Abstract
Oesophageal adenocarcinoma (OAC) is an inflammation-driven cancer with poor prognosis and incidence is increasing rapidly. OAC arises from gastro-oesophageal reflux disease (GORD) and reflux-induced Barrett oesophagus (BO). The role of T cells in this disease progression is not yet fully understood. We have previously demonstrated higher proportions of pro-tumour Th2 cells in BO tissue, implicating them in its pathogenesis. While a Th2 immune profile is thought to underlie the metaplastic transformation in BO and promote OAC development, our studies suggest that the abundance of Th2 cells in BO tissue is likely to occur through altered T cell recruitment. This study examined the chemokine networks governing T cell migration to oesophageal tissue during disease progression. Here, we have identified that circulating T cells in OAC patients, exhibit impaired migratory capacity with decreased frequencies of Th1-associated CXCR3+ and Th17-associated CCR6+ cells. Despite the abundance of Th1 chemokines RANTES (CCL5) and MIP-1α (CCL3) in OAC tumour, enrichments of intratumoural T cells expressing corresponding receptors were not observed. These data suggest that T cell infiltration of oesophageal tissue is compromised in OAC and suggest that future therapies targeting T cell trafficking should occur at the pre-neoplastic stage. This is supported by the finding that antagonism of Th2-biased CCR4 significantly reduces T cell migration in BO but not OAC patients. Since we have previously reported a predominant Th2 immune profile in BO, we suggest that chemokine receptor antagonism may be a viable treatment option to alleviate Th2-predominance in BO and interrupt progression to OAC.
Keywords: T cells, Chemokines, Oesophageal cancer, Barrett Oesophagus, Immunotherapy
Introduction
Oesophageal adenocarcinoma (OAC) is an inflammatory driven malignancy, with chronic gastro-oesophageal reflux disease (GORD) and Barrett oesophagus (BO) defined as major risk factors for its development [1]. BO is characterised pathologically by specialised intestinal metaplasia of the distal oesophagus, which arises from a background of chronic GORD, with a prevalence of 1–2% in the general population [2, 3]. Once patients progress to malignancy, they are faced with a paucity of treatment options and dismal survival rates of approximately 18%, due to late presentation of disease and poor responses to treatment [www.ncri.ie]. Therefore, novel therapeutic approaches to prevent or slow disease progression to malignancy are required. Here, we address the potential of chemokine receptor antagonism and uncover the optimal timing for such an immunotherapeutic intervention in the disease sequence.
We have previously identified a Th1 bias in the initiation of oesophageal inflammation followed by a switch to a Th2 profile in BO [4], which is not only thought to drive the metaplastic transformation [5], but is also pro-tumourigenic [6], thus promoting progression to OAC. In brief, we identified higher frequencies of IL-4 producing T-cells and secreted levels of IL-6, confirming a Th2 phenotype in BO, while a mix of both pro- and anti-inflammatory cytokines were secreted in the tumour microenvironment in OAC [4]. In addition, CD4+ T-cells infiltrating tumour tissue displayed a decreased activation profile, compared with normal tissue. While our group have reported the key role of specific chemokine networks in inflammatory T cell migration to the liver and adipose tissue in OAC patients [7–9], the chemokine profiles active in the oesophagitis-metaplasia-carcinoma sequence have not been studied in detail. Furthermore, the pathways governing Th2 cell accumulation in oesophageal tissues in BO and a Th1:Th2 mixed profile in OAC are yet to be elucidated. Chemokine receptor antagonism represents a promising intervention therapy for inflammatory disease and novel targets are warranted in the space of OAC. So far, CCR5 inhibitor, Maraviroc and CXCR4 inhibitor, Plerixafor are approved for use in HIV-1 and stem cell mobilization, respectively, while Poteligeo, a CCR4 monoclonal antibody is FDA-approved for cutaneous T cell lymphoma (CTCL) [10, 11] [www.fda.gov].
This study firstly examines the T cell chemokine profile in the serum and oesophageal microenvironment at each disease stage from oesophagitis to OAC to elucidate the pathways guiding T cell chemotaxis across the disease sequence and secondly, explores the potential for chemokine receptor antagonism as an immunotherapeutic strategy to skew T cell migration.
Materials and Methods
Subjects
A total of 92 consecutive consenting patients, attending the National Oesophageal and Gastric Centre at St. James Hospital, Dublin were enrolled in this study. The patient group included 17 oesophagitis patients, 16 healthy controls, 30 Barrett Oesophagus patients and 29 patients with OAC. The study comprised 57 males and 35 females, with an average age of 56.8 years.
Sample Preparation
Peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation using Ficoll-Paque™ Plus (GE Healthcare). Oesophageal tissue biopsies, collected over a 4 year period, were taken from oesophagitis, BO and OAC patients. Normal tissue adjacent to BO tissue was also obtained as a source of normal control tissue. Tissue was digested as previously described [4]. Serum was isolated from peripheral blood by collecting and centrifuging whole blood in SerumClotActivator tubes (Greiner) at 3000 RPM for 10 min at 4 °C. Tissue conditioned media (CM) was prepared as previously described [4].
Quantification of Chemokine Levels
The V-PLEX™ and single-plex human chemokine plates (Meso Scale Discovery) were used to detect the levels of MIP-1α (CCL3), MIP-1β (CCL4), TARC (CCL17), IP-10 (CXCL10), MDC (CCL22), RANTES (CCL5), ITAC (CXCL11), Fractalkine (CX3CL1) and MIP-3α (CCL20) in oesophageal tissue conditioned media and patient serum from a total of 8 oesophagitis, 10 BO, and 10 OAC patients and serum from 10 healthy controls, and read using a Meso Scale Diagnostics Sector S600. Chemokine levels (pg/ml) in CM were normalised to total protein content in biopsy and were expressed as pg/μg.
Quantification of Chemokine Receptor Expressing T Cells in Blood and Oesophageal Tissue
Whole blood and single cell suspensions from oesophageal tissue from 4 oesophagitis patients, 8 BO patients and 5 OAC patients and whole blood from a total of 8 healthy controls were stained with monoclonal antibodies (mAbs) specific for human surface markers CD3 (eBioscience), CD4, CD8, CCR5, CCR6 (BD Biosciences), and CCR3, CCR4 (Biolegend) and CXCR3 (Miltenyi Biotec). Cells were acquired using CyAn ADP flow cytometer (Beckman Coulter) and analysed with FloJo software (TreeStar Inc.).
T Cell Chemotaxis Assays
Recombinant chemokines were used at a concentration of 10 ng/ml in M199 media for migration studies. All chemokines were purchased from Cell guidance systems, except for CCL3 which was purchased from Biolegend. For antagonism experiments, PBMC were pretreated with chemokine receptor antagonists for 1 h at 37 °C at the following concentrations: 100 nM AMG 487 (CXCR3 antagonist) (Bio-techne), 10 nM Vicriviroc Malate (CCR5 antagonist) (Merck Millipore), 140 nM C021 dihydrochloride (CCR4 antagonist) (R&D systems), 50 nM SB 328437 (CCR3 antagonist) (Tocris) or 50 nM SCH 527123 (CXCR1/CXCR2 antagonist) (Stratech). Chemotaxis in response to recombinant chemokines was measured using Corning Transwell® plates (6.5 mm diameter, 5 μm pore size, Corning,). The bottom well was loaded with 600 μl M199 media alone or 10 ng/ml recombinant chemokine, 20% FBS was used as a positive control. The top chamber was loaded with 2 × 105 cells in 100 μl M199 media. Following 2 h incubation at 37 °C and 5% CO2, inserts were removed and cells which had migrated to the bottom chamber were stained for analysis by flow cytometry and counted using CountBright™ absolute counting beads as per manufacturer’s instructions (Life technologies).
Statistical Analyses
Statistical analysis was carried out using Prism GraphPad Version 5.0. Differences between multiple groups were assessed using the One way ANOVA with Tukey’s post-hoc test or paired tests (Wilcoxon Sign Rank) where appropriate. p values of <0.05 were considered significant. Any non-reported p values were not significant p > 0.05.
Results
Significantly Diminished Frequencies of Circulating Th1-Biased CXCR3+ and Th17-Biased CCR6+ T Cells in OAC, Compared to Earlier Stages of the GORD, BO, OAC Disease Sequence
Flow cytometric analysis of circulating T cells from patients across the disease sequence revealed that OAC patients displayed significantly diminished frequencies of circulating Th1-biased CD4+CXCR3+ cells (1.79 ± 0.28%) compared to oesophagitis (38.7 ± 4.32%, p < 0.01) and BO patients (30.11 ± 2.42%, p < 0.001, Fig. 1). Similarly within the CD8+ population, OAC patients displayed significantly lower frequencies of circulating CXCR3+ cells (1.36 ± 0.28%) compared to healthy (35.08 ± 8.80%, p < 0.05), oesophagitis (59.83 ± 5.17%, p < 0.01) and BO patients (45.053 ± 3.33%, p < 0.001, Fig. 1).
Fig. 1.
Significantly lower frequencies of circulating CXCR3+and CCR6+T cells in OAC patients while CCR3+T cells are significantly higher in the circulation of Barrett Oesophagus and OAC patients. Whole blood from healthy control (Healthy), oesophagitis, Barrett’s and OAC and oesophageal biopsies from normal tissue adjacent to Barrett Oesophagus tissue (Normal), oesophagitis, Barrett’s and OAC patients were immunophenotyped by flow cytometry by staining for CD3, CD4, CD8, CXCR3, CCR5, CCR4, CCR3 and CCR6. Scatter plots showing percentage frequencies of CD4+ or CD8+ T cells expressing CCR3, CCR4, CCR5, CCR6 and CXCR3 in healthy control (Healthy), oesophagitis, Barrett’s and OAC patient blood (Blood) and normal tissue adjacent to Barrett Oesophagus tissue (Normal), oesophagitis, Barrett’s and OAC patient tissue (Tissue). All data is presented as mean ± SEM, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, T test (Mann Whitney), (Blood: Normal = 7–8, Oesophagitis = 3–4, Barrett’s = 7–8, OAC = 4–5, Tissue: Normal = 4–6, Oesophagitis = 3, Barrett’s = 4, OAC = 4)
Interestingly, our data also showed significantly lower frequencies of circulating T cells expressing the Th17-biased receptor CCR6 in OAC patients (CD4: 0.43 ± 0.84%; CD8: 17.28 ± 1.36%) compared to healthy (CD4:13.6 ± 4.11%, p < 0.05; CD8: 32.41 ± 4.63%, p < 0.05), oesophagitis (CD4: 16.2 ± 13.63%, p < 0.05; CD8 NS) and BO patients (CD4: 16.10 ± 3.371%, p < 0.01; CD8: NS) (Fig. 1).
Significantly Higher Frequencies of T Cells Expressing the Th2 Receptor CCR3 in the Blood of BO and OAC Patients while, there Is an Abundance CD4+ T Cells Expressing the Th1-Biased Receptor CCR5 in Oesophageal Tissue at these Disease Stages
Flow cytometric analysis of circulating T cells from patients across the disease sequence revealed that BO patient blood contained significantly larger populations of CD4+ and CD8+ T cells expressing the predominantly Th2-biased receptor CCR3 (CD4: 6.57 ± 4.74%; CD8: 4.01 ± 0.88%) compared to healthy (CD4: 0.45 ± 0.16%, p < 0.01; CD8: 0.91 ± 0.45%, p < 0.05), suggesting a systemic bias to Th2 cells in BO. Similarly OAC patients displayed significantly higher frequencies of CD8+CCR3+ (4.69 ± 0.73%) compared to healthy (0.91 ± 0.45%, p < 0.05) (Fig. 1). Flow cytometric analysis of T cells in oesophageal tissue demonstrated significantly higher proportions of CD4+CCR5+ cells within BO tissue compared to normal adjacent to BO tissue, and BO blood (Fig. 1, p < 0.001). Furthermore, our data revealed that the Th1-biased CD4+CCR5+ cells were enriched in oesophageal tissue of OAC patients compared to the peripheral blood (Fig. 1, p < 0.001). Interestingly, the majority of T cells across the disease sequence did not co-express CCR5 and CXCR3, suggesting that these T cells are composed of mixed populations with divergent expression patterns of CCR5 and CXCR3 [data not shown]. The percentage of cells expressing the Th2-associated receptor CCR4 was found to be similar across all patient groups (Fig. 1).
Significantly Higher Concentrations of RANTES, MIP-1α and MIP-3α in OAC Patient-Derived Oesophageal Tissue
Chemokines were measured by ELISA in oesophageal tissue conditioned media (CM) and serum from patients across the disease sequence and in serum from healthy controls.
Our data identified significantly higher levels of the Th1-biased chemokine MIP-1α (CCL3) in CM from OAC patients (TCM) compared to CM derived from normal tissue adjacent to BO tissue (NCM) (TCM vs NCM: 229.32 pg/μg v 21.01 pg/μg, p = 0.04). Our analysis of serum chemokine levels revealed significantly lower levels of MIP-1α (CCL3) in oesophagitis (11.70 ± 2.13 pg/ml, p < 0.01) and BO (9.31 ± 0.6 pg/ml, p < 0.001) compared to healthy donor (27.64 ± 2.17 pg/ml) and OAC (23.33 ± 2.3 pg/ml,) (Fig. 2). Furthermore, there were substantially but not significantly higher levels of the Th1 chemokine IP-10 (CXCL10) in OAC tumour compared to earlier in the disease sequence (Fig. 2).
Fig. 2.
Significantly higher concentrations of RANTES (CCL5), MIP-1α (CCL3) and MIP-3α (CCL20) in tumour conditioned media of OAC patients. Serum was collected from healthy controls (Healthy), oesophagitis, Barrett Oesophagus and OAC patients. Oesophageal biopsies were cultured in X-vivo media for 24 h to generate conditioned media from normal tissue adjacent to Barrett Oesophagus tissue (Normal), oesophagitis tissue (Oesophagitis), Barrett Oesophagus tissue (Barrett’s) and tumour tissue (OAC) (Tissue). IP-10 (CXCL10), ITAC (CXCL11), Fractalkine (CX3CL1), MIP-1α (CCL3), MIP-1β (CCL4), MIP-3α (CCL20), TARC (CCL17) and MDC(CCL22) levels (pg/ml) were measured using MSD multiplex and single plex ELISAs and are shown as pg/ml for serum (Serum) but have been normalised to total protein in the biopsy and are shown as pg/μg for tissue (Tissue). All data is presented as mean ± SEM, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, T test (Mann Whitney) (Serum: Normal = 10, Oesophagitis = 8, Barrett’s = 10, OAC = 10, Tissue: Normal = 7, Oesophagitis = 3, Barrett’s = 7, OAC = 7)
Another striking finding was the significant enrichment of RANTES (CCL5) in TCM compared to NCM (228.16 pg/μg vs 12.62 pg/μg, p = 0.004), while serum levels were similar across the disease sequence (Fig. 2). RANTES (CCL5) binds both Th1-biased and Th2-biased chemokine receptors and therefore, demonstrates bias for both helper T cell populations. It is therefore likely that RANTES (CCL5) and MIP-1alpha (CCL3) are leading to accumulations of CCR5+ CD4+ T cells observed in OAC tumour.
The Th17-biased chemokine MIP-3α (CCL20) was also significantly enriched in the TCM compared to BO tissue-derived CM (BCM) (TCM vs BCM: 162.72 pg/μg vs 9.33 pg/μg, p = 0.04), while only detectable at low levels in the serum across the sequence (Fig. 2).
The Th1 chemokine MIP-1β (CCL4) exhibited significantly lower levels in oesophagitis and BO serum (Oes: 51.63 ± 4.036 pg/ml; BO: 52.51 ± 4.036 pg/ml) compared to healthy (134.2 ± 18.25 pg, p < 0.01) and OAC serum (125.5 ± 19.11 pg, p < 0.05) (Fig. 2). TARC (CCL17) is a potent Th2 chemokine that binds to the receptor CCR4, selectively recruiting Th2 T cells. Interestingly, the secreted levels of TARC (CCL17) were significantly lower in serum from BO patients (127 ± 12.02 pg/ml) compared to healthy (457.6 ± 100.8 pg/ml, p < 0.05) and OAC (437.6 ± 83.58 pg/ml, p < 0.05) (Fig. 2). Similarly levels of MDC (CCL22) were significantly higher in the serum of healthy donors and OAC patients compared to oesophagitis and BO patients (p < 0.01, Fig. 2). Levels of ITAC (CXCL11) and Fractalkine (CX3CL1) were similar across all groups with no significant differences noted (Fig. 2).
Overall, serum from healthy donors and OAC patients contain higher concentrations of MIP-1α (CCL3), MIP-1β (CCL4), MDC (CCL22) and TARC (CCL17) compared to oesophagitis and BO patients suggesting that these disease stages exhibit systemically altered chemokine signalling and differ in their ability to retain associated T cells in circulation. Interestingly, these data suggest that there are significantly enhanced chemotactic signals in oesophageal tissue from OAC patients compared to earlier in the disease sequence and implicate MIP-1α (CCL3), MIP-3α (CCL20) and RANTES (CCL5) in T cell infiltration at the malignant stage (Fig. 2).
Circulating T Cells from OAC Patients Have Significantly Lower Migratory Capacity to Recombinant Chemokines, Compared to those Derived from BO Patients
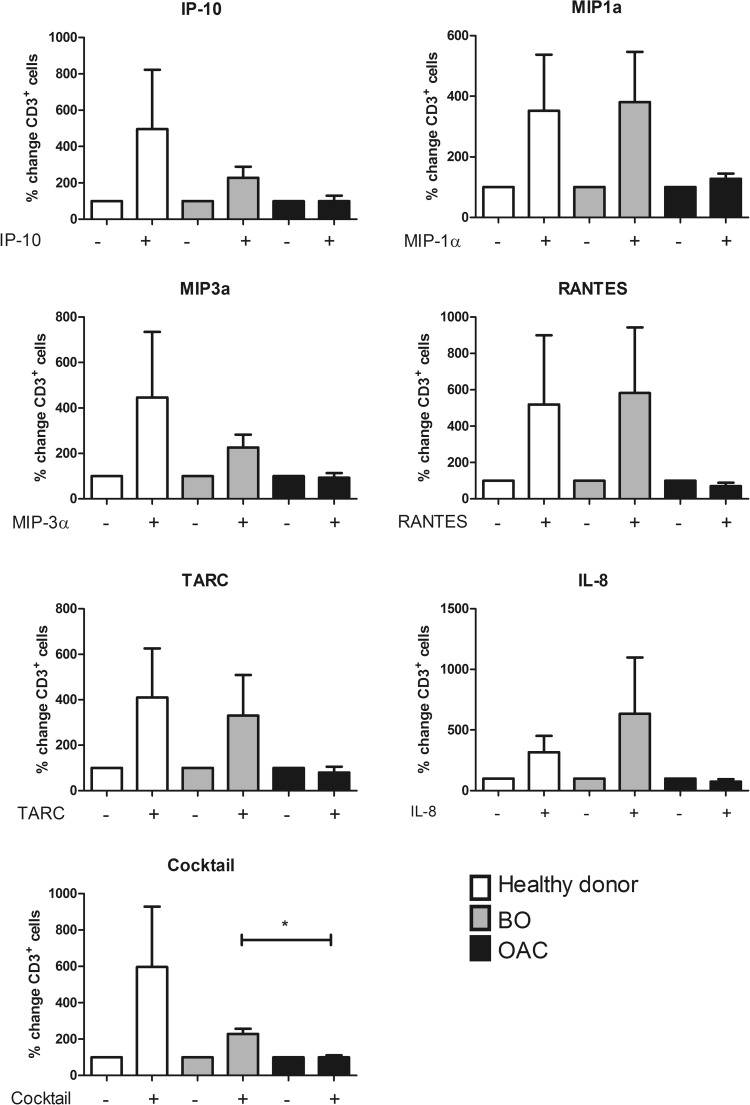
The migratory capacity of circulating T cells was examined across the disease sequence. PBMC were isolated from the blood of healthy donors, BO and OAC patients, plated in a transwell plate and their migration toward the recombinant chemokines IP-10 (CXCL10), MIP-1α (CCL3), MIP-3α (CCL20), RANTES (CCL5), TARC (CCL17) and IL-8 (CXCL8) (at a concentration of 10 ng/ml), or a cocktail of all 6 chemokines (composed of 10 ng/ml of each) was measured. The migratory capacity of T cells from OAC patients to the chemokine cocktail was significantly lower than that of BO patients and substantially lower than that of healthy donors (OAC vs BO vs Healthy (% change); 100.395 vs 227.908 vs 596.825, p = 0.04, Fig. 3). These data suggest that T cells from BO patients may be more amenable to chemokine-targeted therapies than those from OAC patients.
Fig. 3.
Migratory capacity of circulating CD3+T cells to recombinant chemokines is impaired in OAC, compared to BO patients. PBMC from healthy donors (white), BO (grey) and OAC (black) patients were plated in top chamber of transwell plate. Recombinant chemokines (10 ng/ml) were placed in the bottom chamber, and were incubated for 2 h. Cocktail was composed of 10 ng/ml each of IP-10 (CXCL10) MIP-1α (CCL3), MIP-3α (CCL20), RANTES (CCL5), TARC (CCL17) and IL-8 (CXCL8). Bar charts show T cell migration expressed as a percentage of migrated cells to media only control (set to 100%). All data is presented as mean ± SEM, n = 3. Paired t tests were carried out between treatments (between - and + bars of same colour) and unpaired t tests were carried out between disease stages. *p ≤ 0.05
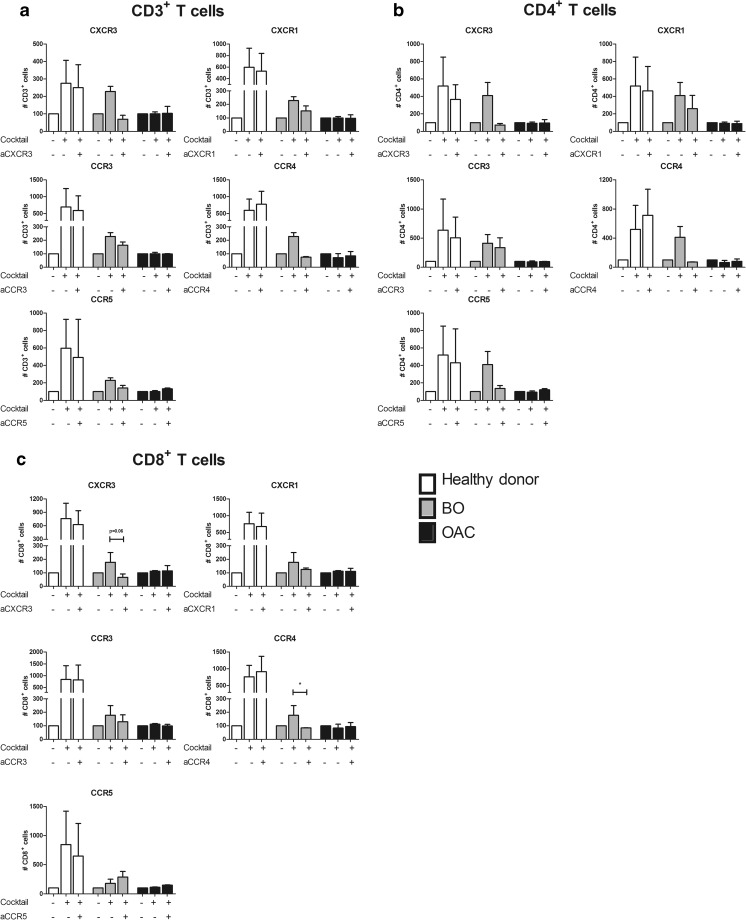
CCR4 Antagonism Significantly Reduces Migration of BO-Patient Derived CD8+ T Cells to Recombinant Chemokines
To examine whether chemokine receptor antagonism could represent a viable therapeutic option to alter T cell accumulation and to identify the optimal disease stage to target T cell migration within diseased oesophageal tissue, PBMC were pre-treated with CXCR3, CXCR1, CCR3, CCR4 and CCR5 antagonists and the effect on T cell migration to the chemokine cocktail was examined. Healthy controls were used as a comparator and not a positive control, as such treatments would not be considered for healthy individuals. Interestingly, cells from BO patients appeared to be the most responsive to antagonism, while cells from OAC patients showed little effect. In PBMC from BO patients, pre-treatment with C021 dihydrochloride, the CCR4 antagonist significantly reduced BO-derived CD8+ T cell migration to recombinant chemokine cocktail (243.0 ± 128.0 cells to 82.43 ± 29.19 cells, p = 0.02) and substantially reduced that of their CD4+ counterparts (410.14 ± 150.6 to 71.583 ± 2.77) (Fig. 4). T cells obtained from OAC patient blood did not demonstrate any response to receptor antagonism. These results suggest that cells from BO patients are the most amenable to receptor blockade, and CCR4 may be a viable therapeutic option to prevent Th2 cell migration to tissue in BO.
Fig. 4.
CCR4 antagonism significantly reduces BO patient-derived T cell migration to recombinant chemokine cocktail. PBMC from healthy donors (white), BO (grey) and OAC (black) patients were pre-treated with chemokine receptor antagonists for 1 h prior to chemotaxis assay using a transwell system and a cocktail of recombinant chemokines (composed of 10 ng/ml each of IP-10 (CXCL10), MIP-1α (CCL3), MIP-3α (CCL20), RANTES (CCL5) and TARC (CCL17) were placed in the bottom chamber and allowed to migrate for 2 h. Bar charts show CD3+ (A), CD4+ (B) and CD8+ (C) T cell migration expressed as a percentage of migrated cells to media only control (set to 100%). All data is presented as mean ± SEM, n = 3. Paired t test was carried out between treatments (between --, − + and ++ bars of same colour), unpaired t test was carried out between disease stages. *p ≤ 0.05
Discussion
Novel therapeutic strategies are required to improve outcomes for patients with the inflammatory-driven malignancy OAC. Since GORD and BO have been identified as major risk factors for this malignancy, therapeutic intervention at the pre-neoplastic stage is a desirable option and our group have examined the potential of chemokine targeted therapy in this disease setting. While chemokines are now accepted to play a role in the initiation and maintenance of inflammation in GORD, the chemokine profile in the oesophagitis-metaplasia-carcinoma sequence has not yet been assessed [12–14]. We and others have described a role for T cells in this disease sequence with GORD being characterised by a pro-inflammatory or Th1 response, BO by a predominantly Th2 profile and OAC having a mixed inflammatory profile [4]. It is likely that the production of specific chemokines in the local oesophageal microenvironment contributes to distinct T cell recruitment and this has been observed in other malignancies, including Hodgkin’s lymphoma and Kaposi’s sarcoma, in which chemokines recruit Th2 cells and cause suppression of Th1 responses to establish a tumour promoting environment [15, 16]. This study has examined the chemokine pathways shaping the T cell profiles in the GORD-BO-OAC disease sequence in an effort to identify novel prognostic and therapeutic candidates.
Our data revealed that T cell chemokines RANTES (CCL5) and MIP-1α (CCL3) were significantly abundant in OAC tumour, and their secretion was accompanied by infiltrations of T cells expressing their shared Th1 receptor CCR5. However, antagonism of CCR5 could not reduce in vitro T cell migration. Interestingly, our results demonstrated that CCR4 antagonism could significantly reduce BO-derived T cell migration. While CCR4 has been implicated in dermatitis, asthma, gastric and renal cell carcinoma and CCR4 monoclonal antibody Poteligeo has been FDA approved for CTCL, our data indicates its possible involvement in the oesophagitis-BO-OAC sequence for the first time [16–22]. Since T cells from OAC patients displayed lower responsiveness to chemokines and proved less amenable to chemokine receptor antagonism, targeting CCR4 might offer a safe and beneficial means of reversing the Th2 cell accumulation at the BO stage and may prevent progression to OAC. Since our data indicate that the T cell migratory capacity to chemotactic signals in tumour is deficient by the time the disease has progressed to the malignant stage, chemokine-targeted approaches at the pre-neoplastic stage presents a more viable prophylactic treatment strategy. Such impaired migratory capacity at tumour stage would also explain why intratumoural CCR5+ T cell infiltrations in OAC patients were lower than BO, despite the abundance of their ligands.
The CCR6:MIP-3α pathway is reported to play a significant role in Th17 cell recruitment and pathological inflammation in ulcerative colitis [23–26]. While our data revealed that there are significantly higher levels of the Th17 chemokine MIP-3α (CCL20) in OAC tumour, enrichments of intratumoural CCR6+ T cells are not observed. The previously reported internalization of CCR6 upon ligand binding may lead to reduced detection of such T cells within OAC tumour and therefore, further work is needed to elucidate the role of MIP-3α (CCL20) in OAC [27].
Increased expression of the chemokines IP-10 (CXCL10), ITAC (CXCL11) and MIG (CXCL9) have been shown to correlate to a better prognosis in colorectal cancer [28]. While the Th1 chemokine IP-10 (CXCL10) was detected at highest levels in the OAC tumour conditioned media, there was a significant absence of T cells expressing its receptor (CXCR3) in the blood of OAC patients and no significant infiltration of such cells in OAC tumour tissue. The decrease in circulating CXCR3+ T cells in OAC patients, together with reduced T cell migratory capacity reflects our finding that CXCR3 antagonism did not affect OAC-derived T cell migration to recombinant chemokine cocktail containing IP-10 (CXCL10). Since IP-10 (CXCL10) has been shown to promote Th1 responses, the lack of its receptor-bearing T cells in OAC might represent further immune dysfunction at the malignant disease stage [29]. It is important to note that observed incongruences between chemokine levels and their responding T cells in this study are also likely due to the redundancy in the chemokine system. Furthermore, it is well reported that many chemokine receptors are internalised upon ligand binding [9, 27]. Studies have shown that high levels of chemokines lead to lower levels of their receptors [30, 31]. This complex process is influenced by the signals in the external microenvironment and the differentiation status of the cell [9]. These dynamic processes were not assessed within the scope of this observational study as the principal aim was to assess the expression pattern of chemokines and their receptors in blood and tissue of these patient cohorts.
For the first time, this study has identified a number of pathways that may potentially play a role in immune cell recruitment to oesophageal tissue in the GORD-BO-OAC sequence. However, the anti-tumour potential of targeting these pathways will have to be assessed using in vivo models. Our data identified impaired migratory capacity and reduced susceptibility to chemokine receptor antagonism in OAC patient-derived T cells but not BO patient-derived T cells and suggest that therapeutic intervention at the pre-neoplastic stage might be preferable. Overall our data indicate that chemokine-targeted therapies could represent an exciting approach to attenuate inflammation in BO patients and potentially prevent progression to OAC. Future large-scale studies are warranted to elucidate the benefit of such approaches to a cohort in urgent need of new and improved treatments.
Acknowledgements
The authors would like to thank all of the patients and staff at the endoscopy and oesophageal unit at St. James’s Hospital for their participation in this study.
Funding
This work was funded by a Health Research Board of Ireland’s Health Research Award HRA_POR/2012/18.
Compliance with Ethical Standards
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethical Approval
The study received ethical approval from the St James’s Hospital Ethics Review Board.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maria E. Kavanagh and Melissa J. Conroy contributed equally to this work.
References
- 1.Vakil N, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 2.Anand O, Wani S, Sharma P. When and how to grade Barrett's columnar metaplasia: the Prague system. Best Pract Res Clin Gastroenterol. 2008;22(4):661–669. doi: 10.1016/j.bpg.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Solaymani-Dodaran M, et al. Risk of oesophageal cancer in Barrett's oesophagus and gastro-oesophageal reflux. Gut. 2004;53(8):1070–1074. doi: 10.1136/gut.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavanagh ME, Conroy MJ, Clarke NE, Gilmartin NT, O'Sullivan KE, Feighery R, MacCarthy F, O'Toole D, Ravi N, Reynolds JV, O'Sullivan J, Lysaght J. Impact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett. 2016;370(1):117–124. doi: 10.1016/j.canlet.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Shan J, Oshima T, Farre R, Fukui H, Watari J, Miwa H. IL-4 induces columnar-like differentiation of esophageal squamous epithelium through JAK/PI3K pathway: possible role in pathogenesis of Barrett's esophagus. Am J Physiol Gastrointest Liver Physiol. 2014;306(8):G641–G649. doi: 10.1152/ajpgi.00386.2013. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto T, Saito H, Tatebe S, Tsujitani S, Ozaki M, Ito H, Ikeguchi M. Interleukin-10 expression significantly correlates with minor CD8+ T-cell infiltration and high microvessel density in patients with gastric cancer. Int J Cancer. 2006;118(8):1909–1914. doi: 10.1002/ijc.21598. [DOI] [PubMed] [Google Scholar]
- 7.Lysaght J, Allott EH, Donohoe CL, Howard JM, Pidgeon GP, Reynolds JV. T lymphocyte activation in visceral adipose tissue of patients with oesophageal adenocarcinoma. Br J Surg. 2011;98(7):964–974. doi: 10.1002/bjs.7498. [DOI] [PubMed] [Google Scholar]
- 8.Conroy MJ, Galvin KC, Kavanagh ME, Mongan AM, Doyle SL, Gilmartin N, O'Farrelly C, Reynolds JV, Lysaght J. CCR1 antagonism attenuates T cell trafficking to omentum and liver in obesity-associated cancer. Immunol Cell Biol. 2016;94(6):531–537. doi: 10.1038/icb.2016.26. [DOI] [PubMed] [Google Scholar]
- 9.Conroy MJ, Maher SG, Melo AM, Doyle SL, Foley E, Reynolds JV, Long A, Lysaght J. Identifying a novel role for Fractalkine (CX3CL1) in memory CD8(+) T cell accumulation in the Omentum of obesity-associated Cancer patients. Front Immunol. 2018;9:1867. doi: 10.3389/fimmu.2018.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H, Iida S, Imada K, Uchiyama T, Akinaga S, Shitara K, Ueda R. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 2010;16(5):1520–1531. doi: 10.1158/1078-0432.CCR-09-2697. [DOI] [PubMed] [Google Scholar]
- 11.Ishida T, et al. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-cell leukemia/lymphoma. Clin Cancer Res. 2004;10(22):7529–7539. doi: 10.1158/1078-0432.CCR-04-0983. [DOI] [PubMed] [Google Scholar]
- 12.Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K, Murata I, Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol. 2003;98(3):551–556. doi: 10.1111/j.1572-0241.2003.07303.x. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187(6):875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19(12):568–574. doi: 10.1016/S0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 15.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99(12):4283–4297. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 16.Sozzani S, et al. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood. 1998;92(11):4036–4039. [PubMed] [Google Scholar]
- 17.Yang YM, Feng AL, Zhou CJ, Liang XH, Mao HT, Deng BP, Yan S, Sun JT, du LT, Liu J, Wang QJ, Neckenig MR, Yang QF, Qu X. Aberrant expression of chemokine receptor CCR4 in human gastric cancer contributes to tumor-induced immunosuppression. Cancer Sci. 2011;102(7):1264–1271. doi: 10.1111/j.1349-7006.2011.01934.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q, Rexiati M, Yang Y, Wang WG, Azhati B, SaiMaiti W, Wang YJ. Expression of chemokine receptor 4 was associated with poor survival in renal cell carcinoma. Med Oncol. 2014;31(4):882. doi: 10.1007/s12032-014-0882-y. [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka K, Mizutani H. The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr Probl Dermatol. 2011;41:80–92. doi: 10.1159/000323299. [DOI] [PubMed] [Google Scholar]
- 20.Pease JE, Horuk R. Recent progress in the development of antagonists to the chemokine receptors CCR3 and CCR4. Expert Opin Drug Discovery. 2014;9(5):467–483. doi: 10.1517/17460441.2014.897324. [DOI] [PubMed] [Google Scholar]
- 21.Solari R, Pease JE. Targeting chemokine receptors in disease--a case study of CCR4. Eur J Pharmacol. 2015;763(Pt B):169–177. doi: 10.1016/j.ejphar.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagami Y, Kawase Y, Yonekubo K, Nosaka E, Etori M, Takahashi S, Takagi N, Fukuda T, Kuribayashi T, Nara F, Yamashita M. RS-1748, a novel CC chemokine receptor 4 antagonist, inhibits ovalbumin-induced airway inflammation in Guinea pigs. Biol Pharm Bull. 2010;33(6):1067–1069. doi: 10.1248/bpb.33.1067. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, et al. CCR6 is a prognostic marker for overall survival in patients with colorectal cancer, and its overexpression enhances metastasis in vivo. PLoS One. 2014;9(6):e101137. doi: 10.1371/journal.pone.0101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frick VO, et al. Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal malignancy: an overview. World J Gastroenterol. 2016;22(2):833–841. doi: 10.3748/wjg.v22.i2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapur N, Mir H, Clark III CE, Krishnamurti U, Beech DJ, Lillard JW, Singh S. CCR6 expression in colon cancer is associated with advanced disease and supports epithelial-to-mesenchymal transition. Br J Cancer. 2016;114(12):1343–1351. doi: 10.1038/bjc.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nandi B, et al. Stromal CCR6 drives tumor growth in a murine transplantable colon cancer through recruitment of tumor-promoting macrophages. Oncoimmunology. 2016;5(8):e1189052. doi: 10.1080/2162402X.2016.1189052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meissner A, et al. CC chemokine ligand 20 partially controls adhesion of naive B cells to activated endothelial cells under shear stress. Blood. 2003;102(8):2724–2727. doi: 10.1182/blood-2003-01-0007. [DOI] [PubMed] [Google Scholar]
- 28.Kistner L, et al. Interferon-inducible CXC-chemokines are crucial immune modulators and survival predictors in colorectal cancer. Oncotarget. 2017;8(52):89998–90012. doi: 10.18632/oncotarget.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocchi F, DeVico AL, Yarchoan R, Redfield R, Cleghorn F, Blattner WA, Garzino-Demo A, Colombini-Hatch S, Margolis D, Gallo RC. Higher macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels from CD8+ T cells are associated with asymptomatic HIV-1 infection. Proc Natl Acad Sci U S A. 2000;97(25):13812–13817. doi: 10.1073/pnas.240469997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]