The Importance of Sleep for Workplace Health
Alongside healthy diet, sufficient physical activity, smoking cessation, and moderation of alcohol intake, healthy sleep has emerged as a key element in health. Getting at least 7 hours of quality sleep per night (or 7–9 hours if there is an upper limit) is a recommendation put forth by the American Academy of Sleep Medicine and Sleep Research Society,1–4 NSF,5,6 American Thoracic Society,7 and American Heart Association.8 These recommendations have been adopted by the Centers for Disease Control and Prevention (CDC; https://www.cdc.gov/sleep) and the National Institutes of Health (https://www.nhlbi.nih.gov/health-topics/sleep-deprivation-and-deficiency). Increasing the number of Americans who achieve adequate sleep was a goal of Healthy People 2020.9
Still, American society demonstrates unhealthy sleep practices. Regarding sleep duration, recent data suggest that just 56% of US adults get 7 to 8 hours of sleep on a regular basis, with about 36% getting about 6 hours or less.10 In addition to insufficient sleep duration, insomnia symptoms are also prevalent in the US population. It has been well documented that about 30% of the population experience significant insomnia symptoms, with approximately 10% likely meeting criteria for insomnia disorder.11 Sleep apnea (SA) is also highly prevalent. As can be seen in data from the Wisconsin Sleep Cohort, prevalence of SA is high, especially in men and especially in adults who are obese.12 Nationally representative data from National Health and Nutrition Examination Survey also suggest that nonrestorative sleep is experienced “often” or “almost always” by approximately 29% of the US population and excessive daytime sleepiness is experienced “often” or “almost always” by about 19% of the population.13 Although insufficient sleep, insomnia, and SA are the most prevalent sleep problems, they are not the only ones. Taken together, it is estimated that approximately 70 million Americans suffer from some sort of disordered sleep.14
The reasons for these unhealthy sleep practices are still unclear. But modern society tends to promote the idea that sleep is unproductive time. The purpose of this review is to demonstrate how sleep is not only time well spent in terms of health, but also for productivity.
Sleep and Health
For over 50 years, and across over 50 studies, habitual sleep has been known to be associated with mortality risk.15,16 Overall, there is a U-shaped relationship such that sleep outside the normative range (typically 7–8 hours) is associated with increased mortality. All of the reasons for why those experiencing shorter or longer sleep die sooner are not fully explicated. The hypotheses regarding long sleep have been articulated, but this issue still remains controversial.2,17,18 Regarding insufficient sleep, though, much more evidence has accumulated.
A growing literature has shown that insufficient sleep is associated with a number of factors closely related to health. For example, many studies have now shown that short sleep is associated with higher body mass index (BMI)/obesity.19 Further, a meta-analysis of longitudinal studies shows that short sleep is associated with an approximately 55% increased likelihood of developing obesity.20 Laboratory studies have shown that sleep deprivation leads to increased caloric intake,21–26 resulting in up to 1 kg of weight gain in just 1 week. Other studies have shown in real-world samples that less sleep is associated with incident weight gain.
Several studies showed that insufficient sleep was associated with increased likelihood of cardiovascular risk factors,27,28 including hypercholesterolemia,29,30 coronary artery calcification,31 and other cardiovascular risk factors, including hypertension.32 A meta-analysis found that short sleep was associated with a 21% increased incidence of hypertension.33,34 Diabetes has also been associated with insufficient sleep in a number of studies10 and is supported by meta-analyses showing an approximately 30% increased likelihood of developing diabetes in the presence of short sleep.35–37
Several potential mechanisms of these relationships have been explored. A number of studies have shown that insufficient sleep is associated with a pro-inflammatory state,38 both in the laboratory and in population samples. In addition, sleep loss has been shown to alter insulin sensitivity and promote insulin resistance,39,40 as well as alterations in metabolic hormones leptin and ghrelin,41,42 cannabinoids,43 testosterone,44,45 cortisol,46–48 and other biomarkers of metabolic processes associated with metabolic risk.49 Behavioral risk factors have also been explored. Insufficient sleep has also been associated with smoking50–54 and alcohol use,54–56 as well as unhealthy dietary patterns21,57–59 and increased sedentary behaviors.52,60–62 Mental health has also been shown to be related to insufficient sleep, which has been shown to be related to depression and anxiety.63–65
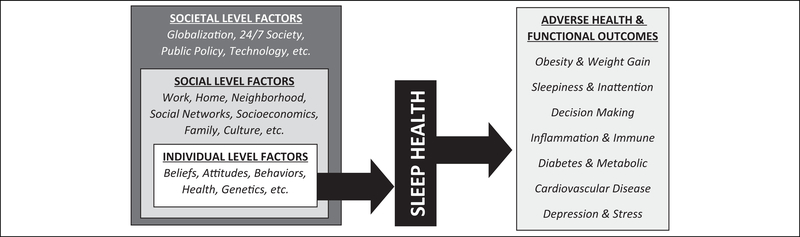
To conceptualize these relationships, the Social Ecological Model of Sleep Health has been described.2,15,27 Briefly, this model describes how sleep plays an important role at the interface of upstream social, environmental, and behavioral influences and downstream health outcomes. Briefly, the model (summarized in Figure 1) describes how an individual’s sleep health is a product of that individual’s own characteristics, status, and choices. But, the model describes that the individual is embedded within a social level that includes work, home, neighborhood, and other factors that exist outside of the individual and exert a powerful influence. The model describes how these factors are still embedded within a larger context of societal factors such as globalization, technology, and 24/7 society, which influence the factors at the social level and, eventually, an individual’s behavior around sleep. For example, an individual may make a choice to stay up late or have an attitude about sleep being something that is a low priority or that an individual may have a health condition that physiologically influences their sleep. But those beliefs, attitudes, practices, and choices (and to some degree the health conditions) are highly influenced by factors such as work (which can dictate commute times, shift schedules, workplace culture) and even larger societal issues, such as globalization (which can influence work schedules); and technology (which can influence mobile device and e-mail use). Recognizing that these issues exist on multiple levels is important for understanding the multidimensional influences on sleep health and developing interventions that are likely to work in real-world situations.
Figure 1.
Social-ecological model of sleep and health.
Sleep Disorders
In addition to insufficient sleep, sleep disorders such as insomnia and SA are important to consider. Insomnia (which represents difficulty sleeping irrespective of sleep quantity) is itself associated with cardiovascular disease,28 as well as both obesity and diabetes.66 An extensive literature describes the role of insomnia as a key risk factor for anxiety and depression.67,68 In addition, a growing literature has identified insomnia as a key risk factor for suicide.69–75 Sleep apnea is also a well-characterized health risk factor. It is a major risk factor for cardiovascular disease76–78 and is strongly related to obesity.12 Sleep apnea, through intermittent hypoxia and/or sleep fragmentation, has been shown to be related to oxidative stress,79,80 neuronal injury,81 inflammation,76,82–84 and other adverse outcomes.
Sleep and Cognitive Functioning
Many previous studies have documented the effects of sleep loss on aspects of cognitive functioning. Several have especially salient applicability to workplace situations, such as sleep propensity, vigilant attention, and decision-making. Sleep propensity refers to the homeostatic sleep pressure that can lead an individual to be able to fall asleep quickly or sometimes inadvertently. It is well documented that sleep loss leads to increased sleep propensity.85–87 Functionally, this means that less sleep places an individual at risk of being unable to maintain wakefulness. This is a critical safety concern and is a major source of accidents and errors, including motor vehicle accidents.88–91 A previous study at the population level found that not only did shorter sleep duration predict drowsy driving, but this persisted in individuals who reported that they felt completely well rested.92 This suggests that the increased sleep propensity that could lead someone to doze in even a safety-sensitive scenario is not always perceived by the individual.
Many studies have evaluated the role of sleep loss in impaired vigilant attention.93–101 This refers to the ability of an individual to maintain their attention over time, in the absence of stimulation, like a guard on duty.96 The psychomotor vigilance task is the gold-standard assessment tool for vigilant attention and has been shown to be sensitive to sleep loss. It has demonstrated a dose-dependent relationship to sleep, such that as sleep duration decreases, attentional lapses increase.102 In addition, deficits in attention are cumulative over time103,104 and do not seem to stop increasing. This is in contrast to self-reports of impairment, which show a levelling off after a few days.97,105 Thus, impairments increase over time, even if the individual believes that they are acclimating to less sleep. This has profound workplace safety implications. Not only attentional lapses and errors more likely in the context of sleep loss, but the individual is often unaware of their impairments, leading to some potentially unsafe situations.
Several studies have examined the role of sleep loss in decisionmaking. In a now classic study, Killgore and colleagues showed that sleep loss impaired function on a decision-making task with a monetary component (participants had to maximize the amount they were paid by strategically completing a task).106 The study found that under sleep deprivation conditions, participants were much less able to make the optimal decisions and ended up with less money at the end of the task. Further, when given stimulants including caffeine, amphetamines, or modafinil, performance was not rescued. This is especially relevant to the workplace, where caffeine is often seen as a solution to impaired decision-making due to sleep loss—the existing studies suggest that though these stimulants increase speed and focus, they do not mitigate these impairments. Other studies have examined sleep and decision-making and have shown that sleep loss leads to a number of alterations in executive function,93,94,107–116 such as more hedonic decision-making, difficulty planning, increased risky decisions, and difficulty managing complex tasks.
Sleep and Workplace Factors
Healthy sleep is increasingly recognized as an important aspect of workplace health and functioning. Several domains in which this is relevant are injuries/disability, absenteeism, presenteeism/productivity, and health-care expenditures. A meta-analysis by Uehli and colleagues showed that sleep problems were associated with a 62% increased likelihood of workplace injuries.117 In a recent study,118 data from a large organization were used to show that poor sleep quality was associated with absenteeism—compared to those that reported good quality sleep, those who reported frequent poor sleep (comprising 11% of the total sample) were 171% more likely to miss 1 to 2 of the past 30 days of work, 548% more likely to miss 3 to 6 of the past 30 days of work, and 1052% more likely to miss 7 or more of the past 30 days of work. Similarly, poor sleep was associated with comparable increased likelihood of missed partial days of work.
Regarding presenteeism/productivity, a study by Rosekind and colleagues showed that a typical company loses $1293 per year of productivity per employee, but this increases by 79% for employees who are at risk for poor sleep, by 116% for employees who are getting insufficient sleep, and by 144% for employees with insomnia.119 Another study found that poor sleep was associated with worse self-rated work performance, as well as lower performance relative to a typical person in that position.118 The study by Rosekind and colleagues found that productivity was lost due to increased problems with time management, mental/interpersonal demands, output/performance demands, and physical job demands.119 A recent study expanded on this work examining productivity loss across all sources relative to sleep characteristics.120 Compared to individuals sleeping 7 to 8 hours, those sleeping 4 hours or less reported 29% more productivity loss, while sleeping 5 to 6 hours reported 19% more productivity loss, and those sleeping 9 hours or more reported 24% more productivity loss. Compared to those without insomnia, those with mild insomnia reported 58% more productivity loss and those with moderate-severe insomnia reported 107% more. Daytime sleepiness was associated with a 50% more lost productivity than those without sleepiness, and those that reported snoring (a possible SA risk factor) reported 19% to 34% more productivity loss, across all sources.
Hui and Grandner also examined health-care dollars spent as a result of poor sleep.118 They found that poor sleep quality was associated with approximately $3400 to $5200 additional dollars spent per person on health care. When participants were followed up after a year, if sleep quality worsened, health-care expenditures increased.
In another study, Hui and Grandner showed that sleep quality impacted the stages of change associated with other health behaviors, including stress management, weight management, physical activity, alcohol use, and smoking.56 This refers to whether an individual is likely to make a change in a behavior related to their health and is conceptualized as precontemplation (has not yet considered change), contemplation (considered change but has not yet chosen to act), preparation (chose to act but has not taken the first step), action (currently engaging in behavior), and maintenance (maintaining healthy behavior). Overall, it was found that generally, poor sleep quality was associated with increased likelihood of being in a contemplation or preparation or action stage of behavior change, relative to precontemplation; however, poor sleep quality was generally associated with a decreased likelihood of being in a maintenance stage. This may be because poor sleep may help motivate an individual to decide to act on health but may—through functional limitations—make it more difficult to maintain healthy behavior. This is particularly relevant for workplace wellness programs that wish to promote healthy behavior change.
Conclusions and Recommendations for Workplace Health Promotion
In summary, healthy sleep has been increasingly recognized as an important aspect of health. Workplace health interventions should address sleep, especially if they address other dimensions of health such as diet, physical activity, smoking, or stress. Sleep has been shown to be a key predictor of shorter lifespan, as well as weight gain and obesity, hypertension, hypercholesterolemia, atherosclerosis risk, diabetes, poor mental health, smoking, alcohol misuse, unhealthy diet, and sedentary activity. In addition, sleep is an important factor in brain functions that are important for the workplace, including sleepiness, attention, and decision-making. For these reasons, insufficient sleep in workplace settings has been shown to be related to workplace injuries, absenteeism, presenteeism/productivity loss, and health-care costs.
Workplace health initiatives should promote the idea that sleep is not unproductive time. Rather, it is an investment of time that has been shown to produce improved productivity and less productivity loss. The available evidence shows that rather than more productive, individuals who are sleeping less are actually less productive, even with more time. The culture of sleep being only “rest” and therefore a sign of weakness or lack of endurance needs to change. In addition, workplace health programs should recognize that needs assessment regarding sleep should reflect 3 layers: (1) assessment of sleep disorders that need treatment by a licensed professional, since sleep disorders are often undiagnosed, (2) assessment of sleep deficiencies that are associated with poor outcomes, and (3) assessment of ways in which otherwise healthy sleep might be optimized. These 3 pathways are each important, since (1) sleep disorders such as SA and insomnia should be addressed with evidence-based therapies, (2) poor sleep quality and insufficient sleep duration—even if they are not sleep disorders—should be addressed as important workplace factors, and (3) even those with otherwise healthy sleep may be able to benefit from a sleep optimization program.
The importance of a sleep intervention has relevance beyond reducing health-care spending, improving morale, and improving productivity. Because it is known that sleep loss leads to workplace injuries and accidents, the issues of shift work, fatigue, and overscheduling are important from a liability perspective. These are often inherent in business operations, but because they carry known risks, it is often up to the employer to mitigate these risks as much as possible in the context of workers’ compensation and other legal matters. A single accident or injury can prove very expensive. These issues and others should be considered when weighing the cost of a workplace sleep health program.
Biography

References
- 1.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. 2015;11(8): 931–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38(8):1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11(6):591–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirshkowitz M, Whiton K, Alpert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. [DOI] [PubMed] [Google Scholar]
- 6.Hirshkowitz M, Whiton K, Alpert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee S, Patel SR, Kales SN, et al. An official American Thoracic Society Statement: the importance of healthy sleep. Recommendations and future priorities. Am J Respir Crit Care Med. 2015;191(12):1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Onge MP, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Office of Disease Prevention and Health Promotion. Healthy People 2020 Objective Topic Areas. Washington, DC: US Department of Health and Human Services; 2011. [Google Scholar]
- 10.Grandner MA, Seixas A, Shetty S, Shenoy S. Sleep duration and diabetes risk: population trends and potential mechanisms. Curr Diab Rep. 2016;16(11):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lederhendler I, Ancoli-Israel S, Atkins D, et al. NIH State-of-the-science conference statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements. 2005;22(2):1–30. [PubMed] [Google Scholar]
- 12.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grandner MA, Petrov MER, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med. 2013;9(9):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colten HR, Altevogt BM; Institute of Medicine Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: Institute of Medicine: National Academies Press; 2006. [PubMed] [Google Scholar]
- 15.Grandner MA, Patel NP, Hale L, Moore M. Mortality associated with sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14(3):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu TZ, Xu C, Rota M, et al. Sleep duration and risk of all-cause mortality: a flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev. 2017;32:28–36. [DOI] [PubMed] [Google Scholar]
- 17.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8(3):159–174. [DOI] [PubMed] [Google Scholar]
- 18.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11(5):341–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandner MA. Sleep and obesity risk in adults: possible mechanisms; contextual factors; and implications for research, intervention, and policy. Sleep Health. 2017;3(5):393–400. [DOI] [PubMed] [Google Scholar]
- 20.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shechter A, Grandner MA, St-Onge MP. The role of sleep in the control of food intake. Am J Lifestyle Med. 2014;8(6):371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med. 2013;9(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013; 110(14):5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spaeth AM, Dinges DF, Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr. 2014;100(2):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36(7):981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandner MA. Sleep, health, and society. Sleep Med Clin. 2017; 12(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grandner MA, Alfonso-Miller P, Fernandez-Mendoza J, Shetty S, Shenoy S, Combs D. Sleep: important considerations for the prevention of cardiovascular disease. Curr Opin Cardiol. 2016; 31(5):551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012;13(10):1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhaga-vatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandner M, Mullington JM, Hashmi SD, Redeker NS, Watson NF, Morgenthaler TI. Sleep duration and hypertension: analysis of >700,000 adults by age and sex. J Clin Sleep Med. 2018;14(6): 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36(11):985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grandner MA, Perlis ML. Short sleep duration and insomnia associated with hypertension incidence. Hypertens Res. 2013; 36(11):932–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2015;30:11–24. [DOI] [PubMed] [Google Scholar]
- 36.Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–537. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Bi Y, Zhang Q, Pan F. Obstructive sleep apnoea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Respirology. 2013;18(1):140–146. [DOI] [PubMed] [Google Scholar]
- 38.Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Upala S, Sanguankeo A, Congrete S, Romphothong K. Sleep duration and insulin resistance in individuals without diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2015;109(3):e11–e12. [DOI] [PubMed] [Google Scholar]
- 40.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157(8):549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovas JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6(6): 648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern JH, Grant AS, Thomson CA, et al. Short sleep duration is associated with decreased serum leptin, increased energy intake, and decreased diet quality in postmenopausal women. Obesity (Silver Spring). 2014;22(5):E55–E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanlon EC, Tasali E, Leproult R, et al. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep. 2016;39(3):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cote KA, McCormick CM, Geniole SN, Renn RP, MacAulay SD. Sleep deprivation lowers reactive aggression and testosterone in men. Biol Psychol. 2013;92(2):249–256. [DOI] [PubMed] [Google Scholar]
- 45.Schmid SM, Hallschmid M, Jauch-Chara K, Lehnert H, Schultes B. Sleep timing may modulate the effect of sleep loss on testosterone. Clin Endocrinol (Oxf). 2012;77(5):749–754. [DOI] [PubMed] [Google Scholar]
- 46.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5): 2119–2126. [DOI] [PubMed] [Google Scholar]
- 47.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(suppl 1): S23–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188): 1435–1439. [DOI] [PubMed] [Google Scholar]
- 49.Koren D, Taveras EM. Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metabolism. 2018;84:67–75. [DOI] [PubMed] [Google Scholar]
- 50.Patterson F, Grandner MA, Lozano A, Satti A, Ma G. Transitioning from adequate to inadequate sleep duration associated with higher smoking rate and greater nicotine dependence in a population sample. Addict Behav. 2018;77:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patterson F, Grandner MA, Malone SK, Rizzo A, Davey A, Edwards DG. Sleep as a target for optimized response to smoking cessation treatment [published online October 22, 2017]. Nicotine Tob Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patterson F, Malone SK, Lozano A, Grandner MA, Hanlon AL. Smoking, screen-based sedentary behavior, and diet associated with habitual sleep duration and chronotype: data from the UK Biobank. Ann Behav Med. 2016;50(5):715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhee JU, Haynes P, Chakravorty S, et al. Susceptibility to smoking during the day and its relationship with insomnia and sleep duration. Sleep. 2016;39(Abstract suppl):A189. [Google Scholar]
- 54.Grandner MA, Jackson NJ, Izci-Balserak B, et al. Social and behavioral determinants of perceived insufficient sleep. Front Neurol. 2015;6:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller MB, Donahue ML, Carey KB, Scott-Sheldon LAJ. Insomnia treatment in the context of alcohol use disorder: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;181: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hui SK, Grandner MA. Associations between poor sleep quality and stages of change of multiple health behaviors among participants of employee wellness program. Prev Med Rep. 2015;2: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji X, Grandner MA, Liu J. The relationship between micronutrient status and sleep patterns: a systematic review. Public Health Nutr. 2017;20(4):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite. 2013;64:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patterson F, Malone SK, Grandner MA, Lozano A, Perkett M, Hanlon A. Interactive effects of sleep duration and morning/evening preference on cardiovascular risk factors. Eur J Public Health. 2018;28(1):155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao Q, Keadle SK, Hollenbeck AR, Matthews CE. Sleep duration and total and cause-specific mortality in a large US cohort: interrelationships with physical activity, sedentary behavior, and body mass index. Am J Epidemiol. 2014;180(10):997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grandner MA, Patel NP, Perlis ML, et al. Obesity, diabetes, and exercise associated with sleep-related complaints in the American population. J Public Health. 2011;19(5):463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. [DOI] [PubMed] [Google Scholar]
- 64.Patel SR, Sotres-Alvarez D, Castaneda SF, et al. Social and health correlates of sleep duration in a US Hispanic population: results from the Hispanic community health study/study of Latinos. Sleep. 2015;38(10):1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson NF, Harden KP, Buchwald D, et al. Sleep duration and depressive symptoms: a gene-environment interaction. Sleep. 2014;37(2):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21(4):427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011; 135(1–3):10–19. [DOI] [PubMed] [Google Scholar]
- 68.Baglioni C, Riemann D. Is chronic insomnia a precursor to major depression? Epidemiological and biological findings. Curr Psychiatry Rep. 2012;14(5):511–518. [DOI] [PubMed] [Google Scholar]
- 69.Chakravorty S, Grandner MA, Mavandadi S, Perlis ML, Sturgis EB, Oslin DW. Suicidal ideation in veterans misusing alcohol: relationships with insomnia symptoms and sleep duration. Addict Behav. 2014;39(2):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chakravorty S, Siu HY, Lalley-Chareczko L, et al. Sleep duration and insomnia symptoms as risk factors for suicidal ideation in a nationally representative sample. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.13m01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perlis ML, Grandner MA, Brown GK, et al. Nocturnal wakefulness as a previously unrecognized risk factor for suicide. J Clin Psychiatry. 2016;77(6):e726–e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perlis ML, Grandner MA, Chakravorty S, Bernert RA, Brown GK, Thase ME. Suicide and sleep: is it a bad thing to be awake when reason sleeps? Sleep Med Rev. 2016;29:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pigeon WR, Britton PC, Ilgen MA, Chapman B, Conner KR. Sleep disturbance preceding suicide among veterans. Am J Public Health. 2012;102(suppl 1):S93–S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pigeon WR, Caine ED. Insomnia and the risk for suicide: does sleep medicine have interventions that can make a difference? Sleep Med. 2010;11(9):816–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–e1167. [DOI] [PubMed] [Google Scholar]
- 76.Baltzis D, Bakker JP, Patel SR, Veves A. Obstructive sleep apnea and vascular diseases. Compr Physiol. 2016;6(3):1519–1528. [DOI] [PubMed] [Google Scholar]
- 77.Levy P, Ryan S, Oldenburg O, Parati G. Sleep apnoea and the heart. Eur Respir Rev. 2013;22(129):333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Golbidi S, Badran M, Ayas N, Laher I. Cardiovascular consequences of sleep apnea. Lung. 2012;190(2):113–132. [DOI] [PubMed] [Google Scholar]
- 79.Ntalapascha M, Makris D, Kyparos A, et al. Oxidative stress in patients with obstructive sleep apnea syndrome. Sleep Breath. 2013;17(2):549–555. [DOI] [PubMed] [Google Scholar]
- 80.Tasci I Oxidative stress, obstructive sleep apnea and cardiovascular disease. Sleep Breath. 2012;16(3):585. [DOI] [PubMed] [Google Scholar]
- 81.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pak VM, Grandner MA, Pack AI. Circulating adhesion molecules in obstructive sleep apnea and cardiovascular disease. Sleep Med Rev. 2014;18(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014; 370(24):2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang SX, Khalyfa A, Wang Y, et al. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes (Lond). 2014; 38(4):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rupp TL, Wesensten NJ, Balkin TJ. Trait-like vulnerability to total and partial sleep loss. Sleep. 2012;35(8):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singareddy R, Bixler EO, Vgontzas AN. Fatigue or daytime sleepiness? J Clin Sleep Med. 2010;6(4):405. [PMC free article] [PubMed] [Google Scholar]
- 87.Short M, Lack L, Wright H. Does subjective sleepiness predict objective sleep propensity? Sleep. 2010;33(1):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burks SV, Anderson JE, Bombyk M, et al. Nonadherence with employer-mandated sleep apnea treatment and increased risk of serious truck crashes. Sleep. 2016;39(5):967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herman J, Kafoa B, Wainiqolo I, et al. Driver sleepiness and risk of motor vehicle crash injuries: a population-based case control study in Fiji (TRIP 12). Injury. 2014;45(3):586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bakiri S, Galera C, Lagarde E, et al. Distraction and driving: results from a case-control responsibility study of traffic crash injured drivers interviewed at the emergency room. Accid Anal Prev. 2013;59:588–592. [DOI] [PubMed] [Google Scholar]
- 91.Powell NB, Chau JKM. Sleepy driving. Med Clin North Am. 2010;94(3):531–540. [DOI] [PubMed] [Google Scholar]
- 92.Maia Q, Grandner MA, Findley J, Gurubhagavatula I. Short and long sleep duration and risk of drowsy driving and the role of subjective sleep insufficiency. Accid Anal Prev. 2013;59:618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goel N, Basner M, Dinges DF. Phenotyping of neurobehavioral vulnerability to circadian phase during sleep loss. Methods Enzymol. 2015;552:285–308. [DOI] [PubMed] [Google Scholar]
- 94.Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mole Biol Transl Sci. 2013;119:155–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dinges DF, Banks S. Sleep deprivation: cognitive performance In: Amlaner CJ, Fuller PM, eds. Basics of Sleep Guide. 2nd ed. Westchester, IL: Sleep Research Society; 2009:257–264. [Google Scholar]
- 96.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. [DOI] [PubMed] [Google Scholar]
- 97.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 98.Dinges DF. The state of sleep deprivation: from functional biology to functional consequences. Sleep Med Rev. 2006;10(5): 303–305. [DOI] [PubMed] [Google Scholar]
- 99.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–129. [DOI] [PubMed] [Google Scholar]
- 100.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep. 2005;28(4):479–496. [DOI] [PubMed] [Google Scholar]
- 101.Rogers NL, Dorrian J, Dinges DF. Sleep, waking and neurobehavioural performance. Front Biosci. 2003;8:s1056–s1067. [DOI] [PubMed] [Google Scholar]
- 102.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010; 136(3):375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27(3):423–433. [PubMed] [Google Scholar]
- 104.Van Dongen HP, Maislin G, Dinges DF. Dealing with interindividual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Environ Med. 2004;75(suppl 3):A147–A154. [PubMed] [Google Scholar]
- 105.Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33(8): 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Killgore WD, Grugle NL, Balkin TJ. Gambling when sleep deprived: don’t bet on stimulants. Chronobiol Int. 2012;29(1): 43–54. [DOI] [PubMed] [Google Scholar]
- 107.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fraser M, Conduit R, Phillips JG. Effects of sleep deprivation on decisional support utilisation. Ergonomics. 2013;56(2):235–245. [DOI] [PubMed] [Google Scholar]
- 109.Jackson ML, Gunzelmann G, Whitney P, et al. Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev. 2013;17(3):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Killgore WDS. Effects of sleep deprivation on cognition In: Kerkhof GA, Van Dongen HPA, eds. Human Sleep and Cognition, Part I: Basic Research. Amsterdam, Netherlands: Elsevier; 2010:105–129. [DOI] [PubMed] [Google Scholar]
- 111.Killgore WD, Grugle NL, Reichardt RM, Killgore DB, Balkin TJ. Executive functions and the ability to sustain vigilance during sleep loss. Aviat Space Environ Med. 2009;80(2):81–87. [DOI] [PubMed] [Google Scholar]
- 112.Killgore WD, Kahn-Greene ET, Grugle NL, Killgore DB, Balkin TJ. Sustaining executive functions during sleep deprivation: a comparison of caffeine, dextroamphetamine, and modafinil. Sleep. 2009;32(2):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Killgore WD, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 2008;9(5):517–526. [DOI] [PubMed] [Google Scholar]
- 114.Spaeth AM, Goel N, Dinges DF. Managing neurobehavioral capability when social expediency trumps biological imperatives. Prog Brain Res. 2012;199:377–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drummond SP, Anderson DE, Straus LD, Vogel EK, Perez VB. The effects of two types of sleep deprivation on visual working memory capacity and filtering efficiency. PLoS One. 2012;7(4): e35653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McKenna BS, Dicjinson DL, Orff HJ, Drummond SP. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res. 2007;16(3):245–252. [DOI] [PubMed] [Google Scholar]
- 117.Uehli K, Mehta AJ, Miedinger D, et al. Sleep problems and work injuries: a systematic review and meta-analysis. Sleep Med Rev. 2014;18(1):61–73. [DOI] [PubMed] [Google Scholar]
- 118.Hui SK, Grandner MA. Trouble sleeping associated with lower work performance and greater health care costs: longitudinal data from Kansas state employee wellness program. J Occup Environ Med. 2015;57(10):1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosekind MR, Gregory KB, Mallis MM, Brandt SL, Seal B, Lerner D. The cost of poor sleep: workplace productivity loss and associated costs. J Occup Environ Med. 2010;52(1):91–98. [DOI] [PubMed] [Google Scholar]
- 120.Yang R, Hale L, Branas C, et al. Work productivity associated with sleep duration, insomnia severity, sleepiness, and snoring. Sleep. 2018;41(Abstract Supplement):A74. [Google Scholar]