Abstract
Grain-rich diets often lead to subacute ruminal acidosis (SARA) impairing rumen and systemic cattle health. Recent data suggest beneficial effects of a clay mineral (CM)- based product on the rumen microbiome of cattle during SARA. This study sought to investigate whether the CM supplementation can counteract SARA-induced perturbations of the bovine systemic health. The study used an intermittent diet-induced SARA-model with eight dry Holstein cows receiving either no additive as control or CM via concentrates (n=8 per treatment). Cows received first a forage diet (Baseline) for 1 week, followed by a 1-week SARA-challenge (SARA 1), a 1-week recovery phase (Recovery) and finally a second SARA-challenge for 2 weeks (SARA 2). Cows were monitored for feed intake, reticular pH and chewing behavior. Blood samples were taken and analyzed for metabolites related to glucose and lipid metabolism as well as liver health biomarkers. In addition, a targeted electrospray ionization-liquid chromatography-MS-based metabolomics approach was carried out on the plasma samples obtained at the end of the Baseline and SARA 1 phase. Data showed that supplementing the cows’ diet with CM improved ruminating chews per regurgitated bolus by 16% in SARA 1 (P=0.01) and enhanced the dry matter intake during the Recovery phase (P=0.05). Moreover, the SARA-induced decreases in several amino acids and phosphatidylcholines were less pronounced in cows receiving CM (P≤0.10). The CM-supplemented cows also had lower concentrations of lactate (P=0.03) and biogenic amines such as histamine and spermine (P<0.01) in the blood. In contrast, the concentration of acylcarnitines with key metabolic functions was increased in the blood of treated cows (P≤0.05). In SARA 2, the CM-cows had lower concentrations of the liver enzymes aspartate aminotransferase and γ-glutamyltransferase (P<0.05). In conclusion, the data suggest that supplementation of CM holds the potential to alleviate the negative effects of high-grain feeding in cattle by counteracting multiple SARA-induced perturbations in the systemic metabolism and liver health.
Keywords: clay minerals, dairy cow, subacute ruminal acidosis, metabolomics, chewing behavior
Implications
Subacute ruminal acidosis (SARA) is a common metabolic disorder in cows fed high-grain diets and has been reported to elicit perturbations of the blood metabolome. Feed additives, such as clay minerals (CM), might be a management tool to reduce the negative consequences of high-grain feeding in dairy cows. This study showed that cows experiencing SARA and receiving a CM-based product had a higher concentration of several amino acids (AA) and phosphatidylcholines (PC) in blood, while several harmful biogenic amines (BA) and liver enzymes were lowered compared to control cows. This implies that a CM-based product holds the potential to counteract SARA-induced perturbations of systemic health.
Introduction
High-grain diets have become common in the feeding of cattle worldwide. These diets are characterized by their high content of rapidly fermentable carbohydrates in the rumen, thereby stimulating the generation of short-chain fatty acids that provide metabolic fuels. However, the inclusion of high amounts of grain occurs at the expense of fiber, which decreases chewing activity, salivary buffer supply and rumen motility (Humer et al., 2018a). Consequently, these diets enhance the risk of disrupted acid–base regulation in the rumen, resulting in long-lasting intermittent drops of ruminal pH below 5.8, designated as SARA (Zebeli and Metzler-Zebeli, 2012). The SARA has evolved to be one of most common digestive disorders causing major economic losses in dairy farming worldwide (Enemark, 2008). Besides local alterations in the rumen metabolome (Saleem et al., 2012), more recent research has also revealed that SARA is associated with major perturbations in the systemic metabolism (Zebeli and Metzler-Zebeli, 2012; Guo et al., 2017), increasing the risk of metabolic disorders and being a serious welfare concern.
The use of high-throughput technologies such as metabolomics has recently improved both diagnosis and our understanding in the etiopathogenesis of metabolic disorders in cattle (Hailemariam et al., 2014; Kenéz et al., 2016). A recent study conducted by our team exploring the effects of grain-induced SARA on the blood metabolomic profile revealed large shifts in the plasma metabolome of dairy cows toward a decrease in lysophosphatidylcholines, PC, sphingomyelins and most AA (Humer et al., 2018b).
Despite the research progress made in the etiopathogenesis and diagnostic tools of SARA in dairy cattle, both prevention strategies and the consequences of SARA for systemic cow health are still largely unresolved (Humer et al., 2018a and 2018c). Supplementation of feed additives has been suggested as a tool to reduce negative effects of high-grain feeding on health and to optimize rumen fermentation in cows at high risk of SARA (Krause and Oetzel, 2006; Humer et al., 2018a). One of these feed additives which are less explored in cattle but long used to improve human health, including gut and systemic health are CMs (Carretero, 2002). In cattle, Sulzberger et al. (2016) recently showed that supplementation of CMs holds potential to improve ruminal pH and energy-corrected milk yield after receiving a grain challenge. One potential mode of action of CMs in cattle could be their potential in binding pathogenic microorganisms and toxic compounds that are associated with perturbed rumen metabolism during high-grain feeding (Saleem et al., 2012), likely due to their high adsorption capacity (Slamova et al., 2011). Indeed, in a recent study, we observed modulating effects of a CM-based product on AA-, starch- and fiber-fermenting bacteria in the ruminal microbiome during high-concentrate feeding in cows. Furthermore, some methane- and sulfate-utilizers in the epimural microbiome were affected (Neubauer et al., 2017), suggesting potentials to improve metabolic health during these feeding stress conditions. As an appropriate health status is a prerequisite for sufficient feed intake and rumination, the CM might also pose positive effects on the feeding behavior of cows experiencing SARA. Therefore, the aim of this study was to evaluate the effects of the supplementation with CM on feed intake and chewing behavior, ruminal pH and the blood metabolic profile in cows. We used a diet-induced challenge model of SARA and targeted metabolomics combined with the monitoring of health and welfare of dairy cattle by using chewing behavior, ruminal pH, liver enzymes and blood metabolites. Our working hypothesis stated that the supplementation of CM to ruminant diets could exert positive effects on rumen and systemic health under SARA-conditions, as indicated by an improved chewing behavior, systemic metabolism and liver health.
Material and methods
Animals
The trial was conducted at the research dairy farm of Vetmeduni Vienna (VetFarm Kremesberg, Pottenstein, Austria). The study was part of a broad research project called ‘Advancement of Dairying in Austria,‘ whereby other data of this project have been published previously (Kröger et al., 2017; Neubauer et al., 2018a; Humer et al., 2018b). The experiment involved eight rumen-cannulated, dry, nonpregnant Holstein cows (average BW=863±65 kg, average lactation number=2.75±1.04) that were housed in a loose-housing stable.
Experimental design and feeding
Cows were subjected to a change-over design. To simulate an intermittent SARA-challenge, cows were switched two times from a pure forage diet to a high-concentrate diet (65% grains on DM-basis) during the experimental period lasting for 43 days. More specifically, cows were fed a hay and grass silage mix (50% each on DM basis) for 1 week (days 1 to 7, termed Baseline feeding). Thereafter, the concentrate level was gradually increased from days 8 to 14 up to 65%. From days 14 to 20 cows received the high-concentrate level to induce SARA (SARA 1). Afterward, the cows were fed the pure forage diet for 1 week (Recovery, days 21 to 27). Finally, after a short adaptation to the 65% concentrate diet (days 28 to 30) a 2-week SARA challenge was conducted (SARA 2, days 30 to 43). Afterward, cows were subjected to a 3-week washout phase with forage-only feeding to avoid carry-over effects.
The diets used in this study were similar to those reported previously (e.g. Kröger et al., 2017; Neubauer et al., 2018a). Details of diets are shown in Supplementary Table S1. The concentrates fed to cows to induce SARA contained either no additive as control (CON) or were mixed with a CM (Mycofix® Plus 3.E; BIOMIN Holding GmbH, Getzersdorf, Austria; 50 g per cow per day) and pelleted before being offered to the cows separately from forages.
The forage and concentrates were given in individual feeding troughs (Insentec B.V., Marknesse, The Netherlands) that enabled individual controlling of feed intake (Kröger et al., 2017).
Reticular pH
The reticular pH was continuously measured using indwelling wireless pH-transmitting units (eCow Farmer bolus; eCow, Dekon, UK) as described recently (Neubauer et al., 2018b). The position of the boli in the reticulum was confirmed throughout the experiment. The pH was measured every minute and summarized to 15-min intervals, which were used for calculating the daily mean and minimum pH as well as the pH <6.0 (min/day). Using the SARA-definition of Neubauer et al. (2018b) when using a reticular pH bolus, a reticular pH <6.0 for longer than 5 h/day was considered as indicative of SARA conditions.
Chewing activity
The chewing activity was determined using noseband-sensors (RumiWatch System; ITIN + Hoch GmbH, Liestal, Switzerland) as described recently by Kröger et al. (2017) during 2 consecutive days in Baseline (days 2, 3), SARA 1 (days 15, 16) and SARA 2 (days 35, 36). Chewing data included the duration of eating, ruminating and total chewing (min/day), the number of ruminating boli per day and the chews per bolus. Moreover, the feed intake data of the respective days were used for calculation of the chewing indices (i.e. eating, ruminating and total chewing per kilogram of dry matter intake (DMI) and kilogram of NDF intake).
Blood sampling and analysis
Blood samples were collected shortly before the morning feeding from the jugular vein on day 6 (Baseline), day 18 (SARA 1), day 28 (Recovery) and day 38 (SARA 2). Serum was obtained using 9 ml serum vacutainer tubes (Vacuette; Greiner Bio-One, Kremsmünster, Austria) and analyzed for ß-hydroxybutyrate (BHBA), non-esterified fatty acids (NEFA), cholesterol and the liver enzymes aspartate aminotransferase (AST), glutamate dehydrogenase (GLDH) and γ-glutamyltransferase (GGT). Samples for lactate and glucose analysis were collected in 6 ml fluoride plasma tubes (Vacuette). Analyses were conducted with a fully automated autoanalyzer for clinical chemistry (Cobas 6000/c501; Roche Diagnostics GmbH, Vienna, Austria).
Metabolome profiling was carried out in plasma samples collected on day 6 (Baseline) and day 18 (SARA 1) in 9 ml heparin-containing vacutainer tubes (Vacuette). A targeted metabolomics approach using electrospray ionization-liquid chromatography-MS (ESI-LC-MS/MS) was carried out by Biocrates Life Sciences AG (Innsbruck, Austria). The fully automated assay of the Absolute-IDQ p180 platform (Kit p180; Biocrates Life Sciences AG, Innsbruck, Austria) enabled the quantification of 176 metabolites out of 10 µl blood plasma. Detailed information regarding the applied technique is given elsewhere (Ramsay et al., 2012; Humer et al., 2018b).
Statistical analyses
Statistical analysis was performed using the MIXED procedure of SAS (SAS, version 9.2). The model included the fixed effects of the treatment (i.e. CON, CM), feeding phase and their interaction. Random effects were the experimental period, cow and sequence The measurements conducted on the same cow but at different times were considered as repeated measures in the model with a first-order autoregressive variance–covariance matrix. Comparisons among treatments were evaluated by the pdiff option and degrees of freedom were estimated with the method of Kenward–Roger. The significance level was set at P≤0.05, and trends were considered at 0.05<P≤0.10 level.
The MetaboAnalyst 4.0 software (http://www.metaboanalyst.ca) was used for multivariate analysis. Partial least-squares discriminant analysis (PLS-DA) was conducted to identify characteristic trends or grouping among cows fed the Baseline v. the SARA-diets and pertaining to group CON or CM. Data were normalized by the Baseline metabolite concentrations by averaging all samples collected during the pure forage feeding. Autoscaling was conducted for compound normalization. The variables obtained on the basis of the PLS-DA results were plotted according to their importance in separating the dietary treatments based on the variable importance in the projection (VIP) scores. Moreover, hierarchical clustering analysis (HCA) with clustered heat maps using Euclidean distance measures and Ward’s clustering algorithm was carried out to explore the presence of clustering patterns among the blood metabolites affected by CM as well as reticular pH, forage and grain intake during Baseline and SARA 1. Overall correlation pattern analyses were conducted between blood metabolites as well as reticular pH, forage and grain intake.
Results
Feed intake and reticular pH
While the overall DMI during the SARA-phases did not differ among groups (Table 1), cows receiving the CM had an on average 11% increased DMI during the Recovery phase (P=0.05). Overall, similar grain intake was found among groups, while the forage intake was higher in the Recovery phase in CM-cows (P=0.01), where no concentrates were fed.
Table 1.
Effect of a clay mineral (CM)-based product on feed intake and reticular pH in dairy cows subjected to two intermittent subacute ruminal acidosis (SARA)-feeding phases with 65% concentrates for 1 week (SARA 1) or 2 weeks (SARA 2), separated by a 1-week feeding with 100% forages (Recovery)
| SARA 1 | Recovery | SARA 2 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | CON | CM | CON | CM | CON | CM | SEM | SARA 1 | Recovery | SARA 2 |
| DM intake (kg/day) | 10.3 | 10.0 | 9.47b | 10.5a | 13.0 | 13.3 | 0.64 | 0.57 | 0.05 | 0.45 |
| Forage intake (kg DM/day) | 3.52 | 3.53 | 9.47b | 10.5a | 4.56 | 4.62 | 0.375 | 0.98 | 0.01 | 0.82 |
| Grain intake (kg DM/day) | 6.71 | 6.42 | 0 | 0 | 8.45 | 8.66 | 0.315 | 0.32 | 0.99 | 0.30 |
| Reticular pH | ||||||||||
| Mean pH | 6.31 | 6.33 | 6.69 | 6.75 | 6.52 | 6.52 | 0.049 | 0.74 | 0.23 | 0.87 |
| Minimal pH | 5.46 | 5.40 | 6.33 | 6.37 | 5.81 | 5.81 | 0.069 | 0.49 | 0.64 | 0.97 |
| Time pH <6.0 (min/day) | 339 | 404 | 71.2 | 56.4 | 184 | 145 | 35.61 | 0.16 | 0.77 | 0.28 |
CON=control.
Different superscripts represent differences among CON- and CM-groups within a feeding phase with P⩽0.05.
During SARA 1 mean reticular pH measurements revealed that cows in both groups experienced SARA (>5 h/day below pH 6.0), while this criterion was not met during the Recovery and SARA 2. Overall, no differences in the mean pH, min pH, as well as the time the pH was below the SARA-threshold were observed among the feeding groups (P⩾0.16).
Chewing behavior
In agreement with the unaffected feed intake during SARA 1 and SARA 2, the eating time did not differ between CON and CM-cows (Table 2, P⩾0.24). Similarly, the time spent ruminating per day as well as the total chewing time was not affected by the supplementation of CM. Among the eating, ruminating, and total chewing indexes related to the intake of DM or NDF, a tendency toward a 22% higher eating time per kilogram NDF intake was observed in cows receiving CM during SARA 2 (P=0.10). Furthermore, during SARA 1 these cows had a 16% higher number of ruminating chews per regurgitated bolus (P=0.01).
Table 2.
Effect of a clay mineral (CM)-based product on chewing behavior in cows subjected to two intermittent subacute ruminal acidosis (SARA) challenges with 65% concentrates for 1 week (SARA 1) or 2 weeks (SARA 2)
| SARA 1 | SARA 2 | P-value | |||||
|---|---|---|---|---|---|---|---|
| Variables | CON | CM | CON | CM | SEM | SARA 1 | SARA 2 |
| Eating | |||||||
| min/day | 196 | 222 | 216 | 220 | 35.4 | 0.24 | 0.86 |
| min/kg of DM intake | 17.3 | 19.1 | 16.5 | 19.5 | 3.47 | 0.45 | 0.19 |
| min/kg of NDF intake | 59.5 | 68.4 | 59.0z | 71.7y | 10.19 | 0.26 | 0.10 |
| Ruminating | |||||||
| min/day | 167 | 212 | 327 | 295 | 40.3 | 0.36 | 0.51 |
| min/kg of DM intake | 13.9 | 18.6 | 25.4 | 24.8 | 4.28 | 0.23 | 0.88 |
| min/kg of NDF intake | 48.3 | 65.4 | 90.3 | 89.1 | 13.38 | 0.20 | 0.93 |
| Ruminating boli | 174 | 201 | 312 | 289 | 39.3 | 0.57 | 0.61 |
| Chews per bolus | 49.9b | 57.8a | 57.6 | 55.2 | 2.90 | 0.01 | 0.42 |
| Total chewing | |||||||
| min/day | 360 | 432 | 543 | 514 | 55.9 | 0.20 | 0.61 |
| min/kg of DM intake | 32.5 | 38.8 | 43.0 | 45.2 | 6.48 | 0.21 | 0.64 |
| min/kg of NDF intake | 108 | 134 | 149 | 160 | 19.96 | 0.12 | 0.49 |
CON=control.
Different superscripts represent differences among CON- and CM-groups within a feeding phase with P⩽0.05.
Different superscripts represent differences among CON- and CM-groups within a feeding phase with 0.05<P⩽0.10.
Blood parameters
The concentration of glucose, cholesterol and BHBA measured during SARA 1, Recovery and SARA 2 did not differ among groups (Table 3). In SARA 1, the cows of the CM group showed higher NEFA (P=0.03), while the lactate concentration in blood was 29% lower in the respective cows (P=0.03). Among the measured liver enzymes, AST and GLDH were reduced in the cows receiving CM in SARA 2 (−25% for AST, P=0.01 and −47% for GLDH, P=0.02).
Table 3.
Effect of a clay mineral (CM)-based product on the concentration of blood metabolites in dairy cows subjected to two intermittent subacute ruminal acidosis (SARA)-feeding phases with 65% concentrates for 1 week (SARA 1) or 2 weeks (SARA 2), separated by a 1-week SARA recovery with 100% forages
| SARA 1 | Recovery | SARA 2 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | CON | CM | CON | CM | CON | CM | SEM | SARA 1 | Recovery | SARA 2 |
| Glucose (mg/dl) | 72.4 | 70.8 | 67.4 | 66.9 | 74.0 | 74.9 | 2.09 | 0.47 | 0.82 | 0.70 |
| Cholesterol (mg/dl) | 39.3 | 40.5 | 72.9 | 69.9 | 61.4 | 59.4 | 6.53 | 0.81 | 0.57 | 0.70 |
| BHBA (mmol/l) | 0.355 | 0.405 | 0.343 | 0.330 | 0.255 | 0.320 | 0.0393 | 0.36 | 0.82 | 0.24 |
| NEFA (mmol/l) | 0.075b | 0.135a | 0.081 | 0.069 | 0.055 | 0.033 | 0.0209 | 0.03 | 0.64 | 0.41 |
| Lactate (mmol/l) | 0.79a | 0.56b | 0.56 | 0.60 | 0.72 | 0.56 | 0.089 | 0.03 | 0.70 | 0.13 |
| AST (U/l) | 102 | 93.8 | 102 | 88.1 | 135a | 101b | 19.81 | 0.53 | 0.32 | 0.01 |
| GLDH (U/l) | 21.3 | 12.8 | 20.5 | 14.9 | 48.6a | 25.8b | 9.28 | 0.39 | 0.57 | 0.02 |
| GGT (U/l) | 25.9 | 25.6 | 27.0 | 25.3 | 30.5 | 27.9 | 2.36 | 0.90 | 0.39 | 0.20 |
CON=control; BHBA=β-hydroxybutyrate; NEFA=non-esterified fatty acids; AST=aspartate aminotransferase; GLDH=γ-glutamyltransferase; GGT=glutamate dehydrogenase.
Different superscripts represent differences among CON- and CM-groups within a feeding phase with P≤0.05.
Table 4 provides a list of the blood metabolites analyzed with the targeted metabolomics approach during SARA 1 and being affected by the addition of CM, while the remaining metabolites are summarized in Supplementary Table S2. Supplementation of CM resulted in an increase in several AA up to 41% compared to CON. More specifically, glycine and the essential AA isoleucine, lysine, methionine and threonine were increased by on average 32% (P≤0.05) and arginine and histidine tended to be on average 27% higher in CM-cows compared to CON (P≤0.09). Among the BA, asymmetric dimethylarginine and dopamine were increased in CM cows (P≤0.04), while the opposite effect was found on dihydroxyphenylalanine, histamine and spermine (P≤0.01). Moreover, methionine-sulfoxide (Met-SO) tended to be reduced in CM-cows (P=0.09). Several acylcarnitines (i.e. C2, C3, C3OH, C4, C5, C5 : 1, C10 : 2, C16 : 1OH) were higher in CM-cows (P≤0.05), while the opposite was noticed for C10 (P=0.02) and C12 (P=0.08). Among PC, an effect of the CM supplementation was only found for those with diacyl-residues (i.e. PC aa C34 : 2, PC aa C36 : 4, PC aa C38 : 5, PC aa C38 : 6), showing an increase by on average 28% (P≤0.09).
Table 4.
Effect of a clay mineral (CM)- based product on the concentration of blood metabolites in dairy cows subjected to a subacute ruminal acidosis (SARA)-feeding phase with 65% concentrates for 1 week
| Metabolites | CON | CM | SEM | P-value |
|---|---|---|---|---|
| Amino acids (µM) | ||||
| Arginine | 75.0 | 101 | 6.86 | 0.09 |
| Glycine | 507 | 620 | 23.0 | 0.01 |
| Histidine | 86.8 | 103 | 6.09 | 0.07 |
| Isoleucine | 116 | 163 | 14.9 | 0.04 |
| Lysine | 102 | 138 | 12.2 | 0.04 |
| Methionine | 20.3 | 26.6 | 1.68 | 0.04 |
| Threonine | 65.6 | 86.4 | 6.33 | 0.05 |
| Biogenic amines (µM) | ||||
| Asymmetric dimethylarginine | 0.66 | 0.84 | 0.068 | 0.04 |
| Dihydroxyphenylalanine | 0.086 | 0.071 | 0.004 | 0.01 |
| Dopamine | 0.15 | 0.28 | 0.002 | <0.01 |
| Histamine | 0.18 | 0.13 | 0.001 | <0.01 |
| Methionine-sulfoxide | 1.32 | 0.83 | 0.213 | 0.09 |
| Spermine | 0.12 | 0.02 | 0.020 | <0.01 |
| Acylcarnitines (µM) | ||||
| C2 | 3.29 | 5.90 | 0.8760 | 0.02 |
| C3 | 0.34 | 0.44 | 0.0400 | 0.02 |
| C3OH | 0.013 | 0.014 | 0.0006 | 0.03 |
| C4 | 0.129 | 0.162 | 0.0172 | 0.02 |
| C5 | 0.069 | 0.092 | 0.0091 | 0.02 |
| C5 : 1 | 0.033 | 0.044 | 0.0017 | <0.01 |
| C10 | 0.058 | 0.046 | 0.0021 | 0.02 |
| C10 : 2 | 0.023 | 0.025 | 0.0007 | 0.05 |
| C12 | 0.042 | 0.036 | 0.0020 | 0.08 |
| C16 : 1OH | 0.005 | 0.006 | 0.0003 | 0.01 |
| Phosphatidylcholines (µM) | ||||
| PC aa C34 : 2 | 28.1 | 36.9 | 4.09 | 0.07 |
| PC aa C36 : 4 | 7.63 | 9.87 | 0.766 | 0.09 |
| PC aa C38 : 5 | 9.71 | 12.42 | 1.146 | 0.10 |
| PC aa C38 : 6 | 1.92 | 2.34 | 0.151 | 0.04 |
CON=control; C=carnitine; PC=phosphatidylcholine; aa=diacyl.
Only a subset of metabolites (with P≤0.10) are presented.
Multivariate analyses
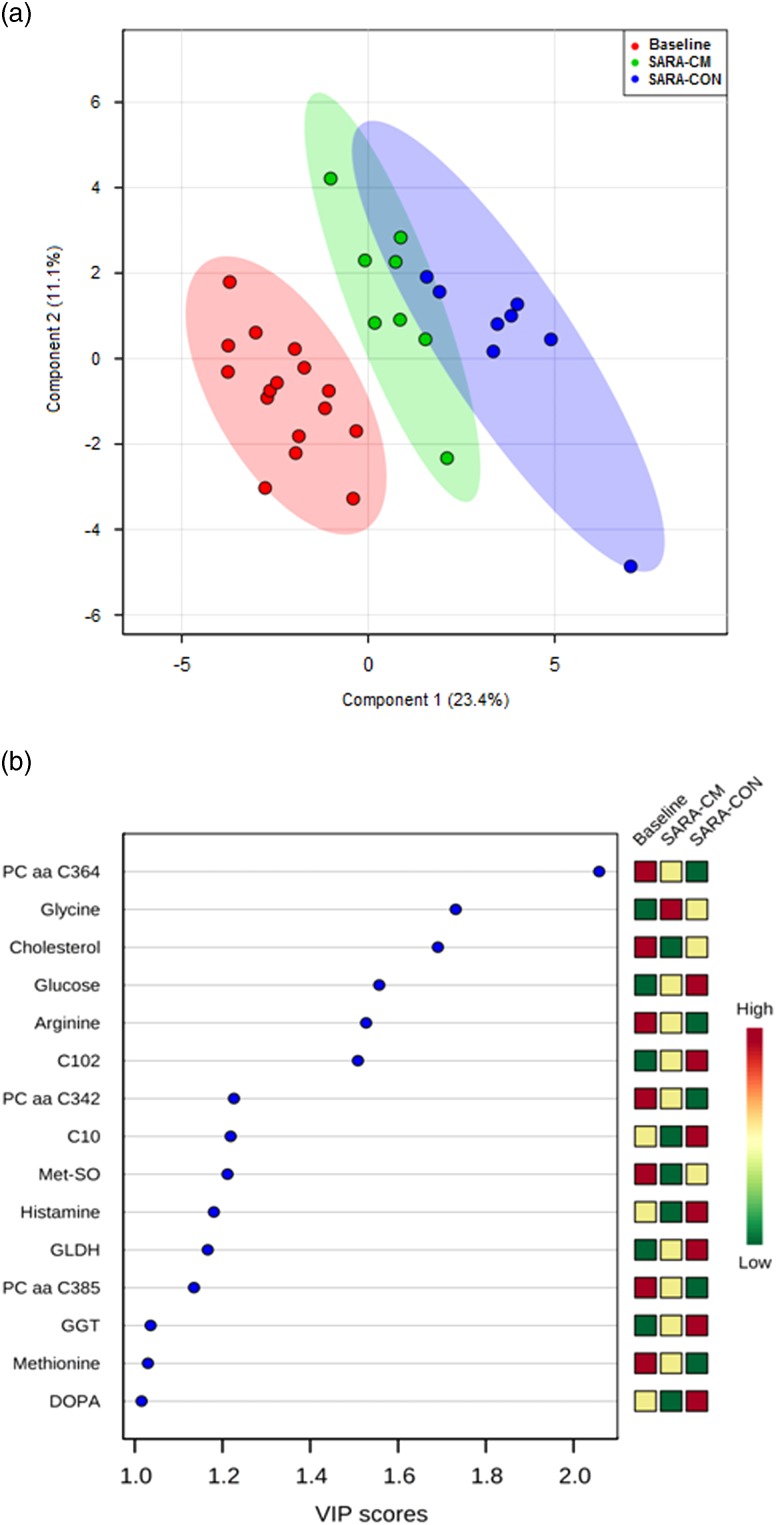
The PLS-DA in Figure 1a visualizes the differences in the data among feeding phases and treatment groups. The major part of the variation in the sample set can be explained by the first principal component. Overall, the SARA samples clustered separately from the Baseline samples, whereby the cows receiving CM clustered closer to the Baseline than CON-cows. As demonstrated in Figure 1b, the most influential variables in the PLS-DA model were PCaaC364, glycine and cholesterol. The P-value for 1000 permutations was P<0.001.
Figure 1.
A partial least-squares discriminant analysis (PLS-DA) of the blood metabolites that were affected by the feed additive (a). The two-dimensional score plot distinguishes the metabolic profiles of cows fed either a pure forage diet (Baseline; red) or a 65% concentrate diet (subacute ruminal acidosis, SARA) without feed additive (control, CON; blue), or a clay mineral-based product (CM; green). Variable importance in the projection (VIP) scores of 15 most influential variables for PLS-DA discriminating along principal components (b). aa=diacyl; C=carnitine; DOPA=dihydroxyphenylalanine; GGT=γ-glutamyltransferase; GLDH=glutamate dehydrogenase; Met-SO=methionine-sulfoxide; PC=phosphatidylcholine.
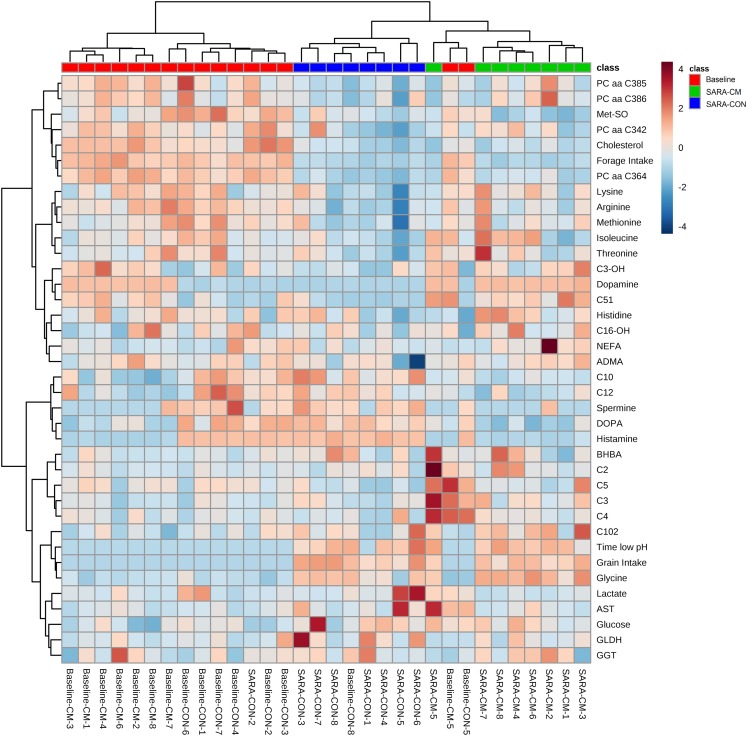
In addition, HCA with a clustered heat map visualization was conducted on all metabolomics variables that were affected by the CM, all classical blood metabolites as well as the feed intake and pH data to depict the relationship and differences in the concentrations among the respective variables. The HCA in Figure 2 reveals the presence of a subcluster consisting of a group of PC, Met-SO, cholesterol and forage intake. Further clusters were formed by AA and the liver enzymes, glucose and lactate. Overall, the decrease in the variables belonging to the first cluster in the SARA-diets was less pronounced in several cows receiving CM. Moreover, the increase in BA was less pronounced in CM-cows. Altogether, during the SARA-phase CON and CM-cows showed a clear separate clustering. Interestingly, two Baseline-cows even clustered within the CM-cows that received the SARA-diets.
Figure 2.
Hierarchical clustering analysis for blood metabolites that were affected by the feed additive, grain and forage intake, as well as reticular pH below the subacute ruminal acidosis (SARA)-threshold, measured in dairy cows receiving a pure forage diet (Baseline; red) or 65% concentrates (SARA) and receiving either no additive (control, CON; blue) or a clay mineral-based product (CM; green) during the SARA-feeding regimen. ADMA=asymmetric dimethylarginine; AST=aspartate aminotransferase; BHBA=ß-hydroxybutyrate; C=carnitine; DOPA=dihydroxyphenylalanine; GLDH=glutamate dehydrogenase; GGT=γ-glutamyltransferase; Met-SO=methionine-sulfoxide; NEFA=non-esterified fatty acids; PC=phosphatidylcholine; aa=diacyl.
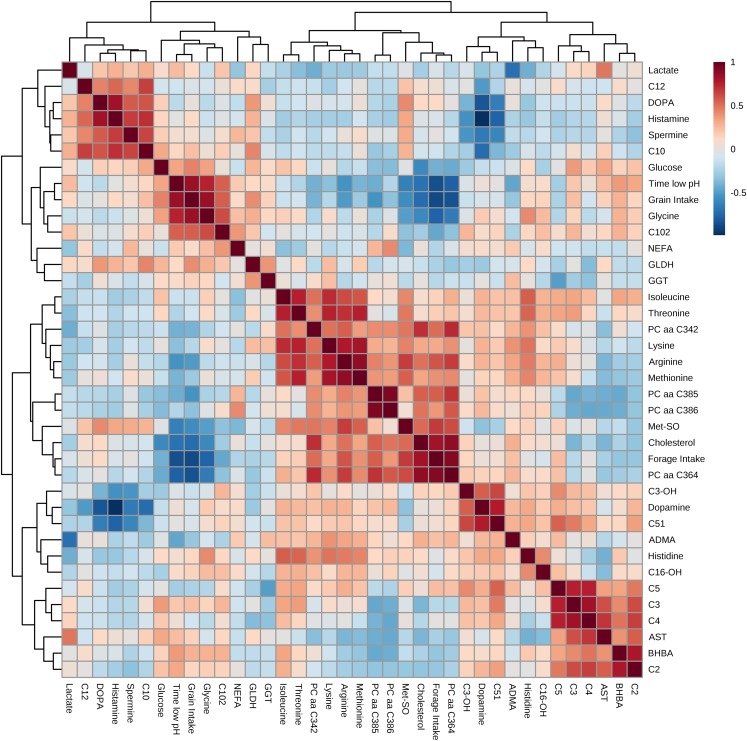
Correlation pattern analysis revealed a cluster comprising of glucose, NEFA, glycine, GLDH, GGT, C10 : 2, grain intake and the time the pH was below the SARA-threshold, which showed a strong negative association with Met-SO, cholesterol, the forage intake and PC aa C36 : 4 (Figure 3). A further cluster comprising of AA (isoleucine, threonine, lysine, arginine and methionine) as well as PC aa C34 : 2 showed high positive associations with the forage intake, cholesterol and PC (i.e. PC aa C36 : 4, PC aa C38 : 5, PC aa C38 : 6) and MetSO. The latter cluster was negatively associated with a cluster of acylcarnitines (C2, C3, C4, C5), BHBA and AST.
Figure 3.
Correlation of the different blood metabolites that were affected by the feed additive, as well as forage and grain intake and reticular pH below the subacute ruminal acidosis (SARA)-threshold, measured during a pure forage feeding regimen or a 65% concentrate-challenge. A heat map of Spearman correlation coefficients was generated for different concentration levels. The red color indicates a positive correlation coefficient, and the blue color represents a negative coefficient. ADMA=asymmetric dimethylarginine; AST=aspartate aminotransferase; BHBA=ß-hydroxybutyrate; C=carnitine; DOPA=dihydroxyphenylalanine; GLDH=glutamate dehydrogenase; GGT=γ-glutamyltransferase; Met-SO=methionine-sulfoxide; NEFA=non-esterified fatty acids; PC=phosphatidylcholine; aa=diacyl.
Discussion
In this research, we used a diet-induced intermittent SARA model and targeted metabolomics as well as traditional methods of monitoring health and welfare to establish the role of CM supplementation in cattle experiencing SARA. Our working hypothesis stated that the supplementation of CM to ruminant diets could exert positive effects on rumen and systemic health under such SARA-conditions. Accordingly, the study revealed that the supplementation with CM to a high-concentrate diet increased the feed intake and chewing behavior as well as altered the blood metabolomic profile in dairy cows subjected to an intermittent SARA-challenge. More specifically, the DMI was increased by 11% in cows receiving the CM during the Recovery phase, which might be indicative of an improved rumen health status due to a faster recovery from SARA, as cows experiencing SARA typically reduce their feed intake until pH returns to physiological values (Enemark, 2008). Moreover, during the phases in which the cows underwent the severest pH drops (SARA 1), the number of chews per bolus was enhanced from 49.9 to 57.8 in groups supplemented with CM. Overall, the number of chews per bolus is often used as a health and SARA indicator in the practice, with a common threshold of 50 chews per bolus to differentiate between healthy and diseased cows (Humer et al., 2018c). Therefore, the higher number of chews per bolus in cows receiving CM might be a regulatory mechanism of the cows to counteract the low ruminal pH by increased saliva secretion (Krause et al., 2002). Moreover, changes in the chewing behavior might become evident in cows experiencing SARA. In this regard, CM-cows enhanced the chewing index in terms of time spent eating per kilogram of NDF intake during SARA 2. Thus it seems that the effectiveness of forage in promoting eating chews was higher in cows receiving CM. The enhanced eating chews might have been an adaptive response to attenuate the decline in rumen pH, as previous studies have reported that chewing time per unit of NDF intake was higher for low-NDF diets compared to diets richer in NDF (Maulfair and Heinrichs, 2013).
Another important finding of this study was that cows receiving CM had lower concentrations of liver enzymes AST and GLDH in SARA 2. Aspartate aminotransferase and GLDH represent markers for hepatocyte integrity of dairy cows (Bobe et al., 2004), and the lower concentration in CM-cows likely suggests a less affected liver function in the respective cows during SARA 2. The exact mechanisms behind the beneficial effects of CM on liver health and the increase of liver enzymes following SARA are not clear. We speculate that the release of toxic compounds in the rumen and their translocation into systemic circulation (Wang et al., 2013) might have played a role in the increase of liver enzymes, either directly by an increased detoxification in the liver as well as indirectly by an increased oxidative stress to the liver cells in response to toxic load (Guo et al., 2017). Moreover, beneficial effects of CM supplementation on improved liver health can be explained by a lower toxic load in the rumen and systemic circulation because CM are known to adsorb toxins (Slamova et al., 2011). Our finding of increased liver enzymes during SARA 2 indicate that effects SARA on liver enzymes are retarded compared with effects of ruminal pH which was more pronounced during SARA 1.
SARA might be associated with an increase in lactate in the rumen, that can cause absorption of lactate into the bloodstream and thus metabolic acidosis (Enemark, 2008). In agreement, we found increasing ruminal lactate during the SARA compared to non-SARA (Neubauer et al., 2018a) and increased concentrations of lactate in the blood have been previously measured in cows experiencing SARA (Li et al., 2012). Interestingly, lactate in the plasma was lower in CM-cows in SARA 1, which might be indicative of a lower absorption of lactate into the bloodstream and therefore might suggest improved health status of the respective cows.
In our companion study, we reported that SARA had a profound effect on the concentration of several AA, showing lowering effects in the plasma of affected cows (Humer et al., 2018b). In addition, the feature selection based on VIP scores revealed that some AA (i.e. glycine and arginine) were instrumental for the separate clustering of the groups (Baseline, SARA-CON, SARA-CM). Interestingly, cows receiving CM showed higher concentrations of several AA during SARA than CON-cows. Besides the importance of AA for protein synthesis and carbohydrate metabolism, they are also involved in immunological processes (Suliman et al., 2005). As inflammatory responses trigger protein catabolism, their depletion during the SARA-phase likely derives from the release of AA from muscle proteins to serve as a substrate for acute phase proteins (Grimble, 2001). In this regard, it has been reported that the concentration of glycine, among others, changes differently between healthy cows and cows experiencing diseases (Hailemariam et al., 2014). Moreover, arginine has been reported to improve the functional activities of immune cells (Newsholme, 2001). An increased synthesis of inflammatory markers was previously associated with decreasing arginine in the systemic circulation, assuming that decreased AA might be attributable to an increased synthesis of acute phase proteins (Humer et al., 2018b). Moreover, the higher concentration of threonine might derive from an improved immune status in CM-cows, as this essential AA is the most abundant AA in immunoglobulin proteins and is also involved in maintaining the integrity of nonspecific defenses of the gut wall (Le Floc’h et al., 2004). It can also be speculated that a lower concentration of reactive oxygen species in the CM-cows might have contributed to the less pronounced decrease in several AA, as enhanced oxidation of AA due to the activation of pro-inflammatory cytokines might affect their concentration in the blood (Suliman et al., 2005).
Besides AA, also their decarboxylation products differed between CON- and CM-cows. Biogenic amines have been discussed for their deleterious effects on cow’s health when entering the systemic circulation (Wang et al., 2013). In general, increasing amounts of BA are produced during high-grain feeding (Saleem et al., 2012; Wang et al., 2013), which can be attributed to a shift in the ruminal microbiome as well as to effects of low ruminal pH on AA-decarboxylase (Mao et al., 2016). More specifically, high-grain diets result in an increased accumulation of fermentation acids and promote the growth of starch- and sugar degrading bacteria such as Lactobacillus spp. and Streptococcus bovis, which produce BA (Bailey et al., 2002). Indeed, in our companion study, the abundance of Lactobacillus increased by 100% during SARA (Neubauer et al., 2018a). Interestingly, cows receiving CM showed changes in the ruminal microbiome compared to CON-cows (Neubauer et al., 2017), such as a lower abundance of the genus Lactobacillus in SARA 1. Moreover, the present study revealed lower concentrations of histamine, Met-SO, and spermine in the blood in the respective cows during SARA 1. This lowering effect might be either attributable to a lower production of BA in the rumen of the cows receiving CM, or a lower translocation of them into the systemic circulation. Overall, the 28% decreased concentration of plasma histamine is a positive outcome, as this BA is considered as a major toxic factor, not only in terms of acidosis but also regarding its speculated role in the pathogenesis of laminitis (Zebeli and Metzler-Zebeli, 2012). As Met-SO, the oxidized form of methionine has been considered to represent a biomarker for oxidative stress in vivo (Tarrago et al., 2015), the lowered concentration of Met-SO in CM-cows might derive from suspected lower oxidative protein damage (Sadri et al., 2017). Moreover, the lower concentration of spermine can be interpreted as improved health status, as spermine in the blood can be oxidized into aldehyde and hydrogen in ruminants, thereby causing oxidative stress as well as toxicological properties on eukaryotic cells (Ronchi et al., 2000).
Overall, cows experiencing SARA showed a strong decrease in PC (Humer et al., 2018b), which is in agreement with Saleem et al. (2012), who demonstrated reduced concentrations of ruminal PC with increasing amounts of grain in the diet. As it is assumed that most of the ruminal PC derive from protozoa, the decreasing concentrations of plasma PC likely reflect the common decrease in protozoa counts in the rumen when grain-rich diets are fed (Saleem et al., 2012). Moreover, PC are used for the assembly or export of very low-density lipoprotein (VLDL) from the liver to transport the triacylglycerols (TAG). Therefore, the higher concentration of some PC in CM-cows in SARA 1 could be also related to their higher NEFA level, as NEFA taken up by the liver can be esterified into TAG and exported into to bloodstream as VLDL, as supported by the positive association between several PC and NEFA.
Furthermore, we found a counteracting effect on the SARA-associated decrease in several acylcarnitines in cows supplemented with CM. In general, acylcarnitines are involved in the transport of fatty acids from the cytoplasm across the inner mitochondrial membrane into the mitochondrial matrix (Stanley et al., 1992). As the removal of excess of acylcarnitines from the mitochondria was hypothesized to represent a protective mechanism, it has been recently suggested that higher levels of blood acylcarnitines reflect a greater ability of hepatocytes to release any surplus of acylcarnitines from the mitochondria, thereby avoiding mitochondria damage (Huber et al., 2016). Since properly functioning hepatic mitochondria are of vital importance for metabolic health (Kenéz et al., 2016), the higher concentrations of acylcarnitines in the plasma of CM-cows may reflect their ability to adapt mitochondrial functions properly to changing metabolic situations, which concurs with the observed lowering effect on liver enzymes.
In conclusion, CM showed potential to alleviate the negative consequences of high-grain feeding in dairy cows by improving chewing behavior and liver health and counteracting multiple SARA-induced perturbations in the systemic metabolism.
Acknowledgements
The authors acknowledge the staff of the VetFarm Kremesberg (Pottenstein, Austria), S. Aditya, T. Braid, N. Kraft, P. Pourazad and A. Stauder (Institute of Animal Nutrition and Functional Plant Compounds, Vetmeduni Vienna, Austria) and M. Valera Rojas (Institute of Animal Science, Carretera Central, San José de las Lajas, Mayabeque, Cuba) for their valuable help in the experiment. Further thanks go to Königshofer Futtermittel, Königshofer GmbH (Ebergassing, Austria) for providing the concentrate. This research was funded by the project ‘ADDA – Advancement of Dairying in Austria’ of the Austrian Research Promotion Agency (FFG, Vienna, Austria) and BIOMIN Holding GmbH under the scope of the Competence Centers for Excellent Technologies (COMET) program (Grant No. 843543).
Declaration of interest
N. R. is employed by Biomin Holding GmbH, a company that manufactures and commercializes feed additives.
Ethics statement
All procedures involving animal handling and treatment were in accordance with national regulations for animal use in research and the national authority approved the study according to §26 of the Law for Animal Experiments (GZ: 68.205/0023-WF/V/3b/2015).
Software and data repository resources
Data are available from the corresponding author on reasonable request.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S1751731118002665.
click here to view supplementary material
References
- Bailey SR, Rycroft AN and Elliott J 2002. Production of amines in equine cecal bacteria contents in an in vitro model of carbohydrate overload. Journal of Animal Science 80, 2656–2662. [DOI] [PubMed] [Google Scholar]
- Bobe G, Young JW and Beitz DC 2004. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. Journal of Dairy Science 87, 3105–3124. [DOI] [PubMed] [Google Scholar]
- Carretero MI 2002. Clay minerals and their beneficial effects upon human health. Applied Clay Science 21, 155–163. [Google Scholar]
- Enemark J 2008. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): a review. Veterinary Journal 176, 32–43. [DOI] [PubMed] [Google Scholar]
- Guo J, Chang G, Zhang K, Xu L, Jin D, Bilal MS and Shen X 2017. Rumen-derived lipopolysaccharide provoked inflammatory injury in the liver of dairy cows fed a high-concentrate diet. Oncotarget 8, 46769–46780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimble RF 2001. Nutritional modulation of immune function. Proceedings of the Nutrition Society 60, 389–397. [DOI] [PubMed] [Google Scholar]
- Hailemariam D, Mandal R, Saleem F, Dunn SM, Wishart DS and Ametaj BN 2014. Metabolomics approach reveals altered plasma amino acid and sphingolipid profiles associated with pathological state in transition dairy cows. Current Metabolomics 2, 184–195. [Google Scholar]
- Huber K, Dänicke S, Rehage J, Sauerwein H, Otto W, Rolle-Kampzyk U and von Bergen M 2016. Metabotypes with properly functioning mitochondria and anti-inflammation predict extended productive life span in dairy cows. Scientific Reports 6, 24642–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humer E, Aschenbach JR, Neubauer V, Kröger I, Khiaosa-ard R, Baumgartner W and Zebeli Q 2018. c. Signals for identifying cows at risk of subacute ruminal acidosis in dairy veterinary practice. Journal of Animal Physiology and Animal Nutrition 102, 380–392. [DOI] [PubMed] [Google Scholar]
- Humer E, Kröger I, Neubauer V, Schedle K, Reisinger N and Zebeli Q 2018. b. Supplementing phytogenic compounds or autolyzed yeast modulates ruminal biogenic amines and plasma metabolome in dry cows experiencing subacute ruminal acidosis. Journal of Dairy Science 101, 9559–9574. [DOI] [PubMed] [Google Scholar]
- Humer E, Petri RM, Aschenbach JR, Bradford BJ, Penner GB, Tafaj M, Südekum K-H and Zebeli Q 2018. a. Invited review: Practical feeding management recommendations to mitigate the risk of subacute ruminal acidosis in dairy cattle. Journal of Dairy Science 101, 872–888. [DOI] [PubMed] [Google Scholar]
- Kenéz A, Dänicke S, Rolle-Kampczyk U, von Bergen M and Huber K 2016. A metabolomics approach to characterize phenotypes of metabolic transition from late pregnancy to early lactation in dairy cows. Metabolomics 12, 165, 10.1007/s11306-016-1112-8. [DOI] [Google Scholar]
- Krause KM, Combs DK and Beauchemin KA 2002. Effects of forage particle size and grain fermentability in midlactation cows. II. Ruminal pH and chewing activity. Journal of Dairy Science 85, 1947–1957. [DOI] [PubMed] [Google Scholar]
- Krause KM and Oetzel GR 2006. Understanding and preventing subacute ruminal acidosis in dairy herds: a review. Animal Feed Science and Technology 126, 215–236. [Google Scholar]
- Kröger I, Humer E, Neubauer V, Reisinger N, Aditya S and Zebeli Q 2017. Modulation of chewing behavior and reticular pH in nonlactating cows challenged with concentrate-rich diets supplemented with phytogenic compounds and autolyzed yeast. Journal of Dairy Science 100, 9702–9714. [DOI] [PubMed] [Google Scholar]
- Le Floc’h N, Melchior D and Obled C 2004. Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livestock Production Science 87, 37–45. [Google Scholar]
- Li S, Gozho G, Gakhar N, Khafipour E, Krause D and Plaizier J 2012. Evaluation of diagnostic measures for subacute ruminal acidosis in dairy cows. Canadian Journal of Animal Science 92, 353–364. [Google Scholar]
- Mao SY, Huo W.J and Zhu WY 2016. Microbiome-metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environmental Microbiology 18, 525–541. [DOI] [PubMed] [Google Scholar]
- Maulfair DD and Heinrichs AJ 2013. Effects of varying forage particle size and fermentable carbohydrates on feed sorting, ruminal fermentation, and milk and component yields of dairy cows. Journal of Dairy Science 96, 3085–3097. [DOI] [PubMed] [Google Scholar]
- Neubauer V, Humer E, Kröger I, Braid T, Wagner M and Zebeli Q 2018. b. Differences between pH of indwelling sensors and pH of fluid and solid phase in the rumen of dairy cows fed varying concentrate levels. Journal of Animal Physiology and Animal Nutrition 102, 343–349. [DOI] [PubMed] [Google Scholar]
- Neubauer V, Petri R, Humer E, Kröger I, Mann E, Reisinger N, Wagner M and Zebeli Q 2018. a. High-grain diets supplemented with phytogenic compounds or autolyzed yeast modulate ruminal bacterial community and fermentation in dry cows. Journal of Dairy Science 101, 2335–2349. [DOI] [PubMed] [Google Scholar]
- Neubauer V, Petri R, Humer E, Kröger I, Reisinger N and Zebeli Q 2017. Supplementation of a clay-mineral based mix modulates the digesta- and epithelial-associated microbiome of cows challenged with high-grain diets. In Proceedings of the 21st European Society of Veterinary and Comparative Nutrition Congress, 20–23 September 2017, Cirencester, UK, 123 pp.
- Newsholme P 2001. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? The Journal of Nutrition 131, 2515–2522. [DOI] [PubMed] [Google Scholar]
- Ramsay SL, Stoeggl WM, Weinberger KM, Graber A and Guggenbichler W 2012. Apparatus and method for analyzing a metabolite profile. Patent No. US 8,265,877 B2, 11 September 2012.
- Ronchi B, Bernabucci U, Lacetera N and Nardone A 2000. Oxidative and metabolic status of high yielding dairy cows in different nutritional conditions during the transition period. In Proceedings of the 51st Annual Meeting of the European Association for Animal Production (EAAP), 21–24 August, The Hague, The Netherlands, 125 pp.
- Sadri H, von Soosten D, Meyer U, Kluess J, Dänicke S, Saremi B and Sauerwein H 2017. Plasma amino acids and metabolic profiling of dairy cows in response to a bolus duodenal infusion of leucine. Public Library of Science one 12, e0176647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem F, Ametaj BN, Bouatra S, Mandal R, Zebeli Q, Dunn SM and Wishart DS 2012. A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. Journal of Dairy Science 95, 6606–6623. [DOI] [PubMed] [Google Scholar]
- Slamova R, Trckova M, Vondruskova H, Zraly Z and Pavlik I 2011. Clay minerals in animal nutrition. Applied Clay Science 51, 395–398. [Google Scholar]
- Stanley CA, Hale DE, Berry GT, Deleeuw S, Boxer J and Bonnefont JP 1992. A deficiency of carnitine acylcarnitine translocase in the inner mitochondrial membrane. New England Journal of Medicine 327, 19–23. [DOI] [PubMed] [Google Scholar]
- Suliman ME, Qureshi AR, Stenvinkel P, Pecoits-Filho R, Bárány P, Heimbürger O, Anderstam B, Ayala ER, Filho JCD, Alverstrand A and Lindholm B 2005. Inflammation contributes to low plasma amino acid concentrations in patients with chronic kidney disease. The American Journal of Clinical Nutrition 82, 342–439. [DOI] [PubMed] [Google Scholar]
- Sulzberger SA, Kalebich CC, Melnichenko S and Cardoso FC 2016. Effects of clay after a grain challenge on milk composition and on ruminal pH, blood and fecal pH in Holstein cows. Journal of Dairy Science 99, 8028–8040. [DOI] [PubMed] [Google Scholar]
- Tarrago L, Peterfi Z, Lee BC, Michel T and Gladyshev VN 2015. Monitoring methionine sulfoxide with stereospecific mechanism-based fluorescent sensors. Natural Chemical Biology 11, 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Zhang RY, Zhu WY and Mao SY 2013. Effects of subacute ruminal acidosis challenges on fermentation and biogenic amines in the rumen of dairy cows. Livestock Science 155, 262–272. [Google Scholar]
- Zebeli Q and Metzler-Zebeli BU 2012. Interplay between rumen digestive disorders and diet-induced inflammation in dairy cattle. Research in Veterinary Science 93, 1099–1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S1751731118002665.
click here to view supplementary material