Abstract
Post-transcriptional regulation underlies the circadian control of gene expression and animal behaviors. However, the role of mRNA surveillance via the nonsense-mediated mRNA decay (NMD) pathway in circadian rhythms remains elusive. Here, we report that Drosophila NMD pathway acts in a subset of circadian pacemaker neurons to maintain robust 24 h rhythms of free-running locomotor activity. RNA interference-mediated depletion of key NMD factors in timeless-expressing clock cells decreased the amplitude of circadian locomotor behaviors. Transgenic manipulation of the NMD pathway in clock neurons expressing a neuropeptide PIGMENT-DISPERSING FACTOR (PDF) was sufficient to dampen or lengthen free-running locomotor rhythms. Confocal imaging of a transgenic NMD reporter revealed that arrhythmic Clock mutants exhibited stronger NMD activity in PDF-expressing neurons than wild-type. We further found that hypomorphic mutations in Suppressor with morphogenetic effect on genitalia 5 (Smg5 ) or Smg6 impaired circadian behaviors. These NMD mutants normally developed PDF-expressing clock neurons and displayed daily oscillations in the transcript levels of core clock genes. By contrast, the loss of Smg5 or Smg6 function affected the relative transcript levels of cAMP response element-binding protein B (CrebB ) in an isoform-specific manner. Moreover, the overexpression of a transcriptional repressor form of CrebB rescued free-running locomotor rhythms in Smg5-depleted flies. These data demonstrate that CrebB is a rate-limiting substrate of the genetic NMD pathway important for the behavioral output of circadian clocks in Drosophila.
Keywords: circadian rhythms, CrebB, Drosophila, nonsense-mediated mRNA decay
INTRODUCTION
Organisms have endogenous circadian clocks that maintain daily rhythmic activities despite environmental alterations. The circadian clock system consists of three main components: an input pathway, a central oscillator, and an output pathway (Agostino et al., 2011). The input pathway transmits external timing information (e.g., light or temperature cycles) to the brain. In the central oscillator, the timing information cues 24 h rhythm generators (e.g., circadian gene expression and/or neural activities) to govern circadian output pathways. The output circuitry is comprised of downstream genes and neurons that relay clock signals to overt behaviors and physiology, such as locomotor activity and metabolism.
The central molecular oscillators in Drosophila run in a network of approximately 150 pacemaker neurons, which are organized into several groups (Beckwith et al., 2015; Peschel et al., 2011). The cell-autonomous molecular clocks comprise transcription-translation feedback loops (TTFLs) of circadian transcription factors that are conserved among fruit flies and other species. The first TTFL in Drosophila is driven by PERIOD (PER) and TIMELESS (TIM) (Konopka et al., 1971; Sehgal et al., 1994), which accumulate in the cytoplasm as mRNA levels of per and tim increase throughout the day, reaching the maximum level in the early evening. TIM stabilizes PER (Price et al., 1995; Suri et al., 1999; Vosshall et al., 1994), and the PER-TIM heterodimer complex enters the nucleus at around midnight where it inhibits the CLOCK (CLK) and CYCLE (CYC) complex, the transcriptional activator of per and tim (Allada et al., 1998; Rutila et al., 1998). This negative autoregulatory feedback is sustained until TIM is degraded by the photoreceptor CRYPTOCHROME (CRY) in the early morning (Emery et al., 1998; Stanewsky et al., 1998). The heterodimeric CLK-CYC complex drives additional TTFLs by binding E-box sequences in genomic loci of several clock genes and promoting their transcription, such as PAR-domain protein 1 (Pdp1 ), encoding an activator of Clk transcription (Cyran et al., 2003); vrille (vri ), encoding a repressor of Clk transcription (Blau et al., 1999), and clockwork orange (cwo), encoding a transcriptional repressor that competes with the CLK-CYC complex for E-box binding (Kadener et al., 2007; Lim, Chung, et al., 2007; Matsumoto et al., 2007; Richier et al., 2008; Zhou et al., 2016).
Although the central oscillators running endogenous clocks have been thoroughly studied at the transcription level, the significance of their post-transcriptional control has begun to emerge (Brunner et al., 2006; Kojima et al., 2011; Lim et al., 2013). Nonsense-mediated mRNA decay (NMD) is a post-transcriptional regulatory mechanism via which mRNAs with premature translation termination codons (PTCs) are recognized and removed. The precise mechanisms that initiate the assembly of NMD effectors differ very little among species (Behm-Ansmant et al., 2007; Eulalio et al., 2007; Muhlemann, 2008; Popp et al, 2014). The conventional model involves an exon junction complex (EJC) that is removed by ribosomes in translation of normal transcript. However, PTCs generated from DNA defect, transcription errors, or alternative splicing, cause the ribosomes to stall upstream of EJC. The distance between the ribosomes and EJC triggers the recruitment of a NMD complex, which consists of a number of UP-FRAMESHIFT (UPF) and SUPPRESSOR WITH MORPHOGENETIC EFFECT ON GENITALIA (SMG) proteins. UPF1 is the core RNA helicase that composes the complex together with UPF2 and UPF3 and is phosphorylated by SMG1, a phosphoinositide-3-kinase-related protein kinase. Dephosphorylation of UPF1 is facilitated by SMG5/7 or SMG6 nucleases.
While it has been shown that NMD regulates 5–10% of the transcriptome (Chan et al., 2007; Mendell et al., 2004; Yepiskoposyan et al., 2011), only a few target genes implicated in circadian rhythms have been identified. In Arabidopsis, splice variants of the genes encoding timing of cab expression 1 and early flowering 3 are degraded via NMD (Kwon et al., 2014). Other clock-regulated glycine-rich RNA-binding proteins, GRP7 and CCR1, form a feedback loop in which unproductive splicing of each transcript is coupled to NMD (Schoning et al., 2007; 2008). Alternative splice variants of a Jumonji C domain-containing gene, JMJC5, in Medicago truncatula also show NMD sensitivity, though the strength of the sensitivity varies depending on the tissue and other exogenous conditions (Shen et al., 2016). Neurospora crassa has a shortened periodicity as a result of Upf1 mutation (Morgan et al., 1997), and NMD was recently found to regulate its major clock gene, frequency, for the transcriptional control of white collar genes (Aronson et al., 1994; Cheng et al., 2001; Merrow et al., 2001; Wu et al., 2017).
There is abundant experimental evidence implicating ATF/CREB (activating transcription factor/cAMP response element-binding protein) and its transcriptional coactivators in the generation of behavioral rhythms through cell-autonomous molecular clocks (Belvin et al., 1999; Ginty et al., 1993; Kim et al., 2016; Koyanagi et al., 2011; Lee et al., 2010; Lim et al., 2007; O’Neill et al., 2008; Obrietan et al., 1999; Scheving et al., 1998; Sun et al., 2015; Travnickova-Bendova et al., 2002). However, few studies have demonstrated the regulatory mechanism of ATF/CREB expression for clock function (Hendricks et al., 2001; Kako et al., 1996; Maurer et al., 2016; Williams et al., 2001). In this study, we provide strong genetic evidence that NMD acts in Drosophila circadian pacemaker neurons to sustain circadian locomotor behaviors and identify CrebB as a rate-limiting target of the clock-relevant NMD.
MATERIALS AND METHODS
Fly stocks
All flies were reared at 25°C and 60% humidity on a 12 h light/dark (LD) cycle and with standard cornmeal-yeast food. The wild-type controls were iso31 (Bloomington Drosophila Stock Center #5905). Smg5 RNAi (#31090, JF01554), Smg5 e04233 (#25167), PBac{IT.GAL4}Smg5 1409-G4 (#65708, Smg5-GAL4), Df(2L)BSC345 (#24369, Smg5 deficiency), Df(3R)BSC494 (#24998, Smg6 deficiency), UAS-Upf2.A (#60579), UAS-Upf2.FVRR (#60583), UAS-Upf2.E801R (#60581), UAS-CrebB-a (#9232), and UAS-CrebB-c (#7219) were all from the Bloomington Drosophila Stock Center. Smg6 RNAi#1 (6369R-1) and Smg6 RNAi#2 (6369R-3) were from Fly Stocks of National Institute of Genetics, Japan. Smg6 2a and UAS-nlsDsRed2:: SV40-3′UTR were kindly provided by Dr. Metzstein (Frizzell et al., 2012), and UAS-Upf1 D45B was provided by Dr. Singh, who received it from the original generator, Dr. Palumbo (Micale et al., 2009). Pdf-GeneSwitch-GAL4 was a gift from Dr. Ceriani (Depetris-Chauvin et al., 2011), and UAS-CrebB-b was from Dr. Davis (Yu et al., 2006). The UAS-Smg6 -V5 transgenic line was made by cloning the full cDNA of Smg6 at the EcoRI and XhoI sites of the 8.5 kb pUAST-AttB-V5 vector.
Fly behavioral assay
The food for behavioral testing was 5% sucrose (31365-1201; Junsei Chemical Co.) and 2% agar (24440-1201; Junsei Chemical Co.). Pdf-GeneSwitch was activated by feeding the flies food containing 62.5 μM RU486 (H110-01; Sigma-Aldrich) dissolved in ethanol. The vehicle control was ethanol. The circadian rhythms of the flies were analyzed with the Drosophila Activity Monitoring system (TriKinetics) using MATLAB-based ClockLab (Actimetrics). The rhythm peak was calculated from the first to the seventh dark/dark (DD) cycle with a confidence interval of 0.05. The power of rhythmicity was calculated by subtracting the value of “significance” from the value of “power.” Morning and evening anticipation indices were calculated using an Excel-based Counting Macro (Pfeiffenberger et al., 2010), as follows: morning index = total activity 3 h prior to lights on/total activity 6 h prior to lights on – 0.5; evening index = total activity 3 h prior to lights off/total activity 6 h prior to lights off – 0.5.
Immunofluorescence assay
Flies were entrained to the LD cycle for at least 72 h before dissection between Zeitgeber time 2 (ZT2) and ZT3 for PDF staining or DsRed detection. Briefly, a 30 min fixation in 3.7% formaldehyde and washes in phosphate-buffered saline with 0.5% Tween 20 were followed by two nights of primary antibody staining, as described previously (Lim et al., 2011; Park et al., 2014), with mouse anti-PDF (a-PDF C7-s; Developmental Studies Hybridoma Bank) at a dilution of 1:600. The secondary antibodies were either Alexa Fluor 488 (A21202; Invitrogen) or Alexa Fluor 594 (A21203; Invitrogen) donkey anti-mouse IgG. The samples were scanned at a pixel resolution of 1,024 × 1,024 with an LSM780 confocal microscope (Carl Zeiss) using LSM image browser ZEN software (Carl Zeiss).
RNA isolation and quantitative real-time PCR
Flies were entrained to a 12 h LD cycle for at least 72 h before they were harvested at 4 h intervals (at ZT3, ZT7, ZT11, ZT15, ZT19, and ZT23). The heads of 10–30 flies per genotype were fresh frozen at −80°C. RNA was extracted with TRIzol (15596018; Ambion), digested with RQ1 DNase to remove genomic DNA, and purified with phenol-chloroformisoamyl alcohol (25:24:1, M3803; Sigma-Aldrich). Purified RNA was reverse transcribed with M-MLV reverse transcriptase (M1705; Promega) and random primers (C1181; Promega). Quantitative PCR was performed with the synthesized cDNA using Prime Q-Mastermix (9200; Genetbio). Real-time PCR results were analyzed with the 2−ΔΔCT method (Livak et al., 2001). Relative mRNA levels were normalized to the peak value of the respective control and then calculated as a percentage of the peak value (set to 100%).
RESULTS
Smg5 depletion in circadian pacemaker neurons dampens circadian locomotor rhythm
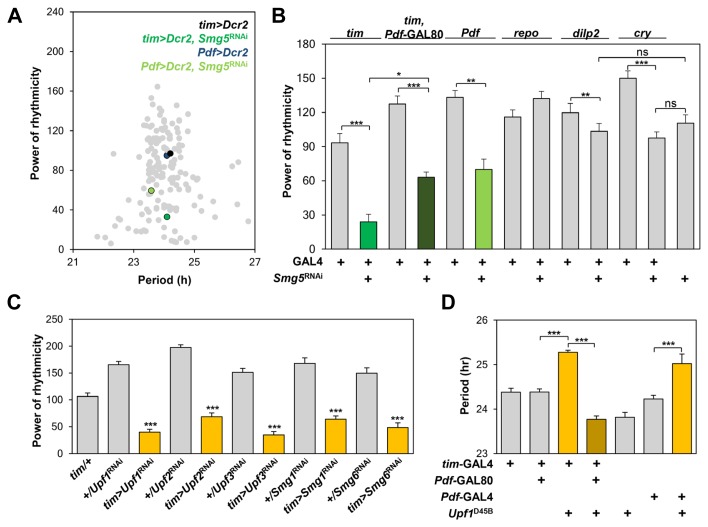
To identify post-transcriptional regulators with circadian clock function, we conducted a behavioral screen of transgenic flies harboring RNAi against genes encoding individual components of processing bodies (Decker et al., 2012; Zheng et al., 2011). We carried out a primary genetic screen using a tim-GAL4 line that drives the overexpression of each RNAi transgene in all clock-relevant cells expressing tim and depletes its target gene expression. Any phenotype in circadian behaviors was then confirmed using a Pdf-GAL4 driver that limits the RNAi expression to PDF-expressing neurons. PDF is a neuropeptide expressed in small and large ventral lateral neurons (s-LNvs and l-LNvs, respectively) of the adult fly brain and is crucial for synchronizing clock phases among different groups of circadian pacemaker neurons (Lin et al., 2004; Peng et al., 2003). After entrainment to LD cycles, wild-type flies display robust 24 h rhythms of their locomotor activity in free-running constant dark (DD) conditions. Abnormalities in circadian rhythms are thus observed as an altered periodicity or rhythm amplitude of DD behaviors compared with that of wild-type flies.
Of the approximately 40 post-transcriptional regulatory genes, the RNAi-mediated depletion of several affected circadian locomotor behaviors (Fig. 1A). In particular, depletion of a NMD-relevant gene, Smg5, in either tim-expressing cells or PDF-expressing neurons dampened circadian locomotor rhythms, whereas the inhibition of tim-GAL4 activity by PDF-specific GAL80 (Stoleru et al., 2004) partially suppressed the Smg5 RNAi phenotype (Fig. 1B, Supplementary Table S1). We confirmed that tim-GAL4-driven expression of Smg5 RNAi resulted in Smg5 mRNA levels in adult heads that were ~60% of wild-type levels (data not shown). By contrast, transgenic flies displayed normal rhythmicity when Smg5 was depleted in other brain regions by repo–GAL4 (glial cells), dilp2 (Drosophila insulin-like peptide 2 )-GAL4 (pars intercerebralis), or cry13-GAL4 (cry -expressing cells).
Fig. 1. Genetic suppression of NMD in circadian pacemaker neurons disrupts free-running locomotor rhythms.
(A) Results of a genetic screen of transgenic lines harboring RNAi against ~40 post-transcriptional regulators that constitute processing bodies. The RNAi transgenes were individually overexpressed along with the RNAi-enhancing Dicer-2 (Dcr2 ) in all tim-expressing clock cells (tim > Dcr2 ) or in PDF-expressing pacemaker neurons (Pdf > Dcr2 ). Circadian periodicity (x axis) and the power of rhythmicity (y axis) in free-running locomotor activity were calculated in individual flies and averaged for each RNAi line (gray dots). Smg5 RNAi flies and their controls are indicated by the colored dots. (B) Powers of rhythmicity seen in Smg5 -depleted flies by different GAL4 drivers (shown at top). *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA with Tukey’s post hoc test. Error bars indicate SEMs (n = 18–57). (C) Depletion of each NMD factor in tim-expressing clock cells significantly decreases behavioral rhythmicity. ***p < 0.001 versus heterozygous tim -GAL4 or RNAi controls by one-way ANOVA. Error bars indicate SEMs (n = 20–47). (D) Overexpression of the dominant-negative UPF1D45B transgene in PDF-expressing neurons was necessary and sufficient to lengthen the periodicity of circadian locomotor rhythms. Pdf-GAL80 represses the UPF1D45B overexpression in PDF-expressing neurons among other tim-expressing cells. ***p < 0.001 by one-way ANOVA, Tukey’s post hoc test. Error bars indicate SEMs (n = 19–57).
Genetic manipulation of the NMD pathway in PDF-expressing neurons alters behavioral rhythms
We next examined if other NMD components similarly influence circadian behaviors. Indeed, transgenic depletion of each NMD factor (e.g., Upf1, Upf2, Upf3, Smg1, or Smg6 ) in tim-expressing cells caused behavioral arrhythmicity comparable to that observed with Smg5 depletion (Fig. 1C, Supplementary Table S2). Furthermore, the individual overexpression of these NMD factors (e.g., Upf1, Upf2, Smg5, or Smg6 ) in tim-expressing cells disrupted free-running locomotor rhythms (Supplementary Table S3), indicating that a specific dosage of NMD activity is required for clock function. RNA helicase UPF1 is a key component of the NMD machinery, whereas UPF2 and UPF3 act as adaptors/recruiters of the complex. Transgenic overexpression of Upf2.E801R, which disrupts UPF2–UPF3 interaction but rescues lethality in the Upf2 mutant (Avery et al., 2011), induced arrhythmicity in circadian behaviors comparable to the overexpression of wild-type Upf2. By contrast, transgenic overexpression of Upf2.FVRR, which disrupts UPF1–UPF2 interaction and cannot rescue lethality in the Upf2 mutant, negligibly affected circadian behaviors. The UPF1–UPF2 interaction might thus be limiting for clock function in Drosophila, as it is important for NMD and adult fly viability. Additionally, we observed that the overexpression of UPF1D45B, a dominant-negative form of UPF1 (Micale et al., 2009), in PDF-expressing neurons was necessary and sufficient to lengthen the free-running periods in locomotor rhythms by >1 h (Fig. 1D, Supplementary Table S4). These results document the importance of the NMD pathway in PDF-expressing neurons for sustaining circadian behaviors.
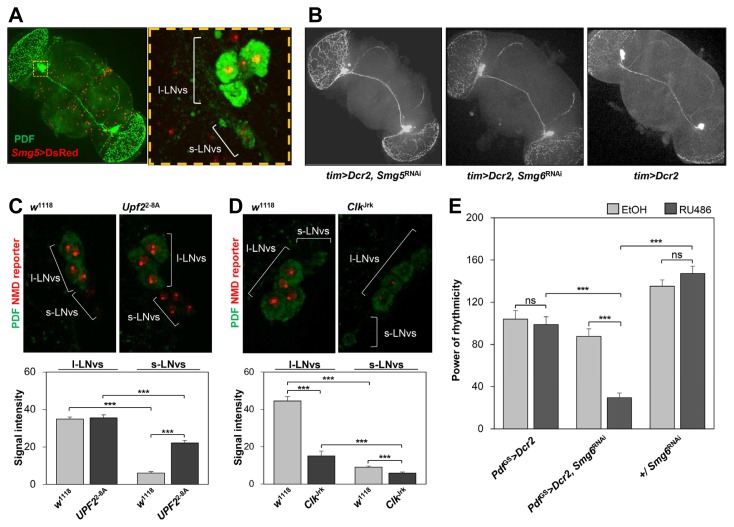
NMD activity in PDF-expressing neurons does not affect neural development but is elevated by Clock mutation
To validate that PDF-expressing neurons express NMD-relevant genes and display NMD activity, we performed imaging analyses using confocal microscopy. We first employed an enhancer-trapping line for the Smg5 locus (PBac{IT.GAL4}Smg5 1409-G4) and visualized its enhancer activity in the adult fly brain by driving the expression of a fluorescent reporter protein. In this way, we indirectly detected Smg5 expression in PDF-expressing s-LNvs and l-LNvs as well as in cells over a broad region of the fly brain (Fig. 2A). To further assess NMD activity in PDF-expressing neurons, we utilized a transgenic RNA reporter harboring the SV40 3′ untranslated region (UTR)(UAS-nlsDsRed ::SV40-3′UTR) (Metzstein et al., 2006). The SV40 3′ UTR is a known target of NMD activity; therefore, the relative expression levels of the DsRed reporter protein are inversely proportional to endogenous NMD activity (Metzstein et al., 2006). We established transgenic flies expressing the NMD reporter in PDF-expressing neurons and compared their levels of DsRed expression among different genetic backgrounds. As expected, confocal imaging revealed that DsRed signals were higher in weak hypomorphic mutants of Upf2 (Upf2 2-8A) than in the wild type, validating the functionality and sensitivity of the NMD reporter in PDF-expressing neurons (Fig. 2C). Importantly, we found that the NMD reporter protein was expressed at very low levels in both s-LNvs and l-LNvs of arrhythmic Clk Jrk mutants (Fig. 2D). These data suggest that NMD activity in PDF-expressing neurons is suppressed by wild-type Clk. Since the Clk Jrk allele generates Clk transcripts harboring NMD-inducible PTCs, we cannot rule out the other possibility that the Clk Jrk mutation potentiates the overall activity of the NMD pathway to promote the decay of its own transcripts.
Fig. 2. NMD activity in PDF-expressing neurons does not affect their neural development but is elevated by Clock mutation.
(A) Expression of DsRed by an enhancer-trapping transgene from an Smg5 locus in the brain of an adult fly (Smg5 > DsRed); both l- and s-LNvs were immunostained with an anti-PDF antibody (green). Yellow dashed box in the left panel indicates the region shown at higher magnification on the right. (B) Representative confocal images of whole-mount brains stained with the anti-PDF antibody. All posterior optic tracts from l-LNvs and dorsal projections from s-LNvs in transgenic flies depleted of Smg5 or Smg6 are intact compared with those in control flies (tim > Dcr2 ). (C, D) Representative images (top) and quantitation (bottom) showing that the Pdf > nlsDsRed ::SV40-3′UTR signal is increased in s-LNvs of Upf2 2-8A flies and decreased in both l- and s-LNvs of Clk Jrk flies compared with that in the wild type w1118. ***p < 0.001 by Student’s t tests. Error bars indicate SEMs (n > 16). (E) Conditional depletion of Smg6 from PDF-expressing neurons dampened DD locomotor rhythms. EtOH (ethanol) indicates a vehicle control for the oral administration of 62.5 μM RU486 during the behavioral tests. ***p < 0.001 by one-way ANOVA, Bonferroni’s post hoc test. Error bars indicate SEMs (n = 10–57).
Developmental defects in PDF-expressing neurons and their projections could be responsible for the abnormal circadian behaviors observed in some clock mutants. Although the Smg5 or Smg6 RNAi flies showed behavioral arrhythmicity, the cell bodies and axonal projections of their PDF-expressing neurons were intact (Fig. 2B). To further exclude developmental effects of NMD on circadian behaviors, we conditionally depleted Smg6 expression in PDF neurons by Pdf GeneSwitch-Gal4 (Depetris-Chauvin et al., 2011). In this experimental condition, Smg6 RNAi expression was suppressed during development but oral administration of RU486 to adult flies induced GeneSwitch-Gal4 activity and the RNAi expression. The adult-specific depletion of Smg6 was sufficient to dampen the rhythmicity in circadian locomotor behaviors, consistent with the behavioral phenotypes caused by constitutive depletion (Fig. 2E, Supplementary Table S5). Collectively, these data suggest that the effects of the NMD pathway are not developmental but they maintain behavioral circadian rhythms in adult flies.
Hypomorphic mutations in Smg5 or Smg6 weaken morning anticipation and free-running locomotor rhythms
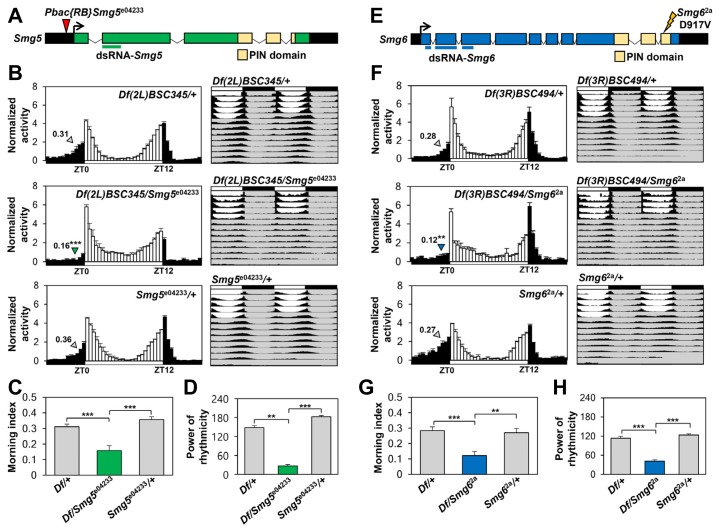
To elucidate the molecular mechanisms underlying NMD effects on circadian behaviors, we established mutant flies hypomorphic for either of the two NMD factors and analyzed their behavioral circadian phenotypes. The Smg5 e04233 mutant allele harbors a piggyBac insertion in the 5′ UTR of the Smg5 locus (Fig. 3A) and reduces Smg5 mRNA levels to 57% of the wild-type (data not shown). The Smg6 2a mutant allele possesses an ethyl methanesulfonate-induced missense mutation in the PilT N-terminal (PIN) domain (Fig. 3E) (Frizzell et al., 2012). This catalytic domain for nuclease activity is conserved between SMG5 and SMG6 proteins, implicating both factors in mRNA degradation at the last step of NMD pathway.
Fig. 3. Hypomorphic mutations in Smg5 or Smg6 dampen morning anticipation and free-running locomotor rhythms.
(A) Schematic diagram of the Smg5 locus. Green, yellow, and black boxes correspond to coding regions, the PIN domain, and untranslated regions of Smg5 transcript, respectively. The green line indicates the coding region targeted by Smg5 RNAi; the red arrowhead depicts the piggy-Bac insertion site in the 5′ UTR of the Pbac{RB}Smg5 e04233 allele. (B, F) Normalized activity profiles from three LD cycles were averaged per genotype. Averaged morning index values are shown above arrowheads. Error bars indicate SEMs (n = 51–159 for Smg5 mutants; n = 45–156 for Smg6 mutants). **p < 0.01, ***p < 0.001 compared with either heterozygous control by one-way ANOVA with Tukey’s post hoc test. White/black bars, LD cycles. Averaged actograms throughout the behavioral assessments were double plotted (n = 37–58 for Smg5 mutants; n = 17–50 for Smg6 mutants that remained alive after five LD and seven DD cycles). (C, G) Trans-heterozygous mutations in Smg5 or Smg6 decreased activity anticipatory to lights-on in LD cycles to lights-on in LD cycles. **p < 0.01, ***p < 0.001 compared with either heterozygous control by one-way ANOVA with Tukey’s post hoc test. Error bars indicate SEMs (n = 51–159 for Smg5 mutants; n = 45–156 for Smg6 mutants). (D, H) Trans-heterozygous mutations in Smg5 or Smg6 decreased the power of rhythmicity in DD locomotor behaviors. **p < 0.01, ***p < 0.001 by one-way ANOVA with Tukey’s post hoc test. Error bars indicate SEMs (n = 37–58 for Smg5 mutants; n = 17–50 for Smg6 mutants). (E) Schematic diagram of the Smg6 locus. Blue, yellow, and black boxes correspond to coding regions, the PIN domain with nuclease activity, and untranslated regions of Smg6 transcript, respectively. Blue lines indicate coding regions targeted by Smg6 RNAi. Smg6 2a allele has an aspartate-to-valine missense mutation in the indicated residue of the PIN domain.
During the LD cycle, wild-type flies gradually increase their locomotor activity prior to the light transitions as a part of clock-dependent anticipatory activities to lights-on (i.e., morning anticipation) or lights-off (i.e., evening anticipation). By contrast, Smg5 mutants trans-heterozygous for Smg5 e04233 over the chromosomal deficiency of the entire Smg5 locus (i.e., Df(2L)BSC345 ) showed significantly weaker morning anticipation than either of the heterozygous controls (Figs. 3B and 3C). Smg5 mutants also exhibited less robust DD locomotor rhythms than their heterozygous controls (Figs. 3B and 3D, Supplementary Table S6). Dampened morning anticipation in LD cycles and low-amplitude rhythms in DD locomotor behaviors were similarly observed in mutants trans-heterozygous for Smg6 2a and chromosomal deficiency of the Smg6 locus (i.e., Df(3R)BSC494 ) (Figs. 3F–3H, Supplementary Table S7). These behavioral phenotypes in NMD mutants are thus consistent with their RNAi phenotypes.
Daily oscillations in levels of clock-relevant mRNAs are comparable between Smg6 mutants and heterozygous controls
PDF-expressing s-LNvs are a well-established neural locus important for morning anticipation and DD locomotor rhythms (Grima et al., 2004; Stoleru et al., 2004). However, we did not detect any gross defects in the cell bodies or axonal projections of these neurons in either Smg5 or Smg6 mutants (Fig. 4A). We reasoned that aberrant NMD of a specific mRNA substrate, but not neuronal defects, might underlie the observed circadian phenotypes. Such targets are expected to have elevated mRNA levels in NMD mutants due to the inefficient degradation of their transcripts, as has been observed for other NMD targets (Chapin et al., 2014; Johansson et al., 2007). Since it is technically difficult to quantify transcript levels in PDF-expressing neurons, we examined the mRNA levels of clock-relevant genes in Smg6 mutant heads. This analysis proved no significant differences between Smg6 mutants and heterozygous controls in the circadian expression of core clock transcripts (e.g., per, tim, vri, and Pdp1)(Fig. 4B). Smg6 mutation rather reduced Clk or cry expression only at a specific time of the day in LD cycles.
Fig. 4. PDF-expressing neuron development and daily oscillations in clock-relevant mRNA levels are comparable between Smg6 mutants and heterozygous control.
(A) Representative confocal images of whole-mount brains stained with anti-PDF antibody. All posterior optic tracts from l-LNvs and dorsal projections from s-LNvs appear intact. (B) Quantitative analyses of daily rhythmic expression of clock-relevant transcripts in Smg6 mutants. Adult flies were collected at the indicated time points in LD cycles (lights on at Zeitgeber time zero [ZT0]; lights off at ZT12). Total RNAs were purified from head extracts, and the level of each clock-relevant transcript relative to that of cyc was quantified by real-time PCR. The y axis indicates the percent expression relative to the peak value in the heterozygous control (set as 100%). Data represent averages ± SEMs (n = 3). *p < 0.05 by Student’s t test. White/black bars, LD cycles.
CrebB is a rate-limiting target of NMD to support robust behavioral rhythms
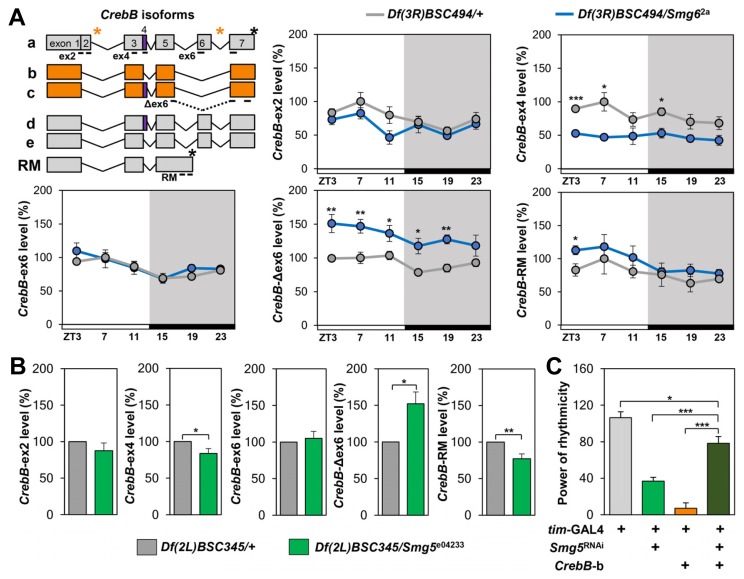
Mammalian ATF/CREB family genes are known targets of NMD (Hatano et al., 2013; Mendell et al., 2004). Moreover, a number of studies link ATF/CREB-dependent transcription with circadian clocks (Belvin et al., 1999; Ginty et al., 1993; Kim et al., 2016; Koyanagi et al., 2011; Lee et al., 2010; Lim et al., 2007; O’Neill et al., 2008; Obrietan et al., 1999; Scheving et al., 1998; Sun et al., 2015; Travnickova-Bendova et al., 2002). Accordingly, we examined the expression of the Drosophila ATF/CREB homolog, CrebB, in Smg6 mutants. The CrebB locus encodes several isoforms that are generated by alternative splicing and differentially include PTCs (Fig. 5A). A quantitative exon-specific analysis of these CrebB isoforms revealed that the levels of CrebB transcripts lacking exon 6 (CrebB-Δex6) were higher in Smg6 mutant heads than in those of the heterozygous controls. By contrast, the expression of CrebB transcripts containing exon 4 (CrebB-ex4) was lower in Smg6 mutants. The relative abundance of CrebB-Δex6 and CrebB-ex4 was similarly affected by Smg5 mutation (Fig. 5B), suggesting that specific isoforms of CrebB are bona fide targets of Drosophila NMD. In addition, modest but opposing effects of Smg5 and Smg6 on CrebB-RM levels may indicate their non-redundant roles in the post-transcriptional expression of this specific CrebB isoform.
Fig. 5. CrebB is a rate-limiting target of NMD to support robust behavioral rhythms.
(A) Schematic diagram of CrebB isoforms. Black bars indicate approximate annealing regions of exon-specific primers. Transcripts including either exon 4 or exon 6 were detected using the ex4 or ex6 primer sets, respectively. Transcripts in orange were detected using the Δex6 primer set and designated collectively as CrebB-Δex6. Exon 2 has not been annotated by FlyBase, but we designed the ex2 primer set based on the previously predicted sequence (Yin et al., 1995b). Black asterisks indicate authentic stop codons in CrebB transcripts; orange asterisks mark potential PTCs. Quantitative transcript analyses of CrebB isoforms in Smg6 mutants [Df(3R)BSC494/Smg6 2a, blue lines] and the heterozygous controls [Df(3R)BSC494/+, gray lines] were performed as described for Fig. 4B. Data represent averages ± SEMs (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t tests. White/black bars, LD cycles. (B) Quantitative transcript analyses of CrebB isoforms in Smg5 mutants [Df(2L)BSC345/Smg5 e04233, green bars] and heterozygous control [Df(2L)BSC345/+, gray bars]. Data represent averages ± SEMs (n = 3–6). *p < 0.05, **p < 0.01 by Student’s t tests. (C) Overexpression of CrebB-b isoform rescues disrupted locomotor rhythms in Smg5-depleted flies. Power of rhythmicity in DD locomotor activity was significantly different in flies with Smg5 depletion and CrebB-b overexpression (p < 0.001, by two-way ANOVA). *p < 0.05, ***p < 0.001 by Sidak’s multiple comparison test. Error bars indicate SEMs (n = 30–65).
To functionally validate that NMD-dependent regulation of CrebB is responsible for sustaining circadian behaviors, we transgenically overexpressed the CrebB-b isoform (Yu et al., 2006) in Smg5 RNAi or control flies and examined the effect on DD locomotor rhythms. Overexpression of the CrebB-b isoform rescued free-running locomotor rhythms in Smg5-depleted flies while it strongly dampened circadian behaviors in control flies (Fig. 5C, Supplementary Table S8). These results suggest that CrebB is rate-limiting for the NMD pathway to sustain high-amplitude behavioral rhythms.
DISCUSSION
NMD is one of the major RNA quality control mechanisms for degrading nonfunctional transcripts with PTCs and ensuring the translation of error-free mRNAs. In addition, NMD monitors the stability of normal mRNAs by targeting their selective cis-elements, such as upstream open-reading frames, long 3′ UTRs, introns in 3′ UTRs, and frame-shifting signals (Alonso, 2005; Chang et al., 2007; Hug et al., 2016; Nickless et al., 2017). It has been previously shown that NMD contributes to the circadian control of gene expression, though only a few clock genes have been identified as direct targets (Filichkin et al., 2014; Hatano et al., 2013; Kwon et al., 2014; Mendell et al., 2004; Shaul, 2015; Wu et al., 2017). In this report, we show that NMD is necessary to sustain circadian behaviors in Drosophila and localize this control to PDF-expressing pacemaker neurons. We further provide molecular and genetic evidence that NMD targets specific isoforms of CrebB to sustain circadian locomotor rhythms.
Daily rhythmic oscillations in cAMP levels are observed in a diversity of species (Eckel-Mahan et al., 2008; Fukuhara et al., 2004; O’Neill et al., 2008; Palacios-Munoz et al., 2018) and linked to the circadian clocks in part via the ATF/CREB family of transcriptional regulators (Ginty et al., 1993; Koyanagi et al., 2011; Obrietan et al., 1999; Scheving et al., 1998). In mammals, these transcription factors promote per transcription (Shimizu et al., 2007; Travnickova-Bendova et al., 2002), and the CLOCK-ARNTL (BMAL) heterodimer in mice negatively regulates ATF5 expression (Lemos et al., 2007). In Drosophila, CRE-dependent transcription exhibits both ultradian and circadian rhythms while these rhythms are disrupted by CrebB mutation (Belvin et al., 1999). As expected, transcriptional coactivators of the ATF/CREB family members, such as CREB-binding protein or CREB-regulated transcriptional coactivator support circadian rhythms in Drosophila and mammals via either CLK-CYC- or CREB-dependent transcription of circadian clock genes (Hosoda et al., 2009; Hung et al., 2007; Kim et al., 2016; Lee et al., 2010; Lim et al., 2007; Sakamoto et al., 2013).
Our genetic analyses revealed that the loss of NMD function potently dampened circadian locomotor rhythms without impairing the circadian expression of clock genes. Thus, the NMD-dependent clock function maps to the clock output pathway, which relays the timing information from molecular clocks (i.e., daily rhythmic gene expression) to behavioral rhythms. Given the isoform-specific effects of NMD on CrebB expression, we reason that NMD tunes the balance of CrebB isoforms with transcriptional activator or repressor function rather than silencing the overall activity of CrebB. There are two potential PTCs in CrebB transcripts: one in the intron after exon 2 and the other after exon 6. CrebB transcripts including exons 4 and 6 encode a transcriptional activator if their translation starts from the second start codon after the PTC following exon 2 (Fropf et al., 2013; Tubon et al., 2013; Yin et al., 1995a; Yin et al., 1995b). On the other hand, CrebB transcripts without exons 2, 4, or 6, such as CrebB-b, encode a transcriptional repressor (Davis et al., 1996; Fropf et al., 2013; Yin et al., 1994; Yin et al., 1995b) while the transcriptional activities of other splice variants have not been characterized (Fropf et al., 2013; Perazzona et al., 2004). Since our transcript analysis in LD cycles did not show any strong time-of-the-day-effects of Smg6 on the relative levels of CrebB-Δex6 and CrebB-ex4, it is less likely that NMD controls CrebB expression in a light- or circadian clock-dependent manner. As reported in a number of species (Barberan-Soler et al., 2008; Lewis et al., 2003; Tabrez et al., 2017; Traunmuller et al., 2014), we speculate that NMD is constitutively coupled to the alternative splicing of CrebB for the differential expression of CrebB isoforms, thereby sustaining robust behavioral rhythms in constant dark.
The transcriptional activity of two mammalian ATF/CREB homologs (i.e., ATF4 and ATF5) is implicated in circadian gene expression (Koyanagi et al., 2011; Lemos et al., 2007), and their transcript stability is regulated by NMD (Hatano et al., 2013; Mendell et al., 2004). Accordingly, we propose that NMD-dependent regulation of ATF/CREB expression is evolutionarily conserved among diverse species and represents an ancestral clock mechanism that sustains circadian rhythms. Future studies will elucidate the isoform-specific regulation of CrebB by NMD in Drosophila and whether there is feedback from the circadian clocks to control NMD activity.
Supplementary Information
ACKNOWLEDGMENTS
We thank Bloomington Drosophila Stock Center, Kyoto Stock Center, Vienna Drosophila Resource Center, Dr. Mark Metzstein, Dr. Ronald Davis, Dr. Anand Singh, and Dr. Maria Fernanda Ceriani for providing the fly stocks and Dr. Young-Joon Kim for generating the UAS-Smg6 transformants. This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (no. 2012H1A2A1016929 to H.R., no. 2016R1A6A3A1193 2215 to J.L., no. 2018R1A5A1024261 to C.L., and no. 2016R1A2B4011111 to J.C.).
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Agostino P.V., Golombek D.A., Meck W.H. Unwinding the molecular basis of interval and circadian timing. Front Integr Neurosci. 2011;5:64. doi: 10.3389/fnint.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R., White N.E., So W.V., Hall J.C., Rosbash M. A mutant Drosophila homolog of mammalian clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. doi: 10.1016/S0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- Alonso C.R. Nonsense-mediated RNA decay: a molecular system micromanaging individual gene activities and suppressing genomic noise. Bioessays. 2005;27:463–466. doi: 10.1002/bies.20227. [DOI] [PubMed] [Google Scholar]
- Aronson B.D., Johnson K.A., Loros J.J., Dunlap J.C. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- Avery P., Vicente-Crespo M., Francis D., Nashchekina O., Alonso C.R., Palacios I.M. Drosophila Upf1 and Upf2 loss of function inhibits cell growth and causes animal death in a Upf3-independent manner. RNA. 2011;17:624–638. doi: 10.1261/rna.2404211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberan-Soler S., Zahler A.M. Alternative splicing regulation during C. elegans development: splicing factors as regulated targets. PLoS Genet. 2008;4:e1000001. doi: 10.1371/journal.pgen.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith E.J., Ceriani M.F. Experimental assessment of the network properties of the Drosophila circadian clock. J Comp Neurol. 2015;523:982–996. doi: 10.1002/cne.23728. [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant I., Kashima I., Rehwinkel J., Sauliere J., Wittkopp N., Izaurralde E. mRNA quality control: an ancient machinery recognizes and degrades mRNAs with nonsense codons. FEBS Lett. 2007;581:2845–2853. doi: 10.1016/j.febslet.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Belvin M.P., Zhou H., Yin J.C. The Drosophila dCREB2 gene affects the circadian clock. Neuron. 1999;22:777–787. doi: 10.1016/S0896-6273(00)80736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau J., Young M.W. Cycling vrille expression is required for a functional Drosophila clock. Cell. 1999;99:661–671. doi: 10.1016/S0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
- Brunner M., Schafmeier T. Transcriptional and posttranscriptional regulation of the circadian clock of cyanobacteria and Neurospora. Genes Dev. 2006;20:1061–1074. doi: 10.1101/gad.1410406. [DOI] [PubMed] [Google Scholar]
- Chan W.K., Huang L., Gudikote J.P., Chang Y.F., Imam J.S., MacLean J.A., 2nd, Wilkinson M.F. An alternative branch of the nonsense-mediated decay pathway. Embo J. 2007;26:1820–1830. doi: 10.1038/sj.emboj.7601628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.F., Imam J.S., Wilkinson M.F. The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem. 2007;76:51–74. doi: 10.1146/annurev.biochem.76.050106.093909. [DOI] [PubMed] [Google Scholar]
- Chapin A., Hu H., Rynearson S.G., Hollien J., Yandell M., Metzstein M.M. In vivo determination of direct targets of the nonsense-mediated decay pathway in Drosophila. G3 (Bethesda) 2014;4:485–496. doi: 10.1534/g3.113.009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P., Yang Y.H., Liu Y. Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc Natl Acad Sci USA. 2001;98:7408–7413. doi: 10.1073/pnas.121170298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyran S.A., Buchsbaum A.M., Reddy K.L., Lin M.C., Glossop N.R., Hardin P.E., Young M.W., Storti R.V., Blau J. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112:329–341. doi: 10.1016/S0092-8674(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Davis G.W., Schuster C.M., Goodman C.S. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron. 1996;17:669–679. doi: 10.1016/S0896-6273(00)80199-3. [DOI] [PubMed] [Google Scholar]
- Decker C.J., Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol. 2012;4:a012286. doi: 10.1101/cshperspect.a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depetris-Chauvin A., Berni J., Aranovich E.J., Muraro N.I., Beckwith E.J., Ceriani M.F. Adult-specific electrical silencing of pacemaker neurons uncouples molecular clock from circadian outputs. Curr Biol. 2011;21:1783–1793. doi: 10.1016/j.cub.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan K.L., Phan T., Han S., Wang H., Chan G.C., Scheiner Z.S., Storm D.R. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., So W.V., Kaneko M., Hall J.C., Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/S0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Filichkin S.A., Cumbie J.S., Dharmawadhana J.P., Jaiswal P., Chang J.H., Palusa S.G., Reddy A.S., Megraw M., Mockler T.C. Environmental stresses modulate abundance and timing of alternatively spliced circadian transcripts in Arabidopsis. Mol Plant. 2015;8:207–227. doi: 10.1016/j.molp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Frizzell K.A., Rynearson S.G., Metzstein M.M. Drosophila mutants show NMD pathway activity is reduced, but not eliminated, in the absence of Smg6. RNA. 2012;18:1475–1486. doi: 10.1261/rna.032821.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fropf R., Tubon T.C., Jr, Yin J.C. Nuclear gating of a Drosophila dCREB2 activator is involved in memory formation. Neurobiol Learn Mem. 2013;106:258–267. doi: 10.1016/j.nlm.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara C., Liu C., Ivanova T.N., Chan G.C., Storm D.R., Iuvone P.M., Tosini G. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neurosci. 2004;24:1803–1811. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty D.D., Kornhauser J.M., Thompson M.A., Bading H., Mayo K.E., Takahashi J.S., Greenberg M.E. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Grima B., Chelot E., Xia R.H., Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Hatano M., Umemura M., Kimura N., Yamazaki T., Takeda H., Nakano H., Takahashi S., Takahashi Y. The 5′-untranslated region regulates ATF5 mRNA stability via nonsense-mediated mRNA decay in response to environmental stress. FEBS J. 2013;280:4693–4707. doi: 10.1111/febs.12440. [DOI] [PubMed] [Google Scholar]
- Hendricks J.C., Williams J.A., Panckeri K., Kirk D., Tello M., Yin J.C., Sehgal A. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat Neurosci. 2001;4:1108–1115. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- Hosoda H., Kato K., Asano H., Ito M., Kato H., Iwamoto Suzuki A., Masushige S., Kida S. CBP/p300 is a cell type-specific modulator of CLOCK/BMAL1-mediated transcription. Mol Brain. 2009;2:34. doi: 10.1186/1756-6606-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug N., Longman D., Caceres J.F. Mechanism and regulation of the nonsense-mediated decay pathway. Nucleic Acids Res. 2016;44:1483–1495. doi: 10.1093/nar/gkw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung H.C., Maurer C., Kay S.A., Weber F. Circadian transcription depends on limiting amounts of the transcription coactivator nejire/CBP. J Biol Chem. 2007;282:31349–31357. doi: 10.1074/jbc.M702319200. [DOI] [PubMed] [Google Scholar]
- Johansson M.J., He F., Spatrick P., Li C., Jacobson A. Association of yeast Upf1p with direct substrates of the NMD pathway. Proc Natl Acad Sci USA. 2007;104:20872–20877. doi: 10.1073/pnas.0709257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadener S., Stoleru D., McDonald M., Nawathean P., Rosbash M. Clockwork orange is a transcriptional repressor and a new Drosophila circadian pacemaker component. Genes Dev. 2007;21:1675–1686. doi: 10.1101/gad.1552607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kako K., Wakamatsu H., Ishida N. c-fos CRE-binding activity of CREB/ATF family in the SCN is regulated by light but not a circadian clock. Neurosci Lett. 1996;216:159–162. doi: 10.1016/0304-3940(96)13018-4. [DOI] [PubMed] [Google Scholar]
- Kim M., Lee H., Hur J.H., Choe J., Lim C. CRTC Potentiates light-independent timeless transcription to sustain circadian rhythms in Drosophila. Sci Rep. 2016;6:32113. doi: 10.1038/srep32113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S., Shingle D.L., Green C.B. Post-transcriptional control of circadian rhythms. J Cell Sci. 2011;124:311–320. doi: 10.1242/jcs.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka R.J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi S., Hamdan A.M., Horiguchi M., Kusunose N., Okamoto A., Matsunaga N., Ohdo S. cAMP-response element (CRE)-mediated transcription by activating transcription factor-4 (ATF4) is essential for circadian expression of the Period2 gene. J Biol Chem. 2011;286:32416–32423. doi: 10.1074/jbc.M111.258970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y.J., Park M.J., Kim S.G., Baldwin I.T., Park C.M. Alternative splicing and nonsense-mediated decay of circadian clock genes under environmental stress conditions in Arabidopsis. BMC Plant Biol. 2014;14:136. doi: 10.1186/1471-2229-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Li A., Hansen K.F., Cao R., Yoon J.H., Obrietan K. CREB influences timing and entrainment of the SCN circadian clock. J Biol Rhythms. 2010;25:410–420. doi: 10.1177/0748730410381229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lee J., Kwon I., Nakajima Y., Ohmiya Y., Son G.H., Lee K.H., Kim K. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J Cell Sci. 2010;123:3547–3557. doi: 10.1242/jcs.070300. [DOI] [PubMed] [Google Scholar]
- Lemos D.R., Goodspeed L., Tonelli L., Antoch M.P., Ojeda S.R., Urbanski H.F. Evidence for circadian regulation of activating transcription factor 5 but not tyrosine hydroxylase by the chromaffin cell clock. Endocrinology. 2007;148:5811–5821. doi: 10.1210/en.2007-0610. [DOI] [PubMed] [Google Scholar]
- Lewis B.P., Green R.E., Brenner S.E. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc Natl Acad Sci USA. 2003;100:189–192. doi: 10.1073/pnas.0136770100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Allada R. Emerging roles for post-transcriptional regulation in circadian clocks. Nat Neurosci. 2013;16:1544–1550. doi: 10.1038/nn.3543. [DOI] [PubMed] [Google Scholar]
- Lim C., Chung B.Y., Pitman J.L., McGill J.J., Pradhan S., Lee J., Allada R. Clockwork orange encodes a transcriptional repressor important for circadian-clock amplitude in Drosophila. Curr Biol. 2007;17:1082–1089. doi: 10.1016/j.cub.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Lee J., Choi C., Kilman V.L., Kim J., Park S.M., Jang S.K., Allada R., Choe J. The novel gene twenty-four defines a critical translational step in the Drosophila clock. Nature. 2011;470:399–403. doi: 10.1038/nature09728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Lee J., Choi C., Kim J., Doh E., Choe J. Functional role of CREB-binding protein in the circadian clock system of Drosophila melanogaster. Mol Cell Biol. 2007;27:4876–4890. doi: 10.1128/MCB.02155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Stormo G.D., Taghert P.H. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Ukai-Tadenuma M., Yamada R.G., Houl J., Uno K.D., Kasukawa T., Dauwalder B., Itoh T.Q., Takahashi K., Ueda R., et al. A functional genomics strategy reveals clockwork orange as a transcriptional regulator in the Drosophila circadian clock. Genes Dev. 2007;21:1687–1700. doi: 10.1101/gad.1552207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer C., Winter T., Chen S.W., Hung H.C., Weber F. The CREB-binding protein affects the circadian regulation of behaviour. FEBS Lett. 2016;590:3213–3220. doi: 10.1002/1873-3468.12336. [DOI] [PubMed] [Google Scholar]
- Mendell J.T., Sharifi N.A., Meyers J.L., Martinez-Murillo F.M., Dietz H.C. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- Merrow M., Franchi L., Dragovic Z., Gorl M., Johnson J., Brunner M., Macino G., Roenneberg T. Circadian regulation of the light input pathway in Neurospora crassa. Embo J. 2001;20:307–315. doi: 10.1093/emboj/20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzstein M.M., Krasnow M.A. Functions of the nonsense-mediated mRNA decay pathway in Drosophila development. PLoS Genet. 2006;2:e180. doi: 10.1371/journal.pgen.0020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micale L., Muscarella L.A., Marzulli M., Augello B., Tritto P., D’Agruma L., Zelante L., Palumbo G., Merla G. VHL frameshift mutation as target of nonsense-mediated mRNA decay in Drosophila melanogaster and human HEK293 cell line. J Biomed Biotechnol. 2009;2009 doi: 10.1155/2009/860761. 860761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L.W., Feldman J.F. Isolation and characterization of a temperature-sensitive circadian clock mutant of Neurospora crassa. Genetics. 1997;146:525–530. doi: 10.1093/genetics/146.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlemann O. Recognition of nonsense mRNA: towards a unified model. Biochem Soc Trans. 2008;36:497–501. doi: 10.1042/BST0360497. [DOI] [PubMed] [Google Scholar]
- Nickless A., Bailis J.M., You Z. Control of gene expression through the nonsense-mediated RNA decay pathway. Cell Biosci. 2017;7:26. doi: 10.1186/s13578-017-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J.S., Maywood E.S., Chesham J.E., Takahashi J.S., Hastings M.H. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K., Impey S., Smith D., Athos J., Storm D.R. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem. 1999;274:17748–17756. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- Palacios-Munoz A., Ewer J. Calcium and cAMP directly modulate the speed of the Drosophila circadian clock. PLoS Genet. 2018;14:e1007433. doi: 10.1371/journal.pgen.1007433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Sonn J.Y., Oh Y., Lim C., Choe J. SIFamide and SIFamide receptor defines a novel neuropeptide signaling to promote sleep in Drosophila. Mol Cells. 2014;37:295–301. doi: 10.14348/molcells.2014.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Stoleru D., Levine J.D., Hall J.C., Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazzona B., Isabel G., Preat T., Davis R.L. The role of cAMP response element-binding protein in Drosophila long-term memory. J Neurosci. 2004;24:8823–8828. doi: 10.1523/JNEUROSCI.4542-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel N., Helfrich-Forster C. Setting the clock-by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 2011;585:1435–1442. doi: 10.1016/j.febslet.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C., Lear B.C., Keegan K.P., Allada R. Processing circadian data collected from the Drosophila activity monitoring (DAM) system. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5519. pdb prot5519. [DOI] [PubMed] [Google Scholar]
- Price J.L., Dembinska M.E., Young M.W., Rosbash M. Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless. Embo J. 1995;14:4044–4049. doi: 10.1002/j.1460-2075.1995.tb00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp M.W., Maquat L.E. The dharma of nonsense-mediated mRNA decay in mammalian cells. Mol Cells. 2014;37:1–8. doi: 10.14348/molcells.2014.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richier B., Michard-Vanhee C., Lamouroux A., Papin C., Rouyer F. The clockwork orange Drosophila protein functions as both an activator and a repressor of clock gene expression. J Biol Rhythms. 2008;23:103–116. doi: 10.1177/0748730407313817. [DOI] [PubMed] [Google Scholar]
- Rutila J.E., Suri V., Le M., So W.V., Rosbash M., Hall J.C. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell. 1998;93:805–814. doi: 10.1016/S0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Norona F.E., Alzate-Correa D., Scarberry D., Hoyt K.R., Obrietan K. Clock and light regulation of the CREB coactivator CRTC1 in the suprachiasmatic circadian clock. J Neurosci. 2013;33:9021–9027. doi: 10.1523/JNEUROSCI.4202-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheving L.A., Gardner W. Circadian regulation of CREB transcription factor in mouse esophagus. Am J Physiol. 1998;274:C1011–1016. doi: 10.1152/ajpcell.1998.274.4.C1011. [DOI] [PubMed] [Google Scholar]
- Schoning J.C., Streitner C., Meyer I.M., Gao Y., Staiger D. Reciprocal regulation of glycine-rich RNA-binding proteins via an interlocked feedback loop coupling alternative splicing to nonsense-mediated decay in Arabidopsis. Nucleic Acids Res. 2008;36:6977–6987. doi: 10.1093/nar/gkn847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoning J.C., Streitner C., Page D.R., Hennig S., Uchida K., Wolf E., Furuya M., Staiger D. Auto-regulation of the circadian slave oscillator component AtGRP7 and regulation of its targets is impaired by a single RNA recognition motif point mutation. Plant J. 2007;52(6):1119–1130. doi: 10.1111/j.1365-313X.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- Sehgal A., Price J.L., Man B., Young M.W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994;263:1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- Shaul O. Unique aspects of plant nonsense-mediated mRNA decay. Trends Plant Sci. 2015;20:767–779. doi: 10.1016/j.tplants.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Shen Y., Wu X., Liu D., Song S., Liu D., Wang H. Cold-dependent alternative splicing of a Jumonji C domain-containing gene MtJMJC5 in Medicago truncatula. Biochem Biophys Res Commun. 2016;474:271–276. doi: 10.1016/j.bbrc.2016.04.062. [DOI] [PubMed] [Google Scholar]
- Shimizu F., Fukada Y. Circadian phosphorylation of ATF-2, a potential activator of Period2 gene transcription in the chick pineal gland. J Neurochem. 2007;103:1834–1842. doi: 10.1111/j.1471-4159.2007.04900.x. [DOI] [PubMed] [Google Scholar]
- Stanewsky R., Kaneko M., Emery P., Beretta B., Wager-Smith K., Kay S.A., Rosbash M., Hall J.C. The cry(b) mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/S0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Stoleru D., Peng Y., Agosto J., Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Sun X., Dang F., Zhang D., Yuan Y., Zhang C., Wu Y., Wang Y., Liu Y. Glucagon-CREB/CRTC2 signaling cascade regulates hepatic BMAL1 protein. J Biol Chem. 2015;290:2189–2197. doi: 10.1074/jbc.M114.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri V., Lanjuin A., Rosbash M. TIMELESS-dependent positive and negative autoregulation in the Drosophila circadian clock. Embo J. 1999;18:675–686. doi: 10.1093/emboj/18.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrez S.S., Sharma R.D., Jain V., Siddiqui A.A., Mukhopadhyay A. Differential alternative splicing coupled to nonsense-mediated decay of mRNA ensures dietary restriction-induced longevity. Nat Commun. 2017;8:306. doi: 10.1038/s41467-017-00370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traunmuller L., Bornmann C., Scheiffele P. Alternative splicing coupled nonsense-mediated decay generates neuronal cell type-specific expression of SLM proteins. J Neurosci. 2014;34:16755–16761. doi: 10.1523/JNEUROSCI.3395-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travnickova-Bendova Z., Cermakian N., Reppert S.M., Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci USA. 2002;99:7728–7733. doi: 10.1073/pnas.102075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubon T.C., Jr, Zhang J., Friedman E.L., Jin H., Gonzales E.D., Zhou H., Drier D., Gerstner J.R., Paulson E.A., Fropf R., et al. dCREB2-mediated enhancement of memory formation. J Neurosci. 2013;33:7475–7487. doi: 10.1523/JNEUROSCI.4387-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall L.B., Price J.L., Sehgal A., Saez L., Young M.W. Block in nuclear localization of period protein by a second clock mutation, timeless. Science. 1994;263:1606–1609. doi: 10.1126/science.8128247. [DOI] [PubMed] [Google Scholar]
- Williams J.A., Su H.S., Bernards A., Field J., Sehgal A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science. 2001;293:2251–2256. doi: 10.1126/science.1063097. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang Y., Sun Y., Yu J., Wang P., Ma H., Chen S., Ma L., Zhang D., He Q., et al. Up-frameshift protein UPF1 regulates neurospora crassa circadian and diurnal growth rhythms. Genetics. 2017;206:1881–1893. doi: 10.1534/genetics.117.202788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepiskoposyan H., Aeschimann F., Nilsson D., Okoniewski M., Muhlemann O. Autoregulation of the nonsense-mediated mRNA decay pathway in human cells. RNA. 2011;17:2108–2118. doi: 10.1261/rna.030247.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J.C., Del Vecchio M., Zhou H., Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Yin J.C., Wallach J.S., Del Vecchio M., Wilder E.L., Zhou H., Quinn W.G., Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Yin J.C., Wallach J.S., Wilder E.L., Klingensmith J., Dang D., Perrimon N., Zhou H., Tully T., Quinn W.G. A Drosophila CREB/CREM homolog encodes multiple isoforms, including a cyclic AMP-dependent protein kinase-responsive transcriptional activator and antagonist. Mol Cell Biol. 1995;15:5123–5130. doi: 10.1128/MCB.15.9.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Akalal D.B., Davis R.L. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D., Chen C.Y., Shyu A.B. Unraveling regulation and new components of human P-bodies through a protein interaction framework and experimental validation. RNA. 2011;17:1619–1634. doi: 10.1261/rna.2789611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yu W., Hardin P.E. Clockwork orange enhances period mediated rhythms in transcriptional repression by antagonizing E-box binding by clock-cycle. PLoS Genet. 2016;12:e1006430. doi: 10.1371/journal.pgen.1006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.