Abstract
A robust regimen for inducing allogeneic transplantation tolerance involves pre-emptive recipient treatment with donor splenocytes (SP) rendered apoptotic by 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide(ECDI) treatment. However, such a regimen is limited by availability of donor cells, cost of cell procurement, and regulatory hurdles associated with cell-based therapies. Nanoparticles (NP) delivering donor antigens are a promising alternative for promoting transplantation tolerance. Here, we used a B6.C-H-2bm12(bm12) to C57BL/6(B6) skin transplant model involving a defined major histocompatibility antigen mismatch to investigate design parameters of poly(lactide-coglycolide) (PLG) NPs delivering peptides containing the donor antigen for optimizing skin allograft survival. We showed that an epitope-containing short peptide (P1) was more effective than a longer peptide (P2) at providing graft protection. Importantly, the NP and P1 complex (NP-ECDI-P1) resulted in a significant expansion of graft-infiltrating Tregs. Interestingly, in comparison to donor ECDI-SP that provided indefinite graft protection, NP-ECDI-P1 targeted different splenic phagocytes and skin allografts in these recipients harbored significantly more graft-infiltrating CD8+IFN-γ+ cells. Collectively, the current study provides initial engineering parameters for a cell-free and biocompatible NP-peptide platform for transplant immunoregulation. Moreover, it also provides guidance to future NP engineering endeavors to recapitulate the effects of donor ECDI-SP as a goal for maximizing tolerance efficacy of NP formulations.
Keywords: Poly(lactide-co-glycolide) (PLG), Nanoparticles, Skin transplantation, Transplantation tolerance, bm-12
1. Introduction
Organ transplantation is currently the most effective treatment for end-stage organ diseases and is often the only available therapeutic option (1–3). Allogeneic organs (i.e., from another person) are commonly used for transplantation, yet the number of individuals awaiting transplantation in the United States is over 121,000, and is growing annually at a rate far greater than the donor growing rate (4). Confounding this unmet need, transplantation generally requires the recipient to take immunosuppressive drugs indefinitely to prevent acute graft rejection, but such a requirement often comes with an increased risk of subsequent neoplasia and infections (5–7). Ideally, antigen-specific tolerance therapies would replace broad-scale immunosuppressants and eliminate their undesirable side effects (8, 9).
One promising antigen-specific therapy developed by us involves intravenous infusion of 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide(ECDI) fixed apoptotic donor splenocytes (ECDI-SP) to induce donor-specific tolerance (10, 11). This approach takes advantage of the body’s natural ability to maintain self-tolerance through daily non-inflammatory clearance of billions of self apoptotic cells generated by physiological turnover and applies it to the clearance of donor apoptotic cells, thereby inducing tolerance to donor (10, 12). This approach has proven to be highly effective in full major histocompatibility complex (MHC) mismatched transplantation models (13, 14), including in non-human primates (15). Mechanistic studies reveal that when delivered in vivo, ECDI-SP mediate anergy and deletion of alloreactive T cells via PD-L1/PD-1 signaling, production of immunosuppressive cytokines, and induction of regulatory T cells (Tregs), which collectively facilitate indefinite graft survival (11, 13, 16). Leveraging the concept of apoptotic cell clearance as a tolerogenic process, ECDI-fixed apoptotic syngeneic splenocytes coupled to donor cell lysate also resulted in comparable allogeneic islet cell survival in comparison to apoptotic donor splenocytes themselves(14). While powerful, these cell-based therapies are limited by the high cost of cell harvesting, processing, administration, and an increased regulatory hurdle to clinical translation(17). A non-cell-based delivery platform that is more easily sourced and that leverages the tolerogenic features of apoptotic cell clearance would be a desirable alternative to cell-based therapies.
One such promising alternative is nanoscale polymeric particles composed of poly(lactide-co-glycolide) (PLG) which is FDA-approved in several biodegradable drug delivery applications (18). PLG has non-toxic degradation byproducts and allows precise control of characteristics such as surface charge, size, and degradation time (19, 20). PLG nanoparticles (PLG-NPs) covalently coupled to antigenic peptides have been shown to have promising tolerance efficacies in pre-clinical models of autoimmune and allergic diseases. This platform exploits tolerogenic pathways similar to apoptotic donor cell treatment (21–23). In models of allergy and autoimmunity such as experimental autoimmune encephalomyelitis (EAE), it has been shown that factors including nanoparticle surface charge, size, shape, and route of administration are key parameters of this platform for achieving peripheral tolerance (24–30). Translating to allogeneic transplantation tolerance, MHCs of donor origin are the primary donor antigens recognized as “non-self”, and therefore can elicit anti-donor immune responses after being processed and presented as donor antigenic MHC peptides by recipient MHCs to activate recipient T cells (31). Consequently, immune tolerance to such appropriately selected donor antigenic MHC peptides that encompass the entire spectrum of donor-recipient MHC mismatches would allow donor-specific tolerance to be established in the recipient. Based on the above-mentioned published data in autoimmunity and allergy, this could be accomplished by NPs coupled to such donor antigenic MHC peptides. However, additional engineering parameters including antigenic peptide length for MHC presentation, chemical method of covalent conjugation, and location of antigen within the polymeric carrier will likely require further characterization in their contributions to transplantation tolerance induction.
Here, we investigate these engineering parameters and their contribution to inducing transplantation tolerance in a bm12 to B6 skin transplant model using PLG NPs. In this model, three point mutations in the MHC class II molecule IAb of the bm12 donor in comparison to the IAb of the B6 recipient is sufficient to induce T cell-mediated immunity in the recipient and to prompt acute rejection of the skin allograft within two to three weeks (32, 33). The current study aims to use the bm12 to B6 skin transplant model to identify key engineering parameters regarding antigen selection and method of conjugation to polymeric particles, and to investigate their efficacy in enhancing skin allograft survival. Our study uncovers the effective NP formulations for partial graft protection in this model and their preponderance for inducing intragraft CD4+Foxp3+ Treg accumulation. However, in comparison to donor ECDI-SP that provide indefinite graft protection, the NP formulations target different splenic phagocyte populations, and are suboptimal in suppressing intragraft CD8+IFNγ+ effector cell accumulation. A broader goal is to investigate the potential of PLG-NPs coupled with either donor antigenic peptides or donor cell lysate derived antigens as a replacement for donor ECDI-SP cell therapy for transplantation tolerance. Our results thus not only form the basis from which investigators can now examine the cellular mechanisms of NP-based transplant tolerance, but will also direct future rational design of polymeric platforms eventually to be used in human transplantation.
2. Materials and Methods
2.1. Mice
8–10-week-old male bm12 (H-2bm12) and C57BL/6 (B6, H-2b) mice were purchased from the Jackson Laboratory and Harlan. ABM (H2b) mice (T cell receptor transgenic mice with CD4 T cells reactive to the I-Ab bm12 epitope) were provided by Dr. Mohammed Javeed I Ansari from the Northwestern University (NU). All mice were housed under specific pathogen free conditions at NU. All protocols were approved by NU IACUC.
2.2. Skin transplantation
The bm12 tail skin grafts were transplanted onto the dorsum of B6 mice on day 0. Following engraftment, rejection was determined when 80% of the graft area was necrotic.
2.3. Donor antigen preparations
Antigenic peptides KSQPEFLEQKRAEL (P1), KLGRPDAEYWNSQPEFLEQKRAELDTVCR (P2) and CSQPEFLEQKRAEL (P3), and P1-Fluorescein isothiocyanate (FITC) were synthesized by Peptide 2.0 (Chantilly, VA). To prepare donor cell lysate, bm12 splenocytes were processed into single cell suspensions and erythrocytes were lysed. Up to 1 × 109 cells were sonicated in PBS at an amplitude of 60% for 10s, followed by 10s on ice for a total of 6 cycles (Cole– Parmer). Total protein was quantified by Coomassie Plus (Bradford) Protein assay (Thermo Fisher Scientific Inc.) prior to coupling to PLG particles.
2.4. PLG particle synthesis
Single emulsion, antigen-free (blank) poly(lactide-co-glycolide) (PLG) particles to be used in surface-coupling experiments were synthesized with poly(ethylene-alt-maleic acid) (PEMA) as a surfactant as previously described (23). Briefly, acid terminated PLG (50% D,L-lactide/50% glycolide) (Sigma Aldrich) was dissolved in dichloromethane (DCM) to make a 20% (w/v) solution. This solution was sonicated (Cole–Parmer) at an amplitude of 100% in 1% w/v PEMA (Polysciences, Inc.) for 30s to create particles. After overnight stirring, particles were collected by centrifugation, washed 3 times with distilled water, and lyophilized overnight.
2.5. Antigenic peptide encapsulation in PLG nanoparticles
Antigen-polymer nanoparticles encapsulating the bm12 peptide were synthesized as described previously with minor modifications (34). Briefly, PLG polymer was dissolved at 2% w/v in dimethylformamide (DMF). ECDI (5× to PLG, mole ratio) was dissolved at 2% w/v in DMF and added dropwise to the PLG solution. N-Hydroxysuccinimide (NHS, 5× to PLG, mole ratio) was dissolved at 1% w/v in DMF and added dropwise to the solution. The reaction was stirred for 15 minutes at room temperature. Antigenic peptide (1.2× to PLG, mole ratio) was dissolved at 2% w/v in DMF and stirred at 400 RPM. Triethylamine (5× to peptide, mole ratio) was added to peptide solution and the mixture was added dropwise to the stirring PLG solution. The reaction was allowed to proceed overnight at room temperature. The resulting polymer was purified by dialysis using 3,500 molecular weight cut-off membrane against distilled water. The dialyzed polymer was collected and washed with MilliQ water three times, and centrifuged at 7000 × g before lyophilization. Coupling efficiency of peptide to PLG was determined by 1H-NMR analysis in DMSO-d6 (Supplementary Figure 1). The NMR endgroup analysis suggested a coupling efficiency of 66.9%.
2.6. Antigenic peptide conjugation to PLG nanoparticles
For carbodiimide conjugation, 10 mg of blank single-emulsion PLG nanoparticles (NPs) were incubated with 30.75 mg of 1-ethyl-3-(3′ dimethylaminopropyl) carbodiimide (ECDI, Calbiochem) and 1 mg of P1, 2 mg of P2 or 1 mg of bm12 lysate in 1 ml of PBS (Life Technologies Inc.) on ice for 1 hour while shaking (Labline Instruments Inc., Melrose Park, IL). For maleimide conjugation, 10 mg of PLG NPs were incubated with 2.74 mg of ECDI and 6.58 mg of N-hydroxysulfosuccinimide (NHS, Sigma-Aldrich) in 1ml of 2-(N-Morpholino) ethanesulfonic acid (Sigma Aldrich) for 15 minutes, followed by washing. The NPs were then incubated with 1.70 mg of N-β-maleimidopropionic acid hydrazide (BMPH, Sigma Aldrich) in 3 ml of PBS for 2 h at room temperature followed by a PBS wash and incubation with 1 mg of P3 in 1 ml of PBS for 5 minutes. To prepare B6 splenocytes coupled with peptides, 3.2 × 108 B6 cells were incubated with 30.75 mg ECDI and 1 mg of P1, 2 mg of P2 or 1 mg of bm12 lysate in 1 ml of PBS on ice for 1 hour on a shaker followed by washing. To prepare donor bm12 ECDI-SP, 3.2 × 108 bm12 splenocytes were incubated with 30.75 mg ECDI in 1 ml PBS on ice for 1 hour on a shaker followed by washing.
2.7. Particle characterization
Particles were imaged with a scanning transmission electron microscope (LEO Gemini 1525) operating at 200 kV. Z-average diameter and surface ζ-potential were obtained using a Zetasizer Nano ZSP (Malvern Instruments Ltd). The release of P1 from NPs was measured over 196 hours. Briefly, 3 mg of NPs were dispersed in 1 mL of PBS with 10% FBS on a rocker at 37°C. At predetermined time points, the NPs were centrifuged at 7000 RPM for 6 minutes and 0.5 mL of supernatant collected. Each time the particles were resuspended, replenished with 0.5 mL PBS with 10% FBS and further incubated until 196 hours were reached. All supernatant samples were stored at −20°C until the end of the experiment. Upon experiment completion, a CBQCA protein quantification assay (Molecular Probes) was used to determine the remaining peptide amount for each supernatant sample. 3 mgs of lyophilized NP formulations were dissolved in DMSO and a CBQCA assay was used to determine the initial amount of loaded peptide before release. Peptide released at each time point was calculated as cumulative peptide release at that time point over the initial peptide loading amount before release (“Percent of Initial Loading”). For fluorescent imaging of NPs with surface conjugated peptide versus encapsulated peptide, P1-FITC was conjugated using the protocol in 2.6 or encapsulated using the protocol in 2.5. Rhodamine dye was encapsulated in NPs to label the NPs. Images were taken at 60× magnification on a confocal microscope and fluorescent channel intensity profiles were calculated (Nikon A1C, Nikon Elements).
2.8. In vivo biodistribution study
Donor bm12 ECDI-SPs were labeled with 2 μM PKH67 (Sigma–Aldrich). Briefly, 2×107 cells were suspended in 1 ml of diluent C mixed with 1 ml of diluent C containing 4 μM of PKH67 for 5 minutes before washing with RPMI 1640 (Gibco) supplemented with 10% FBS (Gibco). Rhodamine B conjugated nanoparticles were fabricated following the single emulsion protocol described in 2.4, with the only modification being that per 100mg of PLG polymers, 0.5 mg of rhodamine B was dissolved simultaneously in DCM. 1×108 PKH67 labelled bm12 ECDI-SPs, 3 mg of blank rhodamine-labeled PLG NPs, or 3 mg of P1-coupled rhodamine-labeled PLG NPs were injected intravenously into B6 mice. At 24 and 96 hours post injection, mice were sacrificed and organs collected, processed into single cell suspensions, and PKH67/rhodamine fluorescence analyzed by flow cytometry (BD FACSCantoII).
2.9. Recipient treatment schemes
PLG NPs coupled with peptides or bm12 cell lysate (3.0 mg per dose), blank PLG NPs (3.0 mg per dose), B6 SPs coupled with peptides (1×108 per dose) or bm12 ECDI-SP (1×108 per dose) were injected i.v. into recipient B6 mice on day −7 and day +1 with day 0 being the day of skin transplant.
2.10. Generation of bone marrow derived macrophages (BMDMs)
The femur and tibia of B6 mice were isolated. Marrow was flushed out with PBS using a 21G needle and subsequently centrifuged. Erythrocytes were lysed and remaining cells were resuspended into single cell suspensions. Bone marrow derived cells were plated in 100 mm petri dishes (Sigma Aldrich) in RPMI-1640 (Gibco) supplemented with 10% fetal bovine serum (Gibco) and 20 ng/ml recombinant mouse macrophage colony-stimulating factor (M-CSF, R&D Systems) for 7 days with a media change on day 3.
2.11. ABM T cell proliferation in vitro
96 well U-shape plates (Fisher Scientific) were pre-coated with 5 μg/ml hamster antimouse CD3e (clone 145–2C1) and 2 μg/ml hamster anti-mouse CD28 (clone 37.51) from BD Biosciences. T cell receptor transgenic (TCR Tg) ABM CD4+ T cells were purified from the spleen of ABM mice using CD4+ cell negative isolation kit (Miltenyi). ABM CD4+ T cells were labeled with 1 μM violet proliferation dye (BD Biosciences). 4×104 B6 BMDMs and 4×104 ABM CD4+ T cells were plated in the precoated plates with the addition of either 30 μg of indicated NP preparations or 1.2×105 indicated SP preparations in RPMI-1640 supplemented with 10% FCS for 3 days. The rationale for using 30μg NP preparations or 1.2×105 SP preparations in this assay was that according to the CBQCA quantification in Figure 1D, these doses would provide approximately the same amount of peptide antigen to the co-cultured BMDMs. Cells were then harvested for determination of ABM CD4+ T cell proliferation by dilution of the violet proliferation dye.
Figure 1. PLG NP characterization and quantification of antigen coupling.
(A) Nanoparticles size (486.5 ± 26.4 nm) and (B) surface Zeta Potential distribution (−40.95 ± 2.66 mV) were obtained using dynamic light scattering (n = 3). (C) Representative scanning Electron Micrograph (SEM) image of PLG NPs used for antigen coupling. (D) Loading amount. After peptide coupling by different methods, the amount of peptide loaded on each platform was determined by CBQCA assay. Measurements were standardized to 1 mg of PLG NPs or 3.3×107 splenocytes (n = 5), as 3 mg of PLG NPs or 1×108 antigen-coupled splenocytes per dose were injected for subsequent in vivo experiments. (E) Loading efficiency. Calculated as the percentage of bound peptide following the conjugation/encapsulation reaction over the input peptide at the beginning of the conjugation/encapsulation reaction. For (A) – (C), data are representative of 3 independent experiments. For (D) and (E), data shown are average from 3 independent experiments.
2.12. Analysis of skin graft infiltrating cells and draining lymph node (DLNs) cells
PLG NPs coupled with P1 (3.0 mg per dose), blank PLG NPs (3.0 mg per dose), or bm12 ECDI-SP (1×108 per dose) were injected i.v. into recipient B6 mice on day −7 and day +1 with day 0 being the day of bm12 skin transplant. At day 8 post-transplant, mice were sacrificed, skin grafts and DLNs were collected, processed into single cell suspensions using collagenase IV (Worthington), and stained for analysis by flow cytometry (BD FACSCantoII). At the time of sacrifice, whole blood was also collected for measurement of levels of critical blood electrolytes on the Bioanalyzer system (Agilent Technologies).
2.13. Luminex liquichip microplate cytokine assays
We measured levels of IFN-ɣ, IL-12p70, IL-1β, and IL-5, GM-CSF, IL-2, IL-6, and TNF-α using a Luminex LiquiChip (BioSource International Inc.) platform, which is a solid phase bead-based immunoassay platform. Cell culture supernatants from the 3-day MLR reactions of various particle and cell formulations and known standards were incubated with cytokine specific beads in a microplate for 90 minutes. The excess beads were then washed away and a biotinylated detection antibody specific for the cytokine analyte was added and incubated for 30 minutes followed by washing. A streptavidin fluorophore specific for the detection antibody was then added and incubated for 30 minutes prior to determining cytokine levels on the Luminex 100™ system.
2.14. Antibodies and flow analysis
Flow cytometry was utilized to determine cell phenotype and cell proliferation. Cells were stained for surface markers using fluorochrome conjugated antibodies for 30 minutes on ice, washed, acquired using FACSCantoII (BD Biosciences) and analyzed using FlowJo v10.4.2 (Tree Star LLC). Antibodies and their specific clones used were: CD3-FITC (17A2, eBioscience), CD3-PerCPCy5.5 (145–2C11, Tonbo Biosciences), CD4-Pacific Blue (RM4–5, eBioscience), CD4-PerCPCy5.5 (RM4–5, Tonbo Biosciences), CD8α-APC-Cy7 (53–6.7, eBioscience), CD8α-eFluor450 (53–6.7, eBioscience), CD11b-PercpCy5.5 (M1/70, Tonbo Biosciences), CD11c-APC-eFluor780 (N418, eBioscience), B220-PECy7 (RA3–6B2, eBioscience), CD44-PECy7(IM7, eBioscience), Foxp3-FITC (FJK-16s, eBioscience), I-Ab-APC(AF6–120.1, eBioscience), interferon-γ-APC (XMG1.2, eBioscience), violet proliferation dye (VPD450, BD Biosciences), and aqua live/dead dye (Molecular Probes).
2.15. Statistics
Statistical analyses were performed using Graphpad Prism (Graphpad, La Jolla, CA). One-way ANOVA with Sidak’s test for multiple comparisons and Student t-test were used to determine significance among data subsets. Log-rank (Mantel-Cox) statistic was used for comparison of skin graft survival in Kaplan Meier curves. All results were displayed as a mean with standard error of the mean, with significance determined by a p-value less than 0.05.
3. Results
Characterization of NPs conjugated or encapsulated with bm12 peptides
Three peptides spanning the bm12 IAb region containing the three point mutations of bm12 were conjugated to PLG NP or B6 SP carriers. The exact amino acid sequences of the peptides are detailed in Materials and Methods. Surface carboxyl groups of PLG NPs or SPs served as the functional site for peptide binding. Peptide 1 (P1) is a 14-amino acid (AA) peptide containing the bm12/B6 mismatched epitopes and a lysine at the N-terminus which was used as the functional group to bind to surface carboxyl groups of NPs (NP-ECDI-P1) or SPs (SP-ECDI-P1) via ECDI coupling. Peptide 2 (P2) is a longer 29-AA peptide also containing the bm12/B6 mismatched epitopes and a lysine at the N-terminus for ECDI-mediated coupling to NPs (NP-ECDIP2) or SPs (SP-ECDI-P2). Peptide 3 (P3) is a 14-AA peptide similar to P1 with the exception of a cysteine residue at the N-terminus substituting the lysine residue in P1. The cysteine thiol side chain of P3 now allows BMPH crosslinking of the peptide to NPs (NP-BMPH-P3). P1 and P2 allowed comparison of peptide length on graft survival. P1 and P3 allowed comparison of lysine-derived amine-mediated conjugation versus cysteine-derived thiol-mediated conjugation on graft survival. We further manufactured NPs “encapsulating” P1. The encapsulation method we used was based on a novel bioconjugation process previously described by us, with first conjugating P1 peptide to PLG in the solution followed by subsequently formulation of NPs, enabling controllable loadings with reduced surface display of the antigen relative to other formulations (34). Specifically, this method of encapsulation has been shown to reduce antigen burst release in comparison to traditional oil-in-water encapsulation techniques while limiting surface exposure of the antigen which may alter particle physicochemical properties (34). From here onward, we referred to this NP formulation as “NP-Encap-P1”. This formulation allowed comparison between such encapsulated peptide (“NP-Encap-P1”) and surface-conjugated peptide (NP-ECDI-P1) on graft survival.
Size (Figure 1A) and surface zeta potential (Figure 1B) of all four NP formulations (NP-ECDI-P1, NP-ECDI-P2, NP-BMPH-P3 and NP-Encap-P1) were measured by dynamic light scattering and zetasizer, respectively. All NP formulations followed a tight size distribution with an average diameter of 486.5 ± 26.4 nm and highly negative zeta potential of −40.95 ± 2.66 mV. Highly anionic NPs with diameters of approximately 500 nm have been previously suggested to be important for immune tolerance (25). Size and morphology of the PLG NPs were confirmed by scanning electron microscopy (SEM) displaying a homogenous distribution (representative image is shown in Figure 1C, and detailed images of all four formulations are provided in Supplementary Figure 2). Stability of nanoparticle preparations was demonstrated by minimal-to-no aggregation in PBS in the time between resuspension of the formulations and their subsequent i.v. injection (Supplementary Figure 3). A CBQCA assay was used to quantify the peptide coupled/loaded on various formulations of NPs, and compared to that coupled to SPs. As shown in Figure 1D, on a per dose bases rationalized and used in our studies below (i.e., 3mg of NP preparations and 1×108 cells of SP preparations), NPs carried a significantly lower amount of peptides than their SP counterparts (~75–150 μg/3mg NP vs. ~450 μg/108 SP). Similarly, the loading efficiency (calculated as the percentage of bound peptide following the conjugation/encapsulation reaction over the input peptide at the beginning of the conjugation/encapsulation reaction) was also significantly lower for NP than SP carriers (Figure 1E).
Peptide length and skin allograft survival
We first examined the effect of length of peptide conjugated to the NPs on skin allograft survival. Peptides P1 and P2 were conjugated to NPs (NP-ECDI-P1, NP-ECDI-P2) using ECDI chemistry, and injected to B6 recipients on day −7 and +1 at 3mg per dose, with day 0 being the date of bm12 skin transplantation. For comparison, the same peptides were similarly conjugated to B6 splenocytes (SP-ECDI-P1, SP-ECDI-P2), injected on day −7 and +1 at 1×108 cells per dose, and transplanted with bm12 skin allografts on day 0. The doses chosen for our studies (3mg NP or 1×108 cells per dose) were based on safety profile and efficacy of tolerance from our previous studies of PLG NP and SP administrations (11, 21). Skin allograft survival was assessed over a period of 90 days. Images of skin allografts at different stages of rejection are provided in Supplementary Figure 4, with rejection defined as greater than or equal to 80% graft area being necrotic. It is reported in the literature that while peptide processing for MHC class II presentation may vary, they are usually loaded at a length of 13–17 AAs (35, 36). Our synthesized peptides represent both the common peptide presentation length (i.e. the 14-AA P1), as well as a much longer peptide (i.e. the 29-AA P2). Treatment with blank NP (NP-blank) was used as a negative control. Treatment with the “goldstandard” donor ECDI-SP (i.e. bm12 ECDI-SP) according to our published data (13) was used as a positive control. As expected, bm12 ECDI-SP provided indefinite skin allograft protection in 100% of the recipients (Figure 2). Remarkably, regardless of the carrier (NPs or SPs), preemptive P1 treatment (NP-ECDI-P1 or SP-ECDI-P1) partially recapitulated the graft protective effect of bm12 ECDI-SP, resulting in a significantly delayed graft rejection kinetics and a prolonged graft survival in comparison to the negative control (p<0.001). Furthermore, 20–35% of the grafts in both groups survived beyond 90 days post transplantation (Figure 2). Interestingly, the graft survival was not significantly different between the SP-ECDI-P1 and the NP-ECDI-P1 groups (Figure 2) despite that 1×108 SP-ECDI-P1 carried a significantly higher amount of the P1 peptide than 3mg of NP-ECDI-P1 (Figure 1D). These findings suggest that at the doses chosen, the amount of the peptide antigen delivered was no longer a determining factor for the degree of graft protective effect evoked by the two different delivery platforms. In contrast, regardless of the carrier, preemptive P2 treatment (NP-ECDI-P2 or SP-ECDIP2) did not have any demonstrable graft protective effect in comparison to the negative control (median graft survival was 12 days and 13 days for NP-ECDI-P2 and SP-ECDIP2, respectively; median graft survival was 10 days for NP-blank), and all grafts were rejected by 20 days post transplantation (Figure 2).
Figure 2. The effect of peptide length on skin allograft survival.
Indicated preparations of NPs (3mg/dose) or SPs (1×108/dose) were injected (i.v.) on day −7 and +1 to mice receiving tail to back bm12 to B6 skin transplantation on day 0. Skin grafts were monitored and rejection was determined when 80% of the graft area was necrotic. Statistical significance was determined by Log-rank (Mantel-Cox) tests. ***p<0.001, ****p<0.0001.
Collectively, our in vivo studies demonstrate that the shorter P1 peptide provides better graft protection than the longer P2 peptide. Furthermore, we demonstrate that despite a significant difference in the amount of peptide delivered, NP and SP carriers provide comparable graft protections.
Biodistribution of NP-ECDI-P1
We next examined the biodistribution pattern of NP-ECDI-P1 that demonstrated superior graft protecting efficacy in the study described above. Rhodamine-labeled NP-ECDI-P1 were injected i.v. to B6 mice. 24 or 96 hours later, mice were sacrificed and the spleen, peripheral lymph nodes (LNs), and the lungs were collected and examined. As shown in Supplementary Figure 5, at 24 hours post injection, the injected NP-ECDIP1 (marked by rhodamine positivity) were most prominently visualized in the spleen, followed by the lungs and peripheral LNs. At 96 hours post injection, rhodamine positivity significantly diminished in all organs examined, demonstrating efficient NP clearance at this later time point.
As the spleen has been shown to be an important organ for tolerance (37, 38) and was the predominant site of NP entrapment in our study (Supplementary Figure 5), we next examined the splenic cell populations that interacted with NP-ECDI-P1. For comparisons, rhodamine-labeled blank NPs (“NP-blank”) and PKH67-labeled bm12 ECDI-SP were similarly injected and examined (11). We focused on splenic phagocytes that have engulfed the NP-ECDI-P1 (i.e. cells double positive for the recipient (B6) MHC class II molecule I-Ab and rhodamine, namely I-Ab+rhodamine+ cells) (37, 39), and compared to those engulfing NP-blank (I-Ab+rhodamine+ cells) or bm12 ECDI-SP (I-Ab+PKH67+ cells). As shown in Figure 3A (representative dot plots) and 3B (summary bar graphs), we found that in comparison to bm12 ECDI-SP, NP-blank and NP-ECDI-P1 were up-taken by a significantly smaller percentage of CD11c+CD8α+ and CD11c+B220+ dendritic cells (DCs). The NP formulations also showed a trend of being up-taken by a smaller percentage of CD11c+CD11b+ conventional DCs compared to bm12 ECDI-SPs. CD11c+CD8α+ DCs have been shown to be the predominant antigen presenting cells (APCs) implicated in antigen cross presentation, while CD11c+B220+ DCs have been implicated in mediating oral tolerance and inducing T cell anergy (40, 41). In contrast to DCs, a comparable percentage of macrophages in the spleen (enumerated as CD11c−CD11b+ cells in Figure 3A) phagocyted NP-blank, NP-ECDI-P1 or bm12 ECDI-SP (Figure 3B). Consequently, NP formulations were relatively more avidly internalized by splenic macrophages, whereas bm12 ECDI-SP were relatively more avidly internalized by splenic DC subpopulations. When the total number of cells of various splenic DC and macrophage populations uptaking NP vs. bm12 ECDI-SP were compared, we again observed a predominance of splenic macrophages uptaking NPs but not bm12 ECDI-SP (Supplementary Figure 6). Of note, our studies also revealed that splenic B cells (B220+CD11c− cells) were a predominant splenic cell population uptaking both NPs and bm12 ECDI-SP, although no discernable differences between the groups could be observed (data not shown).
Figure 3. Biodistribution of NP-blank, NP-ECDI-P1, or bm12 ECDI-SP in the spleen 24 hours post i.v. injection.
(A) MHC I-Ab and Rhodamine/PKH67 double positive cells in the spleen of injected mice were gated out using the spleen from un-injected mice as a negative control (left dot plots). IAb+Rhodamine/PKH67+ cells were further analyzed for CD11c, CD8α, B220 and CD11b expressions (right dot plots). Plots shown are representative of 3 mice in each group. (B) Percentages of each splenic cell population uptaking NP-blank, NP-ECDI-P1 or bm12 ECDI-SP of total IAb+Rhodamine/PKH67+ cells. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons test. *p<0.05, **p<0.01. Data shown were averaged from 3 mice in each group.
Collectively, our data demonstrate that NPs were predominantly phagocytosed by splenic macrophages, whereas bm12 ECDI-SP were preferentially phagocytosed by splenic DCs, particularly DC subpopulations implicated in mediating immune tolerance. Such differences between the biodistribution of NP-ECDI-P1 and bm12 ECDI-SP may partially contribute to the difference in their efficacy in graft protection observed in Figure 2.
Graft-infiltrating cells in NP-ECDI-P1 treated recipients
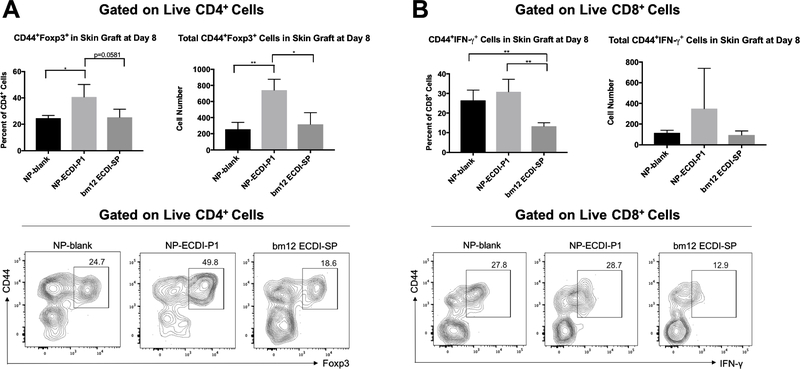
We next examined graft-infiltrating cells in skin allografts from NP-ECDI-P1 treated recipients. For comparison, grafts from NP-blank or bm12 ECDI-SP treated recipients were also analyzed. Skin grafts were harvested on day 8 post-transplant, digested with collagenase and examined by flow cytometry for antigen-experienced (CD44+) regulatory or effector T cell infiltrations. As shown in Figure 4A, compared to grafts from the other two groups, grafts from NP-ECDI-P1 treated recipients harbored a significantly higher total number of antigen-experienced CD4+CD44+Foxp3+ regulatory T cells (Tregs). Such antigen-experienced CD44+ Tregs have been shown to possess greater suppressive function than antigen-inexperienced CD44− Tregs (42). Interestingly, when graft-infiltrating CD8+CD44+IFN-γ+ effector T cells were examined, a different pattern emerged: grafts from both NP-ECDI-P1 and NP-blank treated recipients harbored a significantly higher percentage of CD8+CD44+IFN-γ+ cells compared to those from bm12 ECDI-SP treated recipients (Figure 4B), although the difference in total number of such graft-infiltrating CD8+CD44+IFN-γ+ cells did not reach statistical significance. Of note, there were no significant differences in CD4+CD44+Foxp3+ Tregs or CD8+CD44+IFN-γ+ cells in the draining lymph nodes (DLNs) across the three groups (Supplementary Figure 7). Collectively, these data demonstrate that NP-ECDI-P1 treatment results in an increase of graft-infiltrating CD4+CD44+Foxp3+ Tregs, but is not able to effectively suppress graft-infiltrating CD8+CD44+IFN-γ+ effector T cells. These changes combined ultimately result in only a moderate partial graft protection conferred by NP-ECDI-P1 treatment.
Figure 4. Graft-infiltrating CD4+CD44+Foxp3+ and CD8+CD44+IFN-γ+ cells in skin allografts from recipient mice treated with NP-blank, NP-ECDI-P1 or bm12 ECDI-SP.
(A) Left bar graph: Percentage of graft-infiltrating CD44+Foxp3+ cells among graft-infiltrating CD4+ cells; Right bar graph: Total number of CD4+CD44+Foxp3+ cells in skin allografts. Representative contour plots show gating for CD44+Foxp3+ cells in skin allografts from recipients of each treatment group. (B) Left bar graph: Percentage of graft-infiltrating CD44+IFN-γ+ cells among graft-infiltrating CD8+ cells; Right bar graph: Total number of CD8+ CD44+IFN-γ+ cells in skin allografts. Representative contour plots show gating for CD44+IFN-γ+ cells in skin allografts from recipients of each treatment group. For (A) and (B), skin allografts were harvested and examined on day 8 post transplantation. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons test. *p<0.05, **p<0.01. Data is representative or averaged from 3 mice per group.
To rule out obvious chemical toxicities from NP injections, Bioanalyzer testing of whole blood for common electrolytes was performed at the time of recipient sacrifice, and showed normal values across all three treatment groups (Supplementary Figure 8).
Bm12-specific T cell proliferation and cytokine production
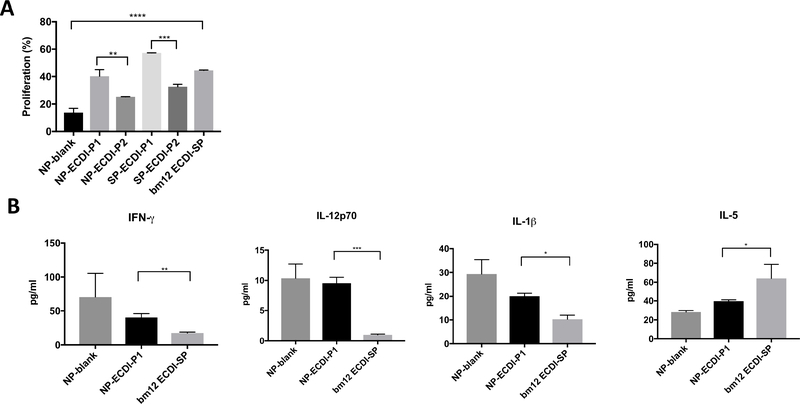
The varied efficacy between P1 and P2 formulations observed in Figure 2 may be due to disparities in their ability to deliver bm12 antigen to APCs for processing and presentation to stimulate bm12-specific T cell proliferation. To investigate this possibility, we took advantage of the CD4+ T cells from ABM mice which carry a transgenically-expressed T cell receptor (TCR) that responds to the mutated bm12 IAb and set up in vitro T cell proliferation assay. In this in vitro assay, we used B6 bone marrow derived macrophages (BMDMs) as APCs, rather than bone marrow derived dendritic cells (BMDCs), because our biodistribution data from Figure 3 showed that NPs were significantly more proficiently phagocytosed by splenic macrophages than by splenic DCs. We co-cultured VPD450-labeled ABM CD4+ T cells with B6 BMDMs pulsed with various NP and SP formulations of P1 and P2 as the source of bm12 antigen as detailed in Materials and Methods. After 3 days of co-cultures, we measured ABM CD4+ T cell proliferation by VPD450 dilution. As shown in Figure 5A, both P1 formulations (NP-ECDI-P1 and SP-ECDI-P1) elicited a more robust ABM CD4+ T cell proliferative response in comparison to the P2 formulations (NP-ECDI-P2 and SP-ECDIP2), consistent with P1 treatment providing superior graft protection than P2 treatments (Figure 2). However, bm12 ECDI-SP elicited an ABM CD4+ T cell proliferative response here intermediate to those seen with the P1 or P2 formulations (Figure 5A), and yet provided the best bm12 skin allograft protection (Figure 2), suggesting that other aspects of tolerogenic delivery (for instance, the type of phagocytes engaged as demonstrated in Figure 3) in addition to antigen processing and presentation by macrophages also play a role in the ultimate tolerance efficacy of these formulations. Indeed, as shown in Supplementary Figure 9, when BMDCs pulsed with for bm12 ECDI-SP were used as APCs, a more rigorous ABM T cell proliferation was observed in comparison to when BMDMs were used as APCs. In contrast, for NP-ECDI-P1, BMDMs as APCs stimulated more rigorous ABM T cell proliferation than BMDCs as APCs. These findings are therefore consistent with the observed differences in bm12 ECDI-SP and NP-ECDI-P1 biodistribution in splenic phagocytes in Figure 3.
Figure 5. In vitro bm12-specific CD4 T cell proliferation and cytokine production in response to stimulation by APCs pulsed with different bm12 antigen sources.
(A) ABM CD4+ T cell proliferation. ABM CD4+ T cells were co-cultured with B6 BMDMs pulsed with various NP and SP formulations of P1 and P2 as the source of bm12 antigen. % proliferation of ABM CD4+ T cells was determined by % of ABM CD4+ T cells with VPD450 fluorescence lower than that of undivided ABM CD4+ T cells. (B) Cytokine production from ABM CD4+ T cells co-cultured with B6 BMDMs pulsed with NP-blank, NP-ECDI-P1 or bm12 ECDI-SP. IFN-γ, IL-12p70, IL-1β, and IL-5 levels were determined by Luminex liquichip microplate reader. For both (A) and (B), statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons test or student’s t-test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Data is averaged from n=2 biological replicates per group.
We next determined cytokine release of ABM CD4+ T cells in the above cocultures. We focused on conditions in which B6 BMDMs were pulsed with either NPECDI-P1 or bm12 ECDI-SP, and compared to the NP-blank pulsed condition. Culture media following the 3-day ABM CD4+ T cell proliferation assays shown in Figure 5A was collected and examined for cytokine production by Luminex liquichip microplate reader. As shown in Figure 5B, NP-ECDI-P1 and bm12 ECDI-SP conditions exhibited distinct cytokine production patterns: in comparison to co-culturing with bm12 ECDI-SP, coculturing with NP-ECDI-P1 resulted in significantly higher levels of IFN-γ, IL-12p70, and IL-1β; but a decreased level of IL-5. Additional cytokines tested included GM-CSF, IL-2, IL-6 and TFN-α, where no difference was observed among different groups (data not shown). These differences in in vitro cytokine production (Figure 5B), together with differences in in vivo targeting of splenic phagocytes (Figure 3) as well as graft-infiltrating cells (Figure 4), are consistent with only a partial graft protection by NP-ECDI-P1 in comparison to that by bm12 ECDI-SP (Figure 2). More importantly, these findings will now guide future NP engineering endeavors to recapitulate similar patterns seen with bm12 ECDI-SP as a goal for further enhancing the tolerance efficacy of NP formulations.
Peptide conjugation method and skin allograft survival
Following determination that a shorter peptide afforded better graft protection, we next examined the efficacy of two commonly used peptide-NP conjugation schemes on graft protection. Scheme 1 utilized ECDI to conjugate the carboxyl groups on PLG NPs to the primary amine of the N-terminus lysine residue of peptide P1 (NP-ECDI-P1). Scheme 2 utilized BMPH as a linker to form a thioether bond between PLG NPs and the N-terminus cysteine residue of peptide P3 (NP-BMPH-P3). Except for a lysine residue at the N-terminus of P1 compared to a cysteine residue at the N-terminus of P3, these two peptides were otherwise identical (Figure 1D). Our rationale for antigen conjugation was that it has been previously demonstrated that antigens conjugated to particles exhibit significantly slower release relative to antigens absorbed to particles (43, 44). We therefore used antigen conjugation to ensure that our antigen payload was not released prematurely prior to their successful delivery to the intended target organ (e.g, the spleen as shown in Figure 3).
Following the same treatment schedule for NP-ECDI-P1, NP-BMPH-P3 was injected to B6 recipients on day −7 and +1 (relative to day 0 of bm12 skin transplantation), and skin graft survival was monitored over a period of 90 days. As shown in Figure 6A, NP-BMPH-P3 treatment showed a trend towards a slightly worse graft outcome than NP-ECDI-P1 treatment, with a median graft survival of 23 days for NP-BMPH-P3 and 36 days for NP-ECDI-P1, although this difference did not reach statistical significance (p=0.169). We next performed a similar T cell proliferation assay using ABM CD4+ T cells and B6 BMDMs pulsed with NP-ECDI-P1 or NP-BMPH-P3 as the source of bm12 antigen. As shown in Figure 6B, ABM CD4+ T cells demonstrated a significantly lower degree of T cell proliferation when incubated with NP-BMPH-P3 compared to NP-ECDI-P1. Collectively, our data demonstrate that compared to lysine-based ECDI conjugation of bm12 peptide to NPs, cysteine-based BMPH conjugation is associated with less efficient antigenic peptide processing and presentation by the interacting APCs, and has a trend towards an inferior skin allograft protective effect.
Figure 6. The effect of peptide conjugation chemistry on skin allograft survival.
(A) Indicated preparations of NPs (3mg/dose) were injected (i.v.) on day −7 and +1 to mice receiving tail to back bm12 to B6 skin transplantation on day 0. Skin grafts were monitored and rejection was determined as in Figure 2. Similarly, the bm12 ECDI-SP treated group served as a positive control whereas the NP-blank treated group served as a negative control. Statistical significance was determined by Log-rank (Mantel-Cox) tests. (B) ABM CD4+ T cell proliferation. ABM CD4+ T cells were co-cultured with B6 BMDMs pulsed with either NP-ECDI-P1 or NP-BMPH-P3 as the source of bm12 antigen. % proliferation of ABM CD4+ T cells was similarly determined as in Figure 5. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons tests. Data is averaged from n=2 biological replicates per group. For both (A) and (B), *p<0.05, ***p<0.001, ****p<0.0001, ns = no significance.
Antigen incorporation method and skin allograft survival
We next examined whether antigenic peptide incorporation into NPs by surface conjugation or by encapsulation would influence skin allograft survival. Surface conjugation allows a covalent linkage between the antigenic peptide and the carrier particle, therefore reduces peptide disassociation from its carrier, but may increase risks of particle agglomeration and undesired immune responses. On the other hand, peptide encapsulation reduces these risks, and our novel bioconjugate encapsulation approach further serves to avoid a burst release of the peptide cargo typically observed with encapsulated peptides (34). The most effective surface-conjugated NP formulation, NP-ECDI-P1, was compared with NPs encapsulating the same peptide (NP-Encap-P1). Although a similar peptide loading was achieved on both NP-ECDI-P1 and NP-Encap-P1 (Figure 1D, 1E), our peptide release study showed that NP-Encap-P1 exhibited a slightly decreased peptide release over time in comparison to NP-ECDI-P1 (Figure 7A). Confocal microscopy of rhodamine-labelled NPs either surface-conjugated or encapsulated with FITC-labeled P1 peptide confirmed their respective peptide localization, namely surface peptide localization on NP-ECDI-P1 versus internal peptide localization within NP-Encap-P1 (Figure 7B). Consistent with confocal images, a representative fluorescence intensity profile across a singular NP-ECDP-P1 showed two FITC peaks at the edges of a broader rhodamine peak demonstrating surface location of the peptide; whereas a representative fluorescence intensity profile across a singular NP-Encap-P1 showed a single FITC peak within the bounds of rhodamine demonstrating internal location of the peptide (Figure 7C).
Figure 7. Surface conjugation or encapsulation of P1 peptide provides equivalent skin allograft survival.
(A) Percent of peptide release over 196 hours of NP-ECDI-P1 and NP-Encap-P1, as described in Materials and Methods. Note that the error bars are smaller than the size of symbols for the NP-Encap-P1 data points. (B) Confocal images of a single representative NP-ECDI-P1 particle and a single representative NP-Encap-P1 particle. Magnification: ×60. (C) Fluorescence intensity profiles calculated across the gray arrow in (B) of a single representative NP-ECDI-P1 particle and a single representative NP-Encap-P1 particle. (D) Indicated preparations of NPs (3mg/dose) were injected (i.v.) on day −7 and +1 to mice receiving tail to back bm12 to B6 skin transplantation on day 0. Skin graft monitoring, determination of rejection, positive and negative controls were similar to Figure 2. Statistical significance was determined by Log-rank (Mantel-Cox) tests. (E) ABM CD4+ T cell proliferation. ABM CD4+ T cells were co-cultured with B6 BMDMs pulsed with either NP-ECDI-P1 or NP-Encap-P1 as the source of bm12 antigen. % proliferation of ABM CD4+ T cells was similarly determined as in Figure 5. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons tests. Data is averaged from n=2 biological replicates per group. For both (D) and (E), ***p<0.001, ****p<0.0001, ns = no significance.
Next, NP-Encap-P1 was injected on day −7 and +1, relative to day 0 of bm12 skin transplantation. As shown in Figure 7D, NP-Encap-P1 and NP-ECDI-P1 demonstrated completely overlapping skin allograft survival, with a median graft survival time of 42.5 days for NP-Encap-P1 and 36 days for NP-ECDI-P1. We next performed a similar T cell proliferation assay using ABM CD4+ T cells and B6 BMDMs pulsed with NP-ECDI-P1 or NP-Encap-P1 as the source of bm12 antigen. As shown in Figure 7E, ABM CD4+ T cells demonstrated equivalent T cell proliferation when incubated with either NP-ECDI-P1 or NP-Encap-P1. Collectively, our data demonstrate that there is no significant difference between surface conjugation (NP-ECDI-P1) and internal encapsulation (NP-Encap-P1) as the delivery method for the antigenic bm12 peptide in terms of skin allograft protection or stimulating AMB CD4+ T cell proliferation.
Whole donor cell lysate containing antigenic epitopes for tolerance delivery
In the bm12 to B6 skin transplant model, the antigenic epitope is well defined as described above. Therefore, both bm12 whole donor cell lysate as well as bm12 peptides used in our experiments above should contain the bm12 antigen necessary for antigen-specific tolerance. To test if the lysate and peptide can function similarly or if additional proteins or molecules in the whole donor cell lysate other than the bm12 antigen also contribute to skin allograft protection, we compared the skin allograft protection provided by bm12 whole donor cell lysate to that by P1. Bm12 splenocyte lysate was generated by sonication of bm12 splenocytes, and coupled to NPs or B6 SPs via ECDI (NP-ECDI-lysate or SP-ECDI-lysate) as described in Materials and Methods. NP-ECDI-lysate or SP-ECDI-lysate were injected on day −7 and +1 relative to day 0 of bm12 skin transplantation. Comparison was made to recipients treated with NP-ECDI-P1. As shown in Figure 8A, no significant difference in skin allograft survival was observed between either of the lysate formulations and NP-ECDI-P1. Similarly, coculture of ABM CD4+ T cells and B6 BMDMs pulsed with either lysate formulations or NP-ECDI-P1 yielded similar proliferation rates (Figure 8B). Collectively, these data suggest that when the donor antigenic epitopes are clearly defined, peptides containing the epitope(s) can provide equal donor graft protection as whole donor cell lysate in this skin transplant model. Moreover, together with data shown in Figure 2, these results again support the notion that NP carriers are equally efficacious at providing graft protection as SP carriers, thus further validating the use of NPs as a more clinically translatable tolerogenic platform.
Figure 8. The antigenic peptide P1 provides comparable skin allograft protection as donor cell lysate.
(A) Indicated preparations of NPs (3mg/dose) or SPs (1×108/dose) were injected (i.v.) on day −7 and +1 to mice receiving tail to back bm12 to B6 skin transplantation on day 0. Skin grafts were monitored and rejection was determined as in Figure 2. Statistical significance was determined by Log-rank (MantelCox) tests. (B) ABM CD4+ T cell proliferation. ABM CD4+ T cells were co-cultured with B6 BMDMs pulsed with either NP-ECDI-lysate or SP-ECDI-lysate as the source of bm12 antigen. % proliferation of ABM CD4+ T cells was similarly determined as in Figure 5. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons tests. Data is averaged of n=3 biological replicates per group. For (A) and (B), ns = no significance.
4. Discussion
Our results demonstrate that polymeric PLG NPs engineered to deliver relevant donor antigens can significantly delay allograft rejection in a murine model of skin transplantation. The significant protection afforded by donor antigen-coupled PLG NPs in this transplant model advances the notion that highly anionic PLG NPs with approximately 500 nm diameter in size indeed provide a robust platform for immune engineering for transplantation tolerance as previously demonstrated for autoimmunity and allergy (45, 46). This platform is particularly promising and clinically translatable given the well-accepted safety profile of PLG (19).
Importantly, in the current study we have interrogated several commonly modifiable engineering parameters relevant to immune tolerance, including antigenic peptide length, chemistry of surface peptide conjugation, and antigen encapsulation versus surface conjugation. A shorter peptide length (P1) resulted in enhanced graft protection despite delivering an equimolar quantity of the relevant antigen as the longer peptide (P2) (Figure 2). This result is consistent with previous work demonstrating that the usual length of peptides loaded and presented by MHC class II is between 13–17 amino acids (35). We hypothesize that this size preference exhibited in MHC II loading of peptides may be a major contributing factor to the improved efficacy of P1 over P2 as it is possible that P1 is capable of successful loading with fewer requisite processing steps(47, 48). It is important to note, however, that numerous other factors including proteolytic susceptibility of the peptide, degree of peptide folding, and MHC groove geometry play critical roles in the presentation of peptides and were likely also contributing to the relative efficacy of each peptide (47, 49).
To address if antigen accessibility could contribute to graft protection, both amide and thioether conjugation were attempted to represent relatively labile and non-labile conjugations (50). Interestingly, amide conjugation resulted in a slightly enhanced median graft survival (Figure 6), suggesting that peptide clipping and subsequent MHC loading may be affected by the nature of the peptide bond to the polymer. This trend may merit consideration in studies that utilize full length proteins for tolerance, as the amine group of lysine residues within a protein are likely more readily available to participate in conjugation reactions compared with the thiol group of cysteine residues which often form disulfide bonds and are less-readily accessible for reactions (51). In addition, numerous follow-up studies are currently being conducted to: (1) interrogate the specific intracellular fate of these particles and (2) tease out the more subtle differences in the efficacies of P1 and P3 formulations in order to identify mechanisms by which these particles differentially exert their graft-protective effects.
Antigen surface conjugation can result in increased agglomeration, antigen denaturation, and increased particle size; whereas traditional antigen encapsulation can result in antigen loss due to PLG bulk hydrolysis and antigen burst release (52, 53). For our study, we utilized a novel bioconjugate encapsulation method of pre-conjugating the peptide to PLG prior to formation of nanospheres to eliminate the problem of antigen burst release (52). Our comparison of surface conjugation and encapsulation revealed similar graft protection (Figure 7), suggesting that neither surface conjugation nor encapsulation significantly impair antigen processing or presentation for the purpose of tolerizing T cells. Our study further demonstrated a similar initial peptide loading for NP-ECDI-P1 and NP-Encap-P1 (Figure 1D and 1E), but a slightly delayed peptide release over time from NP-Encap-P1 compared to NP-ECDI-P1 (Figure 7A). However, equivalent graft survival was observed in recipients treated with either NP-Encap-P1 or NP-ECDI-P1 (Figure 7D), suggests that the slightly greater peptide release from NP-ECDI-P1 did not impact graft protection by PLG-NP carriers.
In order to investigate whether the nature of the carrier platform affected tolerance efficacy, we compared NPs with SPs as carriers for antigens. We found that there was not a significant difference in graft protection provided by NP-ECDI-P1 and SP-ECDI-P1 (Figure 2), or by NP-ECDI-lysate and SP-ECDI-lysate (Figure 8). These findings indicate that carrier platforms for tolerance induction can be versatile. Therefore, it is feasible to replace cell-based carriers with the more clinically translatable polymeric carriers for delivering tolerogenic antigens.
Donor cell lysate is an attractive antigen source since it contains the entire repertoire of donor MHC class I and II proteins relevant to allogeneic immune responses (14). However, preparation of donor cell lysate requires donor cell procurement and processing, which can be cumbersome. On the other hand, epitope-containing peptides can be easily manufactured, although their processing and presentation by recipient APCs may differ from that of natural proteins present in the lysate. Our previous work utilizing donor cell lysate coupled to PLG NPs in a full MHC-mismatched allogeneic islet transplantation model demonstrated a partial islet allograft protection via modulating indirect allo-specific T cell responses (21). Here, the use of bm12 cell lysate in this skin transplant model did not result in superior graft protection compared to that conferred by the epitope-containing peptide P1 (Figure 8). This result is likely due to bm12 epitope being well-defined and entirely encompassed within P1. One important set of subsequent studies is to thoroughly compare dose responses of donor lysate and P1, since an equal weight of lysate undoubtedly contains a much lower dose of immunologically-relevant antigen compared to the same weight of purified, epitope-containing peptide P1. Nonetheless, these data suggest that when antigenic epitopes are known, it is possible to replace total donor cell lysate with a high dose of epitope-containing peptides. Translating this concept to clinical transplantation will require consideration of the diverse disparities between donor and recipients MHCs and their often less well-defined epitopes (54, 55). However, it is also possible that only certain selective dominant epitopes will need to tolerized to sufficiently establish transplantation tolerance (56, 57). Our current study has provided support for continued development of this novel technology for application in transplantation tolerance as our understanding of relevant immunodominant epitopes in donor/recipient pairings improves.
One potential cellular mechanism contributing to the partial protection seen by NP-ECDI-P1 treatment is the expansion and/or accumulation of antigen experienced Tregs at the graft site (Figure 4A). Tregs are well known to play a crucial role in the induction and maintenance of immune tolerance, and increased intragraft Foxp3 expression has been correlated with better graft outcomes (58, 59). Previous studies in islet transplant models have demonstrated an ability of donor ECDI-SP to expand Tregs both in secondary lymphoid organs and at the graft site, which serves as one crucial mechanism for promoting long-term graft tolerance (11). Importantly, antigen conjugated PLG-NPs have also been shown to induce and activate Tregs in murine models of EAE and allergy (22, 60). More specifically, it has been reported that antigen-experienced CD44+ Tregs, in comparison to antigen-naïve CD44− Tregs, are more proliferative, produce a higher amount of IL-10 and are more potent suppressors of effector T cell populations(42). Our data in the current study suggest that antigen-experienced CD44+ Treg expansion and/or accumulation at the graft site is an important mechanism in mediating graft protection provided by antigen conjugated NPs, thus identifying a subset of regulatory T cells to target for future NP engineering for enhancing antigen-specific tolerance. It has been reported in the literature that regulatory T cells employ diverse mechanisms of suppression(61), including production of soluble factors such as IL-10 (62) and TGF-β (13), conversion of extracellular ATP to adenosine via CD39 and CD73, IL-2 consumption via CD25, and downregulation of CD80 and CD86 via CTLA-4, among others. Elucidating the mechanism(s) of action by Tregs by NP-ECDI-P1 will be beyond the scope of our current study.
However, despite delivery of a high dose of epitope-containing peptides, the best performing NP formulation, namely NP-ECDI-P1, did not recapitulate the complete tolerance efficacy seen with bm12 ECDI-SPs (Figure 2). Therefore, in the current study we focused on comparing and contrasting NP-ECDI-P1 and bm12 ECDI-SPs, with an intended goal of providing a platform for future rational design of NP formulations for transplantation tolerance. To this end, our data revealed that skin allografts from NP-ECDI-P1 treated recipients harbored an increased percentage of CD8+CD44+IFN-γ+ cells compared to those from bm12 ECDI-SP treated recipients (Figure 4B). This suggests that antigen conjugated NPs are less capable of effectively attenuating IFN-γ-producing effector CD8 T cells crucial to both acute and chronic rejection (31, 63). A local interferon-rich environment may further increase CD8+ T cell trafficking to promote graft rejection(64). Consistent with this in vivo observation, we also detected a dramatically different cytokine production pattern in vitro by ABM T cells co-cultured with APCs pulsed with either NP-ECDI-P1 or bm12 ECDI-SP. Bm12 ECDI-SP promoted Th2 cytokine IL-5 but exhibited a significantly lower production of proinflammatory cytokines IFN-γ, IL-12p70 and IL-1β; on the other hand, NP-ECDI-P1 promoted the production of such proinflammatory cytokines (Figure 5). It has been reported that PLG platforms can induce proinflammatory cytokines IL-12, IL-1β and TNF-α (65, 66), which in turn impair Treg function and promote allograft rejection (67, 68). The observed cytokine response by NP-ECDI-P1 could be one contributing factor to the suboptimal graft outcome by NP-ECDI-P1 treatment in comparison to bm12 ECDI-SP treatment. Furthermore, our in vivo biodistribution experiment also demonstrated that bm12 ECDISP were more preferentially phagocytosed by splenic DC subsets, whereas NP-ECDIP1 were predominantly phagocytosed by splenic macrophages (Figure 3). Splenic CD8α+ DCs are a crucial cell population for cross-presentation that can modulate allo-specific cytotoxic T cells, whereas CD11c+B220+ DCs have been implicated in mediating oral tolerance and inducing T cell anergy (69). The ability of bm12 ECDI-SPs to preferentially target these DC populations could be a possible tolerogenic event not recapitulated by NP-ECDI-P1. Thus, mirroring the bm12 ECDI-SP cytokine response and biodistribution may be used in the future as a screening and validating tool for further developing and optimizing antigen conjugated NP formulations for immune tolerance.
Peptide conjugated NP formulations exclusively target T cells with indirect allo-specificities where donor MHC peptides are presented by recipient APCs to stimulate recipient T cells (70). We have previously shown that in addition to depleting recipient T cells of indirect allo-specificities such as seen with NP formulations (70), donor ECDI-SP further retain the ability to anergize recipient T cells of direct allo-specificities (11), likely rendering them more effective in abrogating rejection. It has been reported that polymorphic residues on allogeneic MHC can be recognized by recipient allo-specific T cells regardless of the bound peptide (71). Similarly, acid washing of peptides bound to foreign MHC does not diminish their ability to trigger allo-specific T cell reactivities. On the other hand, mutations in HLA-DR or HLA-B27 have been associated with loss of allo-specific T cell reactivities (72–74). These data suggest that sites within intact donor allo-MHC molecules themselves can be crucial for donor recognition and consequently for tolerance induction. Taken together, the presence of intact allo-MHC molecules within donor ECDI-SP may serve to tolerize T cells with direct allo-specificities that would otherwise engage passenger leukocytes via the direct allo-recognition pathway immediately following transplantation. Thus, the ability to modulate T cells with direct allo-specificities will be an intriguing additional target for future NP engineering, with an ultimate goal of enhancing their tolerogenic capacity to that comparable to donor ECDI-SP.
5. Conclusions
The present study highlights the utility of PLG NPs as a viable platform for donor antigen delivery for transplant tolerance induction in a bm12 to B6 allogeneic skin transplant model. Different engineering parameters were examined including peptide design, antigen selection, and method of antigen conjugation to NPs. We have demonstrated that selection of appropriate peptide length was critical to the success of this platform, and that one possible mechanism of protection by donor peptide conjugated NPs was promotion of accumulation of antigen-experienced Treg at the graft site. Conjugation of the peptide to NPs by amide versus thioether chemistry was found to be equivalent in providing graft protection, as was peptide surface conjugation versus internal encapsulation. We have also demonstrated that delivery of epitope-containing peptides provided equivalent graft protection compared to delivery of whole donor cell lysate. Importantly, throughout our studies, NP carriers performed comparably to SP carriers when delivering the same form of antigen. As donor ECDI-SP treatment remains the most effective and superior modality for transplant tolerance induction, in the current study we revealed that in comparison to donor peptide conjugated NPs, donor ECDI-SP targeted different splenic phagocytes and more effectively inhibited CD8+CD44+IFN-γ+ effector T cell accumulation at the graft site. These differences not only explain the superior graft protection by bm12 ECDI-SP in comparison to NP-ECDI-SP, but also provide guidance for future engineering of cell-free, biocompatible and “off-the-shelf” NP platforms with a goal of maximizing tolerance efficacy for transplantation.
Supplementary Material
Acknowledgments
The authors would like to thank the EPIC facility (NUANCE Center-Northwestern University), which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCD-1542205); MRSEC program (NSF DMR-1121262) at the Materials Research Center, the Nanoscale Science and Engineering Center (EEC-0118025/003); the International Institute for Nanotechnology (IIN); and the State of Illinois, through the IIN. Cellular assays were performed in the Flow Cytometry Core Facility of the Interdepartmental ImmunoBiology Center at Northwestern University. The authors would also like to thank Dr. Mohammed Javeed I Ansari for providing the ABM mice used in this study. The work was supported by NIH R01EB009910 (S.D., L.S., X.L.) and JDRF 2-SRA-2016–313-S-B (S.S., X.L.).
Footnotes
Conflict of Interest: The authors have no competing interests to disclose.
Data availability
The raw and processed data required to reproduce these findings are available to download from: https://northwestern.box.com/s/ovw6z0n06×333adlg2pzabaw75w6fizf.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Platt JL. New directions for organ transplantation. Nature. 1998;392(6679 Suppl):11–7. [DOI] [PubMed] [Google Scholar]
- 2.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4(4):259–68. [DOI] [PubMed] [Google Scholar]
- 3.Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, Friedewald JJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQI (TM)) conference. Clin J Am Soc Nephro. 2008;3(2):471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girlanda R Deceased organ donation for transplantation: Challenges and opportunities. World J Transplant. 2016;6(3):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan MD, Wilkes DS. Transplant-related immunosuppression: a review of immunosuppression and pulmonary infections. Proc Am Thorac Soc. 2005;2(5):449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–14. [DOI] [PubMed] [Google Scholar]
- 7.Zafar SY, Howell DN, Gockerman JP. Malignancy after solid organ transplantation: an overview. Oncologist. 2008;13(7):769–78. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56(1):23–46. [DOI] [PubMed] [Google Scholar]
- 9.Pearson RM, Casey LM, Hughes KR, Miller SD, Shea LD. In vivo reprogramming of immune cells: technologies for induction of antigen-specific tolerance. Advanced Drug Delivery Reviews. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo XR, Pothoven KL, McCarthy D, DeGutes M, Martin A, Gettss DR, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. P Natl Acad Sci USA. 2008;105(38):14527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kheradmand T, Wang S, Bryant J, Tasch JJ, Lerret N, Pothoven KL, et al. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol. 2012;189(2):804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy DP, Bryant J, Galvin JP, Miller SD, Luo X. Tempering Allorecognition to Induce Transplant Tolerance With Chemically Modified Apoptotic Donor Cells. Am J Transplant. 2015;15(6):1475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105(38):14527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Zhang X, Zhang L, Bryant J, Kheradmand T, Hering BJ, et al. Preemptive Tolerogenic Delivery of Donor Antigens for Permanent Allogeneic Islet Graft Protection. Cell Transplant. 2015;24(6):1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei J, Kim JI, Shi S, Zhang X, Machaidze Z, Lee S, et al. Pilot Study Evaluating Regulatory T Cell-Promoting Immunosuppression and Nonimmunogenic Donor Antigen Delivery in a Nonhuman Primate Islet Allotransplantation Model. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(10):2739–49. [DOI] [PubMed] [Google Scholar]
- 16.Dilek N, van Rompaey N, Le Moine A, Vanhove B. Myeloid-derived suppressor cells in transplantation. Curr Opin Organ Transplant. 2010;15(6):765–8. [DOI] [PubMed] [Google Scholar]
- 17.Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, ten Brinke A, et al. Hurdles in therapy with regulatory T cells. Sci Transl Med. 2015;7(304):304ps18. [DOI] [PubMed] [Google Scholar]
- 18.Lu JM, Wang XW, Marin-Muller C, Wang H, Lin PH, Yao QZ, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9(4):325–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers-Basel. 2011;3(3):1377–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res-Dordr. 2016;33(10):2373–87. [DOI] [PubMed] [Google Scholar]
- 21.Bryant J, Hlavaty KA, Zhang X, Yap WT, Zhang L, Shea LD, et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials. 2014;35(31):8887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nature biotechnology. 2012;30(12):1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8(3):2148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neef T, Miller SD. Tolerogenic Nanoparticles to Treat Islet Autoimmunity. Curr Diab Rep. 2017;17(10):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Getts DR, Shea LD, Miller SD, King NJ. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015;36(7):419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serra P, Santamaria P. Nanoparticle-based approaches to immune tolerance for the treatment of autoimmune diseases. Eur J Immunol. 2018. [DOI] [PubMed] [Google Scholar]
- 27.Casey LM, Pearson RM, Hughes KR, Liu JMH, Rose JA, North MG, et al. Conjugation of Transforming Growth Factor Beta to Antigen-Loaded Poly(lactide- co-glycolide) Nanoparticles Enhances Efficiency of Antigen-Specific Tolerance. Bioconjug Chem. 2018;29(3):813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kishimoto TK, Maldonado RA. Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance. Frontiers in Immunology. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad S, Xu D, Miller SD. Tolerance strategies employing antigen-coupled apoptotic cells and carboxylated PLG nanoparticles for the treatment of type 1 diabetes. Rev Diabet Stud. 2012;9(4):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy DP, Yap JW, Harp CT, Song WK, Chen J, Pearson RM, et al. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomedicine. 2017;13(1):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingulli E Mechanism of cellular rejection in transplantation. Pediatric Nephrology. 2010;25(1):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mcintyre KR, Seidman JG. Nucleotide-Sequence of Mutant I-a-Beta-Bm12 Gene Is Evidence for Genetic Exchange between Mouse Immune-Response Genes. Nature. 1984;308(5959):551–3. [DOI] [PubMed] [Google Scholar]
- 33.Schmaler M, Broggi MA, Rossi SW. Transplantation of tail skin to study allogeneic CD4 T cell responses in mice. J Vis Exp. 2014(89):e51724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson RM, Casey LM, Hughes KR, Wang LZ, North MG, Getts DR, et al. Controlled Delivery of Single or Multiple Antigens in Tolerogenic Nanoparticles Using Peptide-Polymer Bioconjugates. Mol Ther. 2017;25(7):1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang ST, Ghosh D, Kirschner DE, Linderman JJ. Peptide length-based prediction of peptide-MHC class II binding. Bioinformatics. 2006;22(22):2761–7. [DOI] [PubMed] [Google Scholar]
- 36.Meydan C, Otu HH, Sezerman OU. Prediction of peptides binding to MHC class I and II alleles by temporal motif mining. Bmc Bioinformatics. 2013;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buettner M, Bornemann M, Bode U. Skin Tolerance is Supported by the Spleen. Scand J Immunol. 2013;77(4):238–45. [DOI] [PubMed] [Google Scholar]
- 38.Lin HH, Faunce DE, Stacey M, Terajewicz A, Nakamura T, Zhang-Hoover J, et al. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. Journal of Experimental Medicine. 2005;201(10):1615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gagliani N, Jofra T, Valle A, Stabilini A, Morsiani C, Gregori S, et al. Transplant Tolerance to Pancreatic Islets Is Initiated in the Graft and Sustained in the Spleen. Am J Transplant. 2013;13(8):1963–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29(3):464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin P, del Hoyo GM, Anjuere F, Arias CF, Vargas HH, Fernandez-L A, et al. Characterization of a new subpopulation of mouse CD8 alpha(+) B220(+) dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100(2):383–90. [DOI] [PubMed] [Google Scholar]
- 42.Liu T, Soong L, Liu G, Konig R, Chopra AK. CD44 expression positively correlates with Foxp3 expression and suppressive function of CD4(+) T-reg cells. Biol Direct. 2009;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem Rev. 2016;116(4):2602–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doane T, Burda C. Nanoparticle mediated non-covalent drug delivery. Adv Drug Deliv Rev. 2013;65(5):607–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hlavaty KA, McCarthy DP, Saito E, Yap WT, Miller SD, Shea LD. Tolerance induction using nanoparticles bearing HY peptides in bone marrow transplantation. Biomaterials. 2016;76:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A. 2015;112(2):E156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wieczorek M, Abualrous ET, Sticht J, Alvaro-Benito M, Stolzenberg S, Noe F, et al. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front Immunol. 2017;8:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15(4):203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rock KL, Reits E, Neefjes J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016;37(11):724–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu QY, Berti F, Adamo R. Towards the next generation of biomedicines by site-selective conjugation. Chem Soc Rev. 2016;45(6):1691–719. [DOI] [PubMed] [Google Scholar]
- 51.Spicer CD, Davis BG. Selective chemical protein modification. Nat Commun. 2014;5. [DOI] [PubMed] [Google Scholar]
- 52.Pearson RM, Casey LM, Hughes KR, Wang LZ, North MG, Getts DR, et al. Controlled Delivery of Single or Multiple Antigens in Tolerogenic Nanoparticles Using Peptide-Polymer Bioconjugates. Mol Ther. 2017;25(7):1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Marco M, Shamsuddin S, Razak KA, Aziz AA, Devaux C, Borghi E, et al. Overview of the main methods used to combine proteins with nanosystems: absorption, bioconjugation, and encapsulation. Int J Nanomed. 2010;5:37–49. [PMC free article] [PubMed] [Google Scholar]
- 54.Takemoto S, Port FK, Claas FHJ, Duquesnoy RJ. HLA matching for kidney transplantation. Hum Immunol. 2004;65(12):1489–505. [DOI] [PubMed] [Google Scholar]
- 55.Loveland B, Simpson E. The Non-Mhc Transplantation Antigens - Neither Weak nor Minor. Immunology Today. 1986;7(7–8):223–9. [DOI] [PubMed] [Google Scholar]
- 56.Waldmann H, Adams E, Fairchild PJ, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev. 2006;212:301–13. [DOI] [PubMed] [Google Scholar]
- 57.Cobbold S, Waldmann H. Infectious tolerance. Current Opinion in Immunology. 1998;10(5):518–24. [DOI] [PubMed] [Google Scholar]
- 58.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6(10):577–83. [DOI] [PubMed] [Google Scholar]
- 59.Mansour H, Homs S, Desvaux D, Badoual C, Dahan K, Matignon M, et al. Intragraft Levels of Foxp3 mRNA Predict Progression in Renal Transplants with Borderline Change. J Am Soc Nephrol. 2008;19(12):2277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smarr CB, Yap WT, Neef TP, Pearson RM, Hunter ZN, Ifergan I, et al. Biodegradable antigen-associated PLG nanoparticles tolerize Th2-mediated allergic airway inflammation pre- and postsensitization. P Natl Acad Sci USA. 2016;113(18):5059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plitas G, Rudensky AY. Regulatory T Cells: Differentiation and Function. Cancer Immunol Res. 2016;4(9):721–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, et al. Tolerance Induced by Apoptotic Antigen-Coupled Leukocytes Is Induced by PD-L1+ and IL-10-Producing Splenic Macrophages and Maintained by T Regulatory Cells. J Immunol. 2011;187(5):2405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischbein MP, Yun J, Laks H, Irie Y, Fishbein MC, Bonavida B, et al. Role of CD8(+) lymphocytes in chronic rejection of transplanted hearts. J Thorac Cardiov Sur. 2002;123(4):8039. [DOI] [PubMed] [Google Scholar]
- 64.Bhat P, Leggatt G, Waterhouse N, Frazer IH. Interferon-gamma derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicolete R, dos Santos DF, Faccioli LH. The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int Immunopharmacol. 2011;11(10):1557–63. [DOI] [PubMed] [Google Scholar]
- 66.Ding TT, Sun J, Zhang P. Immune evaluation of biomaterials in TNF-alpha and IL-1 beta at mRNA level. J Mater Sci-Mater M. 2007;18(11):2233–6. [DOI] [PubMed] [Google Scholar]
- 67.Zhao JX, Zhao JC, Perlman S. Differential Effects of IL-12 on Tregs and Non-Treg T Cells: Roles of IFN-gamma, IL-2 and IL-2R. Plos One. 2012;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhai Y, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Th1 and Th2 cytokines in organ transplantation: Paradigm lost? Crit Rev Immunol. 1999;19(2):155–72. [PubMed] [Google Scholar]
- 69.Schnorrer P, Behrens GMN, Wilson NS, Pooley JL, Smith CM, EI-Sukkari D, et al. The dominant role of CD8(+) dendritic cells in cross-presentation is not dictated by antigen capture. P Natl Acad Sci USA. 2006;103(28):10729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bryant J, Hlavaty KA, Zhang X, Yap WT, Zhang L, Shea LD, et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials. 2014;35(31):8887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ingulli E Mechanism of cellular rejection in transplantation. Pediatr Nephrol. 2010;25(1):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lombardi G, Barber L, Sidhu S, Batchelor JR, Lechler RI. The Specificity of Alloreactive T-Cells Is Determined by Mhc Polymorphisms Which Contact the T-Cell Receptor and Which Influence Peptide Binding. Int Immunol. 1991;3(8):769–75. [DOI] [PubMed] [Google Scholar]
- 73.Smith PA, Brunmark A, Jackson MR, Potter TA. Peptide-independent recognition by alloreactive cytotoxic T lymphocytes (CTL). Journal of Experimental Medicine. 1997;185(6):1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villadangos JA, Galocha B, Decastro JAL. Unusual Topology of an Hla-B27 Allospecific T-Cell Epitope Lacking Peptide Specificity. J Immunol. 1994;152(5):2317–23. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.