Abstract
Background
Social media is a potential source of information on postmarketing drug safety surveillance that still remains unexploited nowadays. Information technology solutions aiming at extracting adverse reactions (ADRs) from posts on health forums require a rigorous evaluation methodology if their results are to be used to make decisions. First, a gold standard, consisting of manual annotations of the ADR by human experts from the corpus extracted from social media, must be implemented and its quality must be assessed. Second, as for clinical research protocols, the sample size must rely on statistical arguments. Finally, the extraction methods must target the relation between the drug and the disease (which might be either treated or caused by the drug) rather than simple co-occurrences in the posts.
Objective
We propose a standardized protocol for the evaluation of a software extracting ADRs from the messages on health forums. The study is conducted as part of the Adverse Drug Reactions from Patient Reports in Social Media project.
Methods
Messages from French health forums were extracted. Entity recognition was based on Racine Pharma lexicon for drugs and Medical Dictionary for Regulatory Activities terminology for potential adverse events (AEs). Natural language processing–based techniques automated the ADR information extraction (relation between the drug and AE entities). The corpus of evaluation was a random sample of the messages containing drugs and/or AE concepts corresponding to recent pharmacovigilance alerts. A total of 2 persons experienced in medical terminology manually annotated the corpus, thus creating the gold standard, according to an annotator guideline. We will evaluate our tool against the gold standard with recall, precision, and f-measure. Interannotator agreement, reflecting gold standard quality, will be evaluated with hierarchical kappa. Granularities in the terminologies will be further explored.
Results
Necessary and sufficient sample size was calculated to ensure statistical confidence in the assessed results. As we expected a global recall of 0.5, we needed at least 384 identified ADR concepts to obtain a 95% CI with a total width of 0.10 around 0.5. The automated ADR information extraction in the corpus for evaluation is already finished. The 2 annotators already completed the annotation process. The analysis of the performance of the ADR information extraction module as compared with gold standard is ongoing.
Conclusions
This protocol is based on the standardized statistical methods from clinical research to create the corpus, thus ensuring the necessary statistical power of the assessed results. Such evaluation methodology is required to make the ADR information extraction software useful for postmarketing drug safety surveillance.
International Registered Report Identifier (IRRID)
RR1-10.2196/11448
Keywords: social media, drug-related side effects and adverse reactions, natural language processing, data mining, MedDRA, Racine Pharma
Introduction
Background
The detection of new adverse drug reactions (ADRs) has been based on postmarketing surveillance by government agencies (national authorities) derived from spontaneous reporting by health care professionals and patients. The US Food and Drug Administration (FDA) and the European Medicines Agency collect ADR case reports through the FDA’s Adverse Event Reporting System (FAERS) [1-4] and the EudraVigilance system, respectively. These systems are useful tools for drug agencies, which mine these huge amounts of structured data to look for new safety concerns that might be related to a marketed product [5-8].
In the last 20 years, internet and social media have become an integral part of people’s daily life. Social media is now often used to communicate with other persons having the same health concerns and share information regarding their health conditions, feelings, medications, and many other aspects [9]. Social media is, therefore, a potential provider of information on ADRs. In 2005, the International Society of Drug Bulletins already recognized such a use: Patient reporting systems should periodically sample and evaluate the scattered drug experiences patients report on the internet [10]. This new source of knowledge captured the interest from health informatics, statistics, and public health researchers. Although in its infancy, related scientific literature increased in the last decade [4,11-13].
Objectives
In this context, the French Ministry of Industry funded and launched the Adverse Drug Reactions from Patient Reports in Social Media (ADR-PRISM) project. The objective of ADR-PRISM was to make available the contents about ADR, informal and embedded in forums and discussions on the Web, to the actors involved in drug safety (drug companies, agencies, and pharmacovigilance experts). In the end, the tool developed in the ADR-PRISM project should generate hypotheses about new or poorly documented adverse events (AEs). To reach its goals, the ADR-PRISM consortium gathers a company developing text mining softwares (Expert System), a company specialized in pharmaco-epidemiology (Kappa Santé), 3 academic research groups providing expertise in medical informatics and statistics (National Institute of Health and Medical Research & Cordeliers Research Centre: umrs 1138 team 22 dedicated to Information Sciences to support Personalized Medicine and Laboratory in Medical Informatics and Knowledge Engineering in e-Health, and Biomedicine informatics, Service Catalogue and Index of French Language medical websites SIBM-CISMeF), 2 experts in pharmacovigilance (regional center of pharmacovigilance), as well as Vidal group that supplies the drug database used in most drug prescription systems in France.
From a natural language processing (NLP) perspective, we considered ADR as relationships between drug and AE concepts. On the basis of that, the NLP-based Skill Cartridge for pharmacovigilance developed by Expert System for ADR-PRISM includes a relation extraction module based on (named) entity recognition combined with rules and regular expressions. Before applying it to large collections of forum discussions, we designed a protocol to assess the performance of this ADR information extraction module. The objective of this study is to present this protocol.
Methods
Synopsis
With the objective of sharing a methodology that guarantees the confidence in the results in ADR-PRISM, we followed a way of reasoning inspired by the standards widely adopted in clinical research such as the Standards for Reporting Diagnostic Accuracy studies [14]. These items are displayed in Textbox 1.
Synopsis of the protocol.
Rationale
Nowadays, patients extensively use social media. They report on the adverse events they feel because of their medications (further called adverse drug reaction [ADR]) on health forums. Promising studies exist on the extraction of ADR information from social media with natural language processing (NLP) or machine learning tools.
The consortium Adverse Drug Reaction from Patient Reports in Social Media (ADR-PRISM) has been constituted to create a tool extracting the ADR information from social media. Teams specialized in text mining NLP and pharmacovigilance participate in the consortium.
Before applying the ADR-PRISM’s tool on a larger scale (millions of posts) to draw conclusions on ADRs, our goal was to adapt the principles adopted in clinical research to assess the ADR-PRISM. For example, evaluation was done against a gold standard based on manual annotation of the posts.
Primary objective
To estimate robustly the performance of the ADR information extraction tool against gold standard.
Primary expected results
Precision, recall, and f-measure for ADR extraction.
Secondary objectives
To verify the quality of the gold standard and to evaluate the impact of various conditions (eg, different granularities and sentence constraint of the tool) on the performance of the ADR information extraction tool.
Secondary expected results
Kappa metrics for interannotator agreement; and precision, recall, and f-measure for ADR extraction in various conditions.
Database
Posts from the Kappa Santé Detec’t database published between January 1, 2007, and October 28, 2016.
Eligibility criteria
Posts from the Kappa Santé Detec’t database that contain at least one drug’s or molecule’s (active substance) name, and posts randomly selected from the rest of the Kappa Santé Detec’t database; a list of drugs and adverse events (AEs) of interest is established by drug safety experts; and ADR: any explicit and positive relationship between a drug and a potential AE where either the drug or the AE or both belong to the list.
Index test method
ADR information extraction tool: this tool classifies each co-occurrence of drug and AE in a post as positive ADR or negative ADR or no ADR, and maps the drug and the AE expression to Racine Pharma and Medical Dictionary for Regulatory Activities (MedDRA), respectively.
Reference method
Gold standard: manual annotations of the co-occurrence of drug and AE as positive ADR or negative ADR or no ADR including the mapping of the drug and the AE expression with a Racine Pharma and a MedDRA entity, respectively. Manual annotations will be provided by 2 annotators with experience in medical terminology.
Sample size
A 95% CI, with a total width of 0.1, is used to determine the sample size.
Statistical analyses
Recall, precision, and f-measure calculations for evaluation of the performances of the ADR extraction information tool and interannotator agreement for the evaluation of the gold standard with a Cohen kappa.
Design and Ethics
Design
This project is based on retrospective data collected among threads of discussion accessible on social media (messages from French health forums). A total of 2 experts in pharmacovigilance helped delineating the project. The objective is twofold: (1) focus on certain pharmaceutical products of interest and identify the related ADRs and (2) detect the emergence of general potential problem in public health. On this basis, the extraction of ADR information is expected to (1) perform well on specific concepts for use case study and (2) be able to extract all potential concepts correctly for screening purpose.
To evaluate the ADR information extraction, we therefore build up the global process in 2 phases (Figure 1). In phase 1, we implemented an iterative process of validation and improvement of the Skill Cartridge for pharmacovigilance. The objectives were (1) to correct the most frequent errors and to train the ADR information extraction module and (2) to estimate the performance indicators and the time required for manual annotation for phase 2 sample size determination. Then, in phase 2, we will conduct a definite assessment of the performance of the ADR information extraction module.
Figure 1.
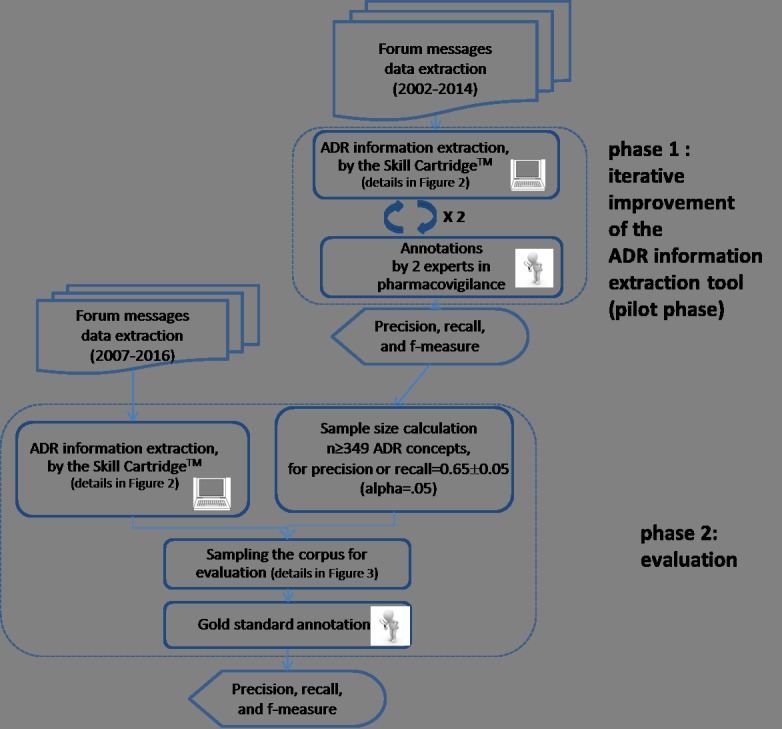
Study overview. ADR: adverse drug reaction.
In the rest of the paper, we use the term AE for medical events that are present in text for a potential ADR, whereas the term ADR is used when a relation between a drug and an AE is established.
Ethics
This research did not involve experiment on either humans or animals. Ethics and guarantee of data privacy constituted an integral and dedicated working group set up for the ADR-PRISM project. To comply with national regulations, we first registered data collection to the French Data Protection Agency (CNIL for Commission Nationale Informatique et Liberté), which is known as a normal notification in technical terminology, on December 23, 2015. We later submitted an authorization request on March 30, 2016, regarding data analysis and validation of the approach adopted about ethics and confidentiality to the same agency. ADR-PRISM’s consortium detailed approach is explained in the study by Bousquet et al [15].
The ADR-PRISM project was supported by an ethics advisory board, which was composed of scientists with different scientific expertise: drug safety, public health, and ethics. Their role was to give independent advice regarding ethical issues to the project consortium. The board approved the whole study design, including the protocol presented in the paper.
Adverse Drug Reactions From Patient Reports in Social Media Adverse Drug Reaction Extraction
Resources
We used 3 lexicons to represent drug information and AEs. We codified AEs with the Medical Dictionary for Regulatory Activities (MedDRA) v15.1 [16] classification. Racine Pharma, maintained by the SIBM-CISMeF [17], provided an extensive source of drug names that covered all medications available on the French market, including brand names and active ingredients. Racine Pharma entries were mapped to the Anatomical Therapeutic Chemical (ATC) system [18], which was used as a classification system for drugs.
The corpus of messages was extracted from the Detec’t database [19], a database developed by Kappa Santé that collects messages from several French forums using a Web crawler. We limited ourselves to a list of 5 major health forums in French obtained using netscoring [9]. Message extraction was based on a named entity recognition module using a drug lexicon designed by Kappa Santé and a fuzzy matching algorithm. The lexicon was based on Racine Pharma, the ATC classification, and a list of medications extracted from the French National Health Insurance database.
Drugs, Adverse Events, and Adverse Drug Reaction Extraction
ADR extraction was performed in 2 steps: first, named entity recognition modules were used to identify drug names and AEs in posts, and then, a relation extraction algorithm was applied to these entities (Figure 2).
Figure 2.
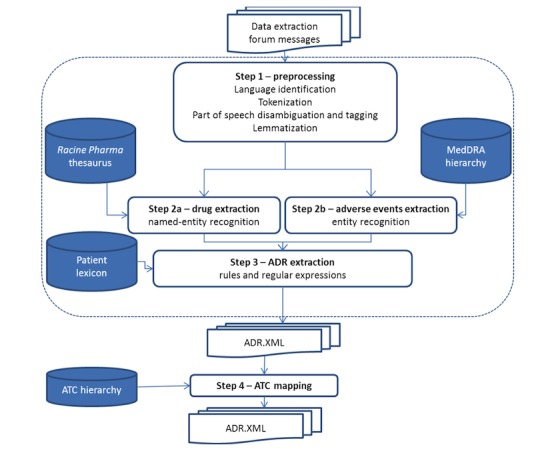
Adverse drug reaction pipeline. ADR: adverse drug reaction; ATC: Anatomical Therapeutic Chemical (in ATC classification); MedDRA: Medical Dictionary for Regulatory Activities.
Drugs and Adverse Events Extraction
Regarding drugs, Expert System has developed a named entity recognition module capable of identifying words or tokens (occurrences) listed in Racine Pharma and extracting their positions from forum posts.
We mapped the extracted expressions from Racine Pharma to the chemical substance level (5th level) of the ATC (last updated version: December 19, 2016) [18]. Considering that the same active ingredient could be found under different trade names, we pooled all mapped expressions within the same ATC chemical subgroup (4th level) to define the drug concepts.
Expert System has developed another module based on MedDRA to identify the AEs and extract their position in the posts. Fuzzy-matching and enrichment of thesaurus with colloquial terms enabled to take into account the characteristics of the posts on health forums.
Drug and Adverse Event Relationship Extraction (Skill Cartridge for Pharmacovigilance)
An NLP module has been developed by Expert System to capture the specific information regarding the relationship established between a drug and an AE by the post’s author. The module combined a set of rules and regular expressions, with a Patient lexicon constituted to ensure that the post’s author set out a situation of a person taking the drug and experiencing the symptom and excludes general information regarding a drug or an AE. This lexicon contains terms such as I, me, my, cause, test, take, feeling, because of, provoke, intolerance, and allergies. This NLP module also included negation detection.
The algorithm can be summarized as follows:
Text was split into sentences based on the punctuation mark.
For each sentence, (named) entity recognition modules extracted drugs and AEs.
-
For each pair of drug and AE co-occurring in a sentence, if the NLP module extracted specific information regarding a relation, then the co-occurrence was classified as follows:
Explicit and positive ADR (eg, Abilify causes me such fears that I cannot concentrate to read, work, etc...);
Explicit and negative ADR (eg, I took some Doliprane and I didn’t feel any nausea);
If no specific information was identified, the co-occurrence was classified as no ADR (eg, Usually, Focalin’s adverse effects are loss of appetite, insomnia, and naso-pharyngitis).
Phase 1: Iterative Improvement of the Adverse Drug Reaction Information Extraction Module
Phase 1 consisted of an iterative process of validations and improvements of the tool. The review on the corpus was constituted by manual annotation of the messages by 2 experts in pharmacovigilance. The manual annotation process used a tool developed by Expert System for in-house testing purposes [20]. This tool did not integrate functionalities for blind manual annotation. No gold standard was established at this step. All along the process, both annotators could see the drugs, AEs, and ADRs extracted by the tool, and their task consisted of validating or invalidating these extractions and, if needed, complementing the annotations performed by the system. The annotators were asked to (1) check all drugs and all AEs in the post, irrespective of whether they have been involved in an ADR relationship, and (2) identify all drug-AE relationships in the same sentence. The output of phase 1 was a first estimation of precision, recall, and f-measure, assessing the capacity to extract information regarding drugs, AEs, and ADRs. More details about the corpus construction, evaluating concept selection, sample size determination, and the preliminary results of this phase were reported in the study by Chen et al [21].
Phase 2: Evaluation
Corpus for Evaluation
Evaluation Dataset
For phase 2, the selection of datasets is given in Figure 3.
Figure 3.
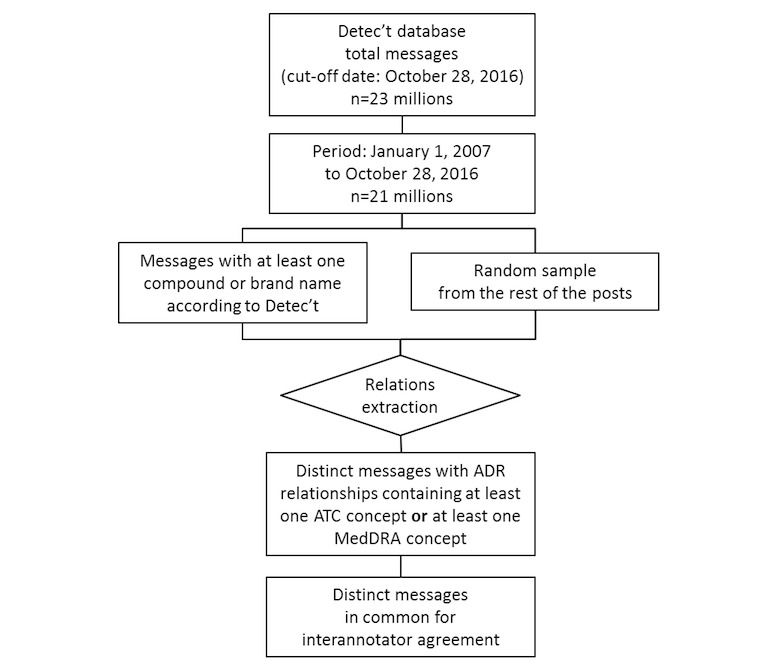
Phase 2 messages selection flowchart. ADR: adverse drug reaction; ATC: Anatomical Therapeutic Chemical (in ATC classification); MedDRA: Medical Dictionary for Regulatory Activities.
On October 28, 2016, Kappa Santé Detec’t database contained about 23 millions of posts, and approximately 21 million posts were published after January 1, 2007. Messages about drugs, for which marketing authorization has been withdrawn before the cut-off date, were considered as some AEs might appear at long term or patients might discuss about old drugs and their safety.
The software developed by Kappa Santé for purpose of Web discussions collection offered the possibility of preidentifying the posts containing at least one pharmaceutical or molecule name. We considered the dataset of all posts containing at least one preidentified compound or brand name and combined it with a complementary set of posts randomly sampled from the remainder of the Kappa Santé Detec’t database.
The drug, AE, and ADR extraction modules developed for ADR-PRISM were executed on both sets of posts, and the output of the extraction module was considered as the evaluation dataset.
Drug Selection
The evaluation focused on 3 categories of drugs: (1) the most frequently extracted drugs in the corpus, (2) the most sold drugs in France, and (3) the drugs of interest of pharmacovigilance because of recent safety alerts. For the last 2 categories, the frequencies of the recognized named entities were also taken into account to cope with the necessary sample size.
The most sold drugs in 2013 in France are listed below, in their French commercial names, including the 10 most sold drugs with mandatory medical prescription for dispensation and the 10 most sold drugs with optional medical prescription for dispensation [22]:
Mandatory medical prescription for dispensation: Levothyrox, Uvedose, Lamaline, Dafalgan codéiné, Méthadone, Crestor, Pivalone, Seresta, Emla patch, and Seroplex
Optional medical prescription for dispensation: Doliprane, Dafalgan, Efferalgan, Kardégic, Spasfon, Gaviscon, Dexéryl, Météospasmyl, Biseptine, and Eludril.
The 2 experts additionally worked out a list of pairs of 1 drug concept and 1 AE concept corresponding to alerts that emerged in the last years (Table 1). This list of use cases has been further employed as a basis for the concept selection for evaluation. The concept selection in phase 1 (iterative improvement) [21] was based on the same consideration; therefore, those concepts on which the extraction tool performed well should be excluded from the selection for evaluation.
Table 1.
List of drug and adverse event concepts selected as pharmacovigilance use cases.
| Active drug ingredient: French names (date of marketing in cases where drug has been withdrawn) | Adverse events of interest | Corresponding Medical Dictionary for Regulatory Activities term | Media coverage or alert date |
| Gardasil | Autoimmune disease, complex regional pain syndrome, and postural orthostatic tachycardia syndrome | Autoimmune disorders (HLGTa) + complex regional pain syndrome (PTb) + postural orthostatic tachycardia syndrome (PT) | 2013 |
| Meningitec | Quality defect and any adverse event | All terms | 2015 |
| Methylphénidate: Ritaline, Concerta, Medikinet, Quasym, and Ritaline | Neurological and cardiac effects | Cardiac disorders (SOCc) + nervous system disorders (SOC) | 2009 (recommendation) |
| Acétate de cyprotérone: Diane 35, Cypropharm (2010-2013), Elleacnelle (2010-2013), Evepar, Holgyme (2002-2015), Lumalia (2003-2013), Minerva, and Arielle (1997-2011) | Thromboembolic risk | Embolism and thrombosis (HLGT) | 2012 (alert) |
| Fluoxétine: Prozac, Elindra (2001-2008), Fluctine (1998-2008), and Fontex (2001-2008) | All adverse events | All terms | Mostly 2013 |
| Méthadone | Prolonged time from the start of the Q wave to the end of the T wave during electrocardiogram (approximates the time taken from when the cardiac ventricles start to contract to when they finish relaxing) | QT interval prolongation | 2007 |
| Sofosbuvir: Harvoni, Epclusa, and Sovaldi | Bradycardia | Bradycardia (PT) | 2013 |
| Codeine: Codenfan, Codoliporane, Migralgine, and Néocodion et Prontalgine | Respiratory disorders | Respiratory disorders (HLGT) | 2012 re-evaluation |
| Hydroxyzine: atarax | Rhythm disorders | Cardiac arrhythmias (HLGT) | 2014 |
| Nicorandil: Adancor and Ikorel | Skin ulceration | Skin ulcer (HLTd) | 2012 |
| Midodrine: Gutron | Hypertension | Blood pressure increased (PT) | 2013 |
| Galantamine: Reminyl, Galanthen (2012-2015), and Galema (2011-2015) | Skin reaction | Skin and subcutaneous tissue disorders (SOC) | 2014 |
| Crizotinib: Xalkori | Heart failure | Heart failures HLGT | 2014 |
| Valproate de sodium: Depakine, Imaslav, and Micropakine | Teratogenic effects | Congenital, familial, and genetic disorders (SOC) | 2012? |
| Isotrétinoine: Curacné, Acnetrait, Contracné, Procuta, and Roaccutane | Teratogenic effects + psychiatric disorders | Congenital, familial, and genetic disorders (SOC) and psychiatric disorders (SOC) | 2002 |
| Fingolimod: Gilenya | Leukoencephalopathy | Toxic leukoencephalopathy (PT) | 2014 |
| Aripiprazole: Abilify | Suicidal behavior | Suicidal and self-injurious behavior (HLGT) | 2013 (words of warning: 2016) |
aHLGT: high-level group term.
bPT: preferred term.
cSOC: system organ class.
dHLT: high-level term.
Adverse Event Concepts Selection
The AE concepts for evaluation corresponded to preferred term (PT) level in the MedDRA hierarchy, and we focused similarly on 2 categories: (1) the most frequent extracted PTs and (2) the PTs of interest of pharmacovigilance (Table 1), guided also by the frequencies of the recognized entities in the corpus to cope with the necessary sample size.
Adverse Drug Reaction Definition
We defined an ADR as any explicit and positive relationship between a drug and an AE where the drug (respectively the AE) belonged to the list of concepts previously selected.
Corpus for the Evaluation (Random Sample From Evaluation Dataset)
Finally, a random sample from the evaluation dataset (ie, among all the ADR extracted by the ADR information extraction module) constituted our corpus for evaluation.
Gold Standard
Adverse Drug Reactions From Patient Reports in Social Media Manual Annotation Platform
We developed a Web application dedicated to manual annotations valuable as gold standard, for the project purposes in phase 2.
This application was based on Java Servlets and JavaScript libraries and connected in Java Database Connectivity to the dataset of posts selected for the gold standard annotation. We used a self-completion mechanism to attach portions of message to Racine Pharma and MedDRA terms. We used drag-and-drop operations to fill in a table containing the manual review of the co-occurrences, where each line was dedicated to 1 co-occurrence. For each co-occurrence, the drug and the AE were dragged-and-dropped in the first and the second column, respectively. In the third column, the manual annotator was given a drop-down menu that presented 3 possibilities for defining the co-occurrence: explicit positive ADR, explicit negative ADR, or no ADR. We finally offered the possibility to export this table containing the manual annotations obtained via the application. The interface of this application is shown in Figure 4.
Figure 4.
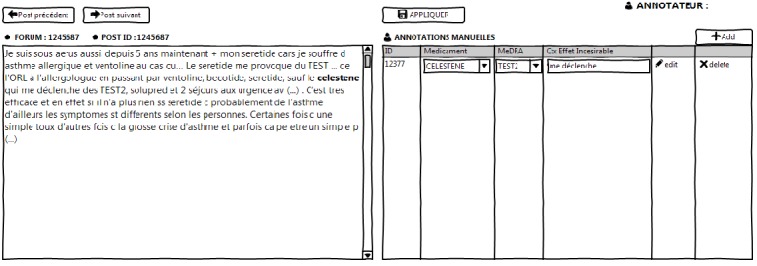
Interface of the manual annotation platform. ID: identifier; MedDRA: Medical Dictionary for Regulatory Activities.
Gold Standard Construction
An annotator guideline has been established to standardize manual annotations. A total of 2 experts in medical terminologies have annotated all ADR relationships for each post in the evaluation corpus, and then, the manual annotation was considered as gold standard. Different from phase 1, here we expected that the annotators annotated all causal relationships between a drug and an AE, even if the drug and AE were not in the same sentence, which will allow us further evaluate the impact of the sentence boundary constraint of the extraction tool.
Each annotator annotated 55.1% (261/474) of the posts in the evaluation corpus; thus, a subset of 10.1% (48/474) of the posts was annotated by both of them. The interannotator agreement could be assessed on these double-annotated posts. In case of disagreements, the 2 annotators discussed to achieve a consensus. If a lot of disagreements had occurred, the annotators would have been asked to learn again the guideline and revise their annotations. All along the process, both annotators were double-blinded: first, from the ADRs identified by the ADR information extraction module, and second, from the other annotator’s annotations.
Statistical Analyses
Primary Analysis
To assess the efficacy of the ADR information extraction module as compared with the gold standard, we will globally calculate the recall, precision, and f-measure.
In a post, if the ADR information extraction module identifies the same expression from Racine Pharma at the same position; and the same AE expression at PT level from MedDRA hierarchy at the same position; and the same type of relationship, as did the gold standard, then we will count the extracted ADR as a true positive (Figure 5).
Figure 5.
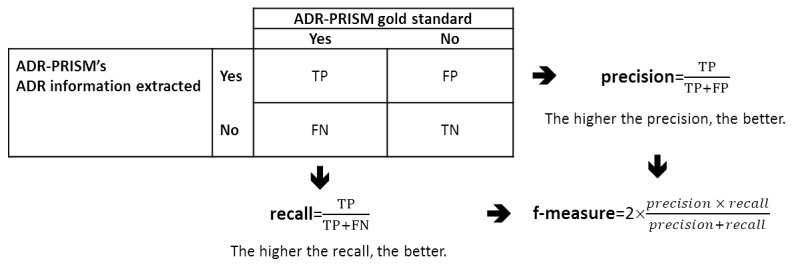
Recall, precision, and f-measure definitions. ADR: adverse drug reaction; ADR-PRISM: Adverse Drug Reaction from Patient Reports in Social Media; FN: false negative, manually annotated ADR by the gold standard that is not extracted by the ADR information extraction tool; FP: false positive, positive explicit relationship extracted by the ADR information extraction tool that is not manually annotated by gold standard; TN: true negative; TP: true positive.
F-Measure Gold Standard’s Evaluation
The interannotator agreement will be assessed by a hierarchical Kappa [23]. A hierarchical Kappa will enable to take into account the situation where the 2 annotators will disagree in either the level or the expression in ATC or MedDRA terminologies but will agree on higher levels than the annotated ones. Separate calculations for interannotator agreements by hierarchical Kappa on drug and AE expressions will be provided as well.
Secondary Analyses
We will complete these principal results with the following analyses.
We will take into account MedDRA and ATC granularities by reproducing precision, recall, and f-measure at each level of the hierarchies (system organ class [SOC], high-level group term [HLGT], high-level term [HLT], and preferred term [PT] of MedDRA and anatomical main group, therapeutic subgroup, pharmacological subgroup, and chemical subgroup of ATC).
We will provide a relaxed definition of true-positive ADRs combining the following 3 conditions: (1) the positions in the post of the extracted expressions for drug and AE both match the positions of the drug and AE expressions manually annotated, (2) the extracted and the manually annotated expressions for AE from MedDRA hierarchy are found in the same SOC levels, and (3) the classification of the identified relationship match the classification of the manually annotated relationship. We will calculate recall, precision, and f-measure with this definition for ADRs, drug, and AE identification separately.
We will provide a global estimation of the performance of ADR information extraction module by taking into account other types of messages. These messages are those without information on at least one ADR concept, that is, messages in which (1) ADR information is extracted but is not concerned by the drug- or AE-selected concepts, (2) no ADR information is extracted but only co-occurrences of information about 1 drug and 1 AE, (3) only AE information is extracted, (4) only drug information is extracted, and (5) neither drug nor AE information is extracted. After having sampled each type of message described above, we will then calculate global recall or precision or f-measure on all types of messages by marginal calibration.
All analyses will be performed in R software [24].
Sample Size Calculation
Recall can be estimated from a random sample of ADR concepts annotated by the gold standard, whereas precision can be estimated from a random sample of ADR concepts extracted by the ADR information extraction module. However, creating the gold standard on the whole evaluation dataset before sampling in it for the recall was an overwhelming task. At the same time, a vast majority of messages did not contain any information on the drug concept. Thus, we expected a very low proportion of ADR concepts identified by the ADR information extraction module in the evaluation dataset. Therefore, the recall estimated from a random sample of ADR concepts annotated by the gold standard was mathematically approximated by the recall estimated on ADR concepts sampled for the precision.
The sample size required for different expected precision or recall is provided in Table 2. By hypothesizing a global recall of 0.5, we needed at least 384 identified ADR concepts to obtain a 95% CI, having a total width of 0.1. The final determination of sample size was based on the previously estimated precision and recall.
Table 2.
Sample size required according to the expected precision and recall and total width of confidence intervals (CIs).
| Range of CI | Expected precision or recall values | ||||||||
|
|
0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 |
| 90% CI | 35 | 61 | 81 | 92 | 96 | 92 | 81 | 61 | 35 |
| 95% CI | 138 | 246 | 323 | 369 | 384 | 369 | 323 | 246 | 138 |
Differences Between Phase 1 and Phase 2
The main differences between phase 1 and phase 2 are threefold (Table 3). First, in phase 1, the annotations targeted all drugs and AEs even if not involved in an ADR relation, whereas in phase 2, annotation focuses on the ADR relations. Second, in phase 2 (and only in phase 2) a gold standard based on blind manual annotation by 2 experts in medical terminologies was established and the interannotator agreement was measured. In contrast, the manual annotations of phase 1 were not blind, and most of the work consisted of validating the automatic annotations; thus, the interannotator agreement was not addressed. Finally, in phase 2, additional parameters such as the granularity and the segmentation of the messages (sentence boundaries) will be analyzed. The precision, recall, and f-measure will be calculated with a gold standard at the message level and might be compared with precisions, recalls and f-measures calculated at the sentence level and for all MedDRA and ATC granularities.
Table 3.
Common features and differences between phase 1 and phase 2.
| Issues | Phase 1 | Phase 2 |
| Evaluation focus | Drugs and adverse events entity recognition | Adverse drug reaction relationships recognition |
| Gold standard (manual annotations) | Not blind | Blind |
| Gold standard annotators | Pharmacovigilance experts | Experts in medical terminologies |
| Interannotator agreement | No | Yes |
| Granularity issue | No | Yes |
| Sentence boundary issue | No | Yes |
Results
At the time of publishing this evaluation protocol, several steps of this project would have been completed. The corpus for evaluation has been constituted, and the NLP tools have identified and extracted the information about ADR inside this corpus. We selected the ADR concepts and constituted the samples of the entities necessary to set up the gold standard. The 2 annotators completed the annotations process.
Data analyses for assessing the interannotator agreement and the performance of the ADR information extraction module as compared with the gold standard are ongoing, and the study results are expected internally before the end of 2018.
Discussion
Summary
With the objective of using messages on health forums as a new source for drug safety, the systems developed to mine the messages must follow strict evaluation rules. This is even more important as these systems might be used to support decision making. The protocol presented in this paper has been designed to evaluate the ADR information extraction Skill Cartridge developed in ADR-PRISM in a pharmacovigilance perspective.
Study Strengths
The protocol presented in this paper is an attempt to apply clinical research–level guidelines [14] to the assessment of such systems.
First, we paid particular attention to the establishment and the validation of the gold standard. The gold standard was established by 2 trained specialists of medical terminologies who annotated selected messages. Manual annotation was performed in a double-blind manner, namely, from both the other annotator and the ADR identified by the ADR information extraction module. The annotated corpus, therefore, constituted a valuable gold standard. On the basis of the study by Sarker et al [4] (see Multimedia Appendix 1), we could find 13 studies where a gold standard, that is, manual annotations used for evaluation, was implemented [25-37]. Conversely, in 8 studies, there was no gold standard [38-45], and the extracted ADRs were compared with already known AEs from FAERS [39-41,43,44] or drug label declared to FDA [38,45] or even AE described in websites [42]. Moreover, in the ADR-PRISM protocol, a common subset of randomly selected messages was annotated in a blind manner by the 2 experts; interannotator agreement evaluation will also ensure ourselves about the quality of this gold standard. In most of studies, the authors gauged the interannotators agreement [25,27,30-32,34,35]. However, it is not systematic [26,28,29,33,36,37].
Second, by calculating a sample size of messages collected from social media, to assess recall, precision, and f-measure, we guaranteed the statistical power to place reliance on our study results.
The chosen terminologies are another crucial aspect of this work. On the one hand, the Racine Pharma thesaurus, with 5164 entries, exhaustively covers a large range of drug names and active ingredients. On the other hand, the MedDRA hierarchy is used daily by drug safety experts and considered expressive for this task. By using these terminologies, we expect to increase the sensitivity of the ADR-PRISM Skill Cartridge for pharmacovigilance. Our choice to map Racine Pharma to ATC was guided by 2 aspects. First, ATC like MedDRA has a hierarchical structure. Thus, we will be able to evaluate the ADR information extraction tool based on different hierarchical levels of these terminologies. Second, ATC and MedDRA are widely used and internationally agreed reference terminologies. Hence, we expect to provide strong and reproducible results.
The ADR information extraction module was not only based on drug and AE information identification but also on rules and regular expressions. As such, we expect to discard noninformative sentences, addressing general information about the drug or drug indications. We would also be able to identify unexpected positive effects, as for example, headaches that would be reduced by a drug without indication for the treatment of this kind of pain. Only few studies have been able to take this aspect into account [27,29,30,32,34,46].
Study Limitations
Despite its positive aspects, the study exhibits several limitations.
The mapping between the terms listed in the drug thesaurus Racine Pharma and the terms referenced in the chemical substance in the ATC hierarchy was incomplete. Among the 5164 terms in Racine Pharma, 852 (16.5%, 852/5164) could not be mapped to ATC, for example, some phytotherapies (St John’s wort herbal tea, Silver birch juice, extract of licorice root, arum triphyllum compound, arnica, etc). This could have a negative impact on the recall scores calculated according to the ATC levels.
In social media’ posts, slangs and colloquial languages are frequent; likewise, syntactic rules are approximate. We chose to use fuzzy-matching and thesaurus enrichment to take into account this bias. However, this approach is inherently nonexhaustive with negative impact on the recall of the ADR information extraction module.
Regarding the gold standard, 2 experts in medical terminologies trained in drug safety performed manual annotations. However, contrary to the phase 1 manual review, none of them could be considered as a pharmacovigilance expert.
The medical informatics community needs shared open corpora to evaluate their methods and tools. Recent efforts have led to making several datasets more accessible and the evaluation of the methods more standardized, for example, the Multiparameter Intelligent Monitoring in Intensive Care corpus [47] and the Informatics for Integrating Biology and the Bedside challenges [48]. Drug safety could benefit from open-access validation datasets made available for research purposes [25,35]. However, several obstacles remain. For example, the ADR-PRISM consortium got approval to reuse posts for research purposes, but the approval is restricted to the project.
Conclusions
The objective of this study is to present the scientific approach developed in the first stage of the ADR-PRISM project. At this stage, our principal objective was to evaluate the performance of the ADR information extraction Skill Cartridge against a gold standard constituted by human annotations. To address this question, our goal was to adapt the principles adopted in clinical research to assess sound and trustworthy measurements of precision, recall, and f-measure.
With the statistical theory, we calculated a sample size. This guaranteed enough ADR information and sufficient narrowness of the confidence interval, to scientifically conclude on our principal objective. We also avoided unnecessarily large extractions for which the process of manual annotations is nothing but both wasteful and time-consuming.
Acknowledgments
This work was labeled in 2013 by the competitiveness cluster Cap Digital and funded by the French General Direction for Enterprises (Direction Générale des Entreprises) and territorial collectivities (Ile de France and Haute Normandie) under the 16th French Unique Interministerial Fund request for proposal through the ADR-PRISM project.
Abbreviations
- ADR
adverse drug reaction
- ADR-PRISM
Adverse Drug Reaction from Patient Reports in Social Media
- AE
adverse event
- ATC
Anatomical Therapeutic Chemical (in ATC classification)
- FAERS
Food and Drug Administration’ adverse event reporting system
- FDA
Food and Drug Administration
- HLGT
high-level group term
- HLT
high-level term
- MedDRA
Medical Dictionary for Regulatory Activities
- NLP
natural language processing
- PT
preferred term
- SIBM-CISMeF
Biomedicine informatics service catalogue and index of French language medical websites (French Service d’Informatique Biomédicale-Catalogue et Index des Sites Médicaux de langue Française)
- SOC
system organ class
Designs of articles related to ADR information extraction on social media (replicated from Sarker et al).
Footnotes
Conflicts of Interest: None declared.
References
- 1.Bates DW, Evans RS, Murff H, Stetson PD, Pizziferri L, Hripcsak G. Detecting adverse events using information technology. J Am Med Inform Assoc. 2003;10(2):115–28. doi: 10.1197/jamia.M1074. http://jamia.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=12595401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golomb BA, McGraw JJ, Evans MA, Dimsdale JE. Physician response to patient reports of adverse drug effects: implications for patient-targeted adverse effect surveillance. Drug Saf. 2007;30(8):669–75. doi: 10.2165/00002018-200730080-00003.3083 [DOI] [PubMed] [Google Scholar]
- 3.Hazell L, Shakir SA. Under-reporting of adverse drug reactions : a systematic review. Drug Saf. 2006;29(5):385–96. doi: 10.2165/00002018-200629050-00003.2953 [DOI] [PubMed] [Google Scholar]
- 4.Sarker A, Ginn R, Nikfarjam A, O'Connor K, Smith K, Jayaraman S, Upadhaya T, Gonzalez G. Utilizing social media data for pharmacovigilance: a review. J Biomed Inform. 2015 Apr;54:202–12. doi: 10.1016/j.jbi.2015.02.004. http://linkinghub.elsevier.com/retrieve/pii/S1532-0464(15)00036-2 .S1532-0464(15)00036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, De Freitas RM. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998 Jun;54(4):315–21. doi: 10.1007/s002280050466. [DOI] [PubMed] [Google Scholar]
- 6.DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. The American Statistician. 1999 Aug;53(3):177. doi: 10.2307/2686093. [DOI] [Google Scholar]
- 7.Szarfman A, Machado SG, O'Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA's spontaneous reports database. Drug Saf. 2002;25(6):381–92. doi: 10.2165/00002018-200225060-00001.250601 [DOI] [PubMed] [Google Scholar]
- 8.van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10. doi: 10.1002/pds.668. [DOI] [PubMed] [Google Scholar]
- 9.Katsahian S, Simond ME, Leprovost D, Lardon J, Bousquet C, Kerdelhué G, Abdellaoui R, Texier N, Burgun A, Boussadi A, Faviez C. Evaluation of internet social networks using net scoring tool: a case study in adverse drug reaction mining. Stud Health Technol Inform. 2015;210:526–30. doi: 10.3233/978-1-61499-512-8-526. [DOI] [PubMed] [Google Scholar]
- 10.ISDB . International Society of Drug Bulletins. ISDB EU; 2005. Jan, [2018-06-29]. ISDB EU: Berlin Declaration on Pharmacovigilance https://www.akdae.de/Arzneimittelsicherheit/Weitere/Archiv/2005/85_200501252.pdf . [Google Scholar]
- 11.Ho T, Le L, Thai DT, Taewijit S. Data-driven approach to detect and predict adverse drug reactions. Curr Pharm Des. 2016;22(23):3498–526. doi: 10.2174/1381612822666160509125047.CPD-EPUB-75526 [DOI] [PubMed] [Google Scholar]
- 12.Wong A, Plasek JM, Montecalvo SP, Zhou L. Natural language processing and its implications for the future of medication safety: a narrative review of recent advances and challenges. Pharmacotherapy. 2018 Jun 09;38(8):822–41. doi: 10.1002/phar.2151. [DOI] [PubMed] [Google Scholar]
- 13.Tricco AC, Zarin W, Lillie E, Pham B, Straus SE. Utility of social media and crowd-sourced data for pharmacovigilance: a scoping review protocol. BMJ Open. 2017 Dec 19;7(1):e013474. doi: 10.1136/bmjopen-2016-013474. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=28104709 .bmjopen-2016-013474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, Kressel HY, Rifai N, Golub RM, Altman DG, Hooft L, Korevaar DA, Cohen JF, STARD Group STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015 Oct 28;351:h5527. doi: 10.1136/bmj.h5527. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=26511519 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bousquet C, Dahamna B, Guillemin-Lanne S, Darmoni SJ, Faviez C, Huot C, Katsahian S, Leroux V, Pereira S, Richard C, Schück S, Souvignet J, Lillo-Le LA, Texier N. The adverse drug reactions from patient reports in social media project: five major challenges to overcome to operationalize analysis and efficiently support pharmacovigilance process. JMIR Res Protoc. 2017 Sep 21;6(9):e179. doi: 10.2196/resprot.6463. http://www.researchprotocols.org/2017/9/e179/ v6i9e179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Board I. Medical Dictionary for Regulatory Activities. [2018-06-27]. https://www.meddra.org/
- 17.Catalog and Index of Medical Sites French-language. [2018-06-27]. http://www.chu-rouen.fr/cismef/outils/outils-et-services/
- 18.World Health Organization. [2018-06-27]. ATC/DDD Index 2017 https://www.whocc.no/atc_ddd_index/
- 19.Kappa Santé. [2018-06-27]. Pharmaco-epidemiology Interventions in public and numeric health https://www.kappasante.com/
- 20.Kostadinov F. GitHub. [2018-06-27]. TEMIS LUXID 7.0.1 SKILL CARTRIDGE DEVELOPMENT CYCLE http://fabian-kostadinov.github.io/2015/10/04/temis-luxid701-skill-cartridge-development-cycle/
- 21.Chen X, Deldossi M, Aboukhamis R, Faviez C, Dahamna B, Karapetiantz P, Guenegou-Arnoux A, Girardeau Y, Guillemin-Lanne S, Lillo-Le-Louët A, Texier N, Burgun A, Katsahian S. Mining adverse drug reactions in social media with named entity recognition and semantic methods. Stud Health Technol Inform. 2017;245:322–6. [PubMed] [Google Scholar]
- 22.Cavalié P, Djeraba A. ANSM [National Agency for the Safety of Medicines and Health Products] 2014. [2018-06-27]. [Analysis of drug sales in France in 2013] https://ansm.sante.fr/var/ansm_site/storage/original/application/3df7b99f8f4c9ee634a6a9b094624341.pdf .
- 23.Kiritchenko S, Matwin S, Nock R, Famili A. Learning Evaluation in the Presence of Class Hierarchies: Application to Text Categorization. 19th Conference of the Canadian Society for Computational Studies of Intelligence; June 7-9, 2006; Québec City, Québec, Canada. Berlin, Heidelberg: Springer; 2006. pp. 395–406. https://www.svkir.com/papers/Kiritchenko-et-al-hierarchical-AI-2006.pdf . [Google Scholar]
- 24.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.r-project.org/ [Google Scholar]
- 25.Ginn R, Pimpalkhute P, Nikfarjam A, Patki A, O'Connor K, Sarker A, Smith K, Gonzalez G. Arizona State University. 2014. [2019-04-01]. Mining Twitter for adverse drug reaction mentions: a corpus and classification benchmark http://www.nactem.ac.uk/biotxtm2014/papers/Ginnetal.pdf .
- 26.Hadzi-Puric J, Grmusa J. Automatic Drug Adverse Reaction Discovery from Parenting Websites Using Disproportionality Methods. IEEE/ACM International Conference on Advances in Social Networks Analysis and Mining; August 26-29, 2012; Istanbul, Turkey. 2012. pp. 792–797. [DOI] [Google Scholar]
- 27.Leaman R, Wojtulewicz L, Sullivan R, Skariah A, Yang J, Gonzalez G. Towards internet-age pharmacovigilance: extracting adverse drug reactions from user posts to health-related social networks. Workshop on Biomedical Natural Language Processing (BioNLP 2010) ACL 2010; July 15, 2010; Uppsala, Sweden. 2010. pp. 117–125. https://www.aclweb.org/anthology/W10-1915 . [Google Scholar]
- 28.Liu X, Chen H. AZDrugMiner: an information extraction system for mining patient-reported adverse drug events in online patient forums. International Conference on Smart Health; August 3-4, 2013; Beijing, China. 2013. pp. 134–50. [DOI] [Google Scholar]
- 29.Nikfarjam A, Gonzalez GH. Pattern mining for extraction of mentions of adverse drug reactions from user comments. AMIA Annu Symp Proc. 2011;2011:1019–26. http://europepmc.org/abstract/MED/22195162 . [PMC free article] [PubMed] [Google Scholar]
- 30.Nikfarjam A, Sarker A, O'Connor K, Ginn R, Gonzalez G. Pharmacovigilance from social media: mining adverse drug reaction mentions using sequence labeling with word embedding cluster features. J Am Med Inform Assoc. 2015 May;22(3):671–81. doi: 10.1093/jamia/ocu041. http://jamia.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=25755127 .ocu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Connor K, Pimpalkhute P, Nikfarjam A, Ginn R, Smith KL, Gonzalez G. Pharmacovigilance on Twitter? Mining tweets for adverse drug reactions. AMIA Annu Symp Proc. 2014;2014:924–33. http://europepmc.org/abstract/MED/25954400 . [PMC free article] [PubMed] [Google Scholar]
- 32.Patki A, Sarker A, Pimpalkhute P, Nikfarjam A, Ginn R, Oconnor K, Smith K, Gonzalez G. Arizona State University. 2014. [2019-04-01]. Mining Adverse Drug Reaction Signals from Social Media: Going Beyond Extraction https://tinyurl.com/y4xwmjco .
- 33.Sampathkumar H, Chen X, Luo B. Mining adverse drug reactions from online healthcare forums using hidden Markov model. BMC Med Inform Decis Mak. 2014;14:91. doi: 10.1186/1472-6947-14-91. http://www.biomedcentral.com/1472-6947/14/91 .1472-6947-14-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarker A, Gonzalez G. Portable automatic text classification for adverse drug reaction detection via multi-corpus training. J Biomed Inform. 2015 Feb;53:196–207. doi: 10.1016/j.jbi.2014.11.002. http://linkinghub.elsevier.com/retrieve/pii/S1532-0464(14)00231-7 .S1532-0464(14)00231-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segura-Bedmar I, Revert R, Martínez P. Detecting drugs and adverse events from Spanish social media streams. Proceedings of the 5th International Workshop on Health Text Mining and Information Analysis (Louhi); 5th International Workshop on Health Text Mining and Information Analysis (Louhi); April 26-30, 2014; Gothenburg, Sweden. 2014. pp. 106–15. https://www.aclweb.org/anthology/W14-1117 . [Google Scholar]
- 36.Yang M, Wang X, Kiang M. Identification of Consumer Adverse Drug Reaction Messages on Social Media. PACIS 2013 Proceedings; The Pacific Asia Conference on Information Systems; June 18-22, 2013; Jeju Island, Korea. 2013. https://aisel.aisnet.org/pacis2013/193 . [Google Scholar]
- 37.Yates A, Goharian N. ADRTrace: detecting expected and unexpected adverse drug reactions from user reviews on social media sites. European Conference on Information Retrieval; March 24-27, 2013; Moscow, Russia. 2013. https://link.springer.com/chapter/10.1007/978-3-642-36973-5_92 . [DOI] [Google Scholar]
- 38.Benton A, Ungar L, Hill S, Hennessy S, Mao J, Chung A, Leonard CE, Holmes JH. Identifying potential adverse effects using the web: a new approach to medical hypothesis generation. J Biomed Inform. 2011 Dec;44(6):989–96. doi: 10.1016/j.jbi.2011.07.005. http://linkinghub.elsevier.com/retrieve/pii/S1532-0464(11)00123-7 .S1532-0464(11)00123-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bian J, Topaloglu U, Yu F. Towards large-scale Twitter mining for drug-related adverse events. SHB12 (2012) 2012 Oct 29;2012:25–32. doi: 10.1145/2389707.2389713. http://europepmc.org/abstract/MED/28967001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chee BW, Berlin R, Schatz B. Predicting adverse drug events from personal health messages. AMIA Annu Symp Proc. 2011;2011:217–26. http://europepmc.org/abstract/MED/22195073 . [PMC free article] [PubMed] [Google Scholar]
- 41.Freifeld CC, Brownstein JS, Menone CM, Bao W, Filice R, Kass-Hout T, Dasgupta N. Digital drug safety surveillance: monitoring pharmaceutical products in Twitter. Drug Saf. 2014 May;37(5):343–50. doi: 10.1007/s40264-014-0155-x. http://europepmc.org/abstract/MED/24777653 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang K, Zheng Y. Mining Twitter Data for Potential Drug Effects. The 9th International Conference on Advanced Data Mining and Applications (ADMA ); 2013; Hangzhou, China. 2013. [DOI] [Google Scholar]
- 43.Yang CC, Yang H, Jiang L. Postmarketing drug safety surveillance using publicly available health-consumer-contributed content in social media. ACM Trans Manage Inf Syst. 2014 Apr 01;5(1):1–21. doi: 10.1145/2576233. [DOI] [Google Scholar]
- 44.Yang C, Yang H, Jiang L, Zhang M. Social media mining for drug safety signal detection. Proceedings of the international workshop on Smart healthwellbeing; Octiber 29, 2012; Maui, Hawaii, USA. 2012. http://www.pages.drexel.edu/~hy95/publications/2012SHB.pdf . [Google Scholar]
- 45.Yeleswarapu S, Rao A, Joseph T, Saipradeep VG, Srinivasan R. A pipeline to extract drug-adverse event pairs from multiple data sources. BMC Med Inform Decis Mak. 2014;14:13. doi: 10.1186/1472-6947-14-13. http://www.biomedcentral.com/1472-6947/14/13 .1472-6947-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bollegala D, Maskell S, Sloane R, Hajne J, Pirmohamed M. Causality patterns for detecting adverse drug reactions from social media: text mining approach. JMIR Public Health Surveill. 2018 May 09;4(2):e51. doi: 10.2196/publichealth.8214. http://publichealth.jmir.org/2018/2/e51/ v4i2e51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saeed M, Villarroel M, Reisner AT, Clifford G, Lehman L, Moody G, Heldt T, Kyaw TH, Moody B, Mark RG. Multiparameter intelligent monitoring in intensive care II: a public-access intensive care unit database. Crit Care Med. 2011 May;39(5):952–60. doi: 10.1097/CCM.0b013e31820a92c6. http://europepmc.org/abstract/MED/21283005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu X, Cherry C, Kiritchenko S, Martin J, de Bruijn B. Detecting concept relations in clinical text: insights from a state-of-the-art model. J Biomed Inform. 2013 Apr;46(2):275–85. doi: 10.1016/j.jbi.2012.11.006. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(12)00180-3 .S1532-0464(12)00180-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Designs of articles related to ADR information extraction on social media (replicated from Sarker et al).


