Abstract
Objective
Zinc oxide (ZnO) nanoparticles can exhibit toxicity towards organisms and oxidative stress is often hypothesized to be one of the most important factors. Nevertheless, the detailed mechanism of toxicity‐induced by ZnO nanoparticles has not been completely addressed. The present study aimed to investigate the toxic effects of ZnO nanoparticles on the expression and activity of Na+/K+‐ATPase and on potassium channel block.
Materials and methods
In the present study, we explored the cytotoxic effect of ZnO nanoparticles on murine photoreceptor cells using lactate dehydrogenase (LDH) release assay, reactive oxygen species (ROS) determination, mitochondrial membrane potential (Δφm) measurement, delayed rectifier potassium current recordings and Na+/K+‐ATPase expression and activity monitoring.
Results
The results indicated that ZnO nanoparticles could increase the LDH release in medium, aggravate the ROS level within cells, collapse the Δφm, block the delayed rectifier potassium current, and attenuate the expressions of Na+/K+‐ATPase at both mRNA and protein levels and its activity, and thus exert cytotoxic effects on murine photoreceptor cells, finally damaging target cells.
Conclusion
Our findings will facilitate the understanding of the mechanism involved in ZnO nanoparticle‐induced cytotoxicity in murine photoreceptor cells via potassium channel block and Na+/K+‐ATPase inhibition.
Keywords: delayed rectifier potassium current, murine photoreceptor cell, Na+/K+‐ATPase, reactive oxygen species, zinc oxide nanoparticle
1. Introduction
Nowadays, the rapid development of nanotechnology provides novel materials with superior properties and these nanomaterials have been widely applied in the biomedical field such as gene transfection,1, 2 diagnostic tool,3, 4 drug delivery,5, 6 cosmetic products,7 anti‐cancer agents8, 9, 10 and so on. The risks of nanomaterials to the organism, to some extent, remain a point of concern, mostly due to the lack of systematic evaluation and scientific knowledge concerning to their potential to affect health. Concerns have been raised on how the exposure to nanomaterials would influence human health.11, 12, 13 Some investigators have performed experiments to explore the relationship between nanomaterials and human as well as ecosystem safety.
Recently, researchers have revealed that zinc oxide (ZnO) nanoparticles could exert potential genotoxicity to organisms.14, 15, 16 The ZnO nanomaterials release into the aquatic ecosystems through domestic and industrial wastewaters has the potential to induce pernicious effects on fish and other organisms.17 Studies have also found that the genotoxicity is linked to the elevated level of reactive oxygen species (ROS),18 disrupted calcium homeostasis,19 the activation of the relevant signalling pathways, such as the mitogen‐activated protein kinase signalling pathway,20 the caspase‐dependent signalling pathway21 and so on. Nevertheless, the underlying mechanisms have not been well addressed.
Mitochondria are small ubiquitous organelles which play important roles in electron transport, mitochondrial transmembrane potential, cellular oxidation‐reduction, especially in terms of ROS production and ROS scavenging.22 Excessive production of ROS in mitochondria will damage many mitochondrial macromolecules, such as lipids, proteins and DNA. Overproduction of ROS will also enhance the release of cytochrome c (caspase activator), resulting in the impaired mitochondria leading to cellular damage or even cell death.23 Therefore, it is crucial for the normal physiological conditions to maintain mitochondrial homeostasis within cells.
The ATP‐dependent Na+/K+ pump can transport three Na+ out of cells in exchange for two K+ into the cell per molecule of ATP hydrolysed, against their respective electrochemical gradients. The appropriate Na+ and K+ gradients generated via Na+/K+ pump can maintain the membrane potential and are essential for cellular electric excitability.24, 25 Therefore, Na+/K+‐ATPase will create a gradient of Na+ and K+, and is necessary for all living mammalian cells. Thus, dysfunction of Na+/K+‐ATPase will induce loss of cell ion gradients, resulting in the damage of cells. Na+/K+‐ATPase is a P‐type ATPase composed of at least two subunits: a large catalytic α subunit and a regulatory, single‐transmembrane‐domain β subunit.26 So far, four α isoforms (α1‐4) and four β isoforms (β1‐4) have been identified, and found to be encoded by four different genes, Atp1a1‐4 and Atp1b1‐4 respectively.26, 27, 28 The α1‐isoform (encoded by Atp1a1 gene) is regarded to be uniformly expressed in all cells,29, 30, 31 and β2‐isoform (encoded by Atp1b2 gene) of the Na+/K+‐ATPase is predominantly expressed distinct neuronal cell types including photoreceptor cells in the retina.32, 33 Currently, the retina has been established as having a high level of “free” (loosely bound) zinc in the region of the photoreceptor terminals where it has been proposed to play a role in the modulation of glutamate release.34
Our previous investigation has revealed that ZnO nanoparticles can inhibit murine photoreceptor‐derived cell proliferation and migration via reducing TGF‐β and MMP‐9 expression.35 In the current study, to investigate the cytotoxic effects of ZnO nanoparticles on murine photoreceptor cells (661W), we have explored the influence of ZnO nanoparticles on the lactate dehydrogenase (LDH) release of murine photoreceptor cells, mitochondrial membrane potential (Δφm) and ROS levels, potassium channel block, and Na+/K+‐ATPase expression at both mRNA and protein levels and its activity. Our investigation will facilitate the understanding of the mechanism that is associated with the potassium channel block and decreased Na+/K+‐ATPase expression and activity based on ZnO nanoparticle‐induced cytotoxicity in murine photoreceptor cells.
2. Materials and methods
2.1. ZnO nanoparticles and preparation of ZnO nanoparticle solutions
Zinc oxide nanoparticles were obtained from Shanghai Fortunebio‐tech Co., Ltd (Shanghai, China) and were modified without any stabilizer molecules. The size distribution of ZnO nanoparticles characterized by a field emission scanning electron microscope ranged from 15 to 50 nm and the mean diameter was about 30 nm.36 For the application of ZnO nanoparticles during experiments, the ZnO nanoparticle suspension was prepared by directly adding a definite amount of ZnO nanoparticles to 5 mL Dulbecco's modified Eagle's medium (DMEM) to be as a stock suspension (6250 μmol/L). Furthermore, the stock suspension was dispersed by a probe sonicator (BILON96‐II; Xi'an Bilon Biotechnology Co. Ltd, Xi'an, China) for 30 minutes on ice. Subsequently, various concentrations (31.25, 62.5 and 125.0 μmol/L respectively) of ZnO nanoparticle suspensions (dissolved in DMEM) were prepared and sonicated with a probe sonicator on ice for 10 minutes, followed by vortexing for 60 seconds prior to experiments.
2.2. Cell culture
A murine photoreceptor cell line (661W) used in the present study was provided by Dr. Muayyad R. Al‐Ubaidi (University of Oklahoma Health Sciences Center, USA). In this study, 661W cells were cultured in DMEM (Life technologies, Oklahoma City, OK 73190, USA) supplemented with 1.0 g/L of glucose, 10% foetal bovine serum (FBS Gaithersburg, MD) (HyClone, Logan, UT, USA), 100 μg/mL streptomycin and 100 U/mL penicillin. All cells were cultured at 37°C in a water‐saturated incubator containing 5% CO2 plus 95% air. Cell counts were performed using an automated cell counter (TC10; Bio‐Rad, Hercules, CA, USA).
2.3. LDH release
Lactate dehydrogenase is a cytoplasmic enzyme that exists in all living cells. If the cell membrane is damaged, LDH will be released into extracellular medium. Thus, the LDH release assay can be applied to accurately quantify the cytotoxicity of chemicals via the measurement of LDH released from the damaged cells. In the present study, we measured the LDH release level in extracellular medium of ZnO nanoparticle‐exposed 661W cells grown in DMEM supplemented with 10% FBS using an LDH Cytotoxicity Assay Kit (Beyotime Institute of Biotechnology, Nantong, China). Briefly, 661W cells (5.0 × 105 cells per well) were seeded in a six‐well plate and cultured overnight at 37°C in a 5% CO2‐containing incubator, then cells were exposed to various concentrations of ZnO nanoparticles (0, 31.25, 62.5 and 125.0 μmol/L respectively). After 24‐hour exposure to ZnO nanoparticles, 120 μl of supernatant under different treatment conditions was applied to react with 120 μL of the reaction mixture from the LDH Cytotoxicity Assay Kit for 30 minutes at room temperature. Supernatant from 661W cells not exposed to ZnO nanoparticles served as negative control. All procedures were performed in accordance with the manufacturer's instructions. The absorbance of reacted LDH was assessed at 490 nm using a UV‐Vis spectrophotometer (4802S; Unico [Shanghai] Instrument Co., Ltd., Shanghai, China).
2.4. Intracellular ROS
To obtain further evidence for direct actions of ZnO nanoparticles on murine photoreceptor cells, we monitored the alterations in ROS levels after treatment with different concentrations of ZnO nanoparticles. The production of intracellular ROS was measured using 2′,7′‐dichlorofluorescin diacetate (DCFH‐DA; Invitrogen, Carlsbad, CA, USA) by a flow cytometer (Accuri C6; Accuri Cytometers Inc., Ann Arbor, MI, USA). Briefly, cells (5 × 105 cells/well) were seeded in a six‐well plate and grown overnight, then incubated with 0, 31.25, 62.5 and 125.0 μmol/L of ZnO nanoparticles for 6 hours, respectively. After harvest of cells, the cells were incubated with DCFH‐DA solution (10 μmol/L) in the dark at 37°C for 30 minutes. Then cells were washed with phosphate‐buffered saline (PBS) and analysed within 30 minutes using flow cytometry. The specific fluorescence signals corresponding to DCFH‐DA were collected with a 525‐nm band pass filter. As a rule, 2.0 × 104 cells were counted in each determination.
2.5. Mitochondrial membrane potential (Δφm)
Maintenance of the Δφm is essential for the normal performance and survival of cells. Usually, 5,5′,6,6′‐tetrachloro‐1,1′,3,3′‐tetraethyl benzimidazolyl carbocyanine iodide (JC‐1) accumulates in the mitochondria as aggregates (whose fluorescence is red) and also in the cytoplasm as monomers (whose fluorescence is green) in healthy cells. During early apoptosis, the Δφm collapses. As a consequence, JC‐1 aggregates cannot accumulate within the mitochondria and dissipate into JC‐1 monomers leading to loss of red fluorescence.18 Thus, red fluorescence responds linearly to an increase in membrane potential.37 In the present study, the alterations of Δφm in cells were measured using JC‐1 probe (Beyotime Institute of Biotechnology, Nantong, China). Briefly, 661W cells (5.0 × 104) were seeded in a six‐well plate and grown overnight, then incubated with 0, 31.25, 62.5 and 125.0 μmol/L of ZnO nanoparticles for 6 hours respectively. After digestion with 0.25% trypsin, cells were harvested and washed twice with cold PBS, followed by the incubation with JC‐1 probe solution at 37°C for 30 minutes in the dark and washing with cold PBS. Finally, the Δφm was measured by a flow cytometer (BDVerse; BD Biosciences, Franklin Lakes, NJ, USA).
2.6. Whole‐cell patch clamp
Voltage‐dependent potassium ion (K+) channels (Kv channels) can conduct K+ across the cell membrane in response to changes in the membrane voltage, and regulate target cell excitability by modulating the shape and frequency of action potentials, thereby having both physiological and pathophysiological implications.38 In the current study, an automated patch clamp experiment was performed using a Nanion chip‐based port‐a‐patch system (EPC‐10, HEKA; Nanion Technologies, Munich, Germany) that can enable completely automated patch clamp recordings at room temperature (23‐25°C). Briefly, 661W cells were cultured in a six‐well plate overnight before patch clamp experiments. Upon the day of experiments, cells were rinsed with PBS, dissociated by exposure to 0.25% trypsin containing 1 mmol/L EDTA. After treatment with various concentrations (ie, 0, 31.25, 62.5, 125.0 μmol/L) of ZnO nanoparticles for 5 minutes, cells were used to determine the delayed rectifier K+ current. The electrolyte solutions used had the following compositions: extracellular solutions: 10 mmol/L NaCl, 75 mmol/L CsCl, 2 mmol/L MgCl2, 70 mmol/L CsF, 10 mmol/L Hepes/KOH, pH 7.2 and intracellular solutions: 10 mmol/L KCl, 75 mmol/L NaCl, 70 mmol/L NaF, 2 mmol/L MgCl2, 2 mmol/L CaCl2, 5 mmol/L d‐glucose monohydrate, 2 mmol/L EGTA, 10 mmol/L Hepes/NaOH, pH 7.4. The delayed rectifier K+ currents were determined at a depolarizing potential of +40 mV from a holding potential of −80 mV. The Patchmaster software (Nanion Technologies, Munich, Germany) was used to record the delayed rectifier outward K+ current.
2.7. Quantitative PCR analysis of Na+/K+‐ATPase mRNA levels
To investigate the effect of different concentrations of ZnO nanoparticles on the expressions of Na+/K+‐ATPase (ie, Atp1a1, Atp1b2) mRNA, quantitative PCR (Q‐PCR) was performed. Briefly, 2.0 × 105 661W cells were seeded in every well in a six‐well plate and grown overnight, and were then treated with different concentrations (0, 31.25, 62.50 and 125.0 μmol/L respectively) of ZnO nanoparticles for 2 hours. After incubation, cells were harvested by trypsinization, and total cellular RNA was then extracted from 661W cells using Trizol reagent (Aidlab Biotech, Beijing, China) according to the manufacturer's instructions. After quantification of total RNA using a micro‐spectrophotometer (K5600; Beijing Kaiao Technology Development Co., Ltd, Beijing, China), single‐stranded cDNA was synthesized with 600 ng of total RNA. Furthermore, cDNA templates obtained by reverse transcription were used to quantify the gene level by Q‐PCR technique with 2×Syb Green qPCR Mix (Aidlab Biotech). The target‐specific primers are listed in Table 1, and the PCR programme was set as follows: 5 minutes at 95°C, followed by 50 cycles at 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds. The fold changes were calculated using the 2ΔΔCt method after normalization to respective endogenous GAPDH control.39
Table 1.
Primers in amplification of target genes by quantitative PCR
| Gene | Primer sequences |
|---|---|
| Atp1a1 | F: 5′‐GAGGCAGCCCAGAAACCCCAAAAC‐3′ |
| R: 5′‐TCGGCCCACTGCACTACCACAATA‐3′ | |
| Atp1b2 | F: 5′‐GGCAGCCCTGTGTCTTCATCAAA‐3′ |
| R: 5′‐TGCGGCATTCAACATTCACCTC‐3 | |
| GAPDH | F: 5′‐ACGGCAAATTCAACGGCACAGTCA‐3′ |
| R: 5′‐CGGCAGAAGGGGCGGAGATG‐3′ |
2.8. Protein level and activity of intracellular Na+/K+‐ATPase
The levels of intracellular Na+/K+‐ATPase and their activities in murine photoreceptor cells were further assessed before and after treatment with various concentrations of ZnO nanoparticles. In brief, 661W cells (4 × 105 cells/well) were seeded in six‐well plates and cultured at 37°C in an incubator overnight, then the supernatant was discarded and further supplemented with various concentrations (0, 31.25, 62.50 and 125.0 μmol/L respectively) of ZnO nanoparticles for 6 hours (final volume: 2 mL). At the indicated time, cells were harvested after digestion with 0.25% trypsin containing 1% EDTA, then the cells were collected by centrifugation at 3500 g for 15 minutes, followed by resuspension in cold PBS. Next, cells were centrifuged at 3500 g for 15 minutes, and the cell pellet was vortexed in 0.5 mL of cold PBS, sonicated on ice for 10 minutes. After centrifugation at 5000 g for 10 minutes, the levels of Na+/K+‐ATPase from cell extracts in 100 μL of the supernatant were measured by Mouse ATPase (ATPase, Na+/K+ transporting) ELISA Kit in accordance with the manufacturer's instructions (Wuhan ColorfulGene Biological Technology Co., Ltd, Wuhan, China). Meanwhile, Na+/K+‐ATPase activity (50 μL for each sample) was also measured using Na+/K+‐ATPase Activity Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. The value of optical density was determined using a 4802S UV/Vis Double Beam Spectrophotometer (Unico [Shanghai] Inc.) at 636 nm, and every experiment was repeated for three times.
2.9. Statistical analysis
Data are presented as mean ± SD (standard deviation) from at least three independent experiments. One‐way analyses of variance and post hoc procedures based on Newman‐Keuls tests were used for significant test and P<.05 was considered significant.
3. Results
3.1. Measurement of LDH release
Figure 1 shows the profile of LDH release from DMEM‐grown 661W cells after exposure to various concentrations of ZnO nanoparticles for 24 hours. It was observed that compared with those observed in negative control sample (ie, cells not exposed to ZnO nanoparticles), exposure 661W cells to ZnO nanoparticles for 24 hours led to apparent increases in the levels of LDH release into DMEM, and the increases of LDH release were closely correlated with concentrations of ZnO nanoparticles incubated with cells.
Figure 1.
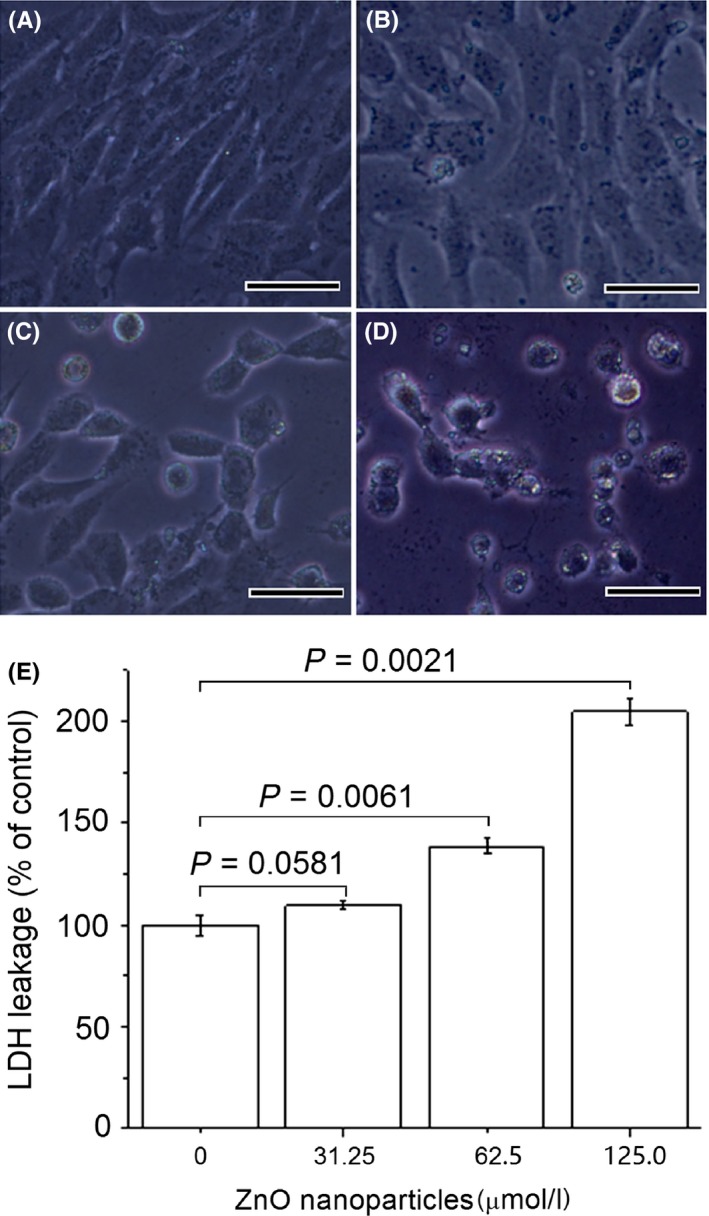
Effects of zinc oxide (ZnO) nanoparticles on cell morphology and lactate dehydrogenase (LDH) release from DMEM supplemented with 10% foetal bovine serum‐grown 661W cells. Cells were exposed to different concentrations of ZnO nanoparticles (ie, 0, 31.25, 62.5, 125.0 μmol/L) for 24 h before assessing morphology and LDH release. Negative control was 661W cells not exposed to ZnO nanoparticles. (A) untreated cells; (B) cells treated with 31.25 μmol/L of ZnO NPs; (C) cells treated with 62.5 μmol/L of ZnO NPs; (D) cells treated with 125.0 μmol/L of ZnO NPs; and (E) the results of histogram analysis for LDH release. P<.05 is considered significant compared with negative control, NPs=nanoparticles and bar=20 μm
3.2. Determination of intracellular ROS
After the cells exposure to 0, 31.25, 62.5 and 125.0 μmol/L of ZnO nanoparticles for 6 hours, the ROS generation rose from 1.8 ± 0.35% to 31.7 ± 2.74%, 43.8 ± 2.97% and 61.5 ± 5.36% (Figure 2) respectively. These facts demonstrated that with the increase of concentrations of ZnO nanoparticles incubated with cells, the levels of ZnO nanoparticle‐induced ROS were also elevated, indicating that the ROS production increased in a concentration‐dependent manner. In addition, there existed a significant difference for the amounts of ZnO nanoparticle‐induced ROS compared to that of control samples.
Figure 2.
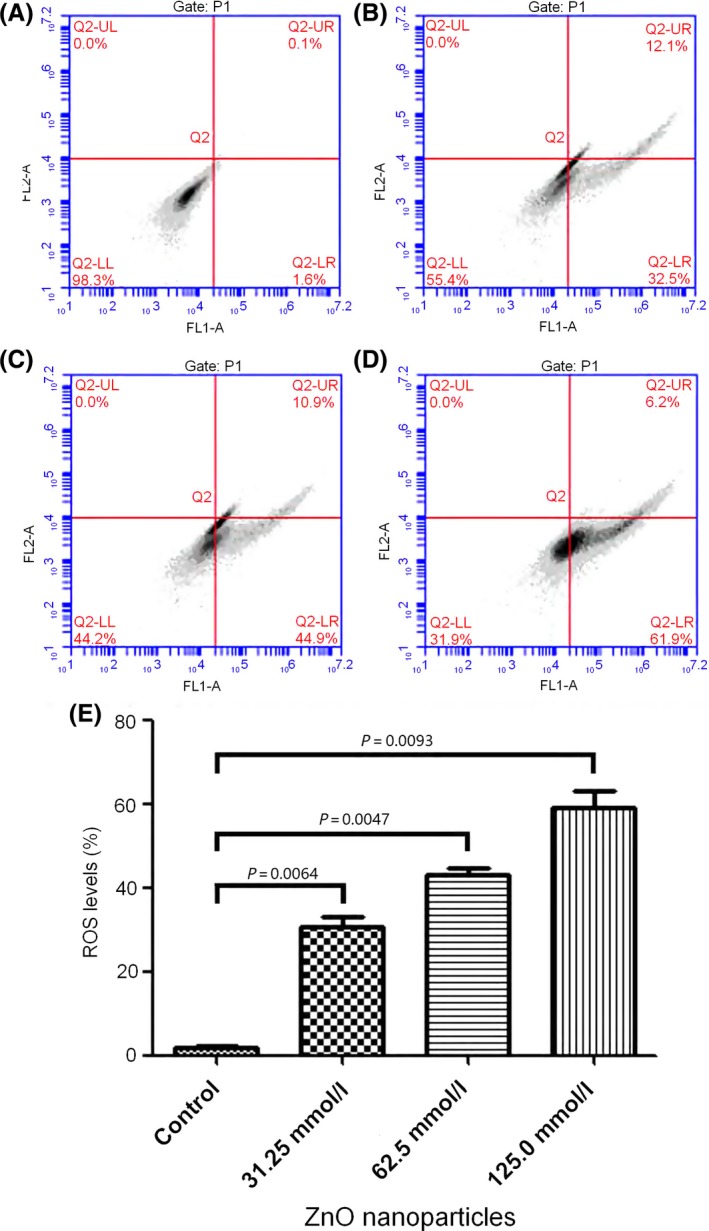
The reactive oxygen species (ROS) levels of 661W cells after exposure to different concentrations of zinc oxide (ZnO) NPs for 24 h assessed by flow cytometry. (A) Normal cells; (B) cells treated with 31.25 μmol/L of ZnO NPs; (C) cells treated with 62.5 μmol/L of ZnO NPs; (D) cells treated with 125.0 μmol/L of ZnO NPs; (E) result of histogram analysis. Data are from three independent experiments performed by flow cytometry. NPs=nanoparticles
3.3. Alterations in Δφm
Treatment with ZnO nanoparticles appeared to decrease the red fluorescence intensity of 661W cells. As shown in Figure 3, after treatment with 31.25, 62.50 and 125.0 μmol/L of ZnO nanoparticles for 6 hours, the red fluorescence intensity decreased from 94.6 ± 1.2% (control) to 83.5 ± 1.6%, 75.1 ± 2.3% and 64.3 ± 1.7% respectively.
Figure 3.
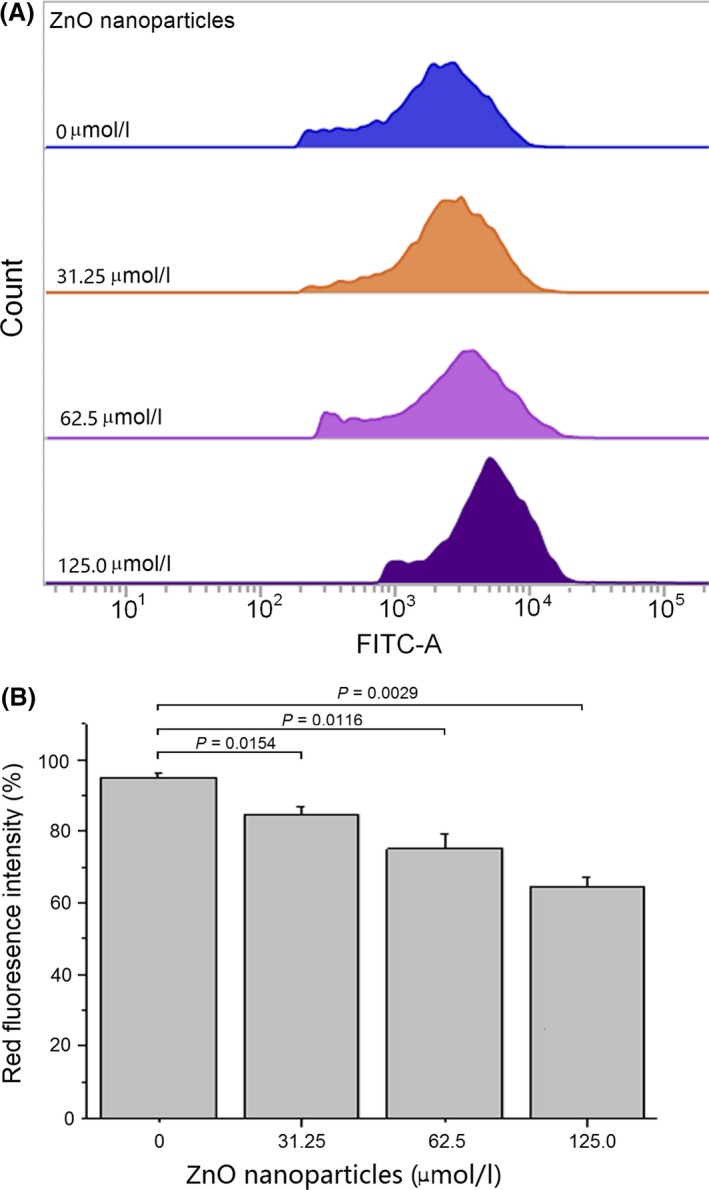
Alterations of mitochondrial membrane potential (Δφm) in murine photoreceptor cells after treatment with different concentrations of zinc oxide (ZnO) nanoparticles for 6 h. Red fluorescence represents the J‐aggregates and indicates the degree of integrity of mitochondrial membrane in living cells. (A) Diagrams obtained from flow cytometry; (B) result of histogram analysis. Data are from three independent experiments performed by flow cytometry
3.4. Inhibition of the delayed rectifier outward K+ current
As shown in Figure 4, murine photoreceptor cells alone possessed larger delayed rectifier outward K+ current. However, after cells exposure to different ZnO nanoparticles, the delayed rectifier outward K+ currents were decreased in a ZnO nanoparticle‐dependent manner, ie, the higher the concentration of ZnO nanoparticles incubated with target cells, the less delayed rectifier outward K+ currents of cells determined by whole‐cell patch clamp.
Figure 4.
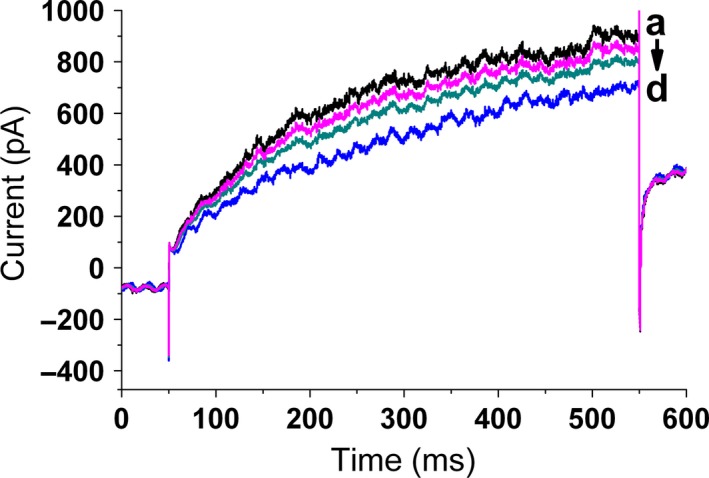
Zinc oxide (ZnO) nanoparticles induces Kv current decrease in murine photoreceptor cells. Cells were treated with different concentrations (ie, 0, 31.25, 62.5, 125.0 μmol/L) of ZnO nanoparticles for 5 min, then Kv currents were measured by using the Nanion chip‐based port‐a‐patch system. (A) Actual traces of Kv currents in a ZnO nanoparticle‐free cell; (B) representative Kv current trace of a cell after treatment with 31.25 μmol/L of ZnO nanoparticles; (C) representative Kv current trace of a cell after treatment with 62.5 μmol/L of ZnO nanoparticles; and (D) representative Kv current trace of a cell after treatment with 125.0 μmol/L of ZnO nanoparticles
3.5. Decrease of Na+/K+‐ATPase
After incubation with different concentrations of ZnO nanoparticles, the intracellular Na+/K+‐ATPase at the both mRNA and protein levels was investigated by either Q‐PCR or ELISA techniques. Figure 5A shows that the mRNA levels of intracellular Na+/K+‐ATPase were reduced after exposure of murine photoreceptor cells to ZnO nanoparticles. The mRNA levels of intracellular Na+/K+‐ATPase were reduced to 45.7%, 62.5% and 33.6% for Atp1a1 and 19.2%, 31.9% and 26.8% for Atp1b2, respectively, and significant differences were observed between untreated cells and ZnO nanoparticle‐treated subjects.
Figure 5.
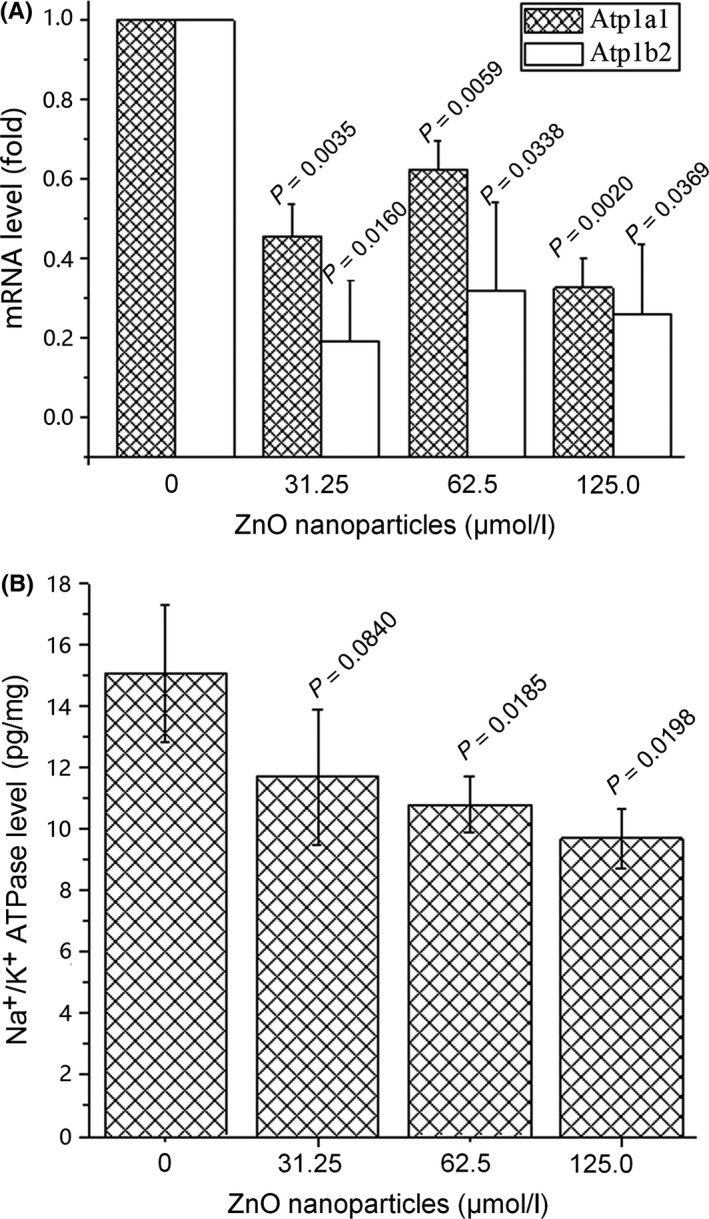
Expressions of Na+/K+‐ATPase at mRNA and protein levels after treatment with different concentrations of zinc oxide (ZnO) nanoparticles. Cells were seeded in six‐well plates and cultured overnight, then treated with different concentrations (ie, 0, 31.25, 62.5, 125.0 μmol/L) of ZnO nanoparticles either for 2 h (for mRNA measurement) or for 6 h (for protein measurement). Next, alterations of Na+/K+‐ATPase in both mRNA (A) and protein (B) levels were determined using quantitative PCR and ELISA techniques respectively. Results were presented as mean ± SD (n=3)
Using the ELISA technique, we also assessed the alterations of intracellular Na+/K+‐ATPase in protein level after murine photoreceptor cells exposure to various concentrations of ZnO nanoparticles. Figure 5B demonstrates that the expression levels of Na+/K+‐ATPase protein were reduced after cells exposure to different concentrations of ZnO nanoparticles. We observed that the protein levels of intracellular Na+/K+‐ATPase within photoreceptor cells were reduced from 15.03 to 11.88, 10.77 and 9.81 pg/mg after exposure to various concentrations of ZnO nanoparticles, respectively, and accompanied by a concentration‐dependent manner.
3.6. Decrease of intracellular Na+/K+‐ATPase activity
To explore the effect of ZnO nanoparticles on the Na+/K+‐ATPase activity, we determined the Na+/K+‐ATPase activity following digestion. We observed that, in the supernatant obtained following trypsin digestion of the cells, the Na+/K+‐ATPase activity was also decreased after cells exposure to different concentrations of ZnO nanoparticles compared with that of untreated murine photoreceptor cells. As shown in Figure 6, ZnO nanoparticle‐treatment could result in the decreased Na+/K+‐ATPase activity. We noted that the Na+/K+‐ATPase activity was decreased from 0.428 to 0.302, 0.344 and 0.324 U/mg, respectively, confirming that ZnO nanoparticles could inhibit the Na+/K+‐ATPase activity.
Figure 6.
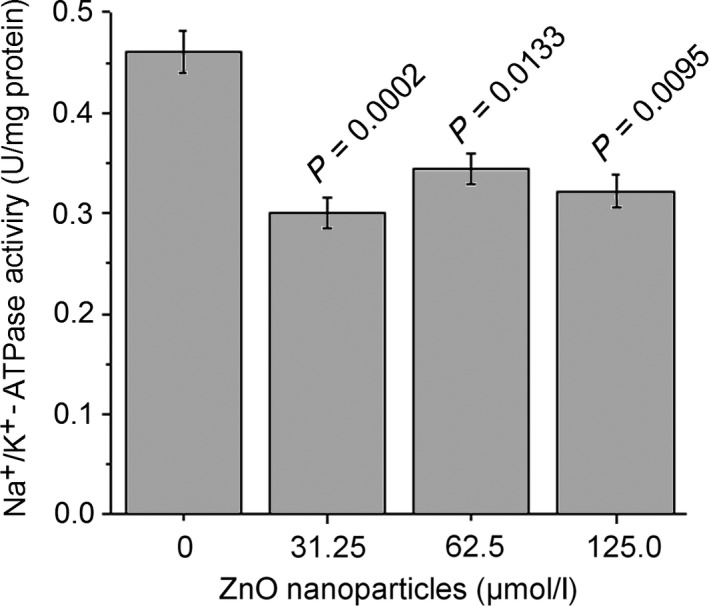
Measurement of Na+/K+‐ATPase activity of murine photoreceptor cells after treatment with different concentrations of ZnO nanoparticles. Cell were treated with different concentrations (0, 31.25, 62.5 and 125.0 μmol/L respectively) of ZnO nanoparticles for 6 h, then cells were digested with 0.25% trypsin, washed with PBS, sonicated on the ice for 15 min, and centrifuged at 5000 g at 4°C for 10 min, finally the supernatant was used to determine Na+/K+‐ATPase activity. Data were presented as mean ± SD of n=3
4. Discussion
Zinc oxide nanoparticles have offered great promise in many industrial and biomedical applications. However, investigations have also shown that ZnO nanoparticles could exert cytotoxic effect on target cells, and the underlying mechanism is involved in oxidative stress.10, 18 Currently, LDH release assay is an appropriate and possibly preferable means of measuring cellular cytotoxic reactions, which can measure the activity of LDH released into the medium from dead cells.40 In the present study, we observed that ZnO nanoparticles could induce LDH release into the medium in a concentration‐dependent manner (Figure 1), causing an elevation of LDH level in medium, indicating that ZnO nanoparticles can apparently induce murine photoreceptor cell damage.
Metabolism of oxygen within cells generates potentially deleterious ROS. Usually, the rate and magnitude of oxidant formation is balanced by the rate of oxidant elimination under normal physiological conditions.41 It has been confirmed that numerous environmental factors, including engineered nanoparticles, can lead to overproduction of ROS and oxidative stress.42 Studies have demonstrated that metal oxide nanoparticle‐induced toxicity is primarily mediated by increased ROS production. Fu et al.43 found that compared to their bulk‐size counterparts, engineered nanomaterials will lead to the production of higher levels of ROS and induction of oxidative damage because of their small size, high specific surface area and high surface reactivity. Regarding ZnO nanoparticles, our investigation revealed that exposure of murine photoreceptor cells to ZnO nanoparticles could lead to the overproduction of ROS (Figure 2). Similarly, Yoo et al.44 observed that treatment with positively charged ZnO nanoparticles made Hela cells generate excessive ROS via adsorption and endocytosis of the nanoparticles and further induce cell death, suggesting the strong inducible role of ZnO nanoparticles in the production of ROS to the organisms. Thus, the overgeneration of ROS will trigger endoplasmic reticulum stress and subsequently cause cell damage, finally inducing target cell apoptosis and/or necrosis.21
Mitochondria are critical subcellular components that play a crucial role in energy generation (adenosine triphosphate, ATP), intermediary metabolism and cell death. Δφm is critical for maintaining the physiological function of the respiratory chain for mitochondria to generate ATP. Normally, a healthy cell has higher Δφm. Mitochondrial dysfunction has been proven to cause the collapse of Δφm, lead to mitochondrial depolarization and release of several apoptogenic proteins into the cytosol, participate in the induction of apoptosis, which may be crucial to the apoptotic pathway.45 In the meantime, mitochondria are also major sources of ROS within the cell. Therefore, the ability to determine Δφm and ROS can provide important clues about the physiological status of the cell and the function of the mitochondria.46 In this paper, we found that exposure to ZnO nanoparticles causes a collapse of mitochondrial inner transmembrane potential, and the higher concentrations of ZnO nanoparticles incubated with target cells, the lower Δφm of target cells possessed (Figure 3). This result indicated that Δφm collapse is a critical event in inducing murine photoreceptor cell death after exposure to ZnO nanoparticles.
Voltage‐dependent potassium channels allow for the selective permeability of K+ in a membrane potential‐dependent manner, playing critical roles in maintaining normal physiological status of cells.47 Zhao et al.48 observed that using the whole‐cell patch clamp technique, ZnO nanoparticles could increase the current‐voltage curve of delayed rectifier potassium current from +20 to +90 mV on rat hippocampal CA3 pyramidal neurons. By contrast, results from the electrophysiological experiments demonstrated that ZnO nanoparticles could block the ion permeation pathway of voltage‐dependent potassium channels, influence the physiological exchange between intracellular sodium ions and extracellular potassium ions, collapse the membrane potential, and disrupt the balance of intracellular microenvironment, finally resulting in the cell damage. These findings indicate that the electrophysiological behaviour varied from the tissues. The electrophysiological property of a single cell may be more sensitive than that of tissue.
Na+/K+‐ATPase is essential for establishing and maintaining hyperpolarized membrane potentials, and the activity of Na+/K+‐ATPase is responsible for transmembrane ion gradients and is fundamental to cell function and survival. Failure of Na+/K+‐ATPase activity has been recognized in the pathogenesis of neurodegeneration.49 At present, it is known that loss of Na+/K+‐ATPase not only eradicates visual function but also leads to age‐dependent degeneration in photoreceptors.50 As a kind of enzyme, Na+/K+‐ATPase is sensitive to alterations in the redox status of cells.51 In our study, we observed that cells exposed to ZnO nanoparticles could cause the elevation of LDH levels and the increment of ROS level, resulting in the occurrence of oxidative stress within cells. If excessive oxidative stress within cells cannot be eliminated in time, it will inhibit both the expressions and activities of Na+/K+‐ATPase, disrupt the balance of intracellular and extracellular ions between Na+ and K+ and block the delayed rectifier outward K+ currents as well as membrane potential, cause the imbalance of physiological homeostasis within cells, and finally damage the cells. However, Sawosz et al.52 found that injection of silver nanoparticles into chicken embryos at the beginning of embryogenesis could increase the expressions of Na+/K+‐ATPase, affecting cell differentiation; meanwhile, Bessemer et al.53 also revealed that exposure of freshwater fish to ZnO nanoparticles could increase the gill Na+/K+‐ATPase activity, and this result may be attributed to the increased epithelial permeability or structural remodelling. Based on above‐mentioned findings, we infer that the differences of Na+/K+‐ATPase activity after exposure to nanoparticles may be interpreted as the difference of tissues exposed to ZnO nanoparticles and the variance of nanomaterials.
5. Conclusions
In conclusion, ZnO nanoparticles can apparently promote the intracellular LDH release into medium, elevate the ROS levels within cells and collapse the Δφm of murine photoreceptor cells. Exposure of murine photoreceptor cells to ZnO nanoparticles will decrease the delayed rectifier potassium currents and attenuate the expressions of Na+/K+‐ATPase and its activity, and thus disrupt the balance of intracellular microenvironment, finally exhibiting cytotoxic effect on murine photoreceptor cells. Taken together, our findings facilitate the understanding of cytotoxic effect of ZnO nanoparticles mediated by potassium channel block and Na+/K+‐ATPase inhibition on murine photoreceptor cells.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the Open Fund Program of Jiangsu Provincial Key Laboratory for Interventional Medical Devices (jr1602) and by the Synergistic Innovative Center for Antivirus with Integrated Traditional Chinese and Western Medicine in Universities of Shandong Province (XTCX2014A04).
Chen C, Bu W, Ding H, et al. Cytotoxic effect of zinc oxide nanoparticles on murine photoreceptor cells via potassium channel block and Na+/K+‐ATPase inhibition. Cell Prolif. 2017;50:e12339 10.1111/cpr.12339
Contributor Information
Hongsheng Bi, Email: azuresky1999@163.com.
Dadong Guo, Email: dadonggene@163.com.
References
- 1. Shahbazi B, Taghipour M, Rahmani H, Sadrjavadi K, Fattahi A. Preparation and characterization of silk fibroin/oligochitosan nanoparticles for siRNA delivery. Colloids Surf B Biointerfaces. 2015;136:867–877. [DOI] [PubMed] [Google Scholar]
- 2. Yu Q, Cao J, Chen B, et al. Efficient gene delivery to human umbilical cord mesenchymal stem cells by cationized porphyra yezoensis polysaccharide nanoparticles. Int J Nanomed. 2015;10:7097–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ching CT, van Hieu N, Cheng TY, et al. Liver cancer detection by a simple, inexpensive and effective immunosensor with zinc oxide nanoparticles. Sensors (Basel). 2015;15:29408–29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savaliya R, Shah D, Singh R, et al. Nanotechnology in disease diagnostic techniques. Curr Drug Metab. 2015;16:645–661. [PubMed] [Google Scholar]
- 5. Zhu D, Tao W, Zhang H, et al. Docetaxel (DTX)‐loaded polydopamine‐modified TPGS‐PLA nanoparticles as a targeted drugdelivery system for the treatment of liver cancer. Acta Biomater. 2016;30:144–154. [DOI] [PubMed] [Google Scholar]
- 6. Thomas SC, Harshita, Mishra PK, Talegaonkar S. Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des. 2015;21:6165–6188. [DOI] [PubMed] [Google Scholar]
- 7. Contado C. Nanomaterials in consumer products: a challenging analytical problem. Front Chem. 2015;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo D, Wu C, Jiang H, Li Q, Wang X, Chen B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. J Photochem Photobiol B. 2008;93:119–126. [DOI] [PubMed] [Google Scholar]
- 9. Guo D, Wu C, Li X, Jiang H, Wang X, Chen B. In vitro cellular uptake and cytotoxic effect of functionalized nickel nanoparticles on leukemia cancer cells. J Nanosci Nanotechnol. 2008;8:2301–2307. [DOI] [PubMed] [Google Scholar]
- 10. Akhtar MJ, Ahamed M, Kumar S, Khan MM, Ahmad J, Alrokayan SA. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomed. 2012;7:845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jain K, Mehra NK, Jain NK. Nanotechnology in drug delivery: safety and toxicity issues. Curr Pharm Des. 2015;21:4252–4561. [DOI] [PubMed] [Google Scholar]
- 12. Martirosyan A, Schneider YJ. Engineered nanomaterials in food: implications for food safety and consumer health. Int J Environ Res Public Health. 2014;11:5720–5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meng H, Xia T, George S, Nel AE. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS Nano. 2009;3:1620–1627. [DOI] [PubMed] [Google Scholar]
- 14. Yin H, Casey PS, McCall MJ, Fenech M. Size‐dependent cytotoxicity and genotoxicity of ZnO particles to human lymphoblastoid (WIL2‐NS) cells. Environ Mol Mutagen. 2015;56:767–776. [DOI] [PubMed] [Google Scholar]
- 15. Condello M, De Berardis B, Ammendolia MG, et al. ZnO nanoparticle tracking from uptake to genotoxic damage in human colon carcinoma cells. Toxicol In Vitro. 2016;35:169–179. [DOI] [PubMed] [Google Scholar]
- 16. Boran H, Ulutas G. Genotoxic effects and gene expression changes in larval zebrafish after exposure to ZnCl2 and ZnO nanoparticles. Dis Aquat Organ. 2016;117:205–214. [DOI] [PubMed] [Google Scholar]
- 17. Beegam A, Prasad P, Jose J, et al. Environmental fate of zinc oxide nanoparticles: risks and benefits Toxicology‐New Aspects to This Scientific Conundrum 2016:81 p. Editor: Larramendy Marcelo L. and Soloneski Sonia. Publisher: InTech Publisher, Rijeka, Croatia. [Google Scholar]
- 18. Guo D, Bi H, Liu B, Wu Q, Wang D, Cui Y. Reactive oxygen species‐induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol In Vitro. 2013;27:731–738. [DOI] [PubMed] [Google Scholar]
- 19. Guo D, Bi H, Wang D, Wu Q. Zinc oxide nanoparticles decrease the expression and activity of plasma membrane calcium ATPase, disrupt the intracellular calcium homeostasis in rat retinal ganglion cells. Int J Biochem Cell Biol. 2013;45:1849–1859. [DOI] [PubMed] [Google Scholar]
- 20. Senapati VA, Kumar A, Gupta GS, Pandey AK, Dhawan A. ZnO nanoparticles induced inflammatory response and genotoxicity in human blood cells: a mechanistic approach. Food Chem Toxicol. 2015;85:61–70. [DOI] [PubMed] [Google Scholar]
- 21. Guo D, Bi H, Wu Q, Wang D, Cui Y. Zinc oxide nanoparticles induce rat retinal ganglion cell damage through bcl‐2, caspase‐9 and caspase‐12 pathways. J Nanosci Nanotechnol. 2013;13:3769–3777. [DOI] [PubMed] [Google Scholar]
- 22. Demarquoy J, Le Borgne F. Crosstalk between mitochondria and peroxisomes. World J Biol Chem. 2015;6:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. [DOI] [PubMed] [Google Scholar]
- 24. Figtree GA, Liu CC, Bibert S, et al. Reversible oxidative modification: a key mechanism of Na+‐K+ pump regulation. Circ Res. 2009;105:185–193. [DOI] [PubMed] [Google Scholar]
- 25. Bhavsar SK, Hosseinzadeh Z, Brenner D, et al. Energy‐sensitive regulation of Na+/K+‐ATPase by Janus kinase 2. Am J Physiol Cell Physiol. 2014;306:C374–C384. [DOI] [PubMed] [Google Scholar]
- 26. Blanco G. The NA/K‐ATPase and its isozymes: what we have learned using the baculovirus expression system. Front Biosci. 2005;10:2397–2411. [DOI] [PubMed] [Google Scholar]
- 27. Blanco G. Na K‐ATPase subunit heterogeneity as a mechanism for tissue‐specific ion regulation. Semin Nephrol. 2005;25:292–303. [DOI] [PubMed] [Google Scholar]
- 28. Wen X, Lacruz RS, Smith CE, Paine ML. Gene‐expression profile and localization of Na+/K+‐ATPase in rat enamel organ cells. Eur J Oral Sci. 2014;122:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weber P, Bartsch U, Schachner M, Montag D. Na, K‐ATPase subunit beta1 knock‐in prevents lethality of beta2 deficiency in mice. J Neurosci. 1998;18:9192–9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Henriksen C, Kjaer‐Sorensen K, Einholm AP, et al. Molecular cloning and characterization of porcine Na+/K+‐ATPase isoforms α1, α2, α3 and the ATP1A3 promoter. PLoS ONE. 2013;8:e79127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edwards IJ, Bruce G, Lawrenson C, et al. Na+/K+ ATPase α1 and α3 isoforms are differentially expressed in α‐ and γ‐motoneurons. J Neurosci. 2013;33:9913–9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Magyar JP, Bartsch U, Wang ZQ, et al. Degeneration of neural cells in the central nervous system of mice deficient in the gene for the adhesion molecule on glia, the β2 subunit of the murine Na K‐ATPase. J Cell Biol. 1994;127:835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wetzel RK, Arystarkhova E, Sweadner KJ. Cellular and subcellular specification of Na, K‐ATPase alpha and beta isoforms in the postnatal development of mouse retina. J Neurosci. 1999;19:9878–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu SM, Qiao X, Noebels JL, Yang XL. Localization and modulatory actions of zinc in vertebrate retina. Vision Res. 1993;33:2611–2616. [DOI] [PubMed] [Google Scholar]
- 35. Guo DD, Li QN, Li CM, Bi HS. Zinc oxide nanoparticles inhibit murine photoreceptor‐derived cell proliferation and migration via reducing TGF‐β and MMP‐9 expression in vitro. Cell Prolif. 2015;48:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo DD, Li Q, Tang HY, Su J, Bi HS. Zinc oxide nanoparticles inhibit expression of manganese superoxide dismutase via amplification of oxidative stress, in murine photoreceptor cells. Cell Prolif. 2016;49:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smiley ST, Reers M, Mottola‐Hartshorn C, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J‐aggreagate‐forming lipophilic cation JC‐1. Proc Natl Acad Sci USA. 1991;88:3671–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Long SB, Campbell EB, MacKinnon R. Crystal structure of a mammalian voltage‐dependent Shaker family K+ channel. Science. 2005;309:897–903. [DOI] [PubMed] [Google Scholar]
- 39. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 40. Abe K, Matsuki N. Measurement of cellular 3‐(4, 5‐dimethylthiazol‐2‐yl)‐2, 5‐diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci Res. 2000;38:325–329. [DOI] [PubMed] [Google Scholar]
- 41. Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. [DOI] [PubMed] [Google Scholar]
- 42. Natarajan V, Wilson CL, Hayward SL, Kidambi S. Titanium dioxide nanoparticles trigger loss of function and perturbation of mitochondrial dynamics in primary hepatocytes. PLoS ONE. 2015;10:e0134541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoo HJ, Yoon TH. Flow cytometric assessment of reactive oxygen species generations that are directly related to cellular ZnO nanoparticle uptake. J Nanosci Nanotechnol. 2014;14:5395–5401. [DOI] [PubMed] [Google Scholar]
- 45. Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (Δφm) in apoptosis; an update. Apoptosis. 2003;8:115–128. [DOI] [PubMed] [Google Scholar]
- 46. Joshi DC, Bakowska JC. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J Vis Exp. 2011;51:e2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nozaki T, Ozawa SI, Harada H, Kimura T, Osawa M, Shimada I. Disulfide mapping the voltage‐sensing mechanism of a voltage‐dependent potassium channel. Sci Rep. 2016;6:37303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao J, Xu L, Zhang T, Ren G, Yang Z. Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicology. 2009;30:220–230. [DOI] [PubMed] [Google Scholar]
- 49. Johar K, Priya A, Wong‐Riley MT. Regulation of Na+/K+‐ATPase by neuron‐specific transcription factor Sp4: implication in the tight coupling of energy production, neuronal activity and energy consumption in neurons. J Neurosci. 2014;39:566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luan Z, Reddig K, Li HS. Loss of Na+/K+‐ATPase in Drosophila photoreceptors leads to blindness and age‐dependent neurodegeneration. Exp Neurol. 2014;261:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saini‐Chohan HK, Hryshko L, Xu YJ, Dhalla NS. Modification of Ca2+‐handling in cardiomyocytes by redox sensitive mechanisms in response to ouabain. Can J Physiol Pharmacol. 2013;91:45–55. [DOI] [PubMed] [Google Scholar]
- 52. Sawosz F, Pineda L, Hotowy A, et al. Nano‐nutrition of chicken embryos. The effect of silver nanoparticles and ATP on expression of chosen genes involved in myogenesis. Arch Anim Nutr. 2013;67:347–355. [DOI] [PubMed] [Google Scholar]
- 53. Bessemer RA, Butler KM, Tunnah L, et al. Cardiorespiratory toxicity of environmentally relevant zinc oxide nanoparticles in the freshwater fish Catostomus commersonii . Nanotoxicology. 2015;9:861–870. [DOI] [PubMed] [Google Scholar]


