Menstrual pain without an underlying medical condition, known as primary dysmenorrhea (PD), is the leading cause of school and work absences [9], and up to 50% of adolescent girls with dysmenorrhea report impaired functioning because of their symptoms [15]. Despite this high prevalence, there is limited research on how and why many adolescent girls and young women experience disabling menstrual pain.
Past research has demonstrated enhanced pain sensitivity in women with dysmenorrhea, both in areas of referred pain [3; 5; 12; 21; 35; 44; 46] and remote body regions [1; 2; 4; 5; 12; 13; 17; 20; 21; 23; 35; 46]. These data provide support for the concept that PD is associated with lasting changes in pain processing. However, little is known about potential mechanisms in this episodic condition. New test paradigms such as temporal summation (TS) and conditioned pain modulation (CPM) examine both excitatory and inhibitory central pain processes, respectively. It is hypothesized that the presence of TS reflects central sensitization, where by pain responses become exaggerated over time despite the pain stimulus remaining at a constant level [16; 32; 40]. Chronic pain populations show elevated TS compared to healthy populations, a difference that suggests overactive excitatory pain responses [33; 36]. In women with PD, one study also found evidence of TS following repeated cervical distensions – a pattern that was not evident in women without PD [3]. CPM, on the other hand, is a measure of inhibitory pain processes, and deficits in CPM may reflect impairments in central descending inhibitory systems posited as an underlying mechanism in chronic pain [45; 52]. CPM has been evaluated in other episodic pain conditions (i.e., migraine headaches), with some studies showing deficient CPM compared to healthy controls [26], and other studies showing no group differences [22; 27; 38]. Extant research suggests changes in inhibitory mechanisms across the menstrual cycle in healthy women [34; 39], and several studies have found dysmenorrhea is associated with changes in central pain processing that persist beyond the time of menstruation [41; 42; 46]. However, research using standardized test paradigms to evaluate excitatory and inhibitory pain mechanisms has not been conducted in PD [29]. Identifying excitatory and inhibitory pain processing deficits in PD may help identify young women with dysmenorrhea at risk for developing other chronic pain conditions [46] and can provide data on the developmental trajectory of pain and disability in PD.
The current study aimed to test differences in experimental pain sensitivity (intensity and threshold) and excitatory (TS) and inhibitory (CPM) pain processes in a sample of young women with and without PD across three menstrual cycle phases: ovulatory, mid luteal, and menstrual. We hypothesized that participants with PD would demonstrate increased pain intensity and lower pain threshold, compared to control participants, across all menstrual cycle phases. Based on limited evidence suggesting deficient CPM in other episodic pain conditions, we also hypothesized that participants with PD would demonstrate elevated TS and deficient CPM, compared to control participants, across all menstrual cycle phases.
Methods
Participants
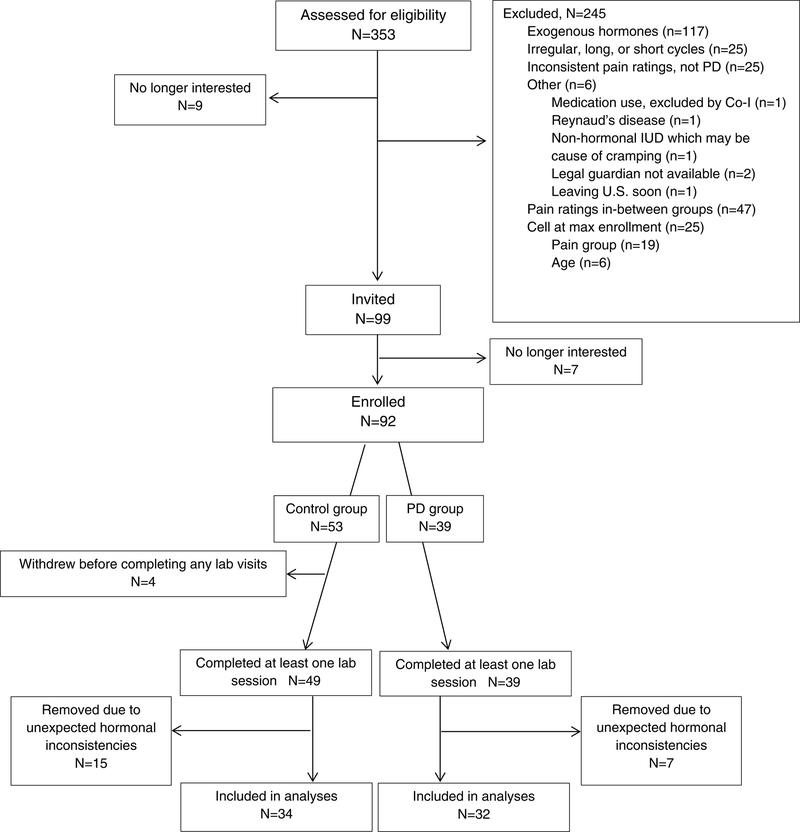
Participants included in this study were selected from a larger group of 92 adolescent girls and young adult women (53 controls, 39 with PD), ages 16–24 years (see Table 1 for demographic data). Three hundred fifty-three individuals were initially screened for eligibility by telephone. Nine individuals (2.5% of total screened) were not interested in participating, and 245 (69.4%) were excluded due to meeting other exclusionary criteria such as use of exogenous hormones in the prior 3 months (see Figure 1 for complete list). We selectively recruited participants with either very little or no menstrual pain or severe or very severe menstrual pain in order to create more distinct groups. Forty-seven individuals were additionally excluded due to having mild to moderate menstrual pain (i.e., > 2/10 and < 6/10 self-reported pain). Of the 99 eligible individuals who were invited to participate, 7 (7.1%) declined participation mainly because of lack of interest or scheduling difficulties. See Figure 1 for a flow chart depicting study enrollment.
Table 1.
Demographic information for study participants
| Measure | PD (n=32) | Control (n=34) | P value |
|---|---|---|---|
| M (SD) | M (SD) | ||
| Age (years) | 21.36 (2.10) | 20.61 (2.14) | 0.157 |
| Age at menarche | 11.81 (1.23) | 12.00 (1.41) | 0.570 |
| BSI – somatization | 2.94 (3.08) | 1.44 (1.33) | 0.052* |
| BSI – depression | 3.69 (3.75) | 3.77 (4.41) | 0.939 |
| BSI – anxiety | 3.19 (2.98) | 2.35 (3.34) | 0.289 |
| BSI – global | 9.81 (7.68) | 7.56 (8.11) | 0.252 |
| n (% of group) | n (% of group) | ||
| Race | |||
| White | 16 (50.0) | 13 (38.2) | 0.451 |
| African-American | 4 (12.5) | 3 (8.8) | |
| Asian | 12 (37.5) | 18 (52.9) | |
| Ethnicity | 0.034 | ||
| Not Hispanic/Latino | 20 (62.5) | 29 (85.3) | |
| Hispanic/Latino | 12 (37.5) | 5 (14.7) |
Note. Analyzed with Wilcoxon two sample test, otherwise two-sample t-test was used.
PD = Primary Dysmenorrhea; BSI = Brief Symptom Inventory 18-Item Version.
Figure 1.
Flow-chart of study procedures.
Four enrolled participants (all from the control group) dropped out of the study without completing a laboratory visit (i.e., after completing just the intake visit). In addition, 7 participants (6 control, 1 PD), did not get a positive ovulation test kit result after 3 attempts and were brought in for only the menstrual phase visit, and were included in the analyses. The control group was over-recruited to compensate for participants who were anovulatory and only completed the menstrual phase visit and those who dropped out of the study. Lastly, 22 individuals (15 control, 7 PD) completed all three lab sessions but were removed from analyses because of unexpected hormonal inconsistencies. Levels of estradiol and progesterone were reviewed by first and fourth authors and a reproductive endocrinologist and it was determined that the hormonal patterns in these individuals deviated significantly enough from the typical hormonal pattern during the menstrual cycle to warrant removal from analyses. The final sample used for analyses included 66 girls and young women (34 control, 32 PD).
Written informed consent forms were completed by young adult participants, and written assent and parental permission were completed by adolescent participants and a legal guardian. The study was approved by the UCLA Institutional Review Board. Each participant received $25 for the intake visit, $50 for the first lab visit, $75 for the second lab visit, and $100 for the third lab visit.
Procedure
The majority of participants (89.1%) were recruited via mass emails sent to female university students. Additional recruitment methods included: posts on local websites (2.2%), word of mouth referrals (5.4%), and participants from previous studies (3.3%).
Eligibility was confirmed by telephone. A trained research coordinator asked potential participants whether they met any of the following exclusion criteria: 1) acute illness or injury that would potentially impact pain task performance (e.g., fever, flu symptoms) or that affect sensitivity of the extremities (e.g., Reynaud’s disease); 2) daily use of opioids at the time of study participation (participants who use other analgesics were included but were requested to not take these analgesics on the day of the laboratory session until after the session); 3) developmental delay, diagnosis of autism, or significant cognitive impairment that may preclude understanding of study procedures; 4) use of hormonal contraceptives in the previous 3 months; 5) irregular menstrual cycles (<24 or >32 days); or 6) currently pregnant. Participants were categorized as having PD if they reported an average menstrual pain rating of “4” or higher on a 0–10 (0=none; 10=worst pain possible) numeric rating scale (NRS). Healthy participants rated average menstrual pain as “3” or below on the same scale. However, we attempted to recruit individuals who rated their menstrual pain as “6” or higher for the PD group or “2” or lower for the healthy group. Eligible individuals were scheduled for an intake visit during which they provided informed assent/consent and completed questionnaires via a website (see self-report measures below). The order of the laboratory sessions for each phase (menstrual, ovulatory, mid-luteal) was counterbalanced across participants within each group. Over the course of study participation, all enrolled participants were sent text messages daily asking them to rate pain in their pelvic area on the 0 – 10 NRS to determine whether they were experiencing significant pain on non-bleeding days, which may be indicative of secondary dysmenorrhea.
For the menstrual phase session, participants were instructed to contact the study team at the start of menstruation and the lab session was scheduled to occur within 48 hours. The ovulatory session was scheduled for within 48 hours of receiving a positive result on the ovulation predictor kit. For the mid-luteal session, participants were scheduled for between 5 and 9 days after positive ovulation kit test result. If a participant did not ovulate for 3 menstrual cycles (i.e., she did not get a positive ovulation kit result after 3 attempts), she was brought in for just the menstrual phase session.
Ovulation predictor kits.
Participants completed urinary LH surge ovulation predictor kits according to manufacturer’s instructions. Start date was determined by the start date of participant’s last reported menstrual period and average cycle length. Two different brands of ovulation predictor kits were used in this study: OvuQuick One-Step Ovulation Predictor (Vitrolife, San Diego, CA, USA), and Clearblue Digital Ovulation Test (Swiss Precision Diagnostics GmbH, Geneva, Switzerland). Both kits have been used in previous menstrual phase studies [4; 50].
Laboratory pain sessions.
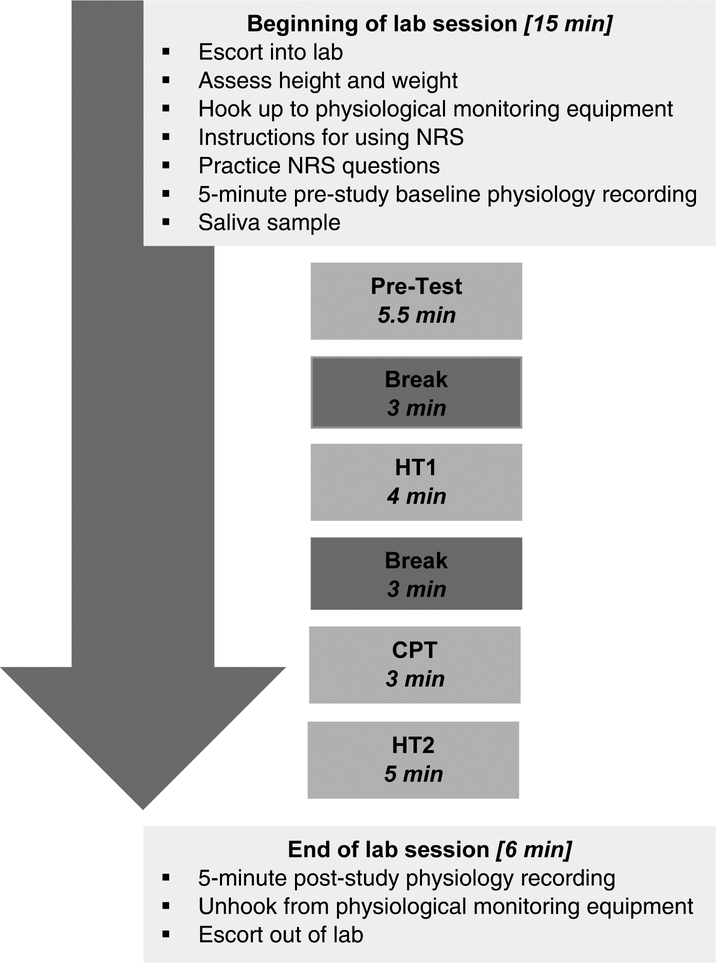
Each laboratory session was conducted in the same manner by the same female research associate. Participants completed a brief set of questionnaires and were then escorted into the laboratory where height and weight were recorded and leads for physiological recording were attached. Participants were shown the 0 (none) to 100 (worst possible) NRS (described below) and instructed on its use. Participants were told that they would use the NRS to indicate how they felt at different times during the study. Practice items for the NRS were administered to ensure participants understood the scale. Then a 5-minute habituation period for recording of baseline physiology was obtained during which participants were instructed to sit quietly and watch a neutral nature video with no sound. The pain task procedures followed those outlined by Tousignant-Laflamme et al. [40], which allow for assessment of pain sensitivity, TS, and CPM in the same laboratory session (see pain measures below). Laboratory pain session procedures are depicted in Figure 2.
Figure 2.
Laboratory pain session procedures.
Before the start of each task, participants were given instructions and the opportunity to ask questions. After the completion of the final laboratory task, there was another 5-minute period during which participants were instructed to sit quietly for recording of post-task physiology. Physiological recording equipment was them removed and participants were escorted out of the laboratory and paid for their participation.
Pretest.
A pretest using the Medoc TSA-II Neurosensory Analyzer (Medoc Ltd., Ramat Yishai, Israel) was used to familiarize participants with the thermode and to assess pain sensitivity. The TSA-II is a precise, computer-controlled device capable of generating and documenting response to highly repeatable thermal stimuli, such as heat-induced pain. The 30mm × 30mm probe is placed against participants’ skin, is heated by a Peltier thermode, and actively cooled by circulating water. The pretest consisted of four trials, during which the thermode increased in heat from 32 degrees C at a rate of 0.3 degrees C per second. Thermode placement for the pretest trials was on the right medial forearm, beginning proximal to the wrist and moving toward the elbow after each trial so as not to overlap with previous stimulation areas. For Trials 1 and 2, participants verbally indicated when they first felt the heat as painful (pain threshold), and when they could no longer keep the heat on their arm (pain tolerance). The thermal stimulus was immediately stopped when the participant indicated pain tolerance. For Trials 3 and 4, participants continuously rated pain levels using a computerized visual analog scale (COVAS; described below). The temperature at which the COVAS began moving was considered the pain threshold, and the temperature at which each participant rated pain levels as 50/100 was recorded. The average temperature to achieve a pain score of 50/100 (Pain50; see below) across Trials 3 and 4 was the temperature used for subsequent heat pain tasks. The pretest was followed by a 3-minute break during which the participant watched a neutral nature video.
Heat Task 1 (HT1).
During the first heat pain test (HT1), the thermode was applied to the participant’s left medial forearm approximately 1 inch distal to the elbow. The temperature began at 32 degrees C and rose at a rate of 0.3 degrees C per second until it reached Pain50. At that point the temperature remained constant for 120 seconds. Participants used the COVAS to continually rate the perceived pain intensity throughout the task. Heat Task 1 was followed by a 3-minute break during which the participant watched a neutral nature video.
Cold Pressor Task (CPT).
The cold pressor unit consisted of a commercial cold pressor measuring 26” wide, 32” long, and 16” deep with a screen that separates the machine components from the arm immersion area (Techne TE-10D Thermoregulator, B-8 Bath, and RU-200 Dip Cooler; Techne, Burlington, NJ, USA; now Cole-Parmer, Vernon Hills, IL, USA). Water temperature was kept constant by a pump that circulated the water to prevent local warming around the hand. Participants were instructed to immerse their right hand to a depth of 2 inches above the wrist in 7 degree C water for two minutes, but that if they absolutely couldn’t keep their hand in for the full two minutes that they may take it out. Every 15 seconds throughout the immersion, participants were prompted to verbally rate the amount of pain they felt from the cold water stimulus using the 0–100 NRS (see below).
Heat Task 2 (HT2).
Immediately following the CPT, the TSA-II thermode was placed back on the participant’s left arm (this time 2 inches distal to the elbow), and the task was repeated identically as in HT1.
Saliva Samples.
Saliva samples were collected at various time points throughout each lab session using the passive drool technique. Participants were prompted to imagine their favorite food, let the saliva collect in their mouth, and then spit the saliva through a piece of straw and into a 2.0mL cryovial. Each sample was collected until a volume of 1.5mL had been obtained or until 5 minutes had passed, whichever came first. Measures of salivary estradiol and progesterone were assayed from the first sample (S1) at Arizona State University’s Institute for Interdisciplinary Salivary Bioscience Research (IISBR, Tempe, AZ, USA) and at Salimetrics, Inc. (Carlsbad, CA, USA).
Self-report Measures.
Psychosocial functioning was assessed using the 18-item version of the Brief Symptom Inventory (BSI-18) [10]. The BSI-18 consists of three subscales: depression, anxiety, and somatization. Menstrual pain characteristics were assessed through a short menstrual history questionnaire designed for the purposes of this study. This questionnaire was completed during the intake visit and inquired about the severity of their menstrual pain (NRS), as well as age at menarche age.
Laboratory Pain Measures
Numeric rating scale (NRS).
A 0 (none) to 100 (worst possible) NRS was used by participants to rate pain levels throughout the laboratory assessment. Ratings were made either verbally (as in during CPT), or using a computerized visual analog scale (COVAS). This sliding bar (variable assessment transducer, TSD-115, BIOPAC Systems, Inc., Goleta, CA, USA) was anchored from 0 (no pain) to 100 (worst pain possible) and was used throughout the lab session for participants to rate heat pain levels. Output from the COVAS was displayed and recorded on the lab laptop in real time during Pretest, HT1, and HT2.
Heat pain tolerance.
Pain tolerance for the pretest was determined during Pretest Trials 1 and 2 and was defined as the temperature, in degrees C, at which the participant requested that the stimulus be stopped. Before the task, participants were instructed to keep the heat on their arm for as long as they could and that they should say “done” when they could no longer keep the heat on their arm. The heat stimulus was immediately stopped by the researcher when the participant said “done.” If participant reached the 51 degrees C safety limit before reaching tolerance, the heat was stopped by the researcher and the participant was informed that that part of the task was over. Pain tolerance was not assessed during Trials 3 and 4; during these trials, the researcher stopped the stimulus approximately 5 seconds after reaching 50/100 on the COVAS.
Average Pain50 (P50).
P50 was defined as the average temperature during Pretest Trials 3 and 4 at which the participant rated the pain as 50 out of 100 on the COVAS.
Cold pain tolerance.
Pain tolerance for the CPT task was defined as the amount of time, in seconds, elapsed from the onset of the pain stimulus to participants’ withdrawal from the stimulus. Before the task, participants were instructed to keep their hand in the cold water for two minutes (i.e., 2-minute informed ceiling), but that if they absolutely could not keep their hand in any longer that they may take it out at any time. Participants were instructed to remove their hand from the cold water after two minutes had passed if they had not already done so.
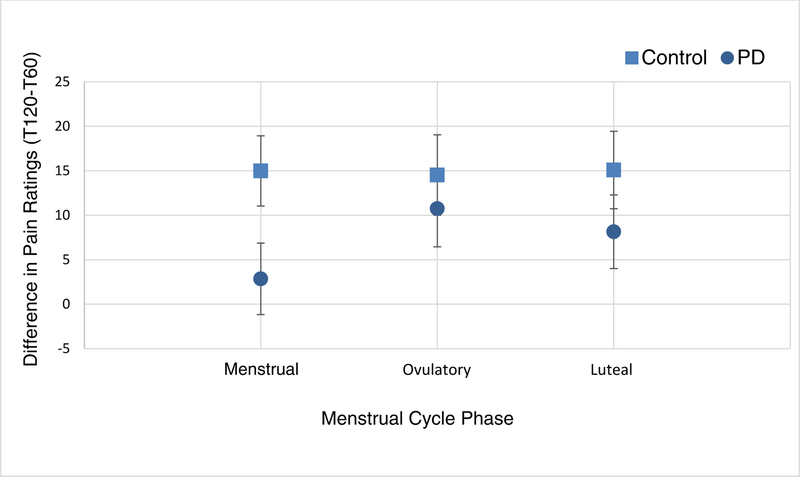
Temporal Summation (TS).
The difference in COVAS pain ratings between the 120-second point (T120) and the 60-second point (T60; i.e., T120-T60) of HT1 [40].
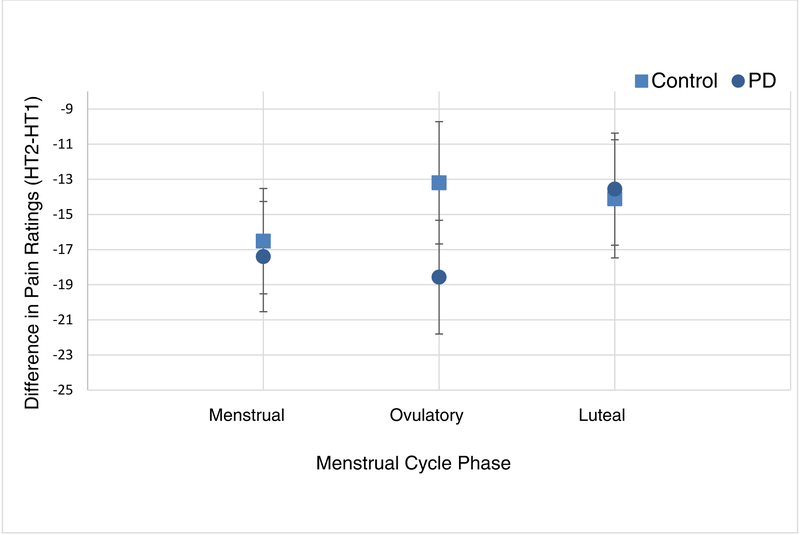
Conditioned Pain Modulation (CPM).
The difference in maximum COVAS pain ratings during the first 30 seconds at the destination temperature (i.e., the difference between pain ratings at HT2 and HT1; HT2-HT1) [40].
Statistical Analysis
A priori sample size calculation was based on a comparison of mean suprathreshold pain intensity ratings in response to the heat task between the PD and control groups [17]. Using a two-sided two-sample t-test with a 0.05 significance level, a sample size of 24 in each group will provide 85% power to detect a significant difference in pain intensity. Univariable analyses were conducted to examine demographic differences between the groups. Validation of group assignment was evidenced by significant differences on self-reported average menstrual pain without medication (control: 0.63 ± 0.74, PD: 7.24 ± 1.61; P<.0001). There were no significant differences in demographic variables (age, age at menarche, and race/ethnicity) or self-reported somatization, depression, or anxiety (as measured by the BSI-18) between the two groups, with the exception of rates of participants identifying as Hispanic/Latino (control 14.7% vs PD 37.5%) (see Table 1). Additionally, in the PD group, the average self-reported pelvic pain during non-bleeding days was 0.17 (SD = .65) on the 0 – 10 NRS, which is well below the average reported daily pain for women with endometriosis [37].
Baseline demographic characteristics were compared using a two-sample t test or Wilcoxon two-sample test for continuous variables and chi square tests for categorical variables. As a first step to examine whether there was a difference between control group and PD group and whether there is a change in laboratory pain measures (NRS, Heat pain tolerance, P50, Cold pain tolerance, TS and CPM) across the three menstrual cycle phases, we plotted the course of each laboratory measurement for each group over the three phases.
After the visual data exploration, we analyzed each of the laboratory pain measures as an outcome using mixed effect models with random intercept. The initial model included age, age at menarche, BMI, race, menstrual cycle phase at time of testing, and group (PD vs. control) as covariates. The final models for each laboratory pain measure that included phase and group as covariates were obtained after carefully examining the model fits and doing likelihood ratio tests. All tests were 2 sided, and all analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA)
Results
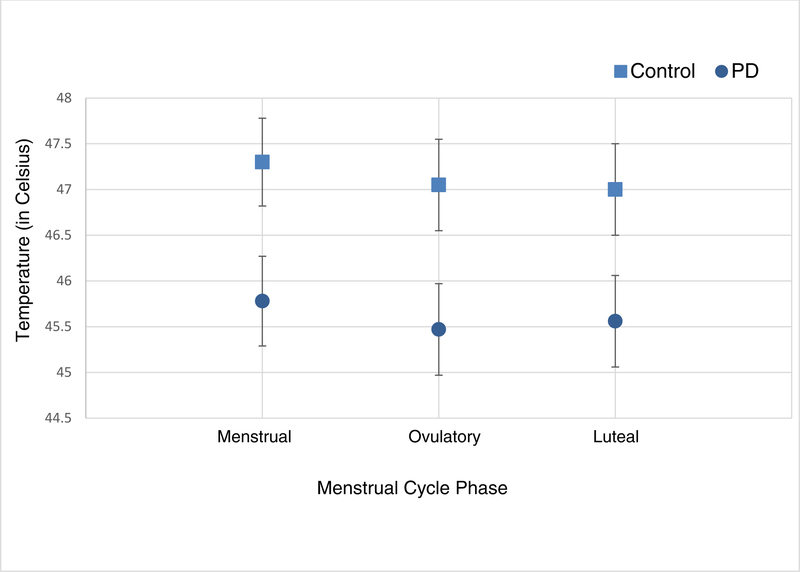
As show in Table 2, the PD group demonstrated significantly lower (by 1.4 degrees C) heat pain tolerance compared to the control group when adjusting for menstrual cycle phase. The heat pain tolerance was higher at mid-luteal phase compared to menstrual and ovulatory phases, but the difference was not statistically significant. Similarly, throughout the three different phases, the PD group consistently showed significantly (P=0.022) lower average pain50 scores by −1.52 (SE: 0.64) and the values did not significantly differ across 3 different phases (P=0.376) (Table 2 and Figure 3). Average pain50 was highest in the menstrual phase compared to either the ovulatory or mid-luteal phase but the difference was not statistically significant.
Table 2.
Estimated means and standard errors from mixed effect models comparing Control and PD groups on laboratory pain measures across menstrual cycle phases
| Heat Pain Tolerance | Average Pain50 | Cold Pain Tolerance | Temporal Summation | Conditioned Pain Modulation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phases | Control | PD | Control | PD | Control | PD | Control | PD | Control | PD |
| Menstrual | 47.46 (0.40) | 46.30 (0.41) | 47.30 (0.48) | 45.78 (0.49) | 96.95 (7.04) | 80.99 (7.25) | 14.98 (3.95) | 2.85 (4.02) | −16.52 (3.00) | −17.40 (3.14) |
| Ovulatory | 47.74 (0.42) | 46.07 (0.42) | 47.05 (0.50) | 45.47 (0.50) | 93.99 (7.30) | 81.32 (7.32) | 14.52 (4.52) | 10.74 (4.28) | −13.20 (3.48) | −18.57 (3.24) |
| Luteal | 47.67 (0.42) | 46.19 (0.42) | 47.00 (0.50) | 45.56 (0.50) | 95.42 (7.27) | 87.15 (7.32) | 15.08 (4.35) | 8.14 (4.14) | −14.11 (3.36) | −13.56 (3.19) |
| (P values) | Control vs PD: (0.011) Across phases: (0.975) |
Control vs PD: (0.022) Across phases: (0.376) |
Control vs PD: (0.193) Across phases: (0.413) |
Control vs PD: (0.056) Across phases: (0.548) |
Control vs PD: (0.559) Across phases: (0.535) |
|||||
Note. PD = Primary dysmenorrhea.
Figure 3.
Estimated means and standard errors from mixed effect model for temperature required to achieve an average pain rating of 50/100. Note. PD = primary dysmenorrhea.
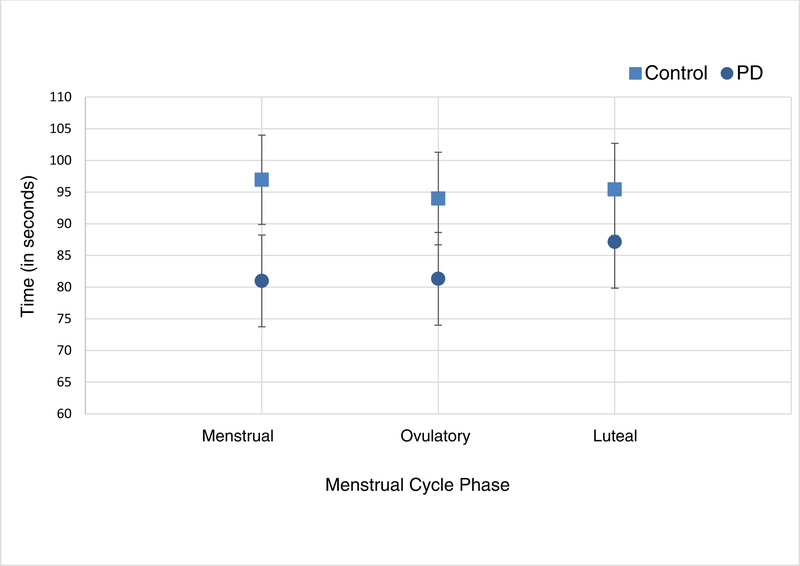
Across the three different menstrual cycle phases no statistical difference in cold pain tolerance was observed between PD and control groups (P=0.193), or among menstrual cycle phases (P=0.413) (Table 2 and Figure 4). Though the difference cold pain tolerance between the PD and control groups was greatest during the menstrual phase and smallest in the mid-luteal phase, those findings were not statistically significant (P=0.401) as tested by the interaction term of group by phase in the mixed effects model.
Figure 4.
Estimated means and standard errors from mixed effect model for average cold pain tolerance (in seconds) by group across menstrual cycle phases. Note. PD = primary dysmenorrhea.
Though not statistically significant after Tukey’s multiple comparison adjustment, some difference (P=0.03) in the amount of TS was observed at the menstrual phase between PD and control groups ((80.99 (SE:7.25) and 96.95 (SE:7.04), respectively) (Table 2 and Figure 5). Such difference was not observed during ovulatory (p=0.545) and mid-luteal (p=0.251) phases. Overall, TS was not significantly different between PD and control groups (P=0.057) or among the three menstrual cycle phases (P=0.548). The group by phase interaction term was not significant (P=0.516) in the mixed effect model showing no significant difference in TS trajectory over the three menstrual cycle phases between the groups.
Figure 5.
Estimated means and standard errors from mixed effect model for average temporal summation by group across menstrual cycle phases. Note. PD = primary dysmenorrhea.
No statistical difference was observed in CPM between PD and control groups (P=0.559) during any of the three menstrual cycle phases, and no significant fluctuation was observed across menstrual cycle phases (P=0.535). The group by phase interaction term was not significant (P=0.602) in the mixed effect model showing no significant difference in CPM over the three cycle phases between the groups (Table 2 and Figure 6).
Figure 6.
Estimated means and standard errors from mixed effect model for average conditioned pain modulation (CPM) by group across menstrual cycle phases. Note. PD = primary dysmenorrhea.
Discussion
The current study is the first published report of experimental excitatory and inhibitory pain testing in adolescent girls and young adult women with and without PD. Results demonstrated that participants with PD showed heightened pain sensitivity, as indicated by significantly lower heat pain tolerance, as compared to participants without PD. This difference held true across the menstrual, ovulatory, and mid-luteal phases. However, contrary to hypotheses, girls with PD did not show differences in measures of cold pain tolerance, TS, or CPM compared to girls without PD, during any of the three menstrual cycle phases. These results may have important implications for understanding the mechanisms of pain in primary dysmenorrhea.
Our findings of enhanced pain sensitivity irrespective of cycle phase supports much of the existing research in adult women with PD. Although not all results consistently show differences in pain sensitivity between women with and without PD (see [30] for review), generally there are group differences across multiple modes of stimuli including, heat [4; 46; 49], pressure [4; 18; 35], and cold [35; 53] pain. However, a major limitation of existing research is that it remains unclear whether enhanced pain sensitivity develops as a function of repeated episodes of pain or whether these differences in girls with PD may reflect a more stable, central sensitization to pain. Ongoing research has begun to identify genetic [11; 48], physiological [28], and symptom-based phenotypes [6] in women with PD; however, further research in this area is warranted, particularly with adolescents within the first 2–3 yeas of starting menstruation, to clearly determine how, when, and for whom these alterations develop.
Contrary to our hypotheses, we did not find any group differences on measures of TS or CPM – potential indicators of the body’s excitatory and inhibitory pain processes. This is inconsistent with the very small literature on these measures examining adult women with PD. One study found that women with PD showed increasing levels of pain over the course of a prolonged period of distension of the uterine cervix, as compared to adult women without PD [3]. However, this study was examining TS in the painful area (uterine cervix) whereas our study focused on remote areas of pain testing (forearm), which may explain the different findings. No studies to our knowledge have examined CPM in girls or women with menstrual pain.
There may be a number of reasons for this finding. First, given that our study sample size was powered using established group differences in heat pain sensitivity only, it is possible that the study was underpowered to detect differences in cold pain tolerance, TS, and CPM. Sample heterogeneity due to potential inclusion of participants with secondary dysmenorrhea may have also prevented group differences from emerging. Other explanations may be that, although central changes as evidenced by measures of brain structure and function may exist in adolescents and younger women with PD as they do in adult women [24; 25; 41–43; 46; 47; 51], these changes are not reflected in behavioral measures of TS and CPM.
Another intriguing possibility is that enhanced TS and deficient CPM develop as a function of increased repeated episodes of pain and therefore are not evident yet in younger populations. One recent study found adolescents ages 12–17 with migraine and family history of migraine showed enhanced pain sensitivity compared to healthy controls, with no differences in CPM [27]. The authors suggest that CPM may not be a phenotypic mechanism of migraine, which may also be true of PD. Alternatively, if altered pain processing as evidenced by enhanced pain sensitivity is a potential mechanism of chronic pain in adolescents, the development of early interventions to prevent pain episodes in young populations is still warranted. Women with fibromyalgia and comorbid dysmenorrhea have shown elevated muscle pain sensitivity compared to women with fibromyalgia only; although after dysmenorrhea was treated hormonally, levels of pain sensitivity significantly decreased [7]. These data highlight the role of menstrual pain and other visceral pain comorbidities as a factor that potentially intensifies central sensitization in those with chronic pain. But perhaps what is even more significant is that effective treatment of visceral pain conditions early on may actually help reduce triggers for chronic pain. If menstrual pain is a visceral trigger for central sensitization of pain, girls at risk must be treated quickly and effectively to prevent the development of chronic pain.
Another potential explanation is that CPM differences exist only in a subgroup of girls. Though deficient CPM has been demonstrated in many chronic pain populations, research suggests that this difference is not consistent across all individuals with chronic pain. One recent study found that a subgroup of patients with fibromyalgia experienced pain facilitation during a CPM procedure, while another subgroup experienced pain inhibition [31]. Other chronic pain conditions such as irritable bowel syndrome and provoked vestibulodynia also show altered CPM, but only in a subgroup of patients [14]. In PD, the clear next step is to gather detailed phenotypic data to predict the behavioral, hormonal, and neural signatures that are associated with ongoing pain and, if feasible, follow these young women over time. Another possibility is that central sensitization is present in adolescent girls with PD, but our current way of assessing this phenomenon (through TS and CPM) is incomplete. The current study used an established protocol that would allow for assessment of TS and CPM in a single session [40] and has been used to assess differences in pain responsivity across the menstrual cycle [39]. However, it did not assess pain responses in multiple body areas (such as the lower abdomen) or with multiple methods, both of which may be important to more conclusively identify efficient or deficient pain modulation. Future research should continue to develop novel methods across a range of body sites to assess central sensitization.
Consistent with the majority of other reports [2; 12; 13; 17; 20; 21; 23; 44; 49], we found significant group differences in heat pain responses across all cycle phases. Although some recent studies have shown enhanced pain modulation (indicative of successful pain inhibition) in healthy women during the ovulatory phase [34], most laboratory research in women with menstrual pain has demonstrated either no group differences or heightened pain sensitivity in women with PD across the menstrual cycle (for review see [30]), suggesting the stability of group differences independent of hormonal variations. It is possible that our assessment of the menstrual cycle phase was not accurate and consistent across all study participants, such that menstrual cycle-related changes were obscured. However, given the relative consistency of these findings in previous studies [30], this appears to be a less likely explanation.
There are a number of limitations to the study. First, PD was determined based on self-reported menstrual history and did not include a pelvic exam or ultrasound. Although this is typically how PD is diagnosed, a physical exam by a clinician may have identified other factors that would suggest a diagnosis of secondary dysmenorrhea due to endometriosis or other pelvic pain condition. Our screening questions attempted to gain information that would suggest secondary dysmenorrhea instead of PD, and no participants reported significant pelvic pain outside of menstruation. However, these measures are not diagnostic. Second, ovulation predictor kits were used to determine ovulation, but the rise in luteinizing hormone (LH) may occur after ovulation has already happened. A number of girls may have unintentionally been assessed post-ovulation, and this imprecise period of assessment may have introduced too much variability in pain responses. This could explain why no differences in CPM across the menstrual cycle were identified, in contrast some previous studies [19]. Additionally, the study population included a relatively wide age range, that may have introduced additional variation. Although we controlled for age and age of menarche in our analyses, it is possible that additional factors such as consistency and frequency of menstrual pain across puberty may be important variables, because some girls and women have experienced more repeated episodes of pain. Due to inclusion and exclusion criteria, we obtained a very “clean” sample of participants that did not report having any other chronic or recurrent pain conditions. Our exclusionary criteria therefore excluded participants with other chronic pain problems who would have demonstrated enhanced TS or deficient CPM [8]. Our heterogenous sample, as well as potential overrepresentation of Asian participants, may limit generalizability to the population of the United States.
The current study found enhanced pain sensitivity in adolescent girls and young adult women with PD across all phases of the menstrual cycle consistent with evidence of central sensitization. However, measures of excitatory and inhibitory pain processing did not reveal any group differences. Further research in this population is warranted, as central changes in how the brain processes pain may be important in better managing pain of PD and also serve as a risk factor for chronic pain problems. Identifying those at risk for developing chronic pain would allow for the delivery of targeted treatments.
Acknowledgements
This research was supported by grants from the National Institute of Child Health and Human Development (K23HD077042; PI: Laura A. Payne), and the National Center for Advancing Translational Sciences UCLA Clinical and Translational Science Institute (KL2TR000122; PI: Laura A. Payne).
Footnotes
The authors have no conflicts of interest to declare.
References
- [1].Aberger EW, Denney DR, Hutchings DF. Pain sensitivity and coping strategies among dysmenorrheic women: much ado about nothing. Behav Res Ther 1983;21(2):119–127. [DOI] [PubMed] [Google Scholar]
- [2].Amodei N, Nelson-Gray RO. Reactions of dysmenorrheic and nondysmenorrheic women to experimentally induced pain throughout the menstrual cycle. J Behav Med 1989;12(4):373–385. [DOI] [PubMed] [Google Scholar]
- [3].Arendt-Nielsen L, Madsen H, Jarrell J, Gregersen H, Drewes AM. Pain evoked by distension of the uterine cervix in women with dysmenorrhea: evidence for central sensitization. Acta Obstet Gynecol Scand 2014;93(8):741–748. [DOI] [PubMed] [Google Scholar]
- [4].Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clin J Pain 2002;18(3):180–190. [DOI] [PubMed] [Google Scholar]
- [5].Brinkert W, Dimcevski G, Arendt-Nielsen L, Drewes AM, Wilder-Smith OH. Dysmenorrhoea is associated with hypersensitivity in the sigmoid colon and rectum. Pain 2007;132 Suppl 1:S46–51. [DOI] [PubMed] [Google Scholar]
- [6].Chen CX, Ofner S, Bakoyannis G, Kwekkeboom KL, Carpenter JS. Symptoms-Based Phenotypes Among Women With Dysmenorrhea: A Latent Class Analysis. West J Nurs Res 2018;40(10):1452–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Costantini R, Affaitati G, Wesselmann U, Czakanski P, Giamberardino MA. Visceral pain as a triggering factor for fibromyalgia symptoms in comorbid patients. Pain 2017;158(10):1925–1937. [DOI] [PubMed] [Google Scholar]
- [8].Damien J, Colloca L, Bellei-Rodriguez CE, Marchand S. Pain Modulation: From Conditioned Pain Modulation to Placebo and Nocebo Effects in Experimental and Clinical Pain. Int Rev Neurobiol 2018;139:255–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Davis AR, Westhoff CL. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J Pediatr Adolesc Gynecol 2001;14(1):3–8. [DOI] [PubMed] [Google Scholar]
- [10].Derogatis LR. Brief Symptom Inventory (BSI) 18 Administration, Scoring, and Procedures Manual: Pearson, 2001. [Google Scholar]
- [11].Dogru HY, Ozsoy AZ, Karakus N, Delibas IB, Isguder CK, Yigit S. Association of Genetic Polymorphisms in TNF and MIF Gene with the Risk of Primary Dysmenorrhea. Biochem Genet 2016;54(4):457–466. [DOI] [PubMed] [Google Scholar]
- [12].Giamberardino MA, Berkley KJ, Iezzi S, de Bigontina P, Vecchiet L. Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non-dysmenorrheic women, dysmenorrheic women and men. Pain 1997;71(2):187–197. [DOI] [PubMed] [Google Scholar]
- [13].Goolkasian P An ROC analysis of pain reactions in dysmenorrheic and nondysmenorrheic women. Percept Psychophys 1983;34(4):381–386. [DOI] [PubMed] [Google Scholar]
- [14].Gougeon V, Gaumond I, Goffaux P, Potvin S, Marchand S. Triggering Descending Pain Inhibition by Observing Ourselves or a Loved-One in Pain. Clin J Pain 2016;32(3):238–245. [DOI] [PubMed] [Google Scholar]
- [15].Grandi G, Ferrari S, Xholli A, Cannoletta M, Palma F, Romani C, Volpe A, Cagnacci A. Prevalence of menstrual pain in young women: what is dysmenorrhea? J Pain Res 2012;5:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Granot M, Granovsky Y, Sprecher E, Nir RR, Yarnitsky D. Contact heat-evoked temporal summation: tonic versus repetitive-phasic stimulation. Pain 2006;122(3):295–305. [DOI] [PubMed] [Google Scholar]
- [17].Granot M, Yarnitsky D, Itskovitz-Eldor J, Granovsky Y, Peer E, Zimmer EZ. Pain perception in women with dysmenorrhea. Obstet Gynecol 2001;98(3):407–411. [DOI] [PubMed] [Google Scholar]
- [18].Haman JO. Pain threshold in dysmenorrhea. Am J Obstet Gynecol 1944;47:686–691. [Google Scholar]
- [19].Hermans L, Van Oosterwijck J, Goubert D, Goudman L, Crombez G, Calders P, Meeus M. Inventory of Personal Factors Influencing Conditioned Pain Modulation in Healthy People: A Systematic Literature Review. Pain Pract 2016;16(6):758–769. [DOI] [PubMed] [Google Scholar]
- [20].Iacovides S, Avidon I, Baker FC. Women with dysmenorrhoea are hypersensitive to experimentally induced forearm ischaemia during painful menstruation and during the pain-free follicular phase. Eur J Pain 2014. [DOI] [PubMed] [Google Scholar]
- [21].Iacovides S, Baker FC, Avidon I, Bentley A. Women with dysmenorrhea are hypersensitive to experimental deep muscle pain across the menstrual cycle. J Pain 2013;14(10):1066–1076. [DOI] [PubMed] [Google Scholar]
- [22].Kisler LB, Granovsky Y, Coghill RC, Sprecher E, Manor D, Yarnitsky D, Weissman-Fogel I. Do patients with interictal migraine modulate pain differently from healthy controls? A psychophysical and brain imaging study. Pain 2018;159(12):2667–2677. [DOI] [PubMed] [Google Scholar]
- [23].Lee LC, Tu CH, Chen LF, Shen HD, Chao HT, Lin MW, Hsieh JC. Association of brain-derived neurotrophic factor gene Val66Met polymorphism with primary dysmenorrhea. PLoS One 2014;9(11):e112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li WC, Tu CH, Chao HT, Yeh TC, Chen LF, Hsieh JC. High prevalence of incidental brain findings in primary dysmenorrhoea. Eur J Pain 2015;19(8):1071–1074. [DOI] [PubMed] [Google Scholar]
- [25].Liu P, Yang J, Wang G, Liu Y, Liu X, Jin L, Liang F, Qin W, Calhoun VD. Altered regional cortical thickness and subcortical volume in women with primary dysmenorrhoea. Eur J Pain 2016;20(4):512–520. [DOI] [PubMed] [Google Scholar]
- [26].Nahman-Averbuch H, Granovsky Y, Coghill RC, Yarnitsky D, Sprecher E, Weissman-Fogel I. Waning of “conditioned pain modulation”: a novel expression of subtle pronociception in migraine. Headache 2013;53(7):1104–1115. [DOI] [PubMed] [Google Scholar]
- [27].Nahman-Averbuch H, Leon E, Hunter BM, Ding L, Hershey AD, Powers SW, King CD, Coghill RC. Increased pain sensitivity but normal pain modulation in adolescents with migraine. Pain 2019. [DOI] [PubMed] [Google Scholar]
- [28].Oladosu FA, Tu FF, Farhan S, Garrison EF, Steiner ND, Roth GE, Hellman KM. Abdominal skeletal muscle activity precedes spontaneous menstrual cramping pain in primary dysmenorrhea. Am J Obstet Gynecol 2018;219(1):91 e91–91 e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oladosu FA, Tu FF, Hellman KM. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol 2018;218(4):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Payne LA, Rapkin AJ, Seidman LC, Zeltzer LK, Tsao JC. Experimental and procedural pain responses in primary dysmenorrhea: a systematic review. J Pain Res 2017;10:2233–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Potvin S, Marchand S. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. Pain 2016;157(8):1704–1710. [DOI] [PubMed] [Google Scholar]
- [32].Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. J Invest Dermatol 1977;69(1):167–171. [DOI] [PubMed] [Google Scholar]
- [33].Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 2002;99(1–2):49–59. [DOI] [PubMed] [Google Scholar]
- [34].Rezaii T, Hirschberg AL, Carlstrom K, Ernberg M. The influence of menstrual phases on pain modulation in healthy women. J Pain 2012;13(7):646–655. [DOI] [PubMed] [Google Scholar]
- [35].Slater H, Paananen M, Smith AJ, O’Sullivan P, Briggs AM, Hickey M, Mountain J, Karppinen J, Beales D. Heightened cold pain and pressure pain sensitivity in young female adults with moderate-to-severe menstrual pain. Pain 2015;156(12):2468–2478. [DOI] [PubMed] [Google Scholar]
- [36].Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ Jr. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain 2003;102(1–2):87–95. [DOI] [PubMed] [Google Scholar]
- [37].Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, Diamond MP, Surrey E, Johnson NP, Watts NB, Gallagher JC, Simon JA, Carr BR, Dmowski WP, Leyland N, Rowan JP, Duan WR, Ng J, Schwefel B, Thomas JW, Jain RI, Chwalisz K. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N Engl J Med 2017;377(1):28–40. [DOI] [PubMed] [Google Scholar]
- [38].Teepker M, Kunz M, Peters M, Kundermann B, Schepelmann K, Lautenbacher S. Endogenous pain inhibition during menstrual cycle in migraine. Eur J Pain 2014;18(7):989–998. [DOI] [PubMed] [Google Scholar]
- [39].Tousignant-Laflamme Y, Marchand S. Excitatory and inhibitory pain mechanisms during the menstrual cycle in healthy women. Pain 2009;146(1–2):47–55. [DOI] [PubMed] [Google Scholar]
- [40].Tousignant-Laflamme Y, Page S, Goffaux P, Marchand S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Res 2008;1230:73–79. [DOI] [PubMed] [Google Scholar]
- [41].Tu CH, Niddam DM, Chao HT, Chen LF, Chen YS, Wu YT, Yeh TC, Lirng JF, Hsieh JC. Brain morphological changes associated with cyclic menstrual pain. Pain 2010;150(3):462–468. [DOI] [PubMed] [Google Scholar]
- [42].Tu CH, Niddam DM, Chao HT, Liu RS, Hwang RJ, Yeh TC, Hsieh JC. Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. Neuroimage 2009;47(1):28–35. [DOI] [PubMed] [Google Scholar]
- [43].Tu CH, Niddam DM, Yeh TC, Lirng JF, Cheng CM, Chou CC, Chao HT, Hsieh JC. Menstrual pain is associated with rapid structural alterations in the brain. Pain 2013;154(9):1718–1724. [DOI] [PubMed] [Google Scholar]
- [44].Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A noninvasive bladder sensory test supports a role for dysmenorrhea increasing bladder noxious mechanosensitivity. Clin J Pain 2013;29(10):883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain 2010;11(5):408–419. [DOI] [PubMed] [Google Scholar]
- [46].Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain 2011;152(9):1966–1975. [DOI] [PubMed] [Google Scholar]
- [47].Wei SY, Chao HT, Tu CH, Li WC, Low I, Chuang CY, Chen LF, Hsieh JC. Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain 2016;157(1):92–102. [DOI] [PubMed] [Google Scholar]
- [48].Wei SY, Chao HT, Tu CH, Lin MW, Li WC, Low I, Shen HD, Chen LF, Hsieh JC. The BDNF Val66Met polymorphism is associated with the functional connectivity dynamics of pain modulatory systems in primary dysmenorrhea. Sci Rep 2016;6:23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wei SY, Chen LF, Lin MW, Li WC, Low I, Yang CJ, Chao HT, Hsieh JC. The OPRM1 A118G polymorphism modulates the descending pain modulatory system for individual pain experience in young women with primary dysmenorrhea. Sci Rep 2017;7:39906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Williams CD, Boggess JF, LaMarque LR, Meyer WR, Murray MJ, Fritz MA, Lessey BA. A prospective, randomized study of endometrial telomerase during the menstrual cycle. J Clin Endocrinol Metab 2001;86(8):3912–3917. [DOI] [PubMed] [Google Scholar]
- [51].Wu TH, Tu CH, Chao HT, Li WC, Low I, Chuang CY, Yeh TC, Cheng CM, Chou CC, Chen LF, Hsieh JC. Dynamic Changes of Functional Pain Connectome in Women with Primary Dysmenorrhea. Sci Rep 2016;6:24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yarnitsky D Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23(5):611–615. [DOI] [PubMed] [Google Scholar]
- [53].Ye R, Wang S, Li Y, Wu R, Pei J, Wang J, Zhao Z. Primary dysmenorrhea is potentially predictive for initial orthodontic pain in female patients. Angle Orthod 2014;84(3):424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]