SUMMARY
Helping B cells and antibody responses is a major function of CD4+ T cells. It has been 10 years since the publication of Bcl6 as the lineage defining transcription factor for T follicular helper (Tfh) differentiation and the requirement of Tfh cells as the specialized subset of CD4+ T cells needed for germinal centers (the microanatomical sites of B cell mutation and antibody affinity maturation) and related B cell responses. A great deal has been learned about Tfh cells in the past 10 years, particularly regarding their roles in a surprising range of diseases. Advances in the understanding of Tfh cells differentiation and function are discussed, as are the understanding of Tfh cells in infectious diseases, vaccines, autoimmune diseases, allergies, atherosclerosis, organ transplants, and cancer. This includes discussion of Tfh cells in the human immune system. Based on the discoveries to date, the next decade of Tfh research surely holds many more surprises.
eTOC blurb
Tfh cells are the CD4+ T cells specialized for helping B cells. Bcl6 was identified as the Tfh lineage defining transcription factor 10 years ago. Crotty reviews the extensive progress made in understanding Tfh cells since then, including surprising roles of Tfh cells in a range of diseases.
Introduction
While T cell help to B cells was one of the very first defined functions of T cells (Crotty, 2015), the identification of the CD4+ T cell subset responsible for T cell-dependent humoral immune responses developed much later. The term T follicular helper (Tfh) cell, was coined in a series of studies on human tonsillar germinal center (GC) CD4+ T cells and CXCR5+ CD4+ T cells in blood (Crotty, 2011). Early converts rightly point to those papers as seminal for establishing the field, but it was not until Bcl6 was determined to be a lineage defining transcription factor (TF) of Tfh cells that Tfh cells were broadly accepted among immunologists as a distinct lineage of helper CD4+ T cells and required for GCs. That trifecta of papers was published 10 years ago (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009), including the demonstration that the TFs Blimp1 and Bcl6 are potent reciprocal antagonists (Johnston et al., 2009) (Figure 1). Now is a good time to review what has been accomplished over the past decade of Tfh research. By any metric, the field of Tfh research has exploded in that time. Not all of the discoveries can be covered here. This review will highlight key findings in major areas of research that provide insights into what Tfh cells do, how they do it, and how proper and improper Tfh biology impacts diseases.
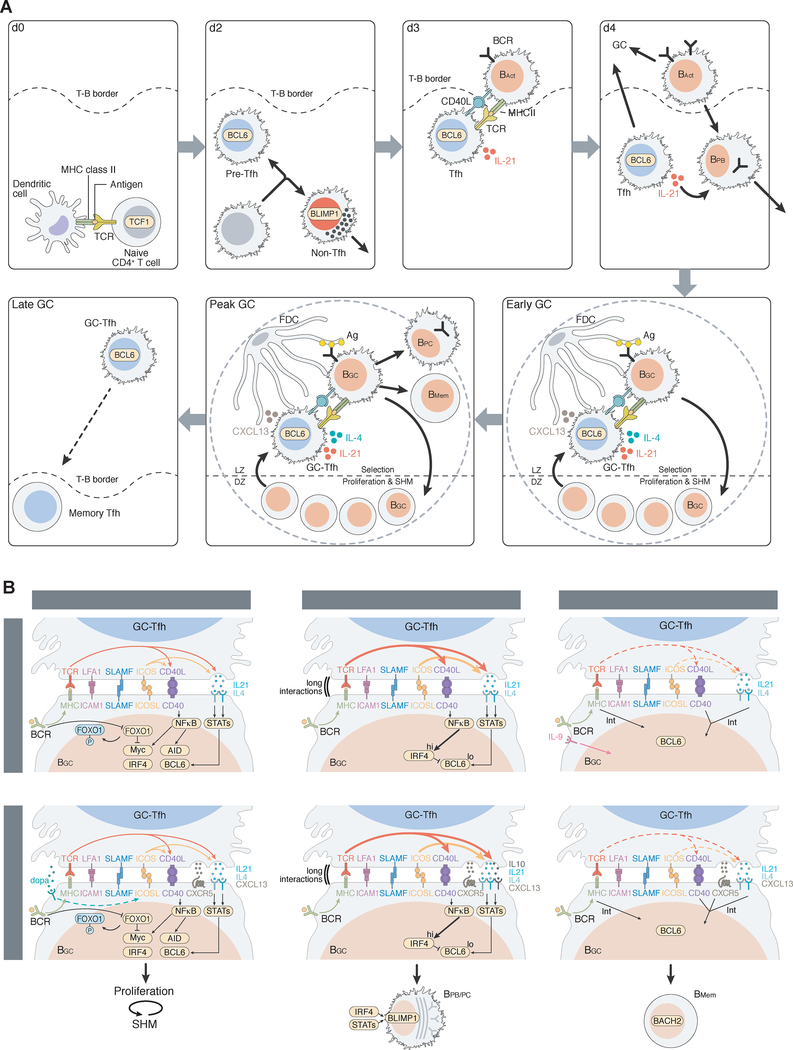
Figure 1. Features of Tfh cells.
Tfh cell differentiation is induced by DCs in the presence of antigen and signals from the microenvironment. Tfh cell versus non-Tfh cell differentiation bifurcates early, based on the balance of inductive and inhibitory signals. Some intermediate cell fates are also possible (grey). Early Tfh cells then subsequently differentiate to GC-Tfh cells.
Molecular and cellular biology of Tfh cells
Tfh differentiation usually starts with interaction of a naive CD4+ T cell with a myeloid professional antigen-presenting cell (APC) such as a dendritic cell (DC). Tfh differentiation can largely be modelled as an early cell fate decision between becoming a Tfh cell and a non-Tfh effector cell, e.g. Th1, Th2, or Th17 cell. The Tfh versus non-Tfh cell fate decision is predominantly made within the first two cell divisions (Choi et al., 2011; 2013; DiToro et al., 2018). Tfh cells stay resident in lymph nodes (LNs) and spleen because their purpose is to help B cells, while non-Tfh effector cells such as Th1, Th2, Th17, cytotoxic CD4 T cells, or Th9 cells are destined to predominantly leave lymphoid tissue and traffic to sites of infection or inflammation, as actions at those sites are the primary purposes of most non-Tfh cells. Interleukin-6 (IL-6) and the costimulatory molecule ICOSL are early factors involved in Tfh differentiation, in mice (Crotty, 2014; Vinuesa et al., 2016). IL-2 is the most potent inhibitor of Tfh differentiation, and IL2R signaling can be dose limiting for Tfh differentiation (Ballesteros-Tato et al., 2012; DiToro et al., 2018; Johnston et al., 2012). This is due to IL-2 induction of Blimp-1 expression and signal transducer and activator of transcription-5 (STAT5) activity (Johnston et al., 2012) and is associated with metabolic differences between Tfh and non-Tfh cells (Ray et al., 2015). T cell receptor (TCR) signal strength plays an important role in Tfh differentiation (DiToro et al., 2018; Fazilleau et al., 2009; Tubo et al., 2013), but the relationship appears to be non-linear (Tubo et al., 2013) and likely depends on which alternative differentiation pathway is available (e.g. Th1 or Th2), the cytokine environment, and the strength of available costimulatory receptor signaling (Figure 1).
Tfh differentiation can be primed by several classes of conventional DCs (Kato et al., 2015; Krishnaswamy et al., 2017) (Figure 2, 3). Murine monocytes can affect Tfh differentiation via cytokine production (IL-6) but not as APCs (Chakarov and Fazilleau, 2014). The diversity of DCs capable of priming Tfh differentiation likely reflects the fact that antibody (Ab) responses are of value for protection against almost all viral, bacterial, fungal, and parasite pathogens (Figure 4), and are of value at all body surfaces and in most body organs.
Figure 2. Signals to and from Tfh cells.
Tfh cells receive signals from a number of cell types that can regulate Tfh differentiation and/or function. Tfh cells function by providing help to B cells at a series of stages of antigen-specific B cell differentiation. BAct, activated B cells. BGC, GC B cells. BPC, PC. Bpb, plasmablasts. BMem, memory B cells.
Figure 3. Tfh and B cell response kinetics and interactions.
A. A time series of Tfh differentiation and function. Details of the steps are summarized in the main text. For simplicity, only one TF is indicated in each cell. TCF1 expression is retained in Tfh cells and not non-Tfh effector cells. An animated versions is included. B. Tfh functions and signals that regulate BGC cycling, BGC➔BPC differentiation, and BGC➔BMem differentiation. “Int” = intermediate.
Figure 4. Relationships of Tfh cells to diseases.
Over the past decade Tfh cells have been associated with a wide range of diseases, with the Tfh cells contributing protective or pathogenic roles in different contexts, discussed in the main text.
DC priming is generally required for Tfh differentiation but is not sufficient. Tfh differentiation is a multistage, multifactorial process. Normal Tfh cell differentiation usually requires two APCs: DCs and B cells (Crotty, 2014). DCs are generally required for early Tfh differentiation, while B cells are required for later events and full GC-Tfh maturation (Figure 3A, Movie). Similar signals are provided by both DCs and B cells (Figure 2), but B cells are usually incapable of priming CD4+ T cell responses because of the rarity of antigen-specific (and thus antigen-presenting) B cells early in an immune response, in contrast to DCs. Additionally, DCs predominantly localize to T cell zones and thus have the greatest opportunity to prime CD4+ T cells. Nevertheless, B cells can prime Tfh cell responses under certain conditions (Hong et al., 2018). B cells are excellent producers of a wide range of cytokines, including IL-6 which can facilitate murine Tfh cell differentiation (Karnowski et al., 2012).
B cells are outstanding APCs for Tfh cells downstream of the initiating event for Tfh differentiation. Antigen-specific B cells undergo exponential expansion and thus become more abundant APCs; activated B cells also upregulate major histocompatibility complex class II proteins (MHCII) and costimulatory molecules, such as CD80 and CD86, which make the activated B cells more potent APCs for Tfh cells. Additionally, activated B cells migrate to the T cell:B cell (T-B) border to enhance their likelihood of interaction with Tfh cells (Figure 3A).
Upon priming by DCs, CD4+ T cells receiving Tfh cell-inductive signals upregulate Bcl6 (Choi et al., 2011; DiToro et al., 2018). This facilitates expression of the chemokine receptor CXCR5 and repression of CCR7 among other molecules, allowing for migration to the T-B border. These differentiating Tfh cells migrate and interact with B cells in an ICOS:ICOSL and peptide:MHC-dependent manner. The additional signals from cognate B cells drive further GC-Tfh differentiation and the formation of GCs. The migration of Tfh cell into GCs is facilitated by repression of the migration-associated receptor PSGL1 and the chemoattractant receptor Ebi2, and changes in the expression of S1P receptors, SLAM family receptors, and integrins (Meli et al., 2016; Vinuesa et al., 2016). ICOS is particularly fascinating, as it functions as both a costimulatory molecule and a migration receptor for Tfh cells (Pedros et al., 2016; Xu et al., 2013). Signaling through ICOS is complex (Leavenworth et al., 2015; Weber et al., 2015) and is a major factor for Tfh differentiation in many contexts, including an elegant study elucidating transformational progression of Tfh cells to angioimmunoblastic T cell lymphoma (AITL) (Cortes et al., 2018).
The TF Bcl6 is essential for Tfh differentiation, and constitutive ectopic expression of Bcl6 enhances Tfh differentiation (Hollister et al., 2013; Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009). Bcl6 acts on multiple aspects of Tfh differentiation and function (Hatzi et al., 2015). Most available data indicate that Bcl6 is an obligate repressor of gene expression (Béguelin et al., 2016; Hatzi et al., 2015; 2013). Multiple TFs in addition to Bcl6 play important roles in Tfh differentiation (Vinuesa et al., 2016). TCF1 and LEF1 are involved in induction of Bcl6, as are STAT3 and STAT1. Inhibition of Prdm1 (Blimp1) is required for Tfh differentiation and is accomplished by Bcl6 (Figure 1) in coordination with TCF1 and additional factors (Choi et al., 2015; Wu et al., 2015; Xu et al., 2015). CXCR5 expression, as one example Tfh cell gene, is dependent on E protein TFs such as E2A and Ascl2. E protein activity is inhibited by Id2, and Id2 is inhibited by Bcl6 (Shaw et al., 2016). E2A is present in resting and early-activated CD4+ T cells, with Ascl2 becoming highly expressed in GC-Tfh cells (Liu et al., 2014; Shaw et al., 2016). More encyclopedic reviews of TFs involved in Tfh differentiation are available (Crotty, 2011; Vinuesa et al., 2016). While major advances have been made in our understanding of the transcriptional and epigenetic regulation of Tfh cell differentiation and function, much remains to be learned.
Most GC-Tfh cells are quite similar in their gene expression profile, indicating that ‘vanilla’ Tfh cells predominate in vivo (Crotty, 2018); nevertheless, substantial phenotypic and functional heterogeneity exists among Tfh cells in disease states, which may be important causes or effects of those diseases (Crotty, 2014) (see below). It was clear from early on that Tfh cells can express molecules associated with other CD4+ T cell lineages, such as the TF Tbet and IFNγ (Johnston et al., 2009); however, transcription of those genes is normally potently repressed by Bcl6 in fully differentiated GC-Tfh cells (Crotty, 2014; Hatzi et al., 2015). Additionally, it is unclear what productive biological function those molecules have in GC-Tfh cells, if any (see Infectious Diseases section below).
Human and mouse GC-Tfh cells are similar in surface protein marker phenotype, gene expression profile, and BCL6 expression, indicating robust evolutionary conservation of many aspects of Tfh cell biology; however, cytokines involved in induction of Tfh differentiation appear to be substantially different between the two species. IL-6 is a potent inducer of murine Tfh cell differentiation, and is the most potent inducer of IL-21 expression by newly activated naive murine CD4+ T cells (Crotty, 2014); however, the role of IL-6 in human Tfh differentiation is far less clear. IL-6 has little impact on IL-21 induction in human CD4+ T cells in vitro. IL-12 is the most potent human cytokine at inducing IL-21-expressing CD4+ T cells, and IL-12 signals through different STATs than IL-6 (Schmitt et al., 2014). Curiously, there remains no reproducible in vitro system for murine Tfh cell differentiation. In contrast, in vitro human Tfh cell differentiation can be accomplished via coculture with TGFβ + IL-12, or the cytokines activin A + IL-12, with essentially no impact of IL-6 under equivalent conditions (Locci et al., 2016; Schmitt et al., 2014). TGFβ + IL-12 or activin A + IL-12 do not differentiate murine Tfh cells in vitro (Locci et al., 2016). Human in vitro Tfh cell differentiation is most optimal when IL-2 is blocked (Locci et al., 2016), consistent with IL-2’s potent inhibitory activity repressing murine Tfh differentiation. While substantial differences between human and murine Tfh cells are apparent, conclusions about the magnitude of the differences are clouded by the fact that there are multiple pathways to Tfh cell differentiation. In mice, IL-27 can largely substitute for IL-6 (when expressed) (Harker et al., 2013; Vijayan et al., 2016) and the IL-12 family member IL-23 can largely substitute for IL-12 to induce IL-21 and human Tfh cell differentiation in vitro (Schmitt et al., 2014). This is consistent with the concept that in vivo pathogen detection mechanisms need to induce Tfh cell differentiation to diverse pathogens.
Human genetics has provided valuable insights into Tfh cell biology. While robust conclusions are frequently limited by the rarity of donors, there is substantial evidence of Tfh cell-intrinsic defects in a number of human primary immunodeficiencies, including ICOSL, ICOS, SH2D1A, STAT3, IL21, IL21R, IL12RB1, and PI3KD (Ma et al., 2015; Preite et al., 2018).
Interactions Between Tfh and B cells
Tfh cells depend on B cells in most contexts, and GC B (BGC) and plasma cells (PCs) depend on Tfh cells. These feedback loops are central aspects of proper regulation of humoral immunity. Early in Tfh cell differentiation, the Tfh cells migrate to the T-B border and interact with antigen-specific B cells (Figure 3A). Most B cell responses cannot progress beyond this point without Tfh cell help; the B cells most likely die within 24h of antigen recognition in the absence of T cell help (Akkaya et al., 2018). Tfh cells provide IL-21 and CD40L signals required for B cell proliferation and differentiation towards both GC and extrafollicular fates (Lee et al., 2011) (Figure 3A). IL-4 expression by Tfh cells is only acquired later, upon full differentiation to GC-Tfh cells (Weinstein et al., 2016; Yusuf et al., 2010). B cells compete for Tfh help at the T-B border based on the amount of p:MHCII presented by the B cell to the Tfh cell (Schwickert et al., 2011; Yeh et al., 2018). The success of B cells competing for early Tfh help depends on the frequency of the B cells and their antigen affinity (Abbott et al., 2018; Schwickert et al., 2011). Rare B cells with low affinity may be excluded from immune responses at this early Tfh cell checkpoint, which has implications for vaccines because neutralizing epitope-specific B cells against some pathogens may be quite rare and low affinity (Havenar-Daughton et al., 2017).
GCs are the microanatomical sites of B cell mutation and antibody affinity maturation. GC responses are evolution in miniature. All evolutionary processes require generations (GC cell division), mutation (SHM), and limiting resources that drive evolutionary selection. Tfh help is a key limiting resource in GCs. BGC cells compete for GC-Tfh help. GCs consistent of a light zone (LZ) containing GC-Tfh cells, follicular dendritic cells (FDCs), and Bgc cells, and a dark zone (DZ) of proliferating BGC cells (Figure 3A). Selection occurs in the LZ. FDCs organize the microanatomic structure of GCs and retain and provide antigens to the BGC cells (Heesters et al., 2013; 2016). Deletion of FDCs rapidly impairs GCs (Wang et al., 2011). BGC cells obtain intact antigen from the surface of FDCs by B cell receptor (BCR)-mediated endocytosis and process the antigen into peptides for surface presentation in p:MHCII complexes. GC-Tfh cells preferentially provide help to BGC cells with higher expression of p:MHCII, which serves as an indirect measurement of higher BCR antigen affinity. BGC cells that do not receive GC-Tfh help die. Bgc cells helped by GC-Tfh cells then migrate to the DZ and undergo rounds of division and division-linked SHM commensurate with the magnitude of the GC-Tfh cell help each BGC cell receives (Ersching et al., 2017; Gitlin et al., 2014) (Figure 3A). One model is that GC-Tfh cell help is the only BGC cell selecting event in GCs. If that is the case, changes in MHCII expression by B cells should alter GC selection. However, B cells with MHCII haploinsufficiency compete in GCs equivalently to WT cells under conditions of physiological antigen concentrations (Yeh et al., 2018), indicating that B cell competition for Tfh cell help predominantly occurs at the T-B border, not in the GCs. Bgc cells with alterations in MHCII degradation also have similar GC competitive fitness (Bannard et al., 2016). Thus, considering experimental systems avoiding supraphysiological stimuli, both BCR signaling and Tfh cell help signals appear to be integrated in BGC cells to determine survival and proliferation in a GC (Bannard et al., 2016; Kräutler et al., 2017; Yeh et al., 2018). That model is further supported by findings that BGC cells have rewired BCR and CD40 signaling. Naive B cells can induce Myc TF expression via CD40 or BCR signaling due to cross-regulation of NFκB and PI3K pathways; however, in BGC cells those signaling pathways become siloed, such that CD40L:CD40 engagement triggers NFκB and BCR antigen signaling engages PI3K signaling (Luo et al., 2018) (Fig 3B). In part this is due to synaptic differences in BGC cells, which provides more stringent affinity discrimination by Bgc cell BCR engagement (Nowosad et al., 2016).
The help provided by GC-Tfh cells to BGC cells classically consists of IL-21, IL-4, CD40L, and CXCL13, in addition to production of more widely expressed cytokines such as IL-2 and TNF. The combination of IL-21 and IL-4 expression by GC-Tfh cells maximally supports BGC cells (Weinstein et al., 2016), in addition to CD40L. While GC-Tfh cells express substantial amounts of IL-21 and IL-4 RNA, GC-Tfh cells secrete infinitesimal quanta of IL-21 and IL-4 protein, consistent with GC-Tfh cells constraining GCs as a resource limited environment (Dan et al., 2016; Havenar-Daughton et al., 2016c). GC-Tfh cells may also directly kill BGC cells via FasL-Fas (Butt et al., 2015); however, GC-Tfh cells express little FasL (Bentebibel et al., 2011) and a role of GC-Tfh FasL has not been directly demonstrated in GC selection.
One substantial difference in Tfh cell help between mice and humans is CXCL13 expression (Figure 3B), discussed later. A second substantial difference is the surprising expression of the neurotransmitter dopamine by human GC-Tfh cells (Papa et al., 2017). Dopamine secretion by human GC-Tfh cells enhances rapid ICOSL surface expression by human BGC cells, resulting in a feedforward help loop between the interacting GC-Tfh and BGC cells (Figure 3B). No equivalent dopamine-related process is known in mice (Papa et al., 2017). IL-10 also appears to have species-specific functions. IL-10 is made by some human GC-Tfh cells, and IL-10 has long been known as a PC differentiation factor for human B cells (Bryant et al., 2007). It is unclear if this biology is conserved in mice. IL-10 is almost exclusively an immunosuppressive cytokine in mice, but there are at least certain cases of IL-10+ murine Tfh cells providing help to B cells (Xin et al., 2018).
GC-Tfh cells not only control selection of high affinity BGC cells, GC-Tfh cells also are critical regulators of the development of long-term humoral immunity via BGC cell differentiation to memory B cells and PCs (Figure 3). Early GC-derived plasmablasts (PBs, differentiating PCs still expressing Ki67) are induced by IL-21 from GC-Tfh cells, in synergy with IL-6 provided by stromal cells (Zhang et al., 2018). GC-Tfh cell induction of long-lived PCs requires IL-21. Differentiation of PCs from BGC cells is a multi-step transcriptional reprogramming process requiring reduction of Bcl6, increase of the TF Irf4, and subsequent expression of Blimp1 (Ise et al., 2018; Kallies et al., 2007). Most GC-Tfh cell interactions with BGC cells are brief (minutes) (Liu et al., 2015a; Shulman et al., 2014). Regulation of CD40L availability to BGC is sophisticated in Tfh cells. Strong CD40L signaling from GC-Tfh cells to Bgc cells can transiently induce enhanced SLAM (CD150), ICAM-1, and CD40 expression by a LZ BGC cell (Ise et al., 2018) (Figure 3B). Those BGC cell surface changes can cause extended GC-Tfh:BGC cell synaptic engagement, which can result in elevated Irf4 and reduced Bcl6 expression. This may be facilitated by LFA-1 adhesion enhancing GC-Tfh gene expression (Meli et al., 2016), or feedforward T:B engagement by ICOS enhancing GC-Tfh cell surface CD40L (Liu et al., 2015a). The process may also involve direct BCR signaling (Kräutler et al., 2017). GC-Tfh cell IL-21 is then likely required to induce Blimp1 expression in Irf4hiBcl6lo early PCs to complete cell fate commitment (Ise et al., 2018; Kräutler et al., 2017) (Figure 3B). Selection of memory B cells appears to involve converse BGC cell characteristics. A fraction of LZ BGC cells with lower affinity are prone to progress to resting memory B cells in a Bach2 TF-dependent manner, associated with CCR6 expression (Shinnakasu et al., 2016; Suan et al., 2017; Wang et al., 2017). GC➔memory B cell differentiation appears to occur when antigen-specific BGC cells receive moderate help signals from GC-Tfh cells (Figure 3B), which are stochastically more likely to occur to Bgc cells with low or intermediate affinity in a GC population (Shinnakasu et al., 2016; Suan et al., 2017). While impressive advances have been made in understanding how GC-Tfh cells control GCs—even when focusing on work in the past two years—much remains to be learned, in part due to the sophisticated reciprocal signaling between the cells and the inherent challenges of studying the molecular biology of rapid and repetitive interactions of GC-Tfh and BGC cells in vivo. While current mechanistic models are appealing, and are based on sophisticated experimental approaches, it is unclear if they are general principles or if they selectively apply to particular types of antigens and adjuvants. For example, while most experimental systems have been chosen in the past based on their ease of use and robust GC and Ab responses, the outcome of an infection or immunization can change dramatically depending on the precursor frequency and affinity of the antigen-specific B cells (Abbott et al., 2018; Tarlinton, 2019).
The study of memory recall responses have more limited data, but data suffice to conclude that memory Tfh cells and memory B cells rapidly interact upon re-exposure to antigen (Ise et al., 2014; Suan et al., 2015). IL-9 appears to have a role in memory B cell recall, but there are conflicting accounts (Takatsuka et al., 2018; Wang et al., 2017).
There exist Tfh cell functions outside the GC. Some of these functions are to help B cells or memory B cells at the T-B border early in an immune response. However, those same functions may occur at other times, and GC-Tfh cells can migrate in and out of GCs (Shulman et al., 2013). Tfh cells outside of GCs express PSGL1 and may have heterogenous phenotypes (Bentebibel et al., 2011; Kim et al., 2018b; Suan et al., 2015). In addition to Tfh cells at the T-B border, there can also be Tfh cells that downregulate CXCR5 and become extrafollicular Tfh cells, distant from the follicle or T-B border. In such cases one can distinguish fTfh (follicle, T-B border, and interfollicular zone Tfh cells) and eTfh cells (extrafollicular), if histological data support the conclusion. This nomenclature highlights the ontological relatedness of the cells (both depend on Bcl6 and express IL-21). A detailed gene expression profiling and histological examination of eTfh cells is lacking and would provide additional insights into their relationship to GC-Tfh cells and Tfh-like cells found outside of lymphoid tissues. Memory Tfh cells are an additional population of Tfh cells. Memory Tfh cells appear to be derived from GC-Tfh cells or mTfh cells (Crotty, 2014; Tubo et al., 2016) and constitute a major fraction of human memory CD4+ T cells.
Tfh Cells in Infectious Diseases
The primary function of Tfh cells is to provide protection from infectious diseases. Tfh cells facilitate Ab responses to viral, bacterial, parasite, and fungal infections (Figure 4). As one example, the IgG response to vaccinia virus infection is reduced 98% in the absence of Tfh cells (Xiao et al., 2014). The importance of Tfh cells for Ab responses to infections has been demonstrated in mice, non-human primates (NHPs), and humans (Crotty, 2014). Tfh cells are required for GC responses to infections, as well as at least some pre-GC extrafollicular Ab responses (Lee et al., 2011). Tfh cells have been shown to be important for control of chronic lymphocytic choriomeningitis (LCMV) infection via support of GCs and the slow development of neutralizing Abs (nAbs) over time (Greczmiel et al., 2017; Harker et al., 2011). Similar findings on the importance of Tfh cells for protective Abs have been made for chronic malarial infections (Butler et al., 2012; Ryg-Cornejo et al., 2016).
Antigen-specific GC-Tfh cells are difficult to study because they are exclusively located in lymphoid tissue and they secrete small quantities of cytokines (Dan et al., 2016; Havenar-Daughton et al., 2016c). The highly constrained cytokine secretion may be a direct effect of Bcl6 expression (Hatzi et al., 2015) and is consistent with the primary function of GC-Tfh cells, which is to preferentially help BGC cells during cognate interactions. Extensive secretion of cytokines throughout a GC would likely be counterproductive to the competitive evolution of Bgc cell composition. As a result of the challenge of identifying antigen-specific GC-Tfh cells, most studies have focused on bulk GC-Tfh cells. Antigen-specific GC-Tfh cells have been studied in HIV, SIV, and Streptococcus pyogenes infections. S. pyogenes is the cause of recurrent tonsillitis (RT), a classic childhood disease. It was a longstanding mystery as to why some children are susceptible to RT. Predisposition to RT disease is associated with particular HLA class II alleles and significantly fewer GC-Tfh cells (Dan et al., 2019). An aberrant GC-Tfh cell population is present in RT that expresses granzyme B, and the granzyme B+ GC-Tfh cells are capable of killing B cells (Dan et al., 2019). S. pyogenes targeting of human GC-Tfh cells is an unexpected immune evasion strategy.
Cytotoxic gene expression and Tfh cell-associated gene expression were thought to be exclusive to different differentiation pathways. However, CXCR5+ CD8+ T cells (‘follicular CD8+ T cells’) co-opt Tfh cell-associated gene expression, including Bcl6, in a TCF1-dependent manner, while maintaining some cytotoxic CD8+ T cell functionality (Im et al., 2016; Leong et al., 2016). TCF1 is a key regulator of Tfh differentiation. Id2 inhibits CXCR5+ CD8 T cell development (He et al., 2016b; Leong et al., 2016), and Id2 inhibits Tfh differentiation (Shaw et al., 2016). The CXCR5+ follicular CD8+ T cells may be critical for controlling pathogens located in B cell follicles or infecting Tfh cells, such as EBV and HIV.
HIV- and SIV-specific GC-Tfh cells have been studied both because HIV can infect Tfh cells (Banga et al., 2016) and because Tfh cells are positively associated with broad HIV nAb responses (Locci et al., 2013; Moody et al., 2016). Env-specific GC-Tfh cells develop in SIV infection and are associated with Env-specific IgG responses (Yamamoto et al., 2015). A substantial fraction of GC-Tfh cells in SIV-infected monkeys are CXCR3+ (Velu et al., 2016). CXCR3 expression is primarily regulated by Tbet and is a signature gene of Th1 cells. Many GC-Tfh cells in SIV+ animals are Tbet+, and IFNγ+ by histology, and thus the cells co-express Tfh and Th1 cell-associated gene expression programs and can be considered GC-Tfh1 cells (Velu et al., 2016). The consequences of Tbet+ IFNγ+ GC-Tfh cells is unclear. While IFNγ regulates IgG2 class switch recombination (CSR) in mice, IFNγ is not an important regulator of class switch in humans (Crotty, 2014), and thus the mouse model may be largely uninformative about Tbet biology in human Tfh cells. Tbet does not appear to block human Tfh differentiation (Schmitt et al., 2016), and the impact of Tbet and IFNγ on Tfh cell help to B cells appears to vary depending on the in vitro culture conditions (Morita et al., 2011; Schmitt et al., 2016; Velu et al., 2016). CXCR3 expression by Tfh cells may drive localization away from GCs and towards the T-B border, where CXCR3 ligands are common during inflammatory conditions. Such a location may bias B cell responses towards non-GC destinies, such as PCs. CXCR3+ Tfh have been found to be poor helpers of B cells in a mouse malarial model, and the impairment was reversed by blocking IFNγ, resulting in improved Tfh cell localization, GCs, and Abs (Ryg-Cornejo et al., 2016). The inhibitory receptor PD1 inhibits expression of CXCR3 by Tfh cells, which is important for proper localization to follicles (Shi et al., 2018). Much remains to be learned about the biology of antigen-specific GC-Tfh cells to pathogens, particularly in humans.
T follicular regulatory cells (Tfr. Foxp3+Bcl6+CXCR5+CD4+) are a subset of natural Treg (nTreg) cells that express Tfh cell-associated genes (Sage and Sharpe, 2016). Absence of Foxp3+ Tfr cells results in a surprisingly mild impact on GC and Ab responses to infections. Anti-influenza Abs and BGC cells are essentially identical in the presence or absence of Tfr cells (Botta et al., 2017; Fu et al., 2018). Similarly mild effects have been observed in Tfr-deficient mice administered immunizations (Wu et al., 2016). In contrast, dramatic effects and rapid lethality are observed in Foxp3-deficient mice when total Treg cells are absent. The data from studies of infections indicate that the primary physiological role of Tfr cells is to limit the development of autoreactive B cells (Botta et al., 2017). While Tfr cells are uncommon early (d10) in influenza or LCMV infection, they accumulate substantially over time (d30) and prevent the development of autoreactive (anti-histone) B cells late in GC responses (Botta et al., 2017).
In addition to facilitating protective immunity against pathogens, Tfh cells are important regulators of the microbiota (Figure 4), via the importance of Tfh cells for generation of high affinity IgA responses in GC-rich Peyer’s patches against pathobionts (Bunker et al., 2015; Kubinak et al., 2015; Proietti et al., 2014; Wei et al., 2011) (though not IgA CSR itself (Reboldi et al., 2016)). Vice versa, gut microbiota also affect Tfh cell biology (Macpherson, 2018; Preite et al., 2018). Gut microbiota such as segmented filamentous bacteria (SFB) that reduce IL-2 responses enhance Tfh cell responses (Teng et al., 2016). There are likely multiple mechanisms by which variation in gut microbiota can modify Tfh differentiation or function, and this is an area ripe for future study, given the broad relevance of microbiota in immune system physiology.
Tfh Cells and Vaccines
The important roles of Tfh cells in immune responses to vaccines parallels their roles in development of immune responses to pathogen infections. Tfh cells are important for GC, Ab, and long-lived PC responses to protein immunizations, including the process of affinity maturation (Crotty, 2014; Victora and Nussenzweig, 2012). Understanding the roles of Tfh cells in the development of highly affinity matured Ab responses to vaccines, and in the development of long-lived memory to vaccines, has substantial promise for improving future vaccines.
The role of Tfh cells in human vaccines has been most extensively studied in the context of annual influenza vaccine immunizations. GC-Tfh cells are not present in blood, but a population of recently activated Tfh cells (CXCR5+ICOS+, also Ki67+PD1hi) is present in blood. Tfh cells in blood (CXCR5+) are commonly termed circulating Tfh (cTfh) cells. Resting memory Tfh cells recirculate regularly through blood, like other central memory CD4+ T cells, and therefore it is important to distinguish activated cTfh cells (CXCR5+ICOS+ or Ki67+PD1hi) from resting memory Tfh cells (TCM-Tfh resting CXCR5+CD45RO+ cells) in human blood cTfh cell studies (Heit et al., 2017). Resting memory Tfh cells represent the majority of cTfh cells in blood (Locci et al., 2013). Activated cTfh cells are most likely derived from GC-Tfh cells (Heit et al., 2017; Locci et al., 2013) or fTfh cells (He et al., 2013; Tsai and Yu, 2014). Flu vaccination recalls an oligoclonal memory Tfh cell response and flu-specific activated cTfh cell frequencies increase sharply at d7 (Bentebibel et al., 2013; Herati et al., 2017). Antigen-specific activated cTfh cells in human blood are also observed in response to an adenoviral vector (Heit et al., 2017) and correlated with anamnestic IgG responses. The large majority of flu-specific activated cTfh cells are CXCR3+ and CXCR3+ cTfh cells correlate with acute anti-influenza IgG (Bentebibel et al., 2013); however, CXCR3+ memory cTfh cells are particularly poor providers of B cell help in vitro (Locci et al., 2013; Morita et al., 2011). The observation that most flu-specific activated cTfh cells are CXCR3+ may be connected with the poor efficacy of the flu vaccine. The annual flu vaccine is frequently only ~30% protective and is associated with poor quality Ab responses. CXCR3+ cTfh cells may preferentially provide help for short-lived PB differentiation. It is possible that flu vaccines would be more efficacious if they successfully elicited Tfh cells with other properties.
Adjuvants are key components of vaccines. Live attenuated vaccines are self-adjuvanted, but other vaccines generally depend on adjuvants. Aluminum salts (alum) are the most common adjuvant in human vaccines and have been used for almost 100 years (McKee and Marrack, 2017; Plotkin et al., 2018). The mechanisms of adjuvanticity of alum remains controversial. Alum does stimulate Tfh cell responses, but relatively poorly (Preite et al., 2018; Riteau et al., 2016). Oil-in-water adjuvants, such as MF59, are also used in human vaccines and induce Tfh cell-dominated CD4+ T cell responses via IL-6 stimulation of Bcl6 expression, at least in mice (Mastelic Gavillet et al., 2015; Riteau et al., 2016). The direct mechanism(s) of action of oil-in-water adjuvants remains unclear, but likely involves endogenous danger signals or stress responses (Riteau et al., 2016; Vono et al., 2013). NKT adjuvants are promising and can enhance Tfh cell responses (Polonskaya et al., 2017). The primary mechanism of action of NKT adjuvants may be the provision of early cytokines such as IL-4 or IL-21 by NKT cells to B cells prior to the availability of IL-4 and IL-21 by Tfh cells (Chang et al., 2012; Gaya et al., 2018; King et al., 2012). There is extensive interest in the use of toll-like receptor (TLR) agonists as vaccine adjuvants. TLR4, TLR6, TLR7, TLR8, and TLR9 agonists can all enhance Tfh cell and Ab responses to protein antigens compared to alum (Kasturi et al., 2011; Leroux-Roels et al., 2016; Moon et al., 2012), and human vaccines containing TLR4 and TLR9 agonists are now available. However, it largely remains unclear if TLR agonist adjuvants preferentially induce Tfh cells or work via other mechanisms, such as direct effects on B cells and overall increases in CD4+ T cell response (Liang et al., 2017; Rookhuizen and DeFranco, 2014). In addition, it is unclear, on a case-by-case basis, if the mechanisms of action of adjuvants in mice are conserved in humans for Tfh cell and GC B cell biology. In the case of TLR8, ‘vita-PAMP’ agonists have a direct effect on human Tfh differentiation (Ugolini et al., 2018).
An additional aspect of adjuvants is antigen delivery. GC-Tfh cells require continuous antigen presentation for their maintenance (Baumjohann et al., 2013; Deenick et al., 2010). The magnitude of the Tfh cell response is also dependent on the antigen quantities presented by APCs to prime the CD4+ T cells. Thus, adjuvants and immunization methods that enhance CD4+ T cell priming, or sustain GC-Tfh cells, can substantially improve humoral immune responses (Cirelli et al., 2018; Tam et al., 2016).
Much remains to be learned about Tfh cell responses to vaccines and candidate vaccines and how they could be improved. A major challenge is the limitation of exclusively studying blood in most human and NHP studies. A fundamental attribute of GCs is that they do not exist in blood, they exist in lymphoid tissues. To understand GC-Tfh and Bgc cell biology it is essential to study lymphoid tissue. This is obviously challenging, and efforts to identify surrogate cell types and biomarkers of GCs and GC-Tfh cells in blood are worthwhile (Bentebibel et al., 2013; Havenar-Daughton et al., 2016b; Herati et al., 2017), but it is clear from many areas of immunological research that the cell types in tissue can be dramatically different than what is measured in blood. LN fine needle aspirates (FNA) provide one avenue for minimally invasive direct assessment of GC-Tfh cells and BGC cells in human and NHP LNs (Havenar-Daughton et al., 2016a). Using this approach, it has been determined in a monkey immunization study that nAb development correlates with Tfh quality and the magnitude of the GCs in LNs (Havenar-Daughton et al., 2016a). LN GC magnitude predicted the magnitude of the nAb responses in a subsequent study (Pauthner et al., 2017). A major advantage of LN FNAs is that they can be performed longitudinally on the same LN, allowing for kinetic measurements of GC-Tfh and GC biology in individuals. A first-of-its-kind longitudinal study of antigen-specific BGC cells and GC-Tfh cells in NHPs has revealed that B cell immunodominance is a major factor shaping the GC and Ab response to a candidate vaccine immunogen (Cirelli et al., 2018). LN FNAs are performed daily in most major hospitals for cancer pathology surveillance, and thus infrastructure is in place for utilizing LN FNAs for human GC investigation. This will hopefully result in improved understanding of human GC-Tfh cells and BGC cells in diverse contexts in the coming years.
Tfh Cells in Autoimmune Diseases
Tfh cells are associated with a wide range of autoimmune diseases, both in mice and humans. These fall into three categories: autoantibody-mediated autoimmune diseases, autoantibody-associated autoimmune diseases for which there is no clear causal role for autoantibodies, and autoimmune diseases not associated with autoantibodies. While associations of Tfh cells with autoantibody-mediated autoimmune diseases are expected, Tfh cell associations with other autoimmune diseases have been more surprising (Figure 4). Type 1 diabetes (T1D) is an example of the second category, where disease can be transferred by Tfh cells in a mouse model (Kenefeck et al., 2015). This may be relevant to humans, as increased T1D antigen-specific cTfh (Serr et al., 2016) and activated cTfh (PD1hi) cells are seen in children with recent onset T1D or at risk for T1D (Viisanen et al., 2017). Separately, Tfh cells have also been associated with multiple sclerosis in an EAE mouse model (Quinn et al., 2018). Due to space constraints, I will focus on two major autoimmune diseases here with Tfh cell involvement, SLE and RA.
In humans, perhaps the clearest connection between Tfh cells and an autoantibody-mediated autoimmune disease is systemic lupus erythematosus (SLE). A clear causal role for Tfh cells in disease progression in mice has been shown for autoantibody-associated autoimmunity in the sanroque SLE model (Linterman et al., 2009) and related models (Gensous et al., 2018). SLE can be considered an autoimmune disease for which the autoantibodies have an active role. In humans, the majority of autoantibody-producing B cells are somatically mutated (Tiller et al., 2007), indicating that they are likely GC-derived. Frequencies of activated cTfh cells in blood are increased in SLE patients (Gensous et al., 2018; He et al., 2013). Low dose IL-2 is being considered as a therapeutic treatment for SLE, on the basis that IL-2 is a potent inhibitor of Tfh cells (He et al., 2016a). Lupus nephritis is one of the most severe manifestations of human SLE, frequently requiring kidney transplantation, and ectopic GCs and Tfh cells are frequently found in lupus nephritis patient kidneys (Chang et al., 2011). Thus, it appears that Tfh cells have a pathogenic role in SLE. Interestingly, mice with Ets1-deficient Tfh cells develop spontaneous SLE (Kim et al., 2018a). The Ets1-deficient Tfh cells co-express a Th2 cell-associated gene expression program (Bcl6+GATA3hi) and are thus Tfh2 cells (Kim et al., 2018a). In contrast, Tfh2 cells were not observed in wild-type mice, implying that Tfh2 may be specifically pathological. IgE CSR is dependent on Tfh cells, not Th2 cells or Tfh2 cells, in helminth infections, as shown via deletion of Gata3 or Il4 in Tfh cells (Liang et al., 2012; Meli et al., 2017) (see Allergies, below). Ets1-deficient mice with SLE have greatly exacerbated IgE synthesis (Kim et al., 2018a), and anti-dsDNA IgE is associated with human SLE (Henault et al., 2016). Ets1 simultaneously inhibits signature Tfh- and Th2-associated genes, providing a potential mechanism for Tfh2 cell development. IL-4+IL-13+CXCR5+ Tfh2 cells are present in SLE patients, but are far less abundant in healthy individuals (Kim et al., 2018a). Thus, Tfh cells may be part of the pathogenesis of human SLE via aberrant Tfh2 cell differentiation resulting in unusual anti-DNA IgE. Additionally, human SLE is associated with ectopic lymphoid structures (ELS), which may directly contribute to the disease. Relationships between Tfh cells and ELS are discussed more below.
IL-21 and CD40L are both key Tfh help molecules, and both proteins have been associated with SLE disease. IL-21 or Tfh cells have been shown to be involved in SLE disease pathogenesis in several animal models with relatively diverse disease presentations (Choi et al., 2017; Kim et al., 2017), consistent with Tfh cells being important for SLE development in a range of contexts. In a pristane SLE mouse model, the nucleotide receptor P2rx7 (which is highly expressed on Tfh cells) regulates pathogenic Tfh cells that drive autoantibody responses, consistent with disrupted responsiveness to P2RX7 by cTfh cells from SLE patients (Faliti et al., 2019). Lastly, engagement of OX40 on human CD4+ T cells enhances BCL6 expression and IL-21 expression, and OX40L+ myeloid cells positively correlated with cTfh cell frequencies in SLE patients (Jacquemin et al., 2015).
Rheumatoid arthritis (RA) is strongly associated with autoantibodies, particularly against modified-self citrullinated proteins (Smolen et al., 2018). RA pathogenesis is multifactorial, involving many cell types and multiple organs. ELS in joints are associated with disease in a substantial subset of RA patients (Bombardieri et al., 2017) (Figure 4). These structures contain B cells, CD4+ T cells, and GCs, among other cell types. Other RA patients have ‘diffuse infiltrates’, of which a plurality of the cells are CD4+ T cells. B cells present in the lesions are autoreactive and have SHM (Amara et al., 2013; Humby et al., 2009), indicative of a requirement for Tfh cells in RA progression. The ELSs in joints are particularly associated with CXCL13-expressing CD4+ T cells (Manzo et al., 2008). This is notable, because ectopic CXCL13 expression induces ELS formation and recruits B cells to nonlymphoid tissues in mice (Luther et al., 2000). In human lymphoid tissue, GC-Tfh cells are the primary producers of CXCL13 (Gu-Trantien et al., 2017; Havenar-Daughton et al., 2016b). However, the CXCL13-expressing CD4+ T cells in RA joints are predominantly CXCR5 and BCL6 negative (Kobayashi et al., 2013; Manzo et al., 2008; Rao et al., 2017). Those synovial T cells expressed CD40L and, in technically challenging experiments, have been demonstrated to help synovial B cells in vitro in an IL-21 and SLAMF5 dependent manner, similar to GC-Tfh cells (Rao et al., 2017). Both Tfh cells and CXCR5neg CXCL13+ CD4 T cells co-localize with B cells in synovial ELSs, while in ‘diffuse infiltrate’ RA synovial tissue it is almost exclusively CXCR5neg CXCL13+ CD4+ T cells observed to colocalize with B cells. Thus, given that the CD4+ T cells co-localize with B cells in vivo, express Tfh-associated B cell help molecules, and provide help to B cells in vitro, these cells are clearly B cell helpers in vivo.
These CXCL13+CXCR5negPD1+IL-21+ CD4+ T cells (Rao et al., 2017) appear to be related to the surprising CXCL13+CXCR5neg CD4+ T cells found in ELS of breast cancer (see below). It is unclear whether the CXCL13-expressing CD4+ T cells in breast cancer and RA are Tfh cells with impaired CXCR5 expression, or a more distantly related cell type. Based on the facts that the breast cancer-associated CXCL13+ cells express multiple Tfh cell-associated proteins (including BCL6), tumor extract could inhibit CXCR5 expression by GC-Tfh cells in vitro, and TGFβ could induce CXCR5negCXCL13+ CD4 T cells in vitro (Gu-Trantien et al., 2017; Kobayashi et al., 2016), the breast cancer researchers concluded that the CXCL13+CXCR5neg CD4 T cells were most likely Tfh cells with CXCR5 inhibited by the tumor microenvironment and termed the cells TfhX13 (Gu-Trantien et al., 2017). The connection with TGFβ is consistent with the finding that major components of human Tfh differentiation can be induced by TGFβ or activin A (see above) (Locci et al., 2016; Schmitt et al., 2014), and TGFβ is common in tumor microenvironments. It is plausible that a similar explanation applies to the RA synovial CD4+ T cells, but direct comparisons have not been done. In either case, the non-lymphoid-tissue CD4+ T cells in these disparate diseases possess central attributes of Tfh cells, and thus a clearer understanding of the genesis of these cells will help better understand Tfh cell biology and the immunobiology of these diseases.
The connection between Tfh and Tfh-like cells and ELS structures is intriguing, but poorly understood. The simplest hypotheses for the function of Tfh cells in RA ELSs, and autoimmune ELS biology more generally, are that (1) Tfh cells may help develop or support ELSs that are a recruitment site for other cells that actively engage in autoimmune pathology, or (2) Tfh cells may support autoantibody responses by B cells (Figure 4). In favor of the first model, Tfh cells are robust producers of CXCL13, and CXCL13 and B cells are usually required for ELS development and maintenance (Pitzalis et al., 2014). The role of Tfh cells in ELSs is challenging to assess in animal models. In mice, CXCL13 is predominantly made by stromal cells (including FDCs), not GC-Tfh cells. Additionally, ELSs are associated with chronic disease processes, which are more difficult to model and study than acute events. Nevertheless, ELSs develop in mice and are associated with Tfh cells in some cases (Pitzalis et al., 2014), and the biology of Tfh cell-ELS interactions is certainly worthy of more extensive exploration.
Tfh cells or IL-21 have been shown to be important in multiple RA animal models (Gensous et al., 2018). IL-6 is a signature cytokine of human RA, and blockade of IL-6 signaling can provide therapeutic benefit (Smolen et al., 2018); however, the role of IL-6 in human Tfh differentiation is unclear, as discussed above. Overall, there is substantial evidence for Tfh cell and Tfh-like cell contributions to RA, but mysteries remain regarding the cells themselves, their relationship to RA ELS structures, and the mechanisms by which they regulate autoantibody development.
Tfr Cells in the Regulation of Autoimmunity
The main function of Tfr cells appears to be to prevent or eliminate autoreactive B cells generated by SHM during an antigen-specific immune response (Botta et al., 2017; Wu et al., 2016). This is supported by data on the specificity of Tfr cells. While rare foreign antigen-specific Tfr cells can be detected as a type of induced Treg (iTreg), >99% of Tfr cells have been found to be natural Treg cells (nTreg) (Aloulou et al., 2016; Maceiras et al., 2017), consistent with a role primarily limiting autoreactivity. It is unclear if Tfr cells directly regulate Tfh cells in vivo under physiological conditions. Tfr cells in GCs are rare, so it is difficult to see how Tfr function could be primarily on GC-Tfh cells (Sayin et al., 2018; Wing et al., 2017). However, since most GC-Tfh cell interactions with Bgc cells are brief, it is possible that rare Tfr cells in GCs may suffice to influence GC biology due to the large number of cellular interactions that can occur in a GC in a single day. Murine Tfh cells do not express MHC class II, so there cannot be direct cognate recognition of Tfh cells by Tfr cells. IL-2 consumption by nTreg cells is one important mechanism of action by which Treg cells control CD4+ T cell responses (Liu et al., 2015b). However, IL-2 consumption by Treg cells generally has a positive impact on Tfh cells (León et al., 2014), as Tfh cells are inhibited by IL-2 and generally express low or no IL-2Rα (Ballesteros-Tato et al., 2012; Johnston et al., 2012). Thus, the main actions of Tfr cells in mice may be by direct influence on B cells. In contrast, human CD4+ T cells express MHC class II, and therefore Tfr cells could directly regulate Tfh cells in humans but not mice. Regarding direct regulation of B cells by Tfr cells, one study has found that CTLA4 is the major mechanism of action of Tfr cells, via directly regulating CD80 and/or CD86 on B cells (Wing et al., 2014), though a parallel study suggested other, undetermined, CTLA4 mechanisms were more important (Sage et al., 2014). Of note, there remain differences in defining Tfr cells, between species and between scientific studies, which may underlie some of the differences observed between studies (Ritvo et al., 2017). A major challenge to understanding the importance of Tfr cells in human autoimmune diseases is the limitation of studying blood. As discussed above, GCs and GC-Tfh cells are not present in blood, and thus studies of lymphoid tissue are required to understand the biology of human Tfh cells, Tfr cells, and GCs.
Tfh Cells in Allergies
Tfh cells are required for Ab-mediated allergies. It is still commonly assumed that IgE responses are driven by Th2 cells (Gould and Ramadani, 2018). That is incorrect. It was first shown in 2012, using a combination of experimental approaches in murine helminth infections models, that Tfh cell-derived IL-4, not Th2 cell -derived IL-4, was the source for IgE induction (Liang et al., 2012). More recently, the requirement for Tfh cell-derived IL-4 to develop IgE responses was elegantly shown in a different helminth model using mice engineered to have a specific Il4 deficiency in Tfh cells but not Th2 cells (Meli et al., 2017). Bcl6+ Tfh cells are distinct from GATA3hi Th2 cells (Liang et al., 2012). The majority of IL-4 producing CD4+ T cells in LNs and spleen are Tfh cells, while Th2 cells are found in peripheral tissues (Reinhardt et al., 2009). IL-4 expression by Tfh cells is regulated by distinct signaling pathways and enhancer loci that excludes expression of IL-5 and IL-13 (Vijayanand et al., 2012; Yusuf et al., 2010). Il5 and Il13 are in the same chromosomal locus as Il4 and are specifically expressed in Th2 cells due to Th2 cell-specific enhancers.
While those studies indicate that allergic IgE responses to environmental antigens are likely to also depend on Tfh cells, they did not directly examine allergens. In a mouse allergic airway inflammation model, allergen-induced Tfh cells (CXCR5+Bcl6+IL-4+IL-21+ IL-5−IL-13−GATA3l°) and Th2 cells (GATA3hiIL-4+IL-5+IL-13+ CXCR5−Bcl6−) are again distinct (Kobayashi et al., 2017). IgE responses to allergen are completely lost in the absence of Tfh cells (Bcl6fl/flCrecd4) or ICOS (Kobayashi et al., 2017). Th2 cell responses and lung eosinophil inflammation are essentially unchanged. Those Tfh versus Th2 findings are consistent with a large human GWAS study that found genetic polymorphism associations with serum IgE and asthma are distinct, suggesting distinct pathways (Moffatt et al., 2010). A subsequent, analogous study used the authentic allergen peanut flour and found essentially identical results; IgE responses are dependent on Tfh cells, which were distinct from Th2 cells, and Tfh-deficient mice are protected from peanut exposure IgE anaphylaxis (Dolence et al., 2018).
Tfh cells express both IL-21 and IL-4. Tfh cells most likely induce IgE CSR under conditions of low IL-21 expression by Tfh cells or low IL21R signaling by B cells (Suto et al., 2002; Tangye, 2015). IgE CSR may be negatively regulated by Tfr cells (Yao et al., 2019).
While Tfh and Th2 are distinct cell types, nevertheless most aspects of CD4+ T cell biology can become complex under differing in vivo conditions, potentially due to a requirement for ‘fuzzy logic’ in T cell responses to prevent immune evasion by pathogens (Crotty, 2018). The relationship between Tfh and Th2 cell-associated transcriptional programs is no exception to exceptions. Tfh cell conversion into Th2 cells can be a primary source of Th2 cell effectors, shown in an elegant lung allergic asthma model study (Ballesteros-Tato et al., 2016). However, Tfh cells are not required for Th2 cell responses in similar models (Dullaers et al., 2017; Hondowicz et al., 2016), apparently due to differences in antigen burden (Dullaers et al., 2017).
Tfh Cells in Heart Disease
Atherosclerosis, the leading cause of death in the United States, is an inflammatory disease of coronary arteries. The causes of atherosclerotic inflammation are multifactorial, and recent evidence points to a functional role for Tfh cells. Abs against oxidized low-density lipoprotein (oxLDL) have a role in atherosclerosis, with most human data indicating that IgM is protective and IgG is potentially pathogenic (van den Berg et al., 2018). In a Notch-signaling-deficient B cell mouse model of atherosclerosis, rapid onset atherosclerosis has been observed and is associated with elevated frequencies of splenic GC-Tfh cells (Nus et al., 2017). The arterial disease can be prevented by blocking ICOSL, which prevented the splenic GC-Tfh cell accumulation. In a second atherosclerosis mouse model, a pro-atherosclerotic role for Tfh cells has also been demonstrated (Gaddis et al., 2018). Unexpectedly, nTreg cells de-differentiated (exTreg) and preferentially converted to autoreactive Tfh cells. Atherosclerosis was ameliorated in Tfh cell-deficient mice (Bcl6fl/flcreCd4). OxLDL appears to drive nTreg cell conversion into Tfh cells via reduction of IL2Rα and enhancement of IL6R expression (Gaddis et al., 2018). LDL can also increase IL-6 and IL-27 expression by DCs of atherogenic mice (Ryu et al., 2018).
Interestingly, no obvious role for Abs have been observed in the atherosclerosis mouse models (Nus et al., 2017), suggesting that the pro-atherosclerotic activities of Tfh cells are not due to increased Abs. This is similar to the situation for multiple autoimmune diseases that are not thought to be primarily autoantibody-mediated, wherein Tfh cells are associated with disease development but not via providing help for pathogenic autoantibody responses. Alternative functions for Tfh cells in such situations are largely hypothetical, and this is an area of Tfh cell research that requires rigorous examination in the future. ELSs with Tfh cells are observed in mouse models of atherosclerosis and in human atherosclerosis (Clement et al., 2015), and may have a role in atherosclerosis disease, as has been hypothesized for SLE, multiple sclerosis, and RA.
Tfh Cells in Organ Transplant Diseases
Alloantibodies have long been associated with organ transplant or skin graft rejection. GCs are associated with organ rejection in a murine heart transplant chronic graft-versus-host disease (cGVHD) model (Ali et a l., 2016), and those responses can be driven by alloantigen-specific Tfh cells (Conlon et al., 2012). Tfh cells and GCs in transplanted kidneys have been associated with acute and chronic human kidney transplant rejection (Chenouard et al., 2017; de Graav et al., 2015). cGVHD is a major complication of human allogeneic HSC bone marrow transplantation. In an allogeneic HSC transplant murine model, cGVHD disease is dependent on Tfh cells and lung disease and alloantibody responses can be efficiently blocked by elimination of Cxcr5, or neutralization of IL-21, CD40L, or ICOS (Flynn et al., 2014). In a mouse skin GVHD disease model, disease development was associated with skin Tfh cells (CXCR5+PD1+ICOS+ CD4+) and blocking IL-21 prevents disease (Taylor et al., 2018).
Tfh Cells in Cancer
Perhaps the most surprising connections found between Tfh cells and human diseases in the past decade have come from the cancer field. Tfh-related cells have been positively associated with long term survival of humans with breast cancer or colorectal cancer (Bindea et al., 2013; Gu-Trantien et al., 2013). The strongest correlation with immunoprotective function is CXCL13 expression. In the context of breast cancer, the CXCL13-expressing CD4+ T cells are strongly correlated with ELSs (Gu-Trantien et al., 2017). In human lymphoid organs, GC-Tfh cells are the primary producers of CXCL13 (Gu-Trantien et al., 2017; Havenar-Daughton et al., 2016b); however, while breast cancer tissue-associated CXCL13-expressing CD4+ T cells have multiple attributes of GC-Tfh cells (including BCL6, CD200, PD1, and ICOS), they are predominantly CXCR5neg (Gu-Trantien et al., 2017). Similar cells have been observed in melanoma tumors (Li et al., 2019). Notably, while many breast cancer tumors exhibit ELSs containing B cells, tumors with GCs are less common, and those tumors do contain fully differentiated GC-Tfh cells in addition to CXCR5neg cells (Gu-Trantien et al., 2017). The Tfh-relatedness of the CXCR5neg cells was discussed above.
The simplest hypotheses for the value of Tfh cells in immune responses against tumors parallel the concepts for autoimmunity: (1) Tfh may help develop or support ELSs, which are a site of recruitment for CD8+ T cells, NK cells, and macrophages that engage in anti-tumor immunity. Alternatively, (2) Tfh cells may support anti-tumor Ab responses by B cells. There is little data available regarding Tfh cells supporting anti-tumor Ab responses (Garaud et al., 2018), and more research is warranted at the granular level of the antigen-specificity (or lack thereof) of the B cells co-localizing with Tfh-like cells in tumors. In addition to breast cancer, survival with colorectal cancer has been positively associated with the presence of ELSs and CXCL13 (Becht et al., 2016; Coppola et al., 2011). Finally, given that there are over 100 types of cancers, it is not surprising that there appear to be differences in Tfh cell associations depending on the cancer. Tfh cells are negatively associated with survival in a mouse model of hepatocellular carcinoma with a prominent role of IgA+ PBs (Shalapour et al., 2017). There are clearly interesting complexities to Tfh-associated biology in the context of cancer, and the available data show that much more needs to be learned.
Perhaps the most exciting connection between Tfh cells and cancer biology are the potential implications of PD1 and PDL1 immunotherapies. As discussed above, it has been thought that Tfh cells and cytotoxic transcriptional programs are exclusive, but it has become clear that CD8+ T cells can appropriate Tfh cell-associated gene expression in a TCF1- and Id2-dependent manner to express CXCR5 and other Tfh cell-associated genes, and it is those CXCR5+ CD8+ T cells that respond most robustly to PD1 immunotherapy (Im et al., 2016). While CD8+ T cells are the primary target of PD1 mAbs in cancer immunotherapy, CD4+ T cells will, of course, also be affected. Given that GC-Tfh cells express very high levels of PD1 and are present in lymphoid organs throughout the human body, and there are Tfh-associated cells in multiple tumor types, it is surprising that the phenotypic and functional impact of PD1 and PDL1 immunotherapy on Tfh cells in humans has not been described.
Perspective: Future Directions
It is clear that both broad and deep advances have been made in the scientific understanding of Tfh cells during the past decade of research. Select advances were highlighted here, due to space constraints, and many other interesting Tfh-related findings have been made. Looking at this summary of the past decade of research, the obvious question is, ‘What does the future hold?’ Future directions and open questions have been discussed in numerous places throughout this article. Broadly, emphasis will need to continue to be put on understanding how antigen-specific Tfh cells differentiate and function, and increasing emphasis will surely be placed on how to apply that knowledge to Tfh-related therapeutics. GCs and numerous aspects of Tfh cell-associated biology are quite complex and frequently elude simple universal answers, but the high biomedical and scientific value of understanding these processes is clear and thus worth the effort. There is great interest in the use of Tfh cell-associated biology for enhancement for vaccines. There is also great interest in targeting Tfh cells for therapeutic interventions in human autoimmune diseases, allergies, atherosclerosis, organ transplants, and cancers. Therefore, well-defined causal roles for Tfh cells in disease pathologies remain a high priority research area. Key aspects of these human diseases are difficult to model in mice, such as ELS biology, the importance of CXCL13 production by Tfh cells, Ab isotype biology and CSR, and the cytokines that preferentially regulate human versus mouse Tfh differentiation. These challenges require creative solutions and should not be ignored.
Supplementary Material
Highlights.
Tfh cells are the CD4+ T cells specialized for helping B cells.
Bcl6 was identified as the Tfh lineage defining transcription factor 10 years ago.
Tfh cell differentiation and functions have now been elucidated in great detail.
Tfh cells have been found to be involved in a wide range of disease.
ACKNOWLEDGEMENTS
Thanks to input from Crotty lab members and helpful feedback from multiple experts in the field. This work was funded by grants from the USA National Institutes of Health (NIH), including NIAID U19AI109976, R01AI135193, and UM1AI100663, as well as LJI funds to SC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, Kulp DW, Bhullar D, Kalyuzhniy O, Havenar-Daughton C, et al. (2018). Precursor Frequency and Affinity Determine B Cell Competitive Fitness in Germinal Centers, Tested with Germline-Targeting HIV Vaccine Immunogens. Immunity 48, 133–146.e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaya M, Traba J, Roesler AS, Miozzo P, Akkaya B, Theall BP, Sohn H, Pena M, Smelkinson M, Kabat J, et al. (2018). Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol 19, 871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali JM, Negus MC, Conlon TM, Harper IG, Qureshi MS, Motallebzadeh R, Willis R, Saeb-Parsy K, Bolton EM, Bradley JA, et al. (2016). Diversity of the CD4 T Cell Alloresponse: The Short and the Long of It. Cell Rep 14, 1232–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloulou M, Carr EJ, Gador M, Bignon A, Liblau RS, Fazilleau N, and Linterman MA (2016). Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat Commun 7, 10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara K, Steen J, Murray F, Morbach H, Fernandez-Rodriguez BM, Joshua V, Engström M, Snir O, Israelsson L, Catrina AI, et al. (2013). Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med 210, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ballesteros-Tato A, León B, Graf BA, Moquin A, Adams PS, Lund FE, and Randall TD (2012). Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, and León B (2016). T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity 44, 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, Corpataux J-M, De Leval L, Pantaleo G, and Perreau M (2016). PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nature Medicine 22, 754–761. [DOI] [PubMed] [Google Scholar]
- Bannard O, McGowan SJ, Ersching J, Ishido S, Victora GD, Shin J-S, and Cyster JG (2016). Ubiquitin-mediated fluctuations in MHC class II facilitate efficient germinal center B cell responses. J Exp Med 213, 993–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, and Sallusto F (2013). Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 38, 596–605. [DOI] [PubMed] [Google Scholar]
- Becht E, de Reyniès A, Giraldo NA, Pilati C, Buttard B, Lacroix L, Selves J, Sautès-Fridman C, Laurent-Puig P, and Fridman WH (2016). Immune and Stromal Classification of Colorectal Cancer Is Associated with Molecular Subtypes and Relevant for Precision Immunotherapy. Clinical Cancer Research 22, 4057–4066. [DOI] [PubMed] [Google Scholar]
- Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flaño E, Mejias A, Albrecht RA, Blankenship D, et al. (2013). Induction of ICOS+CXCR3+CXCR5+ TH Cells Correlates with Antibody Responses to Influenza Vaccination. Sci Transl Med 5, 176ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentebibel SE, Schmitt N, Banchereau J, and Ueno H (2011). Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci USA 108, E488–E497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguelin W, Teater M, Gearhart MD, Calvo Fernández MT, Goldstein RL, Cárdenas MG, Hatzi K, Rosen M, Shen H, Corcoran CM, et al. (2016). EZH2 and BCL6 Cooperate to Assemble CBX8-BCOR Complex to Repress Bivalent Promoters, Mediate Germinal Center Formation and Lymphomagenesis. Cancer Cell 30, 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. (2013). Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795. [DOI] [PubMed] [Google Scholar]
- Bombardieri M, Lewis M, and Pitzalis C (2017). Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat Rev Rheumatol 13, 141–154. [DOI] [PubMed] [Google Scholar]
- Botta D, Fuller MJ, Marquez-Lago TT, Bachus H, Bradley JE, Weinmann AS, Zajac AJ, Randall TD, Lund FE, León B, et al. (2017). Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat Immunol 18, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, and Tangye SG (2007). Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol 179, 8180–8190. [DOI] [PubMed] [Google Scholar]
- Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, Ishizuka IE, Dent AL, Wilson PC, Jabri B, et al. (2015). Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity 43, 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, and Harty JT (2012). Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt D, Chan TD, Bourne K, Hermes JR, Nguyen A, Statham A, O’Reilly LA, Strasser A, Price S, Schofield P, et al. (2015). FAS Inactivation Releases Unconventional Germinal Center B Cells that Escape Antigen Control and Drive IgE and Autoantibody Production. Immunity 42, 890–902. [DOI] [PubMed] [Google Scholar]
- Chakarov S, and Fazilleau N (2014). Monocyte-derived dendritic cells promote T follicular helper cell differentiation. EMBO Molecular Medicine 6, 590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, et al. (2011). In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186, 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P-P, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, Cerundolo V, Tangye SG, Bittman R, et al. (2012). Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol 13, 35–43. [DOI] [PubMed] [Google Scholar]
- Chenouard A, Chesneau M, Bui Nguyen L, Le Bot S, Cadoux M, Dugast E, Paul C, Malard-Castagnet S, Ville S, Guérif P, et al. (2017). Renal Operational Tolerance Is Associated With a Defect of Blood Tfh Cells That Exhibit Impaired B Cell Help. Am. J. Transplant. 17, 1490–1501. [DOI] [PubMed] [Google Scholar]
- Choi J-Y, Seth A, Kashgarian M, Terrillon S, Fung E, Huang L, Wang LC, and Craft J (2017). Disruption of Pathogenic Cellular Networks by IL-21 Blockade Leads to Disease Amelioration in Murine Lupus. J Immunol 198, 2578–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, Love PE, Peng W, Xue H-H, and Crotty S (2015). LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol 16, 980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, and Crotty S (2011). ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity 34, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, and Crotty S (2013). Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol 190, 4014–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli KM, Carnathan DG, Nogal B, Rodriguez OL, Martin JT, Upadhyay AA, Enemuo CA, Gebru EH, Choe Y, Viviano F, et al. (2018). Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. bioRxiv 1–56.
- Clement M, Guedj K, Andreata F, Morvan M, Bey L, Khallou-Laschet J, Gaston A-T, Delbosc S, Alsac J-M, Bruneval P, et al. (2015). Control of the T follicular helper-germinal center B-cell axis by CD8+ regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 131, 560–570. [DOI] [PubMed] [Google Scholar]
- Conlon TM, Saeb-Parsy K, Cole JL, Motallebzadeh R, Qureshi MS, Rehakova S, Negus MC, Callaghan CJ, Bolton EM, Bradley JA, et al. (2012). Germinal center alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J Immunol 188, 2643–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, and Mulé JJ (2011). Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am. J. Pathol 179, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JR, Ambesi-Impiombato A, Couronné L, Quinn SA, Kim CS, da Silva Almeida AC, West Z, Belver L, Martin MS, Scourzic L, et al. (2018). RHOA G17V Induces T Follicular Helper Cell Specification and Promotes Lymphomagenesis. Cancer Cell 33, 259–273.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S (2011). Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29, 621–663. [DOI] [PubMed] [Google Scholar]
- Crotty S (2014). T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S (2015). A brief history of T cell help to B cells. Nat Rev Immunol 15, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S (2018). Do Memory CD4 T Cells Keep Their Cell-Type Programming: Plasticity versus Fate Commitment? Complexities of Interpretation due to the Heterogeneity of Memory CD4 T Cells, Including T Follicular Helper Cells. Cold Spring Harb Perspect Biol 10, a032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan JM, Havenar-Daughton C, Kendric K, Al-Kolla R, Kaushik K, Rosales SL, Anderson EL, LaRock CN, Vijayanand P, Seumois G, et al. (2019). Recurrent group A Streptococcus tonsillitis is an immunosusceptibility disease involving antibody deficiency and aberrant TFH cells. Sci Transl Med 11, eaau3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan JM, Lindestam Arlehamn CS, Weiskopf D, Da Silva Antunes R, Havenar-Daughton C, Reiss SM, Brigger M, Bothwell M, Sette A, and Crotty S (2016). A Cytokine-Independent Approach To Identify Antigen-Specific Human Germinal Center T Follicular Helper Cells and Rare Antigen-Specific CD4+ T Cells in Blood. J Immunol 197, 983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graav GN, Dieterich M, Hesselink DA, Boer K, van Groningen MCC, Kraaijeveld R, Litjens NHR, Bouamar R, Vanderlocht J, Tilanus M, et al. (2015). Follicular T helper cells and humoral reactivity in kidney transplant patients. Clinical & Experimental Immunology 180, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, and Tangye SG (2010). Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 33, 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiToro D, Winstead CJ, Pham D, Witte S, Andargachew R, Singer JR, Wilson CG, Zindl CL, Luther RJ, Silberger DJ, et al. (2018). Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 361, eaao2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolence JJ, Kobayashi T, lijima K, Krempski J, Drake LY, Dent AL, and Kita H (2018). Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol 142, 1144–1158.e1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullaers M, Schuijs MJ, Willart M, Fierens K, Van Moorleghem J, Hammad H, and Lambrecht BN (2017). House dust mite-driven asthma and allergen-specific T cells depend on B cells when the amount of inhaled allergen is limiting. J Allergy Clin Immunol 140, 76–88.e77. [DOI] [PubMed] [Google Scholar]
- Ersching J, Efeyan A, Mesin L, Jacobsen JT, Pasqual G, Grabiner BC, Dominguez-Sola D, Sabatini DM, and Victora GD (2017). Germinal Center Selection and Affinity Maturation Require Dynamic Regulation of mTORC1 Kinase. Immunity 46, 1045–1058.e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faliti CE, Gualtierotti R, Rottoli E, Gerosa M, Perruzza L, Romagnani A, Pellegrini G, De Ponte Conti B, Rossi RL, Idzko M, et al. (2019). P2X7 receptor restrains pathogenic Tfh cell generation in systemic lupus erythematosus. J Exp Med 216, 317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, Rosen H, and McHeyzer-Williams MG (2009). The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol 10, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, Freeman GJ, Serody JS, Murphy WJ, Munn DH, et al. (2014). Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood 123, 3988–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Liu X, Lin X, Feng H, Sun L, Li S, Chen H, Tang H, Lu L, Jin W, et al. (2018). Deficiency in T follicular regulatory cells promotes autoimmunity. J Exp Med 215, 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, et al. (2018). Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun 9, 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaud S, Zayakin P, Buisseret L, Rulle U, Silina K, de Wind A, Van den Eyden G, Larsimont D, Willard-Gallo K, and Linē A (2018). Antigen Specificity and Clinical Significance of IgG and IgA Autoantibodies Produced in situ by Tumor-Infiltrating B Cells in Breast Cancer. Front Immunol 9, 2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaya M, Barral P, Burbage M, Aggarwal S, Montaner B, Warren Navia A, Aid M, Tsui C, Maldonado P, Nair U, et al. (2018). Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells. Cell 172, 517–533.e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensous N, Charrier M, Duluc D, Contin-Bordes C, Truchetet M-E, Lazaro E, Duffau P, Blanco P, and Richez C (2018). T Follicular Helper Cells in Autoimmune Disorders. Front Immunol 9, 1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin AD, Shulman Z, and Nussenzweig MC (2014). Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature 509, 637–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, and Ramadani F (2018). Peanut allergen-specific antibodies go public. Science 362, 1247–1248. [DOI] [PubMed] [Google Scholar]
- Greczmiel U, Kräutler NJ, Pedrioli A, Bartsch I, Agnellini P, Bedenikovic G, Harker J, Richter K, and Oxenius A (2017). Sustained T follicular helper cell response is essential for control of chronic viral infection. Science Immunology 2, eaam8686. [DOI] [PubMed] [Google Scholar]
- Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, et al. (2013). CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 123, 2873–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohée S, Garaud S, Noël G, Dang Chi VL, Lodewyckx J-N, Naveaux C, et al. (2017). CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker JA, Dolgoter A, and Zuniga EI (2013). Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4+ T cell responses and viral control during chronic infection. Immunity 39, 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker JA, Lewis GM, Mack L, and Zuniga EI (2011). Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334, 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzi K, Jiang Y, Huang C, Garrett-Bakelman F, Gearhart MD, Giannopoulou EG, Zumbo P, Kirouac K, Bhaskara S, Polo JM, et al. (2013). A hybrid mechanism of action for BCL6 in B cells defined by formation of functionally distinct complexes at enhancers and promoters. Cell Rep 4, 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, and Crotty S (2015). BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med 212, 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenar-Daughton C, Carnathan DG, Torrents de la Peña A, Pauthner M, Briney B, Reiss SM, Wood JS, Kaushik K, van Gils MJ, Rosales SL, et al. (2016a). Direct Probing of Germinal Center Responses Reveals Immunological Features and Bottlenecks for Neutralizing Antibody Responses to HIV Env Trimer. Cell Rep 17, 2195–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenar-Daughton C, Lee JH, and Crotty S (2017). Tfh cells and HIV bnAbs, an immunodominance model of the HIV neutralizing antibody generation problem. Immunol Rev 275, 49–61. [DOI] [PubMed] [Google Scholar]
- Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, Belanger S, Kasturi SP, Landais E, Akondy RS, et al. (2016b). CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci USA 113, 201520112–201522707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, Torrents de la Peña A, Kasturi SP, Dan JM, Bothwell M, Sanders RW, et al. (2016c). Cytokine-Independent Detection of Antigen-Specific Germinal Center T Follicular Helper Cells in Immunized Nonhuman Primates Using a Live Cell Activation-Induced Marker Technique. J Immunol 197, 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al. (2013). Circulating Precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T Cells Indicate Tfh Cell Activity and Promote Antibody Responses upon Antigen Reexposure. Immunity 39, 770–781. [DOI] [PubMed] [Google Scholar]
- He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, et al. (2016a). Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nature Medicine. [DOI] [PubMed] [Google Scholar]
- He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, et al. (2016b). Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature 537, 412–416. [DOI] [PubMed] [Google Scholar]
- Heesters BA, Chatterjee P, Kim Y-A, Gonzalez SF, Kuligowski MP, Kirchhausen T, and Carroll MC (2013). Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity 38, 1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesters BA, van der Poel CE, Das A, and Carroll MC (2016). Antigen Presentation to B Cells. Trends Immunol 37, 844–854. [DOI] [PubMed] [Google Scholar]
- Heit A, Schmitz F, Gerdts S, Flach B, Moore MS, Perkins JA, Robins HS, Aderem A, Spearman P, Tomaras GD, et al. (2017). Vaccination establishes clonal relatives of germinal center T cells in the blood of humans. J Exp Med 214, 2139–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henault J, Riggs JM, Karnell JL, Liarski VM, Li J, Shirinian L, Xu L, Casey KA, Smith MA, Khatry DB, et al. (2016). Self-reactive IgE exacerbates interferon responses associated with autoimmunity. Nat Immunol 17, 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herati RS, Muselman A, Vella L, Bengsch B, Parkhouse K, Del Alcazar D, Kotzin J, Doyle SA, Tebas P, Hensley SE, et al. (2017). Successive annual influenza vaccination induces a recurrent oligoclonotypic memory response in circulating T follicular helper cells. Science Immunology 2, eaag2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister K, Kusam S, Wu H, Clegg N, Mondal A, Sawant DV, and Dent AL (2013). Insights into the role of Bcl6 in follicular Th cells using a new conditional mutant mouse model. J Immunol 191, 3705–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, Keitany GJ, Garza EN, Fraser KA, Moon JJ, et al. (2016). Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity 44, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Zhang Z, Liu H, Tian M, Zhu X, Zhang Z, Wang W, Zhou X, Zhang F, Ge Q, et al. (2018). B Cells Are the Dominant Antigen-Presenting Cells that Activate Naive CD4+ T Cells upon Immunization with a Virus-Derived Nanoparticle Antigen. Immunity 49, 695–708.e4. [DOI] [PubMed] [Google Scholar]
- Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, Spencer J, and Pitzalis C (2009). Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 6, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. (2016). Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ise W, Fujii K, Shiroguchi K, Ito A, Kometani K, Takeda K, Kawakami E, Yamashita K, Suzuki K, Okada T, et al. (2018). T Follicular Helper Cell-Germinal Center B Cell Interaction Strength Regulates Entry into Plasma Cell or Recycling Germinal Center Cell Fate. Immunity 48, 702–715.e704. [DOI] [PubMed] [Google Scholar]
- Ise W, Inoue T, McLachlan JB, Kometani K, Kubo M, Okada T, and Kurosaki T (2014). Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc Natl Acad Sci USA 111, 11792–11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin C, Schmitt N, Contin-Bordes C, Liu Y, Narayanan P, Seneschal J, Maurouard T, Dougall D, Davizon ES, Dumortier H, et al. (2015). OX40 Ligand Contributes to Human Lupus Pathogenesis by Promoting T Follicular Helper Response. Immunity 42, 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Choi YS, Diamond JA, Yang JA, and Crotty S (2012). STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med 209, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, and Crotty S (2009). Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Fairfax K, Pridans C, Emslie D, McKenzie BS, Lew AM, Corcoran LM, Hodgkin PD, Tarlinton DM, et al. (2007). Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity 26, 555–566. [DOI] [PubMed] [Google Scholar]
- Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, D’Costa K, Tarlinton DM, Kallies A, and Corcoran LM (2012). B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med 209, 2049–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. (2011). Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Zaid A, Davey GM, Mueller SN, Nutt SL, Zotos D, Tarlinton DM, Shortman K, Lahoud MH, Heath WR, et al. (2015). Targeting Antigen to Clec9A Primes Follicular Th Cell Memory Responses Capable of Robust Recall. J Immunol 195, 1006–1014. [DOI] [PubMed] [Google Scholar]
- Kenefeck R, Wang CJ, Kapadi T, Wardzinski L, Attridge K, Clough LE, Heuts F, Kogimtzis A, Patel S, Rosenthal M, et al. (2015). Follicular helper T cell signature in type 1 diabetes. J Clin Invest 125, 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Lee C-G, Jung J-Y, Ghosh A, Hasan SN, Hwang S-M, Kang H, Lee C, Kim G-C, Rudra D, et al. (2018a). The Transcription Factor Ets1 Suppresses T Follicular Helper Type 2 Cell Differentiation to Halt the Onset of Systemic Lupus Erythematosus. Immunity 49, 1034–1048.e1038. [DOI] [PubMed] [Google Scholar]
- Kim ST, Choi J-Y, Lainez B, Schulz VP, Karas DE, Baum ED, Setlur J, Gallagher PG, and Craft J (2018b). Human Extrafollicular CD4+ Th Cells Help Memory B Cells Produce Igs. J Immunol 201, 1359–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Schätzle S, Ahmed SS, Haap W, Jang SH, Gregersen PK, Georgiou G, and Diamond B (2017). Increased cathepsin S in Prdm1−/− dendritic cells alters the TFH cell repertoire and contributes to lupus. Nat Immunol 18, 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IL, Fortier A, Tighe M, Dibble J, Watts GFM, Veerapen N, Haberman AM, Besra GS, Mohrs M, Brenner MB, et al. (2012). Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol 13, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Murata K, Shibuya H, Morita M, Ishikawa M, Furu M, Ito H, Ito J, Matsuda S, Watanabe T, et al. (2013). A distinct human CD4+ T cell subset that secretes CXCL13 in rheumatoid synovitis. Arthritis Rheum 65, 3063–3072. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Watanabe T, Suzuki R, Furu M, Ito H, Ito J, Matsuda S, and Yoshitomi H (2016). TGF-β induces the differentiation of human CXCL13-producing CD4(+) T cells. Eur J Immunol 46, 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, lijima K, Dent AL, and Kita H (2017). Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol 139, 300–313.e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräutler NJ, Suan D, Butt D, Bourne K, Hermes JR, Chan TD, Sundling C, Kaplan W, Schofield P, Jackson J, et al. (2017). Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J Exp Med 214, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy JK, Gowthaman U, Zhang B, Mattsson J, Szeponik L, Liu D, Wu R, White T, Calabro S, Xu L, et al. (2017). Migratory CD11b+ conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Science Immunology 2, eaam9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinak JL, Petersen C, Stephens WZ, Soto R, Bake E, O’Connell RM, and Round JL (2015). MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavenworth JW, Verbinnen B, Yin J, Huang H, and Cantor H (2015). A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat Immunol 16, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, et al. (2011). B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med 208, 1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, Minnich M, Meckiff BJ, Wei Y, Hou Z, et al. (2016). CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol 17, 1187–1196. [DOI] [PubMed] [Google Scholar]
- León B, Bradley JE, Lund FE, Randall TD, and Ballesteros-Tato A (2014). FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat Commun 5, 3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux-Roels G, Marchant A, Levy J, Van Damme P, Schwarz TF, Horsmans Y, Jilg W, Kremsner PG, Haelterman E, Clément F, et al. (2016). Impact of adjuvants on CD4(+) T cell and B cell responses to a protein antigen vaccine: Results from a phase II, randomized, multicenter trial. Clin Immunol 169, 16–27. [DOI] [PubMed] [Google Scholar]
- Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, van den Braber M, Rozeman EA, Haanen JBAG, Blank CU, et al. (2019). Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 176, 775–789.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Lindgren G, Sandgren KJ, Thompson EA, Francica JR, Seubert A, De Gregorio E, Barnett S, O’Hagan DT, Sullivan NJ, et al. (2017). Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci Transl Med 9, eaal2094. [DOI] [PubMed] [Google Scholar]
- Liang H-E, Reinhardt RL, Bando JK, Sullivan BM, Ho I-C, and Locksley RM (2012). Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol 13, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, and Vinuesa CG (2009). Follicular helper T cells are required for systemic autoimmunity. J Exp Med 206, 561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, Luo D, and Qi H (2015a). T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature 517, 214–218. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, Nurieva RI, Yan X, Chen P, van der Flier LG, et al. (2014). Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature 507, 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Gerner MY, van Panhuys N, Levine AG, Rudensky AY, and Germain RN (2015b). Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature 528, 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al. (2013). Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39, 758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci M, Wu JE, Arumemi F, Mikulski Z, Dahlberg C, Miller AT, and Crotty S (2016). Activin A programs the differentiation of human TFH cells. Nat Immunol 17, 976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Weisel F, and Shlomchik MJ (2018). B Cell Receptor and CD40 Signaling Are Rewired for Synergistic Induction of the c-Myc Transcription Factor in Germinal Center B Cells. Immunity 48, 313–326.e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Lopez T, Bai W, Hanahan D, and Cyster JG (2000). BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 12, 471–481. [DOI] [PubMed] [Google Scholar]
- Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T, Bustamante J, Okada S, Stoddard JL, Deenick EK, et al. (2015). Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol 136, 993–1006.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceiras AR, Almeida SCP, Mariotti-Ferrandiz E, Chaara W, Jebbawi F, Six A, Hori S, Klatzmann D, Faro J, and Graca L (2017). T follicular helper and T follicular regulatory cells have different TCR specificity. Nat Commun 8, 15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ (2018). Do the Microbiota Influence Vaccines and Protective Immunity to Pathogens? Issues of Sovereignty, Federalism, and Points-Testing in the Prokaryotic and Eukaryotic Spaces of the Host-Microbial Superorganism. Cold Spring Harb Perspect Biol 10, a029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo A, Vitolo B, Humby F, Caporali R, Jarrossay D, Dell’Accio F, Ciardelli L, Uguccioni M, Montecucco C, and Pitzalis C (2008). Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum 58, 3377–3387. [DOI] [PubMed] [Google Scholar]
- Mastelic Gavillet B, Eberhardt CS, Auderset F, Castellino F, Seubert A, Tregoning JS, Lambert P-H, De Gregorio E, Del Giudice G, and Siegrist C-A (2015). MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J Immunol 1402071. [DOI] [PubMed] [Google Scholar]
- McKee AS, and Marrack P (2017). Old and new adjuvants. Curr Opin Immunol 47, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli AP, Fontés G, Avery DT, Leddon SA, Tam M, Elliot M, Ballesteros-Tato A, Miller J, Stevenson MM, Fowell DJ, et al. (2016). The Integrin LFA-1 Controls T Follicular Helper Cell Generation and Maintenance. Immunity 45, 831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli AP, Fontés G, Leung Soo C, and King IL (2017). T Follicular Helper Cell-Derived IL-4 Is Required for IgE Production during Intestinal Helminth Infection. J Immunol 199, 244–252. [DOI] [PubMed] [Google Scholar]
- Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, Mutius, von E, Farrall M, Lathrop M, Cookson WOCM, et al. (2010). A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 363, 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody MA, Pedroza-Pacheco I, Vandergrift NA, Chui C, Lloyd KE, Parks R, Soderberg KA, Ogbe AT, Cohen MS, Liao H-X, et al. (2016). Immune perturbations in HIV-1-infected individuals who make broadly neutralizing antibodies. Science Immunology 1, aag0851–aag0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, and Irvine DJ (2012). Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci USA 109, 1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel S-E, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. (2011). Human blood CXCR5MCD4M T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowosad CR, Spillane KM, and Tolar P (2016). Germinal center B cells recognize antigen through a specialized immune synapse architecture. Nat Immunol 17, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang Y-H, and Dong C (2009). Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nus M, Sage AP, Lu Y, Masters L, Lam BYH, Newland S, Weller S, Tsiantoulas D, Raffort J, Marcus D, et al. (2017). Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nature Medicine 23, 601–610. [DOI] [PubMed] [Google Scholar]
- Papa I, Saliba D, Ponzoni M, Bustamante S, Cañete PF, Gonzalez-Figueroa P, McNamara HA, Valvo S, Grimbaldeston M, Sweet RA, et al. (2017). TFH-derived dopamine accelerates productive synapses in germinal centres. Nature 547, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, et al. (2017). Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 46, 1073–1088.e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedros C, Zhang Y, Hu JK, Choi YS, Canonigo-Balancio AJ, Yates JR, Altman A, Crotty S, and Kong K-F (2016). A TRAF-like motif of the inducible costimulator ICOS controls development of germinal center TFH cells via the kinase TBK1. Nat Immunol 17, 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis C, Jones GW, Bombardieri M, and Jones SA (2014). Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 14, 447–462. [DOI] [PubMed] [Google Scholar]
- Plotkin S, Orenstein W, and Offit P (2018). Plotkin’s vaccines. [Google Scholar]
- Polonskaya Z, Deng S, Sarkar A, Kain L, Comellas-Aragones M, McKay CS, Kaczanowska K, Holt M, McBride R, Palomo V, et al. (2017). T cells control the generation of nanomolar-affinity anti-glycan antibodies. J Clin Invest 127, 1491–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preite S, Cannons JL, Radtke AJ, Vujkovic-Cvijin I, Gomez-Rodriguez J, Volpi S, Huang B, Cheng J, Collins N, Reilley J, et al. (2018). Hyperactivated PI3Kδ promotes self and commensal reactivity at the expense of optimal humoral immunity. Nat Immunol 19, 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, Rigoni R, Radaelli E, Caprioli F, Preziuso S, et al. (2014). ATP-Gated Ionotropic P2X7 Receptor Controls Follicular T Helper Cell Numbers in Peyer’s Patches to Promote Host-Microbiota Mutualism. Immunity 41, 789–801. [DOI] [PubMed] [Google Scholar]
- Quinn JL, Kumar G, Agasing A, Ko RM, and Axtell RC (2018). Role of TFH Cells in Promoting T Helper 17-Induced Neuroinflammation. Front Immunol 9, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, Donlin LT, Henderson LA, Wei K, Mizoguchi F, et al. (2017). Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Staron MM, Shyer JA, Ho P-C, Marshall HD, Gray SM, Laidlaw BJ, Araki K, Ahmed R, Kaech SM, et al. (2015). The Interleukin-2-mTORc1 Kinase Axis Defines the Signaling, Differentiation, and Metabolism of T Helper 1 and Follicular B Helper T Cells. Immunity 43, 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Arnon TI, Rodda LB, Atakilit A, Sheppard D, and Cyster JG (2016). IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 352, aaf4822–aaf4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Liang H-E, and Locksley RM (2009). Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol 10, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riteau N, Radtke AJ, Shenderov K, Mittereder L, Oland SD, Hieny S, Jankovic D, and Sher A (2016). Water-in-Oil-Only Adjuvants Selectively Promote T Follicular Helper Cell Polarization through a Type I IFN and IL-6-Dependent Pathway. J Immunol 197, 3884–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritvo P-GG, Churlaud G, Quiniou V, Florez L, Brimaud F, Fourcade G, Mariotti-Ferrandiz E, and Klatzmann D (2017). Tfr cells lack IL-2Rα but express decoy IL-1R2 and IL-1Ra and suppress the IL-1-dependent activation of Tfh cells. Science Immunology 2, eaan0368. [DOI] [PubMed] [Google Scholar]
- Rookhuizen DC, and DeFranco AL (2014). Toll-like receptor 9 signaling acts on multiple elements of the germinal center to enhance antibody responses. Proc Natl Acad Sci USA 111, E3224–E3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, et al. (2016). Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell Rep 14, 68–81. [DOI] [PubMed] [Google Scholar]
- Ryu H, Lim H, Choi G, Park Y-J, Cho M, Na H, Ahn CW, Kim YC, Kim W-U, Lee S-H, et al. (2018). Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat Immunol 19, 583–593. [DOI] [PubMed] [Google Scholar]
- Sage PT, and Sharpe AH (2016). T follicular regulatory cells. Immunol Rev 271, 246–259. [DOI] [PubMed] [Google Scholar]
- Sage PT, Paterson AM, Lovitch SB, and Sharpe AH (2014). The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41, 1026–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin I, Radtke AJ, Vella LA, Jin W, Wherry EJ, Buggert M, Betts MR, Herati RS, Germain RN, and Canaday DH (2018). Spatial distribution and function of T follicular regulatory cells in human lymph nodes. J Exp Med 215, 1531–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Liu Y, Bentebibel SE, and Ueno H (2016). Molecular Mechanisms Regulating T Helper 1 versus T Follicular Helper Cell Differentiation in Humans. Cell Rep 16, 1082–1095. [DOI] [PubMed] [Google Scholar]
- Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, and Ueno H (2014). The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol 15, 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert TA, Victora GD, Fooksman DR, Kamphorst AO, Mugnier MR, Gitlin AD, Dustin ML, and Nussenzweig MC (2011). A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med 208, 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serr I, FÜrst RW, Ott VB, Scherm MG, Nikolaev A, Gökmen F, Kälin S, Zillmer S, Bunk M, Weigmann B, et al. (2016). miRNA92a targets KLF2 and the phosphatase PTEN signaling to promote human T follicular helper precursors in T1D islet autoimmunity. Proc Natl Acad Sci USA 113, E6659–E6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S, Lin X-J, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, et al. (2017). Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LA, Belanger S, Omilusik KD, Cho S, Scott-Browne JP, Nance JP, Goulding J, Lasorella A, Lu L-F, Crotty S, et al. (2016). Id2 reinforces TH1 differentiation and inhibits E2A to repress TFH differentiation. Nat Immunol 17, 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Hou S, Fang Q, Liu X, Liu X, and Qi H (2018). PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 49, 264–274.e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, Takahashi Y, Fukuyama H, Okada T, and Kurosaki T (2016). Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol 17, 861–869. [DOI] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, and Victora GD (2013). T follicular helper cell dynamics in germinal centers. Science 341, 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Weinstein JS, Lainez B, Esplugues E, Flavell RA, Craft JE, and Nussenzweig MC (2014). Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science 345, 1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, et al. (2018). Rheumatoid arthritis. Nat. Rev. Dis. Primers 4, 18001. [DOI] [PubMed] [Google Scholar]
- Suan D, Kräutler NJ, Maag JLV, Butt D, Bourne K, Hermes JR, Avery DT, Young C, Statham A, Elliott M, et al. (2017). CCR6 Defines Memory B Cell Precursors in Mouse and Human Germinal Centers, Revealing Light-Zone Location and Predominant Low Antigen Affinity. Immunity 47, 1142–1153.e1144. [DOI] [PubMed] [Google Scholar]
- Suan D, Nguyen A, Moran I, Bourne K, Hermes JR, Arshi M, Hampton HR, Tomura M, Miwa Y, Kelleher AD, et al. (2015). T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity 42, 704–718. [DOI] [PubMed] [Google Scholar]
- Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S-I, Seto Y, Hoshimoto A, Saito Y, Foster DC, and Iwamoto I (2002). Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood 100, 4565–4573. [DOI] [PubMed] [Google Scholar]
- Takatsuka S, Yamada H, Haniuda K, Saruwatari H, Ichihashi M, Renauld J-C, and Kitamura D (2018). IL-9 receptor signaling in memory B cells regulates humoral recall responses. Nat Immunol 19, 1025–1034. [DOI] [PubMed] [Google Scholar]
- Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, et al. (2016). Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci USA 201606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG (2015). Advances in IL-21 biology-enhancing our understanding of human disease. Curr Opin Immunol 34, 107–115. [DOI] [PubMed] [Google Scholar]
- Tarlinton D (2019). B cells still front and centre in immunology. Nat Rev Immunol 19, 871. [DOI] [PubMed] [Google Scholar]
- Taylor DK, Mittereder N, Kuta E, Delaney T, Burwell T, Dacosta K, Zhao W, Cheng LI, Brown C, Boutrin A, et al. (2018). T follicular helper-like cells contribute to skin fibrosis. Sci Transl Med 10, eaaf5307. [DOI] [PubMed] [Google Scholar]
- Teng F, Klinger CN, Felix KM, Bradley CP, Wu E, Tran NL, Umesaki Y, and Wu H-JJ (2016). Gut Microbiota Drive Autoimmune Arthritis by Promoting Differentiation and Migration of Peyer’s Patch T Follicular Helper Cells. Immunity 44, 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, and Wardemann H (2007). Autoreactivity in human IgG+ memory B cells. Immunity 26, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LM, and Yu D (2014). Follicular helper T-cell memory: establishing new frontiers during antibody response. Immunol. Cell Biol 92, 57–63. [DOI] [PubMed] [Google Scholar]
- Tubo NJ, Fife BT, Pagán AJ, Kotov DI, Goldberg MF, and Jenkins MK (2016). Most microbe-specific naïve CD4+ T cells produce memory cells during infection. Science 351, 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubo NJ, Pagán AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, and Jenkins MK (2013). Single Naive CD4(+) T Cells from a Diverse Repertoire Produce Different Effector Cell Types during Infection. Cell 153, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini M, Gerhard J, Burkert S, Jensen KJ, Georg P, Ebner F, Volkers SM, Thada S, Dietert K, Bauer L, et al. (2018). Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nat Immunol 19, 386–396. [DOI] [PubMed] [Google Scholar]
- van den Berg VJ, Haskard DO, Fedorowski A, Hartley A, Kardys I, Caga-Anan M, Akkerhuis KM, Oemrawsingh RM, van Geuns RJ, de Jaegere P, et al. (2018). IgM anti-malondialdehyde low density lipoprotein antibody levels indicate coronary heart disease and necrotic core characteristics in the Nordic Diltiazem (NORDIL) study and the Integrated Imaging and Biomarker Study 3 (IBIS-3). Ebiom 36, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu V, Mylvaganam GH, Gangadhara S, Hong JJ, Iyer SS, Gumber S, Ibegbu CC, Villinger F, and Amara RR (2016). Induction of Th1-Biased T Follicular Helper (Tfh) Cells in Lymphoid Tissues during Chronic Simian Immunodeficiency Virus Infection Defines Functionally Distinct Germinal Center Tfh Cells. J Immunol 197, 1832–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, and Nussenzweig MC (2012). Germinal centers. Annu Rev Immunol 30, 429–457. [DOI] [PubMed] [Google Scholar]
- Viisanen T, Ihantola E-L, Näntö-Salonen K, Hyöty H, Nurminen N, Selvenius J, Juutilainen A, Moilanen L, Pihlajamäki J, Veijola R, et al. (2017). Circulating CXCR5+PD-1+ICOS+ Follicular T Helper Cells Are Increased Close to the Diagnosis of Type 1 Diabetes in Children With Multiple Autoantibodies. Diabetes 66, 437–447. [DOI] [PubMed] [Google Scholar]
- Vijayan D, Mohd Redzwan N, Avery DT, Wirasinha RC, Brink R, Walters G, Adelstein S, Kobayashi M, Gray P, Elliott M, et al. (2016). IL-27 Directly Enhances Germinal Center B Cell Activity and Potentiates Lupus in Sanroque Mice. J Immunol 197, 3008–3017. [DOI] [PubMed] [Google Scholar]
- Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, Huang X, Interlandi J, Djuretic IM, Brown DR, et al. (2012). Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity 36, 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Linterman MA, Yu D, and Maclennan ICM (2016). Follicular Helper T Cells. Annu Rev Immunol 34, 335–368. [DOI] [PubMed] [Google Scholar]
- Vono M, Taccone M, Caccin P, Gallotta M, Donvito G, Falzoni S, Palmieri E, Pallaoro M, Rappuoli R, Di Virgilio F, et al. (2013). The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci USA 110, 21095–21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cho B, Suzuki K, Xu Y, Green JA, An J, and Cyster JG (2011). Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J Exp Med 208, 2497–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shi J, Yan J, Xiao Z, Hou X, Lu P, Hou S, Mao T, Liu W, Ma Y, et al. (2017). Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat Immunol 18, 921–930. [DOI] [PubMed] [Google Scholar]
- Weber JP, Fuhrmann F, Feist RK, Lahmann A, Baz, Al MS, Gentz L-J, Vu Van D, Mages HW, Haftmann C, Riedel R, et al. (2015). ICOS maintains the T follicular helper cell phenotype by down-regulating Kruppel-like factor 2. J Exp Med 212, 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, and Honjo T (2011). Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol 12, 264–270. [DOI] [PubMed] [Google Scholar]
- Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, and Craft J (2016). TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 17, 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JB, Ise W, Kurosaki T, and Sakaguchi S (2014). Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity 41, 1013–1025. [DOI] [PubMed] [Google Scholar]
- Wing JB, Kitagawa Y, Locci M, Hume H, Tay C, Morita T, Kidani Y, Matsuda K, Inoue T, Kurosaki T, et al. (2017). A distinct subpopulation of CD25- T-follicular regulatory cells localizes in the germinal centers. Proc Natl Acad Sci USA 114, E6400–E6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Chen Y, Liu H, Xu L-L, Teuscher P, Wang S, Lu S, and Dent AL (2016). Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur J Immunol 46, 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Shin HM, Moseman EA, Ji Y, Huang B, Harly C, Sen JM, Berg LJ, Gattinoni L, McGavern DB, et al. (2015). TCF1 Is Required for the T Follicular Helper Cell Response to Viral Infection. Cell Rep 12, 2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N, Eto D, Elly C, Peng G, Crotty S, and Liu Y-C (2014). The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat Immunol 15, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin G, Zander R, Schauder DM, Chen Y, Weinstein JS, Drobyski WR, Tarakanova V, Craft J, and Cui W (2018). Single-cell RNA sequencing unveils an IL-10-producing helper subset that sustains humoral immunity during persistent infection. Nat Commun 9, 5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, et al. (2013). Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 496, 523–527. [DOI] [PubMed] [Google Scholar]
- Xu L, Cao Y, Xie Z, Huang Q, Bai Q, Yang X, He R, Hao Y, Wang H, Zhao T, et al. (2015). The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat Immunol 16, 991–999. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Lynch RM, Gautam R, Matus-Nicodemos R, Schmidt SD, Boswell KL, Darko S, Wong P, Sheng Z, Petrovas C, et al. (2015). Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Sci Transl Med 7, 298ra120–298ra120. [DOI] [PubMed] [Google Scholar]
- Yao Y, Wang Z-C, Wang N, Zhou P-C, Chen C-L, Song J, Pan L, Liao B, Zhang X-H, Yang Y-S, et al. (2019). Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J Allergy Clin Immunol. [DOI] [PubMed] [Google Scholar]
- Yeh C-H, Nojima T, Kuraoka M, and Kelsoe G (2018). Germinal center entry not selection of B cells is controlled by peptide-MHCII complex density. Nat Commun 9, 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. (2009). The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468. [DOI] [PubMed] [Google Scholar]
- Yusuf I, Kageyama R, Monticelli L, Johnston RJ, DiToro D, Hansen K, Barnett B, and Crotty S (2010). Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol 185, 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tech L, George LA, Acs A, Durrett RE, Hess H, Walker LSK, Tarlinton DM, Fletcher AL, Hauser AE, et al. (2018). Plasma cell output from germinal centers is regulated by signals from Tfh and stromal cells. J Exp Med 215, 1227–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.