Abstract
Objectives
Previously, we found that long intergenic non‐coding RNA‐p21 (lincRNA‐p21) inhibited the development of human prostate cancer. However, the underlying molecular mechanisms are poorly understood. Here, we attempted to investigate the downstream targets of lincRNA‐p21 in prostate cancer.
Materials and methods
Expression of lincRNA‐p21 and PKM2 was determined by qRT‐PCR and Western blot. Lentivirus expressing shPKM2 or shCtrl was used to explore the role of PKM2 on the enhanced cell proliferation and glycolysis of lincRNA‐p21‐silenced prostate cancer cells. A xenograft mouse model was performed to investigate the effect of PKM2 suppression, glycolytic or mammalian target of rapamycin (mTOR) inhibitor on the tumorigenic capacity of lincRNA‐p21‐silenced prostate cancer cells.
Results
We revealed that lincRNA‐p21 silencing in DU145 and LNCaP cells induced up‐regulation of PKM2 and activation of glycolysis, which could be reversed by PKM2 knockdown or rapamycin treatment. We also found that the proliferation and tumorigenesis of lincRNA‐p21‐silenced prostate cancer cells were significantly inhibited after knocking down PKM2. 3‐bromopyruvate (3‐Brpa) or rapamycin treatment largely decreased the tumour burden. Importantly, PKM2 expression was inversely correlated with the lincRNA‐p21 level and the survival of prostate cancer patients.
Conclusions
We demonstrated that lincRNA‐p21 blunted the prostate cancer cell proliferation and tumorigenic capacity through down‐regulation of PKM2. Therefore, targeting PKM2 or glycolysis might be a therapeutic strategy in prostate cancer patients with lowly expressed lincRNA‐p21.
1. INTRODUCTION
Prostate cancer is one of the most common malignant tumours, accounting for the second most frequently diagnosed cancer and the sixth leading cause of cancer death in males worldwide.1 After response to chemotherapy or surgical castration, patients invariably progress to castration‐resistant prostate cancer and the treatment is limited.2, 3 Although numerous studies have been performed on prostate cancer therapies, the molecular events involved in prostate cancer progression are not well characterized.
Recently, lincRNA‐p21 has been widely investigated in various diseases. It was first reported as a direct transcriptional target of p53,4 and some studies showed that it could regulate gene expression,5 mRNA translation and protein stability.6 The former researchers have confirmed that lincRNA‐p21 is associated with hepatocellular carcinoma,7 skin cancer,8 colorectal cancer9 and chronic lymphocytic leukaemia.10 In addition, our previous study also revealed that lincRNA‐p21 suppressed the development of human prostate cancer.11 However, the mechanisms whereby lincRNA‐p21 inhibits the progression of prostate cancer remain elusive.
Hyperactive aerobic glycolysis is frequently observed in various cancers. Pyruvate kinase M2 (PKM2) is identified as an important isoenzyme of aerobic glycolysis.12 Unlike PKM1 found in normal differentiated tissues, it is preferentially expressed in proliferating cells, including cancer cells.13 Previous studies have established that its expression is up‐regulated during embryonic development and tumour progression.14, 15, 16 Some reports reveal that PKM2 promotes tumorigenesis via phosphorylating MCL2 or histone H3.17, 18 In addition, PKM2 is regulated by mTOR signalling in cervical cancer19, 20 and alterations of PTEN/AKT/PI3K/mTOR signalling are also found in prostate cancer.21, 22 Although PKM2 activation of glycolysis largely contributes to PTEN‐/AKT‐/mTOR‐induced cancer development, its expression and function in prostate cancer and relationship with lincRNA‐p21 are largely unclear.
In this study, we reported that lincRNA‐p21 reduced PKM2 abundance in a PTEN‐/AKT‐/mTOR‐dependent manner. Knockdown of lincRNA‐p21 prominently stimulated Warburg effect, which could be rescued by PKM2 suppression or rapamycin treatment. Furthermore, PKM2 silencing blunted the proliferation and tumorigenesis of prostate cancer cells with lowly expressed lincRNA‐p21. The tumorigenic capacity was also suppressed by rapamycin or 3‐Brpa intervention. Importantly, lincRNA‐p21 level exhibited inverse correlation with PKM2 expression in prostate cancer specimens. PKM2 high expression predicted poor survival in prostate cancer patients. Therefore, PKM2 might be a potential therapeutic target in prostate cancer with silenced lincRNA‐p21.
2. MATERIALS AND METHODS
2.1. Materials
LY294002, 3‐Brpa and rapamycin were purchased from Sigma (St. Louis, MO, USA). Human prostate cancer cell lines DU145 and LNCaP were obtained from the American Type Culture Collection (ATCC). All the cells were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, California, USA) and 1% penicillin and streptomycin solution (Corning, New York, USA). In this study, all the prostate cancer patients undergoing radical prostatectomy were selected from Shanghai General Hospital, School of 43 Medicine, Shanghai Jiaotong University (Shanghai, China). The samples used for analysing the correlation between PKM2 expression and patients’ survival were described previously.11 Radical prostatectomy specimens were collected at the same institute and further evaluated by pathologists. Written informed consent was received from all patients and this study was approved by the Clinical Research Ethics Committee of Shanghai Jiaotong University.
2.2. shRNA
shRNA was designed to target lincRNA‐p21 and PKM2 in prostate cancer cells. ShlincRNA‐p21, shPKM2 and control shRNA (shCtrl) lentivirus particles were obtained from GenePharma. The target sequences are listed as follows: lincRNA‐p21: GGAGGACACAGGAGAGGCA; PKM2: GCTGTGGCTCTAGACACTA.
Lentivirus expressing human lincRNA‐p21 and PKM2 were generated by subcloning their cDNA to the pSLIK lentivirus expression system. 293T cells were used for retroviral packaging. DU145 and LNCap cells were cultured with virus‐containing supernatant in the presence of 8 mg/mL polybrene. After 48 hours, puromycin (1.5 mg/mL) or hygromycin (150 mg/mL) was used to select infected cells.
2.3. Cell proliferation assay
CCK‐8 Cell Proliferation/Viability assay Kit (Sigma) was used to measure cell proliferation rate. LincRNA‐p21‐silenced DU145 cells were seeded in 96‐well plates at a density of 3‐5 × 103 cells per well, followed by treatment with glycolytic inhibitor 3‐Brpa or rapamycin. After 48 hours, 10 μL CCK‐8 solution was added and incubated at 37°C for 2 hours, and then subjected to measurement of spectrometric absorbance at 450 nm. Likewise, equal numbers of lincRNA‐p21‐silenced DU145 or LNCaP cells expressing shPKM2 or shCtrl were seeded in 96‐well plates. Cell viability was measured at 0, 24, 48 and 72 hours. These experiments were performed in triplicate and repeated at least 3 times.
2.4. Colony formation analysis
LincRNA‐p21‐silenced DU145 or LNCaP cells were further transfected with shRNA against negative control or PKM2 and seeded in 10 cm plates and incubated at 37°C with 5% CO2 for 10 days. Then the plates were fixed with methanol, stained with Crystal violet solution and the colony numbers were counted using Photoshop software.
2.5. Glucose, pyruvate and lactate measurement
About 6‐8 × 105 cells were seeded in 12‐well plates and cultured overnight. Cell number of each well was determined before glucose or lactate detection. Cells were subjected to pyruvate concentration analysis using Pyruvate Assay Kit (Abcam, Cambridge, United Kingdom), following the manufacturer's instructions. Culture medium was collected and the glucose or lactate level was determined immediately at Shanghai General Hospital, School of Medicine, Shanghai Jiao Tong University. Glucose or lactate content was measured respectively using an Olympus AU5400 (Japan) and a Dade Behring RXL MAX system (Deerfield, Illinois, USA). The glucose consumption and lactate production were calculated and normalized to cell numbers.
2.6. Western blot
Cell lysates were extracted from indicated cells or tumour tissues using TNTE buffer (150 mmol/L NaCl, 50 mmol/L Tris, pH 7.4, 10 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 1 mmol/L sodium vanadate, 0.5% Triton X‐100 and 25 mmol/L sodium fluoride) containing protease inhibitor cocktail and phosphatase inhibitor cocktail (Roche, Basel, Switzerland). Cell lysates were boiled and centrifuged, and then subjected for immunoblotting. Antibodies against PKM2, PTEN, P‐AKT, AKT, P‐S6 and S6 were obtained from Cell Signaling Technology (Danvers, Massachusetts, USA). Antibody against β‐actin and all the secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody against GAPDH was from Abcam.
2.7. Quantitative real‐time PCR (qRT‐PCR)
Total RNA was extracted from human prostate cancer or adjacent normal tissues or indicated human prostate cancer cells using TRIzol reagent (Invitrogen). One micro gram of total RNA was reverse transcribed using One Step RT‐PCR Kit (Takara, Japan) following the manufacturer's instructions. Quantitative real‐time PCR was performed with SYBR Green qPCR Mix (Takara) on an ABI‐7500 RT‐PCR system (Applied Biosystems, Foster City, CA, USA). β‐actin serves as internal control. All the primer sequences used in this study are listed as follows: PKM2‐F, 5′‐CCATCCTCTACCGGCCCGTTG‐3′,PKM2‐R, 5′‐CCAGCCACAGGATGTTCTCGTC‐3′; lincRNA‐p21‐F, 5′‐CCTGTCCCACTCGCTTTC‐3′, lincRNA‐p21‐R, 5′‐GGAACTGGAGACGGAATGTC‐3′; β‐actin‐F, 5′‐ GACCTGACTGACTACCTCATGAAG‐3′, β‐actin‐R, 5′‐ GTCACACTTCATGATGGAGTTGAAGG‐3′.
2.8. Tumour xenograft experiments
Equal numbers of indicated prostate cancer cells were subcutaneously implanted into the immunodeficient nude mice (6‐week old, male BALB/c). Each cohort contained 8 mice.
For tumour‐free and survival analysis, a total of 3 × 106 lincRNA‐p21‐silenced LNCaP cells expressing shCtrl or shPKM2 lentivirus were injected into nude mice. Three weeks after injection, percentage of tumour‐free and survival were calculated. The time point of 0 day in figure 6A represented 3 weeks after injection. About 2 × 106 shCtrl or shPKM2 lincRNA‐p21‐silenced DU145 cells were implanted into nude mice. Tumour‐free or survival was calculated immediately after implantation. Tumour‐free represented the mice which developed visualize tumours. Mice bearing tumours larger than 1000 mm3 were recognized as “death”. Tumour volume was calculated following the formula: v = 0.5ab 2 (a = long diameter of the tumour, b = short diameter of the tumour and v = volume).
Figure 6.
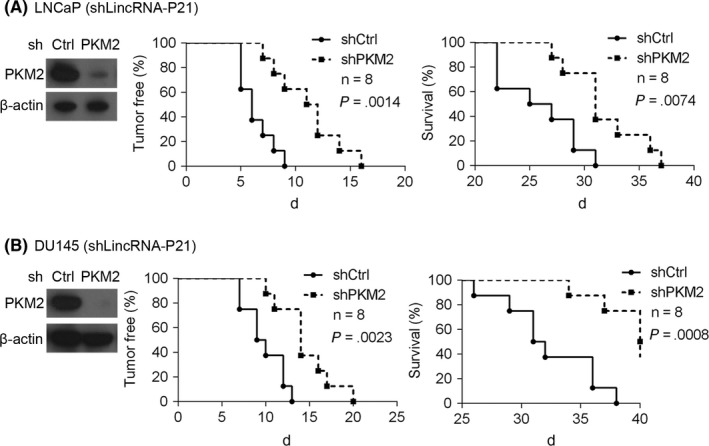
Depletion of pyruvate kinase M2 (PKM2) reduces the tumorigenic capacity of cells with lowly expressed lincRNA‐p21. (A) LincRNA‐p21‐silenced LNCaP cells expressing shPKM2 or shCtrl were implanted into nude mice (n = 8) and tumours developed. The time point of 0 day represented 3 weeks after LNCaP cells injection. (B) LincRNA‐p21‐silenced DU145 cells expressing shPKM2 or shCtrl were implanted into nude mice (n = 8) and tumours developed. Immunoblotting was preformed to examine PKM2 expression (left). Tumour initiation were recorded (middle) and survival was analysed (right)
For the treatment of xenografted tumour, a total of 6 × 106 lincRNA‐p21‐silenced LNCaP cells were injected into nude mice. Beginning on 3 weeks, nude mice bearing tumours derived from lincRNA‐p21‐silenced LNCaP cells were intraperitoneally injected with 2 mg/kg rapamycin or 4 mg/kg 3‐Brpa twice a week. Stock rapamycin dissolved in ethanol was diluted in solvent containing 0.25% PEG and 0.25% Tween‐20. 3‐Brpa was dissolved in saline solution. Solvent injection served as negative control. The animal experiments were approved and carried out in accordance with the Animal Research Ethics Committee of Shanghai Jiaotong University.
2.9. Statistical analysis
All the statistical data were expressed as the mean ± SD at least 3 independent experiments. Two‐tailed Student's t test and 1‐way ANOVA analysis were used to conduct comparisons between 2 groups and multiple groups respectively. Difference was statistically significant when P < .05. *P < .05; **P < .01; ***P < .001; ****P < .0001.
3. RESULTS
3.1. PKM2 expression is inversely correlated with lincRNA‐p21 level in prostate cancer patients and predicts survival
We previously showed that lincRNA‐p21 was dramatically down‐regulated in prostate cancer, and it inhibited the proliferation and tumorigenesis of prostate cancer cells. As PKM2 is highly expressed in tumour cells and promotes tumour progression, we investigated whether lincRNA‐p21 correlated with PKM2 in prostate cancer. Thirty‐six prostate cancer tissues and matched normal prostate tissues were collected and expression of lincRNA‐p21 and PKM2 was examined by qRT‐PCR and Western blot. We found that 67% (24/36) tumour tissues exhibited decreased lincRNA‐p21 level and PKM2 was up‐regulated in 56% (20/36) prostate cancer tissues (Figure 1A‐F). Furthermore, of all the prostate cancer tissues, which had lower level of lincRNA‐p21, PKM2 expression was elevated in 79% (19/24) (Figure 1A‐D). In addition, we also found that 3 (no. 27, 28 and 35) presented no significant change of lincRNA‐p21 expression, among which PKM2 had no obvious change (Figure 1E,F). Surprisingly, there were 9 (no. 25, 26, 29, 30, 31‐34, 36) cancer tissues showing higher lincRNA‐p21 level, among which 6 (no. 26, 29, 31, 32, 33 and 36) exhibited decreased PKM2 expression and 2 (no. 25 and 30) exhibited minimal change of PKM2 level and no. 34 had higher PKM2 expression (Figure 1E,F). Importantly, we quantitatively analysed the tumour/normal expression ratio of lincRNA‐p21 and PKM2 in those tissues and found that there was an inverse correlation between lincRNA‐p21 level and PKM2 expression (Figure 1G). Likewise, we detected the expression of PKM2 in the prostate cancer specimens and separate normal tissues which were used in our previous study.11 The results showed that PKM2 was significantly up‐regulated in those prostate cancer tissues (Figure 1H). Taken together, these data indicate that lincRNA‐p21 expression is inversely correlated with PKM2 expression in prostate cancer patients.
Figure 1.
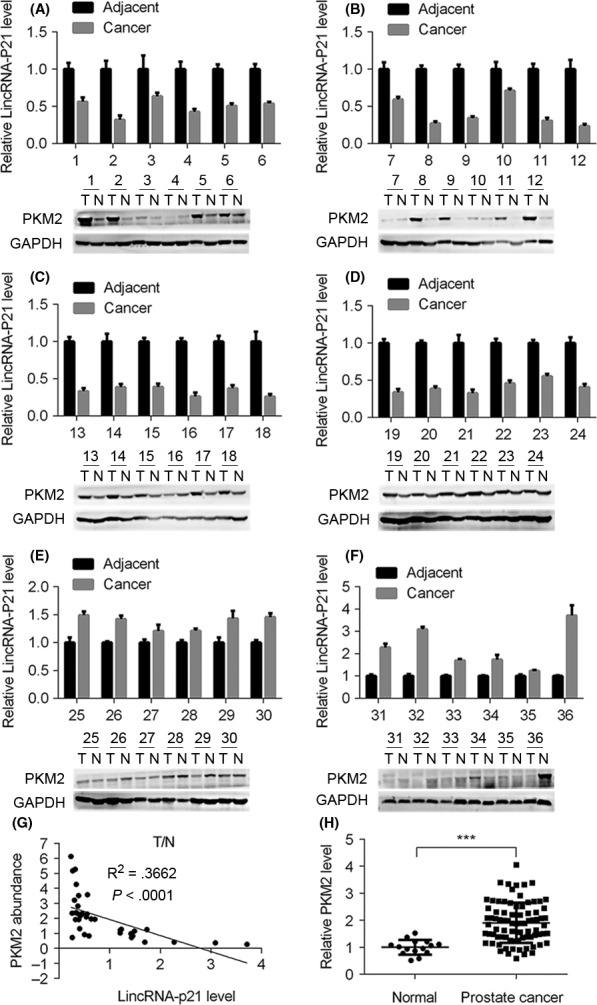
LincRNA‐p21 level is inversely correlated with pyruvate kinase M2 (PKM2) expression in prostate cancer. (A‐F) Relative lincRNA‐p21 level and PKM2 expression in prostate cancer tissues and matched adjacent tissues (n = 36), GAPDH serves as internal control. (G) Statistical analysis of tumour/normal correlation of lincRNA‐p21 and PKM2 in prostate cancer tissues. (H) Prostate cancer tissues (n = 81) and separate normal prostate tissues (n = 15) were subjected to qRT‐PCR analysis of PKM2. ***P< .001
To explore whether highly expressed PKM2 was associated with prostate cancer progression, we next determined the relationship between PKM2 expression and patients’ survival. We divided the patients into PKM2 high expression group (n = 39) and PKM2 low expression (n = 42) group. Our results showed that PKM2 high expression predicted poor survival of prostate cancer patients (Figure 2A,B). Likewise, this correlation was also observed in another separate cohort (Figure 2C,D). These results suggest that up‐regulated PKM2 in prostate cancer may contribute to disease progression.
Figure 2.
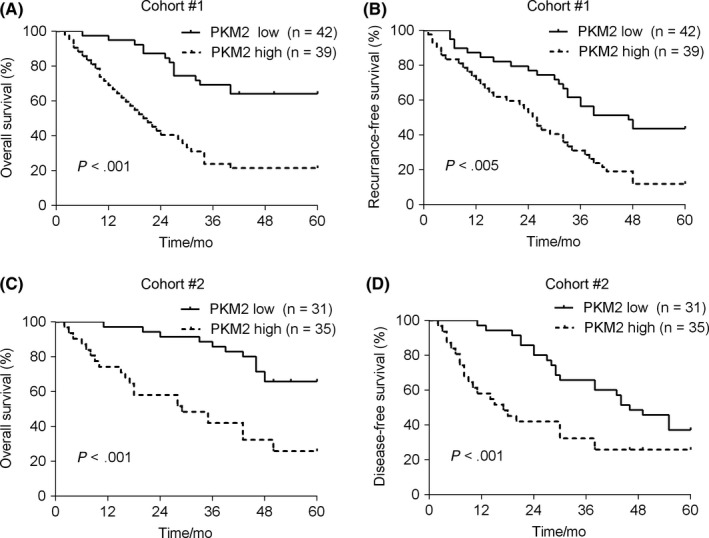
Pyruvate kinase M2 (PKM2) expression predicts prostate cancer patients’ survival. (A and B) In cohort 1, 81 patients were divided into PKM2 high expression group (n = 39) and PKM2 low expression group (n = 42). The overall survival (A) and recurrence‐free survival (B) were analysed. (C and D) In cohort 2, a total of 66 patients was divided into PKM2 high expression group (n = 35) and PKM2 low expression group (n = 31). The overall survival (C) and disease‐free survival (D) were analysed
3.2. Up‐regulation of PKM2 induced by lincRNA‐p21 silencing is dependent on PTEN/AKT/mTOR cascade
To examine whether there is a regulatory relationship between lincRNA‐p21 and PKM2, lincRNA‐p21 was knocked down in DU145 and LNCaP cells. Reduction of lincRNA‐p21 led to enhanced protein and mRNA levels of PKM2 (Figure 3A‐D). PTEN/AKT/mTOR cascade is a key oncogenic signalling pathway in prostate cancer and has been reported to be a stimulator of PKM2.22 We postulated that lincRNA‐p21 silencing up‐regulated PKM2 depended on PTEN/AKT/mTOR cascade. To test this hypothesis, the indicators of this pathway were examined and increased levels of p‐AKT and p‐S6 and decreased level of PTEN were observed in lincRNA‐p21‐silenced DU145 and LNCaP cells (Figure 3A‐D). Moreover, LY294002 or rapamycin treatment reversed the up‐regulation of PKM2 in lincRNA‐p21 knockdown DU145 cells (Figure 3E,F). To further examine whether there is causal relationship between lincRNA‐p21 and PKM2, we overexpressed lincRNA‐p21 in DU145 and LNCaP cells (Figure 3G). As expected, lincRNA‐p21 overexpression in DU145 and LNCaP cells decreased PKM2 abundance (Figure 3H,I). In summary, lincRNA‐p21 is a negative regulator of PKM2 and this regulatory mechanism is dependent on PTEN/AKT/mTOR cascade.
Figure 3.
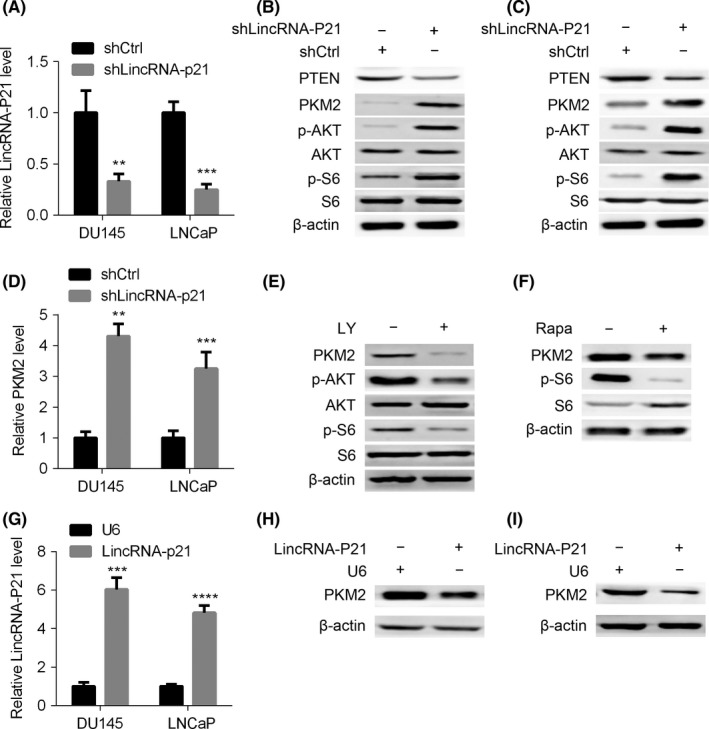
LincRNA‐p21 down‐regulates pyruvate kinase M2 (PKM2) in a PTEN/AKT/mTOR cascade‐dependent manner. (A‐C) LincRNA‐p21 knockdown and control DU145 and LNCaP cells were subjected to qRT‐PCR analysis of lincRNA‐p21, and to Western blot with antibodies as indicated (B and C). (D) Cells described in (A) were used for qRT‐PCR analysis of PKM2. (E and F) LincRNA‐p21‐silenced DU145 cells were treated with LY294002 (LY) (E) or rapamycin (Rapa) (F) and then subjected to Western blot assay. (G‐I) LincRNA‐p21 overexpressed and control DU145 and LNCaP cells were subjected to qRT‐PCR analysis of lincRNA‐p21 (G), and to Western blot analysis of PKM2 (H and I). **P< .01; ***P< .001; ****P< .0001
3.3. LincRNA‐p21 down‐regulation of PKM2 is critical for aerobic glycolysis
Pyruvate kinase M2 promotes cell proliferation and tumour progression at least partly through activation of glycolysis. First, we noticed that glucose consumption, pyruvate concentration and lactate production were enhanced in lincRNA‐p21‐silenced DU145 or LNCaP cells (Figure 4A). As lincRNA‐p21 was a negative regulator of PKM2, we proposed that PKM2 suppression might inhibit aerobic glycolysis in prostate cancer cells with lowly expressed lincRNA‐p21. To test this hypothesis, we knocked down PKM2 in lincRNA‐p21‐silenced DU145 and LNCaP cells. As expected, reduction of PKM2 suppressed the glucose consumption, pyruvate concentration and lactate production (Figure 4B). Furthermore, rapamycin treatment dramatically blunted the hyper‐activated aerobic glycolysis in lincRNA‐p21‐knockdown DU145 cells (Figure 4C). Our previous study showed that p53 was down‐regulated after lincRNA‐p21 knockdown. Because p53 silencing promotes aerobic glycolysis,23 we overexpressed P53 in lincRNA‐p21‐silenced DU145 cells and found that PKM2 level, glucose consumption and lactate production remained no change after P53 overexpression (Figure S1). As reactive oxygen species (ROS) plays an important role in cancer development, we also checked the ROS production and found that it did not change after lincRNA‐p21 knockdown (Figure S2). Therefore, our findings suggest that PKM2 up‐regulation is critical for aerobic glycolysis activation in human prostate cancer cells with silenced lincRNA‐p21.
Figure 4.
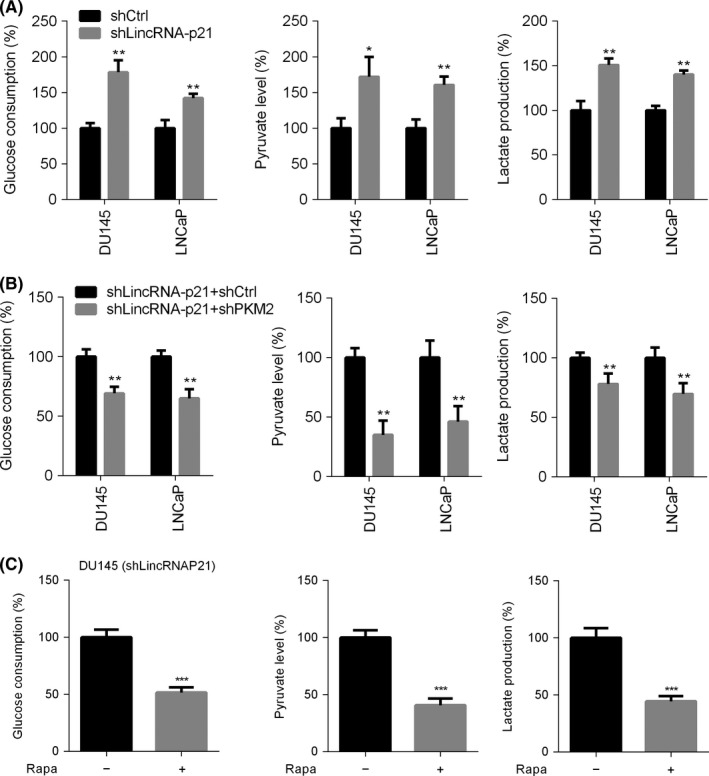
LincRNA‐p21 down‐regulates pyruvate kinase M2 (PKM2) to suppress aerobic glycolysis. (A) Relative glucose consumption, pyruvate concentration and lactate production were quantified in lincRNA‐p21 knockdown or control DU145 or LNCaP cells. (B) Relative glucose consumption, pyruvate concentration and lactate production were quantified in lincRNA‐p21‐silenced DU145 and LNCaP cells expressing shPKM2 or shCtrl. (C) DU145 cells with lowly expressed lincRNA‐p21 were treated with or without rapamycin, and then glucose consumption, pyruvate concentration and lactate production were examined. *P< .05; **P< .01; ***P< .001
3.4. Knockdown of PKM2 suppresses the proliferation of prostate cancer cells with reduced lincRNA‐p21 expression
To identify the effects of PKM2 on prostate cancer cell proliferation, shRNA against PKM2 was stably transfected in DU145 and LNCaP cells harbouring silenced lincRNA‐p21. CCK8 results showed that the viability of DU145 cells was dramatically suppressed after PKM2 knockdown (Figure 5A). Consistently, the colony‐forming capacity of DU145 cells was also inhibited (Figure 5B,C). Likewise, similar results were observed in LNCaP cells (Figure 5D‐F). It is well known that rapamycin and 3‐Brpa are promising drugs specifically against mTOR and glycolysis respectively. Next, we treated shlincRNA‐p21 and shCtrl DU145 cells with rapamycin or 3‐Brpa at various concentrations. We found that lincRNA‐p21‐knockdown DU145 cells were more sensitive to rapamycin and 3‐Brpa treatment (Figure 5G,H). These results strongly suggest that PKM2 or glycolysis inhibition significantly blunts the proliferation of lincRNA‐p21‐silenced prostate cancer cells.
Figure 5.
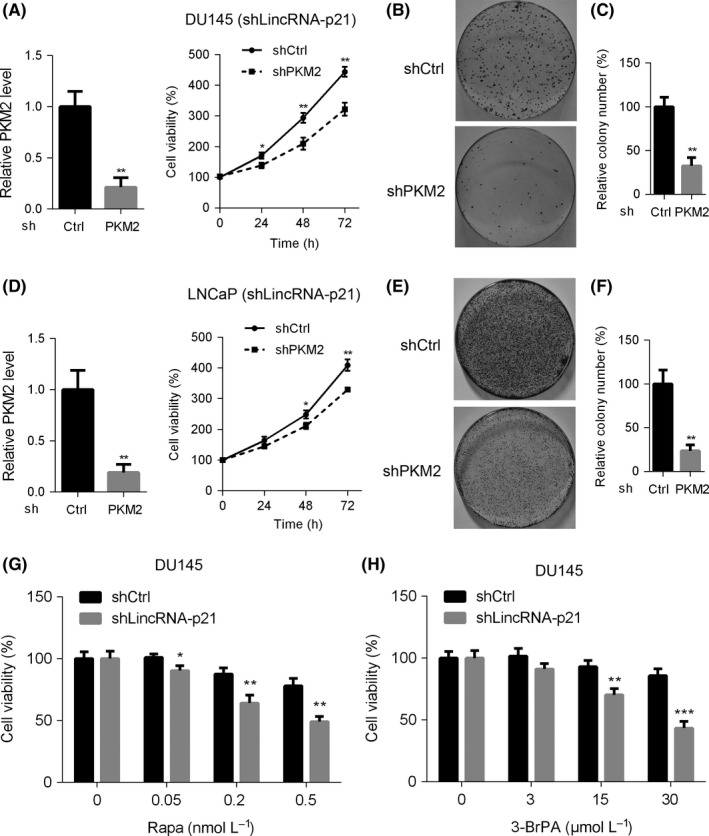
Pyruvate kinase M2 (PKM2) is critical for lincRNA‐p21 suppression of cell proliferation and colony formation of prostate cancer cells. (A) LincRNA‐p21‐silenced DU145 cells expressing shPKM2 or shCtrl were subjected to cell viability analysis at indicated time. (B and C) Cells described in (A) were subjected to colony formation assay. Representative images of cell colonies (B) and relative colony number were calculated (C). (D) LincRNA‐p21‐silenced LNCaP cells expressing shPKM2 or shCtrl were subjected to cell viability analysis at indicated time. (E and F) Cells described in (D) were subjected to colony formation assay. Representative images of cell colonies (E) and relative colony number were calculated (F). (G and H) DU145 cells with lowly expressed lincRNA‐p21 were treated with or without rapamycin (G) or 3‐Brpa (H) at different dosages for 48 hours, and cell viability was examined using CCK8 kit. *P< .05; **P< .01; ***P< .001
3.5. Knockdown of PKM2 inhibits the nude mice tumorigenicity of lincRNA‐p21‐silenced prostate cancer cells
As PKM2 deficiency has been shown to inhibit the proliferation of prostate cancer cell, we next tested whether PKM2 knockdown could suppress tumour growth in vivo. LincRNA‐p21‐silenced DU145 and LNCaP cells expressing shPKM2 or shCtrl were injected into nude mice to establish a xenographic tumour model and tumour growth was assessed. Percentage of tumour‐free and survival was analysed 3 weeks after LNCaP cell implanted into nude mice (details in 2). Our results showed that inhibition of PKM2 prominently attenuated tumour initiation and progression of LNCaP cells in nude mice (Figure 6A). Likewise, targeting PKM2 blunted the tumorigenic capacity of lincRNA‐p21 DU145 cells in nude mice (Figure 6B). Thus, our results showed that PKM2 suppression dramatically inhibited tumour growth and progression in vivo.
3.6. 3‐Brpa or rapamycin reduces the tumour progression of prostate cancer cells in the nude mice
It has been shown that rapamycin or 3‐Brpa suppressed the proliferation of lincRNA‐p21‐silenced prostate cancer cells. Next, we estimated the effect of rapamycin or 3‐Brpa on the tumour development of lincRNA‐p21‐silenced LNCaP cells in nude mice. Three weeks post shlincRNA‐p21 LNCaP cells injection, nude mice bearing tumours were treated with 2 mg/kg rapamycin or 4 mg/kg 3‐Brpa and the tumour volume was evaluated. Our results showed that tumour progression was significantly suppressed in rapamycin‐ or 3‐Brpa‐treated nude mice as compared with those of the control solvent‐treated group (Figure 7A‐D). Moreover, rapamycin treatment inhibited PKM2 expression in vivo (Figure 7E). Cell proliferation was suppressed after rapamycin or 3‐Brpa treatment as indicated by decreased protein level of Ki67 (Figure 7E,F). Because androgen is a stimulator of glycolysis,24 we attempted to clarify whether androgen participate in lincRNA‐p21‐mediated prostate cancer. First, we treated LNCaP cells with androgen and found that androgen had no effect on the expression of lincRNA‐p21 (Figure S3A). Furthermore, we treated shlincRNA‐p21 LNCaP cells with androgen. The results showed that PKM2 expression and glucose consumption in shlincRNA‐p21 LNCaP cells remained unchanged after androgen treatment (Figure S3B). In conclusion, rapamycin or glycolytic inhibitor might be a promising therapeutic strategy for prostate cancer patients with lowly expressed lincRNA‐p21.
Figure 7.
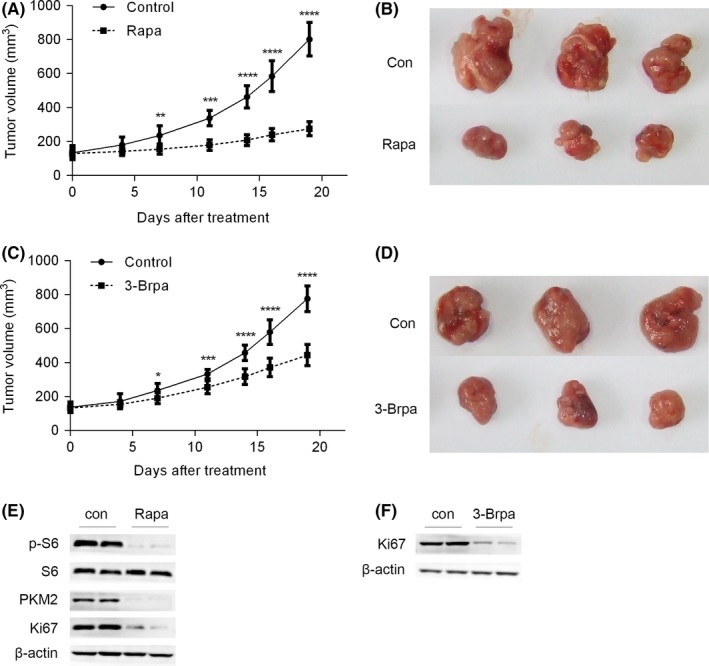
3‐Brpa or rapamycin reduces the tumour burden of prostate cancer cells in nude mice. (A and B) LNCaP cells with lowly expressed lincRNA‐p21 were inoculated into nude mice. Three weeks after injection, the mice bearing tumour were treated with placebo (Con) or rapamycin (Rapa) n = 8. (A) Tumour volume was calculated. (B) Representative photograph of tumours. (C and D) LNCaP cells with lowly expressed lincRNA‐p21 were inoculated into nude mice. 3 weeks after injection, the mice bearing tumour were treated with placebo (Con) or 3‐Brpa (Rapa) n = 8. (A) Tumour volume was calculated. (B) Representative photograph of tumours. (E and F) Tumour tissues described in (A) and (C) were subjected to Western blot. *P< .05; **P< .01; ***P< .001; ****P< .0001
4. DISCUSSION
Increasing evidences show that lincRNA‐p21 plays a vital role in various cancer types, including lung cancer,25 head and neck cancer,26 and liver cancer.7 LincRNA‐p21 is identified as a tumour suppressive long non‐coding RNA (lncRNA) in hepatocellular carcinoma.7 Besides, our previous study showed that lincRNA‐p21 inhibited the development of human prostate cancer. However, the molecular mechanism of lincRNA‐p21 suppressing cancer progression remains less clear. In this work, we found that knockdown of lincRNA‐p21 significantly elevated PKM2 expression and lincRNA‐p21 overexpression decreased PKM2 abundance, revealing that lincRNA‐p21 negatively regulates PKM2 expression. Furthermore, decreasing lincRNA‐p21 level accompanied by increasing PKM2 expression was also observed in a large proportion of human prostate cancer samples, indicating that this inverse regulatory correlation might exist in human prostate cancer patients.
PI3K/AKT/mTOR signalling pathway plays a crucial role in cancer development, including prostate cancer.21 This pathway is a key regulator of cellular metabolism, involved in glucose uptake, glycolysis, fatty acid oxidation and protein synthesis.27 In response to insulin, the activity of PI3K/AKT signalling is enhanced, resulting in hyper‐activation of mTOR.27, 28 Hyper‐activation of mTOR signalling leads to increased mRNA transcription and protein synthesis,29, 30 thus promoting cell proliferation and growth. In addition, activation of this pathway also results in enhanced PKM2 abundance. However, the potential relationship between lincRNA‐p21 and the signalling pathway is less explored. In our study, we found that PKM2 was up‐regulated and AKT or mTOR signalling was activated after lincRNA‐p21 knockdown. Moreover, up‐regulated PKM2 was inhibited by AKT or mTOR inhibitor treatment, suggesting that increased PKM2 expression induced by lincRNA‐p21 reduction depends on PTEN/AKT/mTOR cascade. Also, decreased PTEN level indicated that PTEN might be a direct target of lincRNA‐p21, while the precise regulatory relationship remains to be determined.
It is well known that aerobic glycolysis is a hallmark of cancer, and that increased glucose consumption and elevated lactate production are key characteristics of cancer cell.31 Recently, it was thought that aerobic glycolysis favours various biosynthetic pathways and then provides the metabolic requirements for proliferation. Initially, we observed that the culture medium of lincRNA‐p21‐silenced DU145 and LNCaP cells turned to yellow much faster than that of the control cells. Suspecting that this may be a result of enhanced production of lactate, we analysed the lactate production, as well as the glucose consumption and pyruvate concentration. We found that all of these were enhanced in shlincRNA‐p21 DU145 and LNCaP cells. This suggested that blockade of lincRNA‐p21 prominently enhanced Warburg effect. In addition, we also revealed that the enhanced aerobic glycolysis was rescued by knockdown of PKM2. Therefore, PKM2 up‐regulation is critical for aerobic glycolysis activation in lincRNA‐p21‐silenced prostate cancer cells. mTOR signalling plays an important role in glycolysis and mTOR‐activated glycolysis could be reversed by rapamycin. In our study, rapamycin significantly decreased Warburg effect in DU145 cells with lowly expressed lincRNA‐p21, indicating that mTOR up‐regulation of PKM2 is essential to enhanced aerobic glycolysis in lincRNA‐p21‐silenced prostate cancer cells. It has been reported that p53 silencing promotes aerobic glycolysis23 and our previous study found that p53 was down‐regulated after lincRNA‐p21 knockdown. Thus, we overexpressed p53 in shlincRNA‐p21 DU145 cells and detected the glycolysis. However, the expression of PKM2, glucose consumption and lactate production remained unchanged, suggesting that lincRNA‐p21 negatively regulated aerobic glycolysis independent of p53 signalling. As androgen is a key regulator of prostate cancer development and aerobic glycolysis, we also analysed whether lincRNA‐p21 down‐regulation of PKM2, aerobic glycolysis was dependent of androgen. We first treated LNCaP cells with androgen and found that androgen did not affect the expression of lincRNA‐p21. In addition, enhanced PKM2 expression and glycolysis in shlincRNA‐p21 LNCaP cells remained unchanged after androgen treatment, revealing that the regulation is independent of androgen.
Pyruvate kinase M2 plays a vital role in regulating anabolic metabolism including aerobic glycolysis. Previous studies revealed that PKM2 was commonly up‐regulated in human cancer cells.14, 15, 16 Therefore, targeting PKM2 or aerobic glycolysis is recognized as a promising therapeutic strategy in various human cancer. However, the outcomes are still less satisfactory, mainly due to frequent relapse and severe side effects. Our in vitro results showed that knockdown of PKM2 dramatically suppressed the cell proliferation, colony formation of LNCaP and DU145 cells with lowly expressed lincRNA‐p21. In addition, lincRNA‐p21‐silenced DU145 cells were more sensitive to 3‐Brpa or rapamycin treatment. These results indicated that targeting PKM2 or glycolysis might be specific for human prostate cancer patients with suppressed lincRNA‐p21. Likewise, knockdown of PKM2 also retarded the tumour initiation and progression of lincRNA‐p21‐silenced prostate cancer cells in nude mice. Furthermore, tumour burden was also suppressed after rapamycin or 3‐Brpa treatment.
In summary, PKM2 was first identified as a novel downstream target of lincRNA‐p21. This regulatory correlation was dependent on PTEN/AKT/mTOR signalling. Suppression of PKM2 or aerobic glycolysis effectively blocked proliferation and tumour growth of prostate cancer cells with lowly expressed lincRNA‐p21. Thus, targeting PTEN/AKT/mTOR/PKM2 cascade might be a potential strategy for prostate cancer patients harbouring lower lincRNA‐p21 expression.
AUTHOR CONTRIBUTIONS
Dongliang Xu designed the experiments and wrote the paper. Xiaohai Wang and Yongzhi Xu performed all the experiments. Xingjie Wang, Chenyi Jiang, Sha Han, Kai Dong and Mengjun Shen provided experimental support.
CONFLICT OF INTEREST
None.
Supporting information
Wang X, Xu Y, Wang X, et al. LincRNA‐p21 suppresses development of human prostate cancer through inhibition of PKM2. Cell Prolif. 2017;50:e12395 10.1111/cpr.12395
Funding information
This work was supported by the National Natural Science Foundation of China (Grant 81300625), Shanghai Science and Technology Committee (Grant 13DZ1940602) and Shanghai Municipal Commission of Health and Family Planning (Grant 20134423).
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 2. Nakazawa M, Paller C, Kyprianou N. Mechanisms of therapeutic resistance in prostate cancer. Curr Oncol Rep. 2017;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rathkopf DE, Antonarakis ES, Shore ND, et al. Safety and antitumor activity of apalutamide (ARN‐509) in metastatic castration‐resistant prostate cancer with and without prior abiraterone acetate and prednisone. Clin Cancer Res. 2017;23:3544‐3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huarte M, Guttman M, Feldser D, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dimitrova N, Zamudio JR, Jong RM, et al. LincRNA‐p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol Cell. 2014;54:777‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoon JH, Abdelmohsen K, Srikantan S, et al. LincRNA‐p21 suppresses target mRNA translation. Mol Cell. 2012;47:648‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang N, Fu Y, Zhang H, Sima H, Zhu N, Yang G. LincRNA‐p21 activates endoplasmic reticulum stress and inhibits hepatocellular carcinoma. Oncotarget. 2015;6:28151‐28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall JR, Messenger ZJ, Tam HW, Phillips SL, Recio L, Smart RC. Long noncoding RNA lincRNA‐p21 is the major mediator of UVB‐induced and p53‐dependent apoptosis in keratinocytes. Cell Death Dis. 2015;6:e1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhai H, Fesler A, Schee K, Fodstad O, Flatmark K, Ju J. Clinical significance of long intergenic noncoding RNA‐p21 in colorectal cancer. Clin Colorectal Cancer. 2013;12:261‐266. [DOI] [PubMed] [Google Scholar]
- 10. Isin M, Ozgur E, Cetin G, et al. Investigation of circulating lncRNAs in B‐cell neoplasms. Clin Chim Acta. 2014;431:255‐259. [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Ruan Y, Wang X, et al. Long intragenic non‐coding RNA lincRNA‐p21 suppresses development of human prostate cancer. Cell Prolif. 2017;50(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dayton TL, Jacks T, Vander Heiden MG. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016;17:1721‐1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43:969‐980. [DOI] [PubMed] [Google Scholar]
- 14. Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44:9417‐9429. [DOI] [PubMed] [Google Scholar]
- 15. Mazurek S. Pyruvate kinase type M2: a key regulator within the tumour metabolome and a tool for metabolic profiling of tumours. Ernst Schering Found Symp Proc. 2007;4:99‐124. [DOI] [PubMed] [Google Scholar]
- 16. Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300‐308. [DOI] [PubMed] [Google Scholar]
- 17. Jiang Y, Wang Y, Wang T, et al. PKM2 phosphorylates MLC2 and regulates cytokinesis of tumour cells. Nat Commun. 2014;5:5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang W, Xia Y, Hawke D, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150:685‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu H, Wu J, Zhang W, et al. PKM2 enhances chemosensitivity to cisplatin through interaction with the mTOR pathway in cervical cancer. Sci Rep. 2016;6:30788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuan Y, Guo‐Qing P, Yan T, Hong‐Lin Y, Gong‐Hua H, Cai‐Gao Z. A study of PKM2, PFK‐1, and ANT1 expressions in cervical biopsy tissues in China. Med Oncol (Northwood, London, England). 2012;29:2904‐2910. [DOI] [PubMed] [Google Scholar]
- 21. Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Talesa VN, Ferri I, Bellezza G, Love HD, Sidoni A, Antognelli C. Glyoxalase 2 is involved in human prostate cancer progression as part of a mechanism driven by PTEN/PI3K/AKT/mTOR signaling with involvement of PKM2 and ERalpha. Prostate. 2017;77:196‐210. [DOI] [PubMed] [Google Scholar]
- 23. Ros S, Floter J, Kaymak I, et al. 6‐Phosphofructo‐2‐kinase/fructose‐2,6‐biphosphatase 4 is essential for p53‐null cancer cells. Oncogene. 2017;36:3287‐3299. [DOI] [PubMed] [Google Scholar]
- 24. Vaz CV, Marques R, Alves MG, et al. Androgens enhance the glycolytic metabolism and lactate export in prostate cancer cells by modulating the expression of GLUT1, GLUT3, PFK, LDH and MCT4 genes. J Cancer Res Clin Oncol. 2016;142:5‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castellano JJ, Navarro A, Vinolas N, et al. LincRNA‐p21 impacts prognosis in resected non‐small cell lung cancer patients through angiogenesis regulation. J Thorac Oncol. 2016;11:2173‐2182. [DOI] [PubMed] [Google Scholar]
- 26. Fayda M, Isin M, Tambas M, et al. Do circulating long non‐coding RNAs (lncRNAs) (LincRNA‐p21, GAS 5, HOTAIR) predict the treatment response in patients with head and neck cancer treated with chemoradiotherapy? Tumour Biol. 2016;37:3969‐3978. [DOI] [PubMed] [Google Scholar]
- 27. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353‐361. [DOI] [PubMed] [Google Scholar]
- 29. Laplante M, Sabatini DM. mTOR Signaling. Cold Spring Harb Perspect Biol. 2012;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713‐1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warburg O. On the origin of cancer cells. Science (New York, NY). 1956;123:309‐314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


