Abstract
The convergence between amyloid precursor protein (APP) and its β-secretase β-site APP cleaving enzyme 1 (BACE1) is a prerequisite for the generation of β-amyloid peptide, a key pathogenic agent for Alzheimer’s disease. Yet the underlying molecular mechanisms regulating their convergence remain unclear. Here we show that the polarity protein partitioning-defective 3 (Par3) regulates the polarized convergence between APP and BACE1 in hippocampal neurons. Par3 forms a complex with BACE1 through its first PDZ domain, which is important for regulating BACE1 endosome-to-TGN trafficking. In the absence of Par3, there is an increase in the convergence between internalized APP and BACE1. In hippocampal neurons, loss of Par3 leads to increased APP and BACE1 convergence in axons but not dendrites. This polarized convergence mainly occurs in retrograde or stalled axonal late endocytic organelles, and is likely due to compartment-specific regulation of APP trafficking by Par3. Together, our data show a novel function for Par3 in regulating polarized convergence between APP and BACE1 in hippocampal neurons.
Introduction
A key hallmark of Alzheimer’s disease (AD) is the accumulation of β-amyloid (Aβ) plaques. Aβ is generated by sequential cleavage of the amyloid precursor protein (APP) by β-and γ-secretases (Haass et al., 2012; Tang, 2005). The β-site APP cleaving enzyme 1 (BACE1) is the main β-secretase responsible for Aβ generation, with the cleavage of APP by BACE1 being the rate-limiting step (Cole and Vassar, 2007; Thinakaran and Koo, 2008). Thus, physical convergence of APP and BACE1 is a prerequisite for Aβ generation during AD progression (Das et al., 2013; Das et al., 2016; Sun and Roy, 2018). However, mechanisms regulating the subcellular convergence between APP and BACE1 is unclear.
After biogenesis at the Golgi, both APP and BACE1 are delivered to the plasma membrane through secretory vesicles. At the plasma membrane, APP and BACE1 are internalized through different endocytic pathways. While APP internalization is through a classic clathrin dependent pathway (Kyriazis et al., 2008), BACE1 endocytosis is clathrin-independent (Sannerud et al., 2011). In wild type hippocampal neurons under physiological conditions, APP and BACE1 are largely trafficked through different organelles, which helps preclude large amounts of Aβ generation under normal conditions (Das et al., 2013). Yet the pathological changes that occur in the AD brain that induce the convergence between APP and BACE1 remain unclear.
We recently showed that in the human AD brain, there is a significant loss of the polarity protein Par3 (Sun et al., 2016). In addition, we found that Par3 regulates the subcellular trafficking of both APP and BACE1 (Sun et al., 2016; Sun and Zhang, 2017). Par3 functions together with atypical PKC (aPKC) (Sun and Zhang, 2017), which forms a trimolecular complex with Par3 and another polarity protein Par6 (Joberty et al., 2000). To further understand the underlying molecular mechanisms by which Par3 regulates BACE1 trafficking and determine the effects of Par3 on APP/BACE1 convergence, we examined the domains of Par3 responsible for regulating BACE1 trafficking. Here we show that Par3 forms a complex with BACE1 through its first PDZ domain. In the absence of Par3, we found a significant increase in the convergence of BACE1 with internalized APP. Interestingly, in cultured hippocampal neurons, loss of Par3 results in polarized convergence of BACE1 with internalized APP in axons but not dendrites. Together, our studies show novel mechanisms for regulating the convergence between APP and BACE1.
Materials and Methods
Plasmids and reagents
pcDNA3-APP (human wild type APP 695) was a generous gift from Dr. Sangram S. Sisodia (University of Chicago). pcDNA3-GFP-APP was generated by inserting EGFP near the N-terminus of APP after the signal peptide at the KpnI site (Collin and Martens, 2006). mRFP tagged human BACE1, GFP-tagged Rab7, myc-tagged human full length Par3b and Par3c, and shRNA constructs against luciferase or Par3 have been described previously (Sun et al., 2016; Sun and Zhang, 2017; Zhang and Macara, 2006). Myc-tagged Par3b and Par3c fragments were obtained from Drs. Syed Mukhtar Ahmed and Ian Macara at Vanderbilt University and have been described (Chen and Macara, 2005; Joberty et al., 2000; Zhang and Macara, 2006). Neuro2a cells (N2a) stably expressing wild type human APP695 was a generous gift from Dr. Gopal Thinakaran (University of Chicago).
Hippocampal neuronal culture and transfection
Procedures involving animals have been approved by the Institutional Animal Care and Use Committee (IACUC) at the Rutgers Robert Wood Johnson Medical School (16–022). Primary hippocampal neurons were established from embryonic day 18 Sprague-Dawley rats as described previously (Bernard and Zhang, 2015; Sun et al., 2013; Wu et al., 2017). At DIV6–7, neurons were transfected using calcium phosphate precipitation as described (Sun et al., 2013).
Co-immunoprecipitation and Western blotting
Co-immunoprecipitation was performed as described previously (Sun and Zhang, 2017). Briefly, N2a cells were transfected with the indicated constructs, lysed and incubated with myc 9E10 antibody followed by IgG sepharose beads preblocked with 5% BSA. Beads were washed three times with lysis buffer. Bound proteins were eluted by Laemmli sample buffer and analyzed by Western blotting. Primary antibodies used in Western blotting include: mouse anti-FLAG antibody (1:2000, M2, Sigma-Aldrich, Cat. No. F1804), rabbit anti-FLAG (1:2000, Cell Signaling Technology, Cat. No. 2368), mouse anti-Myc (9E10). Secondary antibodies include horseradish peroxidase conjugated goat anti-mouse and anti-rabbit antibodies (1:10,000, Jackson Immunoresearch).
Immunocytochemistry and live confocal imaging
Immunocytochemistry was performed as described previously (Sun and Zhang, 2017). Briefly, N2a cells were transfected with the indicated constructs and fixed in 4% paraformaldehyde with 4% sucrose in PBS. After permeabilization in 0.2% Triton X-100 and blocking with 5% goat serum, cells were incubated with primary antibodies followed by Alexa fluorophore conjugated secondary antibodies. Primary antibodies used include: mouse anti-APP (1:100, 6E10, Signet, Cat. No. SIG-39320), FLAG (mouse monoclonal, 1:500, Sigma-Aldrich, Cat. No. F1804; rabbit polyclonal, 1:100, Cell Signaling Technology, Cat. No. 2368), TGN38 (mouse monoclonal, 1:800, Thermo Scientific, Cat. No. MA3–063), presenilin 1 (1:200, Millipore, Cat. No. MAB5232). Secondary antibodies used include; Alexa Fluor 405, 488, or 594-conjugated goat anti-mouse or anti-rabbit secondary antibodies (1:500, Invitrogen).
For immunostaining of internalized APP, N2a cells stably expressing WT APP695 were transfected with BACE1-FLAG along with other indicated constructs. 72 hours later live cells were stained with 6E10 for 1 hour at 4 °C, and then incubated at 37°C for 60 min. Hippocampal neurons were transfected with WT APP695, BACE1-GFP, along with other indicated constructs. 72 hours later live neurons were stained with 6E10 for 30 min at 4 °C, and then incubated at 37°C for 60 min. The 60 min time point was chosen because our previous studies indicate that loss of Par3 increases APP trafficking to late endosomes at 60 min after internalization (Sun et al., 2016). The 6E10 antibody reacts with amino acid 1–16 of the Aβ region within the extracellular domain of APP and thus can be used for live labeling of surface APP to follow its internalization (Tang et al., 2015; Xiao et al., 2012). Cells were fixed in 4% paraformaldehyde with 4% sucrose in PBS for 15 min at room temperature, and then blocked by HRP-conjugated secondary antibody for 1 hour at room temperature. After washing by PBS, cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature and then immunostained with the indicated primary or secondary antibodies.
Confocal images were obtained using an Olympus FV1000MPE microscope with a 60× water immersion objective (1.0 numerical aperture) with sequential-acquisition setting. For live cell imaging, neurons were transferred into a 37°C heated chamber. Cells were visualized with 488 nm excitation for eGFP and 559 nm for mRFP. Time-lapse images were collected under 5% of laser intensity with a 100 μm pinhole size. A total of 200 to 500 frames were captured through continuous scanning with a frame rate of around 0.8 frames per second. The stacks of images were imported into ImageJ. For tracing anterograde or retrograde movement of different vesicles in live neurons, single and separated axons were selected and kymographs were generated in ImageJ with the Image Stabilizer and Multi Kymograph plug-ins. The height of kymographs represents time (200–500 frames), while the width represent the length (μm) of the axon imaged. Vesicles movement were counted and quantified by unpaired Student’s t-tests. A membranous organelle was classified as immobile if it was stationary for the entire recording period; a motile one was counted only if the displacement was at least 10 μm. Colocalization of vesicles was quantified by Image J with the Colocalization Pipeline plugin.
Statistics
All experiments were repeated at least three times. The number of biological variables examined and the significance levels are presented in each figure legend. Power analysis was used to determine sample size at 90% power and α=0.05. Two-tailed, unpaired Student’s t-tests or one way ANOVA were used to calculate the p values, using GraphPad Prism. Error bars represent the S.E.M..
Results
Par3 regulates BACE1 trafficking through interaction mediated by its first PDZ domain
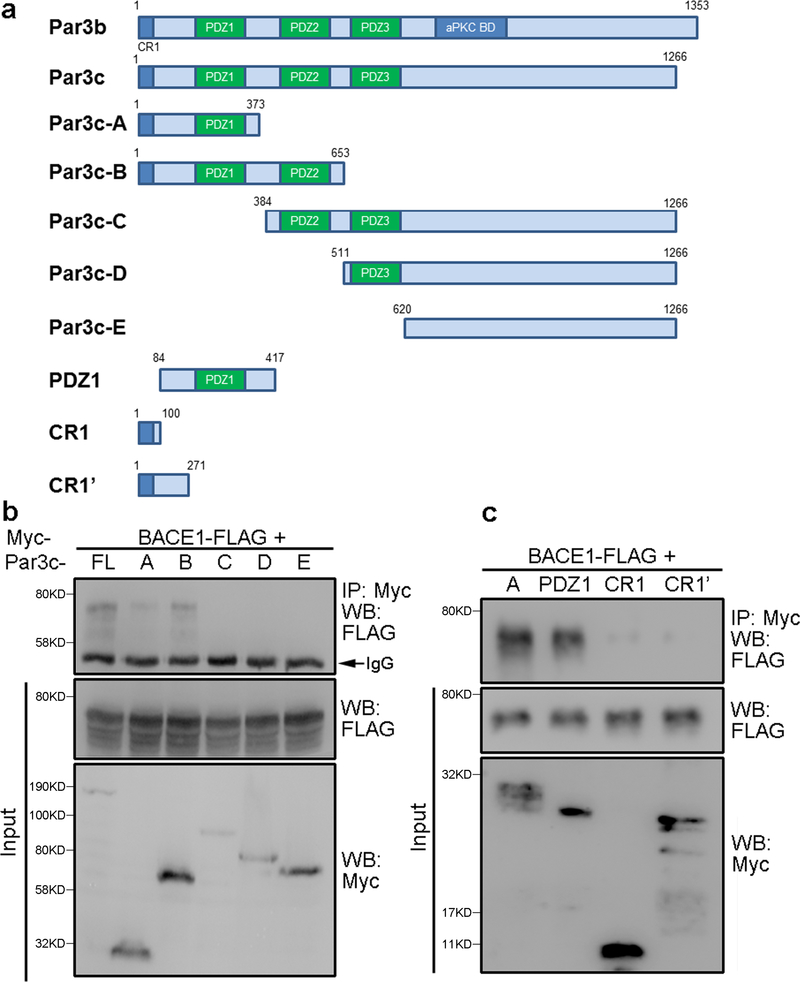
Our recent studies show that Par3 regulates BACE1 trafficking through interacting with BACE1 and recruiting aPKC to promote phosphorylation of Ser498 at the C-terminus of BACE1 (Sun and Zhang, 2017). To further understand the interaction between Par3 and BACE1, we performed co-immunoprecipitation of BACE1 with different fragments of Par3. We found that N-terminal Par3 fragments containing the first PDZ domain was sufficient for mediating the interaction with BACE1, while fragments lacking the first PDZ domain do not interact with BACE1 (Fig. 1a and b). This suggests that the interaction of BACE1 with Par3 is mediated through the first PDZ domain of Par3.
Figure 1. The PDZ1 domain of Par3 mediates the interaction with BACE1.
(a) Schematic diagram for the Par3 constructs. (b, c) N2a cells were transfected with the indicated constructs, lysed and immunoprecipitated with the myc 9E10 antibody.
Immunoprecipitated complexes were analyzed by Western blotting.
Intriguingly, the BACE1 C-terminus does not contain any typical PDZ-binding motifs. Thus, to further confirm that the Par3-BACE1 interaction is through the first PDZ domain, we performed co-immunoprecipitation of BACE1 with smaller N-terminal Par3 fragments. As seen in Fig.1c, Par3-PDZ1, which contains the intact PDZ1 domain, but missing the N-terminal CR1 domain, interacts with BACE1, whereas two other N-terminal fragments that contain the CR1 domain, but lack the PDZ1 domain, do not interact with BACE1. These results further confirms that the interaction between Par3 and BACE1 is through the first PDZ domain.
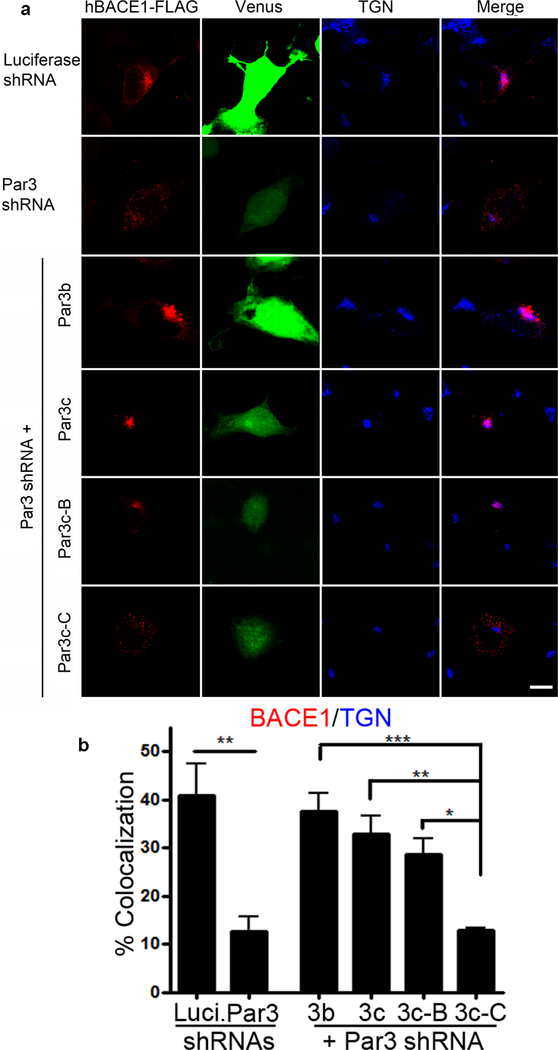
Next, we explored the domains of Par3 that mediate its effects on BACE1 trafficking. We recently showed that Par3 and aPKC promote BACE1 endosome-to-TGN trafficking in primary hippocampal neurons (Sun and Zhang, 2017). In the absence of Par3 or aPKC activity, BACE1 accumulates in the late endosome/lysosome pathway. This effect was observed in hippocampal neurons as well as N2a cells (Sun and Zhang, 2017). To see which domains of Par3 mediate this effect, we depleted endogenous Par3 in N2a cells and re-expressed different Par3 fragments. Both Par3b and Par3c isoforms can efficiently restore the TGN localization of BACE1 (Fig. 2). This suggest that direct interaction between Par3 and aPKC is not necessary, as Par3c lacks the aPKC binding domain (Gao et al., 2002). This is further confirmed by the ability of the N-terminal Par3c-B fragment to rescue the TGN localization of BACE1. By contrast, a C-terminal fragment of Par3, which lacks the N-terminal PDZ1 domain, cannot rescue BACE1 localization (Fig. 2). Together, these data suggest that Par3 interaction with BACE1 through the PDZ1 domain is necessary for its effect on BACE1 trafficking. Since direct interaction between Par3 and aPKC is not necessary for the effects on BACE1 trafficking (Fig. 2), it is likely that Par3 recruits aPKC through Par6, which forms direct interactions with both Par3 and aPKC (Joberty et al., 2000).
Figure 2. The PDZ1 domain of Par3 is necessary for its effect on BACE1 trafficking.
(a) N2a cells were transfected with indicated constructs together with hBACE1-FLAG. Cells were immunostained for FLAG (red) and TGN38 (blue). Venus (green) indicates positive cells. (b) Quantification for percentages of BACE1 colocalized with TGN. Data were expressed as Mean ± SEM with one way ANOVA, *p<0.05; ** p<0.01; *** p<0.001. N (cells) = 15 (Luciferase shRNA), 15 (Par3 shRNA), 15 (Par3b), 15 (Par3c), 19 (Par3c-B), 23 (Par3c-C), from 5 independent experiments. Scale bar: 10μm.
Depletion of Par3 increases colocalization of internalized APP with BACE1
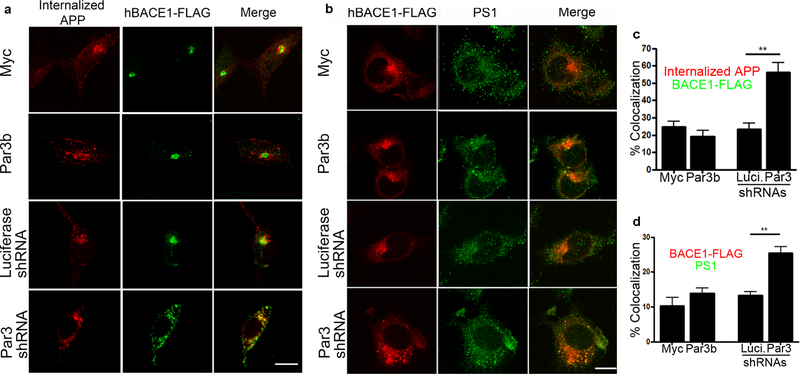
The convergence of APP and BACE1 is a necessary step for Aβ generation. Since APP cleavage by BACE1 is believed to occur after it is internalized from the plasma membrane (Haass et al., 2012; Sun and Roy, 2018), we examined the colocalization of BACE1 with internalized APP by live antibody feeding in N2a cells stably expressing wild type human APP (Thinakaran et al., 1996). We found that the internalized APP show significantly increased convergence with BACE1 upon Par3 depletion (Fig. 3a and c). In addition, we observed a significant increase in the colocalization of BACE1 and the γ-secretase subunit presenilin 1 (PS1) in Par3-depleted cells (Fig. 3b and d). Together, these results suggest that Par3 depletion increases the convergence of internalized APP with BACE1 and PS1, which will lead to increased Aβ generation.
Figure 3. Depletion of Par3 increases colocalization of internalized APP with BACE1.
(a) APPwt N2a cells were transfected with indicated constructs together with hBACE1-FLAG. 72h after transfection, internalized APP (60 min) was immunostained (red) together with FLAG (green) to visualize colocalization of internalized APP and BACE1. (b) N2a cells were transfected with indicated constructs together with hBACE1-FLAG. 72h after transfection, cells were immunostained for FLAG (red) and Presenilin-1 (PS1, green). Scale bar: 10μm. (c) Quantification of colocalization of internalized APP with BACE1-FLAG. N (cells) = 19 (myc), 17 (Par3b), 19 (Luciferase shRNA), 20 (Par3 shRNA) from 5 independent experiments. (d) Quantification of the colocalization between BACE1-FLAG and PS1. N (cells) = 12 (myc), 12 (Par3b), 12(Luciferase shRNA), 11 (Par3 shRNA) from 3 independent experiments. Data were expressed as Mean ± SEM with Student’s t-test: ** p < 0.01.
Depletion of Par3 results in polarized convergence of APP and BACE1 in axons
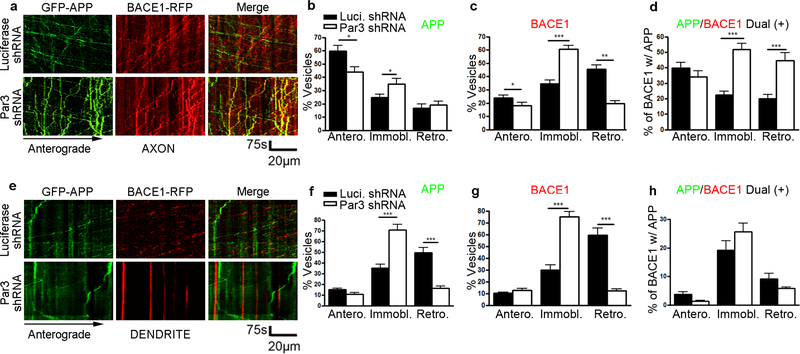
Previous studies have shown that both APP and BACE1 trafficking are differentially regulated in dendrites and axons of neurons (Buggia-Prevot et al., 2014; Buggia-Prevot et al., 2013; Ubelmann et al., 2017). In addition, Aβ generation is known to be polarized with axonal presynaptic terminals being the preferred site (Buggia-Prevot et al., 2013; Buxbaum et al., 1998; DeBoer et al., 2014; Lazarov et al., 2002; Lyckman et al., 1998; Niederst et al., 2015; Yu et al., 2018). Thus, we examined the co-trafficking of APP and BACE1 in dendrites vs. axons of DIV11 hippocampal neurons. In axons under control conditions, we observed limited co-trafficking of APP and BACE1. However, in axons of Par3-depleted neurons, we observed a significant increase in the co-trafficking of BACE1 with APP in retrograde and stalled vesicles (Fig. 4a-d). Remarkably, we observed limited convergence between APP and BACE1 in dendrites with or without Par3 (Fig. 4e-h), suggesting that the effect of Par3 on APP and BACE1 convergence is restricted to axons.
Figure 4. Loss of Par3 increases APP and BACE1 convergence in axons.
(a) Hippocampal neurons were transfected with indicated constructs together with GFP-APP and hBACE1-RFP. Live images were captured on DIV11 and kymographs were generated to trace APP and hBACE1 trafficking in axons. (b) Quantification of the mobility of APP containing vesicles in axons (anterograde, immobile and retrograde). (c) Quantifications of the mobility of BACE1 containing vesicles in axons. (d) Quantifications of the percentage of BACE1 vesicles that are co-trafficking with APP vesicles in axons. N (cells) = 17 (Luciferase shRNA), 14 (Par3 shRNA) from 6 independent experiments, with 20–50 vesicles analyzed per movie. (e) Hippocampal neurons were transfected with indicated constructs together with GFP-APP and hBACE1-RFP. Live images were captured on DIV11 and kymographs were generated to trace APP and hBACE1 trafficking in dendrites. (f) Quantification of the mobility of APP containing vesicles in dendrites (anterograde, immobile and retrograde). (g) Quantifications of the mobility of BACE1 containing vesicles in dendrites. (h) Quantifications of the percentage of BACE1 vesicles that are co-trafficking with APP vesicles in dendrites. N (cells) = 14 (Luciferase shRNA), 16 (Par3 shRNA) from 4 independent experiments, with 10–30 vesicles analyzed per movie. Data were expressed as Mean ± SEM with Student’s t test: *p<0.05; ** p<0.01; *** p<0.001. Scale bar: 75s/20μm.
Interestingly, the effects of Par3 on BACE1 trafficking are similar for both dendrites and axons, with loss of Par3 inhibiting BACE1 retrograde trafficking and increasing the percentage of stalled vesicles (Fig. 4c and g). By contrast, the effects of Par3 on APP trafficking are different in dendrites and axons. While Par3 does not significantly affect APP retrograde trafficking in axons, loss of Par3 significantly inhibits APP retrograde trafficking in dendrites (Fig. 4b and f). Thus, the polarized regulation of APP/BACE1 convergence in axons vs. dendrites is likely due to the differential effects of Par3 on axonal and dendritic APP retrograde trafficking. Taken together, these results suggest that Par3 depletion enhances polarized Aβ generation by increasing the convergence of APP and BACE1 within neuronal axons.
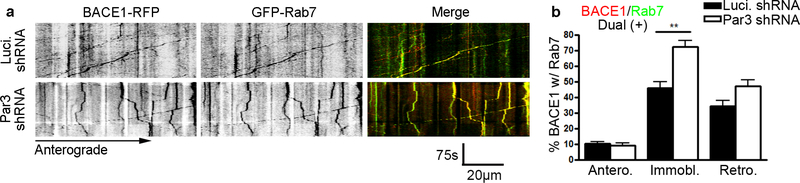
Finally, since the increase in APP and BACE1 convergence was observed in retrograde and immobile vesicles, we would like to determine the identity of these vesicles. Thus, we performed live imaging analysis in DIV 11 hippocampal neurons expressing BACE1-RFP and endosomal markers. In the absence of Par3, there is a significant increase in the percentage of BACE1 containing vesicles that are positive for Rab7, a marker for late endosomes (Yap et al., 2018). In particular, there is a significant increase in stalled vesicles that are dual-positive for BACE1 and Rab7 (Fig. 5). No significant changes were observed in the colocalization of BACE1 and Rab11, a recycling endosome marker (Supplementary Information, Figure S1) (Buggia-Prevot et al., 2014).
Figure 5. Loss of Par3 increases retention of BACE1 in late endocytic organelles in axons.
(a) Hippocampal neurons were transfected with indicated constructs together with GFP-Rab7 and hBACE1-RFP. Live images were captured on DIV11 and kymographs were generated to trace Rab7 and hBACE1 trafficking in axons. Scale bar: 75s/20μm. (b) Quantification of the percentages of BACE1 vesicles colocalized with Rab7 that are trafficking anterograde, immobile or retrograde, respectively. N (cells) = 19 (Luciferase shRNA), 23 (Par3 shRNA) from 8 independent experiments, with 20–50 vesicles analyzed per movie. Data were expressed as Mean ± SEM with Student’s t test: ** p<0.01.
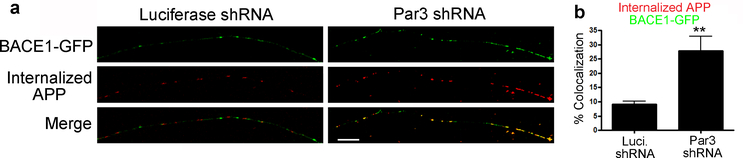
To confirm that BACE1 is converging with internalized APP in the axons, we performed live antibody feeding in hippocampal neurons. Similar to the results in N2a cells, we found that loss of Par3 significantly increased the percentage of internalized APP that converges with BACE1 in the axons (Fig. 6). Taken together, these data suggest that in the absence of Par3, there is an increase in BACE1 trafficking in late endocytic organelles where it converges with internalized APP.
Figure 6. Loss of Par3 increases the convergence of internalized APP with BACE1 in axons.
(a) Hippocampal neurons were transfected with WT APP695, BACE1-GFP and the indicated shRNA constructs. 72 hours after transfection, internalized APP (60 min) was immunostained (red) to visualize colocalization of internalized APP and BACE1 in axons. Scale bar: 10 μm.
(b) Quantification of colocalization of internalized APP with BACE1 in axons. N (cells) = 8 (Luciferase shRNA), 13 (Par3 shRNA), from 3 independent experiments. Data were expressed as Mean ± SEM with Student’s t test: **p<0.01.
Discussion
The convergence of APP and BACE1 is a rate-limiting step for the generation of Aβ in Alzheimer’s disease pathogenesis. Yet the mechanisms regulating APP/BACE1 convergence in the subcellular trafficking pathways remain unclear. Here we reveal mechanistic insight into the role of Par3 in regulating BACE1 trafficking and APP/BACE1 convergence. We show that Par3 regulates BACE1 trafficking by forming a complex with BACE1 through its first PDZ domain. In addition, we show that loss of Par3 leads to the convergence of internalized APP with BACE1 and PS1. Live imaging experiments in primary hippocampal neurons reveal that loss of Par3 leads to APP/BACE1 convergence during endosomal trafficking in axons but not dendrites of hippocampal neurons.
Interestingly, the C-terminus of BACE1 does not contain any known PDZ-binding motifs, which raises the question regarding the nature of Par3-BACE1 interaction. It is possible that the interaction between Par3 and BACE1 is not direct, but rather through another binding partner of Par3, such as Par6, which binds PDZ1 of Par3 (Joberty et al., 2000). Alternatively, BACE1 may form a complex with Par3 through another membrane protein that interacts with PDZ1 of Par3, such as Nectin or junctional adhesion molecules (Ebnet et al., 2003; Itoh et al., 2001; Takekuni et al., 2003).
Our previous studies show that the Par3/aPKC complex regulates retrograde endosometo-TGN trafficking of BACE1 through the phosphofurin acidic cluster sorting protein 1 (PACS1). The Par3/aPKC complex promotes BACE1 phosphorylation at Ser498, which facilitates its interaction with PACS1 and enhances retrograde endosome-to-TGN trafficking (Sun and Zhang, 2017). Together with our current data, these studies point to the following working model for Par3 in regulating BACE1 trafficking: Par3 forms a complex with BACE1 through its first PDZ domain. aPKC is recruited to this complex likely through its interaction with Par6, as direct interaction between Par3 and aPKC is not required (Fig. 2). The Par3/aPKC complex promotes BACE1 phosphorylation at Ser498. Phosphorylated BACE1 binds to PACS1, which facilitates retrograde trafficking of BACE1. In the absence of Par3, there is a reduction in BACE1 phosphorylation, leading to an inhibition of retrograde trafficking.
Our data show that loss of Par3 leads to APP and BACE1 convergence in axons of primary hippocampal neurons. In particular, we found that there is a significant increase in APP/BACE1 dual positive vesicles that are either retrogradely trafficking or immobile. We further found that most of the retrograde or immobile BACE1 vesicles are Rab7 positive showing that they are predominantly late endocytic organelles. Our data are consistent with recent reports suggesting that axonal late endocytic retention of BACE1 leads to increased Aβ generation in the Alzheimer’s neurons (Ye and Cai, 2014; Ye et al., 2017). Together these data point to the late endosomes as an important subcellular site for Aβ generation, along with other reported sites such as early endosomes and recycling endosomes (Das et al., 2013; Das et al., 2016; Grbovic et al., 2003; Kinoshita et al., 2003; Sannerud et al., 2011; Ubelmann et al., 2017). It is likely that Aβ is generated at multiple subcellular locations within the neuron, and that diverse molecular pathways may differentially regulate Aβ generation at these locations. Par3 appears to function at the late endosome level as loss of Par3 does not affect APP or BACE1 localization to early endosomes or recycling endosomes (Sun et al., 2016; Sun and Zhang, 2017). The axonal late endocytic retention of BACE1 is also consistent with our previous data showing increased intracellular Aβ in neurons depleted of Par3 (Sun et al., 2016). It will be interesting to determine whether the observed axonal convergence of APP and BACE1 is occurring at presynaptic terminals.
In addition, we found that in the absence of Par3, there is a polarized convergence between APP and BACE1 in axons but not dendrites. These data add to the growing literature that APP and BACE1 trafficking are differentially regulated in dendrites and axons of neurons. For example, the endocytic regulators BIN1 and CD2AP differentially regulates APP and BACE1 trafficking in dendrites and axons of hippocampal neurons (Guimas Almeida et al., 2018; Ubelmann et al., 2017). Internalized BACE1 undergoes unidirectional retrograde trafficking in dendrites, which is regulated by EHD family proteins, whereas in axons BACE1 undergoes bidirectional trafficking (Buggia-Prevot et al., 2014; Buggia-Prevot et al., 2013). These data are consistent with the observation that Aβ preferentially accumulates in the presynaptic terminals (Yu et al., 2018), and underscores the importance of understanding APP and BACE1 trafficking and convergence within the context of neurons because of their highly compartmentalized nature (Winckler et al., 2018). Since the polarized effects of Par3 is likely due to differential regulation of APP retrograde trafficking in axons vs. dendrites (Fig. 4), it will be interesting to determine whether Par3 functions together with some of the known regulators of polarized APP trafficking, such as CD2AP (Ubelmann et al., 2017).
In summary, our data show a novel function for Par3 in regulating polarized APP and BACE1 convergence in neuronal axons. We also provide further mechanistic insight into the regulation of BACE1 trafficking by Par3. Our data are consistent with a growing body of literature highlighting the compartmentalized nature of APP and BACE1 trafficking and convergence, and provide further mechanistic evidence pointing to a key role for the Par polarity complex in Alzheimer’s disease pathogenesis.
Supplementary Material
Highlights.
Par3 forms a complex with BACE1 through its PDZ1 domain, which is necessary for the effect of Par3 on BACE1 retrograde endosome-to-TGN trafficking.
Loss of Par3 increases convergence between internalized APP and BACE1.
Loss of Par3 increases APP/BACE1 convergence in axons but not dendrites of hippocampal neurons.
Loss of Par3 shows compartment-specific effects on APP trafficking in hippocampal neurons.
Loss of Par3 causes late endocytic retention of BACE1 in axons.
Acknowledgements
We would like to thank Drs. Syed Mukhtar Ahmed and Ian Macara (Vanderbilt University) and Drs. Gopal Thinakaran and Sangram S. Sisodia (University of Chicago) for reagents. This work was supported by National Institutes of Health grant NS089578 to HZ, and National Natural Science Foundation of China grant No.31500845 to HW.
Footnotes
Competing interests
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernard LP, and Zhang H 2015. MARK/Par1 Kinase Is Activated Downstream of NMDA Receptors through a PKA-Dependent Mechanism. PLoS One. 10:e0124816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggia-Prevot V, Fernandez CG, Riordan S, Vetrivel KS, Roseman J, Waters J, Bindokas VP, Vassar R, and Thinakaran G 2014. Axonal BACE1 dynamics and targeting in hippocampal neurons: a role for Rab11 GTPase. Mol Neurodegener. 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggia-Prevot V, Fernandez CG, Udayar V, Vetrivel KS, Elie A, Roseman J, Sasse VA, Lefkow M, Meckler X, Bhattacharyya S, George M, Kar S, Bindokas VP, Parent AT, Rajendran L, Band H, Vassar R, and Thinakaran G 2013. A function for EHD family proteins in unidirectional retrograde dendritic transport of BACE1 and Alzheimer’s disease Abeta production. Cell Rep. 5:1552–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Thinakaran G, Koliatsos V, O’Callahan J, Slunt HH, Price DL, and Sisodia SS 1998. Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J Neurosci. 18:9629–9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, and Macara IG 2005. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 7:262–269. [DOI] [PubMed] [Google Scholar]
- Cole SL, and Vassar R 2007. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin RW, and Martens GJ 2006. The coding sequence of amyloid-beta precursor protein APP contains a neural-specific promoter element. Brain Res. 1087:41–51. [DOI] [PubMed] [Google Scholar]
- Das U, Scott DA, Ganguly A, Koo EH, Tang Y, and Roy S 2013. Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron. 79:447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U, Wang L, Ganguly A, Saikia JM, Wagner SL, Koo EH, and Roy S 2016. Visualizing APP and BACE-1 approximation in neurons yields insight into the amyloidogenic pathway. Nat Neurosci. 19:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer SR, Dolios G, Wang R, and Sisodia SS 2014. Differential release of beta-amyloid from dendrite-versus axon-targeted APP. J Neurosci. 34:12313–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, Meyer zu Brickwedde MK, Suzuki A, Imhof BA, and Vestweber D 2003. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci. 116:3879–3891. [DOI] [PubMed] [Google Scholar]
- Gao L, Macara IG, and Joberty G 2002. Multiple splice variants of Par3 and of a novel related gene, Par3L, produce proteins with different binding properties. Gene. 294:99–107. [DOI] [PubMed] [Google Scholar]
- Grbovic OM, Mathews PM, Jiang Y, Schmidt SD, Dinakar R, Summers-Terio NB, Ceresa BP, Nixon RA, and Cataldo AM 2003. Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. J Biol Chem. 278:31261–31268. [DOI] [PubMed] [Google Scholar]
- Guimas Almeida C, Sadat Mirfakhar F, Perdigao C, and Burrinha T 2018. Impact of late-onset Alzheimer’s genetic risk factors on beta-amyloid endocytic production. Cell Mol Life Sci. 75:2577–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Kaether C, Thinakaran G, and Sisodia S 2012. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2:a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, and Tsukita S 2001. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 154:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, and Macara IG 2000. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2:531–539. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Fukumoto H, Shah T, Whelan CM, Irizarry MC, and Hyman BT 2003. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J Cell Sci. 116:3339–3346. [DOI] [PubMed] [Google Scholar]
- Kyriazis GA, Wei Z, Vandermey M, Jo DG, Xin O, Mattson MP, and Chan SL 2008. Numb endocytic adapter proteins regulate the transport and processing of the amyloid precursor protein in an isoform-dependent manner: implications for Alzheimer disease pathogenesis. J Biol Chem. 283:25492–25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Lee M, Peterson DA, and Sisodia SS 2002. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci. 22:9785–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyckman AW, Confaloni AM, Thinakaran G, Sisodia SS, and Moya KL 1998. Posttranslational processing and turnover kinetics of presynaptically targeted amyloid precursor superfamily proteins in the central nervous system. J Biol Chem. 273:11100–11106. [DOI] [PubMed] [Google Scholar]
- Niederst ED, Reyna SM, and Goldstein LS 2015. Axonal amyloid precursor protein and its fragments undergo somatodendritic endocytosis and processing. Mol Biol Cell. 26:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannerud R, Declerck I, Peric A, Raemaekers T, Menendez G, Zhou L, Veerle B, Coen K, Munck S, De Strooper B, Schiavo G, and Annaert W 2011. ADP ribosylation factor 6 (ARF6) controls amyloid precursor protein (APP) processing by mediating the endosomal sorting of BACE1. Proc Natl Acad Sci U S A. 108:E559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, and Roy S 2018. The physical approximation of APP and BACE-1: A key event in alzheimer’s disease pathogenesis. Dev Neurobiol. 78:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Asghar SZ, and Zhang H 2016. The polarity protein Par3 regulates APP trafficking and processing through the endocytic adaptor protein Numb. Neurobiol Dis. 93:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Bernard LP, Dibona VL, Wu Q, and Zhang H 2013. Calcium phosphate transfection of primary hippocampal neurons. J Vis Exp:e50808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, and Zhang H 2017. Par3 and aPKC regulate BACE1 endosome-to-TGN trafficking through PACS1. Neurobiol Aging. 60:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, Monden M, and Takai Y 2003. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem. 278:5497–5500. [DOI] [PubMed] [Google Scholar]
- Tang BL 2005. Alzheimer’s disease: channeling APP to non-amyloidogenic processing. Biochem Biophys Res Commun. 331:375–378. [DOI] [PubMed] [Google Scholar]
- Tang W, Tam JH, Seah C, Chiu J, Tyrer A, Cregan SP, Meakin SO, and Pasternak SH 2015. Arf6 controls beta-amyloid production by regulating macropinocytosis of the Amyloid Precursor Protein to lysosomes. Mol Brain. 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, and Koo EH 2008. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 283:29615–29619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Teplow DB, Siman R, Greenberg B, and Sisodia SS 1996. Metabolism of the “Swedish” amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the “beta-secretase” site occurs in the golgi apparatus. J Biol Chem. 271:9390–9397. [DOI] [PubMed] [Google Scholar]
- Ubelmann F, Burrinha T, Salavessa L, Gomes R, Ferreira C, Moreno N, and Guimas Almeida C 2017. Bin1 and CD2AP polarise the endocytic generation of beta-amyloid. EMBO Rep. 18:102–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Faundez V, Maday S, Cai Q, Guimas Almeida C, and Zhang H 2018. The Endolysosomal System and Proteostasis: From Development to Degeneration. J Neurosci. 38:9364–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Sun M, Bernard LP, and Zhang H 2017. Postsynaptic density 95 (PSD-95) serine 561 phosphorylation regulates a conformational switch and bidirectional dendritic spine structural plasticity. J Biol Chem. 292:16150–16160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Gil SC, Yan P, Wang Y, Han S, Gonzales E, Perez R, Cirrito JR, and Lee JM 2012. Role of phosphatidylinositol clathrin assembly lymphoid-myeloid leukemia (PICALM) in intracellular amyloid precursor protein (APP) processing and amyloid plaque pathogenesis. J Biol Chem. 287:21279–21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CC, Digilio L, McMahon LP, Garcia ADR, and Winckler B 2018. Degradation of dendritic cargos requires Rab7-dependent transport to somatic lysosomes. J Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, and Cai Q 2014. Snapin-mediated BACE1 retrograde transport is essential for its degradation in lysosomes and regulation of APP processing in neurons. Cell Rep. 6:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Feng T, Tammineni P, Chang Q, Jeong YY, Margolis DJ, Cai H, Kusnecov A, and Cai Q 2017. Regulation of Synaptic Amyloid-beta Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer’s Disease. J Neurosci. 37:2639–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Jans DC, Winblad B, Tjernberg LO, and Schedin-Weiss S 2018. Neuronal Aβ42 is enriched in small vesicles at the presynaptic side of synapses.. Life Science Alliance. 1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, and Macara IG 2006. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol. 8:227–237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.