Abstract
The Colorado potato beetle (Leptinotarsa decemlineata (Say)) is an agricultural pest that threatens the potato industry worldwide. This insect is widely regarded as one of the most difficult-to-control pests, as it can thrive in a wide range of temperature conditions and routinely develops resistance towards various insecticides. The molecular changes associated with response to these challenges have not been fully investigated in L. decemlineata. While differential expression and characterization of heat shock proteins (HSPs) in response to stress have been conducted in several insects, data regarding HSPs in L. decemlineata are limited. The overarching objective of this study consisted of evaluating the expression of various HSPs in L. decemlineata exposed to different temperatures or treated with the insecticides imidacloprid and chlorantraniliprole. Expression levels of HSP60, HSP70, HSP90, and HSP Beta-1 were evaluated by qRT-PCR and insect mortality was assessed using dsRNAs aimed at select HSP targets. Elevated HSP70 and HSP90 transcript levels were observed in heat-exposed L. decemlineata while downregulation of HSP70 transcript levels was measured in insects submitted to cold conditions. Chlorantraniliprole exposure was associated with reduced HSP Beta-1 transcript levels while no change in expression was monitored in insects exposed to imidacloprid. RNAi-based knockdown of HSP60 levels correlated with significant insect mortality 14 days after dsRNA injection. These results highlight the modulation of HSPs that occur in L. decemlineata exposed to fluctuating temperatures and position HSPs as interesting candidates in the identification of novel molecular leads that could be targeted to control this insect.
Electronic supplementary material
The online version of this article (10.1007/s12192-019-00983-3) contains supplementary material, which is available to authorized users.
Keywords: Colorado potato beetle, Heat shock proteins, Cold hardiness, Imidacloprid, Chlorantraniliprole
Introduction
The Colorado potato beetle, Leptinotarsa decemlineata (Say), is an agricultural insect pest that can harm potato crops worldwide (Weber 2003). Adult insects can consume 10 cm2 of potato leaves daily while larvae can consume four times this amount over the same period (Ferro et al. 1985). Efforts to control L. decemlineata have largely relied on various classes of insecticides, towards which L. decemlineata is notorious for developing resistance (Scott et al. 2015). Alternative methods, primarily relying on biological control, are leveraged to target L. decemlineata populations (Alyokhin et al. 2015) but are notably not as widely adopted as insecticides. This insect is also adept at withstanding winter temperatures (Hiiesaar et al. 2001; Izzo et al. 2014) with supercooling points ranging from − 6 °C to − 17 °C observed in overwintering adults (Hiiesaar et al. 2006; Lyytinen et al. 2012). More than one-third of L. decemlineata second instar larvae survived an exposure of 4 h at 43 °C further emphasizing the capacity of this insect to tolerate a wide window of temperatures (Chen et al. 2016). While select targets have been associated with response to these conditions, the molecular basis underlying such adaptation remains to be fully characterized.
The importance of heat shock proteins (HSPs) for stress response has been extensively explored in several insects. Modulation of HSP expression in response to elevated temperatures is well characterized. Pioneering work revealed elevated levels of a heat-inducible HSP70 in the red flour beetle Tribolium castaneum exposed to a heat shock (Mahroof et al. 2005). Such conditions were also associated with upregulation of three HSPs in the maize weevil Sitophilus zeamais (Tungjitwitayakul et al. 2015). HSP70 and HSP90 transcript levels were modulated following heat or cold stress in the orange wheat blossom midge Sitodiplosis mosellana larvae (Cheng et al. 2016). HSP40 and HSP70 protein levels displayed elevated levels in the freeze-tolerant gall fly Eurosta solidaginis when maintained at − 16 °C for 1 day (Zhang et al. 2011). The underlying role of HSPs in moderating insect responses to various insecticides has also been explored. HSP70 mRNA levels were elevated following exposure to beta-cypermethrin in the aphid Rhopalosiphum padi (Li et al. 2017). Increased HSP90 levels were observed in the mirid bug Apolygus lucorum exposed to insecticides and a link between this HSP and adaptation to several chemicals such as cyhalothrin and imidacloprid was proposed (Sun et al. 2014). With a central role in insect stress response, it is not surprising that modulating levels of HSPs via RNA interference (RNAi)-based approaches can lead to insect mortality. Early work highlighted the lethal impact that double-stranded RNA (dsRNA)-mediated knockdown of HSP23 and HSP70 could have on heat shock survival in whitefly Bemisia tabaci females (Lü and Wan 2011). Targeting the heat shock 70-kDa protein cognate 3 using dsRNA in the emerald ash borer Agrilus planipennis resulted in up to 90% mortality in larvae and adult insects (Rodrigues et al. 2018). While the relevance of HSPs for dealing with various stresses has been investigated in multiple insects, information concerning HSP response in stress adaptation in L. decemlineata is sparse and could provide useful targets for RNAi-based pest management approaches.
The current work was performed to profile the expression status of four HSPs in L. decemlineata submitted to heat and cold temperatures as well as exposed to two insecticides. RNAi-mediated knockdown of select HSPs was also performed to assess the impact of such modulation on insect survival. Overall, this study highlights differential expression of various HSPs in response to stress and further reinforces the potential importance of HSPs as molecular targets of interest in the agricultural insect pest L. decemlineata.
Materials and methods
Insect treatments
Adult Colorado potato beetles Leptinotarsa decemlineata (Say) for heat, cold, and imidacloprid treatments were collected in nursery potato fields at the Fredericton Research and Development Centre (FRDC) in the summers of 2016 and 2017 (45° 55′ 17.5″ N, 66° 36′ 01.8″ W, Fredericton, New Brunswick, Canada). Fields had not been previously treated with insecticides prior to sampling. Adult beetles for chlorantraniliprole treatments were obtained from the same nursery fields in the summer of 2017 but reared indoors until December 2017. All insects were deposited in plastic containers that contained potato leaves (var. Kennebec) and that were closed with a screened lid for ventilation. Insects were brought back to Moncton (New Brunswick, Canada).
Heat exposure time-courses were performed by initially acclimating a group of insects to 25 °C for 1 week under a 16 L:8D photoperiod in an incubator (Thermo Fisher Scientific, Waltham, MA, USA). A group of insects was sampled in liquid N2 and used as controls. Temperature was increased to 30 °C and the insects were maintained at this temperature for 2 h. Temperature was further raised to 35 °C and insects were kept for another 2 h. Temperature was ultimately increased to 40 °C and insects were held at this temperature for 2 h. Insect sampling in liquid N2 ensued.
Cold exposure time-courses were conducted as described before (Morin et al. 2017a). Insects were kept for 1 week under a 16 L:8D photoperiod in an incubator set at 15 °C. An initial group of insects was rapidly placed in liquid N2 and subsequently used as controls. Temperature was lowered to 5 °C at which the remaining insects were held for 2 h before another group of insects was placed in liquid N2. Temperature was further lowered to − 5 °C and maintained for another 2 h before insect sampling. Remaining insects were re-acclimated to 15 °C and allowed to recover at this temperature for 24 h before sampling in liquid N2.
Imidacloprid exposure time-courses were conducted as described elsewhere (Morin et al. 2017b). A group of insects was placed in an incubator set at 25 °C for 5 days under 16 L:8D cycles. Topical application of 5 μl (0.5 μg) of imidacloprid (100 μg/ml in acetonitrile, Sigma-Aldrich, St. Louis, MO, USA) was next performed on the abdomen of 15 beetles. An equal volume of acetonitrile was applied on 15 control beetles in parallel. Insects were subsequently returned to the incubator for 24 h before sampling in liquid N2.
Chlorantraniliprole treatment of L. decemlineata was instigated by placing insects in an incubator set at 25 °C for 5 days under 16 L:8D cycles. Following acclimation, 1 μl (1 μg) of chlorantraniliprole solution (Sigma-Aldrich), prepared in acetone, was pipetted topically on the abdomen of 25 beetles. An equal amount of acetone was applied on 25 beetles in parallel which were used as controls. Insects were returned to the incubator for 24 h before sampling as above. All insects, for this treatment and each treatment described above, were stored at − 80 °C until use.
RNA isolation
RNA isolates were prepared from L. decemlineata using the mirVana miRNA Isolation Kit (Thermo Fisher Scientific) following manufacturer’s protocol and as described previously (Morin et al. 2017a). Isolates were prepared using two insects per replicate as starting material. Final products were assessed with a NanoVue Plus Spectrophotometer (GE Healthcare Life Sciences, Mississauga, ON, Canada) and RNA samples were stored at − 80 °C until use.
cDNA synthesis
First strand synthesis for mRNA amplification was conducted by mixing 1 μg of total RNA with 1 μl of oligo dT, 1 μl 10 mM dNTPs, and DEPC-treated water to a volume of 12 μl. Solution was incubated at 65 °C for 5 min and the following reagents were added; 4 μl 5X First Strand Buffer, 2 μl 0.1 M DTT, and 1.5 μl DEPC-treated water. This mixture was placed at 37 °C for 2 min before adding 0.5 μl M-MLV RT. A final incubation at 37 °C for 50 min and 70 °C for 15 min was performed.
qRT-PCR amplification of HSP transcripts
Primers to amplify transcripts were conceived based on L. decemlineata sequences for each respective HSPs. Primers are listed in Table 1. Preliminary amplification reactions contained 5 μl diluted cDNA template (10−1), 1 μl 25 μM forward primer, 1 μl 25 μM reverse primer, 5.5 μl DEPC-treated water, and 12.5 μl 2X Taq FroggaMix. The amplification protocol was comprised of a denaturing step at 95 °C for 5 min, followed by 35 cycles at 95 °C for 15 s, at temperature gradient between 54 and 65 °C for 60 s and 72 °C for 45 s. Electrophoresis of products on a 2% agarose gel to ensure correct size as well as products sequencing at the Université Laval sequencing platform (Quebec City, QC, Canada) were also performed. Targets were also amplified in serial cDNA dilutions by qRT-PCR at different annealing temperatures to assess efficiencies for each primer set. Subsequent qRT-PCR reactions were performed to quantify transcript levels using 2.5 μl of cDNA (10−1), 0.5 μl DEPC-treated water, 1 μl 5 μM forward primer, 1 μl 5 μM reverse primer and 5 μl of iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). The amplification protocol consisted of a denaturing step at 95 °C for 3 min followed by 39 cycles at 95 °C for 15 s and at optimal annealing temperature for 30 s. Transcript levels of α-tubulin were assessed in parallel reactions and used as reference. Significant differences between target expression observed in control insects and insects exposed to heat, imidacloprid, and chlorantraniliprole were evaluated using an unpaired Student’s t test. Significant differences in cold-exposed insects were assessed using one-way analysis of variance (ANOVA). Relative normalized transcript expression data and statistical analysis of data were performed using CFX Maestro software version 1.1 (Bio-Rad).
Table 1.
Primers used to amplify heat shock proteins transcripts of interest by qRT-PCR. Forward (Fwd) and reverse (Rev) primers
| Hsp60 |
Fwd Rev |
5′- GTCACCATGGGTCCAAAGGGC -3′ 5′- TTCTTTGGCAATAGAACGGGC -3′ |
98.7% | 61.0 °C |
| Hsp70 |
Fwd Rev |
5′- GACGAGAAGCAAAGGCAAAG -3′ 5′- CCAATGAGTTGCTGTCTAACCA -3′ |
108.9% | 58.6 °C |
| Hsp90 |
Fwd Rev |
5′- GACCTTGTAAACAACTTGGGT -3′ 5′- ACACCAAACTGTACCAATCAT -3’ |
101.9% | 64.0 °C |
| Hsp Beta-1 |
Fwd Rev |
5′- GAAGAGATTGTAGTTAAAACC -3′ 5′- TTAGTGTTGAATTGGAATAAG -3′ |
95.9% | 61.0 °C |
| α-tubulin |
Fwd Rev |
5′- GARTTCCARACCAACTTGGT -3′ 5′- GCCATGTAWTTGCCGTGSCG -3′ |
104.4% | 55.0 °C |
dsRNA synthesis
Synthesis of dsRNA was performed using the MEGAscript RNAi Kit (Thermo Fisher Scientific) and following manufacturer’s instructions. Fragments coding for HSP60 or HSP70 were chosen as RNAi target-sequences from L. decemlineata sequences. T7 primers designed for HSP60 dsRNA synthesis were forward 5′-TAATACGACTCACTATAGGGAGAATCGTGAAGAGAGCGTTGAAA-3′ and reverse 5′-TAATACGACTCACTATAGGGAGACCTGGCAAACTAGGATATAAA-3′. T7 primers conceived for HSP70 dsRNA synthesis were forward 5′-TAATACGACTCACTATAGGGAGATGTCCAAGCTGCCGTATTGAC-3′ and reverse 5′-TAATACGACTCACTATAGGGAGAATGACTCTCAACTTCCCTCTC-3′. PCR amplification of the target fragments was performed at 95 °C for 5 min, followed by 39 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 45 s. PCR products were purified using the QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany) and sequenced as above. dsRNAs were produced using PCR products as templates and treated subsequently with DNase and RNase. Purified dsRNA was assessed on a 2% agarose gel and quantified on a NanoVue Plus Spectrophotometer. Products were stored at − 20 °C until injection.
dsRNA injection
dsRNA was injected in L. decemlineata using a 10 μl Hamilton Microliter Syringe (Hamilton, Reno, NV, USA). Insects were immobilized on their back using plasticine. Insects were injected with 5 μl of a 600 ng/μl dsRNA solution in the abdomen. Control insects were either not injected or injected with an equal volume of saline solution used to dilute dsRNAs. Insects were kept in an incubator (Thermo Fisher Scientific) and held under a 16 L:8D photoperiod at 25 °C for the duration of the experiment.
HSP silencing analysis
HSP60 and HSP70 transcript expression following dsRNA injection was assessed by qRT-PCR in RNA isolated from insects collected 3 days post-injection using a similar experimental approach as above with the exception that total RNA was isolated using Trizol (Thermo Fisher Scientific) and RNA was treated with DNAse (Thermo Fisher Scientific) following manufacturer’s protocols. Isolates were prepared using one insect per replicate. Primers to verify target silencing were designed outside of the input dsRNA sequences. Primers were conceived towards the 5′ end of the region targeted by the dsRNA. Primers used to verify HSP60 knockdown were forward 5′-GTCACCATGGGTCCAAAGGGC -3′ and reverse 5′-TTCTTTGGCAATAGAACGGGC -3′. Primers used to verify HSP70 knockdown were forward 5′- ATGCAGTCGTCACAGTTCCA – 3′ and reverse 5′ - CGCCCAAATCGAAAATAAGA – 3′. Quantification of targets by qRT-PCR using L. decemlineata cDNA was conducted using an initial cycle at 95 °C for 3 min, followed by 39 cycles of 95 °C for 15 s and at optimal annealing temperature for 30 s. Transcript levels of α-tubulin were used as reference and amplified in parallel reactions using forward 5′- GAGTTCCAGACCAACTTGGT – 3′ and reverse 5′ - GCCATGTACTTGCCGTGACG – 3′primers. Significant differences between target expression observed in control versus dsRNA-injected insects were evaluated using an unpaired Student’s t test.
Results
HSPs transcript expression in heat-exposed L. decemlineata
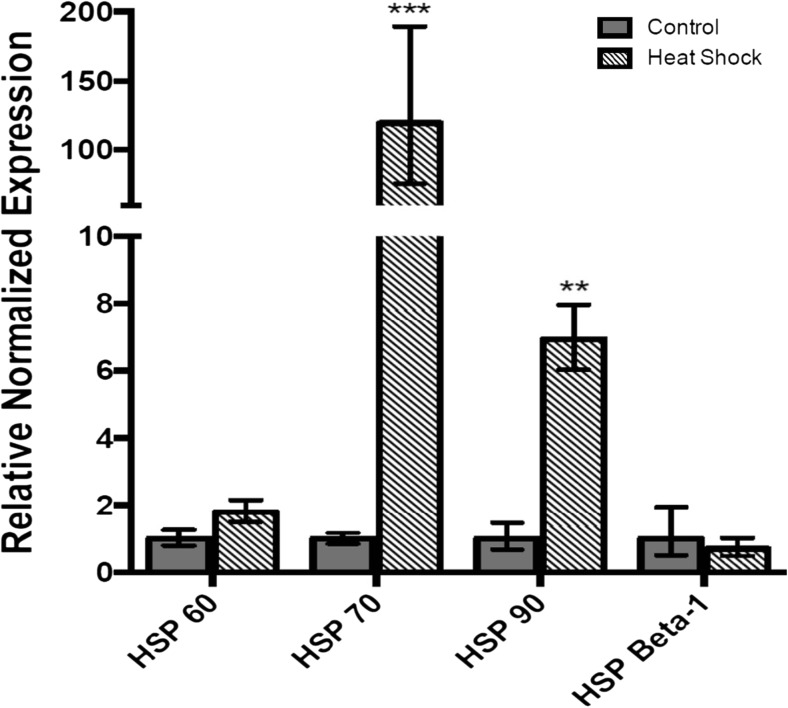
Transcript levels of HSP60, HSP70, HSP90, and HSP Beta-1 were quantified by qRT-PCR in L. decemlineata exposed to heat. α-tubulin transcripts were amplified in each sample and used to normalize target transcripts expression. HSP70 and HSP90 transcript levels were significantly elevated in heat-exposed L. decemlineata with increases of 119.62-fold (P < 0.001) and 6.93-fold (P < 0.01) observed when compared with control samples (Fig. 1). Elevated HSP60 transcript levels, albeit not significantly, were measured as 1.80-fold higher in insects exposed to heat versus control insects. HSP Beta-1 transcript levels were slightly decreased in heat-exposed insects to values that were 0.70-fold the ones measured in control insects.
Fig. 1.
Transcript levels, normalized to α-tubulin, in heat-exposed versus control insects. Data are mean standardized transcript levels (mean ± SEM, n = 3). Asterisks represent results significantly different from control samples (**P < 0.01; *** P < 0.001)
HSPs transcript expression in cold-exposed L. decemlineata
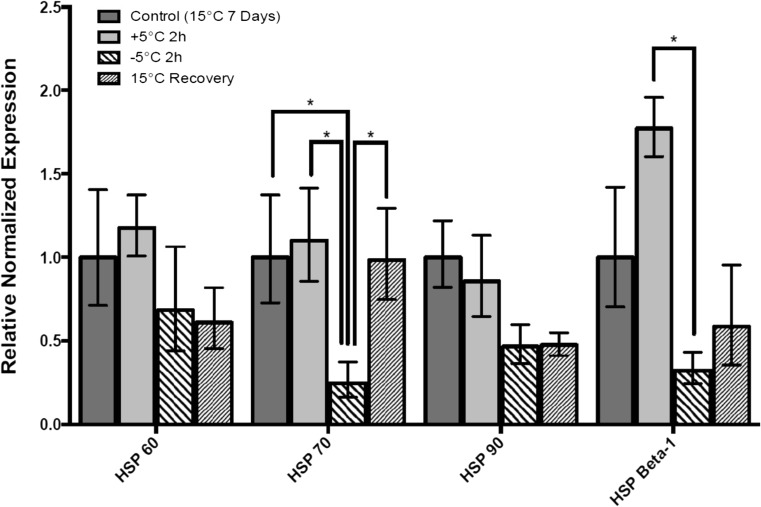
Transcript levels of all four HSPs of interest were measured in control and cold-exposed L. decemlineata using qRT-PCR. HSP70 transcript levels displayed significant reduction in insects exposed to − 5 °C to values that were 0.25-fold (P < 0.05) the ones observed in control insects maintained at 15 °C (Fig. 2). Elevated HSP70 transcript levels were observed in insects kept at 5 °C and insects that were re-acclimated at 15 °C when compared with the − 5 °C-exposed insects with upregulation of 4.43-fold (P < 0.05) and 3.97-fold (P < 0.05), respectively. Significant modulation of HSP Beta-1 transcript levels was also recorded in L. decemlineata exposed to − 5 °C with levels that were 0.18-fold (P < 0.05) the values observed in insects kept at 5 °C. HSP60 and HSP90 transcripts displayed no significant change throughout the temperature time-course even though reduced levels were nevertheless observed for both targets in L. decemlineata kept at − 5 °C and re-acclimated at 15 °C when compared with control insects.
Fig. 2.
Transcript levels, normalized to α-tubulin, in insects submitted to cold conditions versus control insects. Data are mean standardized transcript levels (mean ± SEM, n = 4). Asterisks depict results that are significantly different (*P < 0.05)
HSPs transcript expression in L. decemlineata treated with imidacloprid or chlorantraniliprole
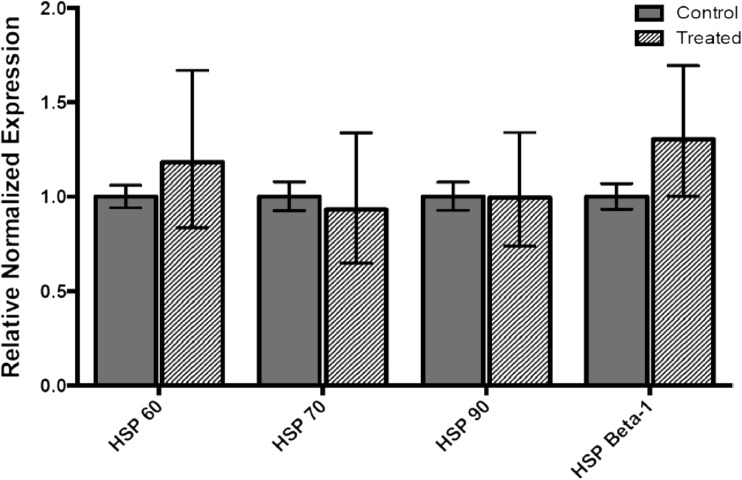
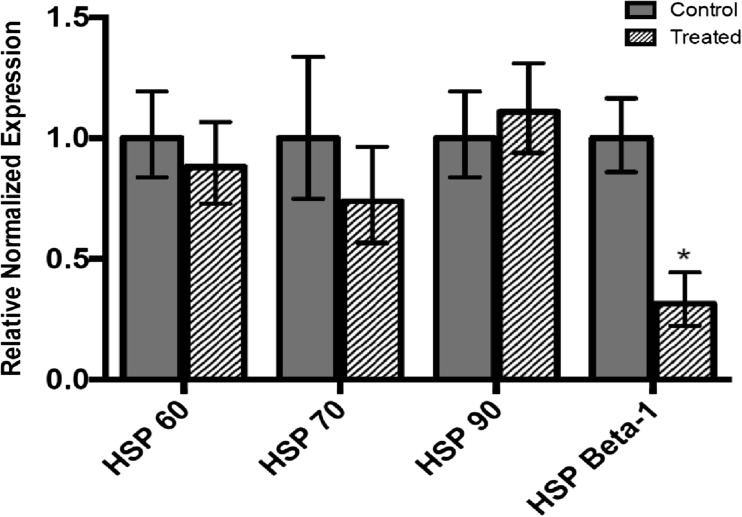
HSP60, HSP70, HSP90, and HSP Beta-1 mRNA levels were measured by qRT-PCR in insecticide-treated and untreated L. decemlineata. HSP60, HSP70, HSP90, and HSP Beta-1 expression remained stable in imidacloprid-treated versus untreated insects as no significant change in transcript levels was observed (Fig. 3). Expression status of the four transcripts of interest was also investigated in insects treated with chlorantraniliprole. HSP Beta-1 transcript levels were downregulated in L. decemlineata exposed to chlorantraniliprole with levels that were 0.31-fold (P < 0.05) the values observed in control insects (Fig. 4). Transcript levels of HSP60, HSP70, and HSP90 did not change significantly following chlorantraniliprole treatment.
Fig. 3.
Expression of transcript levels, normalized to α-tubulin, in imidacloprid-treated versus untreated insects. Data are mean standardized transcript levels (mean ± SEM, n = 3)
Fig. 4.
Transcript levels, normalized to α-tubulin, in chlorantraniliprole-exposed versus untreated insects (mean ± SEM, n = 4–6). Asterisk depict results that are significantly different (*P < 0.05)
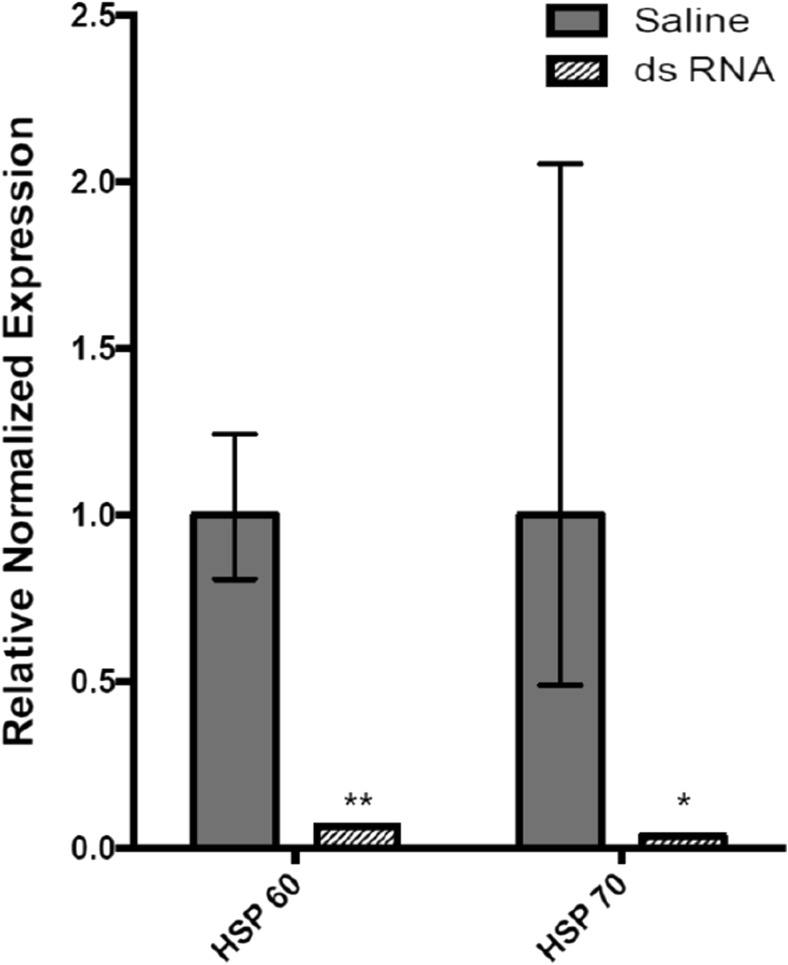
Insect mortality in dsRNA-injected L. decemlineata
HSP60 and HSP70 transcript levels were modulated in L. decemlineata injected with dsRNA and knockdown efficiency was monitored by qRT-PCR. Transcript levels of both targets displayed substantial downregulation in dsRNA-injected insects when compared with control insects (Fig. 5). HSP60 expression levels were reduced to levels that were 0.07-fold the values observed in saline-injected insects. HSP70 levels were downregulated to levels that were 0.03-fold the values observed in saline-injected insects. Similar downregulation of HSP60 and HSP70 transcript expression 3 days following dsRNA injection was also observed in an additional cohort of insects (Fig. S1). Impact of dsRNA injection was monitored post-injection. A significant difference was found between the treatments (chi-square = 16.57, P < 0.01). Insect mortality rates 14 days following injection of dsRNA targeting HSP60 (100%) significantly differed from all other treatments, including control insects that received no injection or that were injected with a saline solution (30% for both conditions; Table 2). Insect mortality following injection of dsRNA targeting HSP70 (20%) was not significantly different from control insects.
Fig. 5.
HSP60 and HSP70 levels in dsRNA-injected L. decemlineata. Histogram presents HSP60 and HSP70 transcript levels in insects injected with dsRNA targeting select HSPs 3 days post-injection. Control insects were injected with saline solution. Data are mean standardized transcript levels (mean ± SEM, n = 2). Asterisks depict results that are significantly from control samples (*P < 0.05; **P < 0.01)
Table 2.
Impact of dsRNA-based knockdown of select HSP targets on insect lethality. Lethality of L. decemlineata was recorded 14 days post-injection
| No injection | 3/10 | 30% |
| Saline | 3/10 | 30% |
| dsRNA-Hsp60 | 10/10 | 100% |
| dsRNA-Hsp70 | 2/10 | 20% |
Discussion
Several studies have highlighted the temperature-mediated modulation of HSPs in insects (King and MacRae 2015; Colinet et al. 2018). On the other hand, data is limited regarding the differential expression of HSPs in L. decemlineata exposed to various stresses including cold and heat shock as well as insecticides. The present work reveals select HSPs that are responsive to temperature fluctuations in L. decemlineata as well as highlights the impact of RNAi-mediated downregulation of HSPs on insect survival.
The current study showed HSP70 differential expression in both heat- and cold-exposed L. decemlineata. HSP90 upregulation following heat shock was also recorded. These results are aligned with other studies that have assessed expression of these HSPs in insects maintained at low or high temperatures. As stress-responsive proteins, HSPs are capable of protecting proteins from various challenges and are modulated in insects exposed to different temperatures (Shi et al. 2013; Zhang et al. 2015; Chen et al. 2016). HSP70 transcript levels upregulation was reported in L. decemlineata larvae submitted to a heat shock of 43 °C for 4 h (Chen et al. 2016). Similar results were obtained when other insects were submitted to heat treatments. Elevated HSP70 expression was shown in the lace bug Corythucha ciliata when kept at high temperatures in the field and in a laboratory setting (Ju et al. 2018). Work that measured transcript levels of several HSPs in the corn earworm Helicoverpa zea also highlighted the heat-inducible properties of HSP70 and, to a lesser extent, HSP90 (Zhang and Denlinger 2010). Cold exposition of L. decemlineata was associated with a downregulation of HSP70 transcript levels. Previous work demonstrated HSP70 cold induction in three different L. decemlineata populations collected in Poland and Russia (Lyytinen et al. 2012). On the other hand, activation of the heat shock transcription factor (HSF) and subsequent increased expression of its target HSP70 were not observed in the fruit fly Drosophila melanogaster during rapid cold hardening (Nielsen et al. 2005). Additional experiments, including HSP70 protein expression measurement as well as HSP70 expression status following cold shock of varying lengths, are envisioned to better characterize its cold-associated function in this insect.
Recent work has also highlighted the potential importance of HSPs in response and resistance to insecticides in various insects. Pioneering work notably evaluated transcript levels in honeybee Apis mellifera larvae exposed to imidacloprid via high-throughput RNA-sequencing and revealed decreased HSP90 expression (Derecka et al. 2013). Treatment of A. lucorum with cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate was associated with elevated HSP90 transcript and protein levels further supporting the involvement of HSP90 in response to different chemicals (Sun et al. 2014). HSP70 modulation has also been observed in insects submitted to insecticides. qRT-PCR-based quantification of HSP70 revealed marked upregulation of HSP70 transcripts in the brown planthopper Nilaparvata lugens treated with imidacloprid (Lu et al. 2017). Transcript levels of all four HSPs investigated in the current study were not altered in insects exposed to imidacloprid suggesting the potential species-specific modulation of HSP transcripts following exposure to this chemical. It is nevertheless important to point out that a HSP70 transcript downregulation, albeit not statistically different, was reported in L. decemlineata larvae treated with imidacloprid for 4 h at 43 °C when compared with control larvae (Chen et al. 2016). While transcript expression of the four HSPs remained unchanged in L. decemlineata following imidacloprid treatment, transcript levels of HSP Beta-1 were shown to be downregulated in chlorantraniliprole-exposed insects. Data are lacking in the literature regarding the potential modulation of HSPs following exposure to this ryanoid. Subsequent work involving longer duration of insecticides treatment or performed at different life stages of L. decemlineata is envisioned to further explore the role, if any, of HSPs in imidacloprid and chlorantraniliprole response in this model.
Several studies have leveraged RNAi-based approaches to modulate HSP levels in insects and evaluate the impact of this variation on diverse phenotypes. Pioneering work in D. melanogaster demonstrated that HSP23 and HSP70 suppression by RNAi approaches could significantly impact the pupa’s ability to cope with cold temperatures (Rinehart et al. 2007). RNAi-based targeting of HSP70 in the firebug Pyrrhocoris apterus altered its ability to recover from a heat shock (Kostál and Tollarová-Borovanská 2009). HSP70/HSC70 downregulation in the haematophagous insect Rhodnius prolixus via dsRNA injection was associated with reduced resistance to starvation and insect mortality approximately 5 weeks following dsRNA injection (Paim et al. 2016). No marked impact was observed on insect mortality in the current work following dsRNA-mediated alteration of HSP70 levels in L. decemlineata. On the other hand, substantial mortality was observed when downregulation of HSP60 levels was performed. While information pertaining to RNAi-based regulation of HSP60 in insects is limited, previous work in D. melanogaster has shown that downregulation of HSP60, a mitochondrial chaperone that underlies mitochondrial protein homeostasis (Ostermann et al., 1989), could interfere with caspase-dependent apoptosis (Arya and Lakhotia 2008). In addition, rat pancreatic tissue isolates administered with HSP60 siRNA and treated with various toxicants revealed a protective effect that HSP60 could have on pancreatic tissues in response to these challenges (Li et al. 2010). These studies, combined with the results gathered here, support the potential impact that an RNAi-based strategy aimed at HSP60 could have on L. decemlineata. RNAi-based approaches are in fact being assessed in multiple insects such as L. decemlineata (Wan et al. 2015) and a closer investigation at RNAi-based strategies directed at HSPs is warranted.
In conclusion, the reported differential expression of HSP70 and HSP90 in insects exposed to heat and cold temperatures positions these HSPs as key molecular nodes involved in temperature adaptation. Characterization of these HSPs is envisioned to better delineate their functions in L. decemlineata exposed to various temperatures. In addition, RNAi-mediated HSP60 knockdown was associated with efficient target downregulation and substantial insect mortality when compared with control insects. Follow-up work is planned to characterize additional phenotypical effects resulting from HSP60 knockdown in this insect. Overall, these results further strengthen the underlying role of select HSPs during stress response in L. decemlineata.
Electronic supplementary material
HSP60 and HSP70 expression in L. decemlineata following dsRNA injection. Histogram shows HSP60 and HSP70 transcript levels in dsRNA- or saline-injected L. decemlineata three days post-injection. Data are mean standardized transcript levels (mean ± SEM, n = 3). (PPTX 60 kb)
Funding information
Support from a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2018-05974) and a Research Grant (EARI 15-007) from the Enabling Agricultural and Research Innovation (EARI) program under the Canada/New Brunswick Growing Forward 2 initiative is acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alyokhin A, Mota-Sanchez D, Baker M, Snyder WE, Menasha S, Whalon M, Dively G, Moarsi WF. The Red Queen in a potato field: integrated pest management versus chemical dependency in Colorado potato beetle control. Pest Manag Sci. 2015;71(3):343–356. doi: 10.1002/ps.3826. [DOI] [PubMed] [Google Scholar]
- Arya R, Lakhotia SC. Hsp60D is essential for caspase-mediated induced apoptosis in Drosophila melanogaster. Cell Stress Chaperones. 2008;13(4):509–526. doi: 10.1007/s12192-008-0051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kitazumi A, Alpuerto J, Alyokhin A, de Los Reyes B. Heat-induced mortality and expression of heat shock proteins in Colorado potato beetles treated with imidacloprid. Insect Sci. 2016;23(4):548–554. doi: 10.1111/1744-7917.12194. [DOI] [PubMed] [Google Scholar]
- Cheng W, Li D, Wang Y, Liu Y, Zhu-Salzman K. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis mosellana. J Insect Physiol. 2016;95:66–77. doi: 10.1016/j.jinsphys.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Colinet H, Rinehart JP, Yocum GD, Greenlee KJ. Mechanisms underpinning the beneficial effects of fluctuating thermal regimes in insect cold tolerance. J Exp Biol. 2018;221(Pt 14):jeb164806. doi: 10.1242/jeb.164806. [DOI] [PubMed] [Google Scholar]
- Derecka K, Blythe MJ, Malla S, Genereux DP, Guffanti A, Pavan P, Moles A, Snart C, Ryder T, Ortori CA, Barrett DA, Schuster E, Stöger R. Transient exposure to low levels of insecticide affects metabolic networks of honeybee larvae. PLoS One. 2013;8(7):e68191. doi: 10.1371/journal.pone.0068191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro DN, Logan JA, Voss RH, Elkinton JS. Colorado potato beetle (Coleoptera: Chrysomelidae) temperature-dependent growth and feeding rates. Environ Entomol. 1985;14(3):343–348. doi: 10.1093/ee/14.3.343. [DOI] [Google Scholar]
- Hiiesaar K, Kuusik A, Jõudu J, Metspalu L, Hermann P. Laboratory experiments on cold acclimation in overwintering Colorado potato beetles, Leptinotarsa decemlineata (Say) Nor J Entomol. 2001;48:87–90. [Google Scholar]
- Hiiesaar K, Metspalu L, Joudu J, Jogar K. Over-wintering of the Colorado potato beetle (Leptinotarsa decemlineata Say) in field conditions and factors affecting its population density in Estonia. Agron Res. 2006;4:21–30. [Google Scholar]
- Izzo VM, Hawthorne DJ, Chen YH. Geographic variation in winter hardiness of a common agricultural pest, Leptinotarsa decemlineata, the Colorado potato beetle. Evol Ecol. 2014;28:505–520. doi: 10.1007/s10682-013-9681-8. [DOI] [Google Scholar]
- Ju RT, Luo QQ, Gao L, Yang J, Li B. Identification of HSP70 gene in Corythucha ciliata and its expression profiles under laboratory and field thermal conditions. Cell Stress Chaperones. 2018;23(2):195–201. doi: 10.1007/s12192-017-0840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AM, MacRae TH. Insect heat shock proteins during stress and diapause. Annu Rev Entomol. 2015;60:59–75. doi: 10.1146/annurev-ento-011613-162107. [DOI] [PubMed] [Google Scholar]
- Kostál V, Tollarová-Borovanská M. The 70 kDa heat shock protein assists during the repair of chilling injury in the insect, Pyrrhocoris apterus. PLoS One. 2009;4(2):e4546. doi: 10.1371/journal.pone.0004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Lu S, Li K, Feng JY, Li YN, Gao ZR, Chen CJ. Down-regulation of HSP60 expression by RNAi increases lipopolysaccharide- and cerulein-induced damages on isolated rat pancreatic tissues. Cell Stress Chaperones. 2010;15(6):965–975. doi: 10.1007/s12192-010-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao Q, Duan X, Song C, Chen M. Transcription of four Rhopalosiphum padi (L.) heat shock protein genes and their responses to heat stress and insecticide exposure. Comp Biochem Physiol A Mol Integr Physiol. 2017;205:48–57. doi: 10.1016/j.cbpa.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Lü ZC, Wan FH. Using double-stranded RNA to explore the role of heat shock protein genes in heat tolerance in Bemisia tabaci (Gennadius) J Exp Biol. 2011;214(Pt 5):764–769. doi: 10.1242/jeb.047415. [DOI] [PubMed] [Google Scholar]
- Lu K, Chen X, Liu W, Zhang Z, Wang Y, You K, Li Y, Zhang R, Zhou Q. Characterization of heat shock protein 70 transcript from Nilaparvata lugens (Stål): its response to temperature and insecticide stresses. Pestic Biochem Physiol. 2017;142:102–110. doi: 10.1016/j.pestbp.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Lyytinen A, Mappes J, Lindstrom L. Variation in Hsp70 levels after cold shock: signs of evolutionary responses to thermal selection among Leptinotarsa decemlineata populations. PLoS One. 2012;7(2):e31446. doi: 10.1371/journal.pone.0031446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahroof R, Yan Zhu K, Neven L, Subramanyam B, Bai J. Expression patterns of three heat shock protein 70 genes among developmental stages of the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae) Comp Biochem Physiol A Mol Integr Physiol. 2005;141(2):247–256. doi: 10.1016/j.cbpb.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Morin MD, Frigault JJ, Lyons PJ, Crapoulet N, Boquel S, Storey KB, Morin PJ. Amplification and quantification of cold-associated microRNAs in the Colorado potato beetle (Leptinotarsa decemlineata) agricultural pest. Insect Mol Biol. 2017;26(5):574–583. doi: 10.1111/imb.12320. [DOI] [PubMed] [Google Scholar]
- Morin MD, Lyons PJ, Crapoulet N, Boquel S, Morin PJ. Identification of differentially expressed miRNAs in Colorado potato beetles (Leptinotarsa decemlineata (Say)) exposed to imidacloprid. Int J Mol Sci. 2017;18(12):2728. doi: 10.3390/ijms18122728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MM, Overgaard J, Sorensen JG, Holmstrup M, Justensen J, Loeschke V. Role of HSF activation for resistance to heat, cold and high-temperature knock-down. J Insect Physiol. 2005;51:1320–1329. doi: 10.1016/j.jinsphys.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ostermann J, Horwich AL, Neupert W, Hartl FU. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989;341:125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Paim RMM, Araujo RN, Leis M, Sant'anna MRV, Gontijo NF, Lazzari CR, Pereira MH. Functional evaluation of heat shock proteins 70 (HSP70/HSC70) on Rhodnius prolixus (Hemiptera, Reduviidae) physiological responses associated with feeding and starvation. Insect Biochem Mol Biol. 2016;77:10–20. doi: 10.1016/j.ibmb.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SA, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci U S A. 2007;104(27):11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues TB, Duan JJ, Palli SR, Rieske LK. Identification of highly effective target genes for RNAi-mediated control of emerald ash borer, Agrilus planipennis. Sci Rep. 2018;8(1):5020. doi: 10.1038/s41598-018-23216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IM, Tolman JH, MacArthur DC. Insecticide resistance and cross-resistance development in Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae) populations in Canada 2008-2011. Pest Manag Sci. 2015;71(5):712–721. doi: 10.1002/ps.3833. [DOI] [PubMed] [Google Scholar]
- Shi M, Wang YN, Zhu N, Chen XX. Four heat shock protein genes of the endoparasitoid wasp, Cotesia vestalis, and their transcriptional profiles in relation to developmental stages and temperature. PLoS One. 2013;8:e59721. doi: 10.1371/journal.pone.0059721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sheng Y, Bai L, Zhang Y, Xiao Y, Xiao L, Tan Y, Shen Y. Characterizing heat shock protein 90 gene of Apolygus lucorum (Meyer-Dür) and its expression in response to different temperature and pesticide stresses. Cell Stress Chaperones. 2014;19(5):725–739. doi: 10.1007/s12192-014-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungjitwitayakul J, Tatun N, Vajarasathira B, Sakurai S. Expression of heat shock protein genes in different developmental stages and after temperature stress in the maize weevil (Coleoptera: Curculionidae) J Econ Entomol. 2015;108(3):1313–1323. doi: 10.1093/jee/tov051. [DOI] [PubMed] [Google Scholar]
- Wan PJ, Fu KY, Lü FG, Wang XX, Guo WC, Li GQ. Knocking down a putative Δ(1)-pyrroline-5-carboxylate dehydrogenase gene by RNA interference inhibits flight and causes adult lethality in the Colorado potato beetle Leptinotarsa decemlineata (Say) Pest Manag Sci. 2015;71:1387–1396. doi: 10.1002/ps.3941. [DOI] [PubMed] [Google Scholar]
- Weber D. Colorado beetle: pest on the move. Pestic Outlook. 2003;14:256–259. doi: 10.1039/b314847p. [DOI] [Google Scholar]
- Zhang Q, Denlinger DL. Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J Insect Physiol. 2010;56(2):138–150. doi: 10.1016/j.jinsphys.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Zhang G, Storey JM, Storey KB. Chaperone proteins and winter survival by a freeze tolerant insect. J Insect Physiol. 2011;57(8):1115–1122. doi: 10.1016/j.jinsphys.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Wang KF, Jing YP, Zhuang HM, Wu G. Identification of heat shock protein genes hsp70s and hsc70 and their associated mRNA expression under heat stress in insecticide-resistant and susceptible diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) Euro J Entomol. 2015;112:215–226. doi: 10.14411/eje.2015.039. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HSP60 and HSP70 expression in L. decemlineata following dsRNA injection. Histogram shows HSP60 and HSP70 transcript levels in dsRNA- or saline-injected L. decemlineata three days post-injection. Data are mean standardized transcript levels (mean ± SEM, n = 3). (PPTX 60 kb)