Abstract
Objective:
Tendinopathy is a major clinical problem in sports medicine and is often difficult to treat. Traditional therapeutic approaches have focused on reducing inflammation, yet research suggests that little to no inflammation is present in the tendons that fail to heal. The purpose of this review was to evaluate the effectiveness of the available treatment options for tendinopathy and to inform best clinical practices.
Design:
A narrative review.
Methods:
A comprehensive search of electronic databases (PubMed, Google Scholar and Web of Science) was conducted to identify relevant studies through June 2016. Studies were deemed relevant if they were published in English and contained original research on the management of tendinopathy in humans.
Results:
Studies varied in methodological quality and were often limited by small sample size and lack of sufficient control groups. Critical evaluation of the literature suggests that physical therapy with or without eccentric exercise should be considered a first-line treatment. Corticosteroids and nonsteroidal anti-inflammatory drugs provide short-term symptomatic relief, but long-term efficacy has not been demonstrated. Inconsistent results do not support the routine use of prolotherapy, platelet-rich plasma injections and topical nitric oxide patches. Operative intervention should be reserved until conservative measures fail or an obvious operative lesion is present.
Conclusions:
While numerous therapeutic modalities exist for tendinopathy in the athlete, the ideal treatment protocol has not been clearly defined. The development of new targeted therapies for tendinopathy is likely to follow a greater understanding of the cellular and molecular mechanisms that underlie its pathogenesis.
Keywords: Tendinopathy, Tendinosis, Tendinitis, Tendon injury, Wound healing
Introduction
Tendinopathy is a frequent and difficult to treat clinical problem in athletes and invariably leads to a negative impact on sport performance. It is often considered a failure of damaged matrix proteins within the tendon to properly heal, but the exact etiology remains largely unknown. Hallmark findings of this disorder include localized pain, swelling and reduced strength and performance of the affected tendon. While frequently thought to be associated with increasing age and participation in sports, tendinopathy is routinely diagnosed in patients of all ages and levels of physical activity 1. Management of this painful condition can be particularly challenging and recovery is usually slow 2. This is especially true for athletes where higher levels of physical activity and minimal rest times make it difficult to return to sports and can lead to re-injury. Numerous treatment options exist for tendinopathy, but overall there is a lack of consensus in the literature regarding their efficacy. Indeed, some tendinopathies respond to simple interventions, while others are refractory to nearly all forms of treatment. A greater understanding of the cellular and molecular mechanisms that underlie the pathogenesis of tendinopathy will likely facilitate the discovery of new targeted therapies to enhance tendon healing and repair.
The objective of this review is to first explore the normal structure and function of tendon followed by an overview of tendinopathy, including its classification, epidemiology and the risk factors associated with its development. The remainder of this review will then provide a cogent and practical approach to the management of tendinopathy with a focus on both non-operative and operative therapeutic interventions.
Tendon Structure and Cell Biology
Macroscopic Overview of Tendon
Tendons connect skeletal muscle to bone and transmit force generated by muscular contraction to permit movement. They are considered an extension of the skeletal muscle extracellular matrix (ECM) and terminate on bone at the enthesis 3. Tendons vary in size and shape with short, thick tendons originating from the powerful muscle groups of the proximal limb, while long, thin tendons execute fine movements of the digits 4. Smooth and efficient motion requires tendon gliding that is not restricted by adjacent tissue. For that reason, synovial sheaths form a closed system around many tendons to provide lubrication and cushion the tendon as it stretches and relaxes 3. Tendons that lack true synovial sheaths are instead surrounded by a loose, fatty and vascularized peritendinous tissue, which allows for free excursion of the tendon within its fascial compartment 4.
The mechanical properties of tendon are determined by the macromolecular structural organization and biochemical composition of the ECM 5. When a tendon is loaded, the extent of tissue deformation is dependent on the rate at which the load is applied 6. At low strain rates, tendons deform more and absorb more energy, while at high strain rates, tendons deform less, become stiffer and are more effective at carrying mechanical load 61. During physical activity, tendons store elastic energy as they are stretched and release that energy as they shorten. As tendons are stretched, the non-covalent bonds between amino acid residues are broken which gives off energy in the form of heat 6. In situations of high-frequency cyclical stretching, certain regions within tendon may not be able to efficiently dissipate this heat 6. This potentially causes an irreversible denaturing of matrix proteins and can lead to tendinopathy or acute tendon rupture 62. In addition to the rate at which tendons are stretched, the degree to which tendons are stretched can also contribute to injury. Strains less than 4% generally allow the tendon to return to its original length once the load is removed, but above 4% strain the collagen fibers can begin to fail, and beyond 8% strain the tendon is susceptible to rupture 2, 4. Lastly, two additional features that highlight the viscoelastic properties of tendon are creep and stress relaxation. Creep refers to time-dependent elongation of the tendon while under constant load, whereas stress relaxation is a time-dependent decline in the load required to maintain constant elongation of the tendon 63.
Microscopic Overview of Tendon
Tendon is a hypocellular tissue composed of elongated fibroblasts interspersed between a complex network of matrix proteins. The organizational pattern of tendon closely resembles that of skeletal muscle with distinct connective tissue layers running parallel to its longitudinal axis 4. The smallest functional unit of tendon is the collagen fibril. Collagen fibrils combine to form larger collagen fibers, and then groups of these fibers coalesce into tendon fascicles. Tendon fascicles are enveloped by the endotenon, which is a delicate reticular network of connective tissue supporting a rich supply of vascular, lymphatic and neural channels 4. The tendon proper is the final structure formed by numerous tendon fascicles held together by a loose epithelial-like tissue layer called the epitenon 7. The epitenon contains larger blood vessels and nerves, and is a potential source of new tendon fibroblasts during periods of tendon growth and repair 8.
During the course of embryonic development, tendons arise from a separate pool of progenitor cells than other limb tissues, including skeletal muscle, cartilage and bone 9. Tendon fibroblasts are rod- or spindle-shaped cells with long cytoplasmic extensions known as fibripositors, whose primary function is to synthesize and secrete collagen fibrils in parallel with the direction of load across the long axis of the tendon 10. Ippolito and colleagues originally described two different populations of tendon fibroblasts, namely immature proliferating tenoblasts and terminally differentiated tenocytes 11. As individuals approach adulthood, the amount of tenoblasts decrease while terminally differentiated tenocytes become the most abundant cell population in tendon 12. The origin and identity of the stem cell population within tendon that gives rise to tenocytes is not fully understood. However, pericytes, which are a population of stem cells that exist in close proximity to the vasculature, appear to be attractive candidates 13. The remaining cells within tendon mostly consist of endothelial and smooth muscle cells of the tendon vasculature, synovial cells within the tendon sheath and chondrocytes at the enthesis 2.
The main structural protein of tendon is type I collagen, which accounts for 65–80% of the tendon dry weight 5. Type I collagen is first synthesized as a procollagen molecule within the tendon fibroblast and typically consists of two α1 and one α2 subunits. Procollagen is then secreted from the cell into the tendon ECM where its amino- and carboxy-terminal ends are cleaved to produce tropocollagen. As the final step in the collagen synthesis pathway, multiple tropocollagen molecules are cross-linked resulting in the formation of the mature collagen fibril. Type I and III collagen are part of the fibrillar collagen family and play an important role in the longitudinal transmission of force during locomotion 3. Compared to type I collagen, type III collagen tends to be smaller in diameter, less organized and has decreased tensile strength 14. Type III collagen is often found at increased levels in aged tendon or at the rupture sites of highly stressed tendons 15. Type V collagen is also a fibrillar collagen, but is present in lesser quantities and regulates the initiation of collagen fibril assembly 16. In addition to the fibrillar collagens, many other types of collagen can be found in tendon. These collagens, such as the network type IV and VI collagens, help to transmit forces laterally between cells and provide structural support for vascular and lymphatic tissue in the endotenon and epitenon 3. Mice deficient in type VI collagen display mechanically weak tendons with an increase in the number of small and aberrant collagen fibrils, indicating that type VI collagen also facilitates collagen fibril organization 17. Aside from collagen, a small but significant portion of the tendon structure in humans is formed by elastin 4. While little is known about its exact function, elastin tends to be localized to the interfascicular matrix and likely contributes to sliding between adjacent tendon fascicles and recoil after mechanical loading 18. Finally, intermixed with the collagen and non-collagen proteins of the tendon ECM is the gelatinous ground substance composed of proteoglycans (PGs) and glycosaminoglycans (GAGs). PGs and GAGs have a fixed net negative charge that allows them to retain water, which in turn helps the tendon to dissipate heat into the surrounding tissues as well as resist high compressive and tensile forces during exercise 19.
In humans, the core of the Achilles tendon will grow until the age of 17, after which protein synthesis and matrix turnover begin to decline 20. This is supported by animal studies in rodents that demonstrate tendon growth mostly occurs in an outward direction from the epitenon 12, 13. However, the lack of a discernible endotenon in rodents makes it difficult to rule out the possibility of intrafascicular growth between tendon fascicles. Together, these findings suggest that the lack of tissue renewal in the core of adult tendon tissue may contribute to the protracted course that is characteristic of many tendinopathies, and perhaps cell-based therapies that selectively utilize a fraction of cells from those connective tissue layers known to be enriched in tendon stem cells may enhance the regeneration of injured tendon tissue.
Epidemiology and Pathogenesis of Tendinopathy
Tendon injuries account for 30–50% of all injuries in sports and the risk of these injuries are increasing as a larger proportion of younger and older age groups participate in recreational activities 1. Greater demands have been placed on athletic performance, requiring that athletes train longer, harder and more often. This type of chronic overuse is associated with an increased risk of developing tendinopathy. For example, Olympic runners had an increased incidence of Achilles tendinopathy with an adjusted odds ratio of 31.2 compared with age-matched controls 21. In addition to the frequency of overuse, activities that prolong the loading of tendons without adequate rest in between sessions also contributes to the risk of developing tendinopathy 22. It has been demonstrated that total hours spent practicing sports is a known risk factor for developing patellar tendinopathy in elite soccer players 23.
Understanding the pathogenesis of tendinopathy has been hindered by a lack of consistent terminology. Traditionally, the term tendinitis referred to symptomatic tendons associated with chronic pain and implied an element of inflammation in its basic etiology, especially in the initial phases of the disease process 24. Repetitive microtrauma from overload or overuse can lead to collagen fibril rupture and activation of the innate immune system 24. However, continued research has demonstrated little to no biochemical or histological evidence of inflammation in biopsies from patients with chronic tendinopathy 25. Given the uncertainty surrounding the role of inflammation in the development of tendon disorders, we prefer to use the term tendinopathy to describe an ongoing process of degeneration and failed regeneration of the tendon ECM in response to injury or disease. In clinical practice, tendinopathy refers to a spectrum of pathologies from intratendinous degenerative lesions that are a source of chronic pain, to spontaneous tendon rupture as a result of mechanical attrition.
Several features are characteristic of tendinopathy at the cellular level (Figure 1). Compared to healthy tendons, tendinopathic tendons have marked disorganization and separation of collagen fibrils with a concomitant increase in mucoid ground substance 26. Collagen fibril diameter is more variable and an increased content of type III collagen contributes to mechanical weakness of the diseased tendon 15. Tendon fibroblasts adopt a rounded rather than flattened appearance and tend to be unevenly distributed throughout the tissue. Another hallmark feature of tendinopathy is neovascularization with budding capillaries invading the tendon from the paratenon 26. These capillaries are usually accompanied by the ingrowth of sensory nerve fibers that release nociceptive substances and trigger pain 27. Finally, there is absence of an inflammatory cell infiltrate in the tendon proper, although inflammation can be observed within the peritendinous tissue 26.
Figure 1.
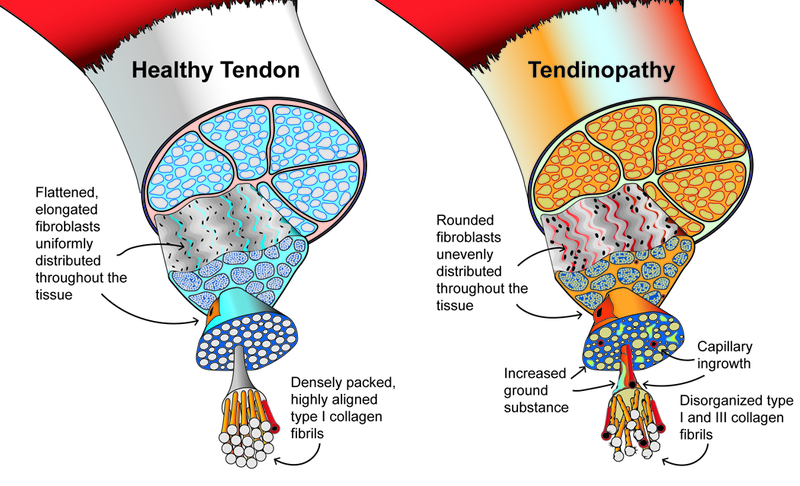
Graphical representation of the morphological features in healthy tendon and tendinopathy. Modified from Scott et al 60.
Non-Operative Management of Tendinopathy
The management of tendinopathy can vary based on the history and extent of the condition, the severity of symptoms, and the physical demands and activity level of an athlete. Conservative measures remain the mainstay of treatment for most tendinopathies, but operative intervention is available for recalcitrant cases, or for athletes with obvious operative lesions (Table 1).
Table 1.
Summary of non-operative and operative therapeutic interventions for the management of tendinopathy.
| Treatment | Proposed Mechanism | Outcome |
|---|---|---|
| Non-Operative | ||
| Eccentric exercises | Promotion of tissue reorganization with improved changes in tendon structure and mechanical properties 28, 31 | Positive outcomes including reduced pain, improved strength and decreased time to return to sport 29, 30 |
| NSAIDs | Reduced pain and inflammation during tendon healing 34 | Positive short-term outcomes for analgesia 32, 33; No significant long-term benefits 26, 34; Some evidence to support that NSAIDs have potential to inhibit normal tendon healing 35, 36 |
| Corticosteroids | Reduced pain and inflammation within the peritendinous tissue 30 | Positive short-term outcomes for analgesia and functional improvement 30, 37–39; Limited or no long-term benefits 41; Potential risk of complications 40 |
| Nitric oxide | Increased tenocyte proliferation and collagen synthesis 43 | Unclear short-term outcomes 46, 47; No significant long-term benefits 48 |
| Prolotherapy | Cellular and tissue irritation to promote inflammation 49 | Insufficient studies to demonstrate short- or long-term benefits 50, 51 |
| PRP | Increased growth factors and cytokines at the side of tendon healing 52 | Insufficient studies to demonstrate short- or long-term benefits 53, 54 |
| Operative | ||
| Percutaneous longitudinal tenotomy | Promotion of tissue regeneration and angiogenesis 56 | Positive long-term outcomes with minimal complications 56 |
| Percutaneous ultrasonic microtenotomy | Removal of pathological tissue and sensory nerve fiber destruction 58 | Reduced pain symptoms at three years 59 |
Eccentric Exercises
Eccentric exercises, defined as exercises in which the muscle and tendon lengthen while under tension, have been well studied and long utilized as a form of treatment in the management of tendinopathies 28. Several studies have demonstrated that eccentric exercises can improve patient-reported outcomes with favorable changes in strength, clinical imaging, and biochemical and histological markers of ECM remodeling 29, 30. While the mechanisms are not completely understood at the molecular level, eccentric exercises result in the production of new type I collagen and increase the density of collagen fibrils 31. Furthermore, mechanical loading induces the expression of genes such as scleraxis and tenomodulin that are associated with the accumulation of new tenocytes and the production of healthy ECM 12, 13. A particular form of eccentric exercise, termed “heavy slow resistance” training, uses heavy weight with slow repetition and has been notably effective for the treatment of patellar tendinopathy 30. The combined efficacy of eccentric training from clinical research studies, the low cost of therapy, and the compelling evidence of improved ECM quality at the cellular and molecular levels, have resulted in eccentric exercises being frequently considered as a first-line therapy in the management of tendinopathy.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs, which function to block the production of prostaglandins from the cyclooxygenase enzymes, remain a popular choice for the management of tendinopathies. Prostaglandins are physiologically active lipid molecules derived from arachidonic acid that play an important role in the development of acute inflammation. Accordingly, both the oral and topical delivery of NSAIDs are generally effective as short-term analgesics for these painful conditions 32, 33. While useful for reducing pain and inflammation, NSAIDs do not appear to alter the course of the disease process, which is consistent with the lack of an inflammatory phenotype in tendinopathic tendons 26, 34. Furthermore, the drugs likely do not accumulate in sufficient quantities within the tendon tissue to change gene transcription 64. There is also some evidence to suggest that NSAIDs may negatively impact tendon healing by impairing the proliferation and differentiation of tenocytes, as well as promoting the formation of adipocytes from mesenchymal stem cells 35, 36. In summary, NSAIDs may have utility in the alleviation of acute pain, but emerging evidence indicates no effect, or potential deleterious effects of NSAIDs, in the management of tendinopathy.
Corticosteroids
Corticosteroids are synthetic analogues of a particular class of steroid hormones produced by the adrenal cortex. These potent anti-inflammatory molecules have been evaluated extensively in many clinical trials of patients with tendinopathies 37, 38. Even though the inflammatory process appears to play little to no role in the pathogenesis of tendinopathy, several studies have demonstrated that corticosteroid therapy often significantly improves patient outcomes in the short term by providing pain relief and enhancing function 39. The positive effect of corticosteroids on pain and function are thought to occur due to a reduction in inflammation within the peritendinous tissue 30. However, there exists the potential risk of iatrogenic tendon rupture and atrophy of the surrounding soft tissues with repeated corticosteroid use 40. A 2015 Cochrane Review investigating the efficacy of injection therapies including corticosteroids for the treatment of painful Achilles tendons also found that there is insufficient evidence to support the routine use of injection therapies in patients with Achilles tendinopathy 41. While corticosteroids have been shown to reduce pain in many tendon disorders, clinicians should be careful against the widespread use of corticosteroids in the management of tendinopathy, due to the lack of long-term benefits and the potential risk of adverse effects.
Nitric Oxide
Nitric oxide therapies have gained popularity within recent years for the management of tendinopathies 42. The most common approach involves the transdermal delivery of nitroglycerin to the involved area where it is converted into bioactive nitric oxide by endogenous nitric oxide synthase 42. At the cellular and molecular levels, nitric oxide increases tenocyte proliferation and collagen synthesis, while the inhibition of nitric oxide synthase decreases the cross-sectional area of healing tendons and reduces the amount of mechanical load required for tendon failure 43. There have been several randomized controlled trials to evaluate the effectiveness of topical nitroglycerin in patients with tendinopathy, but have yielded conflicting results 44, 45. Two meta-analyses have concluded that while additional studies need to be performed, there is evidence to support the use of low-dose nitroglycerin patches for the short-term relief of pain associated with tendinopathy 46, 47. Studies of the long-term efficacy of nitroglycerin patches, however, have generally shown marginal to no improvement in pain and function 48. Side effects of nitroglycerin patches have also been reported, including headache, rash, facial flushing and sweating 45. These potential side effects along with the contradictory short-term results and the lack of long-term efficacy, have limited the enthusiasm for the use of nitroglycerin patches as a first-line therapy in the management of tendinopathy.
Prolotherapy
Prolotherapy refers to the injection of an irritant solution within or around a tendinopathic tendon 49. The irritant effect is thought to promote inflammation and trigger a regenerative response in order to heal the chronically degraded ECM that is characteristic of patients with tendinopathy. There is considerable variability in the type and concentration of irritant solution that can be injected, although most solutions contain either hyperosmolar dextrose, phenol-glycerine-glucose or sodium morrhuate. While the mechanism of action is not completely understood, hyperosmolar dextrose is thought to dehydrate cells until they rupture by creating a large osmotic gradient, phenol-glycerine-glucose causes localized cellular and tissue irritation, and sodium morrhuate functions as a chemoattractant for inflammatory cells and other mediators 49. These irritant solutions can also sclerose small blood vessels and nociceptive neurons that are enriched in tendinopathic tendons. A small number of clinical studies have evaluated the effectiveness of prolotherapy in patients with tendinopathy, but most of these studies are limited by either small sample size or lack of appropriate controls 50, 51. Therefore, while prolotherapy is a relatively safe and low-cost treatment option, there is a need for larger randomized controlled trials to more rigorously assess the use of prolotherapy as a first-line therapy in the management of tendinopathy.
Platelet-Rich Plasma (PRP)
PRP is an autologous blood product consisting of plasma enriched with a concentration of platelets that is greater than that of whole blood, typically at least four times the baseline value 52. PRP has grown in popularity within recent years as a way to accelerate healing in a variety of musculoskeletal conditions, and numerous potential applications have been explored, including the use of PRP as a treatment option for tendinopathy. Platelets are thought to be particularly important for injured tissues to properly heal, as they are enriched with multiple growth factors and cytokines, including transforming growth factor beta (TGF-β), interleukins (ILs), fibroblast growth factors (FGFs), platelet-derived growth factors (PDGFs), vascular endothelial growth factors (VEGFs), connective tissue growth factor (CTGF) and others 52. These proteins are thought to enhance tissue healing when delivered locally 2. However, recent work using wide-scale genome profiling and bioinformatics has reported PRP to work through inducing local inflammation in tissue 52.
There have been several clinical studies to explore the effectiveness of PRP in treating patients with tendinopathy, but have produced mixed results 53, 54. Some of the most encouraging results have come in the treatment of lateral epicondylitis, with PRP demonstrating a near two-fold improvement in outcomes compared to corticosteroids 53. A recent meta-analysis concluded that PRP injections did not provide significantly greater relief compared to placebo or dry needling for the treatment of tendinopathy at six months of follow-up, although there was marginal improvement in patients suffering from rotator cuff tendinopathy 55. Larger clinical studies, conducted on multiple different anatomical locations, are necessary to better inform the evidence-based use of PRP in the management of tendinopathy.
Operative Management of Tendinopathy
Nearly a third of all patients with tendinopathy fail non-operative management 1. For these patients, they either alter or reduce their activity level and sport participation, or seek operative treatments. The goal of operative management for patients with tendinopathy is to either remove the pathological areas of tendon or to induce low-grade trauma to the degenerated tendon in an effort to restart the healing response, similar to prolotherapy or PRP. Several techniques are outlined below.
Percutaneous Longitudinal Tenotomy
Percutaneous longitudinal tenotomy is a procedure in which the surgeon introduces one or multiple stab incisions parallel to the longitudinal axis of the tendon. This has been shown to promote tissue regeneration, increase blood flow and create a local environment more suitable for healing 56. This is a relatively simple procedure which can be performed on an outpatient basis with the use of local anesthesia 57. Ultrasound is used to identify the pathogenic area and helps with placement of the stab incisions. Complications from percutaneous longitudinal tenotomies are reported to be minimal and do not lead to long-term morbidity 57.
Percutaneous Ultrasonic Microtenotomy
Percutaneous ultrasonic microtenotomy has recently become available as a method to treat tendinopathy. Using ultrasonic energy, this minimally-invasive procedure is able to debride pathologic tissue through a needle-like device placed within the tendon. In a case series of 20 patients with recalcitrant lateral epicondylitis, percutaneous ultrasonic microtenotomy demonstrated improvement in pain and function in 95% of patients treated at one year 58. A follow-up on the same patient population demonstrated sustained clinical efficacy at three years, with all patients demonstrating a reduction in tendon thickness and 95% exhibiting a resolution of tendon hypervascularity 59. While studies evaluating the effectiveness of percutaneous ultrasonic microtenotomy have largely been limited to lateral epicondylitis, this treatment has the potential to be used for other refractory cases of tendinopathy.
Conclusions
Despite their prevalence, tendon disorders remain difficult to treat. While numerous therapeutic modalities exist for tendinopathy in the athlete, the ideal treatment protocol has not been clearly defined. New insights into the pathophysiology of these diseases are constantly evolving and more sophisticated treatment strategies continue to emerge. The development of new targeted therapies for tendinopathy is likely to follow a greater understanding of the cellular and molecular mechanisms that underlie its pathogenesis. At the moment, non-operative management is the cornerstone of therapy. Minimally-invasive procedures have shown early promising results, especially in patients with recalcitrant tendinopathy, but high-quality randomized controlled trials are needed before these procedures can be recommended for routine use.
Practical Implications.
Rehabilitation, with a focus on eccentric training, should be a first-line therapy in the treatment of painful tendon disorders.
NSAIDs can be used for short-term symptomatic relief. However, prolonged use should be avoided due to the risk of complications and the lack of proven long-term benefit.
Second-line therapies include prolotherapy, PRP injections and topical nitric oxide patches. As a group, the data for these are inconclusive, but their risks are low and therefore can be recommended for athletes that fail first-line treatment.
Corticosteroids should be used with caution in the treatment of painful tendon disorders, as the underlying cause likely does not involve inflammatory changes and their use may increase the risk of spontaneous tendon rupture.
Surgery should only be pursued when conservative measures have failed or an obvious pathologic lesion is present within the tendon.
Acknowledgements
The authors have no financial interest to declare in relation to the content of this article. This work was supported by NIH grants R01-AR063649, F31-AR065931 and F32-AR067086.
References
- 1.Ackermann PW, Renstrom P. Tendinopathy in sport. Sports health. 2012; 4(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. The Journal of bone and joint surgery. American volume. 2005; 87(1):187–202. [DOI] [PubMed] [Google Scholar]
- 3.Davis ME, Gumucio JP, Sugg KB, et al. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. Journal of applied physiology (Bethesda, Md. : 1985). 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannus P Structure of the tendon connective tissue. Scandinavian Journal of Medicine & Science in Sports. 2000; 10(6):312–320. [DOI] [PubMed] [Google Scholar]
- 5.Kjaer M Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiological reviews. 2004; 84(2):649–698. [DOI] [PubMed] [Google Scholar]
- 6.Paxton JZ, Baar K. Tendon mechanics: the argument heats up. J Appl Physiol (1985). 2007; 103(2):423–424. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SH, Al-Youha S, Van Agtmael T, et al. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS One. 2011; 6(1):e16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugg KB, Lubardic J, Gumucio JP, et al. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2014; 32(7):944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development (Cambridge, England). 2010; 137(17):2807–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canty EG, Lu Y, Meadows RS, et al. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol 2004; 165(4):553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ippolito E, Natali PG, Postacchini F, et al. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. The Journal of bone and joint surgery. American volume. 1980; 62(4):583–598. [PubMed] [Google Scholar]
- 12.Gumucio JP, Phan AC, Ruehlmann DG, et al. Synergist ablation induces rapid tendon growth through the synthesis of a neotendon matrix. J Appl Physiol (1985). 2014; 117(11):1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz AJ, Sarver DC, Sugg KB, et al. p38 MAPK signaling in postnatal tendon growth and remodeling. PLoS One. 2015; 10(3):e0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol 1997; 72(4):352–361. [PubMed] [Google Scholar]
- 15.Eriksen HA, Pajala A, Leppilahti J, et al. Increased content of type III collagen at the rupture site of human Achilles tendon. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002; 20(6):1352–1357. [DOI] [PubMed] [Google Scholar]
- 16.Wenstrup RJ, Florer JB, Brunskill EW, et al. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem 2004; 279(51):53331–53337. [DOI] [PubMed] [Google Scholar]
- 17.Izu Y, Ansorge HL, Zhang G, et al. Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biol 2011; 30(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Screen HR, Berk DE, Kadler KE, et al. Tendon functional extracellular matrix. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2015; 33(6):793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005; 5(1):22–34. [PubMed] [Google Scholar]
- 20.Heinemeier KM, Schjerling P, Heinemeier J, et al. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb (14)C. FASEB J 2013; 27(5):2074–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2005; 15(3):133–135. [DOI] [PubMed] [Google Scholar]
- 22.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. British journal of sports medicine. 2009; 43(6):409–416. [DOI] [PubMed] [Google Scholar]
- 23.Hägglund M, Zwerver J, Ekstrand J. Epidemiology of patellar tendinopathy in elite male soccer players. The American journal of sports medicine. 2011; 39(9):1906–1911. [DOI] [PubMed] [Google Scholar]
- 24.Millar NL, Hueber AJ, Reilly JH, et al. Inflammation is present in early human tendinopathy. The American journal of sports medicine. 2010; 38(10):2085–2091. [DOI] [PubMed] [Google Scholar]
- 25.Alfredson H, Forsgren S, Thorsen K, et al. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. Journal of Orthopaedic Research. 2001; 19(5):881–886. [DOI] [PubMed] [Google Scholar]
- 26.Khan KM. Histopathology of common tendinopathies. Update and implications for clinical management. Sports medicine (Auckland). 1999; 27(6):393–408. [DOI] [PubMed] [Google Scholar]
- 27.Schubert T, Weidler C, Lerch K, et al. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Annals of the rheumatic diseases. 2005; 64(7):1083–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenz D, Reiman M. The role and implementation of eccentric training in athletic rehabilitation: tendinopathy, hamstring strains, and acl reconstruction. International journal of sports physical therapy. 2011; 6(1):27–44. [PMC free article] [PubMed] [Google Scholar]
- 29.Gardin A, Movin T, Svensson L, et al. The long-term clinical and MRI results following eccentric calf muscle training in chronic Achilles tendinosis. Skeletal radiology. 2010; 39(5):435–442. [DOI] [PubMed] [Google Scholar]
- 30.Kongsgaard M, Kovanen V, Aagaard P, et al. Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scand J Med Sci Sports. 2009; 19(6):790–802. [DOI] [PubMed] [Google Scholar]
- 31.Langberg H, Ellingsgaard H, Madsen T, et al. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand J Med Sci Sports. 2007; 17(1):61–66. [DOI] [PubMed] [Google Scholar]
- 32.Mazieres B, Rouanet S, Guillon Y, et al. Topical ketoprofen patch in the treatment of tendinitis: a randomized, double blind, placebo controlled study. The Journal of rheumatology. 2005; 32(8):1563–1570. [PubMed] [Google Scholar]
- 33.Petri M, Hufman SL, Waser G, et al. Celecoxib effectively treats patients with acute shoulder tendinitis/bursitis. The Journal of rheumatology. 2004; 31(8):1614–1620. [PubMed] [Google Scholar]
- 34.Magra M, Maffulli N. Nonsteroidal antiinflammatory drugs in tendinopathy: friend or foe. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2006; 16(1):1–3. [DOI] [PubMed] [Google Scholar]
- 35.Fredriksson M, Li Y, Stalman A, et al. Diclofenac and triamcinolone acetonide impair tenocytic differentiation and promote adipocytic differentiation of mesenchymal stem cells. J Orthop Surg Res. 2013; 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Wang X, Qiu Y, et al. Effect of indomethacin and lactoferrin on human tenocyte proliferation and collagen formation in vitro. Biochem Biophys Res Commun. 2014; 454(2):301–307. [DOI] [PubMed] [Google Scholar]
- 37.Smidt N, van der Windt DA, Assendelft WJ, et al. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002; 359(9307):657–662. [DOI] [PubMed] [Google Scholar]
- 38.Tonks JH, Pai SK, Murali SR. Steroid injection therapy is the best conservative treatment for lateral epicondylitis: a prospective randomised controlled trial. International journal of clinical practice. 2007; 61(2):240–246. [DOI] [PubMed] [Google Scholar]
- 39.Smidt N, Assendelft WJ, van der Windt DA, et al. Corticosteroid injections for lateral epicondylitis: a systematic review. Pain. 2002; 96(1–2):23–40. [DOI] [PubMed] [Google Scholar]
- 40.Knobloch K Drug-Induced Tendon Disorders. Adv Exp Med Biol 2016; 920:229–238. [DOI] [PubMed] [Google Scholar]
- 41.Kearney RS, Parsons N, Metcalfe D, et al. Injection therapies for Achilles tendinopathy. The Cochrane Library. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andres B, Murrell GC. Treatment of Tendinopathy: What Works, What Does Not, and What is on the Horizon. Clin Orthop Relat Res. 2008; 466(7):1539–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murrell GA, Szabo C, Hannafin JA, et al. Modulation of tendon healing by nitric oxide. Inflammation research : official journal of the European Histamine Research Society ... [et al. ]. 1997; 46(1):19–27. [DOI] [PubMed] [Google Scholar]
- 44.Berrazueta JR, Losada A, Poveda J, et al. Successful treatment of shoulder pain syndrome due to supraspinatus tendinitis with transdermal nitroglycerin. A double blind study. Pain. 1996; 66(1):63–67. [DOI] [PubMed] [Google Scholar]
- 45.Paoloni JA, Murrell GA, Burch RM, et al. Randomised, double-blind, placebo-controlled clinical trial of a new topical glyceryl trinitrate patch for chronic lateral epicondylosis. British journal of sports medicine. 2009; 43(4):299–302. [DOI] [PubMed] [Google Scholar]
- 46.Gambito ED, Gonzalez-Suarez CB, Oquinena TI, et al. Evidence on the effectiveness of topical nitroglycerin in the treatment of tendinopathies: a systematic review and meta-analysis. Archives of physical medicine and rehabilitation. 2010; 91(8):1291–1305. [DOI] [PubMed] [Google Scholar]
- 47.Garrick JG. Topical nitroglycerin decreases pain intensity in daily activities: a review. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2011; 21(6):539–540. [DOI] [PubMed] [Google Scholar]
- 48.Paoloni JA, Murrell GA. Three-year followup study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot & ankle international. / American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society. 2007; 28(10):1064–1068. [DOI] [PubMed] [Google Scholar]
- 49.Distel LM, Best TM. Prolotherapy: a clinical review of its role in treating chronic musculoskeletal pain. PM & R : the journal of injury, function, and rehabilitation. 2011; 3(6 Suppl 1):S78–81. [DOI] [PubMed] [Google Scholar]
- 50.Ryan M, Wong A, Taunton J. Favorable outcomes after sonographically guided intratendinous injection of hyperosmolar dextrose for chronic insertional and midportion achilles tendinosis. American Journal of Roentgenology. 2010; 194(4):1047–1053. [DOI] [PubMed] [Google Scholar]
- 51.Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2008; 18(3):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudgens JL, Sugg KB, Grekin JA, et al. Platelet-Rich Plasma Activates Proinflammatory Signaling Pathways and Induces Oxidative Stress in Tendon Fibroblasts. The American journal of sports medicine. 2016; 44(8):1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. The American journal of sports medicine. 2010; 38(2):255–262. [DOI] [PubMed] [Google Scholar]
- 54.Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. The American journal of sports medicine. 2011; 39(10):2130–2134. [DOI] [PubMed] [Google Scholar]
- 55.Tsikopoulos K, Tsikopoulos I, Simeonidis E, et al. The clinical impact of platelet-rich plasma on tendinopathy compared to placebo or dry needling injections: A meta-analysis. Phys Ther Sport. 2016; 17:87–94. [DOI] [PubMed] [Google Scholar]
- 56.Maffulli N, Testa V, Capasso G, et al. Results of percutaneous longitudinal tenotomy for Achilles tendinopathy in middle- and long-distance runners. The American journal of sports medicine. 1997; 25(6):835–840. [DOI] [PubMed] [Google Scholar]
- 57.Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. The Journal of bone and joint surgery. American volume. 2010; 92(15):2604–2613. [DOI] [PubMed] [Google Scholar]
- 58.Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. The American journal of sports medicine. 2013; 41(3):636–644. [DOI] [PubMed] [Google Scholar]
- 59.Seng C, Mohan PC, Koh SB, et al. Ultrasonic Percutaneous Tenotomy for Recalcitrant Lateral Elbow Tendinopathy: Sustainability and Sonographic Progression at 3 Years. The American journal of sports medicine. 2016; 44(2):504–510. [DOI] [PubMed] [Google Scholar]
- 60.Scott A, Huisman E, Khan K. Conservative treatment of chronic Achilles tendinopathy. CMAJ. 2011; 183(10):1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang JHC. Mechanobiology of tendon. Journal of Biomechanics. 2006; 39(9):1563–82. [DOI] [PubMed] [Google Scholar]
- 62.Patterson-Kane JC, Becker DL, Rich T. The pathogenesis of tendon microdamage in athletes: the horse as a natural model for basic cellular research. Journal of Comparative Pathology. 2012; 147(2–3):227–47. [DOI] [PubMed] [Google Scholar]
- 63.Magnusson SP, Hansen P, Kjaer M. Tendon properties in relation to muscular activity and physical training. Scandinavian Journal of Medicine & Science in Sports. 2003; 13(4):211–23. [DOI] [PubMed] [Google Scholar]
- 64.Heinemeier KM, Øhlenschlæger TF, Mikkelsen UR, et al. Effects of anti-inflammatory (NSAID) treatment on human tendinopathic tissue. J Appl Physiol. 2017; 123(5):1397–405. [DOI] [PubMed] [Google Scholar]


