Abstract
In this article, we summarize the role of CD8+ T cells during natural and ART-treated HIV and SIV infections, discuss the mechanisms responsible for their suppressive activity, and review the rationale for CD8+ T cell-based HIV cure strategies. Evidence suggests that CD8+ T cells are involved in the control of virus replication during HIV and SIV infections. During early HIV infection, the cytolytic activity of CD8+ T cells is responsible for control of viremia. However, it has been proposed that CD8+ T cells also use non-cytolytic mechanisms to control SIV infection. More recently, CD8+ T cells were shown to be required to fully suppress virus production in ART-treated SIV-infected macaques, suggesting that CD8+ T cells are involved in the control of virus transcription in latently infected cells that persist under ART. A better understanding of the complex antiviral activities of CD8+ T cells during HIV/SIV infection will pave the way for immune interventions aimed at harnessing these functions to target the HIV reservoir.
Keywords: CD8 T cells, cytotoxicity, HIV, immune response, infectious disease
Introduction
Human immunodeficiency virus (HIV) is the causative agent of the acquired immunodeficiency syndrome (AIDS) [1, 2] and infects an estimated 36.7 million people worldwide. Based on UNAIDS estimates, 1.8 million new HIV infections are projected to occur annually and as well as one million AIDS-related deaths [3]. While antiretroviral therapy (ART), the standard care for HIV infection, has dramatically reduced the mortality and morbidity of HIV infection, there is still no cure for this infection.
The main obstacle in the development of an HIV cure is the presence of a reservoir of HIV-infected cells containing integrated DNA but not expressing the virus, defined as latently infected cells, that seem to persist indefinitely in ART-treated HIV-infected humans [4–7]. This population is termed “HIV viral reservoir” and occurs primarily within resting memory CD4+ T cells [4, 8]. It appears that over time there is a progressive reduction in the size of the HIV viral reservoir around a core of less differentiated memory subsets: central memory (TCM) and stem cell memory (TSCM) [9]. These cells are long-lived, capable of self-renewal (in vitro) and have an estimated half-life of >44 months [7, 10]. It was recently described that the HIV viral reservoir is established early during infection [11, 12] and is responsible for the viral rebound observed after ART interruption [13, 14]. Therefore, strategies additional to ART are necessary to cure HIV, and novel therapies targeting the HIV viral reservoir are of utmost importance.
SIV infection of rhesus macaques (RM) is similar to pathogenic HIV infection of humans with establishment of peak and set point viremia, depletion of CD4+ T cells, onset of AIDS, and suppression of viremia by ART [15]. Therefore, certain experimental limitations of studying HIV infection of humans can be overcome using the nonhuman primate (NHP) with simian immunodeficiency virus (SIV) infection model [reviewed in [15–17]. NHP studies allow for control of the infecting virus strain, timing of infection, more aggressive tissue sampling, selection of specific MHC class I genotypes, and elective necropsies with unlimited tissue collection. Crucially, the NHP/SIV model allows the testing of risky in vivo immune interventions, such as those combining various immunomodulatory approaches, which are virtually impossible to conduct in HIV-infected humans. As such, NHP SIV studies are an important tool used for further insight into HIV pathogenesis, prevention, and treatment in humans.
CD8+ T cells in HIV and SIV pathogenesis
Acute infection
HIV can be transmitted via blood, breast milk, semen or vaginal secretions from infected individuals [18]. Systemic infection is established with the spread of the virus to lymphoid tissues throughout the body including, but not limited to, the thymus, the spleen, peripheral lymphoid organs, mucosal lymphoid tissues, and the brain [19]. Acute HIV infection of humans is characterized by a transient peak in viremia (2-3 weeks) followed by a post-peak decline to a viral set-point level of viremia that is a strong predictor of the ensuing rate of progression to AIDS [20]. Subsequently, HIV-infected patients experience a slow decrease in CD4+ T cells and gradual deterioration of immune function, including exhaustion of CD8+ T-cells, loss of immune function in the lymph nodes and mucosal tissues and chronic immune activation, leading to increased susceptibility to opportunistic infections and cancer [21], [22, 23].
Several lines of evidence suggest that CD8+ T cells play a significant role in the control of virus replication during the acute phase of HIV and SIV infection. First, the post-peak decline of viremia only occurs after the emergence of virus-specific CD8+ T cells, suggesting that CD8+ T cells are involved in the initial control of infection [24, 25]. In support, depletion of CD8+ T cells during acute SIV infection of RM results in the abrogation of the post-peak decline of viremia [26, 27], confirming a critical role in the initial resolution of viral control. In addition, during the first weeks of infection viral mutants capable of escaping the CD8+ T cell response begin to appear and rapidly become fixed in the overall virus population, thus demonstrating a strong evolutionary pressure posed on the virus to escape immunological recognition by CD8+ T cells [28–31]. Overall, these observations indicate that CD8+ T cells play a significant role in the control of acute HIV infection.
Interestingly, unlike with other viral infections, the initial expansion of the effector CD8+ T cell pool is not limited to HIV-specific cells and of the total CD8+ T cell pool expanded during the acute infection, only about 10% are HIV-specific CD8+ T cells [32, 33]. CD8+ T cells specific for persistent pathogens, such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV), and non-persistent pathogens, such as influenza and adenovirus, may reactivate, thus suggesting that CD8+ T cell expansion is capable of occurring through antigen-independent mechanisms [34–36]. The exact cause of such “bystander activation” remains unclear.
Persistent exposure to HIV antigen during the natural course of HIV infection leads to the progressive dysfunction and “exhaustion” of virus-specific T cells. T cell exhaustion is characterized by altered differentiation, impaired function, and decreased proliferation [reviewed in [37]]. Of note, T cell exhaustion begins soon after peak HIV viremia and persists for the remainder of the infection [38, 39]. In the early stages of exhaustion, HIV-specific T cells have an impaired ability to proliferate in response to antigen, as well as reduced expression of interleukin-2 (IL-2), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), chemokine ligand-4/macrophage inflammatory protein-1α (CCL4/MIP-1α) and the degranulation marker CD107a [40]. The upregulation of exhaustion marker programed death-1 (PD-1) on HIV-specific CD8+ T cells from viremic patients is associated with impaired cytokine production, proliferation, survival, and turnover [41–44]. Other markers of T cell exhaustion include co-inhibitory receptors LAG-3, CD160, and Tim-3 [45–47]. It was recently shown that while virus-specific CD8+ T cells are initially capable of cytolytic activity, the potential is significantly reduced after acute infection [48, 49]. Thus, while HIV-specific CD8+ T cells appear to be necessary for the post-peak decline in viremia during the acute infection, persistent exposure to antigen and chronic inflammation results in an exhausted phenotype, in which cells are no longer capable of amounting an appropriate response against HIV and the infection remains.
Chronic infection
CD8+ T cells continue to exert some level of control over HIV and SIV replication after the acute phase of infection, as shown by studies in which depletion of CD8+ T cells during chronic SIV infection results in increased viral replication [50–52]. Additionally, viral escape mutants against CD8+ T cell responses continue to appear during the chronic phase of infection [53]. However, the combination of virus escape and progressive T cell dysfunction and exhaustion makes HIV− or SIV-specific CD8+ cells increasingly less able to successfully control virus replication [40, 54–59]. This loss of CD8+ T cell-mediated control of virus replication is associated with disease progression in chronically HIV-infected individuals [41, 46]. Interestingly, continuous activation of CD8+ T cells in the absence of effective antiviral activity may lead to disease progression [60], as first suggested by the classical observation that the level of CD8+ T cells expressing the activation markers CD38 and HLA-DR are most closely associated with shorter patient survival than viral load or CD4+ T cell count [61].
Natural control of HIV infection
It has long been recognized that a small group of HIV-infected individuals (<1% of the population) are capable of controlling HIV infection independent of ART. These individuals, termed elite controllers (EC) are able to maintain plasma viremia below the limit of detection of standard PCR assays in absence of ART . EC typically have stable CD4 counts without decline and progression to AIDS. Post-treatment controllers (PTC) are HIV-infected individuals who control virus below the limit of detection after interruption of long-term ART [62]. Intriguingly, the non-pathogenic phenotype of natural SIV infection in sooty mangabeys, a natural host species, is associated with relatively low CD8+ T cell responses to the virus [63, 64].
It is now understood that host factors, as opposed to viral factors, largely mediate control of HIV infection in EC and that CD8+ T cells play a prominent role in this phenomenon [reviewed in [65]]. In fact, depletion of CD8+ lymphocyte from controller rhesus macaques resulted in a transient increase in viremia [66]. Another study found that EC have very high levels of escape mutations, suggesting that CD8+ T cells put great selective pressure on the virus [67]. The identification of specific differences in host factors between chronic progressors and EC has defined potential targets for in vivo manipulation of HIV/SIV-specific CD8+ T cell-specific responses to achieve better immunological control of the infection. Among these host factors a key role is played by specific MHC class I alleles whose presence is significantly more frequent in the EC population [49, 68–71]. Specifically, HLA-B*27/*57 EC possess HIV-specific CD8+ T cells restricted by these class-I molecules that throughout chronic infection continue to show in vitro proliferation, whereas the majority of HIV-specific CD8+ T cells restricted by other HLA alleles lose this proliferative capacity [72–74]. Proliferative capacity of CD8 T+ cells in EC is associated with the up-regulation of perforin and therefore associated with enhanced cytotoxic capabilities [72]. In addition, HIV-specific CD8+ T cells from EC synthesize greater amounts of cytotoxic granule components, thus increasing their ability to kill infected cells [75–77] and are found to exceptionally up-regulate T-bet expression, which increases the production of perforin and granzyme B [78, 79].
Of note, EC are not different from CP on the basis of the frequencies of HIV-specific CD8+ T cells in peripheral blood, the antigen specificity or breadth of this response, nor the differences in the functional avidity [32, 80–82]. Together this data strongly suggests that CD8+ T cells play an important role during natural control of HIV and SIV infection.
Cytolytic versus non-cytolytic activities of CD8+ T cells during HIV infection
Cytolytic activities
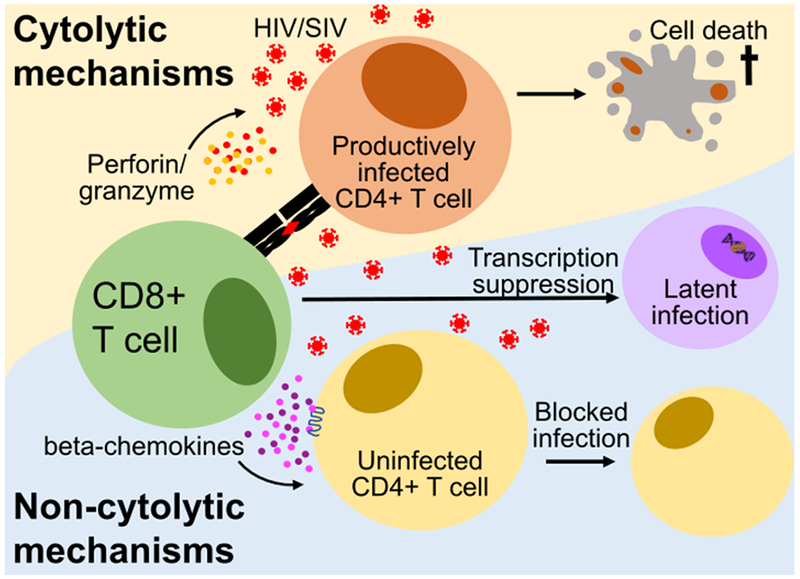
CD8+ T cells have long been characterized by their cytotoxic T lymphocyte (CTL) activity during viral infection. CTL activity is mediated via formation of TCR-dependent immunological synapses in an antigen-dependent manner. CD8+ T cells kill target cells through the secretion of the granule-bound cytolytic molecules perforin and granzyme [83–85]. Granzymes are serine proteases that induce apoptosis by cleaving caspases [86, 87]. Perforin forms pores in the membrane of the cell, which also leads to apoptosis and allows for delivery of granzyme [88, 89]. The balance between the transcription factors Eomes and T-bet seems to dictate the differentiation and CTL functional pathways of the cell [90–94]. Together these transcription factors regulate the differentiation and CTL effector function of CD8+ T cells [95–97]. While T-bet positively regulates perforin and granzyme B expression, as well as genes associated with effector function [78, 98], Eomes positively regulates genes associated the maintenance of memory CD8+ T cells [90, 95, 97, 99].
The specific contribution of CTL responses to the control of HIV infection remains incompletely understood. HIV-specific CD8+ T cells are able to suppress HIV replication in vitro by direct cytotoxicity as well as by secretion of soluble factors [100–102]. During the acute phase of HIV and SIV infections, the CD8+ T cell pool is highly activated and primed for strong cytotoxic effector activity, however, this capacity decreases in the chronic phase of infection [49]. HIV-specific CD8+ T cells lose their ability to upregulate perforin after the resolution of peak viremia, a characteristic that also coincides with reduced expression of T-bet, but not of Eomes [49]. During chronic HIV infection, a T-bethiEomeshi population predominates the HIV-specific CD8+ T cell pool, exhibiting reduced differentiation, decreased functionality, enhanced exhaustion, and little to no expression of perforin [78, 92]. The loss of HIV-specific CD8+ T cell cytolytic function during chronic infection is thought to be a contributing factor to progressive HIV infection [75, 76, 103–105]. As mentioned above in describing the EC phenotype, control of viremia is associated with the ability of CD8+ T cells to proliferate and upregulate granzyme/perforin expression in response to in vitro antigen exposure [76]. In addition, it has also been shown that the ability of CD8+ T cells to upregulate perforin following in vitro stimulation correlates inversely with viral load [75]. Overall this complex set of experimental data suggests that CTL activity by CD8+ T cells is present and likely very important during the acute phase of HIV/SIV infection and in determining the relatively rare EC phenotype, while its role during chronic progressive infection is not clear and possibly much less important.
Non-cytotoxic activities
CD8+ T cells may suppress active HIV replication in vitro via non-cytolytic mechanisms that are related to the secretion of soluble factors [106–111]. Immunological factors able to suppress HIV/SIV replication include the β-chemokines CCL3, CCL4, and CCL5 (also known as MIP-1α, MIP-1β, and RANTES, respectively), which block the entry CCR5-tropic viruses [102, 112–114]. In fact, characterization of CD8+ T cells with a MIP-1β expression profile has been identified as a correlate of virus control and inhibition [115–117]. HIV/SIV-specific CD8+ T-cells also secret IFN-γ, which may play a role in the noncytolytic immune response, however, there is no demonstrable correlation between IFNγ expression and viral load, viral set point, viral clearance, or chronicity, with considerable variation between patients [118–120]. Despite a significant effort in the laboratory of Dr. Jay Levy, relatively little is known about the exact nature or specific identity of another secreted factor termed CD8+ Antiviral Factor (CAF) [121–123] that appears to suppress LTR-mediated gene expression in CD4+ T cells [124]. CAF does not block viral entry, integration, or reverse transcription, nor is it MHC-restricted [122–125]. In addition, CAF is not lentivirus-specific as it was also shown to suppress promotors of other viruses [126] and it is not produced exclusively by CD8+ T cells, which led to the hypothesis that CAF is part of the innate immune response [126, 127]. Of note, CAF lacks identity with IFN-α, IFN-β, TNF-α, IL-4, IL-6, and the β-chemokines [111, 121, 128, 129], and it remains possible that CAF is the activity of multiple factors [127]. The CD8+ T cell-specific noncytolytic mechanisms responsible for the suppression of HIV have yet to be fully understood. Studies have found evidence CD8+ T cells suppress replication by inhibiting viral transcription [130] and proviral gene expression[131, 132].
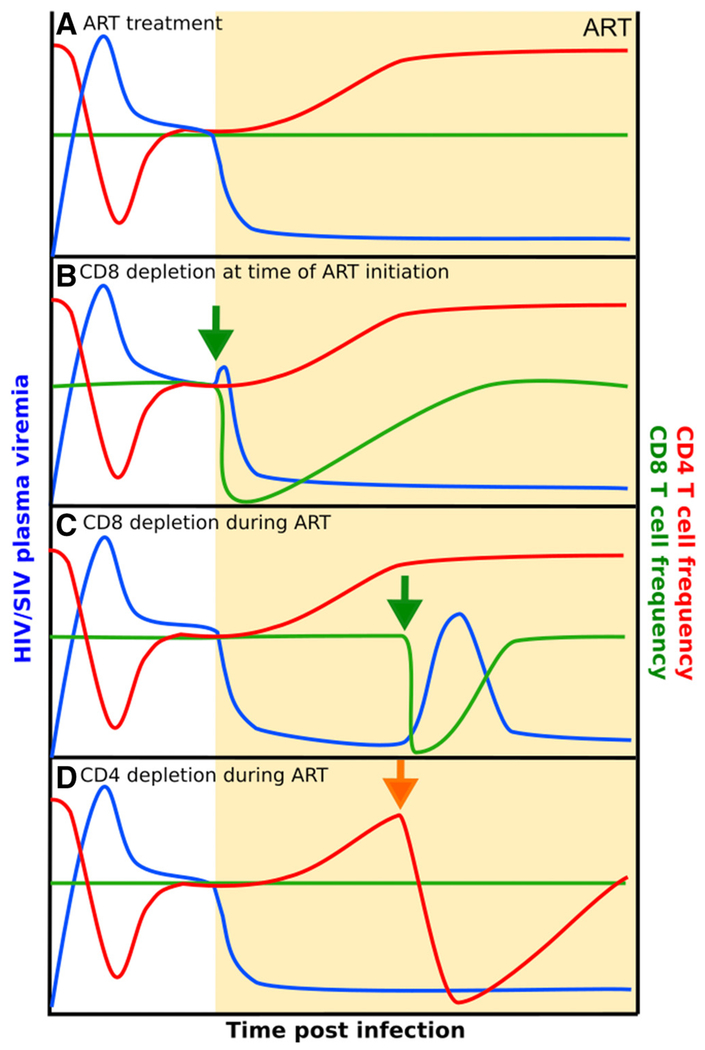
Strong support in favor of the hypothesis that non-cytolytic mechanisms of antiviral activity by CD8+ T cells are important in controlling HIV and SIV replication was provided by two independent studies in which the in vivo lifespan of productively infected cells was measured in CD8+ lymphocyte-depleted versus non-depleted SIV-infected RM [133, 134]. In both studies, SIV-infected RM were initiated on ART immediately after depletion of CD8+ T cells and the in vivo lifespan of productively infected cells was calculated based on the rate of viremia decline under ART using established mathematical models [135, 136]. Interestingly, both studies showed that the viral decay dynamics at the onset of ART was very similar between CD8+ lymphocyte-depleted RM and non-depleted animals, thus demonstrating that the relatively short in vivo lifespan of productively SIV-infected cells cannot be attributed to cytolytic activity of CD8+ T cells (Figure 1 A and B). Instead, the results of both studies are compatible with the hypothesis that non-cytolytic mechanisms that do not impact on the lifespan of a productively infected cell are involved in CD8+ T cell-mediated suppression of SIV replication.
Figure 1:
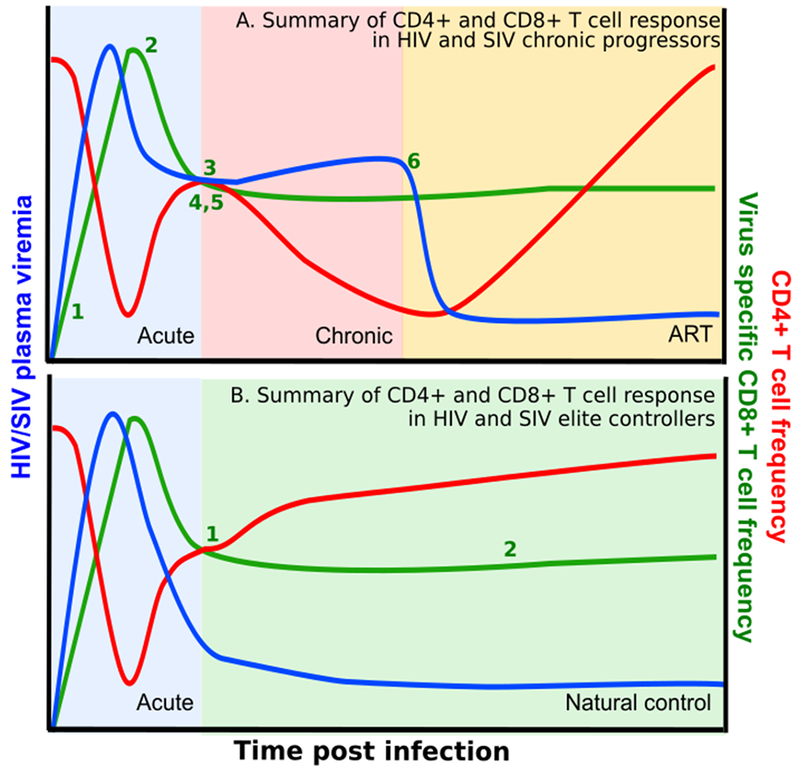
Summary HIV and SIV infection in chronic progressors and elite controllers. A. Summary of CD4+ and CD8+ T cell response in HIV and SIV chronic progressors. 1. Acute HIV infection induces activation and expansion of all CD8+ T cells, including virus-specific CD8+ T-cells. 2. After an initial lag period, 3. expanded CD8+ T cells control peak viremia and chronic infection follows. 4. HIV specific CD8+ T-cells become exhausted and contribute to disease progression during chronic infection. 5. Emergence of CD8 escape mutants indicating selective pressure by CD8+ T-cells. 6. Despite ART, CD8+ T-cell function is not fully restored. (B) Summary of CD4+ and CD8+ T cell response in elite controller. Elite controllers have similar expansion of CD8+ T cells which leads to control of peak viremia and subsequent viral control and restoration of CD4+ T cells. 1. Specific MHC class I alleles are associated with viral control, indicating CD8 selective pressure. 2. HIV specific CD8+ T cells maintain high levels of polyfunctionality, proliferative capacity and maintenance of cytolytic potential throughout infection. Abbreviations: SIV, simian immunodeficiency virus; HIV, human immunodeficiency virus; ART, antiretroviral therapy; MHC, major histocompatibility class.
The main conclusion of these experiments was independently confirmed by three studies. In the first study, al Basatena et al., sough to determine if the consistent observation of viral escape proves that HIV/SIV-specific CD8+ T cells kill infected cells or could this also be the result of a non-cytolytic control [137]. To this end, these authors developed a 3D cellular automaton model of HIV infection that captures both spatial and temporal dynamics, and reproduces in vivo viral dynamics at the cellular and population level. Using this model, al Basatena et al. demonstrated that non-cytolytic effector mechanisms can select for viral escape variants. Intriguingly, those viral variants selected by non-cytolytic mechanisms of suppression have a slower outgrowth and a lower frequency as compared to those escaping from a cytolytic response, thus suggesting that non-cytolytic responses can provide more durable control of HIV/SIV replication. In the second study, Balamurali et al. investigated the mechanisms of virus-specific CD8+ T cell control during immune escape in vivo by using a RT-PCR assay that differentiates wild type (WT) virus from escape mutants (EM) and studying the dynamics of immune escape in early SHIV infection of pigtail macaques. These authors reasoned that for immune escape mediated by cytolysis, the death rate of WT infected cells would be faster than EM-infected cells. However, Balamurali et al. found no significant difference in the rate of decay of WT virus compared with EM virus, thus consistent with an epitope-specific, MHC class I-restricted, noncytolytic mechanism of CD8+ T cell control of both WT and EM variants of SHIV [138]. In the third study, Spits et al., tried to identify correlation(s) between markers of CD8+ T cell function that are associated with CTL activity ex vivo and the calculated in vivo lifespan of productively infected cell as calculated by measuring the kinetics of virus decline under ART. The apparently “negative” result that they obtained, i.e., that the lifespan of productively infected cells is similarly short even in patients with the arguably “worst” CTL responses, is consistent with the hypothesis that non-cytolytic mechanisms are involved in the anti-HIV effect of CD8+ T cells [139].
In conclusion, a number of independent experimental investigations and mathematical analyses suggest that conventional CTL activity does not fully explain the antiviral role of CD8+ T cells in HIV/SIV infection. The possibility that the “CD8 effect” is due to alternative, non-cytolytic mechanisms of viral suppression is quite plausible. However, at this time it remains unclear what specific antiviral mechanisms are involved in this phenomenon, and what is the relative contribution of these non-cytolytic mechanisms to the control of HIV or SIV infection in vivo.
CD8+ T cells during ART-treated infection and HIV reservoir activity
ART does not restore CD8+ T cell compartment to pre-infection state
ART is unable to completely reverse the immune dysfunction bequeathed during the untreated infection, especially in the CD8+ T cell compartment. Although long-term ART results in some restoration of CD8+ T cell polyfunctionality and at least partial downregulation of activation and exhaustion markers, it does not fully restore CD8+ T cell cytotoxic and proliferative capabilities [41, 140–147]. Similarly, the bystander activation and expansion of the CD8+ T cell compartment does not return to normal despite virologic control [148, 149]. Interestingly, initiation of ART during early infection is associated with greater CD8+ T cell count reduction when compared to ART initiation during chronic infection [150, 151].
CD8+ T cells are unable to eliminate the HIV viral reservoir preserved during ART
A number of HIV cure strategies, collectively defined under the term “shock & kill” are based on the premise that, in ART-treated individuals, HIV/SIV-specific CD8+ T cells will recognize and eliminate virus-infected CD4+ T cells in which virus transcription and production has been reactivated by latency reversing agents [152]. While virus specific CD8+ T cells persist under ART, their number remains lower than prior to ART initiation, and the presence of virus immune escape variants as well as persistent dysfunction and/or exhaustion of HIV/SIV-specific CD8+ T cells may negatively affect their ability to clear the reservoir [53, 153–157]. Theoretically, during ART-treated HIV/SIV infections viral evolution ceases and, under this assumption, viral reservoirs preserve the pre-ART quasi-species with their escape mutations [158]. Overtime the ability of CD8+ T cells to recognize viral reservoirs appears to decline at a rate dependent on the time between infection and ART initiation [159]. In fact, a recent study demonstrated that more than 98% of proviruses in patients treated during chronic infection harbored escape mutations in dominant epitopes that were unrecognizable to CD8+ T cells, but subdominant CD8+ T cell responses against non-escaped epitopes were still found in each of the patients [160]. These findings raise the possibility that the epitopes targeted by CD8+ T cells under ART are suboptimal [70, 161–164]. Antigen sequestration has also been postulated to limit the ability of CD8+ T cells to clear the virus reservoir under ART. Most effector CD8+ T cells lack the proper chemokine receptors to enter the B cell follicle of the lymph node [165–169]. In the context of HIV, CD4+ follicular helper (TFH) T cells have been shown to be 30-fold more likely to harbor latently-infected virus than peripheral CD4+ T cells [170], perhaps as a consequence of the inability of CD8+ T cell localization to the germinal center. Another point of discussion is whether and to what extent HIV latency per se poses as a barrier to CD8+ T cell-mediated eradication [159]. CD8+ T cells can detect even a single MHC-peptide complex on a cell surface [171] implying that even small levels of HIV translation can expose latently infected cells to CD8+ T cell killing. However, it is unclear how efficiently RNA transcripts that are often found in low levels in HIV/SIV-infected cells that persist under ART are translated – or, alternatively, their transcription is limited by retention in the nucleus, transcriptional interference, or “read-through” transcription [172] [173].
In conclusion, while CD8+ T cell recognition of the HIV viral reservoir is possible, especially in the setting of interventions that reactivate virus transcription and translation (i.e., latency reversing agents), the effectiveness of these CD8+ T cells may be limited by functional defects and/or residual exhaustion, presence of viral immune escape variants, and limited anatomical access to the latently-infected cell populations.
CD8+ T cells are required for maintenance of HIV viral reservoir suppression under ART
Recent evidence suggests that CD8+ T cells remain an essential component of virus control in ART-treated SIV-infected RMs [174]. In this study, depletion of CD8+ T cells from SIV+ ART-suppressed RM resulted in a rebound of viremia in 13 out 13 depleted animals and the reemergence of viral control was consistently coupled to the reconstitution of CD8+ T cells (Figure 1C). While in this study the depleting antibody used also depleted NK cells, there was no association between reconstitution of the NK cell pool and re-establishment of virus control. As part of this study, longitudinal viral sequencing by single-genome amplification (SGA) of SIVmac239 Env was performed on plasma samples collected during peak viremia (day 10 post-infection), immediately prior to ART initiation (day 56), and at the time of virus rebound after CD8+ lymphocyte depletion. Interestingly, the viral sequences derived from plasma following CD8+ lymphocyte depletion were similar to those obtained at the time of peak viremia and did not include in any case, the mutations that have emerged in the plasma by the time the SIV-infected RMs were started on ART. This observation supports the hypothesis that the source of the rebounding viremia after CD8+ lymphocyte depletion is the reactivation of virus transcription from a pool of long-lived, latently infected cells that were infected prior to ART initiation. In addition, the study found a significant direct correlation between the level of cell-associated SIV DNA in CD4+ T cells before CD8+ lymphocyte depletion and both the peak and the area under the curve of plasma viremia after depletion. This suggests that the size of the viral reservoir maintained under ART before CD8+ T cell depletion is a determinant of the ensuing amount of virus production.
In this study, as well as other experiments involving in vivo depletion of CD8+ lymphocytes, a modest increase in CD4+ T cell proliferation was observed likely as a result of homeostatic proliferation of T cells [50, 63, 174]. These observations raised the possibility that the observed increases in viral load were a passive consequence of this increased level of CD4+ T cell activation and proliferation, as opposed to the removal of a direct antiviral effect of CD8+ lymphocytes. To address this possibility, our group depleted CD4+ T cells from eight ART-treated SIV-infected RM and found that while the CD4+ T cells that survived depletion underwent strong homeostatic proliferation (as measured by increased expression of the proliferation marker Ki67) and increased cellular activation (as measured by increased expression of the markers CD25 and HLA-DR), plasma viremia remained below the limit of detection in all animals and at all time points (Kumar et al., manuscript in preparation) (Figure 1D). Thus, the results of these studies of CD4+ T cell depletion fully support the hypothesis that CD8+ T cells play a previously unappreciated but important direct role in the control of virus production and/or replication in ART-treated SIV-infected RM. Further studies with longer follow-up will determine if this effect of CD8+ T lymphocytes is present only in the first several months of ART or persists for longer periods of time under treatment.
It is important to note that in the natural history of HIV and SIV infections both cytolytic (i.e., CTL) and non-cytolytic (i.e., block of virus entry via beta-chemokines and suppression of virus transcription) activities result in reduction of virus production and replication, thus acting synergistically in promoting better virus control, with the most obvious example represented by the EC phenotype. However, in the setting of ART treatment and in terms of impact on virus persistence and the size of the reservoir, CD8+ T cell mediated CTL activity and CD8+ T cell-mediated suppression of virus transcription may have divergent effects. In particular, while clearance of infected cells via CTL activity will result in a net decrease of the reservoir size, the active suppression of HIV or SIV transcription may paradoxically increase the reservoir size by actively promoting latency. This latter point is of practical importance if we think of ways to manipulate these antiviral roles of CD8+ T cells in ART-treated HIV-infected individuals. In this regard, CTL activity could be enhanced by interventions such as therapeutic vaccinations and/or co-inhibitory blockade. On the other hand, CD8+ lymphocyte depletion could be viewed as a potentially very powerful way to reactivate latent HIV or SIV infection (i.e., latency reversing agent). Further studies aimed at better elucidating the relative in vivo contribution of cytolytic vs. non-cytolytic mechanisms of virus suppression under ART, as well as the molecular pathways that regulate the prevalence of either function of CD8+ T cells, will be crucial to design immune-based interventions that are best suited to reduce the reservoir size in ART-treated HIV-infected individuals.
Conclusion
It is well established from the numerous studies discussed in this review that CD8+ T cells are key players in the antiviral response to HIV and SIV during each stage of infection, including when the infection is treated with ART. Recent studies have shown that (i) CD8+ T cells are required for maintenance of viral suppression under ART, and (ii) that the longitudinal analysis of viral sequences is compatible with a CD8+ T cell-mediated suppression of virus production at the transcriptional level. These findings suggest that CD8+ T cells may paradoxically contribute to persistence of the HIV reservoir and thus pose as a barrier to HIV cure. It is conceivable that while CTL activity occurs early during infection and results in a net reduction of the reservoir size, CD8+ T cells are also capable of maintaining latency via non-cytolytic mechanisms that suppress HIV replication. However, the relative contributions of cytolytic and non-cytolytic activities of CD8+ T cells in suppressing virus production remain unknown. A deeper understanding of these activities would contribute to the design of therapeutic vaccines capable of harnessing and boosting specific antiviral activities or downregulating others in the hopes of targeting and eliminating the HIV viral reservoir. Heightening the ability of CD8+ T cells to recognize and kill virally infected cells, especially during ART treatment, is a promising strategy to eliminate virally infected cells, including the viral reservoir. If the non-cytolytic activities of CD8+ T cells contribute to the establishment and persistence of the viral reservoir via inhibition of viral transcription or translation, strategies aimed at decreasing these capacities could also contribute to the elimination of virally infected cells.
Figure 2:
Changes in viral load and T cell frequencies during CD8 and CD4 depletion studies in SIVmac239 infected, ART-treated rhesus macaques. The initiation of ART in the presence (A) or absence (B) of CD8+ T cells during SIV infection results in similar decay rates of plasma viremia. (C) Viral load increases upon CD8 depletion during short-term ART. (D) Depletion of CD4+ T cells after ART does not result in viral rebound. Key: green arrow represents CD8+ lymphocyte depletion and orange arrow represents CD4+ T cell depletion. Abbreviations: SIV, simian immunodeficiency virus; CD, cluster of differentiation; ART, antiretroviral therapy; P.I., post-infection.
Figure 3:
Schematic representations of the association between CD8+ T cell frequency and SIV viral load. A. The initiation of ART in the absence or presence of CD8+ T cells during SIV infection results in similar decay rates of plasma viremia. B. Viral load increases when CD8+ T cells are absent during short-term ART. C. Viral load does not increase when CD4+ T cells are absent during short-term ART. Abbreviations: SIV: Simian immunodeficiency virus; CD: cluster of differentiation; ART: Antiretroviral therapy; P.I.: post-infection.
Table 1:
Summary of noteworthy studies providing evidence of cytolytic and non-cytolytic antiviral activity of CD8+ T cells during HIV/SIV infection during different phases of infection, including treatment and natural control. Abbreviations: HIV: Human immunodeficiency virus; SIV: Simian immunodeficiency virus; PBMC: peripheral blood mononuclear cell; ART: Antiretroviral therapy; RM: Rhesus macaque; EC: Elite controller; CP: Chronic progressor; CTL: Cytotoxic T lymphocyte; CD: cluster of differentiation; IL: interleukin; IFN: interferon; TNF: tumor necrosis factor; CCL: chemokine (C-C motif) ligand; CCR: chemokine receptor; PD-1: programmed death-1; HLA: human leukocyte antigen; MIP: macrophage inflammatory protein ; CAF: CD8 antiviral factor.
| Phase | Finding | Evidence | Reference |
|---|---|---|---|
| Acute infection | CD8 T cells are required for the initial control of HIV viremia. | Depletion of CD8+ lymphocytes from RM at the time of SIV infection resulted in abrogation of post peak decline. | [26, 175] |
| After initial lag period, HIV-specific CD8+ T cells massively expand and differentiate at the time of peak viremia. | HIV-specific CD8+ T cells exhibit a delay in expansion and differentiation until peak viremia when compartment becomes fully expanded and differentiation in response to systemic proinflammatory cytokine burst, allowing for effective killing of productively-infected cells. | [176] | |
| The emergence of HIV-specific CD8+ T cells is associated with partial control of acute infection. | Increasing frequency of precursor CD8+ T cells specific for HIV-1 gag, pol, and env viral proteins using PBMC from patients experiencing acute HIV infection was correlated with partial resolution of peak viremia. | [24, 25] | |
| CD8+ T cells are capable of exerting significant selective pressure on the HIV viral genome. | Identification of the rapid appearance of specific escape mutations in HIV genome. | [29, 153] | |
| Acute HIV infection induces massive activation and expansion of the entire CD8+ T cell compartment | CD8+ T cell frequencies increase during the course of infection in HIV+ individuals and do not return to normal. | [177] | |
| Activation marker CD38 is up-regulated on Epstein Barr-, Cytomegalovirus- and influenza-specific CD8+ T cells during acute HIV infection, although activation was highest in HIV-specific cells. | [178] | ||
| HIV-specific CD8+ T cells represent less than 10% of the total CD8+ T cell pool expanded during the acute infection. | [32] | ||
| During the acute infection as high as 80%-90% of the entire CD8+ T cell compartment becomes activated. | [55] | ||
| CD8+ T cell expansion can occur through antigen-independent mechanisms. | Microbial products systemically translocated across the gut epithelium contribute to the chronic activation of CD8+ T cells. | [22] | |
| Lipopolysaccharide and inactivated HIV activate monocyte-derived dendritic cells, which are capable of activating CD8+ T cells via transpresentation of IL-15. Therefore, proliferation and activation of the CD8+ T cell pool is initiated by cytokines, most notably IL-15. | [179] | ||
| HIV-specific CD8+ T cells become exhausted during the acute infection and do not recover. | HIV–specific CD8+ T cells proliferate rapidly upon encounter with cognate antigen in acute infection, but lose this capacity with ongoing viral replication. | [73] | |
| HIV-specific CD8+ T cells provide a very early, robust, and highly activated effector response with immediate cytotoxic potential (as measured by perforin expression), but the ability is quickly lost after resolution of peak viremia. | [49] | ||
| After full differentiation and expansion, HIV-specific CD8+ T cells reach a hyperproliferation state that is “too strong for too long” and push them to terminally differentiated effector cells that contributes to exhaustion. | [176] | ||
| Chronic infection | CD8+ T cells contribute to control during chronic HIV infection. | CD8+ T cell depletion during chronic infection results in an increase in viremia that is not controlled until reconstitution of depleted cells. | [50-52] |
| Expanded CD8+ T cell population in chronically HIV-infected patients shows symptoms of immunosenescence. | HIV-specific CD8+ T cells lack of proliferative capability in response to cognate antigen (ex vivo), which could not be overcome by exogenous IL-2 or IL-15. These cells were associated with expression of CD57. | [46] | |
| Ex vivo analysis of virus-specific CD8 T cells shows that HIV disease progression correlates with increased proportions of highly differentiated CD8+ T cells, which exhibit characteristics of replicative senescence: CD57 expression, inability to proliferate in response to antigen, and shortened telomeres. | [55] | ||
| The HIV-specific CD8+ T cell compartment has a skewed differentiation pattern towards effector memory during chronic infection. | 70% of HIV-specific CD8+ T cells were found to be CD45RA-CCR7-, in contrast to cytomegalovirus-specific CD8+ T cells where only 40% are CD45RA-CCR7-. | [180] | |
| Expression of exhaustion markers on HIV-specific CD8+ T cells continues during chronic infection and contributes to disease progression. | Persistent antigen during chronic HIV infection contributes to the impairment of HIV-specific CD8+ T cells. HIV-specific CD8+ T cells show significant upregulation of PD-1. Expression correlates positively with impaired function, viral load and inversely with CD4+ T cell count. | [41, 42, 44] | |
| TIM-3 expression on CD8+ T cells correlates positively with viral load and inversely with CD4 counts during chronic HIV infection. | [140] | ||
| PD-1 expression on HIV-specific CD8+ T cells is correlated with decreased survival, proliferation, and cytokine expression. | Ex vivo anti-PD-L1 treatment of CD8+ T cells from HIV+ donors led to changes in the ability of the cells to survive, expand, and secrete cytokines. | [42] | |
| HIV-specific CD8+ T cells exhibit reduced polyfunctionality during chronic infection. | HIV-specific CD8+ T cells from HIV+ donors exhibit decrease CD107, IFNγ, CCL4, IL-2, and TNFα expression after stimulation. | [40] | |
| HIV-specific CD8+ T cells exhibit impaired cytolytic function during chronic infection | Perforin expression was significantly lower in HIV-specific CD8+ T cells compared to CMV-specific CD8+ T cells of the same donor. | [57] | |
| CD8+ T cells secrete factors that are capable of suppressing replication of HIV through non-cytolytic mechanisms. | CD8+ T cells were found to release β-chemokines (CCL3, CCL4, and CCL5) with suppressive activities capable of blocking entry of M-tropic viruses. | [102, 112, 113] | |
| Replication of HIV in latently infected, resting CD4+ T cell reservoir is effectively suppressed in ex vivo coculture by autologous CD8+ T cells in EC and ART-treated patients but not ART-naïve patients. | [114] | ||
| Identification of the characterization of CD8+ T cells with a MIP-1β expression profile as a correlate of virus control and inhibition. | [115, 116] | ||
| CAF suppresses LTR-mediated HIV gene expression in CD4+ T cells. | [124] | ||
| CD8+ T cells suppress replication by inhibiting viral transcription and proviral gene expression]. | [130-132] | ||
| SIV-infected RM were initiated on ART in the absence or presence of CD8+ T cells. The rates of viral decay did not differ between the two groups, suggesting that CD8+ T cells do not decrease the lifespan of productively infected cells. Thus, the antiviral mechanism of CD8+ T cells may be non-cytolytic. | [133, 134] | ||
| Natural control | CD8+ T cells are important during the control of SIV viral replication during RM controller infection. | Depletion of CD8+ lymphocytes in SIV controller RM resulted in a transient and significant increase in viremia and control was reestablished with the reconstitution of CD8+ T cells. | [66] |
| HIV-specific CD8+ T cells from EC maintain high polyfunctionality. | HIV-specific CD8+ showed increase function via expression of 5 functional markers: CD107 (degranulation), IFNγ, CCL4, IL-2, and TNFα. | [40] | |
| HIV-specific CD8+ T cells from EC show better maintenance of cytolytic potential compared to CP. | HIV-specific CD8+ T cells of EC exhibit greater cytolytic capacity compared to CP. The strong ability of EC to kill HIV-infected CD4+ T cells was mediated by the delivery of granzyme B to target cells, an observation not congruent in CP. | [76] | |
| During chronic infection, cytolytic potential is lost rapidly in most HIV-infected individuals, such that only around 15% of HIV-specific CD8+ T cells express perforin, whereas around 40% express perforin in EC. | [75] | ||
| HIV-specific CD8+ T cells from EC have a higher proliferative capacity as compared to CP. | High proliferative capacity of HIV-specific CD8+ T cells EC is coupled to increases in perforin expression with relative absence of these functions in CP. | [72] | |
| Host factors related to CD8+ T cells contribute to the control of HIV infection observed in EC. | CD8+ T cells restricted by certain protective alleles (HLA-B27 and -B57) can resist replicative defects, which permits expansion and antiviral effector activities. | [74] | |
| HIV-specific CD8+ T cells of EC have a higher functional recall memory than CP. | The expansion of CD8+ T cells producing IFNγ alone or in combination with IL-2 in response to gag peptides presented on monocyte-derived dendritic cells is limited in CP compared to EC. This was not observed by CD8+ cells in response to influenza, cytomegalovirus, and Epstein Barr virus. | [181] | |
| HIV-specific CD8+ T cells put selective pressure on the virus during EC infection. | Sequencing of plasma viremia of EC shows a discordance between the genotypes of the plasma virus and provirus. Specifically, HLA-B*57-restricted Gag epitopes were present in plasma virus but rare in provirus. | [67] | |
| Treated infection | CD8+ T cells are required for the maintenance of viral suppression under ART. | Depletion of CD8+ lymphocytes from SIV+ RM during short-term ART results in a rebound of viremia. | [174] |
| HIV-specific CD8+ T cells decline in peripheral blood after the initiation of ART | The longitudinal responses to 95 HLA class I-restricted HIV epitopes were measured using intracellular staining in HIV+ patients beginning ART. A rapid decline in HIV-specific CD8+ T cell response was observed upon initiation of ART. Discontinuation of ART resulted in a rapid increase in HIV-specific CD8+ T cells. | [149] | |
| Dysfunction of HIV-specific CD8+ T cells is decreased, but not restored during ART. | In a longitudinal study of HIV-infected patients, ART initiation resulted in some restoration of cytokine secretion, increase of IL-7Rα and CD28 expression, and a decline of PD-1 on HIV-specific CD8+ T cells. | [147] | |
| Defective HIV-specific CD8+ T cell polyfunctionality, proliferation, and cytotoxicity are not restored by ART | [182] | ||
| ART does not resolve CD8+ T cell compartment elevation. | ART does not restore ongoing elevation of CD8 counts despite normalized CD4 count, resulting in a persistently low CD4:CD8 ratio even during virological control. This phenotype is correlated with markers of T cell activation and innate immune active, immunosenescence, and serious non-AIDS events and mortality. | [183] | |
| Early initiation of ART during HIV infection, not prolonged duration of ART, contributes to partial normalization of CD8+ T cell counts. | [150] | ||
| ART is able to partially reverse the exhaustion of virus-specific CD8+ T cells observed during chronic HIV infection. | ART-initiation reverses expression of PD-1 on HIV-specific CD8+ T cells, reversing the functional impairment of these cells that had been caused by the constant presence of HIV antigen. | [145] | |
| HIV-specific CD8+ T cells from ART-treated patients expressed significantly lower levels of TIM-3 compared with untreated patients and TIM-3 expression was positively correlated with viral load. | [144] |
Acknowledgements:
Funding: R01-AI90797, R01-AI125064
Abbreviations:
- HIV:
Human immunodeficiency virus
- SIV:
Simian immunodeficiency virus
- AIDS:
Acquired immunodeficiency syndrome
- ART:
Antiretroviral therapy
- RM:
Rhesus macaque
- EC:
Elite controller
- CP:
Chronic progressor
- CTL:
Cytotoxic T lymphocyte
Footnotes
Conflict of interest: The authors declare no commercial or financial conflict of interest.
References:
- 1.Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W and Montagnier L, Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983. 220: 868. [DOI] [PubMed] [Google Scholar]
- 2.WHO, HIV/AIDS (fact sheet 360) 2016.
- 3.UNAIDS, UNAIDS Data 2017. Joint United Nations; Program on HIV/AIDS 2017.
- 4.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA and Fauci AS, Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 1997. 94: 13193–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD and Siliciano RF, Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997. 278: 1295–1300. [DOI] [PubMed] [Google Scholar]
- 6.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA and Richman DD, Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997. 278: 1291–1295. [DOI] [PubMed] [Google Scholar]
- 7.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ and Siliciano RF, Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003. 9: 727–728. [DOI] [PubMed] [Google Scholar]
- 8.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK and Sékaly RP, HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature Medicine 2009. 15: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaafoura S, de Goër de Herve MG, Hernandez-Vargas EA, Hendel-Chavez H, Abdoh M, Mateo MC, Krzysiek R, Merad M, Seng R, Tardieu M, Delfraissy JF, Goujard C and Taoufik Y, Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4(+) memory T Cells. Nature Communications 2014. 5: 5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J and Siliciano RF, Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999. 5: 512–517. [DOI] [PubMed] [Google Scholar]
- 11.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DI, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL and Barouch DH, Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014. 512: 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ananworanich J, Vandergeeten C and Chomchey N, Early ART intervention restricts the seeding of the HIV reservoir in long-lived central memory CD4 T cells. 20th Conference on Retroviruses and Opportunistic Infections 2013. [Google Scholar]
- 13.Davey RT, Bhat N, Yoder C, Chun T-W, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS and Lane HC, HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proceedings of the National Academy of Sciences of the United States of America 1999. 96: 15109–15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun TW, Davey RT Jr., Engel D, Lane HC and Fauci AS, Re-emergence of HIV after stopping therapy. Nature 1999. 401: 874–875. [DOI] [PubMed] [Google Scholar]
- 15.Micci L, McGary CS and Paiardini M, Animal models in HIV cure research. Journal of Virus Eradication 2015. 1: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar N, Chahroudi A and Silvestri G, Animal models to achieve an HIV cure. Curr Opin HIV AIDS 2016. 11: 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans DT and Silvestri G, Nonhuman primate models in AIDS research. Curr Opin HIV AIDS 2013. 8: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Organization), W. W. H., HIV/AIDS (fact sheet 360) 2016.
- 19.Lackner AA, Lederman MM and Rodriguez B, HIV Pathogenesis: The Host. Cold Spring Harbor perspectives in medicine 2012. 2: a007005–a007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellors JW, Rinaldo CR Jr., Gupta P, White RM, Todd JA and Kingsley LA, Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996. 272: 1167–1170. [DOI] [PubMed] [Google Scholar]
- 21.Fauci AS, Schnittman SM, Poli G, Koenig S and Pantaleo G, NIH conference. Immunopathogenic mechanisms in human immunodeficiency virus (HIV) infection. Ann Intern Med 1991. 114: 678–693. [DOI] [PubMed] [Google Scholar]
- 22.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG and Douek DC, Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006. 12: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 23.Deeks SG, Overbaugh J, Phillips A and Buchbinder S, HIV infection. Nature Reviews Disease Primers 2015. 1: 15035. [DOI] [PubMed] [Google Scholar]
- 24.Borrow P, Lewicki H, Hahn BH, Shaw GM and Oldstone MB, Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. Journal of Virology 1994. 68: 6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C and Ho DD, Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. Journal of Virology 1994. 68: 4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matano T, Shibata R, Siemon C, Connors M, Lane HC and Martin MA, Administration of an Anti-CD8 Monoclonal Antibody Interferes with the Clearance of Chimeric Simian/Human Immunodeficiency Virus during Primary Infections of Rhesus Macaques. Journal of Virology 1998. 72: 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz JE, Simon MA, Kuroda MJ, Lifton MA, Ollert MW, Vogel C-W, Racz P, Tenner-Racz K, Scallon BJ, Dalesandro M, Ghrayeb J, Rieber EP, Sasseville VG and Reimann KA, A Nonhuman Primate Model for the Selective Elimination of CD8+ Lymphocytes Using a Mouse-Human Chimeric Monoclonal Antibody. The American Journal of Pathology 1999. 154: 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MBA and Shaw GM, Antiviral pressure exerted by HIV-l-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med 1997. 3: 205–211. [DOI] [PubMed] [Google Scholar]
- 29.Chen ZW, Craiu A, Shen L, Kuroda MJ, Iroku UC, Watkins DI, Voss G and Letvin NL, Simian Immunodeficiency Virus Evades a Dominant Epitope-Specific Cytotoxic T Lymphocyte Response Through a Mutation Resulting in the Accelerated Dissociation of Viral Peptide and MHC Class I. The Journal of Immunology 2000. 164: 6474–6479. [DOI] [PubMed] [Google Scholar]
- 30.McMichael AJ and Phillips RE, Escape of human immunodeficiency virus from immune control Annual Review of Immunology 1997, pp 271–296. [DOI] [PubMed] [Google Scholar]
- 31.Vanderford TH, Bleckwehl C, Engram JC, Dunham RM, Klatt NR, Feinberg MB, Garber DA, Betts MR and Silvestri G, Viral CTL escape mutants are generated in lymph nodes and subsequently become fixed in plasma and rectal mucosa during acute SIV infection of macaques. PLoS Pathog 2011. 7: e1002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA and Picker LJ, Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol 2001. 75: 11983–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, Addo MM, Poon SH, Phillips MN, Robbins GK, Sax PE, Boswell S, Kahn JO, Brander C, Goulder PJR, Levy JA, Mullins JI and Walker BD, Cellular Immune Responses and Viral Diversity in Individuals Treated during Acute and Early HIV-1 Infection. The Journal of Experimental Medicine 2001. 193: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman MA, Kingsley L, Atchison R, Belle S, Breinig M, Ho M and Rinaldo C, Reactivation of Epstein-Barr virus during early infection with human immunodeficiency virus. Journal of clinical microbiology 1991. 29: 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mudd JC and Lederman MM, CD8 T cell persistence in treated HIV infection. Current opinion in HIV and AIDS 2014. 9: 500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bastidas S, Graw F, Smith MZ, Kuster H, Gunthard HF and Oxenius A, CD8+ T cells are activated in an antigen-independent manner in HIV-infected individuals. J Immunol 2014. 192: 1732–1744. [DOI] [PubMed] [Google Scholar]
- 37.Kuchroo VK, Anderson AC and Petrovas C, Coinhibitory receptors and CD8 T cell exhaustion in chronic infections. Current Opinion in HIV and AIDS 2014. 9: 439–445. [DOI] [PubMed] [Google Scholar]
- 38.Radebe M, Gounder K, Mokgoro M, Ndhlovu ZM, Mncube Z, Mkhize L, Van Der Stok M, Jaggernath M, Walker BD and Ndung’u T, Broad and persistent Gag-specific CD8 T-cell responses are associated with viral control but rarely drive viral escape during primary HIV-1 infection. AIDS 2015. 29: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao W, Mehraj V, Kaufmann DE, Li T and Routy JP, Elevation and persistence of CD8 T-cells in HIV infection: the Achilles heel in the ART era. J Int AIDS Soc 2016. 19: 20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M and Koup RA, HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006. 107: 4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJR, Klenerman P, Ahmed R, Freeman GJ and Walker BD, PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006. 443: 350–354. [DOI] [PubMed] [Google Scholar]
- 42.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC and Koup RA, PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. Journal of Experimental Medicine 2006. 203: 2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrovas C, Yamamoto T, Price DA, Rao SS, Klat RN, Brenchley JM, Douek DC, Gostick E, Angermann BR, Grossman Z, Macallan DC, Meier-Schellersheim M and Koupa RA, High production rates sustain in vivo levels of PD-1high simian immunodeficiency virus-specific CD8 t cells in the face of rapid clearance. Journal of Virology 2013. 87: 9836–9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK and Sekaly RP, Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006. 12: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 45.Peretz Y, He Z, Shi Y, Yassine-Diab B, Goulet JP, Bordi R, Filali-Mouhim A, Loubert JB, El-Far M, Dupuy FP, Boulassel MR, Tremblay C, Routy JP, Bernard N, Balderas R, Haddad EK and Sékaly RP, CD160 and PD-1 Co-Expression on HIV-Specific CD8 T Cells Defines a Subset with Advanced Dysfunction. PLoS Pathogens 2012. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC and Koup RA, Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 2003. 101: 2711–2720. [DOI] [PubMed] [Google Scholar]
- 47.Desai S and Landay A, Early immune senescence in HIV disease. Current HIV/AIDS Reports 2010. 7: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts ER, Carnathan DG, Li H, Shaw GM, Silvestri G and Betts MR, Collapse of Cytolytic Potential in SIV-Specific CD8+ T Cells Following Acute SIV Infection in Rhesus Macaques. PLoS Pathogens 2016. 12: e1006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demers KR, Makedonas G, Buggert M, Eller MA, Ratcliffe SJ, Goonetilleke N, Li CK, Eller LA, Rono K, Maganga L, Nitayaphan S, Kibuuka H, Routy J-P, Slifka MK, Haynes BF, McMichael AJ, Bernard NF, Robb ML and Betts MR, Temporal Dynamics of CD8(+) T Cell Effector Responses during Primary HIV Infection. PLoS Pathogens 2016. 12: e1005805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chowdhury A, Hayes TL, Bosinger SE, Lawson BO, Vanderford T, Schmitz JE, Paiardini M, Betts M, Chahroudi A, Estes JD and Silvestri G, Differential Impact of In Vivo CD8+ T Lymphocyte Depletion in Controller versus Progressor Simian Immunodeficiency Virus-Infected Macaques. J Virol 2015. 89: 8677–8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS and Ho DD, Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. Journal of Experimental Medicine 1999. 189: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metzner KJ, Jin X, Lee FV, Gettie A, Bauer DE, Di Mascio M, Perelson AS, Marx PA, Ho DD, Kostrikis LG and Connor RI, Effects of in Vivo CD8 T Cell Depletion on Virus Replication in Rhesus Macaques Immunized with a Live, Attenuated Simian Immunodeficiency Virus Vaccine. The Journal of Experimental Medicine 2000. 191: 1921–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goulder PJR, Phillips RE, Colbert RA, McAdam S, Ogg G, Nowak MA, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael AJ and Rowland-Jones S, Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nature Medicine 1997. 3: 212–217. [DOI] [PubMed] [Google Scholar]
- 54.Appay V, Papagno L, Spina CA, Hansasuta P, King A, Jones L, Ogg GS, Little S, McMichael AJ, Richman DD and Rowland-Jones SL, Dynamics of T Cell Responses in HIV Infection. The Journal of Immunology 2002. 168: 3660. [DOI] [PubMed] [Google Scholar]
- 55.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, Dunbar PR, Shepherd D, Cerundolo V, Emery V, Griffiths P, Conlon C, McMichael AJ, Richman DD, Rowland-Jones SL and Appay V, Immune Activation and CD8(+) T-Cell Differentiation towards Senescence in HIV-1 Infection. PLoS Biology 2004. 2: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pantaleo G, Demarest JF, Soudeyns H, Graziosi C, Denis F, Adelsberger JW, Borrow P, Saag MS, Shaw GM, Sekalytt RP and Fauci AS, Major expansion of CD8+ T cells with a predominant Vβ usage during the primary immune response to HIV. Nature 1994. 370: 463–467. [DOI] [PubMed] [Google Scholar]
- 57.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GMA, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ and Rowland-Jones SL, Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Medicine 2002. 8: 379–385. [DOI] [PubMed] [Google Scholar]
- 58.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ and Pantaleo G, Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 2001. 410: 106–111. [DOI] [PubMed] [Google Scholar]
- 59.Mueller YM, De Rosa SC, Hutton JA, Witek J, Roederer M, Altman JD and Katsikis PD, Increased CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. Immunity 2001. 15: 871–882. [DOI] [PubMed] [Google Scholar]
- 60.Sodora DL and Silvestri G, Immune activation and AIDS pathogenesis. Aids 2008. 22: 439–446. [DOI] [PubMed] [Google Scholar]
- 61.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM and Detels R, Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. Journal of Infectious Diseases 1999. 179: 859–870. [DOI] [PubMed] [Google Scholar]
- 62.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C and Group, A. V. S., Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013. 9: e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barry AP, Silvestri G, Safrit JT, Sumpter B, Kozyr N, McClure HM, Staprans SI and Feinberg MB, Depletion of CD8(+) cells in sooty mangabey monkeys naturally infected with simian immunodeficiency virus reveals limited role for immune control of virus replication in a natural host species. Journal of Immunology 2007. 178: 8002–8012. [DOI] [PubMed] [Google Scholar]
- 64.Dunham R, Pagliardini P, Gordon S, Sumpter B, Engram J, Moanna A, Paiardini M, Mandl JN, Lawson B, Garg S, McClure HM, Xu YX, Ibegbu C, Easley K, Katz N, Pandrea I, Apetrei C, Sodora DL, Staprans SI, Feinberg MB and Silvestri G, The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood 2006. 108: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Connell KA, Bailey JR and Blankson JN, Elucidating the elite: mechanisms of control in HIV-1 infection. Trends Pharmacol Sci 2009. 30: 631–637. [DOI] [PubMed] [Google Scholar]
- 66.Friedrich TC, Valentine LE, Yant LJ, Rakasz EG, Piaskowski SM, Furlott JR, Weisgrau KL, Burwitz B, May GE, León EJ, Soma T, Napoe G, Capuano Iii SV, Wilson NA and Watkins DI, Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. Journal of Virology 2007. 81: 3465–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bailey JR, Williams TM, Siliciano RF and Blankson JN, Maintenance of viral suppression in HIV-1-infected HLA-B*57 + elite suppressors despite CTL escape mutations. Journal of Experimental Medicine 2006. 203: 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Altfeld M, Addo MM, Rosenberg ES, Hecht FM, Lee PK, Vogel M, Yu XG, Draenert R, Johnston MN, Strick D, Allen TM, Feeney ME, Kahn JO, Sekaly RP, Levy JA, Rockstroh JK, Goulder PJ and Walker BD, Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 2003. 17: 2581–2591. [DOI] [PubMed] [Google Scholar]
- 69.Evans DT, Jing P, Allen TM, O’Connor DH, Horton H, Venham JE, Piekarczyk M, Dzuris J, Dykhuzen M, Mitchen J, Rudersdorf RA, Pauza CD, Sette A, Bontrop RE, DeMars R and Watkins DI, Definition of Five New Simian Immunodeficiency Virus Cytotoxic T-Lymphocyte Epitopes and Their Restricting Major Histocompatibility Complex Class I Molecules: Evidence for an Influence on Disease Progression. Journal of Virology 2000. 74: 7400–7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaslow RA, Carrington M, Apple R, Park L, Muñoz A, Saah AJ, Goedert JJ, Winkler C, O’Brien SJ, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H and Mann DL, Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nature Medicine 1996. 2: 405–411. [DOI] [PubMed] [Google Scholar]
- 71.Klein MR, van der Burg SH, Hovenkamp E, Holwerda AM, Drijfhout JW, Melief CJ and Miedema F, Characterization of HLA-B57-restricted human immunodeficiency virus type 1 Gag- and RT-specific cytotoxic T lymphocyte responses. Journal of General Virology 1998. 79: 2191–2201. [DOI] [PubMed] [Google Scholar]
- 72.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Baarle DV, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S and Connors M, HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol 2002. 3: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 73.Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, Cohen D, Robbins GK, Pae E, Alter G, Wurcel A, Stone D, Rosenberg ES, Walker BD and Altfeld M, Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4 + T cells. Journal of Experimental Medicine 2004. 200: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horton H, Frank I, Baydo R, Jalbert E, Penn J, Wilson S, McNevin JP, McSweyn MD, Lee D, Huang Y, De Rosa SC and McElrath MJ, Preservation of T Cell Proliferation Restricted by Protective HLA Alleles Is Critical for Immune Control of HIV-1 Infection. The Journal of Immunology 2006. 177: 7406. [DOI] [PubMed] [Google Scholar]
- 75.Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, Gudonis D, Goepfert PA, Lederman MM, Frank I, Makedonas G, Kaul R, Walker BD and Betts MR, Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog 2010. 6: e1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, Lloyd M, Roby G, Kwan R, McLaughlin M, Stallings S, Rehm C, O’Shea MA, Mican J, Packard BZ, Komoriya A, Palmer S, Wiegand AP, Maldarelli F, Coffin JM, Mellors JW, Hallahan CW, Follman DA and Connors M, Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 2008. 29: 1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang JW, Darko S, Brockman MA, Miura T, Brumme ZL, Schneidewind A, Piechocka-Trocha A, Cesa KT, Sela J, Cung TD, Toth I, Pereyra F, Yu XG, Douek DC, Kaufmann DE, Allen TM and Walker BD, TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nat Immunol 2012. 13: 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hersperger AR, Martin JN, Shin LY, Sheth PM, Kovacs CM, Cosma GL, Makedonas G, Pereyra F, Walker BD, Kaul R, Deeks SG and Betts MR, Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 2011. 117: 3799–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walker BD and Yu XG, Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol 2013. 13: 487–498. [DOI] [PubMed] [Google Scholar]
- 80.Hersperger AR, Migueles SA, Betts MR and Connors M, Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr Opin HIV AIDS 2011. 6: 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J and Connors M, HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proceedings of the National Academy of Sciences of the United States of America 2000. 97: 2709–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Migueles SA, Tilton JC and Connors M, Advances in understanding immunologic control of HIV infection. Curr HIV/AIDS Rep 2004. 1: 12–17. [DOI] [PubMed] [Google Scholar]
- 83.Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschopp J, Slot JW and Geuze HJ, Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. Journal of Experimental Medicine 1991. 173: 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shankar P, Xu Z and Lieberman J, Viral-specific cytotoxic T lymphocytes lyse human immunodeficiency virus-infected primary T lymphocytes by the granule exocytosis pathway. Blood 1999. 94: 3084–3093. [PubMed] [Google Scholar]
- 85.Trambas CM and Griffiths GM, Delivering the kiss of death. Nature Immunology 2003. 4: 399–403. [DOI] [PubMed] [Google Scholar]
- 86.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH and Ley TJ, Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 1994. 76: 977–987. [DOI] [PubMed] [Google Scholar]
- 87.Bots M and Medema JP, Granzymes at a glance. Journal of Cell Science 2006. 119: 5011–5014. [DOI] [PubMed] [Google Scholar]
- 88.Bolitho P, Voskoboinik I, Trapani JA and Smyth MJ, Apoptosis induced by the lymphocyte effector molecule perforin. Current Opinion in Immunology 2007. 19: 339–347. [DOI] [PubMed] [Google Scholar]
- 89.Voskoboinik I, Smyth MJ and Trapani JA, Perforin-mediated target-cell death and immune homeostasis. Nature Reviews Immunology 2006. 6: 940–952. [DOI] [PubMed] [Google Scholar]
- 90.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L and Kaech SM, Inflammation Directs Memory Precursor and Short-Lived Effector CD8+ T Cell Fates via the Graded Expression of T-bet Transcription Factor. Immunity 2007. 27: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Aalderen MC, Remmerswaal EB, Verstegen NJ, Hombrink P, ten Brinke A, Pircher H, Kootstra NA, ten Berge IJ and van Lier RA, Infection history determines the differentiation state of human CD8+ T cells. J Virol 2015. 89: 5110–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buggert M, Tauriainen J, Yamamoto T, Frederiksen J, Ivarsson MA, Michaelsson J, Lund O, Hejdeman B, Jansson M, Sonnerborg A, Koup RA, Betts MR and Karlsson AC, T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog 2014. 10: e1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McLane LM, Banerjee PP, Cosma GL, Makedonas G, Wherry EJ, Orange JS and Betts MR, Differential localization of T-bet and Eomes in CD8 T cell memory populations. Journal of Immunology 2013. 190: 3207–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL and Wherry EJ, Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012. 338: 1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y and Rao A, Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. Journal of Experimental Medicine 2009. 206: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sullivan BM, Juedes A, Szabo SJ, Von Herrath M and Glimcher LH, Antigen-driven effector CD8 T cell function regulated by T-bet. Proceedings of the National Academy of Sciences of the United States of America 2003. 100: 15818–15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA and Reiner SL, Control of Effector CD8+ T Cell Function by the Transcription Factor Eomesodermin. Science 2003. 302: 1041–1043. [DOI] [PubMed] [Google Scholar]
- 98.Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, Glimcher LH and Lord GM, The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proceedings of the National Academy of Sciences of the United States of America 2009. 106: 17876–17881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ and Reiner SL, Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. Journal of Immunology 2010. 185: 4988–4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walker CM, Moody DJ, Stites DP and Levy JA, CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 1986. 234: 1563. [DOI] [PubMed] [Google Scholar]
- 101.Walker BD, Chakrabarti S, Moss B, Paradis TJ, Flynn T, Durno AG, Blumberg RS, Kaplan JC, Hirsch MS and Schooley RT, HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 1988. 328: 345–348. [DOI] [PubMed] [Google Scholar]
- 102.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC and Lusso P, Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995. 270: 1811–1815. [DOI] [PubMed] [Google Scholar]
- 103.Chen H, Piechocka-Trocha A, Miura T, Brockman MA, Julg BD, Baker BM, Rothchild AC, Block BL, Schneidewind A, Koibuchi T, Pereyra F, Allen TM and Walker BD, Differential neutralization of human immunodeficiency virus (HIV) replication in autologous CD4 T cells by HIV-specific cytotoxic T lymphocytes. Journal of Virology 2009. 83: 3138–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saez-Cirion A, HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. PNAS 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, Havlir DV, Richman DD, Waters A, Easterbrook P, McMichael AJ and Rowland-Jones SL, HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med 2000. 192: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mackewicz C and Levy JA, CD8+ Cell Anti-HIV Activity: Nonlytic Suppression of Virus Replication. AIDS Research and Human Retroviruses 1992. 8: 1039–1050. [DOI] [PubMed] [Google Scholar]
- 107.Levy JA, Mackewicz CE and Barker E, Controlling HIV pathogenesis: The role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunology Today 1996. 17: 217–224. [DOI] [PubMed] [Google Scholar]
- 108.Barker TD, Weissman D, Daucher JA, Roche KM and Fauci AS, Identification of multiple and distinct CD8+ T cell suppressor activities: Dichotomy between infected and uninfected individuals, evolution with progression of disease, and sensitivity to gamma irradiation. Journal of Immunology 1996. 156: 4476–4483. [PubMed] [Google Scholar]
- 109.Wiviott LD, Walker CM and Levy JA, CD8+ lymphocytes suppress HIV production by autologous CD4+ cells without eliminating the infected cells from culture. Cellular Immunology 1990. 128: 628–634. [DOI] [PubMed] [Google Scholar]
- 110.Walker CM, Erickson AL, Hsueh FC and Levy JA, Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytotoxic mechanism. Journal of Virology 1991. 65: 5921–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walker CM and Levy JA, A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology 1989. 66: 628–630. [PMC free article] [PubMed] [Google Scholar]
- 112.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM and Berger EA, CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 1996. 272: 1955–1958. [DOI] [PubMed] [Google Scholar]
- 113.Cocchi F, DeVico AL, Yarchoan R, Redfield R, Cleghorn F, Blattner WA, Garzino-Demo A, Colombini-Hatch S, Margolis D and Gallo RC, Higher macrophage inflammatory protein (MIP)-1α and MIP-1β levels from CD8+ T cells are associated with asymptomatic HIV-1 infection. Proceedings of the National Academy of Sciences 2000. 97: 13812–13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chun T-W, Justement JS, Moir S, Hallahan CW, Ehler LA, Liu S, McLaughlin M, Dybul M, Mican JM and Fauci AS, Suppression of HIV replication in the resting CD4(+) T cell reservoir by autologous CD8(+) T cells: Implications for the development of therapeutic strategies. Proceedings of the National Academy of Sciences of the United States of America 2001. 98: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Freel SA, Picking RA, Ferrari G, Ding H, Ochsenbauer C, Kappes JC, Kirchherr JL, Soderberg KA, Weinhold KJ, Cunningham CK, Denny TN, Crump JA, Cohen MS, McMichael AJ, Haynes BF and Tomaras GD, Initial HIV-1 antigen-specific CD8+ T cells in acute HIV-1 infection inhibit transmitted/founder virus replication. Journal of Virology 2012. 86: 6835–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferrari G, Korber B, Goonetilleke N, Liu MKP, Turnbull EL, Salazar-Gonzalez JF, Hawkins N, Self S, Watson S, Betts MR, Gay C, McGhee K, Pellegrino P, Williams I, Tomaras GD, Haynes BF, Gray CM, Borrow P, Roederer M, McMichael AJ and Weinhold KJ, Relationship between functional profile of HIV-1 specific CD8 T cells and epitope variability with the selection of escape mutants in acute HIV-1 infection. PLoS Pathogens 2011. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Freel SA, Lamoreaux L, Chattopadhyay PK, Saunders K, Zarkowsky D, Overman RG, Ochsenbauer C, Edmonds TG, Kappes JC, Cunningham CK, Denny TN, Weinhold KJ, Ferrari G, Haynes BF, Koup RA, Graham BS, Roederer M and Tomaras GD, Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. Journal of Virology 2010. 84: 4998–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lieberman J, Tracking the killers: How should we measure CD8 T cells in HIV infection? AIDS 2004. 18: 1489–1493. [DOI] [PubMed] [Google Scholar]
- 119.Cao J, McNevin J, Holte S, Fink L, Corey L and McElrath MJ, Comprehensive analysis of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-secreting CD8+ T cells in primary HIV-1 infection. Journal of Virology 2003. 77: 6867–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dalod M, Dupuis M, Deschemin JC, Goujard C, Deveau C, Meyer L, Ngo N, Rouzioux C, Guillet JG, Delfraissy JF, Sinet M and Venet A, Weak anti-HIV CD8+ T-cell effector activity in HIV primary infection. Journal of Clinical Investigation 1999. 104: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mackewicz CE, Ortega H and Levy JA, Effect of Cytokines on HIV Replication in CD4+ Lymphocytes: Lack of Identity with the CD8+ Cell Antiviral Factor. Cellular Immunology 1994. 153: 329–343. [DOI] [PubMed] [Google Scholar]
- 122.Vella C and Daniels RS, CD8+ T-cell-mediated non-cytolytic suppression of human immuno-deficiency viruses. Curr Drug Targets Infect Disord 2003. 3: 97–113. [DOI] [PubMed] [Google Scholar]
- 123.Chang TLY, François F, Mosoian A and Klotman ME, CAF-mediated human immunodeficiency virus (HIV) type 1 transcriptional inhibition is distinct from α-defensin-1 HIV inhibition. Journal of Virology 2003. 77: 6777–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Copeland KFT, Leith JG, McKay PJ and Rosenthal KL, CD8+ T cell supernatants of HIV type 1-infected individuals have opposite effects on long terminal repeat-mediated transcription in T cells and monocytes. AIDS Research and Human Retroviruses 1997. 13: 71–77. [DOI] [PubMed] [Google Scholar]
- 125.Mackewicz CE, Patterson BK, Sandra A L and Levy JA, CD8+ cell noncytotoxic anti-human immunodeficiency virus response inhibits expression of viral RNA but not reverse transcription or provirus integration. Journal of General Virology 2000. 81: 1261–1264. [DOI] [PubMed] [Google Scholar]
- 126.Le Borgne S, Février M, Callebaut C, Lee SP and Rivière Y, CD8+-cell antiviral factor activity is not restricted to human immunodeficiency virus (HIV)-specific T cells and can block HIV replication after initiation of reverse transcription. Journal of Virology 2000. 74: 4456–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chang TLY, Mosoian A, Pine R, Klotman ME and Moore JP, A soluble factor(s) secreted from CD8+ T lymphocytes inhibits human immunodeficiency virus type 1 replication through STAT1 activation. Journal of Virology 2002. 76: 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rubbert A, Weissman D, Combadiere C, Pettrone KA, Daucher JA, Murphy PM and Fauci AS, Multifactorial nature of noncytolytic CD8+ T cell-mediated suppression of HIV replication: β-chemokine-dependent and -independent effects. AIDS Research and Human Retroviruses 1997. 13: 63–69. [DOI] [PubMed] [Google Scholar]
- 129.Leith JG, Copeland KFT, McKay PJ, Richards CD and Rosenthal KL, CD8+ T-cell-mediated suppression of HIV/−1 long terminal repeat-driven gene expression is not modulated by the CC chemokines RANTES, macrophage inflammatory protein (MIP)-1α and MIP-1β. AIDS 1997. 11: 575–580. [DOI] [PubMed] [Google Scholar]
- 130.Mackewicz CE, Blackbourn DJ and Levy JA, CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proceedings of the National Academy of Sciences 1995. 92: 2308–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tomaras GD, Lacey SF, McDanal CB, Ferrari G, Weinhold KJ and Greenberg ML, CD8+ T cell-mediated suppressive activity inhibits HIV-1 after virus entry with kinetics indicating effects on virus gene expression. Proceedings of the National Academy of Sciences 2000. 97: 3503–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, De Pierres C, Mncube Z, Mkhwanazi N, Bishop K, Van Der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD and Goulder P, CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nature Medicine 2007. 13: 46–53. [DOI] [PubMed] [Google Scholar]
- 133.Klatt NR, Shudo E, Ortiz AM, Engram JC, Paiardini M, Lawson B, Miller MD, Else J, Pandrea I, Estes JD, Apetrei C, Schmitz JE, Ribeiro RM, Perelson AS and Silvestri G, CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog 2010. 6: e1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wong JK, Strain MC, Porrata R, Reay E, Sankaran-Walters S, Ignacio CC, Russell T, Pillai SK, Looney DJ and Dandekar S, In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog 2010. 6: e1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M and Ho DD, Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 1997. 387: 188–191. [DOI] [PubMed] [Google Scholar]
- 136.Perelson AS, Neumann AU, Markowitz M, Leonard JM and Ho DD, HIV-1 Dynamics in Vivo: Virion Clearance Rate, Infected Cell Life-Span, and Viral Generation Time. Science 1996. 271: 1582. [DOI] [PubMed] [Google Scholar]
- 137.Seich Al Basatena NK, Chatzimichalis K, Graw F, Frost SD, Regoes RR and Asquith B, Can non-lytic CD8+ T cells drive HIV-1 escape? PLoS Pathog 2013. 9: e1003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balamurali M, Petravic J, Loh L, Alcantara S, Kent SJ and Davenport MP, Does cytolysis by CD8+ T cells drive immune escape in HIV infection? J Immunol 2010. 185: 5093–5101. [DOI] [PubMed] [Google Scholar]
- 139.Spits HB, Mudrikova T, Schellens IMM, Wensing AMJ, Prins JM, Feuth T, Spierings E, Nijhuis M, van Baarle D and Borghans JAM, The presence of protective cytotoxic T lymphocytes does not correlate with shorter lifespans of productively infected cells in HIV-1 infection. AIDS 2016. 30: 9–17. [DOI] [PubMed] [Google Scholar]
- 140.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF and Ostrowski MA, Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. Journal of Experimental Medicine 2008. 205: 2763–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES and Walker BD, Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nature Immunology 2007. 8: 1246–1254. [DOI] [PubMed] [Google Scholar]
- 142.Nikolova MH, Muhtarova MN, Taskov HB, Kostov K, Vezenkov L, Mihova A, Boumsell L and Bensussan A, The CD160+ CD8high cytotoxic T cell subset correlates with response to HAART in HIV-1+ patients. Cellular Immunology 2005. 237: 96–105. [DOI] [PubMed] [Google Scholar]
- 143.Grabmeier-Pfistershammer K, Steinberger P, Rieger A, Leitner J and Kohrgruber N, Identification of PD-1 as a unique marker for failing immune reconstitution in HIV-1-infected patients on treatment. Journal of Acquired Immune Deficiency Syndromes 2011. 56: 118–124. [DOI] [PubMed] [Google Scholar]
- 144.Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA and Palmer BE, Suppression of HIV replication by antiretroviral therapy reduces TIM-3 expression on HIV-specific CD8+ T cells. AIDS Research and Human Retroviruses 2011. 27: 1–3. [DOI] [PubMed] [Google Scholar]
- 145.Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, Brumme CJ, Rosenberg ES, Alter G, Allen TM, Walker BD and Altfeld M, Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Medicine 2008. 5: 0790–0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Conrad JA, Ramalingam RK, Duncan CB, Smith RM, Wei J, Barnett L, Simons BC, Lorey SL and Kalams SA, Antiretroviral therapy reduces the magnitude and T cell receptor repertoire diversity of HIV-specific T cell responses without changing T cell clonotype dominance. Journal of Virology 2012. 86: 4213–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rehr M, Cahenzli J, Haas A, Price DA, Gostick E, Huber M, Karrer U and Oxenius A, Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. Journal of Virology 2008. 82: 3391–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Serrano-Villar S, Moreno S, Fuentes-Ferrer M, Sanchez-Marcos C, Avila M, Sainz T, de Villar NGP, Fernandez-Cruz A and Estrada V, The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery. Hiv Medicine 2014. 15: 40–49. [DOI] [PubMed] [Google Scholar]
- 149.Casazza JP, Betts MR, Picker LJ and Koup RA, Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. Journal of Virology 2001. 75: 6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cao W, Mehraj V, Trottier B, Baril J-G, Leblanc R, Lebouche B, Cox J, Tremblay C, Lu W, Singer J, Li T and Routy J-P, Early Initiation Rather Than Prolonged Duration of Antiretroviral Therapy in HIV Infection Contributes to the Normalization of CD8 T-Cell Counts. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 2016. 62: 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jenabian M-A, El-Far M, Vyboh K, Kema I, Costiniuk CT, Thomas R, Baril J-G, LeBlanc R, Kanagaratham C, Radzioch D, Allam O, Ahmad A, Lebouché B, Tremblay C, Ancuta P, Routy J-P, Trottier B, Vézina S, Charest L, Milne M, Huchet E, Lavoie S, Friedman J, Duchastel M, Villielm F, Côté P, Potter M, Lessard B, Charron MA, Dufresne S, Turgeon ME, Rouleau D, Labrecque L, Fortin C, de Pokomandy A, Trottier B, Hal-Gagné V, Munoz M, Deligne B, Martel-Laferrière V, Gilmore N, Fletcher M, Szabo J, Vassal AF, Legault M, Lafontaine V, Massicotte A, Matte S, Pineda R, Sas J, Pankovich J, Gauchat D and Gosselin A, Immunosuppressive Tryptophan Catabolism and Gut Mucosal Dysfunction Following Early HIV Infection. The Journal of Infectious Diseases 2015. 212: 355–366. [DOI] [PubMed] [Google Scholar]
- 152.Deeks SG, HIV: Shock and kill. Nature 2012. 487: 439–440. [DOI] [PubMed] [Google Scholar]
- 153.Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MBA and Shaw GM, Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nature Medicine 1997. 3: 205–211. [DOI] [PubMed] [Google Scholar]
- 154.Koup RA, Virus escape from CTL recognition. Journal of Experimental Medicine 1994. 180: 779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, John A, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD and Goulder PJR, HIV evolution: CTL escape mutation and reversion after transmission. Nature Medicine 2004. 10: 282–289. [DOI] [PubMed] [Google Scholar]
- 156.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N and Haynes BF, The immune response during acute HIV-1 infection: Clues for vaccine development. Nature Reviews Immunology 2010. 10: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Phillips RE, Rowland-Jones S, Nixon DF, Gotch FM, Edwards JP, Ogunlesi AO, Elvin JG, Rothbard JA, Bangham CRM, Rizza CR and McMichael AJ, Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 1991. 354: 453–459. [DOI] [PubMed] [Google Scholar]
- 158.Von Stockenstrom S, Odevall L, Lee E, Sinclair E, Bacchetti P, Killian M, Epling L, Shao W, Hoh R, Ho T, Faria NR, Lemey P, Albert J, Hunt P, Loeb L, Pilcher C, Poole L, Hatano H, Somsouk M, Douek D, Boritz E, Deeks SG, Hecht FM and Palmer S, Longitudinal Genetic Characterization Reveals That Cell Proliferation Maintains a Persistent HIV Type 1 DNA Pool during Effective HIV Therapy. Journal of Infectious Diseases 2015. 212: 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jones RB and Walker BD, HIV-specific CD8(+) T cells and HIV eradication. The Journal of Clinical Investigation 2016. 126: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, Margolick JB, Gurer C, Murphy AJ, Valenzuela DM, Yancopoulos GD, Deeks SG, Strowig T, Kumar P, Siliciano JD, Salzberg SL, Flavell RA, Shan L and Siliciano RF, Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 2015. 517: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, Cozzi-Lepri A, De Luca A, Easterbrook P, Francioli P, Mallal S, Martinez-Picado J, Miro JM, Obel N, Smith JP, Wyniger J, Descombes P, Antonarakis SE, Letvin NL, McMichael AJ, Haynes BF, Telenti A and Goldstein DB, A whole-genome association study of major determinants for host control of HIV-1. Science 2007. 317: 944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carrington M, Nelson GW, Martin MP, Kissner T, Vlahov D, Goedert JJ, Kaslow R, Buchbinder S, Hoots K and O’Brien SJ, HLA and HIV-1: Heterozygote advantage and B*35-Cw*04 disadvantage. Science 1999. 283: 1748–1752. [DOI] [PubMed] [Google Scholar]
- 163.The International, H. I. V. C. S., Writing t., Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PIW, Walker BD, Analysis t., Jia X, McLaren PJ, Ripke S, Brumme CJ, Pulit SL, Telenti A, Carrington M, Kadie CM, Carlson JM, Heckerman D, de Bakker PIW, Study d., Pereyra F, de Bakker PIW, Graham RR, Plenge RM, Deeks SG, Walker BD, Snp genotyping, H. L. A. t., sample m., Gianniny L, Crawford G, Sullivan J, Gonzalez E, Davies L, Camargo A, Moore JM, Beattie N, Gupta S, Crenshaw A, Burtt NP, Guiducci C, Gupta N, Carrington M, Gao X, Qi Y, Yuki Y, recruitment, H. I. V. c., sample m., Pereyra F, Piechocka-Trocha A, Cutrell E, Rosenberg R, Moss KL, Lemay P, O’Leary J, Schaefer T, Verma P, Toth I, Block B, Baker B, Rothchild A, Lian J, Proudfoot J, Alvino DML, Vine S, Addo MM, Allen TM, Altfeld M, Henn MR, Le Gall S, Streeck H, Walker BD, Group, A. C. T., Haas DW, Kuritzkes DR, Robbins GK, Shafer RW, Gulick RM, Shikuma CM, Haubrich R, Riddler S, Sax PE, Daar ES, Ribaudo HJ, team, H. I. V. c. r., Agan B, Agarwal S, Ahern RL, Allen BL, Altidor S, Altschuler EL, Ambardar S, Anastos K, Anderson B, Anderson V, Andrady U, Antoniskis D, Bangsberg D, Barbaro D, Barrie W, The Major Genetic Determinants of HIV-1 Control Affect HLA Class I Peptide Presentation. Science (New York, N.Y.) 2010. 330: 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pereyra F, Heckerman D, Carlson JM, Kadie C, Soghoian DZ, Karel D, Goldenthal A, Davis OB, DeZiel CE, Lin T, Peng J, Piechocka A, Carrington M and Walker BD, HIV control is mediated in part by CD8+ T-cell targeting of specific epitopes. Journal of Virology 2014. 88: 12937–12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, McCarter MD, MaWhinney S, Hage A, White C and Skinner PJ, CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. Journal of Immunology 2007. 178: 6975–6983. [DOI] [PubMed] [Google Scholar]
- 166.Tjernlund A, In situ detection of Gag-specific CD8+ cells in the GI tract of SIV infected Rhesus macaques. Retrovirology 2010. 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vinuesa C and Cyster J, How T cells earn the follicular rite of passage. Immunity 2011. 35: 671–680. [DOI] [PubMed] [Google Scholar]
- 168.Sasikala-Appukuttan AK, Kim HO, Kinzel NJ, Hong JJ, Smith AJ, Wagstaff R, Reilly C, Piatak M Jr, Lifson JD, Reeves RK, Johnson RP, Haase AT and Skinner PJ, Location and dynamics of the immunodominant CD8 T cell response to SIVΔnef immunization and SIVmac251 vaginal challenge. PLoS ONE 2013. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Crotty S, Follicular Helper CD4 T cells (T FH) Annual Review of Immunology 2011, pp 621–663. [DOI] [PubMed] [Google Scholar]
- 170.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M Jr., Estes JD, Lifson JD and Picker LJ, B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015. 21: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Irvine DJ, Purbhoo MA, Krogsgaard M and Davis MM, Direct observation of ligand recognition by T cells. Nature 2002. 419: 845–849. [DOI] [PubMed] [Google Scholar]
- 172.Bullen CK, Laird GM, Durand CM, Siliciano JD and Siliciano RF, New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014. 20: 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lassen KG, Ramyar KX, Bailey JR, Zhou Y and Siliciano RF, Nuclear Retention of Multiply Spliced HIV-1 RNA in Resting CD4(+) T Cells. PLoS Pathogens 2006. 2: e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cartwright EK, Spicer L, Smith SA, Lee D, Fast R, Paganini S, Lawson BO, Nega M, Easley K, Schmitz JE, Bosinger SE, Paiardini M, Chahroudi A, Vanderford TH, Estes JD, Lifson JD, Derdeyn CA and Silvestri G, CD8(+) Lymphocytes Are Required for Maintaining Viral Suppression in SIV-Infected Macaques Treated with Short-Term Antiretroviral Therapy. Immunity 2016. 45: 656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL and Reimann KA, Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 1999. 283: 857–860. [DOI] [PubMed] [Google Scholar]
- 176.Takata H, Buranapraditkun S, Kessing C, Fletcher JL, Muir R, Tardif V, Cartwright P, Vandergeeten C, Bakeman W, Nichols CN, Pinyakorn S, Hansasuta P, Kroon E, Chalermchai T, O’Connell R, Kim J, Phanuphak N, Robb ML, Michael NL, Chomont N, Haddad EK, Ananworanich J, Trautmann L, Rv254/Search and the, R. V. S. S. G., Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection. Sci Transl Med 2017. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Margolick JB, Donnenberg AD, Munoz A, Park LP, Bauer KD, Giorgi JV, Ferbas J and Saah AJ, Changes in T and non-T lymphocyte subsets following seroconversion to HIV-1: stable CD3+ and declining CD3− populations suggest regulatory responses linked to loss of CD4 lymphocytes. The Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 1993. 6: 153–161. [PubMed] [Google Scholar]
- 178.Doisne JM, Urrutia A, Lacabaratz-Porret C, Goujard C, Meyer L, Chaix ML, Sinet M and Venet A, CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. Journal of Immunology 2004. 173: 2410–2418. [DOI] [PubMed] [Google Scholar]
- 179.Bastidas S, Graw F, Smith MZ, Kuster H, Günthard HF and Oxenius A, CD8+ T cells are activated in an antigen-independent manner in HIV-infected individuals. Journal of Immunology 2014. 192: 1732–1744. [DOI] [PubMed] [Google Scholar]
- 180.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Förster R, Rowland-Jones S, Sékaly RP, McMichael AJ and Pantaleo G, Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 2001. 410: 106–111. [DOI] [PubMed] [Google Scholar]
- 181.Arrode G, Finke JS, Zebroski H, Siegal FP and Steinman RM, CD8+ T cells from most HIV-1-infected patients, even when challenged with mature dendritic cells, lack functional recall memory to HIV gag but not other viruses. European Journal of Immunology 2005. 35: 159–170. [DOI] [PubMed] [Google Scholar]
- 182.Migueles SA, Weeks KA, Nou E, Berkley AM, Rood JE, Osborne CM, Hallahan CW, Cogliano-Shutta NA, Metcalf JA, McLaughlin M, Kwan R, Mican JM, Davey RT Jr. and Connors M, Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol 2009. 83: 11876–11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue PY, Van Natta ML, Meinert CL, Lederman MM, Hatano H, Jain V, Huang Y, Hecht FM, Martin JN, McCune JM, Moreno S and Deeks SG, HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality. PLOS Pathogens 2014. 10: e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]