Abstract
Biologic activity of proteases is mainly characterized by the substrate specificity, tissue distribution, and cellular localization. The human metalloproteases meprin α and meprin β share 41% sequence identity and exhibit a similar cleavage specificity with a preference for negatively charged amino acids. However, shedding of meprin α by furin on the secretory pathway makes it a secreted enzyme in comparison with the membrane-bound meprin β. In this study, we identified human meprin α and meprin β as forming covalently linked membrane-tethered heterodimers in the early endoplasmic reticulum, thereby preventing furin-mediated secretion of meprin α. Within this newly formed enzyme complex, meprin α was able to be activated on the cell surface and detected by cleavage of a novel specific fluorogenic peptide substrate. However, the known meprin β substrates amyloid precursor protein and CD99 were not shed by membrane-tethered meprin α. On the other hand, being linked to meprin α, activation of or substrate cleavage by meprin β on the cell surface was not altered. Interestingly, proteolytic activity of both proteases was increased in the heteromeric complex, indicating an increased proteolytic potential at the plasma membrane. Because meprins are susceptibility genes for inflammatory bowel disease (IBD), and to investigate the physiologic impact of the enzyme complex, we performed transcriptome analyses of intestinal mucosa from meprin-knockout mice. Comparison of the transcriptional gene analysis data with gene analyses of IBD patients revealed that different gene subsets were dysregulated if meprin α was expressed alone or in the enzyme complex, demonstrating the physiologic and pathophysiological relevance of the meprin heterodimer formation.—Peters, F., Scharfenberg, F., Colmorgen, C., Armbrust, F., Wichert, R., Arnold, P., Potempa, B., Potempa, J., Pietrzik, C. U., Häsler, R., Rosenstiel, P., Becker-Pauly, C. Tethering soluble meprin α in an enzyme complex to the cell surface affects IBD-associated genes.
Keywords: protease, quaternary structure, chronic intestinal inflammation, meprin β
The cellular localization of proteases is essential for protease regulation by activators and inhibitors and for their access to substrates. The astacin metalloproteases meprin α and meprin β are expressed as membrane-bound dimers in comparison with the other family members bone morphogenetic protein-1, mammalian tolloid (Tll), Tll1, and Tll2, which lack a transmembrane region and cannot form covalently connected dimers. Meprin α is shed by furin on the secretory pathway, oligomerized, and secreted into the extracellular space (1, 2). Meprin β, on the other hand, stays at the cell membrane until it is shed by a disintegrin and metalloproteases (ADAMs) to reach a different subset of substrates (3, 4). Shortly after discovery of meprins, it was shown that both proteases form heterodimers in mice and rats when coexpressed (1, 5). However, the human isoforms and their heterooligomeric connection have not been investigated in detail yet.
Meprin α and meprin β are highly expressed in epithelial cells of the intestine and kidney (6–8). Here, heterodimerization would have a huge impact on meprin α localization. The absence or mislocalization of meprins has been linked to various types of diseases like inflammatory bowel disease (IBD) and renal ischemia-reperfusion (9–11). Therefore, it is important to study the complex formations of these enzymes and the functional consequences for different tissues under pathophysiological conditions. Meprins are expressed as zymogens and require activation by serine proteases (12, 13) or other enzymes exhibiting a trypsin-like specificity (14). The propeptide of membrane-bound meprin β is removed by matriptase-2 (15) or the bacterial protease Arg-gingipain B (RgpB) (4). Additionally, it was shown that activated meprin β could not be shed anymore from the plasma membrane by ADAM10 or ADAM17 (4). However, it is not known whether meprin α in a heterooligomeric complex can be activated itself at the cell surface to gain an additional substrate spectrum or impair meprin β activation or whether shedding of the enzyme complex is altered.
To elucidate the molecular and functional consequences of a meprin α/meprin β-enzyme complex, we employed cell-based assays to demonstrate that human meprin heterodimers are disulfide linked like the murine proteins and locate meprin α to the cell surface. Importantly, meprin α could be activated on the cell surface but did not cleave typical meprin β substrates. We could show that neither shedding by ADAM10 and ADAM17 nor activation of the heterodimer was impaired. However, coexpression of meprins increased proteolytic activity of the protease in the enzyme complex. Finally, gene expression in intestinal tissue of meprin-knockout mice was analyzed by employing transcriptional gene analysis (RNAseq) and compared with gene expression data of IBD patients. The absence or altered localization of meprin α affected different subsets of genes. These findings strengthen the physiologic relevance of meprin heterodimer formation.
MATERIALS AND METHODS
Chemicals
All chemicals obtained were of analytical grade from MilliporeSigma (Burlington, MA, USA), Carl Roth (Karlsruhe, Germany), Merck (Darmstadt, Germany), Roche (Basel, Switzerland), or Thermo Fisher Scientific (Waltham, MA, USA).
Animals
The generation Mep1b−/− and Mep1a−/− mice has been previously described in refs. 9 and 16. Mice were kept under specific pathogen-free conditions in isolated ventilated cages on a 12-h light/dark cycle with food and water ad libitum. Our investigations were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the German Animal Welfare Act on protection of animals. All animal protocols were approved by the relevant German authorities.
Cells, antibodies, plasmids, and transfection
HeLa cells were grown in DMEM supplemented with 10% fetal calf serum (FCS), glutamine, and 1% penicillin and streptomycin (Thermo Fisher Scientific) and cultured at 37°C at 5% CO2 atmosphere and 95% relative humidity. Transient transfection was performed with polyethylenimine according to the manufacturer’s instructions. The following plasmids were used: human (h)Meprinß in PSG5 vector, hMeprinß in pcDNA4/TO-3x-Flag vector, hMeprinα in PSG5 vector, hMeprinα in pcDNA4/TO vector, hMatriptase-2-Myc in pcDNA3.1 vector, APP695 in pCI-neo, hCD99-Myc in pCMV6, and pcDNA3.1 as empty vector control. The following antibodies were used: polyclonal anti–meprin β and polyclonal anti-meprin α (Pineda Antibody-Service, Berlin, Germany), monoclonal anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (2118, 14C10; Cell Signaling Technology, Danvers, MA, USA), polyclonal anti-actin (A2066; MilliporeSigma), monoclonal anti-Flag (M2, F1804; MilliporeSigma), monoclonal anti-Strep (1023944; Qiagen, Hilden Germany), polyclonal anti-transferrin receptor (ab84036; Abcam, Cambridge, United Kingdom), monoclonal anti-Myc (9B11, 2276; Cell Signaling Technology), polyclonal anti-mouse meprin β (R&D Systems, Minneapolis, MN, USA), polyclonal anti–amyloid precursor protein (APP) (Thermo Fisher Scientific), monoclonal anti–N-terminal APP (22C11; MilliporeSigma), and monoclonal anti–soluble APPα (6E10; Covance, Princeton, NJ, USA).
Inhibitor treatment of cells
Transfected HeLa cells were treated 6 h after transfection with brefeldin A (5 µg/ml; BioLegend, San Diego, CA, USA), kifunensine (2 µg/ml; Cayman Chemical, Ann Arbor, MI, USA), swainsonine (2 µg/ml; Cayman Chemical), or control overnight.
Cell lysis, SDS-PAGE, and Western blot analysis
At 24 h after transfection, cells were harvested and lysed in lysis buffer containing 1% Triton X-100 and protease inhibitor tablet with EDTA (Roche) in PBS. Protein concentration was measured using bicinchoninic acid assay (Thermo Fisher Scientific). Total protein lysate (30 µg) was separated by SDS-PAGE and transferred onto nitrocellulose membranes (GE Healthcare, Waukesha, WI, USA). Membranes were blocked with 5% milk in Tris-buffered saline (TBS) for 1 h at room temperature, incubated overnight with primary antibody in milk at 4°C, washed 3 times with TBS, and incubated with horseradish peroxidase–conjugated secondary antibodies in TBS at room temperature. After further washes, membranes were developed with SuperSignal West Femto (Thermo Fisher Scientific) in a chemiluminescence detection system (LAS-3000; Fujifilm, Tokyo, Japan).
Cloning
Human meprin α and human meprin β were cloned into pcDNA4/TO vector with C-terminal 3 times Flag-tag via a 2-primer strategy. An N-terminal 2 times Strep-tag was inserted between the signal peptide and propeptide. Cysteine-mutation variants were cloned via the 2-primer strategy and insertion of a point mutation. Primers were purchased from MilliporeSigma, and plasmid sequences were validated by DNA sequencing (Eurofins Scientific, Luxembourg).
Immunoprecipitation
Cells were grown and transiently transfected as previously described. At 24 h after transfection, cells were harvested and lysed in lysis buffer containing 1% Triton X-100 and protease inhibitor tablet with EDTA (Roche) in PBS. Protein concentration was measured using bicinchoninic acid assay (Thermo Fisher Scientific), and equal amounts of protein per sample were supplemented with anti-Flag or anti-Strep antibody and incubated rolling overnight at 4°C. Protein G Agarose beads (Thermo Fisher Scientific) were centrifuged, washed 3 times with lysis buffer, and blocked overnight at 4°C in 3% bovine serum albumin and H2O. Beads were washed in lysis buffer the next day and added to the samples. After an incubation of 30 min at 4°C, samples were washed 3 times in lysis buffer and denatured with 1 times laemmli buffer at 95°C for 10 min.
Cell surface protein biotinylation
HeLa cells were transiently transfected with meprin α, meprin β, or empty vector and incubated for 24 h. Cells were washed twice with ice-cold PBS–CaCl2 and MgCl2 (CM) (CM: 0.1 mM CaCl2 and 1 mM MgCl2 in PBS) and treated with 1 mg/ml biotin solution (Sulfo-NHS-SS-Biotin; Thermo Fisher Scientific) in PBS-CM for 30 min at 4°C. Biotin solution was removed, and cells were incubated with quenching buffer (50 mM Tris-HCl in PBS-CM, pH 8) for 10 min at 4°C, washed 3 times with PBS-CM, and harvested.
Immunofluorescence microscopy
Tissue from mice was dissected, fixed in 4% (w/v) paraformaldehyde in PBS overnight, paraffin embedded, and sectioned. Tissue sections were rehydrated, boiled in 10 mM citric buffer (pH 5.5), and blocked with 5% FCS and PBS. Primary antibody was diluted in 5% FCS and PBS and applied overnight at 4°C in a humid, dark chamber (rabbit anti–meprin α tier 1, 1:1000; goat anti-meprin β, 1:2000; R&D Systems). Tissue was washed 3 times with PBS and incubated with secondary antibody for 2 h at room temperature (Alexa Fluor 488 donkey anti-rabbit and Alexa Fluor 594 donkey anti-goat, 1:300; Thermo Fisher Scientific). Excessive secondary antibody was removed by 3 washes in PBS. A 5-min incubation with 1 µg/ml DAPI and PBS was used for nuclear staining following an additional 3 washes with PBS. Stained tissue was mounted with Faramount Aqueous Mounting Medium (Agilent Technologies, Santa Clara, CA, USA).
Immunofluorescence staining with cells, which were seeded on coverslips, was performed after 24 h transfection. Cells were washed with PBS and fixed with 4% (w/v) paraformaldehyde in PBS for 10 min at room temperature followed by incubation with 0.12% (w/v) glycine in PBS for 10 min at room temperature. Cells were then blocked and permeabilized with 10% FCS and 0.2% (w/v) saponin in PBS for 30 min at room temperature. For cell surface staining, saponin was excluded from the buffers. Primary antibody was diluted in the same solution and applied for 1 h at room temperature in a humid, dark chamber (mouse anti-Flag, 1:1000; MilliporeSigma; mouse anti-Strep, 1:1000; Qiagen; rabbit anti–meprin α hEcto1, 1:1000; rabbit anti-meprin β hEcto1, 1:1000), following 5 washes in 0.2% (w/v) saponin in PBS. Secondary antibody was applied for 1 h at room temperature (Alexa Fluor 488 donkey anti-rabbit and Alexa Fluor 594 donkey anti-mouse, 1:300; Thermo Fisher Scientific). Excessive antibody was removed by 5 washes in 0.2% (w/v) saponin and PBS and 2 washes in double-distilled H2O. The coverslips were mounted onto slides with a mixture of 17% (w/v) Mowiol, 33% (v/v) glycerol, and 50 mg/ml 1,4-diazabicyclo[2.2.2]octane (MilliporeSigma) supplemented with 1 µg/ml DAPI for nuclear staining.
Images were acquired with a confocal laser scanning microscope (FV1000; Olympus, Tokyo, Japan).
Protein deglycosylation assay
Total protein lysate (50 µg) of transfected HeLa cells was deglycosylated using a protein deglycosylation mix according to the manufacturer’s instructions (P6039; New England Biolabs, Ipswich, MA, USA).
Activity assays
To quantify meprin α and meprin β activity on the cell surface and in the supernatant, specific fluorogenic peptide substrates for meprin α [7-methyloxycoumarin-4-yl (mca)–HVANDPIW–K-ε-2,4-dinitrophenyl (dnp); Genosphere Biotechnologies, Paris, France] and meprin β (mca-EDEDED-dnp; Genosphere Biotechnologies) were used in final concentrations of 10 and 50 µM, respectively. These consisted of a fluorophore (mca), a specific substrate peptide for meprin α (HVANDPIW) or meprin β (EDEDED), and a quencher (dnp). Fluorescence intensity was detected at 37°C every 30 s for 120 min using a spectrophotometer (Tecan, Männedorf, Switzerland). For determination of meprin activity at the cell surface, HeLa cells were transfected and treated with the bacterial protease RgpB (50 nM for 5 h). Cells were counted, and 0.2 × 106 cells were plated onto 48-well plates in a total volume of 300 μl. Equal amounts of corresponding cell supernatants were plated onto 96-well plates, and half of them were also incubated with 10 μg/ml trypsin (420 nm) or 50 nM RgpB for 30 min at 37°C. Remaining cell supernatants and cell lysates were used for Western blot analysis. Activity assays of recombinant active meprin β (1 nM), meprin α (5 nM), RgpB (50 nM), trypsin (10 μg/ml), and intestinal lysates (50 µg) were performed in 100 µl total volume in 96-well plates.
For data analysis, slope of equal linear activity range was compared from 3 individual experiments.
Homology modeling
The model of human meprin α/β heterodimer was built based on the crystal structure of human meprin β [Protein Data Bank (PDB) code: 4gwm; https://www.rcsb.org/] using the Swiss-Model workspace (17). For the analysis of the interaction, the interface PDBsum (18) was used. Structure visualization and analysis was carried out using PyMOL (Schrödinger, New York, NY, USA).
RNAseq data and IBD
Transcriptome sequencing was carried out on a HiSeq 4000 (Illumina, San Diego, CA, USA) employing the TruSeq stranded totalRNA library protocol (Illumina) following the manufacturer’s guidelines. A mean of 25 million 125-nt paired-end reads per sample was obtained. Subsequently, raw reads were subjected to trimming using cutadapt (19) for removal of adapter and low-quality sequences. Alignment to the mm10/Ensemble Genome Reference Consortium Mouse Build 38 patch (GRCm38) reference genome was performed using TopHat2 (20). Quantitative gene expression values were generated using HTSeq (21). Finally, differential gene expression levels were analyzed employing the Bioconductor package DESeq2 (22).
Gene ontology analysis of RNAseq data
Gene ontology analysis was conducted by retrieving ontology terms (http://www.geneontology.org) for differentially expressed genes and a subsequent 2-sided Fisher’s exact test to assess significance of enrichment or depletion as previously described by Tavazoie et al. (23).
Statistical analysis
All statistical analyses were performed with Prism 5 software (GraphPad Software, La Jolla, CA, USA) for 1-way ANOVA followed by Tukey’s posttest, or 2-way ANOVA followed by a Bonferroni posttest. Values are expressed as means ± sd. The null hypothesis was rejected at P < 0.05.
RESULTS
Coexpression and molecular interaction of human meprin α and meprin β
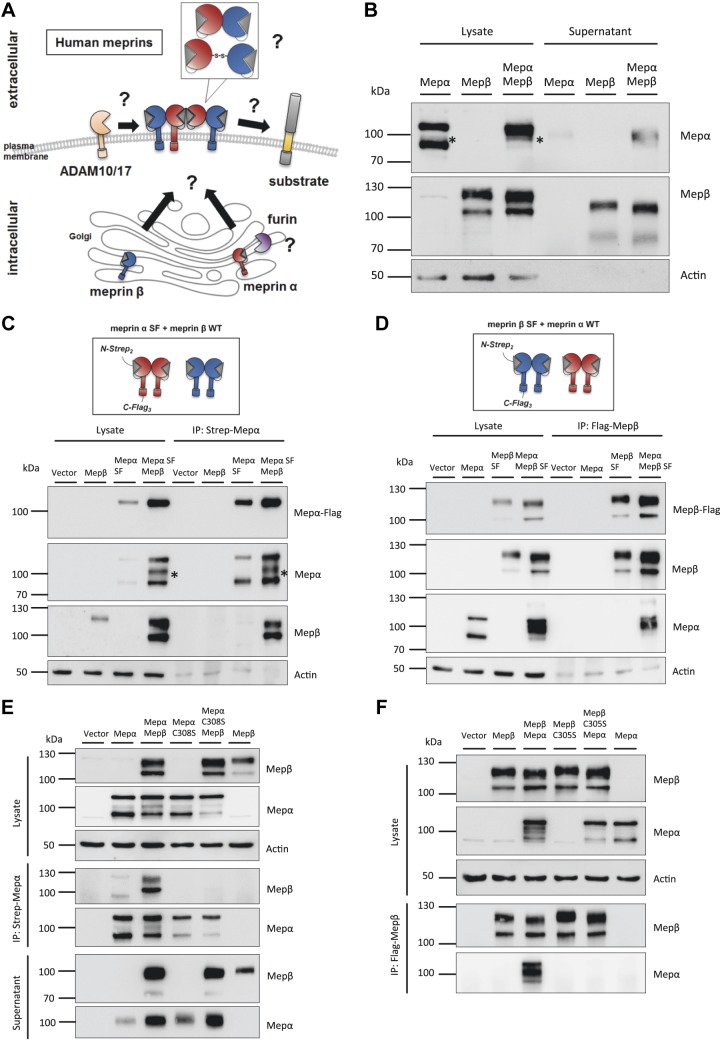
The coexpression of the metalloproteases meprin α and meprin β has been widely studied for the rodent isoforms. Here, meprin α and meprin β monomers are able to form heterodimers, which then oligomerize to heterotetramers at the cell surface (1, 5). However, coexpression and interaction of the human isoforms remains elusive. It is unclear if and where both proteases form a heterodimer and if it is covalently connected. Furthermore, formation of this complex may have an impact on the shedding of meprin α by furin or of meprin β by ADAM10 or ADAM17. Finally, tethering and activation of meprin α at the cell surface might widen its substrate spectrum, which could overlap with meprin β–specific substrates (Fig. 1A).
Figure 1.
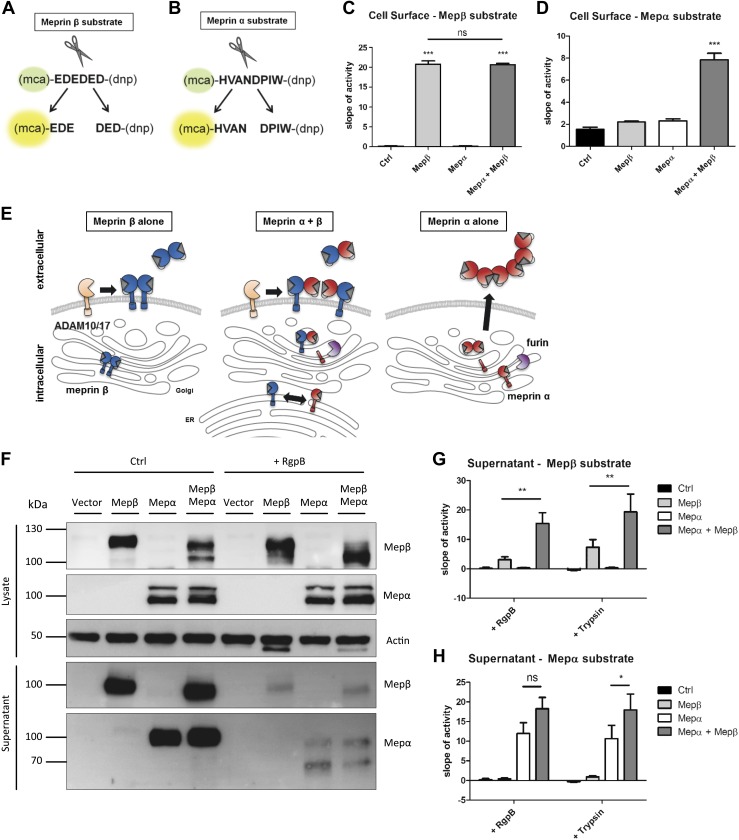
Molecular interaction of the human meprin α and meprin β complex. A) Diagram summarizing possible interaction modes of human meprin α and meprin β with unknown influence on shedding and substrate cleavage. B) Transfection of HeLa cells with human meprin α, human meprin β, or both. Cell lysates and supernatants were analyzed via immunoblotting using specific meprin α or meprin β antibodies. β-Actin served as loading control. The 100-kDa fragment of meprin α is marked with an asterisk. C) Transfection of HeLa cells with meprin β WT and meprin α SF construct. Diagram of double-tagged meprin α construct (upper panel). Coimmunoprecipitation was performed using Strep-tag antibody against the meprin α N terminus and analyzed via immunoblotting (lower panel). The 100-kDa fragment of meprin α is marked with an asterisk. D) Transfection of HeLa cells with meprin α WT and meprin β SF construct. Diagram of double-tagged meprin β construct (upper panel). Coimmunoprecipitation was performed using Flag-tag antibody against the meprin β C terminus and analyzed via immunoblotting (lower panel). E) Transfection of HeLa cells using meprin α SF, meprin α C308S SF variant, and meprin β WT. Coimmunoprecipitation was performed using Strep-tag antibody against the meprin α N terminus and analyzed via immunoblotting. F) Transfection of HeLa cells using meprin β SF, meprin β C305S SF variant, and meprin α WT. Coimmunoprecipitation was performed using Flag-tag antibody against the meprin β C terminus and analyzed via immunoblotting. C-, C terminus; IP, immunoprecipitation; N-, N terminus; Mep, meprin.
We overexpressed both human proteases and observed altered band patterns in Western blot analyses of single or cotransfected HeLa cells. Meprin α single transfection resulted in a double-band pattern of 110- and 90-kDa signals in the cell lysate (Fig. 1B). The presence of meprin β increased the 110-kDa signal and decreased the 90-kDa signal. Interestingly, an additional band for meprin α of 100 kDa appeared in the coexpression with meprin β, which was also found in cell supernatants and, to a lower extent, in the single transfection (Fig. 1B, C). Meprin β signals were obtained as a double band at 120 and 100 kDa (Fig. 1B). The upper band shifted downwards when meprin α was coexpressed. The same shift appeared in the cell supernatants of shed meprin β, which might be an effect of cleavage or other post-translational modification.
To investigate the direct interaction of both proteases further, we utilized variants of meprin α and meprin β containing a 2 times Strep-tag N-terminal of the propeptide and a C-terminal 3 times Flag-tag and named them meprin Strep/Flag (SF) (meprin SF-tagged) (Fig. 1C, D). We performed coimmunoprecipitation experiments to study a direct interaction of both human isoforms. By using a Strep-tag antibody to precipitate meprin α from transfected HeLa cells, 2 meprin α bands were detected after single transfection and 3 bands after cotransfection with meprin β (Fig. 1C). Only 1 band at 110 kDa was detected with the Flag-tag antibody, revealing full-length meprin α with an intact C terminus. Hence, the lower migrating bands detected with a meprin α–specific antibody were products of C-terminal proteolytic processing. Interestingly, both meprin β bands appeared in the pulldown fractions when meprin α was present. In the opposite experiment, when using the tagged meprin β and the Flag-tag antibody for precipitation, both meprin β bands were observed by Western blot (Fig. 1D). Here, mainly the 100-kDa fragment of meprin α was coprecipitated with meprin β, and the 110-kDa fragment to a lesser extent.
Human meprin β homodimer is connected via an intermolecular disulfide bridge at position C305 between the meprin, A-5 protein, and receptor protein-tyrosine phosphatase μ (MAM) domains. For the rat isoform, a previous study showed that the corresponding cysteine residue could also be linked to a meprin α monomer (24). We mutated the C305 to a serine in human meprin β as well as the corresponding C308 in human meprin α and repeated the immunoprecipitation experiment. The meprin α C308S variant appeared as a double band in Western blot and was secreted into the supernatant like the wild-type (WT) form (Fig. 1E). However, WT meprin β was not coprecipitated in that approach. Transfection of the meprin β C305S variant led to the same observation that WT meprin α did not bind to mutated meprin β (Fig. 1F). Furthermore, only 2 bands appeared for meprin α in the cotransfection lysates, whereas the band shift of meprin β was present, although not bound to meprin α. Conclusively, human meprins are able to form heterodimers via a disulfide bond between their MAM domains.
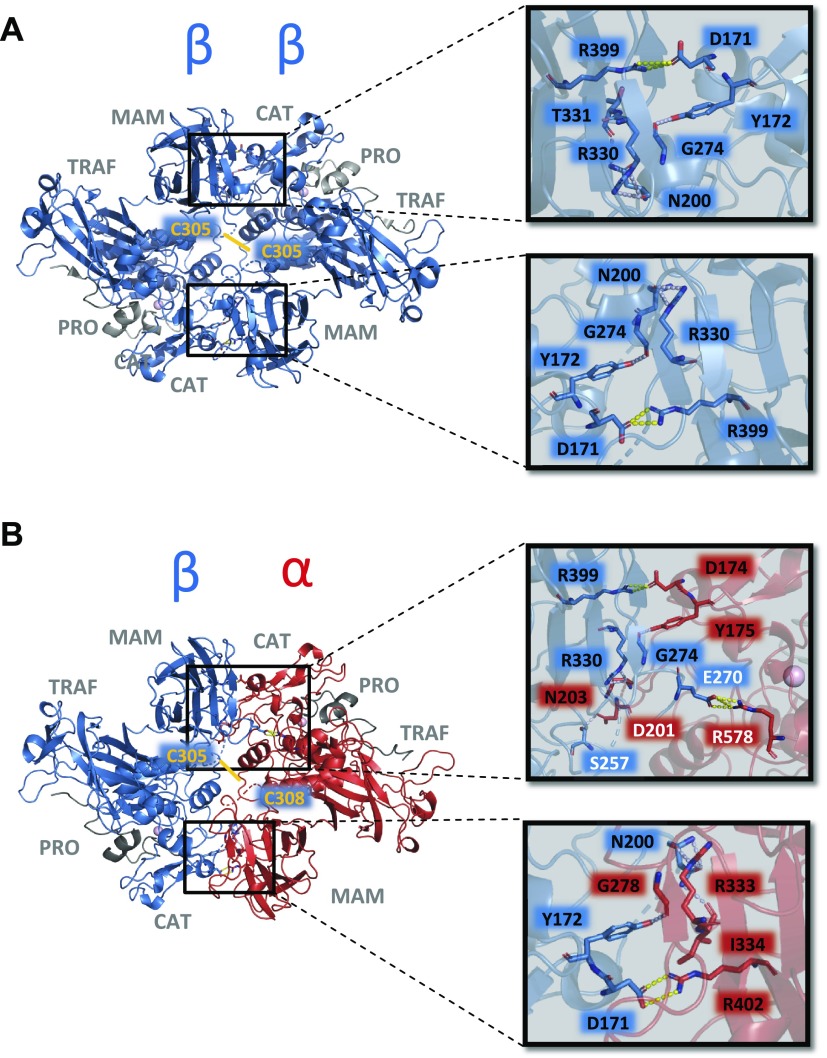
Based on the solved crystal structure of human meprin β homodimer (PDB code: 4gwm) (25) (Fig. 2A), we generated a homology model of the meprin α/meprin β enzyme complex (Fig. 2B). Because the disulfide bond of meprin β homodimer was not resolved, the approximate position in the heterodimer was highlighted via a yellow line. Interestingly, all interaction sites between 2 meprin β monomers were also found in the heterodimer, with an additional salt-bridge formed between E270 (meprin β) and R578 (meprin α) and a hydrogen bond between S257 (meprin β) and D201 (meprin α).
Figure 2.
Structure of human meprin β homodimer and homology model of human meprin α/β heterodimer. A) Structure of human meprin β homodimer with interacting amino acid residues at the interface of both monomers (PDB code: 4gwm). Because the segment containing the intermolecular disulfide bond between C305 residues is disordered in the zymogen structure, disulfide bond is highlighted via a yellow line. B) Structural properties of a meprin α/β heterodimer homology model based on the ectodomain of meprin β (PDB code: 4gwm). The approximate position of the intermolecular disulfide bond between C305 and C308 of the meprin β (blue)/meprin α (red) heterodimer is highlighted via a yellow line. Additional stabilization of the α/β interaction is given by several hydrogen bonds (light-blue dashes) and 3 salt bridges (yellow dashes) as shown in the close-up view (right panel). Binding residues are presented as sticks and the secondary structure is shown as transparent cartoon. Except the salt-bridge formed by E270 and R578 and the hydrogen bond between S257 and D201, all identified interaction sites are conserved between the meprin β monomer and the meprin α/β heterodimer. CAT, catalytic domain; PRO, propeptide; TRAF, tumor necrosis factor receptor-associated factor.
Interaction of meprin heterodimers occurs in the early endoplasmic reticulum
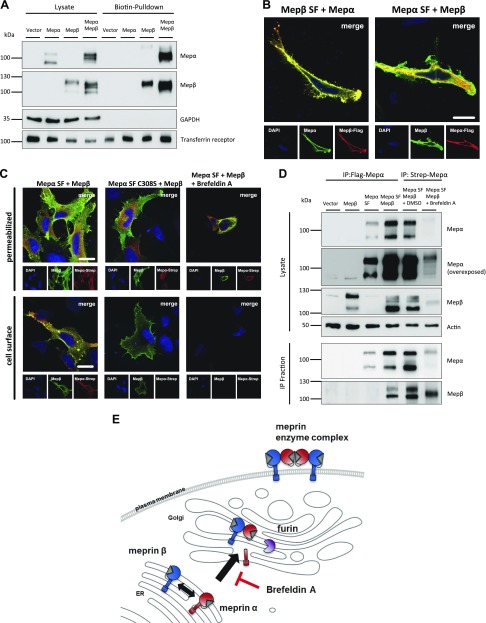
The interaction studies prompted us to further analyze assembly and localization of the human meprin heterodimer. We therefore performed cell surface biotinylation experiments of transfected HeLa cells in order to validate whether the presence of membrane-bound meprin β leads to a localization of meprin α at the cell surface. The biotin-pulldown revealed that no meprin α signal occurred in the single transfection, whereas coexpression with meprin β induced a strong signal for the 100-kDa form of meprin α (Fig. 3A). For meprin β, only the band of higher molecular mass was detected in the biotinylated fraction, whereas the 100-kDa form likely corresponds to immature protein in the endoplasmic reticulum (ER) and Golgi. We confirmed these results employing immunofluorescence microscopy of HeLa cells that were transfected with the tagged meprin constructs. Meprin α and Flag-tagged meprin β showed fluorescence signals on the cell surface as well as intracellularly (Fig. 3B). Use of an antibody against the C-terminal Flag-tag of meprin α revealed that full-length meprin α, including its C terminus, was not located on the plasma membrane. Only meprin β could be detected on the cell surface. Therefore, we concluded that only furin-cleaved meprin α, which appeared as a 100-kDa fragment in Western blot, is located at the cell membrane via interaction with meprin β.
Figure 3.
Meprin interaction on the cell surface and secretory pathway. A) Transfection of HeLa cells with meprin α WT and meprin β WT constructs. Cell surface proteins were labeled by primary amine biotinylation, pulled down with streptavidin sepharose beads, and analyzed via immunoblotting. GAPDH and transferrin receptor served as loading controls. B) Immunofluorescence staining of transfected HeLa cells with meprin β SF and meprin α WT constructs (left panel) or meprin α SF and meprin β WT constructs (right panel). Nuclear staining was visualized using DAPI. Images were taken by confocal fluorescence microscopy. Scale bar, 20 µm. C) Immunofluorescence staining of transfected HeLa cells using meprin α SF, meprin α C308S SF, and meprin β WT constructs. Cells were treated overnight with brefeldin A at 5 µg/ml or DMSO control. Cell surface staining was performed by excluding saponin from buffers. Nuclear staining was visualized using DAPI. Images were taken by confocal fluorescence microscopy. Scale bars, 20 µm. D) Transfection of HeLa cells using meprin α SF and meprin β WT constructs. Cells were treated overnight with brefeldin A at 5 µg/ml or DMSO control. Coimmunoprecipitation was performed using Flag-tag antibody against meprin α C terminus or Strep-tag antibody against meprin α N terminus as indicated. Protein fractions were analyzed by immunoblotting. E) Diagram of meprin α and meprin β interacting in the ER and being transported to the cell surface. Treatment of cells with brefeldin A inhibits the transport from the ER to Golgi apparatus. IP, immunoprecipitation; Mep, meprin.
To investigate whether disulfide linkage of the heterodimer is necessary for cell surface localization, we performed immunofluorescence staining with the cysteine-mutated variants. HeLa cells were cotransfected with meprin β and meprin α WT or C308S variant. As expected, both WT proteases colocalized on the secretory pathway and on the cell surface of nonpermeabilized cells (Fig. 3C). However, the missing disulfide bond in the C308S variant led to intracellular staining of meprin α and to complete absence on the cell surface, although meprin β was coexpressed. Therefore, disulfide linkage of the heterodimers is crucial for meprin α to be located on the cell surface instead of secreted.
Disulfide bonds are mainly formed in the ER after protein translation. However, it is not known where meprin α and meprin β monomers meet at first. Therefore, we used brefeldin A to inhibit the transport of proteins from the ER to the Golgi apparatus in transfected HeLa cells. Immunofluorescence microscopy showed retention of both proteases in the ER of permeabilized cells, whereas no signals could be detected on the cell surface. Subsequent immunoprecipitation of meprin α revealed that meprin β was already connected to meprin α in the ER (Fig. 3D). Additionally, only 1 band was obtained for each of the proteases, confirming that the 110-kDa meprin α fragment is the unprocessed full-length form in the ER as observed before. In summary, meprin α and meprin β are covalently linked in the ER directly after translation and are further transported to the Golgi, where meprin α loses its membrane anchor by furin cleavage (Fig. 3E).
Complex formation of meprin α and meprin β alters post-translational modifications
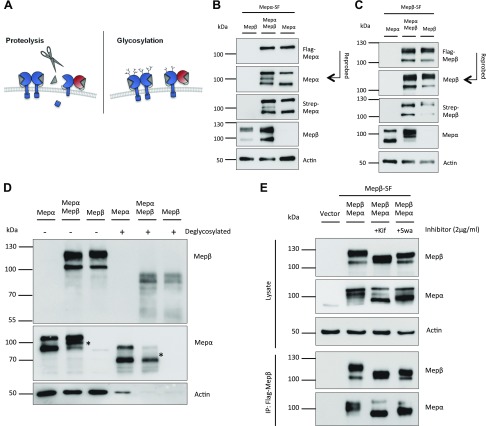
In order to elucidate the different band patterns of meprins observed by Western blot and to investigate whether proteolytic processing or altered glycosylation are responsible (Fig. 4A), we used the N- and C-terminal–tagged meprin constructs (Fig. 1C, D) for the following experiments. We repeated single and cotransfection in HeLa cells and performed Western blot analyses with the lysates. In case of tagged meprin α and untagged meprin β, we again confirmed that the 110-kDa upper band was meprin α still containing its C terminus, whereas the other bands were not visualized with the Flag-tag antibody (Fig. 4B). Those bands reappeared after incubation with the Strep-tag– or meprin α–specific antibody as products of C-terminal proteolytic processing. When expressing the tagged meprin β and untagged meprin α, the meprin β double band was observed using a Flag-tag–, a Strep-tag–, and a meprin β–specific antibody, which excludes that the shift of the upper band is a result of proteolysis at the N or the C terminus (Fig. 4C). We therefore focused on differential glycosylation that could alter the molecular mass of the protein and might occur when both meprins are coexpressed. Indeed, deglycosylation of meprin β led to a similar band pattern in single and cotransfection; therefore, the observed band shift was an effect of altered glycosylation (Fig. 4D). Deglycosylation of meprin α still showed a double band in the single transfection, confirming that the band of lower molecular mass is a result of proteolysis. Interestingly, the 100-kDa fragment of meprin α in the cotransfection vanished after deglycosylation (Fig. 4D). Therefore, the 100-kDa fragment of meprin α might be a higher-glycosylated version of the 90-kDa protein, which is either secreted or held at the cell surface by meprin β. In order to investigate whether glycosylation itself has an influence on the interaction of both meprins, we used 2 compounds that inhibit maturation of sugar trees by α-mannosidases in the ER (kifunensine) or in the Golgi apparatus (swainsonine). Immunoprecipitation of cotransfected meprins showed that both proteases were indeed differentially glycosylated but still interacted after addition of each of the inhibitors (Fig. 4E). Mutation of known glycosylation sites in meprins did not affect protein interaction or it resulted in inappropriate folding and instability of the proteases (unpublished results) (26).
Figure 4.
Post-translational modifications of meprins. A) Diagram of possible post-translational modifications of human meprin β in the meprin enzyme complex. Proteolysis could lead to loss of either C or N terminus (left panel), or altered maturation of sugar trees (right panel) could affect the size of the protein. B) Transfection of HeLa cells with meprin α SF and meprin β WT constructs. Protein fractions were visualized via immunoblotting using meprin-specific antibodies or Strep- and Flag-tag antibodies against N and C terminus of meprin α. C) Transfection of HeLa cells with meprin β SF and meprin α WT constructs. Protein fractions were visualized via immunoblotting using meprin-specific antibodies or Strep- and Flag-tag antibodies against N and C terminus of meprin β. D) Transfection of HeLa cells using meprin α WT and meprin β WT constructs. Protein lysates were treated with deglycosylation mix and compared with untreated lysates by immunoblotting. The 100-kDa fragment of meprin α is marked with an asterisk. E) HeLa cells were transfected with meprin β SF and meprin α WT constructs. Cells were treated overnight with kifunensine (Kif) or swainsonine (Swa) as indicated. Coimmunoprecipitation was performed using Flag-tag antibody against meprin β C terminus and analyzed via immunoblotting. IP, immunoprecipitation; Mep, meprin.
Heteromeric complex of meprin α and meprin β does not change protease activation but increases proteolytic activity
In order to investigate the physiologic consequences of meprin heterodimer formation, we performed experiments to analyze whether the functional properties of each meprin are altered within the heteromeric complex. For meprin β activity, we used an already described specific fluorogenic peptide consisting of negatively charged amino acids (Fig. 5A) (27). For meprin α, we designed a new substrate based on proteomic identification of protease cleavage site specificity (28) and validated its specificity using recombinant active meprin α, meprin β, and trypsin (Fig. 5B and Supplemental Fig. S1A). Of note, only meprin α and not meprin β was able to cleave the peptide HVANDPIW. The specificity for meprin α could even be demonstrated using protein lysates from the small intestine of WT and meprin-knockout mice Supplemental Fig. S1B). With specific substrates for meprin α and meprin β in hands, we measured cell surface protease activities of transfected HeLa cells with each meprin alone or in the heteromeric enzyme complex. Meprin β activities were obtained in single transfection and cotransfection with meprin α, revealing an unaffected proteolytic activity after activation with the soluble pathogenic protease RgpB (Fig. 5C) (4) or membrane-bound protease matriptase-2 Supplemental Fig. S1C, D) (15). Remarkably, meprin α could also be activated with RgpB in the enzyme complex at the cell surface, making it a membrane-tethered protease with possible access to so far unknown membrane-bound substrates (Fig. 5D, E).
Figure 5.
Proteolytic activity and shedding of the meprin heterodimer. A) Representation of the meprin β–specific quenched fluorogenic peptide used for activity assays. B) Representation of the meprin α–specific quenched fluorogenic peptide used for activity assays. C) Cell surface activity of meprin β on transfected HeLa cells. D) Cell surface activity of meprin α on transfected HeLa cells. E) Diagram of meprin β shedding by ADAM10 and ADAM17 as homo- or heterodimer. Meprin α is shed by furin in the Golgi apparatus and secreted into the extracellular space when expressed alone. F) HeLa cells were transfected with meprin α WT and meprin β WT constructs. Cells were stimulated with bacterial protease RgpB for meprin activation as indicated. Cell lysates and supernatants were analyzed via immunoblotting. G) Meprin β activity in either RgpB- or trypsin-stimulated cell supernatants of transfected HeLa cells. H) Meprin α activity in either RgpB or trypsin incubated cell supernatants of transfected HeLa cells. Ctrl, control; Mep, meprin; ns, not significant. Data are presented as means ± sd, and statistical analysis was assessed by 1-way ANOVA followed by Tukey’s posttest (C, D), or 2-way ANOVA followed by a Bonferroni posttest (G, H) from 3 biologic replicates. *P < 0.05, **P < 0.01, ***P < 0.001.
Due to furin cleavage within the inserted domain, meprin α is a soluble protease when expressed alone (Fig. 5E). Meprin β, on the other hand, requires shedding through ADAM10 or ADAM17 to get access to its soluble substrates, which is only possible in its proform (3, 4). Shedding of the meprin enzyme complex has not yet been investigated. Therefore, we performed Western blot analyses of cell lysates and supernatants used for the activity assays and observed both proteases in the supernatants (Fig. 5F). Of note, activation of the proteases by RgpB prevented shedding of both meprin β homodimer and the enzyme complex. Furthermore, cell supernatants were incubated with trypsin or RgpB, and meprin activities were measured. Meprin β showed significantly increased proteolytic activity in the heteromeric enzyme complex compared with the single transfection (Fig. 5G). Similar results were obtained using the meprin α–specific substrate (Fig. 5H).
Membrane-bound meprin α does not cleave meprin β–specific substrates
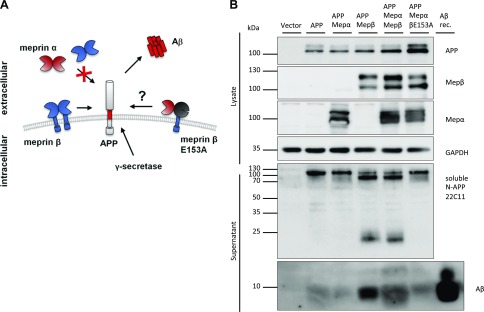
Meprin α and meprin β have a common preference for negatively charged amino acids around the scissile bond (28). However, most likely due to their different localization, substrate specificities differ between the proteases. The APP was described as substrate for membrane-bound meprin β, which cleaves the N terminus and acts as β-secretase, subsequently leading to Aβ formation by the γ-secretase (Fig. 6A) (29, 30). Soluble shed meprin β as well as secreted meprin α are not able to cleave APP at the β-secretase site (unpublished results). Because membrane localization is crucial for APP cleavage by meprin β, we investigated whether APP can also be a substrate for membrane-bound meprin α. We cotransfected APP with each protease or the enzyme complex. To exclude meprin β cleavage, we also used the catalytically inactive meprin β variant E153A for the enzyme complex. APP was processed in all samples transfected with active meprin β, leading to soluble APPβ, N-terminal APP fragments, and Aβ formation (Fig. 6B). Interestingly, meprin α alone or expressed in the enzyme complex was not able to process APP. This indicates that although both proteases can be activated in the hetero-oligomer, their substrate pools clearly differ within the enzyme complex. Equal observations were made with another membrane-bound meprin β substrate, the adhesion molecule CD99 (31), which was only shed by active meprin β and not by meprin α in the enzyme complex (Supplemental Fig. S2).
Figure 6.
APP cleavage by the meprin heterodimer. A) Diagram of APP cleavage by cell surface–bound meprin β and impaired cleavage by soluble meprins. Cleavage of cell surface–bound meprin β and subsequent γ-secretase cleavage leads to generation of accumulating Aβ fragments in the extracellular space. Catalytically inactive meprin β E153A was used for the meprin enzyme complex to exclude meprin β cleavage. B) Transfection of HeLa cells with APP, meprin WT, or meprin β E153A variant. Immunoblotting of cell lysates and supernatants revealed cleavage of APP and Aβ generation by active meprin β, not by membrane-bound meprin α or inactive meprin β. Recombinant Aβ served as positive control. Mep, meprin; N-, N terminus; rec., recombinant.
Localization of meprin α at the cell surface alters gene expression in murine intestinal tissue
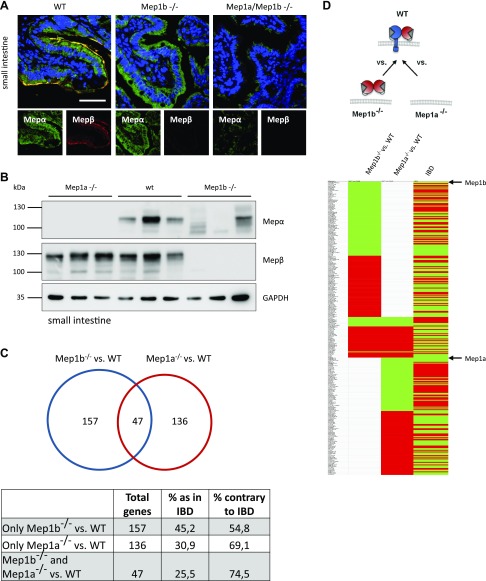
Having investigated the molecular and cellular functions of the human meprin enzyme complex, we were further interested in its biologic relevance in vivo. We first validated cell surface localization of meprin α in murine WT small intestine, which was not detected in meprin β–knockout mice (Fig. 7A). By Western blot analysis of small intestine lysates, we obtained similar results as observed in the in vitro experiments with human meprins (Fig. 7B). In the WT situation, meprin α appeared as a strong distinct band of 110 kDa. Expressed alone in meprin β–knockout mice, the meprin α band pattern looked more heterogeneous, consisting of shed and probably further processed meprin α. For meprin β, a slight shift in MW was observed in meprin α–knockout mice, which might also be a cause of altered glycosylation as seen in the human protein. Because we have not identified a specific membrane-bound substrate of meprin α so far, we performed RNAseq of meprin-knockout mice small intestine samples in order to see the biologic impact of meprin α expression and localization. Total absence or decreased expression of meprin α in murine and human colon and small intestine was associated with a higher risk for IBD progression (9, 32). We wondered whether loss of meprin α membrane localization might also promote and change gene expression with regard to IBD. Therefore, we compared the obtained gene expression data of meprin-knockout mice with data of a recently analyzed IBD patient cohort (32). In total, we identified 340 dysregulated genes in meprin-knockout mice that were found to be altered in the human IBD cohort (Fig. 7C, D and Supplemental Table S1). A total of 157 of these genes were only dysregulated in meprin β–knockout mice compared with WT mice, with 45.2% of genes being dysregulated in the same direction as in IBD patients. Interestingly, 136 genes were differentially expressed in meprin α–knockout mice compared with WT mice, with only 30.9% of genes showing the same tendency as in the IBD cohort. A total of 47 genes were found in both knockout conditions with 25.5% identical regulation as in IBD. Those 47 genes were equally dysregulated in both genotypes, indicating that loss of meprin α at the cell surface in meprin β–knockout mice had the same regulatory effect as the total loss of meprin α for this gene subset. To further contextualize the differentially expressed genes, a gene ontology analysis was performed, resulting in several significantly enriched and depleted biologic processes (Supplemental Fig. S3A, B). A large proportion of biologic processes identified is functionally associated with wound healing, tissue regeneration, innate immunity, and inflammatory mechanisms. In particular, the innate immune response was depleted in genes down-regulated in both Mep1a- and Mep1b-deficient scenarios.
Figure 7.
Expression and transcriptional analysis of meprin heterodimer in vivo. A) Immunofluorescence microscopy of small intestine from WT, Mep1b−/−, and Mep1a−/− mice using specific meprin antibodies. Staining revealed absence of meprin α at the cell surface in Mep1b−/− mice. Scale bar, 40 µm. B) Immunoblotting of small intestine lysates of WT, Mep1b−/−, and Mep1a−/− mice using specific meprin antibodies. GAPDH served as loading control. C) Analysis of RNA sequencing of WT, Mep1b−/−, and Mep1a−/− mice small intestine samples. Obtained data were compared with transcriptome analyses of IBD patients (32), and matching and overlapping genes of each condition were depicted. D) Representation of matching up- and down-regulated genes in WT, Mep1b−/−, and Mep1a−/− mice small intestine samples and IBD patients. Green, down-regulation, red, up-regulation. Mep, meprin.
DISCUSSION
The metalloproteases meprin α and meprin β exhibit unique molecular features among the members of the astacin family, being membrane bound and expressed as disulfide-linked dimers. Although both proteins are found in the same tissues, like small intestine and proximal tubuli of the kidney, their genes are located on different chromosomes and expressed independently from each other because of different promoter regions (33). Nevertheless, coexpression and homology of the proteases raised the question of whether meprins form also heterodimers (1, 2, 5). Indeed, meprin α and meprin β enzyme complexes were observed in rats and mice; however, the type of connection between both monomers was discussed controversially and was not yet shown for human meprins (2, 34–36). We now demonstrate that human meprin α and meprin β also form an enzyme complex that is covalently connected via a disulfide bridge in the MAM domain as described for murine meprins (24). The human meprins are linked between C305 in meprin β and C308 in meprin α, respectively, after translation in the early ER and before glycosylation sites are further modified. The interaction complex is transported to the cell surface, which tethers meprin α at the plasma membrane, although meprin α is shed by furin in the Golgi compartment. Interestingly, the glycosylation pattern of meprin β was altered when coexpressed with meprin α both in vitro for the human and in vivo for the murine isoform. This modification was not a cause of the disulfide-linked enzyme complex per se because altered glycosylation was also observed in the cysteine-mutated variants. Hence, the presence of meprin α within the secretory pathway might have a direct or indirect effect on glycosylation-maturating enzymes such as glycosyltransferases.
Both meprin α and meprin β are expressed as zymogens and require proteolytic activation by tryptic proteases on the cell surface or in the extracellular space (12–14). The meprin β homodimer was shown to be activated on the cell surface by matriptase-2 or the pathologic protease RgpB (4, 15). We observed that activation of meprin β was not impaired and was even significantly elevated for shed meprin β in the enzyme complex using a meprin β–specific fluorogenic substrate. Additionally, shedding of the enzyme complex by ADAM proteases was not impaired. However, once being activated on the cell surface, meprin α and meprin β could not be released into the extracellular space, thus behaving like the meprin β homodimer (4). This regulatory mechanism traps meprin α on the cell surface and is contrary to meprin α single expression, in which the protease is always secreted by the cells. To investigate proteolytic activity of meprin α, we designed a fluorogenic peptide based on a cleavage site specificity screen (28) and validated specificity with recombinant human proteins and knockout murine small intestine lysates. Interestingly, meprin α could also be activated in complex on the cell surface of transfected HeLa cells. Astonishingly, meprin α activity was elevated both on the cell surface and in the supernatant, although protein levels were unchanged as observed in Western blot analysis. Most likely, both proteases benefit from enzyme complex formation regarding increased stability, as predicted from the structure model, and may also promote consecutive substrate processing. Because meprin α and meprin β have a preference for acidic amino acid residues (28), we investigated whether meprin α in complex could also cleave known substrates of meprin β. Both the APP (29, 37) and CD99 (31) could not be shed by membrane-bound meprin α in cell culture, suggesting additional regulatory mechanisms of substrate cleavage besides cellular localization and amino acid sequences. However, cleavage of other substrates needs to be investigated.
The altered proteolytic activity of 2 enzymes has been shown for G-calpain, a noncovalently formed complex of calpain 8 and calpain 9 (38). An active site mutant of calpain 8 compromised proteolytic activity of the whole enzyme complex. However, a comparable covalently linked enzyme complex that increases proteolytic activity of the monomers as shown for meprins has, to our knowledge, not yet been identified.
Being localized on the cell membrane and being able to get activated, meprin α might broaden its substrate spectrum, which would no longer be restricted to soluble substrates. Another way for meprin α to get access to cell surface substrates was shown recently (39). Loss of meprin α binding to heparan sulfate in a noncovalent manner increased inflammatory cell recruitment and neutrophil transmigration in lungs of idiopathic pulmonary arterial hypertension patients. However, cell membrane localization of meprin α in the enzyme complex was not considered in the study.
Absence of meprin α in the gut is a risk for IBD, which was proven in a dextran sulfate sodium mouse model (9). In contrast, meprin β–knockout mice were protected against dextran sulfate sodium colitis because of loss of proinflammatory cytokines (10). Down-regulation of both the MEP1A and the MEP1B genes was observed in transcriptome analyses of IBD patients (32). However, the physiologic role of meprins in the gut with regard to cleavage of substrates needs to be elucidated. It is not known if loss of meprin α at the cell surface might play a role in the meprin β–knockout mouse for the protection or if it is a concomitant. For meprin β, it was also shown that cleavage of mucin 2 and its detachment is important for proper mucus barrier function (40). Additionally, shedding of meprin β by ADAM proteases is crucial for subsequent mucus cleavage, which can be abolished through its activation by the pathogenic protease RgpB (4). Being covalently connected to meprin β in an enzyme complex, meprin α release into the extracellular space would be a regulated process and requires limited proteolysis. We performed transcriptome analysis of meprin-knockout mice in order to compare differences in gene expression between total absence of meprin α and both cellular localizations. The obtained data were matched with transcriptome analyses of IBD patients (32), and we identified different gene subsets when meprin α localization was altered. Interestingly, 47 overlapping genes were found that were equally dysregulated in membrane and total meprin α absence, probably crucial for the protective role of meprin α in IBD. In addition, only ∼31% of the meprin α–knockout mice gene subset matched to observations of up- or down-regulation in IBD patients. This is further supported by our gene ontology analysis indicating that processes functionally associated with wound healing, tissue regeneration, innate immunity, and inflammatory mechanisms are dominating the picture. Although we believe that regulatory miRNA plays a key role as previously illustrated by Ágg et al. (41), our experimental setup does not allow us to draw conclusions on interconnections between the transcripts identified here and previously identified regulatory miRNAs. Hence, further investigations are necessary to demonstrate whether meprins are key factors in the onset and progression of intestinal inflammatory conditions, or if these proteases are only dysregulated as bystander genes.
In summary, we identified human meprin α and meprin β to form a covalently linked heterodimer, which alters post-translational modifications, localization, and proteolytic activity of both enzymes. Furthermore, solubilization of meprin α complexed at the cell surface requires regulated shedding of meprin β and changes transcription of IBD-related genes.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Judith Bond (Pennsylvania State University, State College, PA, USA) for intense discussions on biochemical properties of meprin oligomerization. The authors also thank Lennard Arp (University of Kiel) for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) SFB 877 (Proteolysis as a Regulatory Event in Pathophysiology, Projects A9, A13, and A15) under Germany's Excellence Strategy, EXC 22167-390884018, and BE 4086/2-2 and BE 4086/5-1 (both to C.B.P.). J.P. is supported by the U.S. National Institutes of Health (NIH), National Institute of Dental and Craniofacial Research (NIDCR) (Grant DE 022597). The authors declare no conflicts of interest.
Glossary
- ADAM
a disintegrin and metalloprotease
- APP
amyloid precursor protein
- CM
CaCl2 and MgCl2
- dnp
K-ε-2,4-dinitrophenyl
- ER
endoplasmic reticulum
- FCS
fetal calf serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IBD
inflammatory bowel disease
- MAM
meprin, A-5 protein, and receptor protein-tyrosine phosphatase μ
- mca
7-methyloxycoumarin-4-yl
- meprin-SF
meprin Strep/Flag-tagged
- PDB
Protein Data Bank
- RgpB
Arg-gingipain B
- RNAseq
transcriptional gene analysis
- SF
Strep/Flag
- TBS
Tris-buffered saline
- Tll
tolloid
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
F. Peters, F. Scharfenberg, C. Colmorgen, F. Armbrust, R. Wichert, P. Arnold, and R. Häsler performed research; B. Potempa, J. Potempa, and C. U. Pietrzik provided materials; R. Häsler and P. Rosenstiel performed and analyzed RNA sequencing experiments; and F. Peters and C. Becker-Pauly conceived the experiments and wrote the manuscript.
REFERENCES
- 1.Marchand P., Tang J., Johnson G. D., Bond J. S. (1995) COOH-terminal proteolytic processing of secreted and membrane forms of the alpha subunit of the metalloprotease meprin A. Requirement of the I domain for processing in the endoplasmic reticulum. J. Biol. Chem. 270, 5449–5456 [DOI] [PubMed] [Google Scholar]
- 2.Bertenshaw G. P., Norcum M. T., Bond J. S. (2003) Structure of homo- and hetero-oligomeric meprin metalloproteases. Dimers, tetramers, and high molecular mass multimers. J. Biol. Chem. 278, 2522–2532 [DOI] [PubMed] [Google Scholar]
- 3.Herzog C., Haun R. S., Ludwig A., Shah S. V., Kaushal G. P. (2014) ADAM10 is the major sheddase responsible for the release of membrane-associated meprin A. J. Biol. Chem. 289, 13308–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichert R., Ermund A., Schmidt S., Schweinlin M., Ksiazek M., Arnold P., Knittler K., Wilkens F., Potempa B., Rabe B., Stirnberg M., Lucius R., Bartsch J. W., Nikolaus S., Falk-Paulsen M., Rosenstiel P., Metzger M., Rose-John S., Potempa J., Hansson G. C., Dempsey P. J., Becker-Pauly C. (2017) Mucus detachment by host metalloprotease meprin β requires shedding of its inactive pro-form, which is abrogated by the pathogenic protease RgpB. Cell Rep. 21, 2090–2103 [DOI] [PubMed] [Google Scholar]
- 5.Johnson G. D., Hersh L. B. (1992) Cloning a rat meprin cDNA reveals the enzyme is a heterodimer. J. Biol. Chem. 267, 13505–13512 [PubMed] [Google Scholar]
- 6.Beynon R. J., Shannon J. D., Bond J. S. (1981) Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem. J. 199, 591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterchi E. E., Green J. R., Lentze M. J. (1982) Non-pancreatic hydrolysis of N-benzoyl-l-tyrosyl-p-aminobenzoic acid (PABA-peptide) in the human small intestine. Clin. Sci. (Lond.) 62, 557–560 [DOI] [PubMed] [Google Scholar]
- 8.Barnes K., Ingram J., Kenny A. J. (1989) Proteins of the kidney microvillar membrane. Structural and immunochemical properties of rat endopeptidase-2 and its immunohistochemical localization in tissues of rat and mouse. Biochem. J. 264, 335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee S., Oneda B., Yap L. M., Jewell D. P., Matters G. L., Fitzpatrick L. R., Seibold F., Sterchi E. E., Ahmad T., Lottaz D., Bond J. S. (2009) MEP1A allele for meprin A metalloprotease is a susceptibility gene for inflammatory bowel disease. Mucosal Immunol. 2, 220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee S., Jin G., Bradley S. G., Matters G. L., Gailey R. D., Crisman J. M., Bond J. S. (2011) Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G273–G282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trachtman H., Valderrama E., Dietrich J. M., Bond J. S. (1995) The role of meprin A in the pathogenesis of acute renal failure. Biochem. Biophys. Res. Commun. 208, 498–505 [DOI] [PubMed] [Google Scholar]
- 12.Grünberg J., Dumermuth E., Eldering J. A., Sterchi E. E. (1993) Expression of the alpha subunit of PABA peptide hydrolase (EC 3.4.24.18) in MDCK cells. Synthesis and secretion of an enzymatically inactive homodimer. FEBS Lett. 335, 376–379 [DOI] [PubMed] [Google Scholar]
- 13.Johnson G. D., Bond J. S. (1997) Activation mechanism of meprins, members of the astacin metalloendopeptidase family. J. Biol. Chem. 272, 28126–28132 [DOI] [PubMed] [Google Scholar]
- 14.Ohler A., Debela M., Wagner S., Magdolen V., Becker-Pauly C. (2010) Analyzing the protease web in skin: meprin metalloproteases are activated specifically by KLK4, 5 and 8 vice versa leading to processing of proKLK7 thereby triggering its activation. Biol. Chem. 391, 455–460 [DOI] [PubMed] [Google Scholar]
- 15.Jäckle F., Schmidt F., Wichert R., Arnold P., Prox J., Mangold M., Ohler A., Pietrzik C. U., Koudelka T., Tholey A., Gütschow M., Stirnberg M., Becker-Pauly C. (2015) Metalloprotease meprin β is activated by transmembrane serine protease matriptase-2 at the cell surface thereby enhancing APP shedding. Biochem. J. 470, 91–103 [DOI] [PubMed] [Google Scholar]
- 16.Norman L. P., Jiang W., Han X., Saunders T. L., Bond J. S. (2003) Targeted disruption of the meprin beta gene in mice leads to underrepresentation of knockout mice and changes in renal gene expression profiles. Mol. Cell. Biol. 23, 1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F. T., de Beer T. A. P., Rempfer C., Bordoli L., Lepore R., Schwede T. (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laskowski R. A., Jabłońska J., Pravda L., Vařeková R. S., Thornton J. M. (2018) PDBsum: Structural summaries of PDB entries. Protein Sci. 27, 129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin M. (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 [Google Scholar]
- 20.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D. R., Pimentel H., Salzberg S. L., Rinn J. L., Pachter L. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578; erratum: 9, 2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anders S., Pyl P. T., Huber W. (2015) HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love M. I., Huber W., Anders S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavazoie S., Hughes J. D., Campbell M. J., Cho R. J., Church G. M. (1999) Systematic determination of genetic network architecture. Nat. Genet. 22, 281–285 [DOI] [PubMed] [Google Scholar]
- 24.Chevallier S., Ahn J., Boileau G., Crine P. (1996) Identification of the cysteine residues implicated in the formation of alpha 2 and alpha/beta dimers of rat meprin. Biochem. J. 317, 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arolas J. L., Broder C., Jefferson T., Guevara T., Sterchi E. E., Bode W., Stöcker W., Becker-Pauly C., Gomis-Rüth F. X. (2012) Structural basis for the sheddase function of human meprin β metalloproteinase at the plasma membrane. Proc. Natl. Acad. Sci. USA 109, 16131–16136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishmael S. S., Ishmael F. T., Jones A. D., Bond J. S. (2006) Protease domain glycans affect oligomerization, disulfide bond formation, and stability of the meprin A metalloprotease homo-oligomer. J. Biol. Chem. 281, 37404–37415 [DOI] [PubMed] [Google Scholar]
- 27.Broder C., Becker-Pauly C. (2013) The metalloproteases meprin α and meprin β: unique enzymes in inflammation, neurodegeneration, cancer and fibrosis. Biochem. J. 450, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker-Pauly C., Barré O., Schilling O., Auf dem Keller U., Ohler A., Broder C., Schütte A., Kappelhoff R., Stöcker W., Overall C. M. (2011) Proteomic analyses reveal an acidic prime side specificity for the astacin metalloprotease family reflected by physiological substrates. Mol. Cell. Proteomics 10, M111.009233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bien J., Jefferson T., Causević M., Jumpertz T., Munter L., Multhaup G., Weggen S., Becker-Pauly C., Pietrzik C. U. (2012) The metalloprotease meprin β generates amino terminal-truncated amyloid β peptide species. J. Biol. Chem. 287, 33304–33313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schönherr C., Bien J., Isbert S., Wichert R., Prox J., Altmeppen H., Kumar S., Walter J., Lichtenthaler S. F., Weggen S., Glatzel M., Becker-Pauly C., Pietrzik C. U. (2016) Generation of aggregation prone N-terminally truncated amyloid β peptides by meprin β depends on the sequence specificity at the cleavage site. Mol. Neurodegener. 11, 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bedau T., Peters F., Prox J., Arnold P., Schmidt F., Finkernagel M., Köllmann S., Wichert R., Otte A., Ohler A., Stirnberg M., Lucius R., Koudelka T., Tholey A., Biasin V., Pietrzik C. U., Kwapiszewska G., Becker-Pauly C. (2017) Ectodomain shedding of CD99 within highly conserved regions is mediated by the metalloprotease meprin β and promotes transendothelial cell migration. FASEB J. 31, 1226–1237 [DOI] [PubMed] [Google Scholar]
- 32.Häsler R., Sheibani-Tezerji R., Sinha A., Barann M., Rehman A., Esser D., Aden K., Knecht C., Brandt B., Nikolaus S., Schäuble S., Kaleta C., Franke A., Fretter C., Müller W., Hütt M. T., Krawczak M., Schreiber S., Rosenstiel P. (2017) Uncoupling of mucosal gene regulation, mRNA splicing and adherent microbiota signatures in inflammatory bowel disease. Gut 66, 2087–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahn D., Illisson R., Metspalu A., Sterchi E. E. (2000) Human N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase (human meprin): genomic structure of the alpha and beta subunits. Biochem. J. 346, 83–91 [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson G. D., Hersh L. B. (1994) Expression of meprin subunit precursors. Membrane anchoring through the β subunit and mechanism of zymogen activation. J. Biol. Chem. 269, 7682–7688 [PubMed] [Google Scholar]
- 35.Lottaz D., Hahn D., Müller S., Müller C., Sterchi E. E. (1999) Secretion of human meprin from intestinal epithelial cells depends on differential expression of the alpha and beta subunits. Eur. J. Biochem. 259, 496–504 [DOI] [PubMed] [Google Scholar]
- 36.Lottaz D., Maurer C. A., Noël A., Blacher S., Huguenin M., Nievergelt A., Niggli V., Kern A., Müller S., Seibold F., Friess H., Becker-Pauly C., Stöcker W., Sterchi E. E. (2011) Enhanced activity of meprin-α, a pro-migratory and pro-angiogenic protease, in colorectal cancer. PLoS One 6, e26450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jefferson T., Čaušević M., auf dem Keller U., Schilling O., Isbert S., Geyer R., Maier W., Tschickardt S., Jumpertz T., Weggen S., Bond J. S., Overall C. M., Pietrzik C. U., Becker-Pauly C. (2011) Metalloprotease meprin β generates nontoxic N-terminal amyloid precursor protein fragments in vivo. J. Biol. Chem. 286, 27741–27750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hata S., Kitamura F., Yamaguchi M., Shitara H., Murakami M., Sorimachi H. (2016) A gastrointestinal calpain complex, G-calpain, is a heterodimer of CAPN8 and CAPN9 calpain isoforms, which play catalytic and regulatory roles, respectively. J. Biol. Chem. 291, 27313–27322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biasin V., Wygrecka M., Bärnthaler T., Jandl K., Jain P. P., Bálint Z., Kovacs G., Leitinger G., Kolb-Lenz D., Kornmueller K., Peters F., Sinn K., Klepetko W., Heinemann A., Olschewski A., Becker-Pauly C., Kwapiszewska G. (2018) Docking of meprin α to heparan sulphate protects the endothelium from inflammatory cell extravasation. Thromb. Haemost. 118, 1790–1802 [DOI] [PubMed] [Google Scholar]
- 40.Schütte A., Ermund A., Becker-Pauly C., Johansson M. E. V., Rodriguez-Pineiro A. M., Bäckhed F., Müller S., Lottaz D., Bond J. S., Hansson G. C. (2014) Microbial-induced meprin β cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc. Natl. Acad. Sci. USA 111, 12396–12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ágg B., Baranyai T., Makkos A., Vető B., Faragó N., Zvara Á., Giricz Z., Veres D. V., Csermely P., Arányi T., Puskás L. G., Varga Z. V., Ferdinandy P. (2018) MicroRNA interactome analysis predicts post-transcriptional regulation of ADRB2 and PPP3R1 in the hypercholesterolemic myocardium. Sci. Rep. 8, 10134; erratum: 9, 12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.