for depression, and to interview children about sensitive topics, among other uses. [18–21]
The explosion of computer science research and development has brought about numerous technologies that may be applicable to the future world of pain management. Artificial intelligence and machine learning are already being used to automate and personalize therapy [22], automated emotion recognition in video and audio is in use in commercial and research systems [19], and various machine learning methods are being developed to personalize human-machine interactions in all spheres of life. At the same time, wearable sensors and signal processing algorithms are being developed to utilize a variety of physiologic data (body temperature, vagal tone, blood pressure, heart rate, galvanic skin response, etc.); these are being explored for pain recognition and management [23] These promising systems can be used together with SAR systems to allow a more nuanced human-robot interaction.
Despite the very large body of publications on SAR in various domains, no review has been performed to establish the current scope of SAR interventions for lessening child pain and distress. Therefore, we sought to review the available literature to assess the impact of SAR interaction on pain and distress in children undergoing health-care related treatment.
Methods
Search Strategy
In February 2016 a librarian at the University of Southern California Norris Medical Library (Los Angeles, CA) conducted searches in the following databases using a combination of controlled vocabulary (when possible) and all fields keywords: MEDLINE (see Appendix 1, Supplemental Digital Content 3, http://links.lww.com/CJP/A560), Embase (see Appendix 2, Supplemental Digital Content 3, http://links.lww.com/CJP/A560), Cochrane Library (see Appendix 3, Supplemental Digital Content 3, http://links.lww.com/CJP/A560), CINAHL (see Appendix 4, Supplemental Digital Content 3, http://links.lww.com/CJP/A560), PsycINFO (see Appendix 5), ERIC (see Appendix 6, Supplemental Digital Content 3, http://links.lww.com/CJP/A560), Web of Science (see Appendix 7, Supplemental Digital Content 3, http://links.lww.com/CJP/A560), Scopus (see Appendix 8, Supplemental Digital Content 3, http://links.lww.com/CJP/A560), Engineering Village (see Appendix 9, Supplemental Digital Content 3, http://links.lww.com/CJP/A560), IEEE Xplore (see Appendix 10, Supplemental Digital Content 3, http://links.lww.com/CJP/A560), and Google Scholar (see Appendix 11, Supplemental Digital Content 3, http://links.lww.com/CJP/A560). Proceedings of the Association for Computing Machinery CHI Conference on Human Factors in Computing Systems and Conference on Human-Robot Interaction were identified as relevant and manually searched. References for included studies were screened for additional unique citations. The searches were re-performed and additional citations were retrieved on January 23, 2018 in order to include the most up-to-date studies.
Inclusion criteria
Two investigators independently reviewed abstracts and selected those that met inclusion criteria:
Included children age <18 who were receiving some health care treatment;
Intervention was SAR applied towards the reduction of pain or distress;
Outcomes were measured and reported.
Health care treatment was broadly defined as referral to physical (including dental) or mental care for any reason. Distress encompassed any measures of depression, anxiety, stress, mood, or maladaptive coping. Studies not meeting inclusion criteria were excluded. Non-English studies were included.
Article selection and data extraction
Two authors (MT, AF) independently reviewed and screened all studies for inclusion using a screening tool to increase reproducibility. The following information was extracted and documented from studies that met inclusion criteria: study participant characteristics and setting, description of SAR system studied, duration and frequency of treatment, outcomes (pain, distress, satisfaction with intervention), and any adverse events.
Study quality was assessed using a modified Downs-Black checklist, which has a maximum score of 28 [24, 25]. This tool evaluates methodologic quality for both randomized and non-randomized interventional studies on the basis of reporting, internal and external validity, bias, and power. Two authors (MT, AF) completed quality assessments of all studies meeting inclusion criteria with consensus achieved via review of all authors. For study inclusion and study quality respectively, there was good inter-rater reliability between reviewers (kappa=0.922, 0.864). Disagreements were resolved using a modified Delphi process. The review is registered in PROSPERO (CRD42016043018).
Results:
The study selection process is detailed in Figure 1. Full text evaluation eliminated 23 studies; the majority of these were excluded because they detailed design and development of SAR systems intended for use in populations of interest but without results to date (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CJP/A558). Eight studies met eligibility criteria and represented 206 unique children; only 3 of 8 (37.5%) were referenced in PubMed and the remainder came from engineering literature [26–33]. Study designs included single group intervention with post-exposure survey (n=2), non-randomized controlled (n=3), randomized controlled (n=2), and a mixed-methods analysis of a randomized controlled study (n=1). Three studies (37.5%) used the humanoid robot Nao (Aldebaran Robotics, France), two studies (25%) used the soft seal-like robot PARO (AIST, Japan), two studies (25%) used commercially available dog or cat-type robots [AIBO (Sony, Japan), NeCoRo (Omron, Japan)], and one study used iRobi (Yujin Robot, South Korea) a humanoid robot primarily developed for early childhood education (Figure 2). The clinical settings and populations studied varied from hospitalized inpatients (n=3), general pediatric outpatient (n=1; two studies referenced same population), pediatric oncology clinic (n=1), adoption clinic (n=1), and dental clinic (n=1). There was similarly wide variability in the duration of intervention (from 15 minutes once to 8 one-hour sessions), the outcome measures used, and the quality of the studies (Table 1).
Figure 1:
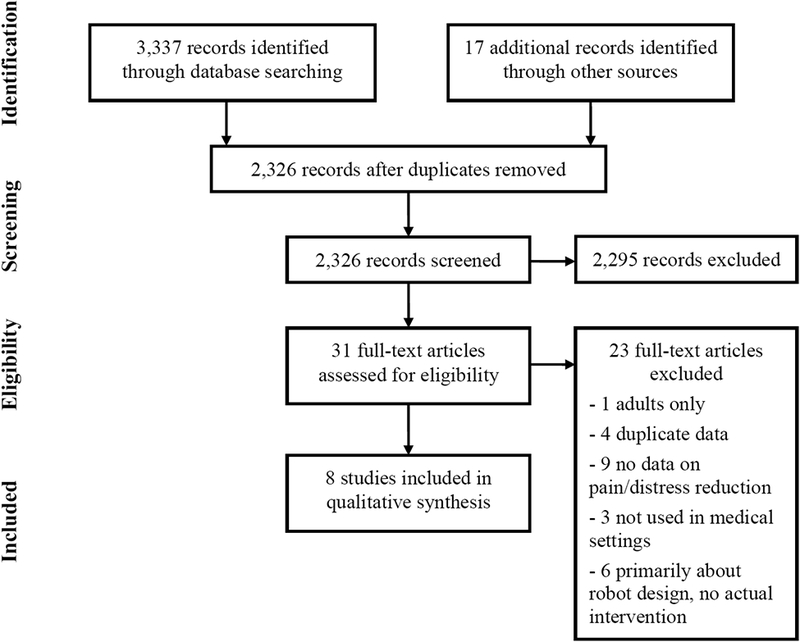
PRISMA Flow Diagram
Figure 2:

Images of the socially assistive robots used in some of the included studies, which show the wide range of shapes and styles. (A) iRobi (B) Paro (C) AIBO (D) Nao
Table 1:
Data extracted from Studies
| Author, Year, Country | Quality: Downs and Black Score (of 28) | Clinical Domain | Participants | Age | Number of Patients | Length of intervention | Intervention | Outcomes related to pain (effective, significant) | Outcomes related to distress (effective, significant) | Outcomes related to satisfaction (effective, significant) | Adverse Effects | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shibata 2001 Japan | 8 | Stress associated with hospitalization |
Inclusion: Short and long term
inpatients, long term inpatient with immune
problems Exclusion: Not given |
2 – 13 years |
Total:
16 Intervention Group: 16 Control Group: 16 |
3 times daily (10:30–11:30 am, 16:30–17:30pm, 18:30-sleep) for 11 days; ON 7 days, OFF 4 days |
Robot: PARO, soft seal
robot Control: PARO turned off to simulate stuffed animal Randomized? No |
Vital Signs, “influenced by medication” (no; thrown out as measure) | Mood; “Face” Scale [1–20; 20=sad]; Mean for PARO on = 3.05 vs. PARO off = 9.85 (yes; no statistics performed) | Comments by Nursing: “The seal robot helped the children to socialize … and relieved their anxiety when not with their parents.” (yes; no statistics performed) | -- | Suggests possible benefit, Pilot-type study with no statistics performed. |
| Kimura 2004 Japan | 9 | Stress associated with hospitalization |
Inclusion: short term inpatients
(<1 week); long term inpatients (>6
months) Exclusion: not given |
1 – 19 years |
Total:
28 Intervention Group: 28 Control Group: none |
Once weekly, 5:00–6:00 pm |
Robot: Multiple- AIBO (dog, 4
series), Necoro (cat), Capriro (cat) Control: none Randomized? No |
N/A | Mood; “Face” Scale [1–5; 5=most negative mood]; 97% had same or improved mood from before to after interaction (yes; no statistics performed) | Influence to Communication: “My child
talked about robotic pet to other children after RAA”, n=18,
“excellent” or “very good” = 28% (no; no
statistics performed) Subjective Evaluation: ““What do you feel about playing with robot in playroom?” n=21, “excellent” or “very good” = 95% (yes; no statistics performed) |
Duration of interest; ~30 minutes if only child-robot, ~60 minutes if child+ another human-robot | Suggests possible benefit, Numbers of participants had to be interpreted from graphs. |
| Trujillo 2011 USA | 21 | Anxiety related to history of adoption |
Inclusion: Adopted through public
child welfare system (assumes historical exposure to abuse, neglect),
residents of Colorado who could travel, had been in home > 6
months Exclusion: Private adoptions, cruel behavior to dog or robot during study |
5 – 11 years |
Total: 43 (24 boys, 19
girls) Intervention: 21 Control: 22 |
15 minutes |
Robot: “Biscuit”,
FurReal dog, commerically available Control: American Humane Association approved therapy dog Randomized? Yes |
N/A | Anxiety; Revised Child Manifest Anxiety
Scale-2 (RCMAS-2) Phyisological Sub-scale (No, paired t-test p=0.67
robot, p=0.14 control) Recognizing emotion; “Reading the Mind Through the Eyes” test (No, paired t-test p=0.78 robot, p=0.76 control) Engagement; video coding for Revised Melson/Trujillo 3-point engagement scale; Kappa=0.89 (No, stated independent t-test not significant but no p-value given) |
Semi-structured interview and survey.
“Would having a friend like this help you deal with new situations?” (no, 62% robot vs. 68% control, no statistics performed) “Do you think having x as a companion would keep you safe?” (no, 90 % robot vs. 91% control) “Social companisonship score” no difference between robot vs. control. “Would you prefer real or robot dog as pet?” (no, robot = 34.9% vs. 65.1% control) |
23% of children had history of cruelty to animals but no cruelty observed in study | No benefit to either robot or real dog. Engagement with either a live or robot dog was similar but real dog was preferred. |
| Beran 2013 Canada | 26 | Pain and distress related to vaccines |
Inclusion: Age 4–9,
inpatients or outpatients referred to Children’s Hospital clinic
for flu vaccine Exclusion: outside of age range |
4 – 9 years | Total: 57 (30 boys, 27
girls) Intervention: 28 (14 boys, 14 girls) Control: 29 (16 boys, 13 girls) |
once |
Robot: Nao, humanoid robot, gave
high-five, talked, blowing activity, waved good-bye
Control: nurse routine care, minimal distraction Randomized? Yes |
Faces Pain Scale-Revised, administered to patient, parent, nurse, and researcher. (yes, effect size 0.49 – 0.62, p<0.05 for all) | Distress, video coded using Behavior Approach-Avoidance Distress Scale, Kappa=0.78, 0.89. (yes, effect size “distress” 0.79, “avoidance” 0.90, p<0.05 for both scales) | Survey to child and parent, 5-point Likert. Children chose “very much” 85.7%, n=24, Parents chose “very much” 85.7%, n=24 (yes, no statistics performed) | -- | Showed reduction in pain and distress, number and variety of distractions in robot condition much greater than in control. |
| Okita 2013 USA | 17 | Pain and distress related to hospitalization |
Inclusion: Female patients
currently hospitalized for any condition Exclusion: Males |
6 – 16 years |
Total: 18 girls and their
mothers Intervention: 9 child only, 9 child and parent Control: none |
30 minutes |
Robot: PARO, soft seal
robot Control: none Randomized? No |
Wong-Baker Faces, both patient’s self-pain and parent’s empathic pain. (yes in “together” condition, p<0.001 by paired sample t-test, no in “alone” condition, p-0.397) | Anxiety; 11 questions from State-Trait Anxiety Index (adult and children’s versions), only when children >9 years old (n=12). (yes in “together” condition, p<0.01 by paired sample t-test, no in “alone” condition no p-value given) | N/A | .-- | Showed reduction in pain and distress, only when both child and parent together interact with the robot. |
| Alemi 2015 Iran | 16 | Anger, anxiety, depression with cancer |
Inclusion: Currently undergoing
treatment, able to make session Exclusion: Completed <6 sessions |
7 – 12 years |
Total: 11 (1 boy, 10
girls) Intervention: 6 Control: 5 |
8 sessions |
Robot: Nao (humanoid robot)
discussed treatment plans, showed empathy, played games
Control: Routine care Randomized? No |
N/A | Anxiety; Multidimensional Anxiety Children
Scale (yes, p=0.002, effect size 0.70 by ANOVA) Anger; Children’s Inventory of Anger (yes, p=0.012, effect size 0.52 by ANOVA) Depression; Kovaks’ (1985) Children’s Depression Inventory (yes, p=0.019, effect size 0.56 by ANOVA) |
N/A | -- | Reduction in distress was shown, very small pilot-type study. |
| Beran* 2013 Canada | 18 | Pain and distress related to vaccines |
Inclusion: Age 4–9,
inpatients or outpatients referred to Children’s Hospital clinic
for flu vaccine Exclusion: outside of age range |
4–9 years |
Total: 57 (30 boys, 27
girls) Intervention: 28 (14 boys, 14 girls) Control: 29 (16 boys, 13 girls) |
once |
Robot: Nao, (humanoid robot) gave
high-five, talked, blowing activity, waved good-bye
Control: nurse routine care, minimal distraction Randomized? Yes |
N/A | Child smiling proportion, video coding, (yes
by ANOVA, p<0.01) Adult smiling proportion (yes, by ANOVA, p<0.001) |
Qualitative, grounded theory, themes “request for robot in the future” “neutral” and “empowering” comments. | Child crying proportion, (did not differ by ANOVA, p>0.05) | Suggests improved mood based on smiling but study not powered for that outcome, unclear how many participated in the qualitative interviews. |
| Yasemin 2016 Turkey | 8 | Pain and distress related to dental work |
Inclusion: First time patients,
accompanied by at least one parent, Agreeing to fill out the questionnaire Exclusion: Any dental work that required sedation or injections, physical or mental disabilities, serious illness, prior dental treatment, chronic oral pain or bleeding |
4–10 years |
Total: 33 (21 boys, 12
girls) Intervention: 16 Control: 17 |
once |
Robot: IRobi, (humanoid robot),
made gestures, interact using LED screen Control: routine care Randomized? No |
Heart rate, pulse oximeter, 68% had no change
or decrease vs. 29.40% in the control group (yes, no statistics performed) |
Facial image scale: Same or improved pre-
post- in 39% of the control vs. 6.25% robot group (yes, no statistics
performed) Frankl behavioral rating scale: change in mean −0.41 in control group vs. +0.31 (yes, no statistics performed) |
Survey: Would you want the robot again next time? 81% most positive response | -- | Suggests improved mood and some pain reduction, however In the control group no topical anesthesia was used vs. in the robot group it was. |
This study was a mixed methods analysis of the other Beran 2013 study
N/A indicates no measures applicable to this domain included in the study. Adverse events not reported for all studies.
Outcomes related to pain
Although four studies used a “face” type scale, only two studies used Wong-Baker’s FACES pain scale while the other two applied the “face” scale to child mood/distress so no meta-analysis was performed. Of the studies using Wong-Baker, one claimed to be effective at reducing child pain (Cohen’s d = 0.49–0.62), while the other reported efficacy only when parents and child interacted with the robot together (mean change 2.11, p<0.001) [26, 27]. Two studies attempted to use heart rate to measure pain, although this is not a reliable proxy for pain and rather a signal indicating general physiologic arousal. Kimura and colleagues abandoned the attempt and reported concern that vital signs were confounded by medication use, however no attempt to control for confounding was made nor was raw data reported [29]. Yasemin et al. also used heart rates as a pain outcome and reported that the majority of children (68%) in the robot intervention arm had the same or reduced heart rate compared to 29% in the control group, however no statistics were performed [33]. In addition, the Yasemin study, which was based in a dental clinic, used topical anesthetic in the intervention arm but not the control arm, which seriously limits any conclusions drawn from this measure.
Outcomes related to distress
Broadly, the concept of distress was evaluated using validated measures in four studies, three of which showed reduction in distress and one compared a robotic dog to a real dog and showed no difference. Specifically, anxiety was reduced in the Alemi study (Multidimensional Anxiety Childrens Scale, p=0.002), and in the Okita study reduced anxiety was seen when parent and child both interacted with the robot (State-trait anxiety index, p<0.01, only children >9 years old tested); in the Trujillo study there was no reduction (Revised Childhood Manifest Anxiety Scale 2, p=0.67) [26, 31, 32]. Only one study measured distress, applying the Behavior Approach-Avoidance Distress Scale to video tapes of the interaction, which showed good inter-rater reliability and significant distress reduction when interacting with the Nao robot (kappa=0.78, 0.89 and p<0.05)[27]. One study included outcome measures for anger and depression and found improvement in those scales after interacting with the robot (effect size 0.52, 0.56 respectively, p<0.05)[32]. The three previously mentioned studies using non-validated “face” scales to judge mood all claimed to show a positive effect in the robot condition but no statistics were reported [29, 30, 33].
Outcomes related to satisfaction
Although child, parent, or medical provider satisfaction was not an outcome measure that defined our article selection, most (n=6, 75%) of included studies performed either quantitative survey or qualitative analysis to evaluate attitudes towards the robot interventions. Satisfaction surveys post-intervention were performed in four studies, all showing that children seemed to like the SAR system and were interested in using it again [27, 30, 31, 33]. One study included a qualitative analysis of comments parents made after their children participated in an interaction attempting to reduce distress associated with vaccines. Although the paper lacked important methodologic details (i.e., it is unclear if all participants gave comments or just some), the comments clustered around three themes: a desire to have the robot the next time, neutral statements, or empowering statements [28]. Another study included comments from nursing who observed the interaction, but did not systematically collect or analyze them; the quotes provided were generally positive [29].
Study quality assessment
Study quality scores based on our assessment ranged from 8–26, on a scale of 1 to 28. The three studies receiving poor (<10) scores suffered from reporting bias (characteristics of patients and/or confounders not clearly described), problems with external validity (non-representative sampling), and issues with internal validity (appropriate statistics not used, unclear patient tracking, see Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/CJP/A559). Only two studies fell into the highest quality category (>20); these studies represented 2 of the only 3 that were randomized. Only the top-scored study explicitly calculated and achieved appropriate sample size, and this same study was the only one to report that the data were interpreted by researchers blinded to the aims of the project [27].
Discussion
In this scoping review, we identified eight studies related to use of socially assistive robots as an intervention for reducing distress and pain in children undergoing health care treatment. Seven of the studies (87.5%) were interpreted by their authors as showing benefit of the SAR experience. Our analysis revealed methodologic problems in several studies. However, the highest quality study showed significant reduction in distress when comparing SAR to control. Based on this review, SAR intervention cannot at this time be recommended for use over the many other well-established non-pharmacological strategies for managing procedural pain and distress. As the associated technologies that constitute SAR systems become more available and affordable, and interdisciplinary teams work together, better informed studies in more realistic environments will become more frequent, as will comparisons to other advanced technologies and matched controls, and randomization to minimize confounding and allow for better comparisons. In addition, more accurate measures of pain will be utilized; for example, some reviewed studies used the Wong-Baker pain scale, whose research validity is questionable [34–36].
Perhaps more interesting than the ultimate conclusions of these studies is that most were published outside of the medical literature. Although SAR as a field is still nascent, there are many potential benefits to applying SAR in pediatric health settings. Mental health researchers have already postulated the idea of SAR as practitioner extenders in areas where there is poor mental health coverage [37]. Similarly, many children are cared for outside of specialized children’s hospitals and could benefit from tools specifically designed to assuage child pain and distress. SAR does not aim to replace providers, but could serve as a way to augment therapies. Finally, SAR has already been identified as a promising therapeutic tool for autism spectrum disorders and related social and developmental disorders [38]. It would be beneficial for physicians and other health-care professionals at academic centers to become familiar with relevant research in departments of computer science and engineering, and to foster partnerships that can evolve into translational research teams.
What would these ideal physician-engineer designed SAR systems ultimately look like? Our literature review gives some insight into how the human-robot interactions are currently designed and where there are opportunities for improvement. SAR methods used in the pain management context can be improved by incorporating pain medicine and psychology tools such as empathy, biofeedback, or coping strategies. For example, in the Beran study the SAR acted as a distraction by giving high-fives and waving to children, but it also blew bubbles with the children because blowing (to simulate deep breathing) is a known coping mechanism. In addition the Okita study identified more success when parent and child engaged with the SAR together as opposed to the parent present but disengaged, which the authors attributed to parental modeling but may indicate that group interactions work better for some children.
Novel and engaging technologies are not necessarily harmless or linked to improved health outcomes. The AAP recommends limited screen time for children due to concerns that excessive use is linked with developmental delays[39]. However, SAR systems are not screens, and many do not include screens at all; instead, SAR systems are embodied like human companions, involving mutual interaction. As noted earlier, in the world of autism therapy, SAR systems have been shown to increase socialization and communication more than similar interactions with other technologies, without significant harms [40]. Three studies in our review made an effort to report adverse events (child cruelty, crying, and disinterest) and found difference in attention spans based on who interacted with the robots (as previously discussed) but no other harms. Possibly, this is because harms were not appropriately selected and measured; SAR-relevant methods for such measurement should be developed and included in studies whenever possible. Potential harms will need to be carefully assessed as rapidly changing SAR technology may create unintended negative effects. In addition, future studies should attempt to evaluate repeated or long-term exposure to SAR-systems, which may have the potential to become less effective as they become less novel.
Even when highly effective SAR systems are developed, more work is needed to determine how they can best be implemented. Although robotics has historically been an expensive field, 3D printing, new materials, commercialization and rapid expansion of consumer market robots, and open-source software have made production of SAR systems less costly. Efforts to make SAR systems sufficiently robust to be fully autonomous will increase their widespread adoption.
Conclusions
Socially assistive robotics is a burgeoning field at the intersection of engineering, cognitive science, social science, and medicine, with numerous potential applications for child health. This scoping review provides some evidence showing future promise of SAR systems reducing distress in children in medical settings, but there is an insufficient evidence base to support any current clinical recommendations. Although the authors of several papers suggested a reduction in pain, better methodology and measures are needed to draw conclusions. Collaborations between health care experts (including physicians, child-life specialists, psychologists, etc.) and engineers early in the development process should be nurtured in order to apply medical principles to the design and operation of such promising technology. In addition, patient and family partners could contribute to user-centered design that may lead to more effective interventions.
Supplementary Material
Acknowledgements
Dr. Trost is an institutionally-funded Clinical and Translational Scholar. This work was supported by grant UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Source: NIH; UL1TR001855
Footnotes
Financial Disclosure: No authors have financial relationships relevant to this article to disclose.
Conflict of Interest: All authors have no conflicts of interest to disclose.
References
- 1.Rennick JE, et al. , Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr, 2002. 23(3): p. 133–44. [DOI] [PubMed] [Google Scholar]
- 2.Childhood Medical Experience and Temperament as Predictors of Adult Functioning in Medical Situations. Children’s Health Care, 1996. 25(4): p. 281–298. [Google Scholar]
- 3.Humphrey GB, et al. , The occurrence of high levels of acute behavioral distress in children and adolescents undergoing routine venipunctures. Pediatrics, 1992. 90(1 Pt 1): p. 87–91. [PubMed] [Google Scholar]
- 4.Kennedy RM, Luhmann J, and Zempsky WT, Clinical implications of unmanaged needle-insertion pain and distress in children. Pediatrics, 2008. 122 Suppl 3: p. S130–3. [DOI] [PubMed] [Google Scholar]
- 5.Bjur KA, et al. , Anesthetic-Related Neurotoxicity and Neuroimaging in Children: A Call for Conversation. J Child Neurol, 2017. 32(6): p. 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aun CS, et al. , Short-Term Changes in Postoperative Cognitive Function in Children Aged 5 to 12 Years Undergoing General Anesthesia: A Cohort Study. Medicine (Baltimore), 2016. 95(14): p. e3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ismail AQ and Gandhi A, Non-pharmacological analgesia: effective but underused. Arch Dis Child, 2011. 96(8): p. 784–5. [DOI] [PubMed] [Google Scholar]
- 8.Pillai Riddell RR, et al. , Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev, 2015(12): p. CD006275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher E, et al. , Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev, 2015(3): p. CD011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold JI and Mahrer NE, Is Virtual Reality Ready for Prime Time in the Medical Space? A Randomized Control Trial of Pediatric Virtual Reality for Acute Procedural Pain Management. J Pediatr Psychol, 2018. 43(3): p. 266–275. [DOI] [PubMed] [Google Scholar]
- 11.Gold JI, et al. , Effectiveness of virtual reality for pediatric pain distraction during i.v. placement. Cyberpsychol Behav, 2006. 9(2): p. 207–12. [DOI] [PubMed] [Google Scholar]
- 12.Burns-Nader S, Joe L, and Pinion K, Computer tablet distraction reduces pain and anxiety in pediatric burn patients undergoing hydrotherapy: A randomized trial. Burns, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Tapus A, The Role of the Physical Embodiment of a Music Therapist Robot for Individuals with Cognitive Impairments: Longitudinal Study. 2009 Virtual Rehabilitation International Conference, 2009: p. 203–203. [Google Scholar]
- 14.Tapus A, Tapus C, and Mataric MJ, The Use of Socially Assistive Robots in the Design of Intelligent Cognitive Therapies for People with Dementia. 2009 Ieee 11th International Conference on Rehabilitation Robotics, Vols 1 and 2, 2009: p. 1077–+. [Google Scholar]
- 15.Wada K, et al. , Effects of robot-assisted activity for elderly people and nurses at a day service center. Proceedings of the IEEE, 2004. 92(11): p. 1780–1788. [Google Scholar]
- 16.Mataric M, et al. , Socially assistive robotics for stroke and mild TBI rehabilitation. Stud Health Technol Inform, 2009. 145: p. 249–62. [PubMed] [Google Scholar]
- 17.Scassellati B, Admoni H, and Mataric M, Robots for use in autism research. Annu Rev Biomed Eng, 2012. 14: p. 275–94. [DOI] [PubMed] [Google Scholar]
- 18.Baxter P, et al. , Robot education peers in a situated primary school study: Personalisation promotes child learning. PLoS One, 2017. 12(5): p. e0178126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogt P, et al. , Child-Robot Interactions for Second Language Tutoring to Preschool Children. Front Hum Neurosci, 2017. 11: p. 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood LJ, et al. , Robot-mediated interviews--how effective is a humanoid robot as a tool for interviewing young children? PLoS One, 2013. 8(3): p. e59448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scassellati B, et al. , Improving social skills in children with ASD using a long-term, in-home social robot. Science Robotics, 2018. 3(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzpatrick KK, Darcy A, and Vierhile M, Delivering Cognitive Behavior Therapy to Young Adults With Symptoms of Depression and Anxiety Using a Fully Automated Conversational Agent (Woebot): A Randomized Controlled Trial. JMIR Ment Health, 2017. 4(2): p. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papi E, Koh WS, and McGregor AH, Wearable technology for spine movement assessment: A systematic review. J Biomech, 2017. 64: p. 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downs SH and Black N, The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health, 1998. 52(6): p. 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trac MH, et al. , Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ, 2016. 188(7): p. E120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okita SY, Self-other’s perspective taking: the use of therapeutic robot companions as social agents for reducing pain and anxiety in pediatric patients. Cyberpsychol Behav Soc Netw, 2013. 16(6): p. 436–41. [DOI] [PubMed] [Google Scholar]
- 27.Beran TN, et al. , Reducing children’s pain and distress towards flu vaccinations: a novel and effective application of humanoid robotics. Vaccine, 2013. 31(25): p. 2772–7. [DOI] [PubMed] [Google Scholar]
- 28.Beran TN, et al. , Humanoid robotics in health care: An exploration of children’s and parents’ emotional reactions. J Health Psychol, 2015. 20(7): p. 984–9. [DOI] [PubMed] [Google Scholar]
- 29.Shibata T, et al. Mental commit robot and its application to therapy of children. in 2001 IEEE/ASME International Conference on Advanced Intelligent Mechatronics. Proceedings (Cat. No.01TH8556) 2001. [Google Scholar]
- 30.Kimura R, et al. Trial of robot assisted activity using robotic pets in children hospital. in SICE 2004 Annual Conference 2004. [Google Scholar]
- 31.Trujillo K, “Developing Emotional Security Among Children Who Have Been Adopted” (2010). Electronic Theses and Dissertations. 940 and http://digitalcommons.du.edu/etd/940. [Google Scholar]
- 32.Alemi M, et al. Effect of utilizing a humanoid robot as a therapy-assistant in reducing anger, anxiety, and depression. in 2014 Second RSI/ISM International Conference on Robotics and Mechatronics (ICRoM) 2014. [Google Scholar]
- 33.Yasemin M, et al. Management of dental anxiety in children using robots. in 2016 24th Signal Processing and Communication Application Conference (SIU) 2016. [Google Scholar]
- 34.Chambers CT and Craig KD, An intrusive impact of anchors in children’s faces pain scales. Pain, 1998. 78(1): p. 27–37. [DOI] [PubMed] [Google Scholar]
- 35.Chambers CT, et al. , A comparison of faces scales for the measurement of pediatric pain: children’s and parents’ ratings. Pain, 1999. 83(1): p. 25–35. [DOI] [PubMed] [Google Scholar]
- 36.Hicks CL, et al. , The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain, 2001. 93(2): p. 173–83. [DOI] [PubMed] [Google Scholar]
- 37.Rabbitt SM, Kazdin AE, and Scassellati B, Integrating socially assistive robotics into mental healthcare interventions: applications and recommendations for expanded use. Clin Psychol Rev, 2015. 35: p. 35–46. [DOI] [PubMed] [Google Scholar]
- 38.Scassellati B, Admoni H, and Mataric M, Robots for Use in Autism Research. Annual Review of Biomedical Engineering, Vol 14, 2012. 14: p. 275–294. [DOI] [PubMed] [Google Scholar]
- 39.Council On C. and Media, Media and Young Minds. Pediatrics, 2016. 138(5). [DOI] [PubMed] [Google Scholar]
- 40.Pennisi P, et al. , Autism and social robotics: A systematic review. Autism Research, 2016. 9(2): p. 165–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


