Abstract
Purpose of review
We review current knowledge regarding HDL and Alzheimer's disease, focusing on HDL's vasoprotective functions and potential as a biomarker and therapeutic target for the vascular contributions of Alzheimer's disease.
Recent findings
Many epidemiological studies have observed that circulating HDL levels associate with decreased Alzheimer's disease risk. However, it is now understood that the functions of HDL may be more informative than levels of HDL cholesterol (HDL-C). Animal model studies demonstrate that HDL protects against memory deficits, neuroinflammation, and cerebral amyloid angiopathy (CAA). In-vitro studies using state-of-the-art 3D models of the human blood–brain barrier (BBB) confirm that HDL reduces vascular Aβ accumulation and attenuates Aβ-induced endothelial inflammation. Although HDL-based therapeutics have not been tested in clinical trials for Alzheimer's disease , several HDL formulations are in advanced phase clinical trials for coronary artery disease and atherosclerosis and could be leveraged toward Alzheimer's disease .
Summary
Evidence from human studies, animal models, and bioengineered arteries supports the hypothesis that HDL protects against cerebrovascular dysfunction in Alzheimer's disease. Assays of HDL functions relevant to Alzheimer's disease may be desirable biomarkers of cerebrovascular health. HDL-based therapeutics may also be of interest for Alzheimer's disease, using stand-alone or combination therapy approaches.
Keywords: Alzheimer's disease, blood–brain barrier, cerebral amyloid angiopathy, cerebrovasculature, dementia, HDL
INTRODUCTION
Alzheimer's disease is the leading cause of senile dementia with over 44 million affected persons and an economic burden of over $600 billion [1]. Beyond the beta-amyloid (Aβ) plaques and neurofibrillary tangles that define Alzheimer's disease, 60–90% of Alzheimer's disease brains have evidence of cerebral vessel disease [2]. No effective disease-modifying drugs for Alzheimer's disease exist despite decades of promising research [3]. This may be due, in part, to the complex interplay of amyloid and tau disorders, neuroinflammation and cerebrovascular compromise, and significant challenges in defining and staging Alzheimer's disease. Studies in humans, animals, and in-vitro models support the hypothesis that circulating HDL, which have established vasoprotective properties, may also provide resilience to cerebrovascular dysfunction in Alzheimer's disease. In this review, we synthesize these data toward a rationale to develop HDL functional assays as potential biomarkers of cerebrovascular health and to consider clinical trials that evaluate HDL-based therapies for Alzheimer's disease.
Box 1.
no caption available
THE CEREBROVASCULATURE AND ITS RELATIONSHIP WITH ALZHEIMER'S DISEASE
Despite constituting only 2% of total body mass, the brain consumes approximately 20% of total cardiac output [4]. The brain's high metabolic activity and lack of glucose stores requires extensive vascularization to enable oxygen and glucose influx, maintain ion balance, and remove neurotoxic waste products [5]. Most dementia cases exhibit vascular disorders that may underlie compromised cerebrovascular function [6]. Histopathological evidence for cerebrovascular dysfunction in Alzheimer's disease includes arteriole and precapillary deformities [7], reduced vascular density [8,9], increased vessel tortuosity [9], and vessel remnants that lack endothelial cells [10–12]. Large-scale autopsy studies by the National Alzheimer's Coordinating Center and the Religious Orders Study and Rush Memory and Aging Project found a greater burden of macroinfarcts and microinfarcts, atherosclerosis, arteriosclerosis, and cerebral amyloid angiopathy (CAA) in Alzheimer's disease compared with other neurodegenerative diseases [6], and increased Alzheimer's disease risk in cases with infarcts and more severe atherosclerosis or arteriosclerosis [13], respectively.
Analysis of 7700 multimodality images from the Alzheimer's Disease Neuroimaging Initiative identified cerebrovascular dysfunction as an early event in Alzheimer's disease. This study compared cerebral blood flow (CBF) alterations measured with arterial spin labelling MRI to the progression of amyloid, structural, metabolic, and functional brain changes in Alzheimer's disease [14▪]. Others have found that dementia risk is higher in subjects with reduced CBF measured with transcranial Doppler [15] and in people with microbleeds observed on MRI [16,17]. Greater arterial stiffness measured by pulse wave velocity associates with greater Aβ burden on PET imaging, lower brain volume in certain brain regions, and more white matter hyperintensities (WMH) on MRI [18]. Dynamic contrast-enhanced MRI shows that hippocampal blood–brain barrier (BBB) breakdown is age-dependent, worsens in mild cognitive impairment (MCI) [19], and occurs in early stages of cognitive impairment independent of Aβ or tau biomarker changes [20▪]. MRI sequences evaluating disrupted CBF and cerebral small vessel disease were proposed as vascular biomarkers for the new amyloid, tau, and neurodegeneration (ATN) research framework developed by the National Institute on Aging and Alzheimer's Association (NIA-AA) to provide a biological definition of Alzheimer's disease [21].
The cerebrovasculature plays a pivotal role in removing Aβ from the brain through active transport across brain endothelial cells in a process involving various receptors including LDL receptor-related protein (LRP1), p-glycoprotein, and LDLR. Aβ is also cleared from the brain via perivascular drainage in mid-sized and large-sized arteries along smooth muscle cell basement membranes [22]. Disruption of Aβ clearance via cerebrovascular pathways may contribute to CAA [23].
Vascular comorbidities in Alzheimer's disease
The importance of the vasculature in Alzheimer's disease is further supported by associations between cardiovascular diseases (CVD) and Alzheimer's disease risk [24–26]. Genetic variations in human apolipoprotein E (apoE) increase Alzheimer's disease risk and reduce age of Alzheimer's disease onset with APOE-ε4 being detrimental, APOE-ε3 neutral and APOE-ε2 protective [27]. In addition to accelerating amyloidogenesis [28], APOE-ε4 contributes to reduced CBF, CAA, cerebrovascular inflammation, altered neurovascular coupling, BBB leakiness, and reduced cerebrovascular resilience to cardiometabolic risk factors (reviewed in [29,30]). Alzheimer's disease and CVD also share many cardiometabolic risk factors including age, sex, smoking, blood pressure, physical activity, blood lipids, and type II diabetes mellitus (T2DM) [31▪,32,33]. Several of these factors have been combined into the Cardiovascular Risk Factors Aging and Dementia risk score, which correlates with executive function, visual perception, and construction, WMH and CSF Aβ and tau in healthy adults [34]. Furthermore, the population-based Rotterdam Study found that an MRI-based cerebral small vessel disease score was associated with greater dementia risk [35] and the Framingham cardiovascular risk profile score predicts conversion from MCI to Alzheimer's disease within 24 months [36].
HDL AND VASCULAR RESILIENCE
Circulating HDL is best known for its pivotal role in reverse cholesterol transport [37]. Only one-third of the identified 95 proteins on HDL [38] have roles in lipid metabolism [39,40] whereas others function in protease inhibition, complement regulation, hemostasis, and inflammation [41]. Known vasoprotective functions of HDL include promoting endothelial nitric oxide (NO) synthase activity, reducing inflammation, and suppressing vascular adhesion molecule expression [42–46]. Importantly, aging and vascular disease can impair these functions [42,47–49].
MIXED GENETIC EVIDENCE ON HDL AND VASCULAR RESILIENCE
Mendelian randomization aims to determine the causality of a modifiable risk factor on disease risk by measuring how disease risk changes based on randomly distributed genetic variants that affect the risk factor [50]. Although it is well accepted that high plasma HDL-C levels associate with reduced heart disease mortality [51], Mendelian randomization questions the causality of this relationship. Several groups observe that genetic variants associated with HDL-C do not alter coronary heart disease (CHD), myocardial infarction, or carotid atherosclerosis risk [52–54], although one study found that an allele score based on all known genetic variants associated with HDL-C was significantly associated with CHD risk [52]. Two Mendelian randomization studies also suggest HDL-C levels are not causal for Alzheimer's disease risk [55,56]. Importantly, these studies address only a causal link between disease risk and elevated HDL-C levels mediated by particular genes; they do not take into account the complex changes to HDL function and composition that can occur in disease and that can be superior predictors of disease risk [47–49,57–62]. Recently, two large genome-wide association studies (GWAS) for Alzheimer's disease found lipoprotein metabolism and HDL particle gene sets to be significantly associated with Alzheimer's disease risk. Genes in these sets encode HDL biogenesis proteins and HDL protein components, such as APOE, ABCA1, APOC1, APOM, APOA2, PON1, CLU, LCAT, CETP, and APOAI[63,64].
EPIDEMIOLOGICAL EVIDENCE FOR A PROTECTIVE EFFECT OF HDL ON ALZHEIMER'S DISEASE
Several studies show that Alzheimer's disease risk is attenuated by higher levels of HDL cholesterol (HDL-C) or apoA-I, the major protein component of HDL [65]. Cross-sectional studies showed serum apoA-I and HDL-C levels are significantly lower in Alzheimer's disease patients and inversely correlated with Mini Mental State Examination (MMSE) scores [66,67]. A role for HDL in Aβ clearance is suggested by positive correlations between plasma apoA-I and Aβ40 in CAA patients [68], and an inverse correlation between plasma HDL-C and brain amyloid burden in cognitively normal people on PET [69]. In people without dementia, positive associations have been found between HDL-C levels and working memory [70,71], MMSE scores [70], and verbal learning scores [71]. The prospective Honolulu-Aging study followed 929 Japanese-American men and found that the highest quartile of plasma apoA-I at baseline correlated with the lowest risk of dementia 16 years later [72]. Similarly, those with the highest baseline HDL-C in a cohort of 1130 elderly people in New York followed for a median of 4 years had reduced Alzheimer's disease risk [73] and higher baseline HDL-C in the Baltimore Longitudinal Study of Aging protected against cognitive impairment and brain volume reductions 20 years later [74▪▪].
However, other cross-sectional studies including the Framingham study of 1100 elderly participants [75] and a small cohort of Spanish nonagenarians [76] and prospective studies including the Adult Changes in Thought study and two studies in cognitively normal elderly women [77–80] found no relationship between HDL-C and cognitive impairment. Baseline age and follow-up length may explain these inconsistencies [72,78]. Indeed, the above studies with follow-up times greater than 10 years found significant associations between HDL-C levels and Alzheimer's disease risk [72,74▪▪] whereas others with less than 10 years of follow-up did not [78,80]. Furthermore, those measuring baseline HDL-C levels at middle age all found significant associations with Alzheimer's disease risk [67,71,72] whereas those with baseline measures in subjects at least 70 years old did not [79,80]. HDL may, therefore, exert its greatest influence on Alzheimer's disease risk at mid-life.
The mechanisms by which HDL influences Alzheimer's disease risk remain unknown. Many HDL-associated proteins, such as apoA-I, apoJ, apoE, apoC-III, apoD, and apoA-IV are present within the brain parenchyma, cerebrospinal fluid (CSF), and cerebrovascular intima of leptomeningeal arteries [81–84]. Except for apoE, the CSF levels of these proteins correlate moderately with their respective levels in plasma, suggesting transport or diffusion from the periphery to the brain. Although it has been reported that HDL can be transported through human brain microvascular endothelial cells via scavenger receptor (SR)-BI [85] and CSF lipoproteins are similar in density to plasma HDL [86], there is currently no evidence that HDL enters the brain as an intact particle in vivo. Therefore, HDL might indirectly influence brain health as a circulating factor primarily acting from the cerebrovascular lumen and intima (Fig. 1).
FIGURE 1.
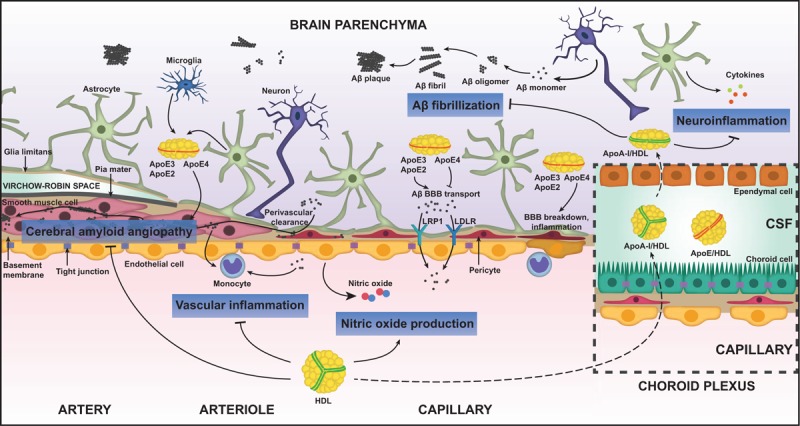
Vasoprotective functions of HDL relevant for Alzheimer's disease. HDL has been shown to have at least four distinctive functions that could protect against Alzheimer's disease. HDL suppresses the pathological accumulation of Aβ in cerebral vessels known as cerebral amyloid angiopathy (CAA). HDL suppresses vascular inflammation induced by Aβ or pro-inflammatory cytokines and global neuroinflammation in Alzheimer's disease. HDL stimulates the production of nitric oxide from brain endothelial cells. HDL delays the fibrillization of Aβ. Although large, spherical HDL is unlikely to cross the blood–brain barrier, apoA-I can gain access to the brain via the blood–CSF barrier at the choroid plexus. HDL-like particles in the brain are mainly apoE-based. ApoE is found in three isoforms in humans; apoE2, apoE3, and apoE4. APOε4 is the major genetic risk factor for late-onset Alzheimer's disease and apoE4 has several detrimental functions including delaying Aβ transport out of the brain, promoting blood–brain barrier breakdown, and increasing neuroinflammation. ApoE is also found in the CSF along with apoA-I. Aβ, amyloid beta; apoA-I, apolipoprotein A-I; apoE, apolipoprotein E; BBB, blood–brain barrier; CSF, cerebrospinal fluid; HDL, high-density lipoprotein; LDLR, low-density lipoprotein receptor; LRP-1, low-density lipoprotein receptor-related protein 1.
VASOPROTECTIVE FUNCTIONS OF HDL IN ALZHEIMER'S DISEASE ANIMAL MODELS
Studies in mice genetically engineered to develop amyloid have explored how HDL levels affect Alzheimer's disease-relevant outcomes. Genetic ablation of apoA-I worsened memory deficits and increased CAA in APP/PS1 mice, a common Alzheimer's disease model [87], without altering parenchymal Aβ plaque load [87,88]. Conversely, APP/PS1 mice with transgenic apoA-I overexpression exhibited attenuated memory deficits, CAA, and neuroinflammation [89]. Treatment of Alzheimer's disease mice with HDL-based therapeutics resulted in similar improvements [90–93].
Although these studies have contributed toward understanding how HDL may protect from cerebrovascular dysfunction in Alzheimer's disease, they may have only modest translational value because of differences in the distribution of circulating lipoproteins between rodents and humans. In mice, circulating lipids are mainly carried by HDL whereas in humans they are mainly carried by LDL [94]. These differences are, in part, governed by the activity of cholesterol ester transfer protein (CETP). CETP facilitates exchange of cholesteryl esters and triglycerides between lipoprotein subclasses and high CETP activity associated with lowered HDL-C levels [95]. However, mice and rats do not express CETP, which may partly underlie their high HDL-C levels [96]. Mice genetically engineered to express human CETP have a moderate dose-dependent reduction of HDL in the presence of both murine, and human apoA-I, but no change in other lipoprotein pools [96,97]. In addition, the murine and human APOE genes are substantially different [98] and extensive efforts have been made to develop targeted replacement or transgenic mice expressing each human APOE isoform [99–105], yet, these models may still under-report cerebrovascular compromise because of the high levels of circulating HDL. To our knowledge, there has not been a concerted effort to produce an animal model combining expression of human apoE, apoA-I, CETP, APP, and tau to improve the predictive power of murine models with respect to the vascular contributions to Alzheimer's disease.
MECHANISTIC STUDIES OF HDL-MEDIATED VASOPROTECTION IN IN-VITRO MODELS
Developing human-based vascular models that retain anatomical and physiological similarities to humans are, therefore, highly desirable to overcome the difficulties of translating research from mice to humans. Many BBB studies have been performed using two dimensional (2D) cell culture of human brain endothelial cells from primary, immortalized, or pluripotent stem cell sources [106–114]. However, as cells behave differently in 3D compared with 2D environments [115], 3D BBB models are considered superior. Trans-well systems offer highly reproducible models for permeability assays [116,117] but lack complex cell–cell and cell–matrix interactions. Multicellular spheroids of human primary brain endothelial cells, pericytes, and astrocytes spontaneously self-organize into a BBB-like structure [118,119] but are not perfusible. Several ‘organ-on-a-chip’ approaches have been developed to overcome these barriers, beginning with microfluidic models culturing primary murine neurons and glia cells with human cerebral endothelial cells [120]. Completely human-based systems have also been developed using iPSC-derived endothelial cells, primary pericytes, and astrocytes [121▪]. Maoz et al.[122▪] developed an innovative microfluidic system linking a BBB chip to a brain chip, however, this model lacks anatomical connections between cells of the neural vascular unit. Our group developed a 3D bioengineered human vessel model using a scaffold-directed dynamic pulsatile flow bioreactor system, populated with primary human endothelial cells, smooth muscle cells, and astrocytes [123▪▪,124▪▪]. These engineered tissues display histological features of native peripheral and cerebral arteries and can be used to model CAA and vascular inflammation. This model can also be used to interrogate four beneficial functions of HDL on cerebral vessels, namely preventing Aβ-induced endothelium activation, reducing Aβ vascular accumulation, maintaining Aβ in a soluble state, and inducing endothelial NO secretion [123▪▪,124▪▪,125] (Fig. 1).
CONSIDERATIONS TO EVALUATE HDL AS A POTENTIAL THERAPEUTIC AGENT FOR THE VASCULAR CONTRIBUTIONS TO ALZHEIMER'S DISEASE
The human, animal, and in-vitro studies discussed above provide support for HDL-based therapeutic approaches to protect or repair the BBB. Several HDL-based therapeutics for CVD have advanced to clinical trials and have both safety and efficacy data (Table 1). The recombinant apoA-I protein CER-001 [126–128], apoA-I mimetics, such as D-4F [129,130] and L-4F [131], the plasma-derived apoA-I formulation CSL-112 [132], and autologous administration of patient-derived apoA-I [133] were all well tolerated in phase I clinical trials for acute coronary syndrome or stable CHD. Although development of many of these agents was halted because of failure to meet primary outcomes of reduced atherosclerosis [126–128] or improved HDL function [131], CSL-112 and autologous apoA-I administration have shown promise and are undergoing phase III trials (NCT03473223, NCT03135184).
Table 1.
HDL-based therapeutics in clinical trials for cardiovascular diseases and under investigation for dementia
| Indication | HDL-targeting approach | Drug type | Drug name | Study population | Safety | Efficacy | References |
| Cardiovascular disease | Direct | Recombinant apoA-I | CER-001 | Acute coronary syndrome | No issues | No improvement to atherosclerosis | [126,127,128] |
| ApoA-I mimetic | D-4F | Coronary heart disease | No issues | Improved anti-inflammatory activity of HDL | [129,130] | ||
| L-4F | Coronary heart disease | No issues | No improvement to HDL function | [131] | |||
| Reconstituted HDL | CSL-112 | Acute coronary syndrome | No issues | May improve cholesterol efflux function of HDL | [132] | ||
| Autologous administration | Acute coronary syndrome | No issues | Tended to reduce atherosclerosis | [133] | |||
| Indirect | ApoA-I transcription inducer | RVX-208 | Atherosclerosis | Elevated liver transaminase levels | No improvement to atherosclerosis | [134,135] | |
| LCAT recombinant protein | ACP-501 | Stable atherosclerotic cardiovascular disease | No issues | Improved HDL metabolism | [136] | ||
| Niacin | Niacin | Cardiovascular disease events | Flushing | Reduced CVD events, may be independent of HDL | [137,138,139] | ||
| CETP inhibitors | Dalcetrapib | Acute coronary syndrome | No issues | No effect on cardiovascular events | [140] | ||
| Evacetrapib | High-risk vascular disease | No issues | No effect on cardiovascular events | [141] | |||
| Torcetrapib | High-risk for coronary events | Increased mortality and morbidity | Increased risk of cardiovascular events | [142] | |||
| Anacetrapib | Atherosclerotic vascular disease | No issues | Reduced major coronary events | [143] | |||
| Dementia | Indirect | Statins | Various | Dementia | Possible short-term memory impairment | Improvements in prospective trials, no improvements in RCT | [150,151,152,153] |
| Niacin | Niacin | Dementia | Flushing | Protective effects in retrospective studies | [154,155] | ||
| ABCA1 modulators | Bexarotene | Dementia | No issues | Raised CSF apoE, no improvements to cognitive function | [168] |
ABCA1, ATP-binding cassette transporter A1; apoA-I, apolipoprotein A-I; apoE, apolipoprotein E; CETP, cholesteryl ester transfer protein; CSF, cerebrospinal fluid; LCAT, lecithin-cholesterol acyltransferase; RCT, randomized control trial.
Indirect HDL-based therapeutics include the apoA-I transcription up-regulator RVX-208, the lecithin-cholesterol acyltransferase (LCAT) recombinant protein ACP-501, niacin, and CETP inhibitors (Table 1). RVX-208 lacked efficacy against atherosclerosis and caused a dose-dependent increase in liver transaminase levels [134,135]. ACP-501 was well tolerated in stable CHD patients [136] and is undergoing a phase II trial evaluating its effects on apolipoprotein B metabolism in CVD patients (NCT03773172). Early trials suggested niacin treatment could reduce cardiovascular events and atherosclerosis [137], however, two large randomized control trials (RCT) were terminated because of lack of efficacy [138,139]. Several trials for CETP inhibitors were terminated early because of futility or safety issues including increased mortality in the case of torcetrapib [140–142]. However, the most recent phase III trial of the potent CETP inhibitor anacetrapib had no adverse effects and reduced major coronary events [143]. CETP inhibitors may be especially useful for repurposing for Alzheimer's disease as certain CETP polymorphisms are associated with Alzheimer's disease risk and memory decline, particularly in APOE4 carriers [144–146].
Evaluation of HDL-based therapeutics on Alzheimer's disease-relevant outcomes in animal models
Although no HDL-based therapeutic strategies have been tested for Alzheimer's disease in clinical trials, several preclinical studies have been performed in Alzheimer's disease mice. Intravenous administration of reconstituted HDL reduced soluble brain Aβ levels in APP/PS1 mice [90] as well as in SAMP8 mice [90], where it also reduced microgliosis and memory deficits [91]. APP23 mice treated intravenously with recombinant apoA-I Milano had reduced microgliosis, Aβ deposition, and CAA [93]. Oral D-4F treatment improved memory, Aβ deposition, microgliosis, astrogliosis, and other markers of inflammation in APPswe/PS1ΔE9 mice [92]. Outside the context of Alzheimer's disease, D-4F treatment after middle cerebral artery occlusion reduced neuroinflammation and white matter damage [147] and D-4F improved cognition and reduced brain arteriole inflammation in atherosclerotic mice [148].
Additional lipid-modifying therapeutics for the prevention and treatment of dementia
Lipid-modifying approaches not directly targeting HDL may also be of interest for Alzheimer's disease (Table 1). Statins inhibit 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase to block cholesterol synthesis and subtly increase the HDL:LDL ratio [149]. Meta-analyses suggest statin use lowers dementia risk in prospective trials [150–152] but not in two large RCTs [150,153]. Retrospective cohort studies on niacin found higher intake during young adulthood improved some measures of cognitive function 25 years later [154], and older adults with higher intake had reduced risk of Alzheimer's disease and cognitive decline over 6 years of follow-up [155], however, these studies lacked direct measurement of blood niacin levels.
Drugs targeting ATP-binding cassette A1 (ABCA1), such as liver-x-receptor (LXR) and retinoid-x-receptor (RXR) agonists, are another potential indirect HDL-based therapy as the rate-limiting step of HDL biogenesis involves ABCA1-mediated efflux [156–158]. Direct LXR and RXR agonists increase plasma HDL-C levels [159–162], central nervous system (CNS) apoE lipidation, and cognitive function in Alzheimer's disease animal models (reviewed in [163]). Significant hepatotoxic and systemic side effects have hampered clinical development of direct LXR/RXR agonists [164–166], although new, LXR-independent ABCA1 modulators may avoid these liabilities [167]. The first ABCA1-targetting compound to reach clinical trials was the RXR agonist bexarotene, which in a phase I trial raised CSF apoE levels but had poor bioavailability [168] (Table 1).
LEVERAGING HDL AS A POTENTIAL THERAPEUTIC TO PROTECT AND REPAIR THE CEREBROVASCULATURE IN ALZHEIMER'S DISEASE
The considerable evidence for the safety of several HDL-based therapeutics in clinical trials suggest these agents could be potentially repurposed for Alzheimer's disease. Specifically, HDL may be of interest to prevent CAA and Alzheimer's disease-related neuroinflammation based on its effects in mouse models [87,89,92,93,169] and 3D bioengineered human arteries [123▪▪,124▪▪,125]. HDL may also be developed as a carrier for drugs and microRNAs to overcome the issue of BBB penetrance in drug delivery. Already, a reconstituted HDL carrying an Aβ-targeting drug has been shown to enter Alzheimer's disease mouse brains, reduce amyloidosis, and improve memory [170].
HDL AS A POTENTIAL PREDICTIVE BIOMARKER FOR VASCULAR COMPROMISE IN ALZHEIMER'S DISEASE
Biomarker research for Alzheimer's disease has rapidly progressed in recent years with the development of imaging techniques to visualize Aβ and tau deposits in living people and breakthroughs in fluid biomarker sensitivity and specificity [171]. As HDL can be isolated from the blood of Alzheimer's disease patients and assayed in-vitro, it may be possible to develop HDL-based assays that specifically report on cerebrovascular health, particularly if they correlate with cerebrovascular disorders, such a CAA, microinfarcts, or WMH. Again, there is currently no evidence that HDL can enter the brain parenchyma as an intact particle in vivo, instead HDL circulating in the lumen of cerebral vessels is proposed to impact brain health through effects on vessel health. It is well understood that HDL composition and function is altered by aging and in T2DM, and CAD patients [47–49,57–60]. Reduced cholesterol efflux and anti-inflammatory activity have also been observed in HDL from Alzheimer's disease subjects [172,173]. Such changes to HDL function, or to other Alzheimer's disease-relevant functions including modifying CAA, attenuating Aβ-induced endothelial activation, maintaining Aβ solubility, and promoting NO secretion [123▪▪,124▪▪,125], have the potential to act as predictive or prognostic biomarkers for Alzheimer's disease.
Predictive biomarkers are used to stratify patient populations into subpopulations that would benefit from certain therapeutic strategies [174]. HDL functional assays reporting on cerebrovascular dysfunction could, therefore, act as predictive biomarkers for Alzheimer's disease patients who may benefit from vascular-specific therapies. Whether HDL functions can predict risk, progression, or resolution of amyloid-related imaging abnormalities (ARIA) resulting from vascular Aβ clearance in response to anti-Aβ immunotherapies may also be interesting to evaluate [175]. HDL functional assays may also work as prognostic biomarkers. Diagnosing Alzheimer's disease before unrepairable neurodegeneration occurs is a major obstacle in treating the disease. Prognostic biomarkers that can predict a patient's progression into Alzheimer's disease earlier than existing biomarkers could be a solution [171]. As vascular dysfunction occurs early in Alzheimer's disease [14▪,20▪,21,176], biomarkers indicating cerebrovascular dysfunction have considerable potential in predicting cognitive decline. It is, therefore, important to evaluate longitudinal changes to HDL function to determine if HDL-based measurements could improve prognostic precision for Alzheimer's disease's vascular components.
It is less clear whether levels of HDL-associated proteins may become Alzheimer's disease biomarkers. Circulating apoA-I levels are negatively associated with risk of future dementia in many [72,73,74▪▪] but not all [77–80] studies. Furthermore, although a panel including serum apoA-I was shown to have high sensitivity and specificity for MCI [177,178], there were no HDL-associated protein hits in a nontargeted proteomic analysis employed to develop a multiprotein Alzheimer's disease biomarker panel [179]. Early work investigating HDL-associated protein levels and cerebrovascular dysfunction found that serum apoA-I levels are significantly lower in Alzheimer's disease, MCI, and control subjects with severe CBF impairments [178]. Other studies found that the levels of HDL particles containing apoE and lacking apoJ predict greater WMH volume in normal and MCI subjects [180], and that plasma apoJ levels are higher in subjects with CAA-related intracerebral hemorrhages compared with Alzheimer's disease subjects [68].
CONCLUSION
A growing body of evidence in humans, mice, and 3D in-vitro models supports a role for HDL in cerebrovascular resilience. As various HDL formulations have already been developed and tested in clinical trials for CVD, repurposing those with attractive safety profiles may offer a novel strategy for preventing or treating the cerebrovascular disorder associated with Alzheimer's disease. Assays of HDL function could also act as biomarkers for cerebrovascular disorder in Alzheimer's disease, which could assist in stratifying Alzheimer's disease patients for more specific therapeutic interventions and providing a wider window for treating patients before irreversible neurodegeneration occurs.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by two Weston Brain Institute Rapid Response grants awarded to Fan and Robert, and support from the Canadian Consortium for Neurodegeneration in Aging to Wellington. Robert was further supported by a Bluma Tischler (UBC) and a Michael Smith for Health Research Foundation/Canadian Consortium for Neurodegenerative Association fellowships. Button was supported by a Canadian Institutes of Health Research (CIHR) Doctoral Research Award and a UBC Four Year Doctoral Fellowship.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2012; 2.: pii: a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease – lessons from pathology. BMC Med 2014; 12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knopman DS. Bad news and good news in Alzheimer's disease, and how to reconcile them. Nat Rev Neurol 2019; 15:61–62. [DOI] [PubMed] [Google Scholar]
- 4.Davison A. Basic neurochemistry: molecular, cellular, and medical aspects. J Neurol Neurosurg Psychiatry 1989; 52:1021. [Google Scholar]
- 5.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57:178–201. [DOI] [PubMed] [Google Scholar]
- 6.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 2013; 136:2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassler O. Vascular changes in senile brains. Acta Neurol Scand 1965; 5:40–53. [DOI] [PubMed] [Google Scholar]
- 8.Bell MA, Ball MJ. Morphometric comparison of hippocampal microvasculature in ageing and demented people: diameters and densities. Acta Neuropathol 1981; 53:299–318. [DOI] [PubMed] [Google Scholar]
- 9.Fischer V, Siddiqi A, Yusufaly Y. Altered angioarchitecture in selected areas of brains with Alzheimer's disease. Acta Neuropathol 1990; 79:672–679. [DOI] [PubMed] [Google Scholar]
- 10.Kalaria R, Hedera P. Differential degeneration of the cerebral microvasculature in Alzheimer's disease. Neuroreport 1995; 6:477–480. [DOI] [PubMed] [Google Scholar]
- 11.Zipser BD, Johanson CE, Gonzalez L, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging 2007; 28:977–986. [DOI] [PubMed] [Google Scholar]
- 12.Challa VR, Thore CR, Moody DM, et al. Increase of white matter string vessels in Alzheimer's disease. J Alzheimers Dis 2004; 6:379–383. [DOI] [PubMed] [Google Scholar]
- 13.Arvanitakis Z, Capuano AW, Leurgans SE, et al. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 2016; 15:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Iturria-Medina Y, Sotero R, Toussaint P, et al. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun 2016; 7:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses fluid biomarkers in combination with 7700 brain images to demonstrate the vascular dysfunction, specifically reduced cerebral blood flow, and glucose metabolism, is an early event in Alzheimer's disease pathogenesis.
- 15.Ruitenberg A, den Heijer T, Bakker SL, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol 2005; 57:789–794. [DOI] [PubMed] [Google Scholar]
- 16.Akoudad S, Wolters FJ, Viswanathan A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol 2016; 73:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shams S, Martola J, Granberg T, et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis—the Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol 2015; 36:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes T, Wagenknecht L, Craft S, et al. Arterial stiffness and dementia pathology: Atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology 2018; 90:e1248–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montagne A. Vascular plasticity and cognition during normal aging and dementia. JAMA Neurol 2015; 72:495–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Nation D, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019; 25:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study finds that people with early cognitive impairment have elevated CSF soluble platelet-derived growth factor receptor-β levels, indicating capillary damage, and blood–brain barrier breakdown in the hippocampus unrelated to tau and Aβ biomarker changes.
- 21.Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction—The disregarded partner of Alzheimer's disease. Alzheimer's Dement 2019; 15:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 2015; 11:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zlokovic BV, Yamada S, Holtzman D, et al. Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med 2000; 6:718–719. [DOI] [PubMed] [Google Scholar]
- 24.de Bruijn RFAG, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer's disease. BMC Med 2014; 12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jellinger KA, Attems J. Neuropathological approaches to cerebral aging and neuroplasticity. Dialogues Clin Neurosci 2013; 15:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 27.Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer's disease. Annu Rev Med 1996; 47:387–400. [DOI] [PubMed] [Google Scholar]
- 28.Zhao N, Liu C, Qiao W, et al. Apolipoprotein E receptors, and modulation of Alzheimer's disease. Biol Psychiatry 2018; 83:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tai LM, Thomas R, Marottoli FM, et al. The role of APOE in cerebrovascular dysfunction. Acta Neuropathol 2016; 131:709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Paule MG, Wang C, et al. Application of microPET imaging approaches in the study of pediatric anesthetic-induced neuronal toxicity. J Appl Toxicol 2013; 33:861–868. [DOI] [PubMed] [Google Scholar]
- 31▪.Gottesman RF, Albert MS, Alonso A, et al. Association0Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol 2017; 74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study followed 15 744 people for 25 years in communites throughout the USA and found that the vascular risk factors during midlife were associated with increased risk of dementia.
- 32.Mosconi L, Walters M, Sterling J, et al. Lifestyle and vascular risk effects on MRI-based biomarkers of Alzheimer's disease: a cross-sectional study of middle-aged adults from the broader New York City area. BMJ Open 2018; 8:e019362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 2014; 13:788–794. [DOI] [PubMed] [Google Scholar]
- 34.Ecay-Torres M, Estanga A, Tainta M, et al. Increased CAIDE dementia risk, cognition, CSF biomarkers, and vascular burden in healthy adults. Neurology 2018; 91:217–226. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz P, Ikram M, Niessen W, et al. Practical small vessel disease score relates to stroke, dementia, and death. Stroke 2018; 49:2857–2865. [DOI] [PubMed] [Google Scholar]
- 36.Viticchi G, Falsetti L, Buratti L, et al. Framingham risk score and the risk of progression from mild cognitive impairment to dementia. J Alzheimers Dis 2017; 59:67–75. [DOI] [PubMed] [Google Scholar]
- 37.Mineo C, Shaul PW. Novel biological functions of high-density lipoprotein cholesterol. Circ Res 2012; 111:1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furtado JD, Yamamoto R, Melchior JT, et al. Distinct proteomic signatures in 16 HDL (high-density lipoprotein) subspecies. Arter Thromb Vasc Biol 2018; 38:2827–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res 2013; 54:2950–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah AS, Tan L, Long JL, et al. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res 2013; 54:2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinecke JW. The HDL proteome: a marker--and perhaps mediator--of coronary artery disease. J Lipid Res 2009; 50 Suppl:S167–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyce G, Button E, Soo S, et al. The pleiotropic vasoprotective functions of high density lipoproteins (HDL). J Biomed Res 2017; 32:164–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuhanna IS, Zhu Y, Cox BE, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 2001; 7:853–857. [DOI] [PubMed] [Google Scholar]
- 44.Nofer JR, van der Giet M, Tolle M, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 2004; 113:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calabresi L, Franceschini G, Sirtori CR, et al. Inhibition of VCAM-1 expression in endothelial cells by reconstituted high density lipoproteins. Biochem Biophys Res Commun 1997; 238:61–65. [DOI] [PubMed] [Google Scholar]
- 46.Cockerill G, Rye K, Gamble J, et al. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol 1995; 15:1987–1994. [DOI] [PubMed] [Google Scholar]
- 47.Holzer M, Trieb M, Konya V, et al. Aging affects high-density lipoprotein composition and function. Biochim Biophys Acta 2013; 1831:1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011; 121:2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorrentino SA, Besler C, Rohrer L, et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2009; 121:110–122. [DOI] [PubMed] [Google Scholar]
- 50.Holmes MV, Ala-korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Publ Gr 2017; 14:577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacMahon S, Duffy S, Rodgers A, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55000 vascular deaths. Lancet 2007; 370:1829–1839. [DOI] [PubMed] [Google Scholar]
- 52.Holmes MV, Asselbergs FW, Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J 2015; 36:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 2012; 380:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah S, Casas JP, Drenos F, et al. Causal relevance of blood lipid fractions in the development of carotid atherosclerosis mendelian randomization analysis. Circ Cardiovasc Genet 2013; 6:63–72. [DOI] [PubMed] [Google Scholar]
- 55.Østergaard SD, Mukherjee S, Sharp SJ, et al. Associations between potentially modifiable risk factors and Alzheimer disease: a Mendelian Randomization study. PLoS Med 2015; 12:e1001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Proitsi P, Lupton MK, Velayudhan L, et al. Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: a Mendelian randomization analysis. PLoS Med 2014; 11: e1001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 2007; 117:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaisar T, Couzens E, Hwang A, et al. Type 2 diabetes is associated with loss of HDL endothelium protective functions. PLoS One 2018; 13:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monette JS, Hutchins PM, Ronsein GE, et al. Patients with coronary endothelial dysfunction have impaired cholesterol efflux capacity and reduced HDL particle concentration. Circ Res 2016; 119:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kopecky C, Genser B, Drechsler C, et al. Quantification of HDL proteins, cardiac events, and mortality in patients with type 2 diabetes on hemodialysis. Clin J Am Soc Nephrol 2014; 10:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohatgi A, Khera AV, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014; 371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khera AV, Cuchel M, de la Llera Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011; 364:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jansen IE, Savage JE, Watanabe K, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet 2019; 51:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kunkle BW, Grenier-Boley B, Sims R, et al. Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 2019; 51:414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zuliani G, Cavalieri M, Galvani M, et al. Relationship between low levels of high-density lipoprotein cholesterol and dementia in the elderly. The InChianti study. J Gerontol A Biol Sci Med Sci 2010; 65:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merched A, Xia Y, Visvikis S, et al. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer's disease. Neurobiol Aging 2000; 21:27–30. [DOI] [PubMed] [Google Scholar]
- 67.Shih Y, Tsai K, Lee C, et al. Apolipoprotein C-III is an amyloid-β-binding protein and an early marker for Alzheimer's disease. J Alzheimers Dis 2014; 41:855–865. [DOI] [PubMed] [Google Scholar]
- 68.Montañola A, de Retana SF, López-Rueda A, et al. ApoA1, ApoJ and ApoE plasma levels and genotype frequencies in cerebral amyloid angiopathy. Neuromolecular Med 2016; 18:99–108. [DOI] [PubMed] [Google Scholar]
- 69.Reed B, Villeneuve S, Mack W, et al. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol 2014; 71:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crichton GE, Elias MF, Davey A, et al. Higher HDL cholesterol is associated with better cognitive function: the Maine-Syracuse study. J Int Neuropsychol Soc 2014; 20:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bates KA, Sohrabi HR, Rainey-Smith SR, et al. Serum high-density lipoprotein is associated with better cognitive function in a cross-sectional study of aging women. Int J Neurosci 2017; 127:243–252. [DOI] [PubMed] [Google Scholar]
- 72.Saczynski JS, White L, Peila RL, et al. The relation between apolipoprotein A-I and dementia: the Honolulu-Asia aging study. Am J Epidemiol 2007; 165:985–992. [DOI] [PubMed] [Google Scholar]
- 73.Reitz C, Tang M-X, Schupf N, et al. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset alzheimer disease. Arch Neurol 2010; 67:1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪▪.Armstrong NM, An Y, Beason-Held L, et al. Predictors of neurodegeneration differ between cognitively normal and subsequently impaired older adults. Neurobiol Aging 2019; 75:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study followed 889 participants in the Baltimore Longitudinal Study of Aging for 20 years and found that higher HDL-C levels were associated with less brain volumetric decline and baseline HDL-C levels were lower in participants that were cognitively impaired at follow-up.
- 75.Tan Z, Seshadri S, Beiser A, et al. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med 2003; 163:1053–1057. [DOI] [PubMed] [Google Scholar]
- 76.Formiga F, Ferrer A, Chivite D, et al. Serum high-density lipoprotein cholesterol levels, their relationship with baseline functional and cognitive status, and their utility in predicting mortality in nonagenarians. Geriatr Gerontol Int 2011; 11:358–364. [DOI] [PubMed] [Google Scholar]
- 77.Marcum ZA, Walker R, Bobb JF, et al. Serum cholesterol and incident Alzheimer's disease: findings from the adult changes in Thought study. J Am Geriatr Soc 2018; 66:2344–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li G, Shofer J, Kukull W, et al. Serum cholesterol and risk of Alzheimer disease: a community-based cohort study. Neurology 2005; 65:1045–1050. [DOI] [PubMed] [Google Scholar]
- 79.Mielke MM, Xue Q, Zhou J, et al. Baseline serum cholesterol is selectively associated with motor speed and not rates of cognitive decline: the Women's Health and Aging Study II. J Gerontol A Biol Sci Med Sci 2008; 63:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yaffe K, Barrett-Connor E, Lin F, et al. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol 2002; 59:378–384. [DOI] [PubMed] [Google Scholar]
- 81.Stukas S, Robert J, Lee M, et al. Intravenously injected human apolipoprotein A-I rapidly enters the central nervous system via the choroid plexus. J Am Hear Assoc 2014; 3:e001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koch S, Donarski N, Goetze K, et al. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res 2001; 42:1143–1151. [PubMed] [Google Scholar]
- 83.Borghini I, Barja F, Pometta D, James RW. Characterization of subpopulations of lipoprotein particles isolated from human cerebrospinal fluid. Biochim Biophys Acta 1995; 1255:192–200. [DOI] [PubMed] [Google Scholar]
- 84.Manousopoulou A, Gatherer M, Smith C, et al. Systems proteomic analysis reveals that Clusterin and tissue inhibitor of metalloproteinases 3 increase in leptomeningeal arteries affected by cerebral amyloid angiopathy. Neuropathol Appl Neurobiol 2017; 43:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fung KY, Wang C, Nyegaard S, et al. SR-BI mediated transcytosis of HDL in brain microvascular endothelial cells is independent of caveolin, clathrin, and PDZK1. Front Physiol 2017; 8:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ladu MJ, Reardon C, Van Eldik L, et al. Lipoproteins in the central nervous system. Ann N Y Acad Sci 2000; 903:167–175. [DOI] [PubMed] [Google Scholar]
- 87.Lefterov I, Fitz NF, Cronican AA, et al. Apolipoprotein A-I deficiency increases cerebral amyloid angiopathy and cognitive deficits in APP/PS1DeltaE9 mice. J Biol Chem 2010; 285:36945–36957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fagan AM, Christopher E, Taylor JW, et al. ApoAI deficiency results in marked reductions in plasma cholesterol but no alterations in amyloid-beta pathology in a mouse model of Alzheimer's disease-like cerebral amyloidosis. Am J Pathol 2004; 165:1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis TL, Cao D, Lu H, et al. Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer. J Biol Chem 2010; 285:36958–36968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robert J, Stukas S, Button E, et al. Reconstituted high-density lipoproteins acutely reduce soluble brain A β levels in symptomatic APP /PS1 mice. Biochim Biophys Acta 2015; 1862:1027–1036. [DOI] [PubMed] [Google Scholar]
- 91.Song Q, Huang M, Yao L, et al. Lipoprotein-based nanoparticles rescue the memory loss of mice with alzheimer's disease by accelerating the clearance of amyloid-beta. ACS Nano 2014; 8:2345–2359. [DOI] [PubMed] [Google Scholar]
- 92.Handattu SP, Garber DW, Monroe CE, et al. Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer's disease. Neurobiol Dis 2009; 34:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fernández-de Retana S, Montañola A, Marazuela P, et al. Intravenous treatment with human recombinant ApoA-I Milano reduces beta amyloid cerebral deposition in the APP23-transgenic mouse model of Alzheimer's disease. Neurobiol Aging 2017; 60:116–128. [DOI] [PubMed] [Google Scholar]
- 94.Gordon SM, Li H, Zhu X, et al. A comparison of the mouse and human lipoproteome: suitability of the mouse model for studies of human lipoproteins. J Proteome Res 2015; 14:2686–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Armitage J, Holmes MV, Preiss D. Cholesteryl ester transfer protein inhibition for preventing cardiovascular events. J Am Coll Cardiol 2019; 73:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agellon LB, Walsh A, Hayek T, et al. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem 1991; 266:10796–10801. [PubMed] [Google Scholar]
- 97.Hayek T, Chajek-Shaul T, Walsh A, et al. An interaction between the human cholesteryl ester transfer protein (CETP) and apolipoprotein A-1 genes in transgenic mice results in a profound CETP-mediated depression of high density lipoprotein cholesterol levels. J Clin Invest 1992; 90:505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maloney B, Ge YW, Alley GM, Lahiri DK. Important differences between human and mouse APOE gene promoters: Limitation of mouse APOE model in studying Alzheimer's disease. J Neurochem 2007; 103:1237–1257. [DOI] [PubMed] [Google Scholar]
- 99.Sullivan PM, Knouff C, Najib J, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human. J Biol Chem 1997; 272:17972–17980. [DOI] [PubMed] [Google Scholar]
- 100.Zhao N, Liu CC, Van Ingelgom AJ, et al. APOE ε2 is associated with increased tau pathology in primary tauopathy. Nat Commun 2018; 9:4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu CC, Zhao N, Fu Y, et al. ApoE4 accelerates early seeding of amyloid pathology. Neuron 2017; 96:1024.e3–1032.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 2017; 549:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alexandra Moser V, Pike CJ. Obesity accelerates Alzheimer-related pathology in APOE4 but not APOE3 mice. eNeuro 2017; 4: pii:ENEURO.0077-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tai LM, Balu D, Avila-Munoz E, et al. EFAD transgenic mice as a human APOE relevant preclinical model of Alzheimer's disease. J Lipid Res 2017; 58:1733–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holtzman DM, Bales KR, Wu S, et al. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer's disease. J Clin Invest 1999; 103:R15–R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jamieson JJ, Searson PC, Gerecht S. Engineering the human blood-brain barrier in vitro. J Biol Eng 2017; 11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mrsulja B, Mrsulja B, Fujimoto T, et al. Isolation of brain capillaries: a simplified technique. Brain Res 1976; 110:361–365. [DOI] [PubMed] [Google Scholar]
- 108.Weksler B, Romero IA, Couraud P. The hCMEC /D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lippmann ES, Al-Ahmad A, Azarin SM, et al. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep 2014; 4:4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sano Y, Shimizu F, Abe M, et al. Establishment of a new conditionally immortalized human brain microvascular endothelial cell line retaining an in vivo blood-brain barrier function. J Cell Physiol 2010; 225:519–528. [DOI] [PubMed] [Google Scholar]
- 111.Cecchelli R, Aday S, Sevin E, et al. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS One 2014; 9:e99733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eigenmann DE, Xue G, Kim KS, et al. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013; 10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bernas MJ, Cardoso FL, Daley SK, et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat Protoc 2010; 5:1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thomsen LB, Burkhart A, Moos T. A triple culture model of the blood-brain barrier using porcine brain endothelial cells, astrocytes and pericytes. PLoS One 2015; 10:e0134765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hutchinson L, Kirk R. High drug attrition rates—where are we going wrong? Nat Rev Clin Oncol 2011; 8:189–190. [DOI] [PubMed] [Google Scholar]
- 116.Man S, Ubogu EE, Williams KA, et al. Human brain microvascular endothelial cells and umbilical vein endothelial cells differentially facilitate leukocyte recruitment and utilize chemokines for T cell migration. Clin Dev Immunol 2008; 2008:384982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hatherell K, Couraud PO, Romero IA, et al. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods 2011; 199:223–229. [DOI] [PubMed] [Google Scholar]
- 118.Urich E, Patsch C, Aigner S, et al. Multicellular self-assembled spheroidal model of the blood brain barrier Sci Rep; 2013; 3:1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cho CF, Wolfe JM, Fadzen CM, et al. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat Commun 2017; 8:15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Adriani G, Ma D, Pavesi A, et al. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 2017; 17:448–459. [DOI] [PubMed] [Google Scholar]
- 121▪.Campisi M, Shin Y, Osaki T, et al. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018; 180:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a blood–brain barrier model composed of human-induced pluripotent stem cell-derived endothelial cells, brain pericytes, and astrocytes grown in a fibrin gel allowing for the self-assembly of a microvascular network.
- 122▪.Maoz BM, Herland A, Fitzgerald EA, et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol 2018; 36:865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a human neurovascular unit model composed of primary human brain microvascular pericytes and endothelial cells, primary human cortical astrocytes, and human neurons differentiated from hippocampal neural stem cells in three coupled chambers arranged such that the individual functions of each cell type can be evaluated.
- 123▪▪.Robert J, Button EB, Yuen B, et al. Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating high- density lipoproteins in bioengineered human vessels. Elife 2017; 6: pii: e29595. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates a novel function of HDL in 3D bioengineered arteries in preventing Aβ vascular accumulation.
- 124▪▪.Robert J, Button EB, Stukas S, et al. High-density lipoproteins suppress Aβ-induced PBMC adhesion to human endothelial cells in bioengineered vessels and in monoculture. Mol Neurodegener 2017; 12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates a novel function of HDL in 3D bioengineered arteries in preventingAβ-induced vascular inflammation.
- 125.Button E, Gilmour M, Cheema H, et al. Vasoprotective functions of high-density lipoproteins relevant to Alzheimer's disease are partially conserved in apolipoprotein B-depleted plasma. Int J Mol Sci 2019; 20: pii: E462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nicholls SJ, Andrews J, Kastelein JJP, et al. Effect of serial infusions of CER-001, a preβ High-density lipoprotein mimetic, on coronary atherosclerosis in patients following acute coronary syndromes in the CER-001 atherosclerosis regression acute coronary syndrome trial: a randomized clinical trial. JAMA Cardiol 2018; 3:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tardif JC, Ballantyne CM, Barter P, et al. Can HDL Infusions Significantly QUicken Atherosclerosis REgression (CHI-SQUARE) Investigators. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J 2014; 35:3277–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nicholls SJ, Puri R, Ballantyne CM, et al. Effect of infusion of high-density lipoprotein mimetic containing recombinant apolipoprotein A-I Milano on coronary disease in patients with an acute coronary syndrome in the MILANO-PILOT trial: a randomized clinical trial. JAMA Cardiol 2018; 3:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bloedon LT, Dunbar R, Duffy D, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res 2008; 49:1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dunbar RL, Movva R, Bloedon LAT, et al. Oral apolipoprotein A-I mimetic D-4F lowers HDL-Inflammatory index in high-risk patients: a first-in-human multiple-dose, randomized controlled trial. Clin Transl Sci 2017; 10:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Watson CE, Weissbach N, Kjems L, et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res 2011; 52:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gibson CM, Korjian S, Tricoci P, et al. Safety and tolerability of CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I, after acute myocardial infarction: the AEGIS-I trial (ApoA-I Event Reducing in Ischemic Syndromes I). Circulation 2016; 134:1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Waksman R, Torguson R, Kent KM, et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol 2010; 55:2727–2735. [DOI] [PubMed] [Google Scholar]
- 134.Nicholls SJ, Gordon A, Johansson J, et al. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol 2011; 57:1111–1119. [DOI] [PubMed] [Google Scholar]
- 135.Nicholls SJ, Puri R, Wolski K, et al. Effect of the BET protein inhibitor, RVX-208, on progression of coronary atherosclerosis: results of the phase 2b, randomized, double-blind, multicenter, ASSURE trial. Am J Cardiovasc Drugs 2016; 16:55–65. [DOI] [PubMed] [Google Scholar]
- 136.Shamburek RD, Bakker-Arkema R, Shamburek AM, et al. Safety and tolerability of ACP-501, a recombinant human lecithin:cholesterol acyltransferase, in a phase 1 single-dose escalation study. Circ Res 2016; 118:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446. [DOI] [PubMed] [Google Scholar]
- 138.Boden W, Probstfield J, et al. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 139.HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, prespecified muscle and liver outcomes, and reasons for stopping study treatment. Eur Hear J 2013; 34:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwartz GG, Olsson AG, Abt M, et al. dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012; 367:2089–2099. [DOI] [PubMed] [Google Scholar]
- 141.Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017; 376:1933–1942. [DOI] [PubMed] [Google Scholar]
- 142.Barter PJ, Caulfield M, Eriksson M, et al. ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122. [DOI] [PubMed] [Google Scholar]
- 143.Bowman L, Hopewell J, Chen F, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017; 377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 144.Sundermann EE, Wang C, Katz M, et al. Cholesteryl ester transfer protein genotype modifies the effect of apolipoprotein (4 on memory decline in older adults. Neurobiol Aging 2016; 41:200.e7–200.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lythgoe C, Perkes A, Peterson M, et al. Population-based analysis of cholesteryl ester transfer protein identifies association between I405V and cognitive decline: the Cache county study. Neurobiol Aging 2015; 36:547.e1–547.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen J-J, Li Y-M, Zou W-Y, Fu JL. Relationships between CETP genetic polymorphisms and alzheimer's disease risk: a meta-analysis. DNA Cell Biol 2014; 33:807–815. [DOI] [PubMed] [Google Scholar]
- 147.Cui X, Chopp M, Zacharek A, et al. D-4F decreases white matter damage after stroke in mice. Stroke 2016; 47:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Buga GM, Frank JS, Mottino GA, et al. D-4F decreases brain arteriole inflammation and improves cognitive performance in LDL receptor-null mice on a Western diet. J LIpid Res 2006; 47:2148–2160. [DOI] [PubMed] [Google Scholar]
- 149.Sirtori CR. The pharmacology of statins. Pharmacol Res 2014; 88:3–11. [DOI] [PubMed] [Google Scholar]
- 150.Larsson SC, Markus HS. Does treating vascular risk factors prevent dementia and Alzheimer's disease? A systematic review and meta-analysis. J Alzheimers Dis 2018; 64:657–668. [DOI] [PubMed] [Google Scholar]
- 151.Zhang X, Wen J, Zhang Z. Statins use and risk of dementia: a dose-response meta analysis. Medicine (Baltimore) 2018; 97:e11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Swiger KJ, Manalac RJ, Blumenthal RS, et al. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc 2013; 88:1213–1221. [DOI] [PubMed] [Google Scholar]
- 153.McGuinness B, Craig D, Bullock R, et al. Statins for the prevention of dementia. Cochrane Database Syst Rev 2016; CD003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Qin B, Xun P, Jacobs DR, Jr, et al. Intake of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr 2017; 106:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Morris MC, Evans DA, Bienias JL, et al. Dietary niacin and the risk of incident Alzheimer's disease and of cognitive decline. J Neurol Neurosurg Psychiatry 2004; 75:1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brooks-Wilson A, Marcil M, Clee SM, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 1999; 22:336–345. [DOI] [PubMed] [Google Scholar]
- 157.Assmann G, Funke H, Brewer HB, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet 1999; 22:352–355. [DOI] [PubMed] [Google Scholar]
- 158.Bodzioch M, Orsó E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet 1999; 22:347–351. [DOI] [PubMed] [Google Scholar]
- 159.Joseph SB, McKilligin E, Pei L, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci USA 2002; 99:7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tangirala RK, Bischoff ED, Joseph SB, et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci USA 2002; 99:11896–11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 2003; 23:1178–1184. [DOI] [PubMed] [Google Scholar]
- 162.Brunham LR, Kruit JK, Pape TD, et al. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ Res 2006; 99:672–674. [DOI] [PubMed] [Google Scholar]
- 163.Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov 2014; 13:433–444. [DOI] [PubMed] [Google Scholar]
- 164.Chu K, Miyazaki M, Man WC, et al. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol 2006; 26:6786–6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Joseph SB, Laffitte BA, Patel PH, et al. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem 2002; 277:11019–11025. [DOI] [PubMed] [Google Scholar]
- 166.Bradley MN, Hong C, Chen M, et al. Ligand activation of LXRβ reverses atherosclerosis and cellular cholesterol overload in mice lacking LXRα and apoE. J Clin Invest 2007; 117:2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fan J, Zhao RQ, Parro C, et al. Small molecule inducers of ABCA1 and apoE that act through indirect activation of the LXR pathway. J Lipid Res 2018; 59:830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ghosal K, Haag M, Verghese PB, et al. A randomized controlled study to evaluate the effect of bexarotene on amyloid-β and apolipoprotein E metabolism in healthy subjects. Alzheimer's Dement (N Y) 2016; 2:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Song Q, Huang M, Yao L, et al. Lipoprotein-based nanoparticles rescue the memory loss of mice with Alzheimer's disease by accelerating the clearance of amyloid-beta. ACS Nano 2014; 8:2345–2359. [DOI] [PubMed] [Google Scholar]
- 170.Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm 2016; 13:3976–3987. [DOI] [PubMed] [Google Scholar]
- 171.Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med 2018; 284:643–663. [DOI] [PubMed] [Google Scholar]
- 172.Khalil A, Berrougui H, Pawelec G, et al. Impairment of the ABCA1 and SR-BI-mediated cholesterol efflux pathways and HDL anti-inflammatory activity in Alzheimer's disease. Mech Ageing Dev 2012; 133:20–29. [DOI] [PubMed] [Google Scholar]
- 173.Camponova P, Le Page A, Berrougui H, et al. Alteration of high-density lipoprotein functionality in Alzheimer's disease patients. Can J Cardiol 2017; 95:894–903. [DOI] [PubMed] [Google Scholar]
- 174.Hampel H, O’Bryant SE, Molinuevo JL, et al. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol 2018; 14:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Banerjee G, Carare R, Cordonnier C, et al. The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice. J Neurol Neurosurg Psychiatry 2017; 88:982–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gottesman RF, Schneider ALC, Zhou Y, et al. Association Between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017; 317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Uchida K, Shan L, Suzuki H, et al. Amyloid-β sequester proteins as blood-based biomarkers of cognitive decline. Alzheimers Dement (Amst) 2015; 1:270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu S, Suzuki H, Ito H, et al. Serum levels of proteins involved in amyloid-β clearance are related to cognitive decline and neuroimaging changes in mild cognitive impairment. Alzheimer's Dement (Amst) 2019; 11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ashton NJ, Nevado-Holgado AJ, Barber IS, et al. A plasma protein classifier for predicting amyloid burden for preclinical Alzheimer's disease. Sci Adv 2019; 5:eaaau7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Koch M, DeKosky ST, Fitzpatrick AL, et al. Apolipoproteins and Alzheimer's pathophysiology. Alzheimer's Demen 2018; 10:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]


