Abstract
Background
Schistosoma mansoni is a parasitic infection common in the tropics and sub‐tropics. Chronic and advanced disease includes abdominal pain, diarrhoea, blood in the stool, liver cirrhosis, portal hypertension, and premature death.
Objectives
To evaluate the effects of antischistosomal drugs, used alone or in combination, for treating S. mansoni infection.
Search methods
We searched MEDLINE, EMBASE and LILACS from inception to October 2012, with no language restrictions. We also searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (The Cochrane Library 2012) and mRCT. The reference lists of articles were reviewed and experts were contacted for unpublished studies.
Selection criteria
Randomized controlled trials of antischistosomal drugs, used alone or in combination, versus placebo, different antischistosomal drugs, or different doses of the same antischistosomal drug for treating S. mansoni infection.
Data collection and analysis
One author extracted data and assessed eligibility and risk of bias in the included studies, which were independently checked by a second author. We combined dichotomous outcomes using risk ratio (RR) and continuous data weighted mean difference (WMD); we presented both with 95% confidence intervals (CI). We assessed the quality of evidence using the GRADE approach.
Main results
Fifty‐two trials enrolling 10,269 participants were included. The evidence was of moderate or low quality due to the trial methods and small numbers of included participants.
Praziquantel
Compared to placebo, praziquantel 40 mg/kg probably reduces parasitological treatment failure at one month post‐treatment (RR 3.13, 95% CI 1.03 to 9.53, two trials, 414 participants, moderate quality evidence). Compared to this standard dose, lower doses may be inferior (30 mg/kg: RR 1.52, 95% CI 1.15 to 2.01, three trials, 521 participants, low quality evidence; 20 mg/kg: RR 2.23, 95% CI 1.64 to 3.02, two trials, 341 participants, low quality evidence); and higher doses, up to 60 mg/kg, do not appear to show any advantage (four trials, 783 participants, moderate quality evidence).
The absolute parasitological cure rate at one month with praziquantel 40 mg/kg varied substantially across studies, ranging from 52% in Senegal in 1993 to 92% in Brazil in 2006/2007.
Oxamniquine
Compared to placebo, oxamniquine 40 mg/kg probably reduces parasitological treatment failure at three months (RR 8.74, 95% CI 3.74 to 20.43, two trials, 82 participants, moderate quality evidence). Lower doses than 40 mg/kg may be inferior at one month (30 mg/kg: RR 1.78, 95% CI 1.15 to 2.75, four trials, 268 participants, low quality evidence; 20 mg/kg: RR 3.78, 95% CI 2.05 to 6.99, two trials, 190 participants, low quality evidence), and higher doses, such as 60 mg/kg, do not show a consistent benefit (four trials, 317 participants, low quality evidence).
These trials are now over 20 years old and only limited information was provided on the study designs and methods.
Praziquantel versus oxamniquine
Only one small study directly compared praziquantel 40 mg/kg with oxamniquine 40 mg/kg and we are uncertain which treatment is more effective in reducing parasitological failure (one trial, 33 participants, very low quality evidence). A further 10 trials compared oxamniquine at 20, 30 and 60 mg/kg with praziquantel 40 mg/kg and did not show any marked differences in failure rate or percent egg reduction.
Combination treatments
We are uncertain whether combining praziquantel with artesunate reduces failures compared to praziquantel alone at one month (one trial, 75 participants, very low quality evidence).
Two trials also compared combinations of praziquantel and oxamniquine in different doses, but did not find statistically significant differences in failure (two trials, 87 participants).
Other outcomes and analyses
In trials reporting clinical improvement evaluating lower doses (20 mg/kg and 30 mg/kg) against the standard 40 mg/kg for both praziquantel or oxamniquine, no dose effect was demonstrable in resolving abdominal pain, diarrhoea, blood in stool, hepatomegaly, and splenomegaly (follow up at one, three, six, 12, and 24 months; three trials, 655 participants).
Adverse events were not well‐reported but were mostly described as minor and transient.
In an additional analysis of treatment failure in the treatment arm of individual studies stratified by age, failure rates with 40 mg/kg of both praziquantel and oxamniquine were higher in children.
Authors' conclusions
Praziquantel 40 mg/kg as the standard treatment for S. mansoni infection is consistent with the evidence. Oxamniquine, a largely discarded alternative, also appears effective.
Further research will help find the optimal dosing regimen of both these drugs in children.
Combination therapy, ideally with drugs with unrelated mechanisms of action and targeting the different developmental stages of the schistosomes in the human host should be pursued as an area for future research.
15 April 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted in April 2019 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review.
Keywords: Adolescent; Adult; Child; Humans; Commiphora; Oxamniquine; Oxamniquine/therapeutic use; Plant Extracts; Plant Extracts/therapeutic use; Praziquantel; Praziquantel/therapeutic use; Randomized Controlled Trials as Topic; Resins, Plant; Schistosomiasis mansoni; Schistosomiasis mansoni/drug therapy; Schistosomicides; Schistosomicides/therapeutic use
Plain language summary
Drugs for treating Schistosoma mansoni infection
Schistosoma mansoni is a parasitic worm common in Africa, the Middle East and parts of South America. The worm larvae live in ponds and lakes contaminated by faeces, and can penetrate a persons’ skin when they swim or bathe. Inside the host, the larvae grow into adult worms; these produce eggs, which are excreted in the faeces. Eggs rather than worms cause disease. Long‐term infection can cause bloody diarrhoea, abdominal pains, and enlargement of the liver and spleen.
In this review, researchers in the Cochrane Collaboration evaluated drug treatments for people infected with Schistosoma mansoni. After searching for all relevant studies, they found 52 trials, including 10,269 people, conducted in Africa, Brazil and the Middle East. Most trials report on whether or not the treatment stops eggs excretion; three reported the persons recovery from symptoms.
The results show that a single dose of praziquantel (40 mg/kg), as recommended by the World Health Organization, is an effective treatment for Schistosoma mansoni infection. Lower doses may be less effective, and higher doses probably have no additional benefit.
Oxamniquine (40 mg/kg), though now rarely used, is also effective. Again, lower doses may be less effective and no advantage has been demonstrated with higher doses.
Only one study directly compared praziquantel 40 mg/kg with oxamniquine 40 mg/kg, and based on this limited evidence, we are uncertain which intervention is more effective. Adverse events were not well reported for either drug, but were mostly described as minor and transient.
In children aged less than 5 years, there is limited evidence that these doses may be less effective, and further research will help optimise the dose for this age‐group.
Summary of findings
Summary of findings for the main comparison. Praziquantel 40 mg/kg for treating S. mansoni infection.
| Praziquantel 40 mg/kg for treating S. mansoni infection | ||||||
| Patient or population: People with S. mansoni infection Settings: Endemic settings Intervention: Praziquantel 40 mg/kg | ||||||
| Outcomes | Comparison | Illustrative comparative risks1 (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||||
| Praziquantel 40 mg/kg | Comparator | |||||
|
Parasitological failure at 1 month |
versus placebo | 22 per 100 | 69 per 100 (23 to 100) | RR 3.13 (1.03 to 9.53) | 414 (2 trials) | ⊕⊕⊕⊝ moderate2,3,4 |
| versus 20 mg/kg | 22 per 100 | 50 per 100 (34 to 72) | RR 2.23 (1.64 to 3.02) | 341 (2 trials) | ⊕⊕⊝⊝ low4,5 | |
| versus 30 mg/kg | 22 per 100 | 33 per 100 (25 to 44) | RR 1.52 (1.15 to 2.01) | 521 (3 trials) | ⊕⊕⊝⊝ low4,5 | |
| versus 60 mg/kg | 22 per 100 | 21 per 100 (16 to 28) | RR 0.97 (0.73 to 1.29) | 783 (4 trials) | ⊕⊕⊕⊝ moderate6,7 | |
| versus split dose | 22 per 100 | 10 per 100 (3 to 37) | RR 0.47 (0.13 to 1.69) | 525 (2 trials) | ⊕⊕⊝⊝ low4,8 | |
| *The basis for the assumed risk is given in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Treatment failure with praziquantel 40 mg/kg ranged from 5% to 48% in the included studies. The risk given here is the median risk in these studies and is given for illustrative purposes. 2 No serious risk of bias. Both studies adequately concealed allocation and blinded participants and investigators. Loss to follow‐up was high in one study. 3 No serious inconsistency: Both trials showed statistically significant benefits with praziquantel but the size of the effect varied. In Kenya in 1999 failure with praziquantel was 43% at one month and in Uganda in 2009 it was 18%. 4 Downgraded by 1 for indirectness: Only two trials from limited settings have evaluated this comparison. 5 Downgraded by 1 for risk of bias: These trials are more than twenty years old and do not provide an adequate description of methods to reduce the risk of bias. 6 No serious risk of bias: The three trials by Olliaro in 2010 adequately concealed allocation and blinded participants and investigators to be considered at low risk of bias. 7 Downgraded by 1 for indirectness: The trials so far do not indicate a benefit with higher doses than 40 mg/kg. However, we cannot be certain that there might not be some benefit in specific settings. 8 Downgraded by 1 for inconsistency: One trial found a significant benefit with splitting the dose and one did not. The trials were of similar size and power.
Summary of findings 2. Oxamniquine 40 mg/kg for treating S. mansoni infection.
| Oxamniquine 40 mg/kg for treating S. mansoni infection | ||||||
| Patient or population: People with S. mansoni infection Settings: Endemic settings Intervention: Oxamniquine 40 mg/kg | ||||||
| Outcomes | Comparison | Illustrative comparative risks1 (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||||
| Oxamniquine 40 mg/kg | Comparator | |||||
|
Parasitological failure at 1 month |
versus placebo2 | 18 per 100 | 100 per 100 (66 to 100) | RR 8.74 (3.74 to 20.43) | 82 (2 trials) | ⊕⊕⊕⊝ moderate3,4 |
| versus 20 mg/kg | 18 per 100 | 68 per 100 (37 to 100) | RR 3.78 (2.05 to 6.99) | 190 (2 trials) | ⊕⊕⊝⊝ low3,5 | |
| versus 30 mg/kg | 18 per 100 | 32 per 100 (21 to 50) | RR 1.78 (1.15 to 2.75) | 268 (4 trials) |
⊕⊕⊝⊝ low3,5 | |
| versus 60 mg/kg | 18 per 100 | 8 per 100 (2 to 38) | RR 0.45 (0.09 to 2.11) | 317 (4 trials) | ⊕⊕⊝⊝ low3,5 | |
| *The basis for the assumed risk is given in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Treatment failure with oxamniquine 40 mg/kg ranged from 5% to 24% in the included studies. The risk given here is the median risk in these studies and is given for illustrative purposes. 2 Parasitological failure for the comparison with placebo was only reported at three months. 3 Downgraded by 1 for serious risk of bias: These studies did not adequately describe any methods to reduce the risk of bias. 4 Only two small studies have assessed this comparison. However, due to the very large effect size we have not downgraded further for indirectness or imprecision. 5 Downgraded by 1 for indirectness: These studies are either too few, too small, or too old to have full confidence that the results can be generalized to widespread control of S. mansoni today.
Summary of findings 3. Oxamniquine 40 mg/kg versus praziquantel 40 mg/kg.
| Praziquantel 40 mg/kg versus oxamniquine 40 mg/kg for treating S. mansoni infection | |||||
|
Patient or population: People with S. mansoni infection
Settings: Endemic settings
Intervention: Oxamniquine 40 mg/kg Control: Praziquantel 40 mg/kg | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
|
Praziquantel 40 mg/kg |
Oxamniquine 40 mg/kg |
||||
|
Parasitological failure at 1 month |
50 per 100 | 20 per 100 (7 to 61) | RR 0.40 (0.13 to 1.22) | 33 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for serious risk of bias: This study did not adequately describe any methods to reduce the risk of bias. 2 Downgraded by 1 for indirectness: This single study is over 20 years old. 3 Downgraded by 1 for imprecision: This trial is underpowered to detect what might be important differences in effect.
Summary of findings 4. Artesunate (12 mg/kg) plus praziquantel (40 mg/kg) versus praziquantel (40 mg/kg) alone.
| Artesunate (12 mg/kg) plus praziquantel (40 mg/kg) versus praziquantel (40 mg/kg) alone for treating S. mansoni infection | |||||
|
Patient or population: People with S. mansoni infection
Settings: Endemic settings
Intervention: Artesunate (12 mg/kg) plus praziquantel (40 mg/kg) Control: Praziquantel (40 mg/kg) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Praziquantel | Artesunate plus praziquantel | ||||
|
Parasitological failure at 1 month |
50 per 100 | 31 per 100 (17 to 55) | RR 0.62 (0.35 to 1.09) | 75 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for serious risk of bias: This study did not adequately describe any methods to reduce the risk of bias. 2 Downgraded by 1 for indirectness: This is a single study and the result is not easily generalized. 3 Downgraded by 1 for imprecision: This trial is underpowered to detect what might be important differences in effect.
Background
Description of the condition
Schistosomiasis is a parasitic blood fluke infection, of which three species commonly infect humans; Schistosoma mansoni (common in the tropics and sub‐tropics), S. haematobium (mostly endemic in Africa and the Middle East) and S. japonicum (endemic in the People's Republic of China and the Philippines) (Engels 2002; WHO 2002; Gryseels 2006; Steinmann 2006; Utzinger 2009). It has been estimated that 779 million people are at risk of schistosomiasis worldwide and 207 million people may be infected (Steinmann 2006). Of these, 120 million people are estimated to be symptomatic and 20 million suffer from long‐term complications (Chitsulo 2000; WHO 2002; van der Werf 2003). In global burden of disease estimates, schistosomiasis causes 1.7 to 4.5 million disability‐adjusted life years (DALYs) (WHO 2002; WHO 2004; Hotez 2006; Steinmann 2006; Utzinger 2009). Some suggest that this value may underestimate the true burden of schistosomiasis (WHO 2002; van der Werf 2003; King 2005; King 2007; King 2008a; King 2010).
People infected with S. mansoni excrete the fluke eggs in their faeces, and faecal contamination of freshwater allows these eggs to hatch into larvae (miracidia) which penetrate a specific freshwater snail (the intermediate host). Within the snail, the miracidia develop into cercariae (the infective larvae), which can penetrate a person’s skin upon contact with contaminated water bodies.
Following infection, the worms migrate through the human venous system, via the right chamber of the heart and the lungs, and through the mesenteric arteries and the liver via the portal vein, before finally settling in the superior mesenteric veins which drain the large intestine. Here, male and female worms mature, pair up and the female worms start to produce eggs (≂ 300 per day) (Davis 2009). An adult worm usually lives for three to five years, but some can live up to 30 years (Gryseels 2006). The eggs produced by the worms traverse the intestinal wall to be excreted in the faeces, and in the process some become trapped and initiate inflammatory reactions, which cause the underlying pathology and symptomatic illness (Richter 2003a; King 2008b). Early symptoms depend on the severity of infection (Gryseels 1987), and if treatment is not provided early, chronic illness and long‐term serious disease can follow.
Symptoms and effects
Schistosomiasis mansoni can present as an acute or chronic illness. The acute illness, or Katayama syndrome, is caused by migrating and maturing schistosomula that may result in a systemic hypersensitivity reaction characterized by fever, feeling of general discomfort (malaise), muscle pain (myalgia), fatigue, non‐productive cough, diarrhoea (with or without blood), and pain in the upper right part of the abdomen just below the rib cage. Chronic and advanced disease results from the host's immune response to schistosome eggs deposited in tissues and the granulomatous reaction evoked by the antigens they secrete and is characterized by non‐specific intestinal symptoms, such as abdominal pain, diarrhoea and blood in the stool (Gryseels 1992; Gray 2011; Gryseels 2012).
Inflammatory reactions in the liver lead to hepatosplenic schistosomiasis, a key feature of chronic infection, which can manifest within a couple of months for heavy infections or many years after light infections. The chronic inflammation produces fibrotic lesions, which in turn lead to liver cirrhosis that progressively occludes the portal system giving rise to portal hypertension. The portal hypertension eventually leads to enlargement of hepatic arteries, and the associated oesophageal varices may rupture with heavy blood loss, haemorrhagic shock and death. The patient may also suffer repeated episodes of variceal bleeding – the primary cause of death in hepatic schistosomiasis (Andersson 2007). Severity of disease depends upon the intensity and duration of infection (Naus 2003), but recent evidence suggests the presence of the infection alone determines morbidity (King 2008a).
S. mansoni infection overlaps in distribution with S. haematobium in some areas of sub‐Saharan Africa resulting in mixed infections (WHO 2002). Unlike S. mansoni, the main early symptoms of S. haematobium infection are blood in urine (haematuria) and painful urination (dysuria). Chronic and advanced disease is insidious and may result in structural damage to the bladder wall which may eventually lead to kidney failure.
Diagnosis
Definitive diagnosis of S. mansoni infection is by microscopy for parasite eggs in the stool. Quantitative methods are recommended for epidemiological purposes because they allow estimation of intensity and evaluation of the impact of control programmes not only in terms of cure rate but also egg reduction rate (WHO 1985; Doenhoff 2004; Bergquist 2009). The Kato‐Katz technique (Katz 1972) is the most common quantitative technique (Booth 2003). Recently, the FLOTAC technique has been applied for the detection and quantification of S. mansoni eggs in stools with promising results and hence warranting further investigation (Glinz 2010).
Egg output can be influenced by several factors, such as day‐to‐day, intra‐stool, and seasonal variations as well as environmental conditions (Braun‐Munzinger 1992; Engels 1996; Engels 1997; Enk 2008). Therefore negative results following microscopic examination of a single stool are unreliable (de Vlas 1992; Kongs 2001; Booth 2003; Enk 2008), and measurement of prevalence and intensity of infection by egg count has shortcomings (Gryseels 1996; de Vlas 1997; Utzinger 2001a). Rectal biopsy is more sensitive than microscopy and is occasionally done when repeated stool examinations are negative for eggs. However, this method is unsuitable for use in population‐based control programmes (Allan 2001).
A monoclonal antibody‐based dipstick is increasingly being used for the diagnosis of the infection with promising results (Polman 2001; Legesse 2007; Legesse 2008; Caulibaly 2011). A more specific and sensitive diagnostic technique based on polymerase chain reaction (PCR) is increasingly being used in some reference laboratories in Europe (Sandoval 2006; Cnops 2012; Enk 2012). Ultrasound is used for diagnosing and assessing infection‐related pathology (Hatz 1990; Mohamed‐Ali 1991; Doehring‐Schwerdtfeger 1992; Hatz 2001; Richter 2003b).
Clinically, intestinal schistosomiasis is diagnosed on the basis of presence of blood in stool, (bloody) diarrhoea, and abdominal pain, but these are non‐sensitive and non‐specific (Gryseels 1992; Utzinger 2000c; Danso‐Appiah 2004) as diarrhoea or blood in stool can be due to other causes such as hookworm infection, dysentery and typhoid fever.
Description of the intervention
Schistosomiasis control measures implemented before the 1970s – when efficacious antischistosomal drugs were not available – focused mainly on interrupting transmission with molluscicides to kill the intermediate host snails (WHO 1985; Sturrock 2001). The 1970s marked the turning point in schistosomiasis control when efficacious drugs that can be applied in a single oral dose were discovered, shifting the control emphasis from transmission control to chemotherapy‐based morbidity control (WHO 1985; Cioli 1995). A body of evidence suggests that morbidity due to schistosomiasis can be prevented and pathology reversed with available antischistosomal treatments (Mohamed‐Ali 1991; Doehring‐Schwerdtfeger 1992; Savioli 2004; Zhang 2007; Webster 2009; Koukounari 2010).
Mass drug administration, or treatment of infected individuals or entire 'at‐risk' populations (eg school‐aged children), usually without prior diagnosis ‐ an approach termed 'preventive chemotherapy', is the control strategy currently pursued by the World Health Organization (WHO) and applied in many endemic countries (WHO 2006). Usually, praziquantel at a single 40 mg/kg oral dose is used (Fenwick 2009), but still there are uncertainties regarding this dose. An exception is Brazil where the national policy adopted since 1995 recommends a single oral dose of 60 mg/kg for children aged between two and 15 years, and 50 mg/kg for adolescents and adults (Favre 2009). The recently adopted policy for schistosomiasis control in Brazil disapproves of treatment without prior diagnosis, and therefore the preventive chemotherapy strategy is no longer applied in Brazil (Favre 2009).
Oxamniquine has also been used extensively for the control of schistosomiasis mansoni in different endemic countries, most notably Brazil, where more than 12 million doses of oxamniquine have been administered by the national schistosomiasis control programme (Katz 2008). There are uncertainties around the standard dose of oxamniquine (Foster 1987; Cioli 1995). Therefore, the WHO recommends total doses of 20 to 60 mg/kg (in divided doses of up to 20 mg/kg) (WHO 2001).
More recently, the artemisinin derivatives used in the treatment of malaria have been shown to have antischistosomal properties, particularly against the immature developing stages of the schistosome parasites (Borrmann 2001; Utzinger 2007). Praziquantel, in contrast, acts against the adult worms and the very young schistosomula just after skin penetration (Sabah 1986; Utzinger 2007).
The current emphasis of schistosomiasis control is to reduce the burden of disease in high endemicity areas and to interrupt transmission in low endemicity areas (WHO 2002). Intensity of infection is highest in school‐aged children and adolescents, therefore preventive chemotherapy is targeted especially to these at‐risk groups (Magnussen 2001; WHO 2002; Savioli 2004; Savioli 2009).
The efficacy of myrrh (Mirazid) in the treatment of intestinal schistosomiasis has been evaluated in Egypt (Barakat 2005 EGY; Botros 2005 EGY).
Why it is important to do this review
Currently, entire control and treatment programmes are based on praziquantel and there is risk of drug resistance and perhaps shortages of praziquantel. There is a need to assess alternative drugs or combinations. Still there are uncertainties around effective and safe dosage of praziquantel and standard doses of oxamniquine. There are also uncertainties about adequacy of current adult doses used in children.
Objectives
To evaluate the effects of antischistosomal drugs, used alone or in combination, for treating S. mansoni infection.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Individuals infected with S. mansoni diagnosed microscopically for the presence of S. mansoni eggs in stool using the Kato‐Katz technique (Katz 1972), or any other quantitative diagnostic method, such as the quantitative oogram and FLOTAC techniques.
Types of interventions
The following comparisons are evaluated in this review:
Antischistosomal drugs alone or in combination versus placebo;
Antischistosomal drugs alone or in combination versus a different dose of the same antischistosomal drug; and
Antischistosomal drugs alone or in combination versus different antischistosomal drugs alone or in combination.
Trials that allocated non‐schistosomal drug or interventions in addition to the treatment and control of interest were eligible provided the same drug was allocated to both treatment and control groups.
Types of outcome measures
Primary outcomes
Parasitological failure, defined as treated individuals who remained positive for S. mansoni eggs in stool using the standard Kato‐Katz or other quantitative techniques (follow‐up: up to one month).
Egg reduction rate, defined as percent reduction in S. mansoni egg count after treatment (follow‐up: up to 12 months).
Secondary outcomes
Parasitological failure (follow‐up: greater than one month).
Resolution of symptoms (eg abdominal pain, diarrhoea and bloody diarrhoea).
Resolution of pathology (eg hepatomegaly, splenomegaly, portal fibrosis, cirrhosis of the liver or colonic polyps) measured by ultrasound, by standard international classification or other standardized methods (CWG 1992).
Adverse events
Non‐serious adverse events.
Serious adverse events (ie any untoward medical occurrence or effect that at any dose: results in death; is life‐threatening; requires hospitalisation or prolongation of existing inpatients' hospitalisation; results in persistent or significant disability or incapacity; is a congenital anomaly or birth defect).
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, under review and in progress).
Electronic searches
Databases
We searched the following databases using the search terms and strategy described in Table 5: Cochrane Infectious Diseases Group Specialized Register (October 2012); Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE (1966 to October 2012); EMBASE (1974 to October 2012); and LILACS (1982 to October 2012). We also searched the metaRegister of Controlled Trials (mRCT) in October 2012 using ’Schisto * mansoni' as the search term.
1. Detailed search strategies.
| Search set | CIDG SR^ | CENTRAL | MEDLINE^^ | EMBASE^^ | LILACS^^ |
| 1 | Schisto* mansoni | SCHISTOSOMA MANSONI | SCHISTOSOMA MANSONI | SCHISTOSOMA MANSONI | Schisto$ mansoni |
| 2 | Esquistossomose | SCHISTOSOMIASIS MANSONI | SCHISTOSOMIASIS MANSONI | SCHISTOSOMIASIS MANSONI | Esquistossomose |
| 3 | 1 or 2 | Intestinal schistosom* ti, ab | Intestinal schistosom* ti, ab | Intestinal schistosom$ ti, ab | 1 or 2 |
| 4 | Bilharzia* | Bilharzia* | Bilharzia$ | ||
| 5 | Esquistossomose ti, ab | Esquistossomose ti, ab | Esquistossomose ti, ab | ||
| 6 | Schistosomicide* | Schistosomicide* | Schistosomicide$ | ||
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 1 or 2 or 3 or 4 or 5 or 6 | 1 or 2 or 3 or 4 or 5 or 6 | ||
| 8 | Limit 7 to humans | Limit 7 to humans | |||
| ^Cochrane Infectious Diseases Group Specialized Register | ^^Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2011); Upper case: MeSH or EMTREE heading; Lower case: free text term |
Searching other resources
Researchers and organizations
We contacted individual researchers working on antischistosomal drugs, pharmaceutical industries and experts from the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) for unpublished data and ongoing trials.
Reference lists
We checked the reference lists of all studies identified by the aforementioned methods for additional relevant studies.
Data collection and analysis
Selection of studies
Vittoria Lutje, the Cochrane Infectious Diseases Group (CIDG) Information Retrieval Specialist, searched the literature and retrieved studies using the search strategy outlined in Table 5. Anthony Danso‐Appiah (ADA) screened the results to identify potentially relevant trials, obtained the full trial reports and assessed the eligibility of trials for inclusion in the review using an eligibility form based on the inclusion criteria. Jürg Utzinger (JU) independently verified the eligibility assessment results.
ADA contacted the authors of potentially relevant trials for clarification if eligibility was unclear. We excluded studies that did not meet our inclusion criteria and we have detailed the reasons for exclusion in the Characteristics of excluded studies. This was verified independently by JU and Piero L. Olliaro (PLO). We resolved any discrepancies through discussion between the authors.
Data extraction and management
ADA extracted trial characteristics such as methods, participants, interventions and outcomes, and recorded on standard forms, which were independently verified by JU. ADA and JU resolved discrepancies through discussion, and where necessary contacted a third author (PLO). ADA contacted trial authors for clarification, or insufficient or missing data when necessary.
We extracted the number of participants randomized and the number of patients followed‐up in each treatment arm. For dichotomous outcomes, we recorded the number of participants experiencing the event in each treatment group of the trial. For continuous outcomes summarized as geometric means, we extracted means and their standard deviations (SD) on the log scale. If the data were summarized as arithmetic mean, we extracted the means and their SDs. We extracted medians or ranges when they were reported to summarize the data.
For each outcome, we extracted data for each follow‐up time reported in the trial report.
Assessment of risk of bias in included studies
ADA assessed the risk of bias of each trial using The Cochrane Collaboration's risk of bias tool (Higgins 2011) and the assessment results were verified independently by Dave Sinclair (DS). Where information in the trial report was unclear, we attempted to contact the trial authors for clarification. We assessed the risk of bias for six domains: sequence generation, allocation concealment, blinding (investigators, outcome assessors and participants), incomplete outcome data, selective outcome reporting and other sources of bias. For each domain, we made a judgment of 'low risk' of bias, 'high risk' of bias or 'unclear'. We resolved any discrepancies by discussion between the authors.
Measures of treatment effect
We presented dichotomous outcomes using risk ratios (RR). Mean differences (MD) were used as the measure of effect for continuous outcomes that were summarized as arithmetic means. We used geometric mean ratios for continuous outcomes that were summarized as geometric means. We presented all results with 95% confidence intervals (CI).
Dealing with missing data
We analysed data based on the number of patients for whom an outcome was recorded (complete case analysis).
Assessment of heterogeneity
We assessed heterogeneity by inspecting the forest plots for overlapping CIs and outlying data; using the Chi2 test with a P value < 0.1 to indicate statistically significant heterogeneity; and using the I2 statistic.
Assessment of reporting biases
We would have attempted to explore publication bias using funnel plots if there were sufficient number of trials in the comparisons.
Data synthesis
We used Review Manager (RevMan) to perform the statistical analyses. We stratified the analyses by: comparison; the dose of the drug; and the length of follow‐up time. We used meta‐analysis to combine the results across trials. When heterogeneity was detected, we used a random‐effects meta‐analysis approach; otherwise a fixed‐effect approach was adopted. We tabulated adverse events and also data that could not be meta‐analysed.
Subgroup analysis and investigation of heterogeneity
When heterogeneity was detected, we planned to carry out subgroup analyses to explore potential causes. Subgroupings would be as follows: patient age (children versus adults); and intensity of infection (< 500 eggs per gram of stool versus > 500 eggs per gram of stool).
We conducted a subsidiary, non‐randomized comparison of failure rates in children with failure rates in adults for the same drug and same dose (mg/kg) to explore issues around dose applicability in children.
Sensitivity analysis
Where data were sufficient we planned to conduct sensitivity analyses to assess the robustness of the results to the risk of bias components.
Results
Description of studies
We identified 52 trials (10,269 participants) which met the inclusion criteria (see Characteristics of included studies). We managed one multicentre trial carried out in Brazil, Mauritania and Tanzania as three separate trials in the analysis (Olliaro 2011 BRA; Olliaro 2011 MRT; Olliaro 2011 TZA), and three papers contained multiple individual studies which we again managed separately (de Clarke 1976a ZWE; de Clarke 1976b ZWE; de Clarke 1976c ZWE; de Clarke 1976d ZWE; Katz 1979a BRA; Katz 1979b BRA; Gryseels 1989a BDI; Gryseels 1989b BDI; Gryseels 1989c BDI).
Of the 52 trials we identified, 19 evaluated praziquantel, 17 evaluated oxamniquine and 12 directly compared praziquantel with oxamniquine. In addition, two compared myrrh (mirazid) with praziquantel, and two compared different brands of praziquantel.
Three trials assessed combination therapies: including praziquantel plus oxamniquine (Creasey 1986 ZWE; Zwingenberger 1987 BRA) and praziquantel plus artesunate (De Clercq 2000 SEN).
For the two primary outcomes, 47 trials reported cure rate or failure rate, 34 trials reported egg reduction rate and 33 trials reported both outcomes. Only Sukwa 1993 ZMB reported reinfection rate.
For secondary outcomes, five trials (Rugemalila 1984 TZA; Gryseels 1989a BDI; Gryseels 1989b BDI; Gryseels 1989c BDI; Sukwa 1993 ZMB) reported clinical improvement or functional indices, but we could not include Rugemalila 1984 TZA and Sukwa 1993 ZMB in the meta‐analysis because of insufficient information. Thirty‐three trials reported adverse events.
In the study by de Jonge 1990 SDN, we excluded the two arms that received metrifonate and placebo respectively from the analysis. Also, we excluded one arm of the study by Ibrahim 1980 SDN involving participants who did not have S. mansoni infection and also one arm each of the trials by Rugemalila 1984 TZA and Taylor 1988 ZWE that did not receive treatment from the analysis.
The trial by Tweyongyere 2009 UGA assessing the effects of praziquantel was a nested cohort study within a larger mother and baby cohort study in which pregnant women found to be infected with S. mansoni were randomized to receive praziquantel or placebo. We obtained data on parasitological failure rate and clinical improvement from figures (Gryseels 1989a BDI; Gryseels 1989b BDI; Gryseels 1989c BDI), but it was not possible to extract egg count data.
Trial setting and participants
The trials were conducted in Africa (n = 36), South America (n = 15; all in Brazil) and the Middle East (n = 1). Eight trials were conducted in the late 1970s, 28 in the 1980s, seven in the 1990s and only nine since the year 2000.
Eighteen trials involved children, 12 trials recruited adults, and 22 recruited whole populations comprising children, adolescents and adults.
Seventeen trials recruited participants from the outpatient clinics, six did not specify the setting whilst one trial (Omer 1981 SDN) consisted of both participants identified in a field survey and those attending the hospital; two trials (Katz 1979a BRA; Katz 1979b BRA) involved military officers in a Barracks who became exposed to the infection during training and another trial (Ibrahim 1980 SDN) recruited university students on campus. The remaining 25 trials recruited participants through community surveys.
Risk of bias in included studies
For risk of bias of included studies see the Characteristics of included studies and summary of the risk of bias graph (Figure 1) and risk of bias summary (Figure 2).
1.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered 16 trials as low risk of bias with regard to the generation of the randomization sequence (Figure 2). In the remaining 36 trials, the methods used to generate the sequence of allocation were not described and therefore the risk of bias is unclear.
Fourteen trials adequately described allocation concealment and had a low risk of bias. One trial did not conceal allocation (Fernandes 1986 BRA); and the methods were unclear in the remaining 37 trials (Figure 2).
Blinding
Twenty‐seven trials employed blinding and stated who was blinded. However, none described the methods of blinding. Nevertheless, the studies were considered to be at low risk of bias. One trial did not employ blinding (Fernandes 1986 BRA) and we therefore classed it at high risk of bias; whereas in 25 trials blinding was unclear (Figure 2).
Incomplete outcome data
We considered the risk of bias for incomplete outcome data to be low in 17 trials (Figure 2). We deemed the risk of bias to be high in 19 trials, and in the remaining 16 trials as unclear.
Selective reporting
All 52 trials had low risk of selective outcome reporting (Figure 2).
Other potential sources of bias
Overall, 42 trials were considered to be free from other biases and the level of bias was unclear in the remaining 10 trials (Figure 2).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Section 1. Monotherapies
Praziquantel
Nineteen trials, conducted in Africa, Brazil and the Arabian Penunsula, evaluated praziquantel. Four studies compared praziquantel with placebo, and 17 trials directly compared different dosing schedules of praziquantel with the standard dose of 40 mg/kg.
Analysis 1: Praziquantel versus placebo
Parasitological failure
Two trials from Kenya and Uganda used the WHO recommended dose of 40 mg/kg. Praziquantel 40 mg/kg achieved parasitological cure in 57% and 82% of the patients respectively, compared to placebo where almost all continued to excrete eggs at one to two months (RR 3.13, 95% CI 1.03 to 9.53, two trials, 414 participants, Analysis 1.1).
1.1. Analysis.
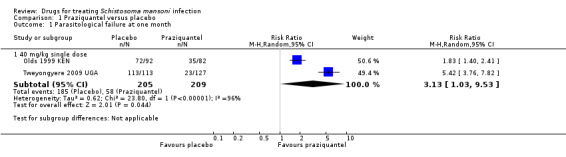
Comparison 1 Praziquantel versus placebo, Outcome 1 Parasitological failure at one month.
In addition, one small trial from Brazil compared three different doses of praziquantel with placebo and presented outcomes at six and 12 months. All patients given 40 mg/kg and 60 mg/kg praziquantel achieved parasitological cure at six months, while two out of five patients given 20 mg/kg and almost all those given placebo continued to excrete eggs (one trial, 40 participants, Analysis 1.2). At 12 months, reinfection was demonstrable in some of those given praziquantel (Analysis 1.3). One further trial from Brazil gave 60 mg/kg praziquantel each day for three days and achieved 100% parasitological cure at six months compared to almost complete failure with placebo (one trial, 55 participants, Analysis 1.2).
1.2. Analysis.

Comparison 1 Praziquantel versus placebo, Outcome 2 Parasitological failure at six months.
1.3. Analysis.

Comparison 1 Praziquantel versus placebo, Outcome 3 Parasitological failure at 12 months.
Egg reduction
None of these trials reported on percentage egg reduction.
Adverse events
No serious adverse events were recorded in these trials but transient dizziness and abdominal pain appeared to be more commonly reported with praziquantel than placebo (seven trials, 1255 participants, Table 6).
2. Adverse events: Praziquantel versus placebo.
| Trial | No. of participants | Praziquantel dose | Remarks |
| Jaoko 1996 KEN | 436 | 40 mg/kg single dose | Adverse events described as minor and transient. Dizziness: Praziquantel 36% versus 6% control Abdominal pain 35% versus 14 % control |
| Katz 1979a BRA | 55 | 20 mg/kg single dose 40 mg/kg: 20 mg/kg twice in one day 60 mg/kg: 20 mg/kg three times in one day |
Adverse events were minor, did not differ between intervention and placebo groups, but were not reported separately for the different dose schedules. |
| Katz 1979b BRA | 61 | 50 mg/kg single dose 60 mg/kg: 20 mg/kg three times in one day |
Adverse events were minor, did not differ between the two intervention groups, but were not reported separately for the two dosing schedules. |
| Olds 1999 KEN | 174 | 40 mg/kg single dose | Abdominal pain: Praziquantel 80% versus 50% control Diarrhoea: Praziquantel 54% versus 25% control |
| Tweyongyere 2009 UGA | 387 | 40 mg/kg single dose | Adverse events were minor and transient. The authors pooled adverse events together over the intervention and placebo groups. Event rates were not reported. |
| Branchini 1982 BRA | 70 | 41.2 to 51.6 mg/kg single dose | No serious adverse events. Dizziness: Praziquantel 46.9% (control group not reported). Abdominal pain: Praziquantel 24.5% versus 17.6% control |
| Ferrari 2003 BRA | 72 | 180 mg/kg: 60 mg/kg once daily for three days | No serious adverse events. Events were mostly headache, dizziness, drowsiness and abdominal pain. Patients from the placebo group also reported having abdominal pain and drowsiness. |
Analyses 2 and 3: Lower doses praziquantel versus 40 mg/kg
Parasitological failure
Lower doses (20 mg/kg to 30 mg/kg) have been evaluated in Zimbabwe, Burundi, Sudan and Brazil. Compared to 40 mg/kg, parasitological failure at one month was more than double with the 20 mg/kg dose, and 50% higher with the 30 mg/kg dose (20 mg/kg: RR 2.23, 95% CI 1.64 to 3.02, two trials, 341 participants; 30 mg/kg: RR 1.52, 95% CI 1.15 to 2.01, three trials, 521 participants; Analysis 2.1). Follow‐up at three months (Analysis 2.2) and at six to 12 months showed a similar pattern (Analysis 2.3).
2.1. Analysis.
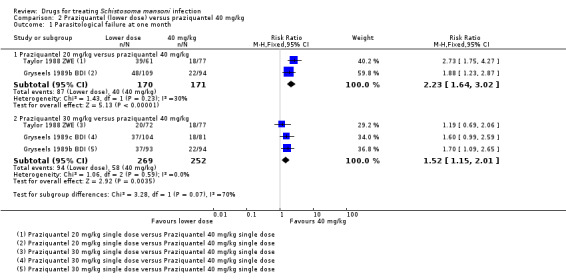
Comparison 2 Praziquantel (lower dose) versus praziquantel 40 mg/kg, Outcome 1 Parasitological failure at one month.
2.2. Analysis.

Comparison 2 Praziquantel (lower dose) versus praziquantel 40 mg/kg, Outcome 2 Parasitological failure at three months.
2.3. Analysis.

Comparison 2 Praziquantel (lower dose) versus praziquantel 40 mg/kg, Outcome 3 Parasitological failure at six to 12 months.
Egg reduction
In one trial from Brazil evaluating 30 mg/kg versus 40 mg/kg, geometric mean egg reductions were high in both groups, at six months (92.5% versus 97.7%, statistical significance not reported (one trial, 138 participants, Analysis 2.4)).
2.4. Analysis.
Comparison 2 Praziquantel (lower dose) versus praziquantel 40 mg/kg, Outcome 4 Percent egg reduction.
| Percent egg reduction | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Geometric mean % egg reduction at 6 months 30 mg/kg | Geometric mean % egg reduction at 6 months 40 mg/kg | P‐value | Comment |
| Katz 1981 BRA | 138 | 92.5% | 97.7% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
Symptom resolution
One trial compared a lower dose of praziquantel at 20 mg/kg with 40 mg/kg and showed no difference in resolving symptoms at three, six, 12 and 24 months of follow‐up: diarrhoea (one trial, 44 participants, Analysis 3.3), blood in stool (one trial, 37 participants, Analysis 3.5), hepatomegaly (one trial, 55 participants, Analysis 3.7) and splenomegaly (one trial, 73 participants, Analysis 3.9), except one study that showed that 40 mg/kg significantly improved abdominal pain at one month (RR 0.59, 95% CI 0.36 to 0.98, one trial, 169 participants, Analysis 3.1).
3.3. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 3 Resolution of diarrhoea: 20 mg/kg versus 40 mg/kg.
3.5. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 5 Resolution of blood in stool: 20 mg/kg versus 40 mg/kg.
3.7. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 7 Resolution of hepatomegaly: 20 mg/kg versus 40 mg/kg.
3.9. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 9 Resolution of splenomegaly: 20 mg/kg versus 40 mg/kg.
3.1. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 1 Resolution of abdominal pain: 20 mg/kg versus 40 mg/kg.
Two trials compared 30 mg/kg with 40 mg/kg and did not show any difference in resolving symptoms at one, three, six, 12 and 24 months of follow‐up: abdominal pain (two trials, 318 participants, Analysis 3.2), diarrhoea (two trials, 48 participants, Analysis 3.4), blood in stool (two trials, 82 participants, Analysis 3.6), hepatomegaly (two trials, 109 participants, Analysis 3.8) and splenomegaly (two trials, 122 participants, Analysis 3.10).
3.2. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 2 Resolution of abdominal pain: 30 mg/kg versus 40 mg/kg.
3.4. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 4 Resolution of diarrhoea: 30 mg/kg versus 40 mg/kg.
3.6. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 6 Resolution of blood in stool: 30 mg/kg versus 40 mg/kg.
3.8. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 8 Resolution of hepatomegaly: 30 mg/kg versus 40 mg/kg.
3.10. Analysis.

Comparison 3 Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg, Outcome 10 Resolution of splenomegaly: 30 mg/kg versus 40 mg/kg.
Adverse events
In the three trials reporting adverse events, consistent differences in frequency or severity between 20, 30 and 40 mg/kg doses have not been shown (three trials, 319 participants, Table 7).
3. Adverse events: praziquantel (lower dose) versus 40 mg/kg.
| Trial | No. of participants | Comparison | Remarks |
| Katz 1979a BRA | 28 | 20 mg/kg single dose 40 mg/kg: 20 mg/kg twice in one day |
No serious adverse events. Minor adverse events, did not differ between intervention and control groups. |
| Katz 1981 BRA | 138 | 30 mg/kg single dose 40 mg/kg single dose |
No serious adverse events. Minor adverse events (lower dose first): Abdominal pain: 42.6% versus 44.4% Giddiness: 14.9% versus 26.7% |
| Omer 1981 SDN | 153 | 30 mg/kg single dose 40 mg/kg single dose |
No serious adverse events. Diarrhoea, vomiting, nausea and abdominal pain were commonly reported but these were transient. |
Analysis 4: Higher doses praziquantel versus 40 mg/kg
Parasitological failure
Higher doses (50 mg/kg to 60 mg/kg) have been evaluated in Brazil (three trials), Mauritania, Senegal and Tanzania. Compared to 40 mg/kg, parasitological failure has not been shown to be improved with higher doses at one month (five trials, 783 participants, Analysis 4.1).
4.1. Analysis.

Comparison 4 Praziquantel (higher dose) versus praziquantel 40 mg/kg, Outcome 1 Parasitological failure at one month.
Egg reduction
Among participants still excreting eggs, percentage egg reductions were similar in both groups at one month (four trials, 786 participants, Analysis 4.4).
4.4. Analysis.
Comparison 4 Praziquantel (higher dose) versus praziquantel 40 mg/kg, Outcome 4 Percent egg reduction at one month.
| Percent egg reduction at one month | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Praziquantel (60 mg/kg) | Praziquantel (40 mg/kg) | P‐value | Comment |
| Guisse 1997 SEN | 130 | 99% | 99% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Olliaro 2011 BRA | 196 | 91.8% | 91.7% | Not reported | EPG (Geometric Mean) of participants excreting eggs and those not excreting eggs at follow‐up |
| Olliaro 2011 MRT | 186 | 89.6% | 89.2% | Not reported | Geometric mean EPG of participants excreting eggs and those not excreting eggs at follow‐up |
| Olliaro 2011 TZA | 271 | 92.3% | 91.9% | Not reported | EPG (Geometric Mean) of participants excreting eggs and those not excreting eggs at follow‐up |
Adverse events
One multi‐country trial reported adverse events and recorded one serious event (a seizure) with the higher dose. At the trial site in Brazil, non‐severe adverse events appeared to be more common with the higher dose but this was not seen consistently at the trial sites in Mauritania or Tanzania (one trial, 653 participants, see Table 8).
4. Adverse events: praziquantel (higher dose) versus 40 mg/kg.
| Trial | No. of participants | Comparison | Remarks |
| Olliaro 2011 BRA | 196 | 60 mg/kg single dose 40 mg/kg single dose |
No serious adverse event. Minor adverse events (highest dose first): Abdominal pain: 48 versus 47.9% Nausea: 20.4% versus 18.4% Dizziness: 20.4% versus 11.2% Headache: 14.3% versus 12.2% Vomiting: 11.2% versus 5.19% Diarrhoea: 8.2% versus 4.1% Rarely sleepiness was also reported. |
| Olliaro 2011 MRT | 186 | 60 mg/kg single dose 40 mg/kg single dose |
One incidence of serious event was recorded in the higher dose (60 mg/kg). The rest of the events were minor. Transient dizziness, abdominal pain, diarrhoea, vomiting and headache were commonly reported (highest dose first): Dizziness: 77.8% versus 9.7% Abdominal pain:79.6% versus 71.0% Diarrhoea: 41.9% versus 49.5% Vomiting: 10.7% versus 32.3% Headache: 9.7% versus 14.0% |
| Olliaro 2011 TZA | 271 | 60 mg/kg single dose 40 mg/kg single dose |
Minor adverse events (highest dose first): Abdominal pain: 88.9% versus 83.8% Diarrhoea: 47.4% versus 49.3% Nausea: 26.7% versus 30.9% Headache: 14.1% versus 9.6% Vomiting: 11.1% versus 16.9% Dizziness: 6.7% versus 9.6% Fever: 0% versus 1.5%. |
Analysis 5: Split dose praziquantel versus 40 mg/kg in a single dose
Splitting 40 mg/kg into divided doses given on the same day was evaluated in the 1980s in three trials in Sudan.
Parasitological failure
At one month, two trials did not demonstrate a statistically significant benefit with the split dose regimen compared to a single 40 mg/kg dose (two trials, 525 participants, Analysis 5.1), but showed benefit at three months (RR 0.31, 95% CI 0.18 to 0.53, two trials, 516 participants, Analysis 5.2).
5.1. Analysis.

Comparison 5 Praziquantel 40 mg/kg divided dose versus praziquantel 40 mg/kg single dose, Outcome 1 Parasitological failure at one month.
5.2. Analysis.

Comparison 5 Praziquantel 40 mg/kg divided dose versus praziquantel 40 mg/kg single dose, Outcome 2 Parasitological failure at three months.
One further small trial, only reported the outcome at six months and found no difference (one trial, 64 participants, Analysis 5.3).
5.3. Analysis.

Comparison 5 Praziquantel 40 mg/kg divided dose versus praziquantel 40 mg/kg single dose, Outcome 3 Parasitological failure at six months.
Egg reduction
In the only trial reporting egg count, the mean percent reduction at one month was higher with the divided dose but statistical significance was not reported (divided dose 93.2% versus single dose 86.5%, one trial, 350 participants, Analysis 5.4).
5.4. Analysis.
Comparison 5 Praziquantel 40 mg/kg divided dose versus praziquantel 40 mg/kg single dose, Outcome 4 Percent egg reduction at one month.
| Percent egg reduction at one month | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Praziquantel (40 mg/kg) in a divided dose of 20 mg/kg | Praziquantel (40 mg/kg) single dose | P‐value | Comment |
| Kardaman 1983 SDN | 350 | 93.2% | 86.5% | P > 0.1 | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
Adverse events
No serious adverse events were reported in these trials. Only one trial reported the frequency of adverse events in each treatment group (Kardaman 1983 SDN). Mild abdominal pain and diarrhoea were less common when the dose was given in divided doses but vomiting was more common (one trial, 350 participants, Table 9).
5. Adverse events: praziquantel (40 mg/kg in a divided dose) versus praziquantel (40 mg/kg) single dose.
| Trial | No. of participants | Comparison | Remarks |
| Kardaman 1983 SDN | 350 | 40 mg/kg: 20 mg/kg twice in a day 40 mg/kg single dose |
No serious adverse events. Events were transient (divided dose first): Abdominal pain: 13.5% versus 24.6% Vomiting: 7.6% versus 4% Diarrhoea: 7.6% versus 12.8% |
| Omer 1981 SDN | 306 | 40 mg/kg: 20 mg/kg twice in a day 40 mg/kg single dose |
No serious adverse events. Adverse events were transient and required no additional intervention. |
Analysis 6: Other praziquantel dosing regimens
Several trials from Brazil have evaluated higher praziquantel dosing regimens with 30 mg/kg to 60 mg/kg given for up to six days (see Analysis 6.1). It is difficult to draw conclusions from these studies as the comparator dose is also a non‐standard regimen, but one trial did demonstrate improved parasitological cure rates with prolonged courses given over three to six days compared to courses lasting one day.
6.1. Analysis.

Comparison 6 Praziquantel alternative dosing (Brazil), Outcome 1 Parasitological failure at six months.
Adverse events
No serious adverse events were reported in these trials, events were mainly transient dizziness and nausea (one trial, 79 participants, Table 10).
6. Adverse events: praziquantel alternative dosing (Brazil).
| Trial | No. of participants | Comparison | Remarks |
| da Cunha 1987 BRA | 79 | 180 mg/kg: 30 mg/kg twice daily for three days 180 mg/kg: 30 mg/kg daily for six days 120 mg/kg: 30 mg/kg twice daily for two days 60 mg/kg: 30 mg/kg twice in one day |
No serious adverse events. Minor and transient events (highest dose first): Dizziness: 65%, 15%, 45% versus 15% Nausea: 55%, 15, 20% versus 20% |
Oxamniquine
Seventeen trials evaluated oxamniquine, with the most recent conducted in the 1980s. Oxamniquine has since fallen out of use in favour of praziquantel. Four trials compared oxamniquine with placebo and 12 trials directly compared different dosing schedules of oxamniquine in different geographical locations in Africa and Brazil. The most common comparator dose was 40 mg/kg.
Analysis 7: Oxamniquine versus placebo
Parasitological failure
In two trials in Brazil, 20 mg/kg was significantly superior to placebo at longer timepoints (RR 3.68, 95% CI 2.53 to 5.36, two trials, 146 participants, Analysis 7.2). In two trials from Ethiopia, oxamniquine achieved parasitological cure rates of > 75% with 30, 40, and 60 mg/kg at three to four months, compared to placebo where almost all participants continued to excrete eggs (30 mg/kg: RR 4.34, 95% CI 2.47 to 7.65, two trials, 82 participants; 40 mg/kg: RR 8.74, 95% CI 3.74 to 20.43, two trials, 82 participants; 60 mg/kg: RR 19.38, 95% CI 5.79 to 64.79, two trials, 89 participants; Analysis 7.1).
7.2. Analysis.

Comparison 7 Oxamniquine versus placebo, Outcome 2 Parasitological failure at six to 10 months.
7.1. Analysis.

Comparison 7 Oxamniquine versus placebo, Outcome 1 Parasitological failure at three to four months.
Egg reduction
Among those still excreting eggs at three to four months, two trials from Ethiopia reported significant reductions in egg numbers in those given oxamniquine (68.1% to 100%), compared to increases of 59 to 80.6% in the placebo groups (two trials, 227 participants, Analysis 7.3).
7.3. Analysis.
Comparison 7 Oxamniquine versus placebo, Outcome 3 Percent egg reduction at three to four months.
| Percent egg reduction at three to four months | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Intervention | Placebo | p‐value | Comment |
| Oxamniquine (40 mg/kg) versus placebo | |||||
| Ayele 1984 ETH | 65 | 100% | 59% increase | Not reported | Geometric mean EPG based on participants excreting eggs at follow‐up |
| Ayele 1986 ETH | 162 | 42.7% | 80.6% increase | Not reported | Geometric mean EPG based on participants excreting eggs at follow‐up |
| Oxamniquine (20 to 30 mg/kg) versus placebo | |||||
| Ayele 1984 ETH | 65 | 73.7% | 59% increase | Not reported | Geometric mean EPG based on participants excreting eggs at follow‐up |
| Ayele 1986 ETH | 162 | 68.1% | 80.6% increase | Not reported | Geometric mean EPG based on participants excreting eggs at follow‐up |
| Oxamniquine (60 mg/kg) versus placebo | |||||
| Ayele 1984 ETH | 65 | 100% | 59% increase | Not reported | Geometric mean EPG based on participants excreting eggs at follow‐up |
| Ayele 1986 ETH | 162 | 82% | 80.6% increase | Not reported | Geometric mean EPG based on participants excreting eggs at follow‐up |
Adverse events
No serious adverse events were reported in these trials. Dizziness was more commonly reported with oxamniquine than placebo but is described as transient, with most resolving within 24 hours (five trials, 425 participants, Table 11).
7. Adverse events: oxamniquine versus placebo.
| Trial | No. of participants | Oxamniquine dose | Remarks |
| Ayele 1984 ETH | 65 | 60 mg/kg: 15 mg/kg twice daily for two days 40 mg/kg: 10 mg/kg twice daily for two days 30 mg/kg: 15 mg/kg twice in one day |
Adverse events were minor and transient. Dizziness: Oxamniquine (15 mg/kg BD for 2 days) 50% versus 38.9% (10 mg/kg BD for two days) versus 30% control. |
| Ayele 1986 ETH | 128 | 60 mg/kg: 15 mg/kg twice daily for two days 40 mg/kg: 20 mg/kg twice in one day 30 mg/kg: 15 mg/kg twice in one day |
All the doses were well tolerated and accepted. Dizziness was the most frequently reported complaint, but this was mild and transient. |
| Lambertucci 1982 BRA | 91 | 20 mg/kg single dose | Adverse events were minor and transient. Dizziness: Oxamniquine 14.6% versus 2.8% control Nausea: Oxamniquine 14.6% versus 5.6% control. |
| Branchini 1982 BRA | 71 | 14 mg/kg single dose | Adverse events were few and minor. Dizziness: Oxamniquine 44.2% (control not reported) Abdominal pain: Oxamniquine 11.5% versus 17.6% control. |
| Ferrari 2003 BRA | 70 | 20 mg/kg: 10 mg/kg twice in one day | No serious adverse events. Adverse events were mild, mostly headache, dizziness, drowsiness and abdominal pain. Patients from the placebo group also had abdominal pain and drowsiness. |
Analyses 8 and 9: Lower doses oxamniquine versus 40 mg/kg
Lower doses of oxamniquine (20 to 30 mg/kg) have been compared to 40 mg/kg in Ethiopia (two trials), Sudan (two trials), Zimbabwe (two trials), Burundi and Malawi.
Parasitological failure
Compared to 40 mg/kg, both 20 mg/kg and 30 mg/kg of oxamniquine resulted in significantly more parasitological failures at one month (20 mg/kg: RR 3.78, 95% CI 2.05 to 6.99, two trials, 190 participants; 30 mg/kg: RR 1.78, 95% CI 1.15 to 2.75, four trials, 268 participants, Analysis 8.1), and at three to four months (20 mg/kg: RR 2.28, 95% CI 1.40 to 3.71, three trials, 209 participants; 30 mg/kg: RR 1.64, 95% CI 1.10 to 2.43, seven trials, 373 participants, Analysis 8.2).
8.1. Analysis.

Comparison 8 Oxamniquine (lower dose) versus oxamniquine 40 mg/kg, Outcome 1 Parasitological failure at one month.
8.2. Analysis.

Comparison 8 Oxamniquine (lower dose) versus oxamniquine 40 mg/kg, Outcome 2 Parasitological failure at three to four months.
At later time points, no statistically significant differences were shown: six months (20 mg/kg: two trials, 163 participants; 30 mg/kg: three trials, 214 participants, Analysis 8.3) and 12 months (20 mg/kg: two trials, 144 participants; 30 mg/kg: one trial, 77 participants, Analysis 8.4).
8.3. Analysis.
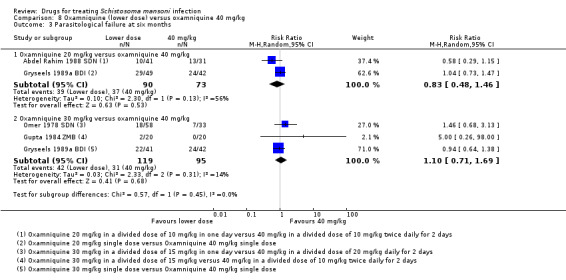
Comparison 8 Oxamniquine (lower dose) versus oxamniquine 40 mg/kg, Outcome 3 Parasitological failure at six months.
8.4. Analysis.

Comparison 8 Oxamniquine (lower dose) versus oxamniquine 40 mg/kg, Outcome 4 Parasitological failure at 12 months.
Egg reduction
Percent egg reduction was evaluated in six of these trials and both lower dose and 40 mg/kg showed a wide range of benefit at one, three and six months: lower dose (57.1% to 99%) and 40 mg/kg (42.7 to 100%) (six trials, 878 participants, Analysis 8.5).
8.5. Analysis.
Comparison 8 Oxamniquine (lower dose) versus oxamniquine 40 mg/kg, Outcome 5 Percent egg reduction.
| Percent egg reduction | ||||||
|---|---|---|---|---|---|---|
| Study | Number of participants | Dose (mg/kg) | Oxamniquine (lower dose) | Oxamniquine (40 mg/kg) | P‐value | Comment |
| One month | ||||||
| Abdel Rahim 1988 SDN | 296 | 20 | 81.7% | 80% | No significant difference with H‐ and Wilcoxon‐tests | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Gupta 1984 ZMB | 60 | 30 | 93.6% | 78.8% | Not reported | Not reported how EPG was calculated |
| Teesdale 1984 MWI | 119 | 30 | 99.4% | 99.1% | Not statistically significant | Not reported how EPG was calculated |
| Three to four months | ||||||
| Abdel Rahim 1988 SDN | 296 | 20 | 78.1% | 67.5% | Not statistically significant with H‐ and Wilcoxon‐ tests | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Ayele 1984 ETH | 65 | 30 | 73.7% | 100% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Ayele 1986 ETH | 162 | 30 | 68.1 | 42.7% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Gupta 1984 ZMB | 60 | 30 | 93.6% | 99.4% | Not reported | Not reported how EPG was calculated |
| Teesdale 1984 MWI | 119 | 30 | 97.6% | 99.1% | Not statistically significant | Not reported how EPG was calculated |
| Six months | ||||||
| Abdel Rahim 1988 SDN | 296 | 20 | 57.1% | 56.8% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Gupta 1984 ZMB | 60 | 30 | 95.2% | 100% | Not reported | Not reported how EPG was calculated |
| Omer 1978 SDN | 176 | 30 | 84% | 89% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
Symptom resolution
One trial compared a lower dose of 20 mg/kg oxamniquine with 40 mg/kg and did not find any difference between the two doses in resolving symptoms at one, three, six, 12 and 24 months of follow‐up: abdominal pain (one trial, 95 participants, Analysis 9.1), diarrhoea (one trial, 16 participants, Analysis 9.3), blood in stool (one trial, 85 participants, Analysis 9.5), hepatomegaly (one trial, 64 participants, Analysis 9.7) and splenomegaly (one trial, 69 participants, Analysis 9.9).
9.1. Analysis.
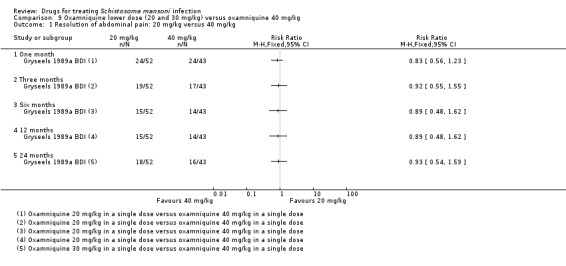
Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 1 Resolution of abdominal pain: 20 mg/kg versus 40 mg/kg.
9.3. Analysis.

Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 3 Resolution of diarrhoea: 20 mg/kg versus 40 mg/kg.
9.5. Analysis.

Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 5 Resolution of blood in stool: 20 mg/kg versus 40 mg/kg.
9.7. Analysis.

Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 7 Resolution of hepatomegaly: 20 mg/kg versus 40 mg/kg.
9.9. Analysis.

Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 9 Resolution of splenomegaly: 20 mg/kg versus 40 mg/kg.
Also, 30 mg/kg did not show any difference statistically compared with 40 mg/kg in resolving symptoms at one, three, six, 12 and 24 months of follow‐up: abdominal pain (one trial, 95 participants, Analysis 9.2), diarrhoea (one trial, 15 participants, Analysis 9.4), blood in stool (one trial, 41 participants, Analysis 9.6), hepatomegaly (one trial, 51 participants, Analysis 9.8) and splenomegaly (one trial, 54 participants, Analysis 9.10).
9.2. Analysis.

Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 2 Resolution of abdominal pain: 30 mg/kg versus 40 mg/kg.
9.4. Analysis.

Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 4 Resolution of diarrhoea: 30 mg/kg versus 40 mg/kg.
9.6. Analysis.

Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 6 Resolution of blood in stool: 30 mg/kg versus 40 mg/kg.
9.8. Analysis.

Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 8 Resolution of hepatomegaly: 30 mg/kg versus 40 mg/kg.
9.10. Analysis.

Comparison 9 Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg, Outcome 10 Resolution of splenomegaly: 30 mg/kg versus 40 mg/kg.
Adverse events
Six trials from Ethiopia (two trials), and one trial each from Malawi, Sudan, Zambia and Zimbabwe assessed adverse events and reported no serious events. Dizziness was most commonly reported, but the event rate and severity did not differ between doses (six trials, 508 participants, Table 12).
8. Adverse events: oxamniquine (lower dose) versus 40 mg/kg.
| Trial | No. of participants | Comparison | Remarks |
| Ayele 1984 ETH | 55 | 30 mg/kg: 15 mg/kg twice in one day. | No serious adverse events were reported. Transient dizziness and nausea were commonly reported (lower dose first): Dizziness: 38.9% versus 42% Nausea: 22.2% versus 26.3% A few mild headaches and abdominal pain were also reported. |
| Ayele 1986 ETH | 96 | 30 mg/kg:15 mg/kg twice in one day. | All doses were well tolerated and no serious event was recorded. Dizziness was more commonly reported, but this was transient. |
| de Clarke 1976b ZWE | 26 | 20 mg/kg: 5 x 2 mg/kg daily for two days 30 mg/kg: 7.5 x 2 mg/kg daily for two days. |
No serious adverse events were recorded. Transient dizziness was more commonly reported and very rarely headache, nausea, and vomiting. Adverse events did not differ between dose. |
| Gupta 1984 ZMB | 60 | 30 mg/kg:15 mg/kg twice in one day 40 mg/kg: 10 mg/kg twice daily for two days |
No serious events were reported. Adverse events were mainly dizziness and nausea, but were minor and transient (lower dose first): Dizziness: 20% versus 25% Nausea: 15% versus 30% A few events of vomiting, headache and abdominal pain were also reported. |
| Omer 1978 SDN | 176 | 30 mg/kg: 15 mg/kg twice in one day 40 mg/kg: 20 mg/kg daily for 2 days |
No serious adverse events were recorded. Asthenia (weakness) was reported among a few receiving 40 mg/kg, but this did not require additional intervention. Transient dizziness was more commonly reported (lower dose first) Dizziness: 3% versus 8% Minor abdominal pain, headache and vomiting also reported. |
| Teesdale 1984 MWI | 95 | 20 mg/kg single dose 30 mg/kg single dose |
Noserious adverse events were recorded. Transient dizziness, nausea and vomiting were most commonly reported. |
Analysis 10: Higher doses oxamniquine versus 40 mg/kg
Higher doses of oxamniquine (50 mg/kg to 60 mg/kg) have been compared to 40 mg/kg in six trials from three countries; Sudan (three trials), Ethiopia (two trials) and Zambia (one trial).
Parasitological failure
Higher doses of oxamniquine have not shown consistent statistically significant benefits over 40 mg/kg at one month (five trials, 349 participants, Analysis 10.1), at three to four months (six trials, 397 participants, Analysis 10.2), or six months (two trials, 177 participants, Analysis 10.3).
10.1. Analysis.

Comparison 10 Oxamniquine (higher dose) versus oxamniquine 40 mg/kg, Outcome 1 Parasitological failure at one month.
10.2. Analysis.

Comparison 10 Oxamniquine (higher dose) versus oxamniquine 40 mg/kg, Outcome 2 Parasitological failure at three to four months.
10.3. Analysis.

Comparison 10 Oxamniquine (higher dose) versus oxamniquine 40 mg/kg, Outcome 3 Parasitological failure at six months.
Losses to follow‐up were high in the trial investigating 50 mg/kg, reaching 76.9% at three months, and heterogeneity between the trials was significant (I2= 64% to 82%).
Egg reduction
Seven trials evaluated egg count and reported a wide range of percent mean reductions among those not cured at one month (86% to 100% versus 56% to 99.1%, four trials, 561 participants, Analysis 10.4), three to four months (82% to 100% versus 42% to 100%, six trials, 791 participants, Analysis 10.4) and six months (62.% to 100% versus 75% to 100%, four trials, 561 participants, Analysis 10.4).
10.4. Analysis.
Comparison 10 Oxamniquine (higher dose) versus oxamniquine 40 mg/kg, Outcome 4 Percent egg reduction.
| Percent egg reduction | ||||||
|---|---|---|---|---|---|---|
| Study | Number of participants | Dose (mg/kg) | Oxamniquine (higher dose) | Oxamniquine (40 mg/kg) | P‐value | Comment |
| One month | ||||||
| Abdel Rahim 1988 SDN | 296 | 60 | 95.7% | 80% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Gupta 1984 ZMB | 60 | 60 | 100% | 78.8% | Not reported | Not reported how EPG was calculated |
| Ibrahim 1980 SDN | 89 | 60 | 86% | 56% | Not reported | EPG (Arithmetic Mean) of participants excreting eggs at follow‐up |
| Teesdale 1984 MWI | 119 | 50 | 99.9% | 99.1% | Not reported | Not reported how EPG was calculated |
| Three to four months | ||||||
| Abdel Rahim 1988 SDN | 296 | 60 | 89.7% | 67.5% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Ayele 1984 ETH | 65 | 60 | 100% | 100% | EPG (Geometric Mean) of participants excreting eggs at follow‐up | |
| Ayele 1986 ETH | 162 | 60 | 82% | 42.7% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Gupta 1984 ZMB | 60 | 60 | 100% | 99.4% | Not reported | Not reported how EPG was calculated |
| Ibrahim 1980 SDN | 89 | 60 | 92% | 65% | Not reported | EPG (Arithmetic Mean) of participants excreting eggs at follow‐up |
| Teesdale 1984 MWI | 119 | 50 | 99.5% | 99.1% | Not reported | Not reported how EPG was calculated |
| Six months | ||||||
| Abdel Rahim 1988 SDN | 296 | 60 | 62.1% | 56.8% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Gupta 1984 ZMB | 60 | 60 | 100% | 100% | Not reported | Not reported how EPG was calculated |
| Ibrahim 1980 SDN | 89 | 60 | 97% | 75% | Not reported | EPG (Arithmetic Mean) of participants excreting eggs at follow‐up |
| Omer 1978 SDN | 176 | 60 | 93% | 89% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
Adverse events
In five trials reporting adverse events, no serious events were recorded. Dizziness and nausea were most commonly reported, but these were transient and did not require additional interventions (one trial, 482 participants, Table 13).
9. Adverse events: oxamniquine (higher dose) versus 40 mg/kg.
| Trial | No. of participants | Comparison | Remarks |
| Ayele 1984 ETH | 55 | 60 mg/kg: 15 mg/kg twice daily for two days 40 mg/kg: 10 mg/kg twice daily for two days |
No serious adverse event was recorded. Dizziness and nausea were commonly reported but these were transient (higher dose first): Dizziness: 50% versus 42% Nausea: 11% versus 26.3% A few mild headaches and abdominal pain were also reported. |
| Ayele 1986 ETH | 96 | 60 mg/kg: 15 mg/kg twice daily for 2 days 40 mg/kg: 20 mg/kg twice in one day |
No serious adverse events were recorded. Dizziness was more commonly reported, but this was transient and did not differ between dose. |
| Gupta 1984 ZMB | 60 | 60 mg/kg: 15 mg/kg twice daily for two days 40 mg/kg: 10 mg/kg twice for daily for two days |
No serious events were reported. Transient dizziness and nausea were more commonly reported (higher dose first): Dizziness: 40% versus 25% Nausea: 25% versus 30% A few events of vomiting, headache and abdominal pain were also reported. |
| Omer 1978 SDN | 176 | 60 mg/kg: 15 mg/kg twice daily for 2 days 40 mg/kg: 20 mg/kg daily for 2 days |
No serious adverse events was recorded. Transient dizziness was more commonly reported (higher dose first): Dizziness: 15% versus 8% Few minor abdominal pain, headache and vomiting were also reported. |
| Teesdale 1984 MWI | 95 | 50 mg/kg single dose 40 mg/kg single dose |
No serious adverse events was recorded. Transient dizziness, nausea and vomiting were most commonly reported and did not differ between dose. |
Analyses 11 and 12: Other oxamniquine dosing regimes
Nine additional trials compared 30 mg/kg oxamniquine with higher and lower doses in Ethiopia (three trials), Zimbabwe (two trials), Burundi (one trial), Nigeria (one trial), Sudan (one trial) and Zambia (one trial).
Lower doses versus 30 mg/kg
Compared to 30 mg/kg, parasitological failure was higher with 15 mg/kg to 20 mg/kg oxamniquine at one month (RR 1.77, 95% CI 1.14 to 2.74, two trials, 230 participants), and at three to four months (RR 2.16, 95% CI 1.40 to 3.32, four trials, 249 participants, Analysis 11.1).
11.1. Analysis.

Comparison 11 Oxamniquine (lower dose) 15 to 20 mg/kg versus oxamniquine 30 mg/kg, Outcome 1 Parasitological failure.
At later follow‐up times, no statistically significant difference were demonstrated (six months: two trials, 179 participants; and 12 months: one trial, 95 participants, Analysis 11.1).
Higher doses versus 30 mg/kg
Compared to 30 mg/kg, 60 mg/kg oxamniquine resulted in significantly fewer parasitological failures at one month (RR 0.04, 95% CI 0.01 to 0.26, two trials, 175 participants, Analysis 12.1), at three to four months (RR 0.17, 95% CI 0.07 to 0.39, four trials, 265 participants, Analysis 12.2) and at six months (RR 0.17, 95% CI 0.06 to 0.50, two trials, 157 participants, Analysis 12.3).
12.1. Analysis.

Comparison 12 Oxamniquine (higher dose) versus oxamniquine 30 mg/kg, Outcome 1 Parasitological failure at one month.
12.2. Analysis.

Comparison 12 Oxamniquine (higher dose) versus oxamniquine 30 mg/kg, Outcome 2 Parasitological failure at three to four months.
12.3. Analysis.
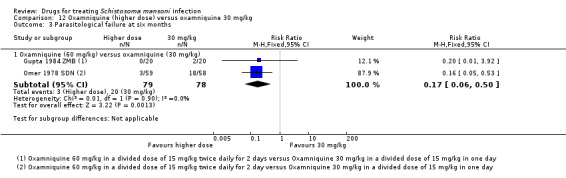
Comparison 12 Oxamniquine (higher dose) versus oxamniquine 30 mg/kg, Outcome 3 Parasitological failure at six months.
No statistically significant differences were seen between 50 mg/kg and 30 mg/kg at one month (one trial, 36 participants, Analysis 12.1) or at three to four months (two trials, 53 participants, Analysis 12.2).
Analysis 13: Praziquantel (40 mg/kg) versus oxamniquine
Eleven trials from different geographical locations directly compared various doses of oxamniquine with praziquantel 40 mg/kg. Dosing schedules commonly applied across different locations are reported in Table 14. The most recent trial, from Sudan, was published in 1990.
10. Commonly used dosing schedule of oxamniquine and praziquantel according to location.
| Trial | Location (country) | Dose | |
| Oxamniquine (mg/kg) | Praziquantel (mg/kg) | ||
| South America | |||
| Branchini 1982 BRA | Brazil | 13.8 | 45.4 |
| da Cunha 1986 BRA | Brazil | 18 | 65 |
| da Cunha 1987 BRA | Brazil | ‐ | 60, 120, 180 |
| da Silva 1986 BRA | Brazil | 15 | 55 |
| Fernandes 1986 BRA | Brazil | 15 | 70 |
| Ferrari 2003 BRA | Brazil | 10 | 180 |
| Katz 1979a BRA | Brazil | ‐ | 20, 40, 60 |
| Katz 1979b BRA | Brazil | ‐ | 50 |
| Katz 1981 BRA | Brazil | ‐ | 30, 30, 50 |
| Katz 1982 BRA | Brazil | 20 | 65 |
| Lambertucci 1982 BRA | Brazil | 20 | ‐ |
| Queiroz 2010 BRA | Brazil | ‐ | 50, 80 |
| Rezende 1985 BRA | Brazil | 15 | 55 |
| Zwingenberger 1987 BRA | Brazil | 15 | 40 |
| North Africa | |||
| Abdel Rahim 1988 SDN | Sudan | 20, 40, 60 | ‐ |
| de Jonge 1990 SDN | Sudan | 60 | 40 |
| Homeida 1989 SDN | Sudan | 40 | |
| Ibrahim 1980 SDN | Sudan | 40, 60 | ‐ |
| Kardaman 1983 SDN | Sudan | ‐ | 40 |
| Omer 1978 SDN | Sudan | 30, 40, 60 | ‐ |
| Omer 1981 SDN | Sudan | ‐ | 30, 40 |
| Barakat 2005 EGY | Egypt | ‐ | 40 |
| Botros 2005 EGY | Egypt | ‐ | 40 |
| Metwally 1995 EGY | Egypt | ‐ | 40 |
| East Africa | |||
| Ayele 1984 ETH | Ethiopia | 30, 40, 60 | ‐ |
| Ayele 1986 ETH | Ethiopia | 30, 40, 60 | ‐ |
| Jaoko 1996 KEN | Kenya | ‐ | 40 |
| Olds 1999 KEN | Kenya | ‐ | 40 |
| Taddese 1988 ETH | Ethiopia | 15, 30 | 40 |
| Teesdale 1984 MWI | Malawi | 30, 40, 50 | 40 |
| Rugemalila 1984 TZA | Tanzania | 15 | 40 |
| Tweyongyere 2009 UGA | Uganda | ‐ | 40 |
| West Africa | |||
| Shafei 1979 NGA | Nigeria | 15, 30 | ‐ |
| De Clercq 2000 SEN | Senegal | ‐ | 40 |
| Guisse 1997 SEN | Senegal | ‐ | 40, 60 |
| Stelma 1997 SEN | Senegal | 20 | 40 |
| Central Africa | |||
| Gryseels 1989a BDI | Burundi | 20, 30, 40 | ‐ |
| Gryseels 1989b BDI | Burundi | ‐ | 20, 30, 40 |
| Southern Africa | |||
| Gupta 1984 ZMB | Zambia | 30, 40, 60 | ‐ |
| Sukwa 1993 ZMB | Zambia | ‐ | 40 |
| de Clarke 1976a ZWE | Zimbabwe | 15, 20 | ‐ |
| de Clarke 1976b ZWE | Zimbabwe | 30, 40 | ‐ |
| de Clarke 1976c ZWE | Zimbabwe | 50, 60 | ‐ |
| Taylor 1988 ZWE | Zimbabwe | ‐ | 10, 20, 30, 40 |
| Middle East | |||
| Al Aska 1990 SAU | Saudi Arabia | 25 | 40 |
Parasitological failure
We did not identify statistically significant differences between oxamniquine (at doses from 10 mg/kg to 60 mg/kg) and praziquantel 40 mg/kg at one month (see Analysis 13.1). No difference was demonstrable at three months between 25 to 30 mg/kg (three trials, 319 participants), 40 mg/kg (one trial, 18 participants) or 50 to 60 mg/kg (one trial, 14 participants, Analysis 13.2). However, 10 to 20 mg/kg of oxamniquine did result in significantly more failures (RR 3.42, 95% CI 1.10 to 10.61, two trials, 135 participants, Analysis 13.2).
13.1. Analysis.

Comparison 13 Oxamniquine versus praziquantel, Outcome 1 Parasitological failure at one month.
13.2. Analysis.

Comparison 13 Oxamniquine versus praziquantel, Outcome 2 Parasitological failure at three months.
In addition, there were no differences between oxamniquine (lower or higher dose) and praziquantel (40 mg/kg) at six months (nine trials, 1167 participants, Analysis 13.3) or 12 months (one trial, 52 participants, Analysis 13.4).
13.3. Analysis.

Comparison 13 Oxamniquine versus praziquantel, Outcome 3 Parasitological failure at six months.
13.4. Analysis.

Comparison 13 Oxamniquine versus praziquantel, Outcome 4 Parasitological failure at 12 months.
Egg reduction
Three trials from Brazil, Ethiopia and Malawi compared oxamniquine 15, 20, 30, 40, and 50 mg/kg with praziquantel 40 mg/kg and measured high percent egg reduction at one month (82.9% to 100% for oxamniquine versus 90% to 92.8% for praziquantel, two trials, 391 participants), three months (70.2% to 99.5% for oxamniquine versus 70% to 100% for praziquantel, three trials, 440 participants), six months (32.5% to 97% for oxamniquine versus 33.6% to 96.8% for praziquantel, three trials, 291 participants), and 12 months (94% for oxamniquine versus 96% for praziquantel, one trial, 91 participants, Analysis 13.5).
13.5. Analysis.
Comparison 13 Oxamniquine versus praziquantel, Outcome 5 Percent egg reduction.
| Percent egg reduction | ||||||
|---|---|---|---|---|---|---|
| Study | Number of participants | Dose (mg/kg) | Oxamniquine | Praziquantel (40 mg/kg) | P‐value | Comment |
| One month | ||||||
| Taddese 1988 ETH | 100 | 15 | 82.9% | 90% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Taddese 1988 ETH | 100 | 30 | 100% | 90% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Teesdale 1984 MWI | 42 | 20 | 91.7% | 92.8% | Not reported | Not reported |
| Teesdale 1984 MWI | 49 | 40 | 99.1% | 92.8% | Not reported | Not reported |
| Teesdale 1984 MWI | 50 | 30 | 99.4% | 92.8% | Not reported | Not reported |
| Teesdale 1984 MWI | 50 | 50 | 99.9% | 92.8% | Not reported | Not reported |
| Three months | ||||||
| Taddese 1988 ETH | 100 | 30 | 83.6% | 70% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Taddese 1988 ETH | 100 | 15 | 70.2% | 70% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Teesdale 1984 MWI | 49 | 40 | 99.1% | 92.7% | Not reported | Not reported who were involved in EPG calculation |
| Teesdale 1984 MWI | 50 | 30 | 97.6% | 92.7% | Not reported | Not reported who were involved in EPG calculation |
| Teesdale 1984 MWI | 50 | 50 | 99.5% | 92.7% | Not reported | Not reported who were involved in EPG calculation |
| Zwingenberger 1987 BRA | 91 | 15 | 46.1‐67.6% | 100% | Not statistically significant | EPG (Range) of participants excreting eggs at follow‐up |
| Six months | ||||||
| Taddese 1988 ETH | 100 | 30 | 85% | 33.6% | Not reported | EPG (Geometric Mean) of participants still excreting eggs at follow‐up |
| Taddese 1988 ETH | 100 | 15 | 73.3% | 33.6% | Not reported | EPG (Geometric Mean) of participants still excreting eggs at follow‐up |
| Zwingenberger 1987 BRA | 91 | 15 | 32.5‐97% | 93.8‐96.8% | Not statistically significant | EPG (Range) of participants still excreting eggs at follow‐up |
| 12 months | ||||||
| Zwingenberger 1987 BRA | 91 | 15 | 94% | 96.9% | Not statistically significant | EPG (Range) of participants still excreting eggs at follow‐up |
Adverse events
In five trials reporting from Brazil, Ethiopia, Malawi, Saudi Arabia and Tanzania that assessed adverse events, only two serious adverse events were recorded (both with oxamniquine) in two trials: one from a moderate endemicity setting in Ethiopia that used 30 mg/kg in a split dose given the same day; and one trial from Saudi Arabia that used a single dose of 25 mg/kg. No further differences were observed in the number and type of adverse events between oxamniquine and praziquantel although dizziness was recorded in excess with oxamniquine and abdominal pain with praziquantel (Table 15).
11. Adverse events: different oxamniquine dose versus praziquantel (40 mg/kg).
| Trial | No. of participants | Comparison | Remarks |
| Al Aska 1990 SAU | 200 | Oxamniquine (25 mg/kg) single dose Praziquantel (40 mg/kg) single dose |
One serious adverse event (seizure) was recorded in the oxamniquine 25 mg/kg group. Transient dizziness, abdominal pain and nausea were most commonly reported (oxamniquine first): Dizziness: 36% versus 20% Abdominal pain: 25% versus 12% Nausea: 10% versus 8 % |
| Branchini 1982 BRA | 101 | Oxamniquine (14 mg/kg) single dose Praziquantel (45 mg/kg) single dose |
No serious adverse events were recorded. Adverse events were minor and transient. Dizziness, abdominal pain and nausea were most frequently reported (oxamniquine first): Dizziness: 44.2% versus 46.9% Abdominal pain: 3.8% versus 24.5% Nausea: 5.8% versus 8.2% |
| Rugemalila 1984 TZA | 72 | Oxamniquine (15 mg/kg) single dose Praziquantel (40 mg/kg) single dose |
No serious adverse events were recorded. Transient abdominal pain and drowsiness were commonly reported (oxamniquine first): Abdominal pain: 16% versus 63% Drowsiness: 25% versus 11% |
| Taddese 1988 ETH | 200 | Oxamniquine (15 mg/kg) single dose Oxamniquine 30 mg/kg :15 mg/kg twice in one day Praziquantel (40 mg/kg) single dose |
One serious adverse event (seizure) was recorded with oxamniquine 30 mg/kg. Adverse events were minor and transient. Dizziness and abdominal pain were commonly reported (oxamniquine lower dose first): Dizziness: 22%, 16% versus 20% Abdominal pain: 20%, 28% versus 24% |
| Teesdale 1984 MWI | 119 | Oxamniquine (30 mg/kg) single dose Oxamniquine (40 mg/kg) single dose Oxamniquine (50 mg/kg): 25 mg/kg twice in one day Praziquantel (40 mg/kg) single dose |
No serious adverse events were recorded. Transient dizziness was commonly reported among participants receiving oxamniquine. There was no difference in events between oxamniquine 30, 40 and 50 mg/kg (oxamniquine lower dose first): Dizziness: 30.8%, 29.2%, 30.8% versus 8.3% |
Analysis 14: Myrrh (Mirazid) versus praziquantel
Parasitological failure
Myrhh (Mirazid) was tested in two trials at a single daily dose of 300 mg for three days, and almost all failed treatment at three to six weeks (RR 4.08, 95% CI 2.87 to 5.78, 236 participants, Analysis 14.1). Consequently, further investigation of this compound was abandoned.
14.1. Analysis.

Comparison 14 Myrrh (Mirazid) 300 mg once daily for three days versus praziquantel 40 mg/kg, Outcome 1 Parasitological failure at three to six weeks.
Egg reduction rate
There were only small reductions in reported percent geometric mean egg reduction in these two studies, but they were not clinically important (Analysis 14.2).
14.2. Analysis.
Comparison 14 Myrrh (Mirazid) 300 mg once daily for three days versus praziquantel 40 mg/kg, Outcome 2 Percent egg reduction three to six weeks.
| Percent egg reduction three to six weeks | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Myrrh (Mirazid: 300 mg) daily for 3 days | Praziquantel (40 mg/kg) | P‐value | Comment |
| Barakat 2005 EGY | 104 | 17.2% | 84% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Botros 2005 EGY | 271 | 8.8% | 63.7% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
Adverse events
No trial reports adverse events.
Section 2. Combination therapies
Analysis 15: Praziquantel plus artesunate versus praziquantel alone
One trial conducted from 1999 to 2000 in a high endemicity setting in Senegal evaluated artesunate plus praziquantel versus praziquantel alone.
Parasitological failure
In this setting, parasitological failure at one month occurred in 50% of participants given praziquantel 40 mg/kg alone. The addition of artesunate 12 mg/kg given in a divided dose of 2.5 mg/kg daily for five days resulted in a lower failure rate at one month but this did not reach statistical significance (one trial, 75 participants, Analysis 15.1). At three and six months no additional benefit with artesunate plus praziquantel was seen.
15.1. Analysis.

Comparison 15 Praziquantel (40 mg/kg) plus artesunate (12 mg/kg total dose) versus praziquantel (40 mg/kg), Outcome 1 Parasitological failure at one month.
Egg reduction
Geometric mean egg reductions appear lower with combination treatment but tests of statistical significance were not reported, and the clinical relevance of this finding are unclear (one trial, 75 participants, Analysis 15.4).
15.4. Analysis.
Comparison 15 Praziquantel (40 mg/kg) plus artesunate (12 mg/kg total dose) versus praziquantel (40 mg/kg), Outcome 4 Percent egg reduction.
| Percent egg reduction | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Artesunate (12 mg/kg) plus Praziquantel (40 mg/kg) | Praziquantel (40 mg/kg) | P‐value | Comment |
| One month | |||||
| De Clercq 2000 SEN | 75 | 83% | 62% | P > 0.2380 | EPG (Range) based on participants excreting eggs and those not excreting eggs at follow‐up |
| Three months | |||||
| De Clercq 2000 SEN | 66 | 89% | 79% | P > 0.5552 | EPG (Range) based on participants excreting eggs and those not excreting eggs at follow‐up |
| Six months | |||||
| De Clercq 2000 SEN | 63 | 87% | 82% | P > 0.5892 | EPG (Range) based on participants excreting eggs and those not excreting eggs at follow‐up |
Adverse events
Adverse events were not reported.
Analysis 16: Praziquantel plus oxamniquine versus praziquantel alone
Only one trial in a high endemicity setting in Brazil published in 1987 has evaluated oxamniquine plus praziquantel versus praziquantel alone.
Parasitological failure
Compared to praziquantel alone (40 mg/kg in two divided doses on one day), a combination of oxamniquine (7.5 mg/kg) plus praziquantel (20 mg/kg) did not demonstrate any statistically significant benefits at three, six or 12 months follow‐up (one trial, 52 participants, Analysis 16.1).
16.1. Analysis.

Comparison 16 Praziquantel (20 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (40 mg/kg), Outcome 1 Parasitological failure at three months.
Egg reduction
The combination treatment was associated with lower geometric mean egg reductions at three, six and 12 months but tests of statistical significance were not reported (one trial, 52 participants, Analysis 16.4).
16.4. Analysis.
Comparison 16 Praziquantel (20 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (40 mg/kg), Outcome 4 Percent egg reduction.
| Percent egg reduction | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Oxamniquine (7.5 mg/kg) plus Praziquantel (20 mg/kg) | Praziquantel (20 mg/kg x 2) in one day | P‐value | Comment |
| Three months | |||||
| Zwingenberger 1987 BRA | 76.9% to 99.5% | 100% | Not statistically significant | EPG (Range) of participants excreting eggs at follow‐up | |
| Six months | |||||
| Zwingenberger 1987 BRA | 87.7% | 93.8% | Not statistically significant | EPG (Range) of participants excreting eggs at follow‐up | |
| 12 months | |||||
| Zwingenberger 1987 BRA | 93.7% | 96.9% | Not statistically significant | EPG (Range) of participants excreting eggs at follow‐up | |
Adverse events
These were not reported.
Analysis 17: Praziquantel (8 mg/kg) plus oxamniquine (4 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg)
One small trial of schoolchildren from a high endemicity setting co‐endemic for S. mansoni and S. haematobium in Zimbabwe investigated different oxamniquine and praziquantel dose combinations.
Parasitological failure
Children aged seven to 16 years and excreting more than 100 eggs per gram of stool were included in this trial. Statistically fewer failures were seen with the higher dose‐combination at one month (RR 6.30, 95% CI 1.60 to 24.75, one trial, 28 participants, Analysis 17.1), but not at three months (one trial, 29 participants, Analysis 17.2) or six months (one trial, 20 participants, Analysis 17.3).
17.1. Analysis.

Comparison 17 Praziquantel (8 mg/kg) plus oxamniquine (4 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg), Outcome 1 Parasitological failure at one month.
17.2. Analysis.

Comparison 17 Praziquantel (8 mg/kg) plus oxamniquine (4 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg), Outcome 2 Parasitological failure at three months.
17.3. Analysis.

Comparison 17 Praziquantel (8 mg/kg) plus oxamniquine (4 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg), Outcome 3 Parasitological failure at six months.
Egg reduction
The percentage egg reduction also appeared to be lower in those receiving the higher dose combination but tests of statistical significance were not reported (one trial, 59 participants, Analysis 17.4).
17.4. Analysis.
Comparison 17 Praziquantel (8 mg/kg) plus oxamniquine (4 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg), Outcome 4 Percent egg reduction.
| Percent egg reduction | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Oxamniquine (4 mg/kg) plus Praziquantel (8 mg/kg) | Oxamniquine (10 mg/kg) plus Praziquantel (20 mg/kg) | P‐value | Comment |
| One month | |||||
| Creasey 1986 ZWE | 59 | 93.7% | 88% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Three months | |||||
| Creasey 1986 ZWE | 59 | 82.4% | 66.3% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Six months | |||||
| Creasey 1986 ZWE | 59 | 98.5% | 96.6% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
Adverse events
No serious adverse events were recorded and the incidence of non‐severe events did not differ between combinations. About 70% of children reported abdominal discomfort but these were transient and had resolved by the following day (Table 16).
12. Adverse events: oxamniquine plus praziquantel versus oxamniquine plus praziquantel.
| Trial | No. of participants | Comparison | Remarks |
| Creasey 1986 ZWE | 59 | Oxamniquine (4 mg/kg) plus praziquantel (8 mg/kg) versus oxamniquine (10 mg/kg) plus praziquantel (20 mg/kg) Oxamniquine (7.5 mg/kg) plus praziquantel (15 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg) |
No serious adverse events. Adverse events were minor and did not differ between combinations. One child reported dizziness five minutes after treatment but required no further treatment and was well the following day. About 70% of children reported abdominal discomfort but these were transient and had resolved the following day. |
Analysis 18: Praziquantel (15 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg)
One trial in Zimbabwe investigated slightly higher oxamniquine and praziquantel dose combinations. The included children had to excrete more than 100 eggs per gram of stool.
Parasitological failure
A statistically significant difference was not demonstrated at one, three and six months (one trial, 48 participants, Analysis 18.1, Analysis 18.2, Analysis 18.3).
18.1. Analysis.

Comparison 18 Praziquantel (15 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg), Outcome 1 Parasitological failure at one month.
18.2. Analysis.

Comparison 18 Praziquantel (15 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg), Outcome 2 Parasitological failure at three months.
18.3. Analysis.

Comparison 18 Praziquantel (15 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg), Outcome 3 Parasitological failure at six months.
Egg reduction rate
Percent egg reductions were high at one, three and six months (82% to 96.1% versus 66.3% to 96.6%, one trial, 59 participants, Analysis 18.4).
18.4. Analysis.
Comparison 18 Praziquantel (15 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg), Outcome 4 Percent egg reduction.
| Percent egg reduction | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Oxamniquine (7.5 mg/kg) plus Praziquantel (15 mg/kg) | Oxamniquine (10 mg/kg) plus Praziquantel (20 mg/kg) | P‐value | Comment |
| One month | |||||
| Creasey 1986 ZWE | 59 | 96.1% | 88% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Three months | |||||
| Creasey 1986 ZWE | 59 | 82% | 66.3% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
| Six months | |||||
| Creasey 1986 ZWE | 59 | 95% | 96.6% | Not reported | EPG (Geometric Mean) of participants excreting eggs at follow‐up |
Adverse events
No serious adverse events were recorded apart from one child who reported dizziness immediately after treatment but required no further treatment (Table 16).
Section 3. Do failure rates vary in children and adults?
Praziquantel
A subgroup analysis conducted in two studies from Burundi raised concern that parasitological failure following 40 mg/kg may be higher in children than in adults. The frequency of parasitological treatment failure was consistently higher in children than adults at one, three, six, and 12 months, and this was also observed for doses of 20 mg/kg and 30 mg/kg (see Table 17).
13. Non‐randomized exploratory analysis of age (praziquantel).
| Dose (mg/kg) | Time point (months) | Study | Number failed/number examined (%) 1 | |
| Children (<20 years) | Adults (≥20 years) | |||
| 20 | 1 | Gryseels 1989b BDI | 48/109 (44) | 26/61 (43) |
| 3 | Gryseels 1989b BDI | 49/100 (49) | 16/54 (30) | |
| 6 | Gryseels 1989b BDI | 61/109 (56) | 19/58 (33) | |
| 12 | Gryseels 1989b BDI | 60/101 (59) | 21/59 (36) | |
| 30 | 1 |
Gryseels 1989b BDI | 37/93 (40) | 2/48 (4) |
| Gryseels 1989c BDI | 37/104 (36) | 12/65 (18) | ||
| 3 |
Gryseels 1989b BDI | 38/91 (42) | 4/40 (10) | |
| Gryseels 1989c BDI | 37/98 (38) | 10/66 (15) | ||
| 6 |
Gryseels 1989b BDI | 41/94 (44) | 6/46 (13) | |
| Gryseels 1989c BDI | 40/94 (43) | 19/61 (31) | ||
| 12 |
Gryseels 1989b BDI | 55/91 (60) | 9/44 (20) | |
| Gryseels 1989c BDI | 73/92 (79) | 19/51 (37) | ||
| 40 | 1 |
Gryseels 1989b BDI | 22/94 (23) | 5/42 (12) |
| Gryseels 1989c BDI | 18/81 (22) | 2/54 (4) | ||
| 3 |
Gryseels 1989b BDI | 23/87 (26) | 1/32 (3) | |
| Gryseels 1989c BDI | 26/83 (31) | 11/54 (20) | ||
| 6 |
Gryseels 1989b BDI | 29/92 (32) | 4/37 (11) | |
| Gryseels 1989c BDI | 23/76 (30) | 10/51 (20) | ||
| 12 | Gryseels 1989b BDI | 34/84 (40) | 10/38 (26) | |
1Number failed/number examined (%) presented for the praziquantel treatment group of each study that presents data for adults and children separately.
Oxamniquine
Similarly, a subgroup analysis of two studies from Burundi and Sudan administering oxamniquine has shown a consistent pattern of higher parasitological treatment failure in children than adults at one to 12 months (see Table 18).
14. Non‐randomized exploratory analysis of age (oxamniquine).
| Dose (mg/kg) | Time point (month) | Study | Number failed/number examined (%) 1 | |
| Children (< 20 years) | Adults (≥ 20 years) | |||
| 20 | 1 |
Abdel Rahim 1988 SDN | 11/41 (26) | 3/55 (6) |
| Gryseels 1989a BDI | 31/57 (60) | 17/95 (17) | ||
| 3 |
Abdel Rahim 1988 SDN | 6/41 (15) | 4/55 (7) | |
| Gryseels 1989a BDI | 30/56 (54) | 22/102 (22) | ||
| 6 |
Abdel Rahim 1988 SDN | 10/41 (24) | 9/55 (16) | |
| Gryseels 1989a BDI | 29/49 (59) | 20/86 (23) | ||
| 12 | Gryseels 1989a BDI | 38/41 (93) | 20/83 (24) | |
| 30 | 1 | Gryseels 1989a BDI | 16/42 (38) | 2/76 (3) |
| 3 | Gryseels 1989a BDI | 12/46 (26) | 8/77 (10) | |
| 6 | Gryseels 1989a BDI | 22/41 (54) | 3/62 (5) | |
| 12 | Gryseels 1989a BDI | 24/39 (62) | 10/67 (15) | |
| 40 | 1 |
Abdel Rahim 1988 SDN | 2/43 (5) | 1/57 (2) |
| Gryseels 1989a BDI | 8/49 (19) | 2/67 (3) | ||
| 3 |
Abdel Rahim 1988 SDN | 5/43 (12) | 3/57 (5) | |
| Gryseels 1989a BDI | 10/51 (20) | 5/65 (8) | ||
| 6 |
Abdel Rahim 1988 SDN | 13/31 (42) | 5/57 (9) | |
| Gryseels 1989a BDI | 24/42 (57) | 11/62 (18) | ||
| 12 | Gryseels 1989a BDI | 25/38 (66) | 11/60 (18) | |
| 60 | 1 | Abdel Rahim 1988 SDN | 2/42 (5) | 0/58 (0) |
| 3 | Abdel Rahim 1988 SDN | 10/42 (24) | 1/58 (2) | |
| 6 | Abdel Rahim 1988 SDN | 16/42 (38) | 1/58 (2) | |
1Number failed/number examined (%) presented for the oxamniquine treatment group of each study that presents data for adults and children separately.
Subgroup analysis of treatment arms receiving 40 mg/kg in the other included studies was not possible given the available data.
Discussion
Summary of main results
Compared to placebo, praziquantel 40 mg/kg substantially reduced parasitological treatment failure at one month post‐treatment (moderate quality evidence). Compared to this standard dose, lower doses of 20 mg/kg and 30 mg/kg were inferior (low quality evidence); and higher doses, up to 60 mg/kg, have not shown any advantage (moderate quality evidence).
Compared to placebo, oxamniquine 40 mg/kg substantially reduced parasitological treatment failure at three months (moderate quality evidence). Lower doses than 40 mg/kg were inferior at one month (low quality evidence), and higher doses such as 60 mg/kg have not shown a consistent benefit (low quality evidence).
Ten trials compared oxamniquine at 20, 30 and 60 mg/kg with praziquantel 40 mg/kg and did not show any convincing differences in failure rate and percent egg reduction. Only one small study directly compared praziquantel 40 mg/kg with oxamniquine 40 mg/kg and did not demonstrate a statistically significant difference in parasitological failure (very low quality evidence).
Combining praziquantel with artesunate has not been shown to have benefits in terms of failure rate compared to praziquantel alone at one month, three or six months (one trial, 75 participants, very low quality evidence). Two trials have also compared combinations of praziquantel and oxamniquine in different doses but did not find statistically significant differences in failure rate.
Compared to 40 mg/kg, no dose effect was demonstrable for clinical improvement with lower doses (20 and 30 mg/kg) of praziquantel or oxamniquine in resolving abdominal pain, diarrhoea, blood in stool, hepatomegaly, and splenomegaly at one, three, six, and 12 months, or up to two years of follow‐up. Adverse events were not well reported but were mostly described as minor and transient.
Overall completeness and applicability of evidence
For praziquantel, the evidence presented is generally supportive of the current WHO recommended dose of 40 mg/kg to treat S. mansoni infection (WHO 2002). Parasitological cure as low as 57% has been reported in Kenya in the 1990s (Olds 1999 KEN), and 52% in Senegal in 1993 (Guisse 1997 SEN). However, higher efficacy has been seen in more recent trials; Tanzania (81%), Mauritania (95%) and Brazil (92%) in 2006/2007 (Olliaro 2011 BRA; Olliaro 2011 MRT; Olliaro 2011 TZA), and Uganda (87%) in 2003/2005 (Tweyongyere 2009 UGA). The lower cure rates from the earlier studies could be expected from the high endemicities where pre‐treatment intensity of infection were very high (prevalence > 80%) compared to the recent studies (prevalence < 30%). In such situations, even at 95% efficacy, a sufficient number of surviving schistosomes would remain, causing sustained egg excretion in most of the treated participants (Danso‐Appiah 2002). Furthermore, as a result of intense transmission, most treated participants might have acquired large numbers of new infections just before treatment and as immature worms are less sensitive to praziquantel most would have escaped drug action and developed into egg‐laying adult worms shortly after treatment to present as failures. The high diagnostic sensitivity (mostly duplicate slides from two or more consecutive stool specimens) and lower dose of praziquantel applied in the earlier studies (except Guisse 1997 SEN) would have also contributed to the observed lower cure rates.
The results in this review appear to be generalizable elsewhere but it should be noted that these trials excluded preschool children under five years and concerns remain that this dose may be less effective in this group. This is because praziquantel works in synergy with host immune status (Sabah 1986) and this is not yet fully developed in very young children. A subgroup analysis conducted in two studies from Burundi with praziquantel at 40 mg/kg and another two studies from Burundi and Sudan with oxamniquine 40 mg/kg raises concern as parasitological failure was consistently higher in children than in adults at one to 12 months of follow‐up. This trend was also observed for doses of 20 mg/kg and 30 mg/kg for both treatments, and a higher dose (60 mg/kg) for oxamniquine.
Higher doses than 40 mg/kg have been national policy in Brazil since 1995: 60 mg/kg for children and 50 mg/kg for adolescents and adults. We found little direct evidence from randomized controlled trials to support or refute this as a policy. Only a single trial from Brazil reported outcomes at one month and this failed to show a statistically significant advantage with 60 mg/kg compared to 40 mg/kg, and excluded children aged less than 10 years (Olliaro 2011 BRA). Several further trials from Brazil have evaluated higher doses and longer regimens but these only reported outcomes at six months or beyond. These do offer some limited evidence that increasing the dose of praziquantel might have parasitological benefits.
There is no justification for using lower doses, even if potentially effective in morbidity control, as sub‐curative doses may eventually select for drug resistant parasites (Doenhoff 1998; Doenhoff 2008).
Praziquantel is known to be less effective on immature schistosomes than adult worms (Sabah 1986), and combination therapy (with drugs with unrelated mechanisms of action and targeting the different developmental stages of the schistosomes), has potential as a future control strategy. Potential partner drugs include oxamniquine and the artemisinin derivatives. Of these, the artemisinin derivatives have been shown to be effective against immature schistosomes in laboratory studies (Utzinger 2001; Utzinger 2002; Utzinger 2003; Utzinger 2007), and there is some indirect evidence for efficacy from non‐randomized studies in urinary schistosomiasis (De Clercq 2002; Inyang‐Etoh 2004; Boulanger 2007; Inyang‐Etoh 2009), and from people with malaria co‐infected S. haematobium (Boulanger 2007). However, to date only a single trial has directly evaluated praziquantel plus artesunate and no additional benefit was observed compared to praziquantel alone (De Clercq 2000 SEN).
For oxamniquine, there is no current consensus on the optimal dosing regimen and it has largely fallen out of use in favour of praziquantel. Although the presented data are now more than 20 years old, and suffers some methodological problems, there is sufficient evidence of its efficacy against S. mansoni to suggest that it could be reinstated as an alternate treatment to decrease the pressure on praziquantel. However, a limitation of oxamniquine is that its effect is restricted to S. mansoni as this is the only species possessing the enzyme which converts oxamniquine to its active metabolite (Cioli 1995). It is therefore unsuitable for use in areas where co‐infection with S. haematobium is common.
The optimal dose of oxamniquine may also be 40 mg/kg but further studies are required to confirm this, preferably in direct comparison with praziquantel, and trials should include and evaluate the efficacy of this dose in young children.
Safety was under reported and inconsistently assessed in most of these clinical trials. Furthermore, only the few studies comparing the intervention versus placebo allow identification of potentially drug‐related events. From these few studies it is therefore not possible to provide a reliable account of treatment tolerability.
Quality of the evidence
The quality of evidence was assessed using the GRADE methodology and displayed in summary of findings (SOF) tables for the main comparisons. The level of quality is judged on a 4‐point scale. High quality evidence implies that level of confidence in the effect estimate is high and that further research is unnecessary. Moderate quality evidence implies lower confidence in the result and further research may have an important impact on the result. Low and very low quality evidence reflect increasing uncertainty in the result and a greater need for further research.
The evidence presented is generally considered to be of moderate or low quality due to concerns related to three key factors: i) the age of the trials, with the majority more than 20 years old, ii) the poor methodological reporting of many of these older trials, and iii) the number and size of the trials being small and often underpowered to reliably detect statistically significant differences. The specific reasons for downgrading the quality of the evidence are given in the footnotes to the SOF tables.
Potential biases in the review process
A few minor difficulties in extracting the data from the available papers should be noted but these are unlikely to have introduced major bias into this review. For three trials (Gryseels 1989a BDI; Gryseels 1989b BDI; Gryseels 1989c BDI), data on parasitological failure were obtained from figures and might not be the exact estimates. One trial (Sukwa 1993 ZMB) actually reported reinfection rate but this is included in this review because this outcome is similar to failure rate. The trial by Tweyongyere 2009 UGA was a nested cohort study within a larger mother and baby cohort study in which pregnant women found to be infected with S. mansoni were randomized to receive praziquantel or placebo. Despite representing a special population, this is not likely to affect the validity of the results.
Agreements and disagreements with other studies or reviews
A non‐Cochrane review compared praziquantel with placebo in two studies in Brazil and showed slightly higher cure rate with praziquantel (Liu 2011). The reliability of the evidence in this review cannot be established given that the two studies that assessed this outcome involved only 25 participants.
The effects of praziquantel and artesunate in urinary schistosomiasis due to S. haematobium have been evaluated in a separate Cochrane review last published in 2008. Praziquantel was found to be effective against S. haematobium with few adverse events, and similarly to this review there was insufficient evidence for the use of artesunate monotherapy or combination therapy (Danso‐Appiah 2008).
Limitations in the design and methodology in schistosomiasis trials identified during the earlier Cochrane review, and consequent future research needs have also been reported elsewhere (Danso‐Appiah 2009).
Authors' conclusions
Implications for practice.
The available evidence supports single dose praziquantel at 40 mg/kg as the standard treatment for S. mansoni infection as recommended by the WHO.
Oxamniquine, a largely discarded alternative, appears efficacious and production and distribution should continue to ease selective pressure on praziquantel. However, its use should be limited to areas without S. haematobium co‐endemicity.
Implications for research.
Further research is necessary to find the optimal dosing regimen of praziquantel and oxamniquine in children under five years, given the observational evidence that failure rates with 40 mg/kg may be higher in this age‐group.
Combination therapy, ideally with drugs with unrelated mechanisms of action and targeting the different developmental stages of the schistosomes in the human host should be pursued as an area for future research; for example; praziquantel plus oxamniquine, praziquantel plus mefloquine, and praziquantel plus an artemisinin derivative.
What's new
| Date | Event | Description |
|---|---|---|
| 6 November 2012 | New citation required and conclusions have changed | This review update has been prepared by new authors (Danso‐Appiah A, Olliaro PL, Donegan S, Sinclair D and Utzinger J). Each section of the review has been rewritten and updated, including the results and conclusions. |
| 6 November 2012 | New search has been performed | This is a new review with a fresh authorship team, replacing a previous version. The previous version included 13 trials and the last search was in 2005 and only reported parasitological failure. The current version includes 52 trials, includes percentage egg reduction as an outcome, and includes new trials evaluating artesunate. All data have been re‐extracted. Each section of the review has been rewritten. Results are summarized using a Summary of Findings table. Data in the intervention arm in relation to cure are explored by age. |
Acknowledgements
We thank Dr. Vittoria Lujte (Information Specialist) for developing the search strategy and doing the search for studies and Christianne Esparza for retrieving hard copies of published studies. We are grateful to Dr. Harriet MacLehose (former Deputy Editor), Anne‐Marie Stephani (Managing Editor), Phil Hinds (Editorial Assistant & Administrator), Reive Robbs (former CIDG Co‐ordinator) and the entire International Health Group for their support during the preparation of this review. Our sincere thanks go to Dr Otavio Pieri and Prof Martin Meremikwu for critically reading this review and for providing useful comments, and to Dr Deirdre Walshe (Associate Editor) for drafting the plain language summary.
This document is funded by the UK Department for International Development (DFID) for the benefit of developing countries. . The views expressed are not necessarily those of DFID. PLO is a staff member of the WHO; the author alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy, or views of WHO.
H. Saconato and A. Atallah prepared the original version of this review (Issue 3, 1999).
Data and analyses
Comparison 1. Praziquantel versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 40 mg/kg single dose | 2 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [1.03, 9.53] |
| 2 Parasitological failure at six months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 20 mg/kg single dose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 40 mg/kg in two divided doses on the same day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 60 mg/kg in 3 divided doses 3 hours apart | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 60 mg/kg daily for 3 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Parasitological failure at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 20 mg/kg single dose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 40 mg/kg in two divided doses on the same day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 60 mg/kg in 3 divided doses 3 hours apart | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Praziquantel (lower dose) versus praziquantel 40 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Praziquantel 20 mg/kg versus praziquantel 40 mg/kg | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.64, 3.02] |
| 1.2 Praziquantel 30 mg/kg versus praziquantel 40 mg/kg | 3 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.15, 2.01] |
| 2 Parasitological failure at three months | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Praziquantel 20 mg/kg versus praziquantel 40 mg/kg | 2 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.66, 2.79] |
| 2.2 Praziquantel 30 mg/kg versus praziquantel 40 mg/kg | 3 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.10, 1.77] |
| 3 Parasitological failure at six to 12 months | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Praziquantel 20 mg/kg versus praziquantel 40 mg/kg | 3 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.35, 4.76] |
| 3.2 Praziquantel 30 mg/kg versus praziquantel 40 mg/kg | 5 | 651 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.19, 1.85] |
| 4 Percent egg reduction | Other data | No numeric data |
Comparison 3. Praziquantel lower dose (20 and 30 mg/kg) versus praziquantel 40 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of abdominal pain: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Resolution of abdominal pain: 30 mg/kg versus 40 mg/kg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 One month | 2 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.55, 1.10] |
| 2.2 Three months | 2 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 2.3 Six months | 2 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.52, 1.08] |
| 2.4 12 months | 2 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.35, 1.01] |
| 2.5 24 months | 2 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.25] |
| 3 Resolution of diarrhoea: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Resolution of diarrhoea: 30 mg/kg versus 40 mg/kg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 One month | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.70, 1.03] |
| 4.2 Three months | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.25] |
| 4.3 Six months | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.22] |
| 4.4 12 months | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.77, 1.37] |
| 4.5 24 months | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.23] |
| 5 Resolution of blood in stool: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Resolution of blood in stool: 30 mg/kg versus 40 mg/kg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 One month | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| 6.2 Three months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.13] |
| 6.3 Six months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.07] |
| 6.4 12 months | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.22] |
| 6.5 24 months | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.40] |
| 7 Resolution of hepatomegaly: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Resolution of hepatomegaly: 30 mg/kg versus 40 mg/kg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 One month | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.83, 1.35] |
| 8.2 Three months | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 8.3 Six months | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 8.4 12 months | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.73, 1.16] |
| 8.5 24 months | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.14] |
| 9 Resolution of splenomegaly: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Resolution of splenomegaly: 30 mg/kg versus 40 mg/kg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 One month | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.65, 1.15] |
| 10.2 Three months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.25] |
| 10.3 Six months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.36] |
| 10.4 12 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.76, 1.46] |
| 10.5 24 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.72, 1.23] |
Comparison 4. Praziquantel (higher dose) versus praziquantel 40 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Praziquantel 60 mg/kg versus praziquantel 40 mg/kg | 4 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 2 Parasitological failure at six months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Praziquantel 50 mg/kg versus praziquantel 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Praziquantel 60 mg/kg versus praziquantel 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Parasitological failure at six to 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Praziquantel 60 mg/kg versus praziquantel 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Percent egg reduction at one month | Other data | No numeric data |
4.2. Analysis.

Comparison 4 Praziquantel (higher dose) versus praziquantel 40 mg/kg, Outcome 2 Parasitological failure at six months.
4.3. Analysis.

Comparison 4 Praziquantel (higher dose) versus praziquantel 40 mg/kg, Outcome 3 Parasitological failure at six to 12 months.
Comparison 5. Praziquantel 40 mg/kg divided dose versus praziquantel 40 mg/kg single dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 2 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.13, 1.69] |
| 2 Parasitological failure at three months | 2 | 516 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.18, 0.53] |
| 3 Parasitological failure at six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Percent egg reduction at one month | Other data | No numeric data |
Comparison 6. Praziquantel alternative dosing (Brazil).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at six months | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Praziquantel 30 mg/kg x 2 daily for 2 days versus praziquantel 30 mg/kg x 2 in one day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Praziquantel 30 mg/kg x 2 daily for 3 days versus praziquantel 30 mg/kg x 2 in one day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Praziquantel 30 mg/kg x 1 daily for 6 days versus 30 mg/kg x 2 in one day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Praziquantel 20 mg/kg x 3, 4 hours apart versus praziquantel 50 mg/kg single dose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Praziquantel 40 mg/kg x 2, 1 hour apart versus praziquantel 50 mg/kg single dose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Percent egg reduction at six months | Other data | No numeric data | ||
| 3 Percent egg reduction at six months | Other data | No numeric data | ||
| 4 Percent egg reduction at six months | Other data | No numeric data |
6.2. Analysis.
Comparison 6 Praziquantel alternative dosing (Brazil), Outcome 2 Percent egg reduction at six months.
| Percent egg reduction at six months | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Geometric mean % egg reduction at 6 months 30 mg/kg x 2 daily for 2 days | Geometric mean % egg reduction at 6 months 30 mg/kg x 2 in one day | p‐value | Comment |
| da Cunha 1987 BRA | 79 | 73.1% | 63.6% | Not reported | EPG (Geometric Mean) of participants with viable eggs found in rectal mucosa biopsies |
6.3. Analysis.
Comparison 6 Praziquantel alternative dosing (Brazil), Outcome 3 Percent egg reduction at six months.
| Percent egg reduction at six months | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Geometric mean % egg reduction 30 mg/kg x 2 daily for 3 days | Geometric mean % egg reduction 30 mg/kg x 2 in one day | p‐value | Comment |
| da Cunha 1987 BRA | 79 | 86.6% | 63.6% | Not reported | EPG (Geometric Mean) of participants with viable eggs found in rectal mucosa biopsies |
6.4. Analysis.
Comparison 6 Praziquantel alternative dosing (Brazil), Outcome 4 Percent egg reduction at six months.
| Percent egg reduction at six months | |||||
|---|---|---|---|---|---|
| Study | Number of participants | Geometric mean % egg reduction 30 mg/kg daily for 6 days | Geometric mean % egg reduction 30 mg/kg x 2 in one day | p‐value | Comment |
| da Cunha 1987 BRA | 79 | 83.8% | 63.6% | Not reported | EPG (Geometric Mean) of participants with viable eggs found in rectal mucosa biopsies |
Comparison 7. Oxamniquine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at three to four months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 30 mg/kg | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.34 [2.47, 7.65] |
| 1.2 40 mg/kg | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.74 [3.74, 20.43] |
| 1.3 60 mg/kg | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.38 [5.79, 64.79] |
| 2 Parasitological failure at six to 10 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 20 mg/kg | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.68 [2.53, 5.36] |
| 3 Percent egg reduction at three to four months | Other data | No numeric data | ||
| 3.1 Oxamniquine (40 mg/kg) versus placebo | Other data | No numeric data | ||
| 3.2 Oxamniquine (20 to 30 mg/kg) versus placebo | Other data | No numeric data | ||
| 3.3 Oxamniquine (60 mg/kg) versus placebo | Other data | No numeric data |
Comparison 8. Oxamniquine (lower dose) versus oxamniquine 40 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oxamniquine 20 mg/kg versus oxamniquine 40 mg/kg | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [2.05, 6.99] |
| 1.2 Oxamniquine 30 mg/kg versus oxamniquine 40 mg/kg | 4 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.15, 2.75] |
| 2 Parasitological failure at three to four months | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oxamniquine 20 mg/kg versus oxamniquine 40 mg/kg | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.40, 3.71] |
| 2.2 Oxamniquine 30 mg/kg versus oxamniquine 40 mg/kg | 7 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.10, 2.43] |
| 3 Parasitological failure at six months | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Oxamniquine 20 mg/kg versus oxamniquine 40 mg/kg | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.46] |
| 3.2 Oxamniquine 30 mg/kg versus oxamniquine 40 mg/kg | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.71, 1.69] |
| 4 Parasitological failure at 12 months | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Oxamniquine 20 mg/kg versus oxamniquine 40 mg/kg | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.32, 2.36] |
| 4.2 Oxamniquine 30 mg/kg versus oxamniquine 40 mg/kg | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.31] |
| 5 Percent egg reduction | Other data | No numeric data | ||
| 5.1 One month | Other data | No numeric data | ||
| 5.2 Three to four months | Other data | No numeric data | ||
| 5.3 Six months | Other data | No numeric data |
Comparison 9. Oxamniquine lower dose (20 and 30 mg/kg) versus oxamniquine 40 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of abdominal pain: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Resolution of abdominal pain: 30 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Resolution of diarrhoea: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Resolution of diarrhoea: 30 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Resolution of blood in stool: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Resolution of blood in stool: 30 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Resolution of hepatomegaly: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Resolution of hepatomegaly: 30 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Resolution of splenomegaly: 20 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Resolution of splenomegaly: 30 mg/kg versus 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 One month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Oxamniquine (higher dose) versus oxamniquine 40 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Oxamniquine (50 mg/kg) versus oxamniquine (40 mg/kg) | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.21, 3.73] |
| 1.2 Oxamniquine (60 mg/kg) versus oxamniquine (40 mg/kg) | 4 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.09, 2.11] |
| 2 Parasitological failure at three to four months | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Oxamniquine (50 mg/kg) versus oxamniquine (40 mg/kg) | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.25, 4.86] |
| 2.2 Oxamniquine (60 mg/kg) versus oxamniquine (40 mg/kg) | 5 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.12, 1.38] |
| 3 Parasitological failure at six months | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Oxamniquine (60 mg/kg) versus oxamniquine (40 mg/kg) | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.12, 3.12] |
| 4 Percent egg reduction | Other data | No numeric data | ||
| 4.1 One month | Other data | No numeric data | ||
| 4.2 Three to four months | Other data | No numeric data | ||
| 4.3 Six months | Other data | No numeric data |
Comparison 11. Oxamniquine (lower dose) 15 to 20 mg/kg versus oxamniquine 30 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 One month | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.14, 2.74] |
| 1.2 Three to four months | 4 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.40, 3.32] |
| 1.3 Six months | 2 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.86, 1.75] |
| 1.4 6 to 12 months | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.02, 2.96] |
Comparison 12. Oxamniquine (higher dose) versus oxamniquine 30 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oxamniquine 50 mg/kg versus oxamniquine 30 mg/kg | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.15, 1.56] |
| 1.2 Oxamniquine 60 mg/kg versus oxamniquine 30 mg/kg | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.26] |
| 2 Parasitological failure at three to four months | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oxamniquine 50 mg/kg versus oxamniquine 30 mg/kg | 2 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.44, 1.53] |
| 2.2 Oxamniquine 60 mg/kg versus oxamniquine 30 mg/kg | 4 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.07, 0.39] |
| 3 Parasitological failure at six months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oxamniquine (60 mg/kg) versus oxamniquine (30 mg/kg) | 2 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.06, 0.50] |
Comparison 13. Oxamniquine versus praziquantel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Oxamniquine 10 to 20 mg/kg versus praziquantel 40 mg/kg | 2 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.08, 14.47] |
| 1.2 Oxamniquine 30 mg/kg versus praziquantel 40 mg/kg | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.45] |
| 1.3 Oxamniquine 40 mg/kg versus praziquantel 40 mg/kg | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.4 [0.13, 1.22] |
| 1.4 Oxamniquine 50 to 60 mg/kg versus praziquantel 40 mg/kg | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.16, 4.84] |
| 2 Parasitological failure at three months | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Oxamniquine (10 to 20 mg/kg) versus praziquantel 40 mg/kg | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 3.42 [1.10, 10.61] |
| 2.2 Oxamniquine (25 to 30 mg/kg) versus praziquantel 40 mg/kg | 3 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.40, 2.12] |
| 2.3 Oxamniquine 40 mg/kg versus praziquantel 40 mg/kg | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.4 [0.14, 1.12] |
| 2.4 Oxamniquine (50 to 60 mg/kg) versus praziquantel 40 mg/kg | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.13, 1.48] |
| 3 Parasitological failure at six months | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Oxamniquine (10 to 20 mg/kg) versus praziquantel 40 mg/kg | 3 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.70, 1.74] |
| 3.2 Oxamniquine (25 to 30 mg/kg) versus praziquantel 40 mg/kg | 2 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.22, 4.49] |
| 3.3 Oxamniquine (50 to 60 mg/kg) versus praziquantel 40 mg/kg | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.11, 4.30] |
| 3.4 Oxamniquine (15 to 20 mg/kg) versus praziquantel 40 mg/kg | 4 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.83, 1.51] |
| 4 Parasitological failure at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Oxamniquine (10 to 20 mg/kg) versus praziquantel 40 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Percent egg reduction | Other data | No numeric data | ||
| 5.1 One month | Other data | No numeric data | ||
| 5.2 Three months | Other data | No numeric data | ||
| 5.3 Six months | Other data | No numeric data | ||
| 5.4 12 months | Other data | No numeric data |
Comparison 14. Myrrh (Mirazid) 300 mg once daily for three days versus praziquantel 40 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at three to six weeks | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Percent egg reduction three to six weeks | Other data | No numeric data |
Comparison 15. Praziquantel (40 mg/kg) plus artesunate (12 mg/kg total dose) versus praziquantel (40 mg/kg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Parasitological failure at three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Parasitological failure at six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Percent egg reduction | Other data | No numeric data | ||
| 4.1 One month | Other data | No numeric data | ||
| 4.2 Three months | Other data | No numeric data | ||
| 4.3 Six months | Other data | No numeric data |
15.2. Analysis.

Comparison 15 Praziquantel (40 mg/kg) plus artesunate (12 mg/kg total dose) versus praziquantel (40 mg/kg), Outcome 2 Parasitological failure at three months.
15.3. Analysis.

Comparison 15 Praziquantel (40 mg/kg) plus artesunate (12 mg/kg total dose) versus praziquantel (40 mg/kg), Outcome 3 Parasitological failure at six months.
Comparison 16. Praziquantel (20 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (40 mg/kg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Parasitological failure at six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Parasitological failure at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Percent egg reduction | Other data | No numeric data | ||
| 4.1 Three months | Other data | No numeric data | ||
| 4.2 Six months | Other data | No numeric data | ||
| 4.3 12 months | Other data | No numeric data |
16.2. Analysis.

Comparison 16 Praziquantel (20 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (40 mg/kg), Outcome 2 Parasitological failure at six months.
16.3. Analysis.

Comparison 16 Praziquantel (20 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (40 mg/kg), Outcome 3 Parasitological failure at 12 months.
Comparison 17. Praziquantel (8 mg/kg) plus oxamniquine (4 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Parasitological failure at three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Parasitological failure at six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Percent egg reduction | Other data | No numeric data | ||
| 4.1 One month | Other data | No numeric data | ||
| 4.2 Three months | Other data | No numeric data | ||
| 4.3 Six months | Other data | No numeric data |
Comparison 18. Praziquantel (15 mg/kg) plus oxamniquine (7.5 mg/kg) versus praziquantel (20 mg/kg) plus oxamniquine (10 mg/kg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Parasitological failure at three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Parasitological failure at six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Percent egg reduction | Other data | No numeric data | ||
| 4.1 One month | Other data | No numeric data | ||
| 4.2 Three months | Other data | No numeric data | ||
| 4.3 Six months | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel Rahim 1988 SDN.
| Methods | Length of follow‐up: one, two, three and six months, with additional examination for children at 8 months | |
| Participants | Number randomized: 296 Inclusion criteria: children and adults with S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: modified Kato‐Katz thick smear (three smears from a single stool sample) |
|
| Interventions | 1. Oxamniquine (60 mg/kg): 15 mg/kg twice daily for two days 2. Oxamniquine (40 mg/kg): 10 mg/kg twice daily for two days 3. Oxamniquine (20 mg/kg): 10 mg/kg twice daily for one day |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate |
|
| Notes | Location: Sudan Date of trial: not stated Endemicity: high (prevalence 80%) Communities studied: not stated Brand of drug: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were stratified according to age, sex and intensity, and randomly allocated to one of three groups', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition did not show a particular trend, but high > 20% |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Al Aska 1990 SAU.
| Methods | Length of follow‐up: three and six months | |
| Participants | Number randomized: 200 Inclusion criteria: patients aged 10 to 63 years (mean: 26 years) with chronic S. mansoni infection with no previous treatment history Exclusion criteria: not stated Diagnostic criteria: Kato‐Katz thick smear, three consecutive stools plus rectal biopsy, infection intensity expressed as geometric mean egg per gram of stool (EPG) |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Oxamniquine (25 mg/kg x 1) One arm consisting of patients with S. haematobium was excluded from this review |
|
| Outcomes | 1. Failure rate 2. Adverse events |
|
| Notes | Location: Saudi Arabia Date of trial: not stated Endemicity: hospital setting Communities studied: N/A; hospital setting Brand of drug: not stated Proctoscopy was done in those patients who were suspected of having schistosomiasis, but in whom frequent stool examination yielded negative findings. Three rectal specimens obtained during proctoscopy were placed between slides and examined under a microscope. The diagnosis was positive if living ova were seen. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomized', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Ayele 1984 ETH.
| Methods | Length of follow‐up: three months | |
| Participants | Number randomized: 65 Inclusion criteria: adolescents and adults aged over 15 years with a geometric mean of 200 EPG or above Exclusion criteria: subjects who had a history of seizure disorder, had received antischistosomal treatment in the last six months or had received any other drugs or were pregnant or lactating Diagnostic criteria: modified Kato‐Katz thick smear (quantity not stated) |
|
| Interventions | 1. Oxamniquine (60 mg/kg): 15 mg/kg twice daily for two days 2. Oxamniquine (40 mg/kg): 10 mg/kg twice daily for two days 3. Oxamniquine (30 mg/kg): 15 mg/kg twice in one day 4. Placebo |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Ethiopia Date of trial: not stated Endemicity: high (prevalence not stated) Communities studied: not stated Brand of drug: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'patients were allocated randomly', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sample size small < 20 participants in each arm: attrition same across arms, but high ( > 20%) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Ayele 1986 ETH.
| Methods | Length of follow‐up: four months | |
| Participants | Number randomized: 162 Inclusion criteria: children below 15 years of age (specific age range not stated) Exclusion criteria: subjects with a history of seizure disorder, geometric mean of less than 200 EPG or who had received antischistosomal treatment in the previous six months Diagnostic criteria: modified Kato‐Katz smear method (three daily consecutive stool samples) |
|
| Interventions | 1. Oxamniquine (60 mg/kg): 15 mg/kg twice daily for two days 2. Oxamniquine (40 mg/kg): 20 mg/kg twice for one day 3. Oxamniquine (30 mg/kg): 15 mg/kg twice for one day 4. Placebo |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Ethiopia Date of trial: 1984 Endemicity: high (prevalence not stated) Communities studied: one Brand of drug: Vansil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Each subject selected for inclusion in the study was randomly assigned to one of four treatment groups', no further details given. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All laboratory technicians were unaware of the different treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses recorded up to six months follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Barakat 2005 EGY.
| Methods | Length of follow‐up: three and six weeks | |
| Participants | Number randomized: 104 Inclusion criteria: S. mansoni‐positive individuals from a whole population Exclusion criteria: not stated Diagnostic criteria: two consecutive stools (duplicate Kato‐Katz thick smear, each 41.7 mg) |
|
| Interventions | 1. Myrrh given in the form of Mirazid capsules (two capsules in three consecutive days which was repeated at three weeks time) regardless of weight or age of the patient as recommended by the manufacturer 2. Praziquantel (40 mg/kg, two doses given at a three‐week interval) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate |
|
| Notes | Location: Egypt Date of trial: not stated Endemicity: moderate (prevalence 14.5%) Communities studied: one Study was conducted during the transmission period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Of the infected person, 104 individuals were randomized in two groups, the first for myrrh and the second for praziquantel, the characteristics of the two groups being comparable', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses recorded, used ITT analysis |
| Selective reporting (reporting bias) | Low risk | No evidence of selecting reporting |
| Other bias | Low risk | No other bias identified |
Botros 2005 EGY.
| Methods | Length of follow‐up: four weeks for children and five to six weeks for adults | |
| Participants | Number randomized: 271 including 30 who did not comply fully with the treatment protocol Inclusion criteria: children and adolescent aged 12 to 18 years and adults aged over 18 years who had S. mansoni eggs in their stool Exclusion criteria: not stated Diagnostic criteria: standard Kato‐Katz thick smear (one stool, 4 slides pre‐treatment), but three consecutive stools and 4 slides per stool (post‐treatment): 41.7 mg of stool |
|
| Interventions | 1. Myrrh (Mirazid; 300 mg/day x 3 days) 2. Praziquantel (40 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate |
|
| Notes | Location: Egypt Date of trial: not stated Endemicity: moderate (prevalence not stated) Communities studied: one Study was conducted during period of low transmission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'All positive eligible subjects were stratified into low, moderate and heavy infection strata. Each stratum was then randomly assigned into two groups. One group received Mirazid while the second group received praziquantel', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'All parasitologists who examined the slides, the technicians who processed them, the clinicians who performed rectal snips, and those responsible for data entry were blinded to the type of treatment given' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were high: 11/66 (16.7%) in myrrh versus 19/51 (37.3%) in praziquantel |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Branchini 1982 BRA.
| Methods | Length of follow‐up: six months | |
| Participants | Number randomized: 101 Inclusion criteria: patients aged 10 to 65 years with chronic intestinal S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: three consecutive stool (3 slides each) from Kato‐Katz and spontaneous sedimentation methods. EPG expressed as geometric mean |
|
| Interventions | 1. Praziquantel (45.4 mg/kg x 1) 2. Oxamniquine (13.8 mg/kg x 1) 3. Placebo |
|
| Outcomes | 1. Cure rate 2. Adverse events |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: hospital setting Communities studied: N/A; hospital setting Brand of drug: not stated Chronic schistosomiasis cases were included in the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomly allocated into three parallel groups, one received praziquantel, one oxamniquine, and one placebo' |
| Allocation concealment (selection bias) | Low risk | The trial was double‐blind placebo control trial. 'The drugs were administered as a single oral dose in conformity to a double‐blind technique' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind placebo controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Creasey 1986 ZWE.
| Methods | Length of follow‐up: one, three and six months | |
| Participants | Number randomized: 107 (59 participants were randomized into S. mansoni treatment and were included in this review) Inclusion criteria: schoolchildren aged 7 to 16 years with both S. mansoni and S. haematobium infections Exclusion criteria: not stated Diagnostic criteria: Kato‐Katz thick smear (three consecutive stools) |
|
| Interventions | 1. Praziquantel (8 mg/kg x 1) plus oxamniquine (4 mg/kg x 1) 2. Praziquantel (15 mg/kg x 1) plus oxamniquine (7.5 mg/kg x 1) 3. Praziquantel (20 mg/kg x 1) plus oxamniquine (10 mg/kg x 1) Three arms consisting of 58 participants infected with S. haematobium was excluded |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Zimbabwe Date of trial: not stated Endemicity: high (prevalence not stated) Communities studied: one Brand of drug: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'The children were randomly assigned to three groups of 10, 30 and 19, respectively, and the combination drug administered at three dosage levels', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were differential: very low in some arms but high reaching >40% in other arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
da Cunha 1986 BRA.
| Methods | Length of follow‐up: six months | |
| Participants | Number randomized: 58 Inclusion criteria: adolescent and adult patients aged 15 to 55 years with chronic S. mansoni infection Exclusion criteria: pregnant and lactating women, with associated kidney, lung, liver or heart disease, acute or sever chronic illness as well marked anaemia or nutritional deficiencies Diagnostic criteria: Quantitative oogram for the estimation of number of viable eggs per gram of tissue found in rectal mucosa biopsies |
|
| Interventions | 1. Praziquantel (65 mg/kg x 1) 2. Oxamniquine (18 mg/kg x 1) |
|
| Outcomes | 1. Adverse events 2. Cure rate 3. Egg reduction rate |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; hospital setting Communities studied: N/A; hospital setting Brand of drug: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Following parallel group design, the patients upon entering the trial were allocated into one of the two groups distributed according to age, sex, body weight, clinical form of the disease and worm burden' |
| Allocation concealment (selection bias) | Low risk | 'The two drugs were dispensed in individually coded bottles and presented in capsules of identical appearance but containing different dosages. The double‐blind code was provided prior to the beginning of the study within sealed envelopes, for each case, to be opened only at the end of the trial' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'Patients were treated in accordance with double‐blind administration' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants across arms very small; 3/27 in the praziquantel arm and 5/27 in the oxamniquine arm were lost to follow‐up at 6 months |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | No other bias identified, but low numbers were included in the study |
da Cunha 1987 BRA.
| Methods | Length of follow‐up: two, four and six months, but only six months follow‐up was used in the analysis | |
| Participants | Number randomized: 80 Inclusion criteria: adolescent and adults patients aged 15 to 55 years with no previous antischistosomal treatment who were infected with S. mansoni Exclusion criteria: children, elderly patients, patients with concomitant acute or serious chronic disease, severe anaemia or nutritional deficiency, pregnant and lactating women, with associated kidney, lung, liver or heart disease Diagnostic criteria: Quantitative oogram for the estimation of number of viable eggs per gram of tissue found in rectal mucosa biopsies |
|
| Interventions | 1. Praziquantel (60 mg/kg, given as 30 mg/kg x 2 for 1 day) 2. Praziquantel (120 mg/kg, given as 30 mg/kg x 2 for 2 days) 3. Praziquantel (180 mg/kg, given as 30 mg/kg x 2 for 3 days) 4. Praziquantel (180 mg/kg, given as 30 mg/kg x 1 for 6 days) Divided doses were given 4 hours apart |
|
| Outcomes | 1. Adverse events 2. Cure rate 3. Egg reduction rate |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; hospital setting Communities studied: N/A; hospital setting Brand: not stated All patients lived away from endemic areas |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomly allocated into four groups with equal number of cases...' The authors make reference to their earlier trial which used parallel group design |
| Allocation concealment (selection bias) | Low risk | Not explicitly stated, but the authors make reference to their earlier parallel double‐blind trial where the two drugs were dispensed in individually coded bottles and presented in capsules of identical appearance but containing different dosages. The double‐blind code was provided prior to the beginning of the study within sealed envelopes, for each case, to be opened only at the end of the trial' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The authors make reference to their earlier trial where they stated 'Patients were treated in accordance with double‐blind administration' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
da Silva 1986 BRA.
| Methods | Length of follow‐up: one, three and six months | |
| Participants | Number randomized: 120 Inclusion criteria: patients with chronic intestinal or hepato‐intestinal S. mansoni infection aged over 14 years Exclusion criteria: clinical form (hepatosplenic cases), patients with associated acute and/or serious disease, pregnant women and those treated in the past six months with antischistosomal drug Diagnostic criteria: Kato‐Katz smear (three slides of three consecutive stools) |
|
| Interventions | 1. Oxamniquine (15 mg/kg) 2. Praziquantel (55 mg/kg x 1) |
|
| Outcomes | 1. Adverse events 2. Cure rate 3. Egg reduction rate |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; hospital setting Communities studied: N/A; hospital setting Brand: not stated Efficacy assessment was based on only those who finished three negative post treatment parasitological follow‐up (one, three and six months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'The patients were randomly allocated into two groups having an equal number of cases', no further details given |
| Allocation concealment (selection bias) | Unclear risk | 'Both drugs were given in a single oral dose in accordance with a double‐blind technique', no further details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'Double‐blind clinical trial' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses did not differ across arms, but around > 20% was high. 'Eefficacy was assessed based on those who finished three negative post treatment parasitological follow‐ups (1, 3 and 6 months)' |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
de Clarke 1976a ZWE.
| Methods | Length of follow‐up: four months | |
| Participants | Number randomized: 30 Inclusion criteria: individuals aged over 5 years who presented with S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: two stools on two consecutive days using the following three methods: sedimentation, hatching and Visser and Pitchford method |
|
| Interventions | 1. Oxamniquine (15 mg/kg x 1) 2. Oxamniquine (20 mg/kg x 1) |
|
| Outcomes | 1. Failure rate 2. Egg reduction rate |
|
| Notes | Location: Zimbabwe Date of trial: 1972 to 1975 Endemicity: N/A; referral cases Communities studied: patients referred to the Blair Research Laboratory Brand: not stated As far as possible those infected with both S. haematobium and S. mansoni were included in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomized', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sample size very small across arms ≤ 15 and attrition was high reaching 33% |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Used very sensitive diagnostic technique: two stools on two consecutive days using a combination of sedimentation, hatching and Visser and Pitchford method. Pretreatment diagnostic sensitivity differed at one time point |
de Clarke 1976b ZWE.
| Methods | Length of follow‐up: four months | |
| Participants | Number randomized: 26 Inclusion criteria: individuals aged over 5 years who presented with S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: two stools on two consecutive days using the following three methods: sedimentation, hatching and Visser and Pitchford method |
|
| Interventions | 1. Oxamniquine (20 mg/kg): 5 x 2 mg/kg daily for 2 days 2. Oxamniquine (30 mg/kg): 7.5 mg/kg x 2 daily for 2 days 3. Oxamniquine (40 mg/kg): 10 x 2 mg/kg daily for 2 days |
|
| Outcomes | 1. Failure rate 2. Egg reduction rate |
|
| Notes | Location: Zimbabwe Date of trial: not stated Endemicity: N/A; referral cases Communities studied: patients referred to the Blair Research Laboratory Brand: not stated As far as possible those infected with both S. haematobium and S. mansoni were included in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomized', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses recorded |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
de Clarke 1976c ZWE.
| Methods | Length of follow‐up: four months | |
| Participants | Number randomized: 30 Inclusion criteria: individuals aged over 5 years who presented with S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: two stools on two consecutive days using the following three methods: sedimentation, hatching and Visser and Pitchford method |
|
| Interventions | 1. Oxamniquine (60 mg/kg): 15 x 2 mg/kg daily for 2 days 2. Oxamniquine (50 mg/kg): 12.5 x 2 mg/kg daily for 2 days |
|
| Outcomes | 1. Failure rate 2. Egg reduction rate |
|
| Notes | Location: Zimbabwe Date of trial: not stated Endemicity: N/A; referral cases Communities studied: patients referred to the Blair Research Laboratory Brand: not stated As far as possible those infected with both S. haematobium and S. mansoni were included in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomized', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses recorded |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Used very sensitive diagnostic technique: 'two stools on two consecutive days using a combination of sedimentation, hatching and Visser and Pitchford method'. Pretreatment diagnostic sensitivity differed at one time point |
de Clarke 1976d ZWE.
| Methods | Length of follow‐up: four months | |
| Participants | Number randomized: 45 Inclusion criteria: individuals aged over 5 years who presented with S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: two stools on two consecutive days using the following three methods: sedimentation, hatching and Visser and Pitchford method |
|
| Interventions | 1. Oxamniquine (50 mg/kg): 12.5 x 2 mg/kg daily for 2 days 2. Oxamniquine (30 mg/kg): 15 x 2 mg/kg in a single day 3. Oxamniquine (30 mg/kg): 10 x 3 mg/kg in a single day |
|
| Outcomes | 1. Failure rate 2. Egg reduction rate |
|
| Notes | Location: Zimbabwe Date of trial: not stated Endemicity: N/A; referral cases Communities studied: patients referred to the Blair Research Laboratory Brand: not stated As far as possible those infected with both S. haematobium and S. mansoni were included in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomized', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses recorded |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Used very sensitive diagnostic technique: 'two stools on two consecutive days using a combination of sedimentation, hatching and Visser and Pitchford method'. Pretreatment diagnostic sensitivity differed at one time point |
De Clercq 2000 SEN.
| Methods | Length of follow‐up: five, 12 and 24 weeks | |
| Participants | Number randomized: 110 Inclusion criteria: individuals positive for S. mansoni Exclusion criteria: children under 1 year, pregnant women and severely ill patients were excluded if they were receiving medication for any other infection or if they had received medication for schistosomiasis within the preceding six months Diagnostic criteria: single stool (duplicate Kato‐Katz thick smear) |
|
| Interventions | 1. Artesunate (12 mg/kg): 2.4 mg/kg x 5 2. Praziquantel (40 mg/kg x 1) 3. Artesunate (12 mg/kg: 2.4 mg/kg x 5) plus praziquantel (40 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate |
|
| Notes | Location: Senegal Date of trial: 1999 to 2000 Endemicity: high (prevalence 60%) Communities studied: one Brand: not stated Compliance with the 5‐day regimen of artesunate was excellent; all completed this regime |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were allocated to one of three groups', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No losses at one month, but losses were high in the artesunate arm (reaching 25%) at six months compared to 5% in the praziquantel arm |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
de Jonge 1990 SDN.
| Methods | Length of follow‐up: one month | |
| Participants | Number randomized: 182 (123 participants were included in the analysis) Inclusion criteria: boys aged 6 to 13 years having both S. haematobium and S. mansoni infections Exclusion criteria: not stated Diagnostic criteria: three consecutive stools and three slides each of modified Kato‐Katz thick smear technique (Teesdale and Amin). Five Kato‐Katz thick smears of 40 mg each were examined daily |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Oxamniquine (60 mg/kg x 1) A third arm (38 patients) that received metrifonate and a fourth arm of 21 patients (control) selected from a nearby village with low prevalence where children without infection were given multivitamin preparation to act as non‐randomized control group, were excluded from this analysis |
|
| Outcomes | 1. Cure rate 2. Egg reduction |
|
| Notes | Location: Sudan Date of trial: not stated Endemicity: high (prevalence not stated) Communities studied: one Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'The patients were randomly divided into four groups', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were similar at one month, but different at three months: 27% in the 40 mg/kg praziquantel arm versus 17% in the 60 mg/kg in the oxamniquine arm |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Only boys aged 6 years were included in the trial, but no reason given |
Fernandes 1986 BRA.
| Methods | Length of follow‐up: six months | |
| Participants | Number randomized: 120 Inclusion criteria: patients aged 15 to 72 years excreting more than 100 to 2,500 EPG of S. mansoni were included Exclusion criteria: pregnant or lactating women, weak patients or those with cardiac, renal or hepatic insufficiency, and patients with other acute or more severe illnesses than schistosomiasis Diagnostic criteria: three consecutive stools using Kato‐Katz thick smear |
|
| Interventions | 1. Praziquantel (70 mg/kg x 1) 2. Praziquantel (35 mg/kg x 2) 3. Oxamniquine (15 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Adverse events 3. Egg reduction rate |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; outpatient setting Communities studied: N/A; hospital setting Chronic schistosomiasis cases were included in the trial Adverse events were evaluated during a 6 to 8 hour period after administration of the drugs, based on clinical observations by the researchers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were assigned randomly to one of three groups', no further details given |
| Allocation concealment (selection bias) | High risk | 'No methods were taken to conceal allocation of participants to the treatment groups' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Outcomes assessment was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Only patients with moderate to heavy infection were included in the study |
Ferrari 2003 BRA.
| Methods | Length of follow‐up: six months | |
| Participants | Number randomized: 106 Inclusion criteria: patients aged 12 to 56 years attending the hospital found to have S. mansoni infection Exclusion criteria: children of pre‐school age, the aged, pregnant and lactating women, suckling infants, patients with acute or chronic severe concomitant diseases, patients with hepatosplenic form of schistosomiasis and those whose water contact put them at risk for reinfection Diagnostic criteria: Kato‐Katz thick smear (three consecutive stools) in addition to quantitative oogram (rectal biopsies) |
|
| Interventions | 1. Praziquantel (60 mg/kg per day for 3 consecutive days) 2. Oxamniquine (10 mg/kg x 1, followed by starch in days 2 and 3) 3. Placebo (starch) |
|
| Outcomes | 1. Cure rate | |
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; hospital setting Communities studied: N/A; hospital setting Brand: not stated Very sensitive diagnosis was applied. Efficacy was assessed as patient testing negative after treatment and remaining negative for up to six months All patients were advised to stay away from the transmission foci, and they reported doing so |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were randomly allocated to one of three groups', no further details given |
| Allocation concealment (selection bias) | Low risk | The identity of each treatment was kept in a sealed envelope and the capsules were identical in shape and appearance as the active drugs |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple masked randomized controlled trial. 'The investigators were blind to which patients were given which treatment, the identity of each was kept in a sealed envelope' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were high: 10/36 (28%) in the praziquantel arm, 3/34 (9%) in the oxamniquine and 7/36 (19%) in the placebo arm |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Gryseels 1989a BDI.
| Methods | Length of follow‐up: 1.5, three, six, 12 and 24 months | |
| Participants | Number randomized: 163 children and 267 adults Inclusion criteria: all individuals excreting eggs for S. mansoni in their stool Exclusion criteria: those with contraindication Diagnostic criteria: duplicate Kato‐Katz thick smear (28 mg) each from one stool |
|
| Interventions | 1. Oxamniquine (40 mg/kg x 1) 2. Oxamniquine (30 mg/kg x 1) 3. Oxamniquine (20 mg/kg x 1) |
|
| Outcomes | 1. Failure rate 2. Resolution of abdominal pain 3. Resolution of diarrhoea 4. Resolution of blood in stool 5. Resolution of hepatomegaly 6. Resolution of splenomegaly |
|
| Notes | Location: Burundi Date of trial: 1983 to 1986 Endemicity: high (prevalence 66%) Communities studied: one Brand: not stated Hepatomegaly was measured under the costal arch and splenomegaly was measured as described by Hackett 1944 Children only were included in the efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'All patients excreting eggs of S. mansoni were treated with one of the randomly allocated schedules', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not stated |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Gryseels 1989b BDI.
| Methods | Length of follow‐up: 1.5, three, six, 12 and 24 months | |
| Participants | Number randomized: 299 children and 153 adults Inclusion criteria: all individuals excreting eggs for S. mansoni in their stool Exclusion criteria: those with contraindication Diagnostic criteria: duplicate Kato‐Katz thick smear (28 mg) each from one stool |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Praziquantel (30 mg/kg x 1) 3. Praziquantel (20 mg/kg x 1) |
|
| Outcomes | 1. Failure rate 2. Resolution of abdominal pain 3. Resolution of diarrhoea 4. Resolution of blood in stool 5. Resolution of hepatomegaly 6. Resolution of splenomegaly |
|
| Notes | Location: Burundi Date of trial: 1983 to 1986 Endemicity: moderate (prevalence 38%) Communities studied: one Brand: not stated Hepatomegaly was measured under the costal arch and splenomegaly was measured as described by Hackett 1944 Children only were included in the efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'All patients excreting eggs of S. mansoni were treated with one of the randomly allocated schedules', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not stated |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Gryseels 1989c BDI.
| Methods | Length of follow‐up: 1.5, three, six, 12 and 24 months | |
| Participants | Number randomized: 193 children and 125 adults Inclusion criteria: all individuals excreting eggs for S. mansoni in their stool Exclusion criteria: those with contraindication Diagnostic criteria: duplicate Kato‐Katz thick smear (28 mg) each from one stool |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Praziquantel (30 mg/kg x 1) |
|
| Outcomes | 1. Failure rate 2. Resolution of abdominal pain 3. Resolution of diarrhoea 4. Resolution of blood in stool 5. Resolution of hepatomegaly 6. Resolution of splenomegaly |
|
| Notes | Location: Burundi Date of trial: 1983 to 1986 Endemicity: moderate (prevalence 42%) Communities studied: one Brand: not stated Hepatomegaly was measured under the costal arch and splenomegaly was measured as described by Hackett 1944 Children only were included in the efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | All patients excreting eggs of S. mansoni were treated with one of the randomly allocated schedules', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not stated |
| Selective reporting (reporting bias) | Low risk | Noevidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Guisse 1997 SEN.
| Methods | Length of follow‐up: three, six and 21 weeks | |
| Participants | Number randomized: 130 Inclusion criteria: children infected with S. mansoni with no previous history of antischistosomal treatment Exclusion criteria: not stated Diagnostic criteria: two consecutive stools (duplicate Kato‐Katz thick smear, 25 mg |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Praziquantel (30 mg/kg x 2) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Senegal Date of trial: 1993 Endemicity: high (100%) Communities studied: one Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients with no history of previous treatment with praziquantel, were selected and randomly allocated into two treatment groups', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Gupta 1984 ZMB.
| Methods | Length of follow‐up: one, two, three and six months | |
| Participants | Number randomized: 60 Inclusion criteria: adults patient with S. mansoni infection Exclusion criteria: pregnant and lactating women, patients with concurrent systematic diseases and those who received antischistosomal treatment within one month before the trial Diagnostic criteria: Stoll/Hauseer’s method |
|
| Interventions | 1. Oxamniquine (60 mg/kg): 15 mg/kg twice daily for two days 2. Oxamniquine (40 mg/kg): 10 mg/kg twice daily for two days 3. Oxamniquine (30 mg/kg): 15 mg/kg twice daily for one day |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Zambia Date of trial: March 1980 to 1982 Endemicity: patients visiting Lusaka hospital Communities studied: not stated Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly allocated to three oxamniquine groups', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size small, losses not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | No other bias identified |
Homeida 1989 SDN.
| Methods | Length of follow‐up: 1.5 and six months | |
| Participants | Number randomized: 885 Inclusion criteria: individuals who were positive for S. mansoni Exclusion criteria: pregnant women, patients who vomited the drug within half an hour or those who reported to have received antischistosomal treatment within the previous 6 months Diagnostic criteria: modified Kato‐Katz thick smear |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1): Biltricide 2. Praziquantel (40 mg/kg x 1): Distocide |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Sudan Date of trial: not stated Endemicity: high (prevalence not stated) Communities studied: one Brand: Biltricide and Distocide |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '1050 infected individuals who agreed to take part were randomly allocated to biltricide or distocide', no further details given |
| Allocation concealment (selection bias) | Unclear risk | 'Tablets were similar in appearance and were dispensed by a doctor', no further details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor of side effects blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was differential loss of participants to follow‐up at six months: Biltricide (7%) versus Distocide (12%), but the sample was large (> 400 patients in each arm) and this is not likely to introduce bias into the results |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Ibrahim 1980 SDN.
| Methods | Length of follow‐up: one, two and three months | |
| Participants | Number randomized: 129 (89 participants were included in the analysis) Inclusion criteria: all university students attending the university hospital found to have S. mansoni infection Exclusion criteria: only one patient who had chronic valvular heart disease was excluded Diagnostic criteria: modified Kato‐Katz thick smear (three daily stool examinations) |
|
| Interventions | 1. Oxamniquine (60 mg/kg): 15 mg/kg twice daily for two days 2. Oxamniquine (40 mg/kg): 10 mg/kg twice daily for two days One arm of 40 participants with no current or previous schistosomiasis was excluded |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate |
|
| Notes | Location: Sudan Date of trial: not stated Endemicity: low (prevalence not stated) Communities studied: university students on campus Brand: Vansil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'Double‐blind random allocation', no further details given |
| Allocation concealment (selection bias) | Low risk | Double‐blind random allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind random allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses low < 10% and did not differ across arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Jaoko 1996 KEN.
| Methods | Length of follow‐up: 24 hours | |
| Participants | Number randomized: 436 Inclusion criteria: schoolchildren aged 7 to 16 years infected with S. mansoni Exclusion criteria: children who were on medication for whatever reason Diagnostic criteria: duplicate modified Kato‐Katz thick smear |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Placebo |
|
| Outcomes | 1. Adverse events | |
| Notes | Location: Kenya Date of trial: not stated Endemicity: high (prevalence > 83%) Communities studied: one Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'patients were randomly assigned to treatment', no further details given |
| Allocation concealment (selection bias) | Low risk | Placebo controlled trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Kardaman 1983 SDN.
| Methods | Length of follow‐up: one month | |
| Participants | Number randomized: 350 Inclusion criteria: all schoolchildren who provided two stool samples positive for S. mansoni or two urine samples positive for S. haematobium were included Exclusion criteria: children aged < 6 years, patients with contraindications, patients with serious or acute disease, those who had received antischistosomal treatment within the preceding six months, pregnant and lactating women Diagnostic criteria: single stool (three slides) from locally developed thick‐smear method, Teesdale & Amin (Teesdale 1976) |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Praziquantel (20 mg/kg x 2) given 4 to 6 hours apart |
|
| Outcomes | 1. Failure rate 2. Adverse events |
|
| Notes | Location: Sudan Date of trial: December 1979 to March 1980 Endemicity: moderate (prevalence not stated) Communities studied: one Brand: Biltricide |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'In one Arab village, 350 patients with S. mansoni were randomly assigned to one of two treatment groups', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were 12% in the single dose (40 mg/kg) versus 9% in the divided dose (20 mg/kg x 2) of praziquantel |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Kardaman 1985 SDN.
| Methods | Length of follow‐up: five weeks and three months | |
| Participants | Number randomized: 237, but only 220 received treatment Inclusion criteria: children aged 7 to 11 years who provided two positive stool samples for S. mansoni and two positive urine samples for S. haematobium Exclusion criteria: children were excluded if they were receiving medication for any other infection or if they had received medication for schistosomiasis within the preceding six months Diagnostic criteria: locally developed thick‐smear method, Teesdale & Amin (Teesdale 1976) |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Praziquantel (20 mg/kg x 2) given 4 to 6 hours apart |
|
| Outcomes | 1. Failure rate 2. Adverse events |
|
| Notes | Location: Sudan Date of trial: not stated Endemicity: high (prevalence not stated) Communities studied: not stated Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'All children who provided two positive stool were then randomly assigned to take either a single 40 mg/ kg dose a divided dose', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Randomized 237 participants but 220 received treatment. Losses were < 10% |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Katz 1979a BRA.
| Methods | Length of follow‐up: six and 12 months | |
| Participants | Number randomized: 55 Inclusion criteria: male patients aged 20 to 48 years from the military police of Minas Gerais who were excreting > 100 EPG of S. mansoni (calculated from a minimum of two stool samples) Exclusion criteria: not stated Diagnostic criteria: Kato‐Katz thick smear (plus hatching test), three consecutive stool (two slides from each stool plus three hatching tests on each stool); EPG expressed as geometric mean |
|
| Interventions | 1. Praziquantel (20 mg/kg x 1) 2. Praziquantel (20 mg/kg x 2) 3. Praziquantel (20 mg/kg x 3, 4 hours apart) 4. Placebo |
|
| Outcomes | 1. Cure rate 2. Adverse events |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; military police Communities studied: N/A Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as 'patients were randomly allocated to treatment', the authors refer to WHO coordinated well‐designed multi‐country trials of which this trial was part |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators, participants and assessors were blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very small sample sizes (≤ 8 participants) across arms, but losses reached over 50% |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Only male patients entered the trial |
Katz 1979b BRA.
| Methods | Length of follow‐up: six and 12 months | |
| Participants | Number randomized: 61 Inclusion criteria: male patients aged 20 to 48 years from the military police of Minas Gerais who were excreting > 100 EPG of S. mansoni (calculated from a minimum of two stool samples). Exclusion criteria: not stated Diagnostic criteria: Kato‐Katz thick smear (plus hatching test), three consecutive stool (two slides from each stool plus three hatching tests on each stool); EPG expressed as geometric mean |
|
| Interventions | 1. Praziquantel (20 mg/kg x 3, 4 hours apart) 2. Praziquantel (50 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Adverse events |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; military police Communities studied: N/A Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as 'patients were randomly allocated to treatment'. the authors refer to WHO coordinated well‐designed multi‐country trials of which this trial was part |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and assessors were blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses > 20% |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Only male patients entered the trial |
Katz 1981 BRA.
| Methods | Length of follow‐up: six months | |
| Participants | Number randomized: 138 Inclusion criteria: patients attending the hospital found to have S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: Kato‐Katz method (plus hatching test), three consecutive stools (two slides from each stool plus three hatching tests on each stool ); EPG expressed as geometric mean |
|
| Interventions | 1. Praziquantel (30 mg/kg x 1) 2. Praziquantel (40 mg/kg x 1) 3. Praziquantel (25 mg/kg x 2, 6 hours apart) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; hospital setting Communities studied: N/A Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were randomly allocated to treatment', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Decribed as single‐blind randomized trial, no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were low |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Only male patients entered the trial |
Katz 1982 BRA.
| Methods | Length of follow‐up: six months | |
| Participants | Number randomized: 120 Inclusion criteria: children aged 8 to 14 years excreting between 90 and < 2,500 EPG, weighing between 17 and 50 kg. Exclusion criteria: not stated Diagnostic criteria: Kato‐Katz thick smear, three consecutive stool samples (2 slides from each stool); EGP expressed as geometric mean |
|
| Interventions | 1. Oxamniquine (20 mg/kg x 1) 2. Praziquantel (65 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Adverse events |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; hospital setting Communities studied: outpatients (coming from two endemic communities) Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'This investigation was designed a double‐blind comparative trial between two parallel groups established by random allocation of patients', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described, but given that it was double‐blind trial, it is more likely allocation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'Double‐blind trial' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate high |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Lambertucci 1982 BRA.
| Methods | Table 15 Length of follow‐up: 10 months |
|
| Participants | Number randomized: 91 Inclusion criteria: children aged 6 to 14 years with chronic S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: duplicate of two consecutive stools using Kato‐Katz thick smear |
|
| Interventions | 1. Oxamniquine (20 mg/kg x 1) 2. Placebo |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Brazil Date of trial: November 1978 to January 1979 Endemicity: low (prevalence 8%) Communities studied: outpatient clinic Brand: not stated Follow‐up comprised 20 quantitative parasitological stool examinations (two each month for 10 months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'double‐blind trial', no further details given |
| Allocation concealment (selection bias) | Low risk | Double‐blind trial. The patients were identified on arrival at the hospital by a code number in relation to oxamniquine or placebo administration, to make it impossible for the doctor in charge to know which child took active drug and which the placebo (double‐blind). The code was broken 8 months after the treatment of the last patient' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition low (< 10%) and similar across arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Metwally 1995 EGY.
| Methods | Length of follow‐up: 1.5 and 2.5 months | |
| Participants | Number randomized: 366 Inclusion criteria: schoolchildren aged 8 to 16 years who were positive for S. mansoni Exclusion criteria: children showing any signs of hepatosplenic disease, those who received antischistosomal treatment within the previous six months Diagnostic criteria: three consecutive stools (three slides per stool) using modified Kato‐Katz thick smear |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1): Biltricide 2. Praziquantel (40 mg/kg x 1): Distocide |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate |
|
| Notes | Location: Egypt Date of trial: not stated Endemicity: high (prevalence not stated) Communities studied: one Brand: Biltricide and Distocide |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Children were stratified into light, moderate and heavy infection. Each stratified group was randomly divided into four groups', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Olds 1999 KEN.
| Methods | Length of follow‐up: five, 12 and 24 weeks, but the authors reported treatment effects for 5 weeks only | |
| Participants | Number randomized: 367 Inclusion criteria: schoolchildren aged 6 to 19 years positive for S. mansoni Exclusion criteria: failure to submit two stool specimens prior to the initial treatment, known allergy to either drug, treatment with either drug within six months, lack of consent, or possible pregnancy Diagnostic criteria: two 50 mg stool slides each were prepared from 2 separate stool samples for Kato‐Katz thick smear |
|
| Interventions | 1. Albandazole (400 mg x 1) + praziquantel (40 mg/kg) 2. Praziquantel (40 mg/kg) + albendazole placebo 3. Albandazole (400 mg x 1) + praziquantel placebo 4. Both placebo |
|
| Outcomes | 1. Cure rate 2. Adverse events |
|
| Notes | Location: Kenya Date of trial: not stated Endemicity: high (prevalence > 80%) Communities studied: one Brand: not stated Losses to follow‐up were not statistically different in terms of treatment, infection status or adverse events |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization lists were prepared by WHO/TDR using a randomized block design with a block size of 80' |
| Allocation concealment (selection bias) | Low risk | Physically identical treatment and placebo were manufactured and packaged on the same equipment, and all bottles were identified only with a letter code. Randomization code was not broken until after the 6‐month results were tabulated and submitted to WHO |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up < 1% at 45 days |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Olliaro 2011 BRA.
| Methods | Length of follow‐up: three weeks | |
| Participants | Number randomized: 196 Inclusion criteria: participants aged 10 to 19 years with S. mansoni infection (≥ 100 EPG) using Kato‐Katz technique, written informed consent, but under 18 years of age, written informed consent from parents/guardians and their verbal assent, able and willing to be examined on follow‐up visits and provide stool samples Exclusion criteria: pregnant or lactating, previous history of adverse reaction associated with praziquantel, history of acute or chronic severe disease including hepato‐splenic schistosomiasis, recent use of praziquantel (within the last 30 days), with symptomatic malaria, currently using other medication or in the past week |
|
| Interventions | 1. Praziquantel (60 mg/kg x 1) 2. Praziquantel (40 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Reinfection rates 4. Hb level 5. Adverse events |
|
| Notes | Location: Brazil Date of trial: March 2006 to Dec 2007 Endemicity: prevalence 25% Communities studied: Brand: DistocideH by Shin‐Poong, Korea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization lists were prepared by WHO/TDR using a randomized block design with a block size of 4' |
| Allocation concealment (selection bias) | Low risk | 'Physically identical treatment and placebo were manufactured and packaged on the same equipment, and all bottles were identified only with a letter code. Sealed and numbered envelopes were kept in a locked cabinet by one responsible person; two different people preparing treatment and evaluating patients; stool specimens read by a technician blinded as to the treatment. Randomization code was not broken until after the 6‐month results were tabulated and submitted to WHO' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, investigators and outcome assessors were blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing or incomplete data considered as missing in ITT and per protocol analyses |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Olliaro 2011 MRT.
| Methods | Length of follow‐up: three weeks | |
| Participants | Number randomized:186 Inclusion criteria: participants aged 10 to 19 years with S. mansoni infection (≥ 100 EPG) using Kato‐Katz technique, written informed consent, but under 18 years of age, written informed consent from parents/guardians and their verbal assent, able and willing to be examined on follow‐up visits and provide stool samples Exclusion criteria: pregnant or lactating, previous history of adverse reaction associated with praziquantel, history of acute or chronic severe disease including hepato‐splenic schistosomiasis, recent use of praziquantel (within the last 30 days), with symptomatic malaria, currently using other medication or in the past week |
|
| Interventions | 1. Praziquantel (60 mg/kg x 1) 2. Praziquantel (40 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Reinfection rates 4. Hb level 5. Adverse events |
|
| Notes | Location: Mauritania Date of trial: August 2005 to December 2006 Endemicity: S. mansoni, prevalence 18.7%, S.haematobium prevalence 30.9%, Coinfection 7.3% and total prevalence 57% Communities studied: not stated Brand: DistocideH by Shin‐Poong, Korea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization lists were prepared by WHO/TDR using a randomized block design with a block size of 4' |
| Allocation concealment (selection bias) | Low risk | 'Physically identical treatment and placebo were manufactured and packaged on the same equipment, and all bottles were identified only with a letter code. Sealed and numbered envelopes were kept in a locked cabinet by one responsible person; two different people preparing treatment and evaluating patients; stool specimens read by a technician blinded as to the treatment. Randomization code was not broken until after the 6‐month results were tabulated and submitted to WHO' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, investigators and outcome assessors were blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing or incomplete data considered as missing in ITT and per protocol analyses |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Olliaro 2011 TZA.
| Methods | Length of follow‐up: three weeks | |
| Participants | Number randomized: 271 Inclusion criteria: participants aged 10 to 19 years with S. mansoni infection (≥ 100 EPG) using Kato‐Katz technique, written informed consent, but under 18 years of age, written informed consent from parents/guardians and their verbal assent, able and willing to be examined on follow‐up visits and provide stool samples Exclusion criteria: pregnant or lactating, previous history of adverse reaction associated with praziquantel, history of acute or chronic severe disease including hepato‐splenic schistosomiasis, recent use of praziquantel (within the last 30 days), with symptomatic malaria, currently using other medication or in the past week |
|
| Interventions | 1. Praziquantel (60 mg/kg x 1) 2. Praziquantel (40 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Reinfection rates 4. Hb level 5. Adverse events |
|
| Notes | Location: Tanzania Date of trial: August 2005 to September 2006 Endemicity: high (prevalent not reported) Communities studied: not stated Brand: DistocideH by Shin‐Poong, Korea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization lists were prepared by WHO/TDR using a randomized block design with a block size of 4'. |
| Allocation concealment (selection bias) | Low risk | 'Physically identical treatment and placebo were manufactured and packaged on the same equipment, and all bottles were identified only with a letter code. Sealed and numbered envelopes were kept in a locked cabinet by one responsible person; two different people preparing treatment and evaluating patients; stool specimens read by a technician blinded as to the treatment. Randomization code was not broken until after the 6‐month results were tabulated and submitted to WHO' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, investigators and outcome assessors were blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing or incomplete data considered as missing in ITT and per protocol analyses. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Omer 1978 SDN.
| Methods | Length of follow‐up: one, two, three and six months | |
| Participants | Number randomized: 176 Inclusion criteria: individuals who were positive for S. mansoni and excreting > 250 EPG Exclusion criteria: those with < 250 EPG, those with severe anaemia, ascites, or poor general health, those who received antischistosomal drug within the last six months, and pregnant women Diagnostic criteria: three consecutive stools using modified Kato‐Katz thick smear, Teesdale & Amin (Teesdale 1976) |
|
| Interventions | 1. Oxamniquine (60 mg/kg): 15 mg/kg x 2 daily for 2 days 2. Oxamniquine (40 mg/kg): 20 mg/kg daily for 2 days 3. Oxamniquine (30 mg/kg): 15 mg/kg x 2 in one day |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Sudan Date of trial: 1975 to 1976 Endemicity: high (prevalence not stated) Communities studied: outpatients Brand: Biltricide Only patients with moderate or heavy infections >250 EPG were included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomly divided into blocks of 15 patients each', no further details were given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses were differential across arms: 14/73 (19%), 3/37 (8%) and 8/66 (12%) in the 60 mg/kg, 40 mg/kg and 30 mg/kg arms at six months' follow‐up, respectively |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Omer 1981 SDN.
| Methods | Length of follow‐up: 3 to 4 months, and six months | |
| Participants | Number randomized: 153 Inclusion criteria: individuals who were positive for S. mansoni and S. haematobium (mixed infection) Exclusion criteria: those < 8 years, with advanced disease, severe anaemia and poor general health Diagnostic criteria: three consecutive stools, using modified Kato‐Katz thick smear (Teesdale & Amin 1976) |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Praziquantel (20 mg/kg x 2) 3. Praziquantel (30 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Adverse events |
|
| Notes | Location: Sudan Date of trial: 1978 to 1979 Endemicity: very high in the community (prevalence not given) Communities studied: patients reporting to the Hospital of Tropical Diseases, Khartoum and those detected during a field survey Brand: Biltricide |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was based on a protocol indicating stratification according to the degree of infection which was determined by the geometric means of three stool sample examinations. Each stratum was then randomly divided into 3 blocks with 15 patients each to receive one of three dosages' |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'Single blind trial, participants and assessors were blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Queiroz 2010 BRA.
| Methods | Follow‐up: one, three and six months | |
| Participants | Inclusion criteria: inhabitants aged ≥ 13 years from Chonin (a district of Governador Valadares) with a positive stool sample for S. mansoni infection Criteria for exclusion from the study included pregnancy, cardiomyopathies and chronic liver and renal diseases; however, no participants were excluded Two parasitological stool examinations (2 slides per stool sample) by the quantitative Kato‐Katz thick smear. Diagnostic criteria same pre‐and post‐ treatment |
|
| Interventions | 1. Praziquantel (80 mg/kg: 2 x 40 given 1 hour apart) 2. Praziquantel (50 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Brazil Date of trial: 2002 Endemicity: moderate (prevalence 22.5%) Communities studied: one Brand: not stated Patients with chronic schistosomiasis mansoni were recruited |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Participants were randomly assigned into two groups using small blocks to achieve balance between them' |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single blind study, did not state who was blinded. 'To keep the study masked, patients who received 50 mg/kg received placebo 1 hour after the first dose' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eighteen participants were lost to follow‐up: 11/156 (7%) from the 80 mg/kg arm versus 7/150 (5%) from the 50 mg/kg arm. Analysed by ITT |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Rezende 1985 BRA.
| Methods | Length of follow‐up: six months | |
| Participants | Number randomized: 539 Inclusion criteria: outpatients of all ages free from previous antischistosomal treatment; whole population who received previous treatment of children and adults with S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: Kato‐Katz thick smear (three smears from three consecutive stool samples) |
|
| Interventions | 1. Oxamniquine (16 mg/kg x 1) 2. Praziquantel (55 mg/kg x 1) |
|
| Outcomes | 1. Failure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: N/A; hospital setting Communities studied: N/A Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized double‐blind parallel group clinical trial |
| Allocation concealment (selection bias) | Low risk | Physically identical capsules were dispensed in individually coded bottles |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind (investigators, participants and assessors were blind) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were high: 80/272 (29%) in oxamniquine 16 mg/kg arm versus 83/267 (31%) in the praziquantel 55 mg/kg arm |
| Selective reporting (reporting bias) | Low risk | No evidence of selecting reporting |
| Other bias | Low risk | No other bias identified |
Rugemalila 1984 TZA.
| Methods | Length of follow‐up: one, two and six months | |
| Participants | Number randomized: 188 (125 included in the analysis) Inclusion criteria: children aged 8 to 14 years attending primary school in Mwanza district infected with S. mansoni Exclusion criteria: not stated Diagnostic criteria: single stool (duplicate slides) from formal‐ether method |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Oxamniquine (15 mg/kg x 1) A third arm consisted of control (63 participants) who received no treatment and were excluded |
|
| Outcomes | 1. Adverse events 2. Resolution of symptoms |
|
| Notes | Location: Tanzania Date of trial: not stated Endemicity: not stated Communities studied: not stated Brand: praziquantel (Biltricide, Bayer) and oxamniquine (Vancil, Pfizer) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomized single‐blind comparative trial. 'The children found positive for egg excretion were stratified for egg output counts before being randomly allocated by their serial numbers to one of the three groups' |
| Allocation concealment (selection bias) | Low risk | 'The investigator giving treatment used case serial numbers to check out the treatment to be given. Case treatment groups were not revealed to the examiners' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only the investigators were blind but this is not likely to introduce bias into the results |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were high > 30% at three months |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Shafei 1979 NGA.
| Methods | Length of follow‐up: one, two and three months | |
| Participants | Number randomized: 45 Inclusion criteria: individuals with S. mansoni infection detected in stool by the McMaster technique Exclusion criteria: not stated Diagnostic criteria: McMaster technique but did not state number of stool and slides |
|
| Interventions | 1. Oxamniquine (15 mg/kg x 1) 2. Oxamniquine (15 mg/kg x 2 given one day) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Nigeria Date of trial: not stated Endemicity: moderate (prevalence not stated) Communities: one Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomized', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size small, losses not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Stelma 1997 SEN.
| Methods | Length of follow‐up: 1.5 months | |
| Participants | Number randomized: 138 Inclusion criteria: patients aged > 5 years with S. mansoni infection Exclusion criteria: pregnant women and children aged < 5 years Diagnostic criteria: Kato‐Katz thick smear (duplicate, two consecutive stools) |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Oxamniquine (20 mg/kg x 1) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate |
|
| Notes | Location: Senegal Date of trial: not stated Endemicity: high (prevalence not stated) Communities studied: one Brand: praziquantel (Distocide, Shin Poong, Seoul Korea) and oxamniquine (Vansil, Pfizer) Cure rate extracted from graph |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly allocated to treatment, no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses were recorded |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Sukwa 1993 ZMB.
| Methods | Length of follow‐up: 12 months | |
| Participants | Number randomized: 377 Inclusion criteria: schoolchildren aged 7 to 19 years infected with S. mansoni Exclusion criteria: not stated Diagnostic criteria: single stool, duplicate slides of modified Kato‐Katz thick smear |
|
| Interventions | 1. Praziquantel (40 mg/kg x 2, given 6 months apart) 2. Praziquantel (40 mg/kg x 1, followed by placebo at 6 months) |
|
| Outcomes | 1. Re‐infection rate 2. Egg reduction rate 3. Resolution of pathology |
|
| Notes | Location: Zambia Date of trial: 1990 to 1991 Endemicity: high (prevalence not stated) Communities studied: one Brand: not stated All children were treated with 40 mg/kg praziquantel at the start. Six months later the children in group A were retreated with 40 mg/kg praziquantel and group B with placebo (multivitamin). They were all followed up for another six months (12 months total follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Double‐blind randomized trial, patients were randomly allocated to treatment A and B, with a 1:1 allocation ratio and the school serving as the block' |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators, assessors and participants were blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Taddese 1988 ETH.
| Methods | Length of follow‐up: one, three and six months | |
| Participants | Number randomized: 200 Inclusion criteria: adolescents and adults aged 17 to 52 years infected with S. mansoni and excreting at least 50 EPG (geometric mean) Exclusion criteria: patients who had received antischistosomal treatment within the last six months, lactating and pregnant women or having concurrent systemic diseases or with a history of seizure disorders Diagnostic criteria: Kato‐Katz thick smear |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Praziquantel (20 mg/kg x 2) 3. Oxamniquine (15 mg/kg x 1) 4. Oxamniquine (15 mg/kg x 2) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate 3. Adverse events |
|
| Notes | Location: Ethiopia Date of trial: 1983 Endemicity: moderate (prevalence <30%) Communities studied: one Brand: praziquantel (Biltricide) and oxamniquine (Vansil) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned to four treatment groups, no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were low in all arms (up to 10%), but losses in one arm reached 18% by six months |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Taylor 1988 ZWE.
| Methods | Length of follow‐up: one, three and six months | |
| Participants | Number randomized: 373 (283 participants were included in the analysis) Inclusion criteria: children aged 10 to 15 years who were all infected with both S. mansoni and S. haematobium Exclusion criteria: not stated Diagnostic criteria: three consecutive stools using Kato‐Katz thick smear (duplicate 41.5 mg) |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Praziquantel (30 mg/kg x 1) 3. Praziquantel (20 mg/kg x 1) 4. Praziquantel (10 mg/kg x 1) A 5th arm consisting of 90 participants not treated was excluded from the analysis |
|
| Outcomes | 1. Failure rate | |
| Notes | Location: Zimbabwe Date of trial: not stated Endemicity: high (prevalence 76%) Communities studied: one Brand: not stated Study was carried out during period of low transmission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Children were randomly assigned to treatment groups', no further details given |
| Allocation concealment (selection bias) | Unclear risk | Described as 'allocation concealed and randomization code not broken until the end of the trial', methods not given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and assessors were blind, only the principal investigator was aware of the groups to which participants had been assigned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Teesdale 1984 MWI.
| Methods | Length of follow‐up: one and three months | |
| Participants | Number randomized: 119 Inclusion criteria: children aged 6 to 14 years with S. mansoni infection Exclusion criteria: not stated Diagnostic criteria: quantitative thick smear (Teesdale 25 mg) from 4 consecutive stools |
|
| Interventions | 1. Oxamniquine (50 mg/kg): 25 mg/kg x 2 2. Oxamniquine (40 mg/kg x 1) 3. Oxamniquine (30 mg/kg x 1) 4. Praziquantel (40 mg/kg x 1) A 5th arm involving 18 participants treated with oxamniquine (20 mg/kg x 1) was excluded because it was not randomized as part of the trial |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate |
|
| Notes | Location: Malawi Date of trial: not stated Endemicity: high (prevalence not stated) Communities studied: one main village with another where only the 20 mg/kg was tested Brand: Vansil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'The positive subjects included in the study were stratified by age and intensity of infection, and then randomized from each stratum to four treatment groups' |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses at three months were high, reaching 77% in the oxamniquine 50 mg/kg arm |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Tweyongyere 2009 UGA.
| Methods | Length of follow‐up: six weeks | |
| Participants | Number randomized: 387 Inclusion criteria: pregnant women found to have S. mansoni infected detected during a population survey Exclusion criteria: those < 8 years, with advanced disease, severe anaemia and poor general health Diagnostic criteria: single stool sample of duplicate Kato‐Katz thick smear for both pre‐ and post‐treatment |
|
| Interventions | 1. Praziquantel (40 mg/kg x 1) 2. Placebo |
|
| Outcomes | 1. Cure rate | |
| Notes | Location: Uganda Date of trial: 2003 to 2005 Endemicity: high (prevalence not stated) Communities studied: not stated Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prepared by statistician with Stata 7 in blocks of 100 |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators, assessors and participants were blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were high in both the praziquantel 59/186 (32%) and placebo 88/201 (44%) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias reported |
Zwingenberger 1987 BRA.
| Methods | Length of follow‐up: three, six and 12 months | |
| Participants | Number randomized: 91 Inclusion criteria: individuals aged 10 to 62 years with S. mansoni diagnosed in a parasitological survey Exclusion criteria: not stated Diagnostic criteria: Kato‐Katz thick smear (single stool) |
|
| Interventions | 1. Praziquantel (20 mg/kg x 2) 2. Oxamniquine (15 mg/kg x 1) 3. Oxamniquine (7.5 mg/kg) plus praziquantel (20 mg/kg) |
|
| Outcomes | 1. Cure rate 2. Egg reduction rate |
|
| Notes | Location: Brazil Date of trial: not stated Endemicity: high (prevalence not stated) Communities studied: one Brand: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly allocated to one of three groups, no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses were high reaching > 40% in one arm |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu‐Elyazeed 1997 | Not randomized, selective treatment |
| Adam 2008 | Praziquantel versus anti‐malaria treatment |
| Almeida 2012 | Randomized controlled trial, but not outcome of interest |
| Assis 1998 | Reported anthropometry, which is not part of this review |
| Boisier 1998 | Not randomized, selective treatment |
| Coura 1980 | Not randomized, selective treatment |
| De Clercq 2000b | Not randomized, selective treatment |
| Doehring 1992 | Randomized, but did not report results of each arm separately. The results of the different arms were rather combined and presented as one |
| Eigege 2008 | Not randomized |
| el Guiniady 1994 | Not randomized |
| el‐Hawey 1991 | Not randomized |
| Friis 1997 | Effect of zinc supplementation on growth and body weight |
| Friis 2003 | Mineral supplementation |
| Gryseels 1987 | Not randomized, selective treatment |
| Homeida 1988 | Not randomized |
| Kabatereine 2003 | Not randomized, selective treatment |
| Katz 1973 | Not randomized |
| Mohamed 2009 | Praziquantel versus anti‐malaria treatment |
| Navaratnam 2012 | The study recruited both children with and without the infection |
| Obonyo 2010 | Praziquantel versus anti‐malaria treatment |
| Odongo‐Aginya 1996 | Not randomized, selective treatment |
| Olsen 2000 | No drug treatment, mineral supplementation |
| Olsen 2003 | No drug treatment, mineral supplementation |
| Pitchford 1978 | No comparison group |
| Polderman 1988 | Not randomized, selective treatment |
| Utzinger 2000a | Artemether given to non‐infected people for the prevention of S. mansoni infection |
| Utzinger 2000b | Not randomized, selective treatment |
| van Lieshout 1994 | Not randomized, selective treatment |
Differences between protocol and review
None
Contributions of authors
ADA, JU and PLO developed the protocol. ADA selected studies, extracted data, assessed risk of bias in the included studies, analysed the data and drafted the review. JU independently verified study selection, data extraction, risk of bias assessment, results of the analysis and edited the draft review. PLO verified study selection, risk of bias assessment and edited the draft review. SD provided statistical advice and edited the methods section. DS helped restructure the review, verified risk of bias assessment and prepared the SOF tables, which were checked by ADA. ADA, JU and PLO interpreted the data, and all authors helped with revisions following the referees' comments.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development, UK.
The World Health Organization, Switzerland.
Swiss National Science Foundation (Project no PPOOB‐102883, PPOOB‐119129), Switzerland.
Declarations of interest
PLO was the lead author in three of the included trials (Olliaro 2011 BRA; Olliaro 2011 MRT; Olliaro 2011 TZA) and helped secure additional financial support from WHO. The rest of the authors have no known conflict of interest.
Unchanged
References
References to studies included in this review
Abdel Rahim 1988 SDN {published data only}
- Abdel Rahim IM, Haridi AA, Abdel‐Hameed AA. Field study of different oxamniquine dose for Schistosoma mansoni in Gezira, Sudan. Journal of Tropical Medicine and Hygiene 1988;91(3):131‐7. [PubMed] [Google Scholar]
- Abdel‐Rahim IM, Haridi AA, Abdel‐Hameed AA. A field evaluation of three dose levels of oxamniquine in Gezira‐ Sudan. East African Medical Journal 1988;65(11):771‐7. [PubMed] [Google Scholar]
Al Aska 1990 SAU {published data only}
- Al‐Aska AK, Al‐Mofleh IA, Al‐Rashed R, Hafez MA, Al‐Nozha M, Abu‐Aisha H, et al. Praziquantel, oxamniquine, and metrifonate in the treatment of schistosomiasis in Riyadh. Annals of Saudi Medicine 1990;10(3):296‐8. [Google Scholar]
Ayele 1984 ETH {published data only}
- Ayele T. Preliminary clinical trial of oral oxamniquine in the treatment of Schistosoma mansoni in Ethiopia. East African Medical Journal 1984;61(8):632‐6. [PubMed] [Google Scholar]
Ayele 1986 ETH {published data only}
- Ayele T. Preliminary clinical trial of oral oxamniquine in the treatment of Schistosoma mansoni in children in Ethiopia. East African Medical Journal 1986;63(4):291‐4. [PubMed] [Google Scholar]
Barakat 2005 EGY {published data only}
- Barakat R, Elmorshedy H, Fenwick A. Efficacy of myrrh in the treatment of human schistosomiasis mansoni. American Journal of Tropical Medicine and Hygiene 2005;73(2):365‐7. [PubMed] [Google Scholar]
Botros 2005 EGY {published data only}
- Botros S, Sayed H, El‐Dusoki H, Sabry H, Rabie I, El‐Ghannam M, et al. Efficacy of mirazid in comparison with praziquantel in Egyptian Schistosoma mansoni‐infected school children and households. American Journal of Tropical Medicine and Hygiene 2005;72(2):119‐23. [PubMed] [Google Scholar]
Branchini 1982 BRA {published data only}
- Branchini ML, Pedro R J de, Dias LC, Deberaldini ER. Double‐blind clinical trial comparing praziquantel with oxamniquine in the treatment of patients with schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de São Paulo 1982;24(5):315‐21. [PubMed] [Google Scholar]
Creasey 1986 ZWE {published data only}
- Creasey AM, Taylor P, Thomas JE. Dosage trial of a combination of oxamniquine and praziquantel in the treatment of schistosomiasis in Zimbabwean schoolchildren. Central African Journal of Medicine 1986;32(7):165‐7. [PubMed] [Google Scholar]
da Cunha 1986 BRA {published data only}
- Cunha AS, Pedrosa RC. Double‐blind therapeutical evaluation based on the quantitative oogram technique, comparing praziquantel and oxamniquine in human Schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de São Paulo 1986;28(5):337‐51. [DOI] [PubMed] [Google Scholar]
da Cunha 1987 BRA {published data only}
- Cunha AS, Cançado JR, Rezende GL. Therapeutical evaluation of different dose regimens of praziquantel in schistosomiasis mansoni, based on the quantitative oogram technique. Revista do Instituto de Medicina Tropical de São Paulo 1987;29(5):295‐304. [DOI] [PubMed] [Google Scholar]
da Silva 1986 BRA {published data only}
- Silva LC, Zeitune JM, Rosa‐Eid LM, Lima DM, Antonelli RH, Christo CH, et al. Treatment of patients with schistosomiasis mansoni: a double blind clinical trial comparing praziquantel with oxamniquine. Revista do Instituto de Medicina Tropical de São Paulo 1986;28(3):174‐80. [DOI] [PubMed] [Google Scholar]
de Clarke 1976a ZWE {published data only}
- Clarke V, Blair DM, Weber MC, Garnett PA. Dose‐finding trials of oral oxamniquine in Rhodesia. South African Medical Journal 1976;50(46):1867‐71. [PubMed] [Google Scholar]
de Clarke 1976b ZWE {published data only}
- Clarke V, Blair DM, Weber MC, Garnett PA. Dose‐finding trials of oral oxamniquine in Rhodesia. South African Medical Journal 1976;50(46):1867‐71. [PubMed] [Google Scholar]
de Clarke 1976c ZWE {published data only}
- Clarke V, Blair DM, Weber MC, Garnett PA. Dose‐finding trials of oral oxamniquine in Rhodesia. South African Medical Journal 1976;50(46):1867‐71. [PubMed] [Google Scholar]
de Clarke 1976d ZWE {published data only}
- Clarke V, Blair DM, Weber MC, Garnett PA. Dose‐finding trials of oral oxamniquine in Rhodesia. South African Medical Journal 1976;50(46):1867‐71. [PubMed] [Google Scholar]
De Clercq 2000 SEN {published data only}
- Clercq D, Vercruysse J, Verle P, Kongs A, Diop M. What is the effect of combining artesunate and praziquantel in the treatment of Schistosoma mansoni infections?. Tropical Medicine and International Health 2000;5(10):744‐6. [DOI] [PubMed] [Google Scholar]
de Jonge 1990 SDN {published data only}
- Doehring E, Ehrich JH, Vester U, Feldmeier H, Poggensee U, Brodehl J. Proteinuria, hematuria, and leukocyturia in children with mixed urinary and intestinal schistosomiasis. Kidney International 1985;28(3):520‐5. [DOI] [PubMed] [Google Scholar]
- Doehring E, Poggensee U, Feldmeier H. The effect of metrifonate in mixed Schistosoma haematobium and Schistosoma mansoni infections in humans. American Journal of Tropical Medicine and Hygiene 1986;35(2):323‐9. [DOI] [PubMed] [Google Scholar]
- Feldmeier H, Gastl GA, Poggensee U, Kortmann C, Daffalla AA, Peter HH. Relationship between intensity of infection and immunomodulation in human schistosomiasis. II. NK cell activity and in vitro lymphocyte proliferation. Clinical and Experimental Immunology 1985;60(2):234‐40. [PMC free article] [PubMed] [Google Scholar]
- Feldmeier H, Nogueira‐Queiroz JA, Peixoto‐Queiroz MA, Doehring E, Dessaint JP, Alencar JE, et al. Detection and quantification of circulating antigen in schistosomiasis by monoclonal antibody. II. The quantification of circulating antigens in human schistosomiasis mansoni and haematobium: relationship to intensity of infection and disease status. Clinical and Experimental Immunology 1986;65(2):232‐43. [PMC free article] [PubMed] [Google Scholar]
- Jonge N, Schommer G, Feldmeier H, Krijger FW, Dafalla AA, Bienzle U, et al. Mixed Schistosoma haematobium and S. mansoni infection: effect of different treatments on the serum level of circulating anodic antigen (CAA). Acta Tropica 1990;48(1):25‐35. [DOI] [PubMed] [Google Scholar]
Fernandes 1986 BRA {published data only}
- Fernandes P, Oliveira CC. Efficacy of two regimes of praziquantel versus oxamniquine [Estudo comparativo da eficacia do praziquantel, em dois esquemas posologicos, e da oxaminiquina no tratamento da esquistossomose mansonica]. Folha Medica 1986;93(5‐6):389‐93. [Google Scholar]
Ferrari 2003 BRA {published data only}
- Ferrari ML, Coelho PM, Antunes CM, Tavares CA, Cunha AS. Efficacy of oxamniquine and praziquantel in the treatment of Schistosoma mansoni infection: a controlled trial. Bulletin of the World Health Organization 2003;81(3):190‐6. [PMC free article] [PubMed] [Google Scholar]
Gryseels 1989a BDI {published data only}
- Gryseels B, Nkulikyinka L. Two‐year follow‐up of Schistosoma mansoni infection and morbidity after treatment with different regimens of oxamniquine and praziquantel. Transactions of the Royal Society of Tropical Medicine and Hygiene 1989;83(2):219‐28. [DOI] [PubMed] [Google Scholar]
Gryseels 1989b BDI {published data only}
- Gryseels B, Nkulikyinka L. Two‐year follow‐up of Schistosoma mansoni infection and morbidity after treatment with different regimens of oxamniquine and praziquantel. Transactions of the Royal Society of Tropical Medicine and Hygiene 1989;83(2):219‐28. [DOI] [PubMed] [Google Scholar]
Gryseels 1989c BDI {published data only}
- Gryseels B, Nkulikyinka L. Two‐year follow‐up of Schistosoma mansoni infection and morbidity after treatment with different regimens of oxamniquine and praziquantel. Transactions of the Royal Society of Tropical Medicine and Hygiene 1989;83(2):219‐28. [DOI] [PubMed] [Google Scholar]
Guisse 1997 SEN {published data only}
- Guisse F, Polman K, Stelma FF, Mbaye A, Talla I, Niang M, et al. Therapeutic evaluation of two different dose regimens of praziquantel in a recent Schistosoma mansoni focus in Northern Senegal. American Journal of Tropical Medicine and Hygiene 1997;56(5):511‐4. [DOI] [PubMed] [Google Scholar]
Gupta 1984 ZMB {published data only}
- Gupta KK. Schistosoma mansoni treatment with oral oxamniquine in Zambia. East African Medical Journal 1984;61(8):641‐4. [PubMed] [Google Scholar]
Homeida 1989 SDN {published data only}
- Homeida MM, Eltom IA, Sulaiman SM, Ali HM, Bennett JL. Tolerance of two brands of praziquantel. Lancet 1989;334(8659):391. [DOI] [PubMed] [Google Scholar]
Ibrahim 1980 SDN {published data only}
- Ibrahim AM. Evaluation of oxamniquine in the treatment of S. mansoni infection among Sudanese patients. East African Medical Journal 1980;57(8):566‐73. [PubMed] [Google Scholar]
Jaoko 1996 KEN {published data only}
- Jaoko WG, Muchemi G, Oguya FO. Praziquantel side effects during treatment of Schistosoma mansoni infected pupils in Kibwezi, Kenya. East African Medical Journal 1996;73(8):499‐501. [PubMed] [Google Scholar]
Kardaman 1983 SDN {published data only}
- Kardaman MW, Amin MA, Fenwick A, Cheesmond AK, Dixon HG. A field trial using praziquantel (Biltricide) to treat Schistosoma mansoni and Schistosoma haematobium infection in Gezira, Sudan. Annals of Tropical Medicine and Parasitology 1983;77(3):297‐304. [DOI] [PubMed] [Google Scholar]
Kardaman 1985 SDN {published data only}
- Kardaman MW, Fenwick A, Igail AB, Tayeb M, Daffalla AA, Dixon HG. Treatment with praziquantel of schoolchildren with concurrent Schistosoma mansoni and S. haematobium infections in Gezira, Sudan. Journal of Tropical Medicine and Hygiene 1985;88(2):105‐9. [PubMed] [Google Scholar]
Katz 1979a BRA {published data only}
- Katz N. Preliminary trials with praziquantel in human infections due to Schistosoma mansoni. Bulletin of the World Health Organization 1979;57(5):781‐5. [PMC free article] [PubMed] [Google Scholar]
Katz 1979b BRA {published data only}
- Katz N. Preliminary trials with praziquantel in human infections due to Schistosoma mansoni. Bulletin of the World Health Organization 1979;57(5):781‐5. [PMC free article] [PubMed] [Google Scholar]
Katz 1981 BRA {published data only}
- Katz N. Clinical trials with praziquantel in schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de São Paulo 1981;23(2):72‐8. [PubMed] [Google Scholar]
Katz 1982 BRA {published data only}
- Katz N, Rocha RS. Double‐blind clinical trial comparing praziquantel with oxamniquine in schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de São Paulo 1982;24(5):310‐4. [PubMed] [Google Scholar]
Lambertucci 1982 BRA {published data only}
- Lambertucci JR, Greco DB, Pedroso ER, Costa Rocha MO, Salazar HM, Lima DP. A double blind trial with oxamniquine in chronic schistosomiasis mansoni. Transactions of the Royal Society of Tropical Medicine and Hygiene 1982;76(6):751‐5. [DOI] [PubMed] [Google Scholar]
Metwally 1995 EGY {published data only}
- Metwally A, Bennett J, Botros S, Ebeid F, attar Gel D. Impact of drug dosage and brand on bioavailability and efficacy of praziquantel. Pharmacological Research 1995;31(1):53‐9. [DOI] [PubMed] [Google Scholar]
Olds 1999 KEN {published data only}
- Olds GR, King CH, Hewlett J, Olveda R, Wu G, Ouma J, et al. Double‐blind placebo‐controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. Journal of Infectious Diseases 1999;179(4):996‐1003. [DOI] [PubMed] [Google Scholar]
Olliaro 2011 BRA {published data only}
- Olliaro P, Vaillant M, Belizario V, Lwambo N, Ouldabdallahi M, Pieri OS, et al. A multicentre randomized controlled trial of the efficacy and safety of single‐dose praziquantel at 40 mg/kg versus 60 mg/kg for treating intestinal schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Neglected Tropical Diseases 2011;5(6):e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olliaro 2011 MRT {published data only}
- Olliaro P, Vaillant M, Belizario V, Lwambo N, Ouldabdallahi M, Pieri OS, et al. A multicentre randomized controlled trial of the efficacy and safety of single‐dose praziquantel at 40 mg/kg versus 60 mg/kg for treating intestinal schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Neglected Tropical Diseases 2011;5(6):e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olliaro 2011 TZA {published data only}
- Olliaro P, Vaillant M, Belizario V, Lwambo N, Ouldabdallahi M, Pieri OS, et al. A multicentre randomized controlled trial of the efficacy and safety of single‐dose praziquantel at 40 mg/kg versus 60 mg/kg for treating intestinal schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Neglected Tropical Diseases 2011;5(6):e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Omer 1978 SDN {published data only}
- Omer AH. Oxamniquine for treating Schistosoma mansoni infection in Sudan. British Medical Journal 1978;2(6131):163‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Omer 1981 SDN {published data only}
- Omer AH. Praziquantel in the treatment of mixed S. haematobium and S. mansoni infections. Arzneimittelforschung 1981;31(3a):605‐8. [PubMed] [Google Scholar]
Queiroz 2010 BRA {published data only}
- Queiroz LC, Drummond SC, Matos ML, Paiva MB, Batista TS, Kansaon AZ, et al. Comparative randomised trial of high and conventional doses of praziquantel in the treatment of schistosomiasis mansoni. Memorias do Instituto Oswaldo Cruz 2010;105(4):445‐8. [DOI] [PubMed] [Google Scholar]
Rezende 1985 BRA {published data only}
- Rezende GL de. Survey on the clinical results achieved in Brazil comparing praziquantel and oxamniquine in the treatment of S. mansoni schistosomiasis. Revista do Instituto de Medicina Tropical de São Paulo 1985;27(6):328‐68. [DOI] [PubMed] [Google Scholar]
Rugemalila 1984 TZA {published data only}
- Rugemalila JB, Asila J, Chimbe A. Randomized comparative trials of single doses of the newer antischistosomal drugs at Mwanza, Tanzania. I. Praziquantel and oxamniquine for the treatment of schistosomiasis mansoni. Journal of Tropical Medicine and Hygiene 1984;87(6):231‐5. [PubMed] [Google Scholar]
Shafei 1979 NGA {published data only}
- Shafei AZ. A preliminary report on the treatment of intestinal schistosomiasis with oxamniquine. Journal of Tropical Medicine and Hygiene 1979;82(1):18‐20. [PubMed] [Google Scholar]
Stelma 1997 SEN {published data only}
- Stelma FF, Sall S, Daff B, Sow S, Niang M, Gryseels B. Oxamniquine cures Schistosoma mansoni infection in a focus in which cure rates with praziquantel are unusually low. Journal of Infectious Diseases 1997;176(1):304‐7. [DOI] [PubMed] [Google Scholar]
Sukwa 1993 ZMB {published data only}
- Sukwa TY. A community‐based randomized trial of praziquantel to control schistosomiasis morbidity in schoolchildren in Zambia. Annals of Tropical Medicine and Parasitology 1993;87(2):185‐94. [DOI] [PubMed] [Google Scholar]
Taddese 1988 ETH {published data only}
- Taddese K, Zein ZA. Comparison between the efficacy of oxamniquine and praziquantel in the treatment of Schistosoma mansoni infections on a sugar estate in Ethiopia. Annals of Tropical Medicine and Parasitology 1988;82(2):175‐80. [DOI] [PubMed] [Google Scholar]
Taylor 1988 ZWE {published data only}
- Taylor P, Murare HM, Manomano K. Efficacy of low doses of praziquantel for Schistosoma mansoni and S. haematobium. Journal of Tropical Medicine and Hygiene 1988;91(1):13‐7. [PubMed] [Google Scholar]
Teesdale 1984 MWI {published data only}
- Teesdale CH, Chitsulo L, Pugh RN. Oxamniquine dosage in Malawi. East African Medical Journal 1984;61(1):40‐4. [PubMed] [Google Scholar]
Tweyongyere 2009 UGA {published data only}
- Tweyongyere R, Mawa PA, Emojong NO, Mpairwe H, Jones FM, Duong T, et al. Effect of praziquantel treatment of Schistosoma mansoni during pregnancy on intensity of infection and antibody responses to schistosome antigens: results of a randomised, placebo‐controlled trial. BMC Infectious Diseases 2009;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweyongyere R, Mawa PA, Ngom‐Wegi S, Ndibazza J, Duong T, Vennervald BJ, et al. Effect of praziquantel treatment during pregnancy on cytokine responses to schistosome antigens: results of a randomized, placebo‐controlled trial. Journal of Infectious Diseases 2008;198(12):1870‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwingenberger 1987 BRA {published data only}
- Zwingenberger K, Queiroz JA, Poggensee U, Alencar JE, Valdegunas J, Esmeralda F, et al. Efficacy of oxamniquine, praziquantel and a combination of both drugs in schistosomiasis mansoni in Brazil. Revista do Instituto de Medicina Tropical de São Paulo 1987;29(5):305‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abu‐Elyazeed 1997 {published data only}
- Abu‐Elyazeed RR, Youssef FG, Merrell BR, El‐Gamal RL, El‐Khoby TA, Hassanein YA, et al. Praziquantel in the treatment of Schistosoma mansoni infection: comparison of 40 and 60 mg/kg bodyweight regimens. American Journal of Tropical Medicine and Hygiene 1997;56(4):404‐7. [DOI] [PubMed] [Google Scholar]
Adam 2008 {published data only}
- Adam I, Elhardello OA, Elhadi MO, Abdalla E, Elmardi KA, Jansen FH. The antischistosomal efficacies of artesunate‐sulfamethoxypyrazine‐pyrimethamine and artemether‐lumefantrine administered as treatment for uncomplicated Plasmodium falciparum malaria. Annals of Tropical Medicine and Parasitology 2008;102(1):39‐44. [DOI] [PubMed] [Google Scholar]
Almeida 2012 {published data only}
- Almeida MCF, Lima GS, Cardoso LS, Souza RPD, Campos RA, Cruz AA, et al. The effect of antihelminthic treatment on subjects with asthma from an endemic area of schistosomiasis: A randomized, double‐blinded, and placebo‐controlled trial. Journal of Parasitology Research 2012;2012(296856):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Assis 1998 {published data only}
- Assis AM, Barreto ML, Prado MS, Reis MG, Parraga IM, Blanton RE. Schistosoma mansoni infection and nutritional status in schoolchildren: a randomized, double‐blind trial in northeastern Brazil. American Journal of Clinical Nutrition 1998;86(6):1247‐53. [DOI] [PubMed] [Google Scholar]
Boisier 1998 {published data only}
- Boisier P, Ramarokoto CE, Ravaoalimalala VE, Rabarijaona L, Serieye J, Roux J, et al. Reversibility of Schistosoma mansoni‐associated morbidity after yearly mass praziquantel therapy: ultrasonographic assessment. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(4):451‐3. [DOI] [PubMed] [Google Scholar]
Coura 1980 {published data only}
- Coura JR, Argento CA, Conceição MJ, Lewis EM, dos Santos ML, Magalhães P. Field experiences with oral oxamniquine in the treatment of schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de São Paulo 1980;22(1 Suppl 4):77‐84. [PubMed] [Google Scholar]
De Clercq 2000b {published data only}
- Clercq D, Vercruysse J, Verlé P, Niasse F, Kongs A, Diop M. Efficacy of artesunate against Schistosoma mansoni infections in Richard Toll, Senegal. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(1):90‐1. [DOI] [PubMed] [Google Scholar]
Doehring 1992 {published data only}
- Doehring‐Schwerdtfeger E, Abdel‐Rahim IM, Kardorff R, Kaiser C, Franke D, Schlake J, et al. Ultrasonographical investigation of periportal fibrosis in children with Schistosoma mansoni infection: reversibility of morbidity twenty‐three months after treatment with praziquantel. American Journal of Tropical Medicine and Hygiene 1992;46(4):409‐15. [DOI] [PubMed] [Google Scholar]
Eigege 2008 {published data only}
- Eigege A, Pede E, Miri E, Umaru J, Ogbu Pearce P, Jinadu MY, et al. Triple drug administration (TDA), with praziquantel, ivermectin and albendazole, for the prevention of three neglected tropical diseases in Nigeria. Annals of Tropical Medicine and Parasitology 2008;102(2):177‐9. [DOI] [PubMed] [Google Scholar]
el Guiniady 1994 {published data only}
- Guiniady MA, Touny MA, Abdel‐Bary MA, Abdel‐Fatah SA, Metwally A. Clinical and pharmacokinetic study of praziquantel in Egyptian schistosomiasis patients with and without liver cell failure. American Journal of Tropical Medicine and Hygiene 1994;51(6):809‐18. [DOI] [PubMed] [Google Scholar]
el‐Hawey 1991 {published data only}
- el‐Hawey AM, Massoud AM, el‐Rakieby A, Royzeik MS, Nassar MO. Study of some aspects of cell mediated immune response in bilharzial children on a field level. Journal of the Egyptian Society of Parasitology 1991;21(2):411‐6. [PubMed] [Google Scholar]
Friis 1997 {published data only}
- Friis H, Ndhlovu P, Mduluza T, Kaondera K, Sandström B, Michaelsen KF, et al. The impact of zinc supplementation on growth and body composition: a randomized, controlled trial among rural Zimbabwean schoolchildren. European Journal of Clinical Nutrition 1997;51(1):38‐45. [DOI] [PubMed] [Google Scholar]
Friis 2003 {published data only}
- Friis H, Mwaniki D, Omondi B, Muniu E, Thiong'o F, Ouma J, et al. Effects on haemoglobin of multi‐micronutrient supplementation and multi‐helminth chemotherapy: a randomized, controlled trial in Kenyan school children. European Journal of Clinical Nutrition 2003;57(4):573‐9. [DOI] [PubMed] [Google Scholar]
Gryseels 1987 {published data only}
- Gryseels B, Nkulikyinka L, Coosemans MH. Field trials of praziquantel and oxamniquine for the treatment of schistosomiasis mansoni in Burundi. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(4):641‐4. [DOI] [PubMed] [Google Scholar]
Homeida 1988 {published data only}
- Homeida M, Abdel‐Gadir AF, Cheever AW, Bennett JL, Arbab BM, Ibrahium SZ, et al. Diagnosis of pathologically confirmed Symmers' periportal fibrosis by ultrasonography: a prospective blinded study. American Journal of Tropical Medicine and Hygiene 1988;38(1):86‐91. [DOI] [PubMed] [Google Scholar]
Kabatereine 2003 {published data only}
- Kabatereine NB, Kemijumbi J, Ouma JH, Sturrock RF, Butterworth AE, Madsen H, et al. Efficacy and side effects of praziquantel treatment in a highly endemic Schistosoma mansoni focus at Lake Albert, Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(5):599‐603. [DOI] [PubMed] [Google Scholar]
Katz 1973 {published data only}
- Katz N, Pellegrino J, Grinbaum E, Chaves A, Zicker F. Preliminary clinical trials with oxamniquine, a new antischistosomal agent. Revisto do Instituto de Medecina Tropical de São Paulo 1973;15(1):25‐9. [PubMed] [Google Scholar]
Mohamed 2009 {published data only}
- Mohamed AA, Mahgoub HM, Magzoub M, Gasim GI, Eldein WN, Ahmed AA, et al. Artesunate plus sulfadoxine/pyrimethamine versus praziquantel in the treatment of Schistosoma mansoni in eastern Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(10):1062‐4. [DOI] [PubMed] [Google Scholar]
Navaratnam 2012 {published data only}
- Navaratnam AMD, Sousa‐Figueiredo JC, Stothard JR, Kabatereine NB, Fenwick A, Mutumba‐Nakalembe MJ. Efficacy of praziquantel syrup versus crushed praziquantel tablets in the treatment of intestinal schistosomiasis in Ugandan preschool children, with observation on compliance and safety. Transactions of the Royal Society of Tropical Medicine and Hygiene 2012;106(7):400‐7. [DOI] [PubMed] [Google Scholar]
Obonyo 2010 {published data only}
- Obonyo CO, Muok MO, Mwinzi NM. Efficacy of artesunate with sulfalene plus pyrimethamine versus praziquantel for treatment of Schistosoma mansoni in Kenyan children: an open‐label randomised controlled trial. Lancet Infectious Diseases 2010;10(9):603‐11. [DOI] [PubMed] [Google Scholar]
Odongo‐Aginya 1996 {published data only}
- Odongo‐Aginya EI, Doehring M, Lakwo TL, Etyono S, Luyinda LB, Roth J, et al. Integrated control trial of schistosomiasis at Nakiwogo fishing village near Entebbe, Uganda. East African Medical Journal 1996;73(8):495‐8. [PubMed] [Google Scholar]
Olsen 2000 {published data only}
- Olsen A, Nawiri J, Friis H. The impact of iron supplementation on reinfection with intestinal helminths and Schistosoma mansoni in western Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(5):493‐99. [DOI] [PubMed] [Google Scholar]
Olsen 2003 {published data only}
- Olsen A, Thiong'o FW, Ouma JH, Mwaniki D, Magnussen P, Michaelsen KF, et al. Effects of multimicronutrient supplementation on helminth reinfection: a randomized, controlled trial in Kenyan schoolchildren. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(1):109‐14. [DOI] [PubMed] [Google Scholar]
Pitchford 1978 {published data only}
- Pitchford RJ, Lewis M. Oxamniquine in the treatment of various schistosome infections in South Africa. South African Medical Journal 1978;53(17):677‐80. [PubMed] [Google Scholar]
Polderman 1988 {published data only}
- Polderman AM, Gryseels B, Caluwe P. Cure rates and egg reduction in treatment of intestinal schistosomiasis with oxamniquine and praziquantel in Maniema, Zaire. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988;82(1):115‐6. [PubMed] [Google Scholar]
Utzinger 2000a {published data only}
- Utzinger J, N'Goran EK, N'Dri A, Lengeler C, Xiao SH, Tanner M. Oral artemether for prevention of Schistosoma mansoni infection: randomised controlled trial. Lancet 2000;355(9212):1320‐5. [DOI] [PubMed] [Google Scholar]
Utzinger 2000b {published data only}
- Utzinger J, N'Goran EK, N'Dri A, Lengeler C, Tanner M. Efficacy of praziquantel against Schistosoma mansoni with particular consideration for intensity of infection. Tropical Medicine and International Health 2000;5(11):771‐8. [DOI] [PubMed] [Google Scholar]
van Lieshout 1994 {published data only}
- Lieshout L, Jonge N, el‐Masry N, Mansour MM, Bassily S, Krijger FW, et al. Monitoring the efficacy of different doses of praziquantel by quantification of circulating antigens in serum and urine of schistosomiasis patients. Parasitology 1994;108(Pt 5):519‐26. [DOI] [PubMed] [Google Scholar]
Additional references
Allan 2001
- Allan RW. The Johns Hopkins Hospital, Department of Pathology. Johns Hopkins Microbiology Newsletter 2001;20:30.
Andersson 2007
- Andersson KL, Chung RT. Hepatic schistosomiasis: Current Treatment Options. Gastroenterology 2007;10(6):504‐12. [DOI] [PubMed] [Google Scholar]
Bergquist 2009
- Bergquist R, Johansen MV, Utzinger J. Diagnostic dilemmas in helminthology: what tools to use and when?. Trends in Parasitology 2009;25(4):151‐6. [DOI] [PubMed] [Google Scholar]
Booth 2003
- Booth M, Vounatsou P, N'Goran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato‐Katz technique in diagnosing Schistosoma mansoni and hookworm co‐infections in rural Côte d'Ivoire. Parasitology 2003;127(Pt 6):525‐31. [DOI] [PubMed] [Google Scholar]
Borrmann 2001
- Borrmann S, Szlezák N, Faucher JF, Matsiegui PB, Neubauer R, Binder RK, et al. Artesunate and praziquantel for the treatment of Schistosoma haematobium infections: a double‐blind, randomized, placebo‐controlled study. Journal of Infectious Diseases 2001;184(10):1363‐6. [DOI] [PubMed] [Google Scholar]
Boulanger 2007
- Boulanger D, Dieng Y, Cisse B, Remoue F, Capuano F, Dieme JL, et al. Antischistosomal efficacy of artesunate combination therapies administered as curative treatments for malaria attacks. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(2):113‐6. [DOI] [PubMed] [Google Scholar]
Braun‐Munzinger 1992
- Braun‐Munzinger RA, Southgate BA. Repeatability and reproducibility of egg counts of Schistosoma haematobium in urine. Tropical Medicine and Parasitology 1992;43(3):149‐54. [PubMed] [Google Scholar]
Caulibaly 2011
- Coulibaly JT, Knopp S, N'Guessan NA, Silué KD, Fürst T, Lohourignon LK, et al. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d'Ivoire. PLoS Neglected Tropical Diseases 2011;5(11):e1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chitsulo 2000
- Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Tropica 2000;77(1):41‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cioli 1995
- Cioli D, Pica‐Mattoccia L, Archer S. Antischistosomal drugs: past, present ... and future?. Pharmacology and Therapeutics 1995;68(1):35‐85. [DOI] [PubMed] [Google Scholar]
Cnops 2012
- Cnops L, Tannich E, Polman K, Clerinx J, Esbroeck M. Schistosoma real‐time PCR as diagnostic tool for international travellers and migrants. Tropical Medical International Health 2012;17(10):1208‐16. [DOI] [PubMed] [Google Scholar]
CWG 1992
- Cairo Working Group (CWG). The use of diagnostic ultrasound in schistosomiasis‐‐attempts at standardization of methodology. Acta Tropica 1992;51(1):45‐63. [DOI] [PubMed] [Google Scholar]
Danso‐Appiah 2002
- Danso‐Appiah A, Vlas SJ. Interpreting low praziquantel cure rates of Schistosoma mansoni infections in Senegal. Trends in Parasitology 2002;18(3):125‐9. [DOI] [PubMed] [Google Scholar]
Danso‐Appiah 2004
- Danso‐Appiah A, Vlas SJ, Bosompem KM, Habbema JDF. Determinants of health‐seeking behaviour for schistosomiasis‐related symptoms in the context of integrating schistosomiasis control within the regular health services in Ghana. Tropical Medicine and International Health 2004;9(7):784–94. [DOI] [PubMed] [Google Scholar]
Danso‐Appiah 2008
- Danso‐Appiah A, Utzinger J, Liu J, Olliaro PL. Drugs for treating urinary schistosomiasis. Cochrane Database of Systematic Reviews 2008, Issue Issue 3. Art. No: CD000053. DOI: 10.1002/14651858.CD000053.pub2. [DOI] [PubMed] [Google Scholar]
Danso‐Appiah 2009
- Danso‐Appiah A, Garner P, Olliaro PL, Utzinger J. Treatment of urinary schistosomiasis: methodological issues and research needs identified through a Cochrane systematic review. Parasitology 2009;136(13):1837‐49. [DOI] [PubMed] [Google Scholar]
Davis 2009
- Davis A. Schistosomiasis [Section 11, Chapter 82: Helminth infections]. Manson's Tropical Diseases. 22nd Edition. WB Saunders, 2009:1425‐60. [Google Scholar]
De Clercq 2002
- Clercq D, Vercruysse J, Kongs A, Verle P, Dompnier JP, Faye PC. Efficacy of artesunate and praziquantel in Schistosoma haematobium infected schoolchildren. Acta Tropica 2002;82:61‐6. [DOI] [PubMed] [Google Scholar]
de Vlas 1992
- Vlas SJ, Gryseels B. Underestimation of Schistosoma mansoni prevalences. Parasitology Today 1992;8:274‐7. [DOI] [PubMed] [Google Scholar]
de Vlas 1997
- Vlas SJ, Engels D, Rabello AL, Oostburg BF, Lieshout L, Polderman AM, et al. Validation of a chart to estimate true Schistosoma mansoni prevalences from simple egg counts. Parasitology 1997;114(Pt 2):113‐21. [DOI] [PubMed] [Google Scholar]
Doehring‐Schwerdtfeger 1992
- Doehring‐Schwerdtfeger E, Abdel‐Rahim IM, Kardorff R, Kaiser C, Franke D, Schlake J, et al. Ultrasonographical investigations of periportal fibrosis in children with Schistosoma mansoni infection: reversibility of morbidity twenty‐three months after treatment with praziquantel. American Journal of Tropical Medicine and Hygiene 1992;46:409‐15. [DOI] [PubMed] [Google Scholar]
Doenhoff 1998
- Doenhoff MJ. Is schistosomacidal chemotherapy sub‐curative? Implications for drug resistance. Parasitology Today 1998;14:434‐5. [DOI] [PubMed] [Google Scholar]
Doenhoff 2004
- Doenhoff MJ, Chiodini PL, Hamilton JV. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies?. Trends in Parasitology 2004;20(1):35‐9. [DOI] [PubMed] [Google Scholar]
Doenhoff 2008
- Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Current Opinion in Infectious Diseases 2008;21(6):659‐67. [DOI] [PubMed] [Google Scholar]
Engels 1996
- Engels D, Sinzinkayo E, Gryseels B. Day‐to‐day egg count fluctuation in Schistosoma mansoni infection and its operational implications. American Journal of Tropical Medicine and Hygiene 1996;54(4):319‐24. [DOI] [PubMed] [Google Scholar]
Engels 1997
- Engels D, Sinzinkayo E, Vlas SJ, Gryseels B. Intraspecimen fecal egg count variation in Schistosoma mansoni infection. American Journal of Tropical Medicine and Hygiene 1997;57(5):571‐7. [DOI] [PubMed] [Google Scholar]
Engels 2002
- Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Tropica 2002;82(2):139‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enk 2008
- Enk MJ, Lima AC, Drummond SC, Schall VT, Coelho PM. The effect of the number of stool samples on the observed prevalence and the infection intensity with Schistosoma mansoni among a population in an area of low transmission. Acta Tropica 2008;108(2‐3):222‐8. [DOI] [PubMed] [Google Scholar]
Enk 2012
- Enk MJ, Oliveira e Silva G, Rodrigues NB. Diagnostic accuracy and applicability of a PCR system for the detection of Schistosoma mansoni DNA in human urine samples from an endemic area. PLoS One 2012;7(6):e38947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Favre 2009
- Favre TC, Pereira AP, Galvão AF, Zani LC, Barbosa CS, Pieri OS. A rationale for schistosomiasis control in elementary schools of the rainforest zone of Pernambuco, Brazil. PLoS Neglected Tropical Diseases 2009;3(3):e395.doi:10.1371/journal.pntd.0000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fenwick 2009
- Fenwick A, Webster JP, Bosque‐Oliva E, Blair L, Fleming FM, Zhang Y, et al. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002‐2008. Parasitology 2009;136(13):1719‐30. [DOI] [PubMed] [Google Scholar]
Foster 1987
- Foster R. A review of clinical experience with oxamniquine. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(1):55‐9. [DOI] [PubMed] [Google Scholar]
Glinz 2010
- Glinz D, Kigbafori SD, Knopp S, Yao PK, Lohourignon LK, Steinmann P, et al. Comparison of Kato‐Katz, koga agar plate, ether‐concentration, and FLOTAC techniques for the diagnosis of Schistosoma mansoni and soil‐transmitted helminths. PLoS Neglected Tropical Diseases 2010;4(7):e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2011
- Gray DJ, Ross AG, Li Y‐S, McManus DP. Diagnosis and management of schistosomiasis. BMJ 2011;342:d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gryseels 1992
- Gryseels B, Polderman AM, Engels D. Experiences with the control of schistosomiasis mansoni in two foci in central Africa. Memorias do Instituto Oswaldo Cruz 1992;87(Suppl 4):187‐94. [DOI] [PubMed] [Google Scholar]
Gryseels 1996
- Gryseels B, Vlas SJ. Worm burdens in schistosome infections. Parasitology Today 1996;12(3):115‐9. [DOI] [PubMed] [Google Scholar]
Gryseels 2006
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet 2006;368(9541):1106‐18. [DOI] [PubMed] [Google Scholar]
Gryseels 2012
- Gryseels B. Schistosomiasis. Infectious Disease Clinics of North America 2012;26(2):383‐97. [DOI] [PubMed] [Google Scholar]
Hackett 1944
- Hackett W. Spleen measurement in malaria. Journal of the Malaria Society 1944;3:121‐3. [Google Scholar]
Hatz 1990
- Hatz CF, Savioli L, Mayombana C, Dhunputh J, Kisumku UM, Tanner M. Measurement of schistosomiasis‐related morbidity at community level in areas of different endemicity. Bulletin of the World Health Organization 1990;68(6):777‐87. [PMC free article] [PubMed] [Google Scholar]
Hatz 2001
- Hatz CF. The use of ultrasound in schistosomiasis. Advances in Parasitology 2001;48:225‐84. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.cochrane‐handbook.org, 2011. [Google Scholar]
Hotez 2006
- Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, Sachs JD. Incorporating a rapid‐impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Medicine 2006;3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inyang‐Etoh 2004
- Inyang‐Etoh PC, Ejezie GC, Useh MF, Inyang‐Etoh EC. Efficacy of artesunate in the treatment of urinary schistosomiasis, in an endemic community in Nigeria. Annals of Tropical Medicine and Parasitology 2004;98(5):491‐9. [DOI] [PubMed] [Google Scholar]
Inyang‐Etoh 2009
- Inyang‐Etoh PC, Ejezie GC, Useh MF, Inyang‐Etoh EC. Efficacy of a combination of praziquantel and artesunate in the treatment of urinary schistosomiasis in Nigeria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103:38‐44. [DOI] [PubMed] [Google Scholar]
Katz 1972
- Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick‐smear technique in Schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de São Paulo 1972;14(6):397‐400. [PubMed] [Google Scholar]
Katz 2008
- Katz N, Coelho PM. Clinical therapy of schistosomiasis mansoni: the Brazilian contribution. Acta Tropica 2008;108(2‐3):72‐8. [DOI] [PubMed] [Google Scholar]
King 2005
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helminthic infection: a meta‐analysis of disability‐related outcomes in endemic schistosomiasis. Lancet 2005;365(9470):1561‐9. [DOI] [PubMed] [Google Scholar]
King 2007
- King CH. Lifting the burden of schistosomiasis‐‐defining elements of infection‐associated disease and the benefits of antiparasite treatment. Journal of Infectious Diseases 2007;196(5):653‐5. [DOI] [PubMed] [Google Scholar]
King 2008a
- King CH, Bertino A‐M. Asymmetries of poverty: why global burden of disease valuations underestimate the burden of neglected tropical diseases. PLoS Neglected Tropical Diseases 2008;2(3):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2008b
- King CH, Dangerfield‐Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illness 2008;4:65‐79. [DOI] [PubMed] [Google Scholar]
King 2010
- King CH. Health metrics for helminthic infections. Advances in Parasitology 2010;73:51‐69. [DOI] [PubMed] [Google Scholar]
Kongs 2001
- Kongs A, Marks G, Verlé P, Stuyft P. The unreliability of the Kato‐Katz technique limits its usefulness for evaluating S. mansoni infections. Tropical Medicine and International Health 2001;6(3):163‐9. [DOI] [PubMed] [Google Scholar]
Koukounari 2010
- Koukounari A, Donnelly CA, Sacko M, Keita AD, Landoure A, Dembele R, et al. The impact of single versus mixed schistosome species infections on liver, spleen and bladder morbidity within Malian children pre‐ and post‐praziquantel treatment. BMC Infectious Diseases 2010;10(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legesse 2007
- Legesse M, Erko B. Field‐based evaluation of a reagent strip test for diagnosis of Schistosoma mansoni by detecting circulating cathodic antigen in urine before and after chemotherapy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(7):668‐73. [DOI] [PubMed] [Google Scholar]
Legesse 2008
- Legesse M, Erko B. Field‐based evaluation of a reagent strip test for diagnosis of schistosomiasis mansoni by detecting circulating cathodic antigen (CCA) in urine in low endemic area in Ethiopia. Parasite 2008;15(2):151‐5. [DOI] [PubMed] [Google Scholar]
Liu 2011
- Liu R, Dong HF, Guo Y, Zhao QP, Jiang MS. Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: a systematic review and meta‐analysis. Parasites and Vectors 2011;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magnussen 2001
- Magnussen P, Ndawi B, Sheshe AK, Byskov J, Mbwana K, Christensen NO. The impact of a school health programme on the prevalence and morbidity of urinary schistosomiasis in Mwera Division, Pangani District, Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(1):58‐64. [DOI] [PubMed] [Google Scholar]
Mohamed‐Ali 1991
- Mohamed‐Ali Q, Doehring‐Schwerdtfeger E, Abdel‐Rahim IM, Schlake J, Kardorff R, Franke D, et al. Ultrasonographical investigations of periportal fibrosis in children with Schistosoma mansoni infection: reversibility of morbidity seven months after treatment with praziquantel. American Journal of Tropical Medicine and Hygiene 1991;44:444‐51. [DOI] [PubMed] [Google Scholar]
Naus 2003
- Naus CW, Booth M, Jones FM, Kemijumbi J, Vennervald BJ, Kariuki CH, et al. The relationship between age, sex, egg‐count and specific antibody responses against Schistosoma mansoni antigens in a Ugandan fishing community. Tropical Medicine and International Health 2003;8(6):561‐8. [DOI] [PubMed] [Google Scholar]
Polman 2001
- Polman K, Stelma FF, Vlas SJ, Sow S, Fathers L, Cessie S, et al. Dynamics of egg counts and circulating antigen levels in a recent Schistosoma mansoni focus in northern Senegal. Tropical Medicine and International Health 2001;6:538‐44. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Richter 2003a
- Richter J. The impact of chemotherapy on morbidity due to schistosomiasis. Acta Tropica 2003;86(2‐3):161‐83. [DOI] [PubMed] [Google Scholar]
Richter 2003b
- Richter J, Hatz C, Häussinger D. Ultrasound in tropical and parasitic diseases. Lancet 2003;362(9387):900‐2. [DOI] [PubMed] [Google Scholar]
Sabah 1986
- Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Experimental Parasitology 1986;61:294‐303. [DOI] [PubMed] [Google Scholar]
Sandoval 2006
- Sandoval N, Siles‐Lucas M, Pérez‐Arellano JL, Carranza C, Puente S, López‐Abán J, et al. A new PCR‐based approach for the specific amplification of DNA from different Schistosoma species applicable to human urine samples. Parasitology 2006;133(Pt 5):581‐7. [DOI] [PubMed] [Google Scholar]
Savioli 2004
- Savioli L, Albonico M, Engels D, Montresor A. Progress in the prevention and control of schistosomiasis and soil‐transmitted helminthiasis. Parasitology International 2004;53(2):103‐13. [DOI] [PubMed] [Google Scholar]
Savioli 2009
- Savioli L, Gabrielli AF, Montresor A, Chitsulo L, Engels D. Schistosomiasis control in Africa: 8 years after World Health Assembly Resolution 54.19. Parasitology 2009;136(13):1677‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinmann 2006
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta‐analysis, and estimates of people at risk. Lancet Infectious Diseases 2006;6(7):411‐25. [DOI] [PubMed] [Google Scholar]
Sturrock 2001
- Sturrock RF. Schistosomiasis epidemiology and control: how did we get here and where should we go?. Memorias do Instituto Oswaldo Cruz 2001;96(Suppl):17‐27. [DOI] [PubMed] [Google Scholar]
Teesdale 1976
- Teesdale CH, Amin MA. A simple thick‐smear technique for the diagnosis of Schistosoma mansoni infection. Bulletin of the World Health Organization 1976;54(6):703‐5. [PMC free article] [PubMed] [Google Scholar]
Utzinger 2000c
- Utzinger J, N'Goran EK, Ossey YA, Booth M, Traoré M, Lohourignon KL, et al. Rapid screening for Schistosoma mansoni in western Côte d'Ivoire using a simple school questionnaire. Bulletin of the World Health Organization 2000;78(3):389‐98. [PMC free article] [PubMed] [Google Scholar]
Utzinger 2001
- Utzinger J, Xiao S, NGoran EK, Bergquist R, Tanner M. The potential of artemether for the control of schistosomiasis. International Journal for Parasitology 2001;31(14):1549‐62. [DOI] [PubMed] [Google Scholar]
Utzinger 2001a
- Utzinger J, Booth M, NGoran EK, Müller I, Tanner M, Lengeler C. Relative contribution of day‐to‐day and intra‐specimen variation in faecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitology 2001;122(Pt 5):537‐44. [DOI] [PubMed] [Google Scholar]
Utzinger 2002
- Utzinger J, Chollet J, Tu Z, Xiao S, Tanner M. Comparative study of the effects of artemether and artesunate on juvenile and adult Schistosoma mansoni in experimentally infected mice. Transactions of the Royal Society of Tropical Medicine Hygiene 2002;96(3):318–23. [DOI] [PubMed] [Google Scholar]
Utzinger 2003
- Utzinger J, Keiser J, Xiao SH, Tanner M, Singer BH. Combination chemotherapy of schistosomiasis in laboratory studies and clinical trials. Antimicrobial Agents and Chemotherapy 2003;47:1487‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Utzinger 2007
- Utzinger J, Xiao SH, Tanner M, Keiser J. Artemisinins for schistosomiasis and beyond. Current Opinion on Investigational Drugs 2007;8(2):105‐16. [PubMed] [Google Scholar]
Utzinger 2009
- Utzinger J, Raso G, Brooker S, Savigny D, Tanner M, Ornbjerg N, et al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology 2009;136(13):1859‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Werf 2003
- Werf MJ, Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JDF, et al. Quantification of clinical morbidity associated with schistosome infection in sub‐Saharan Africa. Acta Tropica 2003;86(2‐3):125‐39. [DOI] [PubMed] [Google Scholar]
Webster 2009
- Webster JP, Koukounari A, Lamberton PH, Stothard JR, Fenwick A. Evaluation and application of potential schistosome‐associated morbidity markers within large‐scale mass chemotherapy programmes. Parasitology 2009;136(13):1789‐99. [DOI] [PubMed] [Google Scholar]
WHO 1985
- WHO. The control of schistosomiasis. Report of WHO expert committee. WHO Technical Report Series No. 728. Geneva. 1985. [PubMed]
WHO 2001
- WHO Expert Committee on the Control of Schistosomiasis (Geneva, Switzerland). Prevention and Control of Schistosomiasis and soil‐transmitted helminthiasis: report of a WHO expert committee. WHO Technical Report Series 912. 2001. [PubMed]
WHO 2002
- WHO. Prevention and control of schistosomiasis and soil‐transmitted helminthiasis. Report of a WHO expert committee WHO Technical Report Series. No 912.1‐57. 2002. [PubMed]
WHO 2004
- WHO. World Health Report, World Health Organization, Geneva, Switzerland. 2004.
WHO 2006
- WHO. Preventive Chemotherapy guidelines. Report of a WHO expert committee, World Health Organization, Geneva, Switzerland. 2006.
Zhang 2007
- Zhang Y, Koukounari A, Kabatereine N, Fleming F, Kazibwe F, Tukahebwa E, et al. Parasitological impact of 2‐year preventive chemotherapy on schistosomiasis and soil‐transmitted helminthiasis in Uganda. BMC Medicine 2007;3:5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]


