Abstract
Background
Stroke is one of the leading causes of disability worldwide and aphasia among survivors is common. Current speech and language therapy (SLT) strategies have only limited effectiveness in improving aphasia. A possible adjunct to SLT for improving SLT outcomes might be non‐invasive brain stimulation by transcranial direct current stimulation (tDCS) to modulate cortical excitability and hence to improve aphasia.
Objectives
To assess the effects of tDCS for improving aphasia in people who have had a stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (June 2018), CENTRAL (Cochrane Library, June 2018), MEDLINE (1948 to June 2018), Embase (1980 to June 2018), CINAHL (1982 to June 2018), AMED (1985 to June 2018), Science Citation Index (1899 to June 2018), and seven additional databases. We also searched trial registers and reference lists, handsearched conference proceedings and contacted authors and equipment manufacturers.
Selection criteria
We included only randomised controlled trials (RCTs) and randomised controlled cross‐over trials (from which we only analysed the first period as a parallel group design) comparing tDCS versus control in adults with aphasia due to stroke.
Data collection and analysis
Two review authors independently assessed trial quality and risk of bias, and extracted data. If necessary, we contacted study authors for additional information. We collected information on dropouts and adverse events from the trials.
Main results
We included 21 trials involving 421 participants in the qualitative synthesis. Three studies with 112 participants used formal outcome measures for our primary outcome measure of functional communication — that is, measuring aphasia in a real‐life communicative setting. There was no evidence of an effect (standardised mean difference (SMD) 0.17, 95% confidence interval (CI) −0.20 to 0.55; P = 0.37; I² = 0%; low quality of evidence; inverse variance method with random‐effects model; higher SMD reflecting benefit from tDCS; moderate quality of evidence). At follow‐up, there also was no evidence of an effect (SMD 0.14, 95% CI −0.31 to 0.58; P = 0.55; 80 participants ; 2 studies; I² = 0%; very low quality of evidence; higher SMD reflecting benefit from tDCS; moderate quality of evidence).
For our secondary outcome measure, accuracy in naming nouns at the end of intervention, there was evidence of an effect (SMD 0.42, 95% CI 0.19 to 0.66; P = 0.0005; I² = 0%; 298 participants; 11 studies; inverse variance method with random‐effects model; higher SMD reflecting benefit from tDCS; moderate quality of evidence). There was an effect for the accuracy in naming nouns at follow‐up (SMD 0.87, 95% CI 0.25 to 1.48; P = 0.006; 80 participants; 2 studies; I² = 32%; low quality of evidence); however the results were not statistically significant in our sensitivity analysis regarding the assumptions of the underlying correlation coefficient for imputing missing standard deviations of change scores. There was no evidence of an effect regarding accuracy in naming verbs post intervention (SMD 0.19, 95% CI −0.68 to 1.06; P = 0.67; I² = 0%; 21 participants; 3 studies; very low quality of evidence). We found no studies examining the effect of tDCS on cognition in people with aphasia after stroke. We did not find reported serious adverse events and the proportion of dropouts and adverse events was comparable between groups (odds ratio (OR) 0.54, 95% CI 0.21 to 1.37; P = 0.19; I² = 0%; Mantel‐Haenszel method with random‐effects model; 345 participants; 15 studies; low quality of evidence).
Authors' conclusions
Currently there is no evidence of the effectiveness of tDCS (anodal tDCS, cathodal tDCS and Dual‐tDCS) versus control (sham tDCS) for improving functional communication in people with aphasia after stroke (low quality of evidence). However, there is limited evidence that tDCS may improve naming performance in naming nouns (moderate quality of evidence), but not verbs (very low quality of evidence) at the end of the intervention period and possibly also at follow‐up. Further methodologically rigorous RCTs with adequate sample size calculation are needed in this area to determine the effectiveness of this intervention. Data on functional communication and on adverse events should routinely be collected and presented in further publications as well as data at follow‐up. Further study on the relationship between language/aphasia and cognition may be required, and improved cognitive assessments for patients with aphasia developed, prior to the use of tDCS to directly target cognition in aphasia. Authors should state total values at post‐intervention as well as their corresponding change scores with standard deviations.
Plain language summary
Direct electrical current to the brain for language difficulties after stroke
Review question
To assess the effects of tDCS for improving language difficulties in people who have had a stroke.
Background
Stroke is one of the leading causes of disability worldwide. Most strokes take place when a blood clot blocks a blood vessel leading to the brain. Without a proper blood supply the brain quickly suffers damage, which can be permanent, and this damage often causes language difficulties (aphasia) among stroke survivors. People with aphasia after stroke have difficulties in communicative settings, i.e. understanding or producing language, or both. Current speech and language therapy (SLT) strategies have limited effectiveness in improving these language difficulties. One possibility for enhancing the effects of SLT might be the addition of non‐invasive brain stimulation provided by a technique known as transcranial direct current stimulation (tDCS). This technique manipulates brain functions and may be used to improve language difficulties. However, the effectiveness of this intervention for improving SLT outcomes is still unknown.
Search date
The search of this review is current to 12 June 2018.
Study characteristics
The review included 21 clinical trials comparing tDCS versus sham tDCS involving 421 participants with aphasia due to first‐time stroke.
Key results
We found no evidence that tDCS may help improve language recovery in terms of everyday communication or thinking abilities. However, there is limited evidence that tDCS may improve a person’s ability to name nouns. We could not identify any serious harmful effects and the number of harmful events and withdrawals from the trials was not increased. Further trials are needed in this area to determine whether this treatment works in routine practice. Authors of future research should adhere to current research quality standards.
Quality of the evidence
The quality of the evidence was very low to moderate.
Summary of findings
Summary of findings for the main comparison. tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia for improving aphasia in patients with aphasia after stroke.
| tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia in patients with aphasia after stroke | ||||||
| Patient or population: patients with improving aphasia in patients with aphasia after stroke Settings: Intervention: tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | TDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia | |||||
| Functional communication post intervention Formal outcome measures of aphasia. Scale from: −infinity to +infinity | The mean functional communication post intervention in the control groups was NA1 | The mean functional communication post intervention in the intervention groups was 0.17 standard deviations higher (0.2 lower to 0.55 higher) | 112 (3 studies) | ⊕⊕⊝⊝ low2,3 | SMD 0.17 (−0.20 to 0.55) | |
| Functional communication at follow‐up formal measures of aphasia. Scale from: −infinity to +infinity Follow‐up: mean 6 months | The mean functional communication at follow‐up in the control groups was NA1 | The mean functional communication at follow‐up in the intervention groups was 0.14 standard deviations higher (0.31 lower to 0.58 higher) | 80 (2 studies) | ⊕⊕⊝⊝ very low2,3,4 | SMD 0.14 (−0.31 to 0.58) | |
| Language impairment: accuracy of naming nouns post intervention Accuracy in naming nouns. Scale from: −infinity to +infinity | The mean language impairment: accuracy of naming nouns post intervention in the control groups was NA1 | The mean language impairment: accuracy of naming nouns post intervention in the intervention groups was 0.42 standard deviations higher (0.19 to 0.66 higher) | 298 (11 studies) | ⊕⊕⊕⊝ moderate2 | SMD 0.42 (0.19 to 0.66) | |
| Language impairment: accuracy of naming nouns at follow‐up Accuracy in naming nouns. Scale from: −infinity to +infinity Follow‐up: mean 6 months | The mean language impairment: accuracy of naming nouns at follow‐up in the control groups was NA1 | The mean language impairment: accuracy of naming nouns at follow‐up in the intervention groups was 0.87 standard deviations higher (0.25 to 1.48 higher) | 80 (2 studies) | ⊕⊕⊝⊝ low2,4 | SMD 0.87 (0.25 to 1.48) | |
| Language impairment: accuracy of naming verbs post intervention Accuracy in verb naming. Scale from: −infinity to +infinity | The mean language impairment: accuracy of naming verbs post intervention in the control groups was NA1 | The mean language impairment: accuracy of naming verbs post intervention in the intervention groups was 0.19 standard deviations higher (0.68 lower to 1.06 higher) | 21 (3 studies) | ⊕⊕⊝⊝ very low2,3,4 | SMD 0.19 (−0.68 to 1.06) | |
| tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia: dropouts post intervention Numbers of dropouts and adverse events | 87 per 1000 | 49 per 1000 (20 to 115) | See comment | 345 (15 studies) | ⊕⊕⊝⊝ low2,3 | Risks were calculated from odds ratio |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 No data can be provided due to the combination of different outcome measures for the same outcome in this analysis 2 Downgraded due to total sample size being < 400 as a rule of thumb 3 Downgraded due to the fact that the 95% CI around the pooled effect estimate includes both 1) no effect and 2) appreciable benefit or appreciable harm (an effect size of 0.5 serves as a surrogate for a minimal clinically important difference/appreciable benefit or harm) 4 Downgraded due to total sample size being < 100
Background
Description of the condition
Every year nearly 15 million people suffer from stroke worldwide (WHO 2011); nearly six million of them die because of their stroke (Mathers 2011). Moreover, approximately five million people annually experience permanent disability due to stroke (WHO 2011). Stroke is one of the main causes of death worldwide and contributes considerably to disease burden (WHO 2011). It is well known that stroke affects activities of daily living (ADL) and quality of life (Pohl 2011). About one‐third of adult stroke patients suffer from aphasia when they are discharged from hospital (Dickey 2010), which means the language system in their brain has been impaired or lost due to brain damage and so they have difficulty comprehending or expressing language (Benson 1996). Other authors have found that almost 20% of all stroke survivors have chronic aphasic symptoms (Pedersen 1995). People with aphasia due to stroke are more likely to stay in hospital longer, have higher odds of dying in hospital, have greater disability (Flowers 2016), and use rehabilitation services more often than stroke patients without aphasia (Dickey 2010; Flowers 2016). Aphasia has not only a remarkable impact on quality of life but in every third patient aphasia is associated with depression 12 months after the stroke (Cruice 2003; Hilari 2010; Kauhanen 2000). Together with functional ADL performance, age, and gender, aphasia appears to lead to reduced long‐term social participation (Dalemans 2010). Another point which is very relevant for people with stroke is to improve their cognition (Pollock 2012). However, given that a lot of clinical tests for measuring cognition after stroke rely on language abilities and people with aphasia after stroke suffer from substantial communication limitations, they are often excluded from studies dealing with this topic and hence experience deprivation of relevant research (Wall 2017). Therefore effective treatment approaches are urgently needed to treat aphasia in people after stroke. There are several approaches to treating aphasia, such as intensive speech and language therapy (SLT), which might improve outcomes for patients affected after their stroke (Bhogal 2003). However, a systematic review found only modest evidence for more intensive treatment and constraint‐induced language therapy for individuals with stroke‐induced aphasia (Cherney 2008). Another systematic review found evidence of an effect of SLT regarding functional communication, that is communication in an everyday situation, together with a dose‐response relationship (Brady 2016). However, the effectiveness of other approaches that might be used as an adjunct to common speech and language therapies should also be considered in order to further improve rehabilitation outcomes.
Description of the intervention
Transcranial direct current stimulation (tDCS) is seen as an approach to modulate cortical excitability (Nitsche 2001). It is usually administered via saline‐soaked surface sponge electrodes attached to the cranium and connected to a direct current stimulator with low intensities (Lang 2005). There are two different means of application: either the anodal electrode (+) is placed over the presumed brain area of interest and the cathodal electrode (−) is placed above the contralateral orbit (anodal stimulation); or the cathodal electrode is placed over the presumed brain area of interest and the anodal electrode is placed above the contralateral orbit (cathodal stimulation) (Hesse 2011).
tDCS is non‐invasive and works by applying a direct current to the brain (Bindman 1964; Nowak 2009; Purpura 1965). It is relatively inexpensive when compared with other approaches such as repetitive transcranial magnetic stimulation or epidural stimulation (Hesse 2011).
Recent research suggests that in people after stroke, tDCS combined with SLT might lead to improvement of aphasia when compared with sham tDCS (Baker 2010; Branscheidt 2018; Fiori 2013; Flöel 2011; Fridriksson 2011; Holland 2011; Kang 2011; Marangolo 2011; Marangolo 2013a; Marangolo 2013b; Marangolo 2013c; Meinzer 2016; Monti 2008a; Shah‐Basak 2015; You 2011), but there are also studies which did not show any evidence of effects (Dos Santos 2017; Fridriksson 2018; Polanowska 2013; Spielmann 2016). One reason for this discrepancy might be due to the fact that the effects of tDCS and the process of language recovery are not completely understood (Wortman‐Jutt 2017). For example, according to the individual location of the brain's lesion and hence modified neurophysiology, seemingly not all patients benefit from tDCS to the same magnitude (Otal 2015; Rosso 2014). A current guideline on the application of tDCS was not able to provide a recommendation for improving aphasia (Lefaucheur 2017).
How the intervention might work
According to some studies tDCS can increase or decrease cortical excitability (Bindman 1964; Purpura 1965). This might be due to a shift of the resting potential of the nerve cells in the brain (Flöel 2010; Purpura 1965). Anodal stimulation may lead to depolarisation of the neuronal membranes and therefore result in greater cortical excitability, whereas cathodal stimulation may lead to polarisation and therefore result in lower cortical excitability (Bindman 1964). Therefore it might be possible that tDCS could generate significant after‐effects, which could last up to several hours, if the stimulation lasted for longer than five minutes (Nitsche 2001; Nitsche 2003).
tDCS may modulate functional reorganisation of language networks after stroke by recruiting neurons near the damaged left‐hemispheric brain area and by reducing interference with the right‐hemispheric language region (Chrysikou 2011).
Pilot studies suggest that tDCS might improve picture naming in both healthy individuals and aphasic patients, and also improve the detection of a violation of written artificial grammar in healthy individuals (De Vries 2010). Furthermore, in healthy individuals tDCS induced an improvement in naming abilities with a concomitant improvement in working memory (Jeon 2012). However optimal dosage, intensity and frequency, and its optimal combination with SLT are still unclear.
Why it is important to do this review
In a recent Cochrane Review, the authors concluded that there is evidence of effects of SLT for improving aphasia after stroke (Brady 2016). tDCS given as an adjunct to therapies for aphasia may be a viable approach to further improve the efficiency of SLT for aphasia after stroke (Marangolo 2017). Regardless of the fact that tDCS in combination with SLT might be beneficial and improve aphasia after stroke, it remains unclear which area of the brain (lesioned or non‐lesioned, language dominant or non‐language dominant), which kind of stimulation (anodal (A‐tDCS), cathodal (C‐tDCS) or both concurrently (Dual‐tDCS)) and at which frequency and intensity tDCS should be combined with SLT in practice. The trials undertaken thus far have used small sample sizes. Moreover, there is no systematic review to compile the effects of all available trials. Thus a systematic review was needed in order to evaluate the available literature on the effectiveness and the acceptability of this treatment approach.
Objectives
To assess the effects of tDCS for improving aphasia in people who have had a stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) and randomised controlled cross‐over trials. We excluded quasi‐randomised controlled trials.
Types of participants
We included people of either gender, aged 18 years and above, who had sustained a stroke according to the World Health Organization (WHO) definition. When the WHO definition was not stated, we used a clinical definition of stroke instead. We did not make any restrictions on inclusion regarding type or level of impairment or time since stroke.
Types of interventions
We compared tDCS alone or tDCS plus SLT or any other approach for improving aphasia versus sham tDCS alone or sham tDCS plus SLT or any other approach for improving aphasia, or no intervention.
Types of outcome measures
Types of outcome measures did not form part of the criteria for the inclusion of studies.
Primary outcomes
Our primary outcomes were measures of aphasia. Measuring aphasia in a real‐life communicative setting (i.e. functional communication) is difficult to define and to evaluate (Brady 2016). Wherever possible we identified formal outcome measures. We prioritised the outcome measures in the following order.
Amsterdam‐Nijmegen Everyday Language Test (ANELT) (Blomert 1994)
Communicative Abilities of Daily Living (CADL) (Holland 1980)
Boston Diagnostic Aphasia Examination (BDAE) (Goodglass 1972)
Scenario Test (Van der Meulen 2010)
Communicative Effectiveness Index (CETI) (Lomas 1989)
Discourse Analysis (DA) (Ulatowska 1983)
Depending on the data provided by the studies and researchers, all the review authors discussed and reached consensus on which measures to be included in the analysis for the primary outcome.
Secondary outcomes
For secondary outcomes we considered surrogate parameters for language impairment such as receptive or expressive language, or both. For this outcome we prioritised outcome measurements as follows.
Aachen Aphasia Test (AAT) (Huber 1991)
Western Aphasia Battery (WAB) (Kertesz 1982)
Porch Index of Communicative Abilities (PICA) (Porch 1967)
Spoken language comprehension (we prioritised according to functional communication i.e. a) discourse comprehension, b) sentence comprehension, and c) single word comprehension)
Other measures of language ability, such as reading or writing
Other secondary outcomes were other domains of cognitive abilities, such as working attention, memory, executive functions, intelligence, visual‐auditory recognition and visual‐spatial abilities. We prioritised outcome measurements as follows.
Cognitive Test Battery for Global Aphasia (Marinelli 2017)
Montreal Cognitive Assessment (Nasreddine 2005)
Clock Drawing Test (Goodglass 1983)
Executive Function (Assessments have been described elsewhere) (Chung 2013)
Other measures of cognitive abilities
Further secondary outcomes were dropouts and adverse events, with their appropriate outcome measurements as reported in the studies.
If other outcome measurements were provided, all review authors discussed and reached consensus about which of them should be included in the secondary outcomes analysis.
Search methods for identification of studies
See the 'Specialized register' information at the Cochrane Stroke Group's website. We searched for relevant trials in all languages and arranged translation of trial reports where necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register and the following electronic bibliographic databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Library, Issue 5, 12 June 2018) (Appendix 1)
MEDLINE Ovid (1948 to 12 June 2018) (Appendix 2)
Embase Ovid (1980 to 12 June 2018) (Appendix 3)
CINAHL EBSCO (1982 to 12 June 2018) (Appendix 4)
AMED OVID (1985 to 12 June 2018) (Appendix 5)
Science Citation Index (1899 to 21 June 2018) (Appendix 6)
Linguistics and Language Behavior Abstracts (LLBA) (1973 to 12 June 2018) (Appendix 7)
Inspec (1969 to 18 June 2018) (Appendix 8)
Compendex (1969 to 21 June 2018) (Appendix 8)
Physiotherapy Evidence Database (PEDro) at www.pedro.org.au/ (24 June 2018) (Appendix 9)
PsycBITE at www.psycbite.com (18 June 2018) (Appendix 10)
speechBITE at www.speechbite.com (18 June 2018) (Appendix 11)
Rehabdata at www.naric.com/?q=REHABDATA (1956 to 12 June 2018)
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and adapted it for the other databases.
We also searched the following ongoing trials and research registers (November 2014).
WHO International Clinical Trials Registry Platform (www.apps.who.int/trialsearch) (Appendix 12)
Stroke Trials Registry (www.strokecenter.org/trials) (Appendix 13)
ClinicalTrials.gov (www.clinicaltrials.gov) (Appendix 14)
Searching other resources
In order to identify further published, unpublished and ongoing trials not available in the aforementioned databases, we undertook the following.
-
Handsearched the following relevant conference proceedings that have not already been searched by the Cochrane Stroke Group.
3rd to 9th World Congress of NeuroRehabilitation (2002, 2006, 2008, 2010, 2012, 2014 and 2016); World Congress of Physical and Rehabilitation Medicine (2001, 2003, 2005, 2007, 2009, 2015, 2016 and 2017)
Deutsche Gesellschaft für Neurotraumatologie und Klinische Neurorehabilitation (2001 to 2018)
Deutsche Gesellschaft für Neurologie (2000 to 2017)
Deutsche Gesellschaft für Neurorehabilitation (1999 to 2018)
Asian Oceania Conference of Physical and Rehabilitation Medicine (2008, 2010, 2012, 2014, 2016)
Screened reference lists from relevant reviews, articles and textbooks
Contacted authors of identified trials and other researchers in the field
Used Science Citation Index Cited Reference Search for forward tracking of important articles
-
Contacted the following equipment manufacturers (latest contact: October 2014)
DJO Global, Vista, USA (www.djoglobal.com)
Grindhouse (www.grindhousewetware.com)
Magstim, Spring Gardens, United Kingdom (www.magstim.com)
Neuroconn, Ilmenau, Germany (www.neuroconn.de)
Neuroelectrics, Barcelona, Spain (www.neuroelectrics.com)
Newronika, Milano, Italy (www.newronika.it)
Soterix Medical, New York City, USA (www.soterixmedical.com)
Trans Cranial Technologies, Hong Kong (www.trans‐cranial.com)
Data collection and analysis
Selection of studies
One review author (BE) read the titles and abstracts of the records identified from the electronic searches and eliminated obviously irrelevant studies. We retrieved the full texts of the remaining studies and two review authors (JK, BE) ranked the studies as relevant, possibly relevant or irrelevant according to our inclusion criteria (types of studies, participants, aims of interventions). Two review authors (JM, MP) then examined whether the possibly relevant publications fitted the population, intervention, comparison, outcome, study type (PICOS) strategy of our study question. We resolved disagreements by discussion with all review authors. If we needed further information, we contacted trial authors.
We listed as excluded studies those that did not match our inclusion criteria regarding the type of study, participants, or type of interventions; those that were not RCTs; and those that did not clearly state or did not utilise proper methods of generating the randomisation schedule or methods of allocation concealment.
Data extraction and management
Two review authors (BE, JM) independently extracted trial and outcome data from the selected trials. If one of the review authors was involved in an included trial, another review author extracted the trial and outcome data from that trial. In accordance with the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we used checklists to independently assess:
methods of random sequence generation;
methods of allocation concealment;
blinding of assessors;
blinding of patients;
use of an intention‐to‐treat analysis (ITT);
adverse effects and dropouts;
important imbalances in prognostic factors at baseline;
participants (country, number of participants, age, gender, type of stroke, time from stroke onset to study entry, inclusion and exclusion criteria, educational background, socioeconomic status, handedness, cognition, pre‐existing neurological impairment(s), neurological history);
comparison (details of interventions in treatment and control groups, duration of treatment, details of co‐interventions in the groups);
outcomes; and
their time point of measurement.
Two review authors (MP, JK) checked the extracted data for agreement. If these two review authors could not reach consensus, a third review author arbitrated. If necessary, we contacted the researchers in order to get more information.
If necessary, we extracted data out of diagrams by using the software WebPlotDigitizer (Rohatgi 2018).
Assessment of risk of bias in included studies
Two review authors assessed the risk of bias in the included trials in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We described the agreement between authors during the assessment of risk of bias, and we resolved disagreement by reaching consensus through discussion. We contacted trialists for clarification and to request missing information.
Measures of treatment effect
For all outcomes representing continuous data, we planned to enter means and standard deviations and calculate a pooled estimate of the mean difference (MD) with 95% confidence intervals (CI). As studies did not use the same outcome, we calculated standardised mean differences (SMD) instead of MD. Some studies presented change scores and other presented total values. Since it is not possible to combine both in an SMD analysis, we reformulated change scores as total values or vice versa in order to ease statistical analysis by combining the two groups. The decision whether our analysis depended on change scores or on total values depended on the number of studies in each category and on the available data. If there were missing standard deviations for change scores, we imputed them by calculating a correlation coefficient from a similar study as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For all binary outcomes we calculated odds ratios (ORs) with 95% CI. For all statistical comparisons we used Cochrane Review Manager 5 software (RevMan 5) (Review Manager 2014).
Unit of analysis issues
In the event that individuals underwent more than one intervention, as in a cross‐over trial, we only used data from the first phase of the study before cross‐over. If outcomes were repeatedly observed in participants (e.g. at the end of intervention, at four and six weeks), we reported the measures at post‐intervention from each study. If outcomes were measured at least three months post intervention, we included them in our follow‐up analyses.
Dealing with missing data
We contacted the relevant principal investigators in order to retrieve missing data.
Assessment of heterogeneity
We used the I² statistic in order to assess heterogeneity. We used a random‐effects model, regardless of the level of heterogeneity. Thus, in the case of heterogeneity we did not violate the preconditions of a fixed‐effect model approach. We regarded an I² value above 50% as substantial heterogeneity.
Assessment of reporting biases
We inspected funnel plots for all outcomes and subgroup analysis in order to assess the risk of publication bias.
Data synthesis
We pooled the results of all eligible studies to present an overall estimate of the effect of tDCS (meta‐analysis). For all statistical analyses, we used the latest version of the Review Manager 5 software (Review Manager 2014). To test the robustness of the results, we did a sensitivity analysis by leaving out studies that we assessed to be of lower or ambiguous methodological quality (with respect to allocation concealment, blinding of assessors, and intention‐to‐treat (ITT) analysis). Clinical diversity and heterogeneity did not contribute to the decision about when to pool trials, but we describe clinical diversity, and variability in participants, interventions, and outcomes studied in Table 2. If studies had three or more intervention groups, for example two treatment groups and one control group, and the results of these intervention groups did not differ significantly, we combined the results of all intervention groups in one combined group and compared this with the results of the control group.
1. Participant characteristics.
| Study ID | Experimental: age, mean (SD) | Control: age, mean (SD) | Experimental: time post‐stroke | Control: time post‐stroke | Experimental: sex | Control: sex | Experimental: affected hemisphere | Control: affected hemisphere | Experimental:education, mean (SD) | Control: education, mean (SD) | Right‐ handedness |
| Baker 2010 | 66 (11) years | 65 (68) months | 5 men; 5 women | 10 (100%) left | 14 (2) years | 10 (100%) | |||||
| Branscheidt 2018 | 61 (10) years | 23 (18) months | 12 men; 4 women | 16 (100%) left | NA | NA | |||||
| Dos Santos 2017 | NA | 13 (100%) left | NA | ||||||||
| Fiori 2013 | 58 (10) years | 33 (28) months | 5 men; 2 women | 7 (100%) left | 13 (4) years | 7 (100%) | |||||
| Flöel 2011 | 52 (9) years | 84 (65) months | 6 men; 6 women | 12 (100%) left | 13 (5) years | 12 (100%) | |||||
| Fridriksson 2018 | 60 (11) | 60 (10) | 44 (45) months | 40 (35) months | 24 men; 10 women | 28 men; 12 women | 34 (100%) left | 40 (100%) left | 15 (2) years | 74 (100%) | |
| Kang 2011 | 62 (9) years | 52 (69) months | 8 men; 2 women | 10 (100%) left | 12 (5) years | 10 (100%) | |||||
| Marangolo 2011 | 66 (3) years | 22 (22) months | 2 men; 1 woman | 3 (100%) left | 14 (2) years | 3 (100%) | |||||
| Marangolo 2013a | 60 (8) years | 37 (22) months | 8 men; 4 women | 12 (100%) left | 13 (4) years | 12 (100%) | |||||
| Marangolo 2013b | 55 (9) years | 29 (24) months | 4 men; 4 women | 8 (100%) left | 12 (4) years | 8 (100%) | |||||
| Marangolo 2013c | 62 (10) years | 41 (27) months | 5 men; 2 women | 7 (100%) left | 13 (6) years | 7 (100%) | |||||
| Marangolo 2018a | 58 (8) years | 22 (7) months | Not described by the authors | 12 (100%) left | 13 (3) years | 12 (100%) | |||||
| Meinzer 2016 | 59 (13) years | 61 (12) years | 54 (22) months | 37 (26) months | 7 men; 6 women |
11 men; 2 women |
13 (100%) left | 13 (100%) left | 10 (2) years | 13 (2) years | 26 (100%) |
| Monti 2008a | 60 (12) years | 47 (23) months | 4 men; 5 women | 7 (88%) left, 1 (22%) both | 11 (5) years | 8 (100%) | |||||
| Polanowska 2013 | 58 (10) years | 61 (12) years | 56 (45) days | 64 (43) days | 11 men; 7 women | 13 men; 6 women | 18 (100%) | 19 (100%) | 15 (4) years | 14 (3) years | 37 (100%) |
| Rosso 2014 | 57 (18) years | 15 (20) months | 12 men; 13 women | 25 (100%) left | 2.6 (1.2) years | Mean EHI (SD) 0.84 (0.37) | |||||
| Shah‐Basak 2015 | 64 (9) years | 31 (30) months | 10 men; 2 women | 12 (100%) left | Not stated | 12 (100%) | |||||
| Spielmann 2016 | 58 (10) years | 60 (10) years | 1.4 (0.5) months | 1.6 months (0.7) | 18 men; 8 women | 22 men; 10 women | Not stated | 12 (2) years | 13 (3) years | Mean EHI (SD) for experimental group 0.99 (0.05) and 0.97 (0.08) for control group | |
| Turkeltaub 2017 | 60 (10) years | 60 (9) years | Not stated | 16 men; 8 women | 5 men; 9 women | 24 (100%) left | 14 (100%) left | Not stated | Not stated | ||
| Volpe 2014 | Between 18 and 65 years (n = 7), above 65 years (n = 8) | At least 6 months | 8 men; 7 women | 15 (100%) left | Not stated | 15 (100%) | |||||
| You 2011 | 68 (11) years | 63 (10) years | 26 (6) days | 25 (9) days | 7 men; 7 women | 5 men; 2 women | 14 (100%) left | 7 (100%) left | 11 (3) years | 11 (4) years | 33 (100 %) |
EHI: Edinburgh Handedness Inventory NA: not applicable SD: standard deviation
Subgroup analysis and investigation of heterogeneity
We conducted a subgroup analysis for the following factors.
A priori: time since stroke, acute or subacute phase (the first week after stroke and the second to the fourth week after stroke, respectively) versus post‐acute phase (from the second to the sixth month after stroke) versus chronic phase (more than six months after stroke)
A priori: location of stimulation (affected or unaffected hemisphere, dominant or non‐dominant hemisphere)
A priori: type of stimulation, cathodal or anodal
Post hoc: type of aphasia (non‐fluent, fluent or mixed populations)
Sensitivity analysis
We performed a planned sensitivity analysis for risk of bias in our included studies in order to test the robustness of our results for our primary outcome, functional communication. We considered concealed allocation, blinding of assessors, and ITT. We also performed a sensitivity analysis regarding the strength of correlation in imputed standard deviations for change scores.
GRADE and 'Summary of findings' table
We assessed the quality of the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), for the following main outcomes of analysis.
Functional communication post intervention
Functional communication at follow‐up
Language impairment: accuracy of naming nouns post intervention
Language impairment: accuracy of naming nouns at follow‐up
Language impairment: accuracy of naming verbs post intervention
Dropouts and adverse events
We created a 'Summary of findings' table and presented the key findings of the review, including a summary of the quantity of data, the magnitude of effect size, and the overall quality of evidence.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies.
Results of the search
We identified a total of 4786 unique records from the searches. After screening the titles and abstracts we excluded 4712 records and obtained the full texts of the remaining 74 articles. After further assessment, 21 studies met the review inclusion criteria (Included studies) and we excluded nine studies (Excluded studies). We identified 23 ongoing trials (Characteristics of ongoing studies). The flow of references is shown in Figure 1.
1.
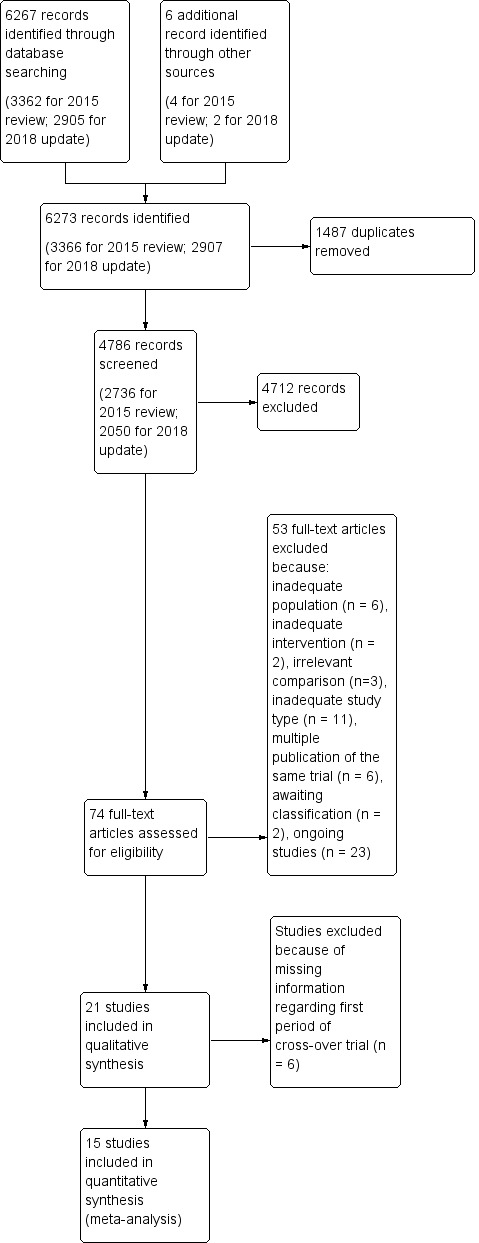
Study flow diagram. Please note, that the numbers of full texts is not necessarily equal to the numbers of included studies, since two of included studies (Meinzer 2016, Shah‐Basak 2015) have been published in two full texts each.
Included studies
We included 21 studies involving a total of 421 participants in the qualitative synthesis of this review (Baker 2010; Branscheidt 2018; Dos Santos 2017; Fiori 2013; Flöel 2011; Fridriksson 2018; Kang 2011; Marangolo 2011; Marangolo 2013a; Marangolo 2013b; Marangolo 2013c; Marangolo 2018a; Meinzer 2016; Monti 2008a; Polanowska 2013; Rosso 2014; Shah‐Basak 2015; Spielmann 2016; Turkeltaub 2017; Volpe 2014; You 2011) (see Characteristics of included studies). All studies investigated the effect of tDCS versus sham tDCS or other active interventions like other forms of tDCS or transcranial magnetic stimulation (TMS). Fourteen of the studies, with a total of 153 analysed participants, were randomised cross‐over trials (Baker 2010; Branscheidt 2018; Dos Santos 2017; Fiori 2013; Kang 2011; Marangolo 2011; Marangolo 2013a; Marangolo 2013b; Marangolo 2013c; Marangolo 2018a; Monti 2008a; Rosso 2014; Shah‐Basak 2015; Volpe 2014), whereas the remaining seven, with 268 analysed participants, were RCTs (Flöel 2011; Fridriksson 2018; Meinzer 2016; Polanowska 2013; Spielmann 2016; Turkeltaub 2017; You 2011). Thirteen studies had one intervention group and one control group (Baker 2010; Branscheidt 2018; Fridriksson 2018; Kang 2011; Marangolo 2011; Marangolo 2013b; Meinzer 2016; Polanowska 2013; Rosso 2014; Shah‐Basak 2015; Spielmann 2016; Turkeltaub 2017; Volpe 2014). Six studies had two intervention groups and one control group (Fiori 2013; Flöel 2011; Marangolo 2013a; Marangolo 2013c; Monti 2008a; You 2011); whereas two studies had two intervention and two control groups, respectively (Dos Santos 2017; Marangolo 2018a). Seven of the included studies were conducted in Italy, five in the USA, three in Germany, two in the Republic of Korea, one in Brazil, one in France, one in the Netherlands, and one in Poland. The experimental groups received anodal tDCS (A‐tDCS) or cathodal tDCS (C‐tDCS), or both (dual or bihemispheric), and the control groups received sham tDCS (S‐tDCS). A widely used outcome was 'accuracy in naming' performance. See Table 2 for a comprehensive summary of patient characteristics, and Table 3 for a comprehensive summary of intervention characteristics, dropouts, and adverse events.
2. Demographics of studies including dropouts and adverse events.
| Study ID | Aphasia severity, mean (SD) | Type of stimulation (polarity) | Electrode position and size | Treatment intensity | Base‐treatment | Dropouts | Reasons for dropouts and adverse events in the experimental group | Reasons for dropouts and adverse events in the control group | Adverse events | Source of information | |
| Baker 2010 | WAB‐AQ: 69.4 (26.0) | A‐tDCS | 25 cm² sponge electrode over the most active area of the left frontal cortex | 1 mA for 20 minutes | Base‐treatment + A‐tDCS and S‐tDCS for 5 days once a day, separated by 7 days intersession interval | Computerised anomia training (picture naming) | 0 | NA | NA | None | Published |
| S‐tDCS | 1 mA for 30 seconds | ||||||||||
| Dos Santos 2017 | NA | Dual tDCS | 2 mA for 20 seconds | 2 mA for 20 minutes | Base treatment + Dual tDCS, TMS and either S‐tDCS or sham TMS with an unknown intersession interval | Boston Naming Test | None | NA | NA | No adverse events | Published |
| S‐tDCS | |||||||||||
| TMS | 1 Hz for 20 seconds | 1 Hz for 20 minutes | |||||||||
| Sham TMS | |||||||||||
| Branscheidt 2018 | AAT‐Naming: 79 (28) |
A‐tDCS | 35 cm² sponge electrode over left motor cortex | 2 mA for 20 minutes | Base‐treatment + A‐tDCS and S‐tDCS once, separated by 7 days intersession interval | Lexical decision tasks with pseudo words and existing words | 0 | NA | NA | Not reported | Published |
| S‐tDCS | 2 mA for 30 seconds | ||||||||||
| Fiori 2013 | Relative accuracy in picture naming in per cent with a higher value reflecting higher accuracy): 8 (7) | A‐tDCS | 35 cm² sponge electrode over Wernicke's area | 1 mA for 20 min | Base‐treatment + A‐tDCS over Wernicke's area, A‐tDCS over Broca's area and S‐tDCS for 10 consecutive sessions once a day, separated by 6 days intersession interval | Computerised anomia training (video naming) | 0 | NA | NA | None | Published |
| A‐tDCS | 35 cm² sponge electrode over Broca's area | ||||||||||
| S‐tDCS | 35 cm² sponge electrode over either Wernicke's or Broca's area | 1 mA for 30 sec | |||||||||
| Flöel 2011 | AAT‐Profile score: 54.8 (8.7) | A‐tDCS | 35 cm² sponge electrode over the right temporo‐parietal cortex (unaffected hemisphere) | 1 mA for 20 minutes | Base‐treatment + A‐tDCS, C‐tDCS and S‐tDCS for 5 days once a day with 3 weeks intersession interval | Anomia training (picture naming) | 0 | NA | NA | None | Published and unpublished |
| C‐tDCS | |||||||||||
| S‐tDCS | 1 mA for 30 seconds | ||||||||||
| Fridriksson 2018 | WAB‐R AQ 60 (19) | A‐tDCS | 25 cm² sponge electrodes over most active cortex during naming identified by fMRI with the cathode over the contralateral supraorbital area | 1 mA for 20 minutes | Base treatment + either A‐tDCS or S‐tDCS 5 times a week over 3 weeks | Computerised anomia training (picture naming) | 3 out of 34 (9%) | Not described (n = 2), withdrew consent (n = 1) | Withdrew consent (n = 1), discontinued treatment owing to adverse events (n = 1) | Dizziness (n = 1), erythema (n = 2) | Published |
| WAB‐R AQ 56 (20) | S‐tDCS | 1 mA for 30 seconds | 2 out of 40 (5%) | Headache (n = 2), Dizziness (n = 2), Convulsion (n = 1), Hypertension (n = 1) | |||||||
| Kang 2011 | WAB‐AQ: 39.5 (8.2) | C‐tDCS | 25 cm² sponge electrode over the right Broca's homologue area (unaffected hemisphere) | 2 mA for 20 minutes | Base‐treatment + C‐tDCS and S‐tDCS for 5 days once a day with 1 week intersession interval | Computerised anomia training (picture naming) | 0 | NA | NA | Not stated | Published |
| S‐tDCS | 1 mA for 1 minute | ||||||||||
| Marangolo 2011 | AAT‐Token test: 19.7 (9.6) | A‐tDCS | 35 cm² sponge electrode over the left inferior frontal gyrus (Broca's area, affected hemisphere) | 1 mA for 20 minutes | Base‐treatment + A‐tDCS and S‐tDCS for 5 days once a day with 6 days intersession interval | Tailored speech and language therapy | 0 | NA | NA | Not stated | Published and unpublished |
| S‐tDCS | 1 mA for 30 seconds | ||||||||||
| Marangolo 2013a | Baseline accuracy of naming (SD): 8 (3) per cent | A‐tDCS | 35 cm² sponge electrode over Wernicke's area | 1 mA for 20 min | Base‐treatment + A‐tDCS over Wernicke's area, A‐tDCS over Broca's area and S‐tDCS for 10 consecutive sessions once a day, separated by 6 days intersession interval | Computerised anomia training (video naming) | 0 | NA | NA | Not stated | Published |
| A‐tDCS | 35 cm² sponge electrode over Broca's area | 1 mA for 20 min | |||||||||
| S‐tDCS | 35 cm² sponge electrode over either Wernicke's or Broca's area | 1 mA for 30 sec | |||||||||
| Marangolo 2013b | AAT token test (SD): 11 (2) out of 36 | Dual tDCS | 35 cm² sponge electrode with the anode over (ipsilesional) Broca's area and the cathode over (contralesional) Broca's homologue area | 2 mA for 20 min | Base‐treatment + Dual tDCS and S‐tDCS in 10 consecutive sessions once a day with 14 days intersession interval | Audiotape based word repetition training | 0 | NA | NA | Not stated | Published |
| S‐tDCS | 2 mA for 30 sec | ||||||||||
| Marangolo 2013c | AAT token test (SD): 14 (6) out of 36 | A‐tDCS | 35 cm² sponge electrode over Wernicke's area | 1 mA for 20 min | Base‐treatment + A‐tDCS over Wernicke's area, A‐tDCS over Broca's area and S‐tDCS for 15 consecutive sessions once a day, separated by 6 days intersession interval | Computerised anomia training (video naming) | 0 | NA | NA | Not stated | Published |
| A‐tDCS | 35 cm² sponge electrode over Broca's area | 1 mA for 20 min | |||||||||
| S‐tDCS | 35 cm² sponge electrode over either Wernicke's or Broca's area | 1 mA for 30 sec | |||||||||
| Marangolo 2018a | Battery for the Analysis of Aphasic Disorders test mean noun naming accuracy (SD): 55% (20%) | C‐tDCS | 35 cm² sponge electrode over right cerebellum, 1 cm under and 4 cm lateral to the inion | 2 mA for 20 minutes | Base‐treatment + C‐tDCS and S‐tDCS once, separated by intersession interval of unknown duration | Verb generation and verb naming task | 0 | NA | NA | None | Published and unpublished |
| S‐tDCS | 2 mA for 30 seconds | ||||||||||
| Meinzer 2016 | Mean AAT naming performance at baseline (SD): 43% (21%) | A‐tDCS | 35 cm² sponge electrode over the left M1 | 1 mA for 20 minutes | Base treatment + A‐tDCS or S‐tDCS at the beginning of each session | Computer‐assisted naming treatment with the 'vanishing cues' approach (2 times for 90 minutes a day, 4 days per week for 2 weeks) | 4 dropouts (2 in experimental and 2 in control group) during follow‐up | n = 1 stroke of partner, n = 1 unavailable due to personal reasons | n = 1 moved abroad, n = 1 extended medical treatment abroad | None | Published |
| S‐tDCS | 1 mA for 30 seconds | ||||||||||
| Monti 2008a | Accuracy in picture naming (0 to 20 points with a higher value reflecting higher accuracy): 12.2 (4.8) | A‐tDCS | 35 cm² electrodes over the left F‐T areas (Broca's area, affected hemisphere) | 2 mA for 10 minutes | Base‐treatment + A‐tDCS, C‐tDCS and S‐tDCS once | Computerised anomia training (picture naming) | 0 | NA | NA | None | NA |
| C‐tDCS | 2 mA for 10 minutes | ||||||||||
| S‐tDCS | 2 mA for 10 seconds | ||||||||||
| Polanowska 2013 | Median severity on the ASRS: 2 | A‐tDCS | 35 cm² electrodes over the left F‐T areas (Broca's area, affected hemisphere) | 1 mA for 10 minutes | Base‐treatment + A‐tDCS and S‐tDCS in 15 consecutive sessions (5 times a week for 3 weeks) | Computerised anomia training (picture naming) | 2 | NA | 2 participants dropped out due to recurrent stroke | No serious side effects, such as seizure | Published |
| S‐tDCS | 10 minutes | ||||||||||
| Rosso 2014 | Baseline picture‐naming accuracy (SD): 28 (13) per cent | C‐tDCS | 35 cm² electrodes over the right Broca's homologue area (positioned by MRI‐based neuronavigation) | 1 mA for 15 minutes | Base‐treatment + C‐tDCS and S‐tDCS once on the same day with 2 hours of intersession interval | Computerised anomia training (picture naming) | No dropouts reported but only 22 out of 25 participants (88%) analysed | NA | NA | No adverse events except itching under the electrodes | Published |
| S‐tDCS | 1 mA for 16 sec | ||||||||||
| Shah‐Basak 2015 | Mean WAB‐AQ (SD): 53 (24) | (1) A‐tDCS | 25 cm² sponge electrodes over the left frontal area (F3) and the reference electrode over the contralateral mastoid | 2 mA for 20 minutes once | In experiment 2 each participant underwent the following interventions in random order: 1 of the active setups (1 to 4), 5 times per week for 2 weeks) and (2) 1 of the sham setups described above (5 or 6), 5 times per week for 2 weeks |
Constraint induced language therapy | CTL group: n = 1 | Declined to participate in active tDCS after crossing over | NA | Not reported | Published |
| (2) C‐tDCS | 25 cm² sponge electrodes over the left frontal area (F3) and the reference electrode over the contralateral mastoid | ||||||||||
| (3) A‐tDCS | 25 cm² sponge electrodes over the right frontal area (F4) and the reference electrode over the contralateral mastoid | ||||||||||
| (4) C‐tDCS | 25 cm² sponge electrodes over the left frontal area (F4) and the reference electrode over the contralateral mastoid | ||||||||||
| (5) S‐tDCS | 25 cm² sponge electrodes over the left frontal area (F3) and the reference electrode over the contralateral mastoid | 2 mA for 1 minute once | |||||||||
| (6) S‐tDCS | 25 cm² sponge electrodes over the right frontal area (F4) and the reference electrode over the contralateral mastoid | ||||||||||
| Spielmann 2016 | Mean aphasia severity according to Shortened token test (SD) 18.8 (7.9) | A‐tDCS | 35 cm² anode over the left IFG (F5) and the cathode over the contralateral orbit | 1 mA for the first 20 minutes of base treatment | Base treatment + either A‐tDCS or S‐tDCS | Each group received word‐finding therapy for 45 minutes per day on 5 consecutive sessions (225 minutes per week) | Experimental group: n = 1 during intervention and n = 3 during follow‐up period; Control group: n = 1 during intervention and n = 1 during follow‐up period | n = 3 due to motivational reasons, n = 1 could not be reached | n = 1 underwent brain surgery, n = 1 due to motivational reasons | None | Published |
| Mean aphasia severity according to Shortened token test (SD) 19.1 (9.0) | S‐tDCS | 1 mA for the first 30 seconds of base treatment | |||||||||
| Turkeltaub 2017 | PNT score 31 (18) | Dual‐tDCS | The anode was placed over the left temple and cathode on the right (electrode size not described) | Dosage not described | Base treatment + either Dual‐tDCS or S‐tDCS | 60 minutes of speech and language treatment 5 days a week for 1 week | None | NA | NA | None | Unpublished only |
| PNT score 32 (24) | S‐tDCS | ||||||||||
| Volpe 2014 | Mean verbal picture‐naming accuracy score (out of 75): 46 | A‐tDCS | Electrode positioning not described | Dosage not described | Base treatment + either Dual‐tDCS or S‐tDCS | Computerised aphasia therapy once (duration not stated) | None | NA | NA | None | Unpublished only |
| Mean verbal picture‐naming accuracy score (out of 75): 45 | S‐tDCS | ||||||||||
| You 2011 | K‐WAB AQ: 22.8 (13.2) | A‐tDCS | 35 cm² saline‐soaked sponge electrodes either over the left supratemporal gyrus (affected hemisphere, for anodal and sham) or over the right supratemporal gyrus (unaffected hemisphere, cathodal) | 2 mA for 30 minutes | Base treatment + 10 consecutive sessions of either A‐tDCS, C‐tDCS or S‐tDCS 5 times a week for 2 weeks | Conventional speech and language therapy | 12 out of 33 (36%) | Not stated groupwise. Reasons were: (1) early discharge of 7 participants (2) 3 participants refused therapy due to uncomfortable sensations and (3) 2 participants were unable to receive therapy due to their sleep habits | None | Published information | |
| C‐tDCS | 2 mA for 30 minutes | ||||||||||
| S‐tDCS | 2 mA for 60 seconds | ||||||||||
AAT: Aachen Aphasia Test ASRS: Six‐point Aphasia Severity Rating Scale A‐tDCS: anodal tDCS C: Coulomb (unit of electric charge; 1C = 1A *1s) C‐tDCS: cathodal tDCS K‐WAB AQ: Korean Western Aphasia Battery Aphasia Quotient mA: milliampere (milliamp) NA: not applicable SD: standard deviation S‐tDCS: sham tDCS tDCS: transcranial direct current stimulation WAB‐AQ: Western Aphasia Battery Aphasia Quotient
We had to exclude six of the 14 included cross‐over trials from the quantitative syntheses (meta‐analyses) because of missing information regarding the first intervention period (Baker 2010; Marangolo 2013a; Marangolo 2013c; Rosso 2014; Shah‐Basak 2015; Volpe 2014).
Excluded studies
We excluded 28 full‐text articles, because they did not meet the inclusion criteria. There were nine trials among them which did not obviously violate our inclusion criteria (Fiori 2011; Fridriksson 2011; Holland 2011; Lee 2013; Monti 2008b; NCT02514044; NCT03486782; Richardson 2015; Vines 2011). Hence, we have listed them in the Characteristics of excluded studies table.
Risk of bias in included studies
We have provided information about the risk of bias in the Characteristics of included studies table. If necessary, we contacted all principal investigators of the included trials and of trials awaiting classification to request further information about methodological issues in order to complete the rating of methodological quality. The contact was via letter and email, including email reminders once a month if we received no response. Some trialists provided all requested information and some did not answer our requests. We used the 'Risk of bias' tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions, to assess risk of bias according to the aspects listed in the Methods section (Review Manager 2014). Two review authors (BE, JM) independently assessed risk of bias in the included trials and the two other authors (JK and MP) checked the extracted data for agreement. We provide information on risk of bias at the study level in Figure 2. All authors discussed disagreements and, if necessary, sought arbitration from another review author (JK). A detailed description of risk of bias can be found in Characteristics of included studies.
2.
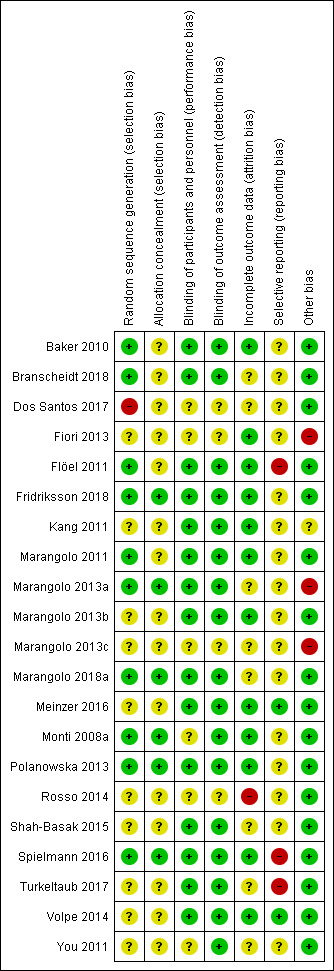
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Ten of the 21 included studies (48%) described a low risk of bias for sequence generation (Baker 2010; Branscheidt 2018; Flöel 2011; Fridriksson 2018; Marangolo 2011; Marangolo 2013a; Marangolo 2018a; Monti 2008a; Polanowska 2013; Spielmann 2016); and six (29%) described a low risk of bias for concealment of allocation by using random number generators (Fridriksson 2018; Marangolo 2013a; Marangolo 2018a; Monti 2008a; Polanowska 2013; Spielmann 2016).
Blinding
Fifteen of the 21 included studies (71%) described a low risk of bias for blinding of participants and personnel (Baker 2010; Branscheidt 2018; Flöel 2011; Fridriksson 2018; Kang 2011; Marangolo 2011; Marangolo 2013a; Marangolo 2013b; Marangolo 2018a; Meinzer 2016; Polanowska 2013; Shah‐Basak 2015; Spielmann 2016; Turkeltaub 2017; Volpe 2014); whereas 17 studies (81%) described a low risk of bias for blinding of outcome assessment (Baker 2010; Branscheidt 2018; Flöel 2011; Fridriksson 2018; Kang 2011; Marangolo 2011; Marangolo 2013a; Marangolo 2013b; Marangolo 2018a; Meinzer 2016; Monti 2008a; Polanowska 2013; Shah‐Basak 2015; Spielmann 2016; Turkeltaub 2017; Volpe 2014; You 2011).
Incomplete outcome data
Twelve of the 21 included studies (57%) were at a low risk of bias for incomplete outcome data (Baker 2010; Fiori 2013; Flöel 2011; Fridriksson 2018; Kang 2011; Marangolo 2011; Marangolo 2013b; Meinzer 2016; Monti 2008a; Polanowska 2013; Spielmann 2016; Volpe 2014); whereas one was at high risk (Rosso 2014).
Selective reporting
Two included studies were at low risk of bias for selective outcome reporting (Meinzer 2016; Volpe 2014); and two studies were at high risk (Flöel 2011; Spielmann 2016).
Other potential sources of bias
Three of the 21 included studies (14%) were at high risk for other biases (Fiori 2013; Marangolo 2013a; Marangolo 2013c), with the remaining 18 studies (86%) having a low risk of bias.
Effects of interventions
See: Table 1
Primary outcome measure: formal outcome measures of aphasia
Outcome 1.1: Functional communication post intervention
Three trials with 112 participants showed no evidence of an effect regarding functional communication at post‐intervention (SMD 0.17, 95% CI −0.20 to 0.55; P = 0.37; I² = 0%; low quality of evidence; inverse variance method with random‐effects model; with a higher SMD reflecting benefit from tDCS; Analysis 1.1) (Meinzer 2016; Spielmann 2016; Turkeltaub 2017).
1.1. Analysis.
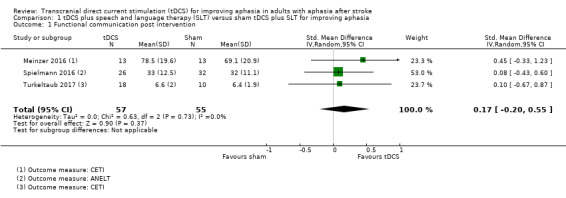
Comparison 1 tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia, Outcome 1 Functional communication post intervention.
Outcome 1.2: Functional communication at follow‐up
Two studies with 80 participants showed no evidence of an effect regarding functional communication at follow‐up (SMD 0.14, 95% CI −0.31 to 0.58; P = 0.55; I² = 0%; very low quality of evidence; inverse variance method with random‐effects model; with a higher SMD reflecting benefit from tDCS; Analysis 1.2) (Meinzer 2016; Spielmann 2016).
1.2. Analysis.
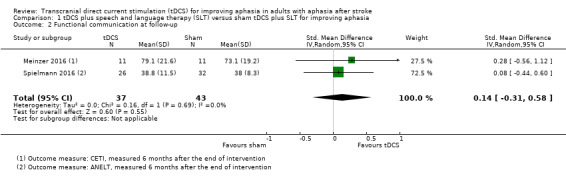
Comparison 1 tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia, Outcome 2 Functional communication at follow‐up.
Secondary outcome measure: language impairment
Outcome 1.3: Language impairment: accuracy of naming nouns post intervention
Eleven trials with 298 participants measured correct noun naming as a surrogate for aphasia (Fiori 2013; Flöel 2011; Fridriksson 2018; Kang 2011; Marangolo 2013b; Meinzer 2016; Monti 2008a; Polanowska 2013; Spielmann 2016; Turkeltaub 2017; You 2011) (Analysis 1.3). We obtained data from the published and unpublished literature. There was evidence of an effect regarding the change in naming accuracy (SMD 0.42, 95% CI 0.19 to 0.66; P = 0.0005; I² = 0%; moderate quality of evidence; inverse variance method with random‐effects model; with a higher SMD reflecting benefit from tDCS). By graphical inspection of the funnel plot of Analysis 1.3 we could not find any evidence of small‐study effects (Figure 3).
1.3. Analysis.
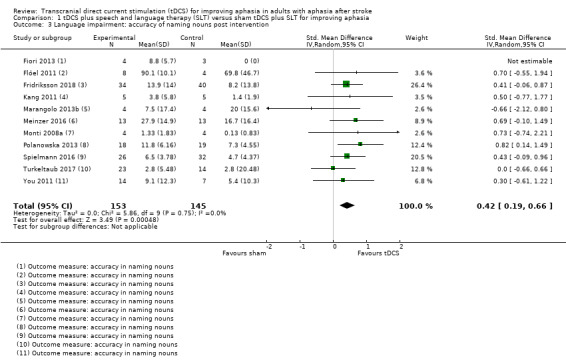
Comparison 1 tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia, Outcome 3 Language impairment: accuracy of naming nouns post intervention.
3.
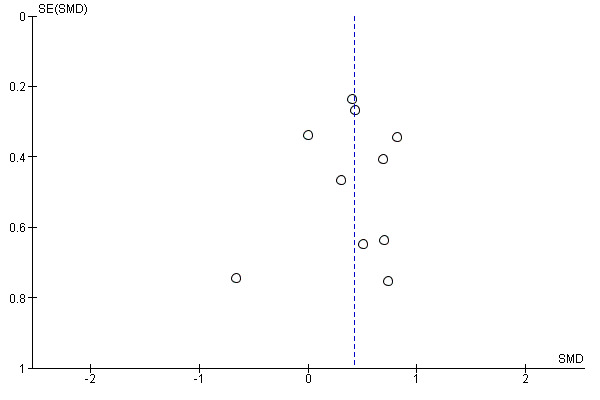
Funnel plot of comparison: 1 tDCS alone or tDCS plus speech and language therapy (SLT) or any other approach for improving aphasia versus sham tDCS alone or sham tDCS plus SLT or any other approach for improving aphasia, or no intervention, outcome: 1.3 Language impairment: accuracy of naming post intervention
Outcome 1.4: Language impairment: accuracy of naming nouns at follow up
Two studies with 80 participants measured correct noun naming at follow‐up as a surrogate for aphasia, yielding evidence of an effect (SMD 0.87, 95% CI 0.25 to 1.48; P = 0.006; I² = 32%; low quality of evidence; inverse variance method with random‐effects model; with a higher SMD reflecting benefit from tDCS; Analysis 1.4) (Meinzer 2016; Spielmann 2016).
1.4. Analysis.
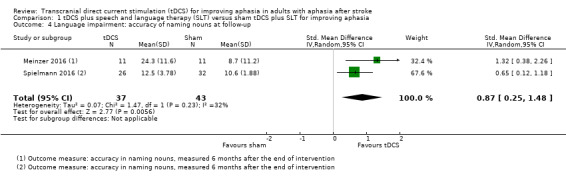
Comparison 1 tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia, Outcome 4 Language impairment: accuracy of naming nouns at follow‐up.
Outcome 1.5: Language impairment: accuracy of naming verbs post intervention
Three trials with 21 participants measured correct verb naming as a surrogate for aphasia by analysing change scores. We obtained data from the published literature and unpublished data. There was no evidence of an effect (SMD 0.19, 95% CI −0.68 to 1.06; P = 0.67; I² = 0%; very low quality evidence; inverse variance method with random‐effects model; with a higher SMD reflecting benefit from tDCS; Analysis 1.5) (Fiori 2013; Marangolo 2013b; Marangolo 2018a). No studies measured accuracy of naming verbs at a follow‐up time point.
1.5. Analysis.
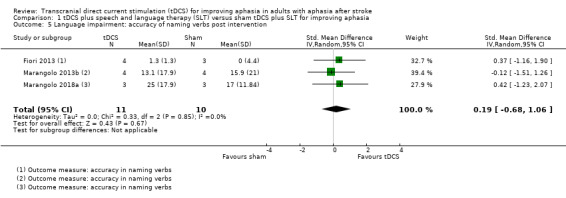
Comparison 1 tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia, Outcome 5 Language impairment: accuracy of naming verbs post intervention.
Secondary outcome measure: cognition
We found no studies examining the effect of tDCS on cognition in stroke patients with aphasia.
Secondary outcome measure: dropouts and adverse events
Outcome 1.6: dropouts and adverse events
Dropouts occurred in only four out of 15 studies (27%) (Fridriksson 2018; Polanowska 2013; Spielmann 2016; You 2011). We obtained data from the published literature. There was no evidence of effect regarding the difference in dropouts between intervention and control groups (OR 0.54, 95% CI 0.21 to 1.37; P = 0.19; 345 participants; 15 studies; I² = 0%; low quality of evidence; Mantel‐Haenszel method with random‐effects model). No serious adverse events were reported and no deaths occurred (Analysis 1.6; Table 3).
1.6. Analysis.
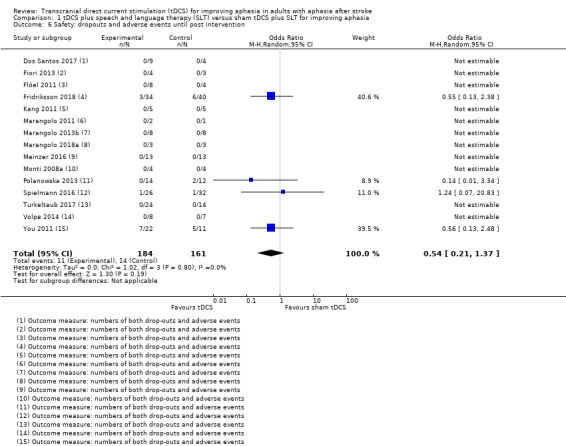
Comparison 1 tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia, Outcome 6 Safety: dropouts and adverse events until post intervention.
Pre‐specified subgroup analyses
Comparison 2: planned subgroup analysis by time since stroke: acute or subacute versus chronic
In a planned subgroup analysis we analysed the effects of tDCS on the relative change in our primary outcome measure, functional communication, in the acute or subacute and chronic phases (Analysis 2.1). There was no evidence for different effects of tDCS between subgroups (P = 0.44, I² = 0%).
2.1. Analysis.
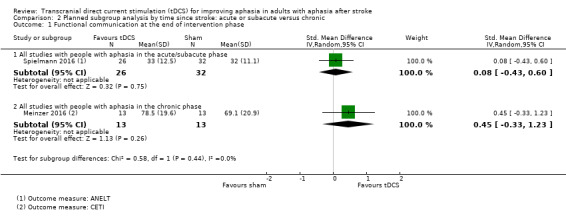
Comparison 2 Planned subgroup analysis by time since stroke: acute or subacute versus chronic, Outcome 1 Functional communication at the end of intervention phase.
Comparison 3: planned subgroup analysis by location of stimulation (lesioned or non‐lesioned hemisphere) and type of stimulation (A‐tDCS, C‐tDCS, S‐tDCS)
We performed a planned subgroup analysis regarding the electrode positioning and hence location of stimulation (Analysis 3.1). There was no evidence of effect between subgroups regarding the difference in functional communication between intervention and control groups regarding the location and the type of stimulation (test for subgroup differences: P = 0.73, I² = 0%).
3.1. Analysis.
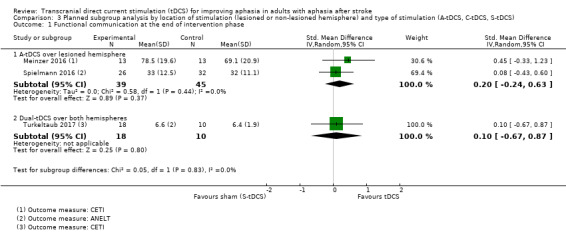
Comparison 3 Planned subgroup analysis by location of stimulation (lesioned or non‐lesioned hemisphere) and type of stimulation (A‐tDCS, C‐tDCS, S‐tDCS), Outcome 1 Functional communication at the end of intervention phase.
Comparison 4: post hoc subgroup analysis regarding type of aphasia (fluent, non‐fluent, or mixed populations)
We performed a post hoc subgroup analysis on our secondary outcome difference in naming nouns regarding the type of aphasia (Analysis 4.1). Whereas tDCS appeared to be effective in mixed populations but not in non‐fluent populations, there was no evidence of a statistically significant difference in treatment effect between subgroups regarding aphasia subtype.
4.1. Analysis.
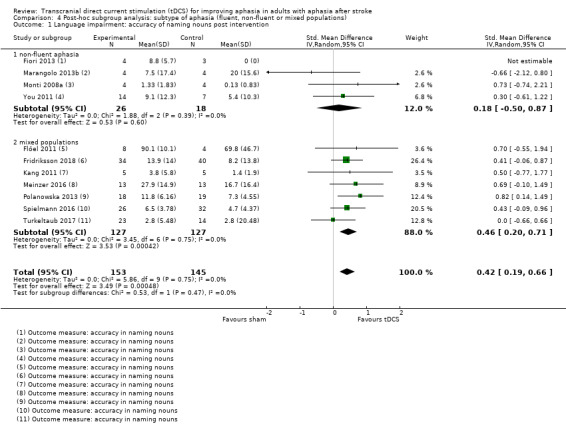
Comparison 4 Post‐hoc subgroup analysis: subtype of aphasia (fluent, non‐fluent or mixed populations), Outcome 1 Language impairment: accuracy of naming nouns post intervention.
Sensitivity analyses
The results of our planned sensitivity analysis for risk of bias in our included studies can be found in Table 4. It shows that the magnitude of effect varies, depending on the choice of studies in respect of their methodological quality that we incorporated in the analysis.
3. Sensitivity analysis for primary outcome functional communication depending on risk of bias.
| Analysis 1.1: Inclusion of: | Analysis results |
| All studies | (SMD 0.17, 95% CI −0.20 to 0.55; 112 participants; 3 studies; I² = 0%) |
| All studies with proper allocation concealment | SMD 0.08, 95% CI −0.43 to 0.60; 58 participants; 1 study I² = 0% |
| All studies with blinded outcome assessors | SMD 0.17, 95% CI −0.20 to 0.55; 112 participants; 3 studies; I² = 0% |
| All studies with intention‐to‐treat analysis | SMD 0.20, 95% CI −0.24 to 0.63; 84 participants; 2 studies; I² = 0% |
All studies with low risk of bias in the corresponding domains were included in this sensitivity analysis.
The sensitivity analysis regarding the strength of correlation in imputed standard deviations for change scores can be found in Table 5. It shows that the results of our analysis regarding the performance in accuracy of naming nouns at the end of intervention (Analysis 1.3), which is based on a calculated correlation coefficient, vary depending on the choice of different assumed correlation coefficients, but remains statistically significant. However, this is not true when considering this outcome measure at follow‐up (Analysis 1.4).
4. Sensitivity analysis for strength of correlation of imputed standard deviations for change scores in our secondary outcome naming nouns.
| Analysis | Studies with imputed SDs for change scores | Strength of mean correlation for experimental and control group | Analysis results |
| 1.3 | Polanowska 2013; Spielmann 2016; Turkeltaub 2017 | 0.976 (observed) | SMD 0.42, 95% CI 0.19 to 0.66; 298 participants; 11 studies; I² = 0% |
| 0.8 | SMD 0.31, 95% CI 0.07 to 0.54; 298 participants; 11 studies; I² = 0% | ||
| 0.6 | SMD 0.29, 95% CI 0.05 to 0.52; 298 participants; 11 studies; I² = 0% | ||
| 1.4 | Spielmann 2016 | 1.0 (observed) | SMD 0.87, 95% CI 0.25 to 1.48; 80 participants; 2 studies; I² = 32% |
| 0.8 | SMD 0.69, 95% CI −0.40 to 1.77; 80 participants; 2 studies; I² = 76% | ||
| 0.6 | SMD 0.66, 95% CI −0.48 to 1.80; 80 participants; 2 studies; I² = 78% |
SD: standard deviation
The correlation coefficients have been obtained from Meinzer 2016, which presented both total values and change scores for our secondary outcome naming performance in naming nouns.
The results of our primary outcome were robust regarding the risk of bias of included studies, and the effects of tDCS regarding accuracy in naming nouns at follow‐up were sensitive regarding assumptions of the underlying correlation coefficient for imputing missing standard deviations of change scores (Analysis 1.4).
Discussion
Summary of main results
The review focused on evaluating the effectiveness of tDCS (A‐tDCS, C‐tDCS, Dual‐tDCS) versus control (S‐tDCS, any other approach for improving aphasia after stroke, or no intervention). We included 21 trials with a total of 421 participants. Three studies with 112 participants addressed our primary outcome measure: investigating the effect of tDCS versus control on functional communication (the ability to communicate in an everyday communicative situation) measured by formal outcome measures of aphasia. There was no evidence of an effect either at post intervention or at follow‐up (low quality of evidence). Regarding our secondary outcome measure — performance in naming nouns and verbs — we found evidence of an effect at post intervention and at follow‐up in naming nouns (moderate quality of evidence), but not in naming verbs (very low quality of evidence). This is true when analysing the effect with combined intervention groups as stated in the protocol, that is A‐tDCS or C‐tDCS or Dual‐tDCS, versus S‐tDCS. Our sensitivity analyses yielded that the results of our secondary outcome — accuracy in naming nouns at follow‐up — were sensitive regarding assumptions of the underlying correlation coefficient for imputing missing standard deviations of change scores; that means that the statistically significant beneficial effect of tDCS could not be observed with a lower correlation coefficient. We found no study examining the effect of tDCS for improving aphasia on cognition. Serious adverse events were not reported and the rate of dropouts was comparable between groups (Analysis 1.6) (low quality of evidence). A summary of this review's main findings can be found in Table 1.
Overall completeness and applicability of evidence
The results of this review seem to be quite generalisable for settings in industrialised countries. However, there are some factors producing uncertainty. These are that:
most of the studies included participants with first‐ever stroke;
the majority of participants suffered from ischaemic stroke;
nearly all of the participants were right‐handed; and
the majority of participants were monolingual.
Hence, the results may be of limited applicability for people with recurrent stroke, haemorrhagic stroke, left‐handed people, and bilingual or multilingual people.
There is currently insufficient high‐quality evidence to make conclusions about the benefits or harms of tDCS. However, as there is no evidence of serious adverse effects and it can be easily administered, further research into tDCS is justified. We found no study examining the effect of tDCS for improving aphasia on cognition. This may be caused by the inherent difficulties in the assessment of cognition in people with aphasia and by the lack of agreement in the literature on the relationship between non‐verbal cognition and language/aphasia (Walker 2018; Wall 2017; Wortman‐Jutt 2017).
Regarding the comparable rate of dropouts between groups, it should not be assumed that the small number of dropouts in the included trials would be transferred into normal practice (Schünemann 2011).
Quality of the evidence
The quality of the evidence was very low to moderate.
We found heterogeneity regarding trial design (parallel group or cross‐over design, two or three intervention groups), therapy variables (type of stimulation, location of stimulation, dosage of stimulation), and participant characteristics (age, time post‐stroke, education and aphasia severity and subtype).
Potential biases in the review process
The methodological rigour of Cochrane Reviews minimises bias in the process of conducting systematic reviews. However, some aspects of this review are open to bias, such as only one review author (BE) eliminated obviously irrelevant publications according to their title and abstracts. This encompasses the possibility of unintentionally ruling out relevant publications. Another possibility is that publication bias could have affected our results. Although the funnel plot for our main outcome did not show evidence of publication bias, measured by visual inspection (Figure 3), this does not mean that publication bias is absent (Sterne 2011).
We had to exclude five included randomised cross‐over trials from the quantitative synthesis (meta‐analysis) because of missing information regarding treatment order (i.e. the first intervention period of the cross‐over trial) (Baker 2010; Marangolo 2013a; Marangolo 2013c; Rosso 2014; Shah‐Basak 2015). However, it is unlikely that the results of these studies would have altered our results substantially.
Agreements and disagreements with other studies or reviews
There is a systematic review of randomised and observational studies about the effects of tDCS on post‐stroke aphasia (Shah‐Basak 2016). The authors included eight studies with 140 participants. Their meta‐analysis showed an effect of tDCS on picture‐naming accuracy (SMD = 0.40, 95% CI 0.28 to 0.51), which is comparable to our results. Another systematic review described 19 studies with an unknown number of included participants and noted that there is emerging evidence regarding tDCS for improving post‐stroke aphasia and that methodological quality of future research should improve, which is consistent with our findings (ALHarbi 2017).
Although we did not find a statistically significant difference regarding adverse events between tDCS and sham tDCS, it should be mentioned that there is an ongoing debate on safety aspects of tDCS regarding cerebral autoregulation. List 2015 recommend the use of a cephalic reference electrode instead of an extracephalic localisation. This might reduce the risk of tDCS‐induced reduction of cerebral blood flow in people with cerebrovascular diseases.
Authors' conclusions
Implications for practice.
There is no evidence of the effectiveness of tDCS (A‐tDCS, C‐tDCS, Dual‐tDCS) versus control (S‐tDCS) for improving functional communication in people with aphasia (low quality of evidence), accuracy in naming verbs (very low quality of evidence), and cognition in stroke patients with aphasia at the present time. However, tDCS improves the accuracy in naming nouns (moderate quality of evidence), measured at the end of the intervention period and possibly also at follow‐up (moderate quality of evidence). Current evidence does not support the routine use of tDCS for aphasia after stroke.
Implications for research.
There is a demand for further randomised controlled trials (RCTs) with a parallel group design and sample‐size estimation in this area. The authors of future RCTs should strictly adhere to the CONSORT Statement (Schulz 2010). Data on functional communication and on adverse events should routinely be collected and presented in further publications, as well as data at follow‐up. Further study on the relationship between language/aphasia and cognition may be required and improved cognitive assessments developed for people with aphasia, prior to the use of tDCS to directly target cognition in aphasia. Authors should state total values at post intervention as well as their corresponding change scores with standard deviations.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2018 | New search has been performed | We have included 9 additional studies, resulting in 21 included studies, involving 421 participants. We found evidence of an effect of tDCS for improving accuracy in naming nouns. |
| 19 December 2018 | New citation required and conclusions have changed | The conclusions of the review have changed. There is moderate evidence of an effect of tDCS on naming nouns at the end of intervention and at follow‐up. |
History
Protocol first published: Issue 4, 2012 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 17 December 2014 | New search has been performed | We included seven new studies. The total number of included studies is now 12, involving 136 participants. We have added cognition as a secondary outcome, and have amended the text throughout |
| 17 December 2014 | New citation required but conclusions have not changed | The conclusions of the review have not changed since the previous version was published in June 2013 |
Acknowledgements
We thank Brenda Thomas for her help with developing the search strategy and Hazel Fraser for giving us helpful support. We also thank Susan Wortman‐Jutt, Julie Gildie and Odie Geiger for their valuable comments.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Aphasia explode all trees
#2 MeSH descriptor Language Disorders explode all trees
#3 MeSH descriptor Speech Disorders explode all trees
#4 MeSH descriptor Anomia explode all trees
#5 MeSH descriptor Speech‐Language Pathology explode all trees
#6 MeSH descriptor Rehabilitation of Speech and Language Disorders explode all trees
#7 (aphasi* or dysphasi* or anomia or anomic)
#8 ((speech or language or linguistic) NEAR/5 (disorder* or impair* or problem* or dysfunction))
#9 ((speech or language or linguistic) NEAR/5 (therap* or train* or rehabilitat* or treat* or remediat* or intervention* or pathol*))
#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#11 MeSH descriptor Electric Stimulation Therapy explode all trees
#12 MeSH descriptor Electric Stimulation explode all trees
#13 MeSH descriptor Electrodes explode all trees
#14 (transcranial NEAR/5 “direct current” NEAR/5 stimulation)
#15 (transcranial NEAR/5 DC NEAR/5 stimulation)
#16 (transcranial NEAR/5 electric* NEAR/5 stimulation)
#17 (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal)
#18 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17
#19 #10 AND #18
Number of records retrieved in 2014 search: 18
Number of records retrieved in updated 2018 search: 112
Appendix 2. MEDLINE Ovid search strategy
exp aphasia/
language disorders/ or speech disorders/ or anomia/
speech‐language pathology/ or exp "rehabilitation of speech and language disorders"/
(aphasi$ or dysphasi$ or anomia or anomic).tw.
((speech or language or linguistic) adj5 (disorder$ or impair$ or problem$ or dysfunction)).tw.
((speech or language or linguistic) adj5 (therap$ or train$ or rehabilitat$ or treat$ or remediat$ or intervention$ or pathol$)).tw.
or/1‐6
Electric Stimulation Therapy/
Electric Stimulation/
Electrodes/
(transcranial adj5 direct current adj5 stimulation).tw.
(transcranial adj5 DC adj5 stimulation).tw.
(transcranial adj5 electric$ adj5 stimulation).tw.
(tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode$ or anode or anodes or anodal or cathode or cathodes or cathodal).tw.
or/8‐14
7 and 15
exp animals/ not humans.sh.
16 not 17
Number of records retrieved in 2014 search: 416
Number of records retrieved in updated 2018 search: 271
Appendix 3. Embase Ovid search strategy
1. exp aphasia/ or dysphasia/
2. language disability/ or speech disorder/
3. exp speech rehabilitation/
4. (aphasi$ or dysphasi$ or anomia or anomic).tw.
5. ((speech or language or linguistic) adj5 (disorder$ or impair$ or problem$ or dysfunction)).tw.
6. ((speech or language or linguistic) adj5 (therap$ or train$ or rehabilitat$ or treat$ or remediat$ or intervention$ or pathol$)).tw.
7. or/1‐6
8. transcranial direct current stimulation/
9. electrostimulation therapy/ or nerve stimulation/ or electrostimulation/
10. electrode/
11. (transcranial adj5 direct current adj5 stimulation).tw.
12. (transcranial adj5 DC adj5 stimulation).tw.
13. (transcranial adj5 electric$ adj5 stimulation).tw.
14. (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode$ or anode or anodes or anodal or cathode or cathodes or cathodal).tw.
15. or/8‐14
16. 7 and 15
17. ((exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/))
18. 16 not 17
Number of records retrieved in 2014 search: 1261
Number of records retrieved in updated 2018 search: 509
Appendix 4. CINAHL EBSCO search strategy
S1 .(MH "Aphasia+") OR (MH "Speech Disorders") OR (MH "Language Disorders") OR (MH "Anomia")
S2 .(MH "Rehabilitation, Speech and Language") OR (MH "Speech‐Language Pathologists") OR (MH "Speech‐Language Pathology") OR (MH "Speech Therapy") OR (MH "Language Therapy")
S3 .TI ( aphasi* or dysphasi* or anomia or anomic ) OR AB ( aphasi* or dysphasi* or anomia or anomic )
S4 .TI ((speech or language or linguistic) N5 (disorder* or impair* or problem* or dysfunction)) OR AB ((speech or language or linguistic) N5 (disorder* or impair* or problem* or dysfunction))
S5 .TI ((speech or language or linguistic) N5 (therap* or train* or rehabilitat* or treat* or remediat* or intervention* or pathol*)) OR AB ((speech or language or linguistic) N5 (therap* or train* or rehabilitat* or treat* or remediat* or intervention* or pathol*))
S6 .S1 or S2 or S3 or S4 or S5
S7 .(MH "Electric Stimulation") OR (MH "Electrical Stimulation, Functional") OR (MH "Electrical Stimulation, Neuromuscular") OR (MH "Electrodes")
S8 .TI (transcranial N5 direct current N5 stimulation) OR AB (transcranial N5 direct current N5 stimulation)
S9 .TI (transcranial N5 electric N5 stimulation) OR AB (transcranial N5 electric N5 stimulation)
S10 .TI (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal) OR AB (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal)
S11 .S7 OR S8 OR S9 OR S10 OR S11
S12 .S6 AND S11
Number of records retrieved in 2014 search: 610
Number of records retrieved in updated 2018 search: 76
Appendix 5. AMED Ovid search strategy
1. exp aphasia/
2. language disorders/ or speech disorders/
3. speech language pathology/ or speech therapy/ or language therapy/
4. (aphasi$ or dysphasi$ or anomia or anomic).tw.
5. ((speech or language or linguistic) adj5 (disorder$ or impair$ or problem$ or dysfunction)).tw.
6. ((speech or language or linguistic) adj5 (therap$ or train$ or rehabilitat$ or treat$ or remediat$ or intervention$ or pathol$)).tw.
7. or/1‐6
8. Electric Stimulation/
9. (transcranial adj5 direct current adj5 stimulation).tw.
10. (transcranial adj5 DC adj5 stimulation).tw.
11. (transcranial adj5 electric$ adj5 stimulation).tw.
12. (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode$ or anode or anodes or anodal or cathode or cathodes or cathodal).tw.
13. or/8‐12
14. 7 and 13
Number of records retrieved in 2014 search: 15
Number of records retrieved in updated 2018 search: 1
Appendix 6. Web of Science ThomsonReuters search strategy
DocType=All document types; Language=All languages;
TS=(aphasia)
TS=(language disorders or speech disorders or anomia)
TS=(speech‐language pathology or "rehabilitation of speech and language disorders")
TS=(aphasi* or dysphasi* or anomia or anomic)
TS=((speech or language or linguistic) NEAR/5 (disorder* or impair* or problem* or dysfunction))
TS=((speech or language or linguistic) NEAR/5 (therap* or train* or rehabilitat* or treat* or remediat* or intervention* or pathol*))
#6 OR #5 OR #4 OR #3 OR #2 OR #1
TS=(Electric Stimulation Therapy)
TS=(Electric Stimulation)
TS=(Electrodes)
TS=(transcranial NEAR/5 "direct current" NEAR/5 stimulation)
TS=(transcranial NEAR/5 "DC" NEAR/5 stimulation)
TS=(transcranial NEAR/5 electric* NEAR/5 stimulation)
TS=(tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal)
#14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8
#15 AND #7
Number of records retrieved in 2014 search: 223
Number of records retrieved in updated 2018 search: 238
Appendix 7. LLBA search strategy
((SU("aphasia") OR SU("brocas aphasia") OR SU("wernickes aphasia") OR SU("language pathology") OR SU("anomia") OR SU("language therapy") OR SU("speech therapy") OR SU("speech/language therapists")) OR (TI(aphasi* OR dysphasi* OR anomia* OR anomic*) OR AB(aphasi* OR dysphasi* OR anomia* OR anomic*)) OR (TI(speech OR language OR linguistic) NEAR/5 TI(disorder* OR impair* OR problem* OR dysfunction OR therap* OR train* OR rehabilitat* OR treat* OR remediat* OR intervention* OR pathol*)) OR (AB(speech OR language OR linguistic) NEAR/5 AB(disorder* OR impair* OR problem* OR dysfunction OR therap* OR train* OR rehabilitat* OR treat* OR remediat* OR intervention* OR pathol*))) AND (TI(transcranial direct current stimulation or transcranial DC stimulation or transcranial electric* stimulation or tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal) or AB(transcranial direct current stimulation or transcranial DC stimulation or transcranial electric* stimulation or tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal))
Number of records retrieved in 2014 search: 136
Number of records retrieved in updated 2018 search: 91
Appendix 8. Inspec and COMPENDEX search strategy
((((aphasi* OR dysphasi* OR anomia* OR anomic* OR speech OR language OR linguistic)) WN KY) AND (((transcranial OR stimulation OR tDCS OR electrode* OR anode OR anodes OR anodal OR cathode OR cathodes OR cathodal)) WN KY)) + (2018 OR 2017 OR 2016 OR 2015 OR 2014) WN YR
Number of records retrieved in 2014 search: 734
Number of records retrieved in updated 2018 search: 1560
Appendix 9. PEDro search strategy
aphasia (Abstract & Title)
anomia (Abstract & Title)
#1 OR #2
Number of records retrieved in 2014 search: 16
Number of records retrieved in updated 2018 search: 18
Appendix 10. PsycBITE search strategy
Neurological Group: Stroke/CVA (Cerebrovascular Accidents)
Target Area: Aphasia/Dysphasia Method: Randomised Controlled Trial Age group: Adults (18+)
Search with AND
Number of records retrieved in 2014 search: 54
Number of records retrieved in updated 2018 search: 22
Appendix 11. SpeechBITE search strategy
Speech Pathology Practice Area: Aphasia Research Design : Randomised Controlled Trial Age group: Adults
Number of records retrieved in 2014: 70
Number of records retrieved in updated 2018 search: 25
Appendix 12. WHO International Clinical Trials Registry Platform search strategy
transcranial direct current stimulation AND aphasia OR tDCS AND aphasia transcranial direct current stimulation AND language OR tDCS AND language
Appendix 13. Stroke Trials Registry search strategy
stroke AND aphasia
Appendix 14. ClinicalTrials.gov search strategy
transcranial direct current stimulation AND ( Aphasia OR Language Disorders OR Speech Disorders ) [DISEASE]
Data and analyses
Comparison 1. tDCS plus speech and language therapy (SLT) versus sham tDCS plus SLT for improving aphasia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional communication post intervention | 3 | 112 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.20, 0.55] |
| 2 Functional communication at follow‐up | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.31, 0.58] |
| 3 Language impairment: accuracy of naming nouns post intervention | 11 | 298 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.19, 0.66] |
| 4 Language impairment: accuracy of naming nouns at follow‐up | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.87 [0.25, 1.48] |
| 5 Language impairment: accuracy of naming verbs post intervention | 3 | 21 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.68, 1.06] |
| 6 Safety: dropouts and adverse events until post intervention | 15 | 345 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.37] |
Comparison 2. Planned subgroup analysis by time since stroke: acute or subacute versus chronic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional communication at the end of intervention phase | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies with people with aphasia in the acute/subacute phase | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.43, 0.60] |
| 1.2 All studies with people with aphasia in the chronic phase | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.33, 1.23] |
Comparison 3. Planned subgroup analysis by location of stimulation (lesioned or non‐lesioned hemisphere) and type of stimulation (A‐tDCS, C‐tDCS, S‐tDCS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional communication at the end of intervention phase | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 A‐tDCS over lesioned hemisphere | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.24, 0.63] |
| 1.2 Dual‐tDCS over both hemispheres | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.67, 0.87] |
Comparison 4. Post‐hoc subgroup analysis: subtype of aphasia (fluent, non‐fluent or mixed populations).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Language impairment: accuracy of naming nouns post intervention | 11 | 298 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.19, 0.66] |
| 1.1 non‐fluent aphasia | 4 | 44 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.50, 0.87] |
| 1.2 mixed populations | 7 | 254 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.20, 0.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baker 2010.
| Methods | Randomised sham‐controlled double‐blind cross‐over trial | |
| Participants | Country: USA
10 participants (5 women) with chronic, stroke‐induced aphasia, age 45 to 81 years (mean ± SD, 65.50 ± 11.44) Inclusion criteria: 1‐time stroke in the left hemisphere, 6 months after stroke onset, <85 years of age, premorbidly right‐handed, native English speaker, and participant in a previous study that included fMRI examination Exclusion criteria: seizures during the previous 36 months, sensitive scalp, previous brain surgery, and medications that raise the seizure threshold |
|
| Interventions | Each participant underwent 1 of the following conditions (A: A‐tDCS 1 mA; B: S‐tDCS; 20 minutes each over the brain area with the highest activation during correct naming as measured by fMRI): ‐ computerised anomia training + 5 days A, 7 days rest period, computerised anomia training + 5 days B ‐ computerised anomia training + 5 days B, 7 days rest period, computerised anomia training + 5 days A |
|
| Outcomes | Outcomes were reported at the end of treatment and at 7 days' follow‐up: ‐ the change in correct picture naming in per cent (continuous; ranging from 0 to 100 with a higher value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not stated by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | For objective outcomes: participants were blinded; blinding of personnel not stated by the study authors |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | For objective outcomes: outcome assessment by 2 speech‐language pathologists blinded to stimulation type with a third speech‐language pathologist arbitrating in case of disagreement |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | For objective outcomes: all participants completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no protocol could be identified |
| Other bias | Low risk | No other bias identified |
Branscheidt 2018.
| Methods | Randomised sham‐controlled double‐blind cross‐over trial | |
| Participants | Country: Germany 16 participants (12 men, 4 women); mean age (SD): 61 (10) years; time since stroke (SD): 23 (18) months; educational level: NA; mean naming accuracy at baseline (SD): 79% (28%) Inclusion criteria: aphasia due to first‐ever ischaemic stroke, native German speaker, integrity of M1 established with CT or MRI scans Exclusion criteria: alexia |
|
| Interventions | Each participant underwent all of the following conditions: single session of either anodal or sham tDCS for 20 minutes with a 7‐day intersession interval between each condition | |
| Outcomes | Outcomes were recorded at baseline, and at the end of each intervention phase: ‐ reaction time ‐ accuracy of naming |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 'RAND' function of Excel was used to create a random number for each patient ..." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[The random] numbers were then sorted by size in descending order and assigned to the patient list created previously." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "tDCS stimulation (anodal and sham) was turned on by a third person not involved in the remainder of the experiment." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome measures reaction time of lexical decision task and accuracy rate |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Objective outcome measures: all participants apparently completed the study. No treatment withdrawals, no trial group changes and no major adverse events stated |
| Selective reporting (reporting bias) | Unclear risk | No study protocol stated |
| Other bias | Low risk | No other bias identified |
Dos Santos 2017.
| Methods | Randomised controlled cross‐over trial | |
| Participants | Country: Brazil 13 participants (7 men, 6 women); mean age (SD): 56 (18) years; time since stroke not described; educational level 11 out of 13 (85%) elementary school; mean naming accuracy at baseline not described Inclusion criteria: left hemispheric stroke at least 6 months prior and Broca or anomic aphasia Exclusion criteria: dysarthria, apraxia of speech, previous speech and language therapy, any clinically significant or unstable medical or psychiatric disorder, history of substance abuse, neuropsychiatric comorbidities |
|
| Interventions | Each participant underwent all of the following conditions before and after the Boston Naming Test with an intersession interval of unknown length between each condition: ‐ dual tDCS (A‐tDCS over Broca's area and concurrent C‐tDCS over homologous Broca's area) with 2 mA for 20 minutes ‐ TMS over homologous Broca's area with 1 Hz for 20 minutes, using 90% of the motor threshold ‐ sham tDCS with 2 mA for 20 seconds ‐ sham TMS with 1 Hz for 20 seconds |
|
| Outcomes | Outcomes were recorded at baseline, and at the end of intervention phase: ‐ performance in picture naming ‐ response time ‐ picture‐naming strategy ‐ response time strategy ‐ total response time |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The randomization was made by statistic orientation in three weeks ..." |
| Allocation concealment (selection bias) | Unclear risk | Not described by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Objective outcome measures: participants apparently were blinded, blinding of personnel not described by the study authors |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not described by the study authors |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Objective outcomes: all participants completed the study. No treatment withdrawals, no trial group changes and no major adverse events stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no published protocol could be identified |
| Other bias | Low risk | No other bias identified |
Fiori 2013.
| Methods | Randomised controlled cross‐over trial | |
| Participants | Country: Italy 7 participants (5 men, 2 women) with single left hemispheric stroke and non‐fluent aphasia were included; mean age (SD): 58 (10) years; time since stroke (SD): 33 (28) months; educational level: 13 (4) years; mean naming accuracy at baseline (SD): 8% (7%) Inclusion criteria: native Italian proficiency, pre‐morbid right‐handedness, single left hemispheric stroke, time since stroke at least 6 months, no neurological symptoms requiring medication, informed consent Exclusion criteria: not stated |
|
| Interventions | Each participant underwent all of the following conditions (20 minutes per day on 10 consecutive sessions; 100 minutes per week) with a 6‐day intersession interval between each condition: ‐ speech therapy plus A‐tDCS over the Broca's area with 1 mA for 20 minutes at the beginning ‐ speech therapy plus A‐tDCS over the Wernicke's area with 1 mA for 20 minutes at the beginning ‐ speech therapy plus S‐tDCS over Broca's or over Wernicke's area with 1 mA for 20 minutes at the beginning |
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase and at 1 and 4 weeks after the end of intervention phase: ‐ mean percentage of correct nouns and verbs (continuous; ranging from 0 to 100 with a higher value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described by the study authors |
| Allocation concealment (selection bias) | Unclear risk | Not described by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Participants and personnel were blinded Quote: "To ensure the double‐blind procedure, both the experimenter and the patients were blinded regarding the experimental and the sham conditions and the stimulator was turned on/off by another person." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not described by the study authors |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Objective outcome measures: all participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no protocol could be identified |
| Other bias | High risk | Risk of multiplicity: 4 out of 7 participants are the same as in Marangolo 2013a |
Flöel 2011.
| Methods | Randomised, double‐blind, sham‐controlled cross‐over trial | |
| Participants | Country: Germany
12 participants (7 men, 5 women) with first‐time single left hemisphere ischaemic stroke, age in years 39 to 67 (mean 52.3) with chronic aphasia, all participants were right‐handed and native speakers of German Inclusion criteria: not explicitly stated Exclusion criteria: severe apraxia of speech |
|
| Interventions | Computerised picture‐naming task + A‐tDCS 1 mA and C‐tDCS 1 mA and S‐tDCS; each for 20 minutes over the right temporo‐parietal cortex for 3 consecutive days, interrupted by 3 weeks of wash‐out period each | |
| Outcomes | Outcomes were reported immediately after training and 2 weeks after the end of the treatment session: ‐ proportional change of correct naming responses (continuous; ranging from 0 to 100 with a higher value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generator with the constraint that identical number of participants had to start/have second/have third session with A‐tDCS/C‐tDCS/sham, respectively (Flöel 2012) |
| Allocation concealment (selection bias) | Unclear risk | Only the person who applied stimulation knew about allocation (Flöel 2012). Hence it is unclear if this person was involved in recruiting participants |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | For objective and subjective outcomes, participants were blinded: "The respective stimulation conditions currents were subsequently turned off slowly out of the field of view of the patients, a procedure that does not elicit perceptions". Personnel were blinded (Flöel 2012) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessor was blinded (Flöel 2012) |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Objective outcome measures: all participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were reported |
| Selective reporting (reporting bias) | High risk | Objective outcome measures: no results have been provided for the Communicative Activity Log and Stroke and Aphasia Quality of Life Scale, which were stated as secondary outcome measures in the protocol |
| Other bias | Low risk | No other bias identified |
Fridriksson 2018.
| Methods | Randomised sham‐controlled double‐blind trial | |
| Participants | Country: USA 74 right‐handed people between 25 and 80 years of age, with aphasia due to left‐hemispheric first‐time ever stroke, more than 6 months post stroke, who are native English speakers Inclusion criteria: willing and able to give informed consent, willing and able to comply with study requirements, at least 65% accuracy on naming task during screening Exclusion criteria: previous brain surgery, seizures during last 12 months, sensitive scalp (self‐report), being able to name more than an average of 140 out of 175 items during the pre‐treatment PNT, inability to overtly name at least an average of 5 out of 80 items during the pre‐treatment fMRI sessions |
|
| Interventions | 2 arms: ‐ A‐tDCS (1 mA) for 20 minutes over the left scalp over the individually most active cortex region identified by naming tasks fMRI during 45 minutes of computerised naming treatment ‐ S‐tDCS for 20 minutes over the left scalp over the individually most active cortex region identified by naming tasks fMRI during 45 minutes of computerised naming treatment |
|
| Outcomes | Outcomes were recorded at baseline and at 1 week after the end of intervention period: Primary outcomes: ‐ change in correctly named objects of the PNT Secondary outcomes: ‐ adverse events during intervention period |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The Biostatistics Core at the Data Coordination Unit (located at Medical University of South Carolina) programmed the randomization algorithm, which used the minimal sufficient balancing method to prevent imbalances in site, baseline age, aphasia type, and aphasia severity." |
| Allocation concealment (selection bias) | Low risk | Quote: "Study participants and all members of the study team (the speech language pathologists [SLPs] who administered clinical testing and treatment, study coordinators, and principal and co‐investigators) were blinded to the intervention assignment." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "To blind patients as to whether they were receiving active or sham tDCS, the same scalp sensation was induced during the start of the S‐tDCS sessions when the tDCS stimulation was applied to the scalp for 30 seconds but then the current was gradually decreased over 15 seconds as the current was shunted to a load resistor. In‐house hardware was used to mask treatment type (A‐tDCS vs S‐tDCS) for both patients as well as the SLPs. The described randomization scheme directed an independent technician to set the position of an internal switch on the sham controller. Neither the patient nor SLP was aware of the position and the SLP did not know which switch position (X or Y) was the sham position. Treatment type was encoded in the software so the SLP only needed to enter a patient and session number to start stimulation without knowing whether those specific numbers were assigned toA‐tDCS or S‐tDCS.Following each individual’s treatment, a technician validated whether the tDCS device was delivering anodal or sham stimulation." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Please see "Allocation concealment". |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | The authors performed an intention‐to‐treat analysis. 33 out of 34 patients (97%) in the experimental group and 39 out of 40 patients (98%) in the control group received the assigned intervention. In the experimental group there were 3 dropouts (9%) until follow‐up (reasons not stated), whereas in the control group there were 2 dropouts (8%) with 1 patient's reasons being put down to adverse events. |
| Selective reporting (reporting bias) | Unclear risk | Results of naming error analysis presented in the protocol (supplementary file) not presented |
| Other bias | Low risk | No other bias identified |
Kang 2011.
| Methods | Randomised double‐blind sham‐controlled cross‐over trial | |
| Participants | Country: Republic of Korea
10 right‐handed Korean participants (2 women) with post‐stroke aphasia due to single left hemispheric infarction, age 46 to 73 years (mean ± SE, 61.9 ± 2.7) with mean full‐time education time 0 to 16 (mean ± SE, 11.6 ± 1.5), mean time from stroke onset to study entry 52.4 ± 21.9 months (range 6.0 to 180.6 months) Inclusion criteria: not clearly stated Exclusion criteria: multiple brain lesions, unstable medical or neurological conditions, metallic foreign body within the brain, pacemaker or artificial cochlear implant, severe depression, history of seizures and inability to perform protocol‐related behavioural tasks |
|
| Interventions | Every participant underwent both of the following treatment conditions, each over right Broca's homologue area: ‐ word retrieval training + 5 days C‐tDCS (2 mA for 20 minutes), at least 7 days' rest period, word retrieval training + 5 days S‐tDCS (20 minutes) ‐ word retrieval training + 5 days S‐tDCS (20 minutes), at least 7 days' rest period, word retrieval training + 5 days C‐tDCS (2 mA for 20 minutes) |
|
| Outcomes | Outcomes were reported at baseline and at the end of each treatment phase: ‐ number of correct responses (0 to 60 with 60 reflecting highest correctness) and ‐ reaction time of an adapted, standardized, validated Korean version of the BNT (0 to infinity with a lower value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated by the study authors |
| Allocation concealment (selection bias) | Unclear risk | Not stated by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Objective outcome measures: 1) personnel: "Word‐retrieval training was provided by a speech and language pathologist who was unaware of the type of stimulation administered (C‐tDCS or sham)"; 2) participants: "This sham procedure does not elicit patient's perceptions and was performed out of the patients' view" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome measures: "A single rater, unaware of stimulation type, administered the BNT" |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Objective outcome measures: all participants apparently completed the study. No treatment withdrawals, no trial group changes and no major adverse events stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no protocol could be identified |
| Other bias | Unclear risk | No other bias identified |
Marangolo 2011.
| Methods | Randomised double‐blinded cross‐over trial | |
| Participants | Country: Italy
3 participants (1 woman) with single left hemispheric stroke, non‐fluent aphasia and no signs of apraxia of speech Inclusion criteria: native Italian proficiency, pre‐morbid right‐handedness, persisting symptoms for at least 6 months Exclusion criteria: acute or chronic neurological symptoms requiring medication |
|
| Interventions | Each participant underwent 2 different treatment conditions (A: A‐tDCS, 1 mA; B: S‐tDCS, 20 minutes over the left inferior frontal gyrus (Broca's area)) in the following order: A: 5 days language therapeutic repetition task + A, 6 days' rest period, 5 days' language therapeutic repetition task + B B: 5 days language therapeutic repetition task + B, 6 days' rest period, 5 days' language therapeutic repetition task + A |
|
| Outcomes | Outcomes were reported at baseline, 1 week, 1 month and 2 months after the end of intervention: ‐ naming accuracy in per cent (continuous; ranging from 0 to 100 with a higher value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list (Marangolo 2012) |
| Allocation concealment (selection bias) | Unclear risk | None (Marangolo 2012) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Objective outcome measures: participants were blinded to stimulation condition, whereas personnel were not |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome measures: outcome assessor was unaware of stimulation type |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Objective outcome measures: all participants apparently completed the study. No treatment withdrawals, no trial group changes and no major adverse events stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no protocol could be identified |
| Other bias | Low risk | No other bias identified |
Marangolo 2013a.
| Methods | Randomised controlled cross‐over trial | |
| Participants | Country: Italy 12 people (8 men, 4 women) with single left hemispheric stroke were included; mean age (SD): 60 (8) years; time since stroke (SD): 37 (22) months; educational level (SD): 13 (4) years; baseline accuracy of naming (SD): 8% (3%) Inclusion criteria: native Italian proficiency, pre‐morbid right‐handedness, single left hemispheric stroke, time since stroke at least 6 months, no neurological symptoms requiring medication, informed consent Exclusion criteria: not stated |
|
| Interventions | Each participant underwent all of the following conditions (2 hours per day on 10 consecutive sessions) with a 14‐day intersession interval between each condition: ‐ speech therapy plus A‐tDCS over the Broca's area with 1 mA for 20 minutes at the beginning ‐ speech therapy plus A‐tDCS over the Wernicke's area with 1 mA for 20 minutes at the beginning ‐ speech therapy plus S‐tDCS over Broca's or over Wernicke's area with 1 mA for 20 minutes at the beginning |
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase and 4 weeks after the end of intervention phase: ‐ amount of stated Content Units ‐ amount of stated verbs ‐ amount of stated sentences (continuous; ranging from 0 to infinity with a higher value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random sequence was generated using sequentially numbered, opaque, sealed envelopes." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization procedure was delivered through allocation concealment. A clinician not involved in the rest of the study assigned each participant to the stimulation’s condition." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "Both the person with aphasia and the clinician were blind with respect to the administration of tDCS." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Both the person with aphasia and the clinician were blind with respect to the administration of tDCS." |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Objective outcome measures: all participants apparently completed the study. No treatment withdrawals, no trial group changes and no major adverse events stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no protocol could be identified |
| Other bias | High risk | Risk of multiplicity: 3 out of 12 participants (25%) apparently are the same as in Marangolo 2013c and 4 out of 12 participants (33%) are the same as in Fiori 2013 |
Marangolo 2013b.
| Methods | Randomised controlled cross‐over trial | |
| Participants | Country: Italy 8 participants (4 men, 4 women) with single left hemispheric stroke and non‐fluent aphasia with concurrent apraxia of speech were included; mean age (SD): 55 (9) years; time since stroke (SD): 29 (24) months; education level (SD): 12 (4) years; token test (SD): 11 (2) out of 36 Inclusion criteria: native Italian proficiency, pre‐morbid right‐handedness, single left hemispheric stroke, time since stroke at least 6 months, no acute or chronic neurological conditions requiring medication Exclusion criteria: not clearly described |
|
| Interventions | Each participant underwent all of the following conditions with a 14‐day intersession interval between each condition: A: patient‐tailored speech therapy plus A‐tDCS over the left inferior frontal gyrus (Broca's area) and C‐tDCS over the contralesional inferior frontal gyrus with 2 mA (20 minutes per weekday on 10 consecutive sessions) B: patient‐tailored speech therapy plus sham tDCS with the electrodes positioned as in (A) (20 minutes per weekday on 10 consecutive sessions) |
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase and 1 week after the end of intervention phase: ‐ accuracy of naming (continuous; ranging from 0 to 100 with a higher value indicating better performance) ‐ vocal reaction time (0 to infinity with a lower value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described by the study authors |
| Allocation concealment (selection bias) | Unclear risk | Not described by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "Both the patient and the clinician were blinded with respect to the administration of tDCS." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Both the patient and the clinician were blinded with respect to the administration of tDCS." |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Objective outcome measures: all participants apparently completed the study. No treatment withdrawals, no trial group changes and no major adverse events stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no protocol could be identified |
| Other bias | Low risk | No other bias identified |
Marangolo 2013c.
| Methods | Randomised controlled cross‐over trial | |
| Participants | Country: Italy 7 participants (5 men, 2 women) with single left hemispheric stroke with non‐fluent aphasia were included, mean age (SD): 62 (10) years; time since stroke (SD): 41 (27) months; educational level (SD): 13 (6) years; token test (SD): 14 (6) out of 36 Inclusion criteria: native Italian proficiency, pre‐morbid right‐handedness, single left hemispheric stroke, time since stroke at least 6 months, no acute or chronic neurological conditions requiring medication, informed consent Exclusion criteria: not clearly described |
|
| Interventions | Each participant underwent all of the following conditions for 5 consecutive days with a 14‐day intersession interval between each condition: ‐ speech therapy plus A‐tDCS over the Broca's area with 1 mA for 20 minutes (100 minutes per week) ‐ speech therapy plus A‐tDCS over the Wernicke's area with 1 mA for 20 minutes (100 minutes per week) ‐ speech therapy plus S‐tDCS over Broca's or over Wernicke's area with 1 mA for 20 minutes (100 minutes per week) |
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase and 1 week after the end of intervention phase: ‐ accuracy of naming (continuous; ranging from 0 to 100 with a higher value indicating better performance) ‐ vocal reaction time (0 to infinity with a lower value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described by the study authors |
| Allocation concealment (selection bias) | Unclear risk | Not described by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not clearly described by the study authors |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not described by the study authors |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Objective outcome measures: all participants apparently completed the study. No treatment withdrawals, no trial group changes and no major adverse events stated. 1 participant was not able to participate in the follow‐up examination due to personal reasons |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no published protocol could be identified |
| Other bias | High risk | Risk of multiplicity: 2 out of 7 participants (29%) are the same participants as in Marangolo 2013a and further 2 participants are the same as in Fiori 2013 |
Marangolo 2018a.
| Methods | Randomised controlled cross‐over trial | |
| Participants | Country: Italy 12 participants; mean age (SD): 58 (8) years; time since stroke (SD): 22 (7) months; educational level: 13 (3) years; mean noun naming accuracy at baseline of Battery for the Analysis of Aphasic Disorders test (SD): 55% (20%) Inclusion criteria: native Italian speaker, premorbid right‐handedness, a single left‐hemispheric stroke at least 6 months before the investigation, mild non‐fluent aphasia with no articulatory difficulties, preserved basic comprehension skills (so as to allow them to be engaged in verbal exchanges with the therapist) Exclusion criteria: attentive or memory deficits that could have biased performance |
|
| Interventions | Each participant underwent all of the following conditions (C‐tDCS with 2 mA on the right cerebellar cortex for 20 minutes once) with an intersession interval of unknown duration between each condition: ‐ right cathodal cerebellar tDCS for verb naming ‐ sham tDCS for verb naming ‐ right cathodal cerebellar tDCS for verb generation ‐ sham tDCS for verb generation |
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase: ‐ accuracy in verb generation ‐ accuracy in verb naming ‐ vocal reaction time in verb generation ‐ vocal reaction time in verb naming |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients underwent the four experimental conditions whose order was randomized across participants [using a computer‐generated list of random numbers (Microsoft Excel)]" (Marangolo 2018b) |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent experimenter provided the randomized allocation sequence through a computer generated randomization list." (Marangolo 2018b) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "To ensure the double‐blind procedure, both the experimenter and the patient were blinded regarding the stimulation condition, and the stimulator was turned on/off by another person." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome measure |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Objective outcome measures: all participants apparently completed the study. No treatment withdrawals, no trial group changes and no major adverse events stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no published protocol could be identified |
| Other bias | Low risk | No other bias identified |
Meinzer 2016.
| Methods | RCT | |
| Participants | Country: Germany 26 participants; 13 in the experimental group and 13 in the control group (18 men, 8 women); mean age (SD): 60 (14) years; time since stroke (SD): 46 (24) months; educational level: 12 (3) years; mean Aachen Aphasia Test naming performance at baseline (SD): 43% (21%) Inclusion criteria: right‐handed, native German speakers with chronic aphasia (> 12 months post stroke), impaired naming ability due to a single infarction or haemorrhage in the left hemisphere Exclusion criteria: contraindications to tDCS (e.g. cardiac pacemaker, history of seizures), a history of alcohol or drug abuse, other severe neurological, psychiatric or medical conditions, antidepressant or antipsychotic medication |
|
| Interventions | 2 arms: ‐ A‐tDCS over the left M1 (1 mA for 20 minutes) at the beginning of computer‐assisted naming treatment session with the 'vanishing cues' approach (2 times for 90 minutes a day, 4 days per week for 2 weeks) ‐ S‐tDCS over the left M1 (1 mA for 30 seconds) at the beginning of computer‐assisted naming treatment session with the 'vanishing cues' approach (2 times for 90 minutes a day, 4 days per week for 2 weeks) |
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase and at 6 months after the end of intervention phase: ‐ mean change in naming ability for trained items ‐ mean change in naming ability for untrained items ‐ confrontation naming of trained items ‐ quality of everyday communication, measured by CETI ‐ quality of everyday communication, measured by Partner Communication Questionnaire |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described by the study authors |
| Allocation concealment (selection bias) | Unclear risk | Not described by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Objective outcome measures: participants were blinded, but personnel was not |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "assessors were fully blinded to the stimulation conditions" |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | 2 participants in the experimental group and 2 in the control group dropped out between the end of study and 6‐month follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the published protocol have been reported; ANELT has been substituted by CETI |
| Other bias | Low risk | No other bias identified |
Monti 2008a.
| Methods | Randomised sham‐controlled cross‐over trial | |
| Participants | Country: Italy 8 right‐handed chronic non‐fluent aphasic patients (4 women), age in years (mean ± SD, 60.38 ± 11.99), education in years (mean ± SD, 10.62 ± 4.86), mean time from stroke onset to study entry 3.93 ± 1.89 years Inclusion criteria: not stated Exclusion criteria: severely impaired auditory verbal comprehension (Token Test < 8), severe apraxia of speech, seizures in the last 12 months, psychiatric disease and dementia |
|
| Interventions | Each participant underwent 2 different treatment conditions (A: A‐tDCS 2 mA; B: C‐tDCS 2 mA; C: S‐tDCS. Each for 10 minutes over the left Broca's region, order of intervention randomised) in the following order: ‐ picture‐naming task + A or C, at least 7 days rest period, picture‐naming task + C or A ‐ picture‐naming task + B or C, at least 7 days rest period, picture‐naming task + C or B |
|
| Outcomes | Outcomes were reported at baseline and at the end of intervention phase: ‐ naming accuracy in per cent (continuous; ranging from 0 to 100 with a higher value indicating better performance) ‐ reaction time for naming pictures (0 to infinity with a lower value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Microsoft Excel random number generator (Priori 2012 [pers comm]) |
| Allocation concealment (selection bias) | Low risk | A third person, uninvolved in the rest of the experiment, assigned participants to their stimulation groups (Priori 2012 [pers comm]) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Objective outcome measures: participants were blinded, whereas personnel were not |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessor was unaware of stimulation type |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Objective outcomes: all participants completed the study. No treatment withdrawals, no trial group changes and no major adverse events stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported; no published protocol could be identified |
| Other bias | Low risk | No other bias identified |
Polanowska 2013.
| Methods | Parallel group randomised double‐blind sham‐controlled trial | |
| Participants | Country: Poland 37 people with stroke‐induced non‐fluent aphasia; mean age (SD) experimental group: 58 (10), control group: 62 (12); time since stroke (SD) experimental group: 56 (45) days, control group: 64 (43) days; level of education (SD) experimental group: 15 (4) years, control group: 14 (3) years; median severity on the 6‐point ASRS experimental group: 2, control group: 2; recruited between May 2009 and June 2012 Inclusion criteria: pre‐morbid right‐handedness, aged 30 to 75 years, native Polish speaker, MRI‐confirmed first‐ever left MCA ischaemic stroke; between 2 and 24 weeks post‐stroke, non‐fluent aphasia (confirmed by BDAE; Polish version), relatively preserved comprehension and speech praxis, functional communication difficulties ranging from 1 to 3 on the 6‐point ASRS Exclusion criteria: unstable medical conditions, concurrent neurological or psychiatric illnesses, epileptiform EEG‐activity, use of medication that could affect cortical excitability |
|
| Interventions | 2 arms: ‐ computerised oral naming task for 45 minutes + A‐tDCS (1 mA for 10 minutes per session with the anode positioned over Broca's area) 5 times a week for 3 weeks ‐ computerised oral naming task for 45 minutes + S‐tDCS (1 mA for the first 25 seconds of every session with the anode positioned over Broca's area) 5 times a week for 3 weeks |
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase and at 3‐month follow‐up: ‐ number of correct naming responses (continuous; ranging from 0 to infinity with a higher value indicating better performance) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... allocation was performed using a computer program for stratified randomization with minimalization to ensure balance between groups ..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Group assignments were made by an independent investigator ..." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Both participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | 2 out of 12 participants (17%) from the control group dropped out due to recurrent stroke and have been excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no protocol could be identified |
| Other bias | Low risk | No other bias identified |
Rosso 2014.
| Methods | Randomised controlled cross‐over trial | |
| Participants | Country: France Participants: 25 people (12 men, 13 women) with stroke‐induced aphasia; mean age (SD): 57 (18) years; time since stroke (SD): 15 (20) months; median educational level: 2 or 3 years university degree; baseline picture‐naming accuracy (SD): 28% (13%) Inclusion criteria: left‐hemispheric first‐ever stroke in the MCA territory, aged between 18 and 85 years, native French speaker, the presence of aphasia based on item 9 of the NIHSS persistent at post‐stroke day 1 (≥ 1 point), no contraindications for MRI or tDCS, being able to walk (Rankin score ≤ 2), no severe white matter lesions (Fazekas score < 3) Exclusion criteria: not explicitly stated |
|
| Interventions | Each participant underwent both of the following conditions once (order randomised; electrodes were positioned by MRI‐based neuronavigation): ‐ C‐tDCS over the ascendant ramus of the lateral sulcus separating the pars triangularis and pars opercularis of the right inferior frontal gyrus (Broca's homologue area) with 1 mA for 15 minutes ‐ S‐tDCS over the ascendant ramus of the lateral sulcus separating the pars triangularis and pars opercularis of the right inferior frontal gyrus (Broca's homologue area) for 15 minutes |
|
| Outcomes | Outcomes were recorded at baseline and at the end of intervention phase: ‐ interhemispheric functional balance (measured by resting state functional MRI) ‐ picture‐naming accuracy (continuous; ranging from 0 to 100 with a higher value indicating better performance) ‐ integrity of language white matter pathways (measured by probabilistic tractography) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | The study authors presented only results regarding behavioural assessment of 19 out of 25 participants (76%) in diagrams and of 22 out of 25 participants (88%) in the text |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no published protocol could be identified |
| Other bias | Low risk | No other bias identified |
Shah‐Basak 2015.
| Methods | Randomised sham‐controlled cross‐over trial | |
| Participants | Country: USA Experiment 1: 12 participants (10 men, 2 women); mean age (SD): 64 (9) years; time since stroke (SD): 31 (30) months; educational level not described; mean WAB‐AQ (SD): 53 (24) Experiment 2: 7 participants who responded with improved naming ability on experiment 1 were enrolled in a randomised sham‐controlled cross‐over trial, 2 participants dropped out during this phase Inclusion criteria of experiment 1: single left‐hemispheric chronic stroke (> 6 months post stroke), mild‐to‐severe non‐fluent aphasia, premorbidly right‐handed, no history of neurological, psychiatric or unstable medical conditions Exclusion criteria of experiment 1: contraindications to either MRI or tDCS Inclusion criteria of experiment 2: positive response in naming ability in experiment 1 with at least 1 active electrode arrangement |
|
| Interventions | Each participant, during constraint‐induced language therapy, once underwent on non‐consecutive days all of the following conditions with a mean (SD) intersession interval of 7 (6) days between each condition in experiment 1 in random order: ‐ 1: A‐tDCS over the left frontal area (F3) and the reference electrode over the contralateral mastoid (2 mA for 20 minutes once) ‐ 2: C‐tDCS over the left frontal area (F3) and the reference electrode over the contralateral mastoid (2 mA for 20 minutes once) ‐ 3: A‐tDCS over the right frontal area (F4) and the reference electrode over the contralateral mastoid (2 mA for 20 minutes once) ‐ 4: C‐tDCS over the left frontal area (F4) and the reference electrode over the contralateral mastoid (2 mA for 20 minutes once) ‐ 5: S‐tDCS over the left frontal area (F3) and the reference electrode over the contralateral mastoid (2 mA for 1 minute once) ‐ 6: S‐tDCS over the right frontal area (F4) and the reference electrode over the contralateral mastoid (2 mA for 1 minute once) In experiment 2 each participant underwent the following interventions in random order: ‐ 1 of the active setups described above (1 to 4) (2 mA for 20 minutes, 5 times per week for 2 weeks) and ‐ 1 of the sham setups described above (5 or 6) (2 mA for 1 minute, 5 times per week for 2 weeks) |
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase and at 2 and 8 weeks after the end of intervention phase: ‐ WAB ‐ WAB‐AQ |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each of the six subjects who entered Phase 2 was randomized to receive either real‐tDCS treatment (n = 3), or sham stimulation followed by real‐tDCS (n = 3)." |
| Allocation concealment (selection bias) | Unclear risk | Not described by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "The order of five conditions was counterbalanced across subjects, who were blinded to real or sham‐tDCS [...] The person administering tDCS was not blinded to tDCS conditions." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Responses were recorded digitally and later scored offline by the investigator who was blinded to the montage." |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Objective outcomes: there were 2 dropouts out of 7 participants (29%) during experiment 2. No treatment withdrawals, no trial group changes and no major adverse events stated. 1 non‐responder was excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the 'Methods' section reported, no published protocol could be identified |
| Other bias | Low risk | No other bias identified |
Spielmann 2016.
| Methods | Multi‐centre RCT | |
| Participants | Country: the Netherlands 58 participants (40 men, 18 women); mean age (SD): 58 (10) years in the experimental group and 60 (10) years in the control group; time since stroke (SD): 1.4 (0.5) months in the experimental group and 1.6 (0.7) months in the control group; educational level: 12 (3) years in the experimental group and 13 (3) in the control group; mean aphasia severity according to shortened token test at baseline (SD):18.8 (7.9) in the experimental group and 19.1 (9.0) in the control group Inclusion criteria: aphasia after stroke, time post onset < 3 months, age 18 to 80 years, native speaker of Dutch, right‐handed Exclusion criteria: subarachnoid haemorrhage, prior stroke resulting in aphasia, brain surgery in the past, epileptic activity in the past 12 months (or anti‐epileptic medications), excessive use of alcohol/drugs, premorbid (suspected) dementia, premorbid psychiatric disease affecting communication, severe non‐linguistic cognitive disturbances impeding language therapy, pace maker, global aphasia, defined as Shortened Token Test < 9 and score 0 on the Aphasia Severity Rating Scale, severe Wernicke's aphasia, defined as Shortened Token Test < 9 and score 0 or 1 on the Aphasia Severity Rating Scale, residual aphasia, defined as Shortened Token Test > 28 and score 4 or 5 on the Aphasia Severity Rating Scale and Boston Naming Test > 150 |
|
| Interventions | 2 arms; each group received word‐finding therapy for 45 minutes per day on 5 consecutive sessions; 225 minutes per week: ‐ A‐tDCS for 1 mA for the first 20 minutes ‐ S‐tDCS for the first 20 minutes |
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase and 6‐month follow‐up: Primary outcome measures: ‐ Boston Naming Test (before and after each intervention week and at 6‐month follow‐up Secondary outcome measures: ‐ naming performance on trained and untrained items (in per cent, after each intervention week) ‐ Aphasia severity rating scale (after the second intervention week and at 6‐month follow‐up) ‐ ANELT (after the second intervention week and at 6‐month follow‐up) ‐ Wong‐Baker Faces 5‐point pain rating scale for assessing adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One of the authors (MHK, epidemiologist), not involved in selecting, testing, or treating participants, performed the randomization using an online random number generator." |
| Allocation concealment (selection bias) | Low risk | Quote: "The random numbers were combined with 5‐number codes from the tDCS manual for active or sham‐tDCS. These codes were concealed in opaque envelopes;[...]" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "[...] a unique code, which did not disclose whether active or sham tDCS would be provided, was used for each individual and was opened at the first therapy session by the speech and language therapists (SLTs)." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | 1 out of 26 participants of the experimental group (4%) and 1 out of 32 (3%) participants of the control group did not receive allocated intervention due to reasons supposed to be unrelated to the intervention |
| Selective reporting (reporting bias) | High risk | In comparison to the published protocol results for the following outcomes were not presented in this publication so far: SAQOL, Euroqol‐5D, care consumption, Werk en zorg vragenlijst (Health care consumption and labour productivity), laterality index, fMRI. Other outcomes: demographic data (socio‐economic status), size and location of the lesion (fMRI), participation: CIQ ‒ overall functioning: Barthel Index |
| Other bias | Low risk | No other bias identified |
Turkeltaub 2017.
| Methods | Randomised controlled double blind trial | |
| Participants | Country: USA 38 people above the age of 18 with aphasia due to left hemisphere stroke (diagnosed by a physician or speech‐language pathologist) Exclusion criteria: skull defect at or near the site of tDCS delivery, history of a significant stroke or traumatic brain injury additional to the event that caused the aphasia, history of other brain conditions that could impact interpretation of results (such as multiple sclerosis, brain tumour, encephalitis, premorbid dementia), presence of metallic devices in the head, psychiatric history, pregnancy, severe comprehension deficits Additional exclusion criteria for the optional MRI portion of the study: presence of metal in the body (except titanium), claustrophobia |
|
| Interventions | 2 arms; either ‐ Dual‐tDCS with the anodal electrode placed over the left hemisphere and the cathodal electrode placed over the right hemisphere at the beginning of each speech and language training session for 5 days a week for 1 week, or ‐ S‐tDCS at the beginning of each speech and language training session for 5 days a week for 1 week during 60 minutes of naming treatment sessions |
|
| Outcomes | Primary outcome measures: WAB‐R: Naming and Word Finding score (change from baseline to 1 day after intervention period) Secondary outcome measures: ‐ WAB‐R: spontaneous speech, repetition, auditory verbal comprehension and overall AQ immediately; 2 weeks after the end of intervention phase; 12 weeks after the end of intervention phase ‐ PNT immediately; 2 weeks after the end of intervention phase; 12 weeks after the end of intervention phase ‐ BDAE: verbal agility subtest immediately; 2 weeks after the end of intervention phase; 12 weeks after the end of intervention phase ‐ Subjective assessments including: CETI, Stroke and Aphasia Quality of Life Scale, and Stroke Aphasic Depression Questionnaire, immediately; 2 weeks after the end of intervention phase; 12 weeks after the end of intervention phase ‐ Cognitive‐Linguistic Quick Test immediately; 2 weeks after the end of intervention phase; 12 weeks after the end of intervention phase ‐ Reading assessments immediately; 2 weeks after the end of intervention phase; 12 weeks after the end of intervention phase ‐ Motricity Index immediately; 2 weeks after the end of intervention phase; 12 weeks after the end of intervention phase |
|
| Notes | Study description and results were published on the clinicaltrials.gov website | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described by the study authors |
| Allocation concealment (selection bias) | Unclear risk | Not described by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "[Blinding:] Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "[Blinding:] Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | 1 out of 24 (4%) participants in the experimental group was lost to follow‐up (reason not stated) and 0 out of 14 (0%) participants in the control group dropped out. 1 out of 23 patients (4%) of the experimental group and 1 out of 14 (7%) have been excluded from analysis (reason not stated) |
| Selective reporting (reporting bias) | High risk | All outcome measures listed in the protocol (except BDAE) have been reported |
| Other bias | Low risk | Results were published on the clinicaltrials.gov website |
Volpe 2014.
| Methods | Randomised cross‐over trial | |
| Participants | Country: USA Actual enrolment: 15 Inclusion criteria: ≥ 18 years of age, first single focal unilateral left hemisphere lesion with diagnosis verified by brain imaging (MRI or CT scans) that occurred at least 6 months prior, pre‐morbidly right handed, pre‐morbidly fluent English speaker, cognitive function sufficient to understand the experiments and follow instructions (per interview with Speech Pathologist), a baseline Aphasia Quotient score between 10 and 94 out of 100 points on the Western Aphasia Battery (neither completely without language comprehension/expression nor fully recovered from aphasia). Exclusion criteria: ongoing use of CNS‐active medications, ongoing use of psychoactive medications, such as stimulants, antidepressants, and anti‐psychotic medications, presence of additional potential tDCS risk factors (damaged skin at the site of stimulation (i.e. skin with ingrown hairs, acne, razor nicks, wounds that have not healed, recent scar tissue, broken skin, etc.), presence of an electrically, magnetically or mechanically activated implant (including cardiac pacemaker), an intracerebral vascular clip or any other electrically sensitive support system, metal in any part of the body, including metal injury to the eye (jewellery must be removed during stimulation), a history of medication‐resistant epilepsy in the family, past history of seizures or unexplained spells of loss of consciousness during the previous 36 months, pregnancy in women, as determined by self‐report |
|
| Interventions | Each participant underwent all of the following conditions, separated by 1 week of wash‐out: ‐ A‐tDCS with 1 mA once for 20 minutes during computerised aphasia therapy ‐ S‐tDCS once for 20 minutes during computerised aphasia therapy |
|
| Outcomes | Outcomes were recorded at baseline and at the end of intervention Primary outcome measure: ‐ mean change in picture‐naming accuracy score |
|
| Notes | Study description and results were published on the clinicaltrials.gov website | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described by the study authors |
| Allocation concealment (selection bias) | Unclear risk | Not described by the study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | All participants completed the study and there were no losses to follow‐up, no treatment withdrawals, no trial group changes and no major adverse events |
| Selective reporting (reporting bias) | Low risk | All outcome measures listed in the protocol have been reported |
| Other bias | Low risk | Results were published on the clinicaltrials.gov website |
You 2011.
| Methods | Parallel group randomised double‐blind sham‐controlled trial | |
| Participants | Country: Republic of Korea
33 participants with subacute left middle cerebral artery ischaemic infarction, confirmed by MRI, age in years (mean ± SD) 66.57 ± 10.76, education in years (mean ± SD) 11.43 ± 3.31, time post‐stroke (unit unknown, most likely in days; mean ± SD) 25.71 ± 7.07 Exclusion criteria: haemorrhagic stroke, history of previous stroke, seizures, multiple stroke lesions, metal implants in the brain, no adherence to speech therapy, medication with Na+, Ca2+ or NMDA receptor antagonists All participants were diagnosed with global aphasia and right‐handed |
|
| Interventions | 3 arms: participants received over 10 consecutive sessions, 5 times a week for 2 weeks, 1 of the following interventions (30 minutes each): ‐ conventional speech and language therapy + A‐tDCS (2 mA) over the left superior temporal gyrus ‐ conventional speech and language therapy + C‐tDCS (2 mA) over the right superior temporal gyrus ‐ conventional speech and language therapy + S‐tDCS over the left superior temporal gyrus |
|
| Outcomes | Outcomes were recorded at baseline and at the end of intervention phase: ‐ aphasia quotient of the Korean Western Aphasia Battery (continuous; from 0 to 100 with a higher value indicating a better result) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Objective outcome measures: blinding of both participants and personnel not stated by the study authors |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "One independent speech and language pathologist, who was blinded to the type of intervention performed, was used for these studies to measure patient outcomes." |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Objective outcome measures: 12 dropouts (36%) were stated without reasons and not included in analysis. However, the proportion of dropouts is relatively balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Objective outcomes: all outcomes listed in the 'Methods' section reported, no protocol could be identified |
| Other bias | Low risk | No other bias identified |
ANELT: Amsterdam‐Nijmegen Everyday Language Test A‐tDCS: anodal tDCS ASRS: Aphasia Severity Rating Scale BDAE: Boston Diagnostic Aphasia Examination BNT: Boston Naming Test CETI: Communicative Effectiveness Index CT: computed tomography C‐tDCS: cathodal tDCS EEG: electroencephalography fMRI: functional magnetic resonance imaging M1: primary motor cortex mA: milliampere (milliamp) MCA: middle cerebral artery MRI: magnetic resonance imaging NMDA: N‐methyl‐D‐aspartate NIHSS: National Institutes of Health Stroke Scale PNT: Philadelphia Naming Test RCT: randomised controlled trial SD: standard deviation S‐tDCS: sham tDCS SE: standard error tDCS: transcranial direct current stimulation TMS: transcranial magnetic stimulation WAB: Western Aphasia Battery WAB‐AQ: Western Aphasia Battery Aphasia Quotient WAB‐R: Western Aphasia Battery Revised
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fiori 2011 | Not a genuine RCT (pseudo‐randomisation of intervention) |
| Fridriksson 2011 | Not a genuine RCT |
| Holland 2011 | Not a genuine RCT (pseudo‐randomisation of intervention) |
| Lee 2013 | Inappropriate comparator intervention (2 different applications of tDCS have been compared) |
| Monti 2008b | In order to test the specificity of findings of Monti 2008a, this trial stimulated a biological implausible area (occipital cortex) to improve aphasia and therefore has been excluded |
| NCT02514044 | Irrelevant comparison: both groups received tDCS |
| NCT03486782 | Irrelevant comparison: all 3 groups received anodal tDCS |
| Richardson 2015 | Irrelevant comparison: both groups received tDCS |
| Vines 2011 | Not a genuine RCT |
RCT: randomised controlled trial tDCS: transcranial direct current stimulation
Characteristics of studies awaiting assessment [ordered by study ID]
Mac Kay 2015.
| Methods | RCT |
| Participants | 14 non‐fluent aphasic participants |
| Interventions | 2 arms: ‐ C‐tDCS with 2 mA for 20 minutes in 5 consecutive days (with the cathode placed over the homologous to Broca's area in the right hemisphere and the anode placed over the right supraorbital region) ‐ S‐tDCS (not described) |
| Outcomes | Outcome measures were assessed at baseline and at the end of intervention period ‐ Boston and Snodgrass naming tests |
| Notes | Conference abstract |
NCT02840370.
| Methods | Randomised cross‐over trial |
| Participants | Actual enrolment: 16 Inclusion criteria: chronic aphasia due to ischaemic or haemorrhagic stroke (> 6 months post‐stroke), French as dominant language, right‐handedness, left hemisphere lesion with intact bilateral prefrontal cortex Exclusion criteria: diagnosed dementia or psychiatric comorbidity, epileptic seizure within the last 12 months, metallic head implants, pacemaker, inability to understand procedures or insufficient language production abilities, pregnancy, strong headache on the days of the tDCS sessions, consumption of alcohol and/or unprescribed drugs on the days of the tDCS sessions or on the day before |
| Interventions | 2 arms: ‐ tDCS over the prefrontal cortex with 1 to 2 mA for 20 minutes ‐ S‐tDCS over the prefrontal cortex for 20 minutes |
| Outcomes | Outcomes will be measured up to 30 minutes after intervention: Primary outcome measure: ‐ picture‐naming task, repetition task and verbal fluency task Secondary outcome measure: ‐ non‐verbal executive functions task |
| Notes | Study completed in September 2017 |
C‐tDCS: cathodal tDCS RCT: randomised controlled trial S‐tDCS: sham tDCS
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IOR‐16010297.
| Trial name or title | Transcranial direct current stimulation (tDCS) combined semantic navigation training to improve aphasia |
| Methods | RCT |
| Participants | Country: China Estimated enrolment: 40 Inclusion criteria: 1) first onset of stroke in the left middle cerebral artery; 2) 2 to 24 weeks after onset; 3) aged 20 to 75 years old; 4) WAB Chinese version: non‐fluent aphasia; 5) Chinese native speakers; 6) right‐handed; 7) did not receive any formal speech training after stroke. Exclusion criteria: 1) dementia; 2) serious comprehension obstacles; 3) severe cognitive dysfunction; 4) dysarthria or speech apraxia; 5) vision and visual space obstacle; 6) hearing disorders; 7) mental disorders; 8) can not sit alone or complete aphasia test; 9) tDCS contraindications; 10) epilepsy |
| Interventions | 4 arms: ‐ A‐tDCS over Broca's area with the cathode on the right shoulder (n = 10) ‐ S‐tDCS with combined semantic navigation training (n = 10) ‐ A‐tDCS over Broca's area with the cathode on the right shoulder + combined semantic navigation training (n = 10) ‐ Dual tDCS (A‐tDCS over Broca's area with the cathode on the Broca's homologue area) + combined semantic navigation training (n = 10) |
| Outcomes | Outcomes will be recorded at unknown time points Primary outcomes: ‐ WAB AQ ‐ Standard Language Test for Aphasia ‐ Mini‐Cal ‐ naming of trained pictures ‐ naming of untrained pictures |
| Starting date | 30 December 2016 (however, study is not recruiting yet) |
| Contact information | Jiang Zhongli +86 13851898370 Jiangzh3721@163.com 300 Guangzhou Road, Nanjing, Jiangsu, China |
| Notes |
ChiCTR‐TRC‐14005072.
| Trial name or title | Effect of transcranial direct current stimulation on apraxia of speech |
| Methods | RCT |
| Participants | Country: China Estimated enrolment: n = 60 Inclusion criteria: 1) 1 to 3 months after the onset of a single left hemispheric stroke; 2) no previous brain injury; 3) a lesion involved in left frontal, temporal and parietal lobes; 4) aged between 15 and 75 years Exclusion criteria: 1) severely impaired auditory verbal comprehension (auditory word‐picture identification less than 6/60); 2) history of seizures; and 3) psychiatric disease or dementia |
| Interventions | 4 arms: ‐ tDCS over Broca's area + speech‐language therapy (n = 15) ‐ tDCS over Primary Sensorimotor area + speech‐language therapy (n = 15) ‐ tDCS over Supplementary Motor area + speech‐language therapy (n = 15) ‐ sham tDCS + speech‐language therapy (n = 15) |
| Outcomes | Outcomes were recorded at unknown time points Primary outcome measures: ‐ BDAE‐Chinese ‐ non‐verbal evaluation ‐ verbal evaluation |
| Starting date | Unknown |
| Contact information | Dongyu WU +86 13911202927 wudongyu73@hotmail.com China‐Japan Friendship Hospital No.2, Yinghuadongjie, Chaoyang District, Beijing |
| Notes |
DRKS00011116.
| Trial name or title | Effects of transcranial direct current stimulation (tDCS) on patients with apraxia of speech: a combined tDCS‐fMRI study |
| Methods | RCT |
| Participants | Estimated enrolment: 40 Inclusion criteria: aged between 18 and 80 years, first‐ever ischaemic stroke of the left cerebral hemisphere; chronic phase of the disease: > 6 months post onset; presence of apraxia of speech as diagnosed by speech and language therapist as well as neurologist; right‐handed; German as native language Exclusion criteria: severe aphasia with language comprehension < 25 in the relevant subtest of the AAT; left‐handedness; contraindications for tDCS (e.g. epilepsy); contraindications for MRI; no/reduced compliance; participation in clinical trial in the last 3 months |
| Interventions | 2 arms: ‐ Dual tDCS with the cathode placed over F7‒F5 right and the anode placed over F7‒F5 left, employing 20 minutes of 2 mA, in addition to conventional speech and language therapy (SLT), every day for 2 weeks ‐ S‐tDCS with the cathode placed over F7‒F5 right and the anode placed over F7‒F5 left. The current will be switched off after 20 seconds, rendering the intervention without effect, every day for 2 weeks |
| Outcomes | Primary outcomes: ‐ improvements in language abilities in the verum cohort as opposed to the sham cohort. Improvements will be measured using the Hierarchical Word Lists in the week prior to the first week of therapy and the week following the last therapeutic session (week 3) Secondary outcomes: ‐ improvements in other linguistic domains in the verum cohort as opposed to the sham cohort. These endpoints will be assessed using the AAT (Aachener Aphasie Test), a novel test measuring apraxia of speech and a formal test of diadochokinesis. Test points will be the week prior and the week after therapy (week 3) ‐ quality of life: the Aachen Quality of Life Inventory will be applied in the week prior to therapy and 20 weeks after therapy ‐ neuronal marker correlating with linguistic improvements. Structural (Diffusion tensor imaging) and fMRI (task based: spontaneous speech, diadochokinesis; resting state fMRI) will be employed prior to therapy and in the week after therapy (week 3) |
| Starting date | 4 August 2017 |
| Contact information | Uniklinik RWTH Aachen, Klinik für Neurologie Mr Dr med Cornelius J Werner Pauwelsstraße 30 52074 Aachen Germany Telephone: 0241‐8089600 E‐mail: cwerner at ukaachen.de |
| Notes |
JPRN‐UMIN000008467.
| Trial name or title | Transcranial direct current stimulation combined with speech therapy among patients with aphasia |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 60 people with aphasia due to stroke Inclusion criteria: time from stroke onset over 6 months, FIM comprehension item score > 5 Exclusion criteria: patients with implanted pacemaker, shunt or other implanted metal, medical history of seizure or other medical complication which inhibits recruitment |
| Interventions | tDCS + speech therapy Sham stimulation + speech therapy |
| Outcomes | Primary outcome measures: ‐ reaction time for naming task, Boston Naming Test Secondary outcome measures: ‐ cerebral blood flow ‐ standard Language Test of Aphasia ‐ communication section of FIM |
| Starting date | Not stated |
| Contact information | Toshiyuki Fujiwara, Keio University School of Medicine Rehabilitation Medicine, 35 Shinanomach, Shinjuku, Tokyo, Japan Email: tofuji@xc5.so‐net.ne.jp |
| Notes |
NCT00854893.
| Trial name or title | Enhance [sic] of language learning with neurostimulation (transcranial direct current stimulation) |
| Methods | Randomised double blind sham‐controlled cross‐over trial |
| Participants | Estimated enrolment: 70 people with aphasia due to ischaemic stroke with intact motor cortex at least 9 months since stroke, aged between 18 and 86 years Exclusion criteria: severe head trauma in the past, seizures, cardial pacemaker [sic], metal implants in the head/neck region, severe comorbidity, especially neurologic and psychiatric diseases, intake of illegal drugs, MMSE < 27, neuroactive substances (e.g. antidepressants), pregnancy |
| Interventions | A‐tDCS or C‐tDCS for 20 minutes or S‐tDCS for 30 seconds during language learning Intensity: 1 mA with the electrodes positioned over the primary motor cortex of language‐dominant hemisphere and the reference electrode over contralateral supraorbital area |
| Outcomes | Primary outcome measure: ‐ relative change to baseline in learning new words in per cent Time point of measurement: ‐ after the end of intervention period and 1 week after study end |
| Starting date | October 2009 |
| Contact information | Gianpiero Liuzzi, MD: +49 40 7410 ext 59278, g.liuzzi@uke.de Friedhelm Hummel, MD: +49 7410 ext 53772, f.hummel@uke.de |
| Notes |
NCT01486654.
| Trial name or title | Transcranial direct current stimulation and aphasia language therapy |
| Methods | Randomised controlled single‐blind trial |
| Participants | Estimated enrolment: 12 right‐handed people with single unilateral left‐hemispheric infarction confirmed by CT or MRI, at least 6 months post stroke, age > 21 years, native English speaker Inclusion criteria: non‐fluent aphasia, minimum education: eighth grade, sufficient visual and auditory acuity Exclusion criteria: other neurologic conditions such as Parkinson's disease, Alzheimer's dementia, TBI, significant psychiatric history, active substance abuse, seizures, lesioned premotor cortex |
| Interventions | 3 arms: either ‐ A‐tDCS ‐ C‐tDCS (1 mA, 5 days a week, for 6 weeks) or ‐ S‐tDCS during the first 13 minutes of 90 minutes of speech language treatment |
| Outcomes | Primary outcome measures: ‐ change from baseline in WAB‐AQ at 6 weeks after the end of intervention period Secondary outcome measures: ‐ change from baseline in functional communication skills at 6 weeks after the end of intervention period (assessed by language sample analyses) ‐ change from baseline in participation in everyday activities at 6 weeks after the end of intervention period (CETI, BOSS, CCRSA) ‐ change from baseline in reading and writing scores of the WAB at 6 weeks after the end of intervention period ‐ change in WAB‐AQ from 6 weeks after the end of intervention period at 12 weeks after the end of intervention period ‐ change in WAB‐reading and writing scores from 6 weeks after the end of intervention period at 12 weeks after the end of intervention period ‐ change from baseline in functional communication from 6 weeks after the end of intervention period in relation to 12 weeks after the end of intervention period (assessed by language sample analyses) ‐ change from baseline in participation in everyday activities from 6 weeks after the end of intervention period in relation to 12 weeks after the end of intervention period (CETI, BOSS, CCRSA) |
| Starting date | March 2010 |
| Contact information | Center for Aphasia Research and Treatment, Rehabilitation Institute of Chicago, Chicago, Illinois, USA Contact: Leora R Cherney PhD (Principal investigator): Tel: +1 312 238 6163 Email: lcherney@ric.org |
| Notes |
NCT01651884.
| Trial name or title | High definition transcranial direct current stimulation (HD‐tDCS) for stroke rehabilitation |
| Methods | Randomised single‐blind cross‐over trial |
| Participants | Estimated enrolment not stated. Right‐handed people, aged 25 to 80 years, with aphasia due to first‐time left‐hemispheric ischaemic stroke, time post‐stroke: at least 6 months, native speaker of English Exclusion criteria: clinically reported history of dementia, alcohol abuse, psychiatric disorder, TBI, or extensive visual acuity or visual‐spatial problems, factors contraindicative of tDCS administration (sensitive scalp, previous brain surgery), seizures during the previous year |
| Interventions | Cross‐over assignment to either: ‐ computerised language training + HD‐tDCS (dosage not stated) and then computerised language training + tDCS (dosage not stated) or ‐ computerised language training + tDCS (dosage not stated) and then computerised language training + HD‐tDCS (dosage not stated) Duration of resting periods not stated |
| Outcomes | Not described |
| Starting date | March 2012 |
| Contact information | Julius Fridriksson, PhD, University of South Carolina, Soterix Medical |
| Notes | Trial was completed in January 2013 |
NCT01701713.
| Trial name or title | Safety study of transcranial direct current stimulation in aphasia therapy in acute and post‐acute stroke |
| Methods | Randomised controlled double‐blind trial |
| Participants | Estimated enrolment: 100 right‐handed people with first‐time ever infarction in the middle cerebral artery and resulting language impairment, aged between 18 and 85 years and with an NIHSS < 20 Exclusion criteria: previous epilepsy or epileptogenic events or epilepsy typical elements in EEG, hypersensitive skin on the head, metal implants in the head, pacemakers or other electronic implants, previous head/brain surgery, medication reducing seizure threshold, psychiatric history |
| Interventions | Behavioural naming therapy + tDCS (polarity not stated) versus behavioural naming therapy + S‐tDCS |
| Outcomes | Primary outcome measures: skin irritation (type of assessment not stated) Secondary outcome measures: improved language, measured by improved picture naming |
| Starting date | June 2009 |
| Contact information | Contact: Gerhard J Jungehuelsing MD; email: jan.jungehuelsing@charite.de, or Isabell Wartenburger, Prof MD; email: isabell.wartenburger@uni‐potsdam.de |
| Notes | Status unknown, last update in October 2012 |
NCT02020421.
| Trial name or title | NOn‐invasive Repeated THerapeutic STimulation for Aphasia Recovery (NORTHSTAR) |
| Methods | RCT |
| Participants | Estimated enrolment: 65 Inclusion criteria: ischaemic stroke in the left MCA territory, between 5 and 30 days post stroke, right‐handedness, English, French, or German as language of daily use, score below the lower limit of the norm on at least 1 of the primary outcome measures Exclusion criteria: prior symptomatic ischaemic or hemorrhagic stroke, severe comprehension deficit that may compromise informed consent or understanding of instructions, contraindications to MRI and/or TMS/tDCS, neurodegenerative or psychiatric disease, epilepsy or EEG‐documented epileptic discharges, chronic renal or liver failure, life‐threatening diseases, auditory or visual deficits that cannot be corrected and might impair testing |
| Interventions | 3 arms: ‐ real rTMS and S‐tDCS: low frequency (1 Hz) rTMS over the centre of the right pars triangularis for 15 minutes (900 pulses) prior to each speech‐language therapy session. Stimulation intensity will be set at 90% of the RMT of the left FDI muscle + sham cathodal tDCS over the right pars triangularis. The anode will be placed on the forehead over the contralateral eye. To elicit the typical skin sensation of real tDCS (tingling sensation on the skin when tDCS is turned on and off), the current will be turned on for 30 seconds and then turned off for the duration of the speech‐language therapy session. The same procedure will be done at the end of the session ‐ real tDCS and sham rTMS: 2 mA cathodal tDCS over the right pars triangularis. The anode will be placed on the forehead over the contralateral eye. tDCS will start immediately before the speech‐language therapy session and last throughout the session + low frequency (1 Hz) rTMS over the vertex for 15 minutes (900 pulses) prior to each speech‐language therapy session. Stimulation intensity will be set at 10% of the RMT of the left FDI muscle ‐ sham rTMS and S‐tDCS: low frequency (1 Hz) rTMS over the vertex for 15 minutes (900 pulses) prior to each speech‐language therapy session. Stimulation intensity will be set at 10% of the RMT of the left FDI muscle + sham cathodal tDCS over the right pars triangularis. The anode will be placed on the forehead over the contralateral eye. To elicit the typical skin sensation of real tDCS (tingling sensation on the skin when tDCS is turned on and off), the current will be turned on for 30 seconds and then turned off for the duration of the speech‐language therapy session. The same procedure will be done at the end of the session |
| Outcomes | Primary outcome measures: ‐ change from baseline in verbal fluency on the Verbal Fluency Test at 1 and 30 days after completion of the treatment period ‐ change from baseline in language comprehension on the Token Test at 1 and 30 days after completion of the treatment period ‐ cumulative number of adverse events and serious adverse events during 10 days of therapy ‐ change from baseline in naming ability on the Boston Naming Test at 1 and 30 days after completion of the treatment period ‐ cumulative number of adverse events and serious adverse events during 30 days following completion of the treatment |
| Starting date | December 2013 |
| Contact information | Alexander Thiel, MD Jewish General Hospital (Montreal, Quebec) Montreal, Quebec, Canada, H3T 1E2 |
| Notes | Study completed in March 2018 |
NCT02101398.
| Trial name or title | Study of the effect of transcranial stimulations in aphasic subject within a year of their stroke |
| Methods | RCT |
| Participants | Estimated enrolment: 5 Inclusion criteria: man or woman of 18 years and older, aphasic patient following a first left hemispheric stroke, BDAE 3.0 aphasia score ≥ to 1, stroke within 3 to 12 months before inclusion in the study, native language French, right handedness, signed informed consent Exclusion criteria: history of other neurologic pathologies, epileptic seizure within 2 months before inclusion, dementia, bilingual patient, history of cranial surgery, presence of intracerebral metallic material, unauthorized drugs at inclusion (sulpiride, rivastigmine, dextromethorphan, carbamazepine, flunarizine, levodopa), pregnant, parturient or lactating woman |
| Interventions | 5 arms (tDCS will be delivered during a 20‐minute speech‐language therapy session): ‐ Dual tDCS with the anodal electrode set on the left Broca's area and cathodal electrode set on its right homologue ‐ Dual tDCS with the cathodal electrode set on the left Broca's area and anodal electrode set on its right homologue ‐ Dual tDCS with the anodal electrode set on the left Wernicke's area and cathodal electrode set on its right homologue ‐ Dual tDCS with the cathodal electrode set on the left Wernicke's area and anodal electrode set on its right homologue ‐ S‐tDCS with the electrodes set on the left Broca's area and its right homologue or electrodes set on the left Wernicke's area and its right homologue |
| Outcomes | Primary outcome measure: ‐ percentage of improvement in picture naming |
| Starting date | Unknown |
| Contact information | Sophie Charveriat sophie.charveriat@rpc.aphp.fr Hôpital Raymond Poincaré Garches, France, 92380 |
| Notes | Status unknown, not yet recruiting |
NCT02226796.
| Trial name or title | Transcranial direct stimulation (tDCS) and behavioral intervention in aphasia |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 10 Inclusion criteria: completion of high school or General Educational Development, normal or corrected‐to‐normal vision, adequate hearing acuity for 1:1 conversational exchanges, use of English as primary language, a vascular lesion in the dominant left hemisphere verified by an MRI scan within 6 months of the start of the study Exclusion criteria: no previous history of neurological‐ or psychiatric‐based illnesses or disease, language or learning disabilities, or alcohol/substance abuse; no history of seizures; no metal implants in the head (except dental fillings); no lesion in the left dorsolateral prefrontal cortex confirmed by MRI; no current pregnancy |
| Interventions | 2 arms: ‐ A‐tDCS for 20 minutes prior to 60 minutes of naming treatment ‐ 40 minutes of naming treatment, followed by additional 20 minutes of naming treatment with concurrent A‐tDCS |
| Outcomes | Primary outcome measure: ‐ Boston naming test Secondary outcome measures: ‐ naming reaction time ‐ working memory |
| Starting date | 1 September 2015 |
| Contact information | Naomi Hashimoto, PhD 715‐425‐3801 naomi.hashimoto@uwrf.edu University of Minnesota Minneapolis, Minnesota, USA, 55455 |
| Notes |
NCT02395874.
| Trial name or title | tDCS and speech therapy to 9mprove aphasia (MP‐LOGA) |
| Methods | RCT |
| Participants | Estimated enrolment: 96 Inclusion criteria: first time stroke (ischaemic or hemorrhagic), either with a total or partial anterior circulation stroke according to the Bamford classification, stroke interval 10 to 45 days, moderate or severe aphasia, i.e. Goodglass‒Kaplan Communication Scale (GKS, 0,1 or 2), native speaker of German, age 18 to 90 years Exclusion criteria: other neurological diseases affecting the CNS, known history of epileptic fits, except for an immediate fit, signs in the EEG of increased cortical excitability, patients with hemicraniectomy, fluent aphasia, i.e. GKS 3,4 or 5, speech apraxia, reduced sensibility of the scalp, previously radiated scalp, metallic parts or implants in the brain, participation in other interventional studies |
| Interventions | 2 arms: ‐ active tDCS with either the anode or cathode placed on the homologous speech area (TACS) in the right hemisphere or on the speech area perilesional in the left hemisphere (PACS) with 2 mA for 20 minutes for 6 weeks + concurrent speech therapy ‐ S‐tDCS + concurrent speech therapy |
| Outcomes | Primary outcome measures: ‐ Goodglass‐Kaplan communication scale (GKS, 0 to 5) ‐ Aphasia Check‐list (ACL, 0 to 148) Secondary outcome measures: ‐ Aphasic depression rating scale (ADRS, 0 to 32) ‐ Alterskonzentrationstest (AKT, 0 to 35) ‐ Barthel‐Index (BI, 0 to 100) ‐ Rivermead Motor Assessment ‐ Arm (RMA, 0 to 15) |
| Starting date | May 2015 |
| Contact information | Dr Cordula Werner +49‐30‐300240 ext 9271 cwerner@reha‐hesse.de Medical Park Berlin Humboldtmuehle Berlin, Germany, 13507 |
| Notes | Status unknown |
NCT02461355.
| Trial name or title | Transcranial direct current stimulation for post‐stroke aphasia |
| Methods | Randomised cross‐over trial |
| Participants | Actual enrolment: 2 Inclusion criteria: age over 21 years, ischaemic left hemispheric stroke verified by imaging (CT or MRI) more than 6 months ago, residual non‐fluent or anomic aphasia, with Western Aphasia Battery‐Revised Aphasia Quotient score < 60, fluent English speaker prior to stroke, right‐handed prior to stroke, ability to give informed consent and understand the tasks involved Exclusion criteria: history of recurrent stroke, either ischaemic or haemorrhagic, in the left middle cerebral artery territory, imaging unavailable, large middle cerebral artery infarct involving entire inferior division (temporo‐parietal) territory, history of dementia prior to the stroke, history of seizure, prior electroconvulsive therapy, deep brain stimulators, or brain surgery, social and/or personal circumstances that interfere with ability to return for therapy and assessment session |
| Interventions | 2 arms: ‐ A‐tDCS over the left posterior language areas during aphasia therapy for 8 × 1‐hour sessions ‐ S‐tDCS over the left posterior language areas during aphasia therapy for 8 × 1‐hour sessions |
| Outcomes | Primary outcome measures: ‐ change in percentage correct of trained scripts from baseline to up to 2 days post training ‐ change in words per minute of trained scripts from baseline to up to 2 days post training Secondary outcome measures: ‐ change in percentage correct of trained scripts from baseline to 2 weeks and 4 weeks post training ‐ change in words per minute of trained scripts from baseline to 2 weeks and 4 weeks post training Other outcome measures: ‐ change in percentage script words omitted from baseline to 2 weeks and 4 weeks post training |
| Starting date | June 2015 |
| Contact information | Tomoko Kitago, MD Adler Aphasia Center Maywood, New Jersey USA, 07607 |
| Notes | Study terminated due to poor recruitment |
NCT02540109.
| Trial name or title | Targeted Electrotherapy for Aphasia Stroke Rehabilitation (TEASER) ‐ phase II multi‐centre study |
| Methods | RCT |
| Participants | Estimated enrolment: 58 Inclusion criteria: 1‐time ischaemic stroke in the left hemisphere, > 6 months post stroke onset, between 25 and 75 years of age, aphasia diagnosis (as determined by pre‐treatment language‐based testing), right‐handed (before the stroke), native speaker of English, ability to provide informed written or verbal consent Exclusion criteria: clinically reported history of dementia, alcohol abuse, psychiatric disorder, traumatic brain injury, or extensive visual acuity or visual‐spatial problems, factors contraindicative of tDCS administration (sensitive scalp, previous brain surgery), prior history of epileptic or unprovoked seizures occurring during the previous 12 months, presence of metal implants or claustrophobia (not able to undergo MRI), pregnancy, presence of any other neurological disease than stroke, childhood history of speech, language, hearing, or intellectual impairment |
| Interventions | 2 arms (prior to treatment MRI and fMRI are acquired to inform the individualised current flow models for optimal targeting): ‐ HD‐tDCS in individualised dosage (number of electrodes and electrode placement) ‐ S‐tDCS in individualised dosage (number of electrodes and electrode placement) |
| Outcomes | Primary outcome measure: ‐ the ability of participants to name objects in a standardised naming task Secondary outcome measures: ‐ naming performance at 4 weeks and 6 months after treatment ‐ improvements in more general discourse performance ‐ screening comparison of HD‐tDCS with historical data on conventional non‐targeted tDCS using sponge electrodes |
| Starting date | July 2015 |
| Contact information | Abhishek Datta, PhD 888‐990‐8327 contact@soterixmedical.com |
| Notes |
NCT02612753.
| Trial name or title | Interest of combining speech therapy [sic]with a non‐invasive brain stimulation (tDCS) for the aphasic patient (Taph) |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 24 Inclusion criteria: aged above 18 years, aphasia due to a brain injury identified by MRI, aphasia severity score > 1 on the Boston Diagnostic Aphasia Examination (BDAE) severity scale, no post‐stroke delay is retained but the patient should be stable from a medical point of view, proficiency in spoken and written French, affiliated to a social security [sic], signed informed consent Exclusion criteria: other previous neurological pathologies, epileptic crisis during the previous 2 months, presence of a cranial flap, intracerebral metal hardware, patient under guardianship, patient unable to understand the study, patient subject to an exclusion period for another experiment |
| Interventions | Each participant will undergo the following interventions: ‐ A‐tDCS (2 mA for 20 minutes) for 3 weeks + speech and language therapy ‐ S‐tDCS for 20 minutes for 3 weeks + speech and language therapy |
| Outcomes | Primary outcome measures: ‐ change of number of names, without error and not repeated in the speech (time frame: baseline measures: start of week 1, 2 and 3. Outcomes measures: 1 assessment at the end of the 5th week, 1 at the beginning of the 7th week, 1 at the end of the 9th week and 1 at the end of the 10th week. Follow‐up measures: 12th, 14th, 16th week) "The participant must answer a simple question "explain to me what your job or your study is". Their response will be recorded and analysed. For evaluate the stability of participant performances before the stimulation period three based line will be propose [sic]. The third base line corresponds to the start of the first stimulation period. After the three week of tDCS coupled with the SLT a new assessment will be realized. One week later begin the new stage of cross‐over. An assessment will be administered just before and just after the second stimulation period. Then, three follow‐up assessments (one all two weeks [sic]) will be proposed during one and half month." Secondary outcome measures: ‐ verbal fluency (time frame: baseline measures: start of Week 1, 2 and 3. Outcome measures: 1 assessment at the end of the 5th week, 1 at the beginning of the 7th week, 1 at the end of the 9th week and 1 at the end of the 10th week. Follow‐up measures: 12th, 14th, 16th week) "The participant has 2 minutes to find the most animal names words beginning by letter P [sic]. Investigator collect the number of correct words and calculate the standard deviation according to published norms." ‐ working memory (time frame: baseline measures: start of week 1, 2 and 3. Outcome measures: 1 assessment at the end of the 5th week, 1 at the beginning of the 7th week, 1 at the end of the 9th week and 1 at the end of the 10th week. Follow‐up measures: 12th, 14th, 16th week) "The participant repeats the numbers in the same order or inverted order. Investigator collect the highest group of number repeated" ‐ visual exploration (time frame: baseline measures: start of week 1, 2 and 3. Outcome measures: 1 assessment at the end of the 5th week, 1 at the beginning of the 7th week, 1 at the end of the 9th week and 1 at the end of the 10th week. Follow‐up measures: 12th, 14th, 16th week). "A paper with a lot of drawing is presented to the participant. The participant must delete all the bells as fast as possible. Investigator collect the number of bell omissions" ‐ everyday life scale (time frame: baseline measures: start of week 1, 2 and 3. Outcome measures: 1 assessment at the end of the 5th week, 1 at the beginning of the 7th week, 1 at the end of the 9th week and 1 at the end of the 10th week. Follow‐up measures: 12th, 14th, 16th week) "A questionnaire is proposed to the participant in order to better understanding how is the communication with their close or with unknown person in a conversation or phone" [sic] ‐ Likert Scale (time frame: at the end of the 9th week, a Likert 5 grade scale was proposed) "Likert scale are proposed to know how the stimulation is tolerated and accepted by the participant, the patient family and the speech therapist" |
| Starting date | November 2015 |
| Contact information | Philippe Azouvi, MD,PhD 0033147107074 philippe.azouvi@aphp.fr |
| Notes |
NCT02622945.
| Trial name or title | Effects of transcranial direct current stimulation in post‐stroke aphasia |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 80 Inclusion criteria: clinically diagnosed with post‐stroke aphasia and word‐retrieval deficits, premorbid speakers of English, diagnosis will be based on neuropsychological testing, language testing (most commonly the Western Aphasia Battery), MRI and clinical assessment, stroke size: any, location: left hemisphere, time since stroke onset: 1 day to 20 years Exclusion criteria: uncorrected visual or hearing impairment by self‐report, other premorbid neurological disorder affecting the brain, any other language‐based learning disorder or other neurodegenerative disorder such as Alzheimer's disease or primary progressive aphasia, premorbidly diagnosed with a developmental language disorder, pregnant women |
| Interventions | Each participant will undergo the following interventions during 45 minutes of speech and language therapy: ‐ active tDCS to a pre‐specified region of the brain not affected by the lesion (perilesional areas, right hemisphere or cerebellum) with 2 mA for 20 minutes ‐ S‐tDCS to a pre‐specified region of the brain not affected by the lesion (perilesional areas, right hemisphere or cerebellum) with 0 mA for 20 minutes |
| Outcomes | Primary outcome measures: ‐ change in picture‐naming scores in trained and untrained items (time frame: before and after 15 sessions of intervention (3 weeks) and at 2 weeks and 2 months follow‐up) Secondary outcome measures: ‐ change in Philadelphia Naming Test: picture naming of everyday objects, different from training set (time frame: before and after 15 sessions of intervention (3 weeks) and at 2 weeks and 2 months follow‐up) ‐ change in written naming of objects and actions (time frame: before and after 15 sessions of intervention (3 weeks) and at 2 weeks and 2 months follow‐up). The investigators will evaluate the absolute number as well as the percent change of the list of objects and actions assigned for intervention as trained and untrained items ‐ change in working memory (digit span) (time frame: before and after 15 sessions of intervention (3 weeks) and at 2 weeks and 2 months follow‐up) ‐ change in verbal fluency (time frame: before and after 15 sessions of intervention (3 weeks) and at 2 weeks and 2 months follow‐up). The investigators will use letter (F, A, S) and semantic fluency measures (animals, fruits and vegetables) and the investigators will measure how many were added or omitted at follow‐up intervals |
| Starting date | February 2014 |
| Contact information | Kyrana Tsapkini, PhD 410‐614‐2464 tsapkini@jhmi.edu Johns Hopkins Medicine Baltimore, Maryland, USA, 21287 |
| Notes | Study withdrawn on 26 November 2018 (study could not recruit any participant) |
NCT02674490.
| Trial name or title | Stimulating Language in Subacute StrokE (SLISSE) |
| Methods | RCT |
| Participants | Estimated enrolment: 50 Inclusion criteria: acute ischaemic left hemisphere stroke, fluent speakers of English by self‐report, being capable of giving informed consent or indicating another to provide informed consent, age 18 or older, premorbidly right handed, within 3 months of onset of stroke, aphasia diagnosis as confirmed by the Western Aphasia Battery‒Revised, at least 65% accuracy on screening task (comparable to treatment task) on 1 of 3 attempts Exclusion criteria: previous neurological or psychiatric disease, including previous symptomatic stroke, seizures during the previous 12 months, uncorrected visual loss or hearing loss by self‐report, use of medications that lower the seizure threshold (e.g. methylphenidate, amphetamine salts), use of NMDA antagonists (e.g. memantine), history of brain surgery or any metal in the head, scalp sensitivity (per participant report) |
| Interventions | 2 arms: ‐ A‐tDCS (1 mA) plus speech and language treatment for 15 sessions (20 minutes per each 45‐minute treatment session) over the course of 3 weeks ‐ S‐tDCS plus speech and language treatment for 15 sessions (20 minutes per each 45‐minute treatment session) over the course of 3 weeks |
| Outcomes | Primary outcome measure: ‐ change in accuracy of naming untrained pictures (Philadelphia Naming Test) pre‐ to post‐treatment Secondary outcome measures: ‐ change in accuracy of naming untrained pictures at 5 and 20 weeks post treatment ‐ change in content of picture description pre‐ to post‐treatment immediately before and within 1 week after treatment ‐ change in efficiency of picture description pre‐ to post‐treatment immediately before and within 1 week after treatment ‐ change in content of picture description pre‐treatment to 5 weeks and 20 weeks post treatment ‐ change in efficiency of picture description pre‐treatment to 5 weeks and 20 weeks post treatment ‐ change in Stroke Impact Scale (SIS) pre‐treatment to post‐treatment |
| Starting date | 1 April 2016 |
| Contact information | Argye B Hillis‐Trupe, MD, MA 410‐614‐2381 argye@jhmi.edu |
| Notes |
NCT02801864.
| Trial name or title | tDCS as an adjuvant to intensive speech therapy for chronic post stroke aphasia |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 6 Inclusion criteria: left hemispheric stroke, > 50% on auditory verbal comprehension section of the WAB Exclusion criteria: any other neurological condition, other medical conditions such as seizures or implants, right hemispheric stroke, receiving teletherapy |
| Interventions | Each participant will undergo the following interventions during 180 minutes of speech and language therapy: ‐ active tDCS with 1 mA for 20 minutes ‐ S‐tDCS for 20 minutes |
| Outcomes | Outcomes will be measured after 8 weeks of treatment Primary outcome measures: ‐ object naming ‐ improvement in naming action verbs as measured by NAVS ‐ improvement in sentence production as measured by NAVS Secondary outcome measures: ‐ improvement in picture description as measured by WAB |
| Starting date | January 2016 |
| Contact information | Austin Speech Labs Austin, Texas, USA, 78757 |
| Notes |
NCT02901574.
| Trial name or title | Cerebellar transcranial direct current stimulation and aphasia treatment |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 50 Inclusion criteria: left hemispheric stroke, fluent speakers of English by self‐report, being capable of giving informed consent or indicating another to provide informed consent, aged above 18, premorbidly right‐handedness, > 6 months post stroke, aphasia diagnosis as confirmed by the BDAE short form, > 65% accuracy screening task (comparable to treatment task) on 1 of 3 attempts Exclusion criteria: participants with lesion involving the right cerebellum, previous neurological or psychiatric disease, seizures during the previous 12 months, uncorrected visual loss or hearing loss by self‐report, use of medications that lower the seizure threshold (e.g. methylphenidate, amphetamine salts), use of NMDA antagonists (e.g. memantine), > 80% (140 out of 175) correct responses on the Philadelphia Naming Test at baseline, history of brain surgery or any metal in the head, scalp sensitivity (per participant report) |
| Interventions | Each participant will undergo all of the following conditions: ‐ A‐tDCS or C‐tDCS with 2 mA for 20 minutes plus computerised naming treatment for 15 sessions (45 minutes each) over the course of 3 to 5 weeks ‐ S‐tDCS for 20 minutes plus computerised naming treatment for 15 sessions (45 minutes each) over the course of 3 to 5 weeks |
| Outcomes | Outcomes will be recorded at baseline, 2 weeks after the end of intervention period, and at 2 months follow‐up: ‐ Primary outcome measure: ‐ change in accuracy of naming of untrained pictures of the Philadelphia naming test (PNT) from baseline to post intervention Secondary outcome measures: ‐ change in accuracy of naming untrained pictures of the PNT from baseline to 2 weeks after the end of intervention period ‐ change in accuracy of naming untrained pictures of the PNT from baseline to 2 months follow‐up ‐ change in functional communication skills (measured by ASHA FACS) from baseline to post intervention ‐ change in fASHA FACS from baseline to 2 weeks after the end of intervention period ‐ change in ASHA FACS from baseline to 2 months follow‐up ‐ change in lexical features of picture description from baseline to post intervention ‐ change in lexical features of picture description from baseline to 2 weeks after the end of intervention period ‐ change in lexical features of picture description from baseline to 2 months follow‐up |
| Starting date | August 2016 |
| Contact information | Johns Hopkins University School of Medicine Baltimore, Maryland, USA, 21287 Rajani Sebastian, PhD, 410‐502‐6045, rsebast3@jhmi.edu Donna C Tippett, MA, MPH, 410‐502‐6045, dtippet@jhmi.edu |
| Notes |
NCT03164213.
| Trial name or title | Facilitation of brain plasticity for language recovery in patients with aphasia due to stroke |
| Methods | RCT |
| Participants | Estimated enrolment: 30 Inclusion criteria: patients post stroke with right hemiplegia and aphasia with cognitive capacity to understand instructions and at the aphasia cut‐off level defined by "Shemesh" assessment Exclusion criteria: unstable clinical state, craniotomy, epilepsy, heart pacer or lack of co‐operation |
| Interventions | 2 arms: ‐ A‐tDCS over the left M1 representation of the hand (C3 of the 10‒20 EEG system) with 1 mA for 20 minutes on 5 days per week for 2 weeks before 45 minutes of speech and language therapy ‐ S‐tDCS over the left M1 representation of the hand (C3 of the 10‒20 EEG system) for 20 minutes on 5 days per week for 2 weeks before 45 minutes of speech and language therapy |
| Outcomes | Outcome measures will be recorded at baseline, post intervention, and at 1‐month follow‐up Primary outcome measures: ‐ change in "Shemesh" 100 nouns test ‐ change in "Shemesh" 100 nouns test Secondary outcome measures: ‐ change in WAB (Hebrew) ‐ change in psycholinguistic assessment of language processing in aphasia |
| Starting date | May 2018 |
| Contact information | Contact: Nachum Soroker MD, 052‐3625193, nachums@clalit.org.il Contact: Corinne R Zarfati MD, 052‐8855626, corinneS@clalit.org.il |
| Notes |
NCT03272906.
| Trial name or title | Clinical feasibility of transcranial direct current stimulation (tDCS) with standard aphasia therapy |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 20 Inclusion criteria: single, unilateral stroke resulting in aphasia, competency to provide written informed consent, ability to participate in standard aphasia therapy Exclusion criteria: serious psychological condition, serious neurological condition other than stroke, serious medical condition, pregnancy, history of seizures, presence of electronic or metal implants (e.g. pacemaker, vagal nerve stimulator, etc.) |
| Interventions | Each participant will undergo all of the following conditions: ‐ A‐tDCS with 2 mA over the ventral inferior frontal gyrus for 20 minutes during 60 minutes of speech and language therapy over the course of 12 weeks ‐ S‐tDCS over the ventral inferior frontal gyrus for 20 minutes during 60 minutes of speech and language therapy over the course of 12 weeks |
| Outcomes | Outcomes will be recorded at the end of intervention period, and at 6‐week follow‐up: Primary outcome measures: ‐ production of Correct Information Units in Cinderella narrative Secondary outcome measures: ‐ WAB‐R ‐ Communication Activities of Daily Living‒2 ‐ PNT |
| Starting date | August 2017 |
| Contact information | Louisiana State University Baton Rouge, Louisiana, USA, 70803 Susan Duncan, PhD |
| Notes |
NCT03297450.
| Trial name or title | tDCS and aphasia therapy in the acute phase after stroke |
| Methods | RCT |
| Participants | Actual enrolment: 1 Inclusion criteria: diagnosed with mild‒moderate aphasia (Token Test Score between 7 and 40), inclusion in the first few days after stroke (acute phase), age 18 to 85 years, being right‐handed, Dutch as native language, being able to undergo functional and specific linguistic testing and therapy in the acute phase following stroke, imaging (CT or MRI) prior to inclusion (standard of care), signed informed consent Exclusion criteria: history of other diseases of the central nervous system, psychological disorders and (developmental) speech and/or language disorders, serious non‐linguistic cognitive disorders (as documented in the patient's medical history and inquired in anamneses), prior brain surgery, excessive use of alcohol or drugs |
| Interventions | 3 arms: ‐ C‐tDCS with 1 mA during the first 20 minutes of aphasia therapy ‐ S‐tDCS during the first 20 minutes of aphasia therapy ‐ S‐tDCS for 20 minutes |
| Outcomes | Outcome measures will be recorded at baseline, at 1‐week follow‐up, 3‐month follow‐up, and at 6 months Primary outcome measures: ‐ change in naming performance (BNT) ‐ changing vital parameters (blood pressure and heart rate) Secondary outcome measures: ‐ change in tolerability (visual analogue scale) ‐ change in spontaneous speech (AAT) ‐ change in ERPs |
| Starting date | October 2017 |
| Contact information | University Hospital Ghent Ghent, East‐Flanders, Belgium, 9000 Prof. Dr .Veerle De Herdt |
| Notes | Terminated in August 2018 (difficult patient recruitment) |
NCT03305614.
| Trial name or title | tDCS and aphasia therapy in the chronic phase after stroke |
| Methods | RCT |
| Participants | Estimated enrolment: 25 Inclusion criteria: diagnosed with mild to moderate aphasia (Token Test Score between 7 and 40) after a first left hemispheric ischaemic or haemorrhagic stroke, inclusion > 6 months post stroke, age 18 to 85 years, being right‐handed (> +8 on the Dutch handedness questionnaire), mother tongue: Dutch, imaging (CT or MRI) prior to inclusion (in patient file), standard of care in the acute phase, signed informed consent Exclusion criteria: history of other diseases of the central nervous system, psychological disorders and (developmental) speech and/or language disorders, serious non‐linguistic, cognitive disorders (as documented in the patients' medical history and inquired in the anamneses), prior brain surgery, excessive use of alcohol or drugs, new neurological symptoms between the acute stage and inclusion |
| Interventions | 2 arms: ‐ C‐tDCS with 1 mA or S‐tDCS (sic) during the first 20 minutes of aphasia therapy ‐ aphasia therapy |
| Outcomes | Outcome measures will be recorded at baseline, at 3‐week follow‐up, and at 3‐month follow‐up Primary outcome measure: ‐ change in naming performance (BNT) Secondary outcome measures: ‐ change in tolerability (visual analogue scale) ‐ change in spontaneous speech (AAT) ‐ change in ERPs ‐ change in quality of life (Dutch version of the Stroke and Aphasia Quality of Life scale) |
| Starting date | November 2017 |
| Contact information | University Hospital Ghent Ghent, East‐Flanders, Belgium, 9000 Veerle De Herdt, Prof. Dr. Contact: Elien De Cock +32(0)478 21 37 84 evedcock.decock@ugent.be |
| Notes |
AAT: Aachen Aphasia Test ASHA FACS: Functional Assessment of Communication Skills for Adults A‐tDCS: anodal tDCS AQ: aphasia quotient BDAE: Boston Diagnostic Aphasia Examination BNT: Boston Naming Test BOSS: Burden of Stroke Scale C‐tDCS: cathodal tDCS CCRSA: Communication Confidence Rating Scale for Aphasia CETI: Communicative Effectiveness Index CT: computerised tomography EEG: electroencephalography ERP: event‐related potential FDI: first dorsal interosseus FIM: Functional Independence Measure fMRI: functional magnetic resonance imaging GKS: Goodglass‐Kaplan‐Communication‐Scale HD‐tDCS: high definition tDCS Hz: Hertz M1: primary motor cortex mA: milliampere (milliamp) MMSE: Mini Mental State Examination MRI: magnetic resonance imaging NAVS: north‐western assessment and sentences NIHSS: National Institute of Health Stroke Scale NMDA: N‐methyl‐D‐aspartate PNT: Philadelphia Naming Test RCT: randomised controlled trial RMT: resting motor threshold r‐TMS: repetitive transcranial magnetic stimulation S‐tDCS: sham tDCS TBI: traumatic brain injury tDCS: transcranial direct current stimulation WAB: Western Aphasia Battery WAB‐AQ: Western Aphasia Battery ‐ Aphasia Quotient WAB‐R: Western Aphasia Battery – Revised
Differences between protocol and review
We discarded the analysis of fatigue due to the diversity and high complexity of neurological symptoms of this outcome. Due to the heterogeneity of outcome measures used for assessing language function we calculated standardised mean differences (SMD) instead of mean differences (MD). We included cognition as a secondary outcome.
Contributions of authors
All review authors contributed to the conception and design of the review and approved the draft. All review authors were involved in all stages of the review. BE was involved in screening titles and abstracts of publications identified by the searches. BE and JM extracted trial and outcome data from the selected trials and analysed outcome data. JM and MP were involved in assessing risk of bias in the included studies. All of the review authors interpreted the results.
Sources of support
Internal sources
Gesundheitswissenschaften/Public Health, Medizinische Fakultät Carl Gustav Carus der TU Dresden, Fetscherstr. 74, 01307 Dresden, Germany.
Wissenschaftliches Institut, Private Europäische Medizinische Akademie der Klinik Bavaria in Kreischa GmbH, An der Wolfsschlucht 1‐2, 01731 Kreischa, Germany.
Professur Therapiewissenschaften, SRH Hochschule für Gesundheit, Neue Straße 28‐30, 07548 Gera, Germany.
External sources
No sources of support supplied
Declarations of interest
Bernhard Elsner: none known Joachim Kugler: none known Marcus Pohl: none known Jan Mehrholz: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Baker 2010 {published data only}
- Baker JM. Using Transcranial Direct Current Stimulation to Treat Aphasia [Dissertation]. University of South Carolina, 2010. [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct‐current stimulation to treat stroke patients with aphasia. Stroke 2010;41(6):1229‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Branscheidt 2018 {published data only}
Dos Santos 2017 {published data only}
- Santos MDD, Cavenaghi VB, Mac‐Kay A, Serafim V, Venturi A, Truong DQ, et al. Non‐invasive brain stimulation and computational models in post‐stroke aphasic patients: single session of transcranial magnetic stimulation and transcranial direct current stimulation. A randomized clinical trial. Sao Paulo Medical Journal 2017; Vol. 135, issue 5:475‐80. [DOI] [PMC free article] [PubMed]
Fiori 2013 {published data only}
- Fiori V, Cipollari S, Paola M, Razzano C, Caltagirone C, Marangolo P. tDCS stimulation segregates words in the brain: evidence from aphasia. Frontiers in Human Neuroscience 2013; Vol. 7:269. [1662‐5161] [DOI] [PMC free article] [PubMed]
Flöel 2011 {published data only}
- Flöel A, Meinzer M, Kirstein R, Nijhof S, Deppe M, Knecht S, et al. Short‐term anomia training and electrical brain stimulation. Stroke 2011;42(7):2065‐7. [DOI] [PubMed] [Google Scholar]
- NCT00822068. Improvement of language disturbances after stroke by intensive training and electrical brain stimulation. clinicaltrials.gov/show/NCT00822068 (first received 14 January 2009).
Fridriksson 2018 {published data only}
- Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L. Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: a randomized clinical trial. JAMA Neurology 2018;75(12):1470‐6. [2168‐6157: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01686373. Brain stimulation and aphasia treatment. clinicaltrials.gov/show/NCT01686373 (first received 18 September 2012).
Kang 2011 {published data only}
- Kang EK, Kim YK, Sohn HM, Cohen LG, Paik NJ. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca's homologue area. Restorative Neurology and Neuroscience 2011;29(3):141‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EK, Sohn HM, Oh MK, Oh BM Jeon JY, Kim DY, et al. Paradoxical facilitatory effect of cathodal transcranial direct current stimulation on poststroke aphasia. Archives of Physical Medicine and Rehabilitation 2007;88(10):e8. [Google Scholar]
Marangolo 2011 {published data only}
- Marangolo P, Marinelli CV, Bonifazi S, Fiori V, Ceravolo MG, Provinciali L, et al. Electrical stimulation over the left inferior frontal gyrus (IFG) determines long‐term effects in the recovery of speech apraxia in three chronic aphasics. Behavioural Brain Research 2011;225(2):498‐504. [DOI] [PubMed] [Google Scholar]
Marangolo 2013a {published data only}
- Marangolo P, Fiori V, Calpagnano MA, Campana S, Razzano C, Caltagirone C, et al. tDCS over the left inferior frontal cortex improves speech production in aphasia. Frontiers in Human Neuroscience 2013;7:539. [1662‐5161] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangolo P, Fiori V, Campana S, Calpagnano MA, Razzano C, Caltagirone C, et al. Something to talk about: enhancement of linguistic cohesion through tdCS in chronic non fluent aphasia. Neuropsychologia 2014a;53(Jan):246‐56. [0028‐3932] [DOI] [PubMed] [Google Scholar]
Marangolo 2013b {published data only}
- Marangolo P, Fiori V, Cipollari S, Campana S, Razzano C, Paola M, et al. Bihemispheric stimulation over left and right inferior frontal region enhances recovery from apraxia of speech in chronic aphasia. European Journal of Neuroscience 2013;38(9):3370‐7. [1460‐9568] [DOI] [PubMed] [Google Scholar]
- Marangolo P, Fiori V, Gelfo F, Shofany J, Razzano C, Caltagirone C, et al. Bihemispheric tDCS enhances language recovery but does not alter BDNF levels in chronic aphasic patients. Restorative Neurology and Neuroscience 2014; Vol. 32, issue 2:367‐79. [0922‐6028] [DOI] [PubMed]
Marangolo 2013c {published data only}
Marangolo 2018a {published data only}
Meinzer 2016 {published data only}
- Darkow R, Martin A, Wurtz A, Flöel A, Meinzer M. Transcranial direct current stimulation effects on neural processing in post‐stroke aphasia. Human Brain Mapping 2017;38(3):1518‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Darkow R, Lindenberg R, Flöel A. Electrical stimulation of the motor cortex enhances treatment outcome in post‐stroke aphasia. Brain 2016;139(Pt 4):1152‐63. [DOI] [PubMed] [Google Scholar]
- NCT01221779. Chronic Aphasia ‐ improved by intensive training and electrical brain stimulation. ClinicalTrials.gov/show/NCT01221779 (first received 15 October 2010).
- NCT01845129. Impact of transcranial direct current stimulation of the motor cortex on language functions in residual aphasia. ClinicalTrials.gov/show/NCT01845129 (first received 3 May 2013).
- NCT01924702. Chronic aphasia ‐ improved by intensive training and electrical brain stimulation (CATS). ClinicalTrials.gov/show/NCT01924702 (first received 16 August 2013).
Monti 2008a {published data only}
- Cappa SF. Current to the brain improves word‐finding difficulties in aphasic patients. Journal of Neurology, Neurosurgery, and Psychiatry 2008;79(4):364. [DOI] [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic‐Sposta S, et al. Improved naming after transcranial direct current stimulation in aphasia. Journal of Neurology, Neurosurgery, and Psychiatry 2008;79(4):451‐3. [DOI] [PubMed] [Google Scholar]
Polanowska 2013 {published data only}
- Polanowska K, Lesniak M, Seniow J. Anodal transcranial direct current stimulation in early treatment of post‐stroke non‐fluent aphasia. Clinical Neurophysiology 2013; Vol. 124 (10):e118‐9. [1388‐2457]
- Polanowska KE, Lesniak M, Seniow JB, Czlonkowska A. No effects of anodal transcranial direct stimulation on language abilities in early rehabilitation of post‐stroke aphasic patients. Neurologia i Neurochirurgia Polska 2013; Vol. 47, issue 5:414‐22. [0028‐3843] [DOI] [PubMed]
- Polanowska KE, Lesniak MM, Seniow JB, Czepiel W, Czlonkowska A. Anodal transcranial direct current stimulation in early rehabilitation of patients with post‐stroke non‐fluent aphasia: A randomized, double‐blind, sham‐controlled pilot study. Restorative Neurology and Neuroscience 2013; Vol. 31, issue 6:761‐71. [0922‐6028] [DOI] [PubMed]
Rosso 2014 {published data only}
Shah‐Basak 2015 {published data only}
- Norise C, Sacchetti D, Hamilton R. Transcranial direct current stimulation in post‐stroke aphasia: the impact of baseline severity and task specificity [69th American Academy of Neurology Annual Meeting, AAN 2017, USA]. Neurology. Netherlands: Lippincott Williams and Wilkins, 2017; Vol. 88, issue 16 Supplement 1. [1526‐632X]
- Norise C, Sacchetti D, Hamilton R. Transcranial direct current stimulation in post‐stroke chronic aphasia: the impact of baseline severity and task specificity in a pilot sample. Frontiers in Human Neuroscience 2017;11:260. [1662‐5161] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah‐Basak PP, Norise C, Garcia G, Torres J, Faseyitan O, Hamilton RH. Individualized treatment with transcranial direct current stimulation in patients with chronic non‐fluent aphasia due to stroke. Frontiers in Human Neuroscience 2015, issue Apr:1‐12. [DOI] [PMC free article] [PubMed]
Spielmann 2016 {published data only}
- NTR4364. tDCS and aphasia. www.trialregister.nl/trialreg/admin/rctview.asp?TC=4364 2014.
- Spielmann K, Sandt‐Koenderman M, Ribbers G. The additional effect of transcranial direct current stimulation (tDCS) in post‐stroke sub‐acute aphasia. Brain Injury 2016;5‐6:755‐6. [Google Scholar]
- Spielmann K, Sandt‐Koenderman WM, Heijenbrok‐Kal MH, Ribbers GM. Transcranial direct current stimulation in post‐stroke sub‐acute aphasia: study protocol for a randomized controlled trial. Trials 2016:380. [DOI] [PMC free article] [PubMed]
- Spielmann K, Sandt‐Koenderman MWME, Ribbers GM. Transcranial direct current stimulation (tDCS) to enhance treatment effects in aphasia. Archives of Physical Medicine and Rehabilitation 2014; Vol. 95 (10):e21. [0003‐9993]
- Spielmann K, Sandt‐Koenderman WME, Heijenbrok‐Kal MH, Ribbers GM. Transcranial direct current stimulation does not improve language outcome in subacute poststroke aphasia. Stroke 2018;49(4):1018‐20. [0039‐2499] [DOI] [PubMed] [Google Scholar]
Turkeltaub 2017 {published data only}
- NCT01709383. Using transcranial direct current stimulation (tDCS) to improve post‐stroke aphasia. clinicaltrials.gov/show/NCT01709383 (first received 6 July 2017).
- Turkeltaub P, Fama M, Desko A, Taylor L, Hussey L, Friedman J, et al. Progress on a randomized trial of transcranial direct current stimulation for poststroke aphasia. Clinical and Translational Science 2014;7(3):219. [1752‐8054] [Google Scholar]
Volpe 2014 {published data only}
- NCT02249819. Evaluating Anodal tDCS Preceding Aphasia Therapy. clinicaltrials.gov/show/nct02249819 (first received 26 September 2014).
You 2011 {published data only}
- You DS, Kim DY, Chun MH, Jung SE, Park SJ. Cathodal transcranial direct current stimulation of the right Wernicke's area improves comprehension in subacute stroke patients. Brain and Language 2011;119(1):1‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fiori 2011 {published data only}
- Fiori V, Coccia M, Marinelli CV, Vecchi V, Bonifazi S, Ceravolo MG, et al. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of Cognitive Neuroscience 2011;23(9):2309‐23. [DOI] [PubMed] [Google Scholar]
Fridriksson 2011 {published data only}
- Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia. Stroke 2011;42:819‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holland 2011 {published data only}
- Holland R, Leff AP, Josephs O, Galea JM, Desikan M, Price CJ, et al. Speech facilitation by left inferior frontal cortex stimulation. Current Biology 2011;21(16):1403‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee SY, Cheon HJ, Yoon KJ, Chang WH, Kim YH. Effects of dual transcranial direct current stimulation for aphasia in chronic stroke patients. Annals of Rehabilitation Medicine 2013; Vol. 37, issue 5:603‐10. [2234‐0645] [DOI] [PMC free article] [PubMed]
Monti 2008b {published data only}
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic‐Sposta S, et al. Improved naming after transcranial direct current stimulation in aphasia. Journal of Neurology, Neurosurgery, and Psychiatry 2008;79(4):451‐3. [DOI] [PubMed] [Google Scholar]
NCT02514044 {published data only}
- NCT02514044. Dextroamphetamine and tDCS to Improve the Fluency. clinicaltrials.gov/show/NCT02514044 (first received 3 August 2015).
NCT03486782 {published data only}
- NCT03486782. Dual site‐dual channel non‐invasive brain stimulation for language and cognitive function in stroke patients. clinicaltrials.gov/show/NCT03486782 (first received 3 April 2018).
Richardson 2015 {published data only}
- Richardson J, Datta A, Dmochowski J, Parra LC, Fridriksson J. Feasibility of using high‐definition transcranial direct current stimulation (HD‐tDCS) to enhance treatment outcomes in persons with aphasia. NeuroRehabilitation 2015, issue 1:115‐26. [DOI] [PMC free article] [PubMed]
Vines 2011 {published data only}
- Vines BW, Norton AC, Schlaug G. Applying transcranial direct current stimulation in combination with melodic intonation therapy facilitates language recovery for Broca's aphasic patients. Stroke 2007;38(2):519. [Google Scholar]
- Vines BW, Norton AC, Schlaug G. Non‐invasive brain stimulation enhances the effects of melodic intonation therapy. Frontiers in Psychology 2011;2:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Mac Kay 2015 {published data only}
- Mac Kay A, Rodrigues Da Silva F, Chao JCT, Devido Dos Santos M, Gagliardi RJ. Effects of transcranial direct current stimulation on naming tasks in aphasic subjects after stroke. Journal of the Neurological Sciences 2015:e354.
NCT02840370 {published data only}
- NCT02840370. Effect of stimulation of the prefrontal cortex on language production in aphasic patients. clinicaltrials.gov/show/nct02840370 (first received 21 July 2016).
References to ongoing studies
ChiCTR‐IOR‐16010297 {published data only}
- ChiCTR‐IOR‐16010297. Transcranial direct current stimulation (tDCS) combined semantic navigation training to improve aphasia. www.chictr.org.cn/hvshowproject.aspx?id=10861 (first received unknown date). [ChiCTR‐IOR‐16010297]
ChiCTR‐TRC‐14005072 {published data only}
- ChiCTR‐TRC‐14005072. Effect of transcranial direct current stimulation on apraxia of speech. www.chictr.org.cn/com/25/showprojen.aspx?proj=4503 (first received unknown date). [ChiCTR‐TRC‐14005072]
DRKS00011116 {published data only}
- DRKS00011116. Effects of transcranial direct current stimulation (tDCS) on patients with apraxia of speech: a combined tDCS‐fMRI study. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00011116 (first received 31 August 2017). [DRKS00011116]
JPRN‐UMIN000008467 {published data only}
- JPRN‐UMIN000008467. Transcranial direct current stimulation combined with speech therapy among patients with aphasia. apps.who.int/trialsearch/Trial.aspx?TrialID=JPRN‐UMIN000008467 (first received unknown date).
NCT00854893 {published data only}
- NCT00854893. Enhance of language learning with neurostimulation. clinicaltrials.gov/show/NCT00854893 (first received 3 March 2009).
NCT01486654 {published data only}
- NCT01486654. Transcranial direct current stimulation and aphasia language therapy. clinicaltrials.gov/show/NCT01486654 (first received 6 December 2011).
NCT01651884 {published data only}
- NCT01651884. Effects of tDCS versus HD‐tDCS for stroke rehabilitation. clinicaltrials.gov/show/NCT01651884 (first received 27 July 2012).
NCT01701713 {published data only}
- NCT01701713. TDCS in acute stroke. clinicaltrials.gov/show/NCT01701713 (first received 5 October 2012).
NCT02020421 {published data only}
- NCT02020421. Non‐invasive repeated therapeutic stimulation for aphasia recovery. clinicaltrials.gov/show/NCT02020421 (first received 24 December 2013).
- Thiel A, Black SE, Rochon EA, Lanthier S, Hartmann A, Chen JL, et al. Non‐invasive repeated therapeutic stimulation for aphasia recovery: a multilingual, multicenter aphasia trial. Journal of Stroke & Cerebrovascular Diseases. 2015/03/05 2015; Vol. 24, issue 4:751‐8. [1532‐8511: (Electronic)] [DOI] [PubMed]
NCT02101398 {published data only}
- NCT02101398. Study of the effect of transcranial stimulations in aphasic subject within a year of their stroke. clinicaltrials.gov/show/nct02101398 (first received 2 April 2014).
NCT02226796 {published data only}
- NCT02226796. Transcranial direct stimulation (tDCS) and behavioral intervention in aphasia. clinicaltrials.gov/show/nct02226796 (first received 27 August 2014).
NCT02395874 {published data only}
- NCT02395874. tDCS and speech therapy to improve aphasia. clinicaltrials.gov/show/nct02395874 (first received 24 March 2015).
NCT02461355 {published data only}
- NCT02461355. Transcranial direct current stimulation for post‐stroke aphasia. clinicaltrials.gov/show/NCT02461355 (first received 3 June 2015).
NCT02540109 {published data only}
- NCT02540109. Targeted Electrotherapy for Aphasia Stroke Rehabilitation (TEASER) ‐ phase II multi‐center study. clinicaltrials.gov/show/nct02540109 (first received 3 September 2015).
NCT02612753 {published data only}
- NCT02612753. Interest of combining speech therapy with a non‐invasive brain stimulation (tDCS) for the aphasic patient. clinicaltrials.gov/show/nct02612753 (first received 24 November 2015).
NCT02622945 {published data only}
- NCT02622945. Effects of transcranial direct current stimulation in post‐stroke aphasia. clinicaltrials.gov/show/nct02622945 (first received 7 December 2015).
NCT02674490 {published data only}
- NCT02674490. Stimulating language in subacute stroke. clinicaltrials.gov/show/nct02674490 (first received 4 February 2016).
NCT02801864 {published data only}
- NCT02801864. tDCS as an adjuvant to intensive speech therapy for chronic post stroke aphasia. clinicaltrials.gov/show/nct02801864 (first received 16 June 2016).
NCT02901574 {published data only}
- NCT02901574. Cerebellar transcranial direct current stimulation and aphasia treatment. clinicaltrials.gov/show/nct02901574 (first received 15 September 2016).
- Sebastian R, Tippett D, Celnik P, Hillis AE. Cerebellar transcranial direct stimulation to augment aphasia therapy. Stroke Conference: American Heart Association/American Stroke Association 2017 International Stroke Conference and State‐of‐the‐Science Stroke Nursing Symposium. USA. 2017.
NCT03164213 {published data only}
- NCT03164213. Facilitation of brain plasticity for language recovery in patients with aphasia due to stroke. clinicaltrials.gov/show/nct03164213 (first received 23 May 2017).
NCT03272906 {published data only}
- NCT03272906. Clinical feasibility of transcranial direct current stimulation with standard aphasia therapy. clinicaltrials.gov/show/nct03272906 (first received 6 September 2017).
NCT03297450 {published data only}
- NCT03297450. tDCS and aphasia therapy in the acute phase after stroke. clinicaltrials.gov/show/nct03297450 (first received 29 September 2017).
NCT03305614 {published data only}
- NCT03305614. tDCS and aphasia therapy in the chronic phase after stroke. clinicaltrials.gov/show/nct03305614 (first received 10 October 2017).
Additional references
ALHarbi 2017
- ALHarbi MF, Armijo‐Olivo S, Kim E S. Transcranial direct current stimulation (tDCS) to improve naming ability in post‐stroke aphasia: a critical review. Behavioural Brain Research 2017;332:7‐15. [DOI] [PubMed] [Google Scholar]
Benson 1996
- Benson DF, Ardila A. Aphasia: A Clinical Perspective. New York: Oxford University Press, 1996. [Google Scholar]
Bhogal 2003
- Bhogal SK, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke 2003;34(4):987‐93. [DOI] [PubMed] [Google Scholar]
Bindman 1964
- Bindman LJ, Lippold OCJ, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long‐lasting after‐effects. Journal of Physiology 1964;172(3):369‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blomert 1994
- Blomert L, Kean ML, Koster C, Schokker J. Amsterdam‐Nijmegen Everyday Language Test: construction, reliability and validity. Aphasiology 1994;8:381‐407. [Google Scholar]
Brady 2016
- Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD000425.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherney 2008
- Cherney LR, Patterson JP, Raymer A, Frymark T, Schooling T. Evidence‐based systematic review: effects of intensity of treatment and constraint‐induced language therapy for individuals with stroke‐induced aphasia. Journal of Speech, Language, and Hearing Research 2008;51(5):1282‐99. [DOI] [PubMed] [Google Scholar]
Chrysikou 2011
- Chrysikou EG, Hamilton RH. Noninvasive brain stimulation in the treatment of aphasia: exploring interhemispheric relationships and their implications for neurorehabilitation. Restorative Neurology and Neuroscience 2011;29(6):375‐94. [DOI] [PubMed] [Google Scholar]
Chung 2013
- Chung CSY, Pollock A, Campbell T, Durward BR, Hagen S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non‐progressive acquired brain damage. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008391.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruice 2003
- Cruice M, Worrall L, Hickson L, Murison R. Finding a focus for quality of life with aphasia: social and emotional health, and psychological well‐being. Aphasiology 2003;17(4):333‐53. [Google Scholar]
Dalemans 2010
- Dalemans RJ, Witte LP, Beurskens AJ, Heuvel WJ, Wade DT. An investigation into the social participation of stroke survivors with aphasia. Disability and Rehabilitation 2010;32(20):1678‐85. [DOI] [PubMed] [Google Scholar]
De Vries 2010
- Vries MH, Barth AC, Maiworm S, Knecht S, Zwitserlood P, Flöel A. Electrical stimulation of Broca's area enhances implicit learning of an artificial grammar. Journal of Cognitive Neuroscience 2010;22(11):2427‐36. [DOI] [PubMed] [Google Scholar]
Dickey 2010
- Dickey L, Kagan A, Lindsay MP, Fang J, Rowland A, Black S. Incidence and profile of inpatient stroke‐induced aphasia in Ontario, Canada. Archives of Physical Medicine and Rehabilitation 2010;91(2):196‐202. [DOI] [PubMed] [Google Scholar]
Flowers 2016
Flöel 2010
- Flöel A, Cohen LG. Recovery of function in humans: cortical stimulation and pharmacological treatments after stroke. Neurobiology of Disease 2010;37(2):243‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flöel 2012
- Flöel A. Re: Cochrane review of tDCS in aphasia due to stroke. Your publication is included and we ask you for your assistance in providing further information. Email to: B Elsner 5 December 2012.
Goodglass 1972
- Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Philadelphia: Lea and Febiger, 1972. [Google Scholar]
Goodglass 1983
- Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Philadelphia: Lea and Febiger, 1983. [Google Scholar]
Hesse 2011
- Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot‐assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabilitation and Neural Repair 2011;25(9):838‐46. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hilari 2010
- Hilari K. The impact of stroke: are people with aphasia different to those without?. Disability and Rehabilitation 2010;33(3):211‐8. [DOI] [PubMed] [Google Scholar]
Holland 1980
- Holland A. Communicative Abilities in Daily Living. Baltimore: University Park Press, 1980. [Google Scholar]
Huber 1991
- Huber W. Aachener Aphasie‐Test: (AAT). Göttingen: Hogrefe, 1991. [Google Scholar]
Jeon 2012
- Jeon SY, Han SJ. Improvement of the working memory and naming by transcranial direct current stimulation. Annals of Rehabilitation Medicine 2012;36(5):585‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kauhanen 2000
- Kauhanen ML, Korpelainen JT, Hiltunen P, Määttä R, Mononen H, Brusin E, et al. Aphasia, depression, and non‐verbal cognitive impairment in ischaemic stroke. Cerebrovascular Diseases 2000;10(6):455‐61. [DOI] [PubMed] [Google Scholar]
Kertesz 1982
- Kertesz A. Western Aphasia Battery. New York: Grune and Stratton, 1982. [Google Scholar]
Lang 2005
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain?. European Journal of Neuroscience 2005;22(2):495‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefaucheur 2017
- Lefaucheur JP, Antal A, Ayache SS. Evidence‐based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology 2017;128(1):56‐92. [DOI] [PubMed] [Google Scholar]
List 2015
Lomas 1989
- Lomas J, Pickard L, Bester S, Elbard H, Finlayson A, Zoghaib C. The communicative effectiveness index: development and psychometric evaluation of a functional communication measure for adults. Journal of Speech and Hearing Disorders 1989;54(1):113‐24. [DOI] [PubMed] [Google Scholar]
Marangolo 2012
- Marangolo P. Re: Cochrane review of tDCS in aphasia due to stroke. Your publication is included and we ask you for your assistance in providing further information. Email to: B Elsner 5 December 2012.
Marangolo 2017
- Marangolo P. The potential effects of transcranial direct current stimulation (tDCS) on language functioning: combining neuromodulation and behavioral intervention in aphasia. Neuroscience Letters 2017;17:[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Marangolo 2018b
- Marangolo P. Re: Cochrane review on tDCS for improving aphasia after stroke: request for further information. Email to: B Elsner 14 August 2018.
Marinelli 2017
- Marinelli CV, Spaccavento S, Craca A, Marangolo P, Angelelli P. Different cognitive profiles of patients with severe aphasia. Behavioural Neurology 2017;2017:3875954. [DOI: 10.1155/2017/3875954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathers 2011
- Mathers CD, Bernard C, Iburg KM, Inoue M, Ma Fat D, Shibuya K, et al. Global burden of disease in 2002: data sources, methods and results. www.who.int/healthinfo/global_burden_disease/en/index.html/2011.
Nasreddine 2005
Nitsche 2001
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001;57:1899‐901. [DOI] [PubMed] [Google Scholar]
Nitsche 2003
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Journal of Physiology 2003;114:600‐4. [DOI] [PubMed] [Google Scholar]
Nowak 2009
- Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabilitation and Neural Repair 2009;23(7):641‐56. [DOI] [PubMed] [Google Scholar]
Otal 2015
- Otal B, Olma M C, Flöel A, Wellwood I. Inhibitory non‐invasive brain stimulation to homologous language regions as an adjunct to speech and language therapy in post‐stroke aphasia: a meta‐analysis. Frontiers in Human Neuroscience 2015;9:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 1995
- Pedersen PM, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Aphasia in acute stroke: incidence, determinants, and recovery. Annuals of Neurology 1995;38(4):659‐66. [DOI] [PubMed] [Google Scholar]
Pohl 2011
- Pohl M, Berger K, Ketter G, Krusch C, Pause M, Puschendorf W, et al. Long‐term course of patients in neurological rehabilitation Phase B. Results of the 6‐year follow‐up in a multicenter study [Langzeitverlauf von Patienten der neurologischen Rehabilitation Phase B. Ergebnisse der 6‐Jahres‐Nachuntersuchung einer Multicenterstudie]. Nervenarzt 2011;82(6):753‐63. [DOI] [PubMed] [Google Scholar]
Pollock 2012
- Pollock A, George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurology 2012;11(3):209. [DOI] [PubMed] [Google Scholar]
Porch 1967
- Porch B. Porch Index of Communicative Ability. Austin, Texas: Pro‐Ed, 1967. [Google Scholar]
Priori 2012 [pers comm]
- Priori A. Re: Cochrane review of tDCS in aphasia due to stroke. Your publication is included and we ask you for your assistance in providing further information. Email to: B Elsner 5 December 2012.
Purpura 1965
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. Journal of Neurophysiology 1965;28(1):166‐85. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rohatgi 2018 [Computer program]
- Rohatgi, Ankit. WebPlotDigitizer. Version 4.1. Austin, TX, USA: automeris.io/WebPlotDigitizer, January 2018.
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c322. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Assessing the quality of a body of evidence. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shah‐Basak 2016
- Shah‐Basak PP, Wurzman R, Purcell JB, Gervits F, Hamilton R. Fields or flows? A comparative metaanalysis of transcranial magnetic and direct current stimulation to treat post‐stroke aphasia. Restorative Neurology and Neuroscience 2016;34(4):537‐58. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ulatowska 1983
- Ulatowska HK, Freedman‐Stern R, Doyel AW, Macaluso‐Haynes S, North AJ. Production of narrative discourse in aphasia. Brain and Language 1983;19(2):317‐34. [DOI] [PubMed] [Google Scholar]
Van der Meulen 2010
- Meulen I, Sandt‐Koenderman WM, Duivenvoorden HJ, Ribbers GM. Measuring verbal and non‐verbal communication in aphasia: reliability, validity, and sensitivity to change of the Scenario Test. International Journal of Language & Communication Disorders 2010;45(4):424‐35. [DOI] [PubMed] [Google Scholar]
Walker 2018
- Walker GM, Hickok G, Fridriksson J. A cognitive psychometric model for assessment of picture naming abilities in aphasia. Psychological Assessment 2018;30(6):809‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wall 2017
- Wall KJ, Cumming TB, Copland DA. Determining the association between language and cognitive tests in poststroke aphasia. Frontiers in Neurology 2017;8:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. The atlas of heart disease and stroke. www.who.int/cardiovascular_diseases/resources/atlas/en/2011.
Wortman‐Jutt 2017
- Wortman‐Jutt S, Edwards DJ. Transcranial direct current stimulation in poststroke aphasia recovery. Stroke 2017;48(3):820‐6. [1524‐4628: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Elsner 2013
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving aphasia in patients after stroke. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009760.pub2] [DOI] [PubMed] [Google Scholar]


