Abstract
Some animal groups associate with the same vertically transmitted microbial symbionts over extended periods of evolutionary time, punctuated by occasional symbiont switches to different microbial taxa. Here we test the oft-repeated suggestion that symbiont switches are linked with host diet changes, focusing on hemipteran insects of the suborder Auchenorrhyncha. These insects include the only animals that feed on plant xylem sap through the life cycle, as well as taxa that feed on phloem sap and plant parenchyma cells. Ancestral state reconstruction provides strong statistical support for a xylem feeding auchenorrhynchan ancestor bearing the dual symbiosis with the primary symbiont Sulcia (Bacteroidetes) and companion symbiont ‘β-Sym’ (β-proteobacteria). We identified seven dietary transitions from xylem feeding (six to phloem feeding, one to parenchyma feeding), but no reversions to xylem feeding; five evolutionary losses of Sulcia, including replacements by yeast symbionts, exclusively in phloem/parenchyma-feeding lineages; and 14–15 losses of β-Sym, including nine transitions to a different bacterial companion symbiont. Our analysis indicates that, although companion symbiont switching is not associated with shifts in host diet, Sulcia is probably required for xylem-feeding. Furthermore, the ancestral auchenorrhynchan bearing Sulcia and β-Sym probably represents the sole evolutionary origin of xylem feeding in the animal kingdom.
Keywords: mutualism, nutritional symbiosis, xylem, sap, symbiont switch, mutualism loss
1. Introduction
Various ecologically important traits of many animals are mediated by symbiotic microorganisms [1,2]. For example, microbial symbionts may produce toxins that protect their animal host from specific pathogens or synthesize nutrients that enable their host to use otherwise inadequate diets [3,4]. Many of these symbioses display high partner fidelity over long periods of evolutionary time, often with strict co-diversification of host and symbiont lineages [5,6]. However, both symbiont loss and symbiont switching, often to a phylogenetically distant microbial taxon, has been identified in many host lineages, especially among insects [7–10], as well as in other invertebrate taxa [11]. It has been suggested that symbiont switching may be linked to changes in host traits, e.g. shifts in habitat or diet [12,13], potentially with major ecological and evolutionary consequences for the host [14]. For instance, symbiont switching has been suggested to have been an important driver of host diversification [15,16]. Yet despite their potential importance, hypotheses of links between diet and symbiont switching have rarely been tested formally, limiting our insight into their general applicability across taxa. In recent years, however, our capacity to evaluate such hypotheses has greatly increased by advances in phylogenetic comparative methods, such as ancestral state reconstructions (ASRs) [17].
The purpose of this study was to investigate the patterns of symbiont switches by analysis of the temporal relationship between evolutionary shifts in host diet and symbiotic partners. Insects of the order Hemiptera are well suited to this problem for two linked reasons. First, uniquely among animals, some hemipterans can use different types of plant sap (i.e. phloem or xylem) through the life cycle; and, second, this trait is correlated with possession of vertically transmitted microbial symbionts that overproduce essential amino acids, nutrients in short supply in plant sap [1,18]. Our specific focus is the suborder Auchenorrhyncha, which includes the only insects that feed on xylem sap through the life cycle, as well as representatives that feed on phloem sap and the more nutritious diet of whole plant cell contents [19]. The Auchenorrhyncha comprises two infra-orders, the Fulgoromorpha (the planthoppers) and Cicadamorpha (including the leafhoppers, cicadas and froghoppers) [20,21]. Many taxa of both infra-orders bear a dual symbiosis with a bacterium Candidatus Sulcia muelleri (Bacteroidetes), also referred to as ‘the primary symbiont’ [1,22], and a β-proteobacterium, which is variously known as: β-Sym, Ca. Nasuia deltocephalinicola in leafhoppers, Ca. Vidania fulgoroideae in planthoppers and Ca. Zinderia insecticola in spittlebugs, or more generally as a ‘companion symbiont’ to Sulcia [23–27]. Some auchenorrhynchans, however, bear Sulcia as the sole symbiont, associate with a companion symbiont other than β-Sym [28–31], or have ascomycete fungal symbionts, generically known as yeast-like symbionts (YLS) [32–35]. This rich diversity of symbionts with functions closely tied to host diet provides a superb basis to test the relationship between switches in diet and symbiont identity.
In this study, we generated a detailed phylogeny of the Auchenorrhyncha. We then mapped key symbiont associations (primary and companion symbiont status) and host diet onto this phylogeny and used a comparative approach to study the association between symbiont and diet. This allowed us to investigate the patterns of association between transitions in symbiont identity—including symbiont losses—and diet across large numbers of clades over long periods of time.
2. Material and methods
(a). Phylogeny
We generated a phylogeny at the genus level for the Auchenorrhyncha using a supermatrix approach [36], using five marker genes that had previously been used to study hemipteran phylogeny (18S, 28S, COI, Wingless and Histone 3) [20,37,38]. We used phyloGenerator (v. 2) [39] to obtain sequences for all auchenorrhynchan genera with any of these markers available in NCBI GenBank. We employed the referenceDownload method to select high-quality representative sequences for each genus, based on reference sequences from [20]. A genus can thus be represented by sequences from different species within the genus. For each marker gene, the selected sequences were aligned using MAFFT (v. 7.310) [40]. We then concatenated the five alignments, and visually verified the quality of the resulting supermatrix of 824 genera and 7243 bp using Aliview (v. 1.20) [41]. We created a maximum-likelihood (ML) phylogeny in RAxML (v. 8.2.8) using a GTRCAT substitution model and rapid bootstrapping (100 replicates) followed by a thorough ML search [42]. The branch lengths of the bootstrap replicates were optimized using GTRGAMMA. Based on [20,21], the genera Ceratocombus (Heteroptera) and Xenophyes (Coleorrhyncha) were used as an outgroup to root the tree, and we used the NCBI taxonomy at the subfamily level to constrain our tree search. We used r8s (v. 1.8), employing the Langley–Fitch method and a truncated newton (TN) algorithm to convert our ML phylogeny to a timetree [43]. To date the phylogeny, we fixed the root to 299 Ma and constrained a further 17 nodes based on previous estimates of divergence times (electronic supplementary material, table S1).
(b). Trait dataset
We conducted a systematic search of the primary literature to compile information on three auchenorrhynchan traits of interest: (i) diet, (ii) primary symbiont association status, and (iii) companion symbiont association status. We obtained data from 34 different studies, covering a total of 162 unique genera (full references in the electronic supplementary material, table S3). We recorded data and performed all our analyses at the genus level because variation in the traits of interest was not evident at the subgenus level. Primary symbiosis was treated as a binary variable: the presence of primary endosymbiont Candidatus Sulcia muelleri versus absence [22]. For the companion symbiosis, we recorded the taxonomic identity of the endosymbiont. We then clustered this into a categorical variable with five levels: (i) α-proteobacteria (Candidatus Hodgkinia cicadicola), (ii) β-proteobacteria (Candidatus Vidania fulgoroideae; Candidatus Zinderia insecticola; Candidatus Nasuia deltocephalinicola), (iii) γ-proteobacteria (Candidatus Purcelliella pentastirinorum; a Sodalis-like bacterial symbiont; Candidatus Baumannia cicadellinicola), (iv) YLS (Ophiocordyceps, Entomomyces delphacidicola); and (v) companion symbionts absent. For the diet variable, each genus was assigned as phloem-feeder, xylem-feeder or parenchyma cell-feeder. Insects within the Fulgoromorpha, Membracidae and Deltocephalinae were inferred as phloem-feeders, and within the Cicadoidea, Cercopoidea, Cicadellinae as xylem-feeders [44–48]. One study reported an absence of both primary and companion symbionts in sap-feeding Auchenorrhyncha [35]. Given the nutritionally highly unbalanced composition of plant sap [1], these cases probably represent false negatives and were therefore not included in our dataset.
(c). Comparative analyses
All our comparative analyses were performed in R (v. 3.4.4.). The overlap of our full phylogeny containing 824 tips (genera), and our full trait database (162 genera) was equal to 145 genera. Of these, we had data on primary symbiont status for 142 genera: this is the dataset we included in our analyses (full analysed genus list is available in the electronic supplementary material, table S4). The full phylogeny was pruned to comprise the 142 tip genera. We first performed ASRs to elucidate the evolutionary history of our three traits of interest. We then compared correlated and uncorrelated models of evolution to study the potential for evolutionary correlations between traits [49].
For our ASRs of both diet (three levels) and companion symbiont association status (five levels), we fitted a model of evolution for categorical traits, using the R-package corHMM (v. 1.22) [50]. We generated equal rates (ER), symmetrical (SYM) and all-rates-different (ARD) models of evolution and used Akaike information criterion corrected for small sample size (AICc)-weights to guide our model selection. In our diet ASR, we treated diet state as missing for our outgroup. For our ASR of the primary symbiosis, we analysed hidden rates models, which allow for potential variation in the rate of evolution of a binary trait [50]. We used marginal likelihoods to estimate ancestral states and employed Yang's method to determine the ancestral root state for diet and companion symbiosis [51] while constraining the root to primary symbiont presence. As before, we calculated the number of transitions between evolutionary states assuming parsimony [13,52].
We used Pagel's method to study potential correlated evolution among binary traits of interest, specifically host diet and symbiotic association status [49]. Given that xylem is under negative pressure and is particularly nutrient poor, using it as a food source requires a highly specialized set of adaptations including an enlarged muscle-filled head to facilitate active pumping of the xylem sap [53]. We therefore analysed diet as a binary variable of xylem feeding versus other food sources (i.e. phloem or parenchyma feeding). For the companion symbiosis, we generated three binary variables, representing the presence or absence of the main types of companion symbionts (i.e. β-proteobacteria, γ-proteobacteria and YLS; we did not analyse α-proteobacteria this way because they are found in only two genera). In all cases, we used R-package phytools (v. 0.6–44) [54] to compare correlated and uncorrelated models of evolution using likelihood ratio tests.
(d). Sensitivity
All results from phylogenetic comparative analyses are subject to uncertainty in the underlying data [55]. We therefore characterized the sensitivity of our key result of correlated evolution among primary endosymbiosis and diet status to (i) phylogenetic, (ii) trait, and (iii) taxon sampling uncertainty. To quantify sensitivity to phylogenetic uncertainty, we regenerated our model across the hundred bootstrap replica that were used to obtain support for our ML phylogeny and characterized the p-value and Δ-AICc for each rerun (electronic supplementary material, figure S6). To study the effects of trait uncertainty, we simulated trait dataset based on our original assignments, assuming separate false-positive and false-negative rates for our data of primary symbiont (Sulcia) presence (electronic supplementary material, figure S7). This allows us to simulate an unbiased but inaccurate dataset (both high false-positive and false-negative), as well as biases in the field towards detection of symbionts (high false-positive, low false-negative) or towards not finding symbionts which are actually there (high false-negative rate, low false-positive). Third, we tested if our key conclusions are robust to the specific genus set sampled (electronic supplementary material, figure S8). This helps address the potential for influential (groups of) genera driving the results. To do this, we used a jack-knifing approach, randomly removing up to 30% of the genera from the analyses [55].
3. Results
(a). Ancestral state reconstructions
In our first analysis of 142 genera of auchenorrhynchan insects that have both phylogenetic information and trait data, we investigated the evolutionary transitions in the diet. The evolutionary model with the best statistical support (electronic supplementary material, table S2) estimates xylem as the most likely ancestral state of the Auchenorrhyncha (likelihood of xylem-feeder: 90.1%; parenchyma-feeder 9.9%). In this model, xylem feeding is subsequently lost at the origin of the Fulgoromorpha (planthoppers), and five times within the Cicadomorpha (comprising leafhoppers, treehoppers, cicadas and spittlebugs) (figure 1; electronic supplementary material, figures S1 and S2). All diet switches are from xylem to phloem sap, apart from a single switch from xylem feeding to parenchyma feeding near the base of the Typhlocybinae (Cicadomorpha: Cicadellidae). All instances of phloem feeding in the Auchenorrhyncha appear to be an evolutionary end state, but the model predicts one further transition, from parenchyma feeding to phloem feeding in the Ledrinae (Cicadomorpha: Cicadellidae).
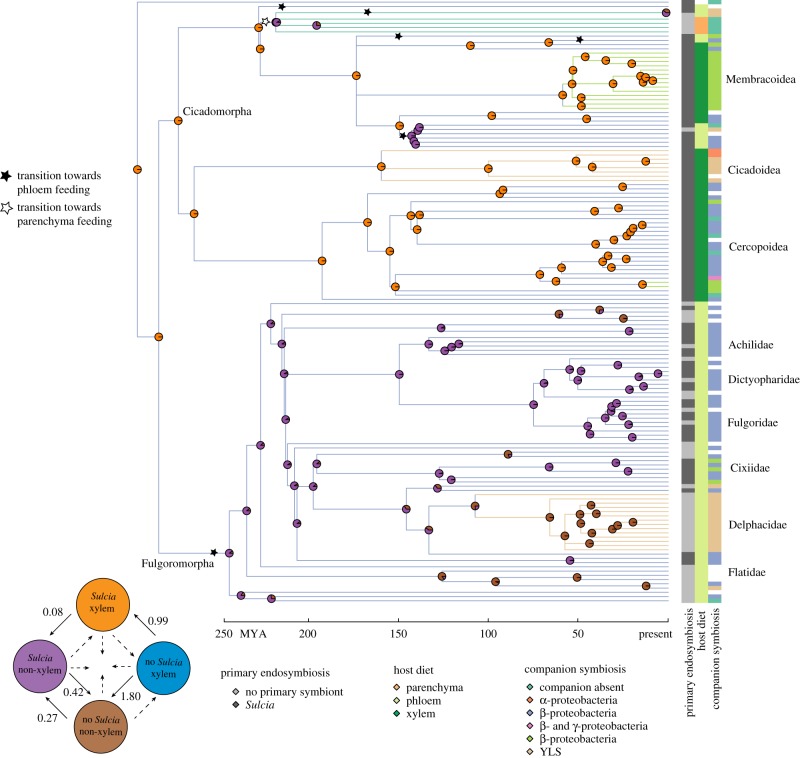
Figure 1.
Analysed phylogeny trait data and key ancestral state reconstructions of the Auchenorrhyncha. Vertical bands at the tips of the 142-genus phylogeny indicate—from left to right—primary endosymbiosis, status host diet and companion symbiosis status. In all bands, missing data (i.e. traits could not be estimated reliably) are represented in white. Branch colours indicate inferred companion symbiont status (full model shown in the electronic supplementary material, figure S5). Stars indicate the branch along which transitions from the ancestral xylem-feeding state towards either phloem feeding (filled stars) or parenchyma feeding (open star) are inferred to have occurred (full model in the electronic supplementary material, figures S1 and S2). The inset figure shows the model of correlated evolution among host diet (xylem versus non-xylem feeding) and primary endosymbiosis status (presence and absence) indicating transition rates between the four potential combinations of these two binary traits. To facilitate interpretation, transition rates are multiplied by hundred and can thus be interpreted as number of transitions per lineage per 100 million years. Transitions that are possible under the model but were inferred to be non-existent are indicated with dashed arrows. The corresponding ancestral state reconstruction is represented on the phylogeny by pie charts. From the ancestral state of feeding on xylem and associating with Sulcia (orange), primary symbiosis loss (transition towards brown) occurred five times and was always preceded by a diet shift away from xylem feeding (purple) sometimes tens of millions of years earlier.
In the best-supported model for the primary symbiont Sulcia, the association is initially in a relatively unstable evolutionary state (electronic supplementary material, figures S3 and S4). Specifically, the symbiosis is lost five times (calculated 4.90 losses) across the Auchenorrhyncha but never regained (calculated 0.19 gains). Four of these losses occur predominantly within the Fulgoromorpha, with a single instance in the Cicadomorpha, in the Ledrinae. In the other lineages of the Cicadomorpha, Sulcia presence is retained, and transitions towards a stable evolutionary state (electronic supplementary material, figure S4). All losses occur in lineages that have switched from xylem to phloem or parenchyma feeding.
For the companion symbionts, the best-supported model is symmetrical, i.e. with equal transition rates between two states in both directions (electronic supplementary material, table S2). The model predicts that the Auchenorrhyncha was ancestrally associated with the β-Sym symbiont (likelihood β-Sym 97.1%; companion absence 2.9%; figure 1 and electronic supplementary material, figure S5), which has been retained in most Fulgoromorpha, as well as many Cercopoidea and Cicadellidae within the Cicadomorpha. In other lineages, however, β-Sym was not stable, with at least nine switches to alternative companion symbionts and a further five or six losses without replacement by a taxonomically different companion symbiont (figure 1). In contrast to the losses of the primary Sulcia symbiosis, these losses take place across all three diets observed in the Auchenorrhyncha.
(b). Correlated evolution
To evaluate the hypothesis that diet changes co-occur with changes in symbiont composition, we compared correlated and uncorrelated models of evolution. This analysis yielded strong evidence of correlated evolution of primary endosymbiont (Sulcia) status and diet (likelihood ratio test: p ≪ 0.01), but no statistically significant evidence of correlated evolution of diet with any group of companion symbionts (β-Sym, p = 0.49), γ-proteobacteria, p = 0.06, YLS, p = 0.70). Specifically, the primary symbiont Sulcia is lost exclusively in insects that have switched from feeding on xylem to consuming phloem or parenchyma (figure 1). The associated ancestral state reconstruction confirms that the loss of Sulcia is invariably preceded by loss of xylem feeding, generally by dozens of millions of years (and in one case by up to 185 Myr). Thus, loss of xylem feeding appears to be a required, but probably not sufficient, condition for Sulcia loss. In summary, these results indicate an indirect evolutionary link between a diet with loss of the Sulcia primary symbiont but not with changes of the companion symbiont.
(c). Sensitivity analyses
We evaluated the sensitivity of our correlated evolution results to phylogenetic, data and sampling uncertainty using a simulation approach [55]. We found that our key conclusions are robust to phylogenetic uncertainty (electronic supplementary material, figure S6). We also simulated errors in our underlying trait data and found that correlated evolution conclusions were robust even to 25% of our underlying trait data being mistaken, including to biased over- or under-detection of symbioses (electronic supplementary material, figure S7). Lastly, we found that our key results are robust to variation in genus sampling (electronic supplementary material, figure S8).
4. Discussion
It has been argued in much of the symbiosis literature that evolutionary acquisition, loss or switching of microbial symbionts have been drivers of major evolutionary changes in host traits, especially diets of animals [1,2,14,56–58]. Despite the seemingly close link between host diet and symbiont function in the Auchenorrhyncha, the correlations identified in this study provide limited support for this proposition. Although our finding of a perfect association between xylem feeding and retention of the primary symbiont Sulcia is consistent with the proposed relationship between symbiont and diet, two other findings of this study are inconsistent. First, we obtain no association between change or loss of companion symbionts and diet switching; both occur across all auchenorrhynchan diets. Second, the loss of Sulcia in some phloem-feeding lineages occurs tens of millions of years after the dietary switch from xylem feeding (figure 1), suggesting that the change in diet is permissive for—and not a consequence of—symbiosis change.
We have also obtained strong statistical support for a xylem-feeding auchenorrhynchan ancestor. This conclusion contrasts with the hypothesis that xylem feeding evolved in the ancestor of the Cicadomorpha, proposed in a recent study [59] that was not based on a formal ancestral trait reconstruction of insect diet and contained only few Auchenorrhyncha species. Our analyses indicate multiple switches from xylem feeding to phloem- and parenchyma feeding, but no reversions back to a diet of xylem sap. Given that no other animal group is known to feed through the life cycle on xylem [60], we conclude that the ancestral auchenorrhynchan represents the sole evolutionary origin of the xylem-feeding habit in the animal kingdom.
This study has provided a quantitative phylogenetic framework for the many instances of symbiont gain, loss and switching reported for the Auchenorrhyncha in the literature [10,27]. In particular, our quantitative data validate the long-held belief that the xylem-feeding habit in the Auchenorrhyncha is perfectly correlated with the possession of bacterial symbionts [1] and confirm the inference from previous studies that the ancestral symbiosis comprised the primary symbiont Sulcia and companion symbiont β-Sym [22,27,28]. The retention of Sulcia in all xylem-feeding lineages, despite multiple switches of the companion symbiont, suggests that Sulcia has unique traits that permit xylem feeding. A possible explanation comes from recent metabolic modelling, which has revealed that Sulcia converts host-derived nitrogen sources into essential amino acids with very high efficiency [61]. This quantitative metabolic trait may be especially valuable for host use of xylem sap, which has a markedly lower total nitrogen content than phloem sap [60]. In other words, the fitness consequences of a symbiont switch from Sulcia to a less-efficient symbiont may be less severe for a phloem-feeding insect, thereby explaining how Sulcia has been replaced by other taxa in some phloem-feeding auchenorrhynchan lineages, but in no xylem-feeding lineages. Intriguingly, most replacements of Sulcia are evolutionarily rather ancient, yielding a pattern of increased stability of the Sulcia symbiosis over time (electronic supplementary material, figure S4). Further research is required to investigate whether other ancient symbionts display a similar pattern of increasing stabilization over time and to identify the contribution of metabolic and other co-evolved host–symbiont interactions to this trait.
What are the factors driving the many instances of symbiont loss and replacement that are not correlated with dietary switches in the Auchenorrhyncha? These losses may be mediated by ecological factors other than diet. For example, many insect symbionts are relatively intolerant of thermal stress [62], and the replacement of thermally intolerant symbionts by tolerant strains has been demonstrated in insects exposed to elevated temperatures in the laboratory [63]. Evolutionary or co-evolutionary processes internal to the symbiosis may also be involved. In particular, these symbionts are subject to genomic decay because obligate vertical transmission of small numbers of symbiont cells results in inefficient selection against deleterious mutations and reduced symbiont functionality [64–66], favouring the replacement of a deteriorating symbiont by an alternative taxon [10,14,28]. The incidence of symbiont replacements may, however, be constrained by the high metabolic cost of maintaining a symbiont of large genome size [61] or by traits of the incoming symbiont that may be deleterious to the host [33].
Is the poor correspondence between evolutionary transitions in symbiont identity and host diet in the Auchenorrhyncha generalizable to other symbioses? A key feature of this system is that the symbiont services to the host relate to insect nutrition, involving the provisioning of essential amino acids and vitamins, and are broadly equivalent for insects feeding on xylem and phloem sap [67]. We predict that, as for the Auchenorrhyncha, the many instances of symbiont switches in other hemipteran groups with nutritional symbioses [10,14] are largely uncoupled to diet-related factors. Symbiont switches in aphids of the subfamily Lachninae (Hemiptera: suborder Sternorrhyncha) are fully consistent with this prediction [68]. Future studies on the patterns of symbiosis switches across larger phylogenetic scales in hemipterans other than the Auchenorrhyncha, as well as in other animal groups with nutritional symbioses, would provide valuable insights into this topic. We hypothesize that the evolutionary co-incidence of symbiont switches and shifts in ecologically important traits of the host may be more evident for protective functions, e.g. symbionts that either produce toxins which confer resistance to natural enemies or detoxify dietary allelochemicals [3,69]. Here, acquisition of a symbiont capable of synthesizing or degrading novel toxins could facilitate the exploitation of novel habitats or diets, potentially with consequences for evolutionary patterns of speciation and adaptive diversification in the host lineage. Other future research could check for symbiont switching between closely related taxa using phylogenomic analysis to test for incongruencies between phylogenies of hosts and both primary and companion endosymbionts [22]. These hypotheses are increasingly becoming testable owing to (i) the routine availability of molecular tools for phylogenetics and symbiont identification, and (ii) development of sophisticated comparative methods to test for large-scale patterns in the relationships between symbiont switches and host divergence and trait evolution.
Supplementary Material
Acknowledgements
We thank Julie Urban for advice and assistance with producing the phylogeny, and two anonymous reviewers for their useful and constructive feedback.
Data accessibility
Our full analysed insect trait database, phylogeny and alignment files, sequence accession table and all R-code to repeat our analyses and generate figures are available on GitHub (https://github.com/gijsbertwerner/aucho_endosymbionts) and from the Dryad Digital Repository: https://doi.org/10.5061/dryad.95p761k [70].
Authors' contributions
L.B.-R., A.E.D. and G.D.A.W. designed the study, L.B.-R. and G.D.A.W. compiled the database, generated the phylogeny and performed the comparative analyses. G.D.A.W. and A.E.D. wrote the manuscript, and all authors commented on the manuscript.
Competing interests
We declare no competing interests.
Funding
This research was funded by a Royal Society Newton International Fellowship and a Junior Research Fellowship (Balliol College Oxford) to G.D.A.W. and by NSF grant no. IOS-1354743 to A.E.D.
References
- 1.Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. New York, NY: John Wiley & Sons. [Google Scholar]
- 2.Douglas AE. 2010. The symbiotic habit. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Flórez LV, Biedermann PHW, Engl T, Kaltenpoth M. 2015. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat. Prod. Rep. 32, 904–936. ( 10.1039/c5np00010f) [DOI] [PubMed] [Google Scholar]
- 4.Douglas AE. 2009. The microbial dimension in insect nutritional ecology. Funct. Ecol. 23, 38–47. ( 10.1111/j.1365-2435.2008.01442.x) [DOI] [Google Scholar]
- 5.Bright M, Bulgheresi S. 2010. A complex journey: transmission of microbial symbionts. Nat. Rev. Microbiol. 8, 218–230. ( 10.1038/nrmicro2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran NA, Munson MA, Baumann P, Ishikawa H. 1993. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc. R. Soc. Lond. B 253, 167–171. ( 10.1098/rspb.1993.0098) [DOI] [Google Scholar]
- 7.Cafaro MJ, et al. 2011. Specificity in the symbiotic association between fungus-growing ants and protective Pseudonocardia bacteria. Proc. R. Soc. B 278, 1814–1822. ( 10.1098/rspb.2010.2118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzano-Marín A, Szabó G, Simon J-C, Horn M, Latorre A. 2017. Happens in the best of subfamilies: establishment and repeated replacements of co-obligate secondary endosymbionts within Lachninae aphids. Environ. Microbiol. 19, 393–408. ( 10.1111/1462-2920.13633) [DOI] [PubMed] [Google Scholar]
- 9.Toju H, Tanabe AS, Notsu Y, Sota T, Fukatsu T. 2013. Diversification of endosymbiosis: replacements, co-speciation and promiscuity of bacteriocyte symbionts in weevils. ISME J. 7, 1378–1390. ( 10.1038/ismej.2013.27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas AE. 2016. How multi-partner endosymbioses function. Nat. Rev. Microbiol. 14, 731–743. ( 10.1038/nrmicro.2016.151) [DOI] [PubMed] [Google Scholar]
- 11.Ozawa G, Shimamura S, Takaki Y, Takishita K, Ikuta T, Barry JP, Maruyama T, Fujikura K, Yoshida T. 2017. Ancient occasional host switching of maternally transmitted bacterial symbionts of chemosynthetic vesicomyid clams. Genome Biol. Evol. 9, 2226–2236. ( 10.1093/gbe/evx166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefèvre C, Charles H, Vallier A, Delobel B, Farrell B, Heddi A. 2004. Endosymbiont phylogenesis in the dryophthoridae weevils: evidence for bacterial replacement. Mol. Biol. Evol. 21, 965–973. ( 10.1093/molbev/msh063) [DOI] [PubMed] [Google Scholar]
- 13.Werner GDA, Cornelissen JHC, Cornwell WK, Soudzilovskaia NA, Kattge J, West SA, Kiers ET. 2018. Symbiont switching and alternative resource acquisition strategies drive mutualism breakdown. Proc. Natl Acad. Sci. USA 115, 5229–5234. ( 10.1073/pnas.1721629115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudakaran S, Kost C, Kaltenpoth M. 2017. Symbiont acquisition and replacement as a source of ecological innovation. Trends Microbiol. 25, 375–390. ( 10.1016/j.tim.2017.02.014) [DOI] [PubMed] [Google Scholar]
- 15.Sudakaran S, Retz F, Kikuchi Y, Kost C, Kaltenpoth M. 2015. Evolutionary transition in symbiotic syndromes enabled diversification of phytophagous insects on an imbalanced diet. ISME J. 9, 1–18. ( 10.1038/ismej.2015.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joy JB. 2013. Symbiosis catalyses niche expansion and diversification. Proc. R. Soc. B 280, 20122820 ( 10.1098/rspb.2012.2820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garamszegi LZ. (ed.). 2014. Modern phylogenetic comparative methods and their application in evolutionary biology. Berlin, Germany: Springer. [Google Scholar]
- 18.Douglas AE. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43, 17–37. ( 10.1146/annurev.ento.43.1.17) [DOI] [PubMed] [Google Scholar]
- 19.Dolling WR. 1991. The hemiptera. London, UK: The Natural History Museum Publications. [Google Scholar]
- 20.Cryan JR, Urban JM. 2012. Higher-level phylogeny of the insect order Hemiptera: is Auchenorrhyncha really paraphyletic? Syst. Entomol. 37, 7–21. ( 10.1111/j.1365-3113.2011.00611.x) [DOI] [Google Scholar]
- 21.Li H, Leavengood JM, Chapman EG, Burkhardt D, Song F, Jiang P, Liu J, Zhou X, Cai W. 2017. Mitochondrial phylogenomics of Hemiptera reveals adaptive innovations driving the diversification of true bugs. Proc. R. Soc. B 284, 20171223 ( 10.1098/rspb.2017.1223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran NA, Tran P, Gerardo NM. 2005. Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl. Environ. Microbiol. 71, 8802–8810. ( 10.1128/AEM.71.12.8802-8810.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett GM, Moran NA. 2013. Small smaller smallest: the origins and evolution of ancient dual symbioses in a phloem-feeding insect. Genome Biol. Evol. 5, 1675–1688. ( 10.1093/gbe/evt118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonella E, et al. 2011. Bacterial endosymbiont localization in Hyalesthes obsoletus the insect vector of Bois Noir in Vitis vinifera. Appl. Environ. Microbiol. 77, 1423–1435. ( 10.1128/AEM.02121-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCutcheon JP, Moran NA. 2010. Functional convergence in reduced genomes of bacterial symbionts spanning 200 My of evolution. Genome Biol. Evol. 2, 708–718. ( 10.1093/gbe/evq055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noda H, Watanabe K, Kawai S, Yukuhiro F, Miyoshi T, Tomizawa M, Koizumi Y, Nikoh N, Fukatsu T. 2012. Bacteriome-associated endosymbionts of the green rice leafhopper Nephotettix cincticeps (Hemiptera: Cicadellidae). Appl. Entomol. Zool. 47, 217–225. ( 10.1007/s13355-012-0110-1) [DOI] [Google Scholar]
- 27.Muller HJ. 1962. Neuere vorstellungen uber verbreitung und phylogenie der endosymbiosen der zikaden. Zeitschrift fur Morphol. und oekologie der Tiere 51, 190–210. ( 10.1007/BF00409635) [DOI] [Google Scholar]
- 28.Koga R, Bennett GM, Cryan JR, Moran NA. 2013. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ. Microbiol. 15, 2073–2081. ( 10.1111/1462-2920.12121) [DOI] [PubMed] [Google Scholar]
- 29.Mao M, Yang X, Poff K, Bennett G. 2017. Comparative genomics of the dual-obligate symbionts from the treehopper Entylia carinata (Hemiptera: Membracidae) provide insight into the origins and evolution of an ancient symbiosis. Genome Biol. Evol. 9, 1803–1815. ( 10.1093/gbe/evx134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCutcheon JP, McDonald BR, Moran NA. 2009. Origin of an alternative genetic code in the extremely small and GC–rich genome of a bacterial symbiont. PLoS Genet. 5, e1000565 ( 10.1371/journal.pgen.1000565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCutcheon JP, Moran NA. 2007. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc. Natl Acad. Sci. USA 104, 19 392–19 397. ( 10.1073/pnas.0708855104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobiałka M, Michalik A, Walczak M, Szklarzewicz T. 2018. Dual ‘bacterial-fungal’ symbiosis in Deltocephalinae leafhoppers (Insecta Hemiptera Cicadomorpha: Cicadellidae). Microb. Ecol. 75, 771–782. ( 10.1007/s00248-017-1075-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuura Y, Moriyama M, Łukasik P, Vanderpool D, Tanahashi M, Meng X-Y, McCutcheon JP, Fukatsu T. 2018. Recurrent symbiont recruitment from fungal parasites in cicadas. Proc. Natl Acad. Sci. USA 115, E5970–E5979. ( 10.1073/pnas.1803245115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noda H, Nakashima N, Koizumi M. 1995. Phylogenetic position of yeast-like symbiotes of rice planthoppers based on partial 18S rDNA sequences. Insect Biochem. Mol. Biol. 25, 639–646. ( 10.1016/0965-1748(94)00107-S) [DOI] [PubMed] [Google Scholar]
- 35.Urban JM, Cryan JR. 2012. Two ancient bacterial endosymbionts have coevolved with the planthoppers (Insecta: Hemiptera: Fulgoroidea). BMC Evol. Biol. 12, 87 ( 10.1186/1471-2148-12-87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Queiroz A, Gatesy J. 2007. The supermatrix approach to systematics. Trends Ecol. Evol. 22, 34–41. ( 10.1016/j.tree.2006.10.002) [DOI] [PubMed] [Google Scholar]
- 37.Cryan JR. 2005. Molecular phylogeny of Cicadomorpha (Insecta: Hemiptera: Cicadoidea Cercopoidea and Membracoidea): adding evidence to the controversy. Syst. Entomol. 30, 563–574. ( 10.1111/j.1365-3113.2004.00285.x) [DOI] [Google Scholar]
- 38.Urban JM, Cryan JR. 2009. Entomologically famous evolutionarily unexplored: the first phylogeny of the lanternfly family Fulgoridae (Insecta: Hemiptera: Fulgoroidea). Mol. Phylogenet. Evol. 50, 471–484. ( 10.1016/j.ympev.2008.12.004) [DOI] [PubMed] [Google Scholar]
- 39.Pearse WD, Purvis A. 2013. phyloGenerator: an automated phylogeny generation tool for ecologists. Methods Ecol. Evol. 4, 692–698. ( 10.1111/2041-210X.12055) [DOI] [Google Scholar]
- 40.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. ( 10.1093/molbev/mst010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson A. 2014. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30, 3276–3278. ( 10.1093/bioinformatics/btu531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. ( 10.1093/bioinformatics/btu033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanderson MJ. 2003. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302. ( 10.1093/bioinformatics/19.2.301) [DOI] [PubMed] [Google Scholar]
- 44.Zahniser JN, Dietrich CH. 2010. Phylogeny of the leafhopper subfamily Deltocephalinae (Hemiptera: Cicadellidae) based on molecular and morphological data with a revised family-group classification. Syst. Entomol. 35, 489–511. ( 10.1111/j.1365-3113.2010.00522.x) [DOI] [Google Scholar]
- 45.Redak RA, Purcell AH, Lopes JRS, Blua MJ, Mizell RF III, Andersen PC. 2004. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 49, 243–270. ( 10.1146/annurev.ento.49.061802.123403) [DOI] [PubMed] [Google Scholar]
- 46.Dietrich C. 2003. Auchenorrhyncha (cicadas spittlebugs leafhoppers treehoppers and planthoppers). In Encyclopedia of insects, pp. 66–74. New York, NY: Academic Press. [Google Scholar]
- 47.Young DA. 1968. Taxonomic study of the Cicadellinae (Homoptera: Cicadellidae) pt. 1: Proconiini. Bull. U.S. Natl Mus. 261, 1–287. ( 10.5479/si.03629236.261.1) [DOI] [Google Scholar]
- 48.Cryan JR, Svenson GJ. 2010. Family-level relationships of the spittlebugs and froghoppers (Hemiptera: Cicadomorpha: Cercopoidea). Syst. Entomol. 35, 393–415. ( 10.1111/j.1365-3113.2009.00520.x) [DOI] [Google Scholar]
- 49.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45. ( 10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 50.Beaulieu JM, O'Meara BC, Donoghue MJ. 2013. Identifying hidden rate changes in the evolution of a binary morphological character: the evolution of plant habit in campanulid angiosperms. Syst. Biol. 62, 725–737. ( 10.1093/sysbio/syt034) [DOI] [PubMed] [Google Scholar]
- 51.Yang Z. 2006. Computational molecular evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 52.Werner GDA, Cornwell WK, Sprent JI, Kattge J, Kiers ET. 2014. A single evolutionary innovation drives the deep evolution of symbiotic N2-fixation in angiosperms. Nat. Commun. 5, 4087 ( 10.1038/ncomms5087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novotny V, Wilson MR. 1997. Why are there no small species among xylem-sucking insects? Evol. Ecol. 11, 419–437. ( 10.1023/A:1018432807165) [DOI] [Google Scholar]
- 54.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 55.Paterno GB, Penone C, Werner GDA. 2018. sensiPhy: an r-package for sensitivity analysis in phylogenetic comparative methods. Methods Ecol. Evol. 9, 1461–1467. ( 10.1111/2041-210X.12990) [DOI] [Google Scholar]
- 56.Hansen AK, Moran NA. 2014. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol. Ecol. 23, 1473–1496. ( 10.1111/mec.12421) [DOI] [PubMed] [Google Scholar]
- 57.Rolshausen G, Dal Grande F, Sadowska-Deś AD, Otte J, Schmitt I. 2018. Quantifying the climatic niche of symbiont partners in a lichen symbiosis indicates mutualist-mediated niche expansions. Ecography (Cop.) 41, 1380–1392. ( 10.1111/ecog.03457) [DOI] [Google Scholar]
- 58.McFall-Ngai M, et al. 2013. Animals in a bacterial world a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236. ( 10.1073/pnas.1218525110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson KP, et al. 2018. Phylogenomics and the evolution of hemipteroid insects. Proc. Natl Acad. Sci. USA 115, 12 775–12 780. ( 10.1073/pnas.1815820115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raven JA. 1983. Phytophages of xylem and phloem: a comparison of animal and plant sap-feeders. Adv. Ecol. Res. 13, 135–234. ( 10.1016/S0065-2504(08)60109-9) [DOI] [Google Scholar]
- 61.Ankrah NYD, Chouaia B, Douglas AE. 2018. The cost of metabolic interactions in symbioses between insects and bacteria with reduced genomes. MBio 9, 1–15. ( 10.1128/mBio.01433-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wernegreen JJ. 2012. Mutualism meltdown in insects: bacteria constrain thermal adaptation. Curr. Opin. Microbiol. 15, 255–262. ( 10.1016/j.mib.2012.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moran NA, Yun Y. 2015. Experimental replacement of an obligate insect symbiont. Proc. Natl Acad. Sci. USA 112, 2093–2096. ( 10.1073/pnas.1420037112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bennett GM, Moran NA. 2015. Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc. Natl Acad. Sci. USA 112, 10 169–10 176. ( 10.1073/pnas.1421388112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campbell MA, Łukasik P, Simon C, McCutcheon JP. 2017. Idiosyncratic genome degradation in a bacterial endosymbiont of periodical cicadas. Curr. Biol. 27, 3568–3575.e3. ( 10.1016/j.cub.2017.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moran NA. 1996. Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc. Natl Acad. Sci. USA 93, 2873–2878. ( 10.1073/pnas.93.7.2873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Douglas AE. 2015. Multiorganismal insects: diversity and function of resident microorganisms. Annu. Rev. Entomol. 60, 17–34. ( 10.1146/annurev-ento-010814-020822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meseguer AS, Manzano-Marín A, Coeur d'Acier A, Clamens A-L, Godefroid M, Jousselin E. 2017. Buchnera has changed flatmate but the repeated replacement of co-obligate symbionts is not associated with the ecological expansions of their aphid hosts. Mol. Ecol. 26, 2363–2378. ( 10.1111/mec.13910) [DOI] [PubMed] [Google Scholar]
- 69.van den Bosch TJM, Welte CU. 2017. Detoxifying symbionts in agriculturally important pest insects. Microb. Biotechnol. 10, 531–540. ( 10.1111/1751-7915.12483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bell-Roberts L, Douglas AE, Werner GDA.2019. Data from: Match and mismatch between dietary switches and microbial partners in plant sap-feeding insects. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bell-Roberts L, Douglas AE, Werner GDA.2019. Data from: Match and mismatch between dietary switches and microbial partners in plant sap-feeding insects. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Our full analysed insect trait database, phylogeny and alignment files, sequence accession table and all R-code to repeat our analyses and generate figures are available on GitHub (https://github.com/gijsbertwerner/aucho_endosymbionts) and from the Dryad Digital Repository: https://doi.org/10.5061/dryad.95p761k [70].