Abstract
Background
Seasonal affective disorder (SAD) is a seasonal pattern of recurrent major depressive episodes that most commonly occurs during autumn or winter and remits in spring. The prevalence of SAD ranges from 1.5% to 9%, depending on latitude. The predictable seasonal aspect of SAD provides a promising opportunity for prevention. This is one of four reviews on the efficacy and safety of interventions to prevent SAD; we focus on psychological therapies as preventive interventions.
Objectives
To assess the efficacy and safety of psychological therapies (in comparison with no treatment, other types of psychological therapy, second‐generation antidepressants, light therapy, melatonin or agomelatine or lifestyle interventions) in preventing SAD and improving person‐centred outcomes among adults with a history of SAD.
Search methods
We searched Ovid MEDLINE (1950‐ ), Embase (1974‐ ), PsycINFO (1967‐ ) and the Cochrane Central Register of Controlled Trials (CENTRAL) to 19 June 2018. An earlier search of these databases was conducted via the Cochrane Common Mental Disorders Controlled Trial Register (CCMD‐CTR) (all years to 11 August 2015). Furthermore, we searched the Cumulative Index to Nursing and Allied Health Literature, Web of Science, the Cochrane Library, the Allied and Complementary Medicine Database and international trial registers (to 19 June 2018). We also conducted a grey literature search and handsearched the reference lists of included studies and pertinent review articles.
Selection criteria
To examine efficacy, we included randomised controlled trials (RCTs) on adults with a history of winter‐type SAD who were free of symptoms at the beginning of the study. To examine adverse events, we intended to include non‐randomised studies. We planned to include studies that compared psychological therapy versus no treatment, or any other type of psychological therapy, light therapy, second‐generation antidepressants, melatonin, agomelatine or lifestyle changes. We also planned to compare psychological therapy in combination with any of the comparator interventions listed above versus no treatment or the same comparator intervention as monotherapy.
Data collection and analysis
Two review authors screened abstracts and full‐text publications against the inclusion criteria, independently extracted data, assessed risk of bias, and graded the certainty of evidence.
Main results
We identified 3745 citations through electronic searches and reviews of reference lists after deduplication of search results. We excluded 3619 records during title and abstract review and assessed 126 articles at full‐text review for eligibility. We included one controlled study enrolling 46 participants. We rated this RCT at high risk for performance and detection bias due to a lack of blinding.
The included RCT compared preventive use of mindfulness‐based cognitive therapy (MBCT) with treatment as usual (TAU) in participants with a history of SAD. MBCT was administered in spring in eight weekly individual 45‐ to 60‐minute sessions. In the TAU group participants did not receive any preventive treatment but were invited to start light therapy as first depressive symptoms occurred. Both groups were assessed weekly for occurrence of a new depressive episode measured with the Inventory of Depressive Syptomatology‐Self‐Report (IDS‐SR, range 0‐90) from September 2011 to mid‐April 2012. The incidence of a new depressive episode in the upcoming winter was similar in both groups. In the MBCT group 65% of 23 participants developed depression (IDS‐SR ≥ 20), compared to 74% of 23 people in the TAU group (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.60 to 1.30; 46 participants; very low quality‐evidence).
For participants with depressive episodes, severity of depression was comparable between groups. Participants in the MBCT group had a mean score of 26.5 (SD 7.0) on the IDS‐SR, and TAU participants a mean score of 25.3 (SD 6.3) (mean difference (MD) 1.20, 95% CI ‐3.44 to 5.84; 32 participants; very low quality‐evidence).
The overall discontinuation rate was similar too, with 17% discontinuing in the MBCT group and 13% in the TAU group (RR 1.33, 95% CI 0.34 to 5.30; 46 participants; very low quality‐evidence).
Reasons for downgrading the quality of evidence included high risk of bias of the included study and imprecision.
Investigators provided no information on adverse events. We could not find any studies that compared psychological therapy with other interventions of interest such as second‐generation antidepressants, light therapy, melatonin or agomelatine.
Authors' conclusions
The evidence on psychological therapies to prevent the onset of a new depressive episode in people with a history of SAD is inconclusive. We identified only one study including 46 participants focusing on one type of psychological therapy. Methodological limitations and the small sample size preclude us from drawing a conclusion on benefits and harms of MBCT as a preventive intervention for SAD. Given that there is no comparative evidence for psychological therapy versus other preventive options, the decision for or against initiating preventive treatment of SAD and the treatment selected should be strongly based on patient preferences and other preventive interventions that are supported by evidence.
Keywords: Humans; Antidepressive Agents; Antidepressive Agents/therapeutic use; Cognitive Behavioral Therapy; Depressive Disorder, Major; Depressive Disorder, Major/therapy; Melatonin; Melatonin/therapeutic use; Phototherapy; Randomized Controlled Trials as Topic; Seasonal Affective Disorder; Seasonal Affective Disorder/prevention & control; Seasonal Affective Disorder/therapy
Plain language summary
Psychological therapies for prevention of winter depression
Why is this review important?
Many people in northern latitudes suffer from seasonal affective disorder (SAD), which occurs as a reaction to reduced sunlight. Three‐quarters of those affected are women. Lethargy, overeating, craving for carbohydrates and depressed mood are common symptoms. In some people, SAD becomes a depression that seriously affects their daily lives. Up to two‐thirds experience depressive symptoms every winter.
Who might be interested in this review?
Anyone who has experienced SAD or who has relatives and friends who have experienced SAD, as well as researchers working in this field might be interested in this review.
What questions does this review aim to answer?
The predictable seasonal aspect of SAD provides a promising opportunity for prevention. However, little is known about the efficacy and potential harms of interventions for preventing SAD. This is one of four reviews conducted to examine the efficacy and side effects of interventions used to prevent SAD; this review focuses on psychological therapy as a preventive intervention in people with a history of SAD who were free of symptoms at the time the preventive intervention was started.
Which studies were included in the review?
We searched databases up to 19 June 2018 for studies on psychological therapies to prevent SAD. Among 3745 records, we found one randomised controlled study including 46 people who received a form of psychological therapy ‐ the so called mindfulness‐based cognitive therapy (MBCT) or treatment as usual. Treatment as usual meant that participants did not receive any preventive treatment but were invited to start light therapy as first depressive symptoms occurred. All individuals in these studies had a history of winter depression and were free of depressive symptoms when the study started.
What does evidence from this review reveal?
The proportion of participants developing a depression in the upcoming winter was similar in both groups as well as the severity of these depressive episodes. However, the quality of the evidence was very low, so we can draw no valid conclusion if MBCT is really ineffective in preventing SAD or not. The included study reported no information on side effects of the intervention. Doctors need to discuss with patients the advantages and disadvantages of MBCT and other potentially preventive treatments for winter depression, such as other psychological therapies, drug treatments, or lifestyle interventions. As no available studies have compared these treatments, treatment selection should be strongly based on patient preferences and other preventive interventions that are supported by evidence.
What should happen next?
Review authors recommend that future studies should evaluate the efficacy of different psychological therapies in preventing SAD in larger study samples and should directly compare these interventions versus other preventive treatments, such as light therapy, antidepressants and agomelatine, to determine the best treatment option for prevention of SAD.
Summary of findings
Summary of findings for the main comparison. Mindfulness‐based cognitive therapy (MBCT) compared with treatment as usual (TAU) for prevention of seasonal affective disorder (SAD).
| Mindfulness‐based cognitive therapy (MBCT) compared with treatment as usual (TAU) for prevention of seasonal affective disorder (SAD) | ||||||
|
Patient or population: adults with a history of SAD but without depressive symptoms when treatment started Settings: outpatient clinic Intervention: mindfulness‐based cognitive therapy Comparison: treatment as usual | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | MBCT | |||||
| Incidence of SAD (IDS‐SR > 20) Follow‐up: 7.5 months |
Low | RR 0.88 (0.60 to 1.30) | 46 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | ||
| 300 per 1000 | 264 per 1000 (180 to 390) | |||||
| Moderate | ||||||
| 500 per 1000 | 440 per 1000 (300 to 650) | |||||
| High | ||||||
| 600 per 1000 | 528 per 1000 (360 to 780) | |||||
| Severity of SAD (measured with IDS‐SR) Follow‐up: 7.5 months |
Mean severity of depression: 25.3 | The mean severity of depression was 1.20 higher (3.44 lower to 5.84 higher) | 32 (1 RCT) |
⊕⊝⊝⊝ Very lowa,c | ||
| Overall discontinuation rate Follow‐up: 7.5 months |
130 per 1000 | 175 per 1000 (44 to 691) | RR 1.33 (0.34 to 5.30) | 46 (1 RCT) |
⊕⊝⊝⊝ Very lowa,c | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IDS‐SR: Inventory of Depressive Symptomology‐Self‐Report (range 0‐90); MBCT: mindfulness‐based cognitive therapy; RCT: randomised controlled trial; RR: risk ratio; SAD: seasonal affective disorder; TAU: treatment as usual | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aThe allocation concealment was unclear and participants who also assessed the outcome were not blinded, therefore we downgraded one point. bThe sample size was very small and did not reach optimal information size, therefore we downgraded two points. cThe sample size was very small and did not reach optimal information size. The confidence interval was very broad, therefore we downgraded two points.
Background
Description of the condition
Seasonal affective disorder (SAD) is a seasonal pattern of recurrent major depressive episodes that most commonly occurs during autumn or winter and remits in spring or summer (Rosenthal 1984). In addition to the predictable seasonal pattern of depression, persons suffering from SAD commonly experience atypical symptoms such as hypersomnia, carbohydrate craving with increased appetite and weight gain and extreme fatigue (Sohn 2005). Prevalence in the USA ranges from 1.5% in southern Florida to 9% in northern regions (Rosen 1990). In northern latitudes, the prevalence of SAD is estimated to be around 10% (Byrne 2008). SAD is a multifactorial condition in which chronobiological mechanisms related to circadian rhythms, melatonin, serotonin turnover and photoperiodism (length of dark hours relative to light hours in a 24‐hour period) are thought to play a role (Ciarleglio 2011; Levitan 2007). A quintessential and especially impairing quality of this illness is its high risk of recurrence and persistence. Approximately two‐thirds of those diagnosed with SAD will face recurrence of these distressing symptoms the following winter (Rodin 1997). In the five to 11 years following initial diagnosis, 22% to 42% of people still suffer from SAD, and 33% to 44% develop a non‐seasonal pattern in subsequent episodes; the disorder resolves completely in only 14% to 18% of people with SAD (Magnusson 2005; Schwartz 1996). Indeed, many people who suffer from SAD experience this type of depression every year, which makes it particularly amenable to preventive treatment (Westrin 2007).
Description of the intervention
In general, psychological treatments for SAD are designed to target the psychological factors that contribute to depression as opposed to the physiological vulnerabilities that lead to this disorder. Several psychological therapies have been evaluated for their effectiveness in treating SAD. Among these are behavioural therapy/behaviour modification, cognitive behaviour therapy (CBT), third‐wave CBT and psychodynamic psychotherapy.
In general, psychological therapy, regardless of the specific modality, may prevent SAD by helping individuals intentionally engage in cognitive or behavioural activities (or both) that directly counter cognitive patterns or behavioural responses that might be inherent sequelae of the worsening mood symptoms commonly associated with SAD.
Other established treatments, such as light therapy, melatonin and second‐generation antidepressants may be used to prevent SAD. Research into the causes of SAD has focused on the role of circadian rhythms and melatonin (Lam 2006). Light therapy, a non‐pharmacological treatment that has proved effective in the treatment of individuals with SAD, is often used as first‐line therapy (Terman 2005). Decreased seasonal exposure to light is thought to be a reason for the development of SAD, which results from phase shifts in circadian rhythms, leading to alterations in serotonin metabolism. Therefore, light therapy has been studied intensively as treatment for SAD (Partonen 1998). Similarly, Lewy et al suggest that relative phase shifting of circadian rhythms as they relate to the timing of sleep‐wake rhythm is responsible for the genesis of SAD (Lewy 1987). As a rhythm‐regulating factor, and as a hormone involved in the regulation of sleep, melatonin is essential for control of mood and behaviour (Srinivasan 2012). Appropriately timed administration of melatonin has chronobiotic properties and can facilitate phase shifting in the circadian system (alone or, more typically, in combination with light exposure) (Hickie 2011). Finally, one of the second‐generation antidepressants (drugs typically used to treat major depressive disorder) ‐ bupropion XL (extended‐release) ‐ is currently licensed for use in preventing SAD (Modell 2005). At the neurochemical level, changes in both serotonergic and catecholaminergic transmitter systems seem to play a key role in SAD (Neumeister 2001). Targeting these systems with serotonin or noradrenaline reuptake inhibition, or both, provides biological plausibility for the mechanism of action of second‐generation antidepressants. The rationale for using second‐generation antidepressants for prevention of SAD is based on the efficacy of second‐generation antidepressants for treatment of SAD (Thaler 2010).
How the intervention might work
Much research into the origin of SAD has focused on the roles of circadian rhythms and melatonin (Lam 2006). Psychological therapies are designed to target maladaptive cognitions, behaviours, interpersonal patterns and communication, as well as coping styles that correlate with SAD, which may contribute to annual relapse and recurrence (Hodges 1998; Levitan 1998; Rohan 2003). Many psychological therapies can be administered individually or as a group. Common characteristics across these treatment modalities include reflective listening; therapeutic support; challenging of distorted thoughts, behaviours or schemata; psychoeducation; normalisation; and empathy.
Behavioural therapy/behaviour modification/CBT interventions
Cognitive‐behavioural interventions have received the most empirical attention (BMJ 2010; Kurlansik 2012). Cognitive interventions focus on depressive cognitive constructs that underlie affective disorders in general, including dysfunctional attitudes, negative automatic thoughts, cognitive reactivity and ruminative response style (Beck 1967; Beck 1976; Nolen‐Hoeksema 1987). Behavioural interventions focus on structured activities and pleasant event scheduling. Many interventions incorporate elements of both cognitive and behavioural therapies, along with basic problem‐solving strategies and psychoeducation (Evans 2013; Rohan 2007Rohan 2009). CBT interventions commonly used to treat individuals with SAD include thought records, activity schedules and a positive data log.
Third‐wave CBT
Mindfulness‐based cognitive therapy (MBCT) utilises traditional methods of CBT combined with mindfulness and mindfulness meditation. As with CBT, MBCT is premised on the notion that negative automatic cognitive processes can lead to depression (Felder 2012). The goal of MBCT is to interrupt these automatic processes while teaching the participant to focus less on reacting to incoming stimuli and accepting and observing them without judgement. This mindfulness practice then allows individuals to notice when automatic processes are occurring and to alter their reactions to show greater reflection and awareness. MBCT interventions employ meditation practices such as breathing and stretching techniques.
Psychodynamic therapies
Psychodynamic therapy helps people gain greater self‐awareness and understanding about their own actions (Leichsenring 2007). It helps individuals identify and explore how their non‐conscious emotions and motivations can influence their behaviour. Elements of psychodynamic therapy are often seen in other forms of psychological therapy, such as CBT or interpersonal therapy. In psychodynamic therapy, individuals learn to examine feelings about which they are aware, as well as those that may not be consciously available, without guidance from the therapist.
Integrative therapies
Interpersonal therapy is a manualised treatment for depression that is based on the idea that improving communication patterns and ways in which people relate to each other will effectively treat depression (DeMello 2005). Interpersonal therapy helps individuals identify how they are interacting with others and change problematic interpersonal behaviours. It may also focus on helping individuals identify painful emotions and their triggers, and it may help them learn to express appropriate emotions in a healthy way. In interpersonal therapy, individuals may examine past relationships that might have been affected by distorted mood and behaviour, with the goal of helping people learn to be more objective about current relationships. Common interpersonal therapy techniques include interpersonal incidents, communication analysis, use of content and process affect and role play.
Other psychologically oriented therapies
Positive psychotherapy is based on the hypothesis that depression can be treated effectively not only by reducing its negative symptoms but also by directly and primarily building positive emotions, character strength and meaning (Seligman 2006). By directly building these positive resources, positive psychotherapy may successfully counteract negative symptoms and may provide a buffer against future reoccurrence. Positive psychology helps individuals focus on their strengths and fosters a sense of gratitude and intentional notice of and appreciation for daily positive events.
Self‐system therapy is a brief, structured psychotherapy that is based on regulatory focus theory. In self‐system therapy, depression is conceptualised as a disorder of motivation and goal pursuit that results from chronic failure to attain personal goals (Strauman 2006). As a result, individuals experience high discrepancy between their actual and ideal selves, together with a high incentive motivation. Self‐system therapy incorporates techniques from several empirically supported psychotherapies, including cognitive, interpersonal and behavioural activation approaches. Self‐system therapy focuses on translating the principles of regulatory focus theory into an intervention that examines and modifies the individual's goals and strategies for pursuing them. This treatment focuses on self‐evaluation and on increasing promotion‐focused behaviour through psychoeducation, helping individuals learn to conduct their own situations and belief analyses, while altering or compensating for one's regulatory style and moving toward a view of self in a broader context.
Why it is important to do this review
The predictable seasonal aspect of SAD provides a specific and promising opportunity for prevention. However, both individuals and clinicians face much uncertainty in their collaborative decisions about the choice of a preventive intervention (Westrin 2007). To date, no Cochrane Reviews have assessed the efficacy, effectiveness and risk of harms of psychological therapies for preventing recurrent SAD.
Our findings were intended to provide insights into (1) available evidence on benefits and harms of competing interventions for prevention of SAD with respect to person‐centred outcomes, and (2) gaps in the evidence base that will inform future research needs.
This is one of four reviews of interventions used to prevent SAD. The others focus on light therapy (Nussbaumer‐Streit 2019), second‐generation antidepressants (Gartlehner 2019), and agomelatine and/or melatonin (Kaminski‐Hartenthaler 2015), as preventive interventions.
Objectives
To assess the efficacy and safety of psychological therapies (in comparison with no treatment, other types of psychological therapy, second‐generation antidepressants, light therapy, melatonin or agomelatine or lifestyle interventions) in preventing SAD and improving person‐centred outcomes among adults with a history of SAD.
Methods
Criteria for considering studies for this review
Types of studies
Efficacy (beneficial effects)
We included randomised controlled trials (RCTs; including cross‐over studies and cluster‐randomised trials) of psychological therapies for prevention of seasonal affective disorder (SAD).
Adverse effects
We planned to include:
RCTs (including cross‐over studies and cluster‐randomised trials) of psychological therapies for prevention of SAD; and
non‐randomised controlled studies such as non‐randomised trials, prospective cohort studies or case control studies of psychological therapies for prevention of SAD.
Types of participants
Participant characteristics
Male and female adults (≥ 18 years of age) of all races, ethnicities and cultural groups with a history of SAD who do not fulfil the criteria for a current major depressive episode.
Diagnosis
We defined SAD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) (APA 2013) as a seasonal pattern of recurrent major depressive episodes. However, we restricted the definition to winter‐type SAD (i.e. major depression in the autumn/winter with full remission in the spring/summer), and we did not include individuals with bipolar disorder and a seasonal pattern. We planned to include studies that used definitions from prior versions of the DSM (APA 1980; APA 1987; APA 2000).
Comorbidities
We excluded studies that enrolled participants with depressive disorder due to another medical condition. We planned to include populations at risk of SAD with common comorbidities (e.g. diabetes, cardiovascular disease) that were not the cause of the depressive episode.
Setting
We included studies conducted in all settings (e.g. outpatient clinics, community, public or private clinics).
Subset data
We planned to include studies that provided data on subsets of participants of interest, as long as the subset met our eligibility criteria. We planned to not include studies with 'mixed' populations if they did not adequately stratify data with respect to our population of interest.
Types of interventions
Experimental interventions
We planned to include the following psychological therapies.
Behavioural therapy/behaviour modification/cognitive behaviour therapy (CBT).
Third‐wave CBT.
Psychodynamic therapies.
Integrative therapies.
Other psychologically oriented interventions.
We also planned to include combination therapies versus any of the comparator interventions listed below.
Comparator interventions
We planned to compare any psychological therapy versus:
no treatment or waiting list;
another therapy from the list above;
second‐generation antidepressants;
light therapy;
melatonin or agomelatine; and
lifestyle interventions (e.g. exercising, making the environment sunnier (opening blinds), spending time outside regularly, adapting nutrition (low‐fat diet, reduction in refined sugars, etc.).
We planned to compare psychological therapies in combination with any of the comparator interventions listed above versus no treatment, or the same comparator intervention as monotherapy (see Data extraction and management).
Types of outcome measures
We included studies that met the above inclusion criteria regardless of whether they reported on the following outcomes. In consultation with clinical experts, we selected the following outcomes a priori.
Primary outcomes
The primary outcome for benefit was the incidence of SAD, measured as the proportion of participants with a Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders (SIGH‐SAD; Williams 2012) score of 20 or higher.
The primary outcome for harm was the overall rate of adverse events (e.g. increased suicidal ideation, worsening of symptoms) related to preventive interventions. We intended to include all information on adverse events, irrespective of the way it was assessed in the study (targeted, structured or spontaneously reported).
Secondary outcomes
Severity of the SAD episode or SAD‐related symptoms, as measured by a validated tool (e.g. Hamilton Depression Rating Scale (Hamilton 1960)).
Quality of life, as measured by a validated quality of life tool (e.g. Short Form (SF)‐36 (Ware 1992)).
Quality of interpersonal and social functioning, as measured by a validated tool (e.g. the Longitudinal Interval Follow‐up Evaluation‐Range of Impaired Functioning Tool (LIFE–RIFT) (Leon 1999)).
Proportion of participants with serious adverse events.
Rates of discontinuation of preventive intervention due to adverse events.
Overall rate of discontinuation.
Timing of outcome assessment
We planned to synthesise outcomes at different time points (e.g. short term (0 to 3 months), medium term (> 3 months to 6 months), long term (> 6 months to 12 months)) throughout an entire six‐month period of risk during an autumn/winter season and during a follow‐up period of one additional autumn/winter period, because psychological therapies appear to have durable effects that may extend the period of risk during the first autumn/winter season.
Hierarchy of outcome measures
Our main focus was person‐centred outcomes, that is, outcomes that people with SAD notice and care about. If several measures assessed the same outcome, we consulted with clinical experts regarding the validity and reliability of individual outcome measures and prioritised accordingly.
Search methods for identification of studies
This is one of four Cochrane Reviews on interventions to prevent SAD in adults with a history of SAD (Gartlehner 2019; Kaminski‐Hartenthaler 2015; Nussbaumer‐Streit 2019). We conducted one search for all four reviews.
The Cochrane Common Mental Disorders Group (CCMD) maintains two clinical trials registers at its editorial base in Bristol, UK: a references‐based register and a studies‐based register. The CCMD Specialised Register (CCMDCTR)‐References contains more than 39,000 reports of randomised controlled trials (RCTs) on common mental disorders. Approximately 60% of these references have been tagged to individual, coded trials. The coded trials are held in the Specialised CCMDCTR‐Studies Register, and records are linked between the two registers through the use of unique study ID tags. Coding of trials is based on the EU‐Psi coding manual and use of a controlled vocabulary (the CCMD Information Specialist can provide further details). Reports of trials for inclusion in the Group Registers are collated from routine (weekly), generic searches of MEDLINE (1950‐), Embase (1974‐) and PsycINFO (1967‐); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL); and review‐specific searches of additional databases. Reports of trials are also obtained from international trials registers through trials portals of the World Health Organization (the International Clinical Trials Registry Platform (ICTRP)) and pharmaceutical companies and by handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCDAN's generic search strategies (used to identify RCTs) can be found on the Group website, with an example of the core MEDLINE search displayed in Appendix 1. This register is current to June 2016 only.
Electronic searches
The searches for this review are up‐to‐date as of 19 June 2018. Details of all searches conducted between April 2013 and June 2018 are described below.
The Information Specialist with the Cochrane Common Mental Disorders (CCMD) Group ran an initial search of their Group's specialised registers (CCMD‐CTR‐Studies and CCMD‐CTR‐References) (all years to 12 April 2013) using terms for condition only. An updated search was performed on 11 August 2015, prior to the first publication of this review.
("seasonal affective disorder*" or "seasonal depression" or "seasonal mood disorder*" or "winter depression" or SIGH‐SAD*).
In addition, we conducted our own searches of the following electronic databases (to 26 May 2014) to ensure that no studies had been missed by the CCMD‐CTR specialised registers (Appendix 2).
International Pharmaceutical Abstracts.
Cumulative Index to Nursing and Allied Health Literature (CINAHL).
Web of Science (includes Web of Science, Current Contents Connect, Conference Proceedings Citation Index, BIOSIS, Derwent Innovations Index, Data Citation Index, SciELO Citation Index) (all available years).
The Cochrane Library.
Allied and Complementary Medicine Database (AMED).
We also searched international trial registries via the World Health Organization trials portal (ICTRP) and ClinicalTrials.gov to identify unpublished or ongoing studies.
We did not restrict searches by date, language or publication status.
In June 2018, CCMD's Information Specialist updated the search for studies on all of the databases listed above (Appendix 3), with the exception of International Pharmaceutical Abstracts. The search of these databases was necessary as the CCMD‐CTR was out of date at the time (current to June 2016 only).
Searching other resources
Grey literature
To detect additional studies, we checked the following sources.
IFPMA (International Federation of Pharmaceutical Manufacturers and Associations) Clinical Trials Portal.
OpenGrey.
National Institute of Health RePORTER.
Health Services Research Projects in Progress (HSRProj).
Hayes Inc. Health Technology Assessment.
The New York Academy of Medicine Grey Literature Index.
Conference Papers Index.
European Medicines Agency.
Drugs@FDA (Food and Drug Administration).
National Registry of Evidence‐Based Programs and Practices (NREPP) (no longer available online).
Reference lists
We handsearched the references of all included studies and pertinent review articles.
Correspondence
We contacted trialists and subject experts to ask for information on unpublished and ongoing studies or to request additional trial data.
Data collection and analysis
Selection of studies
A team of review authors (CF, BN, GG, BG, AG, LM, JW, LL) dually and independently screened the titles and abstracts of all studies identified by these searches. We retrieved full‐text copies of all studies that potentially met the inclusion criteria based on initial assessment; the same review authors then dually screened them independently to determine their eligibility.
If the two review authors did not reach consensus, they discussed and resolved disagreements by involving a third party. We intended to contact study authors when relevant information was missing. We tracked all results in an EndNote X8 database (EndNote X8 2016).
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and a Characteristics of excluded studies table.
Data extraction and management
We used a data collection form. Two review authors (CF and AG) independently extracted study characteristics and outcome data from included studies. We resolved discrepancies by reaching consensus or by involving another review author (BN). We reported whether studies were detected by a search of databases of published studies, by handsearch or by a search of grey literature.
We extracted the following study characteristics.
Methods: study design, study duration, details of any 'run‐in' period, duration of treatment period, number of study centres and locations, study setting, withdrawals and dates of study.
Participants: numbers of participants, mean age, age range, proportion of women, number of prior depressive episodes, diagnostic criteria, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant interventions and excluded interventions.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for studies and notable conflicts of interest of study authors.
We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by reaching consensus or by involving a third person. One review author (CF) transferred data into the Review Manager 5 file (Review Manager 2014). Another review author (AG) double‐checked that data were entered correctly by comparing data presented in the systematic review versus the study reports. Another review author (BN) checked study characteristics for accuracy against the trial report.
Main planned comparisons
Psychological therapy versus no treatment or waiting list.
Psychological therapy versus other psychological therapy.
Psychological therapy versus light therapy.
Psychological therapy versus second‐generation antidepressants.
Psychological therapy versus melatonin or agomelatine.
Psychological therapy versus lifestyle interventions.
Psychological therapy + comparator intervention (as listed in Types of interventions) versus no treatment control group or waiting list.
Psychological therapy + comparator intervention (as listed in Types of interventions) versus the same comparator intervention as monotherapy (e.g. psychological therapy + light therapy versus light therapy alone).
Assessment of risk of bias in included studies
Two review authors (CF, AG) independently assessed risk of bias of included randomised trials by using the Cochrane 'Risk of bias' tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved discrepancies by reaching consensus or by involving another review author (BN). The Cochrane 'Risk of bias' tool enables assessment of random sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting; and other potential threats to validity. Specifically, we assessed attrition in these trials and reasons for attrition, particularly when attrition rates between two groups in a trial differed substantially. In addition, we assessed whether all relevant outcomes for the trial were reported in the published articles. We assessed each domain as having high risk of bias, low risk of bias or unclear risk of bias.
For non‐randomised studies, we would have used the Newcastle‐Ottawa Scale, involving selection of cases or cohorts and controls, adjustment for confounders, methods of outcomes assessment, length of follow‐up and statistical analysis (Wells 2009).
It is not possible for clinicians and participants to be blinded in studies of psychological therapy; therefore all studies would have been assigned high risk of performance bias. When possible, we assessed therapist qualifications and experience, treatment fidelity, researcher allegiance/conflict of interest and therapist allegiance/conflict of interest as other sources of potential bias. In general, risk of bias is probably less when therapists are well trained and are experienced in providing the intervention, when they adhere most closely to the modality employed, when adherence is monitored and evaluated by an objective assessor and when neither the clinician nor the researcher has an inherent allegiance to nor conflict of interest with treatment provided in the study.
Measures of treatment effect
We used data extracted from the original studies to construct 2 × 2 tables for dichotomous outcomes. When multiple studies would allow quantitative analysis, we planned to calculate the risk ratio (RR) with 95% confidence intervals (CIs) for each outcome. We chose the RR as an effect measure because for decision makers, RRs are easier to interpret than odds ratios (ORs), particularly when event rates are high.
We planned to pool continuous data by using the mean difference (MD) if an outcome was measured on the same scale, or the standardised mean difference (SMD) if an outcome was measured on different scales. If available, we would have used final measurements rather than changes from baseline to estimate differences between treatments. When it was considered necessary to use both change and postintervention scores in a comparison, we planned to present these by subgroups by using the MD, rather than the SMD.
For time‐to‐event data, we planned to calculate a pooled hazard ratio (HR) when this was available, or to dichotomise data at multiple time points into response/no response (e.g. at one week, at two weeks, at four weeks).
The same time points as specified under 'Timing of outcomes assessment' in the section Types of outcome measures were intended to form the basis for dichotomisation into response/no response.
For non‐randomised studies, we planned to use adjusted treatment effects when available.
Unit of analysis issues
Cluster‐randomised trials
To incorporate cluster‐randomised trials, we planned to reduce the size of each trial to its 'effective sample size'. If intracluster correlation coefficients were not reported, we intended to find external estimates from similar studies. We planned to undertake sensitivity analyses to assess the impact of including such trials.
Cross‐over trials
To avoid carry‐over effects, we planned to include data only from the first period of cross‐over studies.
Studies with multiple treatment groups
For included trials that had multiple treatment groups (e.g. multiple psychological therapies versus placebo), we planned to include data for the treatment arms and to halve data for the placebo arm.
Dealing with missing data
We used intention‐to‐treat analysis when data were missing for participants who dropped out of trials before completion. When data regarding an outcome of interest were not reported, we contacted the authors of publications to request missing results.
Assessment of heterogeneity
We planned to use the Cochran Chi2 test (Q‐test) to assess heterogeneity. A P value less than 0.10 was considered statistically significant. We planned to use the I2 statistic to estimate the degree of heterogeneity. This measure describes the percentage of total variation across studies that results from heterogeneity rather than chance. We planned to interpret the importance of heterogeneity in terms of its magnitude and direction of effects. We did not intend to consider thresholds; instead we planned to adopt the overlapping bands, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For example, we planned to consider an I2 value between 0% and 40% as probably not important, between 30% and 60% as representing moderate heterogeneity, between 50% and 90% as representing substantial heterogeneity and between 75% and 100% as representing considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
If we found more than 10 studies, we planned to perform a funnel plot analysis. A funnel plot is a graph that is used to detect publication bias. We planned to look at whether the largest studies were near average and small studies spread on both sides of the average. Variations from this assumption can indicate the existence of publication bias, but asymmetry may not necessarily be caused by publication bias. In addition, we planned to use Kendell's tau (Begg 1994), Egger's regression intercept (Egger 1997), and Fail‐Safe N (Rosenthal 1979), to assess reporting biases.
Data synthesis
We analysed data using Review Manager 5 software (Review Manager 2014). We planned to pool data for meta‐analysis when participant groups were similar, when studies assessed the same treatments versus the same comparator and when studies used similar definitions of outcome measures over a similar duration of treatment. In general, we planned to use random‐effects models to combine results because we did not expect the true effect to be the same for all included studies. We intended to weigh studies using the Mantel‐Haenszel method. For comparisons for which fixed‐effect models also seemed viable, we would have employed both models to determine differences between random‐effects and fixed‐effect meta‐analyses. We rated the strength of the evidence using the system developed by the GRADE Working Group (Guyatt 2011).
Subgroup analysis and investigation of heterogeneity
Sex, age, history of non‐seasonal major depressive episodes and psychiatric comorbidities are potential effect measure modifiers for prevention of SAD. If data were sufficient, we planned to conduct subgroup analyses for the primary outcome measures. Subgroup analyses should be performed and interpreted with caution because multiple analyses could lead to false‐positive conclusions. We planned to conduct subgroup analyses based on:
men versus women;
history of non‐seasonal major depressive disorder versus no history of non‐seasonal major depressive disorder;
younger than 65 years of age versus 65 years of age or older.
Axis I, Axis II comorbidities versus no Axis I, Axis II comorbidities.
Sensitivity analysis
The purpose of sensitivity analyses is to test the robustness of decisions made during the review process.
We planned to conduct sensitivity analyses:
excluding small studies (i.e. studies with fewer than 30 participants);
excluding studies with high risk of bias (i.e. studies that have been rated as high risk of bias in one or more domains);
excluding studies published only in abstract form;
comparing adjusted versus unadjusted results; and
excluding cluster‐randomised trials.
'Summary of findings' tables
We assessed the quality of the evidence using the GRADE approach and presented these results in 'Summary of findings' tables for our main comparisons and outcomes (as listed in Types of outcome measures). We did not expect to be able to stratify populations into low‐, medium‐ and high‐risk groups. For 'assumed risk', we used prevalence studies from countries in northern latitudes (e.g. Scandinavia, Canada, northern USA) in which SAD leads to a substantial burden of disease. Grading of the certainty of evidence was done dually (CF, BN).
We used GRADEpro to rate the quality of evidence and to prepare the 'Summary of findings' tables (GRADEpro GDT 2015).
Results
Description of studies
Results of the search
We identified 3745 citations through electronic searches and reviews of reference lists after deduplication of search results. We excluded 3619 records during title and abstract review, and we included 126 articles for full‐text review and assessed them for eligibility. We included one publication for this review. The PRISMA flow chart documents the disposition of the literature in this review (Figure 1).
1.

PRISMA flow chart.
Included studies
We included one randomised controlled trial (RCT) comparing mindfulness‐based cognitive therapy (MBCT) with treatment as usual (TAU) for prevention of seasonal affective disorder (SAD) in 46 participants (Fleer 2014). The study was not funded by external funding sources and did not assess risk of harms. There are no studies awaiting classification nor ongoing studies. We contacted the first author of the included RCT via email. He informed us that no additional outcomes had been measured and no larger study followed this pilot study.
In the following section, we present study characteristics and results in greater detail (see also Characteristics of included studies).
Design
The included RCT was a single‐centre, non‐blinded study, conducted in an outpatient setting. The MCBT took place between May 2011 and June 2011. Depressive symptoms were assessed weekly between September 2011 and April 2012 in both study arms.
Sample size
The study included a total of 46 participants with a history of SAD who had no depressive symptoms when the study started. Initially, investigators recruited 81 outpatients. Twenty of the 81 prospective participants were excluded because they were not interested in participation (even after returning the informed consent form). Nine prospective participants were excluded after an initial telephone screening because they were either already receiving psychological treatment or because they could not be contacted. Six more prospective participants were excluded after completing the Inventory of Depressive Syptomatology‐Self‐Report (IDS‐SR, range 0‐90) because they had an IDS‐SR score > 15 or failed to return the IDS‐SR.
Setting
The study was conducted in the Netherlands and included participants from an outpatient clinic. The authors did not report where the interventions were administered or who administered the interventions.
Participants
Participants were adult outpatients with a history of SAD (formal diagnosis of winter SAD according to DSM‐IV) who received light therapy in the winter of 2010 to 2011. Participants were non‐depressed at the start of the study (IDS‐SR < 15). In all, 46 participants were randomly assigned to either MBCT (n = 23) or TAU (n = 23). Four participants in the MBCT group dropped out after less than four sessions (no reasons described). Three of the participants in the TAU group did not complete any weekly assessments and were lost to follow‐up. There were no significant differences between the MBCT and TAU groups with respect to background characteristics. Mean age of the participants was 40 years and the majority of participants were women (70% to 78%). Most participants were partnered and received higher education (college and beyond). The average onset of SAD was more than 10 years ago and participants reported a SAD episode in four of the past five winters. Both groups reported effective treatment with previous light therapy.
Interventions
The MBCT used in this study was based on a protocol developed by Segal 2002, for preventing the reoccurrence of major depressive disorder in an upcoming winter. Participants in the MBCT group received consecutive weekly 45‐ to 60‐minute individual therapy sessions during a symptom‐free period between April 2011 and June 2011. The authors modified the MBCT protocol from a group‐based intervention to an individually‐based intervention and referred to other studies to explain the theoretical underpinnings. Participants in the TAU group received no treatment until a depressive episode started. If they had a IDS‐SR score ≥ 20 they received five consecutive days of 45 minutes of full spectrum light therapy (without UV, 10,000 lux). Also, participants in the MBCT group were offered light therapy upon first signs of a depressive episode ( IDS‐SR > 20).
Outcomes
The primary outcome for this study was development of depression based on a score of ≥ 20 on the IDS‐SR. Although the primary outcome for this Cochrane Review was defined using SIGH‐SAD (see Types of outcome measures) we also included the incidence rate of SAD based on the IDS‐SR, since SIGH‐SAD was not used in this study. Severity of depression was reported as IDS‐SR mean scores.
Researchers did not assess any other outcomes of interest for this review.
Excluded studies
Overall, we assessed 126 references as full‐text articles and excluded 125 of them. Characteristics of excluded studies show all records that narrowly missed the inclusion criteria for this systematic review. We excluded studies because included participants already had depressive symptoms when the study started. Also, we mentioned in Characteristics of excluded studies those studies that were included in the review on light therapy (Nussbaumer‐Streit 2019), and second‐generation antidepressants (Gartlehner 2019), and we explained why they are not included in this review.
Ongoing studies
We identified no ongoing studies of interest.
Studies awaiting classification
We found no studies currently awaiting classification.
Risk of bias in included studies
For details of the risk of bias judgement, see Characteristics of included studies. We present graphical representations of the overall risk of bias in the included study in Figure 2 and Figure 3.
2.
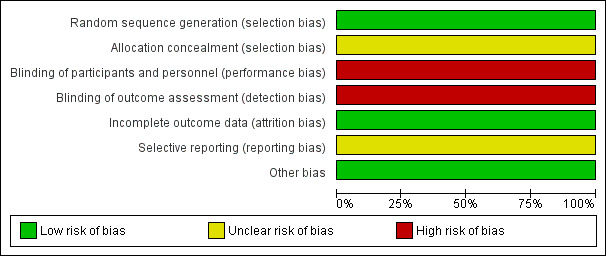
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We rated the risk of bias due to the randomisation process as low because researchers used a computerised randomisation programme. However, no information on the allocation concealment was provided, so we rated this category unclear.
Blinding
Participants were not blinded to the treatment and outcome assessment was done by participants themselves using a self‐reported inventory. Therefore, we rated the risk of performance and detection bias as high.
Incomplete outcome data
The overall attrition rate was moderate (15%) and similar between the groups (MBCT: 17%, TAU: 13%). The authors accounted for the attrition by using intention‐to‐treat (ITT) analysis, therefore we rated this category as low risk of bias.
Selective reporting
We did not identify a protocol, so it remains unclear whether all outcomes have been reported or not.
Other potential sources of bias
We did not identify other potential sources of bias and rated this category as low risk of bias.
Effects of interventions
See: Table 1
1. Comparison: psychological therapy versus no treatment or waiting list
Primary outcomes
1.1 Incidence of SAD
The incidence of a new depressive episode in winter was comparable between the mindfulness‐based cognitive therapy (MBCT) and treatment as usual (TAU) groups over the 33‐week study period. In the MBCT group 15 participants (65%) developed a depression in the upcoming winter compared to 18 (78%) in the TAU group (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.60 to 1.30; 46 participants; very low quality‐evidence; Analysis 1.1; Table 1).
1.1. Analysis.
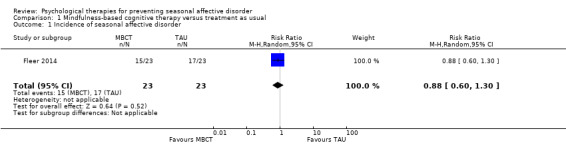
Comparison 1 Mindfulness‐based cognitive therapy versus treatment as usual, Outcome 1 Incidence of seasonal affective disorder.
1.2 Overall rate of adverse events
We found no eligible evidence addressing this outcome.
Secondary outcomes
1.3 Severity of SAD or SAD‐related symptoms
The severity of depressive episodes was similar between the two groups. The mean Inventory of Depressive Syptomatology‐Self‐Report (IDS‐SR) score in the MBCT group was 26.5 (SD 7.0) compared to 25.3 (SD 6.3) in the TAU group (mean difference (MD) 1.20, 95% CI ‐3.44 to 5.84; 32 participants; very low quality‐evidence; Analysis 1.2; Table 1).
1.2. Analysis.
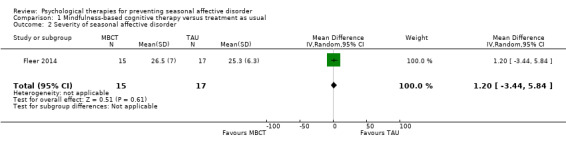
Comparison 1 Mindfulness‐based cognitive therapy versus treatment as usual, Outcome 2 Severity of seasonal affective disorder.
1.4 Quality of life
We found no eligible evidence addressing this outcome.
1.5 Quality of interpersonal and social functioning
We found no eligible evidence addressing this outcome.
1.6 Proportion of participants with serious adverse events
We found no eligible evidence addressing this outcome.
1.7 Rates of discontinuation due to adverse events
We found no eligible evidence addressing this outcome.
1.8 Overall rate of discontinuation
Overall rate of discontinuation was similar in both arms. Four participants in the MBCT group and three in the TAU group discontinued the study (RR 1.33, 95% CI 0.34 to 5.30; 46 participants; very low quality‐evidence; Analysis 1.3; Table 1).
1.3. Analysis.

Comparison 1 Mindfulness‐based cognitive therapy versus treatment as usual, Outcome 3 Overall discontinuation rate.
2. Comparison: psychological therapy versus other psychological therapy
We did not identify any evidence for this comparison.
3. Comparison: psychological therapy versus light therapy
We did not identify any evidence for this comparison.
4. Comparison: psychological therapy versus second‐generation antidepressants
We did not identify any evidence for this comparison.
5. Comparison: psychological therapy versus melatonin or agomelatine
We did not identify any evidence for this comparison.
6. Comparison: psychological therapy versus lifestyle interventions
We did not identify any evidence for this comparison.
7. Comparison: psychological therapy + comparator intervention versus no treatment control group or waiting list
We did not identify any evidence for this comparison.
8. Comparison: psychological therapy + comparator intervention versus the same comparator intervention as monotherapy
We did not identify any evidence for this comparison.
Subgroup analyses
Data were insufficient for subgroup analyses.
Sensitivity analyses
Data were insufficient for sensitivity analyses.
Reporting bias
As we identified only one study, statistical approaches to assess publication bias were not possible. It is unclear whether reporting bias is a problem of the included study, as we could not identify a protocol for the study.
Discussion
Summary of main results
The evidence from the only included study was inconclusive (Fleer 2014). The seasonal affective disorder (SAD) incidence and severity of depression was comparable in both groups. However, the 95% confidence intervals were very broad for both outcomes and included both a possible relevant positive effect of mindfulness‐based cognitive therapy (MBCT) as well as a possible negative effect of MBCT. Also the number of people who discontinued the study was similar.
Table 1 gives an overview of the quality of evidence for all outcomes which was very low.
Overall completeness and applicability of evidence
A major limitation of this report is that the available evidence is limited to one randomised controlled trial (RCT) with a very small number of participants (n = 46) that is rated as having high risk of bias in important domains. Due to the small sample size, results of this RCT can be influenced by lack of power and by random error. The authors provided individual MBCT rather than the group MBCT, the latter of which the authors state has been effective in reducing depressive symptoms in people with major depressive disorder and diabetes (Schroevers 2013; Segal 2002; Tovote 2014). MBCT is a special type of psychological therapy, so no conclusions on the effectiveness of other psychological therapies can be drawn. As no information on adverse events was given, we can not estimate potential harms of MBCT. There seems to be a lack of research on prevention of SAD that was also detected in the other Cochrane Reviews on SAD prevention. The reviews on agomelatine and light therapy for SAD prevention (Kaminski‐Hartenthaler 2015; Nussbaumer‐Streit 2019), also each only identified one small high risk of bias study, precluding us from making conclusions on benefits and harms of these interventions. Only the review on antidepressants for prevention of SAD identified three well‐conducted RCTs that provided moderate‐quality evidence indicating that bupropion extended‐released (bupropion XL) is effective for prevention of recurrence of SAD. Nevertheless, even in a high‐risk population, three out of four people will not benefit from preventive treatment with bupropion XL and will be at risk of harm (Gartlehner 2019). Despite this lack of evidence, preventive treatment in patients with SAD seems to be often implemented in clinical practice. A survey in German‐speaking countries showed that 73% of 81 hospitals reported recommending people with a history of SAD psychotherapy for SAD prevention, 84% recommend antidepressants, 72% light therapy and 47% agomelatine (Nussbaumer‐Streit 2017).
Quality of the evidence
We graded the quality of the evidence for available efficacy of outcomes (incidence of SAD, severity of SAD, and overall discontinuation) as very low. Reasons for downgrading the quality of evidence included high risk of bias of the included study and imprecision due to the small sample size. Other limitations of the included study are that the outcome measure was self‐rated and the implementation of MBCT was altered from its original protocol without a sufficiently reported explanation. In addition, no safety data were reported. Drawing conclusions about psychological therapies in general is not possible because different forms and modes of administration of psychological therapies may lead to different results. However, we did not downgrade the quality of the evidence for indirectness because our conclusions focused on MBCT.
Potential biases in the review process
Publication bias is a threat for any systematic review. Although we searched for grey and unpublished literature, the extent and impact of reporting biases of this body of evidence is impossible to determine.
Agreements and disagreements with other studies or reviews
Fleer 2014 was the only identified study commencing psychological therapy (MBCT) in spring when participants with a history of SAD are still free of symptoms. Rohen et al conducted several studies on the long‐term effect of cognitive behaviour therapy (CBT; Rohan 2009; Rohan 2016). However, they started the interventions in winter when patients had acute depressive symptoms and followed‐up participants over one and two consecutive winters. Therefore, these studies were not included in our review. One RCT including 69 participants showed that after one year 7% in the CBT group, 6% in the CBT + light therapy group and 37% in the light therapy group experienced the onset of a new depressive episode (Rohan 2009). Interestingly, the larger study on 177 participants showed that CBT led to a similar recurrence rate as light therapy in the first upcoming winter (29% versus 25%, P = 0.571), but reduced the recurrence rate in the second winter follow‐up compared to light therapy (27% versus 46%, P = 0.014; Rohan 2016). However, it is hard to compare these results since the mode of psychotherapy differed and the intervention commenced in a different season.
Authors' conclusions
Implications for practice.
The benefits and harms of psychological therapies for preventing seasonal affective disorder (SAD) are uncertain. Methodological limitations and small sample size of the only available study preclude the conclusions of the review authors on benefits and harms of mindfulness‐based cognitive therapy (MBCT) for the prevention of SAD. Evidence on other psychological therapies for the prevention of SAD is missing. Given the lack of comparative evidence for the effectiveness of preventing SAD, the choice of treatment should be based on other preventive interventions that are supported by evidence, such as the antidepressant bupropion XL (Gartlehner 2019), but also strongly on individual preference(s). A recent qualitative study showed that people diagnosed with SAD often have a negative attitude toward antidepressants (Nussbaumer‐Streit 2018).
Implications for research.
SAD is a chronic and disabling condition. Depending on latitude, age, gender and measurement method used, current SAD prevalence estimates vary between 0.4% and 16% in the general adult population (Freed 2010), resulting in the need for effective prevention of the disease. Lack of evidence clearly shows the need for independently funded, high quality, well‐conducted, controlled studies to investigate the effectiveness of psychological interventions to prevent SAD. Ideally, more methodologically sound RCTs of psychological therapies for prevention of SAD would assess the comparative benefits and risks of these interventions against others currently used to treat the disorder such as second‐generation antidepressants, light therapy, lifestyle interventions and melatonin or agomelatine.
What's new
| Date | Event | Description |
|---|---|---|
| 7 May 2019 | New citation required and conclusions have changed | Review updated. New study added |
| 7 May 2019 | New search has been performed | We updated the searches on 19 June 2018; we identified one new study. |
Acknowledgements
We would like to thank Evelyn Auer and Sandra Hummel for providing administrative support during the course of this review. We would also like to thank Julia Hoffmann, and Jeffrey H Sonis for their support when conducting the former version of this Cochrane Review.
The authors and the Cochrane Common Mental Disorders Editorial Team, are grateful to the following peer reviewers and editors for their time and comments: Sarah Chapman, Georgina Cox, Nick Meader and Eleonora Uphoff. They would also like to thank copy editor, Clare Dooley.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: views and opinions expressed herein are those of the review authors and do not necessarily reflect those of NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. CCMDCTR: Core MEDLINE search
The search strategy listed below is the weekly OVID Medline search which was used to inform the Group’s specialised register (to June 2016). It is based on a list of terms for all conditions within the scope of the Cochrane Common Mental Disorders Group plus a sensitive RCT filter.
OVID MEDLINE search strategy, used to inform the Cochrane Common Mental Disorders Group's Specialised Register A weekly search alert based on condition + RCT filter only 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/ 2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf. 3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.) 4. (1 and 2 and 3)
Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record. Similar weekly search alerts are also conducted on OVID Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 2. Database searches 2014
PubMed 26.05.2014
| Search | Query | Items found |
| #1 | Search "Seasonal Affective Disorder"[Mesh] | 1061 |
| #2 | Search "seasonal affective disorder"[All Fields] | 1415 |
| #3 | Search seasonal affective disorder* | 1451 |
| #4 | Search "seasonal depression"[All Fields] | 162 |
| #5 | Search seasonal mood disorder* | 10 |
| #6 | Search "winter depression" | 248 |
| #7 | Search SIGH‐SAD | 61 |
| #8 | Search (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) | 1555 |
| #9 | Search (#8 AND 2013/05:2014[dp]) | 46 |
The Cochrane Library 26.05.2014
| ID | Search | Hits |
| #1 | seasonal affective disorder (Word variations have been searched) | 312 |
| #2 | winter blues (Word variations have been searched) | 25 |
| #3 | seasonal depression | 295 |
| #4 | seasonal mood disorder | 134 |
| #5 | winter depression | 256 |
| #6 | SIGH‐SAD | 39 |
| #7 | {or #1‐#6} Publication Date from 2013 to 2014 | 69 |
EMBASE 26.05.2014
| No. | Query | Results |
| #1 | 'seasonal affective disorder'/exp AND [humans]/lim AND [embase]/lim | 640 |
| #3 | 'seasonal affective disorder'/mj | 484 |
| #4 | #1 OR #3 | 831 |
| #5 | #4 AND [2013‐2014]/py | 79 |
PsycINFO, AMED, IPA, CINAHL (via EBSCO HOST) 26.05.2014
| # | Query | Limiters/Expanders | Last Run Via | Results |
| S1 | seasonal affective disorder | Limiters ‐ Published Date: 20130501‐ | Interface ‐ EBSCOhost Research Databases | 39 |
| Search modes ‐ Boolean/Phrase | Search Screen ‐ Advanced Search | |||
| Database ‐ PsycINFO;AMED ‐ The Allied and Complementary Medicine Database;CINAHL with Full Text;International Pharmaceutical Abstracts |
Web of Science (via UNC) 28.05.2014
| Set | Results | |
| # 5 | 69 | #4 AND #1 |
| Timespan=2013‐2014 | ||
| Search language=English | ||
| # 4 | Approximately | #3 OR #2 |
| 1,107,073 | Timespan=2013‐2014 | |
| Search language=English | ||
| # 3 | Approximately | TOPIC: (treatment) |
| 975,242 | Timespan=2013‐2014 | |
| Search language=English | ||
| # 2 | Approximately | TOPIC: (prevention) |
| 188,628 | Timespan=2013‐2014 | |
| Search language=English | ||
| # 1 | 165 | TOPIC: ("seasonal affective disorder") |
| Timespan=2013‐2014 | ||
| Search language=English |
Appendix 3. Database searches 2018
Summary of searches (19 June 2018)
CCMD Register, n = 8
CENTRAL, n = 30
MEDLINE, n = 233
Embase, n = 301
PsycINFO, n = 154
International Pharmaceutical Abstracts, (database unavailable)
CINHAL, n = 77
Web of Knowledge, n = 489
AMED, n = 1
Total = 1293 Duplicates removed = 607 Number to screen = 686
Database search strategies
CCMD‐CTR (searched via Cochrane CRS) Date searched: Tuesday, 19th June 2018 (Register current to June 2016, only) Hits: 303 (8 in scope for this update) 1"seasonal affective disorder" AND INREGISTER (277) 2seasonal affective disorder* AND INREGISTER (280) 3"seasonal depression" AND INREGISTER34 4 seasonal mood disorder* AND INREGISTER (6) 5 "winter depression" AND INREGISTER (72) 6 SIGH‐SAD AND INREGISTER (48) 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 (303) Cochrane CENTRAL searched the Cochrane Library (Wiley interface) Data parameters: Issue 5 of 12, May 2018 Date searched: Tuesday, 19th June 2018 Hits: 363 (30 in scope for this update) #1 MeSH descriptor: [Seasonal Affective Disorder] explode all trees 172 #2 "seasonal affective disorder" 364 #3 seasonal affective disorder* 397 #4 "seasonal depression" 46 #5 seasonal mood disorder* 199 #6 "winter depression" 85 #7 SIGH‐SAD 55 #8 (#1 or #2 or #3 or #4 or #5 or #6 or #7) 452 Notes: Of 452 returned from searching The Cochrane Library, 363 were records from CENTRAL. Records dating pre‐2015 were visually inspected and manually removed.
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) Data parameters: 1946 to Present Date searched: Tuesday, 19th June 2018 Hits: 233 1 Seasonal Affective Disorder/1180 2 "seasonal affective disorder".ti,ab,kw,ot.1206 3 seasonal affective disorder*.ti,ab,kw,ot.1277 4 "seasonal depression".ti,ab,kw,ot.188 5 seasonal mood disorder*.ti,ab,kw,ot.12 6 "winter depression".ti,ab,kw,ot.272 7 SIGH‐SAD.ti,ab,kw,ot.78 8 (1 or 2 or 3 or 4 or 5 or 6 or 7) 1790 9 (2015* or 2016* or 2017* or 2018*).yr,ed. 4792024 10 (8 and 9) 233
Embase (Ovid Interface) Data parameters: 1974 to 2018 June 18 Date searched: Tuesday, 19th June 2018 Hits: 301 1 Seasonal Affective Disorder/ 1239 2 "seasonal affective disorder".ti,ab,kw,ot. 1528 3 seasonal affective disorder*.ti,ab,kw,ot. 1618 4 "seasonal depression".ti,ab,kw,ot. 246 5 seasonal mood disorder*.ti,ab,kw,ot. 23 6 "winter depression".ti,ab,kw,ot. 334 7 SIGH‐SAD.ti,ab,kw,ot. 92 8 (1 or 2 or 3 or 4 or 5 or 6 or 7) 2297 9 (2015* or 2016* or 2017* or 2018*).yr,ed. 4907927 10 (8 and 9) 301
PsycINFO (Ovid) Data parameters: 2002 to June Week 2 2018 Date searched: Tuesday, 19th June 2018 Hits: 154 1 Seasonal Affective Disorder/ 484 2 "seasonal affective disorder".ti,ab,kw,ot. 511 3 seasonal affective disorder*.ti,ab,kw,ot. 529 4 "seasonal depression".ti,ab,kw,ot. 94 5 seasonal mood disorder*.ti,ab,kw,ot. 6 6 "winter depression".ti,ab,kw,ot. 72 7 SIGH‐SAD.ti,ab,kw,ot. 53 8 (1 or 2 or 3 or 4 or 5 or 6 or 7) 690 9 (2015* or 2016* or 2017* or 2018*).yr,ed. 633488 10 (8 and 9) 154
CINAHL via EBSCOHost Data parameters: 1937‐Current Date searched: Tuesday, 19th June 2018 Hits: 77 S9 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7) Limiters: Published Date (20150101 ‐ 20180631) 77 S8 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7) 454 S7 TI SIGH‐SAD OR AB SIGH‐SAD 15 S6 TI "winter depression" OR AB "winter depression" 24 S5 TI seasonal mood disorder* OR AB seasonal mood disorder* 16 S4 TI "seasonal depression" OR AB "seasonal depression" 43 S3 TI Seasonal Affective Disorder* OR AB Seasonal Affective Disorder* 287 S2 TI "Seasonal Affective Disorder" OR AB "Seasonal Affective Disorder" 276 S1 (MM "Seasonal Affective Disorder") 365
Web of Science (Web of Science Core Collection, BIOSIS, Data citation Index, KCI Korean Journal Database, MEDLINE, Russian Science Citation Database, SciELO Citation Index)* Data parameters: 1900 to Present Date searched: Tuesday, 19th June 2018 Hits: 489 #8 TOPIC ((#6 OR #5 OR #4 OR #3 OR #2 OR #1) Refined by: PUBLICATION YEARS (2018 OR 2017 OR 2016 OR 2015) 489 #7 TOPIC (#6 OR #5 OR #4 OR #3 OR #2 OR #1) 3819 #6 TOPIC (SIGH‐SAD) 84 #5 TOPIC ("winter depression") 790 #4 TOPIC (seasonal mood disorder*) 1525 #3 TOPIC ("seasonal depression") 267 #2 TOPIC (Seasonal Affective Disorder*) 3355 #1 TOPIC ("Seasonal Affective Disorder") 3007
Notes: In the 2015 review, which these searches update, Web of Knowledge was searched. Web of Knowledge (containing Web of Science, Current Contents Connect, Conference Proceedings Citation Index, BIOSIS, Derwent Innovations Index, Data Citation Index, SciELO Citation Index) has been discontinued. This search was the closest representation of the previous search.
Allied and Complementary Medicine Database (AMED) Data parameters: 1985 to June 2018 Date searched: Tuesday, 19th June 2018 Hits: 1 1 Seasonal Affective Disorder/ 0 2 "seasonal affective disorder".ti,ab,kw,ot. 28 3 seasonal affective disorder*.ti,ab,kw,ot. 28 4 "seasonal depression".ti,ab,kw,ot. 2 5 seasonal mood disorder*.ti,ab,kw,ot. 0 6 "winter depression".ti,ab,kw,ot. 4 7 SIGH‐SAD.ti,ab,kw,ot. 2 8 (1 or 2 or 3 or 4 or 5 or 6 or 7) 32 9 (2015* or 2016* or 2017* or 2018*).yr,ed. 22289 10 (8 and 9) 1
Trials registers WHO International Clinical Trials Registry Platform (ICTRP) searched via: http://apps.who.int/trialsearch/Default.aspx search date: Tuesday, 19th June 2018 seasonal n = 113 records for 49 trials. These records were visually inspected and 20 records were retained for screening SIGH‐SAD n = 0 ClinicalTrials.Gov searched via: https://www.clinicaltrials.gov/ct2/home search date: Tuesday, 19th June 2018 Records were visually inspected and records 2015‐current when exported to Endnote. Search field: Condition or Disease seasonal affective n = 3 seasonal depression n = 3 (being duplicates of the above) SIGH‐SAD n = 0
Data and analyses
Comparison 1. Mindfulness‐based cognitive therapy versus treatment as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of seasonal affective disorder | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.60, 1.30] |
| 2 Severity of seasonal affective disorder | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐3.44, 5.84] |
| 3 Overall discontinuation rate | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.34, 5.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fleer 2014.
| Methods | Randomised controlled trial at a single outpatient medical centre in the Netherlands. Mindfulness‐Based Cognitive Therapy (MBCT) took place between April 2011 and June 2011. Participants of the treatment and control arm were assessed for SAD between September 2011 and April 2012 by completing weekly depression questionnaires. Four participants in the MBCT dropped out after < 4 sessions. Three participants in the TAU group were lost to follow‐up. ITT analysis was applied by inputting group means for missing values. | |
| Participants | 46 adult outpatients with a history of SAD, who were symptom‐free at the start of the study and who were not receiving psychological treatment at the time of the study. MBCT group, n = 23; mean age 39.4 (11.5) years, 69.6% female TAU group, n = 23, mean age 41.6 (12.2) years, 78.3% female Average onset of SAD was more than 10 years ago: MBCT 13.0 (11.3); TAU 10.7 (10.6), and participants reported a SAD episode in 4 of the past 5 winters. |
|
| Interventions |
|
|
| Outcomes | Incidence of SAD and severity of SAD, as measured on the IDS‐SR with a score ≥ 20, and overall discontinuation rate | |
| Notes | The study was not funded by external funding sources. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Researchers used a computerised randomisation programme. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants completed the IDS‐SR weekly online. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition rate was moderate (15%). 17% of participants in the MBCT group dropped out after < 4 sessions. 13% of participants in the TAU group were lost to follow‐up. Overall attrition rate was 15%. ITT analysed was applied. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available stating the planned analysis and outcomes. |
| Other bias | Low risk | No other bias suspected. |
IDS‐SR: Inventory for Depressive Symptomatology‐Self‐Report ITT: intention‐to‐treat MBCT: mindfulness‐based cognitive therapy SAD: seasonal affective disorder TAU: treatment as usual
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Meesters 1999 | This study investigated different types of light therapy for prevention of SAD (Nussbaumer‐Streit 2019). This study is included in the systematic review on efficacy and safety of light therapy for SAD prevention; however, as it does not investigate the efficacy nor the safety of psychological therapies as preventive treatment, it is not relevant for inclusion in this systematic review. |
| Modell 2005 | This study investigated the preventive effects of bupropion XL for participants with a history of SAD. This study is included in the systematic review on efficacy and safety of second‐generation antidepressants (Gartlehner 2019); however, as it does not investigate the efficacy nor the safety of psychological therapies as preventive treatment, it is not relevant for inclusion in this systematic review. |
| Rohan 2004 | This study investigated short‐term CBT for SAD alone and in combination with light therapy as compared with solo light therapy. This is a treatment study ‐ not a prevention study. |
| Rohan 2007 | This study investigated the short‐ and long‐term efficacy of CBT for SAD alone and in combination with light therapy as compared with solo light therapy. It included participants who already had symptoms when the interventions were started; therefore, this study was not relevant for inclusion in this systematic review. |
| Rohan 2009 | This study investigated recurrence of SAD after one year of CBT, light therapy and a combination of these. It included participants who already had depressive symptoms when the study started; therefore, this study was not relevant for inclusion in this systematic review. |
| Rohan 2016 | This study investigated relapse prevention in SAD by comparing CBT, and light therapy. It included participants who already had symptoms when the study started. |
| WELL 100006 | This study investigated the preventive effects of bupropion XL in people with a history of SAD. This study is included in the systematic review on efficacy and safety of second‐generation antidepressants (Gartlehner 2019); however, as it did not investigate the efficacy nor the safety of psychological therapies as preventive treatment, it is not relevant for inclusion in this systematic review. |
| WELL AK130930 | This study investigated the preventive effects of bupropion XL in people with a history of SAD. This study is included in the systematic review on efficacy and safety of second‐generation antidepressants (Gartlehner 2019); however, as it did not investigate the efficacy nor the safety of psychological therapies as preventive treatment, it is not relevant for inclusion in this systematic review. |
| WELL AK130936 | This study investigated the preventive effects of bupropion XL in people with a history of SAD. This study is included in the systematic review on efficacy and safety of second‐generation antidepressants (Gartlehner 2019); however, as it did not investigate the efficacy nor the safety of psychological therapies as preventive treatment, it is not relevant for inclusion in this systematic review. |
CBT: cognitive behaviour therapy SAD: seasonal affective disorder
Differences between protocol and review
We defined the primary outcome as "incidence of SAD" based on a SIGH‐SAD score in the protocol. We present "incidence of SAD" based on the IDS‐SR score in the review, because the study authors did not use SIGH‐SAD.
Contributions of authors
CF, BN and GG drafted and revised the review text; MvN ran grey literature searches. BG and DW provided clinical expertise for the background section. CF, BN, GG, BG, AG, LM, JW, LL screened records on title/abstract and full‐text level. CF, AG extracted data, CF, AG assessed the risk of bias of the included study. CF, BN conducted the GRADE assessment. All authors reviewed the manuscript and provided feedback on individual drafts.
Sources of support
Internal sources
Internal funds of Cochrane Austria, Austria, Other.
External sources
No sources of support supplied
Declarations of interest
Catherine A Forneris ‐ no conflict of interest
Barbara Nussbaumer‐Streit ‐ no conflict of interest
Laura C Morgan ‐ no conflict of interest
Amy Greenblatt ‐ no conflict of interest
Megan G Van Noord ‐ no conflict of interest
Bradley N Gaynes ‐ no conflict of interest
Jörg Wipplinger ‐ no conflict of interest
Linda J Lux ‐ no conflict of interest
Dietmar Winkler ‐ has received lecture fees from Angelini Pharmaceuticals, Lundbeck Pharmaceuticals and Pro Mente Austria and has received authorship honoraria from Medizin Medien Austria.
Gerald Gartlehner ‐ no conflict of interest
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Fleer 2014 {published data only}
- Fleer J, Schroevers M, Panjer V, Geerts E, Meesters Y. Mindfulness‐based cognitive therapy for seasonal affective disorder: a pilot study. Journal of Affective Disorders 2014;168:205‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Meesters 1999 {published data only}
- Meesters Y, Beersma DG, Bouhuys AL, Hoofdakker RH. Prophylactic treatment of seasonal affective disorder (SAD) by using light visors: bright white or infrared light?. Biological Psychiatry 1999;46:239‐46. [DOI] [PubMed] [Google Scholar]
Modell 2005 {published data only}
- Modell JG, Rosenthal NE, Harriett AE, Krishen A, Asgharian A, Foster VJ, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biological Psychiatry 2005;58:658‐67. [DOI] [PubMed] [Google Scholar]
Rohan 2004 {published data only}
- Rohan KJ, LIndsey KT, Roecklein KA, Lacy TJ. Cognitive‐behavioral therapy, light therapy, and their combination in treating seasonal affective disorder. Journal of Affective Disorder 2004;80:273‐83. [DOI] [PubMed] [Google Scholar]
Rohan 2007 {published data only}
- NCT00076245. Cognitive behavioral approaches to seasonal depression. clinicaltrials.gov/ct2/show/NCT00076245 (first received 19 January 2004).
- Rohan KJ, Roecklein KA, Tierney Lindsey K, Johnson LG, Lippy RD, Lacy TJ, et al. A randomized controlled trial of cognitive‐behavioral therapy, light therapy, and their combination for seasonal affective disorder. Journal of Consulting and Clinical Psychology 2007;75(3):489‐500. [DOI] [PubMed] [Google Scholar]
Rohan 2009 {published data only}
- Rohan KJ, Roecklein KA, Lacy TJ, Vacek PM. Winter depression recurrence one year after cognitive‐behavioral therapy, light therapy, or combination treatment. Behavior Therapy 2009;40:225‐38. [DOI] [PubMed] [Google Scholar]
Rohan 2016 {published data only}
- NCT01714050. Cognitive‐behavioral therapy vs. light therapy for preventing SAD recurrence. clinicaltrials.gov/ct2/show/NCT01714050 (first received 25 October 2012).
- Rohan KJ, Evans M, Mahon JN, Sitnikov L, Ho S, Nillni YI, et al. Cognitive‐behavioral therapy vs. light therapy for preventing winter depression recurrence: study protocol for a randomized controlled trial. Trials 2013;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan KJ, Mahon JN, Evans M, Ho SY, Meyerhoff J, Postolache TT, et al. Randomized Trial of Cognitive‐Behavioral Therapy Versus Light Therapy for Seasonal Affective Disorder: Acute Outcomes. American Journal of Psychiatry 2015;172(9):862‐9. [DOI: 10.1176/appi.ajp.2015.14101293] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan KJ, Meyerhoff J, Ho SY, Evans M, Postolache TT, Vacek PM. Outcomes one and two winters following cognitive‐behavioural therapy or light therapy for seasonal affective disorder. American Journal of Psychiatry 2016;173(3):244‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
WELL 100006 {published data only}
- GlaxoSmithKline. A 7‐Month, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Comparison of 150‐300mg/day of Extended‐Release Bupropion Hydrochloride (WELLBUTRIN XL) and Placebo for the Prevention of Seasonal Affective Disorder in Subjects with a History of Seasonal Affective Disorder Followed by an 8‐Week Observational Follow‐up Phase. GSK ‐ Clinical Study Register [www.gsk‐clinicalstudyregister.com] 2003.
WELL AK130930 {published data only}
- GlaxoSmithKline. A 7‐Month, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Comparison of 150‐300mg/day of Extended‐Release Bupropion Hydrochloride (WELLBUTRIN XL) and Placebo for the Prevention of Seasonal Affective Disorder in Subjects with a History of Seasonal Affective Disorder Followed by an 8‐Week Observational Follow‐up Phase. GSK ‐ Clinical Study Register [www.gsk‐clinicalstudyregister.com] 2003.
WELL AK130936 {published data only}
- GlaxoSmithKline. A 7‐Month, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Comparison of 150‐300mg/day of Extended‐Release Bupropion Hydrochloride (WELLBUTRIN XL) and Placebo for the Prevention of Seasonal Affective Disorder in Subjects with a History of Seasonal Affective Disorder Followed by an 8‐Week Observational Follow‐up Study. GSK ‐ Clinical Study Register [www.gsk‐clinicalstudyregister.com] 2004.
Additional references
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Beck 1967
- Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. New York: Hoeber, 1967. [Google Scholar]
Beck 1976
- Beck AT. Cognitive Therapy and the Emotional Disorders. New York: International Universities Press, 1976. [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088‐101. [PubMed] [Google Scholar]
BMJ 2010
- BMJ. Management of seasonal affective disorder. Drug and Therapeutics Bulletin 2010;340:1185‐9. [DOI] [PubMed] [Google Scholar]
Byrne 2008
- Byrne B, Brainard GC. Seasonal affective disorder and light therapy. Sleep Medicine Clinics 2008;3(2):307–15. [Google Scholar]
Ciarleglio 2011
- Ciarleglio CM, Resuehr HE, McMahon DG. Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience 2011;197:8‐16. [DOI] [PubMed] [Google Scholar]
DeMello 2005
- Mello MF, Jesus MJ, Bacaltchuk J, Verdeli H, Neugebauer R. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. European Archives of Psychiatry and Clinical Neuroscience 2005;255(2):75‐82. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote X8 2016 [Computer program]
- Clarivate Analytics. EndNote X8. Version accessed prior to 13 May 2019. Philadelphia: Clarivate Analytics, 2016.
Evans 2013
- Evans M, Rohan KJ, Sitnikov L, Mahoon JN, Nillni YI, Tierney‐Lindsey, et al. Cognitive change across cognitive‐behavioral therapy and light therapy treatments for seasonal affective disorder: what accounts for clinical status the next winter?. Cognitive Therapy and Research 2013;37(6):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felder 2012
- Felder JN, Dimidjian S, Segal Z. Collaboration in mindfulness‐based cognitive therapy. Journal of Clinical Psychology 2012;68(2):179‐86. [DOI] [PubMed] [Google Scholar]
Freed 2010
- Freed MC, Osborn RL, Rohan KJ. Estimating the disease burden of seasonal affective disorder. In: Preedy VR, Watson RR editor(s). Handbook of Disease Burdens and Quality of Life Measures. Springer, 2010:1549‐68. [Google Scholar]
Gartlehner 2019
- Gartlehner G, Nussbaumer‐Streit B, Gaynes BN, Forneris CA, Morgan LC, Greenblatt A, et al. Second‐generation antidepressants for preventing seasonal affective disorder in adults. Cochrane Database of Systematic Reviews 2019, Issue 3. [DOI: 10.1002/14651858.CD011268.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hickie 2011
- Hickie IB, Rogers NL. Novel melatonin‐based therapies: potential advances in the treatment of major depression. Lancet 2011;378(9791):621‐31. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hodges 1998
- Hodges S, Marks M. Cognitive characteristics of seasonal affective disorder: a preliminary investigation. Journal of Affective Disorders 1998;50:59‐64. [DOI] [PubMed] [Google Scholar]
Kaminski‐Hartenthaler 2015
- Kaminski‐Hartenthaler A, Nussbaumer B, Forneris CA, Morgan LC, Gaynes BN, Sonis JH, et al. Melatonin and agomelatine for preventing seasonal affective disorder. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011271.pub2] [DOI] [PubMed] [Google Scholar]
Kurlansik 2012
- Kurlansik SL, Ibay AD. Seasonal affective disorder. American Family Physician 2012;86(11):1037‐41. [PubMed] [Google Scholar]
Lam 2006
- Lam RW, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Michalak EE, et al. The Can‐SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. American Journal of Psychiatry 2006;163(5):805‐12. [DOI] [PubMed] [Google Scholar]
Leichsenring 2007
- Leichsenring F, Leibing E. Psychodynamic psychotherapy: a systematic review of techniques, indications and empirical evidence. Psychology and Psychotherapy 2007;80(Pt 2):217‐28. [DOI] [PubMed] [Google Scholar]
Leon 1999
- Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE‐RIFT): a brief measure of functional impairment. Psychological Medicine 1999;29(4):869‐78. [DOI] [PubMed] [Google Scholar]
Levitan 1998
- Levitan RD, Rector NA, Bagby M. Negative attributional style in seasonal affective disorder. American Journal of Psychiatry 1998;155:428‐30. [DOI] [PubMed] [Google Scholar]
Levitan 2007
- Levitan RD. The chronobiology and neurobiology of winter seasonal affective disorder. Dialogues in Clinical Neuroscience 2007;9(3):315‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewy 1987
- Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase‐shifting effects of light. Science 1987;235:352‐4. [DOI] [PubMed] [Google Scholar]
Magnusson 2005
- Magnusson A, Partonen T. The diagnosis, symptomatology, and epidemiology of seasonal affective disorder. CNS Spectrums 2005;10(8):625‐34. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neumeister 2001
- Neumeister A, Konstantinidis A, Praschak‐Rieder N, Willeit M, Hilger E, Stastny J, et al. Monoaminergic function in the pathogenesis of seasonal affective disorder. International Journal of Neuropsychopharmacology 2001;4:409‐20. [DOI] [PubMed] [Google Scholar]
Nolen‐Hoeksema 1987
- Noeln‐Hoeksema S. Sex differences in unipolar depression: evidence and theory. Psychological Bulletin 1987;101:259‐82. [PubMed] [Google Scholar]
Nussbaumer‐Streit 2017
- Nussbaumer‐Streit B, Winkler D, Spies M, Kasper S, Pjrek E. Prevention of seasonal affective disorder in daily clinical practice: results of a survey in German‐speaking countries. BMC Psychiatry 2017;17(1):247. [DOI: 10.1186/s12888-017-1403-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nussbaumer‐Streit 2018
- Nussbaumer‐Streit B, Pjrek E, Kien C, Gartlehner G, Bartova L, Friedrich ME, et al. Implementing prevention of seasonal affective disorder from patients' and physicians' perspectives ‐ a qualitative study. BMC Psychiatry 2018;18(1):372. [DOI: 10.1186/s12888-018-1951-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nussbaumer‐Streit 2019
- Nussbaumer B, Forneris CA, Morgan LC, Noord MG, Gaynes BN, Greenblatt A, et al. Light therapy for preventing seasonal affective disorder. Cochrane Database of Systematic Reviews 2019, Issue 3. [DOI: 10.1002/14651858.CD011269.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Partonen 1998
- Partonen T, Lonnqvist J. Seasonal affective disorder. Lancet 1998;352:1369‐74. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodin 1997
- Rodin I, Thompson C. Seasonal affective disorder. Advances in Psychiatric Treatment 1997;3:352‐9. [Google Scholar]
Rohan 2003
- Rohan KJ, Sigmon ST, Dorhofer DM. Cognitive‐behavioral factors in seasonal affective disorder. Journal of Consulting and Clinical Psychology 2003;71:22‐30. [DOI] [PubMed] [Google Scholar]
Rosen 1990
- Rosen LN, Targum SD, Terman M, Bryant MJ, Hoffman H, Kasper SF. Prevalence of seasonal affective disorder at four latitudes. Psychiatry Research 1990;31:131‐44. [DOI] [PubMed] [Google Scholar]
Rosenthal 1979
- Rosenthal R. The "file‐drawer problem" and tolerance for null results. Psychological Bulletin 1979;86:638‐41. [Google Scholar]
Rosenthal 1984
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Archives of General Psychiatry 1984;41(1):72‐80. [DOI] [PubMed] [Google Scholar]
Schroevers 2013
- Schroevers MJ, Tovote KA, Keers JC, Links TP, Sanderman R, Fleer J. Individual mindfulness‐based cognitive therapy for people with diabetes: a pilot randomized controlled trial. Mindfulness 2015;6(1):99‐110. [Google Scholar]
Schwartz 1996
- Schwartz PJ, Brown C, Wehr TA, Rosenthal NE. Winter seasonal affective disorder: a follow‐up study of the first 59 patients of the National Institute of Mental Health Seasonal Studies Program. American Journal of Psychiatry 1996;153(8):1028‐36. [DOI] [PubMed] [Google Scholar]
Segal 2002
- Segal ZV, Williams JM, Teasdale JD. Mindfulness‐Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: Guilford Press, 2002. [Google Scholar]
Seligman 2006
- Seligman ME, Rashid T, Parks AC. Positive psychotherapy. American Psychologist 2006;61(8):774‐88. [DOI] [PubMed] [Google Scholar]
Sohn 2005
- Sohn CH, Lam RW. Update on the biology of seasonal affective disorder. CNS Spectrums 2005;10(8):635‐46. [DOI] [PubMed] [Google Scholar]
Srinivasan 2012
- Srinivasan V, Berardis D, Shillcutt SD, Brzezinski A. Role of melatonin in mood disorders and the antidepressant effects of agomelatine. Expert Opinion on Investigational Drugs 2012;21(10):1503‐22. [DOI] [PubMed] [Google Scholar]
Strauman 2006
- Strauman TJ, Veith AZ, Kolden GG, Woods TE, Klein MH, Papadakis AA, et al. Self‐system therapy as an intervention for self‐regulatory dysfunction in depression. A randomized comparison with cognitive therapy. Journal of Consulting and Clinical Psychology 2006;74:367‐76. [DOI] [PubMed] [Google Scholar]
Terman 2005
- Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectrums 2005;10:647‐63. [DOI] [PubMed] [Google Scholar]
Thaler 2010
- Thaler K, Delivuk M, Chapman A, Gaynes BN, Kaminski A, Gartlehner G. Second‐generation antidepressants for seasonal affective disorder. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008591.pub2] [DOI] [PubMed] [Google Scholar]
Tovote 2014
- Tovote KA, Fleer J, Snippe E, Peeters AC, Emmelkamp PM, Sanderman R, et al. Individual mindfulness‐based cognitive therapy (MBCT) and cognitive behavior therapy (CBT) for treating depressive symptoms in patients with diabetes: results or a randomized controlled trial. Diabetes Care 2014;37(9):2427‐34. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Wells 2009
- Wells GA, Shea B, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp 2009.
Westrin 2007
- Westrin A, Lam RW. Long‐term and preventative treatment for seasonal affective disorder. CNS Drugs 2007;21(11):901‐9. [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams JBW, Link MJ, Rosenthal NE, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale ‐ Seasonal Affective Disorder version (SIGH‐SAD). New York: New York State Psychiatric Institute, 2012. [Google Scholar]
References to other published versions of this review
Forneris 2014
- Forneris CA, Nussbaumer B, Kaminski‐Hartenthaler A, Morgan LC, Gaynes BN, Sonis JH, et al. Psychological therapies for preventing seasonal affective disorder. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011270] [DOI] [PubMed] [Google Scholar]
Forneris 2015
- Forneris CA, Nussbaumer B, Kaminski‐Hartenthaler A, Morgan LC, Gaynes BN, Sonis JH, et al. Psychological therapies for preventing seasonal affective disorder. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011270.pub2] [DOI] [PubMed] [Google Scholar]


