Abstract
Over 20 genes are involved in the biogenesis and function of the Neisseria Type IV pilus (Tfp). In the pathogenic species, RpoD and the integration host factor (IHF) protein regulate expression of pilE, encoding the Tfp structural subunit. We previously reported that in commensal species, pilE transcription is regulated by RpoN, IHF, and activator Npa. Npa has many hallmarks of response regulators in two‐component regulatory systems, leading us to search for its response regulator partner. We report that Npa partners with sensor kinase Nps to control pilE transcription. Among the genes involved in Tfp biogenesis and function, only pilE is controlled by RpoN and Npa/Nps. We summarize our findings in a model, and discuss the implications of the differential regulation of pilE the context of Neisseria Tfp biogenesis.
Keywords: Neisseria, transcription regulation, two‐component sensors, Type IV fimbriae
1. INTRODUCTION
The Type IV pilus (Tfp) is a multiprotein structure on the surface of many bacteria and archaea (Albers & Jarrell, 2015; Berry & Pelicic, 2015). Tfp of commensal and pathogenic Neisseria promote attachment, motility, biofilm formation, horizontal gene transfer, and host cell signaling (Heckels, 1989; Howie, Glogauer, & So, 2005; Ma et al., 2018; Nassif et al., 1994; Pujol, Eugene, Marceau, & Nassif, 1999; Virji et al., 1995). PilE, the name given to the Neisseria Tfp structural subunit, is assembled into the pilus filament, and anchored to the membrane by means of a complex machinery (Carbonnelle, Helaine, Nassif, & Pelicic, 2006; Parge et al., 1995).
We reported that transcriptional control of pilE differs in pathogenic and commensal species of Neisseria (Rendón, Hockenberry, McManus, & So, 2013). In the pathogens, housekeeping sigma factor RpoD initiates pilE transcription from the conserved −35 and −10 recognition sequences in the pilE promoter (Fyfe, Carrick, & Davies, 1995; Meyer, Billyard, Haas, Storzbach, & So, 1984). In commensals, sigma factor RpoN initiates pilE transcription by binding to a highly conserved motif (GGN10GC) located −24 and −12 bases upstream of pilE (Rendón et al., 2013; Wormann et al., 2016).
RpoN regulation of pilE also requires integration host factor (IHF) and an activator, Npa (Rendón et al., 2013). IHF enhances the efficiency of pilE transcription in both pathogenic and commensal Neisseria species, albeit by different mechanisms (Hill et al., 1997; Rendón et al., 2013). In RpoD‐dependent promoters, such as in Neisseria gonorrhoeae (Ngo) pilE, IHF is thought to bend the DNA, thereby stabilizing the interaction of the promoter with RpoD and enhancing transcription (Giladi et al., 1998; Hill et al., 1997). IHF appears to play a similar role in the RpoN‐dependent pilE promoter of commensal Neisseria. If IHF cannot interact with its binding site, transcription of pilE decreases significantly (Rendón et al., 2013). Finally, Npa is a positive regulator of pilE. Point mutations in its binding site, the upstream activator sequence (UAS), or deletion of npa abolished pilE expression (Rendón et al., 2013).
Npa has motifs typical of response regulators of two‐component regulatory systems (TCRS), namely, a receiver domain in the N terminus and an output domain in the C terminus (Carrick, Fyfe, & Davies, 2000; Jung, Fried, Behr, & Heermann, 2012). A TCRS consists of a membrane‐bound sensor kinase (SK) and a cytoplasmic response regulator (RR). The SK autophosphorylates upon detection of a signal, and transfers the phosphoryl group to the RR, which then activates or represses gene expression. In the RR, the phosphorylated amino acid is a highly conserved aspartate. We previously speculated that aspartate 58 (D58) in Npa is the phosphorylated residue (Rendón et al., 2013). Since Npa has hallmarks of a response regulator, we searched the genome of commensal Neisseria elongata (Nel) for its SK counterpart, and found Open Reading Frame (ORF) (NEIELOOT_00058) whose deduced amino acid sequence has domains typically found in SK. NEIELOOT_00058 is present in all other commensal Neisseria genomes published on the web.
In this work, we tested the hypothesis that NEIELOOT_00058 is the SK that activates Npa to regulate transcription of pilE, using Neisseria elongata (Nel) as the model commensal. We show that Nps is required for pilE transcription, and that Nps and Npa act in concert as a TCRS. Finally, we show that RpoN and Nps/Npa control transcription only of pilE and not the other Tfp biogenesis genes reported to date. The implication of these different control mechanisms for Type IV pilus biogenesis is discussed.
2. EXPERIMENTAL PROCEDURES
2.1. Strains, plasmids, and growth conditions
All strains and plasmids used in this study are listed in Table S1. Neisseria strains were routinely grown at 37°C with 5% CO2 in GCB agar or liquid GCB containing Kellogg's supplements (Kellogg, Peacock, Deacon, Brown, & Pirkle, 1963), unless otherwise indicated. Escherichia coli strains were grown at 37°C in LB media (Bertani, 1951). Antibiotics were added when needed at specified concentrations. Chloramphenicol was added at a final concentration of 5 μg/ml, and kanamycin at 50 μg/ml for Neisseria. For E. coli, chloramphenicol was used at 30 μg/ml and kanamycin at 50 μg/ml.
2.2. Bioinformatics
Various online programs were used to analyze the sequences. To predict conserved domains, NCBI domain (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi/) was used. TMMOD server was used to predict transmembrane domains (Kahsay, Gao, & Liao, 2005) (http://liao.cis.udel.edu/website/servers/TMMOD/scripts/frame.php?p=submit).
Protein sequence alignments were done using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) (Sievers et al., 2011). Protein homology and similarity were determined using the FASTA protein similarity search tool (http://www.ebi.ac.uk/Tools/sss/fasta/) (Pearson, 1990).
2.3. Construction of mutants
The nps and npa ORFs overlap by 11 bases, to ensure deletion of nps would not affect npa, we designed a construct in which 92% of nps was deleted. First, a kanamycin resistance gene from plasmid pHSS6 (Seifert, Chen, So, & Heffron, 1986) was amplified with primers MR327A and MR328A (Table S2). The purified PCR product was transformed into Nel 29315. Transformants were selected in GCB containing 50 mg/ml of kanamycin. Ten kanamycin‐resistant colonies were sequenced to confirm nps replacement. One kanamycin‐resistant clone was selected and transformed with a 321 bp piece of DNA of which 117 bases were from nps. The transformation mix was serially diluted, and plated onto GCB agar. After 24 hr, individual colonies were selected and grown on GCB without antibiotic, and a replica in GCB‐Km. Km‐sensitive clones were selected and analyzed, first by PCR, and then by sequencing to confirm that the kanamycin resistance gene was removed (Figure S7).
2.4. Site directed mutagenesis
A kanamycin resistance gene was cloned between the HincII and HindIII sites in pUC19, to generate plasmid pUC19‐Km. Primers MR313 and MR314 were used to amplify the nps‐npa locus and the amplicon was inserted between the EcoRI and BamHI sites of pUC19‐Km. The site directed mutagenesis protocol in (Heckman & Pease, 2007) was used to generate mutants Nel nps H325A, Nel npa D58A, Nel npaD58E, Nel Δnps‐npa D58E, and Nel nps H325A‐npa D58E. All the primers used are listed in Table S2.
2.5. Complementation of mutant strains
Complemented strains were constructed by placing the wt gene of interest under control of the inducible lac promoter, and inserting the construct into the proB site in Nel. For this protocol, we constructed plasmid pML2 (Figure S8) by modifying plasmid pKH37 (Kohler, Hamilton, Cloud‐Hansen, & Dillard, 2007). Briefly, a 650 bp fragment of the 5′‐end of proB gene, obtained by PCR using primers MR346 and MR347, was used to replace the aspC gene in the FspI and PciI sites generating plasmid pML1. The lctP gene was removed by digestion with SphI and KpnI. A 550 pb fragment of the 3′‐end of proB, generated using primers MR348a and MR349, was cloned into the SphI and KpnI sites of pML1 creating plasmid pML2. The multiple cloning site, lacPO, lacIq, and cat remained intact.
Nps was amplified using primers MR350 and MR 351 and cloned into the PacI and SacI sites of pML2 generating plasmid pML2‐inps. Npa was amplified using primers MR216a and MR216b Nel and cloned into the PacI and XhoI sites of pML2 generating plasmid pML2‐inpa. Nel mutants were transformed with the corresponding plasmid using the colony transformation protocol described in (Dillard, 2011). Transformants were selected in GCB with chloramphenicol (5 μg/ml). Complemented strains were verified by PCR, sequencing, and RT‐PCR.
2.6. RNA extraction and RT‐PCR
Total Neisseria RNA was extracted using Trizol (Invitrogen) and treated with DNAse free (Ambion) to remove DNA, as recommended by the manufacturer. The integrity of the RNA was determined by agarose gel electrophoresis. cDNA was generated with 1,000 μg of RNA using M‐MLV (Promega) per manufacturer's instructions. Reactions without reverse transcriptase were used as negative controls. To ensure that equal amounts of RNA were used, a reaction to amplify 16S rRNA was performed. All primers used, and gene products are listed in Table S2.
2.7. Polyclonal anti‐PilE antibodies
Pili from Nel wt were purified from GCB agar plates as previously described (Rendón et al., 2013). Briefly, Nel was grown for 16 hr on GCB agar. Bacteria were collected in 2 ml of 0.3 M ethanolamine. Pili were sheared off by vortexing the bacteria for 1 min. Pili were precipitated by incubating the solution with 300 μl of saturated ammonium sulfate for 30 min at RT. Precipitated pili were collected by centrifugation (17,000 × g for 30 min). Polyclonal antibodies to this pilus preparation were raised by immunization of a rabbit with 4 weekly doses of purified pili (Alpha Diagnostic Intl. Inc). Nonspecific antibodies were removed from the immune sera by absorbing with intact Nel ΔpilE cells. Finally, specific anti‐PilE antibodies were captured by affinity purification using purified Nel pilin. The antibodies were released by addition of 0.2 M glycine pH 2.5, following by incubation at RT for 5 min. The solution with antibodies was collected and neutralized with 1 M Tris–HCl pH 8. The specificity of the antibodies was tested by western blot (Figure S9).
2.8. Western blot
Equal amounts of bacteria (7 × 108 bacteria) were suspended in sample buffer. Ten microlitre of sample was added to each lane of a 15% acrylamide gel. To ensure a good separation, the gel was run at 90 volts for 2.5 hr. Proteins were transferred onto a nitrocellulose membrane using a Trans‐Blot SD semidry transfer cell (Bio‐Rad). The membrane was dried and then blocked with 5% milk‐TBST for 1 hr. Anti‐PilENel antibodies were diluted 1:10,000 (in 5% milk‐TBST) and incubated with the membrane for 1 hr. The membrane was washed 3× and incubated with secondary goat anti‐rabbit antibodies (LICOR) for 1 hr. The western blot was visualized using an Odyssey Clx Infrared Imaging System (LICOR) instrument.
2.9. Determination of transcription initiation site
Total RNA was purified as described above, followed by mRNA enrichment using the MICROBExpress bacterial mRNA enrichment kit (Ambion) per the manufacturer's instructions. A 5′ Rapid amplification of cDNA ends (RACE) reaction was used to determine the transcription initiation site (TIS) using the SMARTer® RACE 5′/3′ kit (Takara‐Clontech). Primers used to determine TIS and their targets are described in Table S2. The product obtained in each reaction was cloned into pCR2.1 (Life Technologies) and transformed into E. coli DH5. Colonies were selected for sequencing using universal primers M13 forward and M13 reverse.
3. RESULTS
3.1. nps and npa form an operon
We reported that pilE transcription in commensal Neisseria requires RpoN and the activator Npa (Rendón et al., 2013). Several domains characteristic of TCRS response regulators are present in Npa. Upstream of npa is ORF (NEIELOOT_00058, accession number: AJE19474.1) (Figure 1a), whose deduced amino acid sequence contains motifs typically found in SKs. We provisionally named this ORF Neisseria pilus sensor (Nps). Upstream of nps and npa is the DNA topoisomerase IV subunit A, parC (accession number: AJE19475.1); all three ORFs are arranged in the same orientation. This organization of the three genes is identical in the human commensals N. mucosa (Nmu) and N. sicca (Nsi), and in the animal commensals N. musculi (Nmus) and AP206, a Neisseria sp isolated from rhesus macaque (Weyand et al., 2013). In Neisseria weaverii (Nwe), Neisseria shayeganii (Nsh), Neisseria wadsworthii (Nwa), Neisseria subflava (Nsu), Neisseria flavescens (Nfl), Neisseria cinerea (Nci), Neisseria polysaccharea (Npo), and Neisseria lactamica (Nla), npa is followed by a fourth ORF, rsmJ, encoding a putative ribosomal RNA small subunit methyl transferase (locus tag NMA1806 in Nme). In pathogen Neisseria meningitidis (Nme), parC is followed by NMA1803, NMA1805, then rsmJ. NMA1803 is a homolog of Nps that lacks the last 76 bases of the ORF (Figure S1). NMA1805 is a homolog of Npa that lacks the last 1260 bases of the ORF, the region that would have encoded the RpoN‐ and DNA‐binding domains (Rendón et al., 2013). In pathogen Neisseria gonorrhoeae (Ngo), parC is followed by rsp, a fusion of npa and nps (Carrick et al., 2000; Rendón et al., 2013).
Figure 1.
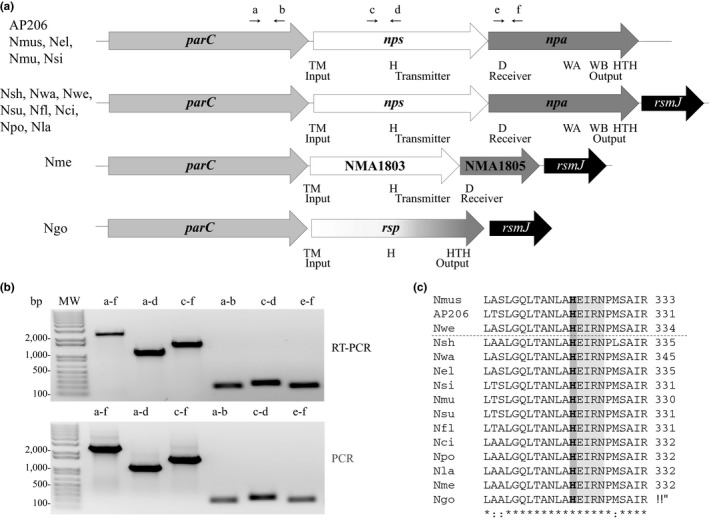
The nps‐npa operon in Neisseria species. (a) The nps‐npa locus in commensal Neisseria, and the analogous locus in pathogens Nme and Ngo. The small black arrows above the genes indicate the binding site of the primers used in the study (b). a, primer MR295, b, primer MR297; c, primer MR299; d, primer MR300; e, primer MR298; f, primer MR296. H, histidine, D, aspartate, WA, walker box A, WB, walker box B, HTH, Helix‐turn‐helix. (b) Top panel: Right three lanes: parC to npa, parC‐nps, and nps‐npa transcripts. Left three lanes: parC, nps and npa transcripts. All transcripts were produced by RT‐PCR using primers shown in (a). Bottom panel: Right three lanes: parC to npa, parC‐nps, and nps‐npa amplicons. Left three lanes: parC, nps and npa amplicons. All amplicons were produced by PCR using primers shown in (a). MW, molecular weight markers. (c) Alignment of the H phosphotransfer domain in Nps from Neisseria species. The phosphorylatable histidine is in bold type and boxed in dark gray. The canonical phosphatase motif (ExxN) in Pseudomonas aeruginosa PilS that is present in the Nps homolog is boxed in light gray. The dashed line separates Nps in animal and human Neisseria. (*) identical residues; (:) highly conserved residues. SAM, S‐adenosyl methyl transferase; Nmus, N. musculi; AP206, Neisseria sp isolated from rhesus macaque; Nwe, N. weaveri; Nsh, N. shayeganii; Nwa, N. wadsworthii; Nel, N. elongata; Nmu, N. mucosa; Nsi, N. sicca; Nsu, N. subflava; Nfl, N. flavescens; Nci, N. cinerea; Npo, N. polysaccharea; Nla, N. lactamica; Nme, N. meningitidis; Ngo, N. gonorrhoeae
The organization of parC, nps, and npa, and the presence of promoter‐like sequences upstream of parC suggest the three ORFs comprise an operon. We determined whether these genes are cotranscribed. Using primers specific for each gene (Table S2) in a RT‐PCR reaction of mRNA from log phase bacteria, we confirmed parC, nps, and npa are part of a large transcript (Figure 1b). In Nme, parC, NMA1803 (nps), NMA1805 (npa), and NMA1806 (rsmJ) are transcribed as an operon (Jamet, Rousseau, Monfort, Nassif, & Martin, 2010).
3.2. Predicted domains of Nps
The closest Nps homologs are PilS of Kingella kingae (Kki) (47.5% identity and 75.5% similarity) and PilS of Pseudomonas aeruginosa (Pae) (28.8% identity and 64.3% similarity). In Kki and Pae, PilS is required for transcription of pilA, encoding the major subunit of their Tfp (Boyd, Koga, & Lory, 1994; Kehl‐Fie, Porsch, Miller, & St Geme, 2009). The N‐terminus of Nps is predicted to span the membrane, and the C terminus to be located in the cytoplasm (TMMOD server) (Kahsay et al., 2005). The N terminus is highly hydrophobic, and harbors the input domain that contains six transmembrane helices interspersed with short loops (residues 21–189) (Figure S1). Residues 313–376 are predicted to function as a transmitter domain, as it contains two motifs, histidine (H) at position 325 and motif E/DxxN/T, that are commonly found in SKs (Figures 1c and S1) (Huynh, Noriega, & Stewart, 2010; Kilmury & Burrows, 2016). When the input domain of a SK detects a signal, the conserved H autophosphorylates, initiating a signaling cascade that activates transcription of the target gene. In Nps, H325 is predicted to participate in the phosphorylation cascade (Figures 1c and S1). These characteristics of Nps lead us to test the hypothesis that Nps is the SK that regulates pilE transcription by activating Npa.
3.3. pilE transcription requires Nps
We created an in‐frame deletion of nps in Nel 29315 (see Methods). NelΔnps, failed to produce detectable amounts of pilE mRNA (Table 1 and Figure 2). The complemented strain, NelΔnps+inps, expressing nps controlled by an Isopropyl β‐D‐1‐thiogalactopyranoside (IPTG)‐inducible promoter, restored pilE mRNA production. IPTG‐inducible promoters are known to be leaky, and Nel Δnps+inps produced low levels of mRNA even in the absence of the inducer (Figure 2a). Nel wt and NelΔnps+inps produced similar amounts of PilE, as detected by western blot (Figure 2b). Consistent with the absence of PilE, NelΔnps produced colonies with a non‐piliated phenotype and did not form microcolonies (Figure S2). npa mRNA levels were identical in both wt and Δnps, indicating the absence of pilE mRNA was due to the absence of nps, and not the absence of npa (Figure S3b). These results show that nps is essential for pilE transcription.
Table 1.
Phenotype of Nel29315 mutants
| Strain | pilE mRNA | PilE protein | Microcolony formation | Reference |
|---|---|---|---|---|
| Nel 29315 (wt) | + | + | + | Marri et al. (2010) |
| Nel 29315 ΔpilE | − | − | − | Higashi et al. (2011) |
| Nel 29315 Δnps | − | − | − | This work |
| Nel 29315 Δnps+inps | + | + | + | This work |
| Nel 29315 nps H325A | − | − | − | This work |
| Nel 29315 nps H325A+inps | + | + | + | This work |
| Nel 29315 Δnpa | − | − | − | Rendón et al. (2013) |
| Nel 29315 Δnpa+inpa | + | + | + | This work |
| Nel 29315 npa D58A | − | − | − | This work |
| Nel 29315 npa D58A+inpa | + | + | + | This work |
| Nel 29315 npa D58E | + | + | + | This work |
| Nel 29315 Δnps‐npa D58E | + | + | + | This work |
| Nel 29315 nps H325A‐npa D58E | + | + | −/+ | This work |
Figure 2.
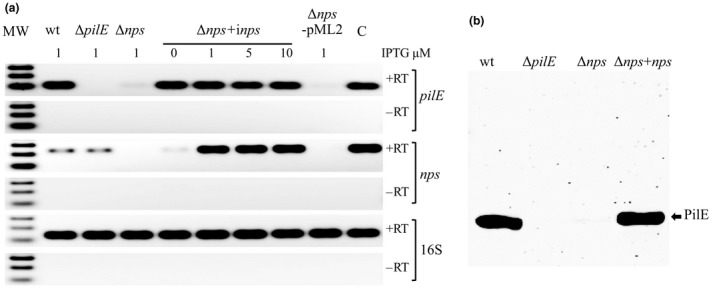
pilE mRNA (a) and PilE protein (b) in Nel 29315 wt, Δnps and Δnpa. (a) Log phase cells were incubated with varying concentrations of IPTG for 4 hr, at 37°C, and pilE or nps mRNA from the lysates was measured by RT‐PCR in the presence (+RT) or absence (−RT) of reverse transcriptase, using nps‐specific primers (see Table S2). The 16S rRNA reaction was used as a loading control and control for mRNA stability. Nel ΔpilE was included as negative control. Lane labeled “C” denotes control for PCR reactions, performed in the presence of DNA (+RT) or H2O (−RT). (b) PilE levels were detected by western blot using polyclonal antibodies raised to Nel PilE. Arrow points to PilE. Nel ΔpilE was included as negative control. Δnps+inps, complemented strain expressing an IPTG‐inducible wt nps; Δnps+pML2, control strain containing a plasmid with the lacZ promoter
3.4. Nps and Npa form a two‐component regulatory system
Upon activation, the SK transfers its phosphoryl group from the conserved H to a conserved aspartate (D) on the RR (Zschiedrich, Keidel, & Szurmant, 2016). We tested the hypothesis that the conserved H325 in Nps and D58 in Npa participate in the phosphorylation cascade, by replacing Nps H325 and Npa D58 with alanine (A) (Figure 3a). npsH325A is predicted not to autophosphorylate, and consequently unable to activate Npa and pilE transcription. As expected, pilE mRNA and PilE levels were undetectable in npsH325A and in npaD58A (Figure 3b,c). These mutants formed colonies with a non‐piliated morphology and did not form microcolonies (Table 1 and Figure S2). Complementation of nps or npa with the cognate IPTG‐inducible wt gene (Nel npsH325A+inps and npaD58A+inpa, respectively) restored pilE transcription, PilE production and microcolony formation (Table 1, Figures 3b,c and S2). Since these strains carry the mutated copy of nps or npa in addition to the inducible copy, the amount of IPTG needed to restore pilE levels was relatively high (5 μM for Nel npsH325 + inps and 10 μM for Nel npaD58A+inpa). We confirmed by RT‐PCR that levels of nps and npa transcripts were not affected by the point mutations in Nel npsH325A and Nel npaD58A (Figure 3b).
Figure 3.
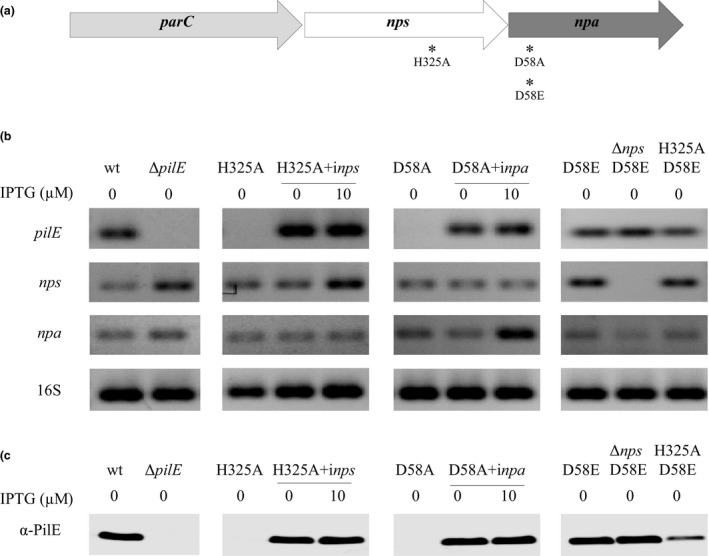
pilE, nps, and npa transcript levels in Nel 29315 wt and nps and npa mutants. (a) Point mutations in the nps and npa genes are indicated by an asterisk. (b) Log phase cells were incubated with varying concentrations of IPTG for 4 hr, at 37°C. pilE, nps, or npa mRNA from the lysates was measured by RT‐PCR in the presence (+RT) or absence (−RT) of reverse transcriptase, using gene‐specific primers (see Table S2). The 16S rRNA reaction was used as a loading control and control for mRNA stability. Name of each mutant appears above each column. Primers targeting 16S rRNA were used as loading control and control of mRNA stability. IPTG concentration used to induce the genes for complementation appears above the columns. (c) Detection of PilE by western blot using anti‐PilE antibodies. H325A+inps, nps H325 complemented with wt nps under control of the IPTG‐inducible promoter; D58A+inpa, npa D58A complemented with wt npa under control of the IPTG‐inducible promoter; H325A D58E, nps‐npa double mutant
Phosphorylation of the response regulator NtrC induces a conformational change (Hwang, Thorgeirsson, Lee, Kustu, & Shin, 1999). To mimic such a change, the activatable aspartate (D) in NtrC was substituted with a glutamate (E) (Klose, Weiss, & Kustu, 1993; Lan & Igo, 1998; Moore, Shiau, & Reitzer, 1993). To confirm that D58 in Npa is the phosphorylated amino acid, we made an analogous mutation and created Nel npa D58E. Nel npaD58E produced pilE mRNA and PilE protein at levels similar to wt (Figure 3b,c) and produced piliated colonies on agar (Figure S4). To further confirm Nel npaD58E behaves like a phosphorylated RR, we created double mutants Nel Δnps‐npa D58E and Nel nps H325A‐npa D58E. As expected, Nel Δnps‐npa D58E and Nel nps H325A‐npa D58E retained the ability to transcribe pilE (Table 1, Figure 3b,c). However, Nel nps H325A‐npa D58E produced lower amounts of pilE mRNA and PilE than the wt parental strain (Figure 3c). The reduction in pilE expression in these double mutants was not caused by a defect in nps or npa expression, since these mutant genes were transcribed at the same level as their wt counterpart.
In sum, the data demonstrate that Nps and Npa work in concert as a TCRS to control pilE expression.
3.5. Tfp genes other than pilE are not controlled by the RpoN/Nps‐Npa regulatory system
In pathogenic Neisseria, assembly and function of the Tfp is a complex process that involves over 20 proteins (Carbonnelle et al., 2006). Tfp biogenesis genes are present in all commensal Neisseria species (Marri et al., 2010), but how these commensal genes are regulated is unknown. We determined whether RpoN/Nps/Npa regulate expression of these genes in addition to pilE.
The Tfp genes in Nel 29315 are clustered in ten regions (Figure 4a). Each cluster is transcribed as a polycistronic unit, as determined by RT‐PCR using a combination of primers (Table S2 and Figure S5). One operon contains pilM, pilN, pilO, pilP, and pilQ (Figures 4a and S5a). A second operon contains ORF NEIELOOT_00092, followed by pilF, pilG, and pilD (Figures 4a and S5b). The genes in this locus are organized differently in pathogenic Neisseria, where pilF is convergently transcribed to the operon formed by pilG‐pilD (Freitag, Seifert, & Koomey, 1995; Lin, Ryan, & Davies, 2011; Tonjum, Freitag, Namork, & Koomey, 1995). A third operon contains six genes (Figures 4a and S5c) only one of which, pilW, is known to function in Tfp biogenesis. A fourth operon contains most of the minor pilin genes (pilH, pilI, pilJ, pilK, and pilX). Another minor pilin gene, pilV, is transcribed as a monocistronic unit (Figures 4a and S5d). Nel has only one copy of pilC, unlike Nme and Ngo (Marri et al., 2010), and its transcript is also monocistronic (Figure 4a). Like Ngo and Nme, Nel pilT and pilU are transcribed as one unit (Figures 4a and S5e). In Ngo and Nme, pilT2 is in a putative operon with pilZ (Brown, Helaine, Carbonnelle, & Pelicic, 2010), but in Nel, pilT2 is in a separate locus (Figure 4a). Finally, pilZ is flanked by ORFs 1 and 2 (NEIELOOT_01544 and NEIELOOT_01542) and the three genes form an operon (Figures 4a and S5f).
Figure 4.
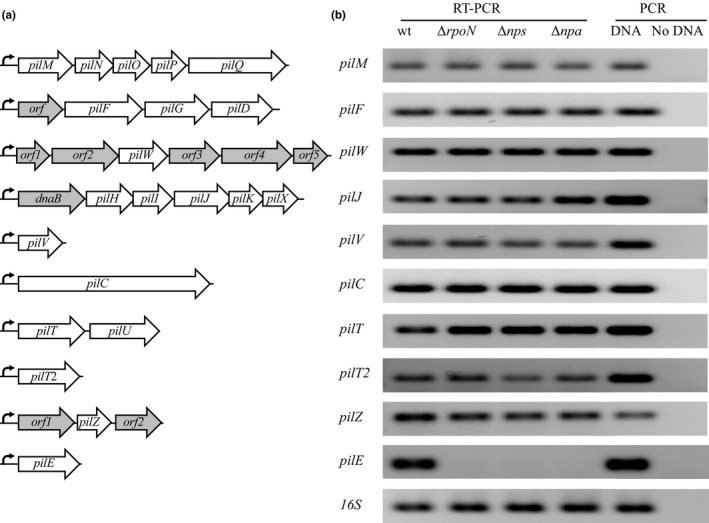
Role of RpoN and Nps/Npa in regulating Tfp biogenesis and function genes. (a) Organization of Tfp biogenesis and function genes in Nel29315. Genes known to play a role in Tfp biogenesis or function are represented by white arrows. Other genes are represented by gray arrows. Dark curved arrow at left indicates the transcriptional initiation site of the mRNA produced at each locus. (b) pilM, pilF, pilW, pilJ, pilV, pilC, pilT, pilT2, and pilZ mRNA was determined in log phase wt, ΔrpoN, Δnps, and Δnpa cells by RT‐PCR. pilE mRNA served as a positive control for rpoN, nps, and npa mutants. 16S rRNA served as the loading control and control of mRNA stability. A PCR reaction in the presence of DNA or in H2O (No DNA) served as the control for the amplification reaction
To determine whether RpoN and Nps‐Npa control transcription of these nine gene clusters, we selected a gene from each operon and examined its expression in ΔrpoN, Δnps, and Δnpa mutants. RT‐PCR was performed on mRNA from mid‐log bacteria using specific primers (Table S2). 16s rRNA was use as the loading control and the control for mRNA integrity. A reaction on DNA was conducted as a control for primers specificity. In contrast to pilE, transcription of the tested genes was not altered in the ΔrpoN, Δnps, and Δnpa mutants when compared to the wt (Figure 4b). The amplicons were not a product of DNA contamination, as judged by lack of a product when RT was excluded from the reaction (data not shown). From these results, we conclude that RpoN and the TCRS Nps‐Npa do not control transcription of these Tfp biogenesis genes other than pilE.
Finally, we mapped the TIS of 4 Tfp loci by 5′ RACE (Rendón et al., 2013). In all cases, the TIS mapped to RpoD recognition sequences (Table S3). This confirms that a sigma factor of the RpoD‐family, and not RpoN, regulates expression of these loci.
In conclusion, of all the Tfp biogenesis and function genes described to date, only pilE is regulated by RpoN and the TCRS Nps‐Npa.
4. DISCUSSION
The genes required for Tfp biogenesis and function are present in animal‐ and human‐dwelling Neisseria species (Marri et al., 2010; Weyand et al., 2013, 2016). Tfp gene expression has been studied mostly in the two pathogenic species. In the pathogens, RpoD regulates transcription of pilE, pilT/U, and pilC (Eriksson et al., 2015; Meyer et al., 1984; Taha, Giorgini, & Nassif, 1996). We previously reported that in commensal species of Neisseria, RpoN, IHF and the activator Npa regulate pilE transcription (Rendón et al., 2013).
Using Neisseria elongata as a model commensal, we showed that Nps and Npa act as the SK and response regulator, respectively, of a TCRS that regulates pilE transcription (Figures 2 and 3), and that Nps H325 and Npa D58 participate in the phosphorylation events. nps H325A and npa D58A failed to transcribe pilE, while the complemented strains had this activity restored. A D58E substitution, which mimics an activated residue, resulted in constitutive expression of pilE. A similar mutation in NtrC altered the conformation of the response regulator, resulting in a constitutively active enzyme (Hwang et al., 1999; Klose et al., 1993; Lan & Igo, 1998; Moore et al., 1993). The npa D58E mutation overcomes the need of nps, as the double mutants Δnps+npa D58E and nps H325A+npaD58E are able to express pilE (Figure 4b). That the double mutant H325A+npaD58E produced significantly lower levels of pilE transcript than the wt is surprising. At the moment we cannot explain this observation. We know that this is not due to reduced expression of nps or npa since expression of these genes was not affected (Figure 3b) and no mutation has occurred in the pilE locus. In the ΔpilE strain used as control, nps and npa transcripts appear to be slightly increased compared to the wt strain. It is unclear why this occurred. Further studies will determine whether this difference is due to a feedback mechanism in which the absence of pilE affects transcription of nps/npa.
Although the signal‐sensing domains of some TCRS response regulators have been characterized (Hulko et al., 2006; Taylor & Zhulin, 1999; Xu & West, 1999), the signals detected by these regulators are unknown. In silico analysis indicates Nps lacks PAS (Per‐Arnt‐Sim), HAMP (domain present in histidine kinases, adenylate cyclases, methyl accepting proteins and phosphatases), and HPT (histidine containing phosphotransfer) motifs. Nps has six membrane‐spanning regions interspersed with very short loops. Pae PilS, the Nps homolog, also has six such transmembrane domains. Pae PilS is both a kinase and phosphatase; it senses the levels of membrane‐bound pilin (PilA) and regulates pilA expression through its phosphatase activity (Boyd et al., 1994; Kilmury & Burrows, 2016). The PilS kinase and phosphatase motifs are highly conserved across all Neisseria Nps (Figures 1 and S1). The amino acids arginine 24 in PilS, and glutamate 5 and proline 22 in PilA are important for PilS/PilA interactions and PilS autoregulation (Kilmury & Burrows, 2016). These residues are also present in all Neisseria Nps and PilE (Figures S1 and S6, respectively). This suggests Nps may, like PilS, sense and respond to membrane‐bound PilE levels.
We attempted unsuccessfully to determine whether Nel Nps detects pH, oxygen, and growth phase (data not shown). Iron depletion affected pilE mRNA levels, but from the data we cannot definitely conclude that iron is the signal detected by Nps. Identifying the signal that Nps recognizes will allow us to understand how commensal Neisseria adapts to different environmental conditions.
Our model for pilE expression in commensal Neisseria is summarized in Figure 5. Upon detecting a signal (left), Nps autophosphorylates and transfers the phosphoryl group to Npa (this work). Phosphorylated Npa binds the UAS, promoting RpoN activation of pilE transcription (Rendón et al., 2013). pilE transcription also requires IHF binding to its cognate sequence, which is situated between the RpoN and Npa recognition sites (Rendón et al., 2013). We hypothesize that this interaction of IHF with DNA bends the DNA, bringing Npa in close proximity to RpoN. In optimal conditions, when the Tfp components necessary for biogenesis are present, PilE would be assembled quickly into pili. If PilE accumulates in the membrane (Figure 5, right panel), Nps would sense this, dephosphorylate Npa, and prevent it from activating RpoN. As a result, pilE transcription is abolished.
Figure 5.
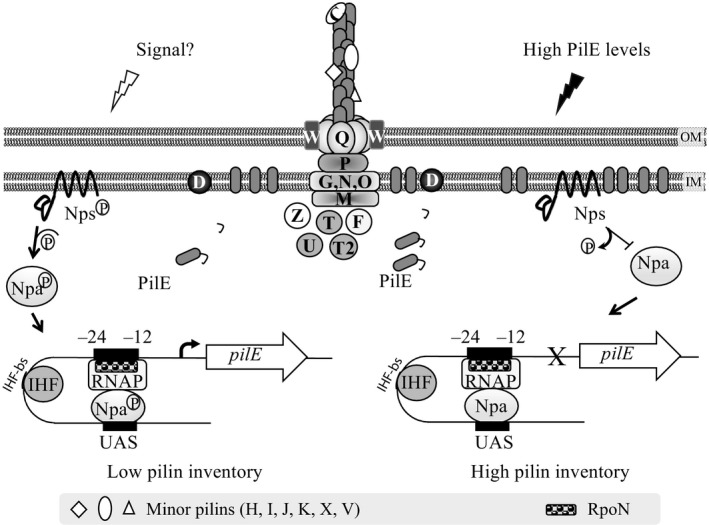
Regulation of pilE transcription by RpoN and Nps/Npa in commensal Neisseria. Left panel. Upon detection of the signal, Nps autophosphorylates (NpsP) and transfers the phosphoryl group to Npa (NpaP). NpaP binds the upstream activator sequence (UAS) and activates RpoN, resulting in pilE transcription. Transcription is assisted by the binding of integration host factor (IHF) to its binding site (IHF‐bs), bending the DNA and bringing the NpaP‐ and RNAP‐DNA complexes into proximity with each other. Right panel. Accumulation of PilE in the inner membrane is detected by Nps. Nps dephosphorylates Npa preventing it from activating RpoN blocking pilE transcription
Our model implies that Tfp biogenesis genes other than pilE are constitutively expressed. Our findings indicate they are transcribed from a RpoD‐dependent promoter (Figure 4), but they do not allow us to draw a conclusion about whether they are under more stringent regulation—for instance by an enhancer or a repressor. Future studies are needed to determine whether these genes are controlled by an intricate regulatory system(s).
Why have commensal and pathogenic Neisseria evolved different mechanisms to regulate pilE? This question might be explained by current information on Neisseria Tfp biogenesis. A large number of proteins situated in the inner membrane, periplasm and outer membrane are involved in the assembly and export of PilE and minor pilins (Brown et al., 2010; Carbonnelle et al., 2006; Winther‐Larsen et al., 2005; Wolfgang, van Putten, Hayes, Dorward, & Koomey, 2000). The PilF ATPase incorporates PilE subunits into the Tfp fiber (Freitag et al., 1995) and the PilT ATPase causes the Tfp fiber to retract, presumably by disassembling these subunits from the fiber base (Wolfgang, Lauer, et al., 1998). If the Tfp assembly/disassembly machinery malfunctions, PilE accumulates in the inner membrane and becomes toxic to the bacterium (Carbonnelle et al., 2006; Wolfgang et al., 2000). It is not unreasonable to speculate that the RpoN/Nps/Npa system serves not only to aid the bacterium to adapt to new niches but also to relieve the toxicity of membrane‐bound PilE.
In the pathogens, pilE is expressed constitutively (Fyfe et al., 1995; Laskos, Dillard, Seifert, Fyfe, & Davies, 1998; Meyer et al., 1984). Excess PilE is secreted as soluble truncated pilins (S pilin) (Jonsson, Pfeifer, & Normark, 1992; Koomey, Bergstrom, Blake, & Swanson, 1991), and this process is proposed to relieve the toxicity of membrane‐bound PilE (Winther‐Larsen et al., 2005; Wolfgang, Park, Hayes, van Putten, & Koomey, 1998; Wolfgang et al., 2000). In addition, Ngo regulates pilE transcription under certain conditions. pilE expression is decreased in a pilF mutant and increased in a pilT mutant (Dietrich, Mollenkopf, So, & Friedrich, 2009), by unknown mechanism(s). Ngo encodes a modified version of the Nps/Npa TCRS, termed Rsp; however, Rsp does not appear to play a role in pilE transcription (Carrick et al., 2000). A number of candidates have been proposed to regulate pilE (De Reuse & Taha, 1997; Deghmane, Giorgini, Larribe, Alonso, & Taha, 2002); however, none of these has been proven conclusively to control pilE transcription in Ngo or Nme. Future studies will allow a better understanding of pilE transcriptional in the pathogens.
CONFLICT OF INTEREST
The authors declare not conflict of interest.
DATA ACCESSIBILITY
The authors adhere to sharing data and materials policies described in the guidelines for authors.
Supporting information
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant R01AI107966 awarded to M.S. We thank A. Hockenberry, W. Kim, and K. Rhodes for helpful discussions and revision of this manuscript.
Rendón MA, Lona B, Ma MC, So M. RpoN and the Nps and Npa two‐component regulatory system control pilE transcription in commensal Neisseria . MicrobiologyOpen. 2019;8:e713 10.1002/mbo3.713
REFERENCES
- Albers, S. V. , & Jarrell, K. F. (2015). The archaellum: How Archaea swim. Frontiers in Microbiology, 6, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, J. L. , & Pelicic, V. (2015). Exceptionally widespread nanomachines composed of type IV pilins: The prokaryotic Swiss Army knives. FEMS Microbiology Reviews, 39, 134–154. 10.1093/femsre/fuu001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani, G. (1951). Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli . Journal of Bacteriology, 62, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, J. M. , Koga, T. , & Lory, S. (1994). Identification and characterization of PilS, an essential regulator of pilin expression in Pseudomonas aeruginosa . Molecular and General Genetics, 243, 565–574. 10.1007/BF00284205 [DOI] [PubMed] [Google Scholar]
- Brown, D. R. , Helaine, S. , Carbonnelle, E. , & Pelicic, V. (2010). Systematic functional analysis reveals that a set of seven genes is involved in fine‐tuning of the multiple functions mediated by type IV pili in Neisseria meningitidis . Infection and Immunity, 78, 3053–3063. 10.1128/IAI.00099-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonnelle, E. , Helaine, S. , Nassif, X. , & Pelicic, V. (2006). A systematic genetic analysis in Neisseria meningitidis defines the Pil proteins required for assembly, functionality, stabilization and export of type IV pili. Molecular Microbiology, 61, 1510–1522. 10.1111/j.1365-2958.2006.05341.x [DOI] [PubMed] [Google Scholar]
- Carrick, C. S. , Fyfe, J. A. , & Davies, J. K. (2000). The genome of Neisseria gonorrhoeae retains the remnants of a two‐component regulatory system that once controlled piliation. FEMS Microbiology Letters, 186, 197–201. 10.1111/j.1574-6968.2000.tb09104.x [DOI] [PubMed] [Google Scholar]
- De Reuse, H. , & Taha, M. K. (1997). RegF, an SspA homologue, regulates the expression of the Neisseria gonorrhoeae pilE gene. Research in Microbiology, 148, 289–303. 10.1016/S0923-2508(97)81585-9 [DOI] [PubMed] [Google Scholar]
- Deghmane, A. E. , Giorgini, D. , Larribe, M. , Alonso, J. M. , & Taha, M. K. (2002). Down‐regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Molecular Microbiology, 43, 1555–1564. 10.1046/j.1365-2958.2002.02838.x [DOI] [PubMed] [Google Scholar]
- Dietrich, M. , Mollenkopf, H. , So, M. , & Friedrich, A. (2009). Pilin regulation in the pilT mutant of Neisseria gonorrhoeae strain MS11. FEMS Microbiology Letters, 296, 248–256. 10.1111/j.1574-6968.2009.01647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard, J. P. (2011). Genetic Manipulation of Neisseria gonorrhoeae . Current Protocols in Microbiology, 23(1), 4A‐2. [DOI] [PubMed] [Google Scholar]
- Eriksson, J. , Eriksson, O. S. , Maudsdotter, L. , Palm, O. , Engman, J. , Sarkissian, T. , … Jonsson, A. B. (2015). Characterization of motility and piliation in pathogenic Neisseria . BMC Microbiology, 15, 92 10.1186/s12866-015-0424-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag, N. E. , Seifert, H. S. , & Koomey, M. (1995). Characterization of the pilF‐pilD pilus‐assembly locus of Neisseria gonorrhoeae . Molecular Microbiology, 16, 575–586. 10.1111/j.1365-2958.1995.tb02420.x [DOI] [PubMed] [Google Scholar]
- Fyfe, J. A. , Carrick, C. S. , & Davies, J. K. (1995). The pilE gene of Neisseria gonorrhoeae MS11 is transcribed from a sigma 70 promoter during growth in vitro . Journal of Bacteriology, 177, 3781–3787. 10.1128/jb.177.13.3781-3787.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi, H. , Koby, S. , Prag, G. , Engelhorn, M. , Geiselmann, J. , & Oppenheim, A. B. (1998). Participation of IHF and a distant UP element in the stimulation of the phage lambda P(L) promoter. Molecular Microbiology, 30, 443–451. 10.1046/j.1365-2958.1998.01079.x [DOI] [PubMed] [Google Scholar]
- Heckels, J. E. (1989). Structure and function of pili of pathogenic Neisseria species. Clinical Microbiology Reviews, 2(Suppl.), S66–S73. 10.1128/CMR.2.Suppl.S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman, K. L. , & Pease, L. R. (2007). Gene splicing and mutagenesis by PCR‐driven overlap extension. Nature Protocols, 2, 924–932. 10.1038/nprot.2007.132 [DOI] [PubMed] [Google Scholar]
- Higashi, D. L. , Biais, N. , Weyand, N. J. , Agellon, A. , Sisko, J. L. , Brown, L. M. , & So, M. (2011). N. elongata produces type IV pili that mediate interspecies gene transfer with N. gonorrhoeae . PLoS ONE, 6, e21373 10.1371/journal.pone.0021373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, S. A. , Samuels, D. S. , Carlson, J. H. , Wilson, J. , Hogan, D. , Lubke, L. , & Belland, R. J. (1997). Integration host factor is a transcriptional cofactor of pilE in Neisseria gonorrhoeae . Molecular Microbiology, 23, 649–656. 10.1046/j.1365-2958.1997.2321612.x [DOI] [PubMed] [Google Scholar]
- Howie, H. L. , Glogauer, M. , & So, M. (2005). The N. gonorrhoeae type IV pilus stimulates mechanosensitive pathways and cytoprotection through a pilT‐dependent mechanism. PLoS Biology, 3, e100 10.1371/journal.pbio.0030100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulko, M. , Berndt, F. , Gruber, M. , Linder, J. U. , Truffault, V. , Schultz, A. , … Coles, M. (2006). The HAMP domain structure implies helix rotation in transmembrane signaling. Cell, 126, 929–940. 10.1016/j.cell.2006.06.058 [DOI] [PubMed] [Google Scholar]
- Huynh, T. N. , Noriega, C. E. , & Stewart, V. (2010). Conserved mechanism for sensor phosphatase control of two‐component signaling revealed in the nitrate sensor NarX. Proceedings of the National Academy of Sciences of the United States of America, 107, 21140–21145. 10.1073/pnas.1013081107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, I. , Thorgeirsson, T. , Lee, J. , Kustu, S. , & Shin, Y. K. (1999). Physical evidence for a phosphorylation‐dependent conformational change in the enhancer‐binding protein NtrC. Proceedings of the National Academy of Sciences of the United States of America, 96, 4880–4885. 10.1073/pnas.96.9.4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet, A. , Rousseau, C. , Monfort, J. B. , Nassif, X. , & Martin, P. (2010). Identification of a novel transcriptional regulator involved in pilC1 regulation in Neisseria meningitidis . FEMS Microbiology Letters, 304, 140–147. 10.1111/j.1574-6968.2009.01894.x [DOI] [PubMed] [Google Scholar]
- Jonsson, A.‐B. , Pfeifer, J. , & Normark, S. (1992). Neisseria gonorrhoeae PilC expression provides a selective mechanism for structural diversity of pili. Proceedings of the National Academy of Sciences of the United States of America, 89, 3204–3208. 10.1073/pnas.89.8.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , Fried, L. , Behr, S. , & Heermann, R. (2012). Histidine kinases and response regulators in networks. Current Opinion in Microbiology, 15, 118–124. 10.1016/j.mib.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Kahsay, R. Y. , Gao, G. , & Liao, L. (2005). An improved hidden Markov model for transmembrane protein detection and topology prediction and its applications to complete genomes. Bioinformatics, 21, 1853–1858. 10.1093/bioinformatics/bti303 [DOI] [PubMed] [Google Scholar]
- Kehl‐Fie, T. E. , Porsch, E. A. , Miller, S. E. , & St Geme, J. W., 3rd (2009). Expression of Kingella kingae type IV pili is regulated by sigma54, PilS, and PilR. Journal of Bacteriology, 191, 4976–4986. 10.1128/JB.00123-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg, D. S. , Peacock, W. L. , Deacon, W. E. , Brown, L. , & Pirkle, C. I. (1963). Neisseria gonorrhoeae. I. Virulence genetically linked to clonal varation. Journal of Bacteriology, 85, 1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmury, S. L. , & Burrows, L. L. (2016). Type IV pilins regulate their own expression via direct intramembrane interactions with the sensor kinase PilS. Proceedings of the National Academy of Sciences of the United States of America, 113, 6017–6022. 10.1073/pnas.1512947113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose, K. E. , Weiss, D. S. , & Kustu, S. (1993). Glutamate at the site of phosphorylation of nitrogen‐regulatory protein NTRC mimics aspartyl‐phosphate and activates the protein. Journal of Molecular Biology, 232, 67–78. 10.1006/jmbi.1993.1370 [DOI] [PubMed] [Google Scholar]
- Kohler, P. L. , Hamilton, H. L. , Cloud‐Hansen, K. , & Dillard, J. P. (2007). AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae type IV secretion system. Journal of Bacteriology, 189, 5421–5428. 10.1128/JB.00531-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey, M. , Bergstrom, S. , Blake, M. , & Swanson, J. (1991). Pilin expression and processing in pilus mutants of Neisseria gonorrhoeae: Critical role of Gly‐1 in assembly. Molecular Microbiology, 5, 279–287. 10.1111/j.1365-2958.1991.tb02108.x [DOI] [PubMed] [Google Scholar]
- Lan, C. Y. , & Igo, M. M. (1998). Differential expression of the OmpF and OmpC porin proteins in Escherichia coli K‐12 depends upon the level of active OmpR. Journal of Bacteriology, 180, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskos, L. , Dillard, J. P. , Seifert, H. S. , Fyfe, J. A. M. , & Davies, J. K. (1998). The pathogenic neisseriae contain an inactive rpoN gene and do not utilize the pilE sigma54 promoter. Gene, 208, 95–102. 10.1016/S0378-1119(97)00664-1 [DOI] [PubMed] [Google Scholar]
- Lin, Y. H. , Ryan, C. S. , & Davies, J. K. (2011). Neisserial Correia repeat‐enclosed elements do not influence the transcription of pil genes in Neisseria gonorrhoeae and Neisseria meningitidis . Journal of Bacteriology, 193, 5728–5736. 10.1128/JB.05526-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, M. , Powell, D. A. , Weyand, N. J. , Rhodes, K. A. , Rendón, M. A. , Frelinger, J. A. , & So, M. (2018). A mouse model for Neisseria colonization. Infection and Immunity, 86, e00839 –17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marri, P. R. , Paniscus, M. , Weyand, N. J. , Rendón, M. A. , Calton, C. M. , Hernandez, D. R. , … So, M. (2010). Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS ONE, 5, e11835 10.1371/journal.pone.0011835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, T. F. , Billyard, E. , Haas, R. , Storzbach, S. , & So, M. (1984). Pilus genes of Neisseria gonorrhoeae: Chromosomal organization and DNA sequence. Proceedings of the National Academy of Sciences of the United States of America, 81, 6110–6114. 10.1073/pnas.81.19.6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. B. , Shiau, S. P. , & Reitzer, L. J. (1993). Alterations of highly conserved residues in the regulatory domain of nitrogen regulator I (NtrC) of Escherichia coli . Journal of Bacteriology, 175, 2692–2701. 10.1128/jb.175.9.2692-2701.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif, X. , Beretti, J. L. , Lowy, J. , Stenberg, P. , O'Gaora, P. , Pfeifer, J. , … So, M. (1994). Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proceedings of the National Academy of Sciences of the United States of America, 91, 3769–3773. 10.1073/pnas.91.9.3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parge, H. E. , Forest, K. T. , Hickey, M. J. , Christensen, D. A. , Getzoff, E. D. , & Tainer, J. A. (1995). Structure of the fibre‐forming protein pilin at 2.6 A resolution. Nature, 378, 32–38. 10.1038/378032a0 [DOI] [PubMed] [Google Scholar]
- Pearson, W. R. (1990). Rapid and sensitive sequence comparison with FASTP and FASTA. Methods in Enzymology, 183, 63–98. 10.1016/0076-6879(90)83007-V [DOI] [PubMed] [Google Scholar]
- Pujol, C. , Eugene, E. , Marceau, M. , & Nassif, X. (1999). The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus‐mediated adhesion. Proceedings of the National Academy of Sciences of the United States of America, 96, 4017–4022. 10.1073/pnas.96.7.4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendón, M. A. , Hockenberry, A. M. , McManus, S. A. , & So, M. (2013). Sigma factor RpoN (sigma54) regulates pilE transcription in commensal Neisseria elongata . Molecular Microbiology, 90, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert, H. S. , Chen, E. Y. , So, M. , & Heffron, F. (1986). Shuttle mutagenesis: A method of transposon mutagenesis for Saccharomyces cerevisiae . Proceedings of the National Academy of Sciences of the United States of America, 83, 735–739. 10.1073/pnas.83.3.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers, F. , Wilm, A. , Dineen, D. , Gibson, T. J. , Karplus, K. , Li, W. , … Higgins, D. G. (2011). Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology, 7, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha, M. K. , Giorgini, D. , & Nassif, X. (1996). The pilA regulatory gene modulates the pilus‐mediated adhesion of Neisseria meningitidis by controlling the transcription of pilC1. Molecular Microbiology, 19, 1073–1084. 10.1046/j.1365-2958.1996.448979.x [DOI] [PubMed] [Google Scholar]
- Taylor, B. L. , & Zhulin, I. B. (1999). PAS domains: Internal sensors of oxygen, redox potential, and light. Microbiology and Molecular Biology Reviews, 63, 479–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonjum, T. , Freitag, N. E. , Namork, E. , & Koomey, M. (1995). Identification and characterization of pilG, a highly conserved pilus‐assembly gene in pathogenic Neisseria . Molecular Microbiology, 16, 451–464. 10.1111/j.1365-2958.1995.tb02410.x [DOI] [PubMed] [Google Scholar]
- Virji, M. , Makepeace, K. , Peak, I. R. , Ferguson, D. J. , Jennings, M. P. , & Moxon, E. R. (1995). Opc‐ and pilus‐dependent interactions of meningococci with human endothelial cells: Molecular mechanisms and modulation by surface polysaccharides. Molecular Microbiology, 18, 741–754. 10.1111/j.1365-2958.1995.mmi_18040741.x [DOI] [PubMed] [Google Scholar]
- Weyand, N. J. , Ma, M. , Phifer‐Rixey, M. , Taku, N. A. , Rendón, M. A. , Hockenberry, A. M. , … So, M. (2016). Isolation and characterization of Neisseria musculi sp. nov., from the wild house mouse. International Journal of Systematic and Evolutionary Microbiology, 66, 3585–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand, N. J. , Wertheimer, A. M. , Hobbs, T. R. , Sisko, J. L. , Taku, N. A. , Gregston, L. D. , … So, M. (2013). Neisseria infection of rhesus macaques as a model to study colonization, transmission, persistence, and horizontal gene transfer. Proceedings of the National Academy of Sciences of the United States of America, 110, 3059–3064. 10.1073/pnas.1217420110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther‐Larsen, H. C. , Wolfgang, M. , Dunham, S. , van Putten, J. P. , Dorward, D. , Lovold, C. , … Koomey, M. (2005). A conserved set of pilin‐like molecules controls type IV pilus dynamics and organelle‐associated functions in Neisseria gonorrhoeae . Molecular Microbiology, 56, 903–917. 10.1111/j.1365-2958.2005.04591.x [DOI] [PubMed] [Google Scholar]
- Wolfgang, M. , Lauer, P. , Park, H. S. , Brossay, L. , Hebert, J. , & Koomey, M. (1998). PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae . Molecular Microbiology, 29, 321–330. 10.1046/j.1365-2958.1998.00935.x [DOI] [PubMed] [Google Scholar]
- Wolfgang, M. , Park, H. S. , Hayes, S. F. , van Putten, J. P. , & Koomey, M. (1998). Suppression of an absolute defect in type IV pilus biogenesis by loss‐of‐function mutations in pilT, a twitching motility gene in Neisseria gonorrhoeae . Proceedings of the National Academy of Sciences of the United States of America, 95, 14973–14978. 10.1073/pnas.95.25.14973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang, M. , van Putten, J. P. , Hayes, S. F. , Dorward, D. , & Koomey, M. (2000). Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO Journal, 19, 6408–6418. 10.1093/emboj/19.23.6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormann, M. E. , Horien, C. L. , Johnson, E. , Liu, G. , Aho, E. , Tang, C. M. , & Exley, R. M. (2016). Neisseria cinerea isolates can adhere to human epithelial cells by type IV pilus‐independent mechanisms. Microbiology, 162, 487–502. 10.1099/mic.0.000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q. , & West, A. H. (1999). Conservation of structure and function among histidine‐containing phosphotransfer (HPt) domains as revealed by the crystal structure of YPD1. Journal of Molecular Biology, 292, 1039–1050. 10.1006/jmbi.1999.3143 [DOI] [PubMed] [Google Scholar]
- Zschiedrich, C. P. , Keidel, V. , & Szurmant, H. (2016). Molecular mechanisms of two‐component signal transduction. Journal of Molecular Biology, 428, 3752–3775. 10.1016/j.jmb.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors adhere to sharing data and materials policies described in the guidelines for authors.


