Abstract
Objectives
The purpose of this study was to investigate effects of adipocytes on osteoclast adhesion‐related molecules.
Materials and methods
ST2 cells, a cloned stromal cell line from mouse bone marrow, able to differentiate into adipocytes, were cultured in serum‐free α‐MEM which was then collected to be used as adipocyte‐conditioned medium (ADIPO CM). RAW264.7 cells were cultured in ADIPO CM in the presence of RANKL, and bone marrow‐derived macrophages were cultured in ADIPO CM in the presence of RANKL and macrophage‐colony stimulating factor to induce osteoclast differentiation. TRAP staining, resorption pit assay, qRT‐PCR and western blotting assays were performed.
Results
ELISAs revealed that CXCL12 was abundant in ADIPO CM and CCK‐8 assay revealed no proliferation of RAW264.7 cells after exogenous CXCL12 treatment. ADIPO CM enhanced osteoclast formation and resorption, both by RAW264.7 cells and BMMs. In addition, exogenous CXCL12 efficiently potentiated formation of TRAP‐positive osteoclast and resorption by RAW264.7 cells. Western blotting and qRT‐PCR suggested that ADIPO CM or combined treatment with exogenous CXCL12 caused significant increase in expression of NFAT2, src and osteoclast adhesion‐related molecules, including β3 integrin, CD44 and osteopontin. However, these promotional effects were largely abrogated on treatment of AMD3100, a CXCR4 antagonist.
Conclusions
Adipocytes promoted osteoclast differentiation, function and expression of adhesion‐related molecules through the CXCL12/CXCR4 signalling pathway.
Keywords: adipocyte, osteoclast, CXCL12/CXCR4, adhesion‐related molecules
1. Introduction
The prevalence of obesity or overweight continues to be unacceptably high and of public health concern worldwide. Clinical evidences indicate that obesity is a risk factor for osteoporosis in humans.1 An inverse relationship between bone mass and marrow adiposity has been observed under pathological and physiological conditions.2, 3, 4 Adipocytes are present in bone marrow niche and are shown to increase greatly with advanced age, obesity and associated abnormal bone metabolism, such as osteoporosis, rheumatoid arthritis and metastatic cancers.5 Therefore, bone marrow adipogenesis has been recently regarded as a therapeutic target for prevention of bone loss. In vitro studies demonstrated that co‐culture of osteoclast precursors with adipocytes or addition of the culture supernatant from bone marrow adipocyte to RAW264.7 cells under low‐dose of RANKL could promote osteoclastogenesis and increase the area of bone resorption,6 indicating that adipocytes could enhance osteoclast differentiation and resorption ability through secretion of pro‐osteoclastogenic cytokines. Up to now, several proinflammatory cytokines and adipocyte‐specific factors such as leptin and adiponectin have been shown to contribute to the adipocytic regulation of osteoclast formation.7
Chemokines have recently been demonstrated to play essential roles in osteoclast differentiation. CXCL12, a member of CXC chemokine family constitutively expressed by murine and human bone marrow stromal cells, has been implicated in mediation of survival for progenitor cells and regulation of their homing, retention, fusion and function.8, 9, 10, 11 Recently, CXCL12 is also shown to be expressed by adipocyte and functions by binding to its major receptor CXCR4, a G‐protein‐coupled receptor which is expressed on many cell types, including osteoclast precursors.12, 13, 14 De Klerck et al.15 reported that CXCL12 potentiates receptor activator of NF‐κB ligand (RANKL)‐induced osteoclast differentiation and activity from splenocytes, which can be counteracted by AMD3100, a specific antagonist for CXCR4. Thus, CXCL12/CXCR4 signalling pathway appears to be closely involved in the process of osteoclast differentiation.
NFAT2, a master transcription factor of osteoclastogenesis, binds to its own gene promoter accelerating its own transcription and further induces the expression of key genes associated with osteoclast activation.16 Active mature osteoclasts are typically multinuclear giant cells that have strong potency to degrade bone matrix. The achievement of bone resorption by osteoclasts requires the tight attachment to bone surfaces and the formation of ruffled border, where they degrade the mineralized bone matrix by releasing several proteolytic enzymes such as matrix metalloproteinase‐9 (MMP‐9) and cathepsin K.17 The process of attachment of osteoclasts to mineral surface requires a series of osteoclast‐derived adhesion molecules including CD44 and integrin β3 sharing a common ligand, osteopontin (OPN), which is also expressed and secreted by osteoclasts.18, 19 The activation of CXCL12/CXCR4 has been noted as one of the key regulatory mechanisms of multiple intracellular changes, such as actin cytoskeletal reorganization and adhesion molecules expression.20 However, much less has been known about the role of CXCL12/CXCR4 in osteoclastic adhesion so far. The objective of this study was to investigate the effect of adipocyte on the expression of osteoclast adhesion‐related molecules and the role of CXCL12 in the process.
2. Materials and methods
2.1. Materials
Murine RAW264.7 cells were purchased from the America Type Culture Collection (Rockville, MD, USA). Minimum essential medium (α‐MEM), foetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco (Rockville, MD, USA). Acid Phosphatase Leukocyte (TRAP) Kit was obtained from Sigma‐Aldrich (St. Louis, MO, USA). Recombinant mouse CXCL12 protein and AMD3100 octahydrochloride were from Abcam (Cambridge, MA, USA). Macrophage‐colony stimulating factor (M‐CSF) and receptor activator of NF‐κB ligand proteins (RANKL) were from R&D Systems (Minneapolis, MN, USA), and mouse CXCL12/SDF‐1 ELISA kit was purchased from 4A Biotech Co., Ltd (Beijing, China). OriCell MSC Adipogenic Differentiation Medium was obtained from Cyagen Biosciences (Guangzhou, China). The Cell Counting Kit‐8 (CCK‐8) was obtained from Solarbio (Beijing, China).
2.2. Adipocytic induction of ST2 cells and preparation of ST2‐adipocyte‐conditioned media (ADIPO CM)
ST2 cells were plated in six‐well plates (1 × 105 cells/well) and treated with OriCell MSC Adipogenic Differentiation Medium for 21 days according to manufacturer's instruction. Differentiated ST2‐adipocyte cells were cultured in serum‐free α‐MEM for 12‐16 h and medium was collected, centrifuged, and stored at −80°C. Prior to use, serum‐free medium collected from adipocyte cultures was diluted 1:1 with α‐MEM appropriate for osteoclast treatment and designated “ADIPO CM.” After rinsing with phosphate‐buffered saline (PBS), cells were fixed with 4% paraformaldehyde in 0.1 mol/L (PBS; pH 7.4) for 30 minutes at room temperature. Oil red O working solution was made up as described by manufacturer's instructions, and after 5 minutes, the Oil Red O solution was washed directly with PBS. The cells were captured using an inverted phase contrast microscope (CKX41; Olympus, Tokyo, Japan).
2.3. ELISA assay for CXCL12 in ADIPO CM
The concentrations of CXCL12 in serum‐free culture medium conditioned by ST2‐adipocytes as mentioned above were detected. Culture medium of serum‐free α‐MEM was used as control. The samples were assayed using commercially available enzyme‐linked immunosorbent assay (ELISA) kit and the data were expressed as pg/mL.
2.4. Cell culture and cell proliferation assay
RAW264.7 cells and BMMs were cultured in α‐MEM supplemented with 10% FBS and 1% penicillin‐streptomycin at 37°C in a humidified 5% CO2 atmosphere. To evaluate the effect of CXCL12 on the proliferation of RAW264.7 cells and BMMs, cell proliferation viability assays were performed using the CCK‐8 according to the manufacturer's instructions. Briefly, RAW264.7 cells plated in 96‐well plates at a density of 5 × 103 cells were grown for 1‐2 days and further treated with CXCL12. BMMs were seeded in 96‐well plates at a density of 1 × 104 cells/well and grown for 1‐2 days in the presence of 30 ng/mL M‐CSF. For cell proliferation assays, cells were incubated in the presence or absence of CXCL12 (20, 50 or 100 ng/mL) for 1, 2, 3 and 4 days. After incubating the cells with CCK‐8 solution for 1 hour, optical density was measured at 450 nm using a GENios microplate reader (Tecan, Austria). Experiments were repeated at least three times each time in triplicate. Cell viability was expressed as a percentage of the control.
2.5. Preparation of bone marrow‐derived macrophages (BMMs)
BMMs were isolated from whole bone marrow of 4‐ to 6‐week old C57BL/6 male mice. Briefly, after killing the mice, total bone marrow cells were flushed from the femora and tibiae with α‐MEM. The cells were layered on a Ficoll‐Hypaque gradient and centrifuged at 440 g for 30 minutes at room temperature. Cells at the gradient interface were collected and plated in α‐MEM, supplemented with 10% FBS, at 37°C in 5% CO2 in the presence of 30 ng/mL M‐CSF. After 3 days, cells were used for osteoclast differentiation assay.
2.6. Osteoclast differentiation assay and tartrate‐resistant acid phosphatase (TRAP) staining
RAW264.7 cells and BMMs were seeded in 24‐well plates at a density of 5 × 104 cells/well and allowed to attach overnight. For osteoclastogenesis assays, RAW264.7 cells and BMMs were cultured with α‐MEM or ADIPO CM, respectively. Meanwhile, RAW264.7 cells were administrated with 10 ng/mL RANKL, while BMMs were cultured with 30 ng/mL M‐CSF and 10 ng/mL RANKL. When indicated, assays were performed in the absence or presence of recombinant proteins CXCL12 (50 ng/mL) or CXCR4 antagonist AMD3100 (650 nmol/L). Every 48 hours, the medium was removed and replenished with fresh media supplemented with M‐CSF, RANKL and appropriate treatment reagents. Data were collected from at least three independent experiments performed in duplicate. Osteoclasts were formed within 4‐6 days, and at the end of the culture period, tartrate‐resistant acid phosphatase (TRAP)‐positive cells with >3 nuclei/cell were counted after staining with Acid Phosphatase Leukocyte (TRAP) Kit.
2.7. Resorption pit assay
RAW264.7 cells or BMMs were seeded in Corning Osteo Assay Surface 96‐well plates coated with calcium phosphate substrate at a density of 5 × 103 cells/well and allowed to attach overnight. The cells were incubated at 37°C in 5% CO2 for 7 days, and the medium was changed every 48 hours. After 7 days, cells were washed with a 10% bleach solution. Images of resorption pits on the plates were captured using a light microscope (CKX41; Olympus) and quantified using Image‐Pro Plus 6 software, and the results were expressed as a percentage of the total field area.21
2.8. Quantitative real‐time PCR analysis
Total RNA was extracted from RAW264.7 cells with RNAiso Plus (Takara Bio Inc, Shiga, Japan) on day 4 after inducing. Total RNA was reverse transcribed with the PrimeScript™RT reagent Kit with gDNA Eraser (Takara Bio Inc). Real‐time polymerase chain reaction amplifications labelled with SYBR Premix Ex Taq (Takara Bio Inc) were performed in a Roche 480 LightCycler (Roche, Mannheim, Germany) at 95°C for 30 seconds, 95°C for 5 seconds and 60°C for 30 seconds for a total of 40 cycles. The appropriate primer sequences were listed in Table 1. Data were normalized against the levels of glyceraldehyde‐3 phosphate dehydrogenase (GAPDH) DNA and were compared with normalized control values.
Table 1.
Sequences of quantitative PCR primers
| Genes | Forward primer | Reverse primer |
|---|---|---|
| β3 integrin | CCCCGATGTAACCTGAAGGAG | GAAGGGCAATCCTCTGAGGG |
| MMP‐9 | GCAGAGGCATACTTGTACCG | TGATGTTATGATGGTCCCACTTG |
| CD44 | TGCAGGTATGGGTTCATAGAAGG | GTGTTGGACGTGACGAGGA |
| c‐src | CAATGCCAAGGGCCTAAATGT | TGTTTGGAGTAGTAAGCCACGA |
| OPN | CCCTCCCGGTGAAAGTGAC | CTTCTGAGATGGGTCAGCA |
| Cathepsin K | TGGAGGGCCAACTCAAGAAG | CCTTTGCCGTGGCGTTATAC |
| GAPDH | TGGCCTTCCGTGTTCCTAC | GAGTTGCTGTTGAAGTCGCA |
2.9. Western blot assay
Cells were lysed and centrifuged and the protein content was quantified by BCA protein assay kit (Pierce, Rockford, USA). Western blot analyses were then performed using NuPAGE 4‐12% Bis‐Tris gradient gels and 0.45 μm Invitrolon polyvinylidene fluoride membranes (Invitrogen, Carlsbad, CA, USA). Proteins were transferred to membranes and immunoblotted with the appropriate primary antibody overnight. The primary antibodies used were rabbit anti‐cathepsin K antibody (ab19027; Abcam), goat anti‐MMP‐9 antibody (AF909; R&D Systems, Inc.), anti‐NFAT2 antibody (ab2796; Abcam), phospho‐src (Tyr416) rabbit mAb (#6943; Cell Signaling Technology, Massachusetts, USA), rabbit anti‐CD44 antibody (15675‐1‐AP; Proteintech Group Inc., Chicago, USA), rabbit anti‐OPN antibody (ab8448; Abcam), integrin beta‐3 polyclonal antibody (18309‐1‐AP; Proteintech) and beta actin monoclonal antibody (60008‐1‐Ig; Proteintech). Following washing and incubating with HRP‐linked goat anti‐mouse IgG (ab102448; Abcam) or swine anti‐rabbit IgG (GP021710/29; Gene Tech, Shanghai, China) or rabbit anti‐goat IgG (Jackson ImmunoResearch Inc., Baltimore, PA, USA) for 1 hour, the signals were then detected with a chemiluminescence detection system (Bio‐Rad, Singapore). The western blot band intensities were measured with ImageJ software. Protein levels were normalized to β‐actin levels and compared to normalized control values.
2.10. Statistical analyses
All data analyses were performed using spss 14.0 software. Data were presented as the mean ± standard deviation. Statistical comparisons were assessed by the unpaired t test between two groups and by the one‐way analysis of variance among more than two groups. P<.05 was considered statistically significant.
3. Results
3.1. CXCL12 was abundantly detected in adipocyte‐conditioned medium
ST2 cells presented with a typical spindle‐shape phenotype (Figure 1A), and cells from passages 4 (21 days) of adipogenic induction were used for the oil red O staining. Cultured ST2 cells treated with adipogenic cocktail exhibited obvious Oil Red O‐positive adipocyte clusters compared with growth medium (Figure 1A,B). After adipogenic induction, the supernatant of adipocyte was collected and was diluted with α‐MEM at 1:1 as mentioned above. FBS‐freed α‐MEM and ADIPO CM were assayed to detect the concentration of CXCL12 by ELISA, and the latter media showed a dramatic increase in CXCL12 levels compared with the α‐MEM vector control (1975 ± 21.21 in the ADIPO CM vs 82.16 ± 5.49 in the α‐MEM, P<.01, Figure 1C). To explore the effect of CXCL12 on the proliferation viability of osteoclast precursor cells, CCK‐8 assay was performed using RAW264.7 cells (Figure 1D) and BMMs (Figure 1E). We found that CXCL12 had no effect on proliferation viability of osteoclast precursor cells at the concentrations used in this study.
Figure 1.
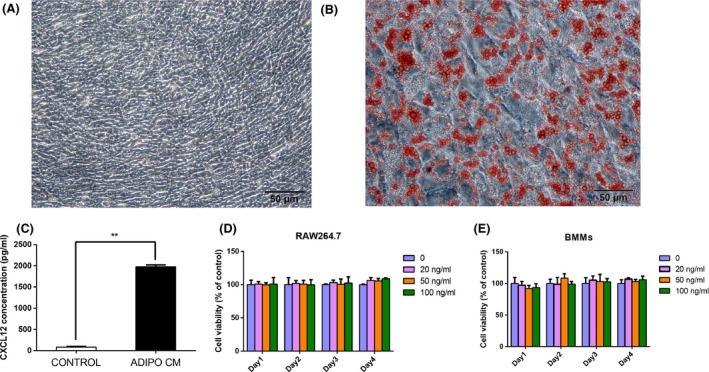
ELISA assay for CXCL12 in ADIPO CM and the effect of CXCL12 on the proliferation viability of RAW264.7 cells and BMMs. The adipogenic effects of ST2 cells under normal and differentiation conditions were measured by Oil Red O staining on day 21 (A, B). ELISA analysis for secreted CXCL12 concentration after adipocyte differentiation compared with α‐MEM culture (C). The proliferation effect of CXCL12 was evaluated using the CCK‐8 method. RAW264.7 cells and BMMs were cultured for 1, 2, 3 and 4 days in the presence or absence of varying concentrations of CXCL12. Optical density was measured at 450 nm. Cell proliferation viability was expressed as a percentage of the control (D, E). Values are mean ± SD of three independent experiments. **P<.01
3.2. ADIPO CM enhanced osteoclast formation and resorption ability
To investigate the direct role of ADIPO CM on osteoclast differentiation and resorption ability, we examined the effect of ADIPO CM on osteoclast differentiation and pit formation from murine RAW 264.7 cells (Figure 2A) and BMMs (Figure 2B). RAW264.7 cells were cultured for 4 days in the presence of 10 ng/mL RANKL with α‐MEM or ADIPO CM. After 4‐day culture in the presence of RANKL, a large number of multinucleated osteoclast (more than three nuclei) could be observed. To confirm the effects of ADIPO CM on osteoclast differentiation and function, similar experiments were performed using another model of osteoclast generation: mouse BMMs cultured in the presence of RANKL and M‐CSF with α‐MEM or ADIPO CM.
Figure 2.
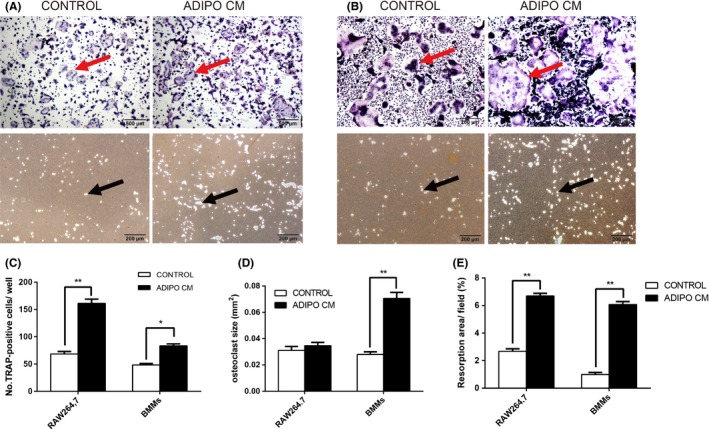
ADIPO CM enhanced osteoclast formation and resorption ability. RAW264.7 cells or BMMs were cultured in the α‐MEM or ADIPO CM. Meanwhile, RAW264.7 cells were treated with 10 ng/mL RANKL while BMMs were administrated with 10 ng/mL RANKL and 30 ng/mL M‐CSF. TRAP staining and resorption pit assay of osteoclasts differentiated from RAW264.7 cells (A) or BMMs (B) were performed. TRAP‐positive cells were counted (C) under a light microscope and results are expressed as number of TRAP‐positive cells per well. The size of osteoclast per field was calculated (D) using Image‐Pro Plus 6 software. Resorption areas were quantified (E) shown as percent of field (%) using Image‐Pro Plus 6 software. Values are mean ± SD of three independent experiments. *P<.05, **P<.01. Red arrows indicate the TRAP‐positive multinucleated cells and black arrows indicate the resorption pits
As shown, treatment for RAW264.7 cells with ADIPO CM resulted in significantly increased number of TRAP‐positive cells in comparison to standard control treatments with RANKL alone (68.33 ± 2.73 in the control group vs 161.31 ± 4.41 in the ADIPO CM group, P<.05). After 4 days (48.33 ± 1.45), TRAP‐positive multinucleated cells were generated from BMMs cultured in the presence of RANKL and M‐CSF, whereas (83.33 ± 2.03) TRAP‐positive multinucleated cells were formed with the addition of adipocyte supernatant (Figure 2C). Notably, the osteoclast size was enlarged significantly upon treatment with ADIPO CM (0.028 ± 0.001 mm2 in the control group vs 0.071 ± 0.003 mm2 in the ADIPO CM group, P<.01, Figure 2D). Besides, the proportion of resorption area is also statistically enhanced after treatment for RAW264.7 cells with ADIPO CM (2.67 ± 0.11% in the control group vs 6.69 ± 0.11% in the ADIPO CM group, P<.01). Similarly, after induction of BMMs, the group of ADIPO CM showed statistically greater resorption area than control (0.98 ± 0.09% in the control group vs 6.08 ± 0.12% in the ADIPO CM group, P<.01, Figure 2E). These results are accordant with data from RAW264.7 cells and the model of BMMs confirming the enhanced effect of ADIPO CM on osteoclast differentiation and resorption ability.
3.3. Adipocyte‐derived CXCL‐12 enhanced osteoclast differentiation and resorption ability in RAW264.7 cells
To understand the effects of adipocyte‐derived CXCL12 on osteoclast differentiation and resorption ability, we incubated RANKL‐treated RAW264.7 cells in the absence or presence of recombinant CXCL12 protein and AMD3100 in culture of ADIPO CM (Figure 3A,B). Then, we performed TRAP staining and evaluated the number of osteoclasts per well (66.33 ± 11.50 in the control group vs 130.72 ± 12.01 in the ADIPO CM group, vs 160.31 ± 11.51 in the ADIPO CM + CXCL12 group vs 94.67 ± 9.82 in the ADIPO CM + AMD3100 group, P<.05, Figure 3C). Treatment with ADIPO CM resulted in significantly increased number of TRAP‐positive cells in comparison to standard control treatment with RANKL alone. Besides, as shown in Figure 3C, it was revealed that the numbers of osteoclasts were significantly increased in the presence of CXCL12 as compared with ADIPO CM only, while addition of AMD3100 lessened this effect. However, although treatment with ADIPO CM and AMD3100 showed more osteoclasts than group of control, there was no obvious distinction between them.
Figure 3.
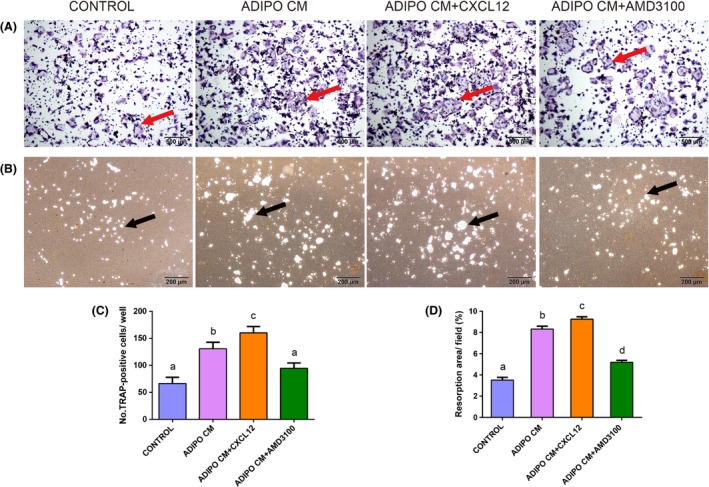
CXCL12 derived from adipocyte promoted osteoclast differentiation and resorption ability. RAW264.7 cells were cultured under control conditions, ADIPO CM or ADIPO CM in the presence of recombinant CXCL12 or AMD3100 with 10 ng/mL RANKL. TRAP staining (A) and resorption pits on the plates (B) of osteoclasts differentiated from RAW264.7 cells were captured using a light microscope. Total number of TRAP‐positive cells per well were counted (C). Resorption areas were quantified (D) shown as percent of field (%). Values are means ± SD of three independent experiments. Means with different letters differ significantly from each other P<.05. Red arrows indicate the TRAP‐positive multinucleated cells and black arrows indicate the resorption pits
In addition, the pit‐forming assay showed that treatment with ADIPO CM clearly enhanced the surface of calcium phosphate resorbed in comparison to control. Statistical analysis revealed that CXCL12 treatment induced an increase in the proportion of resorption area, while after blocking the CXCL12/CXCR4 signalling pathway using AMD3100, the area of bone resorption pits were reduced significantly (3.51 ± 0.21% in the control group vs 8.32 ± 0.21% in the ADIPO CM group, vs 9.26 ± 0.18% in the ADIPO CM + CXCL12 group vs 5.19 ± 0.15% in the ADIPO CM + AMD3100 group, P<.05, Figure 3D). Western blot analysis statistically showed that the level of NFAT2, a master transcription factor, was increased by ADIPO CM, while AMD3100 inhibited this effect. Cathepsin K and MMP‐9 have been convincingly recognized as the most potent proteolytic effectors of osteoclast‐mediated bone resorption. Transcription of cathepsin K and MMP‐9, two bone remodelling genes, were increased in the presence of ADIPO CM, while this effect was disturbed with AMD3100 treatment (Figure 4A). Similarly, protein expression of cathepsin K and MMP‐9 in the culture of ADIPO CM were enhanced with treatment of CXCL12 while partially abolished by AMD3100 (Figure 4B). Besides, statistical analysis revealed that c‐src, as a key factor for the formation of ruffled border, had analogical expression levels of gene in osteoclasts and protein (Figure 4).
Figure 4.
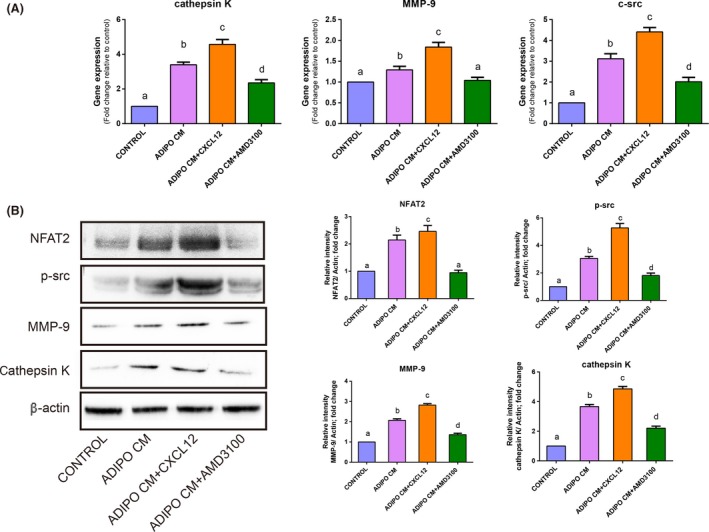
CXCL12 derived from adipocyte contributed to increase the expression of osteoclast functional specific molecules. RAW264.7 cells were cultured for 4 days with control conditions, with ADIPO CM or with ADIPO CM in the presence of recombinant CXCL12 or AMD3100. Quantitative RT‐PCR analysis of osteoclast specific genes: c‐src, cathepsin K and MMP‐9 in osteoclasts were performed (A) and data were graphed as fold change relative to control. Western blot of NFAT2, p‐src, MMP‐9 and cathepsin K in osteoclasts was performed, and band intensity analysis of respective protein levels in osteoclasts was normalized to β‐actin level and represented as % control. Values are mean ± SD of three independent experiments. Means with different letters differ significantly from each other P<.05
3.4. Adipocyte‐derived CXCL‐12 upregulated osteoclast adhesion‐related molecules expression
To further explore the role of adipocyte‐derived CXCL12 in the expression of osteoclast‐related adhesion molecules, we analysed the mRNA and protein expression of osteoclast adhesion‐related molecules such as CD44, β3 integrin and OPN in all groups (Figure 5). qRT‐PCR results statistically showed that the expression of CD44, β3 integrin and OPN mRNA was enhanced in the presence of ADIPO CM and the combination of CXCL12 and ADIPO CM induced a further increase in the expression of these genes. When AMD3100 was added into the ADIPO CM, strong suppression on the expression of adhesion‐related molecules was observed. Western blot analysis revealed a similar expression pattern for the protein of CD44, β3 integrin and OPN corresponding to the results of qRT‐PCR.
Figure 5.
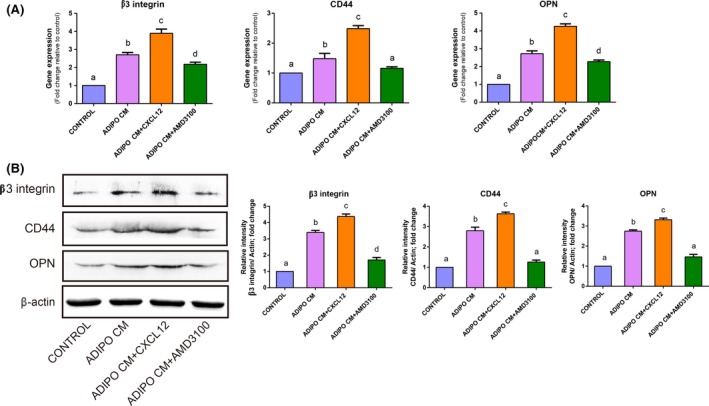
CXCL12 derived from adipocyte upregulated the expression of osteoclast adhesion‐related molecules. RAW264.7 cells were cultured for 4 days with control conditions, with ADIPO CM or with ADIPO CM in the presence of recombinant CXCL12 or AMD3100. Quantitative RT‐PCR analysis of osteoclast adhesion‐related genes: β3 integrin, CD44 and OPN in osteoclasts were performed and data are graphed as fold change relative to control (A). Western blot of above molecules in osteoclasts was performed, and band intensity analysis of respective protein levels in osteoclasts was normalized to β‐actin and represented as % control (B). Values are mean ± SD of three independent experiments. Means with different letters differ significantly from each other P<.05
4. Discussion
Recent studies have shown that body fat mass is negatively correlated with bone mass when the mechanical loading effect of body weight is statistically removed. Therefore, obesity has been identified as a risk factor for osteoporosis.22, 23 In this study, we aimed to examine the contribution of adipocyte‐derived factors to osteoclast differentiation and adhesion‐related molecules in vitro, focusing on the involvement of CXCL12/CXCR4 signalling pathway in osteoclast differentiation and function. The results showed that adipocyte promoted osteoclast differentiation, function and expression of adhesion‐related molecules via secreting CXCL12. Thus, targeting CXCL12/CXCR4 signalling pathway may present a promising therapeutic approach for osteoporosis in obese people.
Several studies have reported a positive relationship of the marrow medullary adipogenesis and osteoclastgenisis.24, 25, 26 In vivo, high‐fat diet caused 2‐fold to 3‐fold in osteoclast formation and bone resorptive capacity compared with low‐fat diet group.27 In vitro, co‐culture of osteoclast precursors with bone marrow‐derived mesenchymal stem cell‐derived adipocytes significantly enhanced osteoclast differentiation with low‐dose RANKL.6 Consistent with these studies, our results showed that adipocyte supernatant effectively enhanced osteoclast differentiation and activity, implying that adipocyte promoted osteoclastogenesis in a paracrine manner. Recent studies suggested that adipocytes might modulate osteoclast differentiation and activity through secreting the factors, such as leptin, adiponectin and pro‐inflammatory cytokines.6, 7, 26, 28, 29 Whether adipocyte can supply additional factor to support and enhance osteoclastogenesis is not well understood.
CXCL12, a chemotactic factor, has been suggested to play potential roles in preosteoclast homing, retention, fusion and function by binding to its major receptor CXCR4.8, 9, 10 Recently, adipocytes have been found to highly express CXCL12 both in vitro and in vivo, a fact proposed CXCL12 as a potential bridging factor linking lipid metabolism and osteoclastic bone resorption.30 In this study, ELISA analyses revealed that CXCL12 was highly upregulated in adipocyte supernatant. We also showed that media conditioned by adipocytes significantly accelerated osteoclast differentiation, a process that can be further augmented by simultaneously treating with recombinant CXCL12 ligands. Notably, treatment with AMD3100 revealed an effect of inhibition on adipocyte‐induced osteoclast differentiation and resorptive ability. Thus, our results suggested that adipocyte enhanced osteoclast differentiation and activity partially through CXCL12/CXCR4 signalling pathway.
It is reported that NFATc1 plays pivotal role in osteoclast formation, mature and activation via upregulation of various genes responsible for osteoclast fusion, adhesion, migration and bone resorption.16, 31 In this study, western blot revealed that treatment with AMD3100 could inhibit the positive role of ADIPO CM in the expression of NFAT2 suggesting that CXCL12‐derived adipocyte contributed to osteoclast development. The initial event in bone resorption is the formation of the ruffled border and osteoclast adhesion to the target matrix. Osteoclasts synthesize several critical adhesion‐related molecules such as β3 integrin and CD44, through which osteoclasts bond to bone matrix and initiate the following resorption. β3 integrin and CD44 may regulate osteoclastic adhesion and migration by interacting with secreted matrix elements such as OPN.32 It has been reported that OPN promoted osteoclastic migration in the metastatic lesion by its high affinity to CD44 and β3 integrin on osteoclast membrane, which leaded to immediate osteolysis.33, 34 In our study, supernatant of ST2‐adipocyte culture enhanced the expression of osteoclastic adhesion molecules. Thus, it appears that some endogenous factors synthesized and secreted by adipocyte may be responsible for this effect.
It has been recently established that by binding to its cell surface receptor CXCR4, CXCL12 contributes to multiple cellular events including activation of integrin, cytoskeletal reorganization and expression of adhesion molecules resulting in tumour cell trafficking.20, 35 In this study, highly escalated level of CXCL12 in supernatant of adipocyte culture detected by ELISA provided a clue to discover the potential pro‐adhesive factor. By blocking CXCL12‐CXCR4 signalling with AMD3100, we confirmed the positive role of CXCL12 in regulating the expression of adhesion factors in osteoclast, as simultaneous treatment with AMD3100 greatly abrogated ADIPO CM‐induced adhesion factors expression by osteoclast. Consistently, similar phenomenon, showing an increased β3 integrin, CD44 and OPN expression upon CXCL12 treatment, has been observed on lung cancer cells, pancreatic cancer cells and other cell types.36, 37 Moreover, phosphorylation src at Tyr416 (p‐src) appears to play an essential role in the dynamics and organization of actin cytoskeleton, as well as the so‐called ruffled border.38, 39 Deletion of c‐src leads to reduced motility and alteration of the cytoskeleton in osteoclast, resulting ultimately in their lack of bone resorbing activity.40 In our study, the fact that the expression of p‐src in osteoclast was increased in the group of ADIPO CM and ADIPO CM + CXCL12, while inhibited in the group of ADIPO CM + AMD3100 revealed the important role of CXCL12 derived from adipocyte. However, osteoclast adhesion is a complex process mediated by several classes of cell adhesion molecules, and it is still not clear enough to explain the mechanism in detail. Thus, further research for effects of CXCL12 derived from adipocytes on osteoclast adhesion is required.
In conclusion, our study indicated the positive effects of adipocytes on osteoclast differentiation and expression of adhesion‐related molecules, which were achieved partially through CXCL12/CXCR4 signalling pathway (Figure 6).
Figure 6.
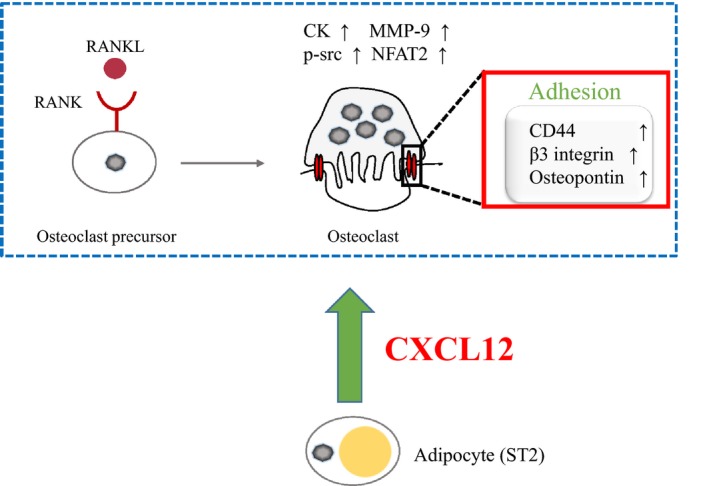
Schematic model for ADIPO CM regulation on the osteoclast differentiation and activation. Adipocytes secrete CXCL12 into extracellular environment. Then, the extracellular CXCL12 binds to its receptor CXCR4 expressed on the preosteoclasts, further to enhance the differentiation, and activity of osteoclasts as well as the expression of osteoclast adhesion‐related molecules such as β3 integrin, CD44 and OPN
Acknowledgement
This study was partially supported by the National Nature Science Foundation of China (grant no. 81271965, 81470719, 81611140133) to M. Li.
Luo T, Liu H, Feng W, et al. Adipocytes enhance expression of osteoclast adhesion‐related molecules through the CXCL12/CXCR4 signalling pathway. Cell Prolif. 2017;50:e12317 10.1111/cpr.12317
Contributor Information
Xianqi Li, Email: li@po.mdu.ac.jp.
Minqi Li, Email: liminqi@sdu.edu.cn.
References
- 1. Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. [DOI] [PubMed] [Google Scholar]
- 2. Hardaway AL, Herroon MK, Rajagurubandara E, et al. Marrow adipocyte‐derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin Exp Metastasis. 2015;32:353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. During A, Penel G, Hardouin P. Understanding the local actions of lipids in bone physiology. Prog Lipid Res. 2015;59:126–146. [DOI] [PubMed] [Google Scholar]
- 4. Jeong BC, Kim TS, Kim HS, et al. Transmembrane protein 64 reciprocally regulates osteoblast and adipocyte differentiation by modulating Wnt/beta‐catenin signaling. Bone. 2015;78:165–173. [DOI] [PubMed] [Google Scholar]
- 5. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mori K, Suzuki K, Hozumi A, et al. Potentiation of osteoclastogenesis by adipogenic conversion of bone marrow‐derived mesenchymal stem cells. Biomed Res. 2014;35:153–159. [DOI] [PubMed] [Google Scholar]
- 7. Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu SL, Pang H, Xu JZ, et al. C/EBPbeta mediates osteoclast recruitment by regulating endothelial progenitor cell expression of SDF‐1alpha. PLoS ONE. 2014;9:e91217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim HR, Kim KW, Kim BM, et al. Reciprocal activation of CD4+ T cells and synovial fibroblasts by stromal cell‐derived factor 1 promotes RANKL expression and osteoclastogenesis in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:538–548. [DOI] [PubMed] [Google Scholar]
- 10. Shahnazari M, Chu V, Wronski TJ, et al. CXCL12/CXCR4 signaling in the osteoblast regulates the mesenchymal stem cell and osteoclast lineage populations. FASEB J. 2013;27:3505–3513. [DOI] [PubMed] [Google Scholar]
- 11. Gronthos S, Zannettino AC. The role of the chemokine CXCL12 in osteoclastogenesis. Trends Endocrinol Metab. 2007;18:108–113. [DOI] [PubMed] [Google Scholar]
- 12. Kincade PW. Plasticity of supporting cells in a stem cell factory. Immunity. 2010;33:291–293. [DOI] [PubMed] [Google Scholar]
- 13. Pramanik R, Sheng X, Ichihara B, et al. Adipose tissue attracts and protects acute lymphoblastic leukemia cells from chemotherapy. Leuk Res. 2013;37:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okada K, Kawao N, Yano M, et al. Stromal cell‐derived factor‐1 mediates changes of bone marrow stem cells during the bone repair process. Am J Physiol Endocrinol Metab. 2016;310:E15–E23. [DOI] [PubMed] [Google Scholar]
- 15. De Klerck B, Geboes L, Hatse S, et al. Pro‐inflammatory properties of stromal cell‐derived factor‐1 (CXCL12) in collagen‐induced arthritis. Arthritis Res Ther. 2005;7:R1208–R1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song I, Kim JH, Kim K, et al. Regulatory mechanism of NFATc1 in RANKL‐induced osteoclast activation. FEBS Lett. 2009;583:2435–2440. [DOI] [PubMed] [Google Scholar]
- 17. Edwards JR, Mundy GR. Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol. 2011;7:235–243. [DOI] [PubMed] [Google Scholar]
- 18. Chabadel A, Banon‐Rodriguez I, Cluet D, et al. CD44 and beta3 integrin organize two functionally distinct actin‐based domains in osteoclasts. Mol Biol Cell. 2007;18:4899–4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merry K, Dodds R, Littlewood A, et al. Expression of osteopontin mRNA by osteoclasts and osteoblasts in modelling adult human bone. J Cell Sci. 1993;104(Pt 4):1013–1020. [DOI] [PubMed] [Google Scholar]
- 20. Peled A, Kollet O, Ponomaryov T, et al. The chemokine SDF‐1 activates the integrins LFA‐1, VLA‐4, and VLA‐5 on immature human CD34 (+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 21. Zeng XZ, He LG, Wang S, et al. Aconine inhibits RANKL‐induced osteoclast differentiation in RAW264.7 cells by suppressing NF‐kappaB and NFATc1 activation and DC‐STAMP expression. Acta Pharmacol Sin. 2016;37:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang CS, Chang YF, Wang MW, et al. Inverse relationship between central obesity and osteoporosis in osteoporotic drug naive elderly females: The Tianliao Old People (TOP) Study. J Clin Densitom. 2013;16:204–211. [DOI] [PubMed] [Google Scholar]
- 23. Zhao LJ, Liu YJ, Liu PY, et al. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holt V, Caplan AI, Haynesworth SE. Identification of a subpopulation of marrow MSC‐derived medullary adipocytes that express osteoclast‐regulating molecules: marrow adipocytes express osteoclast mediators. PLoS ONE. 2014;9:e108920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hozumi A, Osaki M, Goto H, et al. Bone marrow adipocytes support dexamethasone‐induced osteoclast differentiation. Biochem Biophys Res Commun. 2009;382:780–784. [DOI] [PubMed] [Google Scholar]
- 26. Halade GV, El Jamali A, Williams PJ, et al. Obesity‐mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol. 2011;46:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shu L, Beier E, Sheu T, et al. High‐fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif Tissue Int. 2015;96:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elefteriou F, Takeda S, Ebihara K, et al. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA. 2004;101:3258–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamaguchi N, Kukita T, Li YJ, et al. Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans . FEMS Immunol Med Microbiol. 2007;49:28–34. [DOI] [PubMed] [Google Scholar]
- 30. Kim D, Kim J, Yoon JH, et al. CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia. 2014;57:1456–1465. [DOI] [PubMed] [Google Scholar]
- 31. Nakashima T, Takayanagi H. New regulation mechanisms of osteoclast differentiation. Ann N Y Acad Sci. 2011;1240:E13–E18. [DOI] [PubMed] [Google Scholar]
- 32. Chellaiah MA, Hruska KA. The integrin alpha (v) beta(3) and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int. 2003;72:197–205. [DOI] [PubMed] [Google Scholar]
- 33. Li M, Amizuka N, Takeuchi K, et al. Histochemical evidence of osteoclastic degradation of extracellular matrix in osteolytic metastasis originating from human lung small carcinoma (SBC‐5) cells. Microsc Res Tech. 2006;69:73–83. [DOI] [PubMed] [Google Scholar]
- 34. Reufsteck C, Lifshitz‐Shovali R, Zepp M, et al. Silencing of skeletal metastasis‐associated genes impairs migration of breast cancer cells and reduces osteolytic bone lesions. Clin Exp Metastasis. 2012;29:441–456. [DOI] [PubMed] [Google Scholar]
- 35. Zhao E, Wang L, Dai J, et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. 2012;1:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang YC, Hsiao YC, Chen YJ, et al. Stromal cell‐derived factor‐1 enhances motility and integrin up‐regulation through CXCR4, ERK and NF‐kappaB‐dependent pathway in human lung cancer cells. Biochem Pharmacol. 2007;74:1702–1712. [DOI] [PubMed] [Google Scholar]
- 37. Billadeau DD, Chatterjee S, Bramati P, et al. Characterization of the CXCR4 signaling in pancreatic cancer cells. Int J Gastrointest Cancer. 2006;37:110–119. [DOI] [PubMed] [Google Scholar]
- 38. Schwartzberg PL, Xing L, Hoffmann O, et al. Rescue of osteoclast function by transgenic expression of kinase‐deficient Src in src‐/‐ mutant mice. Genes Dev. 1997;11:2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Izawa T, Zou W, Chappel JC, et al. c‐Src links a RANK/alphavbeta3 integrin complex to the osteoclast cytoskeleton. Mol Cell Biol. 2012;32:2943–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanjay A, Houghton A, Neff L, et al. Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin‐mediated signaling, cell adhesion, and osteoclast motility. J Cell Biol. 2001;152:181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]


