Abstract
Individuals differ substantially in their response to pharmacological treatment. Personalized medicine aspires to embrace these inter-individual differences and customize therapy by taking a wealth of patient-specific data into account. Pharmacogenomic constitutes a cornerstone of personalized medicine that provides therapeutic guidance based on the genomic profile of a given patient. Pharmacogenomics already has applications in the clinics, particularly in oncology, whereas future development in this area is needed in order to establish pharmacogenomic biomarkers as useful clinical tools. In this review we present an updated overview of current and emerging pharmacogenomic biomarkers in different therapeutic areas and critically discuss their potential to transform clinical care. Furthermore, we discuss opportunities of technological, methodological and institutional advances to improve biomarker discovery. We also summarize recent progress in our understanding of epigenetic effects on drug disposition and response, including a discussion of the only few pharmacogenomic biomarkers implemented into routine care. We anticipate, in part due to exciting rapid developments in Next Generation Sequencing technologies, machine learning methods and national biobanks, that the field will make great advances in the upcoming years towards unlocking the full potential of genomic data.
Abbreviations: 5caC, 5- Carboxylcytosine; 5fC, 5- Formylcytosine; 5hmC, 5-hydroxymethylcytosine; ABC-HSS, Abacavir hypersensitivity syndrome.; ALL, Acute lymphoblastic leukemia; CAT, Catalase; CFTR, Cystic fibrosis transmembrane conductance regulator; ChIP, Chromatin immunoprecipitation; CNVs, Copy number variations; CPIC, Clinical Pharmacogenetics Implementation Consortium; DHR, Drug hypersensitivity reactions; DIHS, Drug-induced hypersensitivity syndrome.; DILI, Drug-induced liver injury; DNMTs, DNA methyltransferases; DPWG, Dutch Pharmacogenetics Working Group; DRESS, Drug rash with eosinophilia and systemic symptoms; eQTL, Quantitative trait locus; GPCR, G-protein coupled receptor; GST, Glutathione-S-transferase; HDACs, Histone deacetylases; MAF, Minor allele frequencies; MPE, Maculopapular exanthema; MS, Multiple sclerosis; PM, Poor metabolism; oxBS-seq, Oxidative bisulfite sequencing; PRC2, Polycomb repressive complex 2; PTMs, Posttranslational modifications; RA, Retinoic acid; SCAR, Severe cutaneous adverse reaction; SJS, Stevens-Johnson syndrome; SNVs, Single nucleotide variations; TAB-Seq, TET-assisted bisulfite sequencing; TEN, Toxic epidermal necrolysis; UM, Ultrarapid metabolism
1. Introduction
The phenomenon that individuals differ in their response to pharmacological therapy has been known for a long time. The early beginnings of the field can be traced back to the identification of interindividual variability of fava bean poisoning by Pythagoras in the 6th century BC an effect much later shown to be linked to polymorphisms in the G6PD gene. Subsequent important contributions were made by Werner Kalow (Kalow & Gunn, 1957) and Bill Evans (Evans, Manley, & McKusick, 1960) identifying the polymorphism in butyrylcholinesterase and isoniazid metabolism, respectively. Seminal twin studies conducted by Sjöqvist and colleagues found that monozygotic and dizygotic twins differed significantly in nortyptiline pharmacokinetics (Alexanderson, Evans, & Sjoqvist, 1969). Contemporaneously, similar observations were made by Vesell and Page for antipyrine (Vesell & Page, 1968a), dicoumarol (Vesell & Page, 1968b) and phenylbutazone (Vesell & Page, 1968c). While these studies clearly demonstrated the extent of heritability of pharmacokinetic variation, the genetic basis remained elusive.
Another important milestone in pharmacogenetic research was the identification of the genetic polymorphisms underlying differences in debrisoquine and sparteine metabolism by Bob Smith and Michel Eichelbaum in an autosomal locus, which later turned out to be CYP2D6 (Eichelbaum, Spannbrucker, & Dengler, 1979; Eichelbaum, Spannbrucker, Steincke, & Dengler, 1979; Mahgoub, Idle, Dring, Lancaster, & Smith, 1977). Subsequently, characterization of the responsible enzymes and their corresponding genes was only achieved more than a decade later in the 1980s and 1990s. A major development was the true biochemical purification of different cytochrome P450 (CYP) enzymes from liver that allowed the subsequent, often antibody assisted cDNA cloning. These breakthroughs allowed for the identification of the most common polymorphic variants using in vivo phenotype-to-genotype strategies and set the stage for modern pharmacogenetic research. For a comprehensive review about the historical origins of pharmacogenetics, we recommend the review by Lesko and Schmidt (Lesko & Schmidt, 2012).
Completion of the Human Genome Project in the early 2000s opened important new possibilities for pharmacogenetic biomarker discovery and set the stage for a plethora of studies that investigated associations between specific genetic polymorphisms and drug response, drug adverse reactions and disease risks. As a result, >200 pharmacogenomic biomarkers have been identified to date that can provide actionable information for clinicians and guide the choice and dosage of pharmacological therapy tailored for a specific patient. However, the societal benefits of these tests and their socioeconomic impacts are in most cases still uncertain and only nine pharmacogenetic biomarkers have received strict boxed warnings (abacavir, carbamazepine, clopidogrel, codeine, lenalidomide, pegloticase, rasburicase, tramadol and valproic acid). In addition, the literature is overwhelmed with a large number of inconclusive association studies that could not be replicated, primarily due to insufficient power to detect associations using agnostic approaches or incomplete phenotypic characterization of the analyzed patient cohorts.
In order to provide support for the further implementation of pharmacogenomic biomarkers, there is a clear need for more randomized, prospective clinical trials. However, as compared to clinical trials for newly developed medicines, the incentive for financing expensive trials that evaluate the added value of companion diagnostics is often rather low because the drugs in question have lost their patents, reducing the incentive to fund expensive trials that validate their use. The most successful example has been the identification of pharmacogenetic tests prior to initiation of abacavir therapy, funded by GlaxoSmithKline. In addition, few trials have been funded by governmental grants, such as the CoumaGen-II (Anderson et al., 2012), COAG (Kimmel et al., 2013) and EU-PACT (Pirmohamed et al., 2013) trials pertaining to warfarin treatment; however, with mixed results.
In this contribution we first provide a regulatory and clinical perspective of the current status of pharmacogenetic biomarkers (Section 2), highlight and comprehensively review emerging associations and critically reflect on the potential for the clinical implementation of these tests (Section 3), discuss the opportunities and challenges associated with the increasing application of Next Generation Sequencing technologies, and highlight exciting opportunities for pharmacogenomic research enabled by national biobank programs (Section 4). In addition, we provide an update of recent developments in pharmacoepigenetics (Section 5) and lastly give our view of current frontiers of pharmacogenomic research that aim to translate academic findings into clinical and societal benefits (Section 6).
2. Clinical implications of pharmacogenetic biomarkers
2.1. Current status of germline biomarkers
Most pharmacogenetic biomarkers with clinical importance reside in genes involved in drug pharmacokinetics and pharmacodynamics as well as in loci related to immune response. Genetic variability is generally analyzed in the germline genome of the patient of interest using non-invasive or minimally invasive methods to obtain the required DNA. In contrast, in oncological therapy, most biomarkers pertain to mutations within the neoplasm, i.e. the somatic genome, and thus require the genetic analysis of tumor biopsies.
Pharmacogenomic biomarkers in the germline genome mostly relate to genetic variants in loci affecting drug pharmacokinetics, including drug metabolizing enzymes and drug transporters. The clinical use of pharmacokinetic germline variants for preemptive guidance of therapy is most widespread in oncology, where variations in DPYD, TPMT and UGT1A1 are analyzed for the prediction of adverse reactions to fluoropyrimidines, mercaptopurines, and irinotecan, respectively (Lauschke, Milani, & Ingelman-Sundberg, 2017). While the frequency of defective TPMT and DPYD alleles is low, their clinical effects are remarkably high. TPMT genotype-guided dosing is already widely applied in clinical practice and is mandatory before commencing mercaptopurine therapy in childhood leukemia (Lennard, 2014). Also the NUDT15 genotype is recommended by the Clinical Pharmacogenetics Implementation Consortium (CPIC) to be considered in this type of anticancer therapy Relling et al., 2018. Implementation of preemptive DPYD genotyping into routine care is lagging behind despite firm evidence supporting lower incidences of severe toxicities while maintaining fluoropyrimidine exposure levels in the therapeutic range, as well as reduced health care costs (Deenen et al., 2016; Henricks et al., 2018). Furthermore, pharmacogenetic testing is implemented in the clinics for genetic variants in CYP2D6, CYP2C19, CYP2C9 and VKORC1 for guidance of drug treatment in cardiology and psychiatry.
The only germline variation in a pharmacodynamic gene that has received pharmacogenetic labels, pertains to variants in the cystic fibrosis transmembrane conductance regulator (CFTR, ABCC7) gene that cause cystic fibrosis (CF) and genotype-guided CF therapy already constitutes clinical reality. Here, >1900 different genetic variants have been identified that affect CFTR function, 1000 of which occur in fewer than five people in all cohorts studied to date (Oliver, Han, Sorscher, & Cutting, 2017). Depending on the functional consequences of the variants found in a given patients, different drugs can be prescribed including ivacaftor for patients that harbor variants resulting in gating defects (CFTR class III variants) or lumacaftor for patients with CFTR folding defect mutations. Thus, for CF, preemptive pharmacogenetic testing is already of fundamental importance for successful treatment and about 60% of CF patients can benefit from such tailored therapies.
Genetic variability in ADRB2, the gene encoding the β2-adrenergic receptor, has long been considered as a promising biomarker to predict the response to β-agonists in the management of asthma (Kersten & Koppelman, 2017; Ortega & Meyers, 2014). However, results of different trials were conflicting and could, if at all, only explain a minor fraction of the observed variability in drug response (Israel et al., 2004; Wechsler et al., 2009; Wechsler et al., 2015). Thus, the implementation of genotype-guided therapies for asthma utilizing β2-adrenergic receptor variants in the near future appears unlikely. Recent evaluation of sequencing data from 60,000 individuals revealed a surprisingly large number of rare variants in this class of receptors, many potentially important for altered ligand binding or ligand effects (Hauser et al., 2018). Combined, these data indicate the importance to consider such rare receptor variants for drug response predictions.
2.2. Current status of somatic biomarkers
At present oncology is the most important therapeutic area for preemptive prediction of drug outcomes. This area is the subject of very intensive research and in total > 268,000 publications are indexed in PubMed that concern oncological biomarkers, including genomic and epigenomic variants, but the work also encompasses a variety of other molecules, such as non coding RNAs, proteins, peptides and metabolites.
In addition to the aforementioned germline variants in DPYD, TPMT and UGT1A1 that affect the pharmacokinetics of chemotherapeutic agents, somatic mutations in various pharmacodynamic genes open possibilities for the treatment with therapeutics that specifically target the affected pathways. Examples for such targeted cancer drugs that require specific somatic mutations for their effectiveness include the EGFR inhibitors gefitinib, erlotinib and osimertinib, the BRAF inhibitors dabrafenib and vemurafenib and the ERBB2 targeting agents lapatinib, pertuzimab and trastuzumab. In addition, whole genome sequencing (WGS) of the somatic cancer genome is becoming more common, allowing to individualize oncological treatment beyond common mutations. We anticipate that these developments will further accelerate and establish WGS as an integral instrument in the area of anticancer therapy.
Current pharmacogenomic analyses are primarily focused on treatment with small molecules and biomarkers to predict treatment response to emerging biologics constitutes an important frontier. This need is exemplified by treatment outcomes of nivolumab, an antibody-based inhibitor of PD1, in melanoma. While nivolumab significantly improved overall survival compared to conventional dacarbazine chemotherapy, only 20–30% of patients responded to nivolumab and the reasons for the lack of response in the remaining patients remain unknown (Ascierto & Long, 2016). Similar response rates were observed for monoclonal antibodies for CTLA4, such as ipilimumab (Carreau & Pavlick, 2018).
2.3. Pharmacogenomic drug labels and guidelines
One instrument to support the application of genetic variations in the clinics are pharmacogenomic drug labels. These labels are prepared by the drug manufacturers and submitted for approval to the responsible regulatory agency, such as European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) for Europe and the US, respectively. Where applicable, they recommend the genotyping of specific genes or variants to guide drug and dose selection, predict treatment outcomes or adverse reactions, or inform about potential effects on drug-drug interactions. By 2018, FDA has approved a total of 69 labels that carry information regarding indications, contraindications or dosage recommendation in relation to patient genotype, whereas about 107 have correspondingly based labels have been identified by EMA (Table 1 and Fig. 1a). In addition, pharmacogenomic advice is provided by guidelines from pharmacogenetic experts workgroups, such as the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the Dutch Pharmacogenetics Working Group (DPWG).
Table 1.
Comparison of medications with associated pharmacogenomic biomarkers by EMA and FDA. EMA labels were reviewed in Ehmann et al. (Ehmann et al., 2015) and only encompass drugs registered after the foundation of EMA in 1995. FDA labels were extracted from https://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm [Accessed 01.11.2018]. Only the sections describing therapeutic indications, posology and contraindications were considered. BW = boxed warning.
| Compound | Gene | Indication | Posology | Contraindication | Indication |
|---|---|---|---|---|---|
| Abacavir | HLA-B | EMA | FDA | FDA (BW) | HIV infection |
| Abemaciclib | ESR | FDA | Advanced or metastatic breast neoplasms | ||
| ERBB2 | FDA | ||||
| Afatinib | EGFR | EMA & FDA | EMA & FDA | Non-small cell lung cancer | |
| Alectinib | ALK | FDA | FDA | Non-small cell lung cancer | |
| Aliskiren | ABCB1 | EMA | Hypertension | ||
| Anastrozole | ESR, PGR | FDA | Breast neoplasms | ||
| Aripiprazole | CYP2D6 | EMA & FDA | Bipolar disorder, schizophrenia | ||
| CYP3A4 | EMA | ||||
| Arsenic trioxide | PML-RARA | EMA & FDA | Acute promyelotic leukemia | ||
| Atazanavir sulfate | CYP3A4 | EMA | HIV infection | ||
| Atezolizumab | CD274 | FDA | Lung cancer | ||
| Atomoxetine | CYP2D6 | FDA | Attention deficit hyperactivity disorder | ||
| Axitinib | CYP3A4 | EMA | Renal cell carcinoma | ||
| CYP3A5 | EMA | ||||
| Azathioprine | TPMT | FDA | Kidney transplantation, rheumatoid arthritis, Crohn's disease, ulcerative colitis | ||
| Belinostat | UGT1A1 | FDA | T-cell lymphoma | ||
| Binimetinib | BRAF | FDA | FDA | Melanoma | |
| Blinatumomab | BCR-ABL | FDA | Acute lymphoblastic leukemia | ||
| Boceprevir | CYP3A4 | EMA | Chronic hepatitis C | ||
| Bosutinib | BCR-ABL | EMA & FDA | EMA | Myelogenous leukemia | |
| Brentuximab vedotin | CD30 | EMA | Hodgkin disease, non-Hodgkin lymphoma | ||
| Brexpiprazole | CYP2D6 | FDA | Schizophrenia, depression | ||
| Brigatinib | ALK | FDA | Non-small cell lung cancer | ||
| Cabazitaxel | CYP3A4 | EMA | Prostatic neoplasms | ||
| Cabozantinib | CYP3A4 | EMA | Thyroid neoplasms | ||
| Capecitabine | DPYD | EMA | Colorectal neoplasms, colonic neoplasms, stomach neoplasms, breast neoplasms | ||
| Carbamazepine | HLA-B | FDA (BW) | Epilepsy, schizophrenia, bipolar disorder | ||
| Carglumic acid | NAGS | FDA | Hyperammonaemia | ||
| Celecoxib | CYP2C9 | FDA | Treatment of inflammation and pain in various conditions | ||
| Ceritinib | ALK | FDA | FDA | Non-small cell lung cancer | |
| Cerliponase alpha | TPP1 | FDA | Neuronal ceroid lipofuscinosis | ||
| Cetuximab | EGFR | EMA & FDA | FDA | Colorectal neoplasms, head and neck neoplasms | |
| RAS | EMA & FDA | EMA & FDA | EMA | ||
| Citalopram | CYP2C19 | FDA | Major depression | ||
| Clobazam | CYP2C19 | FDA | Epilepsy, acute anxiety | ||
| Clopidogrel | CYP2C19 | FDA (BW) | Peripheral artery disease, stroke prevention | ||
| Clozapine | CYP2D6 | FDA | Schizophrenia | ||
| Cobimetinib | BRAF | FDA | FDA | Melanoma | |
| Codeine | CYP2D6 | FDA (BW) | Treatment of pain | ||
| Crizotinib | ALK | EMA & FDA | EMA & FDA | Non-small cell lung cancer | |
| ROS1 | FDA | FDA | |||
| Dabrafenib | BRAF | EMA & FDA | EMA & FDA | Melanoma | |
| RAS | FDA | ||||
| Darifenacin hydrobromide | CYP2D6 | EMA | Urinary Incontinence, overactive urinary bladder | ||
| CYP3A4 | EMA | ||||
| Darunavir | CYP3A4 | EMA | HIV infection | ||
| Dasatinib | BCR-ABL | EMA & FDA | EMA & FDA | Chronic myelogenous leukemia, precursor cell lymphoblastic leukemia-lymphoma | |
| Denileukin difitox | IL2RA | FDA | Cutaneous T-cell lymphoma | ||
| Deutetrabenazine | CYP2D6 | FDA | Chorea | ||
| Dronedarone | CYP3A4 | EMA | Atrial fibrillation | ||
| Efavirenz | CYP3A4 | EMA | HIV infection | ||
| Eliglustat | CYP2D6 | FDA | FDA | FDA | Gaucher's disease |
| Elosulfase | GALNS | FDA | Morquio-Brailsford syndrome | ||
| Enasidenib | IDH2 | FDA | FDA | Acute myeloid leukemia | |
| Encorafenib | BRAF | FDA | FDA | Melanoma | |
| Erlotinib | EGFR | EMA & FDA | FDA | Non-small cell lung cancer, pancreatic neoplasms | |
| CYP3A4 | EMA | ||||
| Eteplirsen | DMD | FDA | Duchenne muscular dystrophy | ||
| Everolimus | ERBB2 | EMA & FDA | FDA | Renal cell carcinoma, pancreatic neoplasms, breast neoplasms | |
| ESR | FDA | FDA | |||
| Exemestane | ESR, PGR | FDA | FDA | Breast neoplasms | |
| Fampridine | SLC22A2 | EMA | Multiple sclerosis | ||
| Fesoterodine | CYP3A4 | EMA | EMA | Overactive urinary bladder | |
| Fluorouracil | DPYD | FDA | Colorectal neoplasms, stomach neoplasms, pancreatic neoplasms, breast cancer, cervical neoplasms, esophageal neoplasms | ||
| Fosamprenavir | CYP3A4 | EMA | HIV infection | ||
| Fulvestrant | ERBB2 | FDA | Breast neoplasms | ||
| ESR, PGR | FDA | ||||
| Gefitinib | EGFR | EMA & FDA | FDA | Non-small cell lung cancer | |
| CYP2C9 | EMA | ||||
| CYP2D6 | EMA | ||||
| Ibrutinib | Chromosome 17p | FDA | B-cell lymphomas | ||
| Iloperidone | CYP2D6 | FDA | Schizophrenia | ||
| Imatinib | BCR-ABL | EMA & FDA | EMA & FDA | Chronic myelogenous leukemia, myelodysplastic-myeloproliferative diseases, dermatofibrosarcoma, precursor cell lymphoblastic leukemia-lymphoma, hypereosinophilic syndrome |
|
| KIT | EMA & FDA | FDA | |||
| FIP1L1-PDGFRA | EMA & FDA | FDA | |||
| PDGFRB | FDA | FDA | |||
| Indinavir | CYP3A4 | EMA | HIV infection | ||
| Irinotecan | UGT1A1 | FDA | Colorectal neoplasms, pancreatic neoplasms, small cell lung cancer | ||
| Ivabradine | CYP3A4 | EMA | Angina pectoris | ||
| Ivacaftor | CFTR | EMA & FDA | EMA | Cystic fibrosis | |
| CYP3A4 | EMA | ||||
| Lapatinib | ERBB2 | EMA & FDA | EMA & FDA | Breast neoplasms | |
| ESR, PGR | FDA | FDA | |||
| Lenalidomide | Chromosome 5q | FDA | FDA (BW) | Myelodysplastic syndrome, multiple myeloma | |
| Letrozole | ESR, PGR | FDA | Breast neoplasms | ||
| Lomitapide | ABCB1 | EMA | Hypercholesterolemia | ||
| Lurasidone | CYP3A4 | EMA | EMA | Schizophrenia | |
| Maraviroc | CYP3A4 | EMA | HIV infection | ||
| Mercaptopurine | TPMT | FDA | Acute lymphocytic leukemia, chronic myeloid leukemia, Crohn's disease, ulcerative colitis | ||
| NUDT15 | FDA | ||||
| Methylene blue | G6PD | FDA | Methemoglobinemia | ||
| Midostaurin | FLT3 | FDA | FDA | Myelodysplastic syndrome | |
| Nebivolol | CYP2D6 | FDA | Hypertension | ||
| Nelfinavir | CYP3A4 | EMA | HIV infection | ||
| Neratinib | ERBB2 | FDA | Breast neoplasms | ||
| Nilotinib | BCR-ABL | EMA & FDA | FDA | Chronic myelogenous leukemia, acute myeloid leukemia, systemic mastocytosis | |
| Nivolumab | BRAF | FDA | Melanoma, non-small cell lung cancer, renal cell carcinoma | ||
| Olaparib | BRCA | FDA | FDA | Breast neoplasms, ovarian neoplasms, prostate neoplasms | |
| Osimertinib | EGFR | FDA | FDA | Non-small cell lung cancer | |
| Palbociclib | ERBB2 | FDA | Breast neoplasms | ||
| ESR | FDA | ||||
| Panitumumab | RAS | EMA & FDA | EMA & FDA | EMA | Colorectal neoplasms |
| Parathyroid hormone | CASR | FDA | Osteoporosis | ||
| Pegloticase | G6PD | FDA (BW) | Gout | ||
| Pembrolizumab | CD274 | FDA | FDA | Unresectable or metastatic solid tumors | |
| Pertuzumab | ERBB2 | EMA & FDA | EMA | Breast neoplasms | |
| Pimozide | CYP2D6 | FDA | Schizophrenia | ||
| Ponatinib | BCR-ABL | EMA & FDA | Lymphoid leukemia, myeloid leukemia | ||
| Posaconazole | CYP3A4 | EMA | Aspergillosis, coccidioidomycosis, candidiasis, mycoses |
||
| Primaquine | G6PD | FDA | Malaria and Pneumocystis pneumonia | ||
| Propafenone | CYP2D6 | FDA | Arrhythmias | ||
| Quinine sulfate | G6PD | FDA | Malaria and babesiosis | ||
| Ranolazine | CYP3A4 | EMA | EMA | Angina pectoris | |
| Rasburicase | G6PD | FDA (BW) | Tumor lysis syndrome | ||
| CYB5R | FDA (BW) | ||||
| Ribociclib | ERBB2 | FDA | Breast neoplasms | ||
| ESR, PGR | FDA | ||||
| Ritonavir | CYP3A4 | EMA | HIV infection | ||
| Rituximab | MS4A1 | FDA | FDA | Rheumatoid arthritis, hematological cancers | |
| Rucaparib | BRCA | FDA | FDA | Ovarian neoplasms | |
| Ruxolitinib | CYP3A4 | EMA | Myeloproliferative disorders | ||
| Sildenafil | CYP3A4 | EMA | EMA | Pulmonary hypertension | |
| Sirolimus | CYP3A4 | EMA | Kidney transplantation, graft rejection | ||
| Sunitinib | CYP3A4 | EMA | Neuroendocrine tumors, gastrointestinal stromal tumors, renal cell carcinoma | ||
| Tamoxifen | ESR, PGR | FDA | Breast neoplasms | ||
| Telaprivir | CYP3A4 | EMA | Chronic hepatitis C | ||
| Telithromycin | CYP3A4 | EMA | Community-acquired infections, chronic bronchitis, sinusitis, tonsillitis, bacterial pneumonia, pharyngitis | ||
| Tetrabenazine | CYP2D6 | FDA | Hyperkinesia | ||
| Thioguanine | TPMT | FDA | Acute myeloid leukemia, acute lymphocytic leukemia, and chronic myeloid leukemia | ||
| NUDT15 | FDA | ||||
| Thioridazine | CYP2D6 | FDA | Schizophrenia | ||
| Tipranavir | CYP3A4 | EMA | HIV infection | ||
| Tramadol | CYP2D6 | FDA (BW) | Treatment of pain | ||
| Trametinib | BRAF | FDA | EMA & FDA | Melanoma | |
| Trastuzumab | ERBB2 | EMA & FDA | EMA | Stomach neoplasms, breast neoplasms | |
| Trastuzumab emtansine | ERBB2 | EMA & FDA | EMA | Breast neoplasms | |
| Tretinoin | PML-RARA | FDA | Acute promyelocytic leukemia | ||
| Valbenazine | CYP2D6 | FDA | Tardive dyskinesia | ||
| Valproic acid | POLG | FDA (BW) | Epilepsy, bipolar disorder | ||
| Vandetanib | RET | EMA | Thyroid neoplasms | ||
| Vardenafil | CYP3A4 | EMA | EMA | Erectile dysfunction | |
| Vemurafenib | BRAF | EMA & FDA | EMA & FDA | Melanoma | |
| Venetoclax | Chromosome 17p | FDA | FDA | Chronic lymphocytic leukemia | |
| Voriconazole | CYP3A4 | EMA | Aspergillosis, candidiasis, mycoses | ||
| Vortioxetine | CYP2D6 | EMA & FDA | Major depressive disorder | ||
| Warfarin | CYP2C9 | FDA | Deep vein thrombosis, pulmonary embolism, stroke prevention | ||
| VKORC1 | FDA | ||||
| Zonisamide | CYP3A4 | EMA | Partial epilepsies |
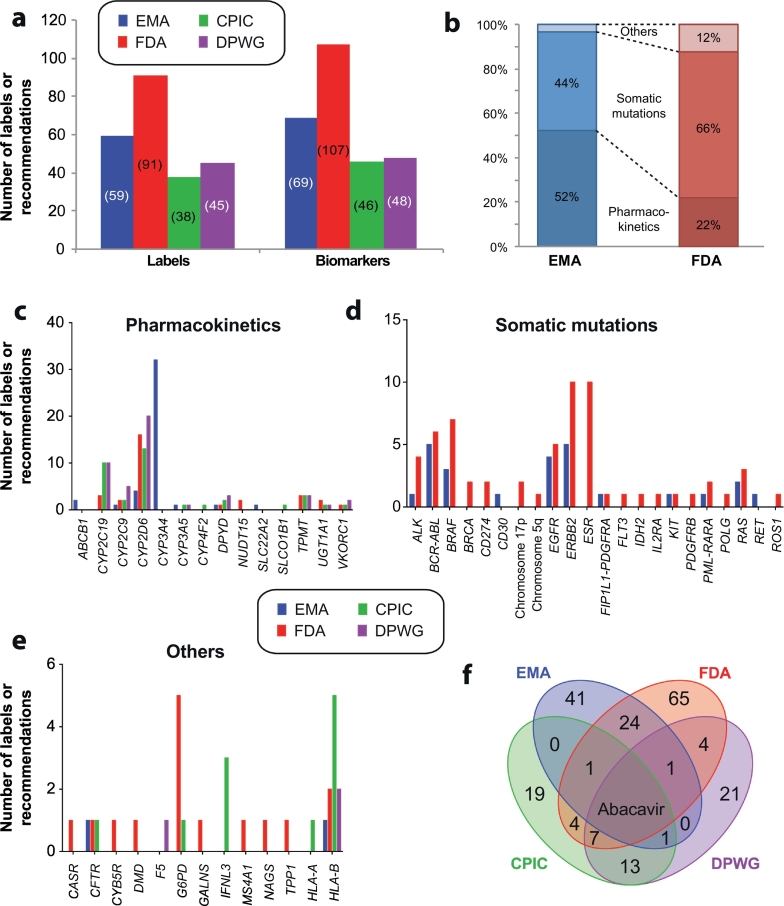
Fig. 1.
Overview of drug labels and pharmacogenetic expert guidelines. a, Overview of the number of drug labels by EMA and FDA and recommendations by CPIC and DPWG, respectively. Note that some labels and guidelines contain references to more than one biomarker. b, The majority of EMA labels refer to pharmacokinetic germline variants, whereas FDA approved labels primarily pertain to variations in the somatic genome. Only the indication, contraindication and posology sections were considered. c-e, Overview of the number of drug labels and pharmacogenetic recommendations, stratified into germline variations that impact drug pharmacokinetics (c), somatic mutations in tumors (d) and other germline variants (e). f, Venn diagram depicting the overlap of pharmacogenetic guidance from EMA (blue) and FDA (red) approved drug labels and recommendations by CPIC (green) and DPWG (purple). EMA label information was reviewed in Ehmann et al. (Ehmann et al., 2015) and only encompasses drugs registered after the foundation of EMA in 1995, which creates some lack of coherence in the comparison. FDA labels were extracted from https://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm. CPIC and DPWG guidelines were obtained from https://cpicpgx.org/guidelines and https://www.pharmgkb.org/guidelines, respectively. All sources were accessed Nov 1st 2018.
There are differences between drug labels and recommendations by the different regulatory agencies and consortia (Fig. 1). The majority of EMA labels (52%) refer to pharmacokinetic genes mainly in oncology, whereas the majority of FDA labels (66%) pertain to mutations or genomic rearrangements in the somatic genome of tumors and only 22% of labels refer to pharmacokinetic germline variations (Fig. 1b). Notably, as published in 2015, several of the EMA labels merely refer to drug-drug interactions rather than to genetic variation. In addition it is important to emphasize that EMA labels only concern drugs approved by EMA, which was founded in 1995, whereas labels in older drugs are provided by the different EU National Medical Product Agencies.
Recommendations from expert consortia are focused exclusively on the genetic variation in the germline genome and a recent comparison between theses therapeutic recommendations concluded that CPIC and DPWG pharmacogenetic guidelines were overall in good agreement (Bank et al., 2018). However, their alignment with drug labels is rather poor. Of 44 EMA labels with pharmacogenetic information referring to germline variants, only four (9%) overlap with CPIC or DPWG recommendations (abacavir and HLA-B, aripiprazole and CYP2D6, capecitabine and DPYD, ivacaftor and CFTR) (Fig. 1f). Alignment for FDA labels is higher and 18 out of 45 labels (40%) are supported by independent expert recommendations. Abacavir constitutes the only drug for which EMA and FDA labels, as well as CPIC and DPWG guidelines concordantly recommend genotype-guided therapy. Overall, there is thus a need to critically reflect upon the different recommendations by regulators and expert groups to reach a consensus view on the role of pre-emptive genotyping in the clinics.
The regulatory agencies also provide guidelines for the integration of pharmacogenomic analyses into early and later phases of drug development (https://www.ema.europa.eu/documents/scientific-guideline/guideline-good-pharmacogenomic-practice-first-version_en.pdf, https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM337169.pdf). Furthermore, EMA and Industry (EBE and EFPIA) have worked out specific guidance concerning the use of NGS as an instrument for pharmacogenomic advice (https://www.ema.europa.eu/documents/scientific-guideline/guideline-good-pharmacogenomic-practice-first-version_en.pdf, https://www.ebe-biopharma.eu/publication/ebe-efpia-position-paper-on-next-generation-sequencing-ngs/). This includes early identification of patients with extreme drug response phenotypes (outlier patients), the possibility to stratify patient groups based on their genetic makeup, methodological advice pertaining to genomic and phenotypic analyses, and planning of follow-up trials based on the pharmacogenomic experience in early phases. During this process also the incorporation of pharmacogenomic advice into the drug label must be considered. In line with a more genetically tailored drug therapy, the number of drugs released on the market with such labels has increased considerably in recent years (Ehmann et al., 2015).
3. Emerging pharmacogenomic biomarkers
In the following section, we synopsize recent promising progress and updates in the field of pharmacogenomic biomarkers to predict safety and efficacy of pharmacological therapies.
3.1. Drug hypersensitivity associated with HLA variations
3.1.1. HLA biomarkers
Drug hypersensitivity reactions (DHR) are the most common idiosyncratic adverse events. DHRs can manifest immediately within the first hours after drug administration or have a delayed onset of weeks to months (Romano et al., 2011). Prospective studies found that DHRs occurred with an overall prevalence of 0.2–0.8% of all hospitalized patients, of which >95% had cutaneous manifestations (Albala et al., 2003; Hernandez-Salazar et al., 2006; Park et al., 2008; Thong, Leong, Tang, & Chng, 2003). Delayed DHRs can manifest as severe cutaneous adverse reactions (SCARs) that encompass Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, the latter involving also internal organs, such as liver (75–94% of patients), kidney (12–40% of patients) and heart (4–27% of patients) (Y.-T. Cho, Yang, & Chu, 2017). Agents most commonly implicated in SCARs are sulfonamides, phenytoin, allopurinol, carbamazepine and non-steroidal anti-inflammatory drugs (NSAIDs) of the oxicam class (Mockenhaupt et al., 2008; Roujeau et al., 1990; Rzany et al., 1996; Schöpf et al., 1991; Yamane, Aihara, & Ikezawa, 2007). In addition, DHRs can manifest as drug-induced liver injury (DILI), with β-lactam antibiotics and NSAIDs as the major culprit drugs. Further manifestations include abacavir systemic hypersensitivity and clozapine-induced agranulocytosis.
Genetic predisposition constitutes the most important risk factor for both immediate and delayed hypersensitivity reactions. Immediate reactions to β-lactams and NSAIDs have been consistently associated with polymorphisms in pro-inflammatory cytokine and IgE signaling (Oussalah et al., 2016). In addition, immediate hypersensitivity to NSAIDs was reproducibly associated with genetic variations in multiple arachidonic acid and leukotriene pathway genes, such as ALOX15, PTGDR, PTGER4, TBXAS1 and CYSLTR1 (Cornejo-García et al., 2012; Kim, Choi, Holloway, et al., 2005; Palikhe et al., 2012; Vidal et al., 2013). Anaphylactic reactions to both β-lactams and NSAIDs have also been associated with polymorphisms in class II HLA genes. A Spanish study with 387 patients that experienced immediate allergic reactions upon treatment with β-lactams and 1124 tolerant controls found multiple significant protective effects of HLA-DRA variations with odds ratios (ORs) around 0.6 that replicated in an Italian cohort of 299 patients and 362 control subjects (Guéant et al., 2015). In contrast, the HLA-DRB1*11 and HLA-DRB1*1302 alleles predisposed patients to NSAID-induced anaphylaxis and urticaria with ORs of 4 to 7.3 (Kim, Choi, Lee, et al., 2005; Quiralte et al., 1999).
For delayed hypersensitivity reactions >25 medications have been associated with MHC variability to date (Table 2, Table 3, Table 4). The most extensively reproduced HLA biomarkers pertain to the antiretroviral abacavir, the antihyperuricemic allopurinol and the antiepileptics carbamazepine, phenytoin and lamotrigine. Hypersensitivity to abacavir is strongly associated with a single genetic risk allele, HLA-B*5701 (Table 2). Prospective genotyping for HLA-B*5701 was found to significantly reduce the incidence of abacavir hypersensitivity syndrome (ABC-HSS) in a single center cohort study with no cases of ABC-HSS among 148 HLA-B*5701 negative patients compared to 5–8% in historic controls (Rauch et al., 2006). These encouraging results were confirmed in the prospective multicenter double-blind randomized PREDICT-1 trial in which the authors confirmed significantly lower incidence of ABC-HSS in the genotype arm (3.4% vs. 7.8% in the control group, p < .001) (Mallal et al., 2008). As a result, testing of HLA-B*5701 has been recommended by both FDA and EMA before commencing abacavir therapy in abacavir-naïve patients.
Table 2.
Overview of genetic variations in the major histocompatibility complex associated with hypersensitivity to antiretrovirals and antibiotics. SJS = Stevens-Johnson syndrome, TEN = toxic epidermal necrolysis, DRESS = drug rash with eosinophilia and systemic symptoms, SCAR = severe cutaneous adverse reaction, DILI = drug-induced liver injury, ABC-HSS = abacavir hypersensitivity syndrome.
| Allele | Ethnicity | Odds ratio | Adverse reaction | Cases | Controls | Study |
|---|---|---|---|---|---|---|
| Abacavir | ||||||
| HLA-B*5701 | Australian | 960 | ABC-HSS | 18 | 230 tolerant controls | (Martin et al., 2004) |
| 117 | ABC-HSS | 18 | 167 tolerant controls | (Mallal et al., 2002) | ||
| White | 55.7 | ABC-HSS | 202 | 486 tolerant controls | CTR Summary for MDC - GSK Clinical Study Register (2007) | |
| 30.4 | ABC-HSS | 61 | 657 tolerant controls | (Mallal et al., 2008) | ||
| Spanish | 44.3 | ABC-HSS | 22 | 70 tolerant controls | CTR Summary for MDC - GSK Clinical Study Register (2007) | |
| 19.1 | ABC-HSS | 26 | 27 tolerant controls | (Rodríguez-Nóvoa et al., 2007) | ||
| Caucasian | 7.9 | ABC-HSS | 13 | 51 tolerant controls | (Hughes et al., 2004) | |
| Self-identified white | 1945 | ABC-HSS | 42 | 202 tolerant controls | (Saag et al., 2008) | |
| Black | 8.4 | ABC-HSS | 21 | 67 tolerant controls | CTR Summary for MDC - GSK Clinical Study Register (2007) | |
| Self-identified black | 900 | ABC-HSS | 5 | 206 tolerant controls | (Saag et al., 2008) | |
| Thai | 263.6 | ABC-HSS | 7 | 102 tolerant controls | CTR Summary for MDC - GSK Clinical Study Register (2007) | |
| Multiethnic group | 23.6 | ABC-HSS | 84 | 113 tolerant controls | (Hetherington et al., 2002) | |
| 6.9 | ABC-HSS | 9 | 41 tolerant controls | (Stekler et al., 2006) | ||
| Nevirapine | ||||||
| HLA-B*1402 | Sardinian | 14.6 | DRESS | 13 | 36 tolerant controls | (Littera et al., 2006) |
| HLA-B*35 | Asian | 3.5 | SCAR | 71 | 227 tolerant controls | (Yuan et al., 2011) |
| Thai | 5.7 | SCAR | 52 | 173 tolerant controls | (Yuan et al., 2011) | |
| HLA-B*3505 | Thai | 19 | CAR | 143 | 181 tolerant controls | (Chantarangsu et al., 2009) |
| HLA-B*5801 | South African | 3.15 | DILI | 53 | 106 tolerant controls | (Phillips et al., 2013) |
| HLA-C*0401 | Sub-Saharan African | 4.8 | SJS/TEN | 267 | 250 tolerant controls | (Carr et al., 2017) |
| Malawian | 17.5 | SJS/TEN | 36 | 155 tolerant controls | (Carr et al., 2013) | |
| HLA-DRB1*0101 | Australian | 17.7 | DRESS | 14 | 221 tolerant controls | (Martin et al., 2005) |
| HLA-DRB1*0102 | South African | 4.3 | DILI | 54 | 103 tolerant controls | (Phillips et al., 2013) |
| HLA-DRB1*01 | French | 70 | CAR | 6 | 15 tolerant controls | (Vitezica et al., 2008) |
| White | 3 | DILI | 57 | 277 tolerant controls | (Yuan et al., 2011) | |
| HLA-Cw*04 | Thai | 3.2 | CAR | 78 | 120 tolerant controls | (Likanonsakul et al., 2009) |
| 2.4 | SCAR | 52 | 179 tolerant controls | (Yuan et al., 2011) | ||
| Asian | 2.6 | SCAR | 71 | 233 tolerant controls | (Yuan et al., 2011) | |
| Black | 5.2 | SCAR | 27 | 77 tolerant controls | (Yuan et al., 2011) | |
| White | 1.9 | SCAR | 77 | 277 tolerant controls | (Yuan et al., 2011) | |
| HLA-Cw*08 | Japanese | 6.2 | DRESS | 12 | 29 tolerant controls | (Gatanaga et al., 2007) |
| Sulfamethoxazole | ||||||
| HLA-A30 | Turkey | 3.9 | Fixed drug eruption | 67 | 2378 general population | (Ozkaya-Bayazit & Akar, 2001) |
| HLA-B*1502 | Thai | 3.9 | SJS/TEN | 43 | 91 tolerant controls | (Kongpan et al., 2015) |
| HLA-B*3801 | European | 4.3 | SJS/TEN | 25 | 1822 general population | (Lonjou et al., 2008) |
| HLA-B*3802 | European | 76 | SJS/TEN | 25 | 1822 general population | (Lonjou et al., 2008) |
| HLA-C*0602 | Thai | 11.8 | SJS/TEN | 43 | 91 tolerant controls | (Kongpan et al., 2015) |
| HLA-C*0801 | Thai | 3.4 | SJS/TEN | 43 | 91 tolerant controls | (Kongpan et al., 2015) |
| Dapsone | ||||||
| HLA-B*1301 | Thai | 60.8 | DRESS | 11 | 29 tolerant controls | (Tempark et al., 2017) |
| 40.5 | SJS/TEN | 4 | 29 tolerant controls | (Tempark et al., 2017) | ||
| Chinese | 122.1 | DRESS | 20 | 102 tolerant controls | (Wang et al., 2013) | |
| 49.6 | DRESS | 7 | 677 general population | (Chen et al., 2018) | ||
| 20.5 | DRESS | 76 | 1034 general population | (Zhang et al., 2013) | ||
| HLA-B*1502 | Thai | 28 | SJS/TEN | 4 | 29 tolerant controls | (Tempark et al., 2017) |
| Amoxicillin-clavulanate | ||||||
| HLA-DRB1*07 | British | 0.18 | DILI | 61 | 40 tolerant controls | (Donaldson et al., 2010) |
| HLA-DRB1*1501 | Scottish | 9.3 | DILI | 20 | 134 tolerant controls | (O'Donohue et al., 2000) |
| Belgian | 7.6 | DILI | 35 | 60 general population | (Hautekeete et al., 1999) | |
| HLA-DQB1*0602 | Belgian | 12 | DILI | 35 | 60 general population | (Hautekeete et al., 1999) |
| European | 4.2 | DILI | 177 | 219 general population | (Lucena et al., 2011) | |
| Flucloxacillin | ||||||
| HLA-B*5701 | European | 80.6 | DILI | 51 | 64 tolerant controls | (Daly et al., 2009) |
| Minocycline | ||||||
| HLA-B*3502 | Caucasian | 29.6 | DILI | 25 | 6835 general population | (Urban et al., 2017) |
| Erythromycin | ||||||
| HLA-A*3301 | European | 10.2 | DILI | 10 | 10,588 general population | (Nicoletti et al., 2017) |
| Terbinafine | ||||||
| HLA-A*3301 | European | 40.5 | DILI | 14 | 10,588 general population | (Nicoletti et al., 2017) |
Table 3.
Overview of genetic variations in the major histocompatibility complex associated with hypersensitivity to antiepileptics. SJS = Stevens-Johnson syndrome, TEN = toxic epidermal necrolysis, MPE = maculopapular exanthema, DRESS = drug rash with eosinophilia and systemic symptoms, SCAR = severe cutaneous adverse reaction, DIHS = drug-induced hypersensitivity syndrome.
| Allele |
Ethnicity |
Odds ratio |
Adverse reaction |
Cases |
Controls |
Study |
|---|---|---|---|---|---|---|
| Carbamazepine | ||||||
| Carbamazepine and HLA-B*1502 | ||||||
| HLA-B*1502 | Thai | 75.4 | SJS/TEN | 34 | 40 tolerant controls | (Kulkantrakorn et al., 2012) |
| 54.8 | SJS/TEN | 42 | 42 tolerant controls | (Tassaneeyakul et al., 2010) | ||
| 25.5 | SJS/TEN | 6 | 50 tolerant controls | (Locharernkul et al., 2008) | ||
| 7.27 | MPE | 17 | 271 tolerant controls | (Sukasem et al., 2018) | ||
| Chinese | 2504 | SJS/TEN | 44 | 101 tolerant controls | (Chung et al., 2004) | |
| 1357 | SJS/TEN | 60 | 144 tolerant controls | (Hung et al., 2006) | ||
| 184 | SJS/TEN | 8 | 50 tolerant controls | (Wu et al., 2010) | ||
| 152 | SJS/TEN | 17 | 21 tolerant controls | (Zhang et al., 2011) | ||
| 114.8 | SJS/TEN | 9 | 80 tolerant controls | (Wang et al., 2011) | ||
| 97.6 | SJS/TEN | 112 | 152 tolerant controls | (Hsiao et al., 2014) | ||
| 89.3 | SJS/TEN | 26 | 135 tolerant controls | (Cheung et al., 2013) | ||
| 58.1 | SJS/TEN | 53 | 72 tolerant controls | (Genin et al., 2014) | ||
| 12.4 | SJS/TEN | 56 | 179 tolerant controls | (Shi et al., 2017) | ||
| Hongkong Chinese | 89.3 | SJS/TEN | 26 | 135 tolerant controls | (Kwan Ng, & Lo, 2014) | |
| Korean | 40.3 | SJS/TEN | 7 | 485 general population | (Kim et al., 2011) | |
| Malaysian | 16.2 | SJS/TEN | 16 | 300 tolerant controls | (Chang Too, Murad, & Hussein, 2011) | |
| Vietnamese | 33.8 | SJS/TEN | 35 | 25 tolerant controls | (Nguyen et al., 2015) | |
| Indian | 71.4 | SJS/TEN | 8 | 10 general population | (Mehta et al., 2009) | |
| Multiethnic group | 168 | SJS/TEN | 6 | 7 tolerant controls | (Then Rani, Raymond, Ratnaningrum, & Jamal 2011) | |
| Carbamazepine and HLA-A*3101 | ||||||
| HLA-A*3101 | European | 57.6 | DRESS | 10 | 257 tolerant controls | (Genin et al., 2014) |
| 25.9 | SJS/TEN | 12 | 257 general population | (McCormack et al., 2011) | ||
| 12.4 | DRESS | 27 | 257 general population | (McCormack et al., 2011) | ||
| 8.3 | MPE | 106 | 257 general population | (McCormack et al., 2011) | ||
| Chinese | 23 | DRESS | 10 | 72 tolerant controls | (Genin et al., 2014) | |
| 17.5 | MPE | 18 | 144 tolerant controls | (Hung et al., 2006) | ||
| 6.4 | DIHS | 13 | 144 tolerant controls | (Hung et al., 2006) | ||
| Japanese | 33.9 | SJS/TEN | 6 | 420 tolerant controls | (Ozeki et al., 2011) | |
| 9.5 | SCAR | 77 | 420 tolerant controls | (Ozeki et al., 2011) | ||
| Korean | 12.4 | HSS | 17 | 485 general population | (Kim et al., 2011) | |
| 10.3 | SCAR | 24 | 485 general population | (Kim et al., 2011) | ||
| 6.5 | SJS | 7 | 485 general population | (Kim et al., 2011) | ||
| Carbamazepine and other class I HLAs | ||||||
| HLA-A*0201 | Chinese | 3.6 | MPE | 40 | 52 tolerant controls | (Li et al., 2013) |
| HLA-A*2402 | Chinese | 2.3 | SJS/TEN | 56 | 178 tolerant controls | (Shi et al., 2017) |
| HLA-A31 | Japanese | 11.2 | SJS/TEN or DIHS | 15 | 33 tolerant controls | (Niihara et al., 2012) |
| HLA-B*1511 | Chinese | 30.8 | SJS/TEN | 56 | 179 tolerant controls | (Shi et al., 2017) |
| Japanese | 9.8 | SJS/TEN | 11 | 493 general population | (Kaniwa et al., 2010) | |
| Korean | 18.4 | SJS | 7 | 485 general population | (Kim et al., 2011) | |
| HLA-B*1521 | Thai | 9.5 | SJS/TEN | 16 | 271 tolerant controls | (Sukasem et al., 2018) |
| HLA-B*4001 | Chinese | 0.16 | DRESS | 23 | 152 tolerant controls | (Hsiao et al., 2014) |
| 0.22 | SJS/TEN | 112 | 152 tolerant controls | (Hsiao et al., 2014) | ||
| HLA-B*4801 | Chinese | 14.4 | DRESS | 23 | 152 tolerant controls | (Hsiao et al., 2014) |
| HLA-B*5101 | Chinese | 4.9 | MPE | 51 | 152 tolerant controls | (Hsiao et al., 2014) |
| 3.9 | DRESS | 23 | 152 tolerant controls | (Hsiao et al., 2014) | ||
| HLA-B*5801 | Thai | 7.6 | DRESS | 5 | 271 tolerant controls | (Sukasem et al., 2018) |
| Chinese | 0.24 | MPE | 40 | 52 tolerant controls | (Li et al., 2013) | |
| HLA-C*0801 | Chinese | 11.8 | SJS/TEN | 55 | 177 tolerant controls | (Shi et al., 2017) |
| Carbamazepine and other class II HLAs | ||||||
| HLA-DRB1*0101 | Chinese | 14 | SJS/TEN | 54 | 176 tolerant controls | (Shi et al., 2017) |
| HLA-DRB1*0301 | Chinese | 0.22 | MPE | 40 | 52 tolerant controls | (Li et al., 2013) |
| HLA-DRB1*1202 | Chinese | 11.4 | SJS/TEN | 60 | 144 tolerant controls | (Hung et al., 2006) |
| 3.4 | SJS/TEN | 54 | 176 tolerant controls | (Shi et al., 2017) | ||
| HLA-DRB1*1405 | Chinese | 22.1 | MPE | 40 | 52 tolerant controls | (Li et al., 2013) |
| HLA-Cw*0801 | Chinese | 86.8 | SJS/TEN | 60 | 144 tolerant controls | (Hung et al., 2006) |
| Phenytoin | ||||||
| HLA-B*1502 | Thai | 18.5 | SJS | 4 | 50 tolerant controls | (Locharernkul et al., 2008) |
| Malaysian | 5.7 | SJS/TEN | 13 | 32 tolerant controls | (Chang et al., 2017) | |
| Multiethnic group | 5 | SJS/TEN | 48 | 130 tolerant controls | (Chung et al., 2014) | |
| HLA-B*5101 | Thai | 4.8 | SJS/TEN | 39 | 92 tolerant controls | (Tassaneeyakul et al., 2016) |
| 5.2 | DRESS | 21 | 92 tolerant controls | (Tassaneeyakul et al., 2016) | ||
| HLA-A*0201 | Thai | 3.9 | SCAR | 60 | 92 tolerant controls | (Tassaneeyakul et al., 2016) |
| Chinese | 11.7 | SJS/TEN | 13 | 40 tolerant controls | (Shi et al., 2017) | |
| HLA-A*2402 | Chinese | 6 | SJS/TEN | 13 | 40 tolerant controls | (Shi et al., 2017) |
| HLA-A*3303 | Thai | 2.7 | SJS/TEN | 39 | 92 tolerant controls | (Tassaneeyakul et al., 2016) |
| HLA-B*1513 | Malaysian | 59 | DRESS | 3 | 32 tolerant controls | (Chang et al., 2017) |
| 11.3 | SJS/TEN | 13 | 32 tolerant controls | (Chang et al., 2017) | ||
| HLA-B*3802 | Thai | 3.2 | SCAR | 60 | 92 tolerant controls | (Tassaneeyakul et al., 2016) |
| HLA-B*5602 | Thai | 8.3 | SCAR | 60 | 92 tolerant controls | (Tassaneeyakul et al., 2016) |
| HLA-B*5801 | Thai | 3.2 | SJS/TEN | 39 | 92 tolerant controls | (Tassaneeyakul et al., 2016) |
| HLA-C*1402 | Thai | 5.9 | SCAR | 60 | 92 tolerant controls | (Tassaneeyakul et al., 2016) |
| Oxcarbazepine | ||||||
| HLA-B*1502 | Thai | 49 | SJS | 3 | 99 general population | (Chen et al., 2017) |
| Chinese | 27.9 | SJS | 17 | 101 tolerant controls | (Chen et al., 2017) | |
| 6.4 | MPE | 9 | 9 tolerant controls | (Hu et al., 2011) | ||
| HLA-B*1501 | Korean | 0.18 | MPE | 40 | 70 tolerant controls | (Moon et al., 2016) |
| HLA-B*3802 | Chinese | 3.2 | MPE | 28 | 56 tolerant controls | (Lv et al., 2013) |
| HLA-B*4002 | Korean | 4.3 | MPE | 40 | 70 tolerant controls | (Moon et al., 2016) |
| HLA-DRB1*0403 | Korean | 14.6 | MPE | 40 | 70 tolerant controls | (Moon et al., 2016) |
| Lamotrigine | ||||||
| HLA-A*0207 | Thai | 7.8 | SCAR | 15 | 50 tolerant controls | (Koomdee et al., 2017) |
| HLA-A*2402 | Spanish | 49 | DRESS | 3 | 10 tolerant controls | (Ramírez et al., 2017) |
| Chinese | 4.5 | SJS/TEN | 22 | 102 tolerant controls | (Shi et al., 2017) | |
| Korean | 4.1 | MPE | 21 | 29 tolerant controls | (Moon et al., 2015) | |
| HLA-A*3001 | Chinese | 14.3 | MPE | 43 | 44 tolerant controls | (Li et al., 2013) |
| HLA-A*3101 | Korean | 11.4 | SCAR | 18 | 29 tolerant controls | (Kim et al., 2017) |
| HLA-B*1502 | Thai | 4.9 | SCAR | 15 | 50 tolerant controls | (Koomdee et al., 2017) |
| Chinese | 3.3 | SJS/TEN | 6 | 30 tolerant controls | (Cheung et al., 2013) | |
| 4.2 | SJS/TEN | 9 | 123 tolerant controls | (Shi et al., 2011) | ||
| HLA-B*1302 | Chinese | 14.3 | MPE | 43 | 44 tolerant controls | (Li et al., 2013) |
Table 4.
Overview of genetic variations in the major histocompatibility complex associated with hypersensitivity to other medications. SJS = Stevens-Johnson syndrome, TEN = toxic epidermal necrolysis, DRESS = drug rash with eosinophilia and systemic symptoms, SCAR = severe cutaneous adverse reaction, DILI = drug-induced liver injury, SLE = systemic lupus erythematosus.
| Allele | Ethnicity | Odds ratio | Adverse reaction | Cases | Controls | Study |
|---|---|---|---|---|---|---|
| Allopurinol | ||||||
| HLA-B*5801 | European | 80 | SJS/TEN | 27 | 1822 general population | (Lonjou et al., 2008) |
| Portuguese | 39.1 | SCAR | 25 | 23 tolerant controls | (Gonçalo et al., 2013) | |
| Thai | 348.3 | SJS/TEN | 27 | 54 tolerant controls | (Tassaneeyakul et al., 2009) | |
| Chinese | 580.3 | SCAR | 51 | 135 tolerant controls | (S.-I. Hung et al., 2005) | |
| Japanese | 65.6 | SJS/TEN or erythemaexudativum multiforme | 7 | 25 tolerant controls | (Niihara et al., 2013) | |
| 40.8 | SJS/TEN | 20 | 986 general population | (Kaniwa et al., 2008) | ||
| Korean | 97.8 | SCAR | 26 | 57 tolerant controls | (Kang et al., 2011) | |
| HLA-B58 | Korean | 179.2 | SCAR | 9 | 432 tolerant controls | (Jung et al., 2011) |
| HLA-A*0201 | Korean | 0.04 | SCAR | 26 | 57 tolerant controls | (Kang et al., 2011) |
| HLA-A*3303 | Korean | 20.5 | SCAR | 26 | 57 tolerant controls | (Kang et al., 2011) |
| HLA-A33 | Korean | 8.3 | SCAR | 9 | 432 tolerant controls | (Jung et al., 2011) |
| HLA-DR3 | Korean | 11.4 | SCAR | 9 | 432 tolerant controls | (Jung et al., 2011) |
| HLA-DR13 | Korean | 5.5 | SCAR | 9 | 432 tolerant controls | (Jung et al., 2011) |
| HLA-Cw3 | Korean | 19.4 | SCAR | 9 | 432 tolerant controls | (Jung et al., 2011) |
| HLA-Cw*0302 | Korean | 82.1 | SCAR | 26 | 57 tolerant controls | (Kang et al., 2011) |
| Lumiracoxib | ||||||
| HLA-DRB1*1501 | Multiethnic | 7.5 | DILI | 137 | 577 tolerant controls | (Singer et al., 2010) |
| HLA-DRB5*0101 | Multiethnic | 7.2 | DILI | 137 | 577 tolerant controls | (Singer et al., 2010) |
| HLA-DQA1*0102 | Multiethnic | 6.3 | DILI | 137 | 577 tolerant controls | (Singer et al., 2010) |
| HLA-DQB1*0602 | Multiethnic | 6.9 | DILI | 137 | 577 tolerant controls | (Singer et al., 2010) |
| Aspirin | ||||||
| HLA-DRB1*0301 | Korean | 9.7 | Asthma | 76 | 73 tolerant controls | (Choi et al., 2004) |
| HLA-DRB1*0901 | Korean | 2.3 | Asthma | 76 | 73 tolerant controls | (Choi et al., 2004) |
| HLA-DRB1*1302 | Korean | 4 | Urticaria | 188 | 152 tolerant controls | (Kim, Choi, Lee, et al., 2005) |
| HLA-DQB1*0609 | Korean | 5.6 | Urticaria | 188 | 152 tolerant controls | (Kim, Choi, Lee, et al., 2005) |
| HLA-DPB1*0301 | Swiss | 5.3 | Asthma | 59 | 57 tolerant controls | (Dekker et al., 1997) |
| Korean | 5.2 | Asthma | 76 | 73 tolerant controls | (Choi et al., 2004) | |
| Feprazone | ||||||
| HLA-B22 | Italian | 48 | Fixed drug eruption | 40 | 215 general population | (Pellicano et al., 1997) |
| HLA-Cw1 | Italian | 13.9 | Fixed drug eruption | 40 | 215 general population | (Pellicano et al., 1997) |
| Oxicam NSAIDs | ||||||
| HLA-B*7301 | European | 152 | SJS/TEN | 14 | 1822 general population | (Lonjou et al., 2008) |
| Clozapine | ||||||
| HLA-B38 | Ashkenazi Jew | 50 | Agranulocytosis | 15 | 32 general population | (Yunis et al., 1995) |
| HLA-B (158 T) | European | 3.1 | Agranulocytosis | 161 | 4300 general population | (Goldstein et al., 2014) |
| HLA-DR4 | Ashkenazi Jew | 23.3 | Agranulocytosis | 15 | 32 general population | (Yunis et al., 1995) |
| HLA-DRB1*0402 | Ashkenazi Jew | 6.8 | Agranulocytosis | 24 | 54 general population | (Yunis et al., 1995) |
| HLA-DRB1*11 | Ashkenazi Jew | 0.06 | Agranulocytosis | 24 | 54 general population | (Yunis et al., 1995) |
| HLA-DQA1*0301 | Ashkenazi Jew | 3.1 | Agranulocytosis | 24 | 54 general population | (Yunis et al., 1995) |
| HLA-DQB1*0302 | Ashkenazi Jew | 4.9 | Agranulocytosis | 24 | 54 general population | (Yunis et al., 1995) |
| HLA-DQB1 (126Q) | European | 0.19 | Agranulocytosis | 161 | 4300 general population | (Goldstein et al., 2014) |
| Sertraline | ||||||
| HLA-A*3301 | European | 29 | DILI | 5 | 10,588 general population | (Nicoletti et al., 2017) |
| Hydralazine | ||||||
| HLA-DR4 | British | 5.6 | SLE | 26 | 113 general population | (Batchelor et al., 1980) |
| Enalapril | ||||||
| HLA-A*3301 | European | 34.8 | DILI | 4 | 10,588 general population | (Nicoletti et al., 2017) |
| Methazolamide | ||||||
| HLA-B*5901 | Chinese | 305 | SJS/TEN | 8 | 30 tolerant controls | (Yang et al., 2015) |
| Korean | 249.8 | SJS/TEN | 5 | 485 general population | (Kim et al., 2010) | |
| Ticlopidine | ||||||
| HLA-A*3301 | European | 163.1 | DILI | 5 | 10,588 general population | (Nicoletti et al., 2017) |
| HLA-A*3303 | Japanese | 13 | DILI | 22 | 85 tolerant controls | (Hirata et al., 2008) |
| Thionamides | ||||||
| HLA-B*3802 | Chinese | 12.3 | Agranulocytosis | 42 | 1202 general population | (Chen et al., 2015) |
| HLA-B*3803 | Chinese | 4.4 | Agranulocytosis | 42 | 1196 general population | (Chen et al., 2015) |
| Lapatinib | ||||||
| HLA-DQA1*0201 | European | 9 | DILI | 24 | 155 tolerant controls | (Spraggs et al., 2011) |
| Flupirtine | ||||||
| HLA-DRB1*1601 | German | 18.7 | DILI | 6 | 39,689 general population | (Nicoletti et al., 2016) |
| Methyldopa | ||||||
| HLA-A*3301 | European | 97.8 | DILI | 4 | 10,588 general population | (Nicoletti et al., 2017) |
| Fenofibrate | ||||||
| HLA-A*3301 | European | 58.7 | DILI | 7 | 10,588 general population | (Nicoletti et al., 2017) |
For antiepileptics, the strongest associations have been identified for carbamazepine-induced SJS/TEN and HLA-B*1502 in South and East Asian populations, including Chinese, Koreans, Thai, Malaysians and Indians, with odds ratios between 10 and 2500 (Table 3). In contrast, HLA-A*3101 predicts SCARs in Koreans, Japanese and Europeans and a recent prospective cohort study with 1130 Japanese patients showed significantly reduced incidence of carbamazepine-induced cutaneous adverse reactions in the genotyped group (2% vs. 3.4–5.1% in historic controls)(Mushiroda et al., 2018). Moreover, HLA-B*1511 and HLA-B*1521 were implicated as additional risk alleles in various Asian populations (Table 3 and (Jaruthamsophon et al., 2017)). HLA biomarkers for phenytoin-induced SCARs have to our knowledge only been reported in Asian populations. The strongest risk factor has been found for HLA-B*1502 with moderate odds ratios between 5 and 20, aligning with pharmacogenetic carbamazepine associations for these populations. However, the largest case-control study published to date in Thailand could not replicate this association and rather identified a multitude of other significantly associated HLA alleles, such as HLA-B*3802, HLA-B*5602 and HLA-C*1402 (Tassaneeyakul et al., 2016). For lamotrigine, various HLA associations have been reported, of which HLA-B*1502 and HLA-A*2402 have been reproduced. Combined, the existing data provide irrefutable evidence for associations between HLA alleles and SCARs related to antiepileptics. Carbamazepine is consistently associated with HLA-B*1502 and HLA-A*3101. In contrast, risk factors for cutaneous adverse reactions to phenytoin and lamotrigine appear more heterogeneous.
Adverse cutaneous reactions following treatment with the xanthine oxidase inhibitor allopurinol, used for the treatment of gout and other conditions associated with an excess of uric acid, have been consistently linked with HLA-B*5801 across ethnicities with odds ratios between 40 and 580 (Table 4). Furthermore, a prospective multicenter study in Taiwan with 2910 Han Chinese participants found that preemptive genotyping eliminated SCARs due to allopurinol when HLA-B*5801 patients were instead referred to an alternate treatment (Ko et al., 2015). In addition, one study in 25 Korean allopurinol SCAR patients and 57 tolerant controls indicated a strong protective effect of HLA-A*0201 (0/25 cases, 17/57 controls; OR = 0.04). However, this interesting observation requires further validations.
Cases of idiosyncratic DILI are generally much more rare than cases of adverse cutaneous reactions, which has made the identification of genetic factors predisposing to DILI difficult. Importantly, the establishment of large networks that collect and consolidate DILI cases, such as DILIN in the US and the DILIGEN study in the UK, have provided a significant step forward, increasing the study power and resulting in the identification of multiple HLA biomarkers in recent years. Notable examples include associations between flucloxacillin and HLA-B*5701 (OR = 80.6) (Daly et al., 2009), terbinafine and HLA-A*3301 (OR = 40.5) (Nicoletti et al., 2017), minocycline and HLA-B*3502 (OR = 29.6) (Urban et al., 2017) and flupirtine with the DRB1*1601-DQB1*0502 haplotype (OR = 18.7)(Nicoletti et al., 2016).
3.1.2. Molecular mechanisms of drug hypersensitivity
The molecular and immunological mechanisms underlying drug hypersensitivity are diverse and drug specific. Abacavir hypersensitivity is restricted exclusively to carriers of the HLA-B*5701 allele with a negative predictive value of 100%. The abacavir parent compound binds specifically to the F-pocket of the peptide-binding groove of HLA-B*5701 and alters the repertoire of presented self-peptides, driving polyclonal alloreactive autoimmune responses (Illing et al., 2012; Norcross et al., 2012; Ostrov et al., 2012). Mechanistically similar immune activation has been suggested for nevirapine in some studies (Hirasawa et al., 2018), whereas others did not observe alterations in the repertoire of presented peptides in nevirapine exposed cells (Pavlos et al., 2017). In contrast, carbamazepine has been shown to activate carbamazepine-reactive CD8+ T-cells in the absence of loaded peptides by directly interacting with the HLA variant HLA-B*1502 (Wei et al., 2012). Similar direct HLA binding and T-cell activation has been reported for ticlopidine (Usui et al., 2018) and the allopurinol metabolite oxypurinol (Yun et al., 2014). Whereas carbamazepine and oxypurinol interact non-covalently with the MHC, hypersensitivity reactions to β-lactam antibiotics involve covalent protein binding. Specifically, flucloxacillin binds covalently to lysine residues on albumin and the resulting flucloxacillin haptens are high affinity binders at HLA-B*5701 (Monshi et al., 2013). Lastly, sulfamethoxazole has been suggested to directly affect T-cell receptor conformation, thereby modulating HLA recognition and autoimmunity (Watkins & Pichler, 2013). For a more detailed overview of the mechanistic underpinnings of drug hypersensitivity, we refer the interested reader to excellent recent reviews on this topic (Bharadwaj et al., 2012; Chen et al., 2018; Pavlos et al., 2015).
3.1.3. Clinical implications
Routine clinical implementation of pharmacogenetic tests requires not only a strong association with severe adverse events but also various other conditions need to be considered, including the availability, efficacy and safety of alternative drugs, supportive clinical and experimental data, permissive environmental factors, sufficiently high prevalence of hypersensitivity and high positive predictive value of the test (Phillips & Mallal, 2010). Furthermore, test rollout depends on monetary considerations and various health economic studies have addressed whether pharmacogenetic testing constitutes a cost-effective use of healthcare resources. Testing of HLA-B*5701 prior to initiation of abacavir is suggested to be cost-effective in the UK (Hughes et al., 2004) and Germany (Wolf et al., 2010). Similarly, genotyping of HLA-A*3101 and HLA-B*1502 before starting carbamazepine therapy is likely cost-effective in the UK (Plumpton et al., 2015; Yip et al., 2012), whereas its cost-effectiveness is dependent on patient ethnicity in Singapore due to differences in population allele frequencies (Dong, Sung, & Finkelstein, 2012). Furthermore, a recent study suggested the cost-effectiveness of restricting long-term hematologic monitoring of patients with treatment-resistant schizophrenia on clozapine to carriers of the HLA-DQB1 (126Q) and HLA-B (158 T) variants (Girardin et al., 2018). In contrast, preemptive testing of HLA-B*5801 and HLA-B*5701 prior to initiation of allopurinol and flucloxacillin therapy, respectively, has not been found to be cost-effective (Phillips & Mallal, 2013; Plumpton, Alfirevic, Pirmohamed, & Hughes, 2017).
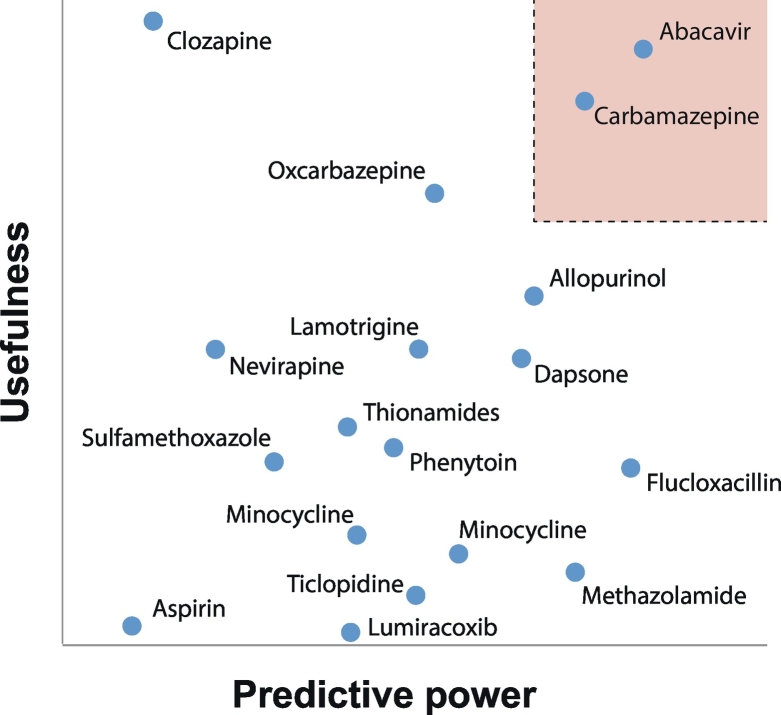
Based on the considerations and data highlighted above, recommendations for pharmacogenetic testing of the respective HLA risk alleles have been incorporated into current guidelines for abacavir (Aberg et al., 2009; Gazzard et al., 2008; Martin et al., 2014) and carbamazepine therapy (Phillips et al., 2018), whereas other associations have not yet been implemented into clinical practice (Fig. 2).
Fig. 2.
Overview of the utility of HLA biomarkers for the prediction of hypersensitivity reactions to different medicines. The abscissa (predictive power) refers to the strength of association between a HLA variant alleles and adverse drug reactions. We refer to Table 2, Table 3, Table 4 for details about the specific variant alleles of importance for the listed medications. The ordinate estimates the usefulness of a test that considers various practical aspects, including cost-effectiveness, availability of alternative treatments and severity of the adverse event. The box shaded in light red highlights the space that supports clinical implementation of the companion diagnostic.
3.2. Anthracycline-induced cardiotoxicity
Anthracyclines are commonly used in chemotherapy regimens for the treatment of a variety of solid tumors and hematological malignancies in both pediatric and adult patients. However, depending on gender, age, cumulative dose and measured endpoints, 9–27% of patients experience cardiotoxicity that manifests in structural changes and left ventricular dysfunction after 1 year of follow-up (Cardinale et al., 2015; Hequet et al., 2004; Thavendiranathan et al., 2013) and up to 5% suffer from congestive heart failure (Swain, Whaley, & Ewer, 2003). Mechanisms underlying anthracycline-induced cardiotoxicity are complex and include oxidative and nitrosative stress, perturbation of myocardial calcium signaling and energy metabolism, as well as DNA damage (Mordente et al., 2009). Identification of biomarkers that can identify patients prone to anthracycline-induced cardiotoxicity therefore represents an important strategy to maximize the clinical utility of anthracyclines and to personalize the choice of chemotherapy-regimen. Recent research implicated variations in >20 genes in anthracyclin-induced cardiotoxicity (Table 5).
Table 5.
Overview of genetic factors associated with anthracycline-induced cardiotoxicity.
| Process | Gene | Variant | Ethnicity | Odds ratio | Study type | Cohort | Study |
|---|---|---|---|---|---|---|---|
| Anthracycline metabolism | CBR3 | rs1056892 (V244M) |
Multiethnic cohort | 8.2 | Candidate gene study | 30 cases and 115 tolerant controls | (Blanco et al., 2008) |
| Multiethnic cohort | 3.3 | Candidate gene study | 170 cases and 317 tolerant controls | (Blanco et al., 2012) | |||
| Anthracycline transport | SLC22A7 | rs4149178 (Intronic) |
Canadian | 0.45 | Candidate gene study | 122 cases and 398 tolerant controls | (Visscher et al., 2015) |
| SLC22A17 | rs4982753 (Regulatory) |
Canadian | 0.5 | Candidate gene study | 122 cases and 398 tolerant controls | (Visscher et al., 2015) | |
| SLC28A3 | rs7853758 (L461 L) |
Multiethnic cohort | 0.35 | Candidate gene study | 121 cases and 319 tolerant controls | (Visscher et al., 2012) | |
| Multiethnic cohort | 0.36 | Candidate gene study | 124 cases and 397 tolerant controls | (Visscher et al., 2013) | |||
| rs885004 (Intronic) |
Multiethnic cohort | 0.34 | Candidate gene study | 124 cases and 397 tolerant controls | (Visscher et al., 2013) | ||
| ABCC1 | rs246221 (V275 V) |
Belgian | 1.6 | Candidate gene study | 153 cases and 724 tolerant controls | (Vulsteke et al., 2015) | |
| rs45511401 (G671 V) |
German | 3.6 | Candidate gene study | 44 cases and 363 tolerant controls | (Wojnowski et al., 2005) | ||
| ABCC2 | rs8187710 (C1515Y) | German | 2.3 | Candidate gene study | 44 cases and 363 tolerant controls | (Wojnowski et al., 2005) | |
| Multiethnic cohort | 4.3 | Candidate gene study | 77 cases and 178 tolerant controls | (Armenian et al., 2013) | |||
| ABCG2 | rs2231142 (Q141K) |
Spanish | 5.3 | Candidate gene study | 45 cases and 180 tolerant controls | (Megías-Vericat et al., 2017) | |
| Redox signaling | CYBA | rs4673 (Y72H) |
German | 2 | Candidate gene study | 44 cases and 363 tolerant controls | (Wojnowski et al., 2005) |
| Spanish | 0.3 | Candidate gene study | 32 cases and 192 tolerant controls | (Megías-Vericat et al., 2018) | |||
| RAC2 | rs13058338 (Intronic) |
Multiethnic cohort | 2.8 | Candidate gene study | 77 cases and 178 tolerant controls | (Armenian et al., 2013) | |
| German | 2.6 | Candidate gene study | 44 cases and 363 tolerant controls | (Wojnowski et al., 2005) | |||
| Multiethnic cohort | 2.3 | Candidate gene study | 56 cases and 94 tolerant controls | (Reichwagen et al., 2015) | |||
| NCF4 | rs1883112 (Regulatory) |
German | 2.5 | Candidate gene study | 44 cases and 363 tolerant controls | (Wojnowski et al., 2005) | |
| Spanish | 5.2 | Candidate gene study | 32 cases and 193 tolerant controls | (Megías-Vericat et al., 2018) | |||
| CAT | rs10836235 (Intronic) |
Caucasian | 0.28 | Candidate gene study | 43 cases and 33 tolerant controls | (Rajić et al., 2009) | |
| Retinoic acid signaling | RARG | rs2229774 (S427 L) |
Multiethnic cohort | 4.7 | GWAS | 73 cases and 383 tolerant controls | (Aminkeng, et al., 2015) |
| Phase II metabolism | UGT1A6 | rs17863783 (V209 V) |
Multiethnic cohort | 4.3 | Candidate gene study | 124 cases and 397 tolerant controls | (Visscher, et al., 2013) |
| GSTM1 | Whole gene | Italian | 0.4 | Candidate gene study | 13 cases and 35 tolerant controls | (Vivenza et al., 2013) | |
| GSTP1 | rs1695 (I105V) | Multiethnic cohort | 9.4 | Candidate gene study | 16 cases and 39 tolerant controls | (Windsor et al., 2012) | |
| Iron transport | HFE | rs1799945 (H63D) |
Multiethnic cohort | 2.5 | Candidate gene study | 77 cases and 178 tolerant controls | (Armenian et al., 2013) |
| rs1800562 (C282Y) | Multiethnic cohort | 9.2 | Candidate gene study | 11 cases and 168 tolerant controls | (Lipshultz et al., 2013) | ||
| CYP regulation | POR | rs2868177 (Intronic) |
Multiethnic cohort | 1.9 | Candidate gene study | 10 cases and 81 tolerant controls | (Lubieniecka et al., 2013) |
| rs13240755 (Intronic) |
Multiethnic cohort | 3.2 | Candidate gene study | 10 cases and 81 tolerant controls | (Lubieniecka et al., 2013) | ||
| Extracellular matrix | HAS3 | rs2232228 (A93A) |
Non-Hispanic white | 56.5 | GWAS | 93 cases and 194 tolerant controls | (Wang et al., 2014) |
| Splicing | CELF4 | rs1786814 (Intronic) |
Non-Hispanic white | 10.2 | GWAS | 112 cases and 219 tolerant controls | (Wang et al., 2016) |
| Golgi homeostasis? | GOLGA6L2 | rs28714259 (Intergenic) |
Multiethnic cohort | 4.2 | GWAS | 24 cases and 298 tolerant controls | (Schneider et al., 2017) |
Carbonyl reductases metabolize anthracyclines to their alcohol metabolites and seminal studies demonstrated that these metabolites are potent inhibitors (up to 80-times more potent then the parent molecule) of sarcoplasmatic calcium handling and mitochondrial F-type proton ATPases that accumulate specifically in the heart after long-term anthracycine treatment (Boucek et al., 1987; Olson et al., 1988). The V244M variant of CBR3 exhibits 2.6-fold reduced metabolism per unit of time and multiple studies have associated the corresponding polymorphism rs1056892 with cardioprotective effects in pediatric (Blanco et al., 2008; Blanco et al., 2012; Volkan-Salanci et al., 2012) and adult patients (Hertz et al., 2016), whereas other studies did not reproduce this association (Aminkeng et al., 2015; Armenian et al., 2013; Lubieniecka et al., 2012; Visscher et al., 2012).
Multiple genes involved in redox signaling and detoxification of reactive oxygen species have been implicated in anthracycline-induced cardiotoxicity risk in multiple cohorts. These include the CYBA, RAC2 and NCF4 subunits of the NADPH oxidase complex, catalase (CAT) as well as the glutathione-S-transferase (GST) GSTP1 (Table 5). Strikingly, NADPH oxidase deficient mice were fully protected from anthracycline-induced cardiotoxicity, further strengthening the link between ROS and myocardial dysfunction (Wojnowski et al., 2005). However, preconditioning of patients with antioxidants, such as coenzyme Q10 or N-acetylcysteine did not result in patient benefits (Iarussi et al., 1994; Myers et al., 1983), and treatment with the iron chelator dexrazoxane remains the only cardioprotective treatment with regulatory approval. Thus, while pharmacogenetic associations between genes involved in redox signaling and anthracycline-induced cardiotoxicity have been consistently reported, their low odds ratios (OR < 6) preclude their application for the guidance of therapy.
In addition to genes involved in anthracycline metabolism and redox signaling, pharmacogenetic studies implicated multiple transporter genes in cardiac dysfunction due to anthracylines, but only associations with ABCC1 (Semsei et al., 2012; Vulsteke et al., 2015), ABCC2 (Aminkeng et al., 2015; Armenian et al., 2013; Wojnowski et al., 2005) and SLC28A3 (Visscher et al., 2012; Visscher et al., 2013) have been replicated. ABCC1 (MRP1) and ABCC2 (MRP2) have been shown to transport anthracyclines (Cole et al., 1994; Folmer, Schneider, Blum, & Hafkemeyer, 2007). Most supportive data are available for rs8187710 in ABCC2 that encodes a C1515Y amino acid exchange in MRP2 and results in reduced uptake of MRP2 substrates (Elens et al., 2011), whereas rs3743527 resides in the untranslated region of ABCC1 and no direct effects of this variant on MRP1 have been reported. SLC28A3 has to our knowledge not been demonstrated to be an anthracycline uptake transporter and thus the pharmacogenetic association lacks mechanistic support.
Retinoic acid (RA) signaling mediated at least in part by its nuclear receptor RARG is essential for cardiac development, coronary vasculogenesis and cardiomyocyte proliferation (Merki et al., 2005; Romeih et al., 2003; Xavier-Neto et al., 2015). Furthermore, levels of Raldh2, the central enzyme in RA biosynthesis, increased in the epicardium and the RA precursor retinol accumulates at the ischemic site in mouse models for myocardial infarction, resulting in significant activation expression of RA target genes (Bilbija et al., 2012; Kikuchi et al., 2011; Zhou et al., 2011). Combined this data suggest that RA signaling might contribute to tissue repair in post ischemic hearts. Importantly, the missense variant rs2229774 encoding an S427L amino acid exchange in RARG is strongly associated with anthracycline-induced cardiotoxicity in cohorts of European, African, Aboriginal Canadian, Hispanic and East Asian ancestry with odds ratios (OR) between 4.1 and 7 (Aminkeng et al., 2015). RARG binds to the TOP2B promoter (Delacroix et al., 2010) and represses its transcription in cardiomyocytes in vitro (Aminkeng et al., 2015). TOP2B is necessary for intercalation of anthracyclines into DNA (Tewey, Rowe, Yang, Halligan, & Liu, 1984) and cardiomyocyte-specific ablation of Top2b protects mice from anthracycline-induced cardiotoxicity (Zhang et al., 2012). Importantly, the repressive effect of RARG on TOP2B expression is diminished when the S427L RARG variant was transfected (Aminkeng et al., 2015), thereby providing a mechanistic link between the identified polymorphism, TOP2B expression and anthracycline-induced cardiotoxicity.
Fueled by these insights and the tremendous clinical relevance of anthracycline-induced cardiotoxicity, a variety of mechanistically diverse cardioprotective adjuvant therapies have been proposed. Of these dexrazoxane (relative risk [RR] = 0.35, p < .00001), inhibition of adrenergic beta receptors (RR = 0.31, p = .001), or HMG-CoA reductase (RR = 0.31, p = .01) and angiotensin antagonists (RR = 0.11, p < .0001) are most extensively studied and were found to significantly prevent cardiotoxicity in a large meta-analysis (Kalam & Marwick, 2013). Furthermore, the substantial available evidence has resulted in the development of clinical practice guidelines that recommend prospective genotyping of pediatric patients with an indication for anthracycline therapy and adjustment of frequency and aggressiveness of monitoring by genotype as well as off-label prescription of the cardioprotective agent dexrazoxane to high-risk patients (Aminkeng et al., 2016).
3.3. Corticosteroid-induced osteonecrosis
The use of glucocorticoids prednisone and dexamethasone in the treatment of acute lymphoblastic leukemia (ALL) constitutes an essential component of ALL chemotherapy regimens and has contributed to significantly increased cure rates (Inaba & Pui, 2010). However, corticosteroid therapy can cause debilitating adverse reactions, including osteonecrosis, which occurs in 6% to 9% of pediatric and up to 20% of adolescent ALL patients and can result in life-long arthritis and pain in cancer survivors (Mattano, Sather, Trigg, & Nachman, 2000; te Winkel et al., 2011). Mechanisms underlying osteonecrosis due to glucocorticoids are believed to be thrombophilia, hyperlipidemia, intraosseous accumulation of lipids and fat embolism, that together result in reduced intramedullary blood flow, bone marrow ischemia and osteonecrosis (Shah, Racine, Jones, & Aaron, 2015). Furthermore, glucocorticoids might directly induce apoptosis of osteoblasts (Yun, Yoon, Jeong, & Chung, 2008).
Pharmacogenomic studies spearheaded primarily by the St. Jude Children's Research Hospital have implicated a variety of genetic factors in corticosteroid-induced osteonecrosis (Table 6). While these candidate studies raised hopes to find genetic biomarkers that could efficiently stratify patients by osteonecrosis risk, results from two agnostic genome-wide association studies (GWAS) were chastening and none of the associations could be replicated. Instead, the first GWAS revealed variants in the ACP1-SH3YL1 locus to be associated with osteonecrosis (p = 1.2*10−6, OR = 5.8), whereas associations with TYMS, VDR and SERPINE1 were again not replicated (Kawedia et al., 2011). While not reaching genome-wide significance (threshold p < 1*10−7), the implication of ACP1 as a key regulator of osteoblast differentiation (Zambuzzi et al., 2008) provides biological plausibility to the role of ACP1 in corticosteroid-induced osteonecrosis. The second and so-far largest GWAS study into corticosteroid adverse reactions encompassing 2285 children in the discovery cohort identified two loci encoding glutamate receptor subunits (GRIN3A and GRIK1) on separate chromosomes as their top two associations and replicated these associations using a candidate approach in two independent cohorts with OR pivoting around 2 and meta-analysis p-values of 2.7*10−8 and 1.3*10−6 (Karol et al., 2015). Thus, while a variety of loci with biologically plausible effects have been identified, the absence of replication in independent cohorts indicates that pharmacogenetic testing of variants, which can predict the risk of developing osteonecrosis following corticosteroid therapy, can currently not result in actionable outcomes and thus do not warrant clinical implementation in the near future.
Table 6.
Overview of genetic factors associated with corticosteroid-induced osteonecrosis. Variant support was defined as follows: Replication = identification of the same association in multiple (≥2) independent cohorts. Mechanistic support = Contextualization of the gene in question with corticosteroid pharmacokinetics, pharmacodynamics or bone development. Pathway = multiple significant associations in the same biological pathway. Experimental = in vitro evidence that the variant alters the functionality of the respective gene product.
| Gene | Variant | Ethnicity | Odds ratio | Study type | Cohort | Study | Support |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Replication | Mechanistic | Pathway | Experimental | |||||||
| VDR | rs2228570 (Altered start codon) | Multiethnic cohort | 4.5 | Candidate gene study | 25 cases and 39 tolerant controls | (Relling et al., 2004) | x | |||
| TYMS | Enhancer tandem repeat | Multiethnic cohort | 7.4 | Candidate gene study | 25 cases and 39 tolerant controls | (Relling et al., 2004) | x | x | ||
| SERPINE1 | rs6092 (A15T) |
Multiethnic cohort | 2.9 | Candidate gene study | 46 cases and 246 tolerant controls | (French, et al., 2008) | x | |||
| ACP1 | rs12714403 & rs10167992 (Intronic) |
Multiethnic cohort | 5.6 | GWAS | 69 cases and 263 tolerant controls | (Kawedia et al., 2011) | x | |||
| GRIN3A | rs10989692 (Regulatory) |
Multiethnic cohort | 2 | GWAS, 2 replication cohorts | 250 cases and 2035 tolerant controls | (Karol et al., 2015) | x | x | x | |
| GRIK1 | rs2154490 (Intronic) |
Multiethnic cohort | 1.3 | GWAS, 2 replication cohorts | 250 cases and 2035 tolerant controls | (Karol et al., 2015) | x | x | x | |
| BCL2L11 | rs2241843 (Intronic) |
Caucasian | 2.4 | Candidate gene study | 32 cases and 272 tolerant controls | (Plesa et al., 2017) | x | x | ||
| rs724710 (I155I) |
Caucasian | 5.5 | Candidate gene study | 14 cases and 166 tolerant controls | (Plesa et al., 2017) | x | x | |||
3.4. L-asparaginase hypersensitivity
In addition to corticosteroids, asparaginase constitutes a cornerstone of the therapy of ALL and other hematologic malignancies since the 1970s. While normal human cells can synthesize asparagine from aspartate and glutamine, many cancers have deficiencies in asparagine biosynthesis and rely on external asparagine supply to fulfill their demands (Balasubramanian, Butterworth, & Kilberg, 2013). Asparaginase exerts its anti-leukemic effect by catalyzing the degradation of asparagine in the circulation, thereby depriving cancer cells of needed asparagine, blunting tumor growth and inducing apoptosis. While treatment is overall effective, 20–30% of patients experience hypersensitivity reactions with anaphylaxis, rash, erythema, urticaria, pruritis, pain, respiratory problems and edema that require modification or discontinuation of the treatment regimen of choice (Panosyan et al., 2004; Pieters et al., 2011).
The glutamate receptor gene GRIA1 was identified as the top hit in a GWAS encompassing 485 children (Chen et al., 2010) and was independently replicated in two additional cohorts of 576 and 146 pediatric patients (Kutszegi et al., 2015; Rajić, Debeljak, Goričar, & Jazbec, 2015). These findings align with the association of the glutamate receptor subunits GRIN3A and GRIK1 with corticosteroid-induced osteonecrosis (compare Table 7 and previous section). In addition, variations in the glutamate receptor GRIA2 and the glutamate decarboxylase GADL1 were also strongly implicated in the pharmacogenetics of lithium therapy in bipolar disorder (Chen et al., 2014; Perlis et al., 2009), providing evidence for an interesting broader implication of glutamate signaling in drug response phenotypes.
Table 7.
Overview of genetic variations associated with asparaginase hypersensitivity.
| Gene | Variant | Ethnicity | Odds ratio | Study type | Cohort | Study |
|---|---|---|---|---|---|---|
| GRIA1 | rs4958351 (Intronic) |
Caucasian | 1.7 | Candidate gene study | 72 cases and 74 tolerant controls | (Rajić et al., 2015) |
| Hungarian | 0.05 | Candidate gene study | 66 cases and 398 tolerant controls | (Kutszegi et al., 2015) | ||
| rs4958381 (Intergenic) |
Multiethnic cohort | 1.8 | Candidate gene study | 204 cases and 281 tolerant controls | (Chen et al., 2010) | |
| rs4958676, rs6889909and rs6890057 (all intronic) |
Caucasian | 1.6 | Candidate gene study | 72 cases and 74 tolerant controls | (Rajić et al., 2015) | |
| rs10070447 (Intronic) |
Caucasian | 1.7 | Candidate gene study | 72 cases and 74 tolerant controls | (Rajić et al., 2015) | |
| rs2055083 (Intronic) |
Hungarian | 0.2 | Candidate gene study | 298 cases and 192 tolerant controls | (Kutszegi et al., 2015) | |
| rs707176 (I187I) |
Hungarian | 3 | Candidate gene study | 292 cases and 185 tolerant controls | (Kutszegi et al., 2015) | |
| HLA-DRB1 | rs17885382 (R54Q) |
Multiethnic cohort | 1.6 | GWAS | 589 cases and 2719 tolerant controls | (Fernandez et al., 2015) |
| HLA-DRB1*0701 | Multiethnic cohort | 1.6 | Candidate gene study | 363 cases and 1844 tolerant controls | (Fernandez et al., 2014) | |
| Hungarian | 2.9 | Candidate gene study | 321 cases and 38 tolerant controls | (Kutszegi et al., 2017) | ||
| ASNS | rs3757676 and rs3832526 (both intronic) |
Caucasian | 0.4 | Candidate gene study | 45 cases and 240 tolerant controls | (Ben Tanfous et al., 2015) |
| NFATC2 | rs6021191 (Intronic) |
Multiethnic cohort | 3.1 | GWAS | 589 cases and 2719 tolerant controls | (Fernandez et al., 2015) |
The adaptive immunity has been strongly implicated in asparaginase hypersensitivity and antibodies against asparaginase are detectable in >50% of patients (Liu et al., 2012). In line with these clinical observations, multiple studies point at associations of immune response-related genetic variations with adverse effects of therapy. The class II HLA allele DRB1*0701 was found to correlate anti-asparaginase antibodies (OR = 2.9) and with incidence of hypersensitivity (OR = 1.6) in GWAS study of a cohort of 1870 pediatric ALL patients from St. Jude Children's Research Hospital (Fernandez et al., 2014). The effect of HLA-DRB1*0701 on hypersensitivity risk was moreover replicated (OR = 2.9) and an additional link with HLA-DQB1*0202 identified (OR = 3) in a Hungarian candidate study encompassing 359 pediatric ALL patients (Kutszegi et al., 2017). Additionally, the largest study published to date in which a total of 3308 patients of diverse ancestry were enrolled, validated HLA-DRB1*0701 as a risk factor with an OR of 1.6 (Fernandez et al., 2015). Furthermore, the authors identified the intronic variant rs6021191 in NFATC2 to be associated with asparaginase hypersensitivity at genome-wide significance with an OR of 3.1 (Fernandez et al., 2015). NFATC2 encodes a transcriptional modulator that impacts on the transcriptional program in regulatory T-cells (Pan, Xiong, & Chen, 2013) and Nfatc2-deficient mice showed reduced cytokine levels in models of experimental chronic inflammation (Weigmann et al., 2008). Thus, the available data provide convincing evidence that genetic variations in both glutamate receptor signaling and immune response modulate asparaginase hypersensitivity risk. However, the predictive power of these associations is generally low, precluding their routine implementation as therapeutic biomarkers.
3.5. Liver injury due to interferon-β
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system, hallmarked by degradation of myelin sheaths and inflammation (Reich, Lucchinetti, & Calabresi, 2018). MS onset and progression is believed to be caused by autoimmune reactions and genetic studies suggest multifactorial etiology with most risk alleles residing in genes related to immune response, such as HLA type II (Haines et al., 1996). No cure for MS is available and therapy is currently restricted to an inhibition of disease progression. Interferon-β constitutes the most widely used agent in MS therapy. However, 30–60% of interferon-β treated patients show increased liver enzyme levels and 1.4% experienced de novo liver injury with aminotransferases elevations >20 the upper limit of normal (Francis et al., 2003; Tremlett, Yoshida, & Oger, 2004).
Importantly, a recent 2-stage GWAS of MS patients of European ancestry identified variant rs2205986, an expression quantitative trait locus (eQTL) for the interferon regulatory factor IRF6, as a genetic risk factor for interferon-β-induced DILI (Kowalec et al., 2018). The IRF gene family encodes transcription factors involved in the regulation of immune responses (Tamura, Yanai, Savitsky, & Taniguchi, 2008) and some evidence had been presented that implicates IRF6 in interferon-β response (Baranzini et al., 2015). The association was robustly detected irrespective of the adjustment for covariates with an OR of 8.3. Furthermore, the authors demonstrate that inclusion of this variant significantly improved the prediction of DILI compared to clinical factors alone. Strength and significance of the presented association suggest that rs2205986 might constitute a promising predictive biomarker for patient stratification in interferon-β therapy. However, further replication studies are needed before clinical implementation can be advocated.
3.6. Vincristine neurotoxicity
The natural vinca alkaloid vincristine is an antineoplastic agent used in multiple chemotherapy regimens for hematologic malignancies and solid tumors. Vinca alkaloids irreversibly bind to microtubule ends and prevent microtubule polymerization (Dumontet & Jordan, 2010). As a result formation of the mitotic spindle is inhibited, which blocks the activation of the anaphase promoting complex, thus causing arrest of dividing cells in metaphase. Furthermore, vinca alkaloids can cause apoptosis independent of cell cycle arrest by activation of NF-κB (Huang et al., 2004). The clinical utility of vincristine is limited by often-irreversible peripheral sensorimotor neuropathies that vary in incidence from 20% in adult patients with myeloma (Johnson et al., 2011) to 70–80% of pediatric ALL patients (Lavoie Smith et al., 2015; Tay et al., 2017).
Multiple cohort studies indicated that the neurotoxic effects of vincristine are dose-related (Desai, van den Berg, Bridges, & Shanks, 1982; Verstappen et al., 2005), prompting a focused search for pharmacogenomic biomarkers in genes related to vincristine pharmacokinetics (Table 8). Vincristine is metabolized by CYP3A isoenzymes and, notably, the intrinsic clearance of CYP3A5 is 10-fold higher than that for CYP3A4 (Dennison, Jones, Renbarger, & Hall, 2007). In individuals of European ancestry, around 90% of individuals are homozygous for the splicing defect CYP3A5*3 and do not express functional CYP3A5 compared to approximately 30% of non-expressers in African populations (Zhou, Ingelman-Sundberg, & Lauschke, 2017). Indeed, presence of the CYP3A5*3 allele was found to impact vincristine clearance and reduced CYP3A5 expression was associated with reduced vincristine-induced neuropathy risk in two multiethnic cohorts of 533 and 107 pediatric ALL patients (Aplenc et al., 2003; Egbelakin et al., 2011). In addition, variants in the vincristine transporter ABCB1 (MDR1) associated with incidence of neurotoxicity (Ceppi et al., 2014). However, other smaller studies did not replicate these associations (Guilhaumou et al., 2011; Hartman et al., 2010; Moore et al., 2011; Plasschaert et al., 2004).
Table 8.
Overview of genetic variations associated with vincristine-induced neuropathies.
| Biological process | Gene | Variant | Ethnicity | Odds ratio | Study type | Cohort | Study |
|---|---|---|---|---|---|---|---|
| Vincristine metabolism | CYP3A5 | rs776746 (CYP3A5*3; Splicing defect) |
Multiethnic cohort | 0.05 | Candidate gene study | 105 cases and 2 tolerant controls | (Egbelakin et al., 2011) |
| Multiethnic cohort | 0.13 | Candidate gene study | 27 cases and 506 tolerant controls | (Aplenc et al., 2003) | |||
| Vincristine transport | ABCB1 | rs4728709 (Intronic) |
Caucasian | 0.3 | Candidate gene study | 63 cases and 214 tolerant controls | (Ceppi et al., 2014) |
| Cytoskeleton | CEP72 | rs924607 (Intronic) |
Multiethnic cohort | 2.4 | GWAS | 64 cases and 158 tolerant controls | (Diouf et al., 2015) |
| ACTG1 | rs1135989 (A310A) |
Caucasian | 2.8 | Candidate gene study | 38 cases and 214 tolerant controls | (Ceppi et al., 2014) | |
| CAPG | rs2229668 (V41I) |
Caucasian | 2.1 | Candidate gene study | 39 cases and 214 tolerant controls | (Ceppi et al., 2014) | |
| rs3770102 (Intronic) |
Caucasian | 0.1 | Candidate gene study | 39 cases and 214 tolerant controls | (Ceppi et al., 2014) |
Besides pharmacokinetic associations, multiple studies implicated cytoskeletal proteins in vincristine neurotoxicity (Table 8). Ceppi et al. found variations in the actin network related genes ACTG1 and CAPG (Ceppi et al., 2014); however the mechanism behind these associations remained elusive. In addition, a recent study in 321 children implicated an eQTL variant (rs924607) in the promoter of the gene encoding the centrosomal protein CEP72 in vincristine-related toxicity (Diouf et al., 2015). The risk variant creates a binding site for the transcriptional repressor NKX6-3 and results in decreased CEP72 expression. Furthermore, knock-down of CEP72 increases vincristine toxicity in human stem cell-derived neurons and primary leukemia cells from homozygous rs924607 carriers showed increased vincristine sensitivity (Diouf et al., 2015), providing strong support for the hypothesis that reduced CEP72 levels sensitize patients to vincristine-related neuropathies.
3.7. CYP2C19 genotype and efficacy of antidepressant and antithrombotic treatment
One of the most polymorphic drug metabolizing enzymes is CYP2C19, which is principally involved in the metabolism of antithrombotic drugs, antidepressants and antipsychotics. The most prevalent CYP2C19 variant alleles are the loss-of-function variant CYP2C19*2 (minor allele frequencies [MAF] between 10 and 35% across populations) and the regulatory variant CYP2C19*17 (MAF 1.5 to 25%) that results in ultrarapid metabolism (UM) (Zhou et al., 2017). In addition, the population-specific stop-gain variant CYP2C19*3 is relevant in East Asians (MAF = 6%).
Much research has been devoted to understand the association between these genotypes and the effectiveness of antidepressant therapy. A recent study leveraged pharmacokinetic data and information pertaining to the switching of antidepressant medication within one year after commencing treatment of >2000 patients (Jukić, Haslemo, Molden, & Ingelman-Sundberg, 2018). Importantly, the authors found that CYP2C19 genotype strongly affected the pharmacokinetics of the commonly used antidepressant escitalopram and only 60% of patients classified as CYP2C19 UM reached recommended therapeutic exposure levels (25 nM). In total 29% of the patients with UM genotype switched antidepressant medicine, likely because of lack of efficacy due to being underdosed. In contrast, CYP2C19 poor metabolizers (PM) experienced serum drug concentrations higher than the recommended range, resulting in 31% of PM patients switching likely due to adverse events. In comparison, only 11–14% of patients with the genotypes encoding normal CYP2C19 enzyme activity (extensive metabolizers) switched medicines. Preemptive CYP2C19 genotyping would allow to adjust the initial doses to 5 mg in PMs and 20 mg in UMs (compared to 10 mg as current standard-of-care), thereby increasing escitalopram treatment efficacy. Given the important role of CYP2C19 in the metabolism of many antidepressants, we anticipate that CYP2C19 genotype-guided dosing might provide patient benefits for a considerable number of the 216 million patients diagnosed with major depressive disorder worldwide (GBD 2015).
A CYP2C19 genotype-dependent outcome was also seen in a recent survey monitoring suicides of Finnish citalopram users (Rahikainen et al., 2018) The study compared the genotypes of 349 citalopram-positive completed suicide cases and 855 general population controls and found that PMs and UMs were significantly enriched in the suicide cases. This finding is in accordance with another study where high CYP2C19 enzymatic capacity was associated with higher suicidality in depressed suicide attempters (Jukić et al., 2017). Based on these findings, we conclude that psychiatry presents a promising and clinically important arena for an increased implementation of preemptive genotyping.
The clinical endpoint of drug switching was also recently used to evaluate the influence of CYP2C19 polymorphisms on antiplatelet treatment in 603 acute coronary syndrome patients (Gross et al., 2018). The authors found that 38% and 67% of patients carrying one or two loss-of-function CYP2C19*2 alleles, respectively, switched from clopidogrel to prasugel due to insufficient platelet inhibition, whereas only 27% and 0% of patients switched medicine that carried one or two copies of the gain-of-function allele CYP2C19*17, respectively. Indeed, it appears evident that monitoring of drug switching might constitute an easily accessible, feasible and relevant endpoint to examine the influence of genetic polymorphisms on the success of drug therapy.
4. Pharmacogenomics and next-generation sequencing
4.1. Genetic determinants of drug disposition and response
Genetic factors are important modulators of the metabolism of medications and can influence their efficacy and toxicity. Overall, 20–30% of the inter-individual differences in drug-response are estimated to be due to genetic variations (Lauschke & Ingelman-Sundberg, 2016a; Sim, Kacevska, & Ingelman-Sundberg, 2013). Yet, seminal twin studies in the 1960s and 70s indicated that this fraction can be even substantially higher with pharmacokinetic heritability estimates ranging from 80%–99% for most evaluated medications, including antipyrine, dicoumarol, nortryptiline and halothane.
However, results from more recent investigations are more heterogeneous. While additive genetic factors explain around 90% of differences in the pharmacokinetics of metoprolol and torsemide (Matthaei et al., 2015), the clearance of metformin (Stage et al., 2015) and talinolol (Matthaei et al., 2016) is mostly governed by environmental factors. Importantly however, even for metoprolol and torsemide whose pharmacokinetics appear hereditary, common genetic polymorphisms in the genes involved in their metabolism and transport, explain only less than half of this heritability (Matthaei et al., 2015). These findings imply that a major fraction of heritable factors governing drug pharmacokinetics are currently missing and remain to be identified.
4.2. Pharmacogenes harbor a plethora of rare population-specific variants
In recent years considerable interest focused on the role of rare variants in the heritability of disease risk and complex traits. Rare genetic variants with minor allele frequencies (MAF) below 1% in the general population are commonly not interrogated in GWAS analyses. However, it has long been postulated that such rare variants with large effect sizes could contribute towards narrowing the gap between explained and expected heritability of complex traits (Manolio et al., 2009).
Only in the last decade or so was it possible to systematically characterize the inventory of rare genetic variants, primarily fueled by spectacular technological advances in Next Generation Sequencing (NGS) methods that allowed comprehensive sequencing of individuals on a population-scale (The 1000 Genomes Project Consortium, 2010; Drmanac et al., 2010). Importantly, these groundbreaking projects revealed a vast repertoire of rare genetic variants across the human genomes. Since these seminal findings, multiple studies focused their analyses on rare genetic variability specifically in genes involved in absorption, distribution, metabolism and excretion (ADME) of drugs (Table 9). In the largest study of its kind published to date encompassing 60,706 unrelated individuals from five global human populations >98% of all identified variants in drug transporters, drug metabolizing enzymes and nuclear receptors were found to be rare with MAF < 1% (Ingelman-Sundberg, Mkrtchian, Zhou, & Lauschke, 2018). Besides single nucleotide variations (SNVs) and small indels (insertions or deletions spanning <50 base pairs), most pharmacogenes harbor moreover copy number variations (CNVs), which account for >5% of all loss-of-function alleles in 87 out of 208 pharmacogenes analyzed (Santos et al., 2018). Similar findings were reported for important human drug targets, such as the G-protein coupled receptor (GPCR) family (Hauser et al., 2018).
Table 9.
Studies evaluating the prevalence of rare pharmacogenetic variants.
| Study | Cohort size | Populations | Number of loci | Sequencing method | Exonic SNVs | Intronic SNVs |
|---|---|---|---|---|---|---|
| (Nelson et al., 2012) | 14,002 | 3 global populations | 2002 | Targeted sequencing | 39,647 | 11,177 |
| (Mizzi et al., 2014) | 482 | 12 global populations | 231 | WGS | 26,807 in exons andproximal regulatorysequences | 382,157 in intronsand surrounding regions |
| (Gordon, et al., 2014) | 6503 | 2 global populations | 12 CYP genes | WES & WGS | 1006 | Not analyzed |
| (Fujikura et al., 2015) | 6503 | 2 global populations | Human CYP genefamily (57 genes) | WES & WGS | 4254 | 1911 |
| (Bush et al., 2016) | 5639 | 5 populations from the US | 82 | Targeted sequencing | 13,194 | 5231 |
| (Kozyra et al., 2017) | 6503 | 5 global populations | 146 | WES | 12,152 | 7176 |
| (Han et al., 2017) | 376 | Koreans | 122 | Targeted sequencing | 4573 | 1079 |
| (Ahn & Park, 2017) | 12,844 | 5 global populations | 48 | WES | Around 9550 | Not analyzed |
| (Zhou & Lauschke, 2018) | 5076 | Ashkenazi Jews | 17 | WES & WGS | 327 | Not analyzed |
| (Wright, Carleton, Hayden, & Ross, 2018) | 2504 | 26 global populations | 120 | WGS | 12,084 | |
| (Ingelman-Sundberg et al., 2018) | 60,706 | Global | 208 | WES | 69,923 | Not analyzed |
| (Zhang & Lauschke, 2018) | 138,632 | 7 global populations | Human SLCO genefamily (11 genes) | WES & WGS | 9811 | 3877 |
Furthermore, around 80% of pharmacogenetic variants were found to be population-specific (Fujikura, Ingelman-Sundberg, & Lauschke, 2015; Kozyra, Ingelman-Sundberg, & Lauschke, 2017; Zhang & Lauschke, 2018). Population-specific variations were of particular importance in populations with pronounced founder effects and repeated bottlenecks, such as Ashkenazi Jews, where their aggregated frequency exceeded 20% (Zhou & Lauschke, 2018). Combined, these studies demonstrate that the pharmacogenetic landscape is complex and that pharmacogenes harbor a plethora of rare variants that are not considered in conventional association studies with potential importance for inter-individual differences in drug disposition and response.
4.2.1. Functional interpretation of rare pharmacogenetic variants
The tremendous genetic complexity and abundance of rare genetic variants in pharmacogenomic loci directly raises the question about the functional and phenotypic relevance variability (Drögemöller, Wright, & Warnich, 2014; Lauschke and Ingelman-Sundberg, 2016b, Lauschke and Ingelman-Sundberg, 2016c, Lauschke and Ingelman-Sundberg, 2018). The functional impact of genetic variants is generally studied using heterologous in vitro expression systems coupled to a quantitative characterization of appropriate endpoints, such as clearance of different substrates per unit of time in the case of metabolic enzymes or activation of downstream signaling cascades for drug targets. Additionally, the functional impacts of variants on drug disposition or response can be analyzed in sufficiently powered cohort studies. Examples for the latter are effects of VKORC1 and CYP2C9 variants on warfarin dose requirements (Johnson & Cavallari, 2015) and impacts of CYP2C19 genotype on escitalopram serum concentrations and treatment efficacy (Jukić et al., 2018).
However, the low throughput and high costs of in vitro assays for the interrogation of variant functionality do not permit a systematic characterization of the tens of thousands of rare variants identified in population-scale sequencing projects. Furthermore, in vivo rare variant association studies are anticipated to fail to identify significant variant-phenotype relationships due to the, by definition, low frequency of allele carriers. Lastly, when applied in a clinical scenario, neither aforementioned in vivo nor in vitro methods would allow to inform about putative functional consequences of the genetic makeup of a given patient sufficiently fast to support pharmacogenetic testing.
Driven by this lack of experimental strategies for the functional assessment of rare pharmacogenetic variants compatible with the sheer scale of the problem, much research has focused on the optimization of computational prediction methodologies. For an overview of methods available for the computational interpretation of pharmacogenomic NGS data we refer the interested reader to a recent comprehensive review (Zhou, Fujikura, Mkrtchian & Lauschke, 2018). Most attention has been centered on the evaluation of variants that result in amino acid exchanges (missense variants). However, this scope widened in recent years to also include the assessment of variations in enhancers, promoters, splice sites and untranslated regions. To infer the functional consequences of missense variations, most currently used algorithms base their predictions on evolutionary conservation of the respective residues, as well as structural information of the corresponding gene product. The most frequently used algorithms for missense variant interpretation include SIFT (Ng & Henikoff, 2001), PolyPhen-2 (Adzhubei et al., 2010), MutationAssessor (Reva, Antipin, & Sander, 2011) and PROVEAN (Choi et al., 2012).
Genetic variation in non-coding regions that account for >99% of the human genome has been proposed to substantially contribute to inter-individual variability in gene expression by modulating the activity of promoter and enhancer elements (Gloss & Dinger, 2018; Zhang & Lupski, 2015). By integrating molecular evolution patterns with functional genomic data, such as genome-wide maps of chromatin accessibility (Boyle et al., 2008), genome segmentation (Ernst & Kellis, 2012; Hoffman et al., 2012), transcription factor binding (Johnson, Mortazavi, Myers, & Wold, 2007) and histone modifications (Zhang et al., 2010), computational methods are now in a position to predict the phenotypic relevance of non-coding variations with acceptable reliability. Notable methods for the functional interrogation of non-coding variation include GWAVA (Ritchie, Dunham, Zeggini, & Flicek, 2014), CADD (Kircher et al., 2014), Basset (Kelley, Snoek, & Rinn, 2016) and LINSIGHT (Huang, Gulko, & Siepel, 2017). As predictions based on the regulatory logic underlying gene expression are highly cell type and context specific they rely on biologically appropriate training sets. In addition, a plethora of focused tools have been presented that analyze the impact of genetic variants on a multitude of diverse features and parameters, including splicing (Harmanci, Sharma, & Mathews, 2011; Mort et al., 2014; Woolfe, Mullikin, & Elnitski, 2010), non-sense mediated decay (Hsu, Lin, & Chen, 2017), miRNA binding (Barenboim, Zoltick, Guo, & Weinberger, 2010; Deveci, Catalyürek, & Toland, 2014; Ryan, Werner, Howard, & Chow, 2016) and translational efficiency (Zhang et al., 2014).
Importantly, functional interpretation of pharmacogenomic variant data is attended by specific challenges. Firstly, the use of conservation as a metric to predict variant functionality might be problematic due to overall low evolutionary constraints in pharmacogenes (Jin et al., 2018). Secondly, most algorithms are not designed to detect functionality but rather pathogenicity and fitness consequences associated with a given variant. Whereas altered functionality and pathogenicity overlap for regions of the genome that are directly associated with human disease, this association is less clear for pharmacogenes in which deleterious variants are generally not pathogenic. Lastly, training of machine learning methods with inaccurately annotated data sets translates into reduced predictive performance. One example of such a problem is the non-curated use of genetic variants that are common in the general population as functionally neutral training data. While these common variants are likely non-pathogenic, they can have pronounced functional consequences, particularly in pharmacogenes, as exemplified by the common functionally important pharmacogenetic polymorphisms rs1057910 (CYP2C9*2), rs4244285 (CYP2C19*2), rs3892097 (CYP2D6*4), rs34983651 (UGT1A1*28) and rs4149056 (SLCO1B1*5). Similarly, utilization of all phenotype associated GWAS polymorphisms as functional training data results is problematic as only 5% of GWAS index SNPs are estimated to be mechanistically responsible for the observed phenotypic consequences, i.e. have direct functional consequences (Farh et al., 2015).
To overcome these problems, we have recently developed and cross-validated an algorithm trained specifically on pharmacogenetic variants with comprehensive and high-quality functional annotations (Zhou, Mkrtchian, Kumondai, Hiratsuka, & Lauschke, 2018). This method outcompeted preexisting methods achieving 93% for both sensitivity and specificity. Importantly, the score provided by this prediction framework not only dichotomously classifies variants into functionally deleterious and neutral variants but rather provides estimates about the quantitative effects of the variant on the function of the gene product in question. We envision that such models can be useful for the prediction of phenotypic consequences pertaining to drug disposition and response in a personalized medicine framework.
4.2.2. Putative impact of rare pharmacogenetic variants on drug metabolism and response
The methodological toolbox presented above provides a sound basis to estimate the consequences of pharmacogenetic variation on drug disposition and response. Rare genetic variations in pharmacokinetic are enriched in variants with putative functional consequences and we (Ingelman-Sundberg et al., 2018; Kozyra et al., 2017) and others (Ramsey et al., 2012) have estimated that rare variants contribute around 10–40% to the entire genetically encoded functional variability in those loci. Importantly, the relevance of rare genetic variations was found to be highly gene-specific (Table 10).
Table 10.
Importance of rare genetic variants in important pharmacokinetic genes. The frequency of functional genetic variants were calculated based on data from 130,000 individuals in the GnomAD database using a computational prediction framework specific for ADME genes (Yitian Zhou, et al., 2018).
| Class | Gene name (Gene product) | Important substrates | Estimated number of individuals that need to be screened to find one rare deleterious variant | Fraction of functional variability allotted to rare variants |
|---|---|---|---|---|
| Transporter | ABCB1 (MDR1, P-gp) | Anthracyclines, vinca alkaloids, methotrexate, etoposide, clozapine, tricyclic antidepressants, selective serotonin reuptake inhibitors, aliskiren, irinotecan, proton pump inhibitors, verapamil, zidovudine, olanzapine | 36 individuals | 28% |
| ABCC1 (MRP1) | Anthracyclines, vinca alkaloids, epipodophyllotoxins | 20 individuals | 39% | |
| ABCC3 (MRP3) | Etoposide, methotrexate | 24 individuals | 37% | |
| ABCG2 (BCRP) | Irinotecan, rosuvastatin, nitrofurantoin, leflunomide, cimetidine, glyburide, sulfasalazine | 50 individuals | 100% | |
| SLC22A1 (OCT1) | Metformin, oxaliplatin, furaminidine, acyclovir, lamivudine | 24 individuals | 3% | |
| SLCO1B1 (OATP1B1) | Statins, meglitinides, rifampicin, angiotensin II receptor antagonists | 36 individuals | 9% | |
| Phase I | CYP1A2 | Olanzapine, theophylline, clozapine, tizanidine, caffeine, flutamide, tacrine | 49 individuals | 2% |
| CYP2C9 | Warfarin, acenocoumarol, phenytoin, sulfonylureas, torasemide, fluoxetine, terbinagine, sildenafil, celecoxib, piroxicam, lesinurad, dronabinol, tolbutamide | 23 individuals | 19% | |
| CYP2C19 | Clopidogrel, tricyclic antidepressants, selective serotonin reuptake inhibitors, proton pump inhibitors, voriconazole, moclobemide. | 18 individuals | 12% | |
| CYP2D6 | Tricyclic antidepressants, selective serotonin reuptake inhibitors, codeine, tramadol, clozapine, risperidone, aripiprazole, venlafaxine, flupentixol, haloperidol, 5-HT3 receptor antagonists, tamoxifen, carvedilol, metoprolol, | 12 individuals | 7% | |
| CYP3A4 | Aripiprazole, gefitinib, erlotinib, sirolimus, cabazitaxel, dronedarone, ivabradine, ranolazine, tlithromycin, posaconazole, simvastatin, enzalutamide, protease inhibitors, ivacaftor, maraviroc, fesoterodine, phosphodiesterase type V inhibitors | 44 individuals | 27% | |
| DPYD | Fluoropyrimidines | 18 individuals | 22% | |
| Phase II | UGT1A1 | Irinotecan, lamotrigine, etoposide, belinostat, carvedilol | 22 individuals | 5% |
| TPMT | Thiopurines | 119 individuals | 6% |
Using this information as a template we estimated the impact of rare genetic variability on pharmacokinetics and response of specific drugs with well-characterized pharmacology (Ingelman-Sundberg et al., 2018). Depending on the genes involved in pharmacokinetics and –dynamics of the respective compounds and their metabolites, the overall functional relevance of rare genetic variants differed substantially across evaluated drugs. Rare genetic variants are expected to only explain a minor part in explaining the inter-individual differences in olanzapine serum levels or simvastatin-induced myopathies. By contrast, rare genetic variations are expected to account for 18.4% of the genetically encoded functional variability in CYP2C9, which is of central importance for warfarin response. Furthermore, >40% of the variability in irinotecan transport was found to be allotted to rare variants (Ingelman-Sundberg et al., 2018). Thus, these analyses can be used to flag medications for which comprehensive NGS-based genotyping instead of candidate SNP interrogations can likely reveal significant additional information for the personalization of pharmacological therapy.
In an elegant study by Hauser et al., the authors characterized the genetic variability in the human GPCR gene family and, by utilizing available crystallographic data and literature information, found >2000 variants in known functional sites (Hauser et al., 2018). Moreover, they experimentally evaluated the effects of selected variants in OPRM1, encoding the μ-opioid receptor, on the response to different ligands. One variant resulted in generally reduced responses to different agonists, whereas other variants had ligand specific effects, exhibiting normal response to endomorphin and morphine but increased response to buprenorphine, a medication used in the treatment of opioid addiction. Most surprisingly, some variants conferred resistance to the opioid receptor antagonist naloxone, resulting in potentially life-threatening lack of efficacy in variant carriers when treated for opioid overdose. Combined, the presented studies indicate that rare genetic variants can have substantial clinically relevant impact on drug disposition and treatment efficacy and underscore the importance of comprehensive pharmacogenetic characterization for personalized medicine.
Notably, genetic variants that are rare globally might be common in specific geographical regions. One example is the functionally defective CYP3A4*20 allele which is found exclusively in parts of Spain, in which the frequency can be as high as 4% (Apellaniz-Ruiz et al., 2015). CYP3A4*20 affects paclitaxel metabolism and thus consideration of this polymorphism is clinically relevant in these specific regions (Apellaniz-Ruiz et al., 2015).
4.2.3. Missing pharmacogenomic heritability
Missing heritability refers to the difference between the estimated heritability of a complex phenotype and the contributions of common genetic variants associated with the trait of interest using a simplistic additive model. One simple explanation could be an overestimation of the phenotype's heritability in twin studies due to a violation of the equal-environment assumption, i.e. monozygotic twins tend to shape an environment for themselves that is more similar than that for dizygotic twins. However, elegant twin studies of antipyrine and theophylline pharmacokinetics showed no differences between twins living in the same household and twins living in different households, regardless of zygosity (Miller, Slusher, & Vesell, 1985; Penno, Dvorchik, & Vesell, 1981).
As discussed above, available data suggest that rare genetic variations indeed explain a considerable fraction of the missing heritability in drug response phenotypes; yet, multiple other effects have been proposed that likely contribute as well. Particularly epistatic phenomena, i.e. the interaction between genetic variations, play important roles for pharmacogenomics. Specifically, we refer here to its classical physiological notion in which a combination of genetic variants gives rise to phenotypic consequences that are different from the additive of the individual variant effects (Cheverud & Routman, 1995). For a comprehensive review of the different notions of epistasis, we refer to a recent comprehensive review by Sackton and Hartl (Sackton & Hartl, 2016). To identify and quantify epistatic interactions in pharmacogenomic data a multitude of machine learning tools are available, including regression trees, random forests, deep neural networks and combinatorial partitioning (Motsinger, Ritchie, & Reif, 2007).
Importantly, epistatic mechanisms are already harnessed in clinical therapy, particularly in the area of oncology. Cancers accumulate genetic variants that drive cancer growth and these mutations can result in unexpected sensitivities to pharmacological interventions. For instance, breast cancers with mutations in BRCA1 or BRCA2 become reliant on the cellular PARP excision repair system and treatment of BRCA-mutation positive breast cancer patients with the PARP inhibitor olaparib resulted in high toxicity specifically in tumors (Fong et al., 2009). We expect that consideration of the genomic context can substantially improve drug response predictions, particularly for medications with complex pharmacology. Thus, systematic epistatic analyses represent an important frontier in contemporary pharmacogenomics.
In addition, missing heritability in complex phenotypes has been postulated to be due to insufficient power of the conducted studies to identify variants with limited effects. When the effects of all genetic variants, including those that do not reach statistical significance, are aggregated, the authors report that for Crohn's disease, bipolar disorder and type I diabetes common genetic variants explained substantially more (25–50%) of the estimated heritability compared to more conservative models (Lee, Wray, Goddard, & Visscher, 2011). Furthermore, based on findings from genetic model organisms, including plants (Undurraga et al., 2012) and flies (Sawyer et al., 1997), as well as human diseases, such as Huntington disease (OMIM identifier 143100), dentatorubro-pallidoluysian atrophy (OMIM 125370), spinal and bulbar muscular atrophy (OMIM 313200) and spinocerebellar ataxias, tandem repeat variations have been suggested as major modulators of gene activity and additional sources of missing heritability of complex human phenotypes (Press, Carlson, & Queitsch, 2014; Quilez et al., 2016). However, further studies are needed to quantify the importance of these postulated factors.
4.3. Opportunities of national biobanks
National biobanks in which clinical, phenotypic and lifestyle data are integrated with longitudinal health registries and extensive omics profiles (primarily genomics but also metabolomic, transcriptomic and epigenomic data sets) provide powerful resources for biomarker discovery and personalized medicine. By now multiple countries have established such biobanks, including Estonia (Leitsalu et al., 2015), Iceland (Gulcher & Stefansson, 1999), Japan (Nagai et al., 2017) and the UK (Bycroft et al., 2018). While these platforms have demonstrated their utility for epidemiological research, implementation of available personalized omics information into primary care still faces multiple important challenges. The most significant obstacles include the sensitivity, privacy and highly distinguishable nature of the data, as well as issues pertaining to insufficient acceptance or lack of knowledge on the part of clinicians (Dankar, Ptitsyn, & Dankar, 2018; Hess, Fonseca, Scott, & Fagerness, 2015; Lauschke & Ingelman-Sundberg, 2016c).
Estonia is among the countries that are spearheading the implementation efforts of NGS-guided therapy. Genome-scale genotype data are available for >44,000 individuals, corresponding to 3.5% of the entire population (Reisberg et al., 2018). Importantly, >99% of these participants were found to harbor at least one pharmacogenetically actionable allele and implementation of genotype-guided prescribing is expected to affect drug choice or dosing for 55 daily drug doses per 1000 individuals in the general population. Furthermore, integrating genomic data with longitudinal health records of the respective individuals provides a powerful tool for the discovery of novel pharmacogenomic associations. In a first proof-of-concept study, Tasa et al. identified associations between CTNNA3 variations and myopathies in biobank participants taking oxicams and were able to replicate this finding in an independent validation cohort (meta-analysis p = 2.4*10−7) (Tasa et al., 2018).
Multiple other countries have launched initiatives intended to facilitate the implementation of NGS-based genotyping into the health care system to utilize genomic information for personalized therapy and diagnostics. Qatar aspires to expand its biobank program (Al Kuwari et al., 2015) that already contains longitudinal clinical data with sequencing data from about one fifth of all Qatari citizens within the next years. Furthermore, Korea promotes a precision medicine initiative focusing mainly on pharmacogenomics (Cho et al., 2010). These efforts include reimbursement of pharmacogenetic tests and the development of clinical decision support (CDS) systems to facilitate the implementation of genotype information into clinical care.
The US has presented strategies for the nationwide implementation of personalized medicine, termed Precision Medicine Initiative. The framework encompasses the All of US program in which >1 million volunteers will be sequenced and followed-up with periodic clinical evaluations. A current status report of the clinical implementation of pharmacogenomic testing in the US has been published recently (Volpi et al., 2018). In addition a large number of national biobanks centered on cancer samples have been established and we refer the interested reader to recent overviews for further information (Krieger & Jahn, 2018; Vaught, Kelly, & Hewitt, 2009).
5. Pharmacoepigenomics
The term “epigenetics” can be interpreted as a cellular or molecular phenomenon (Deans & Maggert, 2015). The former follows the classical Waddingtonian concept of cell state or fate determination, primarily in the context of embryonic development and stem cell biology, whereas the latter can describe any mechanism of gene regulation that can be passed on through cell divisions or, in its widest definition commonly applied particularly in the field of pharmacoepigenetics, alludes to any additional layer transcriptional regulation apart from transcription factors, thus also including non-coding RNA species. In the context of this review, we follow the restricted molecular definition of epigenetics, in which we consider a phenomenon as “epigenetic” if it pertains to chromosome-bound changes of gene expression that can be transmitted through mitosis and that are not caused by alterations in the primary DNA sequence, thus explicitly excluding regulatory RNAs.
5.1. Epigenetic regulation of gene expression
Epigenetic regulation plays an essential role in the modulation of gene expression across eukaryotes. Epigenetic signals can be encoded as modifications of the DNA itself or of associated histones. At the level of DNA, the predominant epigenetic mark is methylation of cytosine–guanine dinucleotides (5mC) affecting 3–5% of all cytosines. In recent years however multiple additional CpG modifications were discovered, including hydroxymethylcytosine (5hmC), formylcytosine (5fC) and carboxylcytosine (5caC) (Ito et al., 2011; Tahiliani et al., 2009). Of these “additional bases” 5hmC is most common, accounting for up to 1% of all cytosines in hmC-rich tissues, such as liver and central nervous system, compared to <0.02% for 5fC and 5caC (Bachman et al., 2015; Globisch et al., 2010; Ivanov et al., 2013).
While these DNA modifications form during the oxidative removal of 5mC marks, they seem to be more than mere demethylation intermediates (Wu & Zhang, 2017); they appear to be temporally stable for multiple weeks in certain contexts and elicit distinct biological responses by changing DNA conformation and selectively binding to specific reader proteins (Iurlaro et al., 2013; Kitsera et al., 2017; Pfaffeneder et al., 2014; Raiber et al., 2015; Spruijt et al., 2013). Generally, 5mC, 5fC and 5caC inhibit transcription factor binding and promote condensation to heterochromatin, whereas 5hmC is commonly associated with actively transcribed genes. Thus, given their antagonistic functional roles and significant abundance in human liver, 5mC and 5hmC have received particular attention in pharmacoepigenetic research.
Compared to DNA marks, epigenetic modifications at the level of histones are more diverse and >35 chemically distinct modifications have been described to date, including acetylation, methylation, phosphorylation, ubiquitinylation, sumoylation, ADP-ribosylation, propionylation, butyrylation and deamination (Lawrence, Daujat, & Schneider, 2016; Zhang, Cooper, & Brockdorff, 2015). Depending on nature and position within the histone tail, histone modifications can associate with transcriptional activation or transcriptional silencing. Arguably the most extensively studied modifications are trimethylation of lysines 4 and 27 in histone 3 (H3K4me3 and H3K27me3, respectively) and acetylation marks in the tails of histones 3 and 4. Actively transcribed genes are generally marked by H3K4me3 and H3 and H4 acetylation in their promoters and gene bodies (Barrera et al., 2007; Guillemette et al., 2011; Liang et al., 2004). H3K4me3 promotes the recruitment of histone acetyltransferases, which entails coordination of different activating histone marks, jointly supporting the formation of a transcriptionally permissive chromatin state (Bian et al., 2011; Hung et al., 2009). In turn, acetylated lysines promote transcription due to specific recognition by proteins containing bromodomains, which are part of many transcriptional regulators (Fujisawa & Filippakopoulos, 2017). Importantly, recruitment of the DNA methyltransferase DNMT3 that catalyzed 5mC formation is blocked by H3K4me3, thus interlocking epigenetic signatures at the level of DNA and histones and resulting in mutual exclusivity of repressive 5mC and activating H3K4me3 marks (Balasubramanian et al., 2012; Ooi et al., 2007; Otani et al., 2009).
In contrast, repressive histone gene signatures feature H3K27me3 and H2AK119ub (ubiquitinylation of lysine 119 in histone H2A). Methylation of H3K27 is catalyzed by the Polycomb repressive complex 2 (PRC2), whereas the PRC1 catalyzes H2AK119ub (Czermin et al., 2002; Endoh et al., 2012). PRC1 components of the CBX gene family recognize PRC2-catalyzed H3K27me3, resulting in largely overlapping maps of H3K27me3 and H2AK119ub modifications (Bernstein et al., 2006; Cao et al., 2002; Kuzmichev et al., 2002). Furthermore, binding of the PRC2 to H3K27me3 provides a positive feedback that reinforces transcriptionally repressive domains (Margueron et al., 2009). For a more detailed overview of the mechanistic underpinnings of the various levels of epigenetic regulation, we refer the interested reader to recent reviews (Allis & Jenuwein, 2016; Chen, Li, Subramaniam, Shyy, & Chien, 2017).
Importantly, many epigenetic alterations appear to be the consequence rather than the cause of the observed phenotype they correlate with. By analyzing epigenetic signatures in whole blood of 3296 individuals phenotyped for fasting blood lipid levels using a Mendelian randomization framework, Dekkers and colleagues found strong evidence that triglycerides and cholesterol cause alterations in DNA methylation patterns and not vice versa (Dekkers et al., 2016). Similar observations were obtained for associations between DNA methylation patterns and adiposity (Li et al., 2018; Mendelson et al., 2017; Richmond et al., 2016; Wahl et al., 2017) or lung cancer risk (Battram et al., 2018). Thus, while more data about the functional role of epigenetic patterns are warranted, these data incentivize analyses of forward and reverse causality in epidemiological studies of epigenetic phenomena.
5.2. Analytical methods
A plethora of protocols has been presented to decode epigenomic profiles. These methods differ by their input requirements, feature resolution, scalability and costs (Clark, Lee, Smallwood, Kelsey, & Reik, 2016; Kurdyukov & Bullock, 2016; Yong, Hsu, & Chen, 2016). For analyses of DNA modifications, approaches are based on bisulfite conversion, enzymatic digestion or affinity enrichment. Bisulfite conversion exploits differences in chemical reactivity of modified and unmodified cytosine variants and constitutes the most widely used method. Bisulfite deaminates unmodified cytosine, as well as 5fC and 5caC to uracil, whereas 5mC and 5hmC are protected. Thus, these conventional bisulfite-based methods cannot distinguish between “repressive” 5mC and “activating” 5hmC marks, which can confound biological conclusions and limits the usefulness of these protocols, particularly for epigenetic studies of liver biology or hepatic metabolism in which 5hmC levels are high.
To overcome these limitations multiple techniques have been presented that change bisulfite sensitivity between 5mC and 5hmC. Oxidative bisulfite sequencing (oxBS-seq) employs chemical oxidation of 5hmC to 5fC before bisulfite conversion (Booth et al., 2012). As a result, 5mC remains protected from bisulfite conversion (thus appearing as cytosine during sequencing), whereas 5hmC is deaminated to uracil during the bisulfite conversion step. In a second method, termed TET-assisted bisulfite sequencing (TAB-Seq), 5hmC is first glycosylated enzymatically by β-glucosyltransferase to 5gmC (Yu et al., 2012). Subsequently, 5mC is oxidized to 5caC by recombinant TET enzymes and deaminated in the bisulfite conversion step. In contrast, 5gmC is protected from TET-mediated oxidation and subsequent bisulfite-mediated deamination. Thus, comparison of results from conventional bisulfite sequencing (5mC and 5hmC are not deaminated and appear as cytosine after bisulfite treatment) with TAB-Seq (5mC appear as uracil, whereas 5hmC appears as cytosine, as it is protected from bisulfite treatment), allows to identify 5hmC positions with base-pair resolution. In a conceptually similar approach, Schutsky and colleagues presented a bilsufite-free enzymatic approach that utilizes cytidine deaminases of the APOBEC gene family, termed APOBEC-coupled epigenetic sequencing (ACE-seq) (Schutsky et al., 2018). In a first step 5hmC is glucosylated to 5gmC as in TAB-Seq. Subsequently, 5mC is deaminated enzymatically by AID/APOBEC enzymes, whereas 5gmC is protected. Notably, as deamination is achieved by enzymes rather than harsh chemical conditions, ACE-Seq requires >1000-fold less input material, thus allowing epigenetic analyses of samples with limited availability.
In addition, single molecule sequencing methods can be used to decode cytosine modifications independent of bisulfite conversion. In SMRT-Seq a single DNA molecule of interest is sequenced by measuring the polymerase-mediated integration of fluorescently labeled nucleotides into the complementary strand (Eid et al., 2009). Importantly, differences in polymerase kinetics as evident from sequencing fluorescence traces allow to directly distinguish cytosine, 5mC and 5hmC (Flusberg et al., 2010). Furthermore, all five different cytosine species (C, 5mC, 5hmC, 5fC and 5caC) can be identified by changes in ionic current signal using nanopore sequencing (Laszlo et al., 2013; Rand et al., 2017; Wescoe, Schreiber, & Akeson, 2014).
In contrast to the analyses of covalent DNA modifications discussed above, methods for studies of posttranslational modifications (PTMs) on histones are primarily antibody based, such as such as Western blot and chromatin immunoprecipitation (ChIP). However, generation of antibodies that specifically recognize a histone modification of interest with suitable sensitivity is difficult and time-consuming (Kidder, Hu, & Zhao, 2011; Wardle & Tan, 2015). Furthermore, these approaches are poorly scalable and can only analyze one or few modifications per experiment. Besides antibody-based detections of histone PTMs, proteomic approaches constitute a quantitative and high-throughput compatible approach to obtain overall histone modification profiles on global chromatin (El Kennani, Crespo, Govin, & Pflieger, 2018; Huang, Lin, Garcia, & Zhao, 2015; Soldi, Bremang, & Bonaldi, 2014).
5.3. Effect of epigenetic gene regulation on pharmacokinetics and drug response
In the past decade a multitude of studies have revealed correlation between epigenetic modifications and expression levels or activity of genes involved in drug ADME and pharmacodynamics and we refer to comprehensive recent reviews for further details (Fisel, Schaeffeler, & Schwab, 2016; Tang & Chen, 2015). Prominent examples for such associations are the correlations between DNA methylation in the promoters of CYP3A4, CYP1A2, CYP2C19 and UGT1A1 with the respective expression levels (Gagnon et al., 2006; Habano et al., 2015; Kacevska et al., 2012; Miyajima, Furihata, & Chiba, 2009). In hepatic cell lines (HepG2) transcriptional activation of CYP3A4 has been shown to require the histone methyltransferase PRMT1, as knock-down of PRMT1 resulted in 20-fold reduced activation of CYP3A4 by rifampicin (Xie et al., 2009). Furthermore, PXR-mediated induction of CYP3A4 by rifampicin results in changes of the histone profile with increased levels of H3K4me3 and H3ac, as well as decreased levels of the repressive histone mark H3K27me3 (Yan et al., 2017). Notably, these changes are the consequence of transcriptional activation as knock-down of PXR prevents both transcriptional activation and epigenetic alterations. In addition, histone modification patterns have been found to correlate with expression levels of multiple drug transporters, such as ABCB1 (MDR1) and ABCG2 (BCRP) (Henrique et al., 2013; To et al., 2008); in light of above-mentioned data, a similar cause-consequence relationship is likely.
The promoters of various CYP genes, including CYP1A1, CYP1B1, CYP2D6 and CYP2E1, are hypermethylated in hepatocyte-like cells derived from embryonic stem cells, correlating with drastically reduced expression (multiple orders of magnitude) of these genes compared to primary human hepatocyte cultures (Park et al., 2015). Notably, pharmacological inhibition of DNA methyltransferases (DNMTs) and histone deacetylases (HDACs) resulted in 10-fold increased expression of CYP1A1 and CYP1B1, whereas changes of CYP2D6 and CYP2E1 were negligible (Park et al., 2015). Combined, these findings provide evidence that epigenetic remodeling and ADME gene expression levels can be directly linked.
Epigenetic changes in ADME genes particularly correlate with expression patterns during embryonic development. While CYP3A7 constitutes the predominant CYP3A isoform in embryonic liver, expression switches to CYP3A4 in postnatal stages and this switching is paralleled by changes in methylation levels of transcription factor binding sites within the CYP3A promoters in mice and humans (Kacevska et al., 2012; Li et al., 2009). A further example is the regulation of CYP2W1 expression. Whereas the gene is expressed in fetal gut, neonatal methylation inhibits further expression in healthy adult tissues (Guo, Johansson, Mkrtchian, & Ingelman-Sundberg, 2016). However, in transformed colon cancer cells and metastases, a critical CpG island in the exon 1 - intron 1 junction is hypomethylated and expression of CYP2W1 is reactivated (Choong et al., 2015). Since the enzyme can bioactivate anticancer prodrugs its specific expression in cancer cells makes it as an interesting target for future anticancer drug development (Travica et al., 2013).
Notably, global hmC content varies by a factor of four between human livers and we could show that hydroxymethylation in coding regions positively correlates with the expression levels of the corresponding human ADME genes (Ivanov et al., 2016). These data suggest that hmC variability contributes to the epigenetic control of hepatic gene expression, possibly by causing chromatin alterations that facilitate gene transcription.
Importantly, epigenomic profiles are highly tissue-specific with each cell type having its unique signature and correlations between the epigenomes of different tissues, particularly blood, are generally poor (Bonder et al., 2014; Hannon, Lunnon, Schalkwyk, & Mill, 2015; Lowe, Slodkowicz, Goldman, & Rakyan, 2015; Lunnon et al., 2016). Yet, most epigenetic association studies are performed using peripheral blood as surrogate tissue. Thus, we emphasize our previously raised concerns (Lauschke, Ivanov, & Ingelman-Sundberg, 2017) that conclusions about epigenetic regulation critically require the analysis of carefully isolated biopsy material from the given tissue of interest to account for this tissue-specificity.
Mutations in epigenetic modifiers, such as DNMT, HDAC, and TET enzymes are found in up to 50% of all cancers, resulting in profound changes of their epigenetic landscape (Ceccacci & Minucci, 2016; Scourzic, Mouly, & Bernard, 2015; Zhang & Xu, 2017). Thus, exploiting these epigenetic changes provides an appealing therapeutic avenue and six small molecule inhibitors of epigenetic modifiers (azacitidine, decitabine, belinostat, panobinostat, romidepsin, and vorinostat), termed epidrugs, have already received regulatory approval for the treatment of various cancers and hundreds of additional clinical trials are currently ongoing. Furthermore, epidrugs are in various stages of development for the treatment of autoimmune disorders, neurodegenerative diseases and type 2 diabetes. For a status update of the clinical implementation of epigenetic therapy and the utilization of epigenetic biomarkers we refer to our recent comprehensive review (Lauschke, Barragan, & Ingelman-Sundberg, 2018).
6. Clinical implementation efforts
Our understanding of the complexity of pharmcogenomic loci has made tremendous advances. As discussed above, only during the last years has it become evident that genes involved in pharmacokinetics and -dynamics harbor a plethora of rare genetic variants and copy number variations that can have important consequences for human drug response. However, today's array-based pharmacogenomic analyses only interrogate common genetic variants. We advocate for a methodological paradigm shift that allows to embrace the entire pharmacogenomic complexity, including rare variants and copy number variations, by comprehensively interrogating loci of interest using NGS-based technologies (Fig. 3). Only then will it be possible to take into account the entire repertoire of genetic variability of a given patient to inform and enhance treatment decisions.
Fig. 3.
Individualization of treatment based on comprehensive NGS-based genotyping data. In conventional care for most indications, treatment is based on clinical parameters without consideration of the patient's genotype (left track in the figure). While these regimens are efficacious and safe in most individuals, some patients do not respond to the prescribed medication or might experience adverse reactions. The utilization of Next Generation Sequencing (NGS) aims to leverage genomic data to predict those outlier patients and pre-emptively provide advice regarding alternative treatments or to flag patients for follow-up monitoring (right track in the figure). To achieve this goal, variations in genes encoding proteins involved in drug absorption, distribution, metabolism and excretion (ADME) and drug targets, as well as their regulatory regions are identified in the NGS data of the given patient. The effects of these variants are interpreted based on available characterization data collected in dedicated databases or the scientific literature. For novel variants, functional effects will be predicted using quantitative computational algorithms specifically developed for pharmacogenomic predictions. Effects of target variations on drug binding are predicted using available structural information. Subsequently, effects of all identified variants are collated and translated into activity scores for all pharmacogenes. Integration of gene activity scores with information about the pharmacology of medications available for the given therapeutic indication, allows to predict their efficacies and risks to cause adverse reactions. These results can provide guidance to the responsible physician regarding choice of drug and its dose, as well as incentivize the scheduling of more frequent follow-ups in at-risk patients, resulting in increased treatment efficacy and safety also for outlier patients. Figure modified with permission from the publisher and authors (Lauschke & Ingelman-Sundberg, 2018).
At present, we recommend to focus pharmacogenetic analyses on loci with importance for drug ADME, adverse reactions or response. Restricting analyses to those genes as well as their surrounding regions with potential regulatory importance (e.g. 50 kb) allows to drastically reduce sequencing costs and analytical complexity with minimal loss of information. Targeted sequencing libraries supporting such focused analyses have already been developed and, when applied to 5000 individuals, revealed >40,000 variants across 82 pharmacogenes (Bush et al., 2016; Gordon et al., 2016). However, little is known about the impact of variants on interindividual variability in drug response that reside in genomic areas beyond well-characterized pharmacogenes and thus library designs might have to be expanded in the future.
Advances in machine learning and artificial intelligence have resulted in a rapid improvement of the methodological toolbox for the functional interpretation of genetic variations identified by NGS-based sequencing. Novel algorithms can predict loss-of-function and functionally neutral missense mutations with reasonable accuracy of >90%. Moreover, tools have been presented that can not only dichotomously distinguish between deleterious and functionally neutral variations but rather provide with quantitative estimates about the extent of functional impact for a given variant. This opens up possibilities to rapidly translate NGS-based sequences into gene activity scores, which can be used in conjunction with existing pharmacogenetic guidelines to advise treatment decisions.
7. Conclusions and future outlook
The translation of pharmacogenomic findings into patient or societal benefits in the past has been relatively slow. However, in light of the rapid methodological developments in the areas of genomics, statistical genetics and machine learning, as well as the multitude of ongoing efforts to quantify the added value of preemptive pharmacogenomic tests, we anticipate that their clinical implementation will accelerate in the near future. With decreasing costs of genetic testing, concerns will shift away from monetary considerations and the main hurdle in the future process will be the accuracy of phenotypic interpretation of a given genotype, particularly pertaining to the rare mutations which are often specific for a given individual. Importantly, computational methods are not (and likely will never be) able to predict alterations of gene function with complete accuracy. However, we believe that they are useful to flag patients with genotypes that indicate an increased likelihood of an outlier response. This information can be of value for the physician who in such cases can adjust follow-up schedules and initiate therapeutic drug monitoring and dose titrations.
Our knowledge of pharmacogenomic variation is rapidly increasing; however, there is still much to learn. Important frontiers include the understanding and functional interpretation of regulatory parts of the genome. Furthermore, non-coding RNAs might be important, currently underappreciated modulators of drug response, as suggested for the response to lithium treatment in bipolar disorder (Hou et al., 2016). Most importantly, there is an urgent need for prospective, randomized clinical trials that evaluate patient benefits and cost effectiveness of preemptive NGS-based genotyping coupled to state-of-the-art computational prediction tools across diseases, medicines and health care systems, as a more wide-spread clinical implementation of personalized medicine can only be achieved by providing a solid base of evidence. Overall, we envision that ongoing development, optimization and validation efforts in the area of pharmacogenomics will pave the way for an increased personalization of drug therapy, resulting in more cost-efficient health care, increased drug development efficiency and improved public health.
Financial & competing interests disclosure
V.M.L. and M.I.-S. are founders and owners of HepaPredict AB. The work in the authors' laboratories is supported by the Swedish Research Council [grant agreement numbers: 2015-02760, 2016-01153 and 2016-01154], by the European Union's Horizon 2020 research and innovation program U-PGx [grant agreement No. 668353], by the Strategic Research Programme in Diabetes at Karolinska Institutet, by the Lennart Philipson Foundation, by the Harald and Greta Jeansson Foundation and by the ERC-AdG project HEPASPHER (grant agreement number 742020).
No writing assistance was utilized in the production of this manuscript.
References
- Aberg J.A., Kaplan J.E., Libman H., Emmanuel P., Anderson J.R., Stone V.E.… . Vol. 49. 2009. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America; pp. 651–681. [DOI] [PubMed] [Google Scholar]
- Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P.…Sunyaev S.R. A method and server for predicting damaging missense mutations. Nature Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn E., Park T. Analysis of population-specific pharmacogenomic variants using next-generation sequencing data. Scientific Reports. 2017;7:8416. doi: 10.1038/s41598-017-08468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kuwari H., Al Thani A., Al Marri A., Al Kaabi A., Abderrahim H., Afifi N.…Elliott P. The Qatar Biobank: Background and methods. BMC Public Health. 2015;15:1208. doi: 10.1186/s12889-015-2522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albala F.F., Auzerie V., Mahe E., Farinotti R., Stocco C.D., Crickx B., Descamps V. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. The British Journal of Dermatology. 2003;149:1018–1022. doi: 10.1111/j.1365-2133.2003.05584.x. [DOI] [PubMed] [Google Scholar]
- Alexanderson B., Evans D.A., Sjoqvist F. Steady-state plasma levels of nortriptyline in twins: Influence of genetic factors and drug therapy. British Medical Journal. 1969;4:764–768. doi: 10.1136/bmj.4.5686.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis C.D., Jenuwein T. The molecular hallmarks of epigenetic control. Nature Reviews Genetics. 2016;17:487–500. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- Aminkeng F., Bhavsar A.P., Visscher H., Rassekh S.R., Li Y., Lee J.W.…The Canadian Pharmacogenomics Network for Drug Safety Consortium A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nature Genetics. 2015;47:1079–1084. doi: 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminkeng F., Ross C.J.D., Rassekh S.R., Hwang S., Rieder M.J., Bhavsar A.P.…Group, CPNDS Clinical Practice Recommendations Group Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. British Journal of Clinical Pharmacology. 2016;82:683–695. doi: 10.1111/bcp.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.L., Horne B.D., Stevens S.M., Woller S.C., Samuelson K.M., Mansfield J.W.…Carlquist J.F. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II) Circulation. 2012;125:1997–2005. doi: 10.1161/CIRCULATIONAHA.111.070920. [DOI] [PubMed] [Google Scholar]
- Apellaniz-Ruiz M., Inglada-Pérez L., Naranjo M.E.G., Sánchez L., Mancikova V., Currás-Freixes M.…Rodríguez-Antona C. High frequency and founder effect of the CYP3A4*20 loss-of-function allele in the Spanish population classifies CYP3A4 as a polymorphic enzyme. The Pharmacogenomics Journal. 2015;15:288–292. doi: 10.1038/tpj.2014.67. [DOI] [PubMed] [Google Scholar]
- Apellaniz-Ruiz M., Lee M.Y., Sanchez-Barroso L., Gutierrez-Gutierrez G., Calvo I., Garcia-Estevez L.…Rodríguez-Antona C. Whole-exome sequencing reveals defective cyp3a4 variants predictive of paclitaxel dose-limiting neuropathy. Clinical Cancer Research. 2015;21:322–328. doi: 10.1158/1078-0432.CCR-14-1758. [DOI] [PubMed] [Google Scholar]
- Aplenc R., Glatfelter W., Han P., Rappaport E., La M., Cnaan A.…Rebbeck T. CYP3A genotypes and treatment response in paediatric acute lymphoblastic leukaemia. British Journal of Haematology. 2003;122:240–244. doi: 10.1046/j.1365-2141.2003.04430.x. [DOI] [PubMed] [Google Scholar]
- Armenian S.H., Ding Y., Mills G., Sun C., Venkataraman K., Wong F.L.…Bhatia S. Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. British Journal of Haematology. 2013;163:205–213. doi: 10.1111/bjh.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascierto P.A., Long G.V. Progression-free survival landmark analysis: a critical endpoint in melanoma clinical trials. Lancet Oncology. 2016;17:1037–1039. doi: 10.1016/S1470-2045(16)30017-1. [DOI] [PubMed] [Google Scholar]
- Bachman M., Uribe-Lewis S., Yang X., Burgess H.E., Iurlaro M., Reik W.…Balasubramanian S. 5-Formylcytosine can be a stable DNA modification in mammals. Nature Chemical Biology. 2015;11:555–557. doi: 10.1038/nchembio.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian D., Akhtar-Zaidi B., Song L., Bartels C.F., Veigl M., Beard L.…Scacheri P.C. H3K4me3 inversely correlates with DNA methylation at a large class of non-CpG-island-containing start sites. Genome Medicine. 2012;4:47. doi: 10.1186/gm346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M.N., Butterworth E.A., Kilberg M.S. Asparagine synthetase: Regulation by cell stress and involvement in tumor biology. American Journal of Physiology - Endocrinology and Metabolism. 2013;304:E789–E799. doi: 10.1152/ajpendo.00015.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank P.C.D., Caudle K.E., Swen J.J., Gammal R.S., Whirl-Carrillo M., Klein T.E.…Guchelaar H.J. Comparison of the guidelines of the clinical pharmacogenetics implementation consortium and the dutch pharmacogenetics working group. Clinical Pharmacology & Therapeutics. 2018;103:599–618. doi: 10.1002/cpt.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranzini S.E., Madireddy L.R., Cromer A., D'Antonio M., Lehr L., Beelke M.…Cree B.A.C. Prognostic biomarkers of IFNb therapy in multiple sclerosis patients. Multiple sclerosis. 2015;21:894–904. doi: 10.1177/1352458514555786. [DOI] [PubMed] [Google Scholar]
- Barenboim M., Zoltick B.J., Guo Y., Weinberger D.R. MicroSNiPer: a web tool for prediction of SNP effects on putative microRNA targets. Human Mutation. 2010;31:1223–1232. doi: 10.1002/humu.21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera L.O., Li Z., Smith A.D., Arden K.C., Cavenee W.K., Zhang M.Q.…Ren B. Genome-wide mapping and analysis of active promoters in mouse embryonic stem cells and adult organs. Genome Research. 2007;18:46–59. doi: 10.1101/gr.6654808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor J.R., Welsh K.I., Tinoco R.M., Dollery C.T., Hughes G.R., Bernstein R.…Russell G.I. Hydralazine-induced systemic lupus erythematosus: Influence of HLA-DR and sex on susceptibility. The Lancet. 1980;1:1107–1109. doi: 10.1016/s0140-6736(80)91554-8. [DOI] [PubMed] [Google Scholar]
- Battram T., Richmond R., Baglietto L., Haycock P., Perduca V., Bojesen S.…Relton C. Appraising the causal relevance of DNA methylation for risk of lung cancer. bioRxiv. 2018:287888. doi: 10.1093/ije/dyz190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Tanfous M., Sharif-Askari B., Ceppi F., Laaribi H., Gagné V., Rousseau J.…Krajinovic M. Polymorphisms of asparaginase pathway and asparaginase-related complications in children with acute lymphoblastic leukemia. Clinical Cancer Research. 2015;21:329–334. doi: 10.1158/1078-0432.CCR-14-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E., Duncan E.M., Masui O., Gil J., Heard E., Allis C.D. Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Molecular and Cellular Biology. 2006;26:2560–2569. doi: 10.1128/MCB.26.7.2560-2569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj M., Illing P., Theodossis A., Purcell A.W., Rossjohn J., McCluskey J. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annual Review of Pharmacology and Toxicology. 2012;52:401–431. doi: 10.1146/annurev-pharmtox-010611-134701. [DOI] [PubMed] [Google Scholar]
- Bian C., Xu C., Ruan J., Lee K.K., Burke T.L., Tempel W.…Min J. Sgf29 binds histone H3K4me2/3 and is required for SAGA complex recruitment and histone H3 acetylation. The EMBO Journal. 2011;30:2829–2842. doi: 10.1038/emboj.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbija D., Haugen F., Sagave J., Baysa A., Bastani N., Levy F.O.…Valen G. Retinoic acid signalling is activated in the postischemic heart and may influence remodelling. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J.G., Leisenring W.M., Gonzalez-Covarrubias V.M., Kawashima T.I., Davies S.M., Relling M.V.…Bhatia S. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:Quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–2795. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- Blanco J.G., Sun C.-L., Landier W., Chen L., Esparza-Duran D., Leisenring W.…Bhatia S. Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes—a report from the Children’s Oncology Group. Journal of Clinical Oncology. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonder M.J., Kasela S., Kals M., Tamm R., Lokk K., Barragan I.…Milani L. Genetic and epigenetic regulation of gene expression in fetal and adult human livers. BMC Genomics. 2014;15:860. doi: 10.1186/1471-2164-15-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth M.J., Branco M.R., Ficz G., Oxley D., Krueger F., Reik W., Balasubramanian S. Quantitative Sequencing of 5-Methylcytosine and 5-Hydroxymethylcytosine at Single-Base Resolution. Science. 2012;336:934–937. doi: 10.1126/science.1220671. [DOI] [PubMed] [Google Scholar]
- Boucek R.J., Olson R.D., Brenner D.E., Ogunbunmi E.M., Inui M., Fleischer S. The major metabolite of doxorubicin is a potent inhibitor of membrane-associated ion pumps. A correlative study of cardiac muscle with isolated membrane fractions. Journal of Biological Chemistry. 1987;262:15851–15856. [PubMed] [Google Scholar]
- Boyle A.P., Davis S., Shulha H.P., Meltzer P., Margulies E.H., Weng Z.…Crawford G.E. High-Resolution Mapping and Characterization of Open Chromatin across the Genome. Cell. 2008;132:311–322. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush W.S., Crosslin D.R., Owusu-Obeng A., Wallace J., Almoguera B., Basford M.A.…Ritchie M.D. Genetic variation among 82 pharmacogenes: The PGRNseq data from the eMERGE network. Clinical Pharmacology and Therapeutics. 2016;100:160–169. doi: 10.1002/cpt.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft C., Freeman C., Petkova D., Band G., Elliott L.T., Sharp K.…Marchini J. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P.…Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- Cardinale D., Colombo A., Bacchiani G., Tedeschi I., Meroni C.A., Veglia F.…Cipolla C.M. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- Carr D.F., Bourgeois S., Chaponda M., Takeshita L.Y., Morris A.P., Castro E.M.C.…Pirmohamed M. Genome-wide association study of nevirapine hypersensitivity in a sub-Saharan African HIV-infected population. The Journal of Antimicrobial Chemotherapy. 2017;72:1152–1162. doi: 10.1093/jac/dkw545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D.F., Chaponda M., Jorgensen A.L., Castro E.C., van Oosterhout J.J., Khoo S.H.…Pirmohamed M. Association of human leukocyte antigen alleles and nevirapine hypersensitivity in a Malawian HIV-Infected Population. Clinical Infectious Diseases. 2013;56:1330–1339. doi: 10.1093/cid/cit021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau N.A., Pavlick A.C. Nivolumab and ipilimumab: Immunotherapy for treatment of malignant melanoma. Future Oncology. 2018;19:21. doi: 10.2217/fon-2018-0607. [DOI] [PubMed] [Google Scholar]
- Ceccacci E., Minucci S. Inhibition of histone deacetylases in cancer therapy: Lessons from leukaemia. British Journal of Cancer. 2016;114:605–611. doi: 10.1038/bjc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceppi F., Langlois-Pelletier C., Gagné V., Rousseau J., Ciolino C., Lorenzo S.D.…Krajinovic M. Polymorphisms of the vincristine pathway and response to treatment in children with childhood acute lymphoblastic leukemia. Pharmacogenomics. 2014;15:1105–1116. doi: 10.2217/pgs.14.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-C., Ng C.-C., Too C.-L., Choon S.-E., Lee C.-K., Chung W.-H.…Murad S. Association of HLA-B*15:13 and HLA-B*15:02 with phenytoin-induced severe cutaneous adverse reactions in a Malay population. The Pharmacogenomics Journal. 2017;17:170–173. doi: 10.1038/tpj.2016.10. [DOI] [PubMed] [Google Scholar]
- Chang C.-C., Too C.-L., Murad S., Hussein S.H. Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. International Journal of Dermatology. 2011;50:221–224. doi: 10.1111/j.1365-4632.2010.04745.x. [DOI] [PubMed] [Google Scholar]
- Chantarangsu S., Mushiroda T., Mahasirimongkol S., Kiertiburanakul S., Sungkanuparph S., Manosuthi W.…Nakamura Y. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenetics and Genomics. 2009;19:139–146. doi: 10.1097/FPC.0b013e32831d0faf. [DOI] [PubMed] [Google Scholar]
- Chen C.-B., Abe R., Pan R.-Y., Wang C.-W., Hung S.-I., Tsai Y.-G., Chung W.-H. An Updated Review of the Molecular Mechanisms in Drug Hypersensitivity. Journal of Immunology Research. 2018;2018:6431694. doi: 10.1155/2018/6431694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-B., Hsiao Y.-H., Wu T., Hsih M.-S., Tassaneeyakul W., Jorns T.P.…For the Taiwan Severe Cutaneous Adverse Reaction Consortium Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians. Neurology. 2017;88:78–86. doi: 10.1212/WNL.0000000000003453. [DOI] [PubMed] [Google Scholar]
- Chen C.-H., Lee C.-S., Lee M.T.M., Ouyang W.-C., Chen C.-C., Chong M.-Y.…For the Taiwan Bipolar Consortium Variant GADL1 and response to lithium therapy in bipolar I disorder. New England Journal of Medicine. 2014;370:119–128. doi: 10.1056/NEJMoa1212444. [DOI] [PubMed] [Google Scholar]
- Chen P.-L., Shih S.-R., Wang P.-W., Lin Y.-C., Chu C.-C., Lin J.-H.…Chang T.-C. Genetic determinants of antithyroid drug-induced agranulocytosis by human leukocyte antigen genotyping and genome-wide association study. Nature Communications. 2015;6:1–8. doi: 10.1038/ncomms8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.H., Pei D., Yang W., Cheng C., Jeha S., Cox N.J.…Relling M.V. Genetic variations in GRIA1 on chromosome 5q33 related to asparaginase hypersensitivity. Clinical Pharmacology and Therapeutics. 2010;88:191–196. doi: 10.1038/clpt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-T., Wang C.-W., Lu C.-W., Chen C.-B., Lee H.-E., Hung S.-I.…Taiwan Severe Cutaneous Adverse Reaction Consortium The function of HLA-B*13:01 involved in the pathomechanism of dapsone-induced severe cutaneous adverse reactions. The Journal of Investigative Dermatology. 2018;138:1546–1554. doi: 10.1016/j.jid.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Chen Z., Li S., Subramaniam S., Shyy J.Y.J., Chien S. Epigenetic regulation: A new frontier for biomedical engineers. Annual Review of Biomedical Engineering. 2017;19:195–219. doi: 10.1146/annurev-bioeng-071516-044720. [DOI] [PubMed] [Google Scholar]
- Cheung Y.-K., Cheng S.H., Chan E.J.M., Lo S.V., Ng M.H.L., Kwan P. HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia. 2013;54:1307–1314. doi: 10.1111/epi.12217. [DOI] [PubMed] [Google Scholar]
- Cheverud J.M., Routman E.J. Epistasis and its Contribution to Genetic Variance-Components. Genetics. 1995;139:1455–1461. doi: 10.1093/genetics/139.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I., Kim J., Kim J.H., Kim H.Y., Kim Y. Design and implementation of a standards-based interoperable clinical decision support architecture in the context of the Korean EHR. International Journal of Medical Informatics. 2010;79:611–622. doi: 10.1016/j.ijmedinf.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Cho Y.-T., Yang C.-W., Chu C.-Y. Drug reaction with eosinophilia and systemic symptoms (DRESS): An interplay among drugs, viruses, and immune system. International Journal of Molecular Sciences. 2017;18 doi: 10.3390/ijms18061243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H., Lee K.W., Oh H.B., Lee K.J., Suh Y.J., Park C.S., Park H.-S. HLA association in aspirin-intolerant asthma. Journal of Allergy and Clinical Immunology. 2004;113:562–564. doi: 10.1016/j.jaci.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Choi Y., Sims G.E., Murphy S., Miller J.R., Chan A.P. Predicting the functional effect of amino acid substitutions and indels. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong E., Guo J., Persson A., Virding S., Johansson I., Mkrtchian S., Ingelman-Sundberg M. Developmental regulation and induction of cytochrome P450 2W1, an enzyme expressed in colon tumors. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.-H., Chang W.-C., Lee Y.-S., Wu Y.-Y., Yang C.-H., Ho H.-C.…Japan Pharmacogenomics Data Science Consortium Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. 2014;312:525–534. doi: 10.1001/jama.2014.7859. [DOI] [PubMed] [Google Scholar]
- Chung W.-H., Hung S.-I., Hong H.-S., Hsih M.-S., Yang L.-C., Ho H.-C.…Chen Y.-T. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- Clark S.J., Lee H.J., Smallwood S.A., Kelsey G., Reik W. Single-cell epigenomics: Powerful new methods for understanding gene regulation and cell identity. Genome Biology. 2016:1–10. doi: 10.1186/s13059-016-0944-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.P., Sparks K.E., Fraser K., Loe D.W., Grant C.E., Wilson G.M., Deeley R.G. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Research. 1994;54:5902–5910. [PubMed] [Google Scholar]
- The 1000 Genomes Project Consortium A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo-García J.A., Jagemann L.R., Blanca-López N., Doña I., Flores C., Guéant-Rodríguez R.M.…Blanca M. Genetic variants of the arachidonic acid pathway in non-steroidal anti-inflammatory drug-induced acute urticaria. Clinical and Experimental Allergy. 2012;42:1772–1781. doi: 10.1111/j.1365-2222.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- Czermin B., Melfi R., McCabe D., Seitz V., Imhof A., Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–196. doi: 10.1016/s0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Daly A.K., Donaldson P.T., Bhatnagar P., Shen Y., Pe'er I., Floratos A.…Day C.P. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nature Genetics. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- Dankar F.K., Ptitsyn A., Dankar S.K. The development of large-scale de-identified biomedical databases in the age of genomics-principles and challenges. Human Genomics. 2018;12:19. doi: 10.1186/s40246-018-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans C., Maggert K.A. What do you mean, "epigenetic". Genetics. 2015;199:887–896. doi: 10.1534/genetics.114.173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenen M.J., Meulendijks D., Cats A., Sechterberger M.K., Severens J.L., Boot H.…Schellens J.H.M. Upfront Genotyping of DPYD* 2Ato Individualize Fluoropyrimidine Therapy: A Safety and cost Analysis. Journal of Clinical Oncology. 2016;34:227–234. doi: 10.1200/JCO.2015.63.1325. [DOI] [PubMed] [Google Scholar]
- Dekker J.W., Nizankowska E., Schmitz-Schumann M., Pile K., Bochenek G., Dyczek A.…Szczeklik A. Aspirin-induced asthma and HLA-DRB1 and HLA-DPB1 genotypes. Clinical and Experimental Allergy. 1997;27:574–577. [PubMed] [Google Scholar]
- Dekkers K.F., van Iterson M., Slieker R.C., Moed M.H., Bonder M.J., van Galen M.…Heijmans B.T. Blood lipids influence DNA methylation in circulating cells. Genome Biology. 2016;17:138. doi: 10.1186/s13059-016-1000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix L., Moutier E., Altobelli G., Legras S., Poch O., Choukrallah M.-A.…Davidson I. Cell-specific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Molecular and Cellular Biology. 2010;30:231–244. doi: 10.1128/MCB.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison J.B., Jones D.R., Renbarger J.L., Hall S.D. Effect of CYP3A5 expression on vincristine metabolism with human liver microsomes. Journal of Pharmacology and Experimental Therapeutics. 2007;321:553–563. doi: 10.1124/jpet.106.118471. [DOI] [PubMed] [Google Scholar]
- Desai Z.R., van den Berg H.W., Bridges J.M., Shanks R.G. Can Severe Vincristine Neurotoxicity be prevented. Cancer Chemotherapy and Pharmacology. 1982;8:211–214. doi: 10.1007/BF00255486. [DOI] [PubMed] [Google Scholar]
- Deveci M., Catalyürek U.V., Toland A.E. mrSNP: Software to detect SNP effects on microRNA binding. BMC Bioinformatics. 2014;15:73. doi: 10.1186/1471-2105-15-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diouf B., Crews K.R., Lew G., Pei D., Cheng C., Bao J.…Evans W.E. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313:815–823. doi: 10.1001/jama.2015.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson P.T., Daly A.K., Henderson J., Graham J., Pirmohamed M., Bernal W.…Aithal G.P. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. Journal of Hepatology. 2010;53:1049–1053. doi: 10.1016/j.jhep.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Dong D., Sung C., Finkelstein E.A. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology. 2012;79:1259–1267. doi: 10.1212/WNL.0b013e31826aac73. [DOI] [PubMed] [Google Scholar]
- Drmanac R., Sparks A.B., Callow M.J., Halpern A.L., Burns N.L., Kermani B.G.…Reid C.A. Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science. 2010;327:78–81. doi: 10.1126/science.1181498. [DOI] [PubMed] [Google Scholar]
- Drögemöller B.I., Wright G.E., Warnich L. Considerations for rare variants in drug metabolism genes and the clinical implications. Expert Opinion on Drug Metabolism & Toxicology. 2014;10:873–884. doi: 10.1517/17425255.2014.903239. [DOI] [PubMed] [Google Scholar]
- Dumontet C., Jordan M.A. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nature Reviews Drug Discovery. 2010;9:790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbelakin A., Ferguson M.J., MacGill E.A., Lehmann A.S., Topletz A.R., Quinney S.K.…Renbarger J.L. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2011;56:361–367. doi: 10.1002/pbc.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann F., Caneva L., Prasad K., Paulmichl M., Maliepaard M., Llerena A.…Papaluca-Amati M. Pharmacogenomic information in drug labels: European Medicines Agency perspective. The Pharmacogenomics Journal. 2015;15:201–210. doi: 10.1038/tpj.2014.86. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Spannbrucker N., Dengler H.J. Influence of the defective metabolism of sparteine on its pharmacokinetics. European Journal of Clinical Pharmacology. 1979;16:189–194. doi: 10.1007/BF00562060. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Spannbrucker N., Steincke B., Dengler H.J. Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. European Journal of Clinical Pharmacology. 1979;16:183–187. doi: 10.1007/BF00562059. [DOI] [PubMed] [Google Scholar]
- Eid J., Fehr A., Gray J., Luong K., Lyle J., Otto G.…Turner S. Real-time dna sequencing from single polymerase molecules. Science. 2009;323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- El Kennani S., Crespo M., Govin J., Pflieger D. Proteomic analysis of histone variants and their ptms: strategies and pitfalls. Proteome. 2018;6 doi: 10.3390/proteomes6030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elens L., Tyteca D., Panin N., Courtoy P., Lison D., Demoulin J.-B., Haufroid V. Functional defect caused by the 4544G>A SNP in ABCC2. Pharmacogenetics and Genomics. 2011;21:884–893. doi: 10.1097/FPC.0b013e32834d672b. [DOI] [PubMed] [Google Scholar]
- Endoh M., Endo T.A., Endoh T., Isono K.-I., Sharif J., Ohara O.…Koseki H. Histone H2A mono-ubiquitination is a crucial step to mediate PRC1-dependent repression of developmental genes to maintain ES cell identity. PLoS Genetics. 2012;8 doi: 10.1371/journal.pgen.1002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J., Kellis M. ChromHMM: Automating chromatin-state discovery and characterization. Nature Methods. 2012;9:215–216. doi: 10.1038/nmeth.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.A., Manley K.A., McKusick V.A. Genetic control of isoniazid metabolism in man. British Medical Journal. 1960;2:485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farh K.K.-H., Marson A., Zhu J., Kleinewietfeld M., Housley W.J., Beik S.…Bernstein B.E. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518:337–343. doi: 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C.A., Smith C., Yang W., Date M., Bashford D., Larsen E.…Relling M.V. HLA-DRB1*07:01 is associated with a higher risk of asparaginase allergies. Blood. 2014;124:1266–1276. doi: 10.1182/blood-2014-03-563742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C.A., Smith C., Yang W., Mullighan C.G., Qu C., Larsen E.…Relling M.V. Genome-wide analysis links NFATC2 with asparaginase hypersensitivity. Blood. 2015;126:69–75. doi: 10.1182/blood-2015-02-628800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Final report from the analysis of candidate gene markers and genome-wide single nucleotide polymorphisms (SNPs) from two retrospective, case-control studies and three controlled clinical studies to investigate genetic polymorphisms in HIV infected subjects who developed hypersensitivity following treatment with abacavir (study RJ2006/00002/00)
- Fisel P., Schaeffeler E., Schwab M. DNA methylation of ADME genes. Clinical Pharmacology and Therapeutics. 2016;99:512–527. doi: 10.1002/cpt.343. [DOI] [PubMed] [Google Scholar]
- Flusberg B.A., Webster D.R., Lee J.H., Travers K.J., Olivares E.C., Clark T.A.…Turner S.W. Direct detection of DNA methylation during single-molecule, real-time sequencing. Nature Methods. 2010;7:461–465. doi: 10.1038/nmeth.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer Y., Schneider M., Blum H.E., Hafkemeyer P. Reversal of drug resistance of hepatocellular carcinoma cells by adenoviral delivery of anti-ABCC2 antisense constructs. Cancer Gene Therapy. 2007;14:875–884. doi: 10.1038/sj.cgt.7701082. [DOI] [PubMed] [Google Scholar]
- Fong P.C., Boss D.S., Yap T.A., Tutt A., Wu P., Mergui-Roelvink M.…de Bono J.S. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. New England Journal of Medicine. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Francis G.S., Grumser Y., Alteri E., Micaleff A., O'Brien F., Alsop J.…Kaplowitz N. Hepatic reactions during treatment of multiple sclerosis with interferon-beta-1a - Incidence and clinical significance. Drug Safety. 2003;26:815–827. doi: 10.2165/00002018-200326110-00006. [DOI] [PubMed] [Google Scholar]
- French D., Hamilton L.H., Mattano L.A., Sather H.N., Devidas M., Nachman J.B.…Group, C. a. s. O A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:4496–4499. doi: 10.1182/blood-2007-11-123885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura K., Ingelman-Sundberg M., Lauschke V.M. Genetic variation in the human cytochrome P450 supergene family. Pharmacogenetics and Genomics. 2015;25:584–594. doi: 10.1097/FPC.0000000000000172. [DOI] [PubMed] [Google Scholar]
- Fujisawa T., Filippakopoulos P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nature Reviews Molecular Cell Biology. 2017;18:246–262. doi: 10.1038/nrm.2016.143. [DOI] [PubMed] [Google Scholar]
- Gagnon J.-F., Bernard O., Villeneuve L., Têtu B., Guillemette C. Irinotecan inactivation is modulated by epigenetic silencing of UGT1A1 in colon cancer. Clinical Cancer Research. 2006;12:1850–1858. doi: 10.1158/1078-0432.CCR-05-2130. [DOI] [PubMed] [Google Scholar]
- Gatanaga H., Yazaki H., Tanuma J., Honda M., Genka I., Teruya K., Tachikawa N., Kikuchi Y., Oka S. HLA-Cw8 primarily associated with hypersensitivity to nevirapine. AIDS. 2007;21:264–265. doi: 10.1097/QAD.0b013e32801199d9. [DOI] [PubMed] [Google Scholar]
- Gazzard B.G., Anderson J., Babiker A., Boffito M., Brook G., Brough G., Churchill D., Cromarty B, Das S., Fisher M., Freedman A.M., Johnson M., Khoo S., Leen C., Nair D., Peters B., Phillips A., Pillay D., Pozniak A., Walsh J., Wilkins E., Williams I., Williams M., Williams I., Youle M., BHIVA Treatment Guidelines Writing Grou British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. 2008;9:563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- GBD (2015). Disease and Injury Incidence and Prevalence Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015, Lancet 388, 2016, 1545-1602. [DOI] [PMC free article] [PubMed]
- Genin E., Chen D.-P., Hung S.-I., Sekula P., Schumacher M., Chang P.-Y.…Roujeau J.-C. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: An international study and meta-analysis. The Pharmacogenomics Journal. 2014;14:281–288. doi: 10.1038/tpj.2013.40. [DOI] [PubMed] [Google Scholar]
- Girardin F.R., Poncet A., Perrier A., Vernaz N., Pletscher M., Samer C F.…Villard J. Cost-effectiveness of HLA-DQB1/HLA-B pharmacogenetic-guided treatment and blood monitoring in US patients taking clozapine. The Pharmacogenomics Journal. 2018;209:385. doi: 10.1038/s41397-017-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globisch D., Münzel M., Müller M., Michalakis S., Wagner M., Koch S.…Carell T. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss B.S., Dinger M.E. Realizing the significance of noncoding functionality in clinical genomics. Experimental and Molecular Medicine. 2018;50:97. doi: 10.1038/s12276-018-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.I., Jarskog L.F., Hilliard C., Alfirevic A., Duncan L., Fourches D.…Sullivan P.F. Clozapine-induced agranulocytosis is associated with rare HLA-DQB1 and HLA-B alleles. Nature Communications. 2014;5:4757. doi: 10.1038/ncomms5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalo M., Coutinho I., Teixeira V., Gameiro A.R., Brites M.M., Nunes R., Martinho A. HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. The British Journal of Dermatology. 2013;169:660–665. doi: 10.1111/bjd.12389. [DOI] [PubMed] [Google Scholar]
- Gordon A.S., Fulton R.S., Qin X., Mardis E.R., Nickerson D.A., Scherer S. PGRNseq: a targeted capture sequencing panel for pharmacogenetic research and implementation. Pharmacogenetics and Genomics. 2016;26:161–168. doi: 10.1097/FPC.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A.S., Tabor H.K., Johnson A.D., Snively B.M., Assimes T.L., Auer P.L.…On Behalf of the NHLBI GO Exome Sequencing Project Quantifying rare, deleterious variation in 12 human cytochrome P450 drug-metabolism genes in a large-scale exome dataset. Human Molecular Genetics. 2014;23:1957–1963. doi: 10.1093/hmg/ddt588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L., Trenk D., Jacobshagen C., Krieg A., Gawaz M., Massberg S.…Geisler T. Genotype-phenotype association and impact on outcomes following guided de-escalation of anti-platelet treatment in acute coronary syndrome patients: The Tropical-ACS genotyping substudy. Thrombosis and Haemostasis. 2018;118:1656–1667. doi: 10.1055/s-0038-1667337. [DOI] [PubMed] [Google Scholar]
- Guéant J.-L., Romano A., Cornejo-Garcia J.-A., Oussalah A., Chery C., Blanca-López N.…Blanca M. HLA-DRA variants predict penicillin allergy in genome-wide fine-mapping genotyping. The Journal of Allergy and Clinical Immunology. 2015;135:253–259. doi: 10.1016/j.jaci.2014.07.047. [DOI] [PubMed] [Google Scholar]
- Guilhaumou R., Solas C., Bourgarel-Rey V., Quaranta S., Rome A., Simon N.…Andre N. Impact of plasma and intracellular exposure and CYP3A4, CYP3A5, and ABCB1 genetic polymorphisms on vincristine-induced neurotoxicity. Cancer Chemotherapy and Pharmacology. 2011;68:1633–1638. doi: 10.1007/s00280-011-1745-2. [DOI] [PubMed] [Google Scholar]
- Guillemette B., Drogaris P., Lin H.-H.S., Armstrong H., Hiragami-Hamada K., Imhof A.…Festenstein R.J. H3 lysine 4 is acetylated at active gene promoters and is regulated by H3 lysine 4 methylation. PLoS Genetics. 2011;7 doi: 10.1371/journal.pgen.1001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcher J., Stefansson K. An Icelandic saga on a centralized healthcare database and democratic decision making. Nature Biotechnology. 1999;17:620. doi: 10.1038/10796. [DOI] [PubMed] [Google Scholar]
- Guo J., Johansson I., Mkrtchian S., Ingelman-Sundberg M. The CYP2W1 enzyme: Regulation, properties and activation of prodrugs. Drug Metabolism Reviews. 2016;48:369–378. doi: 10.1080/03602532.2016.1188939. [DOI] [PubMed] [Google Scholar]
- Habano W., Kawamura K., Iizuka N., Terashima J., Sugai T., Ozawa S. Analysis of DNA methylation landscape reveals the roles of DNA methylation in the regulation of drug metabolizing enzymes. Clinical Epigenetics. 2015:1–11. doi: 10.1186/s13148-015-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J.L., Ter-Minassian M., Bazyk A., Gusella J.F., Kim D.J., Terwedow H.…Oksenberg J.R. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. The Multiple Sclerosis Genetics Group. Nature Genetics. 1996;13:469–471. doi: 10.1038/ng0896-469. [DOI] [PubMed] [Google Scholar]
- Hannon E., Lunnon K., Schalkwyk L., Mill J. Interindividual methylomic variation across blood, cortex, and cerebellum: Implications for epigenetic studies of neurological and neuropsychiatric phenotypes. Epigenetics. 2015;10:1024–1032. doi: 10.1080/15592294.2015.1100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.M., Park J., Lee J.H., Lee S.S., Kim H., Han H., Kim Y., Yi S., Cho J.Y., Jang I.J., Lee M.G. Targeted Next-Generation Sequencing for Comprehensive Genetic Profiling of Pharmacogenes. Clinical Pharmacology & Therapeutics. 2017;101:396–405. doi: 10.1002/cpt.532. [DOI] [PubMed] [Google Scholar]
- Harmanci A.O., Sharma G., Mathews D.H. TurboFold: Iterative probabilistic estimation of secondary structures for multiple RNA sequences. BMC Bioinformatics. 2011;12:108. doi: 10.1186/1471-2105-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman A., van Schaik R.H.N., van der Heiden I.P., Broekhuis M.J.C., Meier M., den Boer M.L., Pieters R. Polymorphisms in genes involved in vincristine pharmacokinetics or pharmacodynamics are not related to impaired motor performance in children with leukemia. Leukemia Research. 2010;34:154–159. doi: 10.1016/j.leukres.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Hauser A.S., Chavali S., Masuho I., Jahn L.J., Martemyanov K.A., Gloriam D.E., Babu M.M. Pharmacogenomics of GPCR drug targets. Cell. 2018;172 doi: 10.1016/j.cell.2017.11.033. 41-54.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautekeete M.L., Horsmans Y., van Waeyenberge C., Demanet C., Henrion J., Verbist L.…Geubel A.P. HLA association of amoxicillin-clavulanate-induced hepatitis. Gastroenterology. 1999;117:1181–1186. doi: 10.1016/s0016-5085(99)70404-x. [DOI] [PubMed] [Google Scholar]
- Henricks L.M., Lunenburg C.A.T.C., de Man F.M., Meulendijks D., Frederix G.W.J., Kienhuis E.…Schellens J.H.M. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncology. 2018;19:1459–1467. doi: 10.1016/S1470-2045(18)30686-7. [DOI] [PubMed] [Google Scholar]
- Henrique R., Oliveira A.I., Costa V.L., Baptista T., Martins A.T., Morais A.…Jerónimo C. Epigenetic regulation of MDR1 gene through post-translational histone modifications in prostate cancer. BMC Genomics. 2013;14:898. doi: 10.1186/1471-2164-14-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hequet O., Le Q.H., Moullet I., Pauli E., Salles G., Espinouse D.…Coiffier B. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. Journal of Clinical Oncology. 2004;22:1864–1871. doi: 10.1200/JCO.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Hernandez-Salazar A., Rosales S.P.-D.-L., Rangel-Frausto S., Criollo E., Archer-Dubon C., Orozco-Topete R. Epidemiology of adverse cutaneous drug reactions. A prospective study in hospitalized patients. Archives of Medical Research. 2006;37:899–902. doi: 10.1016/j.arcmed.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Hertz D.L., Caram M.V., Kidwell K.M., Thibert J.N., Gersch C., Seewald N.J.…Rae J.M. Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines. Pharmacogenomics. 2016;17:231–240. doi: 10.2217/pgs.15.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G.P., Fonseca E., Scott R., Fagerness J. Pharmacogenomic and pharmacogenetic-guided therapy as a tool in precision medicine: Current state and factors impacting acceptance by stakeholders. Genetics Research. 2015;97 doi: 10.1017/S0016672315000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington S., Hughes A.R., Mosteller M., Shortino D., Baker K.L., Spreen W.…Roses A.D. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. The Lancet. 2002;359:1121–1122. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- Hirasawa M., Hagihara K., Abe K., Ando O., Hirayama N. Interaction of Nevirapine with the Peptide Binding Groove of HLA-DRB1*01:01 and its effect on the Conformation of HLA-Peptide complex. International Journal of Molecular Sciences. 2018;19 doi: 10.3390/ijms19061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K., Takagi H., Yamamoto M., Matsumoto T., Nishiya T., Mori K.…Okutani Y. Ticlopidine-induced hepatotoxicity is associated with specific human leukocyte antigen genomic subtypes in Japanese patients: a preliminary case-control study. The Pharmacogenomics Journal. 2008;8:29–33. doi: 10.1038/sj.tpj.6500442. [DOI] [PubMed] [Google Scholar]
- Hoffman M.M., Buske O.J., Wang J., Weng Z., Bilmes J.A., Noble W.S. Unsupervised pattern discovery in human chromatin structure through genomic segmentation. Nature Methods. 2012;9:473–476. doi: 10.1038/nmeth.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Heilbronner U., Degenhardt F., Adli M., Akiyama K., Akula N.…Schulze T.G. Genetic variants associated with response to lithium treatmenjt in bipolar disorder: a genome-wide association study. Lancet. 2016;387:1085–1093. doi: 10.1016/S0140-6736(16)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y.-H., Hui R.C.-Y., Wu T., Chang W.-C., Hsih M.-S., Yang C.-H.…Chung W.-H. Genotype-phenotype association between HLA and carbamazepine-induced hypersensitivity reactions: Strength and clinical correlations. Journal of Dermatological Science. 2014;73:101–109. doi: 10.1016/j.jdermsci.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Hsu M.-K., Lin H.-Y., Chen F.-C. NMD Classifier: A reliable and systematic classification tool for nonsense-mediated decay events. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F.-Y., Wu X.-T., An D.-M., Yan B., Stefan H., Zhou D. Pilot association study of oxcarbazepine-induced mild cutaneous adverse reactions with HLA-B*1502 allele in Chinese Han population. Seizure. 2011;20:160–162. doi: 10.1016/j.seizure.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Huang H., Lin S., Garcia B.A., Zhao Y. Quantitative proteomic analysis of histone modifications. Chemical Reviews. 2015;115:2376–2418. doi: 10.1021/cr500491u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Fang Y., Wu J.M., Dziadyk J.M., Zhu X.M., Sui M.M., Fan W.M. Regulation of Vinca alkaloid-induced apoptosis by NF-kappa B/I kappa B pathway in human tumor cells. Molecular Cancer Therapeutics. 2004;3:271–277. [PubMed] [Google Scholar]
- Huang Y.-F., Gulko B., Siepel A. Fast, scalable prediction of deleterious noncoding variants from functional and population genomic data. Nature Genetics. 2017;49:618–624. doi: 10.1038/ng.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D.A., Vilar F.J., Ward C.C., Alfirevic A., Park B.K., Pirmohamed M. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics. 2004;14:335–342. doi: 10.1097/00008571-200406000-00002. [DOI] [PubMed] [Google Scholar]
- Hung S.-I., Chung W.-H., Liou L.-B., Chu C.-C., Lin M., Huang H.-P.…Chen Y.-T. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proceedings of the National Academy of Sciences. 2005;102:4134–4139. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung S.L., Chung W.-H., Jee S.H., Chen W.C., Chang Y.T., Lee W.R.…Chen Y.T. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenetics and Genomics. 2006;16:297–306. doi: 10.1097/01.fpc.0000199500.46842.4a. [DOI] [PubMed] [Google Scholar]
- Hung T., Binda O., Champagne K.S., Kuo A.J., Johnson K., Chang H.Y.…Gozani O. ING4 Mediates Crosstalk between Histone H3 K4 Trimethylation and H3 Acetylation to Attenuate Cellular Transformation. Molecular Cell. 2009;33:248–256. doi: 10.1016/j.molcel.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iarussi D., Auricchio U., Agretto A., Murano A., Giuliano M., Casale F.…Iacono A. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: Control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Molecular Aspects of Medicine. 1994;15(Suppl):s207–s212. doi: 10.1016/0098-2997(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Illing P.T., Vivian J.P., Dudek N.L., Kostenko L., Chen Z., Bharadwaj M.…McCluskey J. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486:554–558. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- Inaba H., Pui C.-H. Glucocorticoid use in acute lymphoblastic leukaemia. The Lancet Oncology. 2010;11:1096–1106. doi: 10.1016/S1470-2045(10)70114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Mkrtchian S., Zhou Y., Lauschke V.M. Integrating rare genetic variants into pharmacogenetic drug response predictions. Human Genomics. 2018;12:26. doi: 10.1186/s40246-018-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel E., Chinchilli V.M., Ford J.G., Boushey H.A., Cherniack R., Craig T.J.…Blood Institute's Asthma Clinical Research Network Use of regularly scheduled albuterol treatment in asthma: Genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- Ito S., Shen L., Dai Q., Wu S.C., Collins L.B., Swenberg J.A.…Zhang Y. Tet Proteins can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro M., Ficz G., Oxley D., Raiber E.-A., Bachman M., Booth M.J.…Reik W. A screen for hydroxymethylcytosine and formylcytosine binding proteins suggests functions in transcription and chromatin regulation. Genome Biology. 2013;14:R119. doi: 10.1186/gb-2013-14-10-r119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov M., Kals M., Kacevska M., Barragan I., Kasuga K., Rane A.…Ingelman-Sundberg M. Ontogeny, distribution and potential roles of 5-hydroxymethylcytosine in human liver function. Genome Biology. 2013;14:R83. doi: 10.1186/gb-2013-14-8-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov M., Kals M., Lauschke V.M., Barragan I., Ewels P., Käller M.…Ingelman-Sundberg M. Single base resolution analysis of 5-hydroxymethylcytosine in 188 human genes: Implications for hepatic gene expression. Nucleic Acids Research. 2016;44:6756–6769. doi: 10.1093/nar/gkw316. gkw316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaruthamsophon K., Tipmanee V., Sangiemchoey A., Sukasem C., Limprasert P. HLA-B*15:21 and carbamazepine-induced Stevens-Johnson syndrome: Pooled-data and in silico analysis. Scientific Reports. 2017;7:45553. doi: 10.1038/srep45553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Wang J., Bachtiar M., Chong S.S., Lee C.G.L. Architecture of polymorphisms in the human genome reveals functionally important and positively selected variants in immune response and drug transporter genes. Human Genomics. 2018;12:43. doi: 10.1186/s40246-018-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.C., Corthals S.L., Walker B.A., Ross F.M., Gregory W.M., Dickens N.J.…Morgan G.J. Genetic factors underlying the risk of thalidomide-related neuropathy in patients with multiple myeloma. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2011;29:797–804. doi: 10.1200/JCO.2010.28.0792. [DOI] [PubMed] [Google Scholar]
- Johnson D.S., Mortazavi A., Myers R.M., Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- Johnson J.A., Cavallari L.H. Warfarin pharmacogenetics. Trends in Cardiovascular Medicine. 2015;25:33–41. doi: 10.1016/j.tcm.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukić M.M., Haslemo T., Molden E., Ingelman-Sundberg M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: a retrospective study based on 2,087 patients. The American Journal of Psychiatry. 2018;175:463–470. doi: 10.1176/appi.ajp.2017.17050550. appiajp201717050550. [DOI] [PubMed] [Google Scholar]
- Jukić M.M., Opel N., Ström J., Carrillo-Roa T., Miksys S., Novalen M.…Ingelman-Sundberg M. Elevated CYP2C19 expression is associated with depressive symptoms and hippocampal homeostasis impairment. Molecular Psychiatry. 2017;22:1155–1163. doi: 10.1038/mp.2016.204. [DOI] [PubMed] [Google Scholar]
- Jung J.-W., Song W.-J., Kim Y.-S., Joo K.W., Lee K.W., Kim S.-H.…Kang H.-R. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrology, Dialysis, Transplantation. 2011;26:3567–3572. doi: 10.1093/ndt/gfr060. [DOI] [PubMed] [Google Scholar]
- Kacevska M., Ivanov M., Wyss A., Kasela S., Milani L., Rane A., Ingelman-Sundberg M. DNA methylation dynamics in the hepatic CYP3A4 gene promoter. Biochimie. 2012;94:2338–2344. doi: 10.1016/j.biochi.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Kalam K., Marwick T.H. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. European journal of cancer. 2013;49:2900–2909. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- Kalow W., Gunn D.R. The relation between dose of succinylcholine and duration of apnea in man. Journal of Pharmacology and Experimental Therapeutics. 1957;120:203–214. [PubMed] [Google Scholar]
- Kang H.-R., Jee Y.-K., Kim Y.-S., Lee C.H., Jung J.-W., Kim S.-H.…The Adverse Drug Reaction Research Group in Korea Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenetics and Genomics. 2011;21:303–307. doi: 10.1097/FPC.0b013e32834282b8. [DOI] [PubMed] [Google Scholar]
- Kaniwa N., Saito Y., Aihara M., Matsunaga K., Tohkin M., Kurose K.…For the JSAR research group HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia. 2010;51:2461–2465. doi: 10.1111/j.1528-1167.2010.02766.x. [DOI] [PubMed] [Google Scholar]
- Kaniwa N., Saito Y., Aihara M., Matsunaga K., Tohkin M., Kurose K.…Hasegawa R. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens–Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9:1617–1622. doi: 10.2217/14622416.9.11.1617. [DOI] [PubMed] [Google Scholar]
- Karol S.E., Yang W., Van Driest S.L., Chang T.Y., Kaste S., Bowton E.…Relling M.V. Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2015;126:1770–1776. doi: 10.1182/blood-2015-05-643601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawedia J.D., Kaste S.C., Pei D., Panetta J.C., Cai X., Cheng C.…Relling M.V. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117:2340–2347. doi: 10.1182/blood-2010-10-311969. quiz 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D.R., Snoek J., Rinn J.L. Basset: Learning the regulatory code of the accessible genome with deep convolutional neural networks. Genome Research. 2016;26:990–999. doi: 10.1101/gr.200535.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten E.T.G., Koppelman G.H. Pharmacogenetics of asthma: Toward precision medicine. Current Opinion in Pulmonary Medicine. 2017;23:12–20. doi: 10.1097/MCP.0000000000000335. [DOI] [PubMed] [Google Scholar]
- Kidder B.L., Hu G., Zhao K. ChIP-Seq: Technical considerations for obtaining high-quality data. Nature Immunology. 2011;12:918–922. doi: 10.1038/ni.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K., Holdway J.E., Major R.J., Blum N., Dahn R.D., Begemann G., Poss K.D. Retinoic Acid Production by Endocardium and Epicardium is an Injury Response Essential for Zebrafish Heart Regeneration. Developmental Cell. 2011;20:397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.-K., Jung J.-W., Kim T.-B., Chang Y.-S., Park H.-S., Moon J.…Park H.-W. HLA-A*31:01 and lamotrigine-induced severe cutaneous adverse drug reactions in a Korean population. Annals of allergy, asthma and immunology. Asthma & Immunology. 2017;118:629–630. doi: 10.1016/j.anai.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Choi J.-H., Holloway J.W., Suh C.-H., Nahm D.-H., Ha E.H.…Park H.S. Leukotriene-related gene polymorphisms in patients with aspirin-intolerant urticaria and aspirin-intolerant asthma: Differing contributions of ALOX5 polymorphism in Korean population. Journal of Korean Medical Science. 2005;20:926–931. doi: 10.3346/jkms.2005.20.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Choi J.-H., Lee K.-W., Shin E.-S., Oh H.-B., Suh C.-H.…Park H.S. The human leucocyte antigen-DRB1*1302-DQB1*0609-DPB1*0201 haplotype may be a strong genetic marker for aspirin-induced urticaria. Clinical and Experimental Allergy. 2005;35:339–344. doi: 10.1111/j.1365-2222.2004.02197.x. [DOI] [PubMed] [Google Scholar]
- Kim S.-H., Kim M., Lee K.W., Kim S.-H., Kang H.-R., Park H.-W., Jee Y.-K. HLA-B*5901 is strongly associated with methazolamide-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Pharmacogenomics. 2010;11:879–884. doi: 10.2217/pgs.10.54. [DOI] [PubMed] [Google Scholar]
- Kim S.-H., Lee K.W., Song W.-J., Kim S.-H., Jee Y.-K., Lee S.-M.…The Adverse Drug Reaction Research Group in Korea Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Research. 2011;97:190–197. doi: 10.1016/j.eplepsyres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Kimmel S.E., French B., Kasner S.E., Johnson J.A., Anderson J.L., Gage B.F.…Ellenberg J.H. A Pharmacogenetic versus a Clinical Algorithm for Warfarin Dosing. New England Journal of Medicine. 2013;369:2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M., Witten D.M., Jain P., O'Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nature Reviews. Clinical Oncology. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsera N., Allgayer J., Parsa E., Geier N., Rossa M., Carell T., Khobta A. Functional impacts of 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxycytosine at a single hemi-modified CpG dinucleotide in a gene promoter. Nucleic Acids Research. 2017;45:11033–11042. doi: 10.1093/nar/gkx718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko T.-M., Tsai C.-Y., Chen S.-Y., Chen K.-S., Yu K.-H., Chu C.-S.…For the Taiwan Allopurinol-SCAR Consortium Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: National prospective cohort study. BMJ. 2015;351:h4848. doi: 10.1136/bmj.h4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongpan T., Mahasirimongkol S., Konyoung P., Kanjanawart S., Chumworathayi P., Wichukchinda N.…Tassaneeyakul W. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenetics and Genomics. 2015;25:402–411. doi: 10.1097/FPC.0000000000000153. [DOI] [PubMed] [Google Scholar]
- Koomdee N., Pratoomwun J., Jantararoungtong T., Theeramoke V., Tassaneeyakul W., Klaewsongkram J.…Sukasem C. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Frontiers in Pharmacology. 2017;8:813–817. doi: 10.3389/fphar.2017.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalec K., Wright G.E.B., Drögemöller B.I., Aminkeng F., Bhavsar A.P., Kingwell E.…Carleton B.C. Common variation near IRF6 is associated with IFN-β-induced liver injury in multiple sclerosis. Nature Genetics. 2018;50:1081–1085. doi: 10.1038/s41588-018-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozyra M., Ingelman-Sundberg M., Lauschke V.M. Rare genetic variants in cellular transporters, metabolic enzymes, and nuclear receptors can be important determinants of interindividual differences in drug response. Genetics in Medicine. 2017;19:20–29. doi: 10.1038/gim.2016.33. [DOI] [PubMed] [Google Scholar]
- Krieger N., Jahn J.L. Tumor Specimen Biobanks: Data Gaps for Analyzing Health Inequities—the Case of Breast Cancer. JNCI Cancer Spectrum. 2018;2 doi: 10.1093/jncics/pky011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkantrakorn K., Tassaneeyakul W., Tiamkao S., Jantararoungtong T., Prabmechai N., Vannaprasaht S.…Sritipsukho P. HLA-B*1502 strongly predicts carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Thai patients with neuropathic pain. Pain Practice. 2012;12:202–208. doi: 10.1111/j.1533-2500.2011.00479.x. [DOI] [PubMed] [Google Scholar]
- Kurdyukov S., Bullock M. DNA Methylation Analysis: Choosing the right Method. Biology. 2016;5:3–21. doi: 10.3390/biology5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutszegi N., Semsei A.F., Gézsi A., Sági J.C., Nagy V., Csordás K.…Szalai C. Subgroups of paediatric acute lymphoblastic leukaemia might differ significantly in genetic predisposition to asparaginase hypersensitivity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140136. e0140136–0140112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutszegi N., Yang X., Gézsi A., Schermann G., Erdelyi D.J., Semsei A.F.…Szalai C. HLA-DRB1*07:01-HLA-DQA1*02:01-HLA-DQB1*02:02 haplotype is associated with a high risk of asparaginase hypersensitivity in acute lymphoblastic leukemia. Haematologica. 2017;102:1578–1586. doi: 10.3324/haematol.2017.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmichev A., Nishioka K., Erdjument-Bromage H., Tempst P., Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes and Development. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P.K.L., Ng M.H.L., Lo S.V. Association between HLA-B*15:02 allele and antiepileptic drug-induced severe cutaneous reactions in Hong Kong Chinese: a population-based study. Hong Kong Medical Journal. 2014;20(Suppl. 7):16–18. [PubMed] [Google Scholar]
- Laszlo A.H., Derrington I.M., Brinkerhoff H., Langford K.W., Nova I.C., Samson J.M.…Gundlach J.H. Detection and mapping of 5-methylcytosine and 5-hydroxymethylcytosine with nanopore MspA. Proceedings of the National Academy of Sciences. 2013;110:18904–18909. doi: 10.1073/pnas.1310240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauschke V.M., Barragan I., Ingelman-Sundberg M. Pharmacoepigenetics and Toxicoepigenetics: Novel Mechanistic Insights and Therapeutic Opportunities. Annual Review of Pharmacology and Toxicology. 2018;58:161–185. doi: 10.1146/annurev-pharmtox-010617-053021. [DOI] [PubMed] [Google Scholar]
- Lauschke V.M., Ingelman-Sundberg M. The importance of patient-specific factors for hepatic drug response and toxicity. International Journal of Molecular Sciences. 2016;17 doi: 10.3390/ijms17101714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauschke V.M., Ingelman-Sundberg M. Precision Medicine and rare Genetic Variants. Trends in Pharmacological Sciences. 2016;37:85–86. doi: 10.1016/j.tips.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Lauschke V.M., Ingelman-Sundberg M. Requirements for comprehensive pharmacogenetic genotyping platforms. Pharmacogenomics. 2016;17:917–924. doi: 10.2217/pgs-2016-0023. [DOI] [PubMed] [Google Scholar]
- Lauschke V.M., Ingelman-Sundberg M. How to consider rare genetic variants in personalized drug therapy. Clinical Pharmacology and Therapeutics. 2018;103:745–748. doi: 10.1002/cpt.976. [DOI] [PubMed] [Google Scholar]
- Lauschke V.M., Ivanov M., Ingelman-Sundberg M. Pitfalls and opportunities for epigenomic analyses focused on disease diagnosis, prognosis, and therapy. Trends in Pharmacological Sciences. 2017;38:765–770. doi: 10.1016/j.tips.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Lauschke V.M., Milani L., Ingelman-Sundberg M. pharmacogenomic biomarkers for improved drug therapy-recent progress and future developments. The AAPS Journal. 2017;20:4. doi: 10.1208/s12248-017-0161-x. [DOI] [PubMed] [Google Scholar]
- Lavoie Smith E.M., Li L., Chiang C., Thomas K., Hutchinson R.J., Wells E.M.…Renbarger J. Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Journal of the Peripheral Nervous System. 2015;20:37–46. doi: 10.1111/jns.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M., Daujat S., Schneider R. Lateral Thinking: How Histone modifications Regulate Gene Expression. Trends in Genetics. 2016;32:42–56. doi: 10.1016/j.tig.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Wray N.R., Goddard M.E., Visscher P.M. Estimating missing heritability for disease from genome-wide association studies. American Journal of Human Genetics. 2011;88:294–305. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsalu L., Alavere H., Tammesoo M.-L., Leego E., Metspalu A. Linking a population biobank with national health registries-the estonian experience. Journal of Personalized Medicine. 2015;5:96–106. doi: 10.3390/jpm5020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L. Implementation of TPMT testing. British Journal of Clinical Pharmacology. 2014;77:704–714. doi: 10.1111/bcp.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesko L.J., Schmidt S. Individualization of drug therapy: History, present state, and opportunities for the future. Clinical Pharmacology & Therapeutics. 2012;92:458–466. doi: 10.1038/clpt.2012.113. [DOI] [PubMed] [Google Scholar]
- Li L.-J., Hu F.-Y., Wu X.-T., An D.-M., Yan B., Zhou D. Predictive markers for carbamazepine and lamotrigine-induced maculopapular exanthema in Han Chinese. Epilepsy Research. 2013;106:296–300. doi: 10.1016/j.eplepsyres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Li S., Wong E.M., Bui M., Nguyen T.L., Joo J.-H.E., Stone J.…Hopper J.L. Inference about causation between body mass index and DNA methylation in blood from a twin family study. International Journal of Obesity. 2018:1–10. doi: 10.1038/s41366-018-0103-4. [DOI] [PubMed] [Google Scholar]
- Li Y., Cui Y., Hart S.N., Klaassen C.D., Zhong X.b. Dynamic patterns of histone methylation are associated with ontogenic expression of the Cyp3a genes during mouse liver maturation. Molecular Pharmacology. 2009;75:1171–1179. doi: 10.1124/mol.108.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G., Lin J.C.Y., Wei V., Yoo C., Cheng J.C., Nguyen C.T.…Jones P.A. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proceedings of the National Academy of Sciences. 2004;101:7357–7362. doi: 10.1073/pnas.0401866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likanonsakul S., Rattanatham T., Feangvad S., Uttayamakul S., Prasithsirikul W., Tunthanathip P.…Shioda T. HLA-Cw*04 allele associated with nevirapine-induced rash in HIV-infected Thai patients. AIDS Research and Therapy. 2009;6:22. doi: 10.1186/1742-6405-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz S.E., Lipsitz S.R., Kutok J.L., Miller T.L., Colan S.D., Neuberg D.S.…Silverman L.B. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer. 2013;119:3555–3562. doi: 10.1002/cncr.28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littera R., Carcassi C., Masala A., Piano P., Serra P., Ortu F.…Manconi P.E. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS. 2006;20:1621–1626. doi: 10.1097/01.aids.0000238408.82947.09. [DOI] [PubMed] [Google Scholar]
- Liu C., Kawedia J.D., Cheng C., Pei D., Fernandez C.A., Cai X.…Relling M.V. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia. 2012;26:2303–2309. doi: 10.1038/leu.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locharernkul C., Loplumlert J., Limotai C., Korkij W., Desudchit T., Tongkobpetch S.…Shotelersuk V. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008;49:2087–2091. doi: 10.1111/j.1528-1167.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- Lonjou C., Borot N., Sekula P., Ledger N., Thomas L., Halevy S.…Group, R. S A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenetics and Genomics. 2008;18:99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- Lowe R., Slodkowicz G., Goldman N., Rakyan V.K. The human blood DNA methylome displays a highly distinctive profile compared with other somatic tissues. Epigenetics. 2015;10:274–281. doi: 10.1080/15592294.2014.1003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubieniecka J.M., Graham J., Heffner D., Mottus R., Reid R., Hogge D.…Riggs W.K. A discovery study of daunorubicin induced cardiotoxicity in a sample of acute myeloid leukemia patients prioritizes P450 oxidoreductase polymorphisms as a potential risk factor. Frontiers in Genetics. 2013;4:231. doi: 10.3389/fgene.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubieniecka J.M., Liu J., Heffner D., Graham J., Reid R., Hogge D.…Riggs W.K. Single-nucleotide polymorphisms in aldo-keto and carbonyl reductase genes are not associated with acute cardiotoxicity after daunorubicin chemotherapy. Cancer Epidemiology, Biomarkers and Prevention. 2012;21:2118–2120. doi: 10.1158/1055-9965.EPI-12-1037. [DOI] [PubMed] [Google Scholar]
- Lucena M.I., Molokhia M., Shen Y., Urban T.J., Aithal G.P., Andrade R.J.…Spanish DILI Registry, E. D. D. a. I. S Susceptibility to Amoxicillin-Clavulanate-Induced Liver Injury is Influenced by Multiple HLA Class I and II Alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunnon K., Hannon E., Smith R.G., Dempster E., Wong C., Burrage J.…Mill J. Variation in 5-hydroxymethylcytosine across human cortex and cerebellum. Genome Biology. 2016:1–15. doi: 10.1186/s13059-016-0871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y.-D., Min F.-L., Liao W.-P., He N., Zeng T., Ma D.-H., Shi Y.-W. The association between oxcarbazepine-induced maculopapular eruption and HLA-B alleles in a northern Han Chinese population. BMC Neurology. 2013;13:75. doi: 10.1186/1471-2377-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahgoub A., Idle J.R., Dring L.G., Lancaster R., Smith R.L. Polymorphic hydroxylation of Debrisoquine in man. The Lancet. 1977;2:584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Mallal S., Nolan D., Witt C., Masel G., Martin A.M., Moore C.…Christiansen F.T. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. The Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- Mallal S., Phillips E., Carosi G., Molina J.-M., Workman C., Tomazic J.…Team P.-S. HLA-B*5701 screening for hypersensitivity to abacavir. New England Journal of Medicine. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- Manolio T.A., Collins F.S., Cox N.J., Goldstein D.B., Hindorff L.A., Hunter D.J.…Visscher P.M. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R., Justin N., Ohno K., Sharpe M.L., Son J., Drury W.J.…Gamblin S.J. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461:762–767. doi: 10.1038/nature08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A.M., Nolan D., Gaudieri S., Almeida C.A., Nolan R., James I.…Mallal S. Predisposition to abacavir hypersensitivity conferred by HLA-B*5701 and a haplotypic Hsp70-Hom variant. Proceedings of the National Academy of Sciences. 2004;101:4180–4185. doi: 10.1073/pnas.0307067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A.M., Nolan D., James I., Cameron P., Keller J., Moore C.…Mallal S. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS (London, England) 2005;19:97–99. doi: 10.1097/00002030-200501030-00014. [DOI] [PubMed] [Google Scholar]
- Martin M.A., Hoffman J.M., Freimuth R.R., Klein T.E., Dong B.J., Pirmohamed M.…Kroetz D.L. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing: 2014 Update. Clinical Pharmacology and Therapeutics. 2014;95:499–500. doi: 10.1038/clpt.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattano L.A., Sather H.N., Trigg M.E., Nachman J.B. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: A report from the Children's Cancer Group. Journal of Clinical Oncology. 2000;18:3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- Matthaei J., Brockmöller J., Tzvetkov M.V., Sehrt D., Sachse-Seeboth C., Hjelmborg J.B.…Kerb R. Heritability of metoprolol and torsemide pharmacokinetics. Clinical Pharmacology and Therapeutics. 2015;98:611–621. doi: 10.1002/cpt.258. [DOI] [PubMed] [Google Scholar]
- Matthaei J., Tzvetkov M.V., Gal V., Sachse-Seeboth C., Sehrt D., Hjelmborg J.B.…Brockmöller J. Low heritability in pharmacokinetics of talinolol: a pharmacogenetic twin study on the heritability of the pharmacokinetics of talinolol, a putative probe drug of MDR1 and other membrane transporters. Genome Medicine. 2016:1–12. doi: 10.1186/s13073-016-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack M., Alfirevic A., Bourgeois S., Farrell J.J., Kasperavičiūtė D., Carrington M.…Pirmohamed M. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. New England Journal of Medicine. 2011;364:1134–1143. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megías-Vericat J.E., Montesinos P., Herrero M.J., Moscardó F., Bosó V., Rojas L.…Aliño S.F. Impact of ABC single nucleotide polymorphisms upon the efficacy and toxicity of induction chemotherapy in acute myeloid leukemia. Leukemia and Lymphoma. 2017;58:1197–1206. doi: 10.1080/10428194.2016.1231405. [DOI] [PubMed] [Google Scholar]
- Megías-Vericat J.E., Montesinos P., Herrero M.J., Moscardó F., Bosó V., Rojas L.…Aliño S.F. Impact of NADPH oxidase functional polymorphisms in acute myeloid leukemia induction chemotherapy. The Pharmacogenomics Journal. 2018;18:301–307. doi: 10.1038/tpj.2017.19. [DOI] [PubMed] [Google Scholar]
- Mehta T.Y., Prajapati L.M., Mittal B., Joshi C.G., Sheth J.J., Patel D.B.…Goyal R.K. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian Journal of Dermatology, Venereology and Leprology. 2009;75:579–582. doi: 10.4103/0378-6323.57718. [DOI] [PubMed] [Google Scholar]
- Mendelson M.M., Marioni R.E., Joehanes R., Liu C., Hedman Å.K., Aslibekyan S.…Deary I.J. Association of body mass index with DNA methylation and gene expression in blood cells and relations to cardiometabolic disease: A mendelian randomization approach. PLoS Medicine. 2017;14:e1002215–e1002230. doi: 10.1371/journal.pmed.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merki E., Zamora M., Raya A., Kawakami Y., Wang J., Zhang X.…Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proceedings of the National Academy of Sciences. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.A., Slusher L.B., Vesell E.S. Polymorphism of theophylline metabolism in man. Journal of Clinical Investigation. 1985;75:1415–1425. doi: 10.1172/JCI111843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Furihata T., Chiba K. Functional analysis of GC Box and its CpG methylation in the regulation of CYP1A2 gene expression. Drug Metabolism and Pharmacokinetics. 2009;24:269–276. doi: 10.2133/dmpk.24.269. [DOI] [PubMed] [Google Scholar]
- Mizzi C., Peters B., Mitropoulou C., Mitropoulos K., Katsila T., Agarwal M.R.…Patrinos G.P. Personalized pharmacogenomics profiling using whole-genome sequencing. Pharmacogenomics. 2014;15:1223–1234. doi: 10.2217/pgs.14.102. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt M., Viboud C., Dunant A., Naldi L., Halevy S., Bouwes-Bavinck J.-N.…Flahault A. Stevens-Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. The Journal of Investigative Dermatology. 2008;128:35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- Monshi M.M., Faulkner L., Gibson A., Jenkins R.E., Farrell J., Earnshaw C.J.…Naisbitt D.J. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013;57:727–739. doi: 10.1002/hep.26077. [DOI] [PubMed] [Google Scholar]
- Moon J., Kim T.-J., Lim J.-A., Sunwoo J.-S., Byun J.-I., Lee S.-T.…Lee S.K. HLA-B*40:02 and DRB1*04:03 are risk factors for oxcarbazepine-induced maculopapular eruption. Epilepsia. 2016;57:1879–1886. doi: 10.1111/epi.13566. [DOI] [PubMed] [Google Scholar]
- Moon J., Park H.-K., Chu K., Sunwoo J.-S., Byun J.-I., Lim J.-A.…Lee S.K. The HLA-A*2402/Cw*0102 haplotype is associated with lamotrigine-induced maculopapular eruption in the Korean population. Epilepsia. 2015;56:e161–e167. doi: 10.1111/epi.13087. [DOI] [PubMed] [Google Scholar]
- Moore A.S., Norris R., Price G., Nguyen T., Ni M., George R.…Pinkerton R. Vincristine pharmacodynamics and pharmacogenetics in children with cancer: a limited-sampling, population modelling approach. Journal of Paediatrics and Child Health. 2011;47:875–882. doi: 10.1111/j.1440-1754.2011.02103.x. [DOI] [PubMed] [Google Scholar]
- Mordente A., Meucci E., Silvestrini A., Martorana G.E., Giardina B. New developments in anthracycline-induced cardiotoxicity. Current Medicinal Chemistry. 2009;16:1656–1672. doi: 10.2174/092986709788186228. [DOI] [PubMed] [Google Scholar]
- Mort M., Sterne-Weiler T., Li B., Ball E.V., Cooper D.N., Radivojac P.…Mooney S.D. MutPred Splice: Machine learning-based prediction of exonic variants that disrupt splicing. Genome Biology. 2014;15:R19. doi: 10.1186/gb-2014-15-1-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motsinger A.A., Ritchie M.D., Reif D.M. Novel methods for detecting epistasis in pharmacogenomics studies. Pharmacogenomics. 2007;8:1229–1241. doi: 10.2217/14622416.8.9.1229. [DOI] [PubMed] [Google Scholar]
- Mushiroda T., Takahashi Y., Onuma T., Yamamoto Y., Kamei T., Hoshida T.…For the GENCAT Study Group Association of HLA-A*31:01 screening with the incidence of carbamazepine-induced cutaneous adverse reactions in a Japanese population. JAMA Neurology. 2018;75:842–849. doi: 10.1001/jamaneurol.2018.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C., Bonow R., Palmeri S., Jenkins J., Corden B., Locker G.…Epstein S. A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N-acetylcysteine. Seminars in Oncology. 1983;10:53–55. [PubMed] [Google Scholar]
- Nagai A., Hirata M., Kamatani Y., Muto K., Matsuda K., Kiyohara Y.…Kubo M. Overview of the BioBank Japan project: study design and profile. Journal of Epidemiology. 2017;27:S2–S8. doi: 10.1016/j.je.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.R., Wegmann D., Ehm M.G., Kessner D., St Jean P., Verzilli C.…Mooser V. An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science. 2012;337:100–104. doi: 10.1126/science.1217876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P.C., Henikoff S. Predicting deleterious amino acid substitutions. Genome Research. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D.V., Chu H.C., Nguyen D.V., Phan M.H., Craig T., Baumgart K., van Nunen S. HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in Vietnamese. Asia Pacific Allergy. 2015;5:68–77. doi: 10.5415/apallergy.2015.5.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti P., Aithal G.P., Bjornsson E.S., Andrade R.J., Sawle A., Arrese M.…International Serious Adverse Events Consortium Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152:1078–1089. doi: 10.1053/j.gastro.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti P., Werk A.N., Sawle A., Shen Y., Urban T.J., Coulthard S.A.…On behalf of the Task force ‘Genetic predic- tors of drug hypersensitivity’ of the European Network on Drug Allergy (ENDA) of EAACI HLA-DRB1*16: 01-DQB1*05: 02 is a novel genetic risk factor for flupirtine-induced liver injury. Pharmacogenetics and Genomics. 2016;26:218–224. doi: 10.1097/FPC.0000000000000209. [DOI] [PubMed] [Google Scholar]
- Niihara H., Kakamu T., Fujita Y., Kaneko S., Morita E. HLA-A31 strongly associates with carbamazepine-induced adverse drug reactions but not with carbamazepine-induced lymphocyte proliferation in a Japanese population. The Journal of Dermatology. 2012;39:594–601. doi: 10.1111/j.1346-8138.2011.01457.x. [DOI] [PubMed] [Google Scholar]
- Niihara H., Kaneko S., Ito T., Sugamori T., Takahashi N., Kohno K., Morita E. HLA-B*58:01 strongly associates with allopurinol-induced adverse drug reactions in a Japanese sample population. Journal of Dermatological Science. 2013;71:150–152. doi: 10.1016/j.jdermsci.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Norcross, M. A., Luo, S., Lu, L., Boyne, M. T., Gomarteli, M., Rennels, A. D., Woodcock, J., Margulies, D. H., McMurtrey, C., Vernon, S., Hildebrand, W. H., & Buchli, R. (2012). Abacavir induces loading of novel self-peptides into HLA-B*57: 01: An autoimmune model for HLA-associated drug hypersensitivity. AIDS (London, England), 26, F21-29. [DOI] [PMC free article] [PubMed]
- O'Donohue J., Oien K.A., Donaldson P., Underhill J., Clare M., MacSween R.M., Mills P.R. Co-amoxiclav jaundice: Clinical and histological features and HLA class II association. Gut. 2000;47:717–720. doi: 10.1136/gut.47.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K.E., Han S.T., Sorscher E.J., Cutting G.R. ScienceDirect Transformative therapies for rare CFTR missense alleles. Current Opinion in Pharmacology. 2017;34:76–82. doi: 10.1016/j.coph.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson R.D., Mushlin P.S., Brenner D.E., Fleischer S., Cusack B.J., Chang B.K., Boucek R.J. Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proceedings of the National Academy of Sciences. 1988;85:3585–3589. doi: 10.1073/pnas.85.10.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi S.K.T., Qiu C., Bernstein E., Li K., Jia D., Yang Z.…Bestor T.H. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448:714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega V.E., Meyers D.A. Pharmacogenetics: Implications of race and ethnicity on defining genetic profiles for personalized medicine. Journal of Allergy and Clinical Immunology. 2014;133:16–26. doi: 10.1016/j.jaci.2013.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrov D.A., Grant B.J., Pompeu Y.A., Sidney J., Harndahl M., Southwood S.…Peters B. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proceedings of the National Academy of Sciences. 2012;109:9959–9964. doi: 10.1073/pnas.1207934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani J., Nankumo T., Arita K., Inamoto S., Ariyoshi M., Shirakawa M. Structural basis for recognition of H3K4 methylation status by the DNA methyltransferase 3A ATRXandndash;DNMT3andndash;DNMT3L domain. EMBO Reports. 2009;10:1235–1241. doi: 10.1038/embor.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oussalah A., Mayorga C., Blanca M., Barbaud A., Nakonechna A., Cernadas J.…EAACI, T. f. G. p. o. d. h. o. t. E. N. o. D. A. E. o Genetic variants associated with drugs-induced immediate hypersensitivity reactions: a PRISMA-compliant systematic review. Allergy. 2016;71:443–462. doi: 10.1111/all.12821. [DOI] [PubMed] [Google Scholar]
- Ozeki T., Mushiroda T., Yowang A., Takahashi A., Kubo M., Shirakata Y.…Nakamura Y. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Human Molecular Genetics. 2011;20:1034–1041. doi: 10.1093/hmg/ddq537. [DOI] [PubMed] [Google Scholar]
- Ozkaya-Bayazit E., Akar U. Fixed drug eruption induced by trimethoprim-sulfamethoxazole: Evidence for a link to HLA-A30 B13 Cw6 haplotype. Journal of the American Academy of Dermatology. 2001;45:712–717. doi: 10.1067/mjd.2001.117854. [DOI] [PubMed] [Google Scholar]
- Palikhe N.S., Sin H.J., Kim S.-H., Sin H.J., Hwang E.K., Ye Y.M., Park H.-S. Genetic variability of prostaglandin E2 receptor subtype EP4 gene in aspirin-intolerant chronic urticaria. Journal of Human Genetics. 2012;57:494–499. doi: 10.1038/jhg.2012.55. [DOI] [PubMed] [Google Scholar]
- Pan M.-G., Xiong Y., Chen F. NFAT gene family in inflammation and cancer. Current Molecular Medicine. 2013;13:543–554. doi: 10.2174/1566524011313040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panosyan E.H., Seibel N.L., Martin-Aragon S., Gaynon P.S., Avramis I.A., Sather H.…Avramis V.I. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia – Children's Cancer Group Study CCG-1961. Journal of Pediatric Hematology/Oncology. 2004;26:217–226. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- Park C.S., Kim T.-B., Kim S.L., Kim J.Y., Yang K.A., Bae Y.-J.…Moon H.-B. The use of an electronic medical record system for mandatory reporting of drug hypersensitivity reactions has been shown to improve the management of patients in the university hospital in Korea. Pharmacoepidemiology and Drug Safety. 2008;17:919–925. doi: 10.1002/pds.1612. [DOI] [PubMed] [Google Scholar]
- Park H.-J., Choi Y.-J., Kim J.W., Chun H.-S., Im I., Yoon S.…Kim H. Differences in the epigenetic regulation of cytochrome p450 genes between human embryonic stem cell-derived hepatocytes and primary hepatocytes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132992. e0132992–0132917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlos R., Mallal S., Ostrov D., Buus S., Metushi I., Peters B., Phillips E. T cell-mediated hypersensitivity reactions to drugs. Annual Review of Medicine. 2015;66:439–454. doi: 10.1146/annurev-med-050913-022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlos R., McKinnon E.J., Ostrov D.A., Peters B., Buus S., Koelle D.…Phillips E.J. Shared peptide binding of HLA Class I and II alleles associate with cutaneous nevirapine hypersensitivity and identify novel risk alleles. Scientific Reports. 2017:1–12. doi: 10.1038/s41598-017-08876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano R., Lomuto M., Ciavarella G., DiGiorgio G., Gasparini P. Fixed drug eruptions with feprazone are linked to HLA-B22. Journal of the American Academy of Dermatology. 1997;36:782–784. doi: 10.1016/s0190-9622(97)80347-7. [DOI] [PubMed] [Google Scholar]
- Penno M.B., Dvorchik B.H., Vesell E.S. Genetic variation in rates of antipyrine metabolite formation: a study in uninduced twins. Proceedings of the National Academy of Sciences. 1981;78:5193–5196. doi: 10.1073/pnas.78.8.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis R.H., Smoller J.W., Ferreira M.A.R., McQuillin A., Bass N., Lawrence J.…Purcell S. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. The American Journal of Psychiatry. 2009;166:718–725. doi: 10.1176/appi.ajp.2009.08111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffeneder T., Spada F., Wagner M., Brandmayr C., Laube S.K., Eisen D.…Carell T. Tet oxidizes thymine to 5-hydroxymethyluracil in mouse embryonic stem cell DNA. Nature Chemical Biology. 2014;10:574–581. doi: 10.1038/nchembio.1532. [DOI] [PubMed] [Google Scholar]
- Phillips E., Bartlett J.A., Sanne I., Lederman M.M., Hinkle J., Rousseau F.…Haas D.W. Associations between HLA-DRB1*0102, HLA-B*5801, and hepatotoxicity during initiation of nevirapine-containing regimens in South Africa. Journal of Acquired Immune Deficiency Syndromes (1999) 2013;62:e55–e57. doi: 10.1097/QAI.0b013e31827ca50f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips E.J., Mallal S.A. Pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2010;11:973–987. doi: 10.2217/pgs.10.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips E.J., Mallal S.A. HLA-B*5701 and flucloxacillin associated drug-induced liver disease. AIDS. 2013;27:491–492. doi: 10.1097/QAD.0b013e32835ca9d5. [DOI] [PubMed] [Google Scholar]
- Phillips E.J., Sukasem C., Whirl-Carrillo M., Müller D.J., Dunnenberger H.M., Chantratita W.…Pirmohamed M. Clinical pharmacogenetics implementation consortium guideline for hla genotype and use of carbamazepine and oxcarbazepine: 2017 Update. Clinical Pharmacology and Therapeutics. 2018;103:574–581. doi: 10.1002/cpt.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters R., Hunger S.P., Boos J., Rizzari C., Silverman L., Baruchel A.…Pui C.-H. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117:238–249. doi: 10.1002/cncr.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirmohamed M., Burnside G., Eriksson N., Jorgensen A.L., Toh C.H., Nicholson T.…Wadelius M. A randomized trial of genotype-guided dosing of warfarin. New England Journal of Medicine. 2013;369:2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- Plasschaert S.L.A., Groninger E., Boezen M., Kema I., de Vries E.G.E., Uges D.…de Bont E.S.J.M. Influence of functional polymorphisms of the MDR1 gene on vincristine pharmacokinetics in childhood acute lymphoblastic leukemia. Clinical Pharmacology and Therapeutics. 2004;76:220–229. doi: 10.1016/j.clpt.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Plesa M., Gagné V., Glisovic S., Younan M., Sharif-Askari B., Laverdière C.…Krajinovic M. Influence of BCL2L11 polymorphism on osteonecrosis during treatment of childhood acute lymphoblastic leukemia. The Pharmacogenomics Journal. 2017;22:308. doi: 10.1038/s41397-017-0002-4. [DOI] [PubMed] [Google Scholar]
- Plumpton C.O., Alfirevic A., Pirmohamed M., Hughes D.A. Cost effectiveness analysis of HLA-B*58:01 genotyping prior to initiation of allopurinol for gout. Rheumatology. 2017;56:1729–1739. doi: 10.1093/rheumatology/kex253. [DOI] [PubMed] [Google Scholar]
- Plumpton C.O., Yip V.L.M., Alfirevic A., Marson A.G., Pirmohamed M., Hughes D.A. Cost-effectiveness of screening forHLA-A*31:01 prior to initiation of carbamazepine in epilepsy. Epilepsia. 2015;56:556–563. doi: 10.1111/epi.12937. [DOI] [PubMed] [Google Scholar]
- Press M.O., Carlson K.D., Queitsch C. The overdue promise of short tandem repeat variation for heritability. Trends in Genetics. 2014;30:504–512. doi: 10.1016/j.tig.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilez J., Guilmatre A., Garg P., Highnam G., Gymrek M., Erlich Y.…Sharp A.J. Polymorphic tandem repeats within gene promoters act as modifiers of gene expression and DNA methylation in humans. Nucleic Acids Research. 2016;44:3750–3762. doi: 10.1093/nar/gkw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiralte J., Sánchez-García F., Torres M.J., Blanco C., Castillo R., Ortega N.…Carrillo T. Association of HLA-DR11 with the anaphylactoid reaction caused by nonsteroidal anti-inflammatory drugs. Journal of Allergy and Clinical Immunology. 1999;103:685–689. doi: 10.1016/s0091-6749(99)70243-5. [DOI] [PubMed] [Google Scholar]
- Rahikainen A.-L., Vauhkonen P., Pett H., Palo J.U., Haukka J., Ojanperä I.…Sajantila A. Completed suicides of citalopram users-the role of CYP genotypes and adverse drug interactions. International Journal of Legal Medicine. 2018;150:911–935. doi: 10.1007/s00414-018-1927-0. [DOI] [PubMed] [Google Scholar]
- Raiber E.-A., Murat P., Chirgadze D.Y., Beraldi D., Luisi B.F., Balasubramanian S. 5-Formylcytosine alters the structure of the DNA double helix. Nature Structural and Molecular Biology. 2015;22:44–49. doi: 10.1038/nsmb.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajić V., Aplenc R., Debeljak M., Prestor V.V., Karas-Kuželicki N., Mlinaric-Rašcan I., Jazbec J. Influence of the polymorphism in candidate genes on late cardiac damage in patients treated due to acute leukemia in childhood. Leukemia and Lymphoma. 2009;50:1693–1698. doi: 10.1080/10428190903177212. [DOI] [PubMed] [Google Scholar]
- Rajić V., Debeljak M., Goričar K., Jazbec J. Polymorphisms in GRIA1 gene are a risk factor for asparaginase hypersensitivity during the treatment of childhood acute lymphoblastic leukemia. Leukemia and Lymphoma. 2015;56:3103–3108. doi: 10.3109/10428194.2015.1020802. [DOI] [PubMed] [Google Scholar]
- Ramírez E., Bellón T., Tong H.Y., Borobia A.M., de Abajo F.J., Lerma V.…Frías J. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacological Research. 2017;115:168–178. doi: 10.1016/j.phrs.2016.11.027. [DOI] [PubMed] [Google Scholar]
- Ramsey L.B., Bruun G.H., Yang W., Trevino L.R., Vattathil S., Scheet P.…Relling M.V. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Research. 2012;22:1–8. doi: 10.1101/gr.129668.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand A.C., Jain M., Eizenga J.M., Musselman-Brown A., Olsen H.E., Akeson M., Paten B. Mapping DNA methylation with high-throughput nanopore sequencing. Nature Methods. 2017;14:411–413. doi: 10.1038/nmeth.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A., Nolan D., Martin A., McKinnon E., Almeida C., Mallal S. Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV cohort study. Clinical Infectious Diseases. 2006;43:99–102. doi: 10.1086/504874. [DOI] [PubMed] [Google Scholar]
- Reich D.S., Lucchinetti C.F., Calabresi P.A. Multiple Sclerosis. New England Journal of Medicine. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichwagen A., Ziepert M., Kreuz M., Gödtel-Armbrust U., Rixecker T., Poeschel V.…Wojnowski L. Association of NADPH oxidase polymorphisms with anthracycline-induced cardiotoxicity in the RICOVER-60 trial of patients with aggressive CD20 +B-cell lymphoma. Pharmacogenomics. 2015;16:361–372. doi: 10.2217/pgs.14.179. [DOI] [PubMed] [Google Scholar]
- Reisberg S., Krebs K., Lepamets M., Kals M., Mägi R., Metsalu K.…Milani L. Translating genotype data of 44,000 biobank participants into clinical pharmacogenetic recommendations: Challenges and solutions. Genetics in Medicine. 2018;20:1. doi: 10.1038/s41436-018-0337-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relling M.V., Yang W., Das S., Cook E.H., Rosner G.L., Neel M.…Kaste S.C. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. Journal of Clinical Oncology. 2004;22:3930–3936. doi: 10.1200/JCO.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Relling M.V., Schwab M., Whirl-Carrillo M., Suarez-Kurtz G., Pui C.-H., Stein C.M., Moyer A.M., Evans W.E., Klein T.E., Antillon-Klussmann F.G., Caudle K.E., Kato M., Yeoh A.E.J., Schmiegelow K., Yang J.J. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clinical Pharmacology & Therapeutics. 2018 doi: 10.1002/cpt.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva B., Antipin Y., Sander C. Predicting the functional impact of protein mutations: Application to cancer genomics. Nucleic Acids Research. 2011;39 doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R.C., Sharp G.C., Ward M.E., Fraser A., Lyttleton O., McArdle W.L.…Relton C.L. DNA Methylation and BMI: Investigating Identified Methylation Sites at HIF3Ain a Causal Framework. Diabetes. 2016;65:1231–1244. doi: 10.2337/db15-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie G.R.S., Dunham I., Zeggini E., Flicek P. Functional annotation of noncoding sequence variants. Nature Methods. 2014;11:294–296. doi: 10.1038/nmeth.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Nóvoa S., García-Gascó P., Blanco F., González-Pardo G., Castellares C., Moreno V.…Soriano V. Value of the HLA-B*5701 allele to predict abacavir hypersensitivity in Spaniards. AIDS Research and Human Retroviruses. 2007;23:1374–1376. doi: 10.1089/aid.2006.0244. [DOI] [PubMed] [Google Scholar]
- Romano A., Torres M.J., Castells M., Sanz M.L., Blanca M. Diagnosis and management of drug hypersensitivity reactions. The Journal of Allergy and Clinical Immunology. 2011;127:S67–S73. doi: 10.1016/j.jaci.2010.11.047. [DOI] [PubMed] [Google Scholar]
- Romeih M., Cui J., Michaille J.-J., Jiang W., Zile M.H. Function of RARgamma and RARalpha2 at the initiation of retinoid signaling is essential for avian embryo survival and for distinct events in cardiac morphogenesis. Developmental Dynamics. 2003;228:697–708. doi: 10.1002/dvdy.10419. [DOI] [PubMed] [Google Scholar]
- Roujeau J.-C., Guillaume J.C., Fabre J.P., Penso D., Fléchet M.L., Girre J.P. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981-1985. Archives of Dermatology. 1990;126:37–42. doi: 10.1001/archderm.126.1.37. [DOI] [PubMed] [Google Scholar]
- Ryan B.C., Werner T.S., Howard P.L., Chow R.L. ImiRP: a computational approach to microRNA target site mutation. BMC Bioinformatics. 2016;17:190. doi: 10.1186/s12859-016-1057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzany B., Mockenhaupt M., Baur S., Schröder W., Stocker U., Mueller J.…Schöpf E. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990-1992): Structure and results of a population-based registry. Journal of Clinical Epidemiology. 1996;49:769–773. doi: 10.1016/0895-4356(96)00035-2. [DOI] [PubMed] [Google Scholar]
- Saag M., Balu R., Phillips E., Brachman P., Martorell C., Burman W.…Team, S. o. H. t. A. a. P. E. S. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clinical Infectious Diseases. 2008;46:1111–1118. doi: 10.1086/529382. [DOI] [PubMed] [Google Scholar]
- Sackton T.B., Hartl D.L. Genotypic Context and Epistasis in individuals and Populations. Cell. 2016;166:279–287. doi: 10.1016/j.cell.2016.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., Niemi M., Hiratsuka M., Kumondai M., Ingelman-Sundberg M., Lauschke V.M., Rodríguez-Antona C. Novel copy-number variations in pharmacogenes contribute to interindividual differences in drug pharmacokinetics. Genetics in Medicine. 2018;20:622–629. doi: 10.1038/gim.2017.156. [DOI] [PubMed] [Google Scholar]
- Sawyer L.A., Hennessy J.M., Peixoto A.A., Rosato E., Parkinson H., Costa R., Kyriacou C.P. Natural variation in a Drosophila clock gene and temperature compensation. Science. 1997;278:2117–2120. doi: 10.1126/science.278.5346.2117. [DOI] [PubMed] [Google Scholar]
- Schneider B.P., Shen F., Gardner L., Radovich M., Li L., Miller K.D.…Sledge G.W. Genome-wide association study for anthracycline-induced congestive heart failure. Clinical Cancer Research. 2017;23:43–51. doi: 10.1158/1078-0432.CCR-16-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöpf E., Stühmer A., Rzany B., Victor N., Zentgraf R., Kapp J.F. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Archives of Dermatology. 1991;127:839–842. doi: 10.1001/archderm.1991.01680050083008. [DOI] [PubMed] [Google Scholar]
- Schutsky E.K., DeNizio J.E., Hu P., Liu M.Y., Nabel C.S., Fabyanic E.B.…Kohli R.M. Nondestructive, base-resolution sequencing of 5-hydroxymethylcytosine using a DNA deaminase. Nature Biotechnology. 2018;36:1083–1090. doi: 10.1038/nbt.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scourzic L., Mouly E., Bernard O.A. TET proteins and the control of cytosine demethylation in cancer. Genome Medicine. 2015;7:9. doi: 10.1186/s13073-015-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsei A.F., Erdelyi D.J., Ungvari I., Csagoly E., Hegyi M.Z., Kiszel P.S.…Kovacs G.T. ABCC1 polymorphisms in anthracycline-induced cardiotoxicity in childhood acute lymphoblastic leukaemia. Cell Biology International. 2012;36:79–86. doi: 10.1042/CBI20110264. [DOI] [PubMed] [Google Scholar]
- Shah K.N., Racine J., Jones L.C., Aaron R.K. Pathophysiology and risk factors for osteonecrosis. Current Reviews in Musculoskeletal Medicine. 2015;8:201–209. doi: 10.1007/s12178-015-9277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.-W., Min F.-L., Liu X.-R., Zan L.-X., Gao M.-M., Yu M.-J., Liao W.-P. HLA-B alleles and lamotrigine-induced cutaneous adverse drug reactions in the Han Chinese population. Basic and Clinical Pharmacology and Toxicology. 2011;109:42–46. doi: 10.1111/j.1742-7843.2011.00681.x. [DOI] [PubMed] [Google Scholar]
- Shi Y.-W., Min F.-L., Zhou D., Qin B., Wang J., Hu F.-Y.…Liao W.-P. HLA-A*24:02 as a common risk factor for antiepileptic drug-induced cutaneous adverse reactions. Neurology. 2017;88:2183–2191. doi: 10.1212/WNL.0000000000004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S.C., Kacevska M., Ingelman-Sundberg M. Pharmacogenomics of drug-metabolizing enzymes: a recent update on clinical implications and endogenous effects. The Pharmacogenomics Journal. 2013;13:1–11. doi: 10.1038/tpj.2012.45. [DOI] [PubMed] [Google Scholar]
- Singer J.B., Lewitzky S., Leroy E., Yang F., Zhao X., Klickstein L.…Paulding C.A. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nature Genetics. 2010;42:711–714. doi: 10.1038/ng.632. [DOI] [PubMed] [Google Scholar]
- Soldi M., Bremang M., Bonaldi T. Biochemical systems approaches for the analysis of histone modification readout. Biochimica et Biophysica Acta. 2014;1839:657–668. doi: 10.1016/j.bbagrm.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Spraggs C.F., Budde L.R., Briley L.P., Bing N., Cox C.J., King K.S.…Cardon L.R. HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. Journal of Clinical Oncology. 2011;29:667–673. doi: 10.1200/JCO.2010.31.3197. [DOI] [PubMed] [Google Scholar]
- Spruijt C.G., Gnerlich F., Smits A.H., Pfaffeneder T., Jansen P.W.T.C., Bauer C.…Vermeulen M. Dynamic readers for 5-(Hydroxy)methylcytosine and its oxidized derivatives. Cell. 2013;152:1146–1159. doi: 10.1016/j.cell.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Stage T.B., Damkier P., Pedersen R.S., Christensen M.M.H., Christiansen L., Christensen K., Brøsen K. A twin study of the trough plasma steady-state concentration of metformin. Pharmacogenetics and Genomics. 2015;25:259–262. doi: 10.1097/FPC.0000000000000133. [DOI] [PubMed] [Google Scholar]
- Stekler J., Maenza J., Stevens C., Holte S., Malhotra U., McElrath M.J.…Collier A.C. Abacavir hypersensitivity reaction in primary HIV infection. AIDS. 2006;20:1269–1274. doi: 10.1097/01.aids.0000232234.19006.a2. [DOI] [PubMed] [Google Scholar]
- Sukasem C., Chaichan C., Nakkrut T., Satapornpong P., Jaruthamsophon K., Jantararoungtong T.…Aekplakorn W. Association between HLA-B alleles and carbamazepine-induced maculopapular exanthema and severe cutaneous reactions in Thai patients. Journal of Immunology Research. 2018;2018:2780272. doi: 10.1155/2018/2780272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- Tahiliani M., Koh K.P., Shen Y., Pastor W.A., Bandukwala H., Brudno Y.…Rao A. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL Partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Yanai H., Savitsky D., Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annual Review of Immunology. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- Tang X., Chen S. Epigenetic regulation of cytochrome P450 enzymes and clinical implication. Current Drug Metabolism. 2015;16:86–96. doi: 10.2174/138920021602150713114159. [DOI] [PubMed] [Google Scholar]
- Tasa T., Krebs K., Kals M., Mägi R., Lauschke V.M., Haller T.…Milani L. Genetic variation in the Estonian population: Pharmacogenomics study of adverse drug effects using electronic health records. European Journal of Human Genetics. 2018;38:437. doi: 10.1038/s41431-018-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassaneeyakul W., Jantararoungtong T., Chen P., Lin P.-Y., Tiamkao S., Khunarkornsiri U.…Tassaneeyakul W. Strong association between HLA-B*5801 and allopurinol-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenetics and Genomics. 2009;19:704–709. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- Tassaneeyakul W., Prabmeechai N., Sukasem C., Kongpan T., Konyoung P., Chumworathayi P.…Tassaneeyakul W. Associations between HLA class I and cytochrome P450 2C9 genetic polymorphisms and phenytoin-related severe cutaneous adverse reactions in a Thai population. Pharmacogenetics and Genomics. 2016;26:225–234. doi: 10.1097/FPC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Tassaneeyakul W., Tiamkao S., Jantararoungtong T., Chen P., Lin S.-Y., Chen W.-H.…Yodnopaglaw P. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia. 2010;51:926–930. doi: 10.1111/j.1528-1167.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- Tay C.G., Lee V.W.M., Ong L.C., Goh K.J., Ariffin H., Fong C.Y. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatric Blood & Cancer. 2017;64 doi: 10.1002/pbc.26471. [DOI] [PubMed] [Google Scholar]
- Tempark T., Satapornpong P., Rerknimitr P., Nakkam N., Saksit N., Wattanakrai P.…Sukasem C. Dapsone-induced severe cutaneous adverse drug reactions are strongly linked with HLA-B*13: 01 allele in the Thai population. Pharmacogenetics and Genomics. 2017;27:429–437. doi: 10.1097/FPC.0000000000000306. [DOI] [PubMed] [Google Scholar]
- Tewey K.M., Rowe T.C., Yang L., Halligan B.D., Liu L.F. Adriamycin-Induced Dna damage Mediated by Mammalian Dna Topoisomerase-Ii. Science. 1984;226:466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Thavendiranathan P., Grant A.D., Negishi T., Plana J.C., Popović Z.B., Marwick T.H. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. Journal of the American College of Cardiology. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- Then S.-M., Rani Z.Z.M., Raymond A.A., Ratnaningrum S., Jamal R. Frequency of the HLA-B*1502 allele contributing to carbamazepine-induced hypersensitivity reactions in a cohort of Malaysian epilepsy patients. Asian Pacific Journal of Allergy and Immunology. 2011;29:290–293. [PubMed] [Google Scholar]
- Thong B.Y.-H., Leong K.-P., Tang C.-Y., Chng H.-H. Drug allergy in a general hospital: Results of a novel prospective inpatient reporting system. Annals of allergy, asthma and immunology. Asthma & Immunology. 2003;90:342–347. doi: 10.1016/S1081-1206(10)61804-2. [DOI] [PubMed] [Google Scholar]
- To K.K.W., Polgar O., Huff L.M., Morisaki K., Bates S.E. Histone modifications at the ABCG2 promoter following treatment with histone deacetylase inhibitor mirror those in multidrug-resistant cells. Molecular Cancer Research. 2008;6:151–164. doi: 10.1158/1541-7786.MCR-07-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travica S., Pors K., Loadman P.M., Shnyder S.D., Johansson I., Alandas M.N.…Ingelman-Sundberg M. Colon cancer-specific cytochrome P450 2W1 converts duocarmycin analogues into potent tumor cytotoxins. Clinical Cancer Research. 2013;19:2952–2961. doi: 10.1158/1078-0432.CCR-13-0238. [DOI] [PubMed] [Google Scholar]
- Tremlett H.L., Yoshida E.M., Oger J. Liver injury associated with the beta-interferons for MS - A comparison between the three products. Neurology. 2004;62:628–631. doi: 10.1212/wnl.62.4.628. [DOI] [PubMed] [Google Scholar]
- Undurraga S.F., Press M.O., Legendre M., Bujdoso N., Bale J., Wang H.…Queitsch C. Background-dependent effects of polyglutamine variation in the Arabidopsis thaliana gene ELF3. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19363–19367. doi: 10.1073/pnas.1211021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban T.J., Nicoletti P., Chalasani N., Serrano J., Stolz A., Daly A.K.…(iSAEC), I. S. A. E. C Minocycline hepatotoxicity: Clinical characterization and identification of HLA-B∗35:02 as a risk factor. Journal of Hepatology. 2017;67:137–144. doi: 10.1016/j.jhep.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T., Tailor A., Faulkner L., Meng X., Farrell J., Daly A.K.…Naisbitt D.J. HLA-A*33:03-restricted activation of ticlopidine-specific t-cells from human donors. Chemical Research in Toxicology. 2018;31:1022–1024. doi: 10.1021/acs.chemrestox.8b00163. (acs.chemrestox.8b00163) [DOI] [PubMed] [Google Scholar]
- Vaught J., Kelly A., Hewitt R. A review of international biobanks and networks: Success factors and key benchmarks. Biopreservation and Biobanking. 2009;7:143–150. doi: 10.1089/bio.2010.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstappen C.C., Koeppen S., Heimans J.J., Huijgens P.C., Scheulen E., Strumberg D.…Postma T.J. Dose-related vincristine-induced peripheral neuropathy with unexpected off-therapy worsening. Neurology. 2005;64:1076–1077. doi: 10.1212/01.WNL.0000154642.45474.28. [DOI] [PubMed] [Google Scholar]
- Vesell E.S., Page J.G. Genetic control of drug levels in man - antipyrine. Science. 1968;161:72–73. doi: 10.1126/science.161.3836.72. [DOI] [PubMed] [Google Scholar]
- Vesell E.S., Page J.G. Genetic control of dicumarol levels in man. Journal of Clinical Investigation. 1968;47:2657–2663. doi: 10.1172/JCI105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesell E.S., Page J.G. Genetic Control of Drug Levels in Man - Phenylbutazone. Science. 1968;159:1479–1480. doi: 10.1126/science.159.3822.1479. [DOI] [PubMed] [Google Scholar]
- Vidal C., Porras-Hurtado L., Cruz R., Quiralte J., Cardona V., Colás C.…Carracedo A. Association of thromboxane A1 synthase (TBXAS1) gene polymorphism with acute urticaria induced by nonsteroidal anti-inflammatory drugs. The Journal of Allergy and Clinical Immunology. 2013;132:989–991. doi: 10.1016/j.jaci.2013.04.045. [DOI] [PubMed] [Google Scholar]
- Visscher H., Rassekh S.R., Sandor G.S., Caron H.N., van Dalen E.C., Kremer L.C.…CPNDS Consortium Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children. Pharmacogenomics. 2015;16:1065–1076. doi: 10.2217/pgs.15.61. [DOI] [PubMed] [Google Scholar]
- Visscher H., Ross C.J.D., Rassekh S.R., Barhdadi A., Dubé M.-P., Al-Saloos H.…Canadian Pharmacogenomics Network for Drug Safety Consortium Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. Journal of Clinical Oncology. 2012;30:1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- Visscher H., Ross C.J.D., Rassekh S.R., Sandor G.S.S., Caron H.N., van Dalen E.C.…CPNDS Consortium Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatric Blood & Cancer. 2013;60:1375–1381. doi: 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]
- Vitezica Z.G., Milpied B., Lonjou C., Borot N., Ledger T.N., Lefebvre A., Hovnanian A. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008;22:540–541. doi: 10.1097/QAD.0b013e3282f37812. [DOI] [PubMed] [Google Scholar]
- Vivenza D., Feola M., Garrone O., Monteverde M., Merlano M., Lo Nigro C. Role of the renin-angiotensin-aldosterone system and the glutathione S-transferase Mu, Pi and Theta gene polymorphisms in cardiotoxicity after anthracycline chemotherapy for breast carcinoma. The International Journal of Biological Markers. 2013;28:e336–e347. doi: 10.5301/jbm.5000041. [DOI] [PubMed] [Google Scholar]
- Volkan-Salanci B., Aksoy H., Kiratli P.Ö., Tülümen E., Güler N., Öksüzoglu B.…Alikaşifoğlu M. The relationship between changes in functional cardiac parameters following anthracycline therapy and carbonyl reductase 3 and glutathione S transferase Pi polymorphisms. Journal of Chemotherapy. 2012;24:285–291. doi: 10.1179/1973947812Y.0000000037. [DOI] [PubMed] [Google Scholar]
- Volpi S., Bult C.J., Chisholm R.L., Deverka P.A., Ginsburg G.S., Jacob H.J.…Relling M.V. Research directions in the clinical implementation of pharmacogenomics: An overview of us programs and projects. Clinical Pharmacology amd Therapeutics. 2018;103:778–786. doi: 10.1002/cpt.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulsteke C., Pfeil A.M., Maggen C., Schwenkglenks M., Pettengell R., Szucs T.D.…Wildiers H. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Research and Treatment. 2015;152:67–76. doi: 10.1007/s10549-015-3437-9. [DOI] [PubMed] [Google Scholar]
- Wahl S., Drong A., Lehne B., Loh M., Scott W.R., Kunze S.…Chambers J.C. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–86. doi: 10.1038/nature20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yan L., Zhang G., Chen X., Yang J., Li M.…Wang B. Association between HLA-B*1301 and dapsone-induced hypersensitivity reactions among leprosy patients in China. The Journal of Investigative Dermatology. 2013;133:2642–2644. doi: 10.1038/jid.2013.192. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhou J.-Q., Zhou L.-M., Chen Z.-Y., Fang Z.-Y., Chen S.-D.…Wang H.-X. Association between HLA-B*1502 allele and carbamazepine-induced severe cutaneous adverse reactions in Han people of southern China mainland. Seizure. 2011;20:446–448. doi: 10.1016/j.seizure.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Wang X., Liu W., Sun C.-L., Armenian S.H., Hakonarson H., Hageman L.…Bhatia S. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: a report from the children’s oncology group. Journal of Clinical Oncology. 2014;32:647–653. doi: 10.1200/JCO.2013.50.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Sun C.-L., Quiñones-Lombraña A., Singh P., Landier W., Hageman L.…Bhatia S. CELF4 Variant and Anthracycline-Related Cardiomyopathy: A Children’s Oncology Group Genome-Wide Association Study. Journal of Clinical Oncology. 2016;34:863–870. doi: 10.1200/JCO.2015.63.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle, F. C., & Tan, H. (2015). A ChIP on the shoulder? Chromatin immunoprecipitation and validation strategies for ChIP antibodies. F1000Research, 4, 235. [DOI] [PMC free article] [PubMed]
- Watkins S., Pichler W.J. Sulfamethoxazole induces a switch mechanism in T cell receptors containing TCRVβ20-1, altering pHLA recognition. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler M.E., Kunselman S.J., Chinchilli V.M., Bleecker E., Boushey H.A., Calhoun W.J.…Blood Institute’s Asthma Clinical Research Network Effect of beta2-adrenergic receptor polymorphism on response to longacting beta2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial. Lancet. 2009;374:1754–1764. doi: 10.1016/S0140-6736(09)61492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler M.E., Yawn B.P., Fuhlbrigge A.L., Pace W.D., Pencina M.J., Doros G.…BELT Investigators Anticholinergic vs long-acting β-agonist in combination with inhaled corticosteroids in black adults with asthma: The belt randomized clinical trial. JAMA. 2015;314:1720–1730. doi: 10.1001/jama.2015.13277. [DOI] [PubMed] [Google Scholar]
- Wei C.-Y., Chung W.-H., Huang H.-W., Chen Y.-T., Hung S.-I. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. The Journal of Allergy and Clinical Immunology. 2012;129:1562–1569.e1565. doi: 10.1016/j.jaci.2011.12.990. [DOI] [PubMed] [Google Scholar]
- Weigmann B., Lehr H.A., Yancopoulos G., Valenzuela D., Murphy A., Stevens S.…Neurath M.F. The transcription factor NFATc2 controls IL-6-dependent T cell activation in experimental colitis. The Journal of Experimental Medicine. 2008;205:2099–2110. doi: 10.1084/jem.20072484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wescoe Z.L., Schreiber J., Akeson M. Nanopores discriminate among five C5-cytosine variants in DNA. Journal of the American Chemical Society. 2014;136:16582–16587. doi: 10.1021/ja508527b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor R.E., Strauss S.J., Kallis C., Wood N.E., Whelan J.S. Germline genetic polymorphisms may influence chemotherapy response and disease outcome in osteosarcoma: a pilot study. Cancer. 2012;118:1856–1867. doi: 10.1002/cncr.26472. [DOI] [PubMed] [Google Scholar]
- te Winkel M.L., Pieters R., Hop W.C.J., de Groot-Kruseman H.A., Lequin M.H., van der Sluis I.M.…van den Heuvel-Eibrink M.M. Prospective study on incidence, risk factors, and long-term outcome of osteonecrosis in pediatric acute lymphoblastic leukemia. Journal of Clinical Oncology. 2011;29:4143–4150. doi: 10.1200/JCO.2011.37.3217. [DOI] [PubMed] [Google Scholar]
- Wojnowski L., Kulle B., Schirmer M., Schlüter G., Schmidt A., Rosenberger A.…Hasenfuss G. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- Wolf E., Blankenburg M., Bogner J.R., Becker W., Gorriahn D., Mueller M.C.…Stoll M. Cost impact of prospective Hla-B*5701-Screening prior to abacavir/lamivudine fixed dose combination use in Germany. European Journal of Medical Research. 2010;15:145–151. doi: 10.1186/2047-783X-15-4-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfe A., Mullikin J.C., Elnitski L. Genomic features defining exonic variants that modulate splicing. Genome Biology. 2010;11 doi: 10.1186/gb-2010-11-2-r20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G.E.B., Carleton B., Hayden M.R., Ross C.J.D. The global spectrum of protein-coding pharmacogenomic diversity. The Pharmacogenomics Journal. 2018;18:187–195. doi: 10.1038/tpj.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zhang Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nature Reviews Genetics. 2017;18:517–534. doi: 10.1038/nrg.2017.33. [DOI] [PubMed] [Google Scholar]
- Wu X.T., Hu F.Y., An D.M., Yan B., Jiang X., Kwan P.…Zhou D. Association between carbamazepine-induced cutaneous adverse drug reactions and the HLA-B*1502 allele among patients in central China. Epilepsy and Behavior: E and B. 2010;19:405–408. doi: 10.1016/j.yebeh.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Xavier-Neto J., Costa N.M.S., Figueira A.C.M., Caiaffa C.D., do Amaral F.N., Peres L.M.C.…Castillo H.A. Signaling through retinoic acid receptors in cardiac development: Doing the right things at the right times. BBA - Gene Regulatory Mechanisms. 2015;1849:94–111. doi: 10.1016/j.bbagrm.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Ke S., Ouyang N., He J., Xie W., Bedford M.T., Tian Y. Epigenetic Regulation of Transcriptional activity of Pregnane X Receptor by Protein Arginine Methyltransferase 1. The Journal of Biological Chemistry. 2009;284:9199–9205. doi: 10.1074/jbc.M806193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane Y., Aihara M., Ikezawa Z. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to 2006. Allergology International. 2007;56:419–425. doi: 10.2332/allergolint.O-07-483. [DOI] [PubMed] [Google Scholar]
- Yan L., Wang Y., Liu J., Nie Y., Zhong X.-B., Kan Q., Zhang L. Alterations of histone modifications contribute to pregnane X receptor-mediated induction of CYP3A4 by rifampicin. Molecular Pharmacology. 2017;92:113–123. doi: 10.1124/mol.117.108225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Xuan J., Chen J., Zhong H., Luo H., Zhou P.…Xing Q. HLA-B*59:01: a marker for Stevens–Johnson syndrome/toxic epidermal necrolysis caused by methazolamide in Han Chinese. The Pharmacogenomics Journal. 2015;16:83–87. doi: 10.1038/tpj.2015.25. [DOI] [PubMed] [Google Scholar]
- Yip V.L., Marson A.G., Jorgensen A.L., Pirmohamed M., Alfirevic A. HLA genotype and carbamazepine-induced cutaneous adverse drug reactions: a systematic review. Clinical Pharmacology and Therapeutics. 2012;92:757–765. doi: 10.1038/clpt.2012.189. [DOI] [PubMed] [Google Scholar]
- Yong W.-S., Hsu F.-M., Chen P.-Y. Profiling genome-wide DNA methylation. Epigenetics & Chromatin. 2016:1–16. doi: 10.1186/s13072-016-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Hon G.C., Szulwach K.E., Song C.-X., Zhang L., Kim A.…He C. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Guo S., Hall D., Cammett A.M., Jayadev S., Distel M.…the Nevirapine Toxicogenomics Study Team Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS. 2011;25:1271–1280. doi: 10.1097/QAD.0b013e32834779df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J., Marcaida M.J., Eriksson K.K., Jamin H., Fontana S., Pichler W.J., Yerly D. Oxypurinol directly and immediately activates the drug-specific T cells via the preferential use of HLA-B*58:01. Journal of Immunology. 2014;192:2984–2993. doi: 10.4049/jimmunol.1302306. [DOI] [PubMed] [Google Scholar]
- Yun S.-I., Yoon H.-Y., Jeong S.-Y., Chung Y.-S. Glucocorticoid induces apoptosis of osteoblast cells through the activation of glycogen synthase kinase 3β. Journal of Bone and Mineral Metabolism. 2008;27:140–148. doi: 10.1007/s00774-008-0019-5. [DOI] [PubMed] [Google Scholar]
- Yunis J.J., Corzo D., Salazar M., Lieberman J.A., Howard A., Yunis E.J. HLA associations in clozapine-induced agranulocytosis. Blood. 1995;86:1177–1183. [PubMed] [Google Scholar]
- Zambuzzi W.F., Granjeiro J.M., Parikh K., Yuvaraj S., Peppelenbosch M.P., Ferreira C.V. Modulation of Src activity by Low Molecular Weight Protein Tyrosine Phosphatase during Osteoblast Differentiation. Cellular Physiology and Biochemistry. 2008;22:497–506. doi: 10.1159/000185506. [DOI] [PubMed] [Google Scholar]
- Zhang B., Lauschke V.M. Genetic variability and population diversity of the human SLCO (OATP) transporter family. Pharmacological Research. 2018 doi: 10.1016/j.phrs.2018.10.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zhang F., Lupski J.R. Non-coding genetic variants in human disease. Human Molecular Genetics. 2015;24:R102–R110. doi: 10.1093/hmg/ddv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.R., Liu H., Irwanto A., Fu X.A., Li Y., Yu G.Q.…Liu J.J. HLA-B*13:01and the dapsone hypersensitivity syndrome. New England Journal of Medicine. 2013;369:1620–1628. doi: 10.1056/NEJMoa1213096. [DOI] [PubMed] [Google Scholar]
- Zhang S., Liu X., Bawa-Khalfe T., Lu L.-S., Lyu Y.L., Liu L.F., Yeh E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nature Medicine. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- Zhang T., Cooper S., Brockdorff N. The interplay of histone modifications - writers that read. EMBO Reports. 2015;16:1467–1481. doi: 10.15252/embr.201540945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Xu J. DNA methyltransferases and their roles in tumorigenesis. Biomarker Research. 2017;5:1. doi: 10.1186/s40364-017-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Lin H., Zhao H., Hao Y., Mort M., Cooper D.N.…Liu Y. Impact of human pathogenic micro-insertions and micro-deletions on post-transcriptional regulation. Human Molecular Genetics. 2014;23:3024–3034. doi: 10.1093/hmg/ddu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lv J., Liu H., Zhu J., Su J., Wu Q.…Li X. HHMD: The human histone modification database. Nucleic Acids Research. 2010;38:D149–D154. doi: 10.1093/nar/gkp968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang J., Zhao L.-M., Peng W., Shen G.-Q., Xue L.…HE, X.-J., Gong, C.-Y., & Miao, L.-Y. Strong association between HLA-B*1502 and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in mainland Han Chinese patients. European Journal of Clinical Pharmacology. 2011;67:885–887. doi: 10.1007/s00228-011-1009-4. [DOI] [PubMed] [Google Scholar]
- Zhou B., Honor L.B., He H., Ma Q., Oh J.-H., Butterfield C.…Pu W.T. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. Journal of Clinical Investigation. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fujikura K., Mkrtchian S., Lauschke V.M. Computational methods for the pharmacogenetic interpretation of Next Generation Sequencing data. Frontiers in Pharmacology. 2018 doi: 10.3389/fphar.2018.01437. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Ingelman-Sundberg M., Lauschke V.M. Worldwide distribution of cytochrome P450 Alleles: A meta-analysis of population-scale sequencing projects. Clinical Pharmacology and Therapeutics. 2017;102:688–700. doi: 10.1002/cpt.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Lauschke V.M. Comprehensive overview of the pharmacogenetic diversity in Ashkenazi Jews. Journal of Medical Genetics. 2018;55:617–627. doi: 10.1136/jmedgenet-2018-105429. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Mkrtchian S., Kumondai M., Hiratsuka M., Lauschke V.M. An optimized prediction framework to assess the functional impact of pharmacogenetic variants. The Pharmacogenomics Journal. 2018;28:1. doi: 10.1038/s41397-018-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]