Abstract
The purpose of this study was to develop regression equations for estimating the intensity of the exercise work rate (relative peak oxygen uptake-heart rate [%VO2-HR]) and the metabolic energy expenditure (MEE) for exercise prescription and rehabilitation medicine that are specific to children. This study took into account that the specific data in terms of obesity, sex, and pubertal status are currently unavailable. Our hypothesis was that obesity would affect the submaximal exercise the oxygen uptake (VO2), heart rate (HR), and metabolic energy expenditure (MEE), and exercise economy (ExEco). In this retrospective study, the regression analysis was performed on 126 children, matching groups for Tanner pubertal status (prepubertal: 1.8±0.7; postpubertal: 4.1±0.7), BMI-for-age percentile (lean: 50±26; obese: 96±4), and sex (girls: 48%; boys: 52%). Percent peakVO2 was regressed against HR, MEE against work rate (watt), and exercise economy (ExEco, mLO2·kg lean body mass−1·min−1) against work rate. Additionally, stepwise linear regression was used to identify predictors for exercise peak work rate. Prepubertal and postpubertal boys exercise at lower work rates than obese (%peakVO2-HR slope; P=0.01). The reverse was true in girls, lean prepubertal work at lower compared lean postpubertal (%peakVO2-HR slope; P=0.03). Boys expend more calories during exercise compared to girls (MEE-slope; P=0.01), with no effect of puberty or obesity. Obese prepubertal children have poor ExEco compared to lean prepubertal children (ExEco-work rate slopes; P<0.01) but not in postpubertal children. Strong correlations (r=0.92–0.94) for %peakVO2-HR and MEE regressions for boys and girls accounted for 85–92% variation. Height, lean leg, and leg fat mass accounted for 83% of the variance for predicting peak work rate. Obesity, sex, and puberty affect exercise characteristics in children and should be considered for an individualized approach to exercise prescription in children.
Keywords: Pediatrics, oxygen uptake, heart rate, metabolic energy expenditure, exercise economy
INTRODUCTION
Sedentary behavior and overconsumption are primary contributors to obesity, which in the US affects nearly one-third of the general population and 17% of children (38, 41). In the developing world, recent estimates point to 38% of adults and 23% of children and adolescents being overweight or obese (41). It is well established that obesity leads to life-threatening long-term complications such as coronary artery disease, stroke, and type 2 diabetes (43). These complications gradually occur later in life and are often the consequences of early metabolic abnormalities including insulin resistance, hypertension, and dyslipidemia that start in the pediatric obese population (4). The presence of obesity before puberty (i.e., approximately 11–13 years) increases the ability to predict metabolic status and cardiometabolic risk at later ages (16). Once obesity is present, the physiological and psychological processes that can reverse obesity appear resistant to change (16). In fact, early obesity appears to establish a “weight set-point” that the body attempts to preserve after puberty (16). Thus, early infancy to prepuberty is a critical period for understanding metabolic dysfunction and cardiovascular disease in later life (17, 26).
Lifestyle changes such as dietary and behavioral modifications as well as pharmacological interventions are important treatments for obesity. In particular, exercise training and physical activities are important components of the treatment for pediatric obesity (14, 44). In children, many exercise interventions aimed at improving cardiorespiratory fitness and conditions associated with obesity have focused on aerobic exercise training; however, several systemic reviews have reported mixed findings. Some suggest that exercise diminishes percent body fat in overweight and obese children and adolescents, especially when used in conjunction with other lifestyle therapies (27, 44). Others have yielded inconsistent results on whether exercise reduces other measures of adiposity (27). Although the combination of aerobic and resistance training has been shown to improve the glycemic control (37), cardiovascular fitness, and muscle strength in overweight and obese in children (60), many factors such as age, sex, activity level, and age of obesity onset may contribute to the variability of results (30). Obesity diminishes exercise capacity in adults by reducing mitochondrial oxidation or the delivery of oxygen to working skeletal muscle. It may do so through mitochondrial dysfunction (28) and by impairing vascular function and consequently leg blood flow both under resting conditions (25) and during exercise (29). In adults and children, obesity also causes obstructive sleep apnea (OSA) (7), which is associated with weight gain and chronic diseases such as type 2 diabetes, cardiovascular disease, and stroke (35). OSA also affects exercise function in children. Evans et al. reported that children with OSA had lower cardiac output, stroke volume index, heart rate, and oxygen consumption than age-matched children without OSA (15). Additionally, obesity in adults reduces total lung capacity, functional residual capacity, and expiratory reserve capacity; while increasing residual volume, possibly because of the adipose tissue around the rib cage and abdomen (56). The effects of sex, puberty, and obesity on aerobic exercise have not been extensively studied in children. Many report inconsistent results, and it has been suggested that the relationships between other factors such as age, sex, activity level, and age of obesity onset may contribute to the variability of results (30).
In children, boys and girls aged 9–11 years that have V̇O2peak values of less than 46 mL/kg body mass have an increased cardiometabolic risk (1). Boys have 8–18% higher cardiorespiratory fitness than girls that were 8–11 years old; however, whether expressed in absolute values or scaled to body mass, lean body mass (LBM) or allometric scaling, this was dependent on body fat mass (12). Further, it has been found that body fat and maturity explained 47% of the variation in VO2peak in boys, whereas activity energy expenditure and body fat explained 22% of the variation in VO2peak in girls (13). In general, it is well established that sex differences in cardiovascular disease exist which may be due to early life programing (24). In regard to submaximal exercise, there is a gap in the literature examining sex differences in children with obesity.
Regression equations are commonly used in exercise prescription and rehabilitation medicine to estimate the intensity of exercise as a percentage of peak VO2 and heart rate (HR). In addition, exercise tests are used to estimate energy expenditure. The American College of Sports Medicine has frequently used regression equations (45, 46); however, these are derived using adult data and may not be suitable for children with obesity. In addition, children’s biological maturation may affect key physiological aspects of an exercise response and confound the interpretations of the exercise test. (54). Thus, the regression equations derived from pediatric data are needed, though we are unaware of any such equations. Therefore, our objectives were to examine submaximal exercise between sex, age, and obesity. Specifically, we investigated the effect of age (pre- vs. post-puberty), sex (boys vs. girls), and body composition (lean vs. obese) on the VO2 and HR relationship as well as on the metabolic energy expenditure (MEE) and exercise economy. We also performed a regression analysis to model the relationship between the relative percentage of submaximal to peak oxygen uptake (%peak VO2) and peak heart rate (% Peak HR) in lean and obese pre- and postpubertal girls and boys. Finally, we developed the prediction equations for the peak work rate in these groups. We hypothesized that age, sex, and obesity would affect the submaximal energy expenditure, exercise economy and the relative relationship between VO2 and HR.
METHODS
Participants
This was a retrospective study design in which we identified 126 children and adolescents aged 8 to 17 with no serious health conditions from a larger database that involved studies on physical fitness in children in a university setting. Of these, 18% were Asian, 4% African-American, 63% Caucasian, and 15% Mexican-American. All were participants in UC Irvine Institutional Review Board-approved pediatric exercise research studies (focused on physical fitness and childhood obesity), which complied with the Declaration of Helsinki. All children were healthy and not taking medication known to affect body composition or physical activities. Children were grouped by puberty status, body composition (obese vs lean), and sex for the comparison of regressions. Children were excluded if they had asthma or type 1 diabetes or if they were older than 18 years old.
Protocol
Peak aerobic exercise capacity test
The aerobic exercise capacity (peak VO2) was determined by a progressive ramp-type cycle ergometry (SensorMedics ergometrics 800, Yorba Linda, CA, USA) exercise stress test, which was performed to volitional exhaustion. The protocol required participants to cycle at an unloaded work rate for 3 minutes, after which the time wattage was increased every second until reaching volitional fatigue. Respiratory gasses were analyzed using VMAX Encore 229 (Viasys Healthcare, Yorba Linda, CA, USA) after O2 and CO2 gases and air flow were calibrated using known gasses and a 3-L syringe. Heart rate and rhythm were monitored via 5-lead electrocardiography (GE Cardiosoft v. 6.5.1, Viasys Healthcare, Yorba Linda, CA, USA). Notably, no validated and universally accepted criteria for determining the peak VO2 in children have existed until the time of this study (31). For this reason, we used similar standards as those used in adults (23) and deemed the test to be maximal once subjects signaled to stop exercise and at least 3 of the following criteria were met: a respiratory exchange ratio (RER) of ≥ 1.05, a leveling off in VO2 with increasing workloads (less than 2 mL kg−1 min−1), volitional fatigue, final exercise heart rate of 190 bpm or greater, or a final test time between 8 to 12 min. Similar criteria have been used in other studies on exercise in children (47).
Anthropometric and body composition measurements
Dual-energy X-ray absorptiometry (DXA) was performed within 30 days of the exercise protocol to determine body composition. A Hologic QDR 4500 densitometer (Hologic Inc., Bedford, MA, USA) was used to measure body composition. Participants were scanned in light clothing while lying supine. On the day of each test, the densitometer was calibrated using the procedures provided by the manufacturer, and scans were performed and analyzed using the pediatric software. Anthropometric measures collected included weight, height, body mass index (BMI) and BMI percentile for age (BMI%ile). Standard, calibrated scales were used to determine the weight and height of participants. BMI was calculated by dividing the weight in kilograms by the square of height in meters. BMI%ile was computed according to the normative values provided by the Centers for Disease Control and Prevention (CDC), with the obesity being defined as a BMI at or above the 95th percentile for children and teens of the same age and sex (9).
Maturity status
The stage of pubic hair development was assessed by clinical examination, which was performed by a physician/nurse experienced in the protocol described by Tanner (58). We used this protocol to estimate the stage of maturity, as it was not known when participants entered a particular stage or how long they had been in a stage. Boys and girls were considered prepubertal if classified as Tanner stage 1 or 2 and pubertal/postpubertal if classified as Tanner stage 3, 4, or 5.
Submaximal metabolic and exercise economy
The rate of MEE was calculated from oxygen uptake and RER (42), with the energy equivalent of carbohydrate (ec) and fat (ef), set to 21.13 kJ and 19.69 kJ, respectively: M = VO2 × [[(((RER - 0.7)/0.3) × ec) + (((1 - RER)/0.3) × ef)] / 60] × 1000. MEE can also be expressed in joules per minute (1 kJ/min = ~17 W), kilocalories per minute (1 kcal/min ~70 W), or metabolic equivalents (1 MET = 58.2 W/m2). External work rate (watt) was obtained from cycle ergometer work output. Breath-by-breath data was time averaged in 15-second intervals over the duration of exercise, with peak VO2, peak RER, and peak HR determined as the final 15-second average. An analysis was performed on data at 10% increments of total time (ten 15-second mean data points). Submaximal oxygen uptake and heart rates were recorded from the start of the exercise test through the end. Relative percentages were then calculated as a percent of peak values for each oxygen uptake and heart rate. Exercise economy describes the oxygen cost over a given speed or time during submaximal exercise (10, 40). For this study, we use the submaximal oxygen uptake over a given wattage of exercise during the VO2peak test. VO2 was normalized to kilograms of total body mass and lean body mass (LBM).
Statistical Analysis
The data were screened for normality (Shapiro-Wilk test of normality) and outliers. All variables were determined to be appropriate for analysis without transformation, and no outliers were found in the data. Subject characteristics were analyzed using a 3-way multivariate analysis of variance for interactions (age × %fat × sex) and main effects for puberty, percent body fat, and sex. If a significance was found, a factorial analysis of variance with appropriate Holm-Sidak multiple comparison post hoc tests was performed with GraphPad software. Linear regressions using GraphPad software with residuals were used to determine differences in slopes and elevations and Pearson correlation coefficients to investigate relationships. Stepwise linear regression was used to predict exercise peak work rate using sex, age, height, Tanner stage, weight, BMI, BMI%ile, fat mass, lean mass, percent fat, trunk mass, trunk fat, trunk lean, leg mass, leg fat mass, and leg lean mass. The test for heteroskedasticity was performed using McClendon’s Multiple Regression and Causal Analysis for each regression. The data were analyzed by the SPSS Statistics software (Version 22, IBM Corp., Armonk, NY, USA) and the figures and analysis prepared with GraphPad Prism 6.0 (La Jolla, CA, USA). The significance was set at P<0.05. All data are reported as mean ± SD.
RESULTS
The effect of age, percent body fat, and sex on the body morphology and the peak exercise capacity: The BMI%ile and lean mass were the only physical characteristics affected by age, percent body fat, and sex (interaction, P<0.05) (Table 1). For all groups, age affected all body morphology characteristics measured (main effect for each, P<0.05), except the percent body fat. Percent body fat affected all body morphology measures (main effect of fat for each, P<0.05), with the exception of height. Combining all groups, we found that sex affected all body morphology characteristics (main effect for sex, P<0.05), except for the percent body fat. Among the peak exercise capacity variables, only the peak VO2 of both TBM and LBM were found to be affected (age × percent fat × sex interaction, P<0.05). In all groups combined, age (main effect, P<0.05), percent body fat (main effect, P<0.05), and sex (main effect, P<0.05) affected exercise peak work rate and peak MEE.
Table 1.
Subjects physical and exercise capacity characteristics (mean ± SD).
| Lean Groups | Obese Groups | 3-way MANOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Younger | Older | Younger | Older | Interaction | Main effects | |||||||
| Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | A×F×G | Age | %Fat | Gender | |
| Subjects (n) | 15 | 19 | 12 | 22 | 16 | 16 | 17 | 9 | ||||
| Age (y) | 9.8±1 | 10.3±1 | 15.0±2 | 16.3±2 | 10.1±3 | 10.7±1 | 15.4±2 | 14.8±1 | 0.31 | <0.001 | 0.76 | 0.11 |
| Tanner stage | 1.6±0.8 | 1.2±0.4 | 4.2±0.7 | 4.1±0.5 | 2.3±0.9 | 1.9±0.8 | 4.4±0.6 | 3.7±0.8 | 0.32 | <0.001 | 0.04 | <0.001 |
| Body Morphology | ||||||||||||
| Height (cm) | 138.7±10 | 140.6±8 | 163.3±12 | 174.6±10 | 143.8±8 | 148.3±9 | 159.1±16 | 174.4±7 | 0.13 | <0.001 | 0.97 | 0.027 |
| Weight (kg) | 33.7±8 | 36.1±8 | 56.1±13 | 75.8±15 | 55.5±12 | 64.2±15 | 90.3±30 | 111.7±28 | 0.23 | <0.001 | <0.001 | <0.01 |
| BMI (kg·m−2) | 17.2±2 | 18.1±2 | 21.5±3 | 24.7±4 | 26.6±3 | 28.9±5 | 34.5±8 | 36.6±8 | 0.33 | <0.001 | <0.001 | 0.018 |
| BMI (%tile) | 49.8±22 | 49.9±29 | 53.5±28 | 48.9±15 | 96.1±5 | 98.4±1 | 92.5±7 | 98.3±2 | 0.03 | 0.028 | <0.001 | <0.01 |
| Fat (kg) | 9.6±3 | 8.8±3 | 16.2±4 | 17.7±8 | 24.8±7 | 30.2±9 | 41.3±17 | 47.4±11 | 0.17 | <0.001 | <0.001 | 0.033 |
| Fat (%) | 28.1±4 | 24.8±6 | 28.2±3 | 22.1±7 | 43.9±5 | 44.5±6 | 43.7±6 | 42.6±2 | 0.41 | 0.64 | <0.001 | 0.478 |
| Lean (kg) | 23.5±6 | 25.0±5 | 39.3±10 | 57.0±8 | 30.0±5 | 35.2±6 | 48.8±12 | 62.3±16 | <0.01 | <0.001 | <0.001 | <0.001 |
| Peak Exercise Capacity | ||||||||||||
| Peak Watts | 94±21 | 105±27 | 157±44 | 236±44 | 102±17 | 118±45 | 149±25 | 194±34 | 0.46 | <0.001 | 0.004 | <0.001 |
| Peak MEE | 451±101 | 477±114 | 687±172 | 1025±218 | 483±98 | 549±210 | 694±109 | 947±191 | 0.37 | <0.001 | 0.013 | <0.001 |
| Peak VO2 (mLO2·kgTBM·min−1) | 35.3±5 | 38.4±8 | 34.5±5 | 37.5±6 | 25.6 ±5 | 24.6±5 | 21.0±7 | 23.4±3 | <0.01 | 0.46 | <0.001 | 0.138 |
| Peak VO2 (mLO2·kgLBM·min−1) | 55.9±18 | 53.6±10 | 48.7±7 | 49.2±6 | 45.9±6 | 44.4±17 | 40.5±7 | 42.4±5 | <0.01 | 0.89 | <0.001 | 0.336 |
| Peak HR (beats·min1) | 189±8 | 189±8 | 188±12 | 188±12 | 189±12 | 186±15 | 184±9 | 185±12 | 0.3 | 0.4 | 0.112 | 0.674 |
Notes: BMI, body mass index; BMI %ile, body mass index percentile for age; VO2, volume of oxygen; TBM, total body mass; LBM, lean body mass; A, age group; F, % fat group; G, gender group
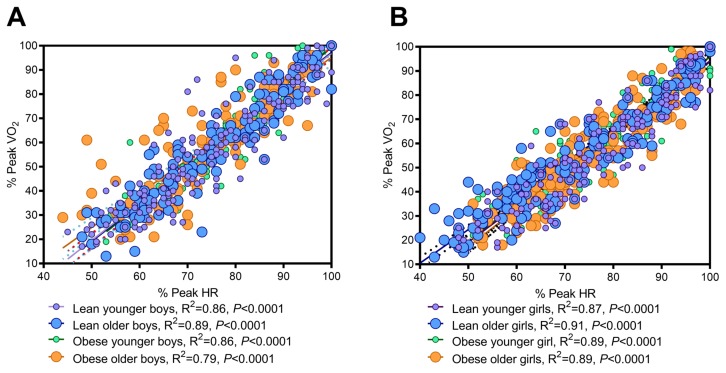
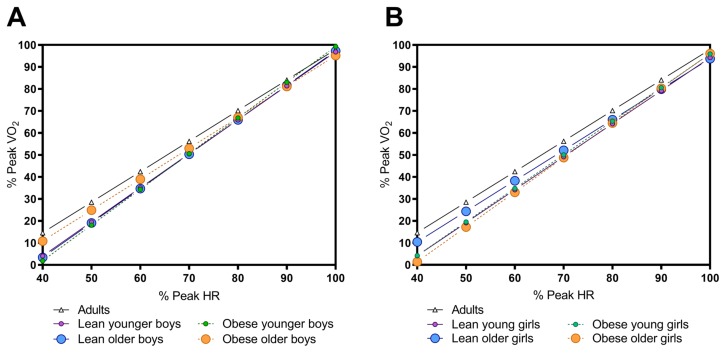
The relationship between the relative peak VO2 and HR is affected by the biological maturation, sex, and percent fat: The regression of the relative peak VO2 against peak HR revealed that the relationship for percent of peak heart rate (% HR) and percent peak oxygen uptake (%PeakVO2) during submaximal exercise did not differ between younger and older lean boys (slopes; P=0.8) (Fig. 1A). However, between younger and older obese boys, obese boys work at a greater effort at lower intensity exercise (greater % of peak HR/VO2) (slope; P=0.012) (Fig. 1A). For girls, the slopes among all groups differed (P=0.002) (Fig. 1B). Younger and older lean girls had different slopes; older girls exercised at a greater percentage of their peak HR/VO2 than younger (Slope; P=0.03), while the younger and older obese girls had similar relative relationships (slope; P=0.4) (Fig. 1B). The relationships and equations for each are presented in Figure 2, with adult regressions shown for comparison (21).
Figure 1.
The relationship between percent peak VO2 and percent peak HR in lean/obese and younger/older boys (A) and girls (B).
Figure 2.
Comparison of adult regressions (27) to regressions for boys (A) and girls (B). Regression equations were as follows: lean younger boys: %VO2 = 1.546 × %HR – 57.76; lean older boys: %VO2 = 1.563 × %HR – 59.1; obese younger boys: %VO2 = 1.623 × %HR – 63.05; obese older boys: %VO2 = 1.406 × %HR – 45.43; lean younger girls: %VO2 = 1.501 × %HR – 55.91; lean older girls: %VO2 = 1.388 × %HR – 45.06; obese younger girls: %VO2 = 1.527 × %HR – 56.85; obese older girls: %VO2 = 1.577 × %HR – 61.66.
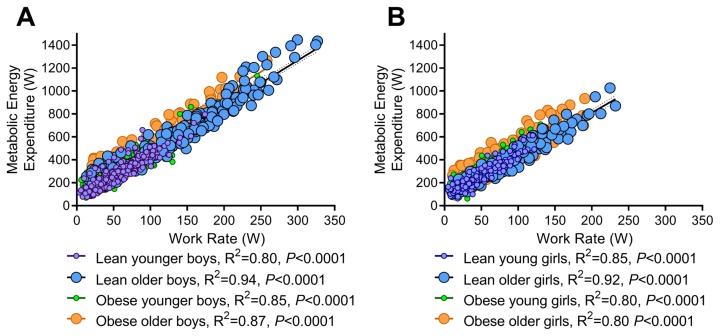
Sex, but not obesity, affects the metabolic energy expenditure during exercise: The regression of MEE against the absolute exercise work rate showed that the lean, obese, younger, and older boys had similar submaximal to maximal oxygen cost during exercise (slope; P=0.12) (Fig. 3A). The same was true for girls (slopes; P=0.36) (Fig. 3B). However, slopes differed between boys and girls (slope; P=0.007), with elevations for each boy and girl group comparison being different (elevation; P<0.0001) suggesting that boys expend more calories for the same given exercise work rate compared to girls.
Figure 3.
Metabolic energy expenditure (MEE) for lean/obese and younger/older boys (A) and girls (B). Regression equations were as follows: lean younger boys: MEE = 3.385 × W + 79.4; lean older boys: MEE = 3.956 × W + 80.17; obese younger boys: MEE = 3.768 × W + 113.9; obese older boys: MEE = 4.049 × W + 156.2; lean younger girls: MEE = 3.886 × W + 75.44; lean older girls: MEE = 3.687 × W + 73.94; MEE = 3.687 × W + 73.94; obese younger girls: MEE = 3.771 × W + 89.07; obese older girls: MEE = 3.561 × W + 136.2.
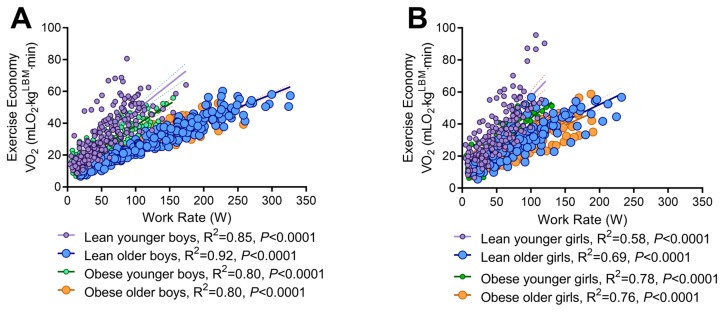
Obesity affects the exercise economy in prepubescent children: The regression analysis of the exercise economy normalized to LBM and exercise work rate showed that the economy was worse in lean compared to obese younger boys; lean boys utilized greater oxygen at similar work rates compared to obese (slopes; P=0.01), while both younger groups had worse economy compared to older boys (slopes; P=0.01). The slopes were comparable between lean and obese older boys (P=0.14) (Fig. 4A). Similarly, lean young girls had greater economy compared to obese younger girls (slopes; P<0.001), and the younger groups had worse economy compared to older groups (slopes; P<0.001). The lean and obese older girls had similar exercise economy (P=0.51) (Fig. 4B). Boys differences were also detected in slopes for boy and girl group comparisons suggesting that boys utilize greater oxygen for the same given exercise work rate, thus have poor exercise economy compared to girls. (P<0.01).
Figure 4.
Exercise economy in lean/obese and younger/older boys (A) and girls (B).
The height, lean leg mass, and leg fat mass account for 83% of the variance of exercise peak work rate in children: The linear stepwise regression models for predicting exercise peak work rate for boys, girls, and all groups combined are shown in Table 2. In the girl group, the strongest predictors were the trunk lean mass, trunk mass, and age, which accounted for 66% of the variance. In the boy’s group, the strongest predictors were the height, leg lean mass, and trunk fat, which accounted for 86% of the variance. In all groups combined, the height, lean leg mass, and the leg fat mass accounted for 83% of the variance. Because these variables were taken from DXA scans, we did an additional analysis with simple subject characteristics (height, weight, age, BMI%ile) and found that, in both girl and boy groups, the height was the strongest predictor, accounting for 66 to 76% of the variance. Each model found very strong Pearson correlations (r =0.67–0.93).
Table 2.
Linear stepwise regression models for predicting peak work rate (W, watt).
| Regression Model Summary | R | Adjusted R2 | P value | |
|---|---|---|---|---|
| Girls | 37.5 + [5.339 × (trunk lean mass, kg)] | 0.69 | 0.46 | <0.0001 |
| 22.4 + [10.6 × (trunk lean mass, kg) + [−2.854 × (trunk mass, kg)] | 0.79 | 0.61 | <0.0001 | |
| −4.85 × [9.089 × (trunk lean mass, kg)] + [−2.686 × (trunk mass, kg)] + [3.765 × (age, yr)] | 0.82 | 0.66 | <0.0001 | |
| −200.7 + [2.16 × (ht, cm)] | 0.67 | 0.45 | <0.0001 | |
| Boys | −326 + [3.053 × (ht, cm)] | 0.87 | 0.76 | <0.0001 |
| −298 + [3.12 × (ht, cm)] + [−119.25 × (%body fat)] | 0.89 | 0.8 | <0.0001 | |
| −148 + [1.484 × (ht, cm)] + [6.643 × (leg lean mass, kg)] + [−2.425 × (trunk fat, kg)] | 0.93 | 0.86 | <0.0001 | |
| −326.3 + [3.053 × (ht, cm)] | 0.87 | 0.76 | <0.0001 | |
| All groups comnbined | −284 + [2.74 × (ht, cm)] | 0.79 | 0.63 | <0.0001 |
| −166 + [1.648 ×(ht, cm)] + 3.839 × (lean leg mass, kg)] | 0.83 | 0.68 | <0.0001 | |
| 16.4 + [2.766 × (trunk lean leg, kg)] + (1.62 × (wt, kg)] + −3.5 × fat mass, kg)] + [ 1.34 × (lean leg mass, kg)] | 0.91 | 0.83 | <0.0001 | |
DISCUSSION
This study tested the hypothesis that obesity would alter the peak VO2-peak HR relationship in pre- and post-pubescent children. This study had the following 4 main findings. 1) The submaximal relative peak VO2-peak HR relationship is affected differently by the body fat in boys and girls. Moreover, in children, this relationship may be different from that described in the commonly used adult equations. 2) The MEE is only affected by body fat in younger boy and girls, in addition to sex differences. 3) Obesity affects the exercise economy in prepubescent boys and girls but not in older children. Further, boys have poor exercise economy compared to girls. 4) The greatest predictors of the exercise peak work rate are the height, lean leg mass, and leg fat mass, which together accounted for 83% of the variation in our combined sample.
Obesity has been reported to affect the physical development in children (36). Evidence suggest that obesity causes girls to mature earlier (11) by affecting the reproductive axis (18, 59). In our sample of 126 children, obesity did not differentially affect most of the body morphology characteristics based on age or sex. The exception was LBM, which was differentially affected according to age, percent fat mass, and sex. With regard to exercise capacity, only peak VO2 was mainly affected by obesity. This is in agreement with other studies reporting the difference with the influence of obesity that uses the standard ratio normalization; normalizing to total body mass (3, 48). All groups of obesity in this study (21–26 ml O2·kg-1·min-1) had very poor (<25 ml O2·kg−1·min−1)-to-poor (25–31 ml O2·kg−1·min−1) range relative non-obese normative children (19, 22).
In the present study, we aimed to tease out the maturity, sex, and obesity differences during submaximal to peak exercise. We found that the percent peak heart rate in relating to percent peakVO2 during exercise was similar in lean younger and older boys; however, obesity resulted in differences between younger and older boys. This suggested that obesity causes boys to work at a greater relative rate (%HR/%VO2). In the girls, the complete opposite was true. That is, between the lean younger and older groups, the older exercise at greater relative HR/VO2, while no differences were seen between obese younger and older girls. Older boys and lean older girls moved closer to adult-derived regressions (33), while the remaining of the groups started at a lower relative work rate for VO2/HR. In general, this suggests that the predictive equation used for adults may not be accurate in children. The use of percent peak HR/VO2 is generally utilized for exercise prescription purposes (52).
Others have reported that energy cost during rest and physical activity is different in children than in adults (20, 50). This may be because resting energy expenditure is affected by age and sex in children (31). Similarly, we found that sex differences also exist for MEE during submaximal exercise. Further, it has been reported that energy expenditure at rest is altered by obesity. (6, 8, 51, 55) In adults overweight women, it was reported that exercise energy expenditure was greater during walking (21, 32). However, this may have been confounded by differences in mass and weight-bearing exercise. Maffis et al (34) found similar results during treadmill exercise, obese children age 9-year-old boys and girls expend more energy at similar exercise workloads as non-obese children. We found similar results in children with obesity but improve upon this understanding by using non-weight bearing exercise and normalizing to lean body mass. Here are several potential explanations for this. In adults, obesity reduces mitochondrial oxidation or the delivery of oxygen to working skeletal muscle. (28) Additionally, obesity may disrupt vascular function and the delivery and disposal of oxygenated blood during rest (25) and exercise (29). Children with obesity also have impaired cardiac function (15) and lung capacity, possibly due to the storage of fat in the rib cage and abdomen (56). Others report relationships between other factors such as age, sex, activity level, and age of obesity onset may also contribute to disparities for exercise response among children (30). Energy expenditure equations of the American College of Sports Medicine (46) and Pandolf et al. (45) are commonly used to predict metabolic work rates. However, these equations were developed in average adult males and are not generalizable to other populations. Here we provide a data set specific to sex and children.
Exercise economy is defined as the oxygen cost required to move at a given work rate. Others report that obese individuals elicit poor exercise economy compared to their non-obese individuals on a treadmill normalized VO2 to total body mass (5, 39). We found similar and when normalized to LBM, exercise economy was unaffected by obesity in older children. We did observe that prepubescent lean children had a worse economy (required greater oxygen uptake for similar exercise work rates) than prepubescent obese children. We also found that pre- to post-puberty differences in exercise economy are similar between the sexes. These results are in agreement with treadmill exercise studies suggesting that children have reduced exercise economy and experience an average decline of 1 mLO2 kg−1 min−1 per year until mid-late adolescence (53). However, obesity during prepuberty affected exercise economy. It is not entirely clear why this occurs as children develop, but their rapid growth and development during puberty as well as fat accumulation may alter endocrine function, metabolism, and cellular metabolic capacity (54), resulting in the economy differences seen between adults (49) and prepubescent obese children.
Estimating an exercise test that lasts between 8–12 minutes involves estimating the starting work rate, which can be under or overestimated by the test administrator. This, taken with the fact that the child may stop too soon or exercise too long, can yield inaccurate test data. Children do not exhibit clear symptoms of fatigue that are supported by maximal heart rates leveling off at about 200 bpm, a respiratory exchange ratio >1.0, and a plateau in VO2 (2). We have provided regression equations for predicting peak work rate to assist in obtaining a more robust test for both girls and boys. Interestingly, when all groups were combined, height, lean leg mass, and fat mass were the strongest predictors (accounting for 78% variance). We found that height was also a strong predictor for peak work rate among all common subject characteristics for all groups (accounting for 63% variance), an important finding given that DXA is sometimes not available. These data can be used for estimating starting work rate with a goal of determining a rate of change of load for a test of about 8–10 minutes (23). (Example: estimated peak 200 W, 200/600 seconds = rate of work rate at 0.33 W/sec). Our prediction equations can be used to estimate starting work rate and thus allow for more accuracy in obtaining maximal exhaustion within the required time.
Limitations
This study has several limitations. First, the relatively small sample size, in addition to the small number of Asian and Mexican American participants in the study, and the data represented here may not be generalizable to these particular ethnic groups. Further, our use of Tanner stages is an indirect measure of puberty. The use of hormones testosterone in both sexes and estradiol in girls would have strengthened the study groups in combination with the Pubertal Development Scale and Tanner assessments (57).
Conclusions
Our data suggest that obesity, sex, and pubertal status affect exercise characteristics in children. Specifically, obesity affects the peak VO2-HR relationship during submaximal and peak exercise. Additionally, MEE is affected by sex, and exercise economy is altered by obesity in only prepubescent children. We present in this study the importance of understanding sex, and age in children for the prescription of exercise. For example, the relative relationships (or example, prepubertal status to economy) were found to differ among sex, obesity, and age have important clinical implications for exercise prescription specific in children. These data highlight the importance of understanding the effect of obesity, sex, and age on exercise characteristics, as these variables can be used to improve and individualize exercise training in children. Future studies using a bigger sample size and including overweight children are necessary in order to generate the regression equations for estimating exercise work rate intensity and MEE.
ACKNOWLEDGEMENTS
We would like to extend our sincere gratitude to the children who participated in the studies and their families as well as the skilled staff for overseeing all patient testing.
Footnotes
Conflict of interest: Pietro Galassetti, M.D., Ph.D. is a Professor Emeritus at the University of California Irvine and a full-time employee of AstraZeneca. The authors have no other competing interests to report. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Agbaje AO, Haapala EA, Lintu N, Viitasalo A, Barker AR, Takken T, Tompuri T, Lindi V, Lakka TA. Peak oxygen uptake cut-points to identify children at increased cardiometabolic risk - the panic study. Scand J Med Sci Sports. 2019;29(1):16–24. doi: 10.1111/sms.13307. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong N, Welsman J, Winsley R. Is peak vo2 a maximal index of children’s aerobic fitness? Int J Sports Med. 1996;17(5):356–359. doi: 10.1055/s-2007-972860. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong N, Welsman JR. Peak oxygen uptake in relation to growth and maturation in 11- to 17-year-old humans. Eur J Appl Physiol. 2001;85(6):546–551. doi: 10.1007/s004210100485. [DOI] [PubMed] [Google Scholar]
- 4.Bao W, Srinivasan SR, Valdez R, Greenlund KJ, Wattigney WA, Berenson GS. Longitudinal changes in cardiovascular risk from childhood to young adulthood in offspring of parents with coronary artery disease: the bogalusa heart study. JAMA. 1997;278(21):1749–1754. [PubMed] [Google Scholar]
- 5.Browning RC, Kram R. Energetic cost and preferred speed of walking in obese vs. normal weight women. Obes Res. 2005;13(5):891–899. doi: 10.1038/oby.2005.103. [DOI] [PubMed] [Google Scholar]
- 6.Byrne NM, Wood RE, Schutz Y, Hills AP. Does metabolic compensation explain the majority of less-than-expected weight loss in obese adults during a short-term severe diet and exercise intervention? Int J Obes (Lond) 2012;36(11):1472–1478. doi: 10.1038/ijo.2012.109. [DOI] [PubMed] [Google Scholar]
- 7.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proceedings of the American Thoracic Society. 2008;5(2):274–282. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carneiro IP, Elliott SA, Siervo M, Padwal R, Bertoli S, Battezzati A, Prado CM. Is obesity associated with altered energy expenditure? Adv Nutr. 2016;7(3):476–487. doi: 10.3945/an.115.008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Bmi percentile calculator for child and teen English version. 2012. https://www.cdc.gov/healthyweight/bmi/calculator.html.
- 10.Daniels JT. A physiologist’s view of running economy. Med Sci Sports Exerc. 1985;17(3):332–338. [PubMed] [Google Scholar]
- 11.Davison KK, Susman EJ, Birch LL. Percent body fat at age 5 predicts earlier pubertal development among girls at age 9. Pediatr. 2003;111(4 Pt 1):815–821. doi: 10.1542/peds.111.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dencker M, Thorsson O, Karlsson MK, Linden C, Eiberg S, Wollmer P, Andersen LB. Gender differences and determinants of aerobic fitness in children aged 8–11 years. Eur J Appl Physiol. 2007;99(1):19–26. doi: 10.1007/s00421-006-0310-x. [DOI] [PubMed] [Google Scholar]
- 13.Ekelund U, Poortvliet E, Nilsson A, Yngve A, Holmberg A, Sjostrom M. Physical activity in relation to aerobic fitness and body fat in 14- to 15-year-old boys and girls. Eur J Appl Physiol. 2001;85(3–4):195–201. doi: 10.1007/s004210100460. [DOI] [PubMed] [Google Scholar]
- 14.Epstein LH, Coleman KJ, Myers MD. Exercise in treating obesity in children and adolescents. Med Sci Sports Exerc. 1996;28(4):428–435. doi: 10.1097/00005768-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Evans CA, Selvadurai H, Baur LA, Waters KA. Effects of obstructive sleep apnea and obesity on exercise function in children. Sleep. 2014;37(6):1103–1110. doi: 10.5665/sleep.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner DS, Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Contribution of early weight gain to childhood overweight and metabolic health: A longitudinal study (earlybird 36) Pediatr. 2009;123(1):e67–73. doi: 10.1542/peds.2008-1292. [DOI] [PubMed] [Google Scholar]
- 17.Gillman MW. Early infancy - a critical period for development of obesity. J Dev Orig Health Dis. 2010;1(5):292–299. doi: 10.1017/S2040174410000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375(9727):1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardee JP, Porter C, Sidossis LS, Borsheim E, Carson JA, Herndon DN, Suman OE. Early rehabilitative exercise training in the recovery from pediatric burn. Med Sci Sports Exerc. 2014;46(9):1710–1716. doi: 10.1249/MSS.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrell JS, McMurray RG, Baggett CD, Pennell ML, Pearce PF, Bangdiwala SI. Energy costs of physical activities in children and adolescents. Med Sci Sports Exerc. 2005;37(2):329–336. doi: 10.1249/01.mss.0000153115.33762.3f. [DOI] [PubMed] [Google Scholar]
- 21.Heden TD, LeCheminant JD, Smith JD. Influence of weight classification on walking and jogging energy expenditure prediction in women. Res Q Exerc Sport. 2012;83(3):391–399. doi: 10.1080/02701367.2012.10599873. [DOI] [PubMed] [Google Scholar]
- 22.Heyward VH, Gibson AL. Advanced fitness assessment and exercise prescription. 7th ed. Champaign, IL: Human Kinetics; 2014. [Google Scholar]
- 23.Howley ET, Bassett DR, Jr, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27(9):1292–1301. [PubMed] [Google Scholar]
- 24.Intapad S, Ojeda NB, Dasinger JH, Alexander BT. Sex differences in the developmental origins of cardiovascular disease. Physiol (Bethesda) 2014;29(2):122–132. doi: 10.1152/physiol.00045.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circ. 1993;88(6):2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 26.Keesey RE, Hirvonen MD. Body weight set-points: determination and adjustment. J Nutr. 1997;127(9):1875S–1883S. doi: 10.1093/jn/127.9.1875S. [DOI] [PubMed] [Google Scholar]
- 27.Kelley GA, Kelley KS. Effects of exercise in the treatment of overweight and obese children and adolescents: a systematic review of meta-analyses. J Obes. 2013;2013 doi: 10.1155/2013/783103. 10 pgs (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: Role of endothelium-dependent vasodilation. Diabetes care. 2003;26(3):899–904. doi: 10.2337/diacare.26.3.899. [DOI] [PubMed] [Google Scholar]
- 30.Lang JE. Exercise, obesity, and asthma in children and adolescents. J Pediatr (Rio J) 2014;90(3):215–217. doi: 10.1016/j.jped.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Lazzer S, Bedogni G, Lafortuna CL, Marazzi N, Busti C, Galli R, De Col A, Agosti F, Sartorio A. Relationship between basal metabolic rate, gender, age, and body composition in 8,780 white obese subjects. Obes. 2010;18(1):71–78. doi: 10.1038/oby.2009.162. [DOI] [PubMed] [Google Scholar]
- 32.LeCheminant JD, Heden T, Smith J, Covington NK. Comparison of energy expenditure, economy, and pedometer counts between normal weight and overweight or obese women during a walking and jogging activity. Eur J Appl Physiol. 2009;106(5):675–682. doi: 10.1007/s00421-009-1059-9. [DOI] [PubMed] [Google Scholar]
- 33.Londeree BR, Ames SA. Trend analysis of the % vo2 max-hr regression. Med Sci Sports. 1976;8(2):123–125. [PubMed] [Google Scholar]
- 34.Maffeis C, Schutz Y, Schena F, Zaffanello M, Pinelli L. Energy expenditure during walking and running in obese and nonobese prepubertal children. J Pediatr. 1993;123(2):193–199. doi: 10.1016/s0022-3476(05)81688-9. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 36.Mamun AA, Hayatbakhsh MR, O’Callaghan M, Williams G, Najman J. Early overweight and pubertal maturation--pathways of association with young adults’ overweight: a longitudinal study. Int J Obes. 2009;33(1):14–20. doi: 10.1038/ijo.2008.220. [DOI] [PubMed] [Google Scholar]
- 37.Marson EC, Delevatti RS, Prado AK, Netto N, Kruel LF. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: a systematic review and meta-analysis. Prev Med. 2016;93:211–218. doi: 10.1016/j.ypmed.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Martinez JA. Body-weight regulation: causes of obesity. Proc Nutr Soc. 2000;59(3):337–345. doi: 10.1017/s0029665100000380. [DOI] [PubMed] [Google Scholar]
- 39.McMurray RG, Ondrak KS. Effects of being overweight on ventilatory dynamics of youth at rest and during exercise. Eur J Appl Physiol. 2011;111(2):285–292. doi: 10.1007/s00421-010-1651-z. [DOI] [PubMed] [Google Scholar]
- 40.Morgan DW, Craib M. Physiological aspects of running economy. Med Sci Sports Exerc. 1992;24(4):456–461. [PubMed] [Google Scholar]
- 41.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishi Y. Measurement of thermal balance in man. In: Cena K, Clark J, editors. Thermal physiology and comfort. New York, NY: Elsevier; 1981. [Google Scholar]
- 43.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP, Summerbell CD. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;(1):CD001872. doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Pandolf KB, Givoni B, Goldman RF. Predicting energy expenditure with loads while standing or walking very slowly. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(4):577–581. doi: 10.1152/jappl.1977.43.4.577. [DOI] [PubMed] [Google Scholar]
- 46.Pescatello LS American College of Sports Medicine. Acsm’s guidelines for exercise testing and prescription. 9th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- 47.Peyer K, Pivarnik JM, Coe DP. The relationship among hrpeak, rerpeak, and vo2peak during treadmill testing in girls. Res Q Exerc Sport. 2011;82(4):685–692. doi: 10.1080/02701367.2011.10599805. [DOI] [PubMed] [Google Scholar]
- 48.Reybrouck T, Weymans M, Vinckx J, Stijns H, Vanderschueren-Lodeweyckx M. Cardiorespiratory function during exercise in obese children. Acta Paediatr Scand. 1987;76(2):342–348. doi: 10.1111/j.1651-2227.1987.tb10472.x. [DOI] [PubMed] [Google Scholar]
- 49.Riddell MC. The endocrine response and substrate utilization during exercise in children and adolescents. J Appl Physiol. 2008;105(2):725–733. doi: 10.1152/japplphysiol.00031.2008. [DOI] [PubMed] [Google Scholar]
- 50.Ridley K, Olds TS. Assigning energy costs to activities in children: A review and synthesis. Med Sci Sports Exerc. 2008;40(8):1439–1446. doi: 10.1249/MSS.0b013e31817279ef. [DOI] [PubMed] [Google Scholar]
- 51.Riou ME, Jomphe-Tremblay S, Lamothe G, Stacey D, Szczotka A, Doucet E. Predictors of energy compensation during exercise interventions: A systematic review. Nutrients. 2015;7(5):3677–3704. doi: 10.3390/nu7053677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivas E, Herndon DN, Cambiaso-Daniel J, Rontoyanni VG, Porter C, Glover S, Suman OE. Quantification of an exercise rehabilitation program for severely burned children: The standard of care at shriners hospitals for children(r)-galveston. J Burn Care Res. 2018;39(6):889–896. doi: 10.1093/jbcr/iry001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowland T. Inferior exercise economy in children: Perpetuating a myth? Pediatr Exerc Sci. 2012;24(4):501–506. doi: 10.1123/pes.24.4.501. [DOI] [PubMed] [Google Scholar]
- 54.Rowland T, Saltin B. Learning from children: The emergence of pediatric exercise science. J Appl Physiol. 2008;105(1):322–324. doi: 10.1152/japplphysiol.90624.2008. [DOI] [PubMed] [Google Scholar]
- 55.Ruohonen S, Valve L, Tuomainen K, Ailanen L, Roytta M, Manz G, Baur N, Joos T, Savontaus E, Scheinin M. Increased energy expenditure, lipolysis, and hyperinsulinemia confer resistance to central obesity and type 2 diabetes in mice lacking alpha2a-adrenoceptors. Neuroendocrinology. 2018;107(4):324–339. doi: 10.1159/000492387. [DOI] [PubMed] [Google Scholar]
- 56.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108(1):206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 57.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanner JM. Growth at adolescence; with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. 2nd ed. Springfield, IL: Oxford, Blackwell Scientific Publications; 1962. [Google Scholar]
- 59.Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-Kraff M, Adler-Wailes DC, Brady S, Reynolds JC, Calis KA, Yanovski JA. Orthopedic complications of overweight in children and adolescents. Pediatr. 2006;117(6):2167–2174. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watts K, Jones TW, Davis EA, Green D. Exercise training in obese children and adolescents: Current concepts. Sports Med. 2005;35(5):375–392. doi: 10.2165/00007256-200535050-00002. [DOI] [PubMed] [Google Scholar]