Abstract
Growing preclinical evidence shows that short-term fasting (STF) protects from toxicity while enhancing the efficacy of a variety of chemotherapeutic agents in the treatment of various tumour types. STF reinforces stress resistance of healthy cells, while tumor cells become even more sensitive to toxins, perhaps through shortage of nutrients to satisfy their needs in the context of high proliferation rates and/or loss of flexibility to respond to extreme circumstances. In humans, STF may be a feasible approach to enhance the efficacy and tolerability of chemotherapy. Clinical research evaluating the potential of STF is in its infancy. This review focuses on the molecular background, current knowledge and clinical trials evaluating the effects of STF in cancer treatment. Preliminary data show that STF is safe, but challenging in cancer patients receiving chemotherapy. Ongoing clinical trials need to unravel if STF can also diminish toxicity and increase efficacy of chemotherapeutic regimes in daily practice.
Keywords: Short-term fasting, Fasting-mimicking diet, Chemotherapy, Differential stress resistance, Differential stress sensitization, Toxicity
Background
Chronic caloric restriction reduces and delays cancer incidence, and inhibits tumor progression and metastasis in rodents [1–5]. Accordingly, cancer incidence and mortality are strongly reduced in chronic calorie restricted non-human primates [6]. Studies of long-term calorie restricted human subjects have shown a reduction of metabolic and hormonal factors associated with cancer risk [7–9]. However, chronic caloric restriction is not a feasible clinical intervention. Evident difficulties, such as the long period required to be effective, and unacceptable weight loss [10, 11], hamper clinical application in cancer patients.
Preclinical studies suggest that short-term fasting (STF) protects rodents from toxic effects of chemotherapy, while simultaneously enhances the efficacy of a variety of chemotherapeutic agents in numerous distinct malignancies, e.g. breast cancer, melanoma, neuroblastoma, pancreatic cancer, and colorectal cancer [12]. In distinct strains of mice bearing xenograft malignancies, tumor growth clearly slows down in response to chemotherapy combined with a 24–60 h fast as compared to treatment with chemotherapy alone [13–17]. STF simultaneously protects mice from chemotoxicity as well, because it reinforces stress resistance of healthy cells [17–24]. The distinct response of healthy versus tumor cells to STF is called differential stress resistance (DSR). During nutrient deprivation, healthy cells re-invest energy in maintenance and repair that contribute to resistance to chemotherapy, while tumor cells are unable to slow down growth due to mutations in tumor suppressor genes and mitogenic pathways [19, 25]. Moreover, low serum levels of glucose during STF impose extra stress on tumor cells, as their energy needs under these circumstances are primarily met by means of glycolysis [14]. As a consequence of these differential responses of healthy versus cancer cells to STF, chemotherapy causes more DNA damage and apoptosis in tumor cells, while leaving healthy cells unharmed when it is combined with STF. Thus, STF protects healthy cells against the toxic properties of chemotherapy and renders tumor cells more sensitive, a phenomenon called differential stress sensitization (DSS).
In contrast to most cancer therapies, STF has only mild side effects, such as headaches, dizziness, nausea, weakness and short-term weight loss in humans [26]. Therefore, STF is a promising strategy to enhance the efficacy and tolerability of chemotherapy in cancer patients, especially as STF is an affordable and accessible approach and is potentially effective in a wide variety of tumors [12]. However, patients with severe weight loss, sarcopenia, cachexia or malnutrition are probably not good candidates for a STF intervention [27, 28]. Recent guidelines recommend to increase protein and fat consumption in patients with cachexia [29, 30]. Thus, STF may be particularly useful for relatively fit patients treated with (neo)adjuvant chemotherapy.
This narrative review will cover the current knowledge of the molecular mechanisms explaining “differential stress resistance” of healthy- and cancer cells in response to STF. Moreover, it summarizes the available clinical data reflecting the impact of STF on the effects of chemotherapy in cancer patients. Finally, ongoing clinical studies of the effects of STF in cancer treatment will be critically reviewed.
Differential stress resistance and sensitization in response to STF
In healthy cells, nutrient deprivation shuts down pathways promoting growth to re-invest energy in maintenance and repair pathways (Fig. 1) [25, 31, 32]. This results in increased cellular protection, contributing to enhanced resistance to distinct stressors including chemotherapy and radiotherapy [19, 33]. In contrast, tumor cells are unable to activate this protective response, due to: 1) uncontrolled activation of growth pathways and self-sufficiency in growth signals caused by oncogenic mutations or autocrine production of growth factors, and 2) loss of anti-proliferative signals due to mutations in tumor suppressor genes [34]. Thus, acquiring the ability to increase growth, tumor cells lose the ability to adapt to extreme environments, including nutrient deprivation. Additionally, the persistent increased growth rate of tumor cells requires abundant nutrients [35]. Therefore, STF increases DSS of tumor cells to several chemotherapeutic agents, radiotherapy and tyrosine kinase inhibitors (TKIs) (Table 1) [12–16, 18–20, 36–40]. Although the exact mechanism of DSR and DSS by STF is unknown, several growth factors and nutrient sensing pathways have been proposed to be key regulators, of which insulin-like growth factor-1 (IGF-1) is the most examined [41–43]. Nutrient sensing pathways are activated or inhibited in response to a low amount of available nutrients and are highly conserved among distinct organisms to overcome periods of famine [44]. During nutrient scarcity, these pathways guide cells to invest energy in repair and maintenance rather than reproduction and growth [45–47], presumably to enhance survival of periods of famine. Analogously, infection-induced anorexia is a common sign of sickness and may be an important strategy for host defence [48, 49].
Fig. 1.
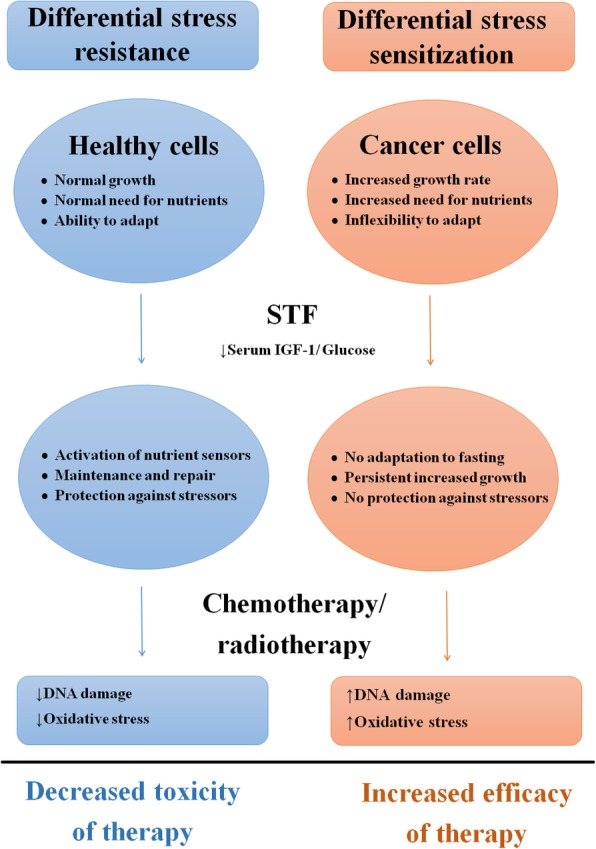
Schematic overview of differential effects of short-term fasting on healthy and cancer cells.
Abbreviations: STF; short term fasting, IGF-1: insulin growth factor-1.
Table 1.
Overview of in vivo studies of the effect of STF on the toxicity and/or efficacy of chemotherapy, radiotherapy and tyrosine kinase inhibitors
| Author | Strain | Treatment | Outcomes of STF |
|---|---|---|---|
| Raffaghello et al. 2008 [19] | A/J, CD-1, nude mice and A/J mice bearing subcutaneous NXS2 neuroblastoma | High dose etoposide ± 48–60 h STF | Decreased mortality (toxicity) after high dose etoposide |
| Lee et al. 2012 [12] | BALB/c, C57BL/6 and nude mice bearing subcutaneous: | ±48–60 h STF and: | |
| 4 T1 breast cancer | Cyclophosphamide |
Increased efficacy of CT, STF alone was as effective as CT alone, Increased survival |
|
| B16 melanoma | Doxorubicin | Increased efficacy of CT, Increased survival, Decreased metastasis | |
| GL26 glioma | Doxorubicin |
Increased efficacy of CT STF alone was as effective as CT alone |
|
| ACN human neuroblastoma | Doxorubicin | Increased efficacy of CT | |
| MDAMB-231 breast cancer | Doxorubicin | Increased efficacy of CT | |
| OVCAR3 ovarian cancer | Doxorubicin | Increased efficacy of CT | |
| NXS2 neuroblastoma | Only STF | STF alone was effective, Increased survival | |
| Safdie et al. 2012 [13] | C57BL/6 N mice bearing subcutaneous or intracranial GL26 glioma | Temozolomide ± 48 h STF |
Increased efficacy of CT, STF alone was as effective as CT alone (subcutaneous model only) |
|
Radiotherapy ± 48 h STF |
Increased efficacy of radiotherapy | ||
| Shi et al. 2012 [37] | CD-1 Nude mice bearing subcutaneous ZL55 mesothelioma and A549 lung carcinoma | Cisplatin ±48 h STF | Increased efficacy of CT, STF alone was more effective as CT alone (mesothelioma only) |
| Kawaguchi et al. 2012 [62] | GFP-LC3 mice | Doxorubicin ±48 h STF | Decreased cardiotoxicity after high dose doxorubicin. |
| Brandhorst et al. 2013 [18] | AIN93G mice | High dose doxorubicin ±60 h STF | Decreased mortality (toxicity) after high dose doxorubicin. |
| Saleh et al. 2013 [38] | BALB/c mice bearing subcutaneous 67NR or NIH3 triple negative breast cancer |
Radiotherapy ± 24 h STF (alternate) |
Increased efficacy of radiotherapy |
| Cheng et al. 2014 [22] | C57BL/6 J mice |
Cyclophosphamide ± 48 h STF |
Decreased mortality (toxicity) after high dose cyclophosphamide. |
| Bianchi et al. 2015 [14] | BALB/c mice bearing subcutaneous CT26 colon cancer | Oxaliplatin ± 48 h STF | Increased efficacy of CT |
| Shim et al. 2015 [15] | C57BL/6 J mice bearing subcutaneous B16 melanoma | Doxorubicin or Cyclophosphamide ± 48 h STF | Increased efficacy of CT STF alone was as effective as CT alone |
| D’Aronzo et al. 2015 [36] | Nu/Nu mice bearing subcutaneous BxPC-3-luc pancreatic cancer | Gemcitabine ± 24 h STF | Increased efficacy of CT |
| Huisman et al. 2015 [20] | FabplCre;Apc15lox/C mice bearing spontaneous intestinal malignancies | Irinotecan ± 48 h STF |
Decreased toxicity to CT No effect on efficacy of CT |
| Tinkum et al. 2015 [21] |
B6(Cg)-Tyrc-2 J/J, Bmi1CreERT/+;Rosa26R/+ HopXCreERT/+;Rosa26R/+ Lgr5EGFP-IRES-CreERT2/+;Rosa26R/+, Lgr5EGFP-IRES-CreERT2/+ mice |
High dose etoposide ± 24 h STF | Decreased mortality (toxicity) after high dose etoposide |
| Caffa et al. 2015 [39] | BALB/c mice bearing subcutaneous H3122 lung cancer or HCT116 colorectal cancer | Crizotinib/regorafinib ± 48 h STF | Increased efficacy of crizotinib/regorafinib |
| Huisman et al. 2015 [23] | BALB/c mice bearing subcutaneous C26 colon cancer | Irinotecan ± 72 h STF |
Decreased toxicity to CT No effect on efficacy of CT |
| Di Biase et al. 2016 [16] | BALB/c, BALB/c-nude and C57BL/6 mice bearing subcutaneous MCF7 and 4T1 breast cancer, B16 melanoma | Doxorubicin and cyclophosphamide ±48 h STF or 96 h FMD | Increased efficacy of CT |
| Pietrocola et al. 2016 [40] | Wild-type C57BL/6 and athymic (nu/nu) mice | Mitoxantrone or oxaliplatin ± 48 h STF | Increased efficacy of CT |
| Di Biase et al. 2017 [17] | C57BL/6 mice | Doxorubicin ±24–72 h STF | Decreased cardiotoxicity after high dose doxorubicin. |
| Jongbloed et al. 2019 [24] | BALB/c mice | Irinotecan ± 72 h STF | Decreased toxicity to CT |
| Authors, site | Subjects | Treatment | Outcome of STF |
| Withers et al. 2014, UC Davis, USA [63] | 20 dogs with lymphoma | Doxorubicin ±24 h STF |
Safe and feasible Reduction in vomiting No reduction in IGF-1 |
STF Short-term fasting, CT Chemotherapy, FMD Fasting mimicking diet
IGF-1 and insulin as key regulators of DSR
IGF-1 and insulin stimulate proliferation and growth and inhibit apoptosis in response to calorie and protein availability through signalling via the IGF-1 receptor (IGF-1R) and insulin receptor isoform A (IR-A), respectively [50–53]. Serum IGF-1 levels decrease during STF [54–56], because low insulin levels cause growth hormone (GH) resistance of the liver, which inhibits hepatic IGF-1 production [54, 57, 58]. Both insulin and IGF-1 activate the Ras/mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/Akt pathways. In healthy cells, inhibition of proliferation and/or investment in maintenance may contribute to increased stress resistance. For example, mice with a liver Igf1 gene deletion (LID), which have decreased IGF-1 levels similar to those during STF [59–61], exhibit increased resistance to high doses of various chemotherapeutic agents [42] and this benefit was nullified through IGF-1 administration [12, 42]. Thus, the IGF-1R pathway seems to be a key mediator of stress resistance in response to STF in healthy cells.
During STF, the Ras/MAPK and PI3K/Akt pathways are down-regulated in cancer cells, whereby proliferation is inhibited [64]. Notably, resistance to the growth limiting effects of STF has been observed in cancer cells carrying mutations that cause a constitutive activation of the PI3K pathway, since these cells proliferate even in the absence of insulin or IGF-1 [65]. Therefore, the IGF-1R pathway is a key mediator of cancer cell growth and cancer resistance to commonly used therapeutics [42, 66, 67]. Thus, the reduction in circulating levels of IGF-1 and insulin during STF may contribute to the anticancer activity as well [68].
AMPK and autophagy
AMP-activated protein kinase (AMPK) may play a major part in DSR due to STF [69]. AMPK monitors cellular energy levels and becomes activated when ADP:ATP or AMP:ATP ratios in the cell increase [70]. AMPK inhibits energy consuming processes, such as cell proliferation and protein synthesis, and activates energy generating processes, such as glycolysis and fatty acid oxidation. It inhibits cell growth and stimulates autophagy [71]. Autophagy (Greek for “self-eating”) is a highly conserved catabolic process among eukaryotes to survive periods of nutrient deprivation. This adaptive response of the cell involves damaged protein and organelle degradation to generate amino acids as an alternative energy source [72, 73]. Activation of AMPK and autophagy seems to play a major part in de protective effects of STF in healthy cells [17].
However, the effects of AMPK activation in distinct tumor cells may vary, as some tumors harbour constitutively active AMPK [74, 75] and others exhibit low AMPK activity [76–78]. Tumors with diminished AMPK activity or autophagy may be highly sensitive to STF, as AMPK activation enhances immune surveillance [40], whereas tumors with highly active AMPK or autophagy may be resistant [77, 79–82].
Glucose metabolism and the “Warburg effect”
During STF, healthy cells, have the metabolic flexibility to cope with nutrient deprivation, since glucose can be replaced by ketone bodies and fatty acids as primary energy source.
In contrast, tumor cells depend on glucose to maintain the high rate of cellular proliferation [83, 84]. Akt stimulates the so called “Warburg effect”, characterized by an increased rate of glycolysis rather than oxidative phosphorylation even in the presence of oxygen [83–85]. STF down-regulates anaerobic glycolysis while up-regulating oxidative phosphorylation in tumor cells, and this “anti-Warburg effect” results in oxidative stress and apoptosis [14]. Also, a counterintuitive increase in protein translation during STF increases unmet energy needs, leading to cell death [12]. Moreover, the 20–40% reduction in circulating glucose during STF may be enough to kill anoxic tumor cells [85]. Thus, a decrease in nutrient availability during STF makes cancer cells more vulnerable to any challenge, including chemotherapy. However, overconsumption after a STF period might accelerate tumor growth, due to high glucose conditions and increased glycolysis [86].
Reactive oxygen species and DNA damage
Chemotherapeutic agents inflict oxidative stress and DNA damage upon healthy cells, which are underlying mechanisms of toxicity [44, 87]. STF dampens oxidative stress in healthy cells by down-regulating metabolic rate and increasing scavenging of reactive oxygen species (ROS), which may contribute to DSR [33, 44]. As serum glucose levels decrease during STF, fatty acids serve as the main energy source. Beta-oxidation of fatty acids produces ketone bodies, which can be used as an alternative/additional fuel. Ketone bodies can also activate pathways involved in protection against ROS [88]. Moreover, STF presumably activates DNA repair processes in healthy cells [22]. For example, in mice fasted for 24 h before high dose infusion of etoposide, less DNA damage was seen in small intestinal stem cells 3 h after the infusion compared to mice who ate ad libitum. As 1.5 h post-treatment DNA damage was similar, DNA repair was likely more efficient in healthy cells due to STF [21].
In contrast, tumor cells exhibit increased ROS production if chemotherapy is combined with STF in vitro [12]. In breast cancer cells cultured in low glucose medium or serum of fasting mice, a 20-fold increase in DNA damage was seen in response to chemotherapy, as compared to cells cultured in regular medium or in serum of ad libitum fed mice [12].
Immune competence
Chemotherapy causes bone marrow toxicity and depletion of circulating immune cells, especially myeloid cell depletion [89, 90]. Fasting protects hematopoietic stem cells and circulating immune cells from the detrimental effects of chemotherapy in mice [22, 91]. Additionally, more efficient immunity as a result of STF presumably causes a lower rate of infections and febrile neutropenia as well [92].
On the other hand, fasting improves the therapeutic effect of chemotherapy on the tumor possibly through cellular immunity in mice, as this effect is nullified in nu/nu mice, which lack T cells [40, 93]. Thus, STF may promote immunity and presentation of tumor-associated antigens (TAA), which promote efficient antitumor immunity contributing to increased efficacy of chemotherapy [94].
From animal models to the clinic
Preclinical data documenting the benefits of STF is abundant and promising. However, words of caution are appropriate regarding its application in patients with cancer. For instance, preclinical studies show severe, albeit transient, weight loss in animal models (20–40% of total bodyweight after 24–48 h of fasting [12, 19, 23, 39, 95]. In contrast, the impact of a few days of fasting on bodyweight of humans appears far more modest (~ 1 kg per day, largely water loss) [26], which is probably explained by metabolic differences between humans and mice [95]. This is reassuring in the context of safety. However, it may also mean that humans need to fast for a (much) longer period of time than mice to obtain the same benefits (see discussion below). Therefore, carefully controlled clinical trials monitoring tumor growth as well as adverse effects of distinct dietary regimes are required before fasting mimicking diets (FMDs) can be applied in clinical practice.
Metabolic risk factors for cancer
Obesity is associated with an increased risk of developing several cancers, such as breast cancer, colon cancer, ovarian cancer, endometrial cancer and thyroid cancer [96, 97] and IGF-1 levels are positively associated with the risk of developing breast and prostate cancer [98, 99]. Moreover, obesity and high levels of insulin and IGF-1, as well as having diabetes mellitus are associated with worse survival in cancer [100–103]. Obese subjects are often hyperglycemic and hyperinsulinemic, as a result of insulin resistance. Although circulating levels of total (free + bound) IGF-1 are normal or even low in obese subjects, levels of free (bioactive) IGF-1 are higher than in lean subjects [104]. Both insulin and free IGF-1 can bind the IGF-1R and IR-A [105] and activate the Ras/MAPK and PI3K/AKT pathway, through which cell proliferation is stimulated and apoptosis is inhibited, respectively [106]. Moreover, preclinically, obesity is associated with macrophage accumulation in adipose tissue resulting in an immune suppressive microenvironment [107]. These metabolic mechanisms may explain the increased risk of cancer as well as the worse prognosis of several cancers in obese subjects.
Clinical studies of fasting
Voluntary fasting has been performed for many centuries and purposes, such as religious, ethical and cosmetic [26, 108]. Hippocrates was probably one of the first advocates of fasting for medical purposes (he recommended to fast during sickness). Since then, several doctors advised their patients to listen to their ‘fasting instinct’ (the natural loss of appetite during disease). Scientific research on the biomedical effects of fasting was performed from the late nineteenth century on, when several non-obese humans fasted for 20–40 days [26]. The first clinical study of medical fasting for the treatment of obesity was performed in 1915 [109]. The authors reported that short periods of four to six days of fasting is a safe and effective method for reducing bodyweight in obese humans. Since that time several studies were performed in obese subjects, with the longest fasting period lasting 382 days (!) [110, 111]. Fasting therapy was observed to be generally safe and well tolerated. Only mild side effects were reported, including headaches, dizziness, nausea, dyspepsia and fatigue [109–114]. However, in rare cases fasting for periods longer than 2 weeks was fatal in obese subjects with comorbidities as cardiac disease or diabetes mellitus [26, 115–117], and in one rare case a 53-day fast caused Wernicke encephalopathy in a patient with a lymphoma [118]. Additionally, fasting is not suitable for patients with rare metabolic illnesses such as glycogen storage disease or disorders of gluconeogenesis [119]. Benefits of fasting are improved cardiovascular risk factors, such as a decrease in blood pressure, improvement of lipid profile and insulin sensitivity, and weight loss in obese and non-obese subjects [114, 120]. The weight loss during STF is approximately 0.9 kg per day and decreases during prolonged fasting to 0.3 kg per day by the third week [26, 121]. Various studies examined the potential of fasting in the treatment of mood disorders, rheumatic diseases, asthma, chronic pain syndromes, hypertension, and metabolic syndrome [122, 123]. For example, a large cohort study of more than 2000 subjects with chronic illness and pain syndromes, who used a very low-calorie diet of 350 kcal per day for 7 days, showed an increase in quality of life without any serious side effect [122]. In healthy subjects, STF by 3 cycles of a fasting mimicking diet (FMD) reduces common risk factors for cardiovascular diseases, diabetes and ageing, such as lowering blood pressure, body weight, glucose, triglycerides and cholesterol [124]. Additionally, STF may improve clinical outcome in patients undergoing a partial liver resection and may prevent acute kidney injury after cardiac surgery [125, 126].
Metabolic changes during STF in humans
STF has profound metabolic effects in humans [127]. Serum glucose levels drop after a few hours and are maintained at a lower level by endogenous glucose production, stimulated by glucagon. Glycogen storage capacity is limited so that stores are virtually depleted after 24 h. From then on, gluconeogenesis provides the brain with glucose as its major fuel source. Fatty acids are the primary fuel for the rest of the body. Beta-oxidation of fatty acids produces ketone bodies, which can serve as auxiliary energy source for the brain and the rest of the body. Insulin levels decrease rapidly and IGF-1 decreases dramatically after 36–72 h [41]. Since the liver is resistant to GH during prolonged fasting, IGF-1 production is profoundly reduced [128]. Diminished negative feedback control through reduction of circulating insulin and IGF-1 causes plasma GH levels to increase [129, 130]. IGF binding proteins, which regulate the bio-availability of IGF-1, change during fasting as well [41, 131, 132]. IGF-BP3 levels decrease, while IGF-BP1 levels increase 5–10-fold [133]. The decrease of IGF-I, downregulates the Ras/MAPK and PI3K/Akt pathways, through which cell proliferation is stimulated and apoptosis inhibited [12, 19]. Moreover, fasting down-regulates the hypothalamus-pituitary-thyroid axis activity. It particularly lowers triiodothyronine (T3), while thyroid stimulating hormone (TSH) and free thyroxine (fT4) are slightly decreased or not affected [134]. Clinical research shows that fasting periods longer than 48 h are required to facilitate a robust decrease in IGF-1 levels [41]. Therefore, it is likely that the positive effects of STF will be enhanced if the period of fasting is prolonged. A low sugar, low protein FMD may be an alternative to ease the burden of fasting, as it mimics the effects of STF on metabolism [91].
Clinical studies of STF during chemotherapy
To date, a few small clinical studies in humans exploring the effects of STF combined with chemotherapy have been published (Table 2) [22, 131, 135–138]. The design and results of these studies in humans are summarized below.
Table 2.
Overview of clinical studies on the effect of STF on the toxicity of chemotherapy
| Authors, site | Human Subjects | Treatment | Outcome |
|---|---|---|---|
| Safdie et al. 2009, USC, USA [136] | 10 human subjects with distinct malignancies | Distinct, + STF varying from 48 to 140 h prior and 5–56 h after CT |
Safe and feasible. Reduction in CT-induced side effects. |
| Badar et al. 2014, KFMC, Saudi Arabia, NCT00757094 [135] | 11 human subjects with distinct malignancies | IF during Ramadan when receiving CT |
Safe and feasible. Reduction in CT-induced side effectsa. |
| Dorff et al. 2016, USC, USA, NCT00936364, [22, 137] | 20 human subjects with distinct malignancies | Platinum based CT + 24 h, 48 h or 72 h STF |
Safe and feasible . Reduces DNA damage in leukocytes (dose response). Reduction of IGF-1 (dose response). |
| de Groot et al. 2015, LUMC, The Netherlands NCT01304251 [131] | 13 women with stage II and III HER2 negative breast cancer | TAC CT ± 48 h STF |
Safe and feasible. Reduction in IGF-1 Beneficial effect on erythrocytes and thrombocytes Possible reduction in DNA damage in healthy cells |
| Bauersfeld et al. 2018, Charite University, Germany, NCT01954836 [138] | 34 women with breast and ovarian cancer | CT ± 60 h STF (cross-over) |
Safe and feasible Beneficial effect on QOL, fatigue and well-being |
USC University of Southern California, KFMC King Fahad Medical City, LUMC Leiden University Medical Center, UC Davis University of California, Davis School of Veterinary Medicine, STF Short-term fasting, IF intermittent fasting, CT Chemotherapy, TAC docetaxel/doxorubicin/cyclophosphamide, IGF-1 insulin-like growth factor-1, QOL Quality of life ano statistics performed
In a case series from the University of Southern California (USC), 10 patients with distinct malignancies fasted in combination with docetaxel, carboplatin, paclitaxel and/or gemcitabine [136, 139]. Seven female and three male patients, with a median age of 61 years, diagnosed with breast (N = 4), prostate (N = 2), esophagus, non-small cell lung cancer, uterus and ovary cancer were described. Patients fasted for 48–140 h prior to, and 5–56 after commencing chemotherapy. Six of the ten patients fasted alternately during the chemotherapy cycles (the other four fasted every cycle) and side effects were compared between cycles combined with STF and chemotherapy alone. Side effects were scored according to the Common Terminology Criteria for Adverse Events (CTCAE) 4.0. Besides hunger and dizziness, fasting had no significant side effects. The authors reported a decrease in chemotherapy-induced side effects, including fatigue, weakness, vomiting and diarrhea, when chemotherapy was combined with STF compared to chemotherapy alone. In five patients the tumor volume (evaluated with PET or PET-CT) or tumor markers (PSA or CA-125) were evaluated. STF did not diminish chemotherapy-induced reduction of tumor volume and tumor markers, suggesting that STF did not interfere with the efficacy of chemotherapy.
In the King Fahad Medical City a clinical trial (NCT00757094) was conducted to evaluate the safety and feasibility of combining chemotherapy and intermittent fasting (including liquids) during the Ramadan [135]. Eleven patients, with distinct types of malignancies, received one gift of chemotherapy. Side effects and blood counts were compared with values measured in response to a similar dose of chemotherapy, given 2 weeks after the end of Ramadan. The authors concluded that combining fasting and chemotherapy during the month of Ramadan was well tolerated and safe. Side effects of chemotherapy tended to be less. However, because the study group was small, no statistics were performed. Moreover, due to the short fasting period (approximately 12 h), major benefits may not be expected, as IGF-1 levels will evidently not be reduced [140].
We performed a randomized pilot study (NCT01304251) to evaluate the effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients in the Leiden University Medical Center (LUMC) [131]. Eligible patients had stage II/III breast cancer and received (neo)-adjuvant TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy. Patients were randomized to fast 24 h before and 24 h after chemotherapy, or to eat according to the guidelines for healthy nutrition. Metabolic parameters (glucose, insulin and IGF-1) at baseline and immediately before chemotherapy infusion –when patients in the STF group had fasted for 24 h- were compared. Toxicity in the two groups was compared as well. Additionally, chemotherapy-induced DNA damage was quantified in peripheral blood mononuclear cells (PBMCs) by the level of γ-H2AX, as determined by flowcytometry. Thirteen patients were included, of whom seven were randomized to the STF arm. STF was well tolerated in our study. Plasma glucose levels increased and insulin levels remained constant in response to STF. We inferred that this phenomenon was the result of the concomitant use of dexamethasone, which was administered as an anti-emetic, for reduction of fluid retention and dampening of hypersensitivity reactions in response to docetaxel. Circulating IGF-1 levels were only modestly reduced in the study, which could be due to the use of dexamethasone as well [141, 142] or to the relatively short duration (24 h) of fasting prior to chemotherapy. Non-hematological toxicity did not differ between the groups. However, mean erythrocyte- and thrombocyte counts 7 days post-chemotherapy were significantly higher in the STF group compared to the non-STF group. Levels of γ-H2AX were significantly increased 30 min post-chemotherapy in CD45 + CD3- cells in non-STF, but not in STF patients [131]. This study provides evidence that STF attenuates bone marrow toxicity in these patients and reduces chemotherapy-induced DNA damage in PBMCs and/or accelerates its recovery.
Moreover, Dorff et al. reported results from a dose-escalating phase I study (NCT00936364), wherein 20 human subjects with distinct malignancies were treated with platinum-based chemotherapy combined with 24, 48 or 72 h STF to identify the optimal fasting duration [22, 137]. Eligible patients had distinct cancer types for which platinum-based combination chemotherapy was given with curative or palliative intent. Metabolic parameters (glucose, insulin, IGF-1 and IGF-BP1) at baseline and immediately before chemotherapy were compared. Moreover, toxicities and chemotherapy-induced DNA damage in PBMCs (determined by the COMET assay) between the three groups were compared. Twenty patients were included, 6 in the 24 h group and 7 in the 48 and 72 h group. The fasting was feasible and fasting-related toxicities were limited to grade 1 according CTCAE 4.0. The authors reported that 72 h of STF was associated with normal lymphocyte counts and maintenance of a normal lineage balance in white blood counts (lymphoid/myeloid ratio) after 2 cycles of chemotherapy, while 24 h STF was not [22]. IGF-1 levels decreased by 30, 33 and 8% in the 24, 48 and 72 h fasting cohorts, respectively, after the first fasting period. Additionally, the COMET assay showed reduced DNA damage 24 h after chemotherapy in leukocytes from subjects who fasted for more than 48 h compared with subjects fasted for 24 h (P = 0.08).
Finally, Bauerfeld et al. published a randomized cross-over trial (NCT01954836) evaluating the effect of STF on quality of life in breast cancer and ovarian cancer patients treated with chemotherapy [138]. Patients were randomized to fast, using an FMD, 36 h before and 24 h after chemotherapy or to eat a normocaloric Mediterranean diet for the first three cycles of chemotherapy. After three cycles the patient crossed over to the other group of nutrition (Mediterranean diet or fasting). The design of the study allows intra-individual comparisons regarding side effects of treatment, but precludes conclusions as efficacy of chemotherapy. In total, 50 patients were included in the study, but only 34 were analyzed because of early study discontinuation. The fasting was safe and feasible and five patients (14.7%) continued fasting after three cycles and did not cross over to the normocaloric diet. The authors concluded that STF led to a better tolerance to chemotherapy with less compromised quality of life (QOL) and reduced fatigue within the 8 days after chemotherapy. Moreover, 31 patients declared that they would fast again during chemotherapy, while only 3 patients declared that they would not fast again during chemotherapy.
These first clinical studies lack enough power to draw definite conclusions. However, the first results suggest that STF is safe, while it reduces toxicity of chemotherapy. Large scale randomized studies are required to get more insight in the benefits of STF in cancer treatment in humans.
Ongoing studies
The first clinical studies have shown that STF combined with chemotherapy is safe and feasible in small patient groups [131, 136, 138]. Moreover, STF may reduce chemotherapy-induced toxicity. Additionally, chemotherapy-induced DNA damage in healthy cells may be decreased due to STF. However, large randomized clinical studies are required to generate (more) insight and validate the possible benefits of STF during chemotherapy. In Table 3, an overview is shown of the ongoing trials with STF combined with cancer treatment.
Table 3.
Overview of ongoing or unpublished clinical trials of STF combined with chemotherapy or radiotherapy
| Trial, site | N | Start | Tumor type and treatment, | STF | Primary endpoint |
|---|---|---|---|---|---|
| Non-randomized trial, NCT01175837, Mayo clinic, USA | 12 | 2010 | Distinct malignancies treated with CT | + 24–48 h prior to chemotherapy (distinct regimens) | Safety and feasibility |
| Phase II randomized trial, NCT01802346, USC, USA | 120 | 2013 | Breast cancer treated with AC and prostate cancer treated with docetaxel | ±96 h (using FMD) during CT | Toxicity of CT |
| Phase II/III Randomized study, NCT02126449, LUMC, the Netherlands | 250 | 2014 | Stage II and III Her2 negative breast cancer treated with AC-T or FEC-T. | ±96 h (using FMD), during AC-T or FEC-T, no corticosteroids in control arm during AC or FEC |
Phase II: toxicity of CT Phase III: pCR |
| Phase Ib non- randomized trial, NCT02379585, Western Regional Medical Center, USA | 10 | 2015 | Breast cancer | CT ± 48 h STF | pCR |
| Randomized trial, NCT02710721, Charite University, Berlin, Germany. | 60 | 2016 | Advanced metastatic prostate cancer | ±60 h (using FMD) during CT vs. Mediterranean diet | QOL |
| Randomized crossover study, NTR5731, Erasmus medical center | 18 | 2016 | Metastatic colorectal cancer or other solid tumors receiving irinotecan | Dietary restriction including STF | 25% reduction of the active irinotecan metabolite, SN38, in healthy liver tissue |
| Randomized trial, NCT03162289 Charite University, Berlin, Germany. | 150 | 2017 | Ovarian or breast cancer | ±60 h (using FMD) during CT | QOL |
| Non-randomized trial, NCT03340935, University of Milan, Italy | 85 | 2017 | Distinct | 5 days (using FMD), 700 kcal a day during cancer treatment | Toxicity of CT |
| Non-randomized trial, NCT03595540, Genova, Italy | 60 | 2017 | Distinct | 5 days (using FMD), 700 kcal a day during cancer treatment | Feasibility |
| Randomized trial, NCT03709147, Milan, Italy | 88 | 2018 | Lung adenocarcinoma | ± 5 days (using FMD) during CT in combination with metformin | PFS |
| Randomized trial. NCT03700437, Indiana University, USA | 40 | 2018 | Non-small cell lung cancer | ± 96 h (using FMD) during carboplatin, pemetrexed and pembrolizumab | DNA damage in and count of circulating tumor cells |
| Studies of STF during radiotherapy | |||||
|
Randomized trial, NCT01754350, Johann Wolfgang Goethe University Hospitals, Germany |
50 | 2013 | Glioblastoma Multiforme | ±72 h during reirradiation | PFS |
USC University of Southern California, CT Chemotherapy, LUMC Leiden University Medical Center, AC-T doxorubicin and cyclophosphamide followed by docetaxel, FEC-T 5-fluorouracil, epirubicin and cyclophosphamide followed by docetaxel, FMD Fasting mimicking diet, pCR pathological complete response, QOL Quality of life, PFS Progression-free survival
One study to date investigates the effects of STF on the effects radiotherapy. This randomized study (NCT01754350) conducted in Johann Wolfgang Goethe University Hospitals, includes patients with recurrent glioblastoma or gliosarcoma. The intervention entails 3 days of STF and 6 days of ketogenic diet during re-irradiation. The primary endpoint of the study is progression free survival.
A phase II study (NCT01802346), ongoing in the University of Southern California, examines the effects of an FMD on toxicity of chemotherapy in patients with breast and prostate cancer.
The phase II/III study (NCT02126449) from the LUMC, investigates the effects of STF using an FMD on toxicity (phase II part) and efficacy (phase III part) of neo-adjuvant AC-T or FEC-T chemotherapy. In this study prophylactic dexamethasone is omitted in the FMD arm during the AC and FEC chemotherapy cycles to reduce its potentially counteractive metabolic effects. Final results of the study are awaited [68]. The same FMD will be used to investigate the effect on circulating tumor cells in non-small cell lung cancer during treatment with carboplatin, pemetrexed and pembrolizumab.
Another FMD, described by Bauerfeld [138], is tested in two studies (NCT02710721, NCT03162289) conducted in the Charité University in Berlin, one in advanced metastatic prostate cancer and another in ovarian or breast cancer. Primary endpoint of both studies is QOL.
Finally, three studies (NCT03340935, NCT03595540 and NCT03709147) investigate the feasibility and effect of a 5-day FMD (approximately 700 kcal a day) on chemotherapy in distinct tumors and distinct chemotherapy regimens.
Discussion and clinical implications
Clinical research evaluating the potential of STF is still in its infancy and more research is needed as the exact mechanism and effects are not established yet. Remaining questions are: is STF clinically effective in patients with solid tumors, in which tumors is STF effective, which markers are useful for prediction and monitoring of efficacy, what is the optimal length and timing of STF and refeeding, is STF safe in all patients, what is the optimal composition of an FMD, how can we increase patient’s compliance?
STF may be an affordable and safe intervention - at least in patients without severe weight loss or malnutrition -, which potentially dampens the side effects of chemotherapy, radiotherapy and TKI’s, while reinforcing their efficacy. Furthermore, it is potentially effective in a wide variety of tumors, although there is evidence that tumors with PI3K mutations or highly active AMPK are not sensitive [65, 82]. Reduction of side effects would improve quality of life and potentially reduce costs of hospitalization and the use of drugs such as anti-emetics or antibiotics. Moreover, STF may broaden the therapeutic window of cancer treatments, allowing for an increase of the dosage of (chemo) therapeutic agents, thereby enhancing their efficacy. However, STF might be only feasible in chemotherapeutic regimens characterized by: 1) bolus infusions on one day, to keep the fasting period short, 2) a long interval between two cycles, to ensure sufficient recovery time between cycles and 3) low dose or no use of corticosteroids, to avoid hyperglycemia, which might interfere with the benefits of STF [131].
Patients at risk for malnutrition or cachexia may not be candidates for STF, as it may be unsafe to further limit nutrient intake in these patients for even a short time [27]. However, notably, in preclinical setting caloric restriction showed even preservation of muscle strength in cancer cachexia [143]. Therefore, robust clinical trials are needed to establish the safety and efficacy of FMD in patients at high risk of cachexia.
Close monitoring of patients by nutritionists with expertise in fasting may be needed to increase compliance in future studies and to prevent patients unacceptable weight loss. Moreover, in our opinion, STF or FMDs should only be applied in the context of clinical research in patients with cancer until there is robust evidence for their safety and benefits.
Conclusion
Abundant and convincing preclinical evidence shows that STF can decrease toxicity and simultaneously increase efficacy of a wide variety of chemotherapeutic agents. Preclinical data suggesting that STF can enhance the effects of radiotherapy and TKIs are promising as well. In clinical studies, STF emerges as a promising strategy to enhance the efficacy and tolerability of chemotherapy. It appears safe as an adjunct to chemotherapy in humans, and it may reduce side effects and DNA damage in healthy cells in response to chemotherapy. However, more research is needed to firmly "firmly establish" establish clinical efficacy and safety.
Acknowledgements
None.
Funding
This work was supported by a grant from Pink Ribbon (2012.WO31.C155).
Availability of data and materials
Not applicable.
Abbreviations
- AMPK
AMP-activated protein kinase
- CTCAE
Common Terminology Criteria for Adverse Events
- DSR
differential stress resistance
- DSS
differential stress sensitization
- FMD
fasting mimicking diet
- fT4
free thyroxine
- GH
growth hormone
- IGF-1
insulin-like growth factor-1
- IGF-1R
IGF-1 receptor
- IR-A
Insulin receptor isoform A
- LID
liver Igf1 gene deletion
- LUMC
Leiden University Medical Center
- MAPK
mitogen-activated protein kinase
- PBMCs
peripheral blood mononuclear cells
- PI3K
phosphatidylinositol-3-kinase
- ROS
reactive oxygen species
- STF
short-term fasting
- T3
lowers triiodothyronine
- TKIs
tyrosine kinase inhibitors
- TSH
thyroid stimulating hormone
- USC
University of Southern California
Authors’ contributions
All the authors contributed for the preparation of this manuscript. S.G. wrote the review article, were responsible for the figure and legend, final editing, and preparation of the manuscript for submission. HP, JH and JK critical revised the manuscript. All authors read and approved the final article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stefanie de Groot, Email: S.de_Groot2@lumc.nl.
Hanno Pijl, Email: H.Pijl@lumc.nl.
Jacobus J. M. van der Hoeven, Email: koos.vanderhoeven@radboudumc.nl
Judith R. Kroep, Phone: +31715263464, Email: J.R.Kroep@lumc.nl
References
- 1.Dirx MJ, Zeegers MP, Dagnelie PC, van den Bogaard T, van den Brandt PA. Energy restriction and the risk of spontaneous mammary tumors in mice: a meta-analysis. Int J Cancer. 2003;106(5):766–770. doi: 10.1002/ijc.11277. [DOI] [PubMed] [Google Scholar]
- 2.TANNENBAUM A, SILVERSTONE H. Effect of limited food intake on survival of mice bearing spontaneous mammary carcinoma and on the incidence of lung metastases. Cancer Res. 1953;13(7:1):532–6. [PubMed]
- 3.De Lorenzo MS, Baljinnyam E, Vatner DE, Abarzua P, Vatner SF, Rabson AB. Caloric restriction reduces growth of mammary tumors and metastases. Carcinogenesis. 2011;32(9):1381–1387. doi: 10.1093/carcin/bgr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mai V, Colbert LH, Berrigan D, Perkins SN, Pfeiffer R, Lavigne JA, et al. Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in Apc(min) mice through different mechanisms. Cancer Res. 2003;63(8):1752–1755. [PubMed] [Google Scholar]
- 5.Lv M, Zhu X, Wang H, Wang F, Guan W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PLoS One. 2014;9(12):e115147. doi: 10.1371/journal.pone.0115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 8.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70(9):1097–1104. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57(6):B211–B224. doi: 10.1093/gerona/57.6.B211. [DOI] [PubMed] [Google Scholar]
- 10.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61(9):943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126(9):913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Lee C., Raffaghello L., Brandhorst S., Safdie F. M., Bianchi G., Martin-Montalvo A., Pistoia V., Wei M., Hwang S., Merlino A., Emionite L., de Cabo R., Longo V. D. Fasting Cycles Retard Growth of Tumors and Sensitize a Range of Cancer Cell Types to Chemotherapy. Science Translational Medicine. 2012;4(124):124ra27–124ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, et al. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One. 2012;7(9):e44603. doi: 10.1371/journal.pone.0044603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi G, Martella R, Ravera S, Marini C, Capitanio S, Orengo A, et al. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget. 2015;6(14):11806–11819. doi: 10.18632/oncotarget.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shim HS, Wei M, Brandhorst S, Longo VD. Starvation promotes REV1 SUMOylation and p53-dependent sensitization of melanoma and breast cancer cells. Cancer Res. 2015;75(6):1056–1067. doi: 10.1158/0008-5472.CAN-14-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Biase S, Longo VD. Fasting-induced differential stress sensitization in cancer treatment. Mol Cell Oncol. 2016;3(3):e1117701. doi: 10.1080/23723556.2015.1117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Biase S, Shim HS, Kim KH, Vinciguerra M, Rappa F, Wei M, et al. Fasting regulates EGR1 and protects from glucose- and dexamethasone-dependent sensitization to chemotherapy. PLoS Biol. 2017;15(3):e2001951. doi: 10.1371/journal.pbio.2001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandhorst S, Wei M, Hwang S, Morgan TE, Longo VD. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp Gerontol. 2013;48(10):1120–1128. doi: 10.1016/j.exger.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105(24):8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huisman SA, Bijman-Lagcher W, IJzermans JN, Smits R, de Bruin RW. Fasting protects against the side effects of irinotecan but preserves its anti-tumor effect in Apc15lox mutant mice. Cell Cycle. 2015;14(14):2333–2339. doi: 10.1080/15384101.2015.1044170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tinkum KL, Stemler KM, White LS, Loza AJ, Jeter-Jones S, Michalski BM, et al. Fasting protects mice from lethal DNA damage by promoting small intestinal epithelial stem cell survival. Proc Natl Acad Sci U S A. 2015;112(51):E7148–E7E54. doi: 10.1073/pnas.1509249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huisman SA, de BP, Ghobadi Moghaddam-Helmantel IM, IJzermans JN, Wiemer EA, Mathijssen RH, et al. Fasting protects against the side-effects of irinotecan treatment but does not abrogate anti-tumor activity in mice. Br. J Pharmacol. 2015. [DOI] [PMC free article] [PubMed]
- 24.Franny Jongbloed SAH, Harry van Steeg, Jeroen L.A. Pennings, Jan N.M. IJzermans, Martijn E.T. Dollé and Ron W.F. de Bruin. The transcriptomic response to irinotecan in colon carcinoma bearing mice preconditioned by fasting. Oncotarget. 2019. [DOI] [PMC free article] [PubMed]
- 25.Schumacher B, Garinis GA, Hoeijmakers JH. Age to survive: DNA damage and aging. Trends Genet. 2008;24(2):77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Kerndt PR, Naughton JL, Driscoll CE, Loxterkamp DA. Fasting: the history, pathophysiology and complications. West J Med. 1982;137(5):379–399. [PMC free article] [PubMed] [Google Scholar]
- 27.Caccialanza R, De Lorenzo F, Gianotti L, Zagonel V, Gavazzi C, Farina G, et al. Nutritional support for cancer patients: still a neglected right? Support Care Cancer. 2017;25(10):3001–3004. doi: 10.1007/s00520-017-3826-1. [DOI] [PubMed] [Google Scholar]
- 28.Caccialanza R, Aprile G, Cereda E, Pedrazzoli P. Fasting in oncology: a word of caution. Nat Rev Cancer. 2019. [DOI] [PubMed]
- 29.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–1196. doi: 10.1016/j.clnu.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8(11):835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 32.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Aronzo M, Vinciguerra M, Mazza T, Panebianco C, Saracino C, Pereira SP, et al. Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget. 2015. [DOI] [PMC free article] [PubMed]
- 37.Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA. Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. BMC Cancer. 2012;12:571. doi: 10.1186/1471-2407-12-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saleh AD, Simone BA, Palazzo J, Savage JE, Sano Y, Dan T, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle. 2013;12(12):1955–1963. doi: 10.4161/cc.25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caffa Irene, Longo Valter D., Nencioni Alessio. Fasting plus tyrosine kinase inhibitors in cancer. Aging. 2015;7(12):1026–1027. doi: 10.18632/aging.100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, et al. Caloric restriction mimetics enhance anticancer Immunosurveillance. Cancer Cell. 2016;30(1):147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15(1):80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 42.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70(4):1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hursting SD, Lavigne JA, Berrigan D, Donehower LA, Davis BJ, Phang JM, et al. Diet-gene interactions in p53-deficient mice: insulin-like growth factor-1 as a mechanistic target. J Nutr. 2004;134(9):2482S–2486S. doi: 10.1093/jn/134.9.2482S. [DOI] [PubMed] [Google Scholar]
- 44.Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30(30):3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- 45.Kirkwood TB, Shanley DP. Food restriction, evolution and ageing. Mech Ageing Dev. 2005;126(9):1011–1016. doi: 10.1016/j.mad.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Kirkwood TL, Kapahi P, Shanley DP. Evolution, stress, and longevity. J Anat. 2000;197(Pt 4):587–590. doi: 10.1046/j.1469-7580.2000.19740587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78(3):361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 48.Murray MJ, Murray AB. Anorexia of infection as a mechanism of host defense. Am J Clin Nutr. 1979;32(3):593–596. doi: 10.1093/ajcn/32.3.593. [DOI] [PubMed] [Google Scholar]
- 49.Exton MS. Infection-induced anorexia: active host defence strategy. Appetite. 1997;29(3):369–383. doi: 10.1006/appe.1997.0116. [DOI] [PubMed] [Google Scholar]
- 50.Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomark Prev. 2003;12(2):84–89. [PubMed] [Google Scholar]
- 51.Prisco M, Romano G, Peruzzi F, Valentinis B, Baserga R. Insulin and IGF-I receptors signaling in protection from apoptosis. Horm Metab Res. 1999;31(2–3):80–89. doi: 10.1055/s-2007-978703. [DOI] [PubMed] [Google Scholar]
- 52.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7(5):681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belfiore A, Malaguarnera R, Vella V, Lawrence MC, Sciacca L, Frasca F, et al. Insulin receptor isoforms in physiology and disease: an updated view. Endocr Rev. 2017;38(5):379–431. doi: 10.1210/er.2017-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ketelslegers JM, Maiter D, Maes M, Underwood LE, Thissen JP. Nutritional regulation of insulin-like growth factor-I. Metabolism. 1995;44(10 Suppl 4):50–57. doi: 10.1016/0026-0495(95)90221-X. [DOI] [PubMed] [Google Scholar]
- 55.Henning PC, Scofield DE, Rarick KR, Pierce JR, Staab JS, Lieberman HR, et al. Effects of acute caloric restriction compared to caloric balance on the temporal response of the IGF-I system. Metabolism. 2013;62(2):179–187. doi: 10.1016/j.metabol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Snel M, Wijngaarden MA, Bizino MB, van der Grond J, Teeuwisse WM, van Buchem MA, et al. Food cues do not modulate the neuroendocrine response to a prolonged fast in healthy men. Neuroendocrinology. 2012;96(4):285–293. doi: 10.1159/000336500. [DOI] [PubMed] [Google Scholar]
- 57.Beauloye V, Willems B. de C, V, frank SJ, Edery M, Thissen JP. Impairment of liver GH receptor signaling by fasting. Endocrinology. 2002;143(3):792–800. doi: 10.1210/endo.143.3.8692. [DOI] [PubMed] [Google Scholar]
- 58.Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85(12):4712–4720. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- 59.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96(13):7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anzo M, Cobb LJ, Hwang DL, Mehta H, Said JW, Yakar S, et al. Targeted deletion of hepatic Igf1 in TRAMP mice leads to dramatic alterations in the circulating insulin-like growth factor axis but does not reduce tumor progression. Cancer Res. 2008;68(9):3342–3349. doi: 10.1158/0008-5472.CAN-07-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62(4):1030–1035. [PubMed] [Google Scholar]
- 62.Kawaguchi T, Takemura G, Kanamori H, Takeyama T, Watanabe T, Morishita K, et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc Res. 2012;96(3):456–465. doi: 10.1093/cvr/cvs282. [DOI] [PubMed] [Google Scholar]
- 63.Withers SS, Kass PH, Rodriguez CO, Jr., Skorupski KA, O'Brien D, Guerrero TA, et al. , Fasting Reduces the Incidence of Delayed-Type Vomiting Associated with Doxorubicin Treatment in Dogs with Lymphoma. Transl. Oncol. 2014. [DOI] [PMC free article] [PubMed]
- 64.Djiogue S, Nwabo Kamdje AH, Vecchio L, Kipanyula MJ, Farahna M, Aldebasi Y, et al. Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer. 2013;20(1):R1–R17. doi: 10.1530/ERC-12-0324. [DOI] [PubMed] [Google Scholar]
- 65.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458(7239):725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Moerkens M, Ramaiahgari S, de BH, Price L, Meerman J, et al. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011;13(3):R52. [DOI] [PMC free article] [PubMed]
- 67.de Groot S, Charehbili A, van Laarhoven HW, Mooyaart AL, Dekker-Ensink NG, van de Ven S, et al. Insulin-like growth factor 1 receptor expression and IGF1R 3129G > T polymorphism are associated with response to neoadjuvant chemotherapy in breast cancer patients: results from the NEOZOTAC trial (BOOG 2010–01). Breast Cancer Res. 2016;18(1):3. [DOI] [PMC free article] [PubMed]
- 68.S de Groot RL, MJ Welters, I Ehsan, MP Vreeswijk, VT Smit, H de Graaf, JB Heijns, JE Portielje, AJ van de Wouw, AL Imholz, LW Kessels, S Vrijaldenhoven, A Baars, E Meershoek-Klein Kranenbarg, M Duijm-de Carpentier, E van Leeuwen-Stok, H Putter, VD Longo, JJ van der Hoeven, JW Nortier, H Pijl and JR Kroep. Abstract P1–15-20: DIetary REstriction as an adjunct to neoadjuvant ChemoTherapy for HER2-negative breast cancer: Final results from the DIRECT trial (BOOG 2013–04). American Association for Cancer Research. 2019.
- 69.Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68(13):5492–5499. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hardie DG. AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem Soc Trans. 2011;39(1):1–13. doi: 10.1042/BST0390001. [DOI] [PubMed] [Google Scholar]
- 71.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 73.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vidal AP, Andrade BM, Vaisman F, Cazarin J, Pinto LF, Breitenbach MM, et al. AMP-activated protein kinase signaling is upregulated in papillary thyroid cancer. Eur J Endocrinol. 2013;169(4):521–528. doi: 10.1530/EJE-13-0284. [DOI] [PubMed] [Google Scholar]
- 75.Zadra G, Batista JL, Loda M. Dissecting the dual role of AMPK in Cancer: from experimental to human studies. Mol Cancer Res. 2015;13(7):1059–1072. doi: 10.1158/1541-7786.MCR-15-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hadad SM, Baker L, Quinlan PR, Robertson KE, Bray SE, Thomson G, et al. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer. 2009;9:307. doi: 10.1186/1471-2407-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leprivier G, Remke M, Rotblat B, Dubuc A, Mateo AR, Kool M, et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell. 2013;153(5):1064–1079. doi: 10.1016/j.cell.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng L, Yang W, Wu F, Wang C, Yu L, Tang L, et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res. 2013;19(19):5372–5380. doi: 10.1158/1078-0432.CCR-13-0203. [DOI] [PubMed] [Google Scholar]
- 79.Wang L, Shang Z, Zhou Y, Hu X, Chen Y, Fan Y, et al. Autophagy mediates glucose starvation-induced glioblastoma cell quiescence and chemoresistance through coordinating cell metabolism, cell cycle, and survival. Cell Death Dis. 2018;9(2):213. doi: 10.1038/s41419-017-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato K, Tsuchihara K, Fujii S, Sugiyama M, Goya T, Atomi Y, et al. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007;67(20):9677–9684. doi: 10.1158/0008-5472.CAN-07-1462. [DOI] [PubMed] [Google Scholar]
- 81.Petibone DM, Majeed W, Casciano DA. Autophagy function and its relationship to pathology, clinical applications, drug metabolism and toxicity. J Appl Toxicol. 2017;37(1):23–37. doi: 10.1002/jat.3393. [DOI] [PubMed] [Google Scholar]
- 82.van Niekerk G, Hattingh SM, Engelbrecht AM. Enhanced therapeutic efficacy in Cancer patients by short-term fasting: the autophagy connection. Front Oncol. 2016;6:242. doi: 10.3389/fonc.2016.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lankelma J, Kooi B, Krab K, Dorsman JC, Joenje H, Westerhoff HV. A reason for intermittent fasting to suppress the awakening of dormant breast tumors. Biosystems. 2015;127:1–6. doi: 10.1016/j.biosystems.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 86.Kusuoka O, Fujiwara-Tani R, Nakashima C, Fujii K, Ohmori H, Mori T, et al. Intermittent calorie restriction enhances epithelial-mesenchymal transition through the alteration of energy metabolism in a mouse tumor model. Int J Oncol. 2018;52(2):413–423. doi: 10.3892/ijo.2017.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007;7(3):147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 88.Veech RL. Ketone ester effects on metabolism and transcription. J Lipid Res. 2014;55(10):2004–2006. doi: 10.1194/jlr.R046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aapro MS, Bohlius J, Cameron DA, Dal LL, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 90.Marij J. Welters, Tetje C. van der Sluis, Hélène van Meir, Nikki M. Loof, Vanessa J. van Ham, Suzanne van Duikeren et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T-cell responses. Sci Transl Med. 2016. [DOI] [PubMed]
- 91.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and Healthspan. Cell Metab. 2015;22(1):86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wing EJ, Stanko RT, Winkelstein A, Adibi SA. Fasting-enhanced immune effector mechanisms in obese subjects. Am J Med. 1983;75(1):91–96. doi: 10.1016/0002-9343(83)91172-5. [DOI] [PubMed] [Google Scholar]
- 93.Englert JM, Powell JD. Hunger pains: stimulating the appetite of the immune system for Cancer. Cancer Cell. 2016;30(1):13–15. doi: 10.1016/j.ccell.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 94.Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell. 2016;30(1):136–146. doi: 10.1016/j.ccell.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jensen TL, Kiersgaard MK, Sorensen DB, Mikkelsen LF. Fasting of mice: a review. Lab Anim. 2013;47(4):225–240. doi: 10.1177/0023677213501659. [DOI] [PubMed] [Google Scholar]
- 96.Bhaskaran K, Douglas I, Forbes H. dos-Santos-Silva I, Leon DA, Smeeth L. body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–14. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer. 2009;124(10):2416–2429. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Endogenous H. Breast Cancer collaborative G, key TJ, Appleby PN, reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferroni P, Riondino S, Laudisi A, Portarena I, Formica V, Alessandroni J, et al. Pretreatment insulin levels as a prognostic factor for breast Cancer progression. Oncologist. 2016;21(9):1041–1049. doi: 10.1634/theoncologist.2015-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duggan C, Wang CY, Neuhouser ML, Xiao L, Smith AW, Reding KW, et al. Associations of insulin-like growth factor and insulin-like growth factor binding protein-3 with mortality in women with breast cancer. Int J Cancer. 2013;132(5):1191–1200. doi: 10.1002/ijc.27753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21(3):433–440. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 103.Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27(7):1082–1086. doi: 10.1200/JCO.2008.19.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomark Prev. 2009;18(10):2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 105.Vella Veronica, Milluzzo Agostino, Scalisi Nunzio, Vigneri Paolo, Sciacca Laura. Insulin Receptor Isoforms in Cancer. International Journal of Molecular Sciences. 2018;19(11):3615. doi: 10.3390/ijms19113615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pollak M. Insulin, insulin-like growth factors and neoplasia. Best Pract Res Clin Endocrinol Metab. 2008;22(4):625–638. doi: 10.1016/j.beem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnstone AM. Fasting - the ultimate diet? Obes Rev. 2007;8(3):211–222. doi: 10.1111/j.1467-789X.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 109.Folin O, Denis W. On starvation and obesity, with special reference to acidosis. J Biol Chem. 1915;21:183–192. [Google Scholar]
- 110.Stewart WK, Fleming LW. Features of a successful therapeutic fast of 382 days' duration. Postgrad Med J. 1973;49(569):203–209. doi: 10.1136/pgmj.49.569.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomson TJ, Runcie J, Miller V. Treatment of obesity by total fasting for up to 249 days. Lancet. 1966;2(7471):992–996. doi: 10.1016/S0140-6736(66)92925-4. [DOI] [PubMed] [Google Scholar]
- 112.BLOOM WL. Fasting as an introduction to the treatment of obesity. Metabolism. 1959;8(3):214–220. [PubMed] [Google Scholar]
- 113.Drenick Ernst J., Smith Roger. Weight Reduction by Prolonged Starvation. Postgraduate Medicine. 1964;36(6):A-95-A-100. doi: 10.1080/00325481.1964.11695378. [DOI] [PubMed] [Google Scholar]
- 114.Franc¸oise Wilhelmi de Toledo FG, Audrey Bergouignan, , Stefan Drinda AM. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS One. 2019. [DOI] [PMC free article] [PubMed]
- 115.Spencer IO. Death during therapeutic starvation for obesity. Lancet. 1968;1(7555):1288–1290. doi: 10.1016/S0140-6736(68)92299-X. [DOI] [PubMed] [Google Scholar]
- 116.Cubberley Peter T., Polster Sheldon A., Schulman Charles L. Lactic Acidosis and Death after the Treatment of Obesity by Fasting. New England Journal of Medicine. 1965;272(12):628–630. doi: 10.1056/NEJM196503252721208. [DOI] [PubMed] [Google Scholar]
- 117.Runcie J, Thomson TJ. Prolonged starvation--a dangerous procedure? Br Med J. 1970;3(5720):432–435. doi: 10.1136/bmj.3.5720.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brazg J, Ruest A, Law S, Bosoy D. A therapeutic fast for lymphoma resulting in Wernicke encephalopathy. J Emerg Med. 2015;49(4):471–474. doi: 10.1016/j.jemermed.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 119.Douillard C, Mention K, Dobbelaere D, Wemeau JL, Saudubray JM, Vantyghem MC. Hypoglycaemia related to inherited metabolic diseases in adults. Orphanet J Rare Dis. 2012;7:26. doi: 10.1186/1750-1172-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goldhamer AC, Lisle DJ, Sultana P, Anderson SV, Parpia B, Hughes B, et al. Medically supervised water-only fasting in the treatment of borderline hypertension. J Altern Complement Med. 2002;8(5):643–650. doi: 10.1089/107555302320825165. [DOI] [PubMed] [Google Scholar]
- 121.Cahill GF, Jr, Owen OE. Starvation and survival. Trans Am Clin Climatol Assoc. 1968;79:13–20. [PMC free article] [PubMed] [Google Scholar]
- 122.Michalsen A, Hoffmann B, Moebus S, Backer M, Langhorst J, Dobos GJ. Incorporation of fasting therapy in an integrative medicine ward: evaluation of outcome, safety, and effects on lifestyle adherence in a large prospective cohort study. J Altern Complement Med. 2005;11(4):601–607. doi: 10.1089/acm.2005.11.601. [DOI] [PubMed] [Google Scholar]
- 123.Han K, Nguyen A, Traba J, Yao X, Kaler M, Huffstutler RD, et al. A pilot study to investigate the immune-modulatory effects of fasting in steroid-naive mild asthmatics. J Immunol. 2018;201(5):1382–1388. doi: 10.4049/jimmunol.1800585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wei Min, Brandhorst Sebastian, Shelehchi Mahshid, Mirzaei Hamed, Cheng Chia Wei, Budniak Julia, Groshen Susan, Mack Wendy J., Guen Esra, Di Biase Stefano, Cohen Pinchas, Morgan Todd E., Dorff Tanya, Hong Kurt, Michalsen Andreas, Laviano Alessandro, Longo Valter D. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Science Translational Medicine. 2017;9(377):eaai8700. doi: 10.1126/scitranslmed.aai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhan C, Dai X, Shen G, Lu X, Wang X, Lu L, et al. Preoperative short-term fasting protects liver injury in patients undergoing hepatectomy. Ann Transl Med. 2018;6(23):449. doi: 10.21037/atm.2018.10.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grundmann F, Muller RU, Reppenhorst A, Hulswitt L, Spath MR, Kubacki T, et al. Preoperative Short-Term Calorie Restriction for Prevention of Acute Kidney Injury After Cardiac Surgery: A Randomized, Controlled, Open-Label, Pilot Trial. J Am Heart Assoc. 2018;7(6). [DOI] [PMC free article] [PubMed]
- 127.Cahill G, Jr, Felig P, Owen O, Wahren J. Metabolic adaptation to prolonged starvation in man. Nord Med. 1970;83(3):89. [PubMed] [Google Scholar]
- 128.Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, et al. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest. 1988;81(4):968–975. doi: 10.1172/JCI113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Norrelund H. The metabolic role of growth hormone in humans with particular reference to fasting. Growth Hormon IGF Res. 2005;15(2):95–122. doi: 10.1016/j.ghir.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 130.Moller L, Dalman L, Norrelund H, Billestrup N, Frystyk J, Moller N, et al. Impact of fasting on growth hormone signaling and action in muscle and fat. J Clin Endocrinol Metab. 2009;94(3):965–972. doi: 10.1210/jc.2008-1385. [DOI] [PubMed] [Google Scholar]
- 131.de Groot S, Vreeswijk MP, Welters MJ, Gravesteijn G, Boei JJ, Jochems A, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC. Cancer. 2015;15:652. [DOI] [PMC free article] [PubMed]
- 132.Maccario M, Aimaretti G, Grottoli S, Gauna C, Tassone F, Corneli G, et al. Effects of 36 hour fasting on GH/IGF-I axis and metabolic parameters in patients with simple obesity. Comparison with normal subjects and hypopituitary patients with severe GH deficiency. Int. J Obes Relat Metab Disord. 2001;25(8):1233–1239. doi: 10.1038/sj.ijo.0801671. [DOI] [PubMed] [Google Scholar]
- 133.Cotterill Andrew M., Holly Jeffrey M. P., Wass John A. H. The regulation of insulin-like growth factor binding protein (IGFBP)-1 during prolonged fasting. Clinical Endocrinology. 1993;39(3):357–362. doi: 10.1111/j.1365-2265.1993.tb02377.x. [DOI] [PubMed] [Google Scholar]
- 134.Boelen A, Wiersinga WM, Fliers E. Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid. 2008;18(2):123–129. doi: 10.1089/thy.2007.0253. [DOI] [PubMed] [Google Scholar]
- 135.Badar T, Ismail A, AlShanqeeti A. Safety and Feasability of Muslim Fasting While Receiving Chemotherapy. IOSR Journal of Pharmacy. 2014.
- 136.Safdie Fernando M., Dorff Tanya, Quinn David, Fontana Luigi, Wei Min, Lee Changhan, Cohen Pinchas, Longo Valter D. Fasting and cancer treatment in humans: A case series report. Aging. 2009;1(12):988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dorff TB, Groshen S, Garcia A, Shah M, Tsao-Wei D, Pham H, et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360. doi: 10.1186/s12885-016-2370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bauersfeld SP, Kessler CS, Wischnewsky M, Jaensch A, Steckhan N, Stange R, et al. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study. BMC Cancer. 2018;18(1):476. doi: 10.1186/s12885-018-4353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Raffaghello L, Safdie F, Bianchi G, Dorff T, Fontana L, Longo VD. Fasting and differential chemotherapy protection in patients. Cell Cycle. 2010;9(22):4474–4476. doi: 10.4161/cc.9.22.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bouhlel E, Zaouali M, Miled A, Tabka Z, Bigard X, Shephard R. Ramadan fasting and the GH/IGF-1 axis of trained men during submaximal exercise. Ann Nutr Metab. 2008;52(4):261–266. doi: 10.1159/000140517. [DOI] [PubMed] [Google Scholar]
- 141.Inder WJ, Jang C, Obeyesekere VR, Alford FP. Dexamethasone administration inhibits skeletal muscle expression of the androgen receptor and IGF-1--implications for steroid-induced myopathy. Clin. Endocrinol. (Oxf). 2010;73(1):126–32. [DOI] [PubMed]
- 142.Veldhuis JD, Lizarralde G, Iranmanesh A. Divergent effects of short term glucocorticoid excess on the gonadotropic and somatotropic axes in normal men. J Clin Endocrinol Metab. 1992;74(1):96–102. doi: 10.1210/jcem.74.1.1727834. [DOI] [PubMed] [Google Scholar]
- 143.Levolger S, van den Engel S, Ambagtsheer G, JNM IJ, de Bruin RWF. Caloric restriction is associated with preservation of muscle strength in experimental cancer cachexia. Aging (Albany NY) 2018;10(12):4213–4223. doi: 10.18632/aging.101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


