Summary
Background:
Spitzoid proliferations range from Spitz nevi to melanomas, and there are few studies describing clinical features and outcomes in the pediatric population.
Objectives:
Determine clinical features and outcomes of a large pediatric cohort with histopathologically-confirmed Spitz tumors.
Methods:
Retrospective cohort study of Boston Children’s Hospital patients younger than 20 years with a histopathologic diagnosis of spitzoid proliferation from 1/1/1994 – 10/23/2012.
Results:
Five hundred ninety-five patients with 622 spitzoid proliferations were identified [median age = 7.4 years, (25th, 75th) quartiles = (4.6, 11.7) years]. Five hundred twelve (82.3%) proliferations were typical, 107 (17.2.%) were atypical, and 3 (0.5%) were melanomas. Median age at biopsy was 7.4, 7.2, and 17.2 years, respectively, and there was a significant difference in age at biopsy for patients with typical or atypical proliferations versus melanoma (p<0.01). Among samples with positive margins (n = 153), 55.1% (54/98) of typical proliferations, 77.4% (41/53) of atypical proliferations, and 100.0% (2/2) of melanomas were re-excised. Six patients had sentinel lymph node biopsy performed, with 3 patients demonstrating nodes positive for melanocytic cells. With median follow-up of 4.1 years for the full cohort, there were no related deaths.
Conclusion:
Spitz tumors have strikingly benign outcomes in the pediatric population, though this study is limited by low number of melanomas and restriction to a single pediatric institution. Aggressive management recommendations should be reconsidered for children and adolescents with banal-appearing Spitz nevi, based upon the clinically indolent behavior in this cohort.
Introduction:
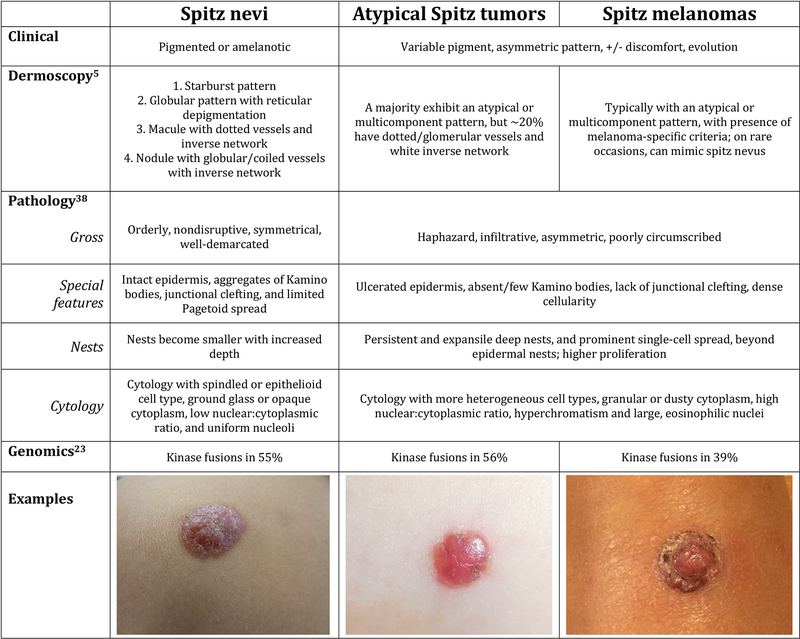
Spitzoid proliferations are spindle and epithelioid cell melanocytic proliferations that pathologist Sophie Spitz first described as “juvenile melanomas” in 1948.(1) Spitzoid proliferations are thought to occur on a biological spectrum, from Spitz nevi to atypical Spitz tumors (AST) and Spitz melanomas (Figure 1), with sequential accumulation of genetic aberrations.(2) Spitz nevi are distinct from other types of nevi and present as flat- or dome-shaped papules and nodules that may be non-pigmented, appearing pink or red, or deeply pigmented, appearing very dark brown. These proliferations can undergo periods of rapid growth before stabilizing and some ultimately involute. ASTs are benign lesions with atypical features on histopathology (Figure 1) that are challenging to diagnose, and Spitz melanomas are malignant lesions. A recent distinction has been made between “Spitz melanoma” and “spitzoid melanoma;” whereas both tumors demonstrate clinical and histologic features reminiscent of Spitz nevi, spitzoid melanomas possess typical melanoma driver mutations, in contrast to the kinase fusions that typify Spitz melanoma and other Spitz tumors.(3) While genetic workup was not utilized for tumor diagnosis in this study and histopathology reports were rendered prior to this classification, we have adopted the WHO terminology for this report.
Figure 1.
Clinical, dermosocopic, pathologic, and genomic features of Spitzoid proliferations.
Sentinel lymph node biopsies were historically utilized to distinguish benign from malignant spitzoid proliferations, but this practice has fallen out of favor as studies show that sentinel lymph node biopsies and completion dissections have limited therapeutic potential, uncertain utility in prognostication, and high associated morbidity.(4–8) FISH, CGH, and next-generation sequencing may offer hope for improved understanding and classification of Spitz tumors, but at present, data is preliminary and sometimes conflicting in the pediatric patient population.(8–20)
The literature provides conflicting guidance on caring for pediatric patients who develop these lesions(21) and there is need for improved understanding and education. Whereas many pediatric dermatologists report comfort with monitoring banal-appearing Spitz nevi,(22) plastic surgeons commonly excise these proliferations,(21) and expert dermoscopists have recommended excision of banal-appearing Spitz nevi in children over age 12 and nodular spitzoid proliferations in children of any age.(23, 24) Excision recommendations are supported by the well-replicated finding that dermoscopy does not reliably distinguish between Spitz nevi, ASTs, and Spitz melanomas,(24–29) and data suggesting that clinically banal-appearing spitzoid proliferations can follow an aggressive disease course.(24, 30) However, these data are derived from predominantly adult cohorts and our experience leads us to question the applicability of these results in regards to the management of pediatric patients.
Spitz nevi and indolence are far more common in the pediatric population than Spitz melanoma or fatal outcome, and aggressive management may result in overtreatment of benign lesions.(30–34) Though histopathology remains the gold standard for diagnosis of spitzoid proliferations, overlapping features are frequent with this modality and distinction is a well-recognized dermatopathologic challenge.(25–29) Downsides of widespread or low-threshold for excision in the pediatric population include undue patient morbidity, high health care costs resulting from procedures, diagnostic testing, and additional dermatopathology consultation, and parental and patient anxiety.
This study aims to characterize clinical features and outcomes of pediatric patients with Spitz tumors, provide improved understanding regarding these proliferations, and address current controversies in care. We evaluate a large pediatric cohort from our institution in this study, and with more than eighteen years of data, it represents the largest histopathologically-confirmed pediatric Spitz tumor cohort to date.
Materials and methods:
We conducted chart review to perform this retrospective cohort study. The study population includes Boston Children’s Hospital patients who were less than 20 years old at time of biopsy and received a histopathologic diagnosis of spitzoid proliferation, (typical Spitz nevus or related proliferation, nevus with spitzoid features, Spitz nevus with atypical features, AST, Spitz melanoma, or related proliferation), and whose pathology accession occurred between 1/1/1994 – 10/23/2012. Six hundred twenty-five proliferations meeting inclusion criteria were identified through database query; three had the unrelated histopathologic diagnosis “plexiform spindle cell nevus” and were excluded from further analysis. Thus, in this cohort, six hundred twenty-two proliferations from 595 unique patients were analyzed. Summary statistics for follow-up times are provided for both the full cohort and patients with clinical follow-up greater than 2 months after diagnosis (n = 398). Follow-up time refers to the interval between the date of diagnosis provided by the Department of Pathology and the date of the most recent clinical contact at our institution, independent of contact type.
In addition to descriptive statistics of the data and non-parametric statistical comparisons between sub-cohorts using the Wilcoxon rank-sum test, multivariate logistic regression models were developed to assess correlations between proliferation type (typical, atypical, and malignant: groups described in Table 2) and various clinical parameters of interest. When multiple categorical variables were examined, appropriate matrices were constructed with one category being treated as the reference. For example, white was used as the reference race and contrast matrices were developed for the remaining racial categories. Gender was modeled as a binary variable (male = 0, female = 1). Separate analyses were performed for detailed and grouped categorizations of lesions’ anatomic locations (groups included extremities, sun-protected, and head/neck), as well as for detailed and grouped categorizations of proliferation type (groups included typical, atypical, and malignant). A statistical significance level of 0.05 was assumed in all analyses. The software Matlab (Mathworks, Inc) was used to analyze the data.
Table 2.
Tumour characteristics (n = 622)
| Tumour characteristics | n (%) of proliferations |
|---|---|
| Typical (n = 512, 82.3%) | |
| Spitz naevus, PSCN, sclerosing or desmoplastic Spitz | 382 (61.4) |
| Pigmented lesion ‘with features of Spitz’ or PSCN | 105 (16.9) |
| Spitzoid tumour with halo effect | 16 (2.6) |
| Multiple diagnosesa | 9 (1.4) |
| Atypical (n = 107, 17.2%) | |
| Spitz with atypia; PSCN with atypia | 99 (15.9) |
| Multiple diagnosesb | 8 (1.3) |
| Melanoma (n = 3, 0.5%) | |
| Spitz melanoma | 3 (0.5) |
| Acral (n = 279, 44.9%) | |
| Leg | 113 (18.2) |
| Arm/shoulder | 107 (17.2) |
| Foot/ankle | 31 (5.0) |
| Hand | 28 (4.5) |
| Sun protected (n = 114, 18.3%) | |
| Back | 66 (10.6) |
| Chest | 14 (2.3) |
| Abdomen | 13 (2.1) |
| Buttocks | 11 (1.8) |
| Genital area | 10 (1.6) |
| Head and neck (n = 225, 36.2%) | |
| Face | 150 (24.1) |
| Ears | 44 (7.1) |
| Scalp | 15 (2.4) |
| Neck | 16 (2.6) |
| Not available | 4 (0.6) |
PSCN, pigmented spindle cell naevus.
Multiple diagnoses for benign lesions included combinations such as ‘compound naevus with some features of spindle and epithelioid cell naevus (Spitz) and halo effect’, ‘lentiginous compound naevus with some features of Spitz naevus and PSCN’ and ‘combined PSCN/spindle and epithelioid cell naevus’.
Multiple diagnoses for atypical lesions included combinations such as ‘atypical, mostly dermal, melanocytic proliferation with spitzoid features, consistent with sclerosing Spitz naevus with some atypical features’, ‘lentiginous compound naevus with some features of pigmented spindle cell naevus and mild atypia of the intraepidermal component’ and ‘lesion with features of Spitz, PSCN, atypical spitzoid and PSCN with disorder/atypia’.
Results:
Clinical features:
Six hundred twenty-two proliferations occurred in a cohort of 595 patients; twenty-four patients had two spitzoid proliferations in the pathology database that was queried and two patients had three such tumors. Proliferations in this cohort were diagnosed between age 3 months to 19.7 years, with median age of 7.4 years, [(25th, 75th) quartiles = (4.6, 11.7) years]. These occurred in 298 males (50.1%) and 297 females (49.9%), and 351 patients (59.0%) self-identified as white (Table 1).
Table 1.
Patient demographics (n = 595)
| Demographics | Number (%) |
|---|---|
| Sex | |
| Male | 298 (50.1) |
| Female | 297 (49.9) |
| Race | |
| White | 351 (59.0) |
| Black | 4 (0.7) |
| Hispanic | 4 (0.7) |
| Asian | 8 (1.3) |
| American Indian | 2 (0.3) |
| Other | 16 (2.7) |
| Not available | 210 (35.3) |
Five hundred twelve (82.3%) proliferations were classified as typical, 107 (17.2%) as atypical, and 3 (0.5%) as Spitz melanomas (Table 2). Less than 1% of typical proliferations and 1.9% of atypical proliferations occurred in patients with substantial medical comorbidities; in contrast, clinically significant medical disease was noted among two of the three patients who were diagnosed with melanomas (Table 2).
The median age at biopsy was 7.4 years for typical proliferations, 7.2 years for atypical proliferations, and 17.2 years for Spitz melanomas. When proliferation diagnoses were grouped as typical, atypical, or melanoma, there was a significant difference in age at biopsy for patients with typical proliferations versus melanoma (p<0.01), and a significant difference in age at biopsy for patients with atypical proliferations versus melanoma (p<0.01), based on the Wilcoxon rank-sum test. Age at biopsy was statistically indistinguishable for patients with typical versus atypical proliferations (p = 0.88). Based on statistical models that included race and patient gender, there were no significant differences between the race or gender of patients presenting with typical, atypical, and melanoma proliferation types (p≥0.7 in all comparisons).
The most common anatomic location for spitzoid proliferations (Table 1) was the face (24.1%), but when proliferation sites were grouped, most occurred on the extremities (44.9%), followed by head/neck (36.2%), and sun-protected regions (18.3%). There were significant differences between the proportion of males and females presenting with proliferations on different anatomic locations: the proportion of males/females with extremity proliferations (41.9% male) was significantly different from the proportion of males/females presenting with head/neck (54.7% male, p = 0.05) and sun-protected region proliferations (61.4% male, p = 0.01).
The three melanoma patients presented distinctly (Table 3), with no death or recurrences at the time of this manuscript.
Table 3.
Details of the three patients with Spitz melanoma
| Characteristics | Patient A | Patient B | Patient C |
|---|---|---|---|
| Age at biopsy | 17.6 years | 17.2 years | 14.7 years |
| Sex | Female | Male | Male |
| Race | White | White | White |
| Fitzpatrick skin type | Not recorded | III | II |
| Associated medical conditions | Metastatic Ewing sarcoma | Suprasellar pilocytic astrocytoma | None |
| Tumour site | Right buttock | Dorsum of left arm | Right perianal region |
| Submitting physician | Dermatologist | Dermatologist | Surgeon |
| Initial biopsy type | Excision | Excision | Excision |
| Depth | 0.7 mm | 0.7 mm | 14 mm |
| Anatomical level | IV | III/IV | V |
| Ulceration | Absent | Absent | Present |
| Regression | Not identified | Present | Present |
| Mitotic rate | 0 mm−2 | 0 mm−2 | 2–3 mm−2 |
| Tumour-infiltrating lymphocytes | Focal, brisk | Present, brisk | Present, not brisk |
| Microscopic satellites | Not identified | Absent | Not recorded |
| Cell type | Epithelioid | Spindle and epithelioid | Epithelioid |
| Lymphatic vascular invasion | Not recorded | Absent | Not recorded |
| Perineural invasion | Not recorded | Absent | Suspicious for |
| Margins positive | No | Yes | Yes |
| Subsequent excision | Yes | Yes | Yes |
| SLNB | No | Yes | Yes |
| Adjuvant therapy | No | No | 8 months of interferon therapy |
| Recurrence | No | No | No |
| Death | No | No | No |
| Follow-up | 17.8 years | 7.6 years | 4.7 years |
SLNB, sentinel lymph node biopsy.
Treatment characteristics:
Excision was the most common initial biopsy type [380 proliferations (61.1%)], followed by shave [51 (8.2%)] and punch [46 (7.4%)]. For the remaining 145 proliferations (23.3%), specimen collection details were not available. Margins were negative in 427 (68.7%) and positive in 153 (24.6%) of specimens, with no report on margin status for 42 (6.8%) of cases. Among samples with positive margins, 98 (64.1%) were typical, 53 (34.6%) were atypical and 2 (1.3%) were melanomas. Of these, a total of 97 (63.4%) proliferations were re-excised, including 54/98 (55.1%) typical spitzoid proliferations, 41/53 (77.4%) atypical spitzoid proliferations, and 2/2 (100.0%) Spitz melanomas.
Sentinel lymph node biopsy was performed on six patients, including patients with 4/107 of the atypical spitzoid proliferations and 2/3 of the Spitz melanomas. Sentinel node was positive for melanocytic cells in 3/4 atypical spitzoid proliferation and 0/2 Spitz melanoma patients. Among the three patients that demonstrated positive sentinel lymph nodes, two continued to receive care at our institution, and both underwent completion lymphadenectomies that demonstrated all nodes negative. None of the 5 patients with sentinel lymph node biopsy, for whom we had follow-up time greater than 2 months (5/6), experienced disease recurrence or fatality with median follow-up time of 5.8 years [(25th, 75th) quartiles = (3.7, 7.4) years). Results of genetic tests were occasionally available from outside consultant work-up, but these studies were not ordered by our institution’s pathologists and were not utilized in management decisions.
Outcomes:
At this referral center, 52 patients (8.7%) had consultation and diagnosis alone, 145 patients (24.4%) had follow-up less than 2 months after diagnosis without subsequent care at our institution, and 398 patients (66.9%) had clinical follow-up in our system for more than 2 months after diagnosis. Median follow-up time for the full patient cohort was 4.1 years [(25th, 75th quartiles) = (0.04, 8.2) years]. For the 398 patients with follow-up greater than 2 months, it was 6.8 years [(25th, 75th quartiles) = (4.1, 10.7) years]. There were no significant differences in follow-up time between patients with different proliferation types (p = 0.1 detailed, p = 0.24 grouped).
Among the full cohort, 5 proliferations recurred (5/622, 0.8%). With initial histopathology records accessible for 4 of these 5 samples, positive margins were found in 50% (2 of 4 tumors). Whereas initial biopsy reports did not indicate atypia in any of the initial samples, histopathologic atypia was noted in 3 of 5 recurrent lesion samples. Tumors with atypical features upon recurrence were classified as atypical proliferations in this study. All patients with recurrence were alive without further disease spread or complication, after median follow-up of 12.6 years, (25th, 75th quartiles) = (6.6, 13.4) years. One patient in the cohort succumbed to unrelated malignancy (1/595, 0.2%).
Conclusions:
Our study demonstrates benign outcomes among a large cohort of pediatric patients with histopathologically-proven Spitz tumors, confirming previous findings that pediatric patients have favorable prognoses(8, 22) distinct from adults.(24, 30) Spitz melanoma is rare among pediatric patients and fatality from Spitz melanoma is even rarer in this population. A retrospective literature review of metastatic Spitz melanoma in patients 17 years of age or younger, between 1949 and 2006, identified 25 fatal cases.(35) Three additional cases of fatal pediatric Spitz melanoma were reported after 2006.(22, 33) Even though death is an uncommon outcome in pediatric patients, prior clinical experiences and fear may influence providers’ tendencies to biopsy or completely excise pigmented lesions. Out of 142 pediatric dermatologists surveyed, two providers reported witnessing fatal outcome from a clinically presumed Spitz nevus/AST.(22)
We put forward for consideration that excision of clinically stable, typical-appearing, dermoscopically banal Spitz tumors -- in the absence of concerning features such as bleeding, evolution, or friability -- may not be required for children and adolescents. Widespread removal may be unnecessary given the overwhelmingly indolent nature of proliferations observed in this cohort, though our retrospective study design cannot rule out the possibility that excision prevented certain patients from experiencing poor outcomes.
While sampling will provide diagnostic certainty, one must balance the pre-test likelihood of a dangerous skin lesion with the down-sides of biopsy including risk of over-diagnosis as melanoma and subsequent over-treatment, trauma from procedure, permanent scar, family and child worry of malignancy, potential need for and risks associated with anesthesia to perform procedures in young children,(36, 37) in addition to procedural and diagnostic costs. We recommend that providers who are not expert in the clinical diagnosis of these proliferations refer patients to a pediatric dermatologist or dermatologist for evaluation, in lieu of sampling or directly referring the patient for excision. Despite this recommendation, we acknowledge that biopsy or excision may be pursued for other important reasons, including cosmesis and other individual patient concerns. Special consideration should be taken for older adolescents and patients with other risk factors.
A reasonable approach may be to monitor closely for interval changes, as aggressive and atypical lesions tend to change over a short time course. Previously, experts have recommended two to three years of dermoscopic follow-up occurring every six months for children under 12 years, followed by annual surveillance; they have also recommended yearly evaluation for children over 12 years old with benign-appearing Spitz nevi.(38) Other investigators question whether flat pigmented Spitz and Reed nevi in children need to be monitored clinically.(39) The general practice at our institution is to monitor lesions until they have achieved stability; prospective studies are required to fully understand the natural history of Spitz tumors in the pediatric population and to determine appropriate monitoring parameters.
In addition to demonstrating the benign natural history of pediatric Spitz tumors, this study offers interesting data about providers’ management strategies following initial biopsy. Research has shown that physicians are mixed in their practice of re-excising histologically proven Spitz nevi that have positive margins,(40) and in our cohort, almost half of patients with residual Spitz nevi did not undergo re-excision. Foregoing re-excision of typical proliferations may be preferable for patients with lesions that are very large or located in cosmetically sensitive areas. However, proliferations that are not re-excised have the potential to recur with mixed histopathologic findings and this practice may result in overtreatment at a later time. Our data highlight both the limitations of histopathology evaluation of margins (given that proliferations recurred in two patients whose initial histopathology report described clear margins) and the potential for atypia to be identified in lesions recurring within a scar. We recommend ample patient and parent education if a conservative approach is pursued, so that any future atypical pigment, nodularity, or unexpected symptoms at the site is evaluated promptly.
Physicians at our institution rarely performed sentinel lymph node biopsies for ASTs, in keeping with the pediatric literature. Many benign tumors, including acquired and congenital nevi, have been associated with benign lymph node deposits of associated cells, and thus the finding of spitzoid cells in patients’ lymph nodes does not necessarily represent conventional metastasis.(41) Large-scale outcomes data and prospective studies on this topic have not yet been performed, and we acknowledge that some may disagree with the interpretation of indolence that we have assigned for patients in this cohort who had favorable outcome despite positive lymph node findings. To mitigate potential risk for all patients given a diagnosis of atypical Spitz tumor, we recommend expert dermatopathology consultation for tumor evaluation and monitoring of patients over time, with clinical examination of regional lymph node basins.(42)
Furthermore, physicians at our institution did not utilize genetic testing despite growing availability. Among available genetic markers, deletion of chromosome 9p21 (expression can also be assessed by immunohistochemical staining for p16) appears to be most consistently associated with distant disease spread;(14, 43, 44) however, recent analyses suggest that ASTs with 9p21 homozygous deletion are associated with a less aggressive clinical behavior than melanomas,(45) and we suggest caution in performing these tests without established interpretation specific to the pediatric population.
In conclusion, we hope that results from this study encourage clinicians to recognize the frequent benign outcomes of Spitz tumors in pediatric patients, and to consider this when deciding whether to perform a skin biopsy in a child or adolescent whose lesion does not exhibit concerning features. Limitations of this study include that the general practice at our institution is to recommend clinical monitoring of typical-appearing Spitz nevi in pediatric patients, thus the study excludes patients who did not undergo biopsy; however, this results in an under-estimation of the number of Spitz nevi and frequency of benign outcomes in our patient population. Findings are further limited by low number of Spitz melanomas, exclusion of dermoscopic data (not available for a majority of patients), restriction to a single pediatric institution (with limited patients in the 18–20 year old range), and the diagnosis of the vast majority of cohort proliferations made by two dermatopathologists. Nevertheless, we hope that results from this study encourage clinicians to recognize the possibility that Spitz tumors in pediatric patients are largely benign, and to consider this when deciding whether to perform a skin biopsy in a child or adolescent whose lesion does not exhibit concerning features.
Bulleted statements:
What’s already known about this topic? Spitz nevi, atypical Spitz tumors, and Spitz melanoma are pigmented lesions that are challenging to diagnose in the pediatric population. Both dermoscopy and genetic tests fail to definitively diagnose these proliferations and histopathology remains the gold standard. Whereas similar lesions in adults may have aggressive outcomes, pediatric-specific data is limited.
What does this study add? This study adds pediatric-specific data from a large cohort, and current management practices are reviewed. With over two decades of data demonstrating benign clinical outcomes in children with biopsy-proven lesions, clinical monitoring may be considered for banal-appearing spitzoid proliferations in pediatric patients.
Acknowledgments
This work was supported by the Dermatology Foundation Fellowship in Pediatric Dermatology and Pediatric Dermatology Career Development Award (EBH), Society for Pediatric Dermatology Weston Career Development Award (EBH). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard.
Footnotes
Disclosures:
This non-human-subjects study was approved by BCH IRB (IRB-P00007219).
EBH discloses non-relevant conflicts of interests: UpToDate, Inc (author, contributor), Foundation Medicine, Inc (stock), and Gritstone Oncology, Inc (spouse employment, stock).
References
- 1.Spitz S Melanomas of childhood. The American journal of pathology. 1948. May;24(3):591–609. PubMed PMID: 18859360. Pubmed Central PMCID: 1942798. [PMC free article] [PubMed] [Google Scholar]
- 2.Wiesner T, Kutzner H, Cerroni L, Mihm MC Jr., Busam KJ, Murali R. Genomic aberrations in spitzoid melanocytic tumours and their implications for diagnosis, prognosis and therapy. Pathology. 2016. February;48(2):113–31. PubMed PMID: 27020384. Pubmed Central PMCID: 4817351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.R B, A B, BC B, KJ B, L C, A dlF, et al. Malignant Spitz tumour and Spitz Naevus In: DR E, D M, R S, R W, editors. WHO Classification of Skin Tumors, Fourth Edition. 4 ed: World Health Organization; 2018. [Google Scholar]
- 4.Berk DR, LaBuz E, Dadras SS, Johnson DL, Swetter SM. Melanoma and melanocytic tumors of uncertain malignant potential in children, adolescents and young adults--the Stanford experience 1995–2008. Pediatric dermatology. 2010. May-Jun;27(3):244–54. PubMed PMID: 20403119. [DOI] [PubMed] [Google Scholar]
- 5.Cerrato F, Wallins JS, Webb ML, McCarty ER, Schmidt BA, Labow BI. Outcomes in pediatric atypical spitz tumors treated without sentinel lymph node biopsy. Pediatric dermatology. 2012. Jul-Aug;29(4):448–53. PubMed PMID: 22211716. [DOI] [PubMed] [Google Scholar]
- 6.Hung T, Piris A, Lobo A, Mihm MC Jr., Sober AJ, Tsao H, et al. Sentinel lymph node metastasis is not predictive of poor outcome in patients with problematic spitzoid melanocytic tumors. Human pathology. 2013. January;44(1):87–94. PubMed PMID: 22939951. [DOI] [PubMed] [Google Scholar]
- 7.Lallas A, Kyrgidis A, Ferrara G, Kittler H, Apalla Z, Castagnetti F, et al. Atypical Spitz tumours and sentinel lymph node biopsy: a systematic review. The Lancet Oncology. 2014. April;15(4):e178–83. PubMed PMID: 24694641. [DOI] [PubMed] [Google Scholar]
- 8.Massi D, Tomasini C, Senetta R, Paglierani M, Salvianti F, Errico ME, et al. Atypical Spitz tumors in patients younger than 18 years. Journal of the American Academy of Dermatology. 2015. January;72(1):37–46. PubMed PMID: 25446807. [DOI] [PubMed] [Google Scholar]
- 9.Ali L, Helm T, Cheney R, Conroy J, Sait S, Guitart J, et al. Correlating array comparative genomic hybridization findings with histology and outcome in spitzoid melanocytic neoplasms. International journal of clinical and experimental pathology. 2010. June 28;3(6):593–9. PubMed PMID: 20661407. Pubmed Central PMCID: 2907121. [PMC free article] [PubMed] [Google Scholar]
- 10.Busam KJ, Kutzner H, Cerroni L, Wiesner T. Clinical and pathologic findings of Spitz nevi and atypical Spitz tumors with ALK fusions. The American journal of surgical pathology. 2014. July;38(7):925–33. PubMed PMID: 24698967. Pubmed Central PMCID: 5042334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMarchis EH, Swetter SM, Jennings CD, Kim J. Fluorescence in situ hybridization analysis of atypical melanocytic proliferations and melanoma in young patients. Pediatric dermatology. 2014. Sep-Oct;31(5):561–9. PubMed PMID: 24924836. Pubmed Central PMCID: 4282368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dika E, Fanti PA, Fiorentino M, Capizzi E, Neri I, Piraccini BM, et al. Spitzoid tumors in children and adults: a comparative clinical, pathological, and cytogenetic analysis. Melanoma research. 2015. August;25(4):295–301. PubMed PMID: 25933206. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara G, De Vanna AC. Fluorescence In Situ Hybridization for Melanoma Diagnosis: A Review and a Reappraisal. The American Journal of dermatopathology. 2016. April;38(4):253–69. PubMed PMID: 26999337. [DOI] [PubMed] [Google Scholar]
- 14.Gerami P, Cooper C, Bajaj S, Wagner A, Fullen D, Busam K, et al. Outcomes of atypical spitz tumors with chromosomal copy number aberrations and conventional melanomas in children. The American journal of surgical pathology. 2013. September;37(9):1387–94. PubMed PMID: 23797719. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Barnhill RL, Dummer R, Dalton J, Wu J, Pappo A, et al. TERT Promoter Mutations Are Predictive of Aggressive Clinical Behavior in Patients with Spitzoid Melanocytic Neoplasms. Scientific reports. 2015. June 10;5:11200 PubMed PMID: 26061100. Pubmed Central PMCID: 4462090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions part I. Background and diagnoses. Journal of the American Academy of Dermatology. 2011. December;65(6):1073–84. PubMed PMID: 22082838. Pubmed Central PMCID: 3217183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrizi A, Fanti PA, Dika E. New data on the use of the FISH technique: the horizon dividing Spitz nevi and melanoma in childhood moves even further away. Dermatologic therapy. 2015. Jul-Aug;28(4):264 PubMed PMID: 25752237. [DOI] [PubMed] [Google Scholar]
- 18.Raskin L, Ludgate M, Iyer RK, Ackley TE, Bradford CR, Johnson TM, et al. Copy number variations and clinical outcome in atypical spitz tumors. The American journal of surgical pathology. 2011. February;35(2):243–52. PubMed PMID: 21263245. [DOI] [PubMed] [Google Scholar]
- 19.Yeh I, Botton T, Talevich E, Shain AH, Sparatta AJ, de la Fouchardiere A, et al. Activating MET kinase rearrangements in melanoma and Spitz tumours. Nature communications. 2015. May 27;6:7174 PubMed PMID: 26013381. Pubmed Central PMCID: 4446791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh I, de la Fouchardiere A, Pissaloux D, Mully TW, Garrido MC, Vemula SS, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. The American journal of surgical pathology. 2015. May;39(5):581–91. PubMed PMID: 25602801. Pubmed Central PMCID: 4398593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzger AT, Kane AA, Bayliss SJ. Differences in treatment of Spitz nevi and atypical Spitz tumors in pediatric patients among dermatologists and plastic surgeons. JAMA dermatology. 2013. November;149(11):1348–50. PubMed PMID: 24026360. [DOI] [PubMed] [Google Scholar]
- 22.Tlougan BE, Orlow SJ, Schaffer JV. Spitz nevi: beliefs, behaviors, and experiences of pediatric dermatologists. JAMA dermatology. 2013. March;149(3):283–91. PubMed PMID: 23553063. [DOI] [PubMed] [Google Scholar]
- 23.Lallas A, Apalla Z, Ioannides D, Lazaridou E, Kyrgidis A, Broganelli P, et al. Update on dermoscopy of Spitz/Reed naevi and management guidelines by the International Dermoscopy Society. The British journal of dermatology. 2017. September;177(3):645–55. PubMed PMID: 28118479. [DOI] [PubMed] [Google Scholar]
- 24.Lallas A, Moscarella E, Longo C, Kyrgidis A, de Mestier Y, Vale G, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. Journal of the American Academy of Dermatology. 2015. January;72(1):47–53. PubMed PMID: 25440960. [DOI] [PubMed] [Google Scholar]
- 25.Barnhill RL, Argenyi ZB, From L, Glass LF, Maize JC, Mihm MC Jr., et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Human pathology. 1999. May;30(5):513–20. PubMed PMID: 10333219. [DOI] [PubMed] [Google Scholar]
- 26.Gerami P, Busam K, Cochran A, Cook MG, Duncan LM, Elder DE, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. The American journal of surgical pathology. 2014. July;38(7):934–40. PubMed PMID: 24618612. [DOI] [PubMed] [Google Scholar]
- 27.Harms KL, Lowe L, Fullen DR, Harms PW. Atypical Spitz Tumors: A Diagnostic Challenge. Archives of pathology & laboratory medicine. 2015. October;139(10):1263–70. PubMed PMID: 26414472. [DOI] [PubMed] [Google Scholar]
- 28.Moscarella E, Al Jalbout S, Piana S, Argenziano G, Lallas A, Longo C, et al. The stars within the melanocytic garden: unusual variants of Spitz naevi. The British journal of dermatology. 2015. April;172(4):1045–51. PubMed PMID: 25123161. [DOI] [PubMed] [Google Scholar]
- 29.Moscarella E, Lallas A, Kyrgidis A, Ferrara G, Longo C, Scalvenzi M, et al. Clinical and dermoscopic features of atypical Spitz tumors: A multicenter, retrospective, case-control study. Journal of the American Academy of Dermatology. 2015. November;73(5):777–84. PubMed PMID: 26475536. Pubmed Central PMCID: 4806681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lott JP, Wititsuwannakul J, Lee JJ, Ariyan S, Narayan D, Kluger HH, et al. Clinical characteristics associated with Spitz nevi and Spitzoid malignant melanomas: the Yale University Spitzoid Neoplasm Repository experience, 1991 to 2008. Journal of the American Academy of Dermatology. 2014. December;71(6):1077–82. PubMed PMID: 25308882. Pubmed Central PMCID: 6133655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnhill RL, Flotte TJ, Fleischli M, Perez-Atayde A. Cutaneous melanoma and atypical Spitz tumors in childhood. Cancer. 1995. November 15;76(10):1833–45. PubMed PMID: 8625056. [DOI] [PubMed] [Google Scholar]
- 32.Herreid PA, Shapiro PE. Age distribution of Spitz nevus vs malignant melanoma. Archives of dermatology. 1996. March;132(3):352–3. PubMed PMID: 8607649. [DOI] [PubMed] [Google Scholar]
- 33.Reguerre Y, Vittaz M, Orbach D, Robert C, Bodemer C, Mateus C, et al. Cutaneous malignant melanoma in children and adolescents treated in pediatric oncology units. Pediatric blood & cancer. 2016. November;63(11):1922–7. PubMed PMID: 27348579. [DOI] [PubMed] [Google Scholar]
- 34.Sepehr A, Chao E, Trefrey B, Blackford A, Duncan LM, Flotte TJ, et al. Long-term outcome of Spitz-type melanocytic tumors. Archives of dermatology. 2011. October;147(10):1173–9. PubMed PMID: 21680758. Pubmed Central PMCID: 3771496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pol-Rodriquez M, Lee S, Silvers DN, Celebi JT. Influence of age on survival in childhood spitzoid melanomas. Cancer. 2007. April 15;109(8):1579–83. PubMed PMID: 17326059. [DOI] [PubMed] [Google Scholar]
- 36.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011. November;128(5):e1053–61. PubMed PMID: 21969289. Pubmed Central PMCID: 3307194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rappaport BA, Suresh S, Hertz S, Evers AS, Orser BA. Anesthetic neurotoxicity--clinical implications of animal models. The New England journal of medicine. 2015. February 26;372(9):796–7. PubMed PMID: 25714157. [DOI] [PubMed] [Google Scholar]
- 38.Brunetti B, Nino M, Sammarco E, Scalvenzi M. Spitz naevus: a proposal for management. Journal of the European Academy of Dermatology and Venereology : JEADV. 2005. May;19(3):391–3. PubMed PMID: 15857482. [DOI] [PubMed] [Google Scholar]
- 39.Lallas A, Apalla Z, Papageorgiou C, Evangelou G, Ioannides D, Argenziano G. Management of Flat Pigmented Spitz and Reed Nevi in Children. JAMA dermatology. 2018. September 12 PubMed PMID: 30208474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaye VN, Dehner LP. Spindle and epithelioid cell nevus (Spitz nevus). Natural history following biopsy. Archives of dermatology. 1990. December;126(12):1581–3. PubMed PMID: 2256684. [PubMed] [Google Scholar]
- 41.Kwon EJ, Winfield HL, Rosenberg AS. The controversy and dilemma of using sentinel lymph node biopsy for diagnostically difficult melanocytic proliferations. Journal of cutaneous pathology. 2008. November;35(11):1075–7. PubMed PMID: 18976401. [DOI] [PubMed] [Google Scholar]
- 42.Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions part II. Natural history and management. Journal of the American Academy of Dermatology. 2011. December;65(6):1087–92. PubMed PMID: 22082839. Pubmed Central PMCID: 3217195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al Dhaybi R, Agoumi M, Gagne I, McCuaig C, Powell J, Kokta V. p16 expression: a marker of differentiation between childhood malignant melanomas and Spitz nevi. Journal of the American Academy of Dermatology. 2011. August;65(2):357–63. PubMed PMID: 21550132. [DOI] [PubMed] [Google Scholar]
- 44.Gammon B, Beilfuss B, Guitart J, Gerami P. Enhanced detection of spitzoid melanomas using fluorescence in situ hybridization with 9p21 as an adjunctive probe. The American journal of surgical pathology. 2012. January;36(1):81–8. PubMed PMID: 21989344. [DOI] [PubMed] [Google Scholar]
- 45.Lee CY, Sholl LM, Zhang B, Merkel EA, Amin SM, Guitart J, et al. Atypical Spitzoid Neoplasms in Childhood: A Molecular and Outcome Study. The American Journal of dermatopathology. 2017. March;39(3):181–6. PubMed PMID: 27391457. [DOI] [PubMed] [Google Scholar]