Abstract
OBJECTIVES: To investigate the predictive value of the pre-operative D-dimer and gamma-glutamyltranspeptidase (GGT) for the prognosis in colorectal liver metastases (CRLM) patients after hepatic resection. METHODS: Two hundred and ninety-two patients between December 2008 and December 2016 and 101 patients at our center from January 2017 to December 2018 were selected as a training set and validation set, respectively. The combination of the pre-operative D-dimer and GGT status (CPDG score) was scored as follows: elevated D-dimer levels with elevated GGT levels was allocated a score of 2, decreased D-dimer levels with decreased GGT levels was allocated a score of 0, and all other combinations were allocated a score of 1. In the training set, a logistic regression was applied to explore potential predictors of major postoperative complications. A Cox proportional hazards analysis was used to analyze survival. We further verified our findings in the validation set. RESULTS: Major complications occurred in 43 (14.7%) and 25 (24.8%) patients in the training set and validation set, respectively. In the training set, multivariate analysis showed that elevated GGT levels and elevated D-dimer levels independently predicted major complications respectively. In the multivariate analyses, elevated pre-operative D-dimer levels remained independently associated with decreased overall survival (OS) (hazard ratio [HR] = 1.751, 95% confidence interval [CI]: 1.139-2.691, P = .01). The CPDG score was an independent prognostic factor for major complications and OS in the multivariate analyses. The predictive ability of the CPDG score was higher than either factor alone. A Kaplan-Meier survival analysis showed that compared with patients with CPDG score = 1 or CPDG score = 0, patients with a CPDG score = 2 had worsened OS. Furthermore, for OS comparisons, the differences between any two groups were significant. In the validation set, elevated GGT and D-dimer were also suggested to predict worse progression-free survival (PFS) and to be independently associated with major complications. CONCLUSIONS: The pre-operative D-dimer levels, GGT levels and CPDG score are reliable biomarkers to predict post-operative major complications or survival in CRLM patients after hepatic resection, which make it useful for CRLM patients in guiding surveillance approaches and prognosis assessments.
Introduction
Colorectal cancer accounts for more than 9% of all cancer incidences and is the third most common cancer reported worldwide, as well as being the fourth most common cause of death [1]. More than half of colorectal cancer patients will develop liver metastases during the course of the disease [2]. Colorectal cancer liver metastasis (CRLM) is the leading cause of death in patients with colorectal cancer [3]. Liver resection is the gold standard treatment for CRLM patients and confers the best prognosis [4], [5]. However, liver resection is associated with significant mortality and morbidity, due to major complications such as liver failure, blood loss and bile leak. Post-operative complications have a reported prevalence of 4% to 53% [6], [7], [8]. In addition, the post-operative 5-year survival rates are only 30% to 50% [3] and more than 70% of patients will have a recurrence after resection for CRLM [9]. Therefore, it is crucial to explore and identify biomarkers that are affordable and technically feasible for predicting post-operative major complications and survival at earlier time points in CRLM patients.
Gamma-glutamyltransferase (GGT) is a membrane bound enzyme that is involved in glutathione (GSH) metabolism [10]. Some studies [11], [12], [13] have demonstrated that GGT modulated the cellular proliferative and apoptotic balance and played an important role in tumor progression, invasion and drug resistance. Recently, pre-operative serum GGT levels have been linked to several human malignancies, including hepatocellular carcinoma (HCC), esophageal squamous cell carcinoma, ovarian cancer and renal cell carcinoma, with GGT acting as a negative prognostic marker [14], [15], [16], [17]. For liver disease, GGT is predominantly used as a diagnostic marker. Latent elevations in GGT are seen to be related to specific pathologies such as liver fibrosis with HBV infection, non-alcoholic fatty liver disease, alcoholic liver disease and other chronic liver disease [18], [19], [20].
Recent studies suggested a process of hemostasis and fibrinolysis was related to tumor angiogenesis, invasion, progression, and metastatic spread [21]. D-dimer, one of the key components in the activation of hemostasis and fibrinolysis, has been found to be associated with poor prognosis in several malignant diseases, including lung cancer, ovarian cancer and gastric cancer [22], [23], [24], [25]. For liver disease, the increased D-dimer correlated well with the higher Child-Pugh scores indicating the aggravation of hepatic impairment. Elevated plasma values of D-dimer in patients with liver cirrhosis are risk factors for a higher incidence in decompensated liver disease and portal vein thrombosis [26], [27].
There has not been a study examining the prognostic significance of serum D-dimer levels and GGT levels in CRLM. In view of the characteristics of these two biomarkers, we speculate that these two biomarkers are of great value in predicting the prognosis of CRLM. The aim of the present study was to explore the association between pre-operative D-dimer levels and GGT levels and prognoses in CRLM patients after liver resection.
Materials and Methods
Patients
Two hundred and ninety-two patients who were admitted to our hospital between December 2008 and December 2016 were identified as a training set. One hundred and one patients at our center from January 2017 to December 2018 were selected as a validation set. This study was approved by the Institutional Review Board of the Cancer Institute & Hospital, Chinese Academy of Medical Sciences.
The inclusion criteria were as follows: (1) the primary tumor site was removed and pathologically diagnosed as colorectal cancer; (2) patients were diagnosed with liver metastasis from colorectal cancer by post-operative pathology; and (3) patients were treated with resection for CRLM, according to the guidelines for the treatment of CRLM [3]. Extrahepatic disease (EHD) was not a contraindication for surgery as long as all of the EHD sites could be radically treated (surgery, radiotherapy, ablation, etc.). Data on patient demographics, clinicopathological characteristics and medical treatment were reviewed.
Patients were not eligible if they presented with pre-treatment comorbidities known to be associated with elevated GGT (i.e. biliary tract-, pancreatic- and heart disease or alcohol abuse) and D-dimer (i.e. deep venous thrombosis, pulmonary embolism or disseminated intravascular coagulation).
Measurement of Serum GGT Levels and D-dimer Levels
Serum GGT levels and D-dimer levels were measured within 1 week before surgery. Blood samples for the evaluation of serum GGT levels and D-dimer levels were obtained by using peripheral venous punctures. Serum GGT concentrations were analyzed with an enzyme kinetic assay (Roche Diagnostics GmbH, Mannheim, Germany). The normal value range of the GGT levels was between 0 and 55 U/L in our hospital. Serum D-dimer concentrations were analyzed with an enzyme-linked fluorescent immunoassay method. The normal value range of the D-dimer levels was between 0 and 0.55 mg/L in our hospital.
To analyze the prognostic value of the combination of the pre-operative D-dimer with GGT levels (CPDG) in major complications and survival, we created a CPDG score construct based on the D-dimer levels and GGT levels. The CPDG score was scored as follows: elevated D-dimer levels with elevated GGT levels was allocated a score of 2, decreased D-dimer levels with decreased GGT levels was allocated a score of 0, and all other combinations were allocated a score of 1.
Treatment
As described previously [28], decisions about the treatment of CRLM were reached by the consensus of a multidisciplinary team (MDT) including surgeons, oncologists and radiologists. Pre-operative chemotherapy was recommended to patients with initially unresectable liver metastases or to patients with multiple high-risk factors [29], [30]. Chemotherapy was mainly comprised of a combination of 5-fluorouracil/capecitabine and oxaliplatin/irinotecan, with or without bevacizumab and cetuximab. Surgical resections included the open and laparoscopic approaches. The resection margin status was defined according to the International Union Against Cancer (UICC) criteria. Liver resections were divided into major and minor resections. Major resections were defined as resections of more than two segments, and other resections were described as being minor resections. Intraoperative RFA was used when a hepatic lesion was located more deeply or proximal to major vascular structures and was especially used for lesions that were less than 3 cm.
Follow-Up and Endpoints
Patients were followed up at regular intervals. The initial post-treatment CT and MRI scans occurred one month after surgery. Afterwards, patients were followed up at 3-month intervals for up to 2 years and every 6 months thereafter. All post-operative complications were graded according to the Clavien-Dindo classification system [5]. Minor complications were classified as Clavien-DindoI-II, and major complications were classified as Clavien-Dindo III -V. Overall survival (OS) was defined as the length of time from the date of hepatic resection for liver metastasis to the last follow-up or death. Progression-free survival (PFS) was defined as the interval between hepatic resection for liver metastasis and a recurrence or progression of the (residual) disease.
Statistical Analysis
Data were compared using Fisher's exact tests for the categorical variables and the non-parametric Mann-Whitney U tests for the continuous variables. A ROC curve was constructed to estimate the predictive value of the pre-operative GGT levels and D-dimer levels. The optimal cutoff values were identified using the highest Youden index (sensitivity+specificity-1) [31]. Prediction accuracy was evaluated with the area under the ROC curve. All predictors with P < .10 by univariate analysis were retained in the multivariate model. A multivariate logistic regression analysis was performed to identify independent factors. A Kaplan-Meier survival analysis was used to calculate OS and PFS, and significant differences between the groups were evaluated by using the log-rank test. Multivariable analyses of OS and PFS were performed by using Cox regression models. Finally, we will verify the findings in the validation cohort. A difference of P < .05 was statistically significant. Statistical analyses were performed using the SPSS, version 22 software (Armonk NV, USA).
Results
Patient and Tumor Characteristics
The clinicopathological characteristics for the training set (n = 292) and validation set (n = 101) are listed in Table 1, Table 2. Of the 393 patients analyzed, 154 (39.2%) were female and 239 (60.8%) were male and the median age (interquartile range [IQR]) was 57.0 (50.0-63.5) years. A total of 45 patients (11.5%) presented with extrahepatic metastases at the initial diagnosis. As to the tumors located in the liver, synchronous metastasis was detected in 235 (80.5%) and 70 (69.3%) patients in the training set and validation set, respectively. Liver lesions located in both lobes of liver were detected in 142 (36.1%) patients. Preoperative chemotherapy was given to 240 (60.1%) patients in the whole cohort. As to the primary site, it was located in the colon among patients in 156 (53.4%) patients from the training set and 58 (57.4%) patients from the validation set and was located in the rectum for other patients. All included patients had received hepatectomy, among which major hepatectomy, heterochronous resection, and RFA was performed in 184 (46.8%), 148 (37.7%), and 44 (11.2%) patients, respectively. R0 resection was achieved in 258 (65.6%) patients.
Table 1.
Patients’ characteristics of the training set and validation set
| Item | Training set (n = 292) (%) |
Validation set (n = 101) (%) |
|---|---|---|
| Age ≥60 years | 108 (37.0) | 45 (44.6) |
| Female | 114 (39.0) | 40 (39.6) |
| Preoperative CEA ≥10 ng/ml | 130 (44.5) | 43 (42.6) |
| Major resection | 124 (42.5) | 60 (59.4) |
| Synchronous metastasis | 235 (80.5) | 70 (69.3) |
| Primary site colon | 156 (53.4) | 58 (57.4) |
| Left hemicolon | 45 (15.4) | 19 (18.8) |
| Pretreatment chemotherapy | 186 (63.6) | 54 (53.5) |
| R0 resection | 195 (66.8) | 63 (62.4) |
| RFA | 36 (12.3) | 8 (7.92) |
| Bilobar distribution | 111 (38.0) | 31 (30.7) |
| Extrahepatic metastases | 26 (8.90) | 19 (18.8) |
| Diameter of metastases ≥3 cm | 138 (47.3) | 45 (44.6) |
| Multiple metastases | 171 (58.6) | 44 (43.6) |
| Operation time ≥ 301.5 min | 127 (43.5) | 38 (37.6) |
| Poorly differentiated | 69 (23.6) | 29 (28.7) |
| T3-T4 | 243 (83.2) | 82 (81.1) |
| Heterochronous resection | 113 (38.7) | 35 (34.7) |
Table 2.
Patient and tumor characteristics in the training set
| Item | D-dimer﹤0.285 (n = 116) (%) |
D-dimer ≥ 0.285 (n = 176) (%) |
P | GGT﹤30.5 (n = 144) (%) |
GGT ≥30.5 (n = 148) (%) |
P | CPDG = 0 (n = 66) (%) | CPDG = 1 (n = 128) (%) | CPDG = 2 (n = 98) (%) | P | All patients (n = 292) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age ≥60 years | 37 (31.9) | 71 (40.3) | 0.144 | 59 (41.0) | 49 (33.1) | 0.164 | 22 (33.3) | 52 (40.6) | 34 (34.7) | 0.515 | 108 (37.0) |
| Female | 43 (37.1) | 71 (40.3) | 0.575 | 63 (43.8) | 51 (34.5) | 0.104 | 24 (36.4) | 58 (45.3) | 32 (32.7) | 0.136 | 114 (39.0) |
| Preoperative CEA ≥10 ng/ml | 45 (38.8) | 85 (48.3) | 0.110 | 58 (40.3) | 72 (48.6) | 0.150 | 24 (36.4) | 55 (43.0) | 51 (52.0) | 0.126 | 130 (44.5) |
| Major resection | 37 (31.9) | 87 (49.4) | 0.003 | 37 (25.7) | 87 (38.8) | <0.001 | 11 (16.7) | 52 (40.6) | 61 (62.2) | <0.001 | 124 (42.5) |
| Synchronous metastasis | 87 (75.0) | 148 (84.1) | 0.055 | 118 (81.9) | 117 (79.1) | 0.533 | 50 (75.8) | 105 (82.0) | 80 (81.6) | 0.544 | 235 (80.5) |
| Primary site Colon | 61 (52.6) | 95 (54.0) | 0.816 | 76 (52.8) | 80 (54.1) | 0.827 | 35 (53.0) | 67 (52.3) | 54 (55.1) | 0.916 | 156 (53.4) |
| Left hemicolon | 16 (13.8) | 29 (16.5) | 0.534 | 29 (20.1) | 16 (10.8) | 0.027 | 13 (19.7) | 19 (14.8) | 13 (13.3) | 0.520 | 45 (15.4) |
| Pretreatment chemotherapy | 53 (45.7) | 133 (75.6) | <0.001 | 78 (54.2) | 108 (73.0) | 0.001 | 25 (37.9) | 81 (63.3) | 80 (81.6) | <0.001 | 186 (63.7) |
| R0 resection | 80(69.0) | 115 (65.3) | 0.520 | 103 (71.5) | 92 (62.2) | 0.089 | 47 (71.2) | 89 (69.5) | 59 (60.2) | 0.231 | 195 (66.8) |
| RFA | 7 (6.0) | 29 (16.5) | 0.008 | 12 (8.3) | 24 (16.2) | 0.041 | 5 (7.6) | 9 (7.0) | 22 (22.4) | 0.001 | 36 (12.3) |
| Bilobar distribution | 30 (25.9) | 81 (46.0) | 0.001 | 36 (25.0) | 75 (50.7) | <0.001 | 13 (19.7) | 40 (31.3) | 58 (59.2) | <0.001 | 111 (38.0) |
| Extrahepatic metastases | 11 (9.5) | 15 (8.5) | 0.778 | 11 (7.6) | 15 (10.1) | 0.454 | 4 (6.1) | 14 (10.9) | 8 (8.2) | 0.502 | 26 (8.9) |
| Diameter of metastases ≥3 cm | 48 (41.4) | 90 (51.1) | 0.102 | 56 (38.9) | 82 (55.4) | 0.005 | 24 (36.4) | 56 (43.8) | 58 (59.2) | 0.009 | 138 (47.3) |
| Multiple metastases | 56 (48.3) | 115 (65.3) | 0.004 | 64 (44.4) | 107 (72.3) | <0.001 | 26 (39.4) | 68 (53.1) | 77 (78.6) | <0.001 | 171 (58.6) |
| Operation time ≥ 301.5 min | 34 (29.3) | 92 (52.3) | <0.001 | 48 (33.3) | 78 (52.7) | 0.001 | 16 (24.2) | 50 (39.1) | 60 (61.2) | <0.001 | 126 (43.2) |
| Poorly differentiated | 27 (23.3) | 42 (23.9) | 0.908 | 29 (20.1) | 40 (27.0) | 0.166 | 16 (24.2) | 24 (18.8) | 29 (29.6) | 0.163 | 69 (23.6) |
| T1-T2 | 13 (11.2) | 36 (20.5) | 0.039 | 17 (11.8) | 32 (21.6) | 0.025 | 8 (12.1) | 14 (10.9) | 27 (27.6) | 0.002 | 49 (16.8) |
| Heterochronous resection | 52 (44.8) | 61 (34.7) | 0.081 | 50 (34.7) | 63 (42.6) | 0.169 | 26 (39.4) | 50 (39.1) | 37 (37.8) | 0.972 | 113 (38.7) |
Predictors for Post-Operative Major Complications in the Training Set
Major complications occurred in 43 (14.7%) and 25 (24.8%) patients in the training set and validation set, respectively.
ROC curves illustrating the ability of the pre-operative D-dimer levels and GGT levels to predict post-operative major complications were performed in the training set. For D-dimer levels, the optimal cutoff level was 0.485 mg/L, and the area under the curve (AUC) was 0.661 (95%CI: 0.572-0.750, P = .001). This value was associated with a sensitivity of 0.651 and a specificity of 0.671 (negative predictive value [NPV]: 0.918; positive predictive value [PPV]: 0.561). One hundred and eighty-two patients (62.3%) had a D-dimer <0.485 mg/L, and 110 patients (37.7%) had a D-dimer ≥0.485 mg/L. For GGT levels, the optimal cutoff level was 35.5 U/L, and the AUC was 0.645 (95%CI: 0.556-0.734, P = .002). This value was associated with a sensitivity of 0.651 and a specificity of 0.639 (NPV: 0.914; PPV: 0.461). One hundred and seventy-four patients (59.6%) had a GGT< 35.5 U/L, and 118 patients (40.4%) had a GGT ≥35.5 U/L.
The predictors for post-operative major complications were analyzed. In the univariate analysis, resection type (P = .024), pretreatment chemotherapy (P = .009), diameters of metastases (P = .011), operation time (P = .005), pre-operative GGT levels (P < .001) and pre-operative D-dimer levels (P < .001) were statistically significant parameters. These statistically significant parameters in the univariate analysis were retained in the multivariate model. In the multivariate analysis, elevated pre-operative D-dimer levels (OR = 3.474, 95% CI: 1.736-6.949, P < .001) and elevated pre-operative GGT levels (OR = 2.977, 95% CI: 1.486-5.965, P = .002) significantly predicted the major complications (Table 3).
Table 3.
Prognostic factors for major complications in CRLM patients after surgery in the training set
| Factor | Univariate analysis |
Multivariate analysis |
|
|---|---|---|---|
| P | OR (95%CI) | P | |
| Age ≥60 years | 0.290 | ||
| Sex (male vs. female) | 0.790 | ||
| Preoperative CEA ≥10 ng/ml | 0.169 | ||
| Major resection | 0.024 | ||
| Synchronous metastasis | 0.278 | ||
| Primary site colon | 0.734 | ||
| Left hemicolon | 0.530 | ||
| Pretreatment chemotherapy | 0.009 | ||
| R0 resection | 0.547 | ||
| Extrahepatic metastases | 0.208 | ||
| Bilobar distribution | 0.574 | ||
| Diameter of metastases ≥3 cm | 0.011 | ||
| Multiple metastases | 0.345 | ||
| Poorly differentiated | 0.652 | ||
| T3-T4 | 0.094 | ||
| Operation time ≥ 301.5 min | 0.005 | ||
| D-dimer ≥0.485 | <0.001 | 3.474 (1.736-6.949) | <0.001 |
| GGT ≥35.5 U/L | <0.001 | 2.977 (1.486-5.965) | 0.002 |
| Heterochronous resection | 0.645 | ||
| the prognostic value on CPDG score | |||
| CPDG = 2 | <0.001 | Reference | - |
| CPDG = 1 | 0.112 (0.045-0.276) | <0.001 | |
| CPDG = 0 | 0.198 (0.090-0.432) | <0.001 | |
Predictors for Survival in the Training Set
In the training set, the median follow-up time was 26.3 months. A total of 204 patients (69.86%) experienced disease recurrence, and 102 patients (34.93%) died. The median OS was 49.7 months (95% CI: 38.04-61.36), and the median PFS was 10.1 months (95% CI: 8.16-12.04). The 1-, 3- and 5-year survival rates were 95.20%, 58.80% and 43.30%, respectively. The 1-, 3-year progression-free survival rates were 44.95%, 25.04% respectively. In the validation set, the median follow-up time was 14.0 months. A total of 51 patients (50.5%) experienced disease recurrence, and only 10 patients (9.90%) died due to a short follow-up period. The median PFS was 14.0 months (95% CI: 6.273-21.727) and the median OS was not reached.
Using the median survival time (49.7 months) as an endpoint, ROC curves were constructed to estimate the optimal cutoff value of the pre-operative D-dimer levels and GGT levels for predicting survival. For D-dimer levels, the optimal cutoff level was 0.285 mg/L, and the AUC was 0.688 (95%CI: 0.592-0.784, P < .001). This value was associated with a sensitivity of 0.702 and a specificity of 0.660 (NPV: 0.805; PPV: 0.525). 116 patients (49.67%) had a D-dimer <0.285 mg/L, and 176 patients (50.33%) had a D-dimer ≥0.285 mg/L. For GGT levels, the optimal cutoff level was 30.5 U/L, and the AUC was 0.676 (95% CI: 0.583-0.768, P = .001). This value was associated with a sensitivity of 0.564 and a specificity of 0.787 (NPV: 0.833; PPV: 0.480). One hundred and forty-four144 patients (49.32%) had a GGT <30.5 U/L, and 148 patients (50.68%) had a GGT ≥30.5 U/L.
The univariate analysis revealed that D-dimer levels ≥0.285 mg/L (P = .005), non R0 resection (P = .002), bilobar distribution (P < .001), multiple metastases (P = .003), operation time ≥301.5 min (P = .026) and GGT levels ≥30.5 U/L (P = .004) were all associated with decreased OS (Table 4). In the multivariate analysis, elevated pre-operative D-dimer levels (HR = 1.751, 95% CI: 1.139-2.691, P = .01), non R0 resection (HR = 1.609, 95%CI: 1.031-2.512, P = .036) and a bilobar distribution (HR = 1.672, 95%CI: 1.068-2.620, P = .025) remained independently associated with decreased OS (Table 4). When regarding PFS, in the univariate analysis, D-dimer levels ≥0.285 mg/L (HR = 1.455, 95% CI: 1.093-1.937, P = .010) and GGT levels ≥30.5 U/L (HR = 1.344, 95%CI: 1.020-1.771, P = .036) significantly decreased PFS. In the multivariate analysis, the elevated pre-operative D-dimer levels and elevated pre-operative GGT levels were not independent factors associated with decreased PFS.
Table 4.
Prognostic factors for OS for CRLM patients after surgery in the training set
| Factor | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| P | HR (95%CI) | P | HR (95%CI) | |
| Age ≥60 years | 0.764 | 1.064 (0.710-1.594) | ||
| Female | 0.660 | 1.093 (0.735-1.625) | ||
| Preoperative CEA ≥10 ng/ml | 0.109 | 1.374 (0.932-2.028) | ||
| Major resection | 0.001 | 1.957 (1.317-2.910) | ||
| Synchronous metastasis | 0.054 | 1.716 (0.991-2.971) | ||
| Primary site Colon | 0.291 | 1.235 (0.835-1.828) | ||
| Left hemicolon | 0.748 | 1.094 (0.632-1.894) | ||
| Pretreatment chemotherapy | 0.073 | 1.467 (0.964-2.231) | ||
| R0 resection | 0.002 | 0.523 (0.349-0.782) | 0.036 | 0.621 (0.398-0.970) |
| RFA | 0.429 | 1.265 (0.706-2.267) | ||
| Bilobar distribution | <0.001 | 2.157 (1.439-3.232) | 0.025 | 1.672 (1.068-2.620) |
| Extrahepatic metastases | 0.142 | 1.571 (0.859-2.872) | ||
| Diameter of metastases ≥3 cm | 0.232 | 1.268 (0.859-1.871) | ||
| Multiple metastases | 0.003 | 1.882 (1.248-2.837) | ||
| Poorly differentiated | 0.932 | 1.022 (0.626-1.667) | ||
| T3-T4 | 0.363 | 1.292 (0.744-2.242) | ||
| Operation time ≥ 301.5 min | 0.026 | 1.565 (1.055-2.324) | ||
| D-dimer ≥0.285 mg/L | 0.005 | 1.817 (1.193-2.769) | 0.011 | 1.751 (1.139-2.691) |
| GGT ≥30.5 U/L | 0.004 | 1.786 (1.199-2.611) | ||
| Heterochronous resection | 0.961 | 0.990 (0.666-1.472) | ||
| The prognostic value on CPDG score | ||||
| CPDG = 2 | - | Reference | - | Reference |
| CPDG = 1 | <0.001 | 0.334 (0.187-0.597) | 0.002 | 0.390 (0.214-0.710) |
| CPDG = 0 | 0.014 | 0.198 (0.374-0.894) | 0.132 | 0.704 (0.446-1.112) |
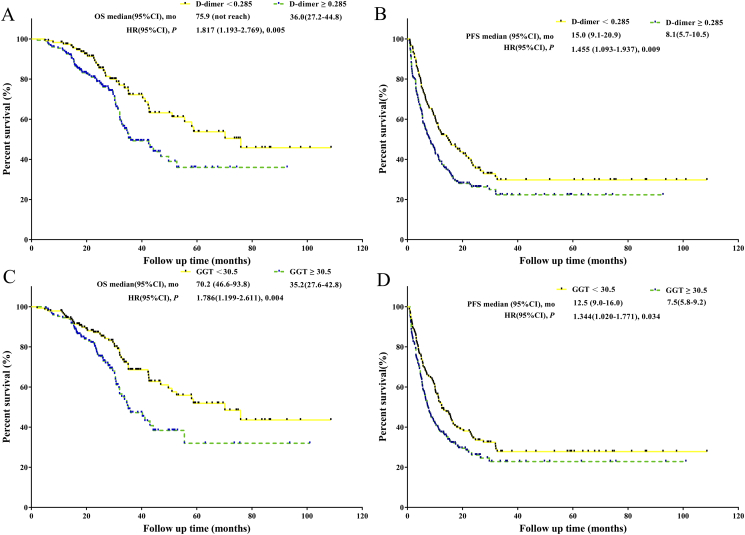
Patients with a D-dimer ≥0.285 mg/L had significantly worse OS and PFS than did patients with a D-dimer <0.285 mg/L (P = .005, mOS: 36.0 months versus 75.9 months; P = .005, mPFS: 8.1 months versus 15.0 months) (Figure 1). Patients with a GGT ≥30.5 U/L had significantly worse OS and PFS than did patients with a GGT <30.5 U/L (P = .004, mOS: 35.2 months versus 70.2 months; P = .034, mPFS: 7.5 months versus 12.5 months) (Figure 1).
Figure 1.
A. Survival analysis of D-dimer <0.285 mg/L versus D-dimer ≥0.285 mg/L in the training set. B. PFS analysis of D-dimer <0.285 mg/L versus D-dimer ≥0.285 mg/L in the training set. C. Survival analysis of GGT <30.5 U/L versus GGT ≥30.5 U/L in the training set. D. PFS analysis of GGT <30.5 U/L versus GGT ≥30.5 U/L in the training set.
Prognostic Value of D-dimer Levels Combined with GGT Levels in the Training Set
In multivariate analysis, two multivariate models were performed separately to account for the fact that the CPDG score is constructed from the D-dimer levels and GGT levels.
In multivariate logistic regression analysis, the CPDG score independently predicted major complications (Table 3). The AUC for the CPDG score was 0.711 (95%CI: 0.622-0.800, P < .001). This value was associated with a sensitivity of 0.588 and a specificity of 0.871 (NPV: 0.908; PPV: 0.550). The AUC for the CPDG score was stronger than the D-dimer levels (P = .040, AUC: 0.711 versus 0.661) and GGT levels (P = .017, AUC: 0.711 versus 0.645) for predicting major complications in patients with CRLM after liver resection.
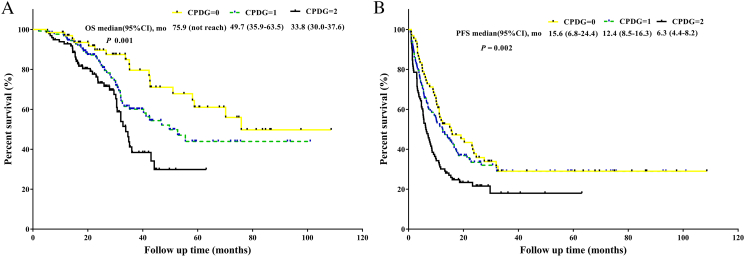
For the prediction of survival, in multivariate analysis the CPDG score was an independent prognostic factor for OS and was not an independent factor for PFS (Table 4). The AUC for the CPDG score was 0.760 (95%CI: 0.680-0.840, P < .001). This value was associated with a sensitivity of 0.526 and a specificity of 0.957 (NPV: 0.952; PPV: 0.506). The AUC for the CPDG score was stronger than the D-dimer levels (P = .017, AUC: 0.760 versus 0.688) and GGT levels (P = .013, AUC: 0.760 versus 0.676) for predicting survival. Kaplan-Meier curve analysis revealed that compared with patients with CPDG = 1 or CPDG = 0, patients with CPDG = 2 were significantly associated with worse OS (P < .001, mOS: 33.8 versus 49.7 months versus 75.9 months, respectively) and worse PFS (P = .002, mPFS: 6.3 months versus 12.4 months versus 15.6 months, respectively) (Figure 2). For OS comparisons, the differences between any two groups were significant (CPDG = 0 versus CPDG = 1, P = .049; CPDG = 0 versus CPDG = 2, P < .001; CPDG = 1 versus CPDG = 2, P = .019).
Figure 2.
A. Survival analysis of CPDG = 0 versus CPDG = 1 versus CPDG = 2 in the training set . B. PFS analysis of CPDG = 0 versus CPDG =1 versus CPDG = 2 in the training set.
Associations of D-dimer, GGT and CPDG with Clinicopathological Characteristics in the Training Set
Based on the cutoff value, all of the patients were dichotomized into either a low value group or a high value group. The clinicopathologic characteristics grouped by D-dimer, GGT and CPDG are summarized in Table 4. Elevated GGT levels were associated with primary sites in the right hemicolon (P = .017), preoperative chemotherapy (P < .001), liver metastases in a bilobar distribution (P < .001), diameters of metastases ≥3 cm (P = .003) and multiple metastases (P < .001). Elevated D-dimer levels were significantly associated with pre-treatment chemotherapy (P < .001), liver metastases in a bilobar distribution (P = .001), multiple metastases (P = .004).
Validation of our Findings in an Independent Cohort
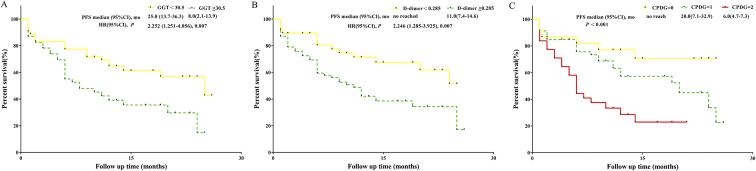
We further verified our findings in the validation set. Elevated D-dimer levels (OR = 4.437, 95%CI: 1.326-14.846, P = .016) and GGT levels (OR = 5.156, 95%CI: 1.547-17.184, P = .008) were independently associated with major complications in the multivariate analysis (Table 5), in addition to the major resection (OR = 4.310, 95% CI: 4.140-16.294, P = .031). The CPDG score was also an independent predictor of major complications. The AUC for the CPDG score was 0.764. This value was associated with a specificity of 0.895 (NPV: 0.791; PPV: 0.467). The AUC for the CPDG score was stronger than the D-dimer levels (AUC: 0.764 versus 0.695) and GGT levels (AUC: 0.764 versus 0.642) for predicting major complications in patients with CRLM after liver resection. In the analysis of survival, elevated D-dimer level (P = .007), GGT level (P = .007) and CPDG score (P < .001) was associated with decreased PFS (Figure 3). Analysis of OS was not conducted due to a short follow-up period.
Table 5.
Prognostic factors for major complications in CRLM patients after surgery in the validation set.
| Factor | Univariate analysis |
Multivariate analysis |
|
|---|---|---|---|
| P | OR (95%CI) | P | |
| Age ≥ 60 years | 0.077 | ||
| Sex (male vs. female) | 0.605 | ||
| Preoperative CEA ≥10 ng/ml | 0.245 | ||
| Major resection | 0.007 | 4.310 (4.140-16.294) | 0.031 |
| Synchronous metastasis | 0.028 | 4.637 (0.953-22.562) | 0.057 |
| Primary site Colon | 0.445 | ||
| Left hemicolon | 0.446 | ||
| Pretreatment chemotherapy | 0.004 | ||
| R0 resection | 0.032 | ||
| Extrahepatic metastases | 0.861 | ||
| Bilobar distribution | 0.010 | ||
| Diameter of metastases ≥3 cm | 0.077 | ||
| Multiple metastases | 0.060 | ||
| Poorly differentiated | 0.522 | ||
| T3-T4 | 0.835 | ||
| Operation time ≥ 301.5 min | <0.001 | 3.017 (0.878-10.366) | 0.079 |
| D-dimer ≥0.485 | 0.001 | 4.437 (1.326-14.846) | 0.016 |
| GGT ≥35.5 U/L | 0.013 | 5.156 (1.547-17.184) | 0.008 |
| Heterochronous resection | 0.083 | ||
| The prognostic value on CPDG score | |||
| CPDG = 2 | Reference | - | |
| CPDG = 1 | 21.529 (2.545->99) | 0.005 | |
| CPDG = 0 | 26.060 (2.618->99) | 0.005 | |
Figure 3.
A. OS analysis of D-dimer <0.285 mg/L versus D-dimer ≥0.285 mg/L in the validation set. B. OS analysis of GGT<30.5 U/L versus GGT ≥30.5 U/L in the validation set. C. OS analysis of CPDG = 0 versus CPDG =1 versus CPDG = 2 in the validation set.
Discussion
To the best of our knowledge, this is the first study to establish a correlation between the outcomes for pre-operative D-dimer levels and GGT levels and the post-operative major complications and survival in patients with CRLM. We found lower pre-operative D-dimer levels and lower pre-operative GGT levels were associated with favorable outcomes, which held true for both post-operative major complications and survival, and then established the CPDG score based on these two biomarkers, as a more accurate prognostic predictor in CRLM patients after resection. Moreover, we have managed to verify the findings in a validation dataset.
The mechanisms of the correlation between elevated D-dimer levels and elevated GGT levels and poor prognoses were unclear. Possible mechanisms are as follows: For the mechanism of D-dimer, the abnormality of hemostasis and fibrinolysis is common in cancer patients, which is related to tumor angiogenesis, invasion, progression and metastatic spread [21]. As a degradation product of fibrin, an elevated D-dimer level is a signal of the abnormality of hemostasis and fibrinolysis. Besides, some studies revealed that D-dimer affected cellular signaling systems to promote cell proliferation and induce angiogenesis [32], and induced the growth and spread of tumors by stimulating the cellular adhesion of tumor cells to endothelial cells [33]. For the mechanism of GGT, GGT is a membrane bound enzyme that is indispensable in GSH metabolism. GSH metabolism is important for protecting cells against oxidants, which is produced during normal metabolism [11]. However, both GGT and GSH are regularly elevated at the same time in pathological states of oxidative stress, and oxidative stress may be related to oncogenic stimulation [11], [34]. In addition, an elevated GGT level plays an important role in carcinogenesis by exerting pro-oxidant effects at both the membrane surface level and the extracellular microenvironment [35]. However, the specific mechanism needs further exploration.
Previous studies have focused on D-dimer levels and GGT levels in relation to metastatic disease and survival, but have not evaluated post-operative complications. The current study is the first to include complication analyses. For patients with CRLM after resection, our study showed that pre-operative elevated D-dimer levels and elevated GGT levels were associated with major complications. This result may be helpful for clinicians in taking corresponding preventive measures. Some studies have shown an association between post-operative inflammatory markers and post-operative complications in CRLM patients after resection. McCluney et al. [36] reported that a high NLR on post-operative days 2, 3 and 4 was associated with major complications. Other studies [37], [38] have shown that a high post-operative CRP can predict the severity of complications following abdominal surgery. Compared with post-operative inflammatory markers, we advanced the prediction of complications. This could provide clinicians with information at earlier time points, in order to allow for the investigation of patients who may be more likely to develop complications before surgery. Whether the GGT levels and D-dimer levels have a particular utility in detecting a particular type of complication (for example, infective complications or anastomotic complications) is an opportunity for a future prospective study in a larger cohort of CRLM patients after resection.
More recently, elevated D-dimer levels have been found to be associated with poor prognosis in several malignant diseases, including lung cancer, ovarian cancer and gastric cancer [22], [23], [24], [25], and elevated serum GGT levels have been associated with a worsened prognosis in many cancers, including HCC, renal cell carcinoma and esophageal squamous cell carcinoma [5], [14], [15], [16]. However, there have been no studies examining the prognostic significance of serum levels of D-dimer and GGT in CRLM after hepatectomy. Our results demonstrated that, in patients with CRLM, compared with pre-operative elevated D-dimer levels and elevated GGT levels, pre-operative decreased D-dimer levels and decreased GGT levels had a better OS, which were consistent with several studies. It is very valuable to be able to predict the prognosis of CRLM patients in the clinic. Some studies have shown that pre-operative inflammatory markers were associated with prognosis in CRLM patients after resection. Giakoustidis et al. [39] reported that pre-operative elevated NLR in CRLM patients with hepatectomy increased the risk of extrahepatic multifocal recurrence and was an independent predictor of overall survival. Neofytou et al. [40] identified pre-operative LMR as an independent prognostic factor for PRS, CSS and OS, but not for DFS, in CRLM patients undergoing hepatectomy. Compared with pre-operative inflammatory markers, pre-operative D-dimer levels predict not only survival but also post-operative major complications. We found that the cut-off values of D-dimer levels for predicting survival (0.285 mg/L) and major complications (0.485 mg/L) increased incrementally. It is a possibility for clinicians to tailor CRLM treatment for individual patients before resection, according to the extent of D-dimer elevation.
In this study, we also established the CPDG score construct by D-dimer levels and GGT levels. Interestingly, our results suggested the CPDG score had a stronger prognostic value than either factor alone in the prediction of major complications and survival. It is worth noting that compared with patients with CPDG score = 1 or CPDG score = 0, patients with a CPDG score = 2 (both the D-dimer levels ≥0.285 mg/L and GGT ≥30.5 U/L) had worsened OS. Furthermore, the differences between any two groups were significant. Therefore, the CPDG score possesses both accurate and clinically meaningful prognostic value for CRLM patients after resection. Clinicians can tailor CRLM treatment for individual patients before resection. For example, patients with a CPDG score = 2 represented a high risk of morbidity and mortality. A closer surveillance and certain protective treatments should be offered to these high-risk patients after resection.
Several studies have suggested that GGT levels may be associated with tumor size, tumor number, vascular invasion and TNM stage in several malignancies [14], [15], [16], [17] and elevated D-dimer levels were demonstrated to be associated with larger tumor, lymph node metastasis, elevated CEA levels and elevated NSE levels [41], [42]. In this study, we assessed the association of D-dimer, GGT and CPDG with clinicopathological characteristics in CRLM patients. It is worth mentioning that elevated D-dimer levels and elevated GGT levels were significantly associated with pretreatment chemotherapy, liver metastases in a bilobar distribution and multiple metastases.
We have noticed that the GGT cutoff value and the D-dimer cutoff value in this study were well below the standard reference range, the reasons of which were as follows: Elevated D-dimer is associated with decompensated liver disease, deep venous thrombosis, pulmonary embolism or disseminated intravascular coagulation [26], [27]. Elevated serum GGT activity can be found in diseases of the liver, biliary system, and pancreas. It also appears to be related to specific pathologies such as metabolic syndrome, alcohol addiction and chronic liver disease [18], [19], [20]. In clinical settings, GGT is predominantly used as a diagnostic marker for liver disease. If the D-dimer value or GGT value of patients exceed the upper limit of reference range, clinicians would postulate that this patient is at a higher risk of before-mentioned diseases. That is how we define a ‘normal range’. However, the outcomes of interest in the current study are the postoperative complications and oncologic outcomes. Patients with GGT value or D-dimer value exceeding cutoffs in this study are thought to be susceptible to worse postoperative and oncologic outcomes, but it did not equal to a higher risk of before-mentioned diseases. In other words, the normal range is defined based on the risk of before-mentioned diseases, whereas the cutoff value in this study is defined based on the risk of worse postoperative and oncologic outcomes. Moreover, the GGT cutoff value and the D-dimer cutoff value largely depend on tumor types and its distribution among patients. Take the HCC for instance, it occurs in the setting of chronic liver inflammation, and is most closely linked to chronic viral hepatitis infection, and thus are frequently associated with elevated GGT value [16]. Unlike HCC, CRLM is not related to liver disease. Patients who presented with pre-existing comorbidities, known to be related with elevation of GGT, were also excluded in the study. Therefore, nearly three-quarters of the included patients are with a normal GGT value. This is also the reason for why the cutoff value is within the normal range.
Pre-operative D-dimer levels and GGT levels are simple and easily assessable clinical biomarkers for the prognostic stratification of CRLM patients after hepatectomy. However, the findings of the current study should be interpreted within its possible limitations. First, as with a typical retrospective study, our study is limited by biases, such as a lack of random assignment and patient selection. Second, peripheral blood samples were performed only once, which might cause bias. Third, the AUCs of single GGT or D-dimer (0.617 and 0.689, respectively) were relatively low, but it increased to more than 0.7 when combined both, which supported the necessity of a composite scoring system. At last, we only internally validated our findings. Examining the prognostic value of CPDG score in the validation set was not available due to a short follow-up period. Multi-institutional and large prospective studies are needed to confirm our findings and to further explore these results.
In conclusion, the pre-operative D-dimer levels and GGT levels are reliable biomarkers that help in predicting post-operative major complications or survival in CRLM patients after hepatic resection. The CPDG score, which is constructed from the preoperative D-dimer levels and GGT levels, is a more accurate prognostic predictor in CRLM patients after resections.
Ethics Approval and Consent to Participate
The research protocol was approved by the Institutional Review Board of the Cancer Hospital of Chinese Academy of Medical Sciences. Informed consent was obtained from all patients.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Funding
This research was supported by CAMS Innovation Fund for Medical Sciences (CIFMS)(Grant No.2017-12 M-3-005).
Footnotes
Fund: CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant No.2017-12M-3-005).
Contributor Information
Wei Cui, Email: wendycuiwei@sina.cn.
Jianguo Zhou, Email: zjgty@hotmail.com.
References
- 1.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, Ychou M, Rougier P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Section of Gastrointestinal Surgery B CMASoCASB, Chinese Medical Association Guideline for the diagnosis and comprehensive treatment of colorectal cancer with liver metastases (2016 edition) Chin J Dig Surg. 2016;(15):755–767. [Google Scholar]
- 4.Kopetz S, Chang GJ, Overman M.J, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucchetti A, Ferrero A, Cescon M, Donadon M, Russolillo N, Ercolani G, Stacchini G, Mazzotti F, Torzilli G, Pinna AD. Cure model survival analysis after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2015;22(6):1908–1914. doi: 10.1245/s10434-014-4234-0. [DOI] [PubMed] [Google Scholar]
- 6.Slakey DP, Simms E, Drew B, Yazdi F, Roberts B. Complications of liver resection: laparoscopic versus open procedures. JSLS. 2013;17(1):46–55. doi: 10.4293/108680812X13517013317716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang TC, Spiro C, Ramacciotti T, Choi J, Drummond M, Sweeney E, Samra JS, Hugh TJ. Complications following liver resection for colorectal metastases do not impact on longterm outcome. HPB. 2015;17(2):185–193. doi: 10.1111/hpb.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tranchart H, Gaillard M, Chirica M, Ferretti S, Perlemuter G, Naveau S, Dagher I. Multivariate analysis of risk factors for postoperative complications after laparoscopic liver resection. Surg Endosc. 2015;29(9):2538–2544. doi: 10.1007/s00464-014-3965-0. [DOI] [PubMed] [Google Scholar]
- 9.D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18(4):1096–1103. doi: 10.1245/s10434-010-1409-1. [DOI] [PubMed] [Google Scholar]
- 10.Orlowski M, Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970;67(3):1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38(4):263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 12.Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006;71(3):231–238. doi: 10.1016/j.bcp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Corti A, Franzini M, Paolicchi A, Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30(4):1169–1181. [PubMed] [Google Scholar]
- 14.Grimm C, Hofstetter G, Aust S, Mutz-Dehbalaie I, Bruch M, Heinze G, Rahhal-Schupp J. Association of gamma-glutamyltransferase with severity of disease at diagnosis and prognosis of ovarian cancer. Br J Cancer. 2013;109(3):610–614. doi: 10.1038/bjc.2013.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofbauer SL, Stangl KI, de Martino M, Lucca I, Haitel A, Shariat SF, Klatte T. Pretherapeutic gamma-glutamyltransferase is an independent prognostic factor for patients with renal cell carcinoma. Br J Cancer. 2014;111(8):1526–1531. doi: 10.1038/bjc.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma H, Zhang L, Tang B, Wang Y, Chen R, Zhang B, Chen Y, Ge N, Wang Y, Gan Y. gamma-Glutamyltranspeptidase is a prognostic marker of survival and recurrence in radiofrequency-ablation treatment of hepatocellular carcinoma. Ann Surg Oncol. 2014;21(9):3084–3089. doi: 10.1245/s10434-014-3724-4. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Zhang S, Yang H, Luo K, Wen J, Hu Y, Hu R, Huang Q, Chen J, Fu J. Prognostic significance of gamma-glutamyltransferase in patients with resectable esophageal squamous cell carcinoma. Diseases of the esophagus : official journal of the International Society for. Dis Esophagus. 2015;28(5):496–504. doi: 10.1111/dote.12227. [DOI] [PubMed] [Google Scholar]
- 18.Dixon JB, Bhathal PS, O'Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006;16(10):1278–1286. doi: 10.1381/096089206778663805. [DOI] [PubMed] [Google Scholar]
- 19.Lu XJ, Li XH, Yuan ZX, Sun HY, Wang XC, Qi X, Zhang X, Sun B. Assessment of liver fibrosis with the gamma-glutamyl transpeptidase to platelet ratio: a multicentre validation in patients with HBV infection. Gut. 2018;67(10):1903–1904. doi: 10.1136/gutjnl-2017-315299. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Cai B, Su Z, Li Y, Xu J, Deng R, Wang L. Aspartate transaminase to platelet ratio index and gamma-glutamyl transpeptidase-to-platelet ratio outweigh fibrosis index based on four factors and red cell distribution width-platelet ratio in diagnosing liver fibrosis and inflammation in chronic hepatitis B. J Clin Lab Anal. 2018;32(4):e22341. doi: 10.1002/jcla.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158–1164. doi: 10.3324/haematol.2011.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadducci A, Cosio S, Tana R, Genazzani AR. Serum and tissue biomarkers as predictive and prognostic variables in epithelial ovarian cancer. Crit Rev Oncol Hematol. 2009;69(1):12–27. doi: 10.1016/j.critrevonc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Tas F, Kilic L, Bilgin E, Keskin S, Sen F, Ciftci R, Yildiz I, Yasasever V. Clinical and prognostic significance of coagulation assays in advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23(2):276–281. doi: 10.1097/IGC.0b013e31827b8796. [DOI] [PubMed] [Google Scholar]
- 24.Inal T, Anar C, Polat G, Unsal I, Halilcolar H. The prognostic value of D-dimer in lung cancer. Clin Respir J. 2015;9(3):305–313. doi: 10.1111/crj.12144. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Yu H, Wu C, Li J, Jiao S, Hu Y, Tao H, Wu B, Li A. Prognostic value of plasma D-dimer levels in patients with small-cell lung cancer. Biomed Pharmacother. 2016;81:210–217. doi: 10.1016/j.biopha.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Hao J, Yang N. Protein C and D-dimer are related to portal vein thrombosis in patients with liver cirrhosis. J Gastroenterol Hepatol. 2010;25(1):116–121. doi: 10.1111/j.1440-1746.2009.05921.x. [DOI] [PubMed] [Google Scholar]
- 27.Saray A, Mesihovic R, Gornjakovic S, Vanis N, Mehmedovic A, Nahodovic K, Glavas S, Papovic V. Association between high D-dimer plasma levels and ascites in patients with liver cirrhosis. Med Arch. 2012;66(6):372–374. doi: 10.5455/medarh.2012.66.372-374. [DOI] [PubMed] [Google Scholar]
- 28.Chen QC, Zhou JG. Advances in research of primary hapatic neuroendocrine tumor. Electron J Liver Tumor. 2018;5(3):22–25. [Google Scholar]
- 29.Kanat O. Current treatment options for patients with initially unresectable isolated colorectal liver metastases. World J Clin Oncol. 2016;7(1):9–14. doi: 10.5306/wjco.v7.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passot G, Soubrane O, Giuliante F, Zimmitti G, Goere D, Yamashita S, Vauthey JN. Recent Advances in Chemotherapy and Surgery for Colorectal Liver Metastases. Liver Cancer. 2016;6(1):72–79. doi: 10.1159/000449349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Dupuy E, Hainaud P, Villemain A, Bodevin-Phedre E, Brouland JP, Briand P, Tobelem G. Tumoral angiogenesis and tissue factor expression during hepatocellular carcinoma progression in a transgenic mouse model. J Hepatol. 2003;38(6):793–802. doi: 10.1016/s0168-8278(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 33.Buller HR, van Doormaal FF, van Sluis GL, Kamphuisen PW. Cancer and thrombosis: from molecular mechanisms to clinical presentations. J Thromb Haemost. 2007;5(Suppl. 1):246–254. doi: 10.1111/j.1538-7836.2007.02497.x. [DOI] [PubMed] [Google Scholar]
- 34.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Dominici S, Pieri L, Comporti M, Pompella A. Possible role of membrane gamma-glutamyltransferase activity in the facilitation of transferrin-dependent and -independent iron uptake by cancer cells. Cancer Cell Int. 2003;3(1):7. doi: 10.1186/1475-2867-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCluney SJ, Giakoustidis A, Segler A, Bissel J, Valente R, Hutchins RR, Abraham AT, Bhattacharya S, Kocher HM. Neutrophil: Lymphocyte ratio as a method of predicting complications following hepatic resection for colorectal liver metastasis. J Surg Oncol. 2018;117(5):1058–1065. doi: 10.1002/jso.24996. [DOI] [PubMed] [Google Scholar]
- 37.McSorley ST, Ramanathan ML, Horgan PG, McMillan DC. Postoperative C-reactive protein measurement predicts the severity of complications following surgery for colorectal cancer. Int J Colorectal Dis. 2015;30(7):913–917. doi: 10.1007/s00384-015-2229-3. [DOI] [PubMed] [Google Scholar]
- 38.Straatman J, Harmsen AM, Cuesta MA, Berkhof J, Jansma EP, van der Peet DL. Predictive Value of C-Reactive Protein for Major Complications after Major Abdominal Surgery: A Systematic Review and Pooled-Analysis. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giakoustidis A, Neofytou K, Khan AZ, Mudan S. Neutrophil to lymphocyte ratio predicts pattern of recurrence in patients undergoing liver resection for colorectal liver metastasis and thus the overall survival. J Surg Oncol. 2015;111(4):445–450. doi: 10.1002/jso.23845. [DOI] [PubMed] [Google Scholar]
- 40.Neofytou K, Smyth EC, Giakoustidis A, Khan AZ, Williams R, Cunningham D, Mudan S. The Preoperative Lymphocyte-to-Monocyte Ratio is Prognostic of Clinical Outcomes for Patients with Liver-Only Colorectal Metastases in the Neoadjuvant Setting. Ann Surg Oncol. 2015;22(13):4353–4362. doi: 10.1245/s10434-015-4481-8. [DOI] [PubMed] [Google Scholar]
- 41.Kilic L, Yildiz I, Sen FK, Erdem MG, Serilmez M, Keskin S, Ciftci R, Karabulut S, Ordu C, Duranyildiz D. D-dimer and international normalized ratio (INR) are correlated with tumor markers and disease stage in colorectal cancer patients. Cancer Biomark. 2015;15(4):405–411. doi: 10.3233/CBM-150477. [DOI] [PubMed] [Google Scholar]
- 42.Jiang X, Mei X, Wu H, Chen X. D-dimer level is related to the prognosis of patients with small cell lung cancer. Ann Transl Med. 2017;5(20):394. doi: 10.21037/atm.2017.07.35. [DOI] [PMC free article] [PubMed] [Google Scholar]