Abstract
Purpose of review
The purpose is to review recent progress in applying the CRISPR/Cas9 system to lipid metabolism and therapeutics.
Recent findings
The CRISPR/Cas9 system has been used to generate knockout animals for lipid genes in multiple species. Somatic genome editing with CRISPR/Cas9 can efficiently disrupt genes in adult animals, including a new strategy for generating atherosclerosis. Refinements to the CRISPR/Cas9 system including epigenetic modulators and base editors offer new avenues to manipulate gene expression. The recent report of germline genome editing in humans highlights the promise as well as perils of this technology.
Summary
CRISPR/Cas9 is a transformative technology that will help advance on our understanding of lipid metabolism and physiology. Somatic genome editing is a particularly promising approach for editing genes in tissues of live organisms, and represents a new means of addressing unmet therapeutic challenges in humans. Educational outreach, public debate, and consideration of ethics and safety must guide the use of genome editing in humans.
Keywords: adeno-associated virus, CRISPR/Cas9, germline genome editing, lipid metabolism, somatic genome editing
INTRODUCTION
Genome engineering has become routine molecular biology practice with the development of the CRISPR/Cas9 system. This article reviews recent advances in applying CRISPR/Cas9 to the study of lipid metabolism – both for research and therapeutic purposes.
CRISPR/Cas9 stands for clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9. Cas9 is a programmable nuclease that generates double strand breaks in DNA in a complex with a guide RNA (gRNA). The gRNA is engineered by the user with the first ∼20–23 nucleotides as an exact match to the target, based on the requirement for a protospacer adjacent motif (PAM) immediately downstream. The two most commonly used CRISPR/Cas9 systems from Streptococcus pyogenes and Staphylococcus aureus use PAM sequences of NGG and NNGRRT (R = G/A) respectively. The requirement for a PAM does limit, which sequences in the genome are targetable. However, many other CRISPR/Cas9 systems have recently been developed [1▪,2▪]. These efforts have been quite successful in either broadening the targetable PAM sequences of the Cas9 nuclease [3▪,4], or conversely further enhancing its specificity [5,6].
Following the creation of a double stranded break, the host cell's DNA repair mechanisms dictate the types of DNA modifications that can be introduced. Nonhomologous end joining (NHEJ) is the preferred DNA repair pathway in postmitotic cells and involves error-prone ligation of blunt-ended DNA. Repeated cycles of NHEJ repair generate small insertion or deletion (indels) of bases at the DSB. The majority of these indels will shift the reading frame, effectively knocking out a gene. NHEJ can also be used to delete entire exons or larger sequences with two gRNAs [7], or to insert foreign DNA in a homology-independent manner [8]. In contrast, homology-directed repair (HDR) is a far more precise repair mechanism that only occurs during the S/G2 phase of the cell cycle. Performing HDR requires that the user supply a donor template that has homology to the 5′ and 3′ regions flanking the cut site. The sequence between the homology arms of the donor replaces the sequence between the homology arms at the endogenous locus [9,10▪,11▪]. This method allows the user to precisely repair mutations or introduce completely new sequences.
CRISPR/Cas9 can be injected directly into fertilized oocytes [12], reducing the time and effort needed to make knockout animals. Cas9 cutting does not occur solely at the single cell stage, and the resulting animals are generally mosaic – composed of a mixture of cells that are either unedited or have one of several different mutations. Founders with the desired modifications can then be backcrossed and bred together to generate homozygous animals. This approach has been used to knockout genes in multiple species, many of which were previously impossible. Recently, Zhao et al. used CRISPR to delete Ldlr and ApoE in rat zygotes and implanted them into pseudopregnant rats. The Ldlr and ApoE double knockout rats had high levels of LDL-cholesterol (LDL-C) and developed atherosclerosis when placed on high-fat diet [13▪]. Huang et al. used somatic cell nuclear transfer (SCNT) to generate a line of rabbits that were susceptible to atherosclerosis. The group used donor nuclei from a CRISPR-modified pig embryonic fibroblast cell line in which ApoE and Ldlr were knocked out. The resulting piglets showed an increase in LDL-C, total cholesterol, triglycerides, and APOB [14▪]. CRISPR/Cas9 was recently used to generate a hamster line deficient in LDLR. Heterozygous hamsters showed increased plasma cholesterol when placed on chow diet. Homozygous mutants spontaneously developed atherosclerosis on chow diet, and surprisingly, died prematurely because of atherosclerosis when maintained on a high-fat/high-cholesterol diet [15▪]. Lu et al. generated Ldlr knockout rabbits through zygote microinjection of CRISPR/Cas9 to model familial hypercholesterolemia. Knockout rabbits spontaneously developed atherosclerosis and hypercholesterolemia on a normal chow diet [16▪]. These are likely only the first examples of how germline genome editing with CRISPR/Cas9 will be applied to generate new animal models for lipid research.
Somatic genome editing (SGE) involves the manipulation of the genome in any cells of an organism other than the germline. SGE has tremendous potential for human gene therapy of lipid disorders, where edits to the patients’ own DNA could provide lasting correction. The first application of SGE to lipid metabolism involved disruption of Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9), a secreted protein that promotes the degradation of LDL receptor. Ding et al.[17] used an Adenoviral vector to deliver Strep. pyogenes Cas9 (SpCas9) and a gRNA targeting the Pcsk9 gene to mouse liver in vivo. They observed increased LDLR protein expression and 35–40% lower plasma cholesterol levels 3–4 days after injection. Studies by the same group in 2016 showed that this approach can specifically reduce human PCSK9 levels through adenoviral delivery of CRISPR/Cas9 to human liver chimeric mice [18]. In the same year, Ran et al. identified a smaller Cas9 ortholog from Staph. aureus (SaCas9) [19] that fits easily into adeno-associated virus (AAV) vectors, currently the leading delivery platform for tissue-directed gene therapy in humans. The authors found that SaCas9 uses a more restrictive PAM than SpCas9, limiting targeting options, but also reducing the likelihood of off-target mutagenesis. Robust editing of murine Pcsk9 (>40%) was achieved in the liver with the use of a single AAV vector. It is clear that PCSK9 is a superb target for LDL-lowering, bolstered by the success of several monoclonal antibodies in humans. The concept of a ‘one shot’ treatment to eliminate PCSK9 from the majority of the liver [20▪] is appealing for patients with heterozygous familial hypercholesterolemia. It remains to be determined if the potential benefits of this approach outweigh the risks, and if such a strategy will be commercially viable. Somatic disruption with CRISPR/Cas9 could be applied to other dyslipidemias, particularly, triglyceride-lowering. As an example, Angptl3 is a promising new candidate, which was recently disrupted in vivo through base editing [21▪▪].
SGE is also a powerful tool to study lipid metabolism and physiology. Jarrett et al.[22▪] used AAV8 vectors to deliver gRNA targeting the Ldlr to SpCas9 transgenic mice. They found that liver-directed disruption of Ldlr was sufficient to produce hypercholesterolemia and atherosclerosis, and that deletion of ApoB provided protection from disease. Follow-up work developed an all-in-one AAV8 vector for liver-directed disruption of Ldlr using the SaCas9 nuclease [23▪]. The authors compared this approach to PCSK9 overexpression as an alternative means of generating atherosclerosis. They found that a single injection of the AAV-CRISPR vector was sufficient to produce severe hypercholesterolemia and atherosclerosis, and that this was superior to PCSK9 overexpression in male mice. Although neither AAV-PCSK9 nor AAV-CRISPR are likely to replace the time-tested germline Ldlr knockout mice, these new strategies would be particularly useful in situations where complex genotypes are involved. Most recently, Fedoseienko et al.[24▪▪] investigated the role of the COMMD-CCDC22-CCDC93 (CCC) complex in LDLR endosomal trafficking in the liver. In this article, the authors used SpCas9 transgenic mice treated with an adenoviral vector expressing gRNAs targeting the Ccdc22 gene, which encodes a core component of the CCC complex. Efficient knockdown of Ccdc22 was obtained, which was accompanied by reductions in the associated COMMD proteins, and a 35% increase in plasma cholesterol levels, establishing the importance of the CCC complex in hepatic lipoprotein clearance. Taken together, these studies show the utility of SGE for studying lipid metabolism, which may be a valuable alternative to antisense oligonucleotides and liver-specific knockout mice.
CRISPR/Cas9 can also be used for epigenetic regulation. Thakore et al.[25▪▪] developed an AAV vector that expresses a catalytically dead SaCas9 fused to a Krüppel-associated box epigenetic repressor motif (KRAB domain). This was co-delivered into mice with another AAV expressing a gRNA targeting promoter elements of Pcsk9. This dual vector system succeeded in transcriptional silencing of Pcsk9 in the liver, which significantly lowered plasma cholesterol and PCSK9 protein levels, albeit less efficiently than disruption approaches [17,19]. Interestingly, the dSaCas9-KRAB protein did appear to elicit an immune response based on gene expression profiles in the liver. Transcriptional activation with CRISPR/Cas9 has been difficult to achieve in vivo, at least partially owing to delivery challenges. To address this limitation, Liao et al.[26▪▪] engineered truncated gRNAs (dgRNAs) containing aptamers that can recruit MS2 domain-containing proteins fused to transcriptional activation domains. This approach uses wild type Cas9, as the shorter dgRNAs (∼15nt) do not allow for DNA cleavage, and the transactivation domains are separately recruited via the dgRNA-aptamer backbone. The authors showed that in-vivo target gene activation could rescue disease in the Mdx mice with overexpression of Klotho and Utrophin, and promote the transdifferentiation of liver cells to insulin producing cells with Pdx1 activation. Although the authors delivered SpCas9 with an AAV9 vector, the vast majority of the work involved the SpCas9 transgenic mice, as efficient delivery of full length SpCas9 with AAV remains challenging. Nonetheless, this work is an impressive example of the power of CRISPR/Cas9 for target gene activation in vivo, and could be applied to lipid genes for therapeutic benefit. In addition to epigenetic regulation at the genomic level, there is also considerable interest in CRISPR effectors for RNA targeting. Konermann et al.[27▪▪] developed a compact RNA-specific nuclease from Ruminococcus flavefaciens XPD3002, termed CasRx. A catalytically dead version of CasRx was fused to a fragment of the negative splicing factor hnRNPa1 to mediate exon skipping in a gRNA-dependent manner. By targeting cis-acting elements in the premRNA of MAPT for exon exclusion, the authors succeeded in reducing the 4R/3R tau ratios to a normal level in human-induced pluripotent stem cell-derived cortical neurons. Although delivery of large CRISPR-based epigenetic regulators is challenging, it is clear that this is an exciting avenue for further innovation and discovery.
Single nucleotide variants (SNVs) are the most common cause of monogenic diseases in humans. Although correcting SNVs with CRISPR/Cas9 is possible with HDR approaches, these are limited by the requirement for cell division, and still involve the generation of DSBs, which can introduce unwanted modifications. David Liu's group developed the first base editor in 2016 – a novel approach to make more precise single nucleotide changes [28]. Base editors allow for the specific conversion of a single base without generating a DSB. A dead Cas9 or Cas9 nickase is fused to a deaminase on its N-terminus, often in conjunction with a uracil glycosylase inhibitor on its C-terminus. Base editors can bind DNA in a gRNA-dependent manner and convert a cytidine to uridine, facilitating a C-T transition [28], or an adenine to an inosine, facilitating an A-G transition [29▪▪], within a small editing window. The occurrence of off-targets with base editing is believed to be higher than that of traditional CRISPR (https://www.biorxiv.org/content/early/2018/11/27/480145). In order to reduce the amount of off-targeting by base editors, several modifications have been made to expand the range of targetable sites and reduce the amount of off-target base switching [3▪,29▪▪,30▪▪,31▪▪,32▪]. As base editors are so large in size, they have been delivered into animals by adenovirus or with AAV using a split-intein system [33▪▪]. Base editors have been used to successfully treat hearing loss [34▪], and dyslipidemias in adult mice [21▪▪,35▪] and in utero [36▪▪].
In 26 November 2018, Dr He Jiankui announced that he had created the first two genetically engineered human beings [37▪▪]. CRISPR/Cas9 was used to disrupt the CCR5 gene in early human embryos with the use of in-vitro fertilization. The CCR5 gene encodes the major receptor for HIV, and individuals with loss of CCR5 are protected from HIV infection. Two of the manipulated embryos were implanted into the female donor, and the pregnancy resulted in a pair of twin girls. On the basis of data presented at the Second International Summit on Human Genome Editing in Hong Kong, one of the twins completely lacks CCR5, whereas the other has at least one normal copy of the gene. Dr Jiankui's announcement provoked widespread condemnation from the scientific community. Many expressed concern that there were simpler ways to prevent HIV transmission, and there were not sufficient grounds to justify this risky procedure. Questions have been raised about the trial itself, and whether regulatory approval and informed consent were properly obtained. Genome editing experts have criticized the secretive manner in which the work was done, particularly the general disregard for the ethical norms of the scientific community.
These developments highlight a problem with emerging technologies like genome editing. The field is moving very fast, easily outpacing the public's understanding of what is happening. Editing the human germline represents a significant departure from everything that has been done up until this point in medicine. By changing our genetic code, we are changing the very essence of what it means to be human. The first and most fundamental question is whether this should be done at all? How can we be certain that germline genome editing is well tolerated, and that debilitating side effects will not occur? How could we deal with the daunting problem of mosaicism, where different edits happen in different cells? What about off-targets? Costly genetic treatments may widen the socioeconomic gap because only the wealthy will be able to prevent their children from having life-altering disease. When does genome editing become enhancement rather than therapeutic? The concern is that this technology will allow us to engineer designer babies with all of our preferred skills and attributes. In its extreme form, this would create two classes of human beings – those who are genetically engineered and those who are not. It is important to realize that everyone has a stake in this issue, not just the patients or families participating in a trial, or scientists passionate about advancing the technology.
In summary, CRISPR/Cas9 is a powerful research tool for studying lipid metabolism and physiology. If important safety and ethical concerns can be addressed, it has tremendous potential for the treatment of lipid disorders and cardiovascular disease. We can expect exciting discoveries and further developments in the coming years from this transformative technology.
Box 1.
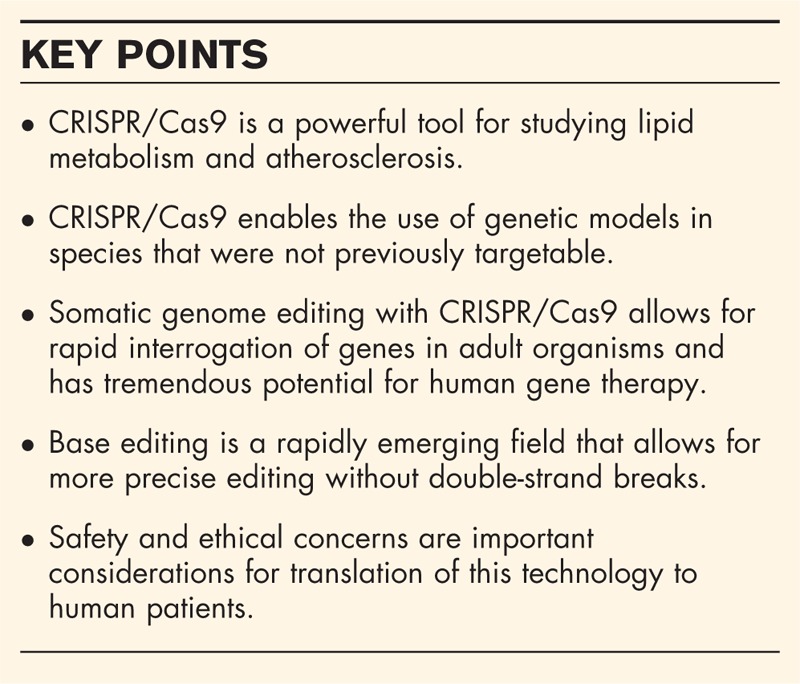
no caption available
Acknowledgements
The authors wrote this article in its entirety without further assistance.
Financial support and sponsorship
This publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL132840. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding for this work was received from the National Institutes of Health (NIH).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Kim E, Koo T, Park SW, et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun 2017; 8:14500. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the first use of a small Cas9 ortholog from Campylobacter jejuni in vivo in the retina.
- 2▪.Edraki A, Mir A, Ibraheim R, et al. A compact, high-accuracy Cas9 with a dinucleotide PAM for in vivo genome editing. Mol Cell 2019; 73:714.e4–726.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates efficient in-vivo genome editing for Pcsk9 using the Neisseria meningitidis Cas9, which has a simple N4CC PAM and can be delivered with AAV vectors.
- 3▪.Hu JH, Miller SM, Geurts MH, et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 2018; 556:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors engineer a new Cas9 variant called xCas9, which can recognize additional PAM sequences including NG, GAA and GAT, improving targeting options.
- 4.Kleinstiver BP, Prew MS, Tsai SQ, et al. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat Biotechnol 2015; 33:1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slaymaker IM, Gao L, Zetsche B, et al. Rationally engineered Cas9 nucleases with improved specificity. Science 2016; 351:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinstiver BP, Pattanayak V, Prew MS, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016; 529:490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson CE, Hakim CH, Ousterout DG, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 2016; 351:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki K, Tsunekawa Y, Hernandez-Benitez R, et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016; 540:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 2014; 32:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪.Pannunzio NR, Watanabe G, Lieber MR. Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J Biol Chem 2018; 293:10512–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a useful review of the NHEJ repair mechanism.
- 11▪.Wright WD, Shah SS, Heyer WD. Homologous recombination and the repair of DNA double-strand breaks. J Biol Chem 2018; 293:10524–10535. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of the mechanisms of double stranded break repair by homologous recombination.
- 12.Wang H, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013; 153:910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Zhao Y, Yang Y, Xing R, et al. Hyperlipidemia induces typical atherosclerosis development in Ldlr and Apoe deficient rats. Atherosclerosis 2018; 271:26–35. [DOI] [PubMed] [Google Scholar]; This article describes the generation of the first rat model of atherosclerosis lacking ApoE.
- 14▪.Huang L, Hua Z, Xiao H, et al. CRISPR/Cas9-mediated ApoE-/- and LDLR-/- double gene knockout in pigs elevates serum LDL-C and TC levels. Oncotarget 2017; 8:37751–37760. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the use of CRISPR/Cas9 and somatic cell nuclear transfer to generate pigs lacking both ApoE and LDLR.
- 15▪.Guo X, Gao M, Wang Y, et al. LDL receptor gene-ablated hamsters: a rodent model of familial hypercholesterolemia with dominant inheritance and diet-induced coronary atherosclerosis. EBioMedicine 2018; 27:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports the generation and characterization of LDLR-deficient hamsters, which share key features of human lipoprotein metabolism including cholesteryl ester transfer protein and intestine-only ApoB editing.
- 16▪.Lu R, Yuan T, Wang Y, et al. Spontaneous severe hypercholesterolemia and atherosclerosis lesions in rabbits with deficiency of low-density lipoprotein receptor (LDLR) on exon 7. EBioMedicine 2018; 36:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports the first genetically engineered model of LDLR deficiency in rabbits, which develop severe hypercholesterolemia and atherosclerosis on a chow diet.
- 17.Ding Q, Strong A, Patel KM, et al. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res 2014; 115:488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Raghavan A, Chen T, et al. CRISPR-Cas9 targeting of PCSK9 in human hepatocytes in vivo-brief report. Arterioscler Thromb Vasc Biol 2016; 36:783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ran FA, Cong L, Yan WX, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015; 520:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Musunuru K. How genome editing could be used in the treatment of cardiovascular diseases. Per Med 2018; 15:67–69. [DOI] [PubMed] [Google Scholar]; Recent review describing potential targets for genome editing as a treatment for cardiovascular diseases.
- 21▪▪.Chadwick AC, Evitt NH, Lv W, Musunuru K. Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation 2018; 137:975–977. [DOI] [PMC free article] [PubMed] [Google Scholar]; Base editing was used to introduce loss-of-function mutations into ANGPTL3 in vivo as an approach to lower plasma triglycerides.
- 22▪.Jarrett KE, Lee CM, Yeh YH, et al. Somatic genome editing with CRISPR/Cas9 generates and corrects a metabolic disease. Sci Rep 2017; 7:44624. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates that liver-directed genome editing can be used to model and correct a systemic metabolic disease, through disruption of Ldlr and Apobi.
- 23▪.Jarrett KE, Lee C, De Giorgi M, et al. Somatic editing of Ldlr with adeno-associated viral-CRISPR is an efficient tool for atherosclerosis research. Arterioscler Thromb Vasc Biol 2018; 38:1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports an all-in-one AAV vector that can be used to model atherosclerosis in mice through disruption of the LDL receptor.
- 24▪▪.Fedoseienko A, Wijers M, Wolters JC, et al. The COMMD family regulates plasma LDL levels and attenuates atherosclerosis through stabilizing the CCC complex in endosomal LDLR trafficking. Circ Res 2018; 122:1648–1660. [DOI] [PubMed] [Google Scholar]; In this study, the authors used somatic genome editing to assess the role of the CCC complex component CCDC22 in endosomal LDLR recycling to clear plasma LDL-C in mice.
- 25▪▪.Thakore PI, Kwon JB, Nelson CE, et al. RNA-guided transcriptional silencing in vivo with S. aureus CRISPR-Cas9 repressors. Nat Commun 2018; 9:1674. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report of targeted gene silencing through transcriptional repression in vivo, using a catalytically dead Cas9 fused to a KRAB domain to silence Pcsk9.
- 26▪▪.Liao HK, Hatanaka F, Araoka T, et al. In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation. Cell 2017; 171:1495.e15–1507.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report of a targeted gene activation in vivo with AAV delivery. This modular vector system uses truncated gRNA and aptamers to recruit a transactivation domain to Cas9 binding sites.
- 27▪▪.Konermann S, Lotfy P, Brideau NJ, et al. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 2018; 173:665–676. e614. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors describe a new CRISPR nuclease that can be used for epigenetic control of RNA splicing
- 28.Komor AC, Kim YB, Packer MS, et al. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016; 533:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪▪.Gaudelli NM, Komor AC, Rees HA, et al. Programmable base editing of A∗T to G∗C in genomic DNA without DNA cleavage. Nature 2017; 551:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes a new base editing enzyme that can convert Adenine to Guanine, greatly expanding the number of disease-causing variants that could be targeted.
- 30▪▪.Kim YB, Komor AC, Levy JM, et al. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat Biotechnol 2017; 35:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work develops new base editors with broadened PAM specificity, increasing the number of targetable sites. Additional modifications narrow the editing window from five to two nucleotides.
- 31▪▪.Rees HA, Komor AC, Yeh WH, et al. Improving the DNA specificity and applicability of base editing through protein engineering and protein delivery. Nat Commun 2017; 8:15790. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article described a high fidelity base editor and uses a novel delivery method of purified ribonucleoproteins to achieve in-vivo base editing of the inner ear.
- 32▪.Koblan LW, Doman JL, Wilson C, et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat Biotechnol 2018; 36:843–846. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article uses ancestral reconstruction to optimize the deaminase component of base editors, and protein engineering to improve the expression and efficiency of these enzymes.
- 33▪▪.Villiger L, Grisch-Chan HM, Lindsay H, et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat Med 2018; 24:1519–1525. [DOI] [PubMed] [Google Scholar]; This article used a split-intein system to deliver C to T base editors with AAV vectors to correct a point mutation that causes phenylketonuria.
- 34▪.Gao X, Tao Y, Lamas V, et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature 2018; 553:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article establishes cationic lipid-mediated in-vivo delivery of CRISPR/Cas9 complexes to the inner ear as a treatment for hearing loss.
- 35▪.Chadwick AC, Wang X, Musunuru K. In vivo base editing of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) as a therapeutic alternative to genome editing. Arterioscler Thromb Vasc Biol 2017; 37:1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article introduces the concept of in-vivo base editing to disrupt a disease-relevant gene by targeting Pcsk9.
- 36▪▪.Rossidis AC, Stratigis JD, Chadwick AC, et al. In utero CRISPR-mediated therapeutic editing of metabolic genes. Nat Med 2018; 24:1513–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article leverages the tyrosine catabolic pathway to promote selective expansion of hepatocytes harboring base edits in Pcsk9.
- 37▪▪.Cyranoski D, Ledford H. Genome-edited baby claim provokes international outcry. Nature 2018; 563:607–608. [DOI] [PubMed] [Google Scholar]; This reference describes the revelation of successful human germline genome editing in 2018.


