Abstract
The β2 adrenergic receptor (β2AR) signals through both Gs and Gi in cardiac myocytes, and the Gi pathway counteracts the Gs pathway. However, Gi coupling is much less efficient than Gs coupling in most cell-based and biochemical assays, making it difficult to study β2AR−Gi interactions. Here we investigate the role of phospholipid composition on Gs and Gi coupling. While negatively charged phospholipids are known to enhance agonist affinity and stabilize an active state of the β2AR, we find that they impair coupling to Gi3 and facilitate coupling to Gs. Positively charged Ca2+ and Mg2+, known to interact with the negative charge on phospholipids, facilitates Gi3 coupling. Mutational analysis suggests that Ca2+ coordinates an interaction between phospholipid and the negatively charged EDGE motif on the amino terminal helix of Gi3. Taken together, our observations suggest that local membrane charge modulates the interaction between β2AR and competing G protein subtypes.
Subject terms: Membrane lipids, G protein-coupled receptors, Hormone receptors
In the healthy heart, the β2 adrenergic receptor (β2AR) signals through Gs and Gi proteins but the mechanism underlying G protein selectivity is not fully understood. Here, the authors show that membrane charge and intracellular cations modulate the β2AR−Gi3 interaction.
Introduction
A third of all FDA-approved pharmaceutical drugs function by modulating the activity of G-protein-coupled receptors (GPCRs)1, a large receptor superfamily. GPCRs catalyze the activation of heterotrimeric G proteins, which in turn initiate a multitude of signaling cascades that alter cellular function.
The β2 adrenergic receptor (β2AR) is a prototypical GPCR that mediates the fight-or-flight response. β2AR signals through both Gs and Gi, and the dual G protein selectivity of β2AR is best characterized in heart muscle (cardiac myocytes). In healthy neonatal cardiac myocytes, epinephrine-stimulated β2AR immediately activates Gs, increasing contraction rate, but after 10−15 min β2AR signals predominantly through Gi 2, which decreases the contraction rate. In cardiac myocytes, β2AR couples to both Gi2 and Gi33. Of interest, Gi activation is impaired if β2AR internalization is blocked4. Also, Gi does not interact with a modified β2AR that internalizes but does not recycle to the plasma membrane5, or with WT β2AR that internalizes but is pharmacologically blocked from recycling6. Taken together, these observations demonstrate that β2AR−Gi interaction is regulated temporally and perhaps spatially.
During heart failure, a condition of chronic, progressive cardiac insufficiency, the Gi pathway counteracts some negative consequences of chronic Gs activation that exacerbate heart failure, namely apoptosis and structural and functional remodeling7–9. However, Gi activation reduces contractility, which can be problematic in certain models of heart failure10. More precise regulation of Gs and Gi activation is a therapeutic aim9.
While β2AR signals through both Gs and Gi, the mechanism that initiates the Gs-to-Gi switch in the healthy heart is not fully understood. Multiple biochemical mechanisms may play a role. PKA phosphorylation of β2AR has been reported to increase Gi1 coupling in vitro11 and Gi coupling in HEK cells12; however, the Gi1 subtype is not expressed in cardiac myocytes, and β2AR−Gi coupling is PKA independent in these cells2. In addition, GRK2 phosphorylation of β2AR has been suggested to increase Gi coupling13, but other investigators have reported that dephosphorylation is critical for β2AR recycling to the plasma membrane, and β2AR−Gi interactions6. Therefore, the mechanisms that modulate β2AR−Gi coupling remain unclear.
In vitro, negatively charged phospholipids stabilize an active conformation of the β2AR and enhance its affinity for the catecholamine isoproterenol14, but the effect of phospholipid charge on G protein coupling is unknown. Negatively charged lipids have previously been shown to facilitate β1AR−Gs interaction15, NTS1−Gq16 interaction, CB2−G protein interaction17, and rhodopsin−Gt18 interaction. In cardiac myocytes, β2AR activates Gs in T-tubules19, deep evaginations of the plasma membrane enriched in L-type calcium channels and negatively charged phospholipids20. β2AR activation of Gi may also occur in T-tubules, after internalization and recycling. However, trafficking events may alter the composition of phospholipids surrounding the β2AR. In addition, the β2AR−Gs signaling that occurs prior to β2AR−Gi signaling greatly increases the Ca2+ concentration near T-tubules21, which may alter the charge properties of the T-tubule lipids22.
Here we examine the effect of phospholipid charge on β2AR interactions with Gs and Gi3. We find that negatively charged lipids enhance β2AR interaction with Gs and impair interaction with Gi3. Further, Ca2+ and Mg2+ facilitate β2AR−Gi3 interaction in negatively charged lipids. Our observations suggest that local membrane charge, tuned by intracellular cations, modulates β2AR interaction with Gi3.
Results
Monitoring G protein coupling by fluorescence spectroscopy
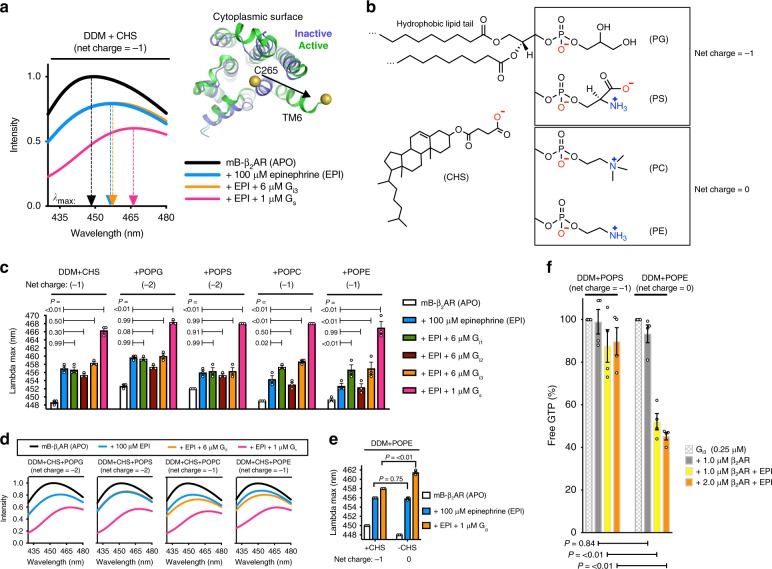
Epinephrine activates β2AR by partially stabilizing the conformation recognized by the G protein. This conformation is fully stabilized upon G protein coupling23–25. A feature of the G-protein-coupled conformation of β2AR is a 14 angstrom outward displacement of the cytoplasmic end of β2AR transmembrane segment 6 (TM6) (Fig. 1a). This conformation can be detected by fluorescence spectroscopy, using a modified β2AR labeled at the cytoplasmic end of TM6 with an environmentally sensitive fluorophore, monobromobimane (mB−β2AR, see Methods and ref. 26). Outward movement of TM6 affects the environmental polarity of mB, going from the hydrophobic receptor core to the solvent, decreasing the intensity of mB and increasing the wavelength where emission intensity is greatest (λmax), from ~447 to ~468 nm (Fig. 1a). We monitor λmax to detect G protein coupling, as λmax increases with coupling (Supplementary Fig. 1).
Fig. 1.
Negatively charged lipids inhibit β2AR−Gi coupling in detergent. a mB−β2AR emission spectra in DDM + CHS micelles (5:1 DDM:CHS mole ratio) in the absence of epinephrine (APO) and in the presence of epinephrine, ± G protein (Gs or Gi3). Arrows point to the lambda max value, i.e. the wavelength where mB emission intensity is greatest. Inset shows how Gs coupling alters the structure of mB−β2AR, highlighting the change in position of monobromobimane (mB) at C265 of transmembrane 6 (TM6) (Inactive: PDB 5JQH74; Active: PDB 3SN669). b Structures of CHS and phospholipids, with net charge indicated. c Effect of phospholipid on mB−β2AR interaction with G protein (Gi1, Gi2, Gi3, and Gs), read out as an increase in lambda max. Interaction was assessed in the absence of lipid (5:1 DDM:CHS mole ratio) and in the presence of POPG, POPS, POPC, or POPE (5:1:1 DDM:CHS:Lipid mole ratio). Multiplicity adjusted P values were computed by two-way ANOVA followed by Dunnett’s post hoc test between indicated groups. d Selected mB−β2AR emission spectra from panel (c). e Effect of CHS on mB−β2AR interaction with 1 μM Gi3. Interaction was assessed in the presence of CHS (5:1:1 DDM:POPE:CHS mole ratio) or in the absence of CHS (5:1 DDM:POPE mole ratio). Multiplicity adjusted P values were computed by two-way ANOVA followed by Sidak’s post hoc test between indicated groups. a–e mB−β2AR concentration is 300 nM. Data are mean of three independent experiments. f β2AR-induced GTP turnover for Gi3 (± 200 μM epinephrine, EPI) in DDM + POPS (5:1 DDM:POPS mole ratio) and in DDM + POPE (5:1 DDM:POPE mole ratio). Free GTP was assayed after 12 min. Luminescence signals were normalized relative to the condition with Gi3 alone (see Supplementary Fig. 4). Data are mean ± s.e.m. of four independent experiments. Multiplicity adjusted P values were computed by two-way ANOVA followed by Sidak’s post hoc test between indicated groups. Source data are provided in the Source Data File
Negatively charged lipids inhibit β2AR-Gi coupling
In the presence of epinephrine, we observe a change in intensity and λmax of mB−β2AR following the addition of Gs in a detergent mixture containing n-dodecyl-β-d-maltopyranoside (DDM) and cholesteryl hemisuccinate (CHS) that is commonly used for biochemical study of GPCR/G protein complexes (Fig. 1a). In contrast, the coupling efficiency of mB−β2AR−Gi3 was relatively weak (Fig. 1a). Next, we compared the coupling efficiency of mB−β2AR−Gi in DDM + CHS mixtures with different phospholipids incorporated (Fig. 1b–d). While we were unable to detect interactions of mB−β2AR with Gi1, Gi2 or Gi3 in the presence of negatively charged lipids phosphatidylserine (POPS) and phosphatidylglycerol (POPG), we observed a weak interaction with Gi1 and Gi3 in neutral lipids phosphatidylethanolamine (POPE) and phosphatidylcholine (POPC) (Fig. 1c, d—Gi1 and Gi3 with epinephrine stabilizes more mB−β2AR in the active conformation than epinephrine alone). This result suggested that negatively charged lipids may repel Gi1 and Gi3 interaction with β2AR.
Indeed, we observed that negatively charged CHS decreased mB-β2AR-Gi3 coupling (Fig. 1e), supporting our hypothesis that negatively charged lipids inhibit coupling. Since CHS is a nonphysiologic cholesterol analog, we omitted negatively charged CHS in subsequent experiments in order to isolate the effect of phospholipid charge on mB−β2AR−Gi interactions.
Given reports that PKA phosphorylation of β2AR increases β2AR−Gi interaction in vitro11, we also tested the effect of PKA phosphorylation, but no enhancement of mB−β2AR interaction with Gi1, Gi2, or Gi3 was observed (Supplementary Fig. 2), suggesting that phosphorylation does not potentiate β2AR−Gi interaction under our experimental conditions, and that other mechanisms may enhance β2AR−Gi interaction.
In contrast to Gi1 and Gi3, we were unable to detect coupling to Gi2 in any of the lipid-detergent mixtures (Fig. 1c), suggesting that the interaction is low affinity. Of interest, Gi1 and Gi3 share higher sequence identity than either subtype shares with Gi2. To confirm the lack of coupling to Gi2 observed by fluorescence analysis, we examined β2AR-stimulated G protein turnover of GTP in net neutral DDM + POPC for Gi2 and Gi3 (Supplementary Fig. 3). As expected from our fluorescence studies, we observed robust β2AR-induced GTP turnover for Gi3, but only weak β2AR-induced GTP turnover for Gi2. In contrast we observed strong coupling of Gi2 to purified neurotensin receptor, indicating that the Gi2 protein was functional. While both Gi2 and Gi3 couple to β2AR in cardiac myocytes3, the coupling efficiency using purified components is substantially different (see Discussion). We therefore focused on β2AR−Gi3 coupling in further studies.
Next we examined how the lipid environment affects β2AR activation of Gi3. In the presence of epinephrine, significantly more β2AR-induced GTP turnover was detected in DDM micelles containing POPE (net neutral lipid) than in DDM micelles containing POPS (net negative) (Fig. 1f). This effect on β2AR-mediated turnover was significant, even though the lipid:DDM mole ratio was only 1:5. POPE did not increase basal GTP turnover by Gi3 in the absence of β2AR (Supplementary Fig. 4). Taken together, these results indicate that the charge property of phospholipids regulates Gi activation by β2AR.
Ca2+ facilitates Gi3 coupling in negatively charged lipids
Ca2+, a ubiquitous second messenger, plays an important role in cardiac myocytes; Ca2+ waves, magnified by Gs activation, drive the cardiac myocyte contraction machinery. Recently Ca2+ was reported to regulate T-cell receptor activation by modulating the charge property of lipids27. Given that Ca2+ interaction with negative charge on phospholipids neutralizes the charge, we tested whether Ca2+ improves mB−β2AR−Gi3 coupling efficiency in negatively charged DDM + POPS. Indeed, Ca2+ improved coupling efficiency in DDM + POPS micelles (Fig. 2a), and this effect required lipid (Fig. 2b). Moreover, Ca2+ had little effect on mB−β2AR−Gs interaction, implicating differences in Gs and Gi3 surface charge.
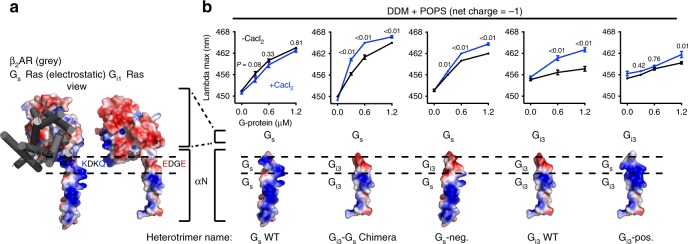
Fig. 2.
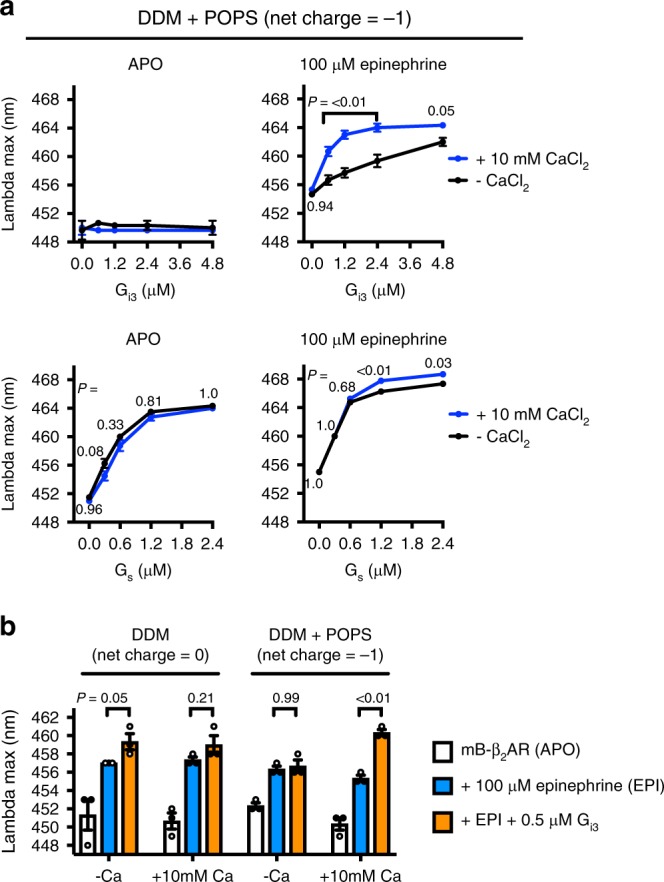
Ca2+ facilitates Gi3 coupling in negatively charged phosphatidylserine. a The effect of 10 mM CaCl2 on mB−β2AR−G protein interaction (Gi3 and Gs) was examined in micelles containing 5:1 DDM:POPS (mole ratio). Data were collected in the absence (APO) and presence (100 μM) of epinephrine. mB−β2AR concentration is 250 nM. Data are mean ± s.e.m. of three independent experiments. Multiplicity adjusted P values were computed by two-way ANOVA followed by Sidak’s post hoc test between CaCl2 conditions. b The effect of 10 mM CaCl2 on mB−β2AR−Gi3 interaction in DDM micelles ± POPS (i.e. DDM alone vs. 2.5:1 DDM:POPS mole ratio). mB−β2AR concentration is 250 nM. Data are mean ± s.e.m. of three independent experiments. Multiplicity adjusted P values were computed by three-way ANOVA followed by Sidak’s post hoc test between indicated groups. Source data are provided in the Source Data File
Ca2+ interacts with the amino terminal helix of Gi3
Next, we sought to determine the mechanism by which Ca2+−POPS interactions increase mB−β2AR coupling to Gi3 but not to Gs. Given that the amino terminal helix (αN) of G protein is adjacent to the membrane when coupled to the β2AR25, and polybasic residues on Gs αN are known to facilitate membrane interaction28, we looked for a possible selectivity determinant within αN. Since αN of Gs and Gi are differentially charged (Fig. 3a), we first replaced αN of Gs with αN of Gi3, creating a Gi3-Gs chimera (Fig. 3b).
Fig. 3.
Ca2+ interacts with the amino terminal helix of Gi3. a Membrane-facing surfaces of Gs and Gi1 Ras domains, with β2AR shown in gray. The membrane-facing surface of Gi1 was modeled by superimposing the Ras domain of Gi1 (PDB: 1GP270) onto the structure of Gs in complex with β2AR (PDB: 3SN669). Red and blue signify negative and positive charge on G protein, respectively. Dashed lines highlight the region in αN where charge differs: The sequence is KDKQ in WT Gs vs. EDGE in WT Gi1 (and in Gi2, Gi3). b mB−β2AR−G protein dose−response curves ± 10 mM CaCl2. Data were generated with the G protein depicted below the curves: mutations were made in αN and corresponding electrostatic models are shown. Epinephrine was not included in experiments titrating Gs WT, Gi3-Gs Chimera, or Gs-neg. to enhance the effect of CaCl2. Epinephrine (100 μM) was included in experiments titrating Gi3 WT and Gi3-pos. mB−β2AR concentration is 250 nM. Data are mean ± s.e.m. of three independent experiments. Multiplicity adjusted P values were computed by two-way ANOVA followed by Sidak’s post hoc test between CaCl2 conditions. Source data are provided in the Source Data File
While Ca2+ does not promote mB−β2AR coupling to WT Gs (Fig. 3b), it did promote mB−β2AR coupling to the Gi3-Gs chimera (Fig. 3b). Next, we compared the membrane-facing charge of Gs WT αN and Gi3 WT αN. Structural analysis revealed that charge differed at the C terminal end of αN: Gi harbors a negatively charged motif (EDGE) at the position where Gs harbors a positively charged motif (KDKQ) (Fig. 3a). To examine whether this motif dictates a differential response to Ca2+, we constructed a Gs mutant (Gs-neg.) containing the negatively charged motif of Gi3 (KDKQ → EDGE). Ca2+ increased mB−β2AR interaction with this mutant (Fig. 3b), suggesting the EDGE motif is responsible for the effect of Ca2+ on Gi3 αN. Taken together, our results imply that Ca2+ coordinates an interaction between the negatively charged EDGE motif on αN of Gi3 and the headgroup of POPS. In the absence of Ca2+, like-charge repulsion decreases mB-β2AR coupling to Gi3.
We also constructed a Gi3 mutant (Gi3-pos.) containing the positively charged motif of Gs (EDGE → KDKQ). The mutations only partially removed the effect of Ca2+ (Fig. 3b), indicating the effect of Ca2+ on Gi3 extends beyond an effect on αN (see Discussion).
Bilayer charge differentially affects Gs and Gi3 coupling
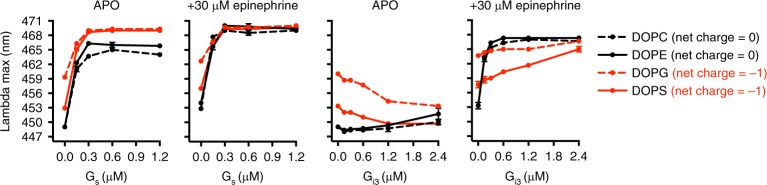
Owing to their geometry and charge, phospholipids might induce changes in the size and shape of micelle assemblies which could also influence mB−β2AR−G protein interaction29,30. To examine the effects of phospholipids in a more native environment, and to restrict the size and shape of lipid ensembles, helping us isolate the effect of membrane charge, we reconstituted mB−β2AR into nanodisc bilayers and purified the nanodiscs to homogeneity using size-exclusion chromatography (Supplementary Fig. 5).
First, we compared the influence of lipid composition in the absence of Ca2+. Negatively charged bilayers (DOPG and DOPS bilayers), previously reported to increase agonist affinity14, expectedly red-shifted the emission spectra of unliganded mB−β2AR, suggesting these lipids stabilize an active conformation. Additionally, unliganded mB-β2AR and Gs could fully couple in negatively charged bilayers but not in neutral bilayers (Fig. 4), suggesting negatively charged lipids might facilitate signaling through Gs. In contrast, negatively charged lipids (especially DOPS bilayers) decreased Gi3 coupling to epinephrine-activated mB−β2AR (Fig. 4).
Fig. 4.
Bilayer charge differentially affects Gs and Gi3 coupling. The effect of Gs (left) and Gi3 (right) concentration on mB−β2AR fluorescence was examined in nanodisc bilayers of varying phospholipid composition (DOPC, DOPE, DOPG, DOPS). Epinephrine was omitted (APO) or included (30 μM). Interaction with G protein is read out as an increase in lambda max. The net charge of the phospholipid is indicated in parentheses. mB−β2AR concentration is 100 nM; maximum stoichiometry is 12:1 (for Gs) and 24:1 (for Gi3). Data are mean ± s.e.m. of three independent experiments. Source data are provided in the Source Data File
In fact, in negatively charged bilayers without epinephrine, Gi3 unexpectedly blue-shifted the emission spectra of mB−β2AR. While this may indicate that Gi3 stabilizes the β2AR in an inactive conformation in negatively charged lipids, it may represent a nonspecific interaction of inactive Gi3 with the β2AR or the lipid bilayer.
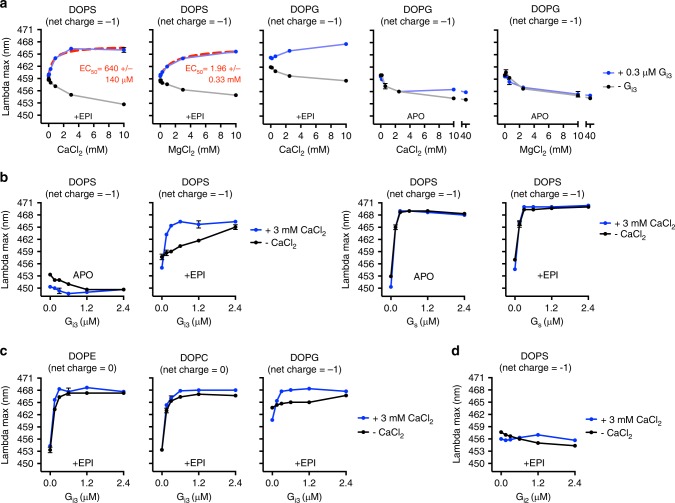
Cations promote Gi3 coupling in negatively charged bilayers
Next we examined the effect of Ca2+ and Mg2+. In the absence of Gi3, both Ca2+ and Mg2+ reversed the active-state stabilizing effect of negatively charged DOPS and DOPG bilayers (Fig. 5a). Despite this, Ca2+ and Mg2+ increased mB-β2AR coupling to Gi3 in negatively charged DOPS bilayers, but only Ca2+ was efficacious at concentrations below 1 mM (Fig. 5a). Ca2+ similarly affected mB−β2AR−Gi3 interaction in negatively charged DOPG bilayers (Fig. 5a), but the magnitude of the effect in DOPG bilayers was less than observed in DOPS bilayers due to the higher baseline effect of DOPG on β2AR conformation.
Fig. 5.
Ca2+ and Mg2+ facilitate Gi3 coupling in negatively charged bilayers a The effect of CaCl2 and MgCl2 concentration on mB−β2AR fluorescence in DOPS and DOPG nanodisc bilayers was examined in the presence and absence of Gi3. Epinephrine was included (30 μM) or omitted (APO). EC50 is mean ± s.e.m. b The effect of G protein concentration (Gi3 and Gs) on mB−β2AR fluorescence ± 3 mM CaCl2 was examined in DOPS nanodiscs in the absence (APO) and in the presence (EPI) of epinephrine (30 μM). c The effect of Gi3 on mB−β2AR fluorescence ± 3 mM CaCl2 was examined in DOPE, DOPC, and DOPG nanodisc bilayers in the presence of 30 μM epinephrine (EPI). d The effect of Gi2 concentration on mB−β2AR fluorescence ± 3 mM CaCl2 was examined in DOPS nanodisc bilayers in the presence of 30 μM epinephrine (EPI). a–d mB−β2AR concentration is 100 nM. The net charge of the phospholipid molecule is indicated in parentheses. Data are mean ± s.e.m. of three independent experiments. Source data are provided in the Source Data File
We also compared the effect of Ca2+ on mB−β2AR interactions with G protein subtypes (Gs versus Gi3) in DOPS bilayers. As observed in micelles, Ca2+ increased mB−β2AR coupling to Gi3 but not to Gs (Fig. 5b). Ca2+ also improved mB−β2AR−Gi3 coupling efficiency in negatively charged DOPG bilayers (Fig. 5c). However, incorporating Ca2+ did not enable detection of mB−β2AR interaction with the Gi2 subtype of Gi (Fig. 5d, see Discussion).
While Ca2+ interacts with net negative PS and PG with relatively high affinity, it also interacts with the negatively charged phosphate group on net neutral PC31 and PE lipids31,32. In neutral DOPE and DOPC bilayers (Fig. 5c and Supplementary Fig. 6), Ca2+ only slightly enhanced mB−β2AR−Gi3 interaction, which could be attributable to weaker Ca2+/DOPE and Ca2+/DOPC interactions that have been reported. Taken together, our observations provide biochemical evidence that local membrane charge can regulate β2AR−G protein interaction.
Discussion
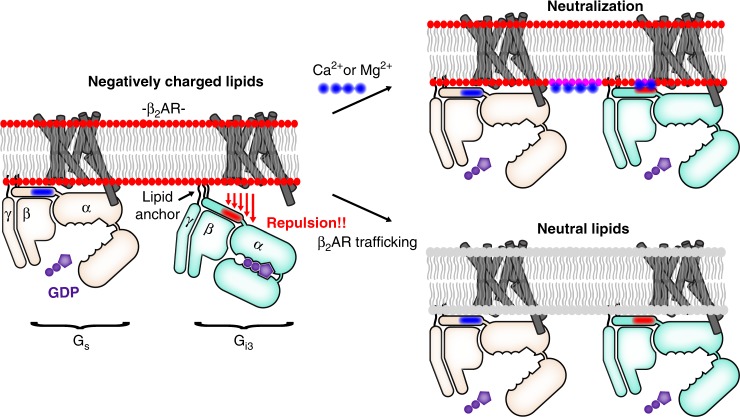
We observed that local membrane charge regulates β2AR−G protein interaction. Negatively charged membrane promotes β2AR−Gs coupling and suppresses β2AR−Gi3 coupling. However, Gs bias is reduced in neutral membrane and in negatively charged membrane in the presence of divalent cations (see model in Fig. 6).
Fig. 6.
Membrane charge is a tunable modulator of β2AR−G protein interaction. Models depict epinephrine-bound β2AR. Left: In negatively charged lipids, β2AR−Gs coupling is efficient, but β2AR−Gi3 coupling is relatively inefficient, in part because β2AR−Gi3 attraction is countered by membrane-Gi3 repulsion. Specifically, negatively charged lipids repel the negatively charged EDGE motif found on the amino terminal helix of Gi3 (shown in red), a region that is positively charged in Gs (shown in blue). Right: Two mechanisms that neutralize membrane charge facilitate β2AR coupling to Gi3. These mechanisms may play a role in Gs-to-Gi switching in cardiac myocytes. Top right: Ca2+ and Mg2+ stabilize a like-charge interaction between the membrane and the EDGE motif. (Note that the effect of Ca2+ and Mg2+ may extend beyond an effect on αN positioning.) Bottom right: Epinephrine-stimulated β2AR traffics to membrane without negatively charged lipids
We have begun to explore the mechanism by which Ca2+ increases mB−β2AR coupling to Gi3 in phospholipids. The effect of Ca2+ was largely dependent on charged groups on Gi3, indicating Ca2+ doesn’t simply affect membrane structure. Although G proteins are membrane tethered via lipidation, the lipid anchor of Gi3 is not sufficient for optimal interaction with β2AR in negatively charged bilayers, possibly due to repulsion of the carboxyl terminal end of the αN helix. We propose that Ca2+ helps orient the carboxyl terminal end of the αN helix of Gi3 near the membrane, thereby facilitating β2AR−Gi3 interactions. More specifically, we propose that Ca2+ facilitates the interaction of lipid with the negatively charged EDGE motif on the αN helix of Gi3. Ca2+ may coordinate a like-charge interaction between the carboxylate groups on the Gi3 EDGE motif and the phosphate group present on all lipids. In addition, Ca2+ may especially stabilize EDGE interaction with PS lipids by coordinating a like-charge interaction between the carboxylate groups on the Gi3 EDGE motif and the carboxylate group on PS (not present on PG) (refer to structures in Fig. 1b), or Ca2+ might coordinate an intramolecular interaction between the phosphate group and the carboxylate group on PS, freeing the amino group (NH3+) on PS to interact with the carboxylate groups on Gi3.
We have previously shown that negatively charged lipids, particularly PG, stabilize the β2AR in an active-like conformation as revealed by changes in mB−β2AR fluorescence and an increased affinity for agonists14. These effects are likely due to interactions between the lipids and positively charged amino acids on the β2AR. Here we observed that the effect of DOPG and DOPS on mB-β2AR can be reversed by both Ca2+ and Mg2+ (Fig. 5a). Yet, these divalent cations do not appear to reduce coupling to Gs.
β2AR signals from caveolin-rich rafts33,34 within T-tubules19. While β2AR preferentially interacts with PG in insect cell membrane14, the phospholipid composition immediately adjacent to β2AR in T-tubules, and how it changes during β2AR trafficking, is currently unknown. Net-neutral PC and PE are the major phospholipids in T-tubules35–37. However, negatively charged PS is enriched in T-tubules relative to other membrane fractions (7.5−12.3% of total phospholipid)35–39. While cytosolic Ca2+ concentrations are typically less than 1 mM40, concentrations of Ca2+ in the mM range may be observed in cardiac myocytes.
Investigators have long speculated about the functional role of Ca2+ in the cleft between the T-tubule membrane (where β2AR is localized) and the juxtaposed sarcoplasmic reticulum (SR)21,22,41. During each action potential, extracellular Ca2+ flows into the cleft through L-type Ca2+ channels (LTCCs) on the plasma membrane and through ryanodine receptors (RyRs) on the sarcoplasmic reticulum40. Cleft Ca2+ concentrations spark to >100 μM in the absence of epinephrine and >1 mM42,43 following epinephrine stimulation, a consequence of Gs activation. Computational models show that negatively charged phospholipids buffer approximately half the Ca2+ released into the cleft42, and experiments have shown that 80% of inner-leaflet bound Ca2+ is bound to negatively charged phospholipids44. Additionally, biochemical investigations show that Ca2+ can cluster negatively charged PS45 and PIP246,47 lipids.
β1AR and β2AR signaling through Gs alters calcium handling in the cardiac myocyte, and increases the magnitude of Ca2+ currents and Ca2+ transients, which stimulate cardiac contraction40,48. However, elevated Ca2+ concentrations also activate the Ca2+/calmodulin-dependent protein kinase II (CaMKII), which promotes apoptosis, and is implicated in structural remodeling that ultimately results in cardiac dysfunction9,49–52. Elevated Ca2+ also activates calcineurin, which exacerbates pathological hypertrophy21. For these reasons, β1AR-selective (and nonselective) beta blockers have proven to be efficacious medicines for treatment of heart failure53. However, β2AR, owing to its dual Gs/Gi selectivity, is functionally distinct from the strictly Gs-coupled β1AR, and several lines of evidence suggest β2AR−Gi signaling functions to keep Gs signaling in check via negative feedback: β2AR−Gi signaling occurs minutes after β1AR−Gs and β2AR−Gs signaling2, β2AR−Gi signaling suppresses changes in calcium handling48,54, and β2AR−Gi signaling is antiapoptotic7,8. While the mechanism that triggers β2AR−Gi signaling is unknown, our biochemical observations suggest elevated Ca2+ concentrations could trigger β2AR coupling to Gi3. It is notable that overexpression of the Ca2+/sodium exchanger facilitates β2AR−Gi suppression of β1AR−Gs signaling55, and overexpression has been cited to increase the inward LTCC Ca2+ current56.
It is also notable that intracellular Ca2+ 57,58 and Mg2+ 58 concentrations rise during ischemia and rise even higher during reperfusion. Whether the rising concentrations facilitate β2AR-Gi signaling is unknown. However, β2AR−Gi signaling can reduce the extent of cardiac necrosis caused by ischemia and reperfusion59.
We were surprised that we did not observe mB−β2AR coupling to Gi2 under conditions where we observed coupling to Gi1 and Gi3 (Fig. 1c, Supplementary Fig. 3). Although Gi2 contains the αN EDGE motif, we did not observe mB−β2AR−Gi2 interaction in the presence of Ca2+ (Fig. 5d). While the sequences of Gαi1 and Gαi3 are 94% identical, Gαi2 shares less sequence identity with Gαi1 and Gαi3, 88% and 86%, respectively. Supplementary Fig. 7 shows the location of the amino acid differences in Gi2 relative to both Gi1 and Gi3 using the recent cryo-electron microscopy structure of the adenosine A1-Gi2 complex (PDB: 6D9H60). It can be seen that these amino acids do not appear to interact directly with the receptor in the nucleotide-free complex. However, these amino acids may form weak interactions with the poorly ordered intracellular loop 3 that is not observed in the structure, or may interact with other domains of the receptor during complex formation. Previous studies provide evidence for at least one transient intermediate state in formation of the β2AR−Gs complex61. Both Gi2 and Gi3 couple to β2AR in cardiac myocytes3. However, during heart failure, Gi2 expression is commonly upregulated62–64. In contrast, Gi3 upregulation, while reported65, is less commonly observed. How selective Gi2 upregulation influences β2AR−Gi signaling is not fully understood. Signaling through Gi2 and Gi3 could play different roles in cardiac physiology. It is possible that β2AR coupling to Gi3 plays a role in preventing myocyte damage during transient ischemia or prolonged periods of adrenergic stimulation, such as during exercise. Both of these conditions would be associated with elevated cytosolic Ca2+. In contrast, β2AR coupling to Gi2 may play a more prominent role in the failing heart.
G proteins are a large superfamily, grouped into four subfamilies (Gs, Gi/o, Gq/11, G12/13) encoded by 16 different genes66. Each subfamily activates distinct signaling pathways, and functional effects are cell-type specific. Most GPCRs can signal through more than one G protein subfamily, and ongoing research attempts to identify mechanisms that regulate G protein selectivity within a cell66. Whether local membrane charge affects Gi interaction with other Gi-coupled GPCRs is not currently known. In cardiac myocytes, β2AR signals from a PS-enriched, Ca2+-enriched microenvironment, which highlights the potential relevance of our biochemical observations. Additionally, the observation that Ca2+ sensing receptor (CaSR) switches from Gq to Gi after cytosolic Ca2+ increases67 is also potentially relevant to our findings. However, knowledge that Ca2+ facilitates Gi interaction with membrane is not sufficient to predict how Ca2+ might affect Gi interaction with other receptors.
In conclusion, we show that local membrane charge differentially modulates β2AR interaction with competing G protein subtypes (Gs and Gi). This discovery expands our knowledge of mechanisms that regulate the G protein coupling selectivity of GPCRs.
Methods
G protein expression and purification
All G proteins were human heterotrimeric G proteins (Gα, Gβ1, Gγ2). The Gβ1 subunit contained an N-terminal 6xHis tag followed by a rhinovirus 3C protease site used for purification61. Gαs was the short splice variant. The Gi3-Gs chimera was created starting with Gαs, replacing residues 1−38 (the αN helix) with the equivalent region of Gαi3 (residues 1−31 of Gαi3)68. Gs-neg. was a Gs mutant with residues 32−35 of Gαs (KDKQ) replaced with the equivalent region in Gαi3 (EDGE). Gi3-pos. was a Gi3 mutant with residues 25−28 of Gαi3 (EDGE) replaced with the equivalent region in Gαs (KDKQ). G protein was expressed in Tni insect cells (Expression Systems Cat. 94-002S) using two recombinant baculoviruses, a virus encoding Gα and a separate virus encoding both the Gβ1 and Gγ2 subunits. Following infection, cells were incubated for 48 h at 27 °C, harvested by centrifugation, and suspended in lysis buffer (10 mM Tris (pH 7.5), 100 μM MgCl2, 5 mM β-mercaptoethanol (BME), 10 μM GDP, and protease inhibitors). The membrane fraction was collected by centrifugation and solubilized using a Dounce homogenizer and buffer comprised of 20 mM HEPES (pH 7.4), 100 mM NaCl, 1% sodium cholate, 0.05% n-dodecyl-β-d-maltopyranoside (DDM), 5 mM MgCl2, 5 mM BME, 10 μM GDP, and protease inhibitors. The soluble fraction was isolated by centrifugation, G protein was captured on Sepharose Fast Flow (GE Healthcare) charged with nickel, and gradually exchanged into SEC buffer (20 mM HEPES (pH 7.4), 100 mM NaCl, 0.05% DDM, 100 μM TCEP, 10 μM GDP, 1 mM MgCl2). 3C protease was added to cleave G protein off the resin, and the G protein was dephosphorylated using calf intestinal alkaline phosphatase, antarctic phosphatase, and lambda protein phosphatase. Subsequently, G protein was isolated in SEC buffer using a Superdex 200 10/300 GL column (GE Healthcare). The main peak corresponding to heterotrimeric G protein was collected, concentrated, and frozen.
Receptor expression, purification, and labeling
The β2AR construct was PN114, where human WT β2AR (R16, Q27 variant) is modified to contain an N-terminal FLAG tag, a C-terminal rho-1D4 tag, a TEV protease cleavage site between V24 and T25, and a 3C protease cleavage site between G365 and Y366. Additionally, mutations were introduced to increase expression (M96T, M98T), to remove a glycosylation site (N187E) and to remove reactive cysteines (C378A, C406A). β2AR was expressed in Sf9 insect cells (Expression Systems Cat. 94-001S) using recombinant baculovirus and media supplemented with 1 μM alprenolol. Cells expressing β2AR were harvested by centrifugation and suspended in lysis buffer (10 mM HEPES (pH 7.4), 1 mM EDTA, 1 μM alprenolol, and protease inhibitors). The membrane fraction was collected by centrifugation and solubilized using a Dounce homogenizer and buffer comprised of 20 mM HEPES (pH 7.4), 100 mM NaCl, 1% n-dodecyl-β-d-maltopyranoside (DDM), 0.03% CHS, 2 mM MgCl2, 1 μM alprenolol, and protease inhibitors. The soluble fraction was isolated by centrifugation and anti-FLAG (ATCC HB-9259) affinity chromatography was used to purify β2AR, remove alprenolol, and adjust detergent concentration (to 0.1% DDM, 0.01% CHS). Monobromobimane (mB, Thermo Fisher Scientific) labeling was then performed overnight with excess mB in the presence of 100 μM TCEP and the reaction was quenched with 5 mM l-cysteine before further purification. All β2AR preparations were functionally purified by alprenolol-Sepharose affinity chromatography and washed on an anti-FLAG column to remove ligand. The eluted β2AR was dialyzed in buffer comprised of 20 mM HEPES (pH 7.4), 100 mM NaCl, 0.1% DDM, and 0.02% CHS, concentrated, and dephosphorylated using lambda protein phosphatase. For experiments assessing phosphorylation, unphosphorylated β2AR was phosphorylated with protein kinase A in the presence of 2 mM ATP. β2AR was stored frozen. Phosphorylation was assessed using the Pro-Q Diamond Phosphoprotein Gel Stain (ThermoFisher Scientific), per the manufacturer’s instructions. Stained acrylamide gels were scanned with a Typhoon 9410 Imager (GE Healthcare). Recombinant human NTSR1 (residues 20−418) contained an A851.54L mutation to increase expression. NTSR1 was purified from Sf9 insect cells into buffer comprised of 20 mM HEPES (pH 7.5), 100 mM NaCl, 5% glycerol, 0.01% lauryl maltose neopentyl glycol (LMNG), 0.001% CHS, and 0.5 µM JMV 449, and stored frozen.
Ligands
(−) epinephrine was purchased from Sigma (purity > 99%). JMV 449 was purchased from Tocris (purity 97.6%).
Micelle composition
n-dodecyl-β-d-maltopyranoside (DDM), cholesteryl hemisuccinate (CHS), and 1-palmitoyl-2-oleoyl-sn-glycero-3-(PE,PC,PG,PS) lipids (Avanti Polar Lipids) were mixed in the indicated ratios and solubilized in chloroform. Chloroform was evaporated, and the films were re-suspended in 20 mM HEPES (pH 7.4), 100 mM NaCl.
Fluorescence spectroscopy
In experiments examining mB-β2AR in micelles, mB−β2AR was preincubated (30 min room temperature) in micelle stock prior to dilution with other reaction components. pH 7.4 HEPES buffer (containing 100 mM NaCl, ± ligand, ± CaCl2 or MgCl2) and G protein were sequentially included. Mixtures were incubated 2.5–3.0 h at room temperature. Final mB−β2AR concentration was 100−300 nM. Emission spectra were read at 22 °C using a Fluorolog-3 spectrofluorometer (Horiba Jobin Yvon Inc.). (Bandpass = 4 nm; Excitation = 370 nm; Emission = 420−500 nm, collected in 1 nm increments). The raw S1c/R1c spectra were smoothed using Prism (GraphPad Software) (n = 15 neighbors, second-order polynomial). Lambda max is defined as the wavelength at which fluorescence emission is maximum. To determine the EC50, data were fit to the agonist vs. response model in Prism 7.0d software.
GTP turnover
Where lipid environments were compared, samples were prepared as they were for fluorescence spectroscopy. Following β2AR incubation with G protein, 1 μM GTP + 5 μM GDP mixtures (final concentration) were added to initiate the GTP turnover reaction. Reaction buffer contained 20 mM HEPES (pH 7.4) and 100 mM NaCl. Where Gi2 and Gi3 were compared, the GTP turnover reaction was initiated by mixing a solution containing 10 μM GTP and ligand-bound receptor (4 μM β2AR + 800 μM epinephrine or 1 μM NTSR1 + 10 μM JMV 449) in equal volume with a solution containing 2.5 μM G protein, 20 μM GDP, 20 mM MgCl2, and 200 μM TCEP. At the indicated timepoints, the GTP remaining was assessed using the GTPase-Glo assay (Promega), which detects GTP using a luminescence readout. Luminescence was detected using a SpectraMax Paradigm plate reader equipped with a TUNE SpectraMax detection cartridge (Molecular Devices). Background luminescence was subtracted from experimental reactions.
Statistics
Two-way and three-way ANOVA were performed using Graphpad Prism 7.0d.
Electrostatic modeling
Structural views and mutant models were generated using PyMOL (Schrödinger, LLC). We selected rotomer positions that most closely matched those seen in PDB 3SN669 (for the Gi3-pos. model) and PDB 1GP270 (for the Gs-neg. model). Continuum electrostatics models were calculated using the APBS71 plugin (MG Lerner, University of Michigan, Ann Arbor) for PyMOL. Atomic charge and radii were calculated using the online PDB2PQR server72 (pH 7.4, PARSE force field, hydrogen bond optimization, clash avoidance).
Nanodisc reagents
1,2-dioleoyl-sn-glycero-3-(PE,PC,PG,PS) lipids (Avanti Polar Lipids) were used because of their low phase transition temperature. Lipids were dissolved in buffer comprised of 20 mM HEPES (pH 7.5), 100 mM NaCl, 50 mM sodium cholate, 1 mM EDTA at 16.6 mM, and were sonicated on ice before use. The MSP belt was MSP1E3D173. pMSP1E3D1 was a gift from Stephen Sligar (Addgene plasmid #20066). The protein was expressed in BL21(DE3) E. coli (Cat. 70235-3 Millipore Sigma), and cells were lysed with sonication in 10 mM Tris-HCl, 100 mM NaH2PO4, 6 M GuHCl, 1% Triton X-100 (pH 8.0). The soluble fraction was isolated by centrifugation and passed through a Sepharose Fast Flow (GE Healthcare) column charged with nickel. Immobilized protein was washed with buffer comprised of 10 mM Tris-HCl, 100 mM NaH2PO4, 6 M GuHCl, and 0.2% Triton X-100 (pH 7.0), and then washed with buffer comprised of 50 mM NaH2PO4, 300 mM NaCl, and 0.2% Triton X-100 (pH 8.0). The protein was eluted using 250 mM imidazole. Impurities were precipitated by two rounds of heating at 70 °C for 1 h; after each round, the soluble fraction was isolate by centrifugation. The final soluble fraction containing MSP1E3D1 was supplemented with 20 mM sodium cholate and purified further using a HiPrep 16/60 Sephacryl S-300 HR size-exclusion column (GE Healthcare) equilibrated with buffer comprised of 20 mM HEPES, 100 mM NaCl, 1 mM EDTA, and 20 mM sodium cholate. The main peak corresponding to MSP1E3D1 was collected, dialyzed in 20 mM HEPES, 100 mM NaCl, 1 mM EDTA, 5 mM sodium cholate, concentrated to 728 μM, and frozen.
Nanodisc reconstitution
Nanodiscs were formed with one lipid type (i.e. 100% DOPS, DOPG, DOPE, or DOPC). Reconstitution was initiated by sequentially mixing water, 20× reconstitution buffer (400 mM HEPES (pH 7.5), 2 M NaCl, 20 mM EDTA), lipid, mB−β2AR, and MSP1E3D1. The volume of mB−β2AR stock added to the mixture was ~13% of the final mixture volume. MSP1E3D1 and mB−β2AR were added 1:10 (molar ratio). Lipid and MSP1E3D1 were added 35:1 (molar ratio). The mixture was incubated at 4 °C for 2 h. Subsequently, Bio-Beads SM-2 resin (107.1 mg per μmol lipid, Bio-Rad) were added (4 °C for 4 h) to remove detergent, which triggers the reconstitution of mB−β2AR in nanodisc bilayers. The soluble fraction was isolated by centrifugation. Bare nanodiscs were separated from nanodiscs containing mB−β2AR using anti-FLAG (ATCC HB-9259) affinity chromatography. The eluate was incubated with 5 mM EDTA at 4 °C for ≥ 1.5 h to remove divalent cations. Subsequently, samples were injected into a Superdex 200 10/300GL size-exclusion column (GE Healthcare) equilibrated in buffer comprised of 20 mM HEPES (pH 7.4), 100 mM NaCl, and the main peak corresponding to nanodisc mB−β2AR was harvested, concentrated, and frozen. The concentration of nanodisc mB−β2AR was approximated by SDS-PAGE, using detergent solubilized β2AR as a protein concentration reference standard.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Betsy White for assistance with G protein and β2AR expression, and Hideaki Kato for providing purified NTSR1. This work was supported by the National Institutes of Health grants R01NS028471 and R01GM083118 (B.K.K.). B.K.K. is supported by the Chan Zuckerberg Biohub. D.H. was supported by the German Academic Exchange Service (DAAD). M.M. was supported by the American Heart Association Postdoctoral fellowship (17POST33410958).
Author contributions
M.J.S. designed, performed, and interpreted the research, and wrote the manuscript. B.K.K. championed the investigation, advised on the project, and edited the manuscript. M.J.S. performed the β2AR and G protein purifications, labeling, nanodisc reconstitutions, and all of the experiments, and S.M., D.H., M.M. and Y.D. provided valuable technical assistance as described: S.M. advised on cloning and provided G protein for pilot experiments. D.H. advised on G protein purification and the GTP turnover assay. M.M. advised on nanodisc reconstitution and provided MSP1E3D1 protein. Y.D. collaborated on pilot experiments not included.
Data availability
The source data underlying Figs. 1a, c−f, 2a, b, 3b, 4, 5a–d, and Supplementary Figs. 1a−c, 2a, b, 3a, b, 4, and 6 are provided as a Source Data File. pMSP1E3D1 is available from Addgene (#20066). All PDB files that were analyzed have been published before and can be obtained from the RCSB Protein Data Bank using the accession codes 5JQH, 3SN6, 1GP2, and 6D9H. A reporting summary for this Article is available as a Supplementary Information file. All other datasets supporting the findings of the study are available from the corresponding author on reasonable request.
Competing interests
B.K.K. is a co-founder of and consultant for ConfometRx, Inc. The other authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Amitabha Chattopadhyay, Sergi Ferre and Anthony Lee for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-10108-0.
References
- 1.Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 2017;16:829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devic E, Xiang Y, Gould D, Kobilka B. beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol. Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 3.Xiao RP, et al. Coupling of beta2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 1999;84:43–52. doi: 10.1161/01.RES.84.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Xiang Y, Devic E, Kobilka B. The PDZ binding motif of the beta 1 adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J. Biol. Chem. 2002;277:33783–33790. doi: 10.1074/jbc.M204136200. [DOI] [PubMed] [Google Scholar]
- 5.Xiang Y, Kobilka B. The PDZ-binding motif of the 2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc. Natl. Acad. Sci. USA. 2003;100:10776–10781. doi: 10.1073/pnas.1831718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, et al. Norepinephrine- and epinephrine-induced distinct beta2-adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. J. Biol. Chem. 2008;283:1799–1807. doi: 10.1074/jbc.M705747200. [DOI] [PubMed] [Google Scholar]
- 7.Zhu WZ, et al. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl. Acad. Sci. USA. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesley A, et al. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3’-kinase. Circ. Res. 2000;87:1172–1179. doi: 10.1161/01.RES.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 9.Schmid E, et al. Cardiac RKIP induces a beneficial beta-adrenoceptor-dependent positive inotropy. Nat. Med. 2015;21:1298–1306. doi: 10.1038/nm.3972. [DOI] [PubMed] [Google Scholar]
- 10.Fajardo G, et al. Deletion of the beta2-adrenergic receptor prevents the development of cardiomyopathy in mice. J. Mol. Cell Cardiol. 2013;63:155–164. doi: 10.1016/j.yjmcc.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system. J. Biol. Chem. 2002;277:31249–31256. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]
- 12.Lefkowitz RJ, Daaka Y, Luttrell LM. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 13.Zhu W, et al. Gi-biased 2AR signaling links GRK2 upregulation to heart failure. Circ. Res. 2011;110:265–274. doi: 10.1161/CIRCRESAHA.111.253260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawaliby R, et al. Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat. Chem. Biol. 2016;12:35–39. doi: 10.1038/nchembio.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen HY, et al. PtdIns(4,5)P2 stabilizes active states of GPCRs and enhances selectivity of G-protein coupling. Nature. 2018;559:423–427. doi: 10.1038/s41586-018-0325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inagaki S, et al. Modulation of the interaction between neurotensin receptor NTS1 and Gq protein by lipid. J. Mol. Biol. 2012;417:95–111. doi: 10.1016/j.jmb.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vukoti K, Kimura T, Macke L, Gawrisch K, Yeliseev A. Stabilization of functional recombinant cannabinoid receptor CB(2) in detergent micelles and lipid bilayers. PLoS ONE. 2012;7:e46290. doi: 10.1371/journal.pone.0046290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaya AI, Thaker TM, Preininger AM, Iverson TM, Hamm HE. Coupling efficiency of rhodopsin and transducin in bicelles. Biochemistry. 2011;50:3193–3203. doi: 10.1021/bi200037j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolaev VO, et al. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 20.Hong T, Shaw RM. Cardiac T-tubule microanatomy and function. Physiol. Rev. 2017;97:227–252. doi: 10.1152/physrev.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houser SR, Molkentin JD. Does contractile Ca2+control calcineurin-NFAT signaling and pathological hypertrophy in cardiac myocytes? Sci. Signal. 2008;1:pe31. doi: 10.1126/scisignal.125pe31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verkleij AJ, Post JA. Membrane phospholipid asymmetry and signal transduction. J. Membr. Biol. 2000;178:1–10. doi: 10.1007/s002320010009. [DOI] [PubMed] [Google Scholar]
- 23.Nygaard R, et al. The dynamic process of beta(2)-adrenergic receptor activation. Cell. 2013;152:532–542. doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manglik A, et al. Structural insights into the dynamic process of beta2-adrenergic receptor signaling. Cell. 2015;161:1101–1111. doi: 10.1016/j.cell.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen SG, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao XJ, et al. The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc. Natl. Acad. Sci. USA. 2009;106:9501–9506. doi: 10.1073/pnas.0811437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi X, et al. Ca2+regulates T-cell receptor activation by modulating the charge property of lipids. Nature. 2013;493:111–115. doi: 10.1038/nature11699. [DOI] [PubMed] [Google Scholar]
- 28.Crouthamel M, Thiyagarajan MM, Evanko DS, Wedegaertner PB. N-terminal polybasic motifs are required for plasma membrane localization of Galpha(s) and Galpha(q) Cell Signal. 2008;20:1900–1910. doi: 10.1016/j.cellsig.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escriba PV, et al. Membrane lipid therapy: modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog. Lipid Res. 2015;59:38–53. doi: 10.1016/j.plipres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez R, et al. G protein-membrane interactions I: Galphai1 myristoyl and palmitoyl modifications in protein-lipid interactions and its implications in membrane microdomain localization. Biochim. et Biophys. Acta. 2015;1851:1511–1520. doi: 10.1016/j.bbalip.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Melcrova A, et al. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016;6:38035. doi: 10.1038/srep38035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Her C, et al. The charge properties of phospholipid nanodiscs. Biophys. J. 2016;111:989–998. doi: 10.1016/j.bpj.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J. Biol. Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 34.Xiang Y. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. J. Biol. Chem. 2002;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- 35.Rosemblatt M, Hidalgo C, Vergara C, Ikemoto N. Immunological and biochemical properties of transverse tubule membranes isolated from rabbit skeletal muscle. J. Biol. Chem. 1981;256:8140–8148. [PubMed] [Google Scholar]
- 36.Lau YH, Caswell AH, Brunschwig JP, Baerwald R, Garcia M. Lipid analysis and freeze-fracture studies on isolated transverse tubules and sarcoplasmic reticulum subfractions of skeletal muscle. J. Biol. Chem. 1979;254:540–546. [PubMed] [Google Scholar]
- 37.Pediconi MF, Donoso P, Hidalgo C, Barrantes FJ. Lipid composition of purified transverse tubule membranes isolated from amphibian skeletal muscle. Biochim. et Biophys. acta. 1987;921:398–404. doi: 10.1016/0005-2760(87)90042-7. [DOI] [PubMed] [Google Scholar]
- 38.Post JA, Langer GA, Op den Kamp JA, Verkleij AJ. Phospholipid asymmetry in cardiac sarcolemma. Analysis of intact cells and ‘gas-dissected’ membranes. Biochim. et Biophys. acta. 1988;943:256–266. doi: 10.1016/0005-2736(88)90557-3. [DOI] [PubMed] [Google Scholar]
- 39.Post JA, Verkleij AJ, Langer GA. Organization and function of sarcolemmal phospholipids in control and ischemic/reperfused cardiomyocytes. J. Mol. Cell Cardiol. 1995;27:749–760. doi: 10.1016/0022-2828(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 40.Fearnley CJ, Roderick HL, Bootman MD. Calcium signaling in cardiac myocytes. Cold Spring Harb. Perspect. Biol. 2011;3:a004242. doi: 10.1101/cshperspect.a004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louch WE, Stokke MK, Sjaastad I, Christensen G, Sejersted OM. No rest for the weary: diastolic calcium homeostasis in the normal and failing myocardium. Physiology (Bethesda, MD) 2012;27:308–323. doi: 10.1152/physiol.00021.2012. [DOI] [PubMed] [Google Scholar]
- 42.Langer GA, Peskoff A. Calcium concentration and movement in the diadic cleft space of the cardiac ventricular cell. Biophys. J. 1996;70:1169–1182. doi: 10.1016/S0006-3495(96)79677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peskoff A, Langer GA. Calcium concentration and movement in the ventricular cardiac cell during an excitation-contraction cycle. Biophys. J. 1998;74:153–174. doi: 10.1016/S0006-3495(98)77776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philipson KD, Bers DM, Nishimoto AY. The role of phospholipids in the Ca2+binding of isolated cardiac sarcolemma. J. Mol. Cell Cardiol. 1980;12:1159–1173. doi: 10.1016/0022-2828(80)90063-2. [DOI] [PubMed] [Google Scholar]
- 45.Boettcher JM, et al. Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry. 2011;50:2264–2273. doi: 10.1021/bi1013694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haverstick DM, Glaser M. Visualization of Ca2+-induced phospholipid domains. Proc. Natl. Acad. Sci. USA. 1987;84:4475–4479. doi: 10.1073/pnas.84.13.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang YH, Slochower DR, Janmey PA. Counterion-mediated cluster formation by polyphosphoinositides. Chem. Phys. Lipids. 2014;182:38–51. doi: 10.1016/j.chemphyslip.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu W, Zeng X, Zheng M, Xiao RP. The enigma of beta2-adrenergic receptor Gi signaling in the heart: the good, the bad, and the ugly. Circ. Res. 2005;97:507–509. doi: 10.1161/01.RES.0000184615.56822.bd. [DOI] [PubMed] [Google Scholar]
- 49.Woo AY, Song Y, Xiao RP, Zhu W. Biased beta2-adrenoceptor signalling in heart failure: pathophysiology and drug discovery. Br. J. Pharmacol. 2015;172:5444–5456. doi: 10.1111/bph.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bayeva M, Sawicki KT, Butler J, Gheorghiade M, Ardehali H. Molecular and cellular basis of viable dysfunctional myocardium. Circ. Heart Fail. 2014;7:680–691. doi: 10.1161/CIRCHEARTFAILURE.113.000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waagstein F, Rutherford JD. The evolution of the use of beta-blockers to treat heart failure: a conversation with Finn Waagstein, MD. Circulation. 2017;136:889–893. doi: 10.1161/CIRCULATIONAHA.117.029934. [DOI] [PubMed] [Google Scholar]
- 52.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernstein D, Fajardo G, Zhao M. The role of beta-adrenergic receptors in heart failure: differential regulation of cardiotoxicity and cardioprotection. Prog. Pedia. Cardiol. 2011;31:35–38. doi: 10.1016/j.ppedcard.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao RP, Ji XW, Lakatta EG. Functional coupling of the beta(2)-adrenoceptor to a Pertussis-toxin-sensitive G-protein in cardiac myocytes. Mol. Pharmacol. 1995;47:322–329. [PubMed] [Google Scholar]
- 55.Sato M, Gong H, Terracciano CM, Ranu H, Harding SE. Loss of beta-adrenoceptor response in myocytes overexpressing the Na+/Ca(2+)-exchanger. J. Mol. Cell Cardiol. 2004;36:43–48. doi: 10.1016/j.yjmcc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Ottolia M, Torres N, Bridge JH, Philipson KD, Goldhaber JI. Na/Ca exchange and contraction of the heart. J. Mol. Cell Cardiol. 2013;61:28–33. doi: 10.1016/j.yjmcc.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy E, Steenbergen C. Ion transport and energetics during cell death and protection. Physiology (Bethesda, MD) 2008;23:115–123. doi: 10.1152/physiol.00044.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong H, Bernstein D, Murphy E, Steenbergen C. The role of beta-adrenergic receptor signaling in cardioprotection. FASEB J. 2005;19:983–985. doi: 10.1096/fj.04-3067fje. [DOI] [PubMed] [Google Scholar]
- 60.Draper-Joyce, C. J. et al. 6D9H. RCSB Protein Data Bank. 10.2210/pdb6D9H/pdb (2018).
- 61.Gregorio GG, et al. Single-molecule analysis of ligand efficacy in beta2AR-G-protein activation. Nature. 2017;547:68–73. doi: 10.1038/nature22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bohm M, et al. Radioimmunochemical quantification of Gi alpha in right and left ventricles from patients with ischaemic and dilated cardiomyopathy and predominant left ventricular failure. J. Mol. Cell Cardiol. 1994;26:133–149. doi: 10.1006/jmcc.1994.1017. [DOI] [PubMed] [Google Scholar]
- 63.Feldman AM, et al. Increase of the 40,000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J. Clin. Investig. 1988;82:189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eschenhagen T, et al. Increased messenger RNA level of the inhibitory G protein alpha subunit Gi alpha-2 in human end-stage heart failure. Circ. Res. 1992;70:688–696. doi: 10.1161/01.RES.70.4.688. [DOI] [PubMed] [Google Scholar]
- 65.Xiao RP. Enhanced Gi signaling selectively negates 2-adrenergic receptor (AR)- but not 1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation. 2003;108:1633–1639. doi: 10.1161/01.CIR.0000087595.17277.73. [DOI] [PubMed] [Google Scholar]
- 66.Flock T, et al. Selectivity determinants of GPCR-G-protein binding. Nature. 2017;545:317–322. doi: 10.1038/nature22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conigrave AD. The calcium-sensing receptor and the parathyroid: past, present, future. Front. Physiol. 2016;7:563. doi: 10.3389/fphys.2016.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maeda S, et al. Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat. Commun. 2018;9:3712. doi: 10.1038/s41467-018-06002-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rasmussen, S. G. F. et al. 3SN6. RCSB Protein Data Bank. 10.2210/pdb3SN6/pdb (2011).
- 70.Wall, M. A. et al. 1GP2. RCSB Protein Data Bank. 10.2210/pdb1GP2/pdb (1997).
- 71.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:W665–W667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Denisov IG, Baas BJ, Grinkova YV, Sligar SG. Cooperativity in cytochrome P450 3A4: linkages in substrate binding, spin state, uncoupling, and product formation. J. Biol. Chem. 2007;282:7066–7076. doi: 10.1074/jbc.M609589200. [DOI] [PubMed] [Google Scholar]
- 74.Staus, D. P. et al. 5JQH. RCSB Protein Data Bank. 10.2210/pdb5JQH/pdb (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source data underlying Figs. 1a, c−f, 2a, b, 3b, 4, 5a–d, and Supplementary Figs. 1a−c, 2a, b, 3a, b, 4, and 6 are provided as a Source Data File. pMSP1E3D1 is available from Addgene (#20066). All PDB files that were analyzed have been published before and can be obtained from the RCSB Protein Data Bank using the accession codes 5JQH, 3SN6, 1GP2, and 6D9H. A reporting summary for this Article is available as a Supplementary Information file. All other datasets supporting the findings of the study are available from the corresponding author on reasonable request.