Abstract
Background
Infection with enterotoxigenic Escherichia coli (ETEC) bacteria is a common cause of diarrhoea in adults and children in developing countries and is a major cause of 'travellers' diarrhoea' in people visiting or returning from endemic regions. A killed whole cell vaccine (Dukoral®), primarily designed and licensed to prevent cholera, has been recommended by some groups to prevent travellers' diarrhoea in people visiting endemic regions. This vaccine contains a recombinant B subunit of the cholera toxin that is antigenically similar to the heat labile toxin of ETEC. This review aims to evaluate the clinical efficacy of this vaccine and other vaccines designed specifically to protect people against diarrhoea caused by ETEC infection.
Objectives
To evaluate the efficacy, safety, and immunogenicity of vaccines for preventing ETEC diarrhoea.
Search methods
We searched the Cochrane Infectious Disease Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, LILACS, and http://clinicaltrials.gov up to December 2012.
Selection criteria
Randomized controlled trials (RCTs) and quasi‐RCTs comparing use of vaccines to prevent ETEC with use of no intervention, a control vaccine (either an inert vaccine or a vaccine normally given to prevent an unrelated infection), an alternative ETEC vaccine, or a different dose or schedule of the same ETEC vaccine in healthy adults and children living in endemic regions, intending to travel to endemic regions, or volunteering to receive an artificial challenge of ETEC bacteria.
Data collection and analysis
Two authors independently assessed each trial for eligibility and risk of bias. Two independent reviewers extracted data from the included studies and analyzed the data using Review Manager (RevMan) software. We reported outcomes as risk ratios (RR) with 95% confidence intervals (CI). We assessed the quality of the evidence using the GRADE approach.
Main results
Twenty‐four RCTs, including 53,247 participants, met the inclusion criteria. Four studies assessed the protective efficacy of oral cholera vaccines when used to prevent diarrhoea due to ETEC and seven studies assessed the protective efficacy of ETEC‐specific vaccines. Of these 11 studies, seven studies presented efficacy data from field trials and four studies presented efficacy data from artificial challenge studies. An additional 13 trials contributed safety and immunological data only.
Cholera vaccines
The currently available, oral cholera killed whole cell vaccine (Dukoral®) was evaluated for protection of people against 'travellers' diarrhoea' in a single RCT in people arriving in Mexico from the USA. We did not identify any statistically significant effects on ETEC diarrhoea or all‐cause diarrhoea (one trial, 502 participants, low quality evidence).
Two earlier trials, one undertaken in an endemic population in Bangladesh and one undertaken in people travelling from Finland to Morocco, evaluated a precursor of this vaccine containing purified cholera toxin B subunit rather than the recombinant subunit in Dukoral®. Short term protective efficacy against ETEC diarrhoea was demonstrated, lasting for around three months (RR 0.43, 95% CI 0.26 to 0.71; two trials, 50,227 participants). This vaccine is no longer available.
ETEC vaccines
An ETEC‐specific, killed whole cell vaccine, which also contains the recombinant cholera toxin B‐subunit, was evaluated in people travelling from the USA to Mexico or Guatemala, and from Austria to Latin America, Africa, or Asia. We did not identify any statistically significant differences in ETEC‐specific diarrhoea or all‐cause diarrhoea (two trials, 799 participants), and the vaccine was associated with increased vomiting (RR 2.0, 95% CI 1.16 to 3.45; nine trials, 1528 participants). The other ETEC‐specific vaccines in development have not yet demonstrated clinically important benefits.
Authors' conclusions
There is currently insufficient evidence from RCTs to support the use of the oral cholera vaccine Dukoral® for protecting travellers against ETEC diarrhoea. Further research is needed to develop safe and effective vaccines to provide both short and long‐term protection against ETEC diarrhoea.
23 April 2019
No update planned
Other
This is not a current question.
Keywords: Adult; Child; Humans; Cholera Toxin; Cholera Toxin/adverse effects; Cholera Toxin/immunology; Cholera Vaccines; Cholera Vaccines/adverse effects; Cholera Vaccines/therapeutic use; Developing Countries; Diarrhea; Diarrhea/microbiology; Diarrhea/prevention & control; Enterotoxigenic Escherichia coli; Enterotoxigenic Escherichia coli/immunology; Randomized Controlled Trials as Topic; Travel; Vaccines, Inactivated; Vaccines, Inactivated/adverse effects; Vaccines, Inactivated/therapeutic use
Plain language summary
Vaccines for preventing diarrhoea caused by enterotoxigenic Escherichia coli bacteria
Enterotoxigenic E. coli (ETEC) is a type of bacteria that can infect both children and adults, causing diarrhoea. In particular, it affects people in developing countries. However, it is also a major cause of 'travellers' diarrhoea' in people visiting or returning from regions where this infection is common. It is transmitted from person to person by eating or drinking unclean food or water. Typically it causes watery diarrhoea, with abdominal pains and vomiting, that can last for several days. Vaccines are being considered as a way to prevent diarrhoea caused by ETEC bacteria. ETEC bacteria share some similarities with the bacteria that cause cholera. In this review, we examined the effectiveness of either vaccines designed to prevent cholera or vaccines designed specifically to prevent ETEC infection for preventing ETEC diarrhoea. We compared these vaccines against the use of a control vaccine (either an inert vaccine or a vaccine normally given to prevent an unrelated infection), no intervention, an alternative ETEC vaccine, or a different dose or schedule of the same ETEC vaccine.
We examined the research published up to 07 December 2012. We included 24 randomized controlled trials and 53,247 participants in this review. Four studies assessed the use of oral cholera vaccines to prevent diarrhoea caused by ETEC and eight trials assessed the use of ETEC‐specific vaccines to prevent diarrhoea. Seven studies presented data from field trials and four studies presented data from studies where people were artificially infected with ETEC bacteria. Also, 13 trials gave safety and immunological data only.
There is currently insufficient evidence to support the use of the oral cholera vaccine Dukoral® to protect travellers against ETEC diarrhoea. Based on a single trial in people travelling from the USA to Mexico, the oral cholera vaccine Dukoral® may have little or no effect in preventing ETEC diarrhoea (one trial, 502 participants, low quality evidence). Two earlier trials, one undertaken in an endemic population in Bangladesh and one undertaken in people travelling from Finland to Morocco, evaluated a precursor of the oral cholera vaccine Dukoral®. Short term protection against ETEC diarrhoea was demonstrated, lasting for around three months (RR 0.43, 95% CI 0.26 to 0.71; two trials, 50,227 participants). However, this vaccine is no longer available.
An ETEC‐specific, killed whole cell vaccine, which also contains the recombinant cholera toxin B‐subunit, was evaluated in people travelling from the USA to Mexico or Guatemala, and from Austria to Latin America, Africa, or Asia. There were no statistically significant differences in ETEC‐specific diarrhoea or all‐cause diarrhoea (two trials, 799 participants) found and the vaccine was associated with increased vomiting (RR 2.0, 95% CI 1.16 to 3.45; nine trials, 1528 participants). The other ETEC‐specific vaccines in development have not yet demonstrated clinically important benefits. Further research is needed to develop safe and effective vaccines to provide both short and long‐term protection against ETEC diarrhoea.
Summary of findings
Summary of findings for the main comparison. Cholera WC‐rCTB vaccine for preventing enterotoxigenic E. coli (ETEC) diarrhoea.
| Cholera killed whole cells plus recombinant B‐subunit vaccine for enterotoxigenic E. coli (ETEC) diarrhoea | ||||||
| Patient or population: People travelling from non‐endemic settings Settings: Endemic settings Intervention: Cholera killed whole cells plus recombinant B‐subunit vaccine (WC‐rCTB Cholera) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vaccine | |||||
| ETEC diarrhoea | 99 per 1000 | 120 per 1000 (72 to 198) | RR 0.93 (0.61 to 1.41) | 502 (1 study) | ⊕⊕⊝⊝ low1,2,3,4 | |
| Severe ETEC diarrhoea | ‐ | ‐ | ‐ | (0 studies) | ‐ | |
| All‐cause diarrhoea | 492 per 1000 | 512 per 1000 (428 to 610) | RR 1.04 (0.87 to 1.24) | 502 (1 study) | ⊕⊕⊝⊝ low1,4,5 | |
| Adverse events | ‐ | ‐ | ‐ | 502 (1 study) | 6 | |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 This single study was conducted in adults travelling from the USA to Mexico (Scerpella 1995). Although the paper does not clearly describe the methods used to prevent selection bias, we have not downgraded the evidence as selection bias is probably unlikely in a trial where everyone is healthy at enrolment. 2 Two older trials evaluated a prototype of this vaccine which contained purified cholera B‐subunit rather than the recombinant subunit contained in this vaccine. Although both trials found some evidence of benefit, the evidence may no longer be applicable due to changes in both composition and dosing of the vaccine. 3 Downgraded by one for indirectness: in this study the vaccine was provided in two doses 10 days apart after the travellers had arrived in Mexico. Most cases of ETEC diarrhoea occurred between doses or within seven days of administration of the second dose. The authors conducted a subgroup analysis of only those participants who had diarrhoea > 7 days after the second dose, which excluded 75% of cases. We did not find a statistically significant difference in our analysis of this data. 4 Downgraded by one for imprecision: this trial was small and underpowered to reliably prove or exclude a clinically important effect with the vaccine. 5 Downgraded by one for indirectness: in this study the vaccine was provided in two doses 10 days apart after the travellers had arrived in Mexico. Further studies are required which assess administration prior to travel to a variety of destinations. 6Scerpella 1995 reported no differences in the frequency of gastrointestinal symptoms, headaches, or febrile illnesses between vaccinees, or placebo recipients but data were not presented.
Background
Description of the condition
Enterotoxigenic Escherichia coli (ETEC) is the most common bacterial cause of diarrhoea in adults and children in developing countries (Qadri 2005; Walker 2007). The annual incidence of this disease is highest in young children and susceptibility to the disease declines with age. Children born in endemic regions are likely to experience two to three episodes of ETEC diarrhoea before their fifth birthday (Wennerås 2004). The practical difficulties associated with making an accurate diagnosis of ETEC in low‐resource settings mean that its significance has often been underestimated (Wennerås 2004; Qadri 2005). However, a review of microbiological studies conducted in endemic regions between 1992 and 2000 found that ETEC was the causative organism in approximately 25% of all diarrhoeal episodes in children aged between one and four years (Wennerås 2004). Many more children were shown to carry the organism asymptomatically in their gut (Walker 2007). A global burden of approximately 280 million clinical episodes and 380,000 deaths annually are estimated (WHO 2009).
Person‐to‐person transmission of ETEC occurs via ingestion of faecally‐contaminated food or water. In developed countries where sanitation standards are usually higher, ETEC infection is rare. However, it remains a major cause of 'travellers' diarrhoea' which occurs in people visiting or returning from ETEC‐endemic regions (Qadri 2005; DuPont 2008; Widermann 2009). Epidemics of ETEC diarrhoea have also occurred during natural disasters, such as floods, where there has been an acute deterioration in the quality of drinking water and sanitation (Schwartz 2006; Harris 2008).
The clinical illness is characterized by a profuse watery diarrhoea that lasts for several days and may be associated with abdominal cramp, malaise, vomiting, and a low grade fever. Without adequate treatment this can lead to dehydration. If people have a prolonged infection or are infected again, this can lead to malnutrition or growth inhibition in young children (Black 1984; Qadri 2005; Qadri 2007).
Following ingestion, ETEC bacteria adhere to the lining of the gut and secrete either one or both types of enterotoxins: the heat labile toxin (LT) and the heat stable toxin (ST). These toxins induce the hypersecretion of fluids and electrolytes, which cause the typical watery diarrhoea (Gill 1980). Different strains of ETEC can be further characterized on the basis of the antigens expressed on the cell surface: the colonization factor (CF), and the 'O' and 'H' antigens (Wolf 1997). Some of these antigens have been shown to be important in inducing natural immunity and therefore represent key targets for vaccine development (Rao 2005; Svennerholm 2008). Over 100 different "O'' antigens can be present on ETEC and therefore have not been considered important for vaccine development. Since both antitoxic and antibacterial antibodies are important for protection, most vaccine formulations have been based on the enterotoxins and CFs of ETEC (Svennerholm 1984; Ahren 1998). Important antigens considered until now for vaccine development include the LT and CFs. Over 25 CFs that have been characterized and most common CFs present on clinical isolates include CFA/I, CS1, CS2, CS3, CS5, and CS6. These CFs have been included as vaccine antigens on ETEC vaccines to date (Harro 2011; Tobias 2011; Tobias 2012).
Improvements in public health and sanitation conditions represent the ideal solution to preventing transmission of ETEC and other faecally‐transmitted organisms. However, this can be difficult to achieve given the financial and logistical constraints in low‐resource regions. Thus prophylactic measures, including vaccines, are being considered as alternative short‐term strategies (Walker 2007).
Description of the intervention
Only one vaccine (Dukoral® produced by SBL Sweden) is currently available for the prevention of ETEC diarrhoea. This vaccine has been recommended to prevent 'travellers' diarrhoea' in people visiting endemic regions from developed countries (Steffen 2005). This vaccine is primarily designed and licensed to prevent diarrhoea due to Vibrio cholerae (cholera), but it contains a recombinant B subunit of the cholera toxin that is antigenically very similar to the LT of ETEC (Walker 2007). In an early clinical trial, using a prototype of this vaccine which contained purified cholera B subunit rather than the recombinant form, significant cross protection against ETEC diarrhoea was demonstrated (Clemens 1988).
Many alternative vaccine candidates designed specifically to protect people against ETEC diarrhoea are now at various stages of clinical development (Table 2). The vaccine candidates can be broadly categorized in to two groups: inactivated vaccines containing killed whole cells, purified CF antigens, or inactivated LT; and live attenuated vaccines containing genetically modified, non‐pathogenic strains of ETEC, or alternative carrier bacteria expressing the important ETEC antigens (Svennerholm 2008). Given the number of antigenically different strains of ETEC, it is likely that a vaccine formulation capable of providing broad protection would need to contain a combination of the most commonly expressed antigens (Walker 2007; Svennerholm 2008).
1. Currently available and experimental ETEC vaccines.
Oral inactivated vaccines
|
Oral live attenuated vaccines
|
Other ETEC vaccines under development include
|
WC/rCTB: whole cell/recombinant cholera toxin B subunit; ETEC: enterotoxigenic E. coli; CFA: colonization factor antigen; CS: E. coli surface antigen; LT: heat labile toxin; CT: cholera toxin; LTB/ST: heat labile toxin B subunit/heat stable toxin.
How the intervention might work
Epidemiological and experimental data suggest that natural immunity to ETEC does occur following natural infection and antibodies against CF antigens and the B subunit of LT have been detected (Qadri 2005; Rao 2005). Vaccine candidates aim to induce similar immunity (without the associated clinical illness) and to provide lasting protection against a broad range of the pathogenic ETEC strains (Svennerholm 2008). Attempts have been made to find immunological markers of protection, including toxin‐ or CF‐specific immune responses, or both. CF‐specific antibodies have been used to determine 'take rates' (the proportion of vaccinations that induce high antibody levels to vaccine) for ETEC vaccines containing CFs as components (Wenneras 1999; Qadri 2003; Rao 2005). However, adequate and lasting protection cannot be assumed without demonstrating reduced incidence of the clinical illness in large, well conducted clinical trials.
The route of administration of a vaccine may influence both its immunogenicity and acceptability. Oral vaccines have the potential to stimulate local immunity within the mucosa of the gut, preventing the colonization and multiplication of the bacteria. ETEC is transmitted through the faecal‐oral route and vaccines designed to be given orally have been developed (Holmgren 2005). Such vaccines are easy to administer in all settings and have a reduced risk of transmitting blood‐borne infections.
Why it is important to do this review
Assessment of the level of mortality and morbidity associated with ETEC diarrhoea and the extent of the global disease burden has resulted in several initiatives to develop effective vaccines (Walker 2007). ETEC vaccine development is at an earlier stage than some other vaccines (eg cholera vaccine) but data from phase II and phase III trials in endemic areas and non‐primed participants are available and these need to be reviewed.
This review aims to evaluate the efficacy, safety, and immunogenicity of current vaccine candidates tested in randomized controlled trials (RCTs), including the oral cholera vaccine Dukoral®, when used to protect against ETEC diarrhoea.
Objectives
To evaluate the efficacy, safety, and immunogenicity of vaccines for preventing enterotoxigenic ETEC diarrhoea.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and quasi‐RCTs, for which the unit of randomization is the individual participant or a cluster of participants.
Types of participants
Healthy adults and children living in endemic regions, intending to travel to endemic regions, or receiving an artificial challenge.
Types of interventions
Intervention
Any vaccine being used to prevent ETEC diarrhoea. Studies evaluating vaccines which have not yet been evaluated for clinical outcomes will be excluded.
Control
No intervention, a control vaccine (either an inert vaccine or a vaccine normally given to prevent an unrelated infection), an alternative ETEC vaccine, or a different dose or schedule of the same ETEC vaccine.
Types of outcome measures
Primary outcomes
Protective efficacy as measured against:
Episodes of ETEC diarrhoea (any severity)
Severe episodes of ETEC diarrhoea
Secondary outcomes
Protective efficacy as measured against:
Episodes of all‐cause diarrhoea
Severe episodes of all‐cause diarrhoea
Safety measured as:
The number of adverse events, including systemic and local reactions.
Immunological outcomes:
Any immunological measure of response to vaccination, eg an increase in CF, or toxin‐specific, immune responses in serum/plasma, or both, or an increase in antibody‐secreting cell responses in lymphocytes.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
Published studies
We searched the Cochrane Infectious Disease Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, LILACS and http://clinicaltrials.gov/, using the search terms detailed in Table 3.
2. Detailed Search Strategy.
| Search set | CIDG SR¹ | CENTRAL | MEDLINE² | EMBASE² | LILACS² |
| 1 | E.coli | Enterotoxigenic Escherichia coli [MeSH] | Enterotoxigenic Escherichia coli [MeSH] | Enterotoxigenic Escherichia coli [Emtree] | E.coli |
| 2 | Enterotoxigenic | ETEC | ETEC ti, ab | ETEC ti, ab | Enterotoxigenic |
| 3 | ETEC | Enterotoxigenic E coli | Enterotoxigenic E coli ti, ab | Enterotoxigenic E coli ti, ab | ETEC |
| 4 | Travel* diarrh* | Travel* diarrh* | Travel* diarrh* ti, ab | Traveller diarrhea [Emtree] | Travel$ diarrh$ |
| 5 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 |
| 6 | Vaccin* | Vaccin* | Vaccin* ti, ab | Vaccin* ti, ab | Vaccin$ |
| 7 | 5 and 6 | 5 and 6 | 5 and 6 | 5 and 6 | 5 and 6 |
| 8 | Escherichia coli vaccines [MeSH] | Escherichia coli vaccines [MeSH] | Escherichia coli vaccine [Emtree] | ||
| 9 | 7 or 8 | 7 or 8 | 7 or 8 | ||
| 10 | Limit 9 to humans | Limit 9 to Human |
¹Cochrane Infectious Diseases Group Specialized Register.
²Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2008).
Ongoing studies
We searched the metaRegister of Controlled Trials (mRCT) and the WHO International Clinical Trials Registry Platform (ICTRP) for ongoing trials using 'Enterotoxigenic' and 'vaccin*' as search terms.
Searching other resources
Reference lists
We searched the reference lists of all included studies for additional references relevant to this review.
Data collection and analysis
Selection of studies
Tanvir Ahmed (TA) and Taufiqur Bhuiyan (TB) independently screened all citations and abstracts identified by the search strategy to identify potentially eligible studies. We obtained full‐text articles of potentially eligible studies. TA and TB independently assessed these articles for inclusion in the review using a pre‐designed eligibility form based on the inclusion criteria.
In the event that it was unclear whether a trial was eligible for the review, we resolved any differences in opinion through discussion with Firdausi Qadri (FQ). We excluded any studies that did not meet the inclusion criteria and we documented the reasons for exclusion.
Data extraction and management
For each included trial, TA and TB independently extracted information on the characteristics of the trial (study design, study dates and duration, study location, setting, and source of funding), the participants recruited (the inclusion and exclusion criteria), and the intervention (the type of vaccine, type of placebo, dose, and immunization schedule), and listed the outcomes presented in the papers using a pre‐tested data extraction form. For all outcomes, we extracted the number of patients randomized to each treatment group and the number of patients for whom an outcome was available. For dichotomous outcomes, we extracted the number of participants that experienced the event and the number of patients in each treatment group. We extracted adverse event data for each individual type of event wherever possible. Where adverse events were reported for more than one dose, the number of people reporting each side‐effect after each dose was recorded. Where trials reported the occurrence of adverse events over time following a single dose, we recorded the proportion of people affected during each time period. If the denominator or total number of people affected for each time period was not clear, then we only recorded the events that occurred in the first time period (typically 72 hours) after each dose. Where data were missing or incomplete, we contacted the authors for clarification. In cases of disagreement, we double‐checked the data extraction and we resolved any disagreements through discussion.
Assessment of risk of bias in included studies
Two authors (TA and TB) independently assessed the risk of bias of each trial using 'The Cochrane Collaboration's tool for assessing the risk of bias' (Higgins 2008). We followed the guidance to assess whether steps were taken to reduce the risk of bias across six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias.
For sequence generation and allocation concealment, we reported the methods used. For blinding, we described who was blinded and the blinding method. For incomplete outcome data, we reported the percentage and proportion lost to follow‐up in each group. For selective outcome reporting, we stated any discrepancies between the methods used and the results, in terms of the outcomes measured or the outcomes reported. For other biases, we described any other trial features that we thought could have affected the trial result (eg if the trial was stopped early).
We categorized our judgements as either low, high, or unclear risk of bias. We used this information to guide our interpretation of the data. Where our judgement was unclear risk of bias, we attempted to contact the trial authors for clarification and we resolved any differences of opinion through discussion.
Measures of treatment effect
We expressed dichotomous outcomes using risk ratios (RR), and presented with 95% confidence intervals (CI).
Unit of analysis issues
We ensured that the same patients were not included in the same meta‐analysis more than once, by grouping or splitting the data in multi‐arm trials as appropriate.
Dealing with missing data
If data from the trial reports were insufficient, unclear, or missing, we attempted to contact the trial authors for additional information. We aimed to carry out an intention‐to‐treat analysis. However if the duration of follow‐up of all patients was not known, we carried out a complete case analysis, in which we only included patients for whom an outcome was available.
Assessment of heterogeneity
We assessed heterogeneity between the trials by examining the forest plot to check for overlapping CIs, by using the Chi2 test for heterogeneity with a 10% level of significance, and by using the I2 statistic with a value of 50% to represent moderate levels of heterogeneity.
Assessment of reporting biases
We did not assess publication bias using funnel plots because the number of trials per comparison were insufficient.
Data synthesis
We analyzed the data using Review Manager (RevMan).
We used the Mantel‐Haenszel method to combine dichotomous data. If there was no heterogeneity present, we used a fixed‐effect model. If there was moderate heterogeneity and it was still appropriate to combine studies, we used a random‐effects model. When it was deemed inappropriate to combine studies due to methodological or statistical heterogeneity, we presented the data in tables. We stratified the primary analysis by vaccine type.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses to investigate causes of heterogeneity but the data were too limited.
Sensitivity analysis
As the number of trials per comparison were insufficient, we did not conduct the pre‐planned sensitivity analysis to assess the robustness of the results against risk of bias judgements.
Assessment of the quality of the evidence
We assessed the quality of evidence using the GRADE approach (Guyatt 2008). The GRADE system considers ‘quality’ to be a judgment of the extent to which we can be confident that the estimates of effect are correct. The level of ‘quality’ is judged on a four‐point scale. Evidence from RCTs is initially graded as high and downgraded by either one, two, or three levels after full consideration of: any limitations in the design of the studies, the directness (or applicability) of the evidence, the consistency and precision of the results, and the possibility of publication bias.
Results
Description of studies
Results of the search
We identified 55 potentially relevant articles for inclusion. We assessed these articles using the pre‐stated inclusion criteria (see Figure 1).
1.
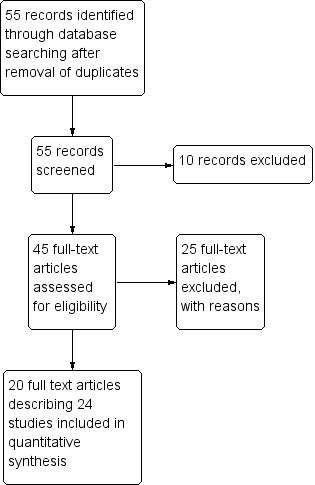
Study flow diagram.
Included studies
We included 20 individual papers describing 24 trials which evaluated eight different vaccines. Of these, seven trials presented efficacy data from field trials (Clemens 1988; Peltola 1991; Scerpella 1995; Wiedermann 2000; Sack 2007; Leyten 2005; Frech 2008), and four trials presented efficacy data from artificial challenge studies (Freedman 1998; Tacket 1999; McKenzie 2007; McKenzie 2008). A summary of the main characteristics of these trials is given in Table 4. For further details see the Characteristics of included studies tables. An additional 13 trials only contributed safety and immunogenicity data.
3. Characteristics of trials assessing clinical efficacy.
| Vaccine details | Study ID | Population details | Challenge | |||||
| Type | Name | Route | Schedule | Age | Group | Country setting | ||
| Inactivated | Cholera WC‐BS | Oral | Three doses, at 6 week intervals | Clemens 1988 | Children aged 2 to 15 years Women aged > 15 years |
Endemic | Bangladesh | Natural |
| Oral | Two doses two weeks apart | Peltola 1991 | Adults | Travellers | From Finland to Morocco | Natural | ||
|
Cholera WC‐rCTB (Dukoral®) |
Oral | Two doses, 10 days apart | Scerpella 1995 | Adults | Travellers | From USA to Mexico | Natural | |
| ETEC WC‐rCTB | Oral | Two doses, 7 to 21 days apart | Sack 2007 | Adults | Travellers | From USA to Mexico or Guatemala | Natural | |
| Oral | Two doses, 7 to 21 days apart | Wiedermann 2000 | All ages | Travellers | From Austria to Latin America, Africa, and Asia. | Natural | ||
| Live attenuated | CVD 103‐HgR | Oral | Single dose | Leyten 2005 | Adults | Travellers | From Holland to Indonesia, Thailand, India, or West Africa | Natural |
| PTL‐003 | Oral | 2 doses, 10 days apart | McKenzie 2008 | Adults | Volunteers | USA | Artificial | |
| Other | Transcutaneous LT‐ETEC patch | Patch | 2 to 3 doses, at 2 to 3 week intervals | Frech 2008 | Adults | Travellers | From USA to Mexico and Guatemala | Natural |
| Patch | 2 to 3 doses, at 2 to 3 week intervals | McKenzie 2007 | Adults | Volunteers | USA | Artificial | ||
| Hyper immune Anti‐E coli. CFA | Oral | 3 times daily for 5 to 7 days | Freedman 1998 | Adults | Volunteers | USA | Artificial | |
| Oral | 3 times daily for 5 to 7 days | Tacket 1999 | Adults | Volunteers | USA | Artificial | ||
WC = killed whole cell, BS = cholera toxin B subunit, rCTB = recombinant cholera toxin B subunit.
Interventions
Three different killed whole cell vaccines were evaluated in efficacy trials: the oral cholera vaccine with purified B‐subunit (Cholera WC‐BS: Clemens 1988; Peltola 1991), the oral cholera vaccine with recombinant B‐subunit (Cholera WC‐rCTB: Scerpella 1995), and an ETEC vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB: Wiedermann 2000; Sack 2007). Two live attenuated vaccines have undergone evaluation of clinical efficacy: one oral cholera vaccine (CVD 103‐HgR: Leyten 2005) and one ETEC‐specific vaccine (PTL‐003: McKenzie 2008). Two additional studies evaluated an LT subunit vaccine delivered by transcutaneous patch (McKenzie 2007; Frech 2008) and two evaluated passive immunization using hyperimmune anti‐E. coli CFA (Freedman 1998; Tacket 1999).
Populations
Only one vaccine was evaluated for use among an endemic population in a low income country and this vaccine is no longer available (Cholera WC‐BS: Clemens 1988). Five vaccines were evaluated among travellers to endemic settings: Cholera WC‐BS (Peltola 1991). Cholera WC‐rCTB (Scerpella 1995), ETEC WC‐rCTB (Wiedermann 2000; Sack 2007), CVD 103‐HgR (Leyten 2005), and the LT transcutaneous patch (Frech 2008). The remaining three vaccines were evaluated among volunteers in artifical challenge studies.
Outcomes
Ten trials reported episodes of ETEC diarrhoea, six reported on severe ETEC diarrhoea, and ten reported on all‐cause diarrhoea. The definitions of these outcomes varied between trials and we have presented these in Table 5.
4. Outcome definitions for primary and secondary measures of clinical efficacy.
| Vaccine | Study ID | Trial definitions | Challenge type | Confirmation of ETEC | |
| DIarrhoea | Moderate or Severe Diarrhoea | ||||
| Cholera WC‐BS | Clemens 1988 | ≥ 3 non‐bloody loose stools in 24 hours, or fewer episodes with signs of dehydration | Severe diarrhoea = absent or feeble pulse plus one other sign of dehydration. | Natural | Faecal culture |
| Peltola 1991 | Any diarrhoea confirmed by the physician as abnormally loose | Not reported. | Natural | Faecal culture | |
|
Cholera WC‐rCTB (Dukoral®) |
Scerpella 1995 | ≥ 4 unformed stools in 24 hours, or ≥3 unformed stools in 8 hours, plus an additional symptom (nausea. pain, fever, urgency, tenesmus) | Not reported. | Natural | Faecal culture |
| ETEC WC‐rCTB | Sack 2007 | ≥ 3 loose stools in 24 hours, plus at least one other symptom, such as abdominal pain, cramps or nausea. | Severe diarrhoea = ≥ 5 loose stools in 24 hours, or illness episodes with abdominal cramps, pain, or vomiting that interfered with daily activities. | Natural | Faecal culture |
| Wiedermann 2000: | ≥ 3 liquid stools | Not reported. | Natural | Faecal culture | |
| CVD 103‐HgR | Leyten 2005 | ≥ 3 unformed stools in 24 hours, or 2 unformed stools accompanied by vomiting, abdominal cramps or subjective fever, | Moderate diarrhoea = 3 to 6 stools per day Severe diarrhoea = ≥ 6 stools per day |
Natural | Faecal culture |
| PTL‐003 | McKenzie 2008 | 1 loose stool weighing ≥ 300 g, or ≥ 2 loose stools in 48 h with a combined weight of ≥200 g, | Moderate diarrhoea = 4 to 5 loose stools, or 401 to 800 g of loose stool, in 24 hours Severe diarrhoea = ≥ 6 loose stools, or > 800 g of loose stools, in 24 hours Or mild diarrhoea plus one of these symptoms rated as moderate or severe: nausea, vomiting, anorexia, abdominal pain, or cramps. |
Artificial | Assumed all |
| Transcutaneous LT‐ETEC patch | Frech 2008 | ≥ 3 loose stools in 24 hours | Moderate diarrhoea = 4 to 5 loose stools Severe diarrhoea = 6 or more loose stools |
Natural | Faecal culture |
| McKenzie 2007 | 1 loose stool weighing ≥ 300 g or ≥ 2 loose stools in 48 hours weighing a total of ≥200 g, within 120 hours after challenge. | Moderate/severe diarrhoea = > 400 g of loose stool in 24 hours, or ≥ 4 loose stools in 24 hours, or ≥ 2 loose stools within a 48 hour period totaling ≥ 200 g, or a single loose stool weighing ≥ 300 g plus one of the following symptoms rated as moderate or severe: nausea, vomiting, abdominal pain, or cramps. | Artificial | Assumed all | |
| Hyperimmune Anti‐E coli. CFA | Freedman 1998 | 1 liquid stool of ≥ 300 mL or 2 liquid stools totaling > 200 mL during any 48 hour period within 120 hours after challenge. | Not reported. | Artificial | Assumed all |
| Tacket 1999 | 1 diarrhoeal stool of > 300 mL or 2 diarrhoeal stools totaling > 200 mL passed during a 48 hour period within 96 hours after challenge. | Not reported. | Artificial | Assumed all | |
Excluded studies
We excluded 35 studies and we listed the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
We summarized the risk of bias assessments in Figure 2.
2.
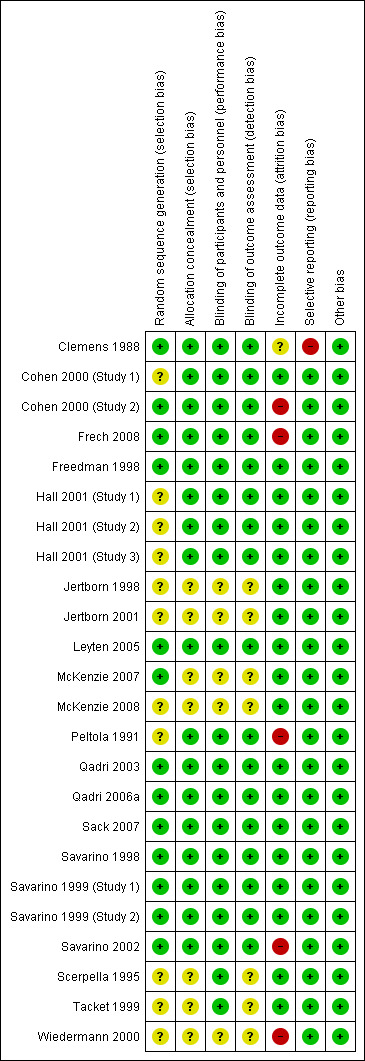
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Efficacy studies
We judged five natural challenge studies to have adequately described allocation concealment and we considered them to be at low risk for selection bias (Clemens 1988; Peltola 1991; Sack 2007; Leyten 2005; Frech 2008). In two natural challenge studies we judged the risk of bias to be unclear (Scerpella 1995; Wiedermann 2000). Of the five artificial challenge studies, only one adequately described a method of allocation concealment (Freedman 1998).
Safety and immunogenicity only studies
We judged 11 of the 13 safety and immunogenicity studies at low risk of selection bias.
Blinding
Efficacy studies
We found that blinding of participants and study personnel was clearly described in eight out of 11 efficacy trials (Clemens 1988; Peltola 1991; Scerpella 1995; Freedman 1998; Tacket 1999; Leyten 2005; Sack 2007; Frech 2008), and was unclear in three. Outcome assessors were blinded to treatment allocation in six trials (Clemens 1988; Peltola 1991; Freedman 1998; Leyten 2005; Sack 2007; Frech 2008), and was unclear in five trials (Scerpella 1995; Tacket 1999; Wiedermann 2000; McKenzie 2007; McKenzie 2008).
Safety and immunogenicity studies
Most studies (11 out of 13) used placebos which were identical in appearance to the vaccine. We considered these studies to be at low risk of bias for safety outcomes. In two studies assessors were not blinded to make a judgement and so we classified these studies at 'unclear' risk of bias (Jertborn 1998; Jertborn 2001).
Incomplete outcome data
Efficacy studies
Seven efficacy trials had low losses to follow‐up and we considered these trials at low risk of attrition bias (Scerpella 1995; Freedman 1998; Tacket 1999; Leyten 2005; McKenzie 2007; Sack 2007; McKenzie 2008). We found that three trials had high losses to follow‐up (Peltola 1991; Wiedermann 2000; Frech 2008) and we judged these trials at high risk of attrition bias. One trial was unclear about the number of participants lost to follow‐up (Clemens 1988).
Safety and immunogenicity studies
Eleven studies out of 13 reported minimal losses to follow‐up. We considered these trials at low risk of bias. Two studies had high losses to follow‐up and we judged these trials at high risk of bias (Cohen 2000 (Study 2); Savarino 2002).
Selective reporting
We found no evidence of selective reporting bias in any of the included studies.
Other potential sources of bias
We found no evidence of other potential sources of bias in the trials.
Effects of interventions
See: Table 1
Cholera killed whole cell vaccines versus placebo
Two oral vaccines containing killed whole cells of V. cholerae have been evaluated. The first contained 1 mg of purified cholera B‐subunit (Cholera WC‐BS). This vaccine was further developed with a recombinant cholera B‐subunit and is commercially available (Cholera WC‐rBS).
Analysis 1: Cholera killed whole cells plus purified B‐subunit (Cholera WC‐BS)
Clinical efficacy
In Bangladesh, in a passive surveillance study in a community endemic with ETEC diarrhoea, Clemens 1988 found that the oral Cholera WC‐BS vaccine provided short‐term protection against LT‐ETEC diarrhoea at three months' follow‐up compared to the same whole cell vaccine without the B‐subunit (RR 0.33, 95% CI 0.13 to 0.84; one trial, 49,612 participants, Analysis 1.1). However, only 24 episodes of ETEC diarrhoea were reported in this study (18 in the placebo group versus six in the vaccine group). Eight episodes of severe ETEC diarrhoea were reported (seven in the placebo group versus one in the vaccine group) and this result was not statistically significant (one trial, 49,612 participants, Analysis 1.2). No protective efficacy was demonstrated at later time points.
1.1. Analysis.
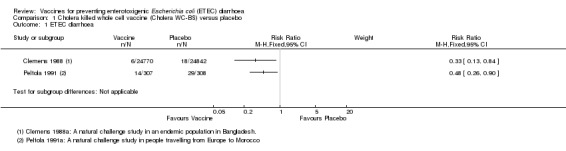
Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 1 ETEC diarrhoea.
1.2. Analysis.
Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 2 Severe ETEC diarrhoea.
One additional trial evaluated the same vaccine given to people intending to travel from Europe to Morocco (Peltola 1991). The authors found a statistically significant reduction in ETEC diarrhoea (RR 0.48, 95% CI 0.26 to 0.90; one trial, 615 participants, Analysis 1.1) and all‐cause diarrhoea (RR 0.77, 95% CI 0.59 to 1.00; one trial, 615 participants, Analysis 1.3).
1.3. Analysis.
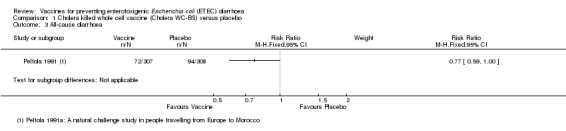
Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 3 All‐cause diarrhoea.
Safety
Safety data were only available from 508 participants in Peltola 1991. 'Gastrointestinal symptoms' were higher in those receiving the placebo than the vaccine during the first three days after vaccination (P = 0.03, Analysis 1.4). No other significant reactogenicity was observed.
1.4. Analysis.
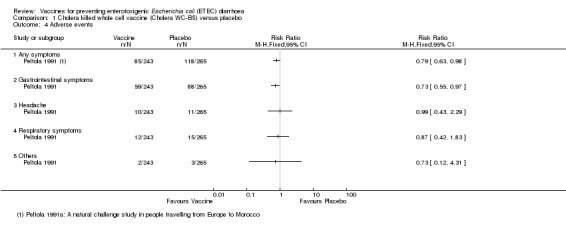
Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 4 Adverse events.
Immunological response
The studies did not report on the outcome of immunological response.
Analysis 2: Cholera killed whole cells plus recombinant B‐subunit (Cholera WC‐rCTB; Dukoral®)
Clinical efficacy
The currently available oral Cholera WC‐rCTB vaccine was evaluated in a single RCT in people arriving in Mexico from the USA (Scerpella 1995). There were no statistically significant differences in episodes of either ETEC‐specific diarrhoea or all‐cause diarrhoea between those receiving vaccine and placebo (one trial, 502 participants, Analysis 2.1; Analysis 2.2). However, in this trial the vaccine was only administered after arrival in Mexico and most episodes of diarrhoea occurred before completion of the two dose regimen or within 7 days of the second dose.
2.1. Analysis.
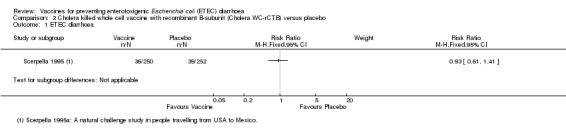
Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 1 ETEC diarrhoea.
2.2. Analysis.
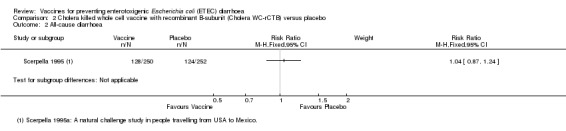
Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 2 All‐cause diarrhoea.
The authors of this paper considered that adequate protection would not be attained until seven days after the second dose. They reported a 50% protective effect in a subgroup analysis of cases occurring after this timepoint (95% CI 14 to 71%, authors' own figures). However, it should be noted that this subgroup analysis excluded 75% of the observed cases of ETEC diarrhoea. Only 19 episodes of ETEC diarrhoea were included (12 with placebo and seven with vaccine), and our re‐analysis of this data suggested that this difference was not statistically significant (Analysis 2.3).
2.3. Analysis.
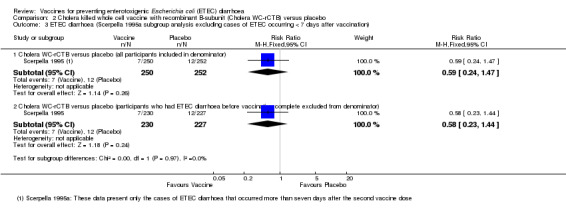
Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 3 ETEC diarrhoea (Scerpella 1995a subgroup analysis excluding cases of ETEC occurring < 7 days after vaccination).
Safety
Scerpella 1995 reported that there were no differences in the frequency of gastrointestinal symptoms, headache, or febrile illnesses between people that received either the vaccine or placebo, but data were not presented.
Immunological response
Toxin‐specific IgG antibody (TSA) responses were available from 281 participants. A greater than four‐fold increase was observed in 87% of the participants who received Cholera WC‐rCTB vaccine compared to 8% in controls (RR 10.54, 95% CI 6.11 to 18.20; one trial, 281 participants, Analysis 2.4).
2.4. Analysis.

Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 4 Immunological response: > 4‐fold increase in toxin‐specific IgG antibody responses in serum/plasma.
ETEC killed whole cell vaccines versus placebo
Analysis 3: ETEC killed whole cells plus recombinant cholera B‐subunit (ETEC WC‐rCTB)
Clinical efficacy
Two studies have evaluated this oral ETEC vaccine (ETEC WC‐rCTB); in people travelling from the USA to Mexico or Guatemala (Sack 2007), and from Austria to one of 44 different countries in Latin America, Africa, or Asia (Wiedermann 2000). There were no statistically significant differences in ETEC‐specific diarrhoea, or all‐cause diarrhoea (two trials, 799 participants, Analysis 3.1; Analysis 3.3).
3.1. Analysis.
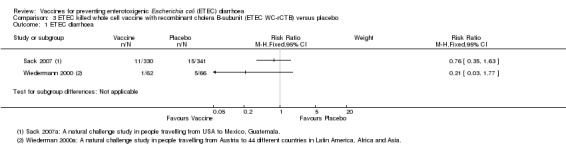
Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 1 ETEC diarrhoea.
3.3. Analysis.
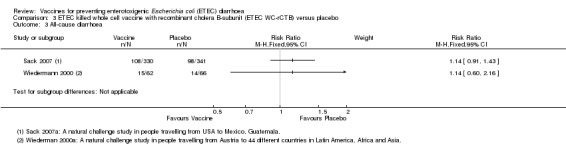
Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 3 All‐cause diarrhoea.
In Sack 2007 a small number of severe ETEC episodes are recorded (two in the vaccine group and nine in the placebo group), and this difference approached statistical significance (RR 0.23, 95% CI 0.05 to 1.05; one trial, 671 participants, Analysis 3.2).
3.2. Analysis.
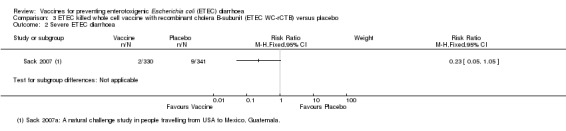
Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 2 Severe ETEC diarrhoea.
Safety
A total of 1695 participants have received ETEC WC‐rCTB or placebo in 11 RCTs. Vomiting was the only symptom significantly more common in those receiving the vaccine compared to placebo (RR 2.0, 95% CI 1.16 to 3.45; nine trials, 1528 participants, Analysis 3.4).
3.4. Analysis.
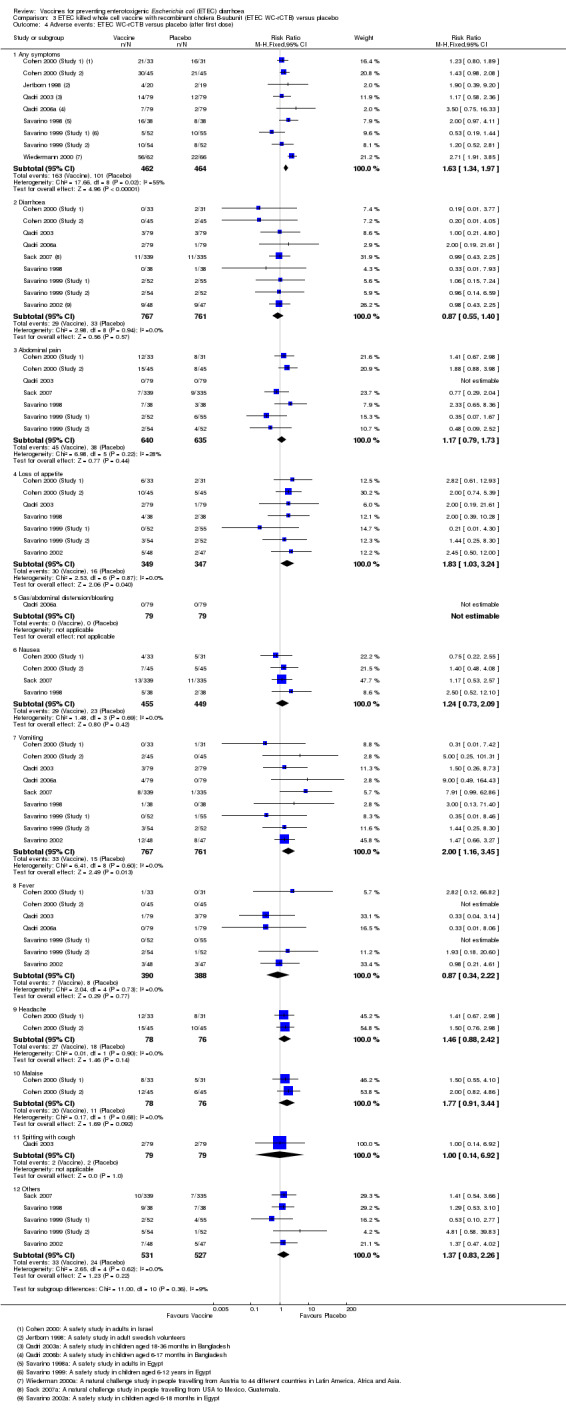
Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 4 Adverse events: ETEC WC‐rCTB versus placebo (after first dose).
Immunological response
CFA/I‐specific antibody response:
Anti‐CFA/I antibody responses were evaluated in 880 participants in 12 RCTs. CFA/I‐specific IgA antibody responses were evaluated in serum, plasma, or antibody secreting cells (ASCs). They were found to be statistically higher in the vaccine group compared to controls (RR 6.78, 95% CI 5.12 to 8.98, P < 0.00001; 12 trials, 880 participants, Analysis 3.5). In individual studies, the proportion of participants with a greater than two‐fold increase following vaccination ranged from: 26% to 94% in adults; 96% to 100% in children aged between 6 to 12 years; 73% to 95% in children aged between 18 months to 5 years; and 59% to 61% in infants aged between 6 to 18 months (Table 6).
3.5. Analysis.
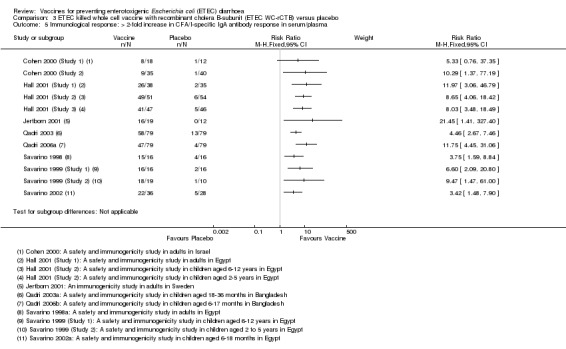
Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 5 Immunological response: > 2‐fold increase in CFA/I‐specific IgA antibody response in serum/plasma.
5. Additional immunological data for responses to CFs in the IgA isotype to ETEC WC‐rCTB vaccine.
| Study ID | Age group | Trial setting | Number of participants with a > 2‐fold increase in immunological response after the second dose of oral ETEC‐rCTB (%) | ||||||||||
| CFA/I | CS1 | CS2 | CS3 | CS4 | Remarks | ||||||||
| V | P | V | P | V | P | V | P | V | P | ||||
| Savarino 1998 | Adults | Egypt | 15/16 (94%) |
4/16 (25%) |
11/16 (69%) |
1/16 (6%) |
13/16 (81%) |
2/16 (13%) |
ND | ND | 16/16 (100%) |
6/16 (38%) |
Serum |
| Cohen 2000 (Study 2) | Adults | Israel | 9/35 (26%) |
1/40 (3%) |
11/35 (31%) |
2/40 (5%) |
ND | ND | ND | ND | ND | ND | Serum |
| Jertborn 2001 | Adults | Sweden | 16/19 (84%) |
0/12 (0%) |
4/19 (21%) |
1/12 (8%) |
10/19 (53%) |
0/12 (0%) |
ND | ND | 12/19 (63%) |
0/12 (0%) |
Serum |
| Hall 2001 (Study 1) | Adults | Egypt | 26/38 (68%) |
2/35 (6%) |
21/38 (56%) |
0/35 (0%) |
12/38 (31%) |
2/35 (6%) |
ND | ND | 26/38 (69%) |
4/35 (12%) |
Serum |
| Savarino 1999 (Study 1) | Children 6‐12 Y |
Egypt | 16/16 (100%) |
2/16 (13%) |
3/8 (38%) |
1/9 (11%) |
12/13 (92%) |
2/12 (17%) |
ND | ND | 14/15 (93%) |
4/16 (25%) |
ASC |
| Hall 2001 (Study 2) | Children 6‐12 Y |
Egypt | 49/51 (96%) |
6/54 (11%) |
47/51 (92%) |
4/54 (7%) |
40/51 (78%) |
5/54 (9%) |
ND | ND | 43/51 (84%) |
3/54 (6%) |
Serum |
| Savarino 1999 (Study 2) | Children 2‐5 Y |
Egypt | 18/19 (95%) |
1/10 (10%) |
ND | ND | 15/18 (83%) |
1/10 (10%) |
ND | ND | ND | ND | ASC |
| Hall 2001 (Study 3) | Children 2‐5 Y |
Egypt | 41/47 (87%) |
5/46 (11%) |
43/47 (91%) |
1/46 (2%) |
37/47 (79%) |
6/46 (12%) |
ND | ND | 33/47 (70%) |
3/46 (7%) |
Serum |
| Qadri 2003 | Children 18‐36 M |
Bangladesh | 58/79 (73%) |
13/79 (16%) |
61/79 (77%) |
10/79 (13%) |
69/79 (87%) |
8/79 (10%) |
ND | ND | 55/79 (70%) |
2/79 (3%) |
Plasma |
| Savarino 2002 | Children 6‐18 M |
Egypt | 22/36 (61%) |
5/28 (18%) |
7/36 (20%) |
1/28 (4%) |
9/36 (26%) |
1/28 (4%) |
ND | ND | 14/36 (39%) |
2/28 (7%) |
Serum |
| Qadri 2006a | Children 6‐17 M |
Bangladesh | 47/79 (59%) |
4/79 (5%) |
53/79 (67%) |
26/79 (33%) |
37/79 (47%) |
16/79 (20%) |
40/79 (50%) |
12/79 (15%) |
ND | ND | Plasma |
V = vaccine, P = placebo, CS = colonization surface antigen, ND = not done , ASC = antibody secreting cell, CFA = colonization factor antibody
CF‐specific IgA antibody responses were also reported to anti‐CS1 (10 trials), anti‐CS2 (10 trials), anti‐CS3 (one study), and anti‐CS4 (eight trials). These data are summarized in Table 6.
Toxin‐specific antibody response:
Either CT or LT toxin‐specific IgA antibody responses were evaluated in a total of 1228 participants in 13 RCTs. In individual studies, the percentage of participants with > two‐fold increases in toxin‐specific antibodies ranged from 50% to 100% in those receiving the vaccine compared to 0% to 33% in controls (RR 5.03, 95% CI 4.25 to 5.96, P < 0.00001; 13 trials, 1228 participants, Analysis 3.6).
3.6. Analysis.
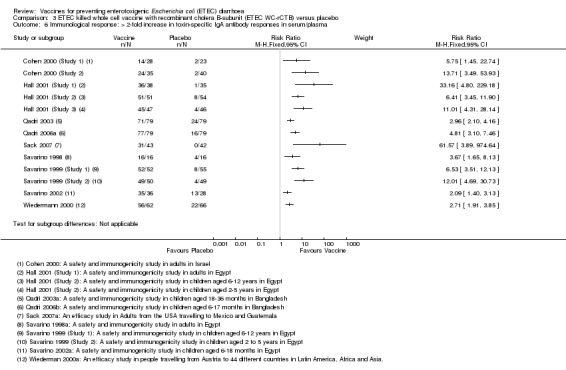
Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 6 Immunological response: > 2‐fold increase in toxin‐specific IgA antibody responses in serum/plasma.
Live attenuated vaccines versus placebo
Two live attenuated vaccines have been evaluated in placebo controlled trials: the oral cholera vaccine CVD 103‐HgR in a natural challenge study in travellers (Leyten 2005) and the oral ETEC vaccine PTL‐003 in a small artificial challenge study (McKenzie 2008).
Analysis 4: Live attenuated cholera vaccine (CVD 103‐HgR)
Clinical efficacy
Leyten 2005 evaluated CVD 103‐HgR, a live oral cholera vaccine, in Dutch volunteers intending to travel to Indonesia, Thailand, the Indian subcontinent, or West Africa. This study reported no significant differences in ETEC diarrhoea, severe ETEC diarrhoea, or all‐cause diarrhoea (one trial, 134 participants, Analysis 4.1; Analysis 4.2; Analysis 4.3).
4.1. Analysis.
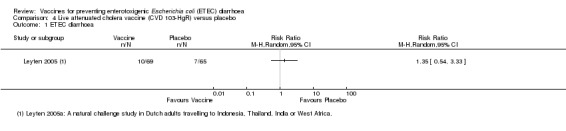
Comparison 4 Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo, Outcome 1 ETEC diarrhoea.
4.2. Analysis.
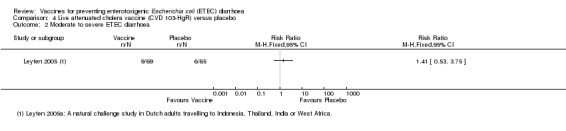
Comparison 4 Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo, Outcome 2 Moderate to severe ETEC diarrhoea.
4.3. Analysis.
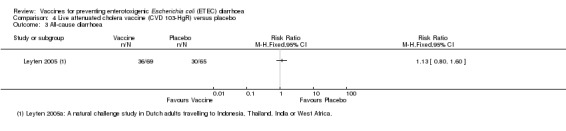
Comparison 4 Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo, Outcome 3 All‐cause diarrhoea.
Safety
This outcome was not reported.
Immunological response
This outcome was not reported.
Analysis 5: Live attenuated ETEC vaccine (PTL‐003)
Clinical efficacy
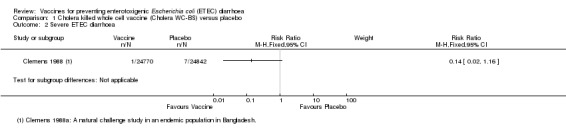
McKenzie 2008 evaluated PTL‐003, a live attenuated ETEC‐specific vaccine, in a small artificial challenge study in North American volunteers. The authors reported no statistically significant reduction in ETEC diarrhoea (one trial, 33 participants, Analysis 5.1; Analysis 5.2).
5.1. Analysis.
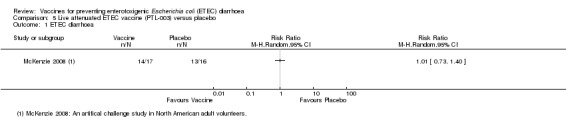
Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 1 ETEC diarrhoea.
5.2. Analysis.
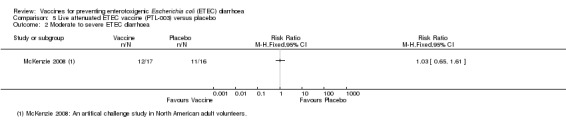
Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 2 Moderate to severe ETEC diarrhoea.
Safety
McKenzie 2008 reported safety data. No statistically significant differences in adverse events were observed between vaccine and control groups (one trial, 33 participants, Analysis 5.3).
5.3. Analysis.
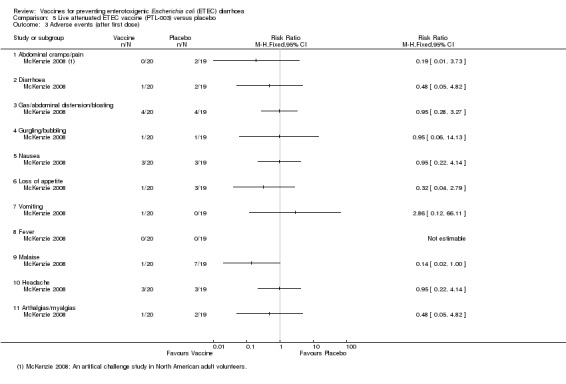
Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 3 Adverse events (after first dose).
Immunological response
McKenzie 2008 reported the proportion of participants with a > two‐fold increase in CF‐specific antibody responses against CS1 and CS3 and found significantly higher IgA titres in vaccinees compared to controls (see Table 7). There were no significant differences in toxin‐specific antibody responses (Analysis 5.4).
6. Additional immunological data for IgA response to CFs to live oral attenuated vaccine.
| Study ID | Number of participants with > 2‐fold increase in immunological response (%) | ||||||||
| CS1 | CS2 | CS3 | CS4 | Remarks | |||||
| V | P | V | P | V | P | V | P | ||
| McKenzie 2008 | 7/17 (41%) |
1/16 (6%) |
ND | ND | 7/17 (41%) |
0/16 (0%) |
ND | ND | Serum |
V = vaccine, P = placebo, CS = colonization surface antigen, ND = not done
5.4. Analysis.
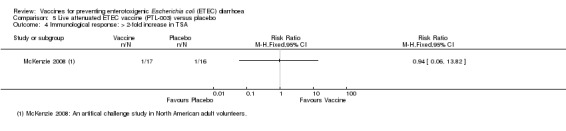
Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 4 Immunological response: > 2‐fold increase in TSA.
Transcutaneous vaccines versus placebo
Analysis 6: Transcutaneous LT patch
Clinical efficacy
An LT‐ETEC vaccine delivered via a transcutaneous patch was evaluated in one natural challenge study in American adults intending to travel to Mexico and Guatemala (Frech 2008) and in one artificial challenge study in North American volunteers (McKenzie 2007). No statistically significant differences were reported for ETEC diarrhoea, severe ETEC diarrhoea, or all‐cause diarrhoea (two trials, 217 participants, Analysis 6.1; Analysis 6.2; Analysis 6.3).
6.1. Analysis.
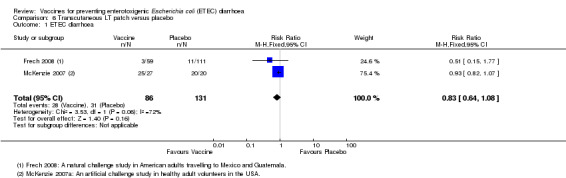
Comparison 6 Transcutaneous LT patch versus placebo, Outcome 1 ETEC diarrhoea.
6.2. Analysis.
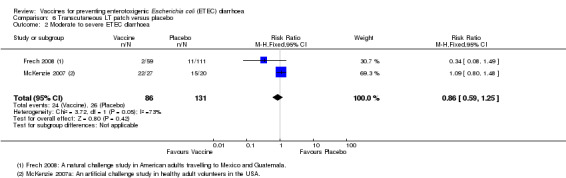
Comparison 6 Transcutaneous LT patch versus placebo, Outcome 2 Moderate to severe ETEC diarrhoea.
6.3. Analysis.
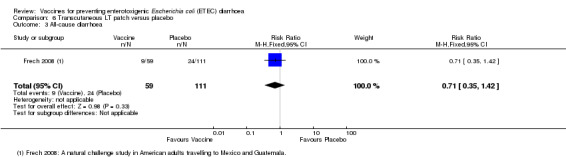
Comparison 6 Transcutaneous LT patch versus placebo, Outcome 3 All‐cause diarrhoea.
Safety
A total of 260 participants from two studies were evaluated for safety data, particularly regarding reactogenicity at the application site and other systemic adverse events (McKenzie 2007; Frech 2008). A significantly higher number of local immune reactions in the form of rash (P < 0.00001), pruritus (P < 0.00001), and skin discolouration (P = 0.0003) were observed in people that received the vaccine compared to placebo recipients (Analysis 6.4). For other events, there were no significant differences (Analysis 6.4).
6.4. Analysis.
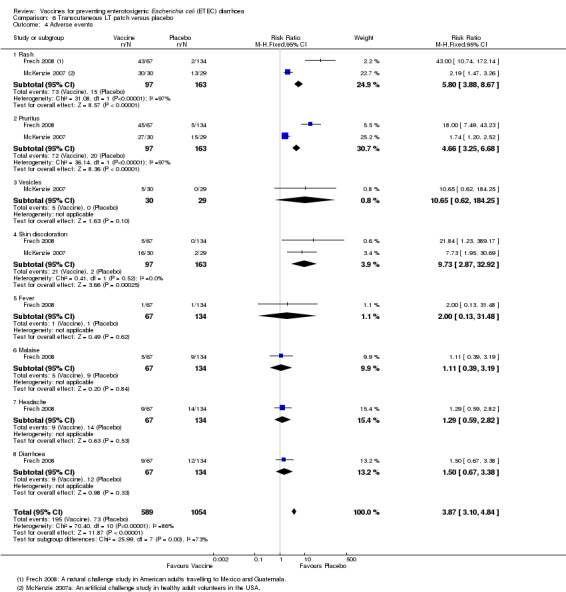
Comparison 6 Transcutaneous LT patch versus placebo, Outcome 4 Adverse events.
Immunological Response
Frech 2008 and McKenzie 2007 reported a > four‐fold increase in toxin‐specific IgA antibody responses in 82% and 93% of people vaccinated, respectively (RR 43.0, 95% CI 12.33 to 149.97, P < 0.00001; two trials, 217 participants, Analysis 6.5).
6.5. Analysis.
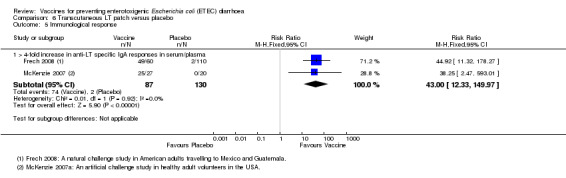
Comparison 6 Transcutaneous LT patch versus placebo, Outcome 5 Immunological response.
Passive immunization versus placebo
Analysis 7: Hyperimmune anti‐ETEC CFA
Clinical efficacy
Two artificial challenge studies reported passive immunization using bovine hyperimmune anti‐ETEC CFA in North American volunteers (Freedman 1998; Tacket 1999). The authors did not find any significant protective efficacy against all‐cause diarrhoea (two trials, 45 participants, Analysis 7.1).
7.1. Analysis.
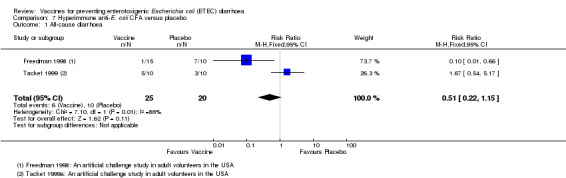
Comparison 7 Hyperimmune anti‐E. coli CFA versus placebo, Outcome 1 All‐cause diarrhoea.
Safety
A total of 45 participants from the studies by Freedman 1998 and Tacket 1999 were evaluated for safety data. A significantly higher number of events occurred in people that were vaccinated compared to those that received a placebo regarding the events of anorexia (P = 0.01) and abdominal pain (P = 0.003) (Analysis 7.2). For other events, there were no significant differences between people that were vaccinated and those that received a placebo (Analysis 7.2).
7.2. Analysis.
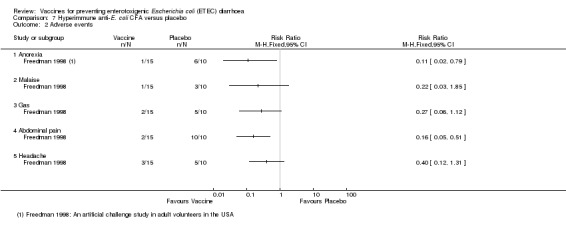
Comparison 7 Hyperimmune anti‐E. coli CFA versus placebo, Outcome 2 Adverse events.
Immunological Response
No immunological data were reported.
Discussion
Summary of main results
In this review, we included 24 trials and 53,247 participants. Four studies assessed the protective efficacy of oral cholera vaccines when used to also prevent diarrhoea due to ETEC and eight trials assessed the protective efficacy of ETEC‐specific vaccines.
Cholera vaccines
A single RCT evaluated the currently available oral cholera killed whole cell vaccine (Dukoral®) for protection against 'travellers' diarrhoea' in people arriving in Mexico from the USA. There were no statistically significant effects on ETEC diarrhoea or all‐cause diarrhoea (one trial (Scerpella 1995), 502 participants, low quality evidence).
Two earlier trials, one in an endemic population in Bangladesh (Clemens 1988) and one in travellers from Finland to Morocco (Peltola 1991), evaluated a precursor of this vaccine containing purified cholera toxin B subunit, rather than the recombinant subunit in Dukoral®. Short term protective efficacy against ETEC diarrhoea was demonstrated lasting for around three months (two trials, 50,227 participants). This vaccine is no longer available.
ETEC vaccines
An ETEC‐specific killed whole cell vaccine, also containing the recombinant cholera toxin B‐subunit, was evaluated in people travelling from the USA to Mexico or Guatemala (Sack 2007), and from Austria to Latin America, Africa, or Asia (Wiedermann 2000). There were no statistically significant differences in ETEC‐specific diarrhoea or all‐cause diarrhoea (two trials, 799 participants) and the vaccine was associated with increased vomiting (nine trials, 1528 participants) (Cohen 2000 (Study 1); Cohen 2000 (Study 2); Qadri 2003; Qadri 2006a; Sack 2007; Savarino 1998; Savarino 1999 (Study 1); Savarino 1999 (Study 2); Savarino 2002). The other ETEC‐specific vaccines in development have not yet demonstrated clinically important benefits.
Overall completeness and applicability of evidence
The use of the oral cholera WC‐rCTB vaccine for preventing 'travellers' diarrhoea' has been based on the findings of two trials that demonstrated some short term protection against ETEC diarrhoea (Clemens 1988; Peltola 1991). The vaccine used in both of these trials contained 1 mg of purified cholera B‐subunit, rather than the recombinant B‐subunit in the current vaccine. It should be noted that the cholera B‐subunit is the only element of this vaccine which could be expected to induce immunity to ETEC. These two vaccines were directly compared in a single trial of 41 Swedish volunteers (Jertborn 1992), which reported comparable cholera‐specific antibody responses but did not evaluate either clinical or immunological protection against ETEC. The finding of limited protective benefit with the cholera WC‐rCTB vaccine (Scerpella 1995) is supported by two further trials evaluating the ETEC WC‐rCTB vaccine which contains the same recombinant cholera B‐subunit, and also found no evidence of clinical protection in travellers (Wiedermann 2000; Sack 2007). These three trials raise concerns that the earlier findings may not be applied to the current vaccine.
In addition, the large study from Bangladesh (Clemens 1988), which contributed over 90% of participants included in this review, aimed primarily to assess the protective efficacy against cholera not ETEC. The assessment of protective efficacy against ETEC therefore represented a post‐hoc analysis. This trial was conducted among an endemic population who were likely to have acquired some natural immunity against ETEC. The results of this study may therefore be poorly applicable to travellers.
The ETEC‐specific vaccines are now primarily being designed for use in developing country settings for prevention of ETEC diarrhoea in infants and young children, although protection of travellers remains important (Holmgren 2012). Promising CFs and toxin‐specific immune responses to the ETEC WC‐rCTB vaccine have been observed. However, following failure to demonstrate clinical protective efficacy and safety concerns, further pre‐clinical development of this vaccine is underway (Tobias 2012). Several additional vaccine candidates not included in this review are currently at early stages of development. In Sweden, an oral inactivated tetravalent ETEC vaccine alone or together with double mutant heat labile toxin (dmLT) adjuvant is undergoing testing in Phase I/II studies. In the USA an oral live attenuated three strain recombinant ETEC vaccine, ACE527, is undergoing testing with plans for moving field sites in developing countries (Darsley 2012).
Quality of the evidence
The quality of the evidence for the oral cholera vaccine (Dukoral®) was assessed using the GRADE approach. Clinically important benefits of this vaccine have not yet been demonstrated and the quality of this evidence was downgraded to 'low'. This means that use of this vaccine may have little or no difference in preventing ETEC diarrhoea but further research may change this result. The quality was downgraded due to concerns about the applicability of the evidence. Most cases of ETEC diarrhoea occurred prior to completion of the vaccine schedule (indirectness) and the sample size was small (imprecision) (Table 1).
Agreements and disagreements with other studies or reviews
A previous review of vaccination to prevent ETEC diarrhoea concluded that the protective effect of Dukoral® was up to 43% and that it should be recommended for travellers (Jelinek 2008). However, this conclusion was based predominantly on positive findings from the older trials assessing prototypes of the Dukoral® vaccine, on subgroup analyses which may or may not have been pre‐planned, or on the findings of non‐randomized retrospective studies.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence from RCTs to support the use of the oral cholera vaccine (Dukoral®) for protecting travellers against ETEC diarrhoea.
Implications for research.
Further research is needed to develop safe, immunogenic, and effective vaccines to provide both short and long term protection against ETEC diarrhoea.
More studies are needed to evaluate the efficacy of new and candidate vaccines for safety and immunogenicity in naive adult travellers, and exposed and primed populations in a developing country setting where children and infants will be the major targets for future vaccination. ETEC vaccine development needs to include plans for overcoming barriers to oral vaccination in children. Also, strategies are needed to deliver the vaccines using the existing national immunization system of these countries, including the EPI, the cold chain facilities, and other national health facilities. In addition, the use of different modes of delivery of vaccines (including use of mucosal adjuvants) needs to be studied to improve immunogenicity and efficacy of ETEC vaccines.
Acknowledgements
The academic editor for this review was Dr Patricia Graves.
The editorial base for the Cochrane Infectious Disease Group is funded by the Department for International Development (DFID), UK, for the benefit of developing countries. This work was also supported by the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) and by funding from the Effective Health Research Consortium, and a grant from the Department for International Development, UK.
Data and analyses
Comparison 1. Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ETEC diarrhoea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Severe ETEC diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 All‐cause diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Any symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Gastrointestinal symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Respiratory symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Others | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ETEC diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 All‐cause diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 ETEC diarrhoea (Scerpella 1995a subgroup analysis excluding cases of ETEC occurring < 7 days after vaccination) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cholera WC‐rCTB versus placebo (all participants included in denominator) | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.47] |
| 3.2 Cholera WC‐rCTB versus placebo (participants who had ETEC diarrhoea before vaccination complete excluded from denominator) | 1 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.44] |
| 4 Immunological response: > 4‐fold increase in toxin‐specific IgG antibody responses in serum/plasma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ETEC diarrhoea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Severe ETEC diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 All‐cause diarrhoea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Adverse events: ETEC WC‐rCTB versus placebo (after first dose) | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Any symptoms | 9 | 926 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.34, 1.97] |
| 4.2 Diarrhoea | 9 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.40] |
| 4.3 Abdominal pain | 7 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.79, 1.73] |
| 4.4 Loss of appetite | 7 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.03, 3.24] |
| 4.5 Gas/abdominal distension/bloating | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Nausea | 4 | 904 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.73, 2.09] |
| 4.7 Vomiting | 9 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.16, 3.45] |
| 4.8 Fever | 7 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.34, 2.22] |
| 4.9 Headache | 2 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.88, 2.42] |
| 4.10 Malaise | 2 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.91, 3.44] |
| 4.11 Spitting with cough | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.92] |
| 4.12 Others | 5 | 1058 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.83, 2.26] |
| 5 Immunological response: > 2‐fold increase in CFA/I‐specific IgA antibody response in serum/plasma | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Immunological response: > 2‐fold increase in toxin‐specific IgA antibody responses in serum/plasma | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 4. Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ETEC diarrhoea | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Moderate to severe ETEC diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 All‐cause diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Live attenuated ETEC vaccine (PTL‐003) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ETEC diarrhoea | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Moderate to severe ETEC diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events (after first dose) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Abdominal cramps/pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Gas/abdominal distension/bloating | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Gurgling/bubbling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Loss of appetite | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Malaise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Arthalgias/myalgias | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immunological response: > 2‐fold increase in TSA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Transcutaneous LT patch versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ETEC diarrhoea | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| 2 Moderate to severe ETEC diarrhoea | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.25] |
| 3 All‐cause diarrhoea | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.42] |
| 4 Adverse events | 2 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.87 [3.10, 4.84] |
| 4.1 Rash | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.80 [3.88, 8.67] |
| 4.2 Pruritus | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [3.25, 6.68] |
| 4.3 Vesicles | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.65 [0.62, 184.25] |
| 4.4 Skin discoloration | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [2.87, 32.92] |
| 4.5 Fever | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.13, 31.48] |
| 4.6 Malaise | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.39, 3.19] |
| 4.7 Headache | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.82] |
| 4.8 Diarrhoea | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.67, 3.38] |
| 5 Immunological response | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 > 4‐fold increase in anti‐LT specific IgA responses in serum/plasma | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 43.00 [12.33, 149.97] |
Comparison 7. Hyperimmune anti‐E. coli CFA versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause diarrhoea | 2 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.22, 1.15] |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Malaise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Gas | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Clemens 1988.
| Methods | Study type: Randomized, natural challenge, efficacy study in an endemic population Trial dates and duration: From January 1985 to May 1986 Surveillance: Surveillance for diarrhoea was done at treatment centres serving the study participants at Matlab for 365 post‐vaccination days |
|
| Participants | Number of participants: 49,612 Inclusion criteria: People aged between 2 to 15 years of age and female subjects > 15 years of age residing in Matlab Exclusion criteria: Persons who were absent or refused to participate, pregnant, or suffering from any other illness |
|
| Interventions | Vaccine: Cholera toxin B subunit plus killed cholera whole cells (BS‐WC) Control: Killed cholera whole cells (WC) Additional details: In this study participants received 3 doses of vaccine at 6 weeks apart, of BS‐WC vaccine, WC vaccine only, or an E. coli K12 strain placebo. However, protective efficacy was calculated based on WC vaccine as control and BS‐WC as study intervention group, because the killed cholera whole cells, which were identical for the BS‐WC and WC vaccines, were not anticipated to have any protective effects against LT‐ETEC |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Matlab, Bangladesh Setting: Three different treatment centres at Matlab, a rural setting of Bangladesh Source of funding: US agency for International Development, the Government of Japan, the Swedish agency for Research Cooperation with Developing Countries and the World Health Organization (WHO) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After computerisation of the census, we assigned every person in the eligible age‐gender categories to letters A, B or C, using simple randomisation" (from additional paper describing this study). |
| Allocation concealment (selection bias) | Low risk | "The agents were identified only by the letters A, B and C" (from an additional paper describing this study). Allocation concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "During the conduct of the study, the identities of these letter...were unknown to all persons connected with the trial in Bangladesh". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "During the conduct of the study, the identities of these letter...were unknown to all persons connected with the trial in Bangladesh". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up were not clearly described. |
| Selective reporting (reporting bias) | High risk | This was a three arm study. It was unclear why the group given the cholera WC vaccine was selected as the control arm rather than the group given a placebo. |
| Other bias | Low risk | None identified. |
Cohen 2000 (Study 1).
| Methods | Study type: Randomized safety and immunogenicity study in volunteers Trial dates and duration: Between May 22 and July 10 1995 |
|
| Participants | Number of participants: 65 Inclusion criteria: Healthy men and women and were recruited among the School of Military Medicine cadets or the Medical Corps Headquarters staff. Exclusion criteria: Not described. |
|
| Interventions | Vaccine: Contained 1.0 mg of rCTB plus a final count of ∼1011 formalin‐inactivated bacteria. Each vaccine dose included the following inactivated ETEC strains: SBL 101 (O78, CFA/I, LT2/ST1), SBL 106 (O6, CS1, LT2/ST2), SBL 107 (OR, CS2, CS3, LT2/ST2), SBL 104 (O25, CS41CS6, LT2/ST2) and SBL 105 (O167, CS51CS6, LT2/ST2). Placebo: Heat‐killed E. coli K12 with an optical density (OD) equivalent to that of the ETEC vaccine, was administered in the same buffered solution as the vaccine. Additional details: Each dose of lot E003 was given in 150 mL of water with a raspberry‐flavoured bicarbonate‐citric acid buffer containing 4 g of sodium bicarbonate per dose (Recip AB, Stockholm, Sweden). |
|
| Outcomes |
Included in review:
Not included in the review:
|
|
| Notes | Location: Israel Setting: Israel Defence Force (IDF), Medical Corps, Army Health Branch Research Unit, and the IDF, Medical Corps, School of Military Medicine Source of funding: US Army Medical Research & Material Command (DAMD 17‐93‐V‐3001) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Group randomization was used so that each group was assigned two letters, and each volunteer was openly allotted to one of the four resulting letter groups". It is unclear if this method was truly random. |
| Allocation concealment (selection bias) | Low risk | "Each volunteer was openly allotted to one of the four resulting letter groups. The association between a letter group and a vaccine/placebo group was determined by a third party and was kept locked from both volunteers and investigators for the duration of the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo preparation, containing a suspension of heat‐killed E. coli K12 with an optical density (OD) equivalent to that of the ETEC vaccine, was administered in the same buffered solution as the vaccine". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were low: 3 out of 33 (9%) in the vaccine group and 2 out of 31 (6%) in controls either dropped out of the study or were not given the second dose. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Cohen 2000 (Study 2).
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Between April 1 and June 18 1997 |
|
| Participants | Number of participants: 90 Inclusion criteria: Healthy men and women and were recruited among the School of Military Medicine cadets or the Medical Corps Headquarters staff Exclusion criteria: Not mentioned |
|
| Interventions | Vaccine: Contained 1.0 mg of rCTB plus a final count of ∼1011 formalin‐inactivated bacteria. Each vaccine dose included the following inactivated ETEC strains: SBL 101 (O78, CFA/I, LT2/ST1), SBL 106 (O6, CS1, LT2/ST2), SBL 107 (OR, CS2, CS3, LT2/ST2), SBL 104 (O25, CS41CS6, LT2/ST2) and SBL 105 (O167, CS51CS6, LT2/ST2) Placebo: Heat‐killed E. coli K12 with an optical density (OD) equivalent to that of the ETEC vaccine, was administered in the same buffered solution as the vaccine Additional details: Each dose of lot E005 was given in 150 mL of water with a raspberry‐flavoured bicarbonate‐citric acid buffer containing 4 g of sodium bicarbonate per dose (Recip AB, Stockholm, Sweden) |
|
| Outcomes |
Included in review:
Not included in the review:
|
|
| Notes | Location: Israel Setting: IDF, Medical Corps, Army Health Branch Research Unit, and the IDF, Medical Corps, School of Military Medicine Source of funding: US Army Medical Research & Material Command (DAMD 17‐93‐V‐3001) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A blocked randomization scheme was constructed off‐site". |
| Allocation concealment (selection bias) | Low risk | "Subjects were assigned a unique participant identification number (101 to 190) at the time of the first dose and received the correspondingly labelled study agent". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo preparation, containing a suspension of heat‐killed E. coli K‐12 with an optical density (OD) equivalent to that of the ETEC vaccine, was administered in the same buffered solution as the vaccine". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigation team remained blinded until all safety and immunogenicity data were generated, computerized, cleaned, and locked". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up were moderate: 8 out of 45 (18%) in the vaccine group and 4 out of 45 (9%) in controls either dropped out of the study or were not given the second dose. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Frech 2008.
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: May 2006 to February 2007 Surveillance: Surveillance was conducted on US travellers who visited to Mexico and Guatemala |
|
| Participants | Number of participants: 201 Inclusion criteria: Healthy adults aged 18 to 64 years, who planned to travel to Cuernavaca, Guadalajara, San Miguel, or Cancun (Mexico), or Antigua (Guatemala) and who had access to one of the 14 US regional vaccination centres Exclusion criteria: History of travellers' diarrhoea and travelled to an endemic country in the previous 12 months, history of taking cholera, LT or ETEC vaccine, significant illness, immunosuppression or if female, pregnant, nursing, or unwilling to use effective form of any contraceptives |
|
| Interventions | Vaccine: LT patch; 37.5 µg of ETEC LT Placebo: All the excipients of LT patch without LT Additional details: Vaccinations with either an LT patch or placebo patch were given to alternate upper arms a minimum of 3 weeks (first vaccination) and 1 week (second vaccination) before departure |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Mexico and Guatemala Setting: University of Texas Health Science Center at Houston (Houston, TX, USA), Universidad Del Valle De Guatemala (Guatemala City, Guatemala), Inovamed Hospital (Cuernavaca, Mexico) and ViroMed Laboratory, Minnetonka, MN, USA Source of funding: IOMAI corporation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study used a web‐based, audit‐trail enabled, centralised randomisation code and allocation system". |
| Allocation concealment (selection bias) | Low risk | "Vaccination sites accessed a web page, entered participants into the system, and received unique patch numbers for every study participant". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Dose information was masked at allocation, as well as on primary and secondary product packaging. Participants and site staff, including those assessing study outcomes, remained masked until database lock". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up were high: 8 out 67 (12%) in the vaccine group and 23 out of 134 (17%) in the placebo group due to failure to: receive second dose of vaccine, provide diary cards, or attend in‐country visit. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Freedman 1998.
| Methods | Study type: Randomized, artifical challenge, efficacy study in volunteers Trial dates and duration: Not given Surveillance: Daily medical rounds were conducted to monitor symptoms during the 7 days of study period. Daily stool samples were taken for bacteriologic examination. Artificial challenge: On day 4 |
|
| Participants | Number of participants: 25 Inclusion criteria: Not described Exclusion criteria: Not described |
|
| Interventions | Vaccine: Each lyophilized dose containing hyperimmune anti‐E. coli bovine milk IgG dissolved in 150 mL of bicarbonate solution Placebo: A single dose of a lactose‐free infant formula Additional details: Three doses/day for 7 days, vaccine, or placebo were administered 15 minutes after meals Artificial challenge: 109 cfu of H10407 (O78:H11), a CFA/I‐bearing ETEC strain suspended in 1 ounce (30 mL) of water containing sodium bicarbonate |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Baltimore, MD, USA Setting: Center for Vaccine Development (University of Maryland School of Medicine) Source of funding: ImmuCell |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated and secured by ImmuCell’s Quality Assurance Supervisor". |
| Allocation concealment (selection bias) | Low risk | "by assigning subject identification numbers to identically packaged foil pouches containing measured doses of each test article". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All investigators and volunteers were blinded to these treatment group assignments throughout the study and during assessment of outcome". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Hall 2001 (Study 1).
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Subjects were randomly assigned to receive two doses of vaccine or placebo 2 weeks apart |
|
| Participants | Number of participants: 76 Inclusion criteria: Adults aged between 21 and 45 years from Benha, Qalyubia Governorate, Egypt Exclusion criteria: Not mentioned |
|
| Interventions | Vaccine: Each 4 mL vaccine dose (lot E003) contained 1 mg of rCTB plus a mixture of ∼2 x 1010 bacteria each of five strains individually expressing CFA/I, CS1, CS2 plus CS3, CS4, and CS5 Placebo: Each 4‐mL placebo dose contained ∼1011 heat‐killed E. coli K12 cells Additional details: Each dose was added to 150 mL of water containing 4 g of sodium bicarbonate plus 1.45 g of citric acid (Recip AB, Stockholm, Sweden) for adult administration. |
|
| Outcomes |
Included in review:
Not included in the review:
|
|
| Notes | Location: Egypt Setting: Benha, Qalyubia Governorate, Egypt Source of funding: Naval Medical Research and Development Command (B69000101. PIX3270), Intragency Agreement Y1‐HD‐0026‐01, the National Institute of Child Health and Human Development and WHO Global Programme for Vaccines and Immunization Research and Development. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After enrollment, subjects were randomized to receive vaccine or placebo in a double‐blind fashion within blocks of 4 sequentially randomized subjects" (Savarino 1998). It is unclear if this was truly random. |
| Allocation concealment (selection bias) | Low risk | "At the time of initial dosing, each subject was assigned a sequential number corresponding to sequentially numbered single‐dose vials of study agent" (Savarino 1998). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "double blind, placebo controlled", and vaccines labelled only with study code. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The code was broken after all clinical and laboratory evaluations were completed". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were low: 2/49 (4%) in the vaccine group and 2/48 (4%) in the placebo group. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Hall 2001 (Study 2).
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Subjects were randomly assigned to receive two doses of vaccine or placebo 2 weeks apart. |
|
| Participants | Number of participants: 107 Inclusion criteria: School children aged between 6 to 12 years old from Benha, Qalyubia Governorate, Egypt Exclusion criteria: Not mentioned |
|
| Interventions | Vaccine: Each 4 mL vaccine dose (lot E003) contained 1 mg of rCTB plus a mixture of ∼2 x 1010 bacteria each of five strains individually expressing CFA/I, CS1, CS2 plus CS3, CS4, and CS5. Placebo: Each 4 mL placebo dose contained ∼1011 heat‐killed E. coli K12 cells. Additional details: Each dose was added to 75 mL of water containing 4 g of sodium bicarbonate plus 1.45 g of citric acid (Recip AB, Stockholm, Sweden) for school children administration. |
|
| Outcomes |
Included in review:
Not included in the review:
|
|
| Notes | Location: Egypt Setting: Benha, Qalyubia Governorate, Egypt Source of funding: Naval Medical Research and Development Command (B69000101. PIX3270), Intragency Agreement Y1‐HD‐0026‐01, the National Institute of Child Health and Human Development and WHO Global Programme for Vaccines and Immunization Research and Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After enrollment, subjects were randomized to receive vaccine or placebo in a double‐blind fashion within blocks of 4 sequentially randomized subjects" (Savarino 1998). It is unclear if this was truly random. |
| Allocation concealment (selection bias) | Low risk | "At the time of initial dosing, each subject was assigned a sequential number corresponding to sequentially numbered single‐dose vials of study agent" (Savarino 1998). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "double blind, placebo controlled", and vaccines labelled only with study code. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The code was broken after all clinical and laboratory evaluations were completed". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Hall 2001 (Study 3).
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Subjects were randomly assigned to receive two doses of vaccine or placebo 2 weeks apart |
|
| Participants | Number of participants: 106 Inclusion criteria: Preschool children aged between 2 to 5 years old from Benha, Qalyubia Governorate, Egypt Exclusion criteria: Not mentioned |
|
| Interventions | Vaccine: Each 4 mL vaccine dose (lot E003) contained 1 mg of rCTB plus a mixture of ∼2 x 1010 bacteria each of five strains individually expressing CFA/I, CS1, CS2 plus CS3, CS4, and CS5 Placebo: Each 4 mL placebo dose contained ∼1011 heat‐killed E. coli K12 cells Additional details: Each dose was added to 37.5 mL of water containing 4 g of sodium bicarbonate plus 1.45 g of citric acid (Recip AB, Stockholm, Sweden) for adult administration |
|
| Outcomes |
Included in review:
Not included in the review:
|
|
| Notes | Location: Egypt Setting: Benha, Qalyubia Governorate, Egypt Source of funding: Naval Medical Research and Development Command (B69000101. PIX3270), Intragency Agreement Y1‐HD‐0026‐01, the National Institute of Child Health and Human Development and WHO Global Programme for Vaccines and Immunization Research and Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After enrollment, subjects were randomized to receive vaccine or placebo in a double‐blind fashion within blocks of 4 sequentially randomized subjects" (Savarino 1998). It is unclear if this was truly random. |
| Allocation concealment (selection bias) | Low risk | 'At the time of initial dosing, each subject was assigned a sequential number corresponding to sequentially numbered single‐dose vials of study agent" (Savarino 1998). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "double blind, placebo controlled", and vaccines labelled only with study code. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The code was broken after all clinical and laboratory evaluations were completed". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant was lost to follow‐up (from the placebo group). |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Jertborn 1998.
| Methods | Study type: Randomized, safety study in volunteers Study 1: Single blinded, placebo controlled, randomized study Study 2: Non‐placebo controlled study (data excluded) Study 3: Non‐placebo controlled study (data excluded) Trial dates and duration: Not mentioned in the article Surveillance: Study 1: 5 consecutive post vaccination days |
|
| Participants | Number of participants: 20 (vaccine) plus 20 (placebo) Inclusion criteria: Adult Swedish volunteers, aged between 20 to 50 years were recruited, with no history of travel to an endemic country for the past 6 months Exclusion criteria: Not mentioned |
|
| Interventions | Vaccine: One single dose of vaccine contained 1.0 mg of rCTB and 1011 formalin‐inactivated enterotoxigenic E. coli bacteria of each of the following strains: O78:H12‐CFA/I ST+, O25:H42‐ CS4+CS6, O167:H5‐CS5+CS6/ST+, O6:H16‐CS2+CS3, and O139:H28‐CS1 Placebo: One single dose of placebo consisted of 150 mL of a sodium bicarbonate solution (Samarin; Cederroths Nordic AB, Upplands Vasby, Sweden) The volunteers were instructed not to eat or drink (except water) for 1 hour before and after intake of the vaccine or placebo preparation |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Goteborg, Sweden Setting: University of Goteborg, Sweden Source of funding: Swedish Research Council (16X‐09084 and 16X‐3382), the Swedish Agency for Research Cooperation with Developing Countries, the WHO and the Medical Faculty, Goteborg University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized". No further details. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "single blind" but no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "single blind" but no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were low: 1 out of 20 in placebo group was excluded due to viral infection. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Jertborn 2001.
| Methods | Study type: Randomized, immunogenicity study in volunteers Study 1: Open study without any control group (excluded from the review)) Study 2: Double blinded, placebo controlled, randomized study Trial dates and duration: Not mentioned in the article Surveillance: Not described |
|
| Participants | Number of participants: Study 1: 36 (excluded from the review) Study 2: 31 Inclusion criteria: Adult Swedish volunteers, aged between 18 to 46 years were recruited, no history of travelling to ETEC endemic areas for 6 months prior to the study Exclusion criteria: Not mentioned |
|
| Interventions | Vaccine: Study 1: One 4 mL dose of vaccine (Lot 003) contained 1.0 mg of rCTB and 1011 formalin‐inactivated E. coli bacteria of each of the following strains: SBL101 (O78:H12; CFA/I ST1), SBL104 (O25:H42; CS4), SBL105 (O167:H5; CS5 ST1), SBL 106 (O6:H16; CS1), and SBL 107 (OR:H6; CS21 CS3) (Data not included in the review) Study 2: Different lot (Lot 005) of vaccine with same formulation except the half the amount of CFA/I and three times more CS2 than lot 003 was used in this study Placebo: The 4 mL placebo dose consisted of ∼1 ×1011 heat‐killed E. coli K12 bacteria Additional details: Each dose of a study agent was administered in 150 mL of a sodium bicarbonate solution (Samarin; Cederroths Nordic AB, Upplands Vasby, Sweden). The volunteers were instructed not to eat or drink (except water) for 1 hour before and after intake of the vaccine or placebo preparation. |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Goteborg, Sweden Setting: University of Goteborg, Sweden Source of funding: Swedish Research Council (16X‐09084), the Swedish Agency for Research Cooperation with Developing Countries, the WHO and the Medical Faculty, Goteborg University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as ‘randomized’, no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as ‘double blind, Placebo controlled" study, no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Leyten 2005.
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: May 1995 to February 1996 Surveillance: All participants kept a diary of their defecation pattern during their stay abroad. On return, they filled out a questionnaire concerning defecation pattern, use of medication and information regarding travel, accommodation, and dietary hygiene. Each participant submitted a stool specimen. Subjects who had experienced an episode of diarrhoea during travel collected a sample during the first diarrhoeal episode, prior to having taken any medication. The remaining travellers collected and submitted a sample within 3 days after returning home. |
|
| Participants | Number of participants: 145 Inclusion criteria: Dutch volunteers, travellers from the travellers clinics of the Leiden University Medical Centre (LUMC), the Netherlands, the Municipal Health Centre at Leiden and the Harbour Hospital at Rotterdam. All adults who were intending to travel to Indonesia, Thailand, the Indian subcontinent or West Africa (Gambia or Senegal) for a period of 1 to 4 weeks were invited to take part in the trial. Exclusion criteria: People suffering from acute or chronic inflammatory disease of the intestinal tract, prior recipients of WC‐BS cholera vaccine or CVD 103‐HgR vaccine, subjects receiving immunosuppressive drugs, persons known to be immunodeficient, subjects participating in other clinical trials women who were either pregnant or breast‐feeding |
|
| Interventions | Vaccine: CVD 103‐HgR, Single dose of 5 x 108 cfu of lyophilized CVD 103‐HgR live oral cholera vaccine Placebo: 5 x 108 heat‐killed E. coli K12 Additional details: Vaccine has been administered orally. Both vaccine and placebo are identical in appearance. |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: The Netherlands Setting: Leiden University Medical Center (LUMC) Source of funding: Berna Biotech AG, Bern, Switzerland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "For randomisation a computer‐generated randomisation list, was used". |
| Allocation concealment (selection bias) | Low risk | "Sachets and suspensions of vaccine and placebo that were identical in appearance, were labelled by a coded number from 1 to 200". Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The key to the coded sachets was stored at the hospital pharmacy in a sealed envelope. The envelope was only to be opened by the investigator in case of an emergency that required knowledge of the identity of the trial medication in order to manage the participant’s condition. At the end of the trial the coded envelope was returned to the Berna Biotech AG and checked to ensure that the seal had remained unbroken". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were low: in vaccine group 4/73 (5%) and in placebo group 7/72 (10 %). |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
McKenzie 2007.
| Methods | Study type: Randomized, artificial challenge, efficacy study in volunteers Trial dates and duration: September 15 2004 to June 30 2005 Surveillance: Post vaccination follow‐up on day 0, 7, 21, 28, 42, 49, and 77; Artificial challenge: on day 55; Post challenge follow‐up for 5 days |
|
| Participants | Number of participants: 59 Inclusion criteria: Healthy adults, between 18 to 45 years of age Exclusion criteria: Clinically significant medical conditions, history of traveller’s diarrhoea in the last 3 years and LT IgG titer > 2000 EU by ELISA |
|
| Interventions | Vaccine: 150 μL of saline containing 50 μg of LT Placebo: 150 μL of saline containing no LT Additional details: All participants were randomized to receive transcutaneous application of 3 doses saline containing LT or saline only, at an interval of 21 days Artificial challenge: 120 mL of sodium bicarbonate buffer containing 6 × 108 CFU of the challenge virulent strain of E. coli E24377A |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: USA Setting: Vaccine Testing Unit, the Center for Immunization Research (CIR), Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; IOMAI Corporation, Gaithersburg, MD, USA; Amarex Clinical Research, Germantown, MD, USA Source of funding: IOMAI Corporation, Gaithersburg, MD, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated by the statistician". |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double blind" but no details given of how this was done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "double blind" but no details given of how this was done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 out of 30 (7%) subjects from vaccine group and 4 out 29 (14%) of subjects from placebo group subsequently withdrew from the study before the inpatient challenge phase. However, once entered into the challenge phase there were no further drop‐outs. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
McKenzie 2008.
| Methods | Study type: Randomized, artificial challenge, efficacy study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Post vaccination follow‐up for 20 days Artificial challenge: On day 27; post challenge follow‐up for 5 days (passive using diary cards) |
|
| Participants | Number of participants: 39 Inclusion criteria: Healthy adults, 18 to 50 years of age Exclusion criteria: Not mentioned in the article |
|
| Interventions | Vaccine: PTL‐003 ( PTL‐003 was derived from the spontaneously toxin‐negative, O139:H28 ETEC strain E1392/75‐2A containing 2 × 109 cfu/mL live attenuated bacteria in 200 mL of CeraVacxTM buffer) Placebo: CereVacx buffer (CeraVacx, Cera Products Inc., Jessup, MD: rice solids, 7.0 g; sodium bicarbonate, 2 g; trisodium citrate, 0.5 g in 200 mL of water) Additional details: All participants were randomized to receive 2 doses, at an interval of 10 days Artificial challenge: 30 mL of sodium bicarbonate buffer containing 3 × 109 CFU of the challenge virulent strain of E. coli E24377 |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: USA Setting: Vaccine Testing Unit, the Center for Immunization Research (CIR), Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA Source of funding: Acambis Research Ltd., Cambridge, UK and by Johns Hopkins University School of Medicine General Clinical Research Center grant number M01‐RR00052 from the National Center for Research Resources, NIH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized", no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double blind", no further details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "double blind", no further details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were low (three participants in each group withdrew before the challenge phase), but as the trial was very small this represented 15% of all participants. However once they entered the challenge phase, there was no further drop‐outs. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Peltola 1991.
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: October 24 1989 to November 21 1989 Surveillance: Any diarrhoea during the trip period and immediately after returning back was recorded and stool samples were collected |
|
| Participants | Number of participants: 615 Inclusion criteria: Travellers from Finland to Morocco Exclusion criteria: Travellers aged less than 15 years and history of taking antimicrobials during the previous 7 days of vaccination days |
|
| Interventions | Vaccine: 1 x 1011 heat‐killed whole cells of V. cholerae with 1 mg of the B‐subunit of cholera toxin Placebo: E. coli K12 Additional details: Two doses of vaccine or placebo identical in appearance, 3 and 1 week before the departure were administered |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Finland and Morocco Setting: Enteric Laboratory, Moroccan Health Authority, Agadir, Morocco; Laboratory of Enteric Pathogen, The National Public Health Institute, Helsinki, Finland Source of funding: National Public Health Authority, Finland, Fintours Company, Sun Tours Company, Moroccan Health Authority, University of Gothenburg, Pohjola Company, and Tapiola Company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized", no further details given. |
| Allocation concealment (selection bias) | Low risk | "The vaccine and placebo were in a similar liquid form, coded blindly and packed in identical 4 mL vials". Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The vaccine code was opened after all demographic, clinical, and microbiological data were available". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up were high (18%) for adverse event data. Losses to follow‐up for clinical outcomes were not clearly stated. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Qadri 2003.
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Surveillance for side‐effects was carried out for 3 days after each vaccination. The child was observed for 1 hour in the field clinic and was then allowed to return home. The trained health workers made house to house visits within 3 hours to assess and record adverse events, thereafter home visits were made every day, for the next 2 days. |
|
| Participants | Number of participants: 158 Inclusion criteria: Healthy children aged 18 to 36 months with both sexes Exclusion criteria: History of gastrointestinal disorder, diarrhoeal illness in the past 2 weeks, febrile illness in the preceding week or antibiotic treatment for at least 7 days prior to enrolment as well as children, weight‐for‐height <−2(S.D.) of the median value of the National Centre of Health Statistics (NCHS) were not enrolled in the study. |
|
| Interventions | Vaccine: One 6 mL dose of vaccine consisted of 1 mg of rCTB plus ∼2 × 1010 CFU of five strains of formalin‐inactivated ETEC expressing CFA/I, CS1, CS2 + CS3, CS4 and CS5 antigens each. Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12. Additional details: The children enrolled in the study were received two doses of the oral CF‐BS‐ETEC vaccine (lot E‐009) or the placebo with a 2 week interval in the health station of the field site. |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Dhaka, Bangladesh Setting: International Centre for Diarrhoeal Disease Research, Bangladesh Source of funding: USAID (HRN‐A‐00‐96‐90005‐00), the Swedish Agency for Research and Economic Cooperation, Sida‐SAREC (1995‐0069) and ICDDR,B. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random code generated by statistician (author communication). |
| Allocation concealment (selection bias) | Low risk | "Each subject was assigned a sequential number at the time of initial dosing, corresponding to numbered and randomized set of two single‐dose vials of study agent". Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The vaccine and placebo formulation appeared similar". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigators including field staff and laboratory personnel were completely blinded to the identity of the study subjects, whether vaccine or placebo". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up was short and no losses to follow‐up occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Qadri 2006a.
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Surveillance for side effects was carried out for 3 days after each vaccination. The children were observed for 1 hour in the field clinic and were then allowed to return home. Local health workers made house‐to‐house visits within 3 hours to assess and record adverse events, thereafter home visits were made every day for the next 2 days. |
|
| Participants | Number of participants: 158 Inclusion criteria: Healthy children aged 6 to 17 months living in same socioeconomic background Exclusion criteria: History of gastrointestinal disorder, diarrhoeal illness in the past 2 weeks, febrile illness in the preceding week or antibiotic treatment at least 7 days prior to enrolment as well as children <−2 S.D. (weight/height) of the National Center of Health Statistics (NCHS) were also not recruited. Children who were found to be asymptomatically positive for any bacterial enteric pathogen including ETEC during the screening and any participant with ETEC infection during the study period was to be excluded. |
|
| Interventions | Vaccine: A quarter dose of vaccine composed of total ∼ 2.5 × 1010 CFU of five strains of ETEC. An 1.5 mL dose contained 0.25 mg of recombinant cholera toxin B subunit (BS) plus ∼ 0.5 × 1010 formalin‐inactivated bacteria of each of five different ETEC strains producing CFA/I, CS1, CS2, CS3, CS4, CS5 (lot E 008). Placebo: ∼2.5 × 1010 CFU of heat‐killed E. coli K12 bacteria Additional details: Each two‐dose (quarter dose) of vaccine regimen was given at intervals of 2 weeks intervals |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Dhaka, Bangladesh Setting: International Centre for Diarrhoeal Disease Research, Bangladesh Source of funding: USAID (HRN‐A‐00‐96‐90005‐00), the Swedish Agency for Research and Economic Cooperation, Sida‐SAREC (2001‐3970) and icddr,b. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random code generated by statistician (author communication). |
| Allocation concealment (selection bias) | Low risk | "Randomized vials of study agents either vaccine or placebo were supplied by the company". The vials were identical in appearance (author communication) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The blinding of the study code was maintained throughout (author communication). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up was short and no losses to follow‐up occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Sack 2007.
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: May 1998 to September 1999 Surveillance: The participants were given vials for collection of faecal samples and daily diaries to record the presence or absence of symptoms each day during the stay, up to 28 days. The participants visited the office at least twice weekly, and turned in their diaries weekly, at which time the diaries were reviewed with the study staff. |
|
| Participants | Number of participants: 685 for safety analysis and 669 for efficacy analysis Inclusion criteria: Travellers from USA to Mexico and Guatemala and planned to stay at least 14 days, travellers more than ≥17 years of age, good health condition, US resident with a telephone, willingness to participate and signed consent, females not pregnant and willing to use reliable birth control during the study period Exclusion criteria: Clinically significant acute or chronic gastrointestinal disease, any serious medical condition, immunodeficiency, planned to use antibiotics during the trip and recent exposure to ETEC within the past 1‐year |
|
| Interventions | Vaccine: 1 x 1011 formalin‐killed 5 strains of enterotoxigenic E. coli expressing CFA1, CS1, CS2, CS3, CS4 and CS5 plus 1 mg of the recombinant B‐subunit of cholera toxin Placebo: Killed non‐pathogenic E. coli K12 Additional details: The participants fasted for one hour before and after vaccination. The first dose was taken about 3 weeks before travel (acceptable range, 11 to 35 days prior to travel) and the second dose was taken about 8 days before travel (acceptable range: between 4 to 10 days before travel). The two doses were separated by between 7 to 21 days. |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: USA, Mexico, and Guatemala Setting: Vaccine Testing Unit (VTU), Johns HopkinsUniversity, Baltimore, MD, USA; Institute of Nutrition of Central America and Panama (INCAP), Guatemala; Hospital del Nino Morelense, Mexico; University of Gothenburg, Sweden Source of funding: SBL VaccineAB, Stockholm, Sweden |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation used blocks of 10 (prepared by the CRO Clinical Data Care in Lund, Sweden) to assure similar distribution throughout the study and the blocks were stratified according to destination (Guatemalaor Mexico)". |
| Allocation concealment (selection bias) | Low risk | "The participants were randomised to receive two doses of the vaccine in a bicarbonate citrate buffer, or an identical appearing placebo". "The vials of vaccine had unique study numbers that were then used as the study number to identify that participant. The volunteers were considered as randomised when they had signed the informed consent". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The complete randomisation list exists in two sealed and identical copies. One was stored at SBL Vaccin AB, and the other at CDC in Lund". "These envelopes were to be opened only if there was a medical and/or ethical need to know the vaccination code, as requested by the DSMB". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were low (< 10%) in each group. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Savarino 1998.
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article. Surveillance: Subjects had oral temperatures taken and underwent a standardized, structured interview on 3 consecutive days after each dose. In addition, subjects were asked to provide 3 venous blood samples, 1 just before the first dose and 1 each 7 days after the first and second doses. |
|
| Participants | Number of participants: 76 Inclusion criteria: Healthy men and women aged 21 to 45 years Exclusion criteria: Participants with a history of chronic gastrointestinal illness, diarrhoea, antidiarrhoeal drug usage, febrile illness in the week before dosing or pregnancy. |
|
| Interventions | Vaccine: Each 4 mL dose of the ETEC/rCTB vaccine (Lot E003) contained 1 mg rCTB plus 2 x 1010 formalin‐inactivated bacteria of each of the following ETEC strains: SBL101 (O78:H12; CFA/I; ST+); SBL104 (O25:H42; CS4+CS6); SBL105 (O167:H5; CS5+CS6; ST+); SBL106 (O6:H16; CS1); and SBL107 (OR:H6; CS2 CS3) Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12 bacteria Additional details: Subjects were offered a two dose schedule of study agent in three rounds at intervals of 2 weeks, with fasting for at least 90 minutes before and after dosing |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Benha, Egypt Setting: Cairo, Egypt. Source of funding: Naval Medical Research and Development Command, the National Institute of Child Health and Human Development Under Interagency Agreement (Y1‐HD‐0026‐01) and WHO Global Programme for Vaccines and Immunization/Vaccine Research and Development. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After enrollment, subjects were randomized to receive vaccine or placebo in a double‐blind fashion within blocks of 4 sequentially randomized subjects". It was unclear if this was truly randomized. |
| Allocation concealment (selection bias) | Low risk | "At the time of initial dosing, each subject was assigned a sequential number corresponding to sequentially numbered single‐dose vials of study agent". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "double blind, placebo controlled", and vaccines labelled only with study code. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The code was broken after all clinical and laboratory evaluations were completed". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only two subjects were excluded from the 2nd dose from placebo group because of absenteeism or intercurrent diarrhoea. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Low risk | None. |
Savarino 1999 (Study 1).
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: June 1996 Surveillance: Subjects were observed for 30 minutes after each dose for occurrence of immediate adverse effects. For the next 3 days after each dose, parents were asked to report to the study centre with their child. During each visit, a trained health care provider obtained the child’s temperature and performed a standardized, structured interview for 24 hour recall of symptoms. |
|
| Participants | Number of participants: 107 Inclusion criteria: Healthy Egyptian children aged between 6 to 12 years were recruited from Benha, Egypt Exclusion criteria: Children with a history of chronic gastrointestinal disorder, diarrhoea, or febrile illness, or some other serious chronic illness |
|
| Interventions | Vaccine: Each 4 mL dose of the ETEC/rCTB vaccine (Lot E003) contained 1 mg rCTB plus 2 x 1010 formalin‐inactivated bacteria of each of the following ETEC strains: SBL101 (O78:H12; CFA/I; ST); SBL104 (O25:H42; CS4); SBL105 (O167:H5; CS5; ST); SBL106 (O6:H16; CS1); and SBL107 (OR:H6; CS2 CS3) Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12 bacteria Additional details: Subjects received a two‐dose schedule of study agent 2 weeks apart, fasting for at least 90 minutes before and after each dose |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Benha, Egypt. Setting: US Naval Medical Research Unit No. 3, Cairo, Egypt. Source of funding: Naval Medical Research and Development Command, the National Institute of Child Health and Human Development Under Interagency Agreement (Y1‐HD‐0026‐01) and WHO Global Programme for Vaccines and Immunization/Vaccine Research and Development. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each child was assigned a sequential number corresponding to serially numbered and randomized sets of two single‐dose vials of study agent". |
| Allocation concealment (selection bias) | Low risk | See above. Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators and subjects were kept blinded as to assignments until all clinical and laboratory evaluations were completed and data files were frozen". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only two subjects were excluded from the 2nd dose because of fever, diarrhoea, or other intercurrent illness. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Savarino 1999 (Study 2).
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: November to December 1996, pre‐school children Surveillance: Subjects were observed for 30 minutes after each dose for occurrence of immediate adverse effects. For the next 3 days after each dose, parents were asked to report to the study centre with their child. During each visit, a trained health care provider obtained the child’s temperature and performed a standardized, structured interview for 24 hour recall of symptoms. |
|
| Participants | Number of participants: 106 Inclusion criteria: Healthy Egyptian children ages, both boys and girls aged between 2 to 5 years were recruited from Benha, Egypt Exclusion criteria: Children with a history of chronic gastrointestinal disorder, diarrhoea, or febrile illness, or some other serious chronic illness |
|
| Interventions | Vaccine: Each 4 mL dose of the ETEC/rCTB vaccine (Lot E003) contained 1 mg rCTB plus 2x1010 formalin‐inactivated bacteria of each of the following ETEC strains: SBL101 (O78:H12; CFA/I; ST); SBL104 (O25:H42; CS4); SBL105 (O167:H5; CS5; ST); SBL106 (O6:H16; CS1); and SBL107 (OR:H6; CS2 CS3). Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12 bacteria. Additional details: Subjects received a two‐dose schedule of study agent 2 weeks apart, fasting for at least 90 minutes before and after each dose. |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Benha, Egypt Setting: US Naval Medical Research Unit No. 3, Cairo, Egypt Source of funding: Naval Medical Research and Development Command, the National Institute of Child Health and Human Development Under Interagency Agreement (Y1‐HD‐0026‐01) and WHO Global Programme for Vaccines and Immunization/Vaccine Research and Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each child was assigned a sequential number corresponding to serially numbered and randomized sets of two single‐dose vials of study agent". |
| Allocation concealment (selection bias) | Low risk | See above. Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators and subjects were kept blinded as to assignments until all clinical and laboratory evaluations were completed and data files were frozen". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were low: Nine subjects were excluded from the 2nd dose because of fever, diarrhoea, or other intercurrent illness. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Savarino 2002.
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: September to October 1997 Surveillance: Subjects were directly observed for 30 minutes after each dose for immediate adverse events. For three days after each dose parents reported daily to the study centre with their child. At each visit a trained health worker took the child’s rectal temperature and performed a standardized, structured interview for 24 hour recall of symptoms. Occurrence of specific gastrointestinal symptoms and other unanticipated complaints was ascertained. |
|
| Participants | Number of participants: 95 Inclusion criteria: Healthy boys and girls aged 6 to 18 months were recruited from Benha, Egypt. Exclusion criteria: Children with a history of chronic gastrointestinal disorder, some other serious chronic illness, or congenital anomaly |
|
| Interventions | Vaccine: Each 4 mL dose of the ETEC/rCTB vaccine (Lot E003) contained 1 mg rCTB plus 2 x 1010 formalin‐inactivated bacteria of each of the following ETEC strains: SBL101 (O78:H12; CFA/I; ST); SBL104 (O25:H42; CS4); SBL105 (O167:H5; CS5; ST); SBL106 (O6:H16; CS1); and SBL107 (OR:H6; CS2 CS3) Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12 bacteria Additional details: Subjects were offered a three dose schedule of study agent in three rounds at intervals of 2 weeks, with fasting for at least 1 hour before and after dosing |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Benha, Egypt Setting: US Naval Medical Research Unit Number 3, Cairo, Egypt Source of funding: Naval Medical Research and Development Command, the National Institute of Child Health and Human Development Under Interagency Agreement (Y1‐HD‐0026‐01) and WHO Global Programme for Vaccines and Immunization/Vaccine Research and Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After enrollment subjects were stratified by age (6‐month bands) and gender and then block randomized to vaccine or control (block size, four) in a 1:1 ratio". |
| Allocation concealment (selection bias) | Low risk | "Within each stratum children were assigned a sequential number corresponding to serially numbered and randomized sets of three single dose vials of study agent". Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators and subjects were kept blinded as to assignments until all clinical and laboratory evaluations were completed, and data files were locked". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up was high: 26% of randomized subjects (33/128) dropped out before receiving any dose of study agent, and 33% of subjects who received at least one dose of study agent did not complete the study (31/95). |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Scerpella 1995.
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: From June 1992 to July 1992 Surveillance: WC/rBS oral cholera vaccine in 502 US college students attending summer educational programs in Mexico |
|
| Participants | Number of participants: 502 healthy adults Inclusion criteria: Full‐time US residence, aged 18 and over, willingness to participate in the study and willingness to sign the consent form Exclusion criteria: Failure to understand the nature and plan of the study, inability to receive adequate follow‐up examinations in Mexico, unwillingness to submit serum specimens, use of oral or parenteral antibiotics in the 7 days previous to enrolment, use of more than two doses of anti‐diarrhoeal medications in the 7 days previous to enrolment, significant abnormalities detected by screening of the medical history and physical exam, history of severe allergic reaction to any vaccine, and in women of childbearing age, a positive urine pregnancy test result and nursing mothers |
|
| Interventions | Vaccine: WC‐rBS, I mg of purified CTB subunit together with 1X1011 inactivated V. cholerae Placebo: Bicarbonate buffer alone. Additional details: Two doses of oral vaccine given, first dose at day 0 and second days 10 days later. Both vaccine and placebo are identical in appearance. |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Mexico Setting: Center for Infectious Diseases of the University of Texas Health Science Center in Houston Source of funding: DOD; DAMD 17‐90‐R‐0048 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized", but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "For the placebo group, the 3 rnL dose of vaccine was not added before administration of the buffer solution. In this fashion, study participants were blinded as to which study group they were in". Personnel appear to be unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were low. Data for 492 of 502 (98%) participants were available for analysis. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Tacket 1999.
| Methods | Study type: Randomized, artificial challenge, efficacy study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Vaccine or placebo administered 3 times daily for 5 days Artificial challenge: on day 2; post challenge follow‐up for 4 days |
|
| Participants | Number of participants: 20 Inclusion criteria: Healthy adults Exclusion criteria: None mentioned |
|
| Interventions | Vaccine: All participants were randomized to receive 690 mg of bovine anti‐E. coli CFA milk immunoglobulin capsule Placebo: All participants were randomized to receive 690 mg of placebo capsule Additional details: Vaccine or placebo were administered 3 times daily, 10 minutes after each meal, for 5 days followed by on day 2 with an applesauce containing artificial challenge: 1 × 108 CFU of the challenge virulent strain of E. coli E24377. Schedule dose of vaccine or placebo was also administered 10 minutes after challenge. |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: USA Setting: Research Isolation Ward, Kernan Hospital, University of Maryland, Baltimore, MD, USA Source of funding: ImmuCell Corporation, Portland, ME |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "‘randomized"’, but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo consisted of an identical preparation from non‐immunized cow. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "‘double blind"’ but no further details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Wiedermann 2000.
| Methods | Study type: RCT, natural challenge, efficacy study in travellers Trial dates and duration: Not mentioned in the article Surveillance: Post‐vaccination symptoms and adverse events were reported after both doses. All volunteers enrolled in the study were equipped with both a daily record diary to monitor episodes of travellers’ diarrhoea during their stay abroad and with transport media (Portagerm® and Cary Blair tubes) for collection of stool samples in case of diarrhoea. Travelers were instructed to hand over their travel diary and transport media tubes immediately after return. |
|
| Participants | Number of participants: 128 travellers (66 placebo group and 62 ETEC vaccine group) Inclusion criteria: Adults and children, who had signed up for a trip to tropical or subtropical destinations (44 different countries in Africa, Asia and Latin‐America) with a duration of stay intended to last 7 to 23 days Exclusion criteria: Not mentioned in the article |
|
| Interventions | Vaccine: ETEC vaccine, containing 1 mg of recombinant B‐subunit of cholera toxin plus 1011 formalin‐killed ETEC bacteria of five ETEC strains expressing the most common CFAs such as CFA I, CFA II (CS1, CS2 and CS3) and CFA IV (CS4, CS5 and CS6); a B‐subunit cholera whole cell vaccine (licensed in Sweden since 1992), containing 1 mg recombinant subunit B cholera toxin and 1011 inactivated whole cells (Inaba,Ogawa; classical and El Tor) Placebo: Approximately 1011 inactivated E. coli K12 Additional details: Two consecutive vaccine or placebo doses given at an interval of between 7 to 21 days, not less than 7 days and not more than 30 days before departure |
|
| Outcomes |
Included in review:
|
|
| Notes | Location: Institute for Specific Prophylaxis and Tropical Medicine, University of Vienna, Austria Setting: Institute for Specific Prophylaxis and Tropical Medicine, University of Vienna, Austria; Department of Medical Microbiology and Immunology, Göteborg University, Sweden; Swedish Bacteriological Laboratory, Vaccin, Sweden Source of funding: Not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized" but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double blind" but no further details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "double blind" but no further details given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seventy‐three recruited participants (29.2%) were excluded from the primary analysis. The reasons for these exclusions were unclear. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2006 | Participants did not receive a vaccine to prevent ETEC. |
| Ahren 1998 | No control group. |
| Carpenter 2006 | Not described as randomized. |
| Clemens 2004 | Randomized placebo controlled Phase III trial but published the data as a cohort study. Clinical efficacy data are unavailable from the article. |
| Coster 2007 | Participants did not receive a vaccine to prevent ETEC. |
| Daley 2007 | No clinical efficacy data for this vaccine is available. |
| Evans 1984 | Not described as randomized. |
| Evans 1988a | No control group. |
| Evans 1988b | Not described as randomized. |
| Glenn 2007 | No control group. |
| Guereña‐Burgueño 2002 | Not described as randomized. |
| Hallander 2002 | No protective efficacy data against travellers' diarrhoea |
| Holmgren 1992 | No outcomes relevant to this review. |
| Katz 2003 | Not described as randomized. |
| Khan 2007 | Not described as randomized. |
| Klipstein 1986 | Not described as randomized. |
| Lapa 2008 | No clinical efficacy data for this vaccine is available. |
| Levine 1982 | Not described as randomized. |
| McKenzie 2006 | No true control arm for safety and immunological data. |
| Qadri 2006b | Participants did not receive a vaccine to prevent ETEC. |
| Sougioultzis 2002 | Topic unrelated to ETEC vaccination. |
| Tacket 1994 | Not described as randomized. |
| Turner 2006 | No clinical efficacy data for this vaccine is available. |
| Wasserman 1993 | Participants did not receive a vaccine to prevent ETEC. |
| Wenneras 1999 | Not described as randomized. |
Differences between protocol and review
Since the publication of the original review, several changes have occurred in standard Cochrane methodology which were not in the original review. Notably, the method of assessing risk of bias has changed, and summary of findings tables incorporating the GRADE methodology for assessing the quality of evidence have been added. We have described the methodology for these additions in the methods section.
Contributions of authors
Tanvir Ahmed (TA), Taufiqur Bhuiyan (TB), K Zaman (KZ), and Firdausi Qadri (FQ) wrote the overall study design, background, and data management plan for this protocol. TA and TB extracted the data; TA, TB and David Sinclair (DS) analysed the data; TA, TB, DS, KZ and FQ wrote the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Department for International Development, UK.
Declarations of interest
We certify that we have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of the review (eg employment, consultancy, stock ownership, honoraria, and expert testimony).
Unchanged
References
References to studies included in this review
Clemens 1988 {published data only}
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Neogy PK, Stanton B, et al. Cross‐protection by B subunit‐whole cell cholera vaccine against diarrhea associated with heat‐labile toxin‐producing enterotoxigenic Escherichia coli: results of a large‐scale field trial. Journal of Infectious Diseases 1988;158(2):372‐7. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Stanton BF, Harris JR, Chakraborty J, Sack DA, Rao MR, et al. Exclusion of clinically atypical or microbiologically mixed diarrhoeal episodes from outcome events in a field trial of oral cholera vaccines. International Journal of Epidemiology 1989;18(2):440‐5. [DOI] [PubMed] [Google Scholar]
Cohen 2000 (Study 1) {published data only}
- Cohen D, Orr N, Haim M, Ashkenazi S, Robin G, Green MS, et al. Safety and immunogenicity of two different lots of the oral, killed enterotoxigenic Escherichia coli‐cholera toxin B subunit vaccine in Israeli young adults. Infection & Immunity 2000;68(8):4492‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2000 (Study 2) {published data only}
- Cohen D, Orr N, Haim M, Ashkenazi S, Robin G, Green MS, et al. Safety and immunogenicity of two different lots of the oral, killed enterotoxigenic Escherichia coli‐cholera toxin B subunit vaccine in Israeli young adults. Infection & Immunity 2000;68(8):4492‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frech 2008 {published data only}
- Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind‐Gerson J, Figueroa JF, et al. Use of a patch containing heat‐labile toxin from Escherichia coli against travellers' diarrhoea: a phase II, randomised, double‐blind, placebo‐controlled field trial. Lancet 2008;371(9629):2019‐25. [DOI] [PubMed] [Google Scholar]
Freedman 1998 {published data only}
- Freedman DJ, Tacket C O, Delehanty A, Maneval DR, Nataro J, Crabb JH. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. Journal of Infectious Diseases 1998;177(3):662‐7. [DOI] [PubMed] [Google Scholar]
Hall 2001 (Study 1) {published data only}
- Hall ER, Wierzba TF, Ahren C, Rao MR, Bassily S, Francis W, et al. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenicEscherichia coli plus cholera toxin B subunit vaccine. Infection & Immunity 2001;69(5):2853‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2001 (Study 2) {published data only}
- Hall ER, Wierzba TF, Ahren C, Rao MR, Bassily S, Francis W, et al. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenicEscherichia coli plus cholera toxin B subunit vaccine. Infection & Immunity 2001;69(5):2853‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2001 (Study 3) {published data only}
- Hall ER, Wierzba TF, Ahren C, Rao MR, Bassily S, Francis W, et al. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenicEscherichia coli plus cholera toxin B subunit vaccine. Infection & Immunity 2001;69(5):2853‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jertborn 1998 {published data only}
- Jertborn M, Ahren C, Holmgren J, Svennerholm AM. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine 1998;16(2‐3):255‐60. [DOI] [PubMed] [Google Scholar]
Jertborn 2001 {published data only}
- Jertborn M, Ahrén C, Svennerholm AM. Dose‐dependent circulating immunoglobulin A antibody‐secreting cell and serum antibody responses in Swedish volunteers to an oral inactivated enterotoxigenic Escherichia coli vaccine. Clinical and Diagnostic Laboratory Immunology 2001;8(2):424‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leyten 2005 {published data only}
- Leyten EM, Soonawala D, Schultsz C, Herzog C, Ligthelm RJ, Wijnands S, et al. Analysis of efficacy of CVD 103‐HgR live oral cholera vaccine against all‐cause travellers' diarrhoea in a randomised, double‐blind, placebo‐controlled study. Vaccine 2005;23(43):5120‐6. [DOI] [PubMed] [Google Scholar]
McKenzie 2007 {published data only}
- McKenzie R, Bourgeois AL, Frech SA, Flyer DC, Bloom A, Kazempour K, et al. Transcutaneous immunization with the heat‐labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double‐blind, placebo‐controlled challenge study. Vaccine 2007;25(18):3684‐91. [DOI] [PubMed] [Google Scholar]
McKenzie 2008 {published data only}
- McKenzie R, Darsley M, Thomas N, Randall R, Carpenter C, Forbes E, et al. A double‐blind, placebo‐controlled trial to evaluate the efficacy of PTL‐003, an attenuated enterotoxigenic E. coli (ETEC) vaccine strain, in protecting against challenge with virulent ETEC. Vaccine 2008;26(36):4731‐9. [DOI] [PubMed] [Google Scholar]
Peltola 1991 {published data only}
- Peltola H, Siitonen A, Kyrönseppä H, Simula I, Mattila L, Oksanen P, et al. Prevention of travellers' diarrhoea by oral B‐subunit/whole‐cell cholera vaccine. Lancet 1991;338(8778):1285‐9. [DOI] [PubMed] [Google Scholar]
Qadri 2003 {published data only}
- Qadri F, Ahmed T, Ahmed F, Bradley Sack R, Sack DA, Svennerholm AM. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi children 18‐36 months of age. Vaccine 2003;21(19‐20):2394‐403. [DOI] [PubMed] [Google Scholar]
Qadri 2006a {published data only}
- Qadri F, Ahmed T, Ahmed F, Begum Y A, Sack DA, Svennerholm AM, et al. Reduced doses of oral killed enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine is safe and immunogenic in Bangladeshi infants 6‐17 months of age: dosing studies in different age groups. Vaccine 2006;24(10):1726‐33. [DOI] [PubMed] [Google Scholar]
Sack 2007 {published data only}
- Sack DA, Shimko J, Torres O, Bourgeois AL, Francia DS, Gustafsson B, et al. Randomised, double‐blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. coli diarrhoea of travellers to Guatemala and Mexico. Vaccine 2007;25(22):4392‐400. [DOI] [PubMed] [Google Scholar]
Savarino 1998 {published data only}
- Savarino SJ, Brown FM, Hall E, Bassily S, Youssef F, Wierzba T, et al. Safety and immunogenicity of an oral, killed enterotoxigenic Escherichia coli‐cholera toxin B subunit vaccine in Egyptian adults. Journal of Infectious Diseases 1998;177(3):796‐9. [DOI] [PubMed] [Google Scholar]
Savarino 1999 (Study 1) {published data only}
- Savarino SJ, Hall ER, Bassily S, Brown FM, Youssef F, Wierzba TF, et al. Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. PRIDE Study Group. Journal of Infectious Diseases 1999;179(1):107‐14. [DOI] [PubMed] [Google Scholar]
Savarino 1999 (Study 2) {published data only}
- Savarino SJ, Hall ER, Bassily S, Brown FM, Youssef F, Wierzba TF, et al. Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. PRIDE Study Group. Journal of Infectious Diseases 1999;179(1):107‐14. [DOI] [PubMed] [Google Scholar]
Savarino 2002 {published data only}
- Savarino SJ, Hall ER, Bassily S, Wierzba TF, Youssef FG, Peruski LF, et al. Introductory evaluation of an oral, killed whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Egyptian infants. Pediatric Infectious Disease Journal 2002;21(4):322‐30. [DOI] [PubMed] [Google Scholar]
Scerpella 1995 {published data only}
- Scerpella EG, Sanchez JL, Mathewson III JJ, Torres‐Cordero JV, Sadoff JC, Svennerholm AM, et al. Safety, immunogenicity, and protective efficacy of the whole‐cell/recombinant B subunit (WC/rBS) oral cholera vaccine against travelers' diarrhea. Journal of Travel Medicine 1995;2(1):22‐7. [DOI] [PubMed] [Google Scholar]
Tacket 1999 {published data only}
- Tacket CO, Losonsky G, Livio S, Edelman R, Crabb J, Freedman D. Lack of prophylactic efficacy of an enteric‐coated bovine hyperimmune milk product against enterotoxigenic Escherichia coli challenge administered during a standard meal. Journal of Infectious Diseases 1999;180(6):2056‐9. [DOI] [PubMed] [Google Scholar]
Wiedermann 2000 {published data only}
- Wiedermann G, Kollaritsch H, Kundi M, Svennerholm AM, Bjare U. Double‐blind, randomized, placebo controlled pilot study evaluating efficacy and reactogenicity of an oral ETEC B‐subunit‐inactivated whole cell vaccine against travelers' diarrhea (preliminary report). Journal of Travel Medicine 2000;7(1):27‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 2006 {published data only}
- Ahmed A, Li J, Shiloach Y, Bobbins JB, Szu SC. Safety and immunogenicity of Escherichia coli O157 O‐specific polysaccharide conjugate vaccine in 2‐5‐year‐old children. Journal of Infectious Diseases 2006;193(4):515‐21. [DOI] [PubMed] [Google Scholar]
Ahren 1998 {published data only}
- Ahren C, Jertborn M, Svennerholm A M. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin a responses in blood. Infection and Immunity 1998;66(7):3311‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2006 {published data only}
- Carpenter CM, Hall ER, Randall R, McKenzie R, Cassels F, Diaz N, et al. Comparison of the antibody in lymphocyte supernatant (ALS) and ELISPOT assays for detection of mucosal immune responses to antigens of enterotoxigenic Escherichia coli in challenged and vaccinated volunteers. Vaccine 2006;24(18):3709‐18. [DOI] [PubMed] [Google Scholar]
Clemens 2004 {published data only}
- Clemens J, Savarino S, Abu‐Elyazeed R, Safwat M, Rao M, Wierzba T, et al. Development of pathogenicity‐driven definitions of outcomes for a field trial of a killed oral vaccine against enterotoxigenic Escherichia coli in Egypt: application of an evidence‐based method . Journal of Infectious Disease 2004;189(12):2299‐307. [DOI] [PubMed] [Google Scholar]
Coster 2007 {published data only}
- Coster TS, Wolf MK, Hall ER, Cassels FJ, Taylor DN, Liu CT, et al. Immune response, ciprofloxacin activity, and gender differences after human experimental challenge by two strains of enterotoxigenic Escherichia coli. Infection and Immunity 2007;75(1):252‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daley 2007 {published data only}
- Daley A, Randall R, Darsley M, Choudhry N, Thomas N, Sanderson IR, et al. Genetically modified enterotoxigenic Escherichia coli vaccines induce mucosal immune responses without inflammation. Gut 2007;56(11):1550‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1984 {published data only}
- Evans DG, Graham DY, Evans DJ Jr. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers. Response to challenge with virulent enterotoxigenic Escherichia coli. Gastroenterology 1984;87(4):934‐40. [PubMed] [Google Scholar]
Evans 1988a {published data only}
- Evans DG, Evans DJ Jr, Opekun AR, Graham DY. Non‐replicating oral whole cell vaccine protective against enterotoxigenic Escherichia coli (ETEC) diarrhea: stimulation of anti‐CFA (CFA/I) and anti‐enterotoxin (anti‐LT) intestinal IgA and protection against challenge with ETEC belonging to heterologous serotypes. FEMS Microbiology Immunology 1988;1(3):117‐26. [DOI] [PubMed] [Google Scholar]
Evans 1988b {published data only}
- Evans DJ, Evans DG, Opekun AR, Graham DY. Immunoprotective oral whole cell vaccine for enterotoxigenic Escherichia coli diarrhea prepared by in situ destruction of chromosomal and plasmid DNA with colicin E2. FEMS Microbiology Immunology 1988;1(1):9‐18. [DOI] [PubMed] [Google Scholar]
Glenn 2007 {published data only}
- Glenn GM, Villar CP, Flyer DC, Bourgeois AL, McKenzie R, Lavker RM, et al. Safety and immunogenicity of an enterotoxigenic Escherichia coli vaccine patch containing heat‐labile toxin: use of skin pretreatment to disrupt the stratum corneum. Infection and Immunity 2007;75(5):2163‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guereña‐Burgueño 2002 {published data only}
- Guereña‐Burgueño F, Hall ER, Taylor DN, Cassels FJ, Scott DA, Wolf MK, et al. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infection & Immunity 2002;70(4):1874‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallander 2002 {published data only}
- Hallander HO, Paniagua M, Espinoza F, Askelöf P, Corrales E, Ringman M, Storsaeter J. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine 2002;22(1‐2):138‐45. [DOI] [PubMed] [Google Scholar]
Holmgren 1992 {published data only}
- Holmgren J, Svennerholm AM, Jertborn M, Clemens J, Sack DA, Salenstedt R, et al. An oral B subunit: whole cell vaccine against cholera. Vaccine 1992;10(13):911‐4. [DOI] [PubMed] [Google Scholar]
Katz 2003 {published data only}
- Katz DE, DeLorimier AJ, Wolf MK, Hall ER, Cassels FJ, Hamont JE, et al. Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli (ETEC) CS6 antigen. Vaccine 2003;21(5‐6):341‐6. [DOI] [PubMed] [Google Scholar]
Khan 2007 {published data only}
- Khan S, Chatfield S, Stratford R, Bedwell J, Bentley M, Sulsh S, et al. Ability of SPI2 mutant of S. typhi to effectively induce antibody responses to the mucosal antigen enterotoxigenic E. coli heat labile toxin B subunit after oral delivery to humans. Vaccine 2007;25(21):4175‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klipstein 1986 {published data only}
- Klipstein FA, Engert RF, Houghten RA. Immunisation of volunteers with a synthetic peptide vaccine for enterotoxigenic Escherichia coli. Lancet 1986;1(8479):471‐2. [DOI] [PubMed] [Google Scholar]
Lapa 2008 {published data only}
- Lapa JA, Sincock SA, Ananthakrishnan M, Porter CK, Cassels FJ, Brinkley C, et al. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat‐labile enterotoxin with mutation R192G. Clinical and Vaccine Immunology 2008;15(8):1222‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 1982 {published data only}
- Levine MM, Black RE, Brinton CC Jr, Clements ML, Fusco P, Hughes TP, et al. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scandinavian Journal of Infectious Diseases 1982;33:83‐95. [PubMed] [Google Scholar]
McKenzie 2006 {published data only}
- McKenzie R, Bourgeois AL, Engstrom F, Hall E, Chang HS, Gomes JG, et al. Comparative safety and immunogenicity of two attenuated enterotoxigenic Escherichia coli vaccine strains in healthy adults. Infection and immunity 2006;74(2):994‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qadri 2006b {published data only}
- Qadri F, Ahmed F, Ahmed T, Svennerholm AM. Homologous and cross‐reactive immune responses to enterotoxigenic Escherichia coli colonization factors in Bangladeshi children. Infection and Immunity 2006;74(8):4512‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sougioultzis 2002 {published data only}
- Sougioultzis S, Lee CK, Alsahli M, Banerjee S, Cadoz M, Schrader R, et al. Safety and efficacy of E coli enterotoxin adjuvant for urease‐based rectal immunization against Helicobacter pylori. Vaccine 2002;21(3‐4):194‐201. [DOI] [PubMed] [Google Scholar]
Tacket 1994 {published data only}
- Tacket CO, Reid RH, Boedeker EC, Losonsky G, Nataro JP, Bhagat H, et al. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine 1994;12(14):1270‐4. [DOI] [PubMed] [Google Scholar]
Turner 2006 {published data only}
- Turner AK, Beavis JC, Stephens JC, Greenwood J, Gewert C, Thomas N, et al. Construction and phase I clinical evaluation of the safety and immunogenicity of a candidate enterotoxigenic Escherichia coli vaccine strain expressing colonization factor antigen CFA/I. Infection and immunity 2006;74(2):1062‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasserman 1993 {published data only}
- Wasserman SS, Kotloff KL, Losonsky GA, Levine MM. Immunologic response to oral cholera vaccination in a crossover study: a novel placebo effect. American Journal of Epidemiology 1993;138(11):988‐93. [DOI] [PubMed] [Google Scholar]
Wenneras 1999 {published data only}
- Wenneras C, Qadri F, Bardhan PK, Sack RB, Svennerholm AM. Intestinal immune responses in patients infected with enterotoxigenic Escherichia coli and in vaccinees. Infection and Immunity 1999;67(12):6234‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Black 1984
- Black RE, Merson MH, Eusof A, Huq I, Pollard R. Nutritional status, body size and severity of diarrhoea associated with rotavirus or enterotoxigenic Escherichia coli. Journal of Tropical Medicine & Hygiene 1984;87(2):8309. [PubMed] [Google Scholar]
Darsley 2012
- Darsley MJ, Chakraborty S, DeNearing B, Sack DA, Feller A, Buchwaldt C, et al. The oral, live attenuated enterotoxigenic Eschericia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clinical and Vaccine Immunology 2012;19(12):1921‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
DuPont 2008
- DuPont HL. Systematic review: prevention of travellers' diarrhoea. Alimentary Pharmacology & Therapeutics 2008;27(9):741‐51. [DOI] [PubMed] [Google Scholar]
Gill 1980
- Gill DM, Richardson SH. Adenosine diphosphate‐ribosylation of adenylate cyclase catalyzed by heat‐labile enterotoxin of Escherichia coli: comparison with cholera toxin. Journal of Infectious Diseases 1980;141(1):64‐70. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2008
- Harris AM, Chowdhury F, Begum YA, Khan AI, Faruque AS, Svennerholm AM, et al. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. American Journal of Tropical Medicine and Hygiene 2008;79(5):708‐14. [PMC free article] [PubMed] [Google Scholar]
Harro 2011
- Harro C, Sack D, Bourgeois AL, Walker R, DeNearing B, Feller A, et al. A combination vaccine consisting of three live attenuated enterotoxigenic Escherichia coli strains expressing a range of colonization factors and heat‐labile toxin subunit B is well tolerated and immunogenic in a placebo‐controlled double‐blind phase I trial in healthy adults. Clinical and Vaccine Immunology 2011;18(12):2118‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Holmgren 2005
- Holmgren J, Adamsson J, Anjuere F, Clemens J, Czerkinsky C, Eriksson K, et al. Mucosal adjuvants and anti‐infection and anti‐immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunology Letters 2005;97(2):181‐8. [DOI] [PubMed] [Google Scholar]
Holmgren 2012
- Holmgren J, Svennerholm A. Vaccines against mucosal infections. Current Opinion in Immunology 2012;24(3):343‐53. [DOI] [PubMed] [Google Scholar]
Jelinek 2008
- Jelinek T, Kollaritsch H. Vaccination with Dukoral against travelers' diarrhea (ETEC) and cholera. Expert Review of Vaccines 2008;7(5):561‐7. [DOI] [PubMed] [Google Scholar]
Jertborn 1992
- Jertborn M, Svennerholm A‐M, Holmgren J. Safety and immunogenicity of an oral recombinant cholera b subunit‐whole cell vaccine in Swedish volunteers. Vaccine 1992;10(2):130‐2. [DOI] [PubMed] [Google Scholar]
Qadri 2005
- Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clinical Microbiology Review 2005;18(3):465‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qadri 2007
- Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infection and Immunity 2007;75(8):3961‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2005
- Rao MR, Wierzba TF, Savarino SJ, Abu‐Elyazeed R, El‐Ghoreb N, Hall ER, et al. Serologic correlates of protection against enterotoxigenic Escherichia coli diarrhea. Journal of Infectious Diseases 2005;191(4):562‐70. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schwartz 2006
- Schwartz BS, Harris JB, Khan AI, Larocque RC, Sack DA, Malek MA, et al. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. American Journal of Tropical Medicine and Hygiene 2006;74(6):1067‐73. [PMC free article] [PubMed] [Google Scholar]
Steffen 2005
- Steffen R, Castelli F, Dieter Nothdurft H, Rombo L, Jane Zuckerman N. Vaccination against enterotoxigenic Escherichia coli, a cause of travelers' diarrhea. Journal of Travel Medicine 2005;12(2):102‐7. [DOI] [PubMed] [Google Scholar]
Svennerholm 1984
- Svennerholm AM, Jertborn M, Gothefors L, Karim AM, Sack DA, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit‐whole cell vaccine. Journal of Infectious Diseases 1984;149(6):884‐93. [DOI] [PubMed] [Google Scholar]
Svennerholm 2008
- Svennerholm AM, Tobias J. Vaccines against enterotoxigenic Escherichia coli. Expert Review of Vaccines 2008;7(6):795‐804. [DOI] [PubMed] [Google Scholar]
Tobias 2011
- Tobias J, Svennerholm AM, Carlin NI, Lebens M, Holmgren J. Construction of a non‐toxigenic Escherichia coli oral vaccine strain expressing large amounts of CS6 and inducing strong intestinal and serum anti‐CS6 antibody responses in mice. Vaccine 2011;29(48):8863‐9. [DOI] [PubMed] [Google Scholar]
Tobias 2012
- Tobias J, Svennerholm AM. Strategies to overexpress enterotoxigenic Escherichia coli (ETEC) colonization factors for the construction of oral whole‐cell inactivated ETEC vaccine candidates. Applied Microbiology and Biotechnology 2012;93(6):2291‐300. [DOI] [PubMed] [Google Scholar]
Walker 2007
- Walker RI, Steele D, Aguado T, Ad Hoc ETEC Technical Expert Committee. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 2007;25(14):2545‐66. [DOI] [PubMed] [Google Scholar]
Wennerås 2004
- Wennerås C, Erling V. Prevalence of enterotoxigenic Escherichia coli‐associated diarrhoea and carrier state in the developing world. Journal of Health, Population and Nutrition 2004;22(4):370‐82. [PubMed] [Google Scholar]
WHO 2009
- WHO. Diarrhoeal diseases (updated February 2009). http://www.who.int/vaccine_research/diseases/diarrhoeal/en/index4.html 2009.
Widermann 2009
- Wagner A, Wiedermann U. Travellers’ diarrhoea – pros and cons of different prophylactic measures. Wiener Klinische Wochenschrift 2009;121(Suppl 3):13‐8. [DOI] [PubMed] [Google Scholar]
Wolf 1997
- Wolf MK. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clinical Microbiology Review 1997;10(4):569‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]


