Abstract
Background
Health professionals frequently recommend fever treatment regimens for children that either combine paracetamol and ibuprofen or alternate them. However, there is uncertainty about whether these regimens are better than the use of single agents, and about the adverse effect profile of combination regimens.
Objectives
To assess the effects and side effects of combining paracetamol and ibuprofen, or alternating them on consecutive treatments, compared with monotherapy for treating fever in children.
Search methods
In September 2013, we searched Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; EMBASE; LILACS; and International Pharmaceutical Abstracts (2009‐2011).
Selection criteria
We included randomized controlled trials comparing alternating or combined paracetamol and ibuprofen regimens with monotherapy in children with fever.
Data collection and analysis
One review author and two assistants independently screened the searches and applied inclusion criteria. Two authors assessed risk of bias and graded the evidence independently. We conducted separate analyses for different comparison groups (combined therapy versus monotherapy, alternating therapy versus monotherapy, combined therapy versus alternating therapy).
Main results
Six studies, enrolling 915 participants, are included.
Compared to giving a single antipyretic alone, giving combined paracetamol and ibuprofen to febrile children can result in a lower mean temperature at one hour after treatment (MD ‐0.27 °Celsius, 95% CI ‐0.45 to ‐0.08, two trials, 163 participants, moderate quality evidence). If no further antipyretics are given, combined treatment probably also results in a lower mean temperature at four hours (MD ‐0.70 °Celsius, 95% CI ‐1.05 to ‐0.35, two trials, 196 participants, moderate quality evidence), and in fewer children remaining or becoming febrile for at least four hours after treatment (RR 0.08, 95% CI 0.02 to 0.42, two trials, 196 participants, moderate quality evidence). Only one trial assessed a measure of child discomfort (fever associated symptoms at 24 hours and 48 hours), but did not find a significant difference in this measure between the treatment regimens (one trial, 156 participants, evidence quality not graded).
In practice, caregivers are often advised to initially give a single agent (paracetamol or ibuprofen), and then give a further dose of the alternative if the child's fever fails to resolve or recurs. Giving alternating treatment in this way may result in a lower mean temperature at one hour after the second dose (MD ‐0.60 °Celsius, 95% CI ‐0.94 to ‐0.26, two trials, 78 participants, low quality evidence), and may also result in fewer children remaining or becoming febrile for up to three hours after it is given (RR 0.25, 95% CI 0.11 to 0.55, two trials, 109 participants, low quality evidence). One trial assessed child discomfort (mean pain scores at 24, 48 and 72 hours), finding that these mean scores were lower, with alternating therapy, despite fewer doses of antipyretic being given overall (one trial, 480 participants, low quality evidence)
Only one small trial compared alternating therapy with combined therapy. No statistically significant differences were seen in mean temperature, or the number of febrile children at one, four or six hours (one trial, 40 participants, very low quality evidence).
There were no serious adverse events in the trials that were directly attributed to the medications used.
Authors' conclusions
There is some evidence that both alternating and combined antipyretic therapy may be more effective at reducing temperatures than monotherapy alone. However, the evidence for improvements in measures of child discomfort remains inconclusive. There is insufficient evidence to know which of combined or alternating therapy might be more beneficial.Future research needs to measure child discomfort using standardized tools, and assess the safety of combined and alternating antipyretic therapy.
Keywords: Child, Humans, Acetaminophen, Acetaminophen/administration & dosage, Antipyretics, Antipyretics/administration & dosage, Body Temperature, Body Temperature/drug effects, Combined Modality Therapy, Combined Modality Therapy/adverse effects, Combined Modality Therapy/methods, Fever, Fever/drug therapy, Ibuprofen, Ibuprofen/administration & dosage, Randomized Controlled Trials as Topic, Time Factors
Alternating and combined antipyretics for treatment of fever in children
When they are ill with infections, children often develop a fever. The fever with common viral illnesses, such as colds, coughs, sore throats and gastrointestinal illness, usually lasts a few days, makes children feel unwell, and is distressing for the children, their parents, or other caregivers.
Paracetamol (also known as acetaminophen) and ibuprofen lower the child's temperature and relieve their discomfort. This review evaluates whether giving both treatments together, or alternating the two treatments, is more effective than giving paracetamol or ibuprofen alone.
In September 2013, we found six studies, involving 915 children, that evaluated combined or alternating paracetamol and ibuprofen to treat fever in children.
Compared to giving ibuprofen or paracetamol alone, giving both medications together is probably more effective at lowering temperature for the first four hours after treatment (moderate quality evidence). However, only one trial assessed whether combined treatment made children less uncomfortable or distressed and found no difference compared to ibuprofen or paracetamol alone.
In practice, caregivers are often advised to initially give a single agent (paracetamol or ibuprofen), and then give a further dose of the alternative if the child continues to have a fever. Giving alternating treatment in this way may be more effective at lowering temperature for the first three hours after the second dose (low quality evidence), and may also result in less child discomfort (low quality evidence)
Only one small trial compared alternating therapy with combined therapy and found no advantages between the two (very low quality evidence).
Background
Description of the condition
Fever is a host response to disease caused by the invasion of the body by pathogens (Kluger 1995). Fevers are triggered by the release of endogenous cytokines by white blood cells, which act on the anterior hypothalamus to raise the thermoregulatory set‐point, leading to elevated levels of prostaglandin E2 (PGE2) and a rise in body temperature (Kwiatkowski 1995). This elevation in body temperature is thought to attenuate the viability of some pathogens by recruiting and enhancing components of the immune system, and to help promote healing of damaged cellular components.
Many physicians and caregivers favour treatment of fever with antipyretics to minimize distress, as a child who feels better is more likely to eat and drink, avoiding complications of dehydration and the effects of poor nutrition. Other reasons for treating fever include improving comfort and normalizing body temperature (Crocetti 2001; Schmitt 1980). Rapid increases in fever can result in seizures, which although usually short‐lived and self‐limiting can lead to significant caregiver anxiety. Despite a lack of supporting clinical studies and proven ineffectiveness of prophylaxis in high risk children, some caregivers administer antipyretics to febrile children with a history of febrile seizures to prevent further seizures (Schnaiderman 1993; van Stuijvenberg 1998). The UK National Institute for Health and Care Excellence (NICE) Guidelines note that antipyretics do not prevent febrile convulsions and should not be used for prophylaxis of this condition (NICE Clinical Guidelines). Patients with underlying cardiac and pulmonary disorders may be at risk from fever, as the metabolic demands can be substantial. However, there are no studies that show antipyretics benefit patients with cardiopulmonary disease by reducing metabolic demand (Mackowiak 1998). Paracetamol (also known as acetaminophen) and ibuprofen are two of the most common antipyretic agents used by physicians and caregivers to treat fever.
Description of the intervention
Paracetamol is a para‐aminophenol derivative that is probably a cyclooxygenase‐3 inhibitor, which inhibits the formation and release of PGE2. It appears to act preferentially within the central nervous system to lower levels of fever‐producing cytokines (Feldberg 1973; Mackowiak 1998). It is absorbed via the gastrointestinal tract, with maximal temperature reduction after approximately two hours (Brown 1992; Kelley 1992). The recommended paediatric dose of paracetamol is 12 to 15 mg/kg every four to six hours orally. Paracetamol is also available in an intravenous formulation, with the recommended dose being 15 mg/kg every six hours with a maximum daily dose of 4 g.
Adverse effects include allergic reaction resulting in a pruritic rash, and hepatotoxicity following overdose, which may in turn lead to organ degeneration and death (Kelley 1992). Although paracetamol is used to treat fever in millions of children every day with few or no adverse effects, the risk of overdose with therapeutic intent remains. A study looking at 47 case reports of overdose with therapeutic intent was conducted and found a mortality rate of 55%, with children less than two years old accounting for half the deaths. In 52% of cases, the overdose was the result of children receiving adult preparations of paracetamol (Heubi 1998).
Recently, concerns have been raised regarding the association between paracetamol and the risk of asthma, rhinoconjunctivitis, and eczema in children and adults. The International Study of Asthma and Allergies in Childhood (ISAAC) programme has examined the association between atopy and both the use of paracetamol for fever in the first year of life and the frequency of use in the past 12 months. They concluded that there was an increased risk of asthma symptoms in childhood with paracetamol use for fever in the first year of life, as well as later on in childhood (Beasley 2008; Del‐Rio‐Navarro 2008). A number of other studies have suggested an association between both paracetamol exposure in utero and usage in childhood and wheezing and atopy (Cohet 2004; Newson 2000). A meta‐analysis published in 2009 found an increase in the risk of asthma and wheezing in both children and adults exposed to paracetamol (Etminan 2009). However, a prospective cohort study concluded that no association could be found between early paracetamol use and risk of subsequent allergic disease after adjustment for respiratory infections or when paracetamol use was restricted to non‐respiratory tract infections (Lowe 2010).
Ibuprofen is a nonsteroidal anti‐inflammatory propionic acid derivative that is a non‐selective cyclooxygenase inhibitor, although it has both central and peripheral effects on the nervous system. It is also absorbed via the gastrointestinal tract, with maximal temperature reduction within three hours. The recommended paediatric dose is 5 to 10 mg/kg every six to eight hours.
Adverse effects include gastrointestinal bleeding and renal failure, although ibuprofen has been reported to be as well tolerated as paracetamol (McIntyre 1996). As for paracetamol, risk of overdose with therapeutic intent remains. In children who have taken doses over 400 mg/kg, adverse effects include seizures, apnoea, hypotension, and renal and hepatic dysfunction.
The risk of acetylsalicylic acid‐induced asthma has been well documented for decades; it was first described in 1922, after aspirin first became available (Widal 1922). Due to the high level of cross reactivity with aspirin, caution in the use of other nonsteroidal anti‐inflammatory drugs (NSAIDs), including ibuprofen, has been suggested in asthmatic patients (Kanabar 2007). However, several studies have suggested that the use of ibuprofen does not exacerbate asthma morbidity in children (Lesko 1999; Lesko 2002; McIntyre 1996) and a recent literature review concluded that the risk of asthma morbidity from the use of ibuprofen is low (Kanabar 2007).
Efficacy of paracetamol and ibuprofen
In terms of efficacy, studies show that both paracetamol and ibuprofen are superior to a placebo (Brewer 1968; Walson 1989; Wilson 1991), and that ibuprofen is superior to paracetamol in the treatment of fever (Kauffman 1992; Perrott 2004). Both antipyretics have been found to be equally safe in children. However, literature reviews often conclude that paracetamol should be used preferentially, due to a lower risk of adverse effects (Canadian Pediatric Society 1998; Drwal‐Klein 1992; L’Italien 2001; Renn 2000). To date, there is no Cochrane systematic review summarizing the available literature on the efficacy of paracetamol compared to that of ibuprofen.
How the intervention might work
As paracetamol and ibuprofen have differing mechanisms of action, it is possible that they are more effective when used together than alone. One regimen option is to give both paracetamol and ibuprofen simultaneously (combined therapy), at regular intervals as needed. Another option may be to start with one antipyretic and then only administer the second medication if the fever does not subside within one to four hours (alternating therapy).
Studies have reported that at least 50% of caregivers give their children both antipyretics, but their method of alternating between the two varies (Li 2000). In addition, inaccurate dosing occurs in about half of cases (Mayoral 2000).
Recent guidelines from the UK state no preference for either drug in the treatment of fever, and recommends considering alternating these agents only if the distress persists or recurs before the next dose is due (NICE Clinical Guidelines).
The Canadian Pediatrics Society states no preference for either drug in the treatment of fever, and recommends that alternating therapy should only be used under professional supervision after considering the possible risks and benefits of exposing a child to two drugs (Canadian Pediatric Society 1998).
The American Academy of Pediatrics states that the primary goal of treating febrile children should be to improve overall comfort rather than focus on the normalization of body temperature. Their conclusion is that there is insufficient evidence to support or refute the routine combination of antipyretics (Sullivan 2011).
The Italian Paediatric Society states that antipyretics should only be used when fever is associated with discomfort, and doesn't recommend combining or alternating ibuprofen and paracetamol (Chiappini 2009).
Why it is important to do this review
Although many studies have investigated the efficacy and safety of paracetamol and ibuprofen on their own, fewer studies have explored the efficacy and safety of combined or alternating therapy. In a clinical setting, the use of antipyretics is widely recommended for treating child discomfort rather than absolute temperature. However, the popularity of combined and alternating antipyretics has increased in the literature and it can be extrapolated that these regimens are also increasingly used in clinical settings, particularly primary care. A systematic summary of available evidence would benefit health practitioners and caregivers in making informed decisions regarding the efficacy and safety of alternating and combined paracetamol and ibuprofen therapy.
As treating fever with antipyretics is mainly recommended for alleviating child discomfort, it is important to study qualitative measurements of child discomfort or stress. Although there are no specific standardized rating scales for these measurements in direct relation to fever, other rating scales have been used and are taken into consideration. Objective temperature measurements are generally not recommended as indications for antipyretic therapy. However, body temperature has been well studied in randomized controlled trials and as child discomfort and/or stress may be associated with fever it is still important to analyze objective measures of temperature. Determining specific clinical endpoints to monitor in terms of treatment efficacy (ie time without fever, wellness scores) would be useful for future research and for both caregivers and clinicians in hospital.
Most febrile periods associated with common infectious illnesses in children last one to three days and it is thus important to observe the effect of treatment during the first 24 to 48 hours after fever onset. Our clinical question is: in paediatric patients, is combined or alternating antipyretic treatment more effective than monotherapy for reducing discomfort and temperature in the first 24 to 48 hours of acute febrile illness? In clinical practice, physicians recommend treatment with antipyretics according to child discomfort and not necessarily based on absolute temperature measurements. Thus, one of our primary outcomes focuses on qualitative measures of child comfort.
Objectives
To assess the effects and side effects of combined and/or alternating paracetamol and ibuprofen versus monotherapy for the treatment of fever in children.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Inclusion Criteria
Children up to 18 years of age, with new fever associated with presumed infectious origin. Fever is defined by individual study authors.
Exclusion Criteria
Children with injury or undergoing surgery at the time of fever.
Types of interventions
Combined therapy versus ibuprofen alone
Combined therapy versus paracetamol alone
Alternating therapy versus ibuprofen alone
Alternating therapy versus paracetamol alone
Combined therapy versus alternating therapy.
Combined therapy is defined as simultaneous administration of paracetamol and ibuprofen at regular intervals. Alternating therapy is defined as one antipyretic (either paracetamol or ibuprofen) administered immediately and the second medication (either paracetamol or ibuprofen) administered only if fever does not subside within one to four hours.
Types of outcome measures
Primary outcomes
Measures of child discomfort, including stress scores; number of doses of medications given; and absences from daycare or school.
Proportion of febrile children at one, four and six hours after administration of initial antipyretic.
Secondary outcomes
Adverse events
Serious adverse events leading to hospitalization
Other adverse events (ie gastrointestinal symptoms, hepatic dysfunction/failure, renal dysfunction/failure).
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status, ie published, unpublished, in press, and ongoing.
Electronic searches
We searched the following databases on 6 September 2013 (all available, no date restrictions) using the search terms detailed in Appendix 1: Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; LILACS; and International Pharmaceutical Abstracts. In addition to the terms noted in the table, we employed the highly sensitive search strategy for identifying RCTs in MEDLINE (sensitivity‐maximizing version) described in the current Cochrane Handbook for Systematic Reviews of Interventions (section 6.4.11; Higgins 2011). We also used the recommended terms for identifying RCTs in EMBASE, as outlined in the current Handbook (section 6.3.2.2).
We searched the metaRegister of Controlled Trials (mRCT) for ongoing trials.
Searching other resources
Conference proceedings of the Pediatric Academic Society / American Academy of Pediatrics and the American Society for Toxicology (2009 to 2013) were handsearched but did not reveal any eligible trials that were not already identified in the initial search. ClinicalTrials.gov was searched and one eligible study (Adding a Second Drug for Febrile Children Treated With Acetaminophen) was found. However, when contacted, the main author reported that the study had to be stopped due to difficulties with recruitment.
We contacted pharmaceutical companies, study authors, and researchers working in the field for unpublished or ongoing trials and did not find any eligible trials.
Reference lists of all included and excluded studies were checked and did not reveal any eligible trials that were not already identified in the initial search.
Data collection and analysis
Selection of studies
In the first phase, one author and two assistants (TW, CC, EM) independently examined citations generated from a search based on Title, Abstract and MeSH headings. Trials were designated as RCT, possible RCT, or non‐RCT. All potentially relevant articles (RCTs and possible RCTs) were retrieved. Multiple copies of the same publication were identified and removed.
In the second phase, copies of the full text articles were reviewed independently by the three reviewers. Decisions on inclusion were based on the criteria as described above. Trial authors were contacted if there was need for clarification of study protocols or data. Disagreements were resolved by discussion between the reviewers. If multiple copies of the same publication were identified, all publications were assessed for differences in data sets. Studies reported in non‐English language journals were translated before assessment.
Data extraction and management
Data extraction was carried out independently by two authors (TW, AT) using standard data extraction forms. Data from each study, including study characteristics (location of study, patient demographics, intervention details etc) and details of outcome measures, were entered onto separate forms. For dichotomous outcomes, the number of events, the number of patients analyzed, and the number of patients randomized for each group were extracted. For continuous outcomes, the mean and standard deviation for each group were extracted. Authors were contacted in order to obtain missing or additional data.
Further information required from the original author was requested by written correspondence. Relevant information obtained in this manner was included in the review. Disagreements in data extraction were resolved by discussion amongst the authors.
Assessment of risk of bias in included studies
Two authors (TW, AT) independently assessed risk of bias for the studies using the Cochrane Risk of Bias tool. The tool assessed six domains: sequence generation, allocation concealment, blinding, missing outcome data, selective outcome reporting, and 'other sources of bias' (eg balance across groups of demographic variables at baseline, inappropriate influence of study sponsor). We followed guidelines in the Cochrane Handbook for applying the Risk of Bias tool (Higgins 2011). Results are presented in the risk of bias graph, summary, and tables.
Measures of treatment effect
We assessed the superiority of combined and alternating antipyretics versus monotherapy in terms of effect on child discomfort, temperature reduction and side effects. We analysed continuous outcomes using mean differences (MD) and 95% confidence intervals (CI). Mean differences were used for outcomes measured on the same scale and we planned to use standardized mean differences for outcomes measured on different scales across the trials. We calculated 95% CI and reported risk ratios for dichotomous data.
Dealing with missing data
Quantitative analyses of outcomes were based on intention‐to‐treat results. An available case analysis that included all patients with a measured outcome was conducted.
Assessment of heterogeneity
The I2 statistic with a value of 50% was taken to indicate moderate heterogeneity; 50% to 75% indicated substantial heterogeneity; and 75% to 100% indicated considerable heterogeneity (Higgins 2002). A Chi2 test was also used to assess heterogeneity using a P value < 0.1 to denote significant heterogeneity. The overlap of CI was also compared.
Assessment of reporting biases
We planned to assess reporting biases by constructing funnel plots using risk ratios. However, we did not have sufficient data to accurately assess funnel plots.
Data synthesis
Review Manager 5 was used to combine and analyze the trial data (Review Manager). The analysis was stratified by comparison (alternating therapy versus monotherapy, combined therapy versus monotherapy, combined therapy versus alternating therapy). A fixed‐effect model was used when there was no heterogeneity and a random‐effects model was used when moderate heterogeneity existed.
Grading the body of evidence
We used the Evidence‐based Practice Centers' Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess overall strength of evidence (Guyatt 2008). We evaluated the following outcomes judged to be most relevant: child discomfort; mean temperature for first 24 to 48 hours; proportion of afebrile children at one, four and six hours after administration of initial antipyretic; and other measures of child comfort or parental perception of antipyretic efficacy. We examined four domains: risk of bias, consistency, directness and precision.
The overall strength of evidence was graded as high (further research is very unlikely to change our confidence in the estimate of effect), moderate (further research may change our confidence in the estimate of effect and may change the estimate), low (further research is likely to change our confidence in the estimate of effect and is likely to change the estimate), or insufficient (evidence either is unavailable or does not permit estimation of an effect).
Two review authors (TW, AS) independently graded the body of evidence using GRADE guidance (GRADEpro 2008) and decision rules adapted to the clinical and research context. For the risk of bias domain, we considered all evidence as high or medium, as we only included RCTs. All decisions were made explicitly and we calculated inter‐rater agreement (available from authors). Two review authors (TW, AS) resolved discrepancies through consensus.
Sensitivity analysis
We had planned to assess the robustness of results using sensitivity analyses for risk of bias components, but were unable to do so due to the limited number of studies.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies
Results of the search
The search strategy identified 3649 citations from electronic databases (Figure 1). After screening titles and abstracts, 53 studies were assessed to be potentially relevant. Ten additional studies were identified for further examination after handsearching abstracts from the Pediatric Academic Society conference proceedings, but none met the inclusion criteria. No additional studies were identified for further examination after contact with experts or handsearching reference lists from previous systematic reviews and included studies.
Figure 1.
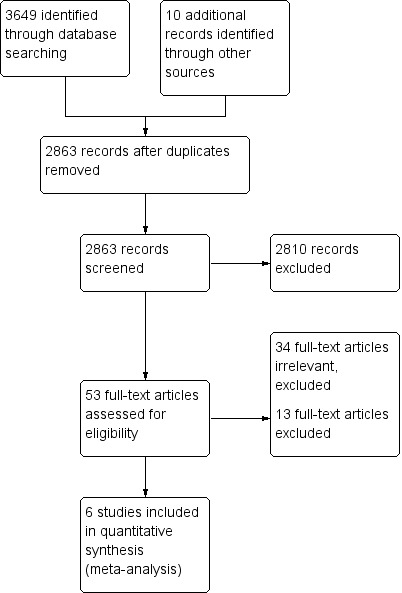
Study flow diagram.
We reviewed the full text of 53 reports using the pre‐defined inclusion criteria: 34 trials were considered irrelevant to this review and excluded; 13 studies were related to the review topic but did not fulfil the inclusion criteria and are excluded for the reasons stated in the Characteristics of excluded studies table.
Included studies
Six studies, enrolling 915 participants, are included in this review.
For detailed information on each study, refer to Characteristics of included studies tables. No distinction was made between inpatients and outpatients.
Four studies considered children with a temperature of 38 °C or above to be febrile (Erlewyn‐Lajeunesse 2006; Kramer 2008; Nabulsi 2006; Paul 2010). Other studies defined fever as a temperature greater than or equal to 37.8 °C (Hay 2008; Sarrell 2006). For temperature measurement, one study used continuous axillary temperature probes (Hay 2008), one used tympanometric thermometers (Erlewyn‐Lajeunesse 2006), one used temporal artery thermometers (Paul 2010), two studies used rectal thermometers (Nabulsi 2006; Sarrell 2006), and one study used a combination of oral thermometers (> two years old) and rectal thermometers (< two years old) (Kramer 2008).
In all six studies, antipyretic medication was administered orally. Five studies used a paracetamol dose of 15 mg/kg orally (Erlewyn‐Lajeunesse 2006; Hay 2008; Kramer 2008; Nabulsi 2006; Paul 2010) and one study used a loading dose of paracetamol of 25 mg/kg with subsequent doses of 12.5 mg/kg (Sarrell 2006). Four studies used an ibuprofen dose of 10 mg/kg (Hay 2008; Kramer 2008; Nabulsi 2006; Paul 2010), one study used an ibuprofen dose of 5 mg/kg (Erlewyn‐Lajeunesse 2006) and one study used an ibuprofen loading dose of 10mg/kg with subsequent doses of 5 mg/kg (Sarrell 2006).
Three studies compared alternating therapy to ibuprofen alone (Nabulsi 2006; Paul 2010; Sarrell 2006) and two studies compared alternating therapy to paracetamol alone (Kramer 2008; Sarrell 2006). Three studies looked at combined therapy versus ibuprofen alone (Erlewyn‐Lajeunesse 2006; Hay 2008; Paul 2010) and two studies looked at combined therapy versus paracetamol alone (Erlewyn‐Lajeunesse 2006; Hay 2008). Only one study compared alternating therapy to combined therapy (Paul 2010). A summary of the drug dosing and timing is shown in Table 4.
Table 1.
Dosing regimens and timing
| Study ID | Time after administration (hours) | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Erlewyn‐Lajeunesse 2006 | P (15 mg/kg) | Ⓣ | Ⓣ | |||||||
| I (5 mg/kg) | Ⓣ | Ⓣ | ||||||||
| P (15 mg/kg) + I (5 mg/kg) | Ⓣ | Ⓣ | ||||||||
| Hay 2008 | P (15 mg/kg) | Ⓣ | Ⓣ P | Ⓣ | Ⓣ | |||||
| I (10 mg/kg) | Ⓣ | Ⓣ | Ⓣ I | Ⓣ | ||||||
| P (15 mg/kg) + I (10 mg/kg) | Ⓣ | Ⓣ P | Ⓣ I | Ⓣ | ||||||
| Kramer 2008 | P (15 mg/kg) | Ⓣ | Ⓣ P | Ⓣ | Ⓣ | |||||
| P (15 mg/kg) | Ⓣ I (10 mg/kg) | Ⓣ | Ⓣ | Ⓣ | ||||||
| Nabulsi 2006 | I (10 mg/kg) | Ⓣ | Ⓣ | Ⓣ | Ⓣ | Ⓣ | ||||
| I (10 mg/kg) | Ⓣ P (15 mg/kg) | Ⓣ | Ⓣ | Ⓣ | Ⓣ | |||||
| Paul 2010 | I (10 mg/kg) | Ⓣ | Ⓣ | Ⓣ | Ⓣ | Ⓣ | Ⓣ | |||
| I (10 mg/kg) | Ⓣ | Ⓣ | Ⓣ P | Ⓣ | Ⓣ | Ⓣ | ||||
| I (10 mg/kg) + P (15 mg/kg) | Ⓣ | Ⓣ | Ⓣ | Ⓣ | Ⓣ | Ⓣ | ||||
| Sarrell 20061 | P or I | P | ||||||||
| P or I | I | |||||||||
| P or I | P | I | ||||||||
Ⓣ = Temperature reported; P = paracetamol; I = ibuprofen
1Sarrell 2006 asked caretakers to record rectal temperatures three times per day.
The primary outcomes of all studies involved temperature measurements, although these measurements were taken at a wide range of time points after initial medication ingestion (one hour to five days). Three studies also attempted to assess child comfort (Hay 2008; Kramer 2008; Sarrell 2006).
Secondary outcomes included: temperature at two hours, time spent in emergency department (Erlewyn‐Lajeunesse 2006); time to temperature first falling below 37.2 °C in the first 24 hours (fever clearance), time spent without fever over 24 hours, proportion of children without fever‐associated symptoms at 48 hours and day five (Hay 2008); symptom checklist at three and four hours, parental perception of efficacy at three and four hours (Kramer 2008); proportions of afebrile children in each group at seven and eight hours from baseline, maximum decline in temperature during study period, time to recurrence of fever, mean temperature changes from baseline at four and eight hours (Nabulsi 2006); total days that a primary caretaker had to stay home from work because the infant could not attend daycare due to illness, recurrence of fever within five and 10 days after initiation of treatment, and number of emergency department visits within ten days of enrolment (Sarrell 2006).
Adverse effects were listed as a secondary outcome in four studies (Hay 2008; Kramer 2008; Nabulsi 2006; Sarrell 2006) and were not reported in two studies (Erlewyn‐Lajeunesse 2006; Paul 2010).
Excluded studies
One study (Lal 2000) met the search criteria for a RCT in the topic of interest. However, relevant data on mean temperature was not reported. The author of the trial was contacted and did not have available access to the desired data. Thus, the trial was excluded. Another study (Pashapour 2009) met the search criteria for a RCT in the topic of interest. However, the comparison was not relevant to this review as patients received only single doses of medication in the alternating group. For detailed information on reasons for exclusion refer to the Characteristics of excluded studies table.
Risk of bias in included studies
Domain‐specific and overall risk of bias assessments are detailed in the Characteristics of included studies table and summarized by outcome and study in Figure 2 and Figure 3, respectively.
Figure 2.
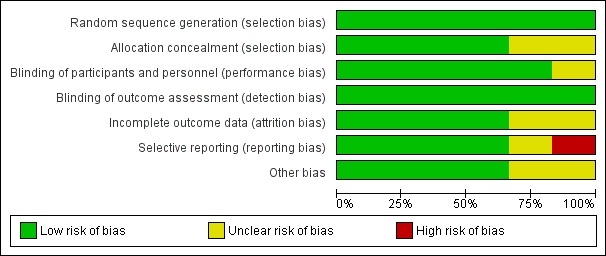
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
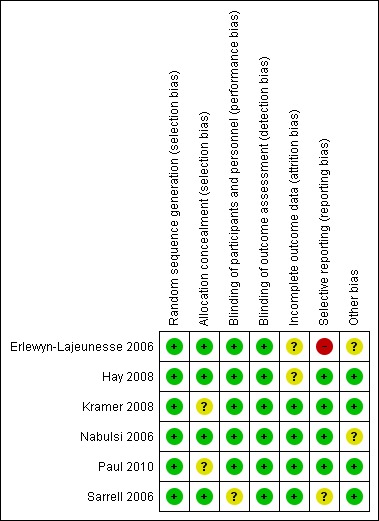
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All six studies had a low risk of bias when reporting a method for generating the randomization sequence (Erlewyn‐Lajeunesse 2006; Hay 2008; Kramer 2008; Nabulsi 2006; Paul 2010; Sarrell 2006).
Four studies had a low risk of bias when concealing the randomization sequence from the investigators and participants (Erlewyn‐Lajeunesse 2006; Hay 2008; Nabulsi 2006; Sarrell 2006). Two studies had an unclear risk of bias when reporting allocation concealment (Kramer 2008; Paul 2010).
Blinding
Five studies had a low risk of bias when addressing blinding of patients and participating personnel (Erlewyn‐Lajeunesse 2006; Hay 2008; Kramer 2008; Nabulsi 2006; Paul 2010). One study had an unclear risk of bias when addressing blinding of patients and participating personnel (Sarrell 2006).
Six studies had a low risk of bias when addressing blinding of outcome assessors (Erlewyn‐Lajeunesse 2006; Hay 2008; Kramer 2008; Nabulsi 2006; Paul 2010; Sarrell 2006; ).
Incomplete outcome data
There was a low risk of bias for incomplete outcome reporting in four studies (Kramer 2008; Nabulsi 2006; Paul 2010; Sarrell 2006) and an unclear risk of bias in two studies (Erlewyn‐Lajeunesse 2006; Hay 2008).
Selective reporting
Four studies had a low risk of bias when reporting outcomes in methods/protocol and results (Hay 2008; Kramer 2008; Nabulsi 2006; Paul 2010), one study had unclear reporting (Sarrell 2006), and one study had a high risk of bias when reporting all outcomes in the methods/protocol and results (Erlewyn‐Lajeunesse 2006).
Other potential sources of bias
The studies were assessed for bias in terms of potential for inappropriate influence of funding agencies and important imbalances in baseline characteristics. Three studies were at low risk of bias for these other sources of bias (Hay 2008; Kramer 2008; Sarrell 2006). Two studies were unclear (Erlewyn‐Lajeunesse 2006; Nabulsi 2006) and two studies were at least partially funded by pharmaceutical companies (Nabulsi 2006; Paul 2010). One study did not disclose sources of funding (Sarrell 2006).
Effects of interventions
Summaries of findings are provided for the following comparisons: alternating versus single agent (Table 5; Table 6), combined versus single agent (Table 7), and alternating versus combined therapy (Table 8).
Table 2.
Summary of findings: Alternating versus single agent for fever in children
| Alternating versus single agent for fever in children | |||||||
| Patient or population: children with fever Intervention: alternating versus single agent | |||||||
| Outcomes | Timepoint | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Single agent | Alternating regimen | ||||||
|
NCCPC score Standardized stress score for non‐verbal children. A score of 7 or more indicates pain. |
Day 1 | The mean NCCPC score in the control group was 11.38 | The mean NCCPC score in the intervention groups was 2.01 lower (2.58 to 1.44 lower) | ‐ | 309 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Day 2 | The mean NCCPC score in the control group was 8.85 | The mean NCCPC score in the intervention groups was 3.76 lower (4.27 to 3.25 lower) | ‐ | 475 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| Day 3 | The mean NCCPC score in the control group was 7.81 | The mean NCCPC score in the intervention groups was 3.63 lower (4.17 to 3.09 lower) | ‐ | 464 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | |||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||||
1 Downgraded by 2 for risk of bias: in this study mothers collected the data on NCCPC scores and were unblinded to allocation. In addition, in this study the mean number of doses of medication was actually lower in the group allocated to alternating treatment. The reasons for this are unclear as logically they should receive more doses.
Table 3.
Summary of findings: Alternating versus single agent for fever in children
| Alternating versus single agent for fever in children | |||||||
| Patient or population: children with fever Intervention: alternating versus single agent | |||||||
| Outcomes | Timepoint | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Single agent | Alternating regimen | ||||||
| Mean Temperature | 1 hour | The mean temperature in the control group was 37.6 °C | The mean temperature in the intervention groups was 0 °C higher (0.28 °C lower to 0.28 °C higher) | ‐ | 40 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4 | Children in the alternating regimen group received a second dose of antipyretic at 3‐4 hours |
| 4 hours | The mean temperature in the control groups ranged from 37.5 °C to 38.0 °C | The mean temperature in the intervention groups was 0.60 °C lower (0.94 °C to 0.26 °C lower) | ‐ | 78 (2 studies) | ⊕⊕⊝⊝ low5,6,7 | ||
| 6 hours | The mean temperature in the control group was 38.5 °C | The mean temperature in the intervention groups was 1.60°C lower (2.27 °C to 0.93 °C lower) | ‐ | 40 (1 study) | ⊕⊝⊝⊝ very low1,3,4 | ||
| Proportion febrile | 1 hour | 20 per 100 | 20 per 100 (6 to 69) | RR 1 (0.29 to 3.45) | 40 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4 | Children in the alternating regimen group received a second dose of antipyretic at 3‐4 hours |
| 4 hours | 30 per 100 | 2 per 100 (0 to 39) | RR 0.08 (0.00 to 1.29) | 40 (1 study) | ⊕⊝⊝⊝ very low1,3,4 | ||
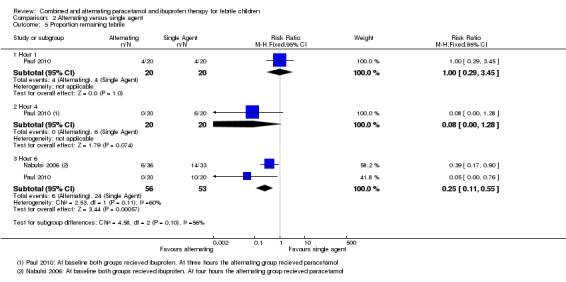
| 6 hours | 45 per 100 | 11 per 100 (5 to 25) | RR 0.25 (0.11 to 0.55) | 109 (2 studies) | ⊕⊕⊝⊝ low8,6,7 | ||
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||||
1 This single study compared a single dose of ibuprofen with ibuprofen plus paracetamol 3 hours later. 2 At this time point both treatment arms had received the same medication so differences would not be expected. 3 Downgraded by 1 for risk of bias: this study was at unclear risk of selection bias as allocation concealment was not described. 4 Downgraded by 2 for very serious imprecision due to the very small sample size. 5Paul 2010 compared ibuprofen at baseline plus paracetamol at 3 hours in the intervention group. Kramer 2008 compared paracetamol at baseline plus ibuprofen at 3 hours in the intervention group. 6 Downgraded by 1 for serious risk of bias: both studies are at unclear risk of selection bias as allocation concealment was not described. 7 Downgraded by 1 for imprecision due to the small sample size. 8Paul 2010 compared ibuprofen at baseline plus paracetamol at 3 hours in the intervention group. Nabulsi 2006 compared ibuprofen at baseline plus paracetamol at 4 hours in the intervention group.
Table 4.
Summary of findings: Combined versus single agent for fever in children
| Combined versus single agent for fever in children | |||||||
|
Patient or population: children with fever Intervention: combined ibuprofen and paracetamol at baseline Control: a single agent alone at baseline | |||||||
| Outcomes | Timepoint | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Single agent | Combined regimen | ||||||
| Mean Temperature | 1 hour | The mean temperature in the control groups ranged from 37.6 °C to 37.9 °C | The mean temperature in the intervention groups was 0.27 °C lower (0.45 °C to 0.08 °C lower) | ‐ | 163 (2 studies) | ⊕⊝⊝⊝ moderate 1,2,3 |
|
| 4 hours | The mean temperature in the control groups ranged from 36.5 °C to 37.5 °C | The mean temperature in the intervention groups was 0.7 °C lower (1.05 °C to 0.35 °C lower) | ‐ | 173 (2 studies) | ⊕⊝⊝⊝ moderate 4,2,3 |
||
| 6 hours | The mean temperature in the control group was 38.5 °C | The mean temperature in the intervention groups was 1.30 °C lower (2.01 °C to 0.59 °C lower) | ‐ | 40 (1 study) | ⊕⊝⊝⊝ very low5,6,7 | ||
| Proportion Febrile | 1 hour | 20 per 100 | 10 per 100 (2 to 49) | RR 0.5 (0.1 to 2.43) | 40 (1 study) | ⊕⊝⊝⊝ very low5,6,7 | |
| 4 hours | 23 per 100 | 2 per 100 (1 to 10) | RR 0.08 (0.02 to 0.43) | 196 (2 studies) | ⊕⊝⊝⊝ moderate 4,2,3 |
||
| 6 hours | 50 per 100 | 5 per 100 (1 to 35) | RR 0.10 (0.01 to 0.71) | 40 participants (1 study) | ⊕⊝⊝⊝ very low5,6,7 | ||
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1 These two studies compared ibuprofen plus paracetamol at baseline with ibuprofen alone (Paul 2010) or ibuprofen or paracetamol alone (Erlewyn‐Lajeunesse 2006). 2 No serious indirectness: these studies were conducted in the UK and the USA in children with mild febrile illness. The studies excluded children with signs of severe illness or contra‐indications to the study drugs. 3 Downgraded by 1 for imprecision due to the small sample size of the studies. 4 These two studies compared ibuprofen plus paracetamol at baseline with ibuprofen alone (Paul 2010 and Hay 2008). 5 This single study was conducted in the USA and compared ibuprofen plus paracetamol at baseline with ibuprofen alone (Paul 2010). 6 Downgraded by 1 for risk of selection bias as allocation concealment was not described. 7 Downgraded by 2 for very serious imprecision: only one very small study.
Table 5.
Summary of findings: Combined versus alternating therapy for fever in children
| Combined versus alternating therapy for fever in children | |||||||
| Patient or population: children with fever Intervention: alternating versus combined therapy | |||||||
| Outcomes | Timepoint | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Alternating therapy | Combinedtherapy | ||||||
| Mean Temperature | 1 hour | The mean temperature in the control group was 37.6 °C | The mean temperature in the intervention groups was 0.2 °C lower (0.48 °C lower to 0.08 °C higher) | ‐ | 40 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |
| 4 hours | The mean temperature in the control group was 36.9 °C | The mean temperature in the intervention groups was 0 °C higher (0.19 °C lower to 0.19 °C higher) | ‐ | 40 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 6 hours | The mean temperature in the control group was 36.9 °C | The mean temperature in the intervention groups was 0.3 °C higher (0.01 °C to 0.59 °C higher) | ‐ | 40 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| Proportion Febrile | 1 hour | 200 per 1000 | 100 per 1000 (20 to 486) | RR 0.5 (0.1 to 2.43) | 40 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |
| 4 hours | ‐ | ‐ | Not estimable | 40 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 6 hours | 0 per 1000 | 0 per 1000 (0 to 0) | RR 3 (0.13 to 69.52) | 40 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||||
1 This single study was conducted in the USA. 2 Downgraded by 1 for risk of bias: this study was at unclear risk of selection bias as allocation concealment was not described. 3 Downgraded by 2 for very serious imprecision due to the very small sample size.
Comparison 1. Combined antipyretics versus single agent
Three studies conducted in the UK and the USA compared giving ibuprofen and paracetamol together at baseline with giving a single agent alone. Two studies compared combined therapy with both paracetamol alone and ibuprofen alone (Erlewyn‐Lajeunesse 2006; Hay 2008), and one study compared combined therapy with ibuprofen alone (Paul 2010).
Measures of child discomfort
One study (Hay 2008) assessed fever‐associated symptoms at 24 hours, 48 hours and five days, but found no consistent evidence showing a benefit of combined therapy over a single agent (data was not presented in the article).
Temperature
Mean temperature was lower after combined treatment at one hour (MD ‐0.27, 95% CI ‐0.45 to ‐0.08, 163 participants, two trials), four hours (MD ‐0.70, 95% CI ‐1.05 to ‐0.35, 173 participants, two trials, Analysis 1.1) and six hours (MD ‐1.30, 95% CI ‐2.01 to ‐0.59, 40 participants, one trial, Analysis 1.1). All reported mean temperatures were 38 °C and below between one and six hours.
Analysis 1.1.
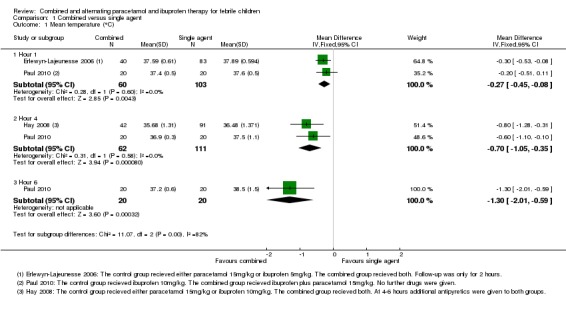
Comparison 1 Combined versus single agent, Outcome 1 Mean temperature (°C).
There was no significant difference in the proportion of patients still febrile at one hour after initial antipyretic administration (RR 0.50, 95% CI 0.10 to 2.43, 40 participants, one trial). However, the proportion remaining febrile was significantly lower following combined treatment at four hours (RR 0.08, 95% CI 0.02 to 0.42, 196 participants, two trials) and six hours (RR 0.10, 95% CI 0.01 to 0.71, 40 participants, one trial, Analysis 1.2).
Analysis 1.2.
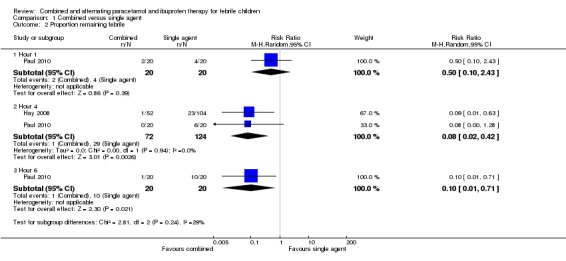
Comparison 1 Combined versus single agent, Outcome 2 Proportion remaining febrile.
Comparison 2. Alternating antipyretics versus single agent
Three studies conducted in the USA, Lebanon and Israel evaluated the benefits of administering a second antipyretic three to four hours after the first dose of a single agent. Two studies administered ibuprofen to both groups at baseline followed by paracetamol to the intervention group at three to four hours (Nabulsi 2006; Paul 2010), and one study administered paracetamol to both groups at baseline followed by ibuprofen to the intervention group at three hours (Kramer 2008).
One additional three‐arm study compared alternating paracetamol and ibuprofen every four hours with paracetamol alone (every six hours) and ibuprofen alone (every eight hours) (Sarrell 2006). The children were followed up for three days through telephone conversations with caregivers, who were asked to measure the rectal temperature three times daily, record the number of doses of medications given, and to assess the non‐communicating children's pain checklist (NCPCC) score. The NCCPC scoring system was designed to be used for children (3 to 18 years) who are unable to speak; a total score of seven or more indicates a child is experiencing pain.
Measures of child discomfort
Sarrell 2006 found that NCPCC scores were lower in children receiving alternating therapy than in those receiving either of the single agents (day one versus paracetamol alone: MD ‐2.51, 95% CI ‐3.08 to ‐1.94, 309 participants, one trial; versus ibuprofen alone: MD ‐2.22, 95% CI ‐2.78 to 1.66, 310 participants, one trial, Analysis 2.1). The benefits of alternating therapy were also apparent on days two and three, although mean NCPCC scores decreased in all groups over time (Analysis 2.1). There were no significant differences between ibuprofen alone and paracetamol alone.
Analysis 2.1.
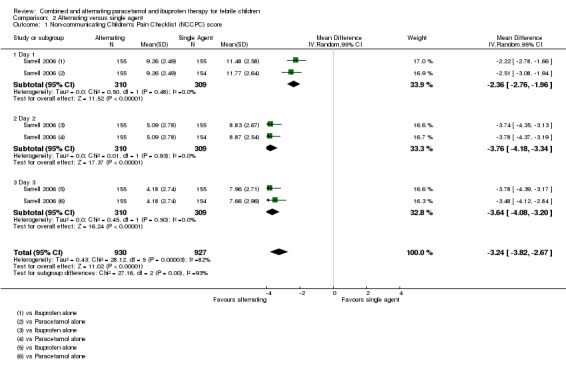
Comparison 2 Alternating versus single agent, Outcome 1 Non‐communicating Children's Pain Checklist (NCCPC) score.
The study also looked at days absent from daycare as a secondary outcome, which also favoured the alternating group (versus paracetamol alone: MD ‐0.88, 95% CI ‐1.02 to ‐0.74, 309 participants, one trial; versus ibuprofen alone: ‐0.82, 95% CI ‐0.96 to ‐0.68, 310 participants, one trial, Analysis 2.2).
Analysis 2.2.
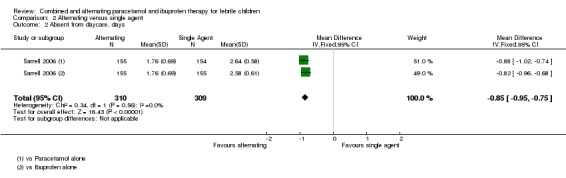
Comparison 2 Alternating versus single agent, Outcome 2 Absent from daycare, days.
However, the trial reports that the alternating group actually received a lower mean number of doses of antipyretic per child. This finding suggests that the allocated regimens were not followed by the caretaker even during the first day. In the light of this, it is difficult to understand the improvement in NCPCC scores and reduction in days absent from childcare.
Temperature
At one hour after administration of ibuprofen to both groups, Paul 2010 found no difference in mean temperature (MD 0.00, 95% CI ‐0.28 to 0.28, 40 participants, one trial, Analysis 2.4). At four hours, one hour after administration of the alternative agent to the intervention group, the mean temperature was significantly lower in the intervention group (MD ‐0.60, 95% CI ‐0.94 to ‐0.26, 78 participants, two trials, Analysis 2.4). One study demonstrated that this difference remained statistically significant at six hours (MD ‐1.60, 95% CI ‐2.27 to ‐0.93, 40 participants, one trial, Analysis 2.4).
Analysis 2.4.
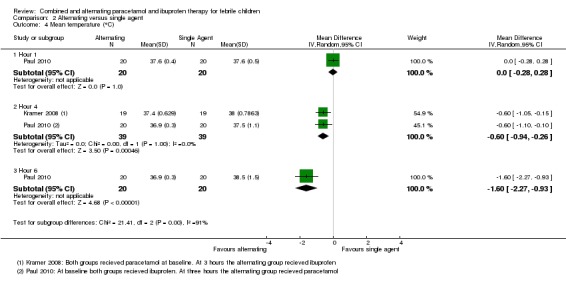
Comparison 2 Alternating versus single agent, Outcome 4 Mean temperature (°C).
In studies administering ibuprofen to both groups at baseline, followed by paracetamol at three to four hours for the intervention group, the proportion remaining febrile was significantly lower in the intervention group at six hours (RR 0.25, 95% CI 0.11 to 0.55, 109 participants, two trials, Analysis 2.5).
Analysis 2.5.
Comparison 2 Alternating versus single agent, Outcome 5 Proportion remaining febrile.
Comparison 3. Combined versus alternating
One study conducted in the USA compared giving combined ibuprofen and paracetamol at baseline, with giving ibuprofen alone followed by paracetamol three hours later (Paul 2010).
Measures of child discomfort.
The study did not address measures of child discomfort.
Temperature
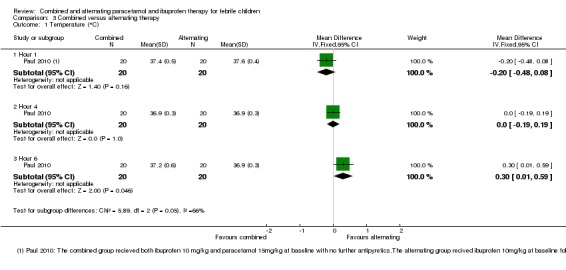
Mean temperature was lower following combined therapy at one hour, although this did not reach statistical significance (MD ‐0.20, 95% CI ‐0.48 to 0.08, 40 participants, one trial, Analysis 3.1). At four hours, one hour after the alternating group received their second antipyretic, there was no difference between the groups (MD 0.00, 95% CI ‐0.19 to 0.19, 40 participants, one trial, Analysis 3.1). At six hours, mean temperature was lower in the group given alternating therapy (MD 0.30, 95% CI 0.01 to 0.59, 40 participants, one trial, Analysis 3.1)
Analysis 3.1.
Comparison 3 Combined versus alternating therapy, Outcome 1 Temperature (°C).
The proportion remaining febrile at one, four and six hours was very low in both groups with no significant differences between them (40 participants, one study, Analysis 3.2).
Analysis 3.2.
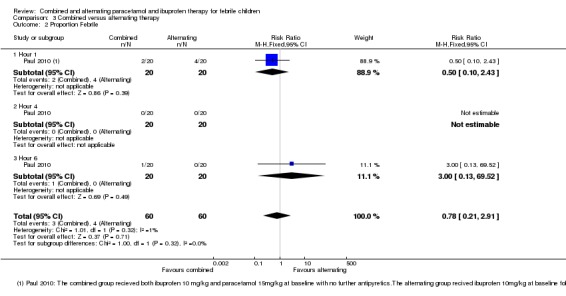
Comparison 3 Combined versus alternating therapy, Outcome 2 Proportion Febrile.
Adverse effects
Overall, there were no serious adverse effects thought to be associated with alternating, combined or monotherapy found in any studies. However, no study had sufficient power in terms of number of participants to make a definitive statement about frequency of severe adverse effects. A table summarizing adverse effect evaluation for each study is available in Table 9.
Table 6.
Adverse Effects
| Comparison | Studies |
N |
Duration of follow up | Serious adverse events | Comments |
| Combined versus single agent | Erlewyn‐Lajeunesse 2006 | 123 | 2 hours | Not reported | |
| Hay 2008 | 156 | 5 days | Five serious AE occurred (Admission to hospital ‐ reasons not reported) with no difference between groups | Non‐severe adverse events (mainly diarrhoea and vomiting) were evenly distributed between groups2 | |
| Paul 2010 | 46 | 6 hours | Not reported | ||
| Alternating versus single agent |
Paul 2010 | 46 | 6 hours | Not reported | |
|
Kramer 2008 |
40 | 6 hours |
None observed |
Non‐severe adverse effects reported in 8/38 (21%) of patients with no difference between groups1 |
|
|
Nabulsi 2009 |
70 | 8 hours | None observed | Rectal temperature < 36.5 °C (range 35.0 °C to 36.2 °C) 5 (13.9%) combined group 6 (18.2%) ibuprofen group |
|
| Sarrell 2006 | 480 | 14 days | None observed | Mild elevated liver enzymes, n=8, mild abnormal renal function, n=14, all normalized by 14 day follow up | |
| Alternating versus combined therapy | Paul 2010 | 46 | 6 hours | Not reported |
1 Non‐severe AE stated as: diarrhoea, flatulence, emesis, decreased appetite, epigastric pain, nausea, headache, insomnia. Symptoms did not prevent any patients from taking study medications.
Discussion
Summary of main results
Six trials, enrolling 915 participants, are included.
Compared to giving a single antipyretic alone, giving combined paracetamol and ibuprofen to febrile children can result in a lower mean temperature at one hour after treatment (moderate quality evidence). If no further antipyretics are given, combined treatment probably also results in a lower mean temperature at four hours (moderate quality evidence), and in fewer children remaining or becoming febrile for at least four hours after treatment (moderate quality evidence).
In practice, mothers are often advised to initially give a single agent (paracetamol or ibuprofen), and then give a further dose of the alternative if the child's fever fails to resolve or recurs. Giving alternating treatment in this way may result in a lower mean temperature at one hour after the additional dose (low quality evidence), and may also result in fewer children remaining or becoming febrile for up to three hours after it is given (low quality evidence).
Only one small trial compared alternating therapy with combined therapy. No statistically significant differences were seen in mean temperature or in the number febrile at one, four or six hours (very low quality evidence).
There were no serious adverse events in the trials that were directly attributed to the medications used.
Overall completeness and applicability of evidence
This review was intended to focus on patient comfort/distress as the primary outcome of, and the primary reason for, administering antipyretics, but information on patient comfort/distress was rarely reported, and reliable conclusions could not be made. 'Discomfort' is not an easily definable outcome and this review was only able to consider a few measurable, albeit potentially indirect, manifestations (ie pain score, amount of medication given, days away from daycare). In fact, comfort is a complex construct, which likely varies across different age groups and may even extend to caregivers of febrile children. The amount of medication given to children by caregivers may be a reflection of their distress over child discomfort. This review does not address the role of caregiver distress in the management of febrile children.
For the effects of antipyretics on temperature, the included studies enrolled children aged six months to 14 years with fever presumed to be viral or bacterial in origin. The trials were from moderate to high income settings in Europe, the USA, and the Middle East, and therefore the findings should not be extrapolated to tropical settings where the common causes of fever may be substantially different.
There was a large amount of variation between the trials in medication dosage, regimens of administration, and frequency and type of assessment. Due to the small number of studies in each comparison, we were unable to assess the impact of these variations.
Similarly, there was large variation in patient factors such as age, aetiology (viral or bacteria), severity of illness, and co‐morbidities that may affect the effectiveness of interventions. Two studies only included children less than five years old. Only one study attempted to distinguish between viral and bacterial infections. Two studies involved patients from emergency departments, three involved patients from outpatient clinics and two involved patients from inpatient wards. Thus, there was insufficient data from the few included trials to allow for subgroup comparisons. Higher temperatures may be related to increasing severity of illness, however this data could not be obtained from the studies. Most trials excluded children with co‐morbidities.
For the analyses looking at mean temperature, some of the mean differences were statistically significant but probably not clinically meaningful in those cases where both treatment groups had mean temperatures within the range of being afebrile
Safety concerns have been raised about caregivers giving inappropriate doses and getting confused over when the next dose is due in alternating and combined therapy. In addition, the effectiveness of administering combined or alternating antipyretics may be very different when administered by parents and caregivers than when administered in a controlled setting with health care professionals supervising.
Current guidelines recommend only monotherapy for febrile children, in order to avoid potential side effects from multiple medication administration. The results from this study do not suggest any serious short term adverse effects from either alternating or combined antipyretic therapy compared with monotherapy. However, none of the included trials was large enough to have the power to detect important differences between treatment arms, nor were they long enough to detect potential adverse events from regular use. From the vast amount of literature on paracetamol and ibuprofen both drugs are regarded as safe with serious side effects being few and infrequent.
Quality of the evidence
The quality of the evidence summarized in this review is presented in Table 5; Table 6; Table 7; Table 8.
For combined therapy versus a single agent, the evidence for a reduction in mean temperature at one and four hours was judged to be of moderate quality, meaning we can have reasonable confidence in this result. Although the studies are generally at low risk of bias, the sample size is small (less than 200 patients overall) and the possibility of chance findings and publication bias is high. The evidence of an effect on patient discomfort could not be graded as the data were not adequately presented. However, the data would likely be of low or very low quality as they come from a single trial.
For alternating therapy versus single agent, the overall sample size is larger, however the largest study with 480 subjects (Sarrell 2006) provided information only on the Non‐communicating Children's Checklist, days absent from daycare and doses of medication per child. When looking at reduction in mean temperature and proportion remaining febrile, the sample size is very small (less than 200 patients overall). The quality of evidence for reductions in mean temperature and the proportions remaining febrile is of low quality at best, meaning we can have little confidence in the results. The evidence for a reduction in mean NCPCC score is also judged to be of low quality.
For combined versus alternating therapy, the evidence was downgraded to 'very low' due to the extremely small study size (40 participants) and the lack of allocation concealment in the single trial. For these reasons, we can have no real confidence in the results of this trial.
Potential biases in the review process
None identified.
Agreements and disagreements with other studies or reviews
Three systematic reviews looking at combined or alternating ibuprofen and paracetamol therapy exist in the literature (Nabulsi 2009; Pereira 2012; Purssell 2011). All three reviews raised similar concerns to those highlighted in this review regarding lack of blinding and reasons for withdrawal from studies, low sample size, and variable drug doses and administration regimens.
Nabulsi 2010 examined five studies that were included in this review (Erlewyn‐Lajeunesse 2006; Hay 2008; Kramer 2008; Nabulsi 2006; Sarrell 2006). The review differs from ours in that the primary outcome measure was temperature reduction and it did not include measures of patient discomfort. Our review also includes one additional study. Nabulsi et al. concluded that, given ongoing uncertainty, either drug alone should be used instead of combined or alternating regimens.
Purssell 2011 examined seven studies (Erlewyn‐Lajeunesse 2006; Hay 2008; Kramer 2008; Lal 2000; Nabulsi 2006; Paul 2010; Sarrell 2006), one of which (Lal 2000) is excluded from this review as no extractable numerical data was published. Purssell concluded that the practice of combining paracetamol and ibuprofen has limited benefit and unclear safety data, and thus should not be encouraged.
Pereira 2012 examined four studies looking at alternating antipyretic therapy only (Hay 2008; Kramer 2008; Paul 2010; Sarrell 2006). It also excluded studies involving hospitalized children, whereas we included studies with hospitalized children. The main measure of the effect of treatment was the mean difference in body temperature among the compared groups and the study did not include measures of child discomfort. Pereira concluded there was not enough evidence to show that alternating antipyretic therapy is more effective than monotherapy and had concerns regarding the safety of using this regimen to treat febrile children.
Other systematic reviews or meta‐analyses in the literature assess studies comparing paracetamol directly with ibuprofen, which was not the focus of this review.
Authors' conclusions
There is some evidence that both alternating and combined antipyretic therapy may be more effective at reducing temperatures than monotherapy alone. However, the evidence for improvements in measures of child discomfort remains inconclusive.
There is insufficient evidence to support the use of alternating antipyretic therapy over combined antipyretic therapy.
Future RCTs should focus on child discomfort using standardized and validated assessment tools.
More research is needed on the safety of alternating and combined antipyretic regimens.
Acknowledgements
The academic editor for this protocol was Professor Paul Garner.
This review is partially funded by the Alberta Children’s Hospital (ACH) Foundation; the views expressed are not necessarily those of the ACH Foundation. We thank Calli Carrington and Emily Macphail for their assistance in article review and inclusion. We would like to thank Dr David Sinclair for his significant contribution to the structure of the review, data analysis, summary of findings tables and general editorial advice. We thank Christianne Esparza for assistance with translation and data extraction. The editorial base for the Cochrane Infectious Diseases Group is funded by the Department for International Development, UK, for the benefit of low‐ and middle‐income countries.
Appendices
Appendix 1. Table 1: Search Strategies for CENTRAL, MEDLINE, EMBASE, and International Pharmaceutical Abstracts
| Search Set | CENTRAL | MEDLINE (1950‐present)[1] | EMBASE (1980‐present)[2] | IPA (1970‐present) |
| 1 | ACETAMINOPHEN[3] | ACETAMINOPHEN | PARACETAMOL | ‐ |
| 2 | acetaminophen or paracetamol or acamol or acenol or acephen or acetaco or acetalgin or acetamidophenol or acetamino phenol or acetaminophene or acetaminophenol or acetamol or acetominophen or acetylaminophenol or algotropyl or alvedon or amadil or anacin or anaflon or apamide or apap or apotel or arthralgen or benuron or calpol or cp 500 or cp500 or dafalgan or datril or depon or disprol or dolal or doliprane or dolprone or duorol or efferalgan or enelfa or eneril or eu med or febrilix or fendon or fibrinol or gelocatil or hedex or hydroxyacetanilide or janupap or letamol or liquiprin or lyteca or medamol or metalid or mexalen or napap or nebs or neocitran or neodalmin or nevral or nobedon or n‐acetyl‐p‐aminophenol or pacemol or pamal or pamol or panadol or panasorb or panodil or para suppo or paracetaminophenol or paracetamole or paralen or paramax or paratabs or pasolind or perfalgan or phenaphen or polarfen or puernol or pyrigesic or rapidol or relaphen or rhodapap or sedes a or sinpro or tabalgin or tachipirin or tachipirina or tapar or tempra or tralgon or tramil or treuphadol or tylenol or valadol or zolben | acetaminophen or paracetamol or acamol or acenol or acephen or acetaco or acetalgin or acetamidophenol or acetamino phenol or acetaminophene or acetaminophenol or acetamol or acetominophen or acetylaminophenol or algotropyl or alvedon or amadil or anacin or anaflon or apamide or apap or apotel or arthralgen or benuron or calpol or cp 500 or cp500 or dafalgan or datril or depon or disprol or dolal or doliprane or dolprone or duorol or efferalgan or enelfa or eneril or eu med or febrilix or fendon or fibrinol or gelocatil or hedex or hydroxyacetanilide or janupap or letamol or liquiprin or lyteca or medamol or metalid or mexalen or napap or nebs or neocitran or neodalmin or nevral or nobedon or n‐acetyl‐p‐aminophenol or pacemol or pamal or pamol or panadol or panasorb or panodil or para suppo or paracetaminophenol or paracetamole or paralen or paramax or paratabs or pasolind or perfalgan or phenaphen or polarfen or puernol or pyrigesic or rapidol or relaphen or rhodapap or sedes a or sinpro or tabalgin or tachipirin or tachipirina or tapar or tempra or tralgon or tramil or treuphadol or tylenol or valadol or zolben | acetaminophen or paracetamol or acamol or acenol or acephen or acetaco or acetalgin or acetamidophenol or acetamino phenol or acetaminophene or acetaminophenol or acetamol or acetominophen or acetylaminophenol or algotropyl or alvedon or amadil or anacin or anaflon or apamide or apap or apotel or arthralgen or benuron or calpol or cp 500 or cp500 or dafalgan or datril or depon or disprol or dolal or doliprane or dolprone or duorol or efferalgan or enelfa or eneril or eu med or febrilix or fendon or fibrinol or gelocatil or hedex or hydroxyacetanilide or janupap or letamol or liquiprin or lyteca or medamol or metalid or mexalen or napap or nebs or neocitran or neodalmin or nevral or nobedon or n‐acetyl‐p‐aminophenol or pacemol or pamal or pamol or panadol or panasorb or panodil or para suppo or paracetaminophenol or paracetamole or paralen or paramax or paratabs or pasolind or perfalgan or phenaphen or polarfen or puernol or pyrigesic or rapidol or relaphen or rhodapap or sedes a or sinpro or tabalgin or tachipirin or tachipirina or tapar or tempra or tralgon or tramil or treuphadol or tylenol or valadol or zolben | acetaminophen or paracetamol or acamol or acenol or acephen or acetaco or acetalgin or acetamidophenol or acetamino phenol or acetaminophene or acetaminophenol or acetamol or acetominophen or acetylaminophenol or algotropyl or alvedon or amadil or anacin or anaflon or apamide or apap or apotel or arthralgen or benuron or calpol or cp 500 or cp500 or dafalgan or datril or depon or disprol or dolal or doliprane or dolprone or duorol or efferalgan or enelfa or eneril or eu med or febrilix or fendon or fibrinol or gelocatil or hedex or hydroxyacetanilide or janupap or letamol or liquiprin or lyteca or medamol or metalid or mexalen or napap or nebs or neocitran or neodalmin or nevral or nobedon or n‐acetyl‐p‐aminophenol or pacemol or pamal or pamol or panadol or panasorb or panodil or para suppo or paracetaminophenol or paracetamole or paralen or paramax or paratabs or pasolind or perfalgan or phenaphen or polarfen or puernol or pyrigesic or rapidol or relaphen or rhodapap or sedes a or sinpro or tabalgin or tachipirin or tachipirina or tapar or tempra or tralgon or tramil or treuphadol or tylenol or valadol or zolben |
| 3 | IBUPROFEN | IBUPROFEN | IBUPROFEN | ‐ |
| 4 | ibuprofen or brufen or propionic acid or advil or aktren or algifor or algofen or analgyl or anco or attritin or balkaprofen or brufort or bufohexal or burana or contraneural or dc 7034 or dc7034 or dg 7034 or dg7034 or dolgit or dolocyl or dolodolgit or ecoprofen or emflam or exidol or femapirin or fenalgic or fenbid or halprin or haltran or ibofen or ibudak or ibufen or ibugel or ibugesic or ibulgan or ibumetin or ibuprin or ibusynth or ibutop or irfen or ibu slow or junifen or kontraneural or lidifen or maxagesic or mcn r 1451 or medipren or mediprin or mensoton or midol 200 or motrin or neobrufen or nerofen or novogent n or nugin or nuprin or nureflex or nurofen or optifen or opturem or paduden or pedea or proflex or rebugen or reuvol or rufen or rufort or seclodin or tabalon or trendar or unipro or urem | ibuprofen or brufen or propionic acid or advil or aktren or algifor or algofen or analgyl or anco or attritin or balkaprofen or brufort or bufohexal or burana or contraneural or dc 7034 or dc7034 or dg 7034 or dg7034 or dolgit or dolocyl or dolodolgit or ecoprofen or emflam or exidol or femapirin or fenalgic or fenbid or halprin or haltran or ibofen or ibudak or ibufen or ibugel or ibugesic or ibulgan or ibumetin or ibuprin or ibusynth or ibutop or irfen or ibu slow or junifen or kontraneural or lidifen or maxagesic or mcn r 1451 or medipren or mediprin or mensoton or midol 200 or motrin or neobrufen or nerofen or novogent n or nugin or nuprin or nureflex or nurofen or optifen or opturem or paduden or pedea or proflex or rebugen or reuvol or rufen or rufort or seclodin or tabalon or trendar or unipro or urem | ibuprofen or brufen or propionic acid or advil or aktren or algifor or algofen or analgyl or anco or attritin or balkaprofen or brufort or bufohexal or burana or contraneural or dc 7034 or dc7034 or dg 7034 or dg7034 or dolgit or dolocyl or dolodolgit or ecoprofen or emflam or exidol or femapirin or fenalgic or fenbid or halprin or haltran or ibofen or ibudak or ibufen or ibugel or ibugesic or ibulgan or ibumetin or ibuprin or ibusynth or ibutop or irfen or ibu slow or junifen or kontraneural or lidifen or maxagesic or mcn r 1451 or medipren or mediprin or mensoton or midol 200 or motrin or neobrufen or nerofen or novogent n or nugin or nuprin or nureflex or nurofen or optifen or opturem or paduden or pedea or proflex or rebugen or reuvol or rufen or rufort or seclodin or tabalon or trendar or unipro or urem | ibuprofen or brufen or propionic acid or advil or aktren or algifor or algofen or analgyl or anco or attritin or balkaprofen or brufort or bufohexal or burana or contraneural or dc 7034 or dc7034 or dg 7034 or dg7034 or dolgit or dolocyl or dolodolgit or ecoprofen or emflam or exidol or femapirin or fenalgic or fenbid or halprin or haltran or ibofen or ibudak or ibufen or ibugel or ibugesic or ibulgan or ibumetin or ibuprin or ibusynth or ibutop or irfen or ibu slow or junifen or kontraneural or lidifen or maxagesic or mcn r 1451 or medipren or mediprin or mensoton or midol 200 or motrin or neobrufen or nerofen or novogent n or nugin or nuprin or nureflex or nurofen or optifen or opturem or paduden or pedea or proflex or rebugen or reuvol or rufen or rufort or seclodin or tabalon or trendar or unipro or urem |
| 5 | 1‐4/or | 1‐4/or | 1‐4/or | 1‐4/or |
| 6 | ‐ | FEVER | FEVER | ‐ |
| 7 | fever* or pyrexia* or hyperthermia* or temperature | fever* or pyrexia* or hyperthermia* or temperature | fever* or pyrexia* or hyperthermia* or temperature | fever* or pyrexia* or hyperthermia* or temperature |
| 8 | 6 or 7 | 6 or 7 | 6 or 7 | 6 or 7 |
| 9 | CHILD | CHILD | CHILD | ‐ |
| 10 | ADOLESCENT | ADOLESCENT | ADOLESCENT | ‐ |
| 11 | INFANT | INFANT | INFANT | ‐ |
| 12 | ‐ | ‐ | NEWBORN | ‐ |
| 13 | PEDIATRICS | PEDIATRICS | PEDIATRICS | ‐ |
| 14 | child* or pediatric* or paediatric* or perinat* or neonat* or newborn* or infan* or bab* or toddler* or boy* or girl* or school?age or juvenil* or adolescen* | child* or pediatric* or paediatric* or perinat* or neonat* or newborn* or infan* or bab* or toddler* or boy* or girl* or school?age or juvenil* or adolescen* | child* or pediatric* or paediatric* or perinat* or neonat* or newborn* or infan* or bab* or toddler* or boy* or girl* or school?age or juvenil* or adolescen* | child* or pediatric* or paediatric* or perinat* or neonat* or newborn* or infan* or bab* or toddler* or boy* or girl* or school?age or juvenil* or adolescen* |
| 15 | infan* or child* or pediatric* or paediatric* or adolescen* (journal title word) | infan* or child* or pediatric* or paediatric* or adolescen* (journal title word) | infan* or child* or pediatric* or paediatric* or adolescen* (journal title word) | infan* or child* or pediatric* or paediatric* or adolescen* (journal name) |
| 16 | 9‐15/or | 9‐16/or | 9‐16/or | 9‐16/or |
| 17 | 5 and 8 and 17 | 5 and 8 and 17 | 5 and 8 and 17 | 5 and 8 and 17 |
[1] In addition to the terms in the table, the MEDLINE search includes the highly sensitive search strategy for identification of RCTs in MEDLINE (sensitivity‐maximizing version) described in the current Cochrane Handbook for Systematic Reviews of Interventions (section 6.4.11).
[2] In addition to the terms in the table, the EMBASE search includes the Cochrane Handbook for Systematic Reviews of Interventions' recommended terms for identifying RCTs in EMBASE (see section 6.3.2.2 of the Handbook).
[3] Terms in all caps indicate a subject heading used by that particular database. Terms in lower‐case are searched as general keywords unless otherwise noted in parentheses after the search string.
Data and analyses
Comparison 1.
Combined versus single agent
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean temperature (°C) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Hour 1 | 2 | 163 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.45, ‐0.08] |
| 1.2 Hour 4 | 2 | 173 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.05, ‐0.35] |
| 1.3 Hour 6 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.01, ‐0.59] |
| 2 Proportion remaining febrile | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Hour 1 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.43] |
| 2.2 Hour 4 | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.02, 0.42] |
| 2.3 Hour 6 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.71] |
Comparison 2.
Alternating versus single agent
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐communicating Children's Pain Checklist (NCCPC) score | 1 | 1857 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐3.82, ‐2.67] |
| 1.1 Day 1 | 1 | 619 | Mean Difference (IV, Random, 95% CI) | ‐2.36 [‐2.76, ‐1.96] |
| 1.2 Day 2 | 1 | 619 | Mean Difference (IV, Random, 95% CI) | ‐3.76 [‐4.18, ‐3.34] |
| 1.3 Day 3 | 1 | 619 | Mean Difference (IV, Random, 95% CI) | ‐3.64 [‐4.08, ‐3.20] |
| 2 Absent from daycare, days | 1 | 619 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐0.95, ‐0.75] |
| 3 Doses of medication per child | 1 | 2166 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐1.69, ‐0.88] |
| 3.1 Day 1 | 1 | 619 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐2.40, 0.22] |
| 3.2 Day 2 | 1 | 619 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.29, ‐0.49] |
| 3.3 Day 3 | 1 | 928 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐1.48, ‐1.30] |
| 4 Mean temperature (°C) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Hour 1 | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.28, 0.28] |
| 4.2 Hour 4 | 2 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.94, ‐0.26] |
| 4.3 Hour 6 | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.27, ‐0.93] |
| 5 Proportion remaining febrile | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hour 1 | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.29, 3.45] |
| 5.2 Hour 4 | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.28] |
| 5.3 Hour 6 | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.11, 0.55] |
Analysis 2.3.
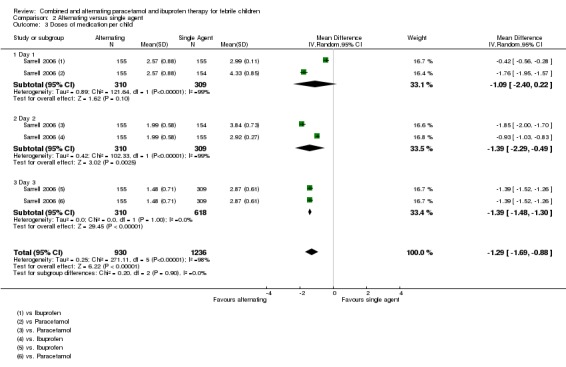
Comparison 2 Alternating versus single agent, Outcome 3 Doses of medication per child.
Comparison 3.
Combined versus alternating therapy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Temperature (°C) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Hour 1 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.48, 0.08] |
| 1.2 Hour 4 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.19, 0.19] |
| 1.3 Hour 6 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.01, 0.59] |
| 2 Proportion Febrile | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.91] |
| 2.1 Hour 1 | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.43] |
| 2.2 Hour 4 | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Hour 6 | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
Differences between protocol and review
The initial protocol focused on decline in temperature measurements over time as a primary outcome. Common clinical practice is to recommend antipyretic therapy for children with fever who are distressed, and not necessarily for lowering absolute temperature. This review has altered the primary outcome by including patient discomfort in addition to temperature measurements. Anna Thomsen was added as an author for her contributions as data extractor.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: open label, three‐arm randomized trial. Study dates and duration: October 2004 to January 2005. Method of temperature measurement: tympanometric thermometer. Time points measured in study: admission, T0 (time medication given), hour 1 and 2 (if patient not discharged). |
|
| Participants | Number: 123 randomized. Number of patients in each intervention: paracetamol n=41, ibuprofen n=42, paracetamol plus ibuprofen n=40. Inclusion criteria: consecutive children between 6 months and 10 years old with a fever of 38 °C. Exclusion criteria: paracetamol or ibuprofen given in the previous six hours, severe or life threatening infection, suspected chicken pox, cellulitis or other spreading skin infection, known to be immunosuppressed, allergy to either ibuprofen or paracetamol, medicated with warfarin, heparin or antihypertensives, symptoms of active gastrointestinal bleeding, known coagulopathy, acute jaundice, likely dehydration, defined as more than four episodes of diarrhoea or vomiting in the previous 24 hours, asthma, defined as a need for regular 'preventer' medication, chronic renal, liver or cardiac failure. Baseline characteristics:
|
|
| Interventions | Group 1: paracetamol 15 mg/kg Group 2: ibuprofen 5 mg/kg Group 3: paracetamol 15 mg/kg + ibuprofen 5 mg/kg Frequency of administration: single dose of each |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Location: Bristol, UK Setting: single centre ‐ Children's Emergency Department, Bristol. Funding: the Anthony Hopkins Memorial Prize, awarded by the Faculty of Accident and Emergency Medicine as an unrestricted award to the Emergency Department. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was block randomized and generated independently of the research team. |
| Allocation concealment (selection bias) | Low risk | Allocations were placed in sequentially numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open label trial, however temperature is an objective measurement that should not be subject to bias from lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This was an open label trial, however temperature is an objective measurement that should not be subject to bias from lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons given for missing data or patients withdrawn. Subjects withdrawn or missing data: combined 9%, ibuprofen 14.7%, paracetamol 9%. |
| Selective reporting (reporting bias) | High risk |
|
| Other bias | Unclear risk | Potential baseline imbalance: higher proportion of patients in combined group were admitted to hospital compared with other groups. |
| Methods | Study design: individually‐randomized, blinded three‐arm trial. Study dates and duration: January 2005 to May 2007. Method of temperature measurement: axillary continuous probe for 24 hours, then standard digital axillary thermometer for home measurements. Time points measured in study: temperature taken every 30 seconds using axillary temperature probe for first 24 hours, then as needed with standard axillary thermometer at home. |
|
| Participants | Number: 156 randomized. Number of patients in each intervention: paracetamol n=52, ibuprofen n=52, paracetamol plus Ibuprofen n=52. Inclusion criteria: children aged 6 months to 6 years in the primary care setting and households in England. Required axillary temperatures of at least 37.8 °C and up to 41 °C. Exclusion criteria: if patients required hospital admission, clinically dehydrated; had recently participated in another trial; had previously participated in PITCH; had a known intolerance, allergy or contraindication to a trial drug; had a chronic neurological, cardiac, pulmonary (except asthma), liver or renal disease; or had parents who could not read or write in English. Baseline characteristics:
|
|
| Interventions | Group A: paracetamol 15 mg/kg every 4‐6 hours Group B: ibuprofen 10 mg/kg every 6‐8 hours Group C: paracetamol + ibuprofen alternating Advice was given to parents to give the study drugs for up to 48 hours |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Location: England Setting: multi‐centre – 35 primary care sites (NHS Direct, one walk‐in centre, 30 general practices, two general practitioner out of hours cooperatives, and the emergency department of the Bristol Royal Hospital for Children) and households. Funding: National Institute for Health Research Health Technology Programme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was generated via a remote, automated telephone service provided by the Health Services Research Unit at the University of Aberdeen. |
| Allocation concealment (selection bias) | Low risk | After written informed consent had been obtained and the baseline questionnaire completed, the research nurse telephoned a remote, automated randomization service. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medication identity was concealed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research nurse was blinded to process. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Article did not address why three patients (one from ibuprofen and two from ibuprofen plus paracetamol group) had missing data for time without fever. "Attrition was minimal." "Thus, children were omitted from analyses only if none of the data required were available, and as these were so few in number the influence of missing data on the intention‐to‐treat analyses was negligible." |
| Selective reporting (reporting bias) | Low risk | All assessed outcomes were reported. |
| Other bias | Low risk | The possibility of receiving either or both drugs combined and the severity of the child's illness may have influenced parental decision to participate. |
| Methods | Study design: prospective, randomized double‐blind placebo control study. Study dates and duration: January 2004 to January 2006. Method of temperature measurement: children > 2 years oral, Children < 2 years rectal. Parents given thermometers for home use. Time points measured in study:temperature measurements at hours 0, 3, 4, 5, 6. |
|
| Participants | Number: 40 randomized. Number of patients in each intervention: paracetamol n=19, paracetamol alternating with ibuprofen n=19. Inclusion criteria: healthy children presenting to the out patient clinic with chief complaint of fever. Fever in clinic > 38 °C Exclusion criteria: history of any antipyretic use in the preceding 4 hours or if they had an allergy or other medical contraindication to the medications. Baseline characteristics:
|
|
| Interventions | Group A: paracetamol (15 mg/kg) alternated with placebo Group B: paracetamol (15 mg/kg) alternating with Ibuprofen (10 mg/kg) Administration regime: Time Group A Group B 0 APAP APAP 3 placebo ibuprofen 4 APAP placebo |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Location: Washington, USA Setting: single centre: pediatric clinic at Madigan Army Medical centre in Tacoma, Washington. Funding: Resident Research Grant from the American Academy of Pediatrics. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were assigned to treatment group A or B using previously generated computer based randomization blocks performed by the Department of Clinical investigation. |
| Allocation concealment (selection bias) | Unclear risk | Each caretaker received a sealed envelope containing their randomization sequence. No mention of the envelope being opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents and investigators remained blinded to the regimen each child had received. Pharmacist was unblinded, but did not assess patients. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents and investigators who measured temperature remained blinded to the regimen each child received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All subjects were accounted for. Loss to follow up: alternating group 0.5%, paracetamol 0.5%. |
| Selective reporting (reporting bias) | Low risk | All assessed outcomes were reported. |
| Other bias | Low risk | |
| Methods | Study design: randomized, double‐blind and placebo‐controlled clinical trial. Study dates and duration: November 2002 to April 2005. Method of temperature measurement: rectal. Each patient used the same thermometer for the whole duration of study (SureTemp 679, Welch Allyn). Time points measured in study: baseline rectal temperature at T0 then at hours 4, 5, 6, 7, 8. |
|
| Participants | Number: 70 randomized Number of patients in each intervention: combined ibuprofen & paracetamol n=37, ibuprofen & placebo n=33. Inclusion criteria: febrile inpatients aged 6 months – 14 years, with rectal temp ≥ 38.8 °C. Exclusion criteria: vomiting, any medical or surgical condition that precluded oral drug administration, acute or chronic hepatic disease, malabsorption syndromes, acute or chronic renal disease with the exception of UTI, chronic metabolic disease, bleeding disorders, asthma, chronic neurological disease that may affect central thermoregulation, cancer, immune suppression, sepsis, critical medical status or known allergy to paracetamol or ibuprofen. Baseline characteristics:
|
|
| Interventions | Control: ibuprofen 10 mg/kg followed by placebo 4 hours later Treatment group: single oral dose ibuprofen 10 mg/kg followed by single oral dose paracetamol 15 mg/kg 4 hours later |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Location: Lebanon Setting: multi‐centre. This study was conducted in the paediatric inpatient services of two hospitals in Beirut: the American University of Beirut Medical Centre (AUBMC), which is a tertiary care facility; and Najjar Hospital, a secondary care facility. Funding: this study was funded by the Medical Practice Plan of the Faculty of Medicine at the American University of Beirut, Grant number 686056. Gulf Pharmaceutical Industries, United Arab Emirates, donated all the drugs investigated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children enrolled in the study were assigned a random number by the hospital pharmacist according to a computer‐generated random‐number list, which was kept with the pharmacist until the end of the study. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was generated by one of the co‐investigators who was not involved in subject recruitment, drug administration or outcome assessment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Subjects, parents and research assistant were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses responsible for drug administration and outcome assessment, treating physicians were blinded to patients' assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intent‐to‐treat analysis was planned. Loss to follow up: combined group 3%, ibuprofen group 0%. |
| Selective reporting (reporting bias) | Low risk | All assessed outcomes were reported. |
| Other bias | Unclear risk | Forced to stop the trial before achieving the calculated sample size. |
| Methods | Study design: three‐arm, randomized, controlled trial. Study dates and duration: March 2006 to July 2009. Method of temperature measurement: temporal artery thermometer. Time points measured in study: hourly for 6 hours. |
|
| Participants | Number: 46 patients; among the 46 patients, 8 participated twice, 3 participated 3 times and 35 participated only once, contributing to 60 febrile episodes that were randomly assigned into the 3 treatment groups. Number of patients in each intervention: 20 episodes per group. Inclusion criteria: 6 months ‐ 8 years with temperature ≥38 °C. Required to demonstrate an ability to cooperate with serial temporal artery temperature measurements and to take medications by mouth Exclusion criteria: Received paracetamol within 6 hours of presentation or ibuprofen, aspirin or other NSAIDs within 8 hours of presentation. Other major exclusions included weight > 60kg (to avoid surpassing 600 mg of ibuprofen or 1000 mg of paracetamol in a single dose), a history of adverse reaction to any study medication ingredient, diabetes mellitus, renal dysfunction, hepatic dysfunction, thrombocytopenia, or presence of moderate or severe dehydration. Children were also excluded if medical judgement determined that the severity of the underlying illness prohibited inclusion or if the child had already participated in the trial on 3 previous occasions. Baseline characteristics:
|
|
| Interventions | Treatment group A: single dose ibuprofen 10 mg/kg (oral suspension 100 mg/5 mL) Treatment group B: single dose APAP 15 mg/kg (oral solution USP 160 mg/5 mL) plus ibuprofen 10 mg/kg Treatment group C: ibuprofen 10 mg/kg at the beginning of the study followed by 15 mg/kg of APAP 3 hours later |
|
| Outcomes | Primary:
|
|
| Notes | Location: USA Setting: one academic medical centre in Hershey, Pennsylvania, USA; patients recruited from outpatient clinics and child day‐care facilities. Funding: this study was supported by a research grant from the George L. Laverty Foundation and in part by a General clinical Research Centre grant from the National Institutes of Health and a CGRC Construction Grant awarded to the Pennsylvania State University College of Medicine. Disclosure: first author has been a paid consultant for the Consumer Healthcare Products Association, McNeil Consumer Healthcare, Novartis Consumer Health, Inc., Procter & Gamble, and Reckitt Benckiser Healthcare International Ltd., but no industry employees were involved in any aspect of the study. 11 patients participated ≥ 2 times (maximum 3 times). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each child was randomly assigned to 1 of 3 treatment groups according to a computer‐generated log. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No attempt was made to blind the participants, however temperature is an objective measurement that should not be subject to bias from lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No attempt was made to blind the research nurses, however temperature is an objective measurement that should not be subject to bias from lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants during the study. |
| Selective reporting (reporting bias) | Low risk | All assessed outcomes were reported. |
| Other bias | Low risk | |
| Methods | Study design: randomized, double‐blind, parallel‐group trial. Study dates and duration: September 2003 to March 2004. Method of temperature measurement: rectal glass and mercury thermometer. Time points measured in study: daily temperature diary (parents asked to measure rectal temp at least 3 times daily during tx then once daily x 10 days), telephone interview at 24 hours and 48 hours, office visit day 3, 5, 10, and 14 day follow up evaluation. |
|
| Participants | Number: 480 Number of patients in each intervention: 160 in each of the 3 groups. Inclusion criteria: all consecutive children aged 6 ‐ 36 months who had rectal temperature ≥38.4 °C. Exclusion criteria: not attending daycare centers, had taken temperature‐altering drugs or antibiotics within 10 days before presentation, known abnormal liver or renal laboratory values, medical history of renal or hepatic impairment, gastrointestinal bleeding, known allergy to any antipyretic, congenital or acquired immunodeficiency, Reye syndrome, asthma, bronchiolitis or malignancy, and children whose caregivers were unable to apply the NCCPC to measure stress. Baseline characteristics:
URI 66(43) 81(52) 80(51) AOM 16(10) 13(8) 17(11) Pharyngitis 10(7) 7(5) 3(2) Bronchiolitis 8(5) 7(5) 9(6) Gastroenteritis 7(5) 7(5) 6(4) Viral illness 47(30) 40(25) 40(26) |
|
| Interventions | Group1: paracetamol (12.5 mg/kg) q6h, max 50 mg/kg/day); half of the group received initial loading with paracetamol (25 mg/kg) and the other half received initial loading with ibuprofen (10 mg/kg) Group 2: ibuprofen (5 mg/kg) q8h, max 20 mg/kg/day; half of the group received initial loading with paracetamol (25 mg/kg) and the other half received initial loading with ibuprofen (10 mg/kg) Group 3: paracetamol (12.5 mg/kg/dose, max 50 mg/kg/d) alternating with ibuprofen (5 mg/kg/dose, max 20 mg/kg/d) q4h; half of the group received initial loading with paracetamol (25 mg/kg) and the other half received loading with ibuprofen (10 mg/kg) |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Location: Central Israel Setting: multi‐Centre; three primary paediatric community ambulatory centers. Funding: None disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerized random number generator to stratify according to the center in blocks of 60 numbers so that each block comprised 20 patients randomly assigned to each treatment group, with 10 patients assigned to each loading medication." |
| Allocation concealment (selection bias) | Low risk | Admitting nurse used a computerized random‐number generator and handed the parent or guardian a sealed opaque folder holding 3 sealed envelopes: 1 containing an advice sheet explaining the physiology of fever and its nonpharmacologic management; 1 containing the prescription for the loading medication; and 1 containing the drug prescription. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Parents were described as being blinded, but the differences in drug regimens and lack of placebos in the single agent arms suggest that blinding is unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All of the children were evaluated and followed up by the same physician (E.M.S.), who was blinded to the group allocations (as were the parents or guardians)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 480 infants met the eligibility criteria, of whom 464 (96.7%) completed the study. Of the 16 infants (3.3%) who withdrew from the study, 7 (1.5%) failed to return for follow‐up visits within the first 10 days, and 9 (1.9%) did not return for laboratory evaluation after symptoms were alleviated. |
| Selective reporting (reporting bias) | Unclear risk | No significant differences found with different loading medications, so patients were grouped according to maintenance medication. Data for outcomes from different loading medication groups were not reported. |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Del Vecchio 2001 | Letter |
| Diez Domingo 2001 | Physician survey |
| Drucker 2009 | Comment on PITCH trial |
| Lal 2000 | No mean temperature or proportion afebrile data published, despite being measured. Thus, study was at high risk for selective outcome reporting bias. Author was contacted and did not have access to original data. No quality of life indices examined. |
| Malik 2007 | Review article |
| Mayoral 2000 | Survey |
| Miller 2007 | Discussion |
| Nabulsi 2010 | Systematic review |
| Pashapour 2009 | Comparison not relevant to this review |
| Pereira 2012 | Systematic review |
| Purssell 2011 | Systematic review |
| Ruiz Lazaro 2009 | Review of PITCH trial |
| Uhl 2008 | Review article |
Contributions of authors
TW contributed to protocol development, relevance and inclusion screening, quality assessment, data extraction, analysis and writing the review. AS contributed to protocol development, relevance and inclusion screening, quality assessment, analysis and editing the review. DWJ contributed to protocol development and editing the review. HG contributed to the development and execution of the search strategy and editing the review. AT contributed to data extraction. LH contributed to protocol development and editing the review. IM contributed to protocol development and editing the review.
Sources of support
Internal sources
-
Alberta Children's Hospital Foundation, Canada.
Initial grant money was recieved for statistical analysis support. Later, permission was granted for funds to be used for travel to Liverpool where statistical support is available for the study.
External sources
-
Cochrane Infectious Diseases Group Fellowship Program, UK.
Funds from the fellowship program were used to assist with travel to Liverpool, UK where editorial advisor and statistical support were available.
Declarations of interest
None declared.
Unchanged
References
References to studies included in this review
- Erlewyn‐Lajeunesse MD, Coppens K, Hunt LP, Chinnick PJ, Davies P, Higginson I M, et al. Randomised controlled trial of combined paracetamol and ibuprofen for fever. Archives of Disease in Childhood 2006;91(5):414‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay AD, Costelloe C, Redmond NR, Montgomery AA, Fletcher M, Hollinghurst S, et al. Paracetamol and ibuprofen for the treatment of fever in children (PITCH): randomised controlled trial. British Medical Journal 2008;337:a1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LC, Richards PA, Thompson AM, Harper DP, Fairchok MP. Alternating antipyretics: antipyretic efficacy of acetaminophen versus acetaminophen alternated with ibuprofen in children. Clinical Pediatrics 2008;47(9):907‐11. [DOI] [PubMed] [Google Scholar]
- Nabulsi M, Tamim H, Mahfoud Z, Itani M, Sabra R, Chamseddine F, et al. Alternating ibuprofen and acetaminophen in the treatment of febrile children: a pilot study. BMC Medicine 2006;4(4):b3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul IM, Sturgis SA, Yang C, Engle L, Watts H, Berlin CM Jr. Efficacy of standard doses of ibuprofen alone, alternating, and combined with acetaminophen for the treatment of febrile children. Clinical Therapeutics 2010;32(14):2433‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrell EM, Wielunsky E, Cohen HA. Antipyretic treatment in young children with fever: acetaminophen, ibuprofen, or both alternating in a randomized, double‐blind study. Archives of Pediatrics & Adolescent Medicine 2006;160(2):197‐202. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Vecchio MT, Sundel ER. Alternating antipyretics: Is this an alternative?. Pediatrics 2001;108(5):1236‐7. [PubMed] [Google Scholar]
- Diez Domingo J, Burgos Ramirez A, Garrido Garcia J, Ballester Sanz A, Moreno Carretero E. Use of alternating antipyretics in the treatment of fever in Spain [Utilizacion de la alternancia de antipireticos en el tratamiento de la fiebre en Espana]. Anales Espanoles de Pediatria December 2001;55(6):503‐10. [PubMed] [Google Scholar]
- Drucker R. Comparison of acetaminophen, ibuprofen, and both agents for treatment of fever in children acetaminophen plus ibuprofen and ibuprofen alone are equally effective in reducing fever. Archives of Disease in Childhood 2009;94(1):30. [Google Scholar]
- Lal A, Gomber S, Talukdar B. Antipyretic effects of nimesulide, paracetamol and ibuprofen‐paracetamol. Indian Journal of Pediatrics 2000;67(12):865‐70. [DOI] [PubMed] [Google Scholar]
- Malik K, Goldman RD. PRETx update: Alternating antipyretics in febrile children. International Pediatrics 2007;22(2):95‐7. [Google Scholar]
- Mayoral CC, Marino RV, Rosenfeld W, Greensher J. Alternating antipyretics: Is this an alternative?. Pediatrics 2000;105(5):1009‐12. [DOI] [PubMed] [Google Scholar]
- Miller AA. Alternating acetaminophen with ibuprofen for fever: Is this a problem?. Pediatric Annals 2007;36(7):384‐6. [DOI] [PubMed] [Google Scholar]
- Nabulsi M. Is combining or alternating antipyretic therapy more beneficial than monotherapy for febrile children?. British Medical Journal 2010;340(7737):92‐3. [DOI] [PubMed] [Google Scholar]
- Pashapour N, Macooei AA, Golmohammadlou S. Alternating ibuprofen and acetaminophen in the treatment of febrile hospitalized children aged 9‐24 months. Iranian Journal of Pediatrics June 2009;19(2):164‐8. [Google Scholar]
- Pereira GL, Dagostini JMC, Silva Dal Pizzol T. Alternating antipyretics in the treatment of fever in children: a systematic review of randomized clinical trials [Uso alternado de antipiréticos para tratamento da febre em crianças: revisão sistemática de ensaios clínicos randomizados]. Jornal de Pediatria 2012;88(4):289‐96. [DOI] [PubMed] [Google Scholar]
- Purssell E. Systematic review of studies comparing combined treatment with paracetamol and ibuprofen, with either drug alone. Archives Of Disease In Childhood 2011;96:1175‐9. [DOI] [PubMed] [Google Scholar]
- Ruiz Lazaro PJ. Ibuprofen, alone or combined with paracetamol, is more effective than paracetamol alone in home treatment of fever in children from 6 months to 6 years of age [Administrar ibuprofeno, solo o combinado con paracetamol, es mas efectivo que utilizar solo paracetamol en el tratamiento domiciliario de la fiebre en ninos de 6 meses a 6 anos de edad]. FMC Formacion Medica Continuada En Atencion Primaria 2009;16(5):311. [Google Scholar]
- Uhl D. Ibuprofen and/or paracetamol for the treatment of fever in children? [Ibuprofen und paracetamol im wechsel geben?]. Deutsche Apotheker Zeitung 2008;148(44):52‐5. [Google Scholar]
Additional references
- Beasley R, Clayton T, Crane J, Mutius E, Lai CKW, Montefort S, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6‐7 years: analysis from Phase Three of the ISAAC programme. Lancet 2008;372:1039‐48. [DOI] [PubMed] [Google Scholar]
- Brewer EJ. A comparative evaluation of indomethacin, acetaminophen and placebo as antipyretic agents in children. Arthritis and Rheumatism 1968;11(5):645‐51. [DOI] [PubMed] [Google Scholar]
- Brown DR, Wilson JT, Kearns GL, Eichler VF, Johnson VA, Bertrand KM. Single‐dose pharmacokinetics of ibuprofen and acetaminophen in febrile children. Journal of Clinical Pharmacology 1992;32:231‐41. [DOI] [PubMed] [Google Scholar]
- Position Paper DT98‐01 of the Drug Therapy and Hazardous Substances Committee. Paediatric Child Health1998; Vol. 3, issue 4:273‐4.
- Chiappini E, Principi N, Longhi R, Tovo PA, Beccheruci P, et al. Management of fever in children: summary of the Italian Pediatric Society guidelines. Clinical Therapeutics 2009;8:1826‐43. [DOI] [PubMed] [Google Scholar]
- Cohet C, Cheng S, MacDonald C, Baker M, Foliaki S, Huntington N, et al. Infections, medication use, and the prevalence of symptoms of asthma, rhinitis, and eczema in childhood. Journal of Epidemiology and Community Health 2004;58:852‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocetti M, Moghbeli N, Serwint J. Fever phobia revisited: have parental misconceptions about fever changed in 20 years?. Pediatrics 2001;107(6):1241‐6. [DOI] [PubMed] [Google Scholar]
- Del‐Rio‐Navarro BE, Ito‐Tsuchiya FM, Berber A, Zepeda‐Ortega B, Sienra‐Monge JJ, Garcia‐Almaraz R, et al. Study of the relationship between acetaminophen and asthma in Mexican children aged 6 to 7 years in 3 Mexican cities using ISAAC methodology. Journal of Investigational Allergology and Clinical Immunology 2008;18(3):194. [PubMed] [Google Scholar]
- Drwal‐Klein LA, Phelps SJ. Antipyretic therapy in the febrile child. Clinical Pharmacology 1992;11:1005‐21. [PubMed] [Google Scholar]
- Etminan M, Sadatsafavi M, Jafari S, Doyle‐Waters M, Aminzadeh K, Fitzgerald JM. Acetaminophen use and the risk of asthma in children and adults: A systematic review and metaanalysis. Chest November 2009;136(5):1316‐23. [DOI] [PubMed] [Google Scholar]
- Feldberg W, Gupta KP. Pyrogen fever and prostaglandin like activity in cerebrospinal fluid. Journal of Physiology 1973;228:41‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows .. 2008.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: Hepatotoxicity after multiple doses in children. Journal of Pediatrics 1998;132:22‐7. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org. The Cochrane Collaboration2011.
- Kanabar D, Dale S, Rawat M. A review of ibuprofen and acetaminophen use in febrile children and the occurrence of asthma‐related symptoms. Clinical Therapeutics 2007;29:2716‐23. [DOI] [PubMed] [Google Scholar]
- Kauffman RE, Sawyer LA, Scheinbaum ML. Antipyretic efficacy of ibuprofen vs acetaminophen. American Journal of Diseases of Children 1992;146:622‐5. [DOI] [PubMed] [Google Scholar]
- Kelley MT, Walson PD, Edge JH, Cox S, Mortensen ME. Pharmacokinetics and pharmacodynamics of ibuprofen isomers and acetaminophen in febrile children. Clinical Pharmacology & Therapeutics 1992;52:181‐9. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Drugs for childhood fever (Letter). Lancet 1992;339:70. [PubMed] [Google Scholar]
- Kwiatkowski D. The biology of malarial fever. Baillieres Clinical Infectious Diseases 1995;2(2):371‐88. [Google Scholar]
- Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics 1999;104:39. [DOI] [PubMed] [Google Scholar]
- Lesko SM, Louik C, Vezina RM, Mitchell AA. Asthma morbidity after the short‐term use of ibuprofen in children. Pediatrics 2002;109:20. [DOI] [PubMed] [Google Scholar]
- Li SF, Lacher F, Crain EF. Acetaminophen and ibuprofen dosing by parents. Pediatric Emergency Care 2000;16:394‐7. [DOI] [PubMed] [Google Scholar]
- Lowe AJ, Carlin JB, Bennett CM, Allen KJ, Robertson CF, Axelrad C, et al. Paracetamol use in early life and asthma: prospective birth cohort study. BMJ September 2010;341:c4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Italien C, Jacqz‐Aigrain E. Risks and benefits of nonsteroidal anti‐inflammatory drugs in children: a comparison with paracetamol. Paediatric Drugs 2001;3:817‐58. [DOI] [PubMed] [Google Scholar]
- Mackowiak PA, Plaisance KI. Benefits and risk of antipyretic therapy. Annals New York Academy of Sciences 1998;856:214‐23. [DOI] [PubMed] [Google Scholar]
- McIntyre J, Hull D. Comparing efficacy and tolerability of ibuprofen and paracetamol in fever. Archives of Disease in Childhood 1996;74:164‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabulsi M. Is combining or alternating antipyretic therapy more beneficial than monotherapy for febrile children?. British Medical Journal 2009;339:92‐4. [DOI] [PubMed] [Google Scholar]
- Newson RB, Shaheen SO, Chinn S, Burney PG. Paracetamol sales and atopic disease in children and adults: An ecological analysis. European Respiratory Journal 2000;16:817‐23. [DOI] [PubMed] [Google Scholar]
- Feverish illness in children: Assessment and initial management in children younger than 5 years. National Institue for Health and Clinical ExcellenceMay 2013. [PubMed]
- Perrott DA, Piira T, Goodenough B, Champion GD. Efficacy and safety of acetaminophen vs ibuprofen for treating children's pain or fever. A meta‐analysis. Archives of Pediatrics & Adolescent Medicine 2004;158:521‐6. [DOI] [PubMed] [Google Scholar]
- Renn E. The antipyretic use of acetaminophen versus ibuprofen in a pediatric care setting. Physical Therapy 2000;25:395‐7. [Google Scholar]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Cochrane Collaboration, 2008.
- Schmitt BC. Fever phobia: Misconceptions of parents about fevers. American Journal of Diseases of Children 1980;134(2):176‐81. [PubMed] [Google Scholar]
- Schnaiderman D, Lahat E, Sheefer T, Aladjem M. Antipyretic effectiveness of acetaminophen in febrile seizures: Ongoing prophylaxis versus sporadic usage. European Journal of Paediatrics 1993;152:747‐9. [DOI] [PubMed] [Google Scholar]
- Sullivan JE, Farrar HC. Fever and antipyretic use in children. Pediatrics 2011;127(3):580‐7. [DOI] [PubMed] [Google Scholar]
- Stuijvenberg M, Derksen‐Lubsen G, Steyerberg EW, Habbema JDF, Moll HA. Randomized controlled trial of ibuprofen syrup administered during febrile illness to prevent febrile seizure recurrences. Pediatrics 1998;102:1‐7. [DOI] [PubMed] [Google Scholar]
- Walson PD, Galletta G, Braden NJ, Alexander L. Ibuprofen, acetaminophen, and placebo treatment of febrile children. Clinical Pharmacology & Therapeutics 1989;46:9‐17. [DOI] [PubMed] [Google Scholar]
- Widal MF, Abrami P, Lenmoyez J. Anaphylaxis and Idiosyncrasy [Anaphylaxie et idiosyncrasie]. La Presse Medicale 1922;30:189‐92. [PubMed] [Google Scholar]
- Wilson JT, Brown D, Kearns GL, Eichler VF, Johnson VA, Bertrand KM, et al. Single‐dose, placebo‐controlled comparative study of ibuprofen and acetaminophen antipyresis in children. Journal of Pediatrics 1991;119:803‐11. [DOI] [PubMed] [Google Scholar]


