Abstract
Background
Severe bleeding and massive transfusion of blood products may be associated with increased morbidity and mortality of cardiac surgery. A transfusion algorithm incorporating thromboelastography (TEG) or rotational thromboelastometry (ROTEM) can help to determine the appropriate time and target for the use of hemostatic blood products, which may thus reduce the quantity of blood loss as well as blood products transfused.
Methods
We conducted meta-analysis and trial sequential analysis to evaluate the effects of TEG or ROTEM-guided transfusion algorithms vs. standard treatments for patients undergoing cardiac surgery with cardiac pulmonary bypass.
Results
Nineteen studies with a total of 15,320 participants, including 13 randomized controlled trials (RCTs), were included. All-cause mortality was not reduced either in overall studies or in RCTs. Blood loss volume was reduced by 132 mL in overall studies [mean difference (MD): −132.46, 95% CI: −207.49, −57.43; I2 =53%, P<0.01], and by 103 mL in RCTs (MD: −103.50, 95% CI: −156.52, −50.48; I2 =0%, P<0.01). The relative risks (RRs) in RCTs were 0.89 (95% CI: 0.80–0.98; I2 =0%, P=0.02) for red blood cells transfusion, 0.59 (95% CI: 0.42–0.82; I2 =55%, P<0.01) for fresh frozen plasma transfusion, and 0.81 (95% CI: 0.74–0.90; I2 =0%, P<0.01) for platelet transfusion, respectively. Trial sequential analysis of continuous data on blood loss and dichotomous outcomes on transfusion of blood products suggested the benefits of a TEG/ROTEM-guided algorithm.
Conclusions
TEG or ROTEM-guided transfusion strategies may reduce blood loss volume and the transfusion rates in adult patients undergoing cardiac surgery.
Keywords: Thromboelastography (TEG), blood transfusion, cardiac surgical procedures, adult
Introduction
Abnormal bleeding is a common complication of cardiac surgery with cardiopulmonary bypass (CPB) (1,2). Excessive bleeding increases the rates of massive transfusion and re-exploration, which are potentially associated with increased morbidity and mortality (3-6). Furthermore, blood transfusion is associated with a prolonged hospital stay as well as increased hospital costs (7). Timely diagnosis and treatment of bleeding diathesis are thus important to prevent adverse events.
Transfusion of hemostatic blood products is traditionally based on standard laboratory tests. However, these have limited use in acute bleeding because of their long turnaround time and poor predictive value of bleeding tendency (8). Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are viscoelastic point-of-care testing. A transfusion algorithm incorporating TEG or ROTEM can help to determine the appropriate time and target for the use of hemostatic blood products, which may thus reduce the quantity of blood loss as well as blood products transfused. The point-of-care testing may also enable clinicians to distinguish coagulopathy from surgical causes (9).
The aim of this article is to assess the effects of TEG/ROTEM-guided transfusion algorithm vs. current standard treatments on all-cause mortality, blood products transfusion and short-term hospitalization outcomes in adult patients undergoing cardiac surgery with CPB.
Methods
We conducted a systematic review with a meta-analysis using Cochrane Collaboration methodology and PRISMA and GRADE guidelines (10-12). We searched the Cochrane Register of Controlled Trials, MEDLINE, EMBASE, BIOSIS, International Web of Science, Latin American Caribbean Health Sciences Literature, The Chinese Biomedical Literature Database, Advanced Google, and Cumulative Index to Nursing & Allied Health Literature from 1980 to August 1, 2017. Search strategies were developed specifically for each database, the main search syntax was “("Thoracic Surgery"[Mesh] OR "Cardiac Surgical Procedures"[Mesh] OR aortic valve replacement OR aortic valve repair OR mitral valve replacement OR mitral valve repair OR coronary artery bypass OR pulmonary valve replacement OR pulmonary valve repair OR aortic artery replacement OR aortic root replacement OR antrectomy OR tricuspid valve replacement OR tricuspid valve repair) AND ("Thrombelastography"[Mesh] OR rotational thromboelastometry OR thromboelastogram)”. We hand-searched the reference lists and reviews and contacted authors and experts in this field for any missed, unreported, or ongoing studies. We searched for ongoing clinical trials and unpublished studies on the following websites: clinical trials registry, ISRCTN registry, Center Watch, and UMIN clinical trials registry.
We included all publications of retrospective cohort studies, matched case-control studies and randomized controlled trials (RCTs) evaluating the effect of a TEG/ROTEM-guided transfusion algorithm vs. the current standard treatments, irrespective of publication status, language of the report, or blinding status. We excluded trials conducted on pediatric patients. Two authors independently screened the search results and selected studies for inclusion. Any disagreements were solved by discussion.
All trials were evaluated for major sources of bias. For parallel groups, the items were random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias, including funder bias. Risk of bias in cluster-randomized trials was assessed as recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomized trials. We graded each domain of bias “high risk”, “low risk”, or “unclear risk”. Following Cochrane guideline, publication bias was assessed for items with more than ten trials included.
The outcomes of this review were: (I) all-cause mortality (longest follow-up data from each trial regardless of the period of follow-up); (II) blood loss including mediastinal drainage and post-operative bleeding; (III) proportion of patients transfused with allogeneic blood products, including red blood cell (RBC) concentrates, fresh frozen plasma (FFP), platelet (PLT) concentrates, cryoprecipitate and some pharmacological agents such as fibrinogen concentrate and prothrombin complex concentrate (PCC); (IV) incidence of massive bleeding or massive transfusion and surgical re-exploration; (V) short-term hospitalization outcomes, including length of hospital stay and intensive care unit (ICU) stay. For each item we conducted analysis in the overall studies and RCTs respectively.
Statistics
Data were summarized as relative risks (RRs) with 95% confidence intervals (CI) for dichotomous variables and mean differences (MD) with 95% CI for continuous variables. The degree of heterogeneity was quantified with the I2 statistic and Chi-square test. I2 values of 50% and more indicate a substantial level of heterogeneity, while I2 values of 25% and less indicates a low level of heterogeneity (12). As we included RCTs, observational and retrospective studies, there are substantial heterogeneity in overall study analyses which made use of a fixed effect model little valuable. Therefore, we only reported results from random effect model in overall studies. As for RCTs, we reported the results from fixed effect models when I2 ≤25%. In case of I2 >25%, we tried to determine the cause of heterogeneity by performing relevant subgroup analyses, and when it failed, we reported the results from random effect models. We used the Chi-square test to provide an indication of heterogeneity between studies, with P value ≤0.1 considered significant. All forest plot and meta-analytic estimates were performed using Review Manager 5.3.5 (The Nordic Cochrane Centre, Rigshospitalet 2008). We considered P<0.05 as significant.
Meta-analysis may result in type I errors because of sparse data or repeated significance testing when updating the meta-analysis with new trials. To avoid this, trial sequential analysis (TSA) was applied in the analysis of RCTs. TSA is a methodology to quantify the statistical reliability of evidence in a cumulative meta-analysis and to adjust the threshold of statistical significance for sparse data and repetitive testing on accumulating data. It may reduce the risk of type I errors resulting from meta-analysis due to random errors arising from repeated significance testing when updating meta-analysis with new trials (13). Using a trial sequential monitoring boundary can also help to determine whether additional trials are needed or whether a trial could be terminated early (14), so it is also used as a second step to verify the findings of meta-analysis. TSA was performed using the TSA Viewer, version 0.9.5.10 Beta (TSA Viewer 0.9.5.10 Beta, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, 2016).
Results
Study characteristics
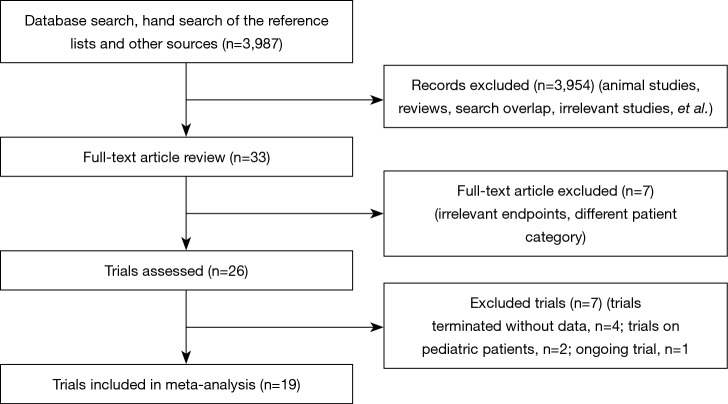
We identified 3,987 publications on the use of TEG or ROTEM, from which 26 publications were selected (Figure 1). Four of the trials were terminated without published data (15-18). We found one ongoing trial but were unable to retrieve any data from the investigators at their current stage (19). Two of the trials (20,21) were excluded because they enrolled pediatric patients only. Altogether, we included 19 studies (20-38) with a total of 15,320 participants, of which 13 (23-26,28-32,34-36,38) were RCTs (Table 1). The trial conducted by Karkouti et al., which enrolled 7,402 patients, was a multicenter stepped-wedge cluster RCT (38). To adjust for the stepped-wedge cluster design, we recalculated the effective sample size for this trial according to the recommendation in the Cochrane Handbook, using the intracluster coefficient calculation of 0.095 stated in the trial methods. In 15 trials (with 9 RCTs), the intervention group applied a transfusion algorithm fully based on TEG or ROTEM, while four trials used TEG or ROTEM in combination with other point-of-care testing devices. The control group in 13 trials adopted a transfusion algorithm based on the clinician’s discretion in combination with a standard laboratory test, while five trials (26,34,35,38,39) adopted a transfusion based on standard laboratory tests only and one (22) based on the clinician’s discretion only. Three (29,34,35) of the trials were published in abstracts only.
Figure 1.
Flow diagram of included and excluded studies.
Table 1. Characteristics of included studies.
| Study | No. of participants | Inclusion criteria | Interventional algorithm | Control group transfusion management | Type of study |
|---|---|---|---|---|---|
| Ak 2009 | 224 | Elective first-time CABG, with CPB | Fully TEG-based algorithm | Clinical judgement and SLTs | RCT |
| Anderson 2006 | 990 | All types of cardiac surgery requiring CPB | Fully ROTEM-based algorithm | Clinical judgement and SLTs | RC |
| Avidan 2004 | 102 | Elective first-time CABG, with CPB | Partly TEG-based algorithm, included the Hepcon and PFA-100 platelet function analyser | SLTs | RCT |
| Fassl 2013 | 62 | Proximal aortic surgery with induced HCA, requiring CPB | Fully ROTEM-based algorithm | Clinical judgement and SLTs | MCC |
| Girdauskas 2010 | 56 | High risk aortic surgery including urgent and emergency surgery with HCA, requiring CPB | Fully ROTEM-based algorithm | Clinical judgement and SLTs | RCT |
| Görlinger 2011 | 3,865 | All types of cardiac surgery requiring CPB | Fully ROTEM-based algorithm | Clinical judgement and SLTs | RC |
| Karkouti 2016 | 7,402 | All types of cardiac surgery requiring CPB | Partly ROTEM-based algorithm | SLTs | RCT |
| Kempfert 2011 | 104 | Adult patients with significant postoperative bleeding (>200 mL/h) following standard elective isolated or combined cardiac surgical procedures | Fully ROTEM-based algorithm | SLTs | RCT |
| Kuiper 2019 | 355 | All types of cardiac surgery requiring CPB | Fully ROTEM-based algorithm | SLTs | PC |
| Kultufan Turan 2006 | 40 | CABG or valve surgery | Fully ROTEM-based algorithm | Clinical judgement and SLTs | RCT |
| Nuttall 2001 | 92 | All types of cardiac surgery requiring CPB, with abnormal microvascular bleeding after CPB | Partly TEG-based algorithm, included point-of-care SLTs | Clinical judgement and SLTs | RCT |
| Paniagua 2011 | 22 | Cardiac surgery requiring CPB, with major postoperative bleeding (>300 mL in the first postoperative hour) | Fully ROTEM-based algorithm | SLTs | RCT |
| Rauter 2007 | 208 | Elective on-pump cardiac surgery | Fully ROTEM-based algorithm | Clinical judgement and SLTs | RCT |
| Royston 2001 | 60 | Surgery requiring CPB, with high risk of requiring hemostatic products (Heart transplantation, revascularization bypass, Ross procedure, multiple valve and revascularization surgery) | Fully TEG-based algorithm | Clinical judgement and SLTs | RCT |
| Shore-Lesserson 1999 | 105 | High risk cardiac procedure with CPB (Single or multiple valve replacement, combined artery bypass plus valvular procedure, cardiac reoperation, thoracic aortic replacement) | Fully TEG-based algorithm | Clinical judgement and SLTs | RCT |
| Spiess 1995 | 1,079 | All types of cardiac surgery requiring CPB | Fully TEG-based algorithm | Clinical judgement | RC |
| St-Onge 2018 | 385 | Cardiac surgery involving aorta with CPB | Fully TEG-based algorithm | Clinical judgement and SLTs | RC |
| Weber 2012 | 100 | Elective complex cardiothoracic surgery with CPB (combined coronary artery bypass graft and valve surgery, double or triple valve procedures, aortic surgery or redo surgery) | Fully ROTEM-based algorithm | Clinical judgement and SLTs | RCT |
| Westbrook 2009 | 69 | All types of cardiac procedures with CPB except lung transplantations | Partly TEG-based algorithm, included Platelet mapping | Clinical judgement and SLTs | RCT |
CPB, cardiopulmonary bypass; CABG, cardiac artery bypass graft; TEG, thromboelastography; ROTEM, rotational thromboelastometry; SLT, standard laboratory tests; RCT, randomized controlled trial; MCC, matched case-control study; RC, retrospective cohort study; PC, prospective cohort study; HCA, hypothermic circulatory arrest.
Four studies gave no data on percentage of selective or emergent surgery, including two RCTs (23,25) and two retrospective cohort studies (22,27). Calculated by the available data, the proportion of elective surgeries in overall studies is 92.8% in TEG/ROTEM group vs. 92.5% in control group, while the proportion in RCTs is 94.8% in TEG/ROTEM group vs. 92.8% in control group.
Only one trial (23) included could be classified as having an overall low risk of bias (Figure 2). The multicenter stepped-wedge cluster RCT conducted by Karkouti and colleagues (38) was judged to be at low risk for the domain recruitment bias, baseline imbalance, loss of clusters, and incorrect analysis. Publication bias were assessed for blood loss, RBC transfusion, FFP transfusion, PLT transfusion and re-exploration in overall studies. The funnel plot of standard error versus risk ratio for RBC transfusion and re-exploration showed a symmetrical distribution that indicated no publication bias, while that for blood loss, FFP transfusion and PLT transfusion showed a relatively higher publication bias.
Figure 2.
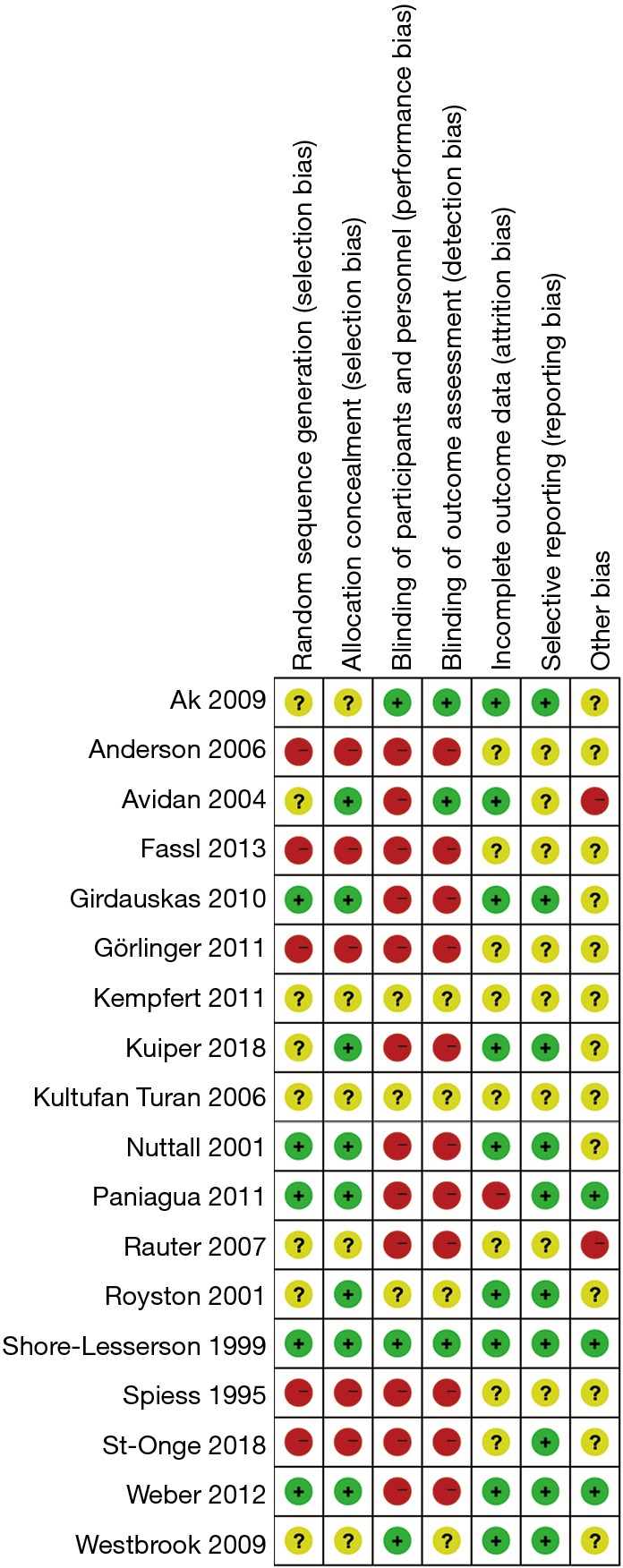
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. A green symbol denotes a low risk of bias, while a red one denotes a high risk of bias. A yellow symbol denotes unclear risk of bias.
Mortality
Eight trials supplied mortality data, including five RCTs (23,30,32,35,36), two retrospective cohort study (33,40) and one prospective cohort study (39). Analyses from both overall studies and RCTs showed no significant effect of the TEG/ROTEM-guided algorithm vs. the control group on the longest follow-up mortality: in all studies it was 135/2,680 (5.0%) in the TEG/ROTEM group compared with 124/2,293 (5.4%) in the control group (RR 0.83, 95% CI: 0.53–1.30; I2 =25%, P=0.4); in RCTs it was 12/270 (4.4%) in the TEG/ROTEM group compared with 23/259 (8.9%) in the control group (RR 0.50, 95% CI: 0.26–0.96, I2 =1%, P=0.04). All trials except three (36,38,40) had the point of hospital discharge as the longest follow-up.
Blood loss
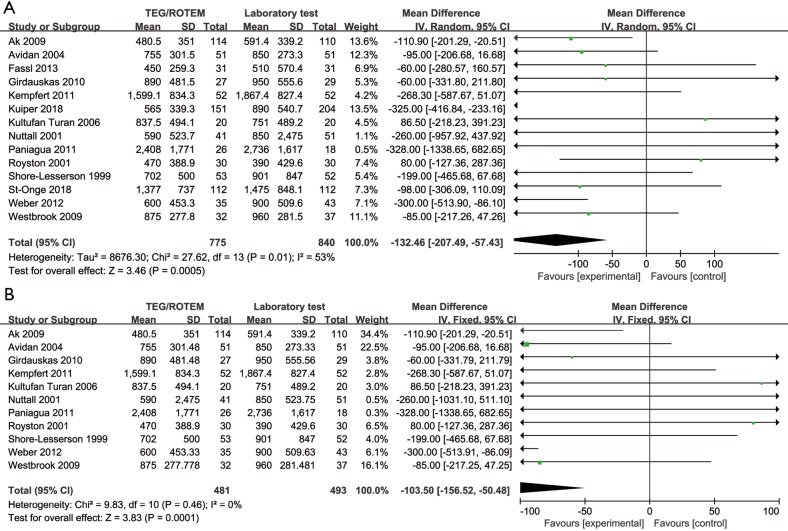
Blood loss volume at 12 or 24 hours after the operation varied from 450±259.3 to 2,408±1,771 mL in the intervention group and 390±429.4 to 2,736±1,617 mL in the control group. The analyses conducted in both overall studies and RCTs showed beneficial effects of the TEG/ROTEM-guided algorithm, indicating reduced bleeding of 132 mL in overall studies (MD: −132.46, 95% CI: −207.49, −57.43, I2 =53%, P<0.01) (Figure 3A), and 103 mL in RCTs (MD: −103.50, 95% CI: −156.52, −50.48, I2 =0%, P<0.01) (Figure 3B).
Figure 3.
Forest plot of blood loss in overall studies (A) and in RCTs (B). RCT, randomized controlled trial; TEG, thromboelastography; ROTEM, rotational thromboelastometry.
Transfusion requirements
Transfusion frequencies of RBC, FFP, and PLT were available in 14 studies, while transfusion of fibrinogen or cryoprecipitate was available in seven studies. Data on the usage of PCC was supplied in six studies. The proportion of patients with each type of blood product transfusion in overall studies and in RCTs is shown in Table 2. In overall studies, the RRs for RBC, FFP and PLT transfusion were 0.87 (95% CI: 0.83–0.91, I2 =11%, P<0.01), 0.50 (95% CI: 0.31–0.80, I2 =93%, P<0.01), and 0.86 (95% CI: 0.73–1.02, I2 =62%, P=0.08), respectively. In RCTs, the RRs for each of the transfusion agents were 0.89 (95% CI: 0.80–0.98, I2 =0%, P=0.02), 0.59 (95% CI: 0.42–0.82, I2 =55%, P<0.01), and 0.81 (95% CI: 0.74–0.90, I2 =0%, P<0.01), respectively. Transfusion frequencies of fibrinogen or cryoprecipitate in overall studies were 376/3,109 (12.1%) in the TEG/ROTEM group vs. 228/2,632 (8.7%) in the control group (RR 1.20, 95% CI: 0.78–1.84, I2 =91%, P=0.4), while the frequency of PCC usage was 236/2,518 (9.4%) vs. 137/2,144 (6.4%) (RR 1.04, 95% CI: 0.48–2.28, I2 =89%, P=0.92). When including RCTs only, the transfusion frequencies of fibrinogen or cryoprecipitate were 53/77 (68.8%) in the TEG/ROTEM group vs. 56/79 (70.9%) in the control group (RR 0.98, 95% CI: 0.80–1.19, I2 =22%, P=0.81), while that of PCC usage was 33/108 (30.6%) vs. 56/110 (50.9%) (RR 0.62, 95% CI: 0.18–2.07, I2 =86%, P=0.4).
Table 2. Transfusion frequencies of allogeneic blood products. Values are events/total.
| Study | RBC | FFP | PLT | Fib or Cry. | PCC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEG/ROTEM | Control | TEG/ROTEM | Control | TEG/ROTEM | Control | TEG/ROTEM | Control | TEG/ROTEM | Control | |||||
| Ak 2009 | 52/114 | 60/110 | 19/114 | 31/110 | 17/114 | 29/110 | – | – | – | – | ||||
| Anderson 2006 | 270/502 | 294/488 | 60/502 | 81/488 | 56/502 | 77/488 | – | – | – | – | ||||
| Avidan 2004 | 34/51 | 35/51 | 2/51 | 0/51 | 2/51 | 1/51 | – | – | 7/31 | 4/31 | ||||
| Fassl 2013 | 15/31 | 22/31 | 10/31 | 20/31 | 9/31 | 7/31 | 23/31 | 24/31 | – | – | ||||
| Girdauskas 2010 | 24/27 | 27/29 | 9/27 | 25/29 | 14/27 | 23/29 | 21/27 | 26/29 | 4/27 | 26/29 | ||||
| Görlinger 2011 | 868/2,147 | 854/1,718 | 24/2,147 | 333/1,718 | 280/2,147 | 173/1,718 | 215/2,147 | 64/1,718 | 191/2,147 | 76/1,718 | ||||
| Karkouti 2016 | 1,749/3,847 | 1,580/3,555 | 915/3,847 | 730/3,555 | 925/3,847 | 938/3,555 | – | – | – | – | ||||
| Kempfert 2011 | – | – | – | – | – | – | – | – | – | – | ||||
| Kuiper 2019 | 39/151 | 56/204 | 7/151 | 19/204 | 16/151 | 20/204 | 17/151 | 9/204 | 0/151 | 0/204 | ||||
| Kultufan Turan 2006 | 7/20 | 12/20 | – | – | 1/20 | 0/20 | – | – | – | – | ||||
| Nuttall 2001 | – | – | – | – | – | – | – | – | – | – | ||||
| Paniagua 2011 | 23/26 | 16/18 | 12/26 | 10/18 | 10/26 | 10/18 | – | – | – | – | ||||
| Rauter 2007 | – | – | – | – | – | – | – | – | – | – | ||||
| Royston 2001 | – | – | – | – | – | – | – | – | – | – | ||||
| Shore-Lesserson 1999 | 22/53 | 31/52 | 4/53 | 16/52 | 7 | 15 | – | – | – | – | ||||
| Spiess 1995 | 437/591 | 406/488 | 156/591 | 176/488 | 285/591 | 289/488 | 39/591 | 44/488 | – | – | ||||
| St-Onge 2018 | 51/112 | 64/112 | 32/112 | 43/112 | 54/112 | 61/112 | 29/112 | 31/112 | 12/112 | 5/112 | ||||
| Weber 2012 | 42/50 | 49/50 | 20/50 | 40/50 | 28/50 | 33/50 | 32/50 | 30/50 | 22/50 | 26/50 | ||||
| Westbrook 2009 | – | – | – | – | – | – | – | – | – | – | ||||
Fib, fibrinogen; Cry., cryoprecipitate; RBC, red blood cell; FFP, fresh frozen plasma; PLT, platelet; PCC, prothrombin complex concentrate; TEG, thromboelastography; ROTEM, rotational thromboelastometry.
Massive bleeding/transfusion and re-exploration
Massive bleeding was reported in three studies and was defined as (I) mediastinal blood loss over 400 mL in the first hour after surgery or over 100 mL/hour for four consecutive hours (30), (II) drainage volumes from chest tubes more than 1,000 mL within the first 24 hours (37), and (III) ≥5 U of RBCs, ≥5 U of plasma, chest tube drainage of ≥1,000 mL within 24 hours of surgery, surgical re-exploration, or administration of recombinant activated factor VII (38). Massive transfusion was reported in four studies and was defined as transfusion of more than 10 U of RBC (33), more than 20 U of any allogeneic blood products (32,40), or both (22). Massive bleeding or transfusion was found in 141 (4.5%) of the 3,149 patients in the TEG/ROTEM group and in 172 (6.6%) of the 2,606 patients in the control group (RR 0.71, 95% CI: 0.54–0.93, I2 =32%, P=0.01). In RCTs, 44 (16.4%) of the 268 patients in the TEG/ROTEM group and 49 (19.1%) of the 257 patients in the control group demonstrated massive bleeding or transfusion (RR 0.86, 95% CI: 0.60–1.24, I2 =0%, P=0.42).
The incidence of surgical re-exploration was reported in 13 studies (22-24,26,27,30,32-36,39,40), including 131 (3.3%) of the 3,917 patients in the TEG/ROTEM group vs. 196 (5.7%) of the 3,423 patients in the control group (RR 0.67, 95% CI: 0.50–0.88, I2 =26%, P<0.01). However, when including RCTs only, the outcome was no longer statistically significant (RR 0.74, 95% CI: 0.50–1.10, I2 =0%, P=0.14).
Short-term hospitalization outcomes
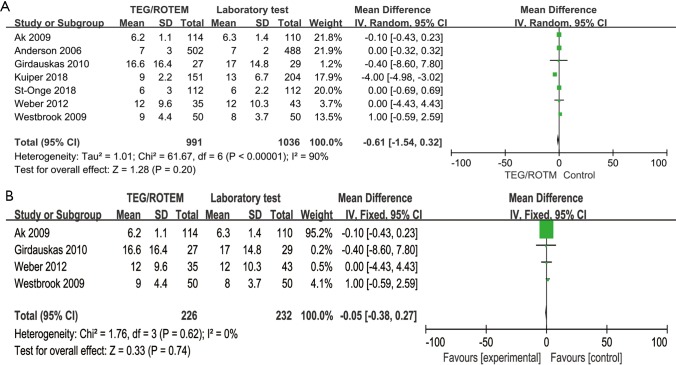
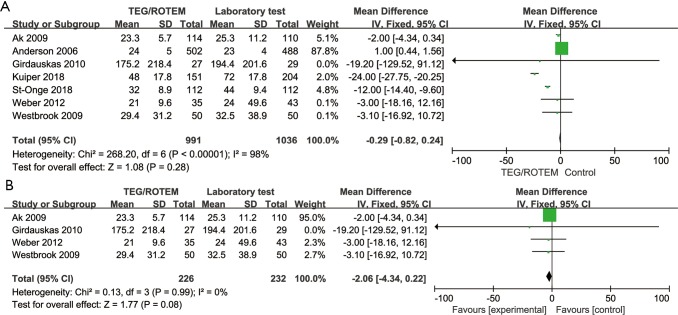
Analyses on the short-term hospitalization outcomes including length of hospital stay and ICU stay also reached no statistically significant difference, either in overall study or in RCTs (see Figures S1,S2 in the supplementary material).
Figure S1.
Forest plot of length of hospital stay. The length of hospital stay (days) reached no statistical significant difference, either in overall study (A) or in RCTs (B). RCT, randomized controlled trial; TEG, thromboelastography; ROTEM, rotational thromboelastometry.
Figure S2.
Forest plot of length of ICU stay. The length of hospital stay (hours) reached no statistical significant difference, either in overall study (A) or in RCTs (B). ICU, intensive care unit; RCT, randomized controlled trial; TEG, thromboelastography; ROTEM, rotational thromboelastometry.
TSA
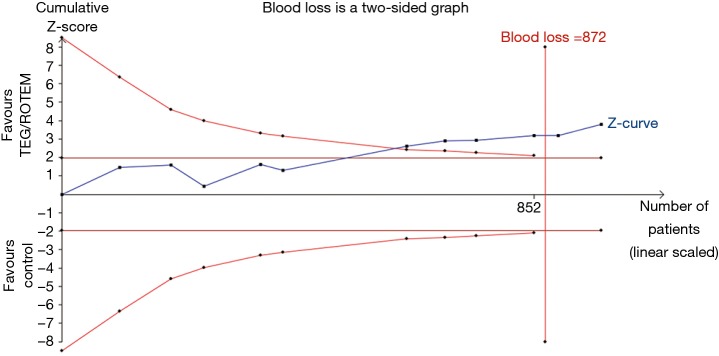
TSA of 11 RCTs on the effect of the transfusion algorithm guided by TEG/ROTEM on blood loss resulted in a statistically significant TSA α-boundary adjusted MD of −102.29 [95% CI: −158.79, −45.79, diversity (D2) =12%, I2 =5%, fixed-effect model, Figure 4]. The cumulative Z-curve crossed the monitoring boundary constructed for a required information size of 872 participants corresponding to a low bias, based on MD and variance, with 80% power and α of 0.05. Only one trial had a low risk of bias. TSA of the effect of the TEG/ROTEM-guided algorithm on the proportion of patients requiring RBC resulted in a TSA α-boundary adjusted RR of 0.87 (95% CI: 0.83–0.91, D2 =0, I2 =0, fixed-effect model) with an intervention event proportion of 55.98% and a control event proportion of 62.9% (based on meta-analysis). The cumulative Z-curve crossed the monitoring boundary constructed for a required information size of 1,246 participants, with 80% power and α of 0.05. TSA on the proportion of patients requiring FFP resulted in a TSA α-boundary adjusted RR of 0.59 (95% CI: 0.42–0.82, D2 =63%, I2 =55%, random-effect model) with continuity adjustment for zero event trials (0.5 in each arm). The cumulative Z-curve crossed the monitoring boundary constructed for an adjusted information size of 693 participants with an intervention event proportion of 21.65% and a control event proportion of 35.74% (based on meta-analysis, with 80% power and α of 0.05). TSA on the proportion of patients requiring PLT resulted in a TSA α-boundary adjusted RR of 0.77 (95% CI: 0.63–0.94, D2 =24%, I2 =14%, fixed-effect model) with continuity adjustment for zero event trials (0.5 in each arm). The cumulative Z-curve crossed the monitoring boundary constructed for a required information size of 1,354 participants with an intervention event proportion of 23.29% and a control event proportion of 30.13% (based on meta-analysis, with 80% power and α of 0.05). The cumulative Z-curve of massive bleeding/transfusion and re-exploration in RCTs did not reach the monitoring boundary constructed for a required information size.
Figure 4.
Trial sequential analysis of blood loss. Trial sequential analysis of 11 RCTs on the effect of transfusion algorithm guided by TEG/ROTEM on blood loss resulted in a statistically significant TSA α-boundary adjusted MD of −102.29 [95% CI: −158.79 to −45.79, diversity (D2) =12%, I2 =5%, fixed effect model]. Cumulative Z-curve crossed the monitoring boundary constructed for a required information size of 872 participants corresponding to low bias based mean difference and variance, with 80% power and α of 0.05. However, only one trial had low risk of bias. RCT, randomized controlled trial; TEG, thromboelastography; ROTEM, rotational thromboelastometry; TSA, trial sequential analysis; MD, mean difference.
Discussion
In this systematic review of 13 RCTs and six observational studies involving adult patients undergoing cardiac surgery with CPB, we found that the mortality rate in the TEG/ROTEM group was lower than that in control group, but without statistically significant difference, either in overall studies or in RCTs. Only six studies, including five RCTs, provided data on mortality.
We found a statistically significant reduction of blood loss in favor of the TEG/ROTEM-guided algorithm in both overall studies and RCTs. Despite a potential benefit of TEG/ROTEM in the estimation and prevention of bleeding after cardiac surgery, no association with improvement of long-term prognosis was found. The use of a TEG/ROTEM-guided algorithm had a significant beneficial effect on the transfusion requirements of RBC and FFP. TSA of continuous data on blood loss and dichotomous outcomes on transfusion of blood products verified the conclusions drawn from the meta-analysis.
Several meta-analyses have been performed on the TEG/ROTEM-guided algorithm, but most were aimed at bleeding patients with no regard to original disease and included both adult and pediatric patients. Most of the analyses found a reduction of blood loss or transfusion rates favoring TEG/ROTEM-guided algorithm (41-45), while few found a beneficial effect on mortality or short-term hospitalization outcomes (44,46). A recent updated meta-analysis (47) focusing on the effectiveness of viscoelastic tests in patients undergoing cardiac surgery reached similar conclusions with our analysis on transfusion of RBCs and FFP, but concluded that the use of viscoelastic testing had no beneficial effects on objective clinical outcomes. Blood loss was not assessed in this study. Our analysis included six more observational studies (22,27,33,37,39,40), including two latest ones (39,40), which provided important complementary information to the existing reviews conducted on RCTs. Separate analyses of RCTs could reduce bias that may be caused by inclusion of the retrospective studies. As pediatric patients have completely different characteristics and surgical procedures with adult patients, we excluded this subgroup of patients to reduce bias.
Though our analysis showed consistent benefits of viscoelastic testing on blood loss and transfusion rates, it failed to reach the same beneficial effects on patients’ outcome including mortality, length of hospital stay and ICU stay, even rates of re-exploration and massive bleeding/transfusion. There are several possible reasons underlying this phenomenon. For one thing, aside from blood loss and transfusion, there are other variables that may affect outcomes of patients undergoing cardiac surgery, such as length of surgical, duration on extracorporeal circulation, duration of cross-clamping, hematocrit level, thrombocyte count, temperature on arrival to the ICU and comorbidities. Not all of the above items are comparable in each of the included trials, especially in observational studies, and standardized transfusion and bleeding protocols in control groups are quite poor in almost all the trials. For another thing, although mortality in overall studies reached no statistical difference, mortality in RCTs is marginally lower in TEG/ROTEM group (P=0.04). Massive bleeding or transfusion was marginally and surgical re-exploration was significantly reduced in the TEG/ROTEM group, though when including RCTs only, neither of the outcome was statistically significant. The result of TSA showed that unlike blood loss and transfusion rates, the cumulative Z-curve of massive bleeding/transfusion and re-exploration in RCTs did not reach the monitoring boundary constructed for a required information size. Therefore, the most likely reason for the inconsistent impact of viscoelastic testing on patient outcomes is the insufficient sample size of RCTs.
Furthermore, clinical complications including infection, thrombosis, allergic reactions, acute kidney or pulmonary injury, which are more related to bleeding and transfusion (48), were not demonstrated in most of the included trials, whether viscoelastic testing can reduce the incidences of these complications is still unknown. Another concern of transfusion is financial and social costs. Whiting and colleagues have concluded in a meta-analysis and review that viscoelastic testing was more cost-saving and effective than standard laboratory testing (43). Their analysis directly informed current National Health Service NICE Guidelines, which recommend routine use of viscoelastic testing in cardiac surgery. We have good reason to believe that more strictly-designed and large-sized RCTs are needed to evaluate the complications and short-term mortality.
Findings and interpretations in this review are limited by the quality and quantity of the available evidence. On one hand, even excluding retrospective and observational studies, most RCTs also have little or no allocation concealment or blinding of clinical personnel, which contributed to the high procedural bias in these trials. Furthermore, control groups in almost all trials had no standard transfusion protocols, random sequence generation, allocation concealment, or blinding. Publication bias are also high for blood loss, FFP transfusion and PLT transfusion. On the other hand, the interventional algorithms and follow-up times described in the trials included in this meta-analysis were not completely consistent, especially the first one. The transfusion algorithms in intervention group are fully or partly based on TEG or ROTEM, while that in control group based on standard laboratory test or the clinician’s discretion, or both. Direct translation to other clinical settings should thus be made with great caution.
In conclusion, though the evidence is limited to surrogate outcomes such as blood loss and transfusion rates, it is still reasonable to use TEG or ROTEM as a tool to guide transfusion in cardiac surgery. Large-sized RCTs with low bias are seriously needed to evaluate the effects of transfusion algorithms based on TEG or ROTEM on the hospitalization outcomes and complications in cardiac surgery setting.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding: This study was supported by National Natural Science Foundation of China (NSFC-81670385 to J Yu); Foundation of Lanzhou University Second Hospital (ynbskyjj2015-2-1 to J Yu) and Cuiying Technology Innovation Project of Lanzhou University Second Hospital (CY2018-MS05 to Q Zhao).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Despotis GJ, Avidan MS, Hogue CW., Jr Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg 2001;72:S1821-31. 10.1016/S0003-4975(01)03211-8 [DOI] [PubMed] [Google Scholar]
- 2.Shander A, Moskowitz D, Rijhwani TS. The safety and efficacy of “bloodless” cardiac surgery. Semin Cardiothorac Vasc Anesth 2005;9:53-63. 10.1177/108925320500900106 [DOI] [PubMed] [Google Scholar]
- 3.Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 2004;44:1453-62. 10.1111/j.1537-2995.2004.04144.x [DOI] [PubMed] [Google Scholar]
- 4.Johnson JL, Moore EE, Kashuk JL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg 2010;145:973-7. 10.1001/archsurg.2010.216 [DOI] [PubMed] [Google Scholar]
- 5.Vamvakas EC, Blajchman MA. Blood still kills: six strategies to further reduce allogeneic blood transfusion-related mortality. Transfus Med Rev 2010;24:77-124. 10.1016/j.tmrv.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isbister JP, Shander A, Spahn DR, et al. Adverse blood transfusion outcomes: establishing causation. Transfus Med Rev 2011;25:89-101. 10.1016/j.tmrv.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 7.Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544-52. 10.1161/CIRCULATIONAHA.107.698977 [DOI] [PubMed] [Google Scholar]
- 8.Hardy JF, de Moerloose P, Samama CM, et al. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth 2006;53:S40-58. 10.1007/BF03022251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol 2005;27:81-90. 10.1111/j.1365-2257.2005.00681.x [DOI] [PubMed] [Google Scholar]
- 10.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, 2011. Available online: http://handbook-5-1.cochrane.org/
- 13.Brok J, Thorlund K, Wetterslev J, et al. Apparently conclusive meta-analyses may be inconclusive--Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38:287-98. 10.1093/ije/dyn188 [DOI] [PubMed] [Google Scholar]
- 14.Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64-75. 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 15.Michael S. Algorithm-Guided Transfusions in Cardiac Surgery Patients for Reduction of Drainage Blood Losses (HEART-PoC). Trial registration NCT01402739 2011. (accessed 31/12/2017). Available online: https://clinicaltrials.gov/ct2/results?term=NCT01402739
- 16.Nathan-Denizot N. Perioperative Coagulation Management in Cardiac Surgery. (ROTEM). Trial registration NCT00772239 2008. (accessed 31/12/2017).Available online: https://clinicaltrials.gov/ct2/results?term=NCT00772239
- 17.Petricevic M. Bleeding Prediction in Patients Following Cardiac Surgery Using Whole Blood Aggregometry and Thromboelastometry. Trial registration NCT01281397 2011. (accessed 31/12/2017). Available online: https://clinicaltrials.gov/show/NCT01281397
- 18.Kim W. The Predictability of Intraoperative Rotational Thromboelastometry on Postoperative Bleeding and Transfusion Requirements. Trial registration NCT02081222 2014. (accessed 31/12/2017). Available online: https://clinicaltrials.gov/show/NCT02081222
- 19.Ibla J. Evaluation of Coagulation Testing in Patients Undergoing Cardiac Surgery. Trial registration NCT02410473 2015. (accessed 31/12/2017). Available online: https://clinicaltrials.gov/show/NCT02410473
- 20.Cui Y, Hei F, Long C, et al. Perioperative monitoring of thromboelastograph on blood protection and recovery for severely cyanotic patients undergoing complex cardiac surgery. Artif Organs 2010;34:955-60. 10.1111/j.1525-1594.2010.01148.x [DOI] [PubMed] [Google Scholar]
- 21.Nakayama Y, Nakajima Y, Tanaka KA, et al. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br J Anaesth 2015;114:91-102. 10.1093/bja/aeu339 [DOI] [PubMed] [Google Scholar]
- 22.Spiess BD, Gillies BSA, Chandler W, et al. Changes in transfusion therapy and reexploration rate after institution of a blood management program in cardiac surgical patients. J Cardiothorac Vasc Anesth 1995;9:168-73. 10.1016/S1053-0770(05)80189-2 [DOI] [PubMed] [Google Scholar]
- 23.Shore-Lesserson L, Manspeizer HE, DePerio M, et al. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 1999;88:312-9. [DOI] [PubMed] [Google Scholar]
- 24.Nuttall GA, Oliver WC, Santrach PJ, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology 2001;94:773-81. 10.1097/00000542-200105000-00014 [DOI] [PubMed] [Google Scholar]
- 25.Royston D, Von Kier S. Reduced haemostatic factor transfusion using heparinase-modified thrombelastography during cardiopulmonary bypass. Br J Anaesth 2001;86:575-8. 10.1093/bja/86.4.575 [DOI] [PubMed] [Google Scholar]
- 26.Avidan MS, Alcock EL, Da Fonseca J, et al. Comparison of structured use of routine laboratory tests or near‐patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth 2004;92:178-86. 10.1093/bja/aeh037 [DOI] [PubMed] [Google Scholar]
- 27.Anderson L, Quasim I, Soutar R, et al. An audit of red cell and blood product use after the institution of thromboelastometry in a cardiac intensive care unit. Transfus Med 2006;16:31-9. 10.1111/j.1365-3148.2006.00645.x [DOI] [PubMed] [Google Scholar]
- 28.Kultufan Turan S, Aydinli B, Ayik I, et al. The role of rotational thromboelastgraphy on decision of blood transfusion in open heart surgery. GKD Anest Yog Bak Dern Derg 2006;12:154-9. [Google Scholar]
- 29.Rauter M, Kastenbauer T, Schwarz S, et al. Reduced number of red blood cell transfusions in cardiac surgery due to perioperative coagulation testing by thromboelastometry. Acta Anaesthesiol Scand 2007;51:40. [Google Scholar]
- 30.Ak K, Isbir CS, Tetik S, et al. Thromboelastography‐based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg 2009;24:404-10. 10.1111/j.1540-8191.2009.00840.x [DOI] [PubMed] [Google Scholar]
- 31.Westbrook AJ, Olsen J, Bailey M, et al. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ 2009;18:277-88. 10.1016/j.hlc.2008.08.016 [DOI] [PubMed] [Google Scholar]
- 32.Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117-24.e2. 10.1016/j.jtcvs.2010.04.043 [DOI] [PubMed] [Google Scholar]
- 33.Görlinger K, Dirkmann D, Hanke AA, et al. First-line Therapy with Coagulation Factor Concentrates Combined with Point-of-Care Coagulation Testing Is Associated with Decreased Allogeneic Blood Transfusion in Cardiovascular SurgeryA Retrospective, Single-center Cohort Study. Anesthesiology 2011;115:1179-91. [DOI] [PubMed] [Google Scholar]
- 34.Kempfert J, Hänsig M, Wobbe P, et al. Thromboelastography-guided blood component therapy after cardiac surgery: a randomised study. Interact Cardiovasc Thorac Surg 2011;13:S106-7. [Google Scholar]
- 35.Paniagua P, Koller T, Requena T, et al. Randomized controled trial to evaluate postoperative coagulation management with bed‐side trombelastometry (Rotem) compared with a transfusion protocol based on laboratory measurements in bleeding patients after cardiac surgery: Preliminary data: 6AP6‐4. Eur J Anaesthesiol 2011;28:94 10.1097/00003643-201106001-00301 [DOI] [Google Scholar]
- 36.Weber CF, Görlinger K, Meininger D, et al. Point-of-Care TestingA Prospective, Randomized Clinical Trial of Efficacy in Coagulopathic Cardiac Surgery Patients. Anesthesiology 2012;117:531-47. 10.1097/ALN.0b013e318264c644 [DOI] [PubMed] [Google Scholar]
- 37.Fassl J, Matt P, Eckstein F, et al. Transfusion of allogeneic blood products in proximal aortic surgery with hypothermic circulatory arrest: effect of thromboelastometry-guided transfusion management. J Cardiothorac Vasc Anesth 2013;27:1181-8. 10.1053/j.jvca.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 38.Karkouti K, Callum J, Wijeysundera DN, et al. Point-of-Care Hemostatic Testing in Cardiac Surgery. Circulation 2016;134:1152-62. 10.1161/CIRCULATIONAHA.116.023956 [DOI] [PubMed] [Google Scholar]
- 39.Kuiper GJAJM, van Egmond LT, Henskens YMC, et al. Shifts of Transfusion Demand in Cardiac Surgery After Implementation of Rotational Thromboelastometry-Guided Transfusion Protocols: Analysis of the HEROES-CS (HEmostasis Registry of patiEntS in Cardiac Surgery) Observational, Prospective Open Cohort Database. J Cardiothorac Vasc Anesth 2019;33:307-17. 10.1053/j.jvca.2018.08.203 [DOI] [PubMed] [Google Scholar]
- 40.St-Onge S, Lemoine É, Bouhout I, et al. Evaluation of the real-world impact of rotational thromboelastometry-guided transfusion protocol in patients undergoing proximal aortic surgery. J Thorac Cardiovasc Surg 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 41.Wikkelsoe AJ, Afshari A, Wetterslev J, et al. Monitoring patients at risk of massive transfusion with Thrombelastography or Thromboelastometry: a systematic review. Acta Anaesthesiol Scand 2011;55:1174-89. 10.1111/j.1399-6576.2011.02534.x [DOI] [PubMed] [Google Scholar]
- 42.Bolliger D, Tanaka KA. Roles of thrombelastography and thromboelastometry for patient blood management in cardiac surgery. Transfus Med Rev 2013;27:213-20. 10.1016/j.tmrv.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 43.Whiting P, Al M, Westwood M, et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19:1-228. 10.3310/hta19580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wikkelsø A, Wetterslev J, Møller AM, et al. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev 2016;(8):CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afshari A, Wikkelsø A, Brok J, et al. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev 2011;(3):CD007871. [DOI] [PubMed] [Google Scholar]
- 46.Wikkelsø A, Wetterslev J, Møller AM, et al. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta‐analysis and trial sequential analysis. Anaesthesia 2017;72:519-31. 10.1111/anae.13765 [DOI] [PubMed] [Google Scholar]
- 47.Serraino GF, Murphy GJ. Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: updated systematic review and meta-analysis. Br J Anaesth 2017;118:823-33. 10.1093/bja/aex100 [DOI] [PubMed] [Google Scholar]
- 48.Carson JL, Triulzi DJ, Ness PM. Indications for and Adverse Effects of Red-Cell Transfusion. N Engl J Med 2017;377:1261-72. 10.1056/NEJMra1612789 [DOI] [PubMed] [Google Scholar]