Abstract
Introduction
The use of betel quid is the most understudied major addiction in the world. The neuropsychological activity of betel quid has been attributed to alkaloids of Areca catechu. With the goal of developing novel addiction treatments, we evaluate the muscarinic and nicotinic activity of the four major Areca alkaloids: arecoline, arecaidine, guvacoline, and guvacine and four structurally related compounds.
Methods
Acetylcholine receptors were expressed in Xenopus oocytes and studied with two-electrode voltage clamp.
Results
Both arecoline- and guvacoline-activated muscarinic acetylcholine receptors (mAChR), while only arecoline produced significant activation of nicotinic AChR (nAChR). We characterized four additional arecoline-related compounds, seeking an analog that would retain selective activity for a α4* nAChR, with diminished effects on mAChR and not be a desensitizer of α7 nAChR. We show that this profile is largely met by isoarecolone. Three additional arecoline analogs were characterized. While the quaternary dimethyl analog had a broad range of activities, including activation of mAChR and muscle-type nAChR, the methyl analog only activated a range of α4* nAChR, albeit with low potency. The ethyl analog had no detectable cholinergic activity.
Conclusions
Evidence indicates that α4* nAChR are at the root of nicotine addiction, and this may also be the case for betel addiction. Our characterization of isoarecolone and 1-(4-methylpiperazin-1-yl) ethanone as truly selective α4*nAChR selective partial agonists with low muscarinic activity may point toward a promising new direction for the development of drugs to treat both nicotine and betel addiction.
Implications
Nearly 600 million people use Areca nut, often with tobacco. Two of the Areca alkaloids are muscarinic acetylcholine receptor agonists, and one, arecoline, is a partial agonist for the α4* nicotinic acetylcholine receptors (nAChR) associated with tobacco addiction. The profile of arecoline activity suggested its potential to be used as a scaffold for developing new tobacco cessation drugs if analogs can be identified that retain the same nicotinic receptor selectivity without muscarinic activity. We report that isoarecolone is a selective partial agonist for α4* nAChR with minimal muscarinic activity and 1-(4-methylpiperazin-1-yl) ethanone has similar nAChR selectivity and no detectable muscarinic action.
Introduction
Betel quids are used by as many as 600 million people in Asia; worldwide they are the fourth most widely used addictive substance, after alcohol, caffeine, and nicotine.1 The primary psychoactive agent in betel quid preparations is believed to be arecoline, from the fruit of the Areca catechu palm.2 In the betel quid Areca nut fragments are wrapped in a leaf of the Piper betle vine with slaked lime, other flavorants, and often tobacco. Although Areca nut users are classifiable as drug dependent3–5 and pay a cost in their personal health with high risk for cancers6–9 and other oral disease,10 the root cause for their addiction has been unclear. It is well known that there are two aspects to drug-taking behavior: short-term reward or euphoria, and ultimately the neurological change that leads to dependence.11 It is likely that for Areca nut users, the short-term euphoric/stimulant effects may be attributed to the muscarinic activity of the Areca alkaloid, arecoline.12 However, the pharmacology of the Areca nut is indeed rich,13,14 rendering it and its key active component arecoline as leads for refinement to minimize undesired pharmacological effects and toxicity.
There are five subtypes of G-protein-coupled muscarinic acetylcholine receptors (mAChR) which mediate the autonomic responses of the parasympathetic nervous system, including the profuse salivation associated with betel quid use. Muscarinic receptors are also important for various aspects of brain function including cognition and memory.15 While muscarine and arecoline are the classical agonists for G-protein acetylcholine receptors, nicotine is the prototypical agonist for a second major class of acetylcholine receptors that are directly coupled to the activation of intrinsic ion channels. These nicotinic acetylcholine receptors (nAChR) mediate synaptic transmission in the periphery and have multiple neuromodulatory actions in the brain. In humans there are nineteen different genes coding for nicotinic acetylcholine subunits, and receptors form as pentameric assemblies of these subunits. Most nAChR are heteromers, containing at least one type of alpha subunit and at least one type of nonalpha subunit,16 although one important nAChR found in both neuronal and non-neuronal cells appears to form as homopentamers of the α7 subunit (for review, see Papke17). The tissue localization, functional, and pharmacological properties of different nAChR depend on the specific subunit composition. Animal models of nicotine addiction have shown that this form of drug use requires receptors contain α4 and/or α6 subunits.18–20 These receptors are sometimes referred to as α4* and α6* nAChR, with the asterisk indicating that they include these subunits along with others such as β2, and β3, and may include both α4 and α6 subunits.20
The muscarinic manifestations of Areca use have long been appreciated, yet a common link in molecular substrates between Areca use and nicotine dependence has only recently been identified.21 Specifically, it was found that arecoline is a partial agonist for α4* and α6* nAChR subtypes. In this regard, arecoline is similar to the smoking cessation drugs cytisine and varenicline,22,23 although it’s efficacy is lower, probably too low to have direct effects on dopamine release as nicotine does, and it has a different spectrum of putative off-target activity.21
While α4* and α6* nAChR can be identified as important molecular targets for the management of nicotine dependence, effects at other nAChR may produce side effects. Chief among the off-target nAChR for smoking cessation therapies are muscle-type α1* receptors, ganglionic α3*, and homomeric α7 receptors. Although cytisine and varenicline are inactive at muscle-type nAChR, they are efficacious activators of α3β4 and α7 receptors24,25 as well as some serotonin receptors.26 In contrast, although arecoline lacks activity on off-target nAChR, as noted, it is a very effective activator of G-protein-coupled mAChR.
Considering the natural history of Areca nut addiction, the discovery of the previously unknown nicotinic partial agonist activity of an Areca nut alkaloid has created an opportunity to investigate an alternative scaffold for drug development that might be useful for managing nicotine addiction and dependence. The potential for further development of such a scaffold is supported by previous behavioral characterization of the related compound, isoarecolone.27 In the present study, we more fully characterize the acetylcholine receptor activity of arecoline and isoarecolone on nicotinic and muscarinic receptors and additionally investigate the pharmacological profile of the other known Areca alkaloids, arecaidine, guvacoline, and guvacine. These compounds have received relatively little attention in the scientific literature. Guvacoline has been reported to be a muscarinic agonist, but less potent than arecoline.28 Arecaidine has no known activity but has been used as a scaffold for developing M2 mAChR selective agonists.29 Likewise, cholinergic activity has not been reported for guvacine, which is known to be a GABA transport inhibitor.30
Our ultimate goal is to further evaluate the potential for the use of these compounds as scaffolds to develop additional compounds and to determine if balance between nicotinic and muscarinic activity could be tipped based on structural modifications toward retaining partial agonist activity for nAChR subtypes relevant to human nicotine addiction while reducing muscarinic receptor activity.
Methods
The supplementary information includes sections devoted to “Chemicals chemicals and reagents, organic synthesis, and heterologous expression of AChRs in Xenopus laevis oocytes.”
Two-Electrode Voltage Clamp Electrophysiology
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Union City, CA) as previously described.31
Nicotinic AChR Experiments
A typical recording for each set of oocytes constituted two initial control applications of ACh, one or more experimental compound applications, and then a follow-up control application(s) of ACh. ACh controls were 10, 30, 60, or 100 µM for α4(2)β2(3) and α4(2)β2(2)α5, α4β2α6β2β3 and α1β1εδ, α7, and α3β4 and α4(3)β2(2), respectively. Note that subunit concatamers32,33 were used in some experiments in order to obtain pentameric receptors with known subunit composition (α4(2)β2(3), α4(2)β2(2)α5, α4β2α6β2β3, and α4(3)β2(2)). The responses were calculated as both peak current amplitudes and net charge, as previously described.34 Net-charge data are reported for α7 and peak current amplitude for all other subtypes. The averages of two initial ACh controls were used for normalization purposes for each oocyte. Statistical comparisons were based on t-tests of the normalized data where indicated. In experiments with α1β1εδ, α7, and α3β4, and in some cases α4β2, RNAs for the subunit monomers were injected. The ACh control for α4β2 when formed from monomers was 30 µM. All experiments on nAChR were conducted in the presence of 1 μM atropine in both the bath and test solutions.
Muscarinic AChR Experiments
All experiments with Xenopus oocytes expressing human mAChRs via RNA microinjection were done in atropine-free Ringer’s solution. The M1 and M3 AChR subtypes are coupled to Gq and produce a rise in intracellular calcium that is sufficient to activate endogenous calcium-dependent chloride currents in the oocytes,35 allowing these currents to serve as a reporter for the activation of these muscarinic receptors. However, a single episode of activation produces sufficient desensitization to prevent oocytes from being used to record multiple responses. Therefore, control responses were obtained with applications of 10 µM ACh to separate sets of oocytes on the same day as results were obtained with experimental compounds. The desensitization phenomenon was also useful as a secondary assessment of receptor activation since active compounds not only stimulated currents when applied, but as noted, also prevented effective activation by a subsequent application of ACh, while the application of inactive compounds, such as nicotine, left the cells still responsive to ACh. Thus, after application of a probative compound to the mAChR, we followed up with an ACh application. Diminution of the subsequent ACh response was a sign of mAChR activity, whereas a response to the follow-up application of ACh response indicated that application of the probative compound had failed to significantly activate/desensitize the mAChR.
Data were collected at 50 Hz, filtered at 20 Hz, and analyzed by Clampfit 9.2 or 10.0 (Molecular Devices) and Excel (Microsoft, Redmond WA). Data are expressed as means ± SEM from at least four oocytes for each experiment and plotted by Kaleidagraph 4.5.2 (Abelbeck Software, Reading PA). Multi-cell averages were calculated for comparisons of complex responses and for display purposes. Averages of the normalized data were calculated for each of the 10322 points in each of the 206.44 s traces (acquired at 50 Hz), as well as the standard errors for those averages.
Results
Characterization of Areca Alkaloid Muscarinic Activity
The Xenopus oocyte expression system was used to test the prototypical nicotinic and muscarinic ligands for their activity on M1 and M3 muscarinic receptors co-expressed in oocytes. In these experiments, uninjected oocytes did not show an ACh response in the absence of atropine. The M1 and M3 receptors were used as a standard survey for muscarinic activity since these subtypes M1 and M3 are very highly expressed in mammalian brain,36 In these studies, we did not examine M2 or M4 type receptors. For the Gq-coupled M1 and M3 receptors, single responses could be obtained for active agents that were mediated by calcium-dependent chloride channels.35,37 As shown in Supplementary Figure S1A, an application of 10 µM ACh to cells expressing both M1 and M3 AChR stimulated currents (average peak current amplitude 930 ± 20 nA, n = 7), that could be used as reference responses for other ligands tested on other oocytes from the same injection set on the same day. Responses to a second application of ACh were reduced by more than 90% (P < .01), desensitization being a second indication of the ACh agonist activity, as noted above. An application of 10 µM nicotine to cells expressing M1 and M3 AChR evoked no detectable responses, and responses to 10 µM ACh after nicotine were not different from the first ACh responses in control cells (P = .11). As expected, an application of 10 µM muscarine to cells expressing M1 and M3 AChR generated large currents, comparable to the ACh controls (P = .21), and smaller responses to a subsequent application of 10 µM ACh (P < .05). These data are summarized in Supplementary Figure S1B. Supplementary Figure S1B also shows that co-application of 10 µM ACh with 100 µM of the muscarinic antagonist atropine effectively decreased responses compared to cells stimulated with ACh alone (P < .01). Responses to ACh after the atropine/ACh co-application were not significantly different from the initial ACh responses in control cells (P = .71).
The approach illustrated in Supplementary Figure S1 was used to characterize the activity of the four known Areca alkaloids on the Gq-coupled muscarinic receptors (Supplementary Figure S2). Application of 10 µM arecoline or 10 µM guvacoline stimulated responses that were not significantly different from the ACh responses in control cells. However, responses to arecoline were significantly larger than those to guvacoline (P < .01). Responses to ACh after arecoline or guvacoline were significantly reduced compared to initial ACh control responses (P < .01). Neither guvacine nor arecaidine produced detectable responses from cells expressing M1 and M3 AChR and responses to subsequent applications of ACh were not decreased. Note that the compounds tested did not stimulate currents in uninjected oocytes (not shown).
Nicotinic Activity of Areca Alkaloids
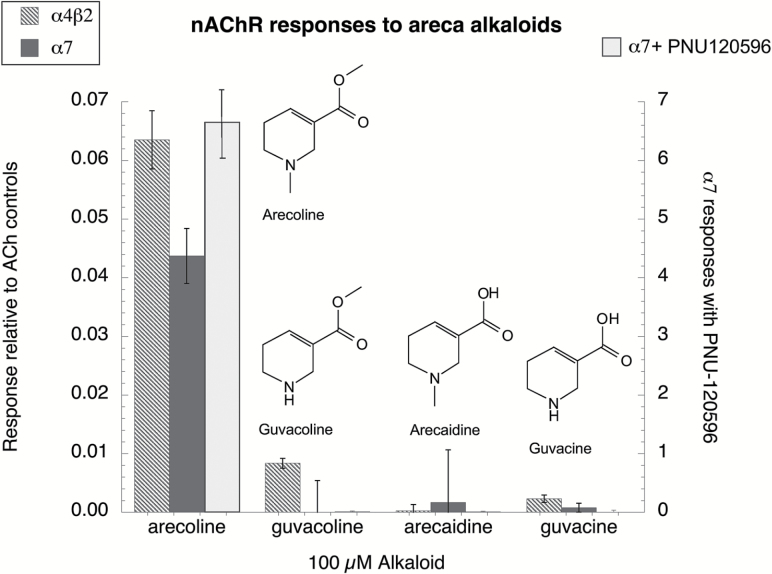
We previously published that arecoline is a partial agonist for α4* nAChR and a silent agonist for α7 receptors.21 We tested the other Areca alkaloids for their activity on these receptors, and as shown in Figure 1, aside from arecoline, only 100 µM guvacoline stimulated detectable responses from the α4β2-expressing cells, and even these small responses were barely above our limit of detection. None of the alkaloids other than arecoline stimulated α7-expressing cells, even when they were co-applied with the strong positive allosteric modulator (PAM), PNU-120596.38
Figure 1.
Nicotinic acetylcholine receptor (nAChR) responses to Areca alkaloids. Oocytes expressing either human α4 and β2 nAChR subunits or α7 subunits were first evaluated for their responses to two control applications to acetylcholine (Ach), 30 µM for the α4β2-expressing cells or 60 µM for the α7-expressing cells and then for their responses to the Areca alkaloids applied at 100 µM. Alkaloid responses were calculated relative to the average of the two initial ACh applications. Responses of the α4β2 receptors were calculated as peak currents and the α7 responses as net charge.34 Additionally, the α7 receptors were also tested with the alkaloids co-applied with 10 µM of the PAM PNU-120596, and those responses are scaled on the right-hand y-axis. In these experiments, the α4 and β2 subunit RNAs were co-injected at a 1:1 ratio.
Muscarinic Activity of Arecoline-Related Compounds
Our data suggest that arecoline is a sort of bridge compound, spanning the pharmacophores for both nicotinic and muscarinic receptors, with an interesting selectivity for α4* nAChR. We investigated structurally related alkaloids to determine whether compounds could be found that retained the α4* nAChR selectivity of arecoline without muscarinic activity. In this article, we report data on four exploratory compounds (Figure 2), starting with isoarecolone, a compound that has previously been shown to have “nicotine-like” properties.39 The structure of isoarecolone is shown in Figure 2 along with three additional compounds that were investigated: 4-acetyl-1,1-dimethylpiperazin-1-ium (DMPA); (4-methylpiperazin-1-yl) ethanone (MPA); and 1-(4-ethylpiperazin-1-yl) ethanone (EPA).
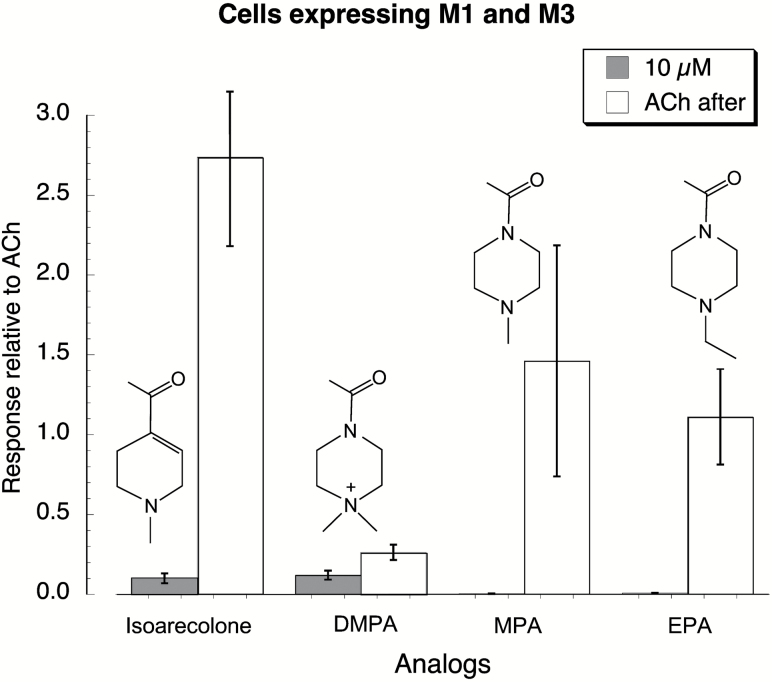
Figure 2.
Characterization of Areca alkaloids analogs on M1 and M3 acetylcholine (AChR) expressed in Xenopus oocytes. The structures of the analogs, 1-(4-methylpiperazin-1-yl) ethanone (MPA), 4-acetyl-1,1-dimethylpiperazin-1-ium (DMPA), and 1-(4-ethylpiperazin-1-yl) ethanone (EPA) are shown accompanying the corresponding data. A two-application protocol as described for Supplementary Figure S1 was used to characterize these ligands for their effects on cells expressing M1 and M3 AChR. Test compounds were applied at 10 µM, and then after a 3-min wash period 10 µM acetylcholine was applied to determine whether the initial application was able to desensitize the receptor/channel system and decrease or eliminate further responses.
Using the protocol illustrated in Supplementary Figure S1, we determined (Figure 2) that 10 µM isoarecolone had much less activity on cells expressing M1 and M3 AChR than arecoline or guvacoline, especially as indicated by its lack of desensitizing effect. In contrast, 10 µM DMPA activated small currents in cells expressing these receptors and produced significant inhibition of subsequent ACh-evoked responses (P < .05). At a concentration of 10 µM, neither MPA nor EPA stimulated any current from the Gq-coupled mAChR, and following the application of these compounds the cells were fully responsive to a 10 µM ACh application. Indeed, we failed to detect a response to MPA even up to concentrations of 300 μM (data not shown).
Nicotinic Activity of Arecoline-Related Compounds
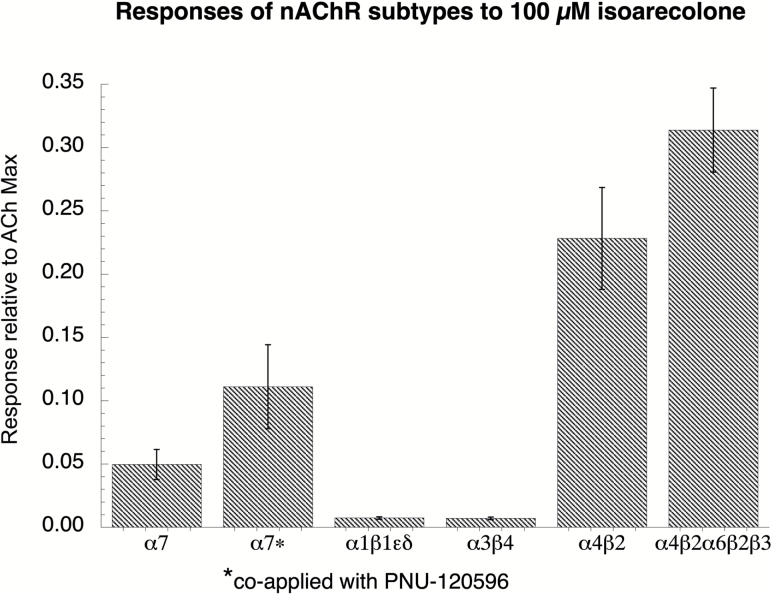
In order to determine whether isoarecolone had the same pattern of α4* nAChR selectivity that was seen with arecoline, we tested 100 µM isoarecolone on oocytes expressing different nAChR subtypes (Figure 3). There were barely detectable responses in α7 expressing cells, similar to what was seen with arecoline. However, arecoline could be characterized as an α7 silent agonist21,40; silent agonists produce little if any partial agonist activity but do induce a PAM-sensitive desensitized state (Figure 1). Such compounds are emerging as interesting anti-inflammatory agents. This was not the case with isoarecolone since the co-application of 10 µM PNU-120596 with 100 µM isoarecolone did not stimulate significantly larger currents than 100 µM isoarecolone alone. Isoarecolone did not effectively activate muscle-type (α1β1εδ) receptors or ganglionic-like α3β4 receptors but was an effective agonist for the α4* and α6* receptors. Full concentration-response studies were conducted with isoarecolone on α4* receptor subtypes that had defined subunit composition by means of an α4β2 concatamer, as previously described32,33 (Supplementary Figure S3A). The EC50 and Imax values are provided in Supplementary Table 1 and compared to those for arecoline. Not unexpectedly, isoarecolone had low potency for α4β2 receptors that contained three α4 and two β2 units, as this is generally found to be a low sensitivity subtype.41 The efficacy of isoarecolone for the other three subtypes tested was 30–40% that of ACh, which is roughly two to three times that of varenicline.25 To further confirm that isoarecolone was not a silent agonist of α7, we tested the effectiveness of isoarecolone as an antagonist of α7 ACh-evoked responses (Supplementary Figure S3B). A range of concentrations was co-applied with 60 µM ACh, and there was only a small inhibition observed at the highest concentration of isoarecolone tested (300 µM).
Figure 3.
Nicotinic acetylcholine receptor (nAChR) responses to isoarecolone. Oocytes expressing human α7, α3β4, α4β2 nAChR subunits, mouse muscle α1β1εδ subunits, or the human α4β2α4β2α3 concatamer33 were first evaluated for their responses to two control applications to ACh, and then for their responses to the analogs applied at 100 µM. Acetylcholine (ACh) controls were 60 µM for the α7-expressing cells, 100 µM for α3β4, and 30 µM for the other subtypes. Responses were normalized relative to the average of the two initial ACh applications from the same cells. Responses of the α7 receptors were calculated as net charge34 and as peak current for the other subtypes. Additionally, the α7 receptors were also tested with isoarecolone co-applied with 10 µM of the PAM PNU-120596, and those data are indicated by the asterisk. (Note that these are scaled on the same left-hand y-axis.)
As an α4β2 partial agonist, a low concentration of isoarecolone should partially inhibit or desensitize responses to phasic application of a full agonist such as ACh, as well as potentially produce low levels of steady-state (smoldering) activation.23,42 We, therefore, applied 1 µM isoarecolone to the bath during a series of ACh applications (10 µM or 1 µM) to cells expressing α4β2 receptors (Supplementary Figure S4). We saw a concentration-dependent inhibition of the ACh responses, evidenced by greater inhibition of currents stimulated by the lower concentration of ACh and the induction of a steady-state current, as indicated by a shift in the baseline holding current.
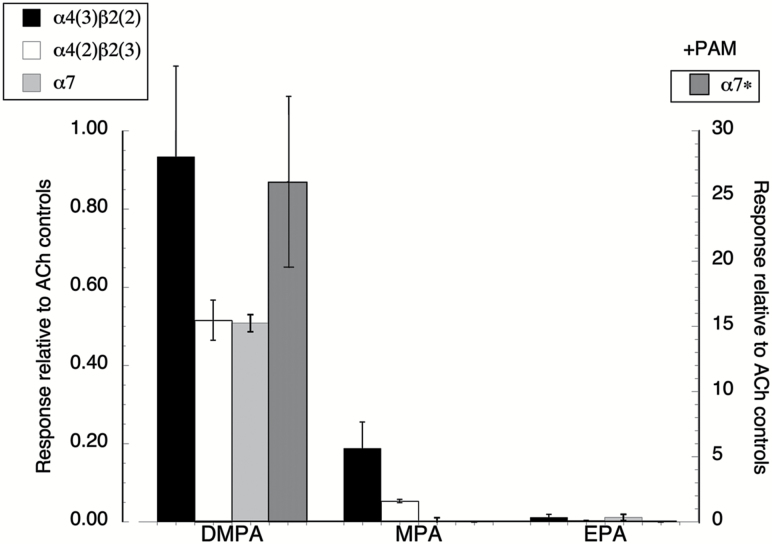
The isoarecolone analogs DMPA, MPA, and EPA were tested for their activity on the two forms of α4β2 and on α7 receptors. DMPA was an effective activator of these receptors (Figure 4 and Supplementary Table 2), and, as expected, the α7 responses to DMPA were strongly potentiated by the PAM PNU-120596. (Note that the PAM-potentiated responses reference the right-hand Y axis, which is expanded 50-fold relative to the left-hand axis.) In contrast to DMPA, which, in addition to having some muscarinic activity, is a strong nicotinic agonist on several nAChR subtypes (Table 1), MPA, which had no detectable muscarinic activity, was a weak partial agonist of the α4* receptors. Note that the α4* efficacy of 100 µM MPA was comparable to that of arecoline (Figure 1), but like isoarecolone and unlike arecoline, MPA showed no agonist or silent agonist activity for α7. It was also confirmed that 100 µM MPA did not activate muscle-type (α1β1εδ) or α3β4 nAChR (not shown). In contrast, DMPA produced small but detectable activation of α3β4 receptors (1.5 ± 0.03% of the ACh controls) and was a very efficacious activator of muscle receptors, with a 100 µM application stimulating 40 ± 4% of an ACh maximum response. The ethyl analog, EPA, which had no detectable muscarinic activity, also lacked activity on α4* or α7 nicotinic receptors when tested at a concentration of 100 µM. With the goal of working toward α4-selective partial agonists, these data indicated that DMPA (Table 1) would be a poor lead, lacking selectivity, as would EPA, lacking any significant efficacy. The preliminary profile of MPA, however, seems promising.
Figure 4.
Nicotinic activity of the isoarecolone analogs (see Figure 3 for structures). After obtaining two acetylcholine control responses, 100 µM applications of 1-(4-methylpiperazin-1-yl) ethanone, 4-acetyl-1,1-dimethylpiperazin-1-ium, and 1-(4-ethylpiperazin-1-yl) ethanone were made to oocytes expressing the low sensitivity form of α4β2 (α4(3)β2(2)), the high sensitivity form of α4β2 (α4(2)β2(3)), or α7. Analog-evoked responses were calculated relative to the peak currents (α4β2 subtypes) or net charge (α7 receptors) of the ACh controls. Control ACh concentrations were 10 µM, 60 µM, and 100 µM for α4(2)β2(3), α7, and (α4(3)β2(2), respectively. An α4β2 dimer was co-expressed with monomeric α4 or β232 to yield the α4β2 subtypes indicated. Additionally, the α7-expressing cells were tested with co-applications of 10 µM PNU-120596 and 100 µM of the analogs. Those data, normalized to the responses of the same cells to 60 µM ACh alone, are plotted relative to y-axis on the right. All points are the averages of at least five oocytes (± SEM).
Table 1.
Responses of nAChR Subtypes to 100 μM DMPA Relative to ACh Maximum Response
| α4(3)β2(2) | 0.47 ± 0.12 |
| α4(2)β2(3) | 0.46 ± 0.05 |
| α4(2)β2(2)α5 | 0.39 ± 0.02 |
| α6β2β3α4β2 | 0.46 ± 0.01 |
| α3β4 | 0.01 ± 0.00 |
| α1β1εδ | 0.40 ± 0.03 |
| α7 | 0.41 ± 0.02 |
| α7a | 20.83 ± 5.29 |
aNet charge response to 100 μM 4-acetyl-1,1-dimethylpiperazin-1-ium plus 10 μM PNU-120596 relative to acetylcholine alone.
Full concentration-response studies were conducted with MPA on α4* and α6-containing receptors, and the results are shown in Supplementary Figure S5. We have previously published similar studies with arecoline, and those data are presented in Table 2 compared to isoarecolone and MPA. Additionally, we have compared arecoline and MPA on receptors containing α5 subunits (α4(2)β2(2)α5), and those results are shown in Supplementary Figure S5B and included in Table 2.
Table 2.
Partial Agonist Imax Values Relative to ACh Maximum
| Receptor | Arecolinea | Isoarecolone | MPA |
|---|---|---|---|
| α4(3)β2(2) | 0.036 ± 0.003 | 0.23 ± 0.03 | 0.28 ± 0.01 |
| α4(2)β2(3) | 0.054 ± 0.004 | 0.32 ± 0.01 | 0.31 ± 0.01 |
| α4(2)β2(2)α5 | 0.16 ± 0.01 | 0.44 ± 0.01 | 0.23 ± 0.01 |
| α6β2β3α4β2 | 0.056 ± 0.003 | 0.45 ± 0.05 | 0.23 ± 0.01 |
| EC50 values (μM) | |||
| α4(3)β2(2) | 75 ± 7 | 440 ± 170 | 253 ± 24 |
| α4(2)β2(3) | 14 ± 3 | 31 ± 5 | 327 ± 7 |
| α4(2)β2(2)α5 | 94 ± 12 | 45 ± 4 | 280 ± 20 |
| α6β2β3α4β2 | 21 ± 4 | 27 ± 1 | 204 ± 18 |
Ach, acetylcholine.
aData on subtypes other than α4(2)β2(2)α5 are taken from Papke et al.,21.
Discussion
Clearly, the betel quid delivers users a complex cocktail of agents based just on the compounds coming from the Areca nut, with even more coming from the P. betle leaves that are used to wrap the quid.43 In our previous work, we reported that the application of Areca nut infusion to α4β2- and α6β2-containing nAChR resulted in a weak partial agonist response and a subsequent refractory period when the receptor became insensitive to ACh, as determined by two-electrode voltage-clamp electrophysiological measurements made in the Xenopus expression system.21 Control applications of Areca extract to uninjected Xenopus oocytes showed no effect. Arecoline, the major pyridine alkaloid from the Areca nut, was also a weak partial agonist of the α4β2 nAChR, but did not show the same level of subsequent inhibition of the ACh response.21 We have since found that the inhibitory component is greater than 10 kDa in molecular weight, and it will be pursued in separate work. The present study has therefore focused on the Areca alkaloids. While the muscarinic activity of Areca comes from both arecoline and guvacoline, the nicotinic activity appears to be due only to arecoline. Although of relatively low potency and efficacy, the selectivity of arecoline for the α4* receptors associated with nicotine addiction is suggestive both of a common link between these two drug dependencies, and of a potentially promising lead toward new smoking cessation medications. An ideal drug for such an indication would be one with focused partial agonism of α4* and α6* nAChR20 without the muscarinic activity and α7 silent agonism of arecoline.
Our data show that a step in this direction is realized with isoarecolone. There is evidence to suggest that isoarecolone displays a unique profile of neurochemical and behavioral effects that justifies further investigation, particularly with regard to its effects on attention. Initial behavioral studies confirmed this nicotinic analogue to possess “nicotine-like” properties as detected by rats in a nicotine discrimination task.39 However, on locomotor activity, isoarecolone produces minimal activation in nicotine-dependent rats,44 which supports the observations from microdialysis studies showing this nicotinic analogue to possess relatively weak dopamine-releasing properties in the nucleus accumbens of rats,45 consistent with the partial agonism we report. In a more extensive biochemical assay, isoarecolone was found to evoke mecamylamine-sensitive dopamine release in a concentration-dependent manner from preloaded cortical or striatal synaptosomes; however, the analogue was reported to be 20 times less potent than nicotine and was less efficacious, producing a maximal response up to 50% of that observed for nicotine,46 again consistent with isoarecolone having partial agonist actions.46
In experiments specifically designed to evaluate abuse liability, graded unit doses of isoarecolone failed to cross-substitute in rats self-administering intravenous nicotine.27 Furthermore, priming doses of isoarecolone had a much lesser effect to reinstate nicotine-seeking behavior.27 However, the selective partial agonism shown by isoarecolone justifies further study as a model agent that might blunt the reinforcing effects of nicotine or ameliorate the symptoms of nicotine withdrawal, in a way analogous to cytisine or varenicline but with a better selectivity profile.
It is interesting to note that isoarecolone has also been considered as a cognitive-enhancer in laboratory animals. In a delayed matching-to-sample paradigm in monkeys, isoarecolone was shown to be effective in enhancing short-term memory.47 More recently, isoarecolone has been evaluated in a rodent model of attention. Using the five-choice serial reaction time task, graded doses of isoarecolone enhanced performance.48 However, unlike nicotine, which enhanced accuracy, omission errors, and latency measures in the attention task, isoarecolone selectively enhanced accuracy without affecting the other measures, which are thought to be primarily dependent on dopaminergic systems.48
Isoarecolone has a chemical structure that could lead to undesired reactions with proteins and other species in vivo, which would not be desirable. We hypothesized that similar compounds with an isosteric carbonyl group, but lacking the ring double bond (ie, MPA) might be suitable analogs. To this end, a piperazine framework seemed appropriate and indeed, older work with MPA49 suggested that it might have weak nicotinic activity on rodent tissues, leading to our work here with human nAChR. DMPA, previously reported as AMP,50 is known to have nicotinic activity but has not been investigated as a muscarinic agent. MPA was published as synthetic intermediate50 for DMPA. Due to our previous work implicating the potential importance of single methyl groups for modulating nAChR agonist activity and selectivity,40,51–53 we also investigated the ethyl analog, EPA.54
Our studies point in several new directions regarding the sculpting of selective nAChR pharmacophores. Features of both isoarecolone and MPA eliminated the α7 nAChR activity present in arecoline and DMPA. In the case of isoarecolone, relative to arecoline, we suggest that moving the carbonyl position to a 1,4 position relative to the charged nitrogen atom selects against α7 activity. The loss of α7 activity for MPA relative to DMPA may center around a preference for α7 to bind dialkylated (or positively charged) species. In any case, the permanent charge of DMPA is not likely to promote blood–brain barrier passage, so MPA would likely be a better compound for central nervous system activity. MPA was superior to its ethyl analog EPA and molecular docking studies suggest a possible explanation. The lowest energy scored poses for MPA versus EPA in a model of the α4β2 ligand binding domain55 (data not shown) reveal that the ethyl group of EPA is in contact distance with F119 of the β2 subunit whereas smaller MPA appears to avoid this clash. Our sequence of compounds has also demonstrated that the muscarinic activity intrinsic to arecoline can be reduced while retaining the pattern of α4*/α6* partial agonism. Thus, isoarecolone relative to arecoline has strongly reduced mAChR activity, and our model compounds exemplified by MPA maintain this strong selectivity against mAChR activation or inhibition. Another significant point about the comparison between MPA and arecoline or isoarecolone is that the results show that the alpha beta unsaturated carbonyl functionality is not required for partial agonism at α4* receptors, validating the isostere approach we pursue.
The analog MPA has a very nice profile of activity for a potential smoking cessation drug; however, its utility might be limited by its relatively low potency. This might in part be due to a relatively low pKa of 6.9 we estimated by titration (data not shown) causing approximately 2/3 of the compound to be uncharged at physiological pH and therefore relatively ineffective at nAChR activation. Further development of the MPA structure might be used to improve potency, involving optimization of the N-acyl group, use of alternate carbonyl isosteres, and other elaborations of the core ring system. The recent accomplishment of the crystal structure for the α4β2 nAChR55 will provide an excellent framework to continue development of new partial agonists as inspired by Areca nut alkaloids. Further, that tobacco and Areca products are often used together leads to the intriguing idea that perhaps therapeutic approaches that are useful for alleviating tobacco addiction could also be useful for those who use Areca products.21
Funding
This work was supported in part by the National Institute of Health grant (R01 GM57481).
Declaration of Interests
Aspects of this work are part of a University of Florida US Provisional Patent Application No. 62/464,326 filed 27 February 2017. Coauthors NAH and RLP are inventors on this application.
Supplementary Material
Acknowledgments
We wish to thank Ryan Hibbs for a critical reading of this manuscript and comments.
References
- 1. Little MA, Papke RL. Betel, the orphan addiction. J Addict Res Ther. 2015;6(3):130–132. [Google Scholar]
- 2. Lord GA, Lim CK, Warnakulasuriya S, Peters TJ. Chemical and analytical aspects of areca nut. Addict Biol. 2002;7(1):99–102. [DOI] [PubMed] [Google Scholar]
- 3. Herzog TA, Murphy KL, Little MA, Suguitan GS, Pokhrel P, Kawamoto CT. The Betel Quid Dependence Scale: replication and extension in a Guamanian sample. Drug Alcohol Depend. 2014;138:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee CH, Chiang SL, Ko AM, et al. Betel-quid dependence domains and syndrome associated with betel-quid ingredients among chewers: an Asian multi-country evidence. Addiction. 2014;109(7):1194–1204. [DOI] [PubMed] [Google Scholar]
- 5. Winstock AR, Trivedy CR, Warnakulasuriya KA, Peters TJ. A dependency syndrome related to areca nut use: some medical and psychological aspects among areca nut users in the Gujarat community in the UK. Addict Biol. 2000;5(2):173–179. [DOI] [PubMed] [Google Scholar]
- 6. Song H, Wan Y, Xu YY. Betel quid chewing without tobacco: a meta-analysis of carcinogenic and precarcinogenic effects. Asia Pac J Public Health. 2015;27(2):NP47–NP57. [DOI] [PubMed] [Google Scholar]
- 7. Kao SY, Lim E. An overview of detection and screening of oral cancer in Taiwan. Chin J Dent Res. 2015;18(1):7–12. [PubMed] [Google Scholar]
- 8. Akhtar S. Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: a meta-analysis of case-control studies. Cancer Causes Control. 2013;24(2):257–265. [DOI] [PubMed] [Google Scholar]
- 9. Franke AA, Lai JF, Kawamoto CT, Pokhrel P, Herzog TA. University of Hawai’i Cancer Center connection: areca (betel) nut consumption: an underappreciated cause of cancer. Hawaii J Med Public Health. 2014;73(12):400–403. [PMC free article] [PubMed] [Google Scholar]
- 10. Lee CH, Ko AM, Warnakulasuriya S, et al. Population burden of betel quid abuse and its relation to oral premalignant disorders in South, Southeast, and East Asia: an Asian Betel-quid Consortium Study. Am J Public Health. 2012;102(3):e17–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8(11):1442–1444. [DOI] [PubMed] [Google Scholar]
- 12. Chu NS. Neurological aspects of areca and betel chewing. Addict Biol. 2002;7(1):111–114. [DOI] [PubMed] [Google Scholar]
- 13. Peng W, Liu YJ, Wu N, et al. Areca catechu L. (Arecaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Ethnopharmacol. 2015;164:340–356. [DOI] [PubMed] [Google Scholar]
- 14. Liu YJ, Peng W, Hu MB, Xu M, Wu CJ. The pharmacology, toxicology and potential applications of arecoline: a review. Pharm Biol. 2016;54(11):2753–2760. [DOI] [PubMed] [Google Scholar]
- 15. Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16(6):710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74(8):1102–1111. [DOI] [PubMed] [Google Scholar]
- 17. Papke RL. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol. 2014;89(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pons S, Fattore L, Cossu G, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28(47):12318–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb Perspect Med. 2013;3(1):a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brunzell DH, McIntosh JM, Papke RL. Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation. Ann N Y Acad Sci. 2014;1327:27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papke RL, Horenstein NA, Stokes C. Nicotinic activity of arecoline, the psychoactive element of “betel nuts”, suggests a basis for habitual use and anti-inflammatory activity. PLoS One. 2015;10(10):e0140907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2007(1):CD006103. [DOI] [PubMed] [Google Scholar]
- 23. Papke RL, Trocmé-Thibierge C, Guendisch D, Al Rubaiy SA, Bloom SA. Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists. J Pharmacol Exp Ther. 2011;337(2):367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–805. [DOI] [PubMed] [Google Scholar]
- 25. Papke RL, Wecker L, Stitzel JA. Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2010;333(2):501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lummis SC, Thompson AJ, Bencherif M, Lester HA. Varenicline is a potent agonist of the human 5-hydroxytryptamine3 receptor. J Pharmacol Exp Ther. 2011;339(1):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shoaib M. Effects of isoarecolone, a nicotinic receptor agonist in rodent models of nicotine dependence. Psychopharmacology (Berl). 2006;188(2):252–257. [DOI] [PubMed] [Google Scholar]
- 28. Wolf-Pflugmann M, Lambrecht G, Wess J, Mutschler E. Synthesis and muscarinic activity of a series of tertiary and quaternary N-substituted guvacine esters structurally related to arecoline and arecaidine propargyl ester. Arzneimittelforschung. 1989;39(5):539–544. [DOI] [PubMed] [Google Scholar]
- 29. Moser U, Lambrecht G, Wagner M, Wess J, Mutschler E. Structure-activity relationships of new analogues of arecaidine propargyl ester at muscarinic M1 and M2 receptor subtypes. Br J Pharmacol. 1989;96(2):319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersen KE, Sørensen JL, Lau J, et al. Synthesis of novel gamma-aminobutyric acid (GABA) uptake inhibitors. 5.(1) Preparation and structure-activity studies of tricyclic analogues of known GABA uptake inhibitors. J Med Chem. 2001;44(13):2152–2163. [DOI] [PubMed] [Google Scholar]
- 31. Papke RL, Stokes C. Working with OpusXpress: methods for high volume oocyte experiments. Methods. 2010;51(1):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23(27):9004–9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuryatov A, Lindstrom J. Expression of functional human α6β2β3* acetylcholine receptors in Xenopus laevis oocytes achieved through subunit chimeras and concatamers. Mol Pharmacol. 2011;79(1):126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papke RL, Porter Papke JK. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol. 2002;137(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miledi R, Parker I, Sumikawa K. Oscillatory chloride current evoked by temperature jumps during muscarinic and serotonergic activation in Xenopus oocyte. J Physiol. 1987;383:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988;8(12):4646–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982;215(1201):491–497. [DOI] [PubMed] [Google Scholar]
- 38. Hurst RS, Hajós M, Raggenbass M, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25(17):4396–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reavill C, Spivak CE, Stolerman IP, Waters JA. Isoarecolone can inhibit nicotine binding and produce nicotine-like discriminative stimulus effects in rats. Neuropharmacology. 1987;26(7A):789–792. [DOI] [PubMed] [Google Scholar]
- 40. Papke RL, Chojnacka K, Horenstein NA. The minimal pharmacophore for silent agonism of the α7 nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 2014;350(3):665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Papke RL, Stokes C, Muldoon P, Imad Damaj M. Similar activity of mecamylamine stereoisomers in vitro and in vivo. Eur J Pharmacol. 2013;720(1–3):264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campling BG, Kuryatov A, Lindstrom J. Acute activation, desensitization and smoldering activation of human acetylcholine receptors. PLoS One. 2013;8(11):e79653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pradhan D, Suri KA, Pradhan DK, et al. Golden heart of the nature: Piper betle L. J Pharmacogn Phytochem. 2013;1(6):147–167. [Google Scholar]
- 44. Reavill C, Stolerman IP. Locomotor activity in rats after administration of nicotinic agonists intracerebrally. Br J Pharmacol. 1990;99(2):273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mirza NR, Pei Q, Stolerman IP, Zetterström TS. The nicotinic receptor agonists (−)-nicotine and isoarecolone differ in their effects on dopamine release in the nucleus accumbens. Eur J Pharmacol. 1996;295(2–3):207–210. [DOI] [PubMed] [Google Scholar]
- 46. Whiteaker P, Garcha HS, Wonnacott S, Stolerman IP. Locomotor activation and dopamine release produced by nicotine and isoarecolone in rats. Br J Pharmacol. 1995;116(3):2097–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buccafusco JJ, Jackson WJ, Gattu M, Terry AV Jr. Isoarecolone-induced enhancement of delayed matching to sample performance in monkeys: role of nicotinic receptors. Neuroreport. 1995;6(8):1223–1227. [DOI] [PubMed] [Google Scholar]
- 48. Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44(8):1054–1067. [DOI] [PubMed] [Google Scholar]
- 49. Manetti D, Bartolini A, Borea PA, et al. Hybridized and isosteric analogues of N1-acetyl-N4-dimethyl-piperazinium iodide (ADMP) and N1-phenyl-N4-dimethyl-piperazinium iodide (DMPP) with central nicotinic action. Bioorg Med Chem. 1999;7(3):457–465. [DOI] [PubMed] [Google Scholar]
- 50. Garcha HS, Thomas P, Spivak CE, Wonnacott S, Stolerman IP. Behavioural and ligand-binding studies in rats with 1-acetyl-4-methylpiperazine, a novel nicotinic agonist. Psychopharmacology (Berl). 1993;110(3):347–354. [DOI] [PubMed] [Google Scholar]
- 51. Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett. 1996;213(3):201–204. [DOI] [PubMed] [Google Scholar]
- 52. Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem. 2007;101(1): 160–167. [DOI] [PubMed] [Google Scholar]
- 53. Horenstein NA, Leonik FM, Papke RL. Multiple pharmacophores for the selective activation of nicotinic alpha7-type acetylcholine receptors. Mol Pharmacol. 2008;74(6):1496–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fraser RR, Swingle RB. The oxidation of triethylamine trichloroacetyl chloride. Tetrahedron. 1969;25(16):3469–3475. [Google Scholar]
- 55. Morales-Perez CL, Noviello CM, Hibbs RE. X-ray structure of the human α4β2 nicotinic receptor. Nature. 2016;538(7625):411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.