Abstract
The mechanisms responsible for the persistence of chemotherapy-induced peripheral neuropathy (CIPN) in a significant proportion of cancer survivors are still unknown. Our previous findings show that CD8+ T cells are necessary for the resolution of paclitaxel-induced mechanical allodynia in male mice. In the present study, we demonstrate that CD8+ T cells are not only essential for resolving cisplatin-induced mechanical allodynia, but also to normalize spontaneous pain, numbness, and the reduction in intra-epidermal nerve fiber density in male and female mice. Resolution of CIPN was not observed in Rag2−/− mice that lack T and B cells. Reconstitution of Rag2−/− mice with CD8+ T cells prior to cisplatin treatment normalized the resolution of CIPN. In vivo education of CD8+ T cells by cisplatin was necessary to induce resolution of CIPN in Rag2−/− mice because adoptive transfer of CD8+ T cells from naïve WT mice to Rag2−/− mice after completion of chemotherapy did not promote resolution of established CIPN. The CD8+ T cell-dependent resolution of CIPN does not require epitope recognition by the T cell receptor (TCR). Moreover, adoptive transfer of cisplatin-educated CD8+ T cells to Rag2−/− mice prevented CIPN development induced by either cisplatin or paclitaxel, indicating that the activity of the educated CD8+ T is not cisplatin-specific.
In conclusion, resolution of CIPN requires in vivo education of CD8+ T cells by exposure to cisplatin. Future studies should examine whether ex vivo CD8+ T cell education could be applied as a therapeutic strategy for treating or preventing CIPN in patients.
Keywords: chemotherapy-induced peripheral neuropathy, cisplatin, CD8+ T cells
1. INTRODUCTION
Chronic pain affects between 11%–40% of Americans [28; 46]. Chronic pain results from abnormal activity of the neurons of the nociceptive pathway within the dorsal root ganglia (DRG) and the central nervous system [64]. Increasing evidence suggests a role for non-neuronal cells in chronic pain [27; 53; 69]. Notably, immune cells have been shown to be important regulators of the transition from acute to chronic pain [53]. Activated macrophages and microglia release pro-inflammatory factors which sensitize neurons in DRG and spinal cord leading to increased pain signaling [5; 68]. Less studied, but likely as important, is the role of anti-inflammatory macrophages, which promote resolution of pain [3; 15; 67]. The contribution of the adaptive immune system to chronic pain is less clear, and the role of T cells in chronic pain is especially debated. Studies have reported that depletion of T cells attenuates allodynia in response to peripheral nerve injury in rodents [10; 14]; in models of neuropathic pain induced by nerve injury or chemotherapy and in a model of inflammatory pain associated with rheumatoid arthritis, we and others have shown that the absence of T cells does not affect the onset of allodynia and even worsens the intensity or duration of allodynia [1; 2; 30; 36; 52; 61]. In addition, evidence suggests that the contribution of T cells to neuropathic and inflammatory pain may be sex-specific [58].
Most of the efforts to study the role of T cells in pain has been focused on CD4+ T cells [1; 14; 61]. However, we demonstrated recently that CD8+ T cells but not CD4+ T cells are necessary for the resolution of mechanical allodynia in mice treated with paclitaxel [30]. In male Rag1−/− mice (lacking mature B and T cells), allodynia was significantly prolonged after paclitaxel treatment. Reconstitution of male Rag1−/− mice with CD8+ T cells before paclitaxel treatment normalized resolution of allodynia.
Understanding the mechanisms underlying resolution of chemotherapy-induced peripheral neuropathy (CIPN) is important, because CIPN does not resolve after treatment cessation in 20%–30% of patients and can persist for years [45; 54]. To study the underlying mechanisms of persistent CIPN, we used a mouse model of CIPN induced by cisplatin, a platinum-based drug used to treat solid tumors [21; 44]. Our mouse model of CIPN shows spontaneous pain, mechanical allodynia and peripheral numbness [31; 39; 65; 70]. At the structural level, cisplatin-induced peripheral neuropathy is associated with a reduction in density of intra-epidermal nerve fibers (IENF) [31; 55].
Here, we first tested the hypothesis that CD8+ T cells are essential for the resolution of all signs of CIPN in female and male mice treated with cisplatin. Second, we tested the hypothesis that CD8+ T cells need to be educated by cisplatin in order to be capable of resolving CIPN.
2. METHODS
2.1. Animal model
Female and male (10–14 weeks old) offspring of wild type (WT), Rag2−/− (no mature T and B cells), Ifng−/−, and OT-I (major histocompatibility complex class I-restricted, ovalbumin-specific CD8+ T cell transgenic) mice in a C57BL/6J background obtained from Jackson Laboratory (Bar Harbor, Maine) were housed and bred at The University of Texas MD Anderson Cancer Center. All procedures were approved by the MD Anderson Animal Care and Use Committee. All protocols conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 86–23) and ARRIVE guidelines.
To induce CIPN, mice were treated intraperitoneally with cisplatin (Teva Pharmaceutical Industries Limited, North Wales, Pennsylvania) for 3 days at a dose of 2 mg/kg (cumulative dose 6 mg/kg) or paclitaxel on day 0 and 2 at a dose of 2 mg/kg. Phosphate-buffered saline (PBS) was used as vehicle. Animals were randomly assigned to the different treatment groups, and measures were taken by investigators blinded to treatment.
2.2. Adoptive transfer
Adoptive transfer of CD8+ T cells was performed before or after chemotherapy injection, as previously described [30; 33]. Spleens were collected from WT, OT-I, or Ifng−/− mice, and single-cell suspensions were obtained by passing spleens though a 70-μm mesh. CD8+ T cells were isolated using negative selection kit II (#130-095-130, Miltenyi Biotec Inc, San Diego, California). Three million CD8+ T cells were intravenously (IV) injected into the tail vein in a 100-μL volume. Control mice received an IV injection of vehicle (PBS plus 0.1% bovine serum albumin). Homing and survival of the adoptively transferred cells were confirmed by flow cytometry and quantitative real-time polymerase chain reaction (qRT-PCR). Educated CD8+ T cells were obtained when WT mice had recovered from CIPN, 8 weeks after 5 daily injections of cisplatin 2 mg/kg (cumulative dose 10 mg/kg).
2.3. Flow cytometry
Blood obtained by cardiac puncture was collected into heparinized tubes. Leukocytes were stained with anti-CD45-APC (#561018; BD Biosciences, San Jose, California) and T cells were labeled with anti-CD3-PE and anti-CD8a-FITC (#561799 and #553031; BD Biosciences). Red blood cells were lysed using lysing buffer (#555899; BD Biosciences) and samples were analyzed by flow cytometry (C6 Accuri; BD Biosciences) as described [30; 33].
2.4. Behavioral assessment
2.4.1. Mechanical allodynia
Mechanical allodynia was monitored using the von Frey method, as previously described [34; 35]. Mice were placed in plastic cages (10 × 10 × 10 cm) on a mesh stand (IITC Life Science, Woodland Hills, California) for 30 minutes for habituation. A series of calibrated filaments were applied to the plantar surface of the hind paw for up to 5 seconds. The “up and down” method was used to calculate the force needed for 50% likelihood of withdrawal [11]. The data presented represent group means of the average of both hind paws for each mouse.
2.4.2. Spontaneous pain
Spontaneous pain was measured using a conditioned place preference (CPP) paradigm [31; 62]. The CPP apparatus consists of 2 chambers (18 × 20 cm each, one dark, one bright) connected by a 15-cm hallway (Stoelting Co., Wood Dale, Illinois). On the first day, each mouse was placed in the hallway and permitted to freely explore the apparatus for 15 minutes. On the next 4 days in the morning, mice were injected intraperitoneally with PBS and 10 minutes later placed individually in the dark chamber for 15 minutes. Four hours later, each mouse received an analgesic dose of retigabine (10 mg/kg in PBS; #R-100; Alomone Labs, Jerusalem, Israel) administered intraperitoneally and was placed in the bright chamber. On the sixth day, mice were allowed to freely explore the apparatus for 15 minutes; the change in the amount of time spent in the bright (previously retigabine-paired) chamber was quantified. The preconditioning and post-conditioning test results were recorded and analyzed using Ethovision XT video tracking software (Noldus Information Technology Inc., Leesburg, Virginia).
2.4.3. Adhesive recognition test
We used a modified version of the adhesive recognition test [6] to assess numbness. A 3/16” round sticker (Teeny Tough-Spot; USA Scientific Inc, Ocala, Florida) was placed on the plantar surface of the hind paw. The mouse was placed in a plastic box (10 × 20 × 10 cm) and was videotaped from below to determine the time until the mouse responds to the sticker, as previously described [31; 41].
2.5. Intra-epidermal nerve fiber staining
Intra-epidermal nerve fiber (IENF) density was quantified in glabrous skin on the plantar surface of the hind paws, as previously described [31; 55]. Briefly, frozen 25-μm sections were incubated with antibodies against the pan-neuronal marker PGP9.5 (rabbit; Bio-Rad AbD Serotec Limited, Oxford, United Kingdom) and Collagen IV (goat; Southern Biotech, Birmingham, Alabama) followed by Alexa-594 donkey anti-rabbit and Alexa-488 donkey anti-goat antibodies (Life Technologies, Carlsbad, California). IENF density was expressed as the number of nerve fibers crossing the basement membrane/length of the basement membrane (mm) and was measured using 4 mice/group and 5 random pictures/mouse.
2.6. Quantitative RT-PCR
Mice were terminated by CO2 exposure and transcardially perfused with ice-cold PBS. Spleen and DRG were quickly harvested and snap-frozen in liquid nitrogen. RNA were isolated using the Trizol (Invitrogen, Carlsbad, California) chloroform method, and qRT-PCR was used to quantify mRNA levels using prime assay primers (Integrated DNA Technologies, Coralville, Iowa).
2.7. Statistical analysis
Differences in behavioral activity and expression levels were assessed by Student’s t-test, one-way or repeated-measure two-way ANOVA followed by Bonferroni correction for multiple tests, depending on experimental design. Significant difference are indicated in graphs as ***=P<0.001, **=P<0.01, *=P<0.05.
3. Results
3.1. CD8+ T cells are necessary for resolution of CIPN
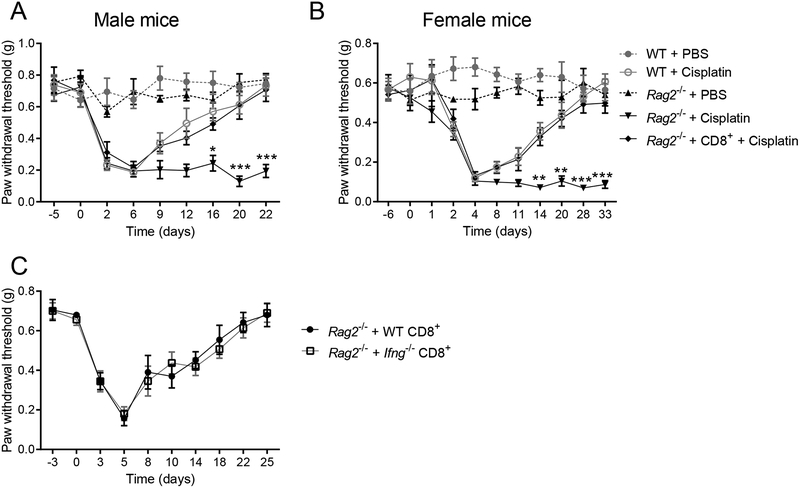
To evaluate the contribution of CD8+ T cells to resolution of mechanical allodynia induced by cisplatin, we compared WT mice with Rag2−/− mice, which lack mature B and T cells. Mice were treated with 3 daily intraperitoneal injections of cisplatin. The onset and intensity of allodynia was similar in cisplatin-treated male and female WT and Rag2−/− mice. Resolution of allodynia was markedly delayed in Rag2−/− mice of both sexes (males: Figure 1A; females: Figure 1B). Adoptive transfer of naïve CD8+ T cells to Rag2−/− mice 10 days before cisplatin injection normalized the resolution of allodynia in both male and female mice (Figure 1). CD8+ T cell reconstitution in the Rag2−/− mice was confirmed by quantifying CD8+ T cells in peripheral blood by flow cytometric analysis, and gene-expression analysis of Cd3e and Cd8a in spleen and DRG (Supplemental Digital Content 1A,B shows results from the reconstitution of Rag2−/− mice with T cells). CD8+ T cells are one of the main producers of interferon (IFN)-γ [48]. We tested whether CD8+ T cells must be capable of producing IFN-γ in order to resolve cisplatin-induced allodynia. We found that resolution of allodynia was similar in Rag2−/− mice reconstituted with CD8+ T cells from Ifng−/− mice or WT mice, indicating that CD8+ T cells promote resolution of allodynia independent of their capability to produce IFN-γ (Figure 1C).
Figure 1. Effects of CD8+ T cells on mechanical allodynia induced by cisplatin in female and male mice.
Cisplatin (2 mg/kg) or PBS was administrated on days 0, 1, and 2 to male and female WT mice, Rag2−/− mice, and Rag2−/− mice reconstituted with CD8+ T cells on day −10. (A) Mechanical sensitivity in male mice. Two-way repeated measures ANOVA interaction (time × genotype): F(32,200)=5.20, P<0.0001 (n=6 mice/group). (B) Mechanical sensitivity in female mice. Two-way repeated measures ANOVA interaction (time × genotype): F(40,280)=6.95, P<0.0001 (n=6 mice/group). ***p<0.001, **p<0.01, *p<0.05 between cisplatin-treated Rag2−/− vs. Rag2−/− mice reconstituted with CD8+ T cells”. (C) Female Rag2−/− mice were reconstituted with CD8+ T cells from WT or Ifng−/− mice (n=5 mice/group). Data are shown as mean ± SEM.
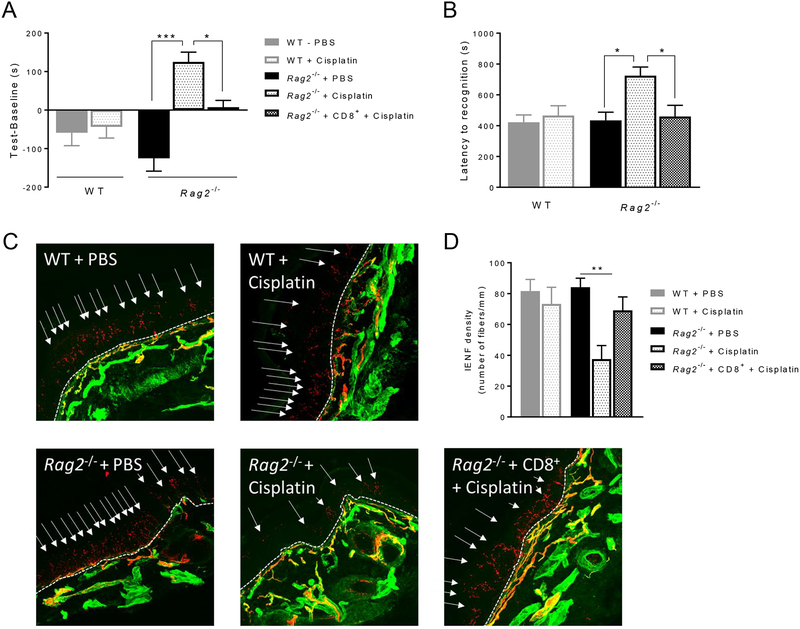
We previously showed that cisplatin induces spontaneous pain and numbness in WT mice [31]. To investigate whether spontaneous pain is prolonged in Rag2−/− mice, we used a CPP paradigm 3 weeks after cisplatin injection. At this time point, mechanical allodynia had already resolved in WT mice, whereas Rag2−/− mice still displayed allodynia (Figure 1). Three weeks after cisplatin, Rag2−/− mice developed a preference for the analgesic-paired chamber, whereas WT mice no longer did. Rag2−/− mice reconstituted with CD8+ T cells behaved like WT mice (Figure 2A). These findings indicate that CD8+ T cells are necessary and sufficient for the resolution of spontaneous pain after cisplatin treatment.
Figure 2. Effects of CD8+ T cells on spontaneous pain, numbness and intra-epidermal nerve fiber (IENF) density after cisplatin treatment.
Female WT mice, Rag2−/− mice, and Rag2−/− mice reconstituted with CD8+ T cells were treated with cisplatin or PBS as in Figure 1. (A) Conditioned place preference (CPP) paradigm was performed 3 weeks after cisplatin or PBS injection (days 21 to 28). Y-axis indicates the change in time spent in the bright (analgesic-paired chamber) between baseline and test. One-way ANOVA followed by Bonferroni’s multiple-comparisons test: F(4,40)=10.9, P<0.0001 (n=9 mice/group). (B) The adhesive recognition test (ART) was performed on day 26 after cisplatin or PBS injection. One-way ANOVA followed by Bonferroni’s multiple-comparisons test: F(4,22)=5.12, P=0.004 (n=6 mice/group). (C) Representative images of IENFs (PGP9.5, red and collagen, green) in the glabrous skin of the plantar surface of the hind paws 3 weeks after cisplatin or PBS. Dashed lines indicate the basement membrane; arrows indicate IENFs. (D) Quantification of IENF density, expressed as the number of nerve fibers crossing the basement membrane/length of the basement membrane (mm). One-way ANOVA followed by Bonferroni’s multiple-comparisons test: F(4,25)=4.87, P=0.005 (n=6 mice/group). Data are shown as mean ± SEM. ***P<0.001, **P<0.01, *P<0.05.
To assess cisplatin-induced peripheral numbness, we performed the adhesive removal test 3 weeks after cisplatin injection. We measured the time it took each mouse to respond to a small sticker placed on the plantar surface of the hind paw [6]. Cisplatin-treated Rag2−/− mice took approximately twice as long to respond to the sticker as compared to PBS-treated Rag2−/− mice, and PBS- or cisplatin-treated WT mice. Rag2−/- mice reconstituted with CD8+ T cells responded to the sticker as fast as cisplatin-treated or PBS-treated WT mice did (Figure 2B). These data indicate that CD8+ T cells are required for the resolution of numbness after cisplatin treatment.
Next, we determined the contribution of CD8+ T cells to normalization of IENF density in the hind paw after completion of cisplatin treatment. In WT mice cisplatin induces a reduction of IENF density as early as 8 days (Supplemental Digital Content 2 shows IENF density reduction at 8 days after cisplatin). Three weeks after cisplatin, IENF density had normalized in cisplatin-treated WT mice (Figure 2C,D). Three weeks after cisplatin, IENF density in Rag2−/− mice was still reduced in comparison to all other groups. Reconstitution of Rag2−/− mice with CD8+ T cells restored the IENF density to a similar level as that found in the WT mice at 3 weeks after cisplatin (Figures 2C and 2D). These data demonstrate that CD8+ T cells are critical for restoration of IENF density.
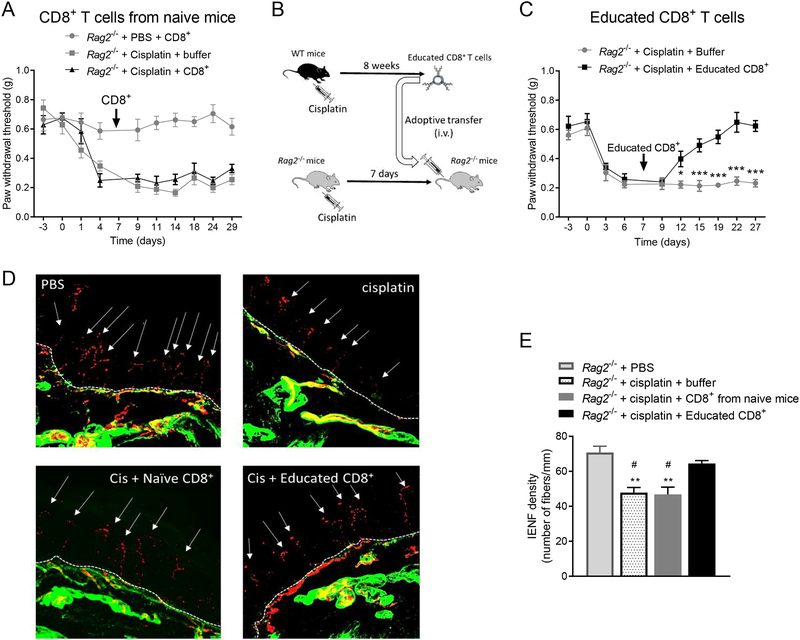
3.2. Resolution of established CIPN requires educated CD8+ T cells
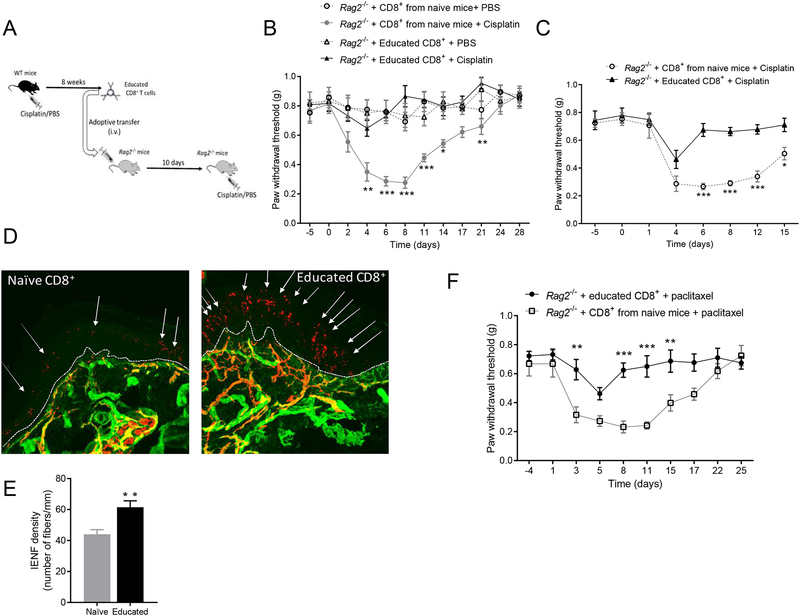
To determine whether adoptive transfer of CD8+ T cells can reverse already established CIPN, we reconstituted Rag2−/− mice with CD8+ T cells 7 days after cisplatin injection (at the peak of allodynia). Adoptive transfer of CD8+ T cells from naïve mice into Rag2−/− mice after cisplatin injection did not reverse established allodynia (Figure 3A). This was not due to insufficient reconstitution of CD8+ T cells, because the number of CD8+ T cells was similar in Rag2−/− mice reconstituted with CD8+ T cells before or after cisplatin (Supplemental Digital Content 1C, shows results from the reconstitution of Rag2−/− mice with T cells). Next, we tested whether CD8+ T cells must be ‘educated’ by prior exposure to cisplatin to be capable of promoting resolution of established CIPN. For this purpose, WT mice were treated with 5 injections of 2 mg/kg cisplatin. Eight weeks after cisplatin injection, their CD8+ T cells were harvested from spleens and adoptively transferred to cisplatin-treated-Rag2−/− mice (Figure 3B). Adoptive transfer of these educated CD8+ T cells into Rag2−/− mice reversed established CIPN (Figure 3C). In addition, adoptive transfer of educated CD8+ T cells, but not of naïve CD8+ T cells, after completion of cisplatin treatment normalized IENF density as well (Figures 3D and 3E).
Figure 3. Effects of CD8+ T cells educated in vivo on established CIPN.
(A) Time course of change in mechanical pain sensitivity. Female Rag2−/− mice were treated with cisplatin or PBS on days 0, 1, and 2. CD8+ T cells from naïve female WT mice were adoptively transferred on day 7 after start of cisplatin treatment (n=7 mice/group). (B) Schematic of in vivo education of CD8+ T cells. (C) Time course of mechanical pain sensitivity. In vivo educated CD8+ T cells obtained from WT female mice 8 weeks after cisplatin treatment were adoptively transferred on day 7 after start of cisplatin treatment into female Rag2−/− mice. Two-way repeated measures ANOVA interaction (time × CD8+ T cells): F(9,108)=7.20, P<0.0001 (n=7 mice/group). (D) Representative images of IENFs (PGP9.5, red and collagen, green) from Rag2−/− mice treated with PBS or cisplatin and reconstituted with CD8+ T cells from naïve mice or with educated CD8+ T cells. Dashed lines indicate the basement membrane; arrows indicate IENFs. (E) Quantification of IENF density, expressed as the number of nerve fibers crossing the basement membrane/length of the basement membrane (mm). One-way ANOVA followed by Bonferroni’s multiple-comparisons test: F(3,16)=13.8, P=0.001 (n=5 mice/group). ** P<0.01, versus the PBS-treated group; # p<0.05 versus the cisplatin + educated CD8+ group. Data are shown as mean ± SEM. ***P<0.001, **P<0.01, *P<0.05.
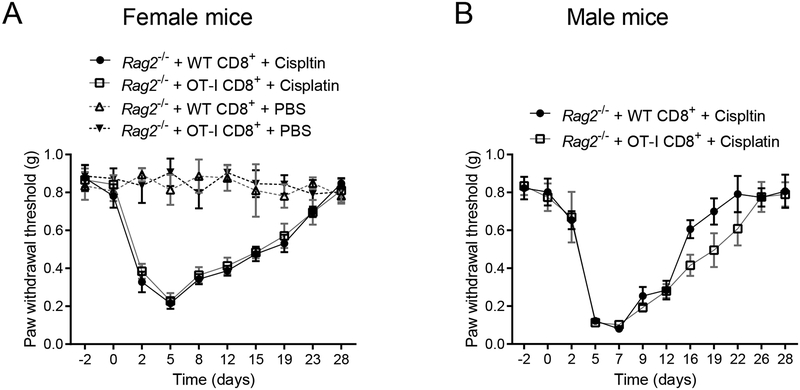
Education of CD8+ T cells can involve antigen recognition by the T-cell receptor (TCR) complex. To assess whether the effect of educated CD8+ cells was epitope-specific involving recognition by the TCR complex that recognizes only the irrelevant chicken ovalbumin antigen [24]. We reconstituted Rag2−/− mice with CD8+ T cells from OT-I or WT mice. Resolution of allodynia was similar in female and male Rag2−/− mice reconstituted with CD8+ T cells from WT or OT-I mice (Figures 4A and 4B). These data indicate that CIPN resolution is independent of specific epitope recognition by the TCR.
Figure 4. Contribution of TCR antigen recognition to resolution of chemotherapy-induced peripheral neuropathy.
Female and male Rag2−/− mice were treated with cisplatin or PBS on days 0, 1, and 2. CD8+ T cells were adoptively transferred 10 days before start of cisplatin treatment. Mechanical pain sensitivity and resolution of allodynia. (A) Female Rag2−/− mice treated with PBS or cisplatin and reconstituted with CD8+ T cells from WT or OT-I mice (n=8 mice/group). (B) Male Rag2−/− mice treated with cisplatin and reconstituted with CD8+ T cells from WT or OT-I mice (n=6 mice/group). Data are shown as mean ± SEM.
3.3. CD8+ T cells educated in vivo can prevent CIPN
We next tested whether adoptive transfer of in vivo educated CD8+ T cells by prior exposure of the donor mice to cisplatin can also prevent development of CIPN. CD8+ T cells were educated by exposing WT mice to cisplatin or PBS for 5 days; 8 weeks after completion of treatment, CD8+ T cells were collected from spleens and adoptively transferred to naïve Rag2−/- mice (Figure 5A). Ten days after reconstitution with CD8+ T cells, Rag2−/− mice were treated with cisplatin or PBS. The development of CIPN was completely prevented in Rag2−/− mice reconstituted with educated CD8+ T cells. This preventive effect of educated CD8+ T cells was observed in both sexes (Figures 5B and 5C). Reconstitution of Rag2−/− mice with educated CD8+ T cells also prevented cisplatin-induced reduction in IENF density (Figures 5D and 5E).
Figure 5. Effects of CD8+ T cells educated in vivo on chemotherapy-induced peripheral neuropathy.
(A) Schematic of experiment to test the effect of reconstitution of Rag2−/− mice with CD8+ T cells educated in vivo before cisplatin or PBS administration to the Rag2−/− mice. (B) Female Rag2−/− mice were treated with cisplatin or PBS on days 0, 1, and 2. CD8+ T cells were adoptively transferred 10 days before start of cisplatin treatment. Mechanical pain sensitivity in female Rag2−/− reconstituted with educated CD8+ T cells or CD8+ T cells from naïve mice before cisplatin or PBS administration. Two-way repeated measures ANOVA interaction: F(44,275)=5.05, P<0.0001 (n=6 mice/group). (C) Mechanical allodynia in male Rag2−/− reconstituted with educated CD8+ T cells or naïve CD8+ T cells before cisplatin administration to the reconstituted Rag2−/− mice. Two-way repeated measures ANOVA interaction: F(7,70) = 5.25, P<0.0001 (n=6 mice/group). (D) Representative images of IENFs (PGP9.5, red and collagen, green) from cisplatin-treated female Rag2−/− mice previously reconstituted with educated CD8+ T cells or CD8+ T cells from naïve mice. Dashed lines indicate the basement membrane; arrows indicate IENFs. (E) Quantification of IENF density, expressed as the number of nerve fibers crossing the basement membrane/length of the basement membrane (mm). T-test (t=3.42, df=8) (n=5 mice/group). (F) Rag2−/− mice were treated with paclitaxel on days 0 and 2. CD8+ T cells from WT mice were adoptively transferred 10 days before start of paclitaxel treatment. Mechanical pain sensitivity I response to paclitaxel treatment in female Rag2−/− reconstituted with educated CD8+ T cells or CD8+ T cells from naïve mice. Two-way repeated measures ANOVA interaction time × education: F(9, 72)= 3.41, p=0.002 (n=5mice/group). Data are shown as mean ± SEM. ***P<0.001, **P<0.01, *P<0.05.
We then assessed whether the preventive effect of educated CD8+ T cells was specific for resolution of cisplatin-induced neuropathy. CD8+ T cells were educated by cisplatin and adoptively transferred to Rag2−/− mice as described above (Figure 5A). Ten days after reconstitution with CD8+ T cells, Rag2−/− mice were treated with paclitaxel. Cisplatin-educated CD8+ T cells were capable of preventing development of allodynia in both paclitaxel- and cisplatin-treated Rag2−/− acceptor mice. (Figure 5B,C,F).
4. DISCUSSION
We demonstrate that CD8+ T cells are essential for resolution of all signs of cisplatin-induced peripheral neuropathy in both male and female mice. Our findings apply to allodynia, spontaneous pain, numbness, and reduction in IENF density in both sexes. All these signs of CIPN are significantly prolonged in T-cell–deficient Rag2−/− mice and are normalized in Rag2−/− mice reconstituted with CD8+ T cells. These original findings strengthen the concept that resolution of CIPN depends on an active endogenous process mediated by CD8+ T cells.
Importantly, we show for the first time that education of CD8+ T cells by cisplatin is a critical step in the resolution of established CIPN. Furthermore, CD8+ T cells educated in vivo by cisplatin are capable of preventing CIPN. Interestingly, cisplatin educated CD8+ T cells not only resolve cisplatin-induced CIPN but also paclitaxel-induced CIPN. These findings indicate that the activity of the educated CD8+ T is not cisplatin-specific and imply that ex vivo education of CD8+ T cells might be a promising future approach to prevent or treat CIPN in cancer patients and survivors.
The contribution of T cells to chronic pain is controversial. In the spared nerve injury model of neuropathic pain, some authors reported that the onset of allodynia was attenuated in Rag1−/− mice [14]; others found no difference in the onset and intensity of allodynia between WT and Rag1−/− mice [52]. In CIPN models, we and others showed that T-cell–deficient mice or mice depleted from T cells have a similar onset of allodynia, compared with WT mice [30; 40]. In contrast, Liu et al. reported an exacerbation of paclitaxel-induced allodynia after transfer of CD8+ T cells into WT mice [38]. A potential explanation for this discrepancy with our findings is that Liu et al. administered the T cells intrathecally; T cells are not present (or are at a very low level) in the spinal cords of control and neuropathic-pain animals [16; 20; 26]. We administered the (educated) CD8+ T cells intravenously and these cells will end up first in the secondary lymphoid organs, allowing the T cells to differentiate and/or to regulate activity of other cell types such as macrophages. Subsequently, these macrophages may migrate from the spleen to DRG or spinal cord to suppress the pain response. Indeed, we and others have shown that macrophages produce IL-10 to promote resolution of inflammatory pain [3; 67].
Our findings are in line with accumulating evidence that T cells play a beneficial role after nerve injury and promote recovery after stroke in a mouse model [42]. It is interesting to note that a reduction in circulating CD8+ T cells has been reported in patients suffering from complex regional pain syndrome and fibromyalgia [29]. This reduction in circulating CD8+ T cells may be sufficient to account for their inability to promote resolution of pain or prevent development of chronic pain. A protective role for T cells has also been described in models of stress; T-cell–deficient mice are less resilient to stress-induced depression-like behavior [7; 37]. In addition, we recently showed that inflammation-induced depression-like behavior is prolonged in T cell-deficient mice [33]. These data point to a common role for T cells in endogenous resolution pathways in comorbid pain and depression.
In the present study, we did not observe differences in onset or severity of CIPN between male and female mice, while resolution tends to be slightly faster in male mice. Importantly, however, in both sexes CD8+ T cells are required for resolution of CIPN. It has been suggested that neuropathic pain after nerve injury or inflammatory pain induced by lipopolysaccharide (LPS) is mostly T-cell dependent in female and microglia dependent in male mice [58]. In the present study, the absence of CD8+ T cells prolonged cisplatin-induced allodynia to the same extent in female and male mice. Likewise, educated CD8+ T cells prevented cisplatin-induced allodynia in both sexes. Unlike nerve injury or intrathecal injection of lipopolysaccharide, cisplatin does not induce activation of microglia [51; 57; 58; 71], which could explain the absence of sex differences as microglia are one of the main drivers of sex difference in the nervous system [63].
Even if resolution of all signs of CIPN requires CD8+ T cells, it remains to be determined what the underlying neuroimmune mechanisms are. It is unlikely that the chemotherapy-induced loss in IENF density and pain are causally related and it may well be possible that their resolution mechanisms differ. With respect to resolution of pain, we examined the contribution of IFN-γ produced by CD8+ T cells. IFN-γ activates GABAergic inhibitory currents [19] which are reduced in the spinal cord for CIPN [8; 9; 12]. However, our finding that CD8+ T cells from Ifng−/− and WT mice both promote resolution of CIPN indicates that CD8+ T cells do not need to produce IFN-γ to resolve CIPN. We also know that CD8+ T cells themselves do not need to produce IL-4 or IL-10 to induce allodynia resolution [30]. However, we did show previously that IL-10 signaling is required for normalization of pain, indicating indirect effects of CD8+ T cells through promoting IL-10 signaling. In addition, CD8+ T cells may have direct or indirect neuroimmune interactions with neurons or release factors to promote normalization of IENF density during resolution of CIPN.
Interestingly, our data indicate that CD8+ T cells need to be educated to develop the capacity to promote resolution of CIPN as only adoptive transfer of educated CD8+ T cells reversed established CIPN. The resolution of CIPN does not require epitope recognition by the TCR complex as CD8+ T cells from OT-I mice, that can only recognize and respond to ovalbumin-derived peptide antigens, were as effective to resolve CIPN as CD8+ T cells from WT mice. A substantial population of CD8+ T cells has been shown to express phenotypical markers of immunological memory in absence of antigen exposure [66]. These CD8+ T cells develop a memory phenotype in response to TCR stimulation independently of epitope recognition [13; 18; 59] and stimulation by cytokines [23; 49; 50]. As IL-10 promotes the maturation of memory-like CD8+ T cells and potentiates the activity of CD8+ T cells [17; 22; 32; 47] it is possible that CD8+ T cells are educated by IL-10 in secondary lymphoid organs toward a regulatory phenotype to promote resolution of CIPN. In accordance with this hypothesis we have already shown that IL-10 is necessary for CIPN resolution but that the CD8+ T cells do not need to produce IL-10 [30]. In addition, cisplatin has immunogenic properties and might induce expression of co-stimulatory molecules on antigen-presenting cells to stimulate CD8+ T cells to exert their regulatory effect [4; 60].
A salient finding of our work is that adoptive transfer of educated CD8+ T cells to Rag2−/− mice prevented CIPN in recipient mice. The protective effects of the educated CD8+ T cells studied here in the context of CIPN are reminiscent of previous observations indicating the capacity of “educated” T cells to transfer stress resilience or alleviate demyelination in a multiple sclerosis model. In these studies, adoptive transfer of lymphocytes from stressed WT mice conferred antidepressant effects on Rag2−/− mice [7] and adoptive transfer of CD4+ T regulatory cells from experimental autoimmune encephalomyelitis (EAE) mice delayed the progression of EAE [43].
We demonstrated here that transfer of cisplatin-educated CD8+ T cells prevent CIPN induced either with cisplatin or paclitaxel again confirming that there is no epitope specificity involved in the education of CD8+ T cells. Cisplatin and paclitaxel have different intracellular anticancer mechanisms: cisplatin induces DNA adducts and paclitaxel promotes stabilization of microtubules although the cellular outcome in peripheral sensory neurons may be similar and includes mitochondrial dysfunction, retraction of IENF, and neuronal hyperexcitability in both cases [39; 56]. Whether the preventive effects of cisplatin educated-CD8+ T cells would apply to all types of chemotherapeutic agents that induce CIPN, remains to be determined.
The educated CD8+ T cells used here were obtained 8 weeks after cisplatin injection; preliminary data indicate that they retain their protective capacity to prevent CIPN even when they are obtained 20 weeks after completion of cisplatin treatment (data not shown). These data suggest that the educated phenotype is long-lasting.
5. CONCLUSION
Our study documents two important findings. First, we show that resolution of CIPN after cisplatin treatment is an active process that includes a critical role for CD8+ T cells in the resolution of allodynia, spontaneous pain, numbness, and reduction of IENF density, regardless of sex. Moreover, CD8+ T cells need to be educated by cisplatin in order to resolve established CIPN. We also demonstrate that cisplatin educates CD8+ T cells which can be used to prevent CIPN induced either by cisplatin or paclitaxel. Our work strengthen the prospect for immune cell-based therapies in neurological disorders [25], such as ex vivo education of autologous peripheral CD8+ T cells, to relieve the burden of CIPN and restore quality of life of cancer survivors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Bianka Ford and Iteeben Mahant for tissue processing and staining and scoring of IENF images.
Financial support
This study is supported by grants from the National Institute of Neurological Disorders and Stroke and the National Cancer Institute of the National Institutes of Health, including R01 NS073939 (AK, RD, CJH), and R01 CA227064 (AK, CJH), a Cyrus Scholar Award (GL) and an American Pain Society Future Leader in Pain Research Award (GL).
Footnotes
Conflicts of interest
R. Dantzer received an honorarium from Danone Nutricia Research for work not related to the present study. All other authors declare no conflict of interest.
REFERENCES
- [1].Austin PJ, Kim CF, Perera CJ, Moalem-Taylor G. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 2012;153(9):1916–1931. [DOI] [PubMed] [Google Scholar]
- [2].Baddack-Werncke U, Busch-Dienstfertig M, Gonzalez-Rodriguez S, Maddila SC, Grobe J, Lipp M, Stein C, Muller G. Cytotoxic T cells modulate inflammation and endogenous opioid analgesia in chronic arthritis. J Neuroinflammation 2017;14(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest 2018;128(8):3568–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beyranvand Nejad E, van der Sluis TC, van Duikeren S, Yagita H, Janssen GM, van Veelen PA, Melief CJ, van der Burg SH, Arens R. Tumor Eradication by Cisplatin Is Sustained by CD80/86-Mediated Costimulation of CD8+ T Cells. Cancer Res 2016;76(20):6017–6029. [DOI] [PubMed] [Google Scholar]
- [5].Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci 2008;28(52):14062–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, Freret T. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc 2009;4(10):1560–1564. [DOI] [PubMed] [Google Scholar]
- [7].Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci 2015;35(4):1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Braz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, Basbaum AI. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron 2012;74(4):663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Braz JM, Wang X, Guan Z, Rubenstein JL, Basbaum AI. Transplant-mediated enhancement of spinal cord GABAergic inhibition reverses paclitaxel-induced mechanical and heat hypersensitivity. Pain 2015;156(6):1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol 2008;38(2):448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [12].Chen SR, Zhu L, Chen H, Wen L, Laumet G, Pan HL. Increased spinal cord Na(+)-K(+)-2Cl(−) cotransporter-1 (NKCC1) activity contributes to impairment of synaptic inhibition in paclitaxel-induced neuropathic pain. J Biol Chem 2014;289(45):31111–31120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity 2010;32(2):214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci 2009;29(46):14415–14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos AR. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol 2015;51(1):19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Denk F, Crow M, Didangelos A, Lopes DM, McMahon SB. Persistent Alterations in Microglial Enhancers in a Model of Chronic Pain. Cell Rep 2016;15(8):1771–1781. [DOI] [PubMed] [Google Scholar]
- [17].Emmerich J, Mumm JB, Oft M. Autochthonous T cells to the rescue: IL-10 directly activates tumor-resident CD8(+) T cells. Oncoimmunology 2012;1(9):1637–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 1999;11(2):173–181. [DOI] [PubMed] [Google Scholar]
- [19].Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC, Gadani SP, Turner SD, Weng Z, Peerzade SN, Chen H, Lee KS, Scott MM, Beenhakker MP, Litvak V, Kipnis J. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 2016;535(7612):425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gattlen C, Clarke CB, Piller N, Kirschmann G, Pertin M, Decosterd I, Gosselin RD, Suter MR. Spinal Cord T-Cell Infiltration in the Rat Spared Nerve Injury Model: A Time Course Study. Int J Mol Sci 2016;17(3):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol 2012;14 Suppl 4:iv45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol 1998;160(7):3188–3193. [PubMed] [Google Scholar]
- [23].Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med 2009;206(2):435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell 1994;76(1):17–27. [DOI] [PubMed] [Google Scholar]
- [25].Hu X, Leak RK, Thomson AW, Yu F, Xia Y, Wechsler LR, Chen J. Promises and limitations of immune cell-based therapies in neurological disorders. Nat Rev Neurol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Janes K, Wahlman C, Little JW, Doyle T, Tosh DK, Jacobson KA, Salvemini D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun 2015;44:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science 2016;354(6312):572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain 2010;11(11):1230–1239. [DOI] [PubMed] [Google Scholar]
- [29].Kaufmann I, Eisner C, Richter P, Huge V, Beyer A, Chouker A, Schelling G, Thiel M. Lymphocyte subsets and the role of TH1/TH2 balance in stressed chronic pain patients. Neuroimmunomodulation 2007;14(5):272–280. [DOI] [PubMed] [Google Scholar]
- [30].Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A. CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci 2016;36(43):11074–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krukowski K, Ma J, Golonzhka O, Laumet GO, Gutti T, van Duzer JH, Mazitschek R, Jarpe MB, Heijnen CJ, Kavelaars A. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain 2017;158(6):1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, Kaech SM. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol 2015;16(8):871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Laumet G, Edralin JD, Chiang AC, Dantzer R, Heijnen CJ, Kavelaars A. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM, Dong Y, Jelinek J, Cesaroni M, Issa JP, Pan HL. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015;18(12):1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Laumet G, Zhou W, Dantzer R, Edralin JD, Huo X, Budac DP, O’Connor JC, Lee AW, Heijnen CJ, Kavelaars A. Upregulation of neuronal kynurenine 3-monooxygenase mediates depression-like behavior in a mouse model of neuropathic pain. Brain Behav Immun 2017;66:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lees JG, Duffy SS, Perera CJ, Moalem-Taylor G. Depletion of Foxp3+ regulatory T cells increases severity of mechanical allodynia and significantly alters systemic cytokine levels following peripheral nerve injury. Cytokine 2015;71(2):207–214. [DOI] [PubMed] [Google Scholar]
- [37].Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, Schwartz M. Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry 2009;65(4):283–288. [DOI] [PubMed] [Google Scholar]
- [38].Liu XJ, Zhang Y, Liu T, Xu ZZ, Park CK, Berta T, Jiang D, Ji RR. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res 2014;24(11):1374–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ma J, Kavelaars A, Dougherty PM, Heijnen CJ. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer 2018;124(11):2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Makker PG, Duffy SS, Lees JG, Perera CJ, Tonkin RS, Butovsky O, Park SB, Goldstein D, Moalem-Taylor G. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PLoS One 2017;12(1):e0170814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mao-Ying QL, Kavelaars A, Krukowski K, Huo XJ, Zhou W, Price TJ, Cleeland C, Heijnen CJ. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS One 2014;9(6):e100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mao L, Li P, Zhu W, Cai W, Liu Z, Wang Y, Luo W, Stetler RA, Leak RK, Yu W, Gao Y, Chen J, Chen G, Hu X. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain 2017;140(7):1914–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol 2005;175(5):3025–3032. [DOI] [PubMed] [Google Scholar]
- [44].McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 2009;8(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev 2014;40(7):872–882. [DOI] [PubMed] [Google Scholar]
- [46].Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 2015;16(8):769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Naing A, Papadopoulos KP, Autio KA, Ott PA, Patel MR, Wong DJ, Falchook GS, Pant S, Whiteside M, Rasco DR, Mumm JB, Chan IH, Bendell JC, Bauer TM, Colen RR, Hong DS, Van Vlasselaer P, Tannir NM, Oft M, Infante JR. Safety, Antitumor Activity, and Immune Activation of Pegylated Recombinant Human Interleukin-10 (AM0010) in Patients With Advanced Solid Tumors. J Clin Oncol 2016;34(29):3562–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005;5(5):375–386. [DOI] [PubMed] [Google Scholar]
- [49].Ramanathan S, Gagnon J, Dubois S, Forand-Boulerice M, Richter MV, Ilangumaran S. Cytokine synergy in antigen-independent activation and priming of naive CD8+ T lymphocytes. Crit Rev Immunol 2009;29(3):219–239. [DOI] [PubMed] [Google Scholar]
- [50].Ramanathan S, Gagnon J, Ilangumaran S. Antigen-nonspecific activation of CD8+ T lymphocytes by cytokines: relevance to immunity, autoimmunity, and cancer. Arch Immunol Ther Exp (Warsz) 2008;56(5):311–323. [DOI] [PubMed] [Google Scholar]
- [51].Robinson CR, Zhang H, Dougherty PM. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 2014;274:308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rosen SF, Ham B, Drouin S, Boachie N, Chabot-Dore AJ, Austin JS, Diatchenko L, Mogil JS. T-Cell Mediation of Pregnancy Analgesia Affecting Chronic Pain in Mice. J Neurosci 2017;37(41):9819–9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10(11):1361–1368. [DOI] [PubMed] [Google Scholar]
- [54].Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014;155(12):2461–2470. [DOI] [PubMed] [Google Scholar]
- [55].Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol 2006;201(2):507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol 2014;10(12):694–707. [DOI] [PubMed] [Google Scholar]
- [57].Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 2011;31(43):15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18(8):1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Takada K, Jameson SC. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med 2009;206(10):2253–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tran L, Allen CT, Xiao R, Moore E, Davis R, Park SJ, Spielbauer K, Van Waes C, Schmitt NC. Cisplatin Alters Antitumor Immunity and Synergizes with PD-1/PD-L1 Inhibition in Head and Neck Squamous Cell Carcinoma. Cancer Immunol Res 2017;5(12):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Verma-Gandhu M, Bercik P, Motomura Y, Verdu EF, Khan WI, Blennerhassett PA, Wang L, El-Sharkawy RT, Collins SM. CD4+ T-cell modulation of visceral nociception in mice. Gastroenterology 2006;130(6):1721–1728. [DOI] [PubMed] [Google Scholar]
- [62].Vichaya EG, Laumet G, Christian DL, Grossberg AJ, Estrada DJ, Heijnen CJ, Kavelaars A, Robert D. Motivational changes that develop in a mouse model of inflammation-induced depression are independent of indoleamine 2,3 dioxygenase. Neuropsychopharmacology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, Lolli F, Marcello E, Sironi L, Vegeto E, Maggi A. Sex-Specific Features of Microglia from Adult Mice. Cell Rep 2018;23(12):3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012;73(4):638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wahlman C, Doyle TM, Little JW, Luongo L, Janes K, Chen Z, Esposito E, Tosh DK, Cuzzocrea S, Jacobson KA, Salvemini D. Chemotherapy-induced pain is promoted by enhanced spinal adenosine kinase levels through astrocyte-dependent mechanisms. Pain 2018;159(6):1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].White JT, Cross EW, Kedl RM. Antigen-inexperienced memory CD8(+) T cells: where they come from and why we need them. Nat Rev Immunol 2017;17(6):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A. Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain 2014;15(5):496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol 1997;121(3):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM. Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J Pain 2016;17(7):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zheng H, Xiao WH, Bennett GJ. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol 2012;238(2):225–234. [DOI] [PubMed] [Google Scholar]
- [71].Zhou W, Kavelaars A, Heijnen CJ. Metformin Prevents Cisplatin-Induced Cognitive Impairment and Brain Damage in Mice. PLoS One 2016;11(3):e0151890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.