Abstract
Potent antiresorptive drugs (bisphosphonate and denosumab) are often used to protect bone health in postmenopausal breast cancer patients. In addition, clinical trials have shown that these drugs increase disease‐free survival, though the mechanism of adjuvant benefit is largely unknown. Here we review the bone health and adjuvant data for both classes of antiresorptive drugs and highlight differences in their pharmacology. Inhibition of bone resorption is vitally important to protect against osteoporotic fractures, and may also contribute to adjuvant survival benefits by making the bone microenvironment less amenable to breast cancer metastasis. After a course of therapy, stoppage of bisphosphonates yields a persistent antiresorptive effect, whereas discontinuation of denosumab causes a rebound increase in bone resorption markers and a loss of bone mineral density to baseline levels. Whether the potential adjuvant benefits of denosumab are also rapidly lost after drug discontinuation deserves further investigation.
Keywords: antiresorptive, bisphosphonates, denosumab, fracture, metastasis, osteoporosis
Introduction
In postmenopausal women with oestrogen receptor‐positive breast cancer, aromatase inhibitors are the standard of care for adjuvant therapy. Because these drugs profoundly lower postmenopausal circulating oestradiol levels to below the lower limit of assay quantification, and since oestrogen inhibits bone resorption, these drugs lead to bone loss 1. Thus, aromatase inhibitors may compound the negative effects of menopause and ageing on bone health 2. Data from a large Austrian cohort suggests that these drugs may increase fracture risk across a wide range of ages and, surprisingly, even in women with normal bone mineral density (BMD) 3. Furthermore, adjuvant aromatase inhibitors may be given for ten years or more. Major osteoporotic fractures can be painful, disabling and are associated with an increased risk for mortality 4. Therefore, potent antiresorptive drugs, namely bisphosphonates and denosumab, are often used to maintain bone health and prevent fractures in aromatase inhibitor‐treated patients 5.
In this review, we summarize differences in pharmacology (particularly offset of action) between bisphosphonates and denosumab, as well as evidence for their protective effects on bone health and tumour activity in postmenopausal women with breast cancer. Breast cancer frequently metastasizes to bone. Bone is constantly remodelled, causing liberation of bone‐derived growth factors embedded in the bone matrix; it has been theorized that increased levels of bone remodelling could attract and promote growth of subclinical bone metastases 6. Thus, antiresorptive drugs were hypothesized to prevent skeletal metastases from developing 7. A multitude of randomized adjuvant bisphosphonate studies have been conducted since the 1990s with data from the most definitive meta‐analysis supporting improved disease‐free and overall survival specific to postmenopausal breast cancer patients 8. Two adjuvant denosumab studies are ongoing. While numerous theories have been proposed to explain how bisphosphonates, and potentially denosumab, provide this adjuvant benefit, results from clinical trials and animal studies, also discussed in this review, suggest a direct relationship with antiresorptive activity.
Pharmacology of antiresorptive drugs
Mechanism of action
Both nitrogen‐containing (zoledronate, pamidronate, alendronate, risedronate and ibandronate) and non‐nitrogen‐containing (clodronate) bisphosphonates have been studied in postmenopausal breast cancer patients. Nitrogen‐containing bisphosphonates reduce bone resorption by inhibiting an enzyme within osteoclasts, farnesyl pyrophosphate synthase (FPPS); this interferes with the localization and function of small GTPases that are essential for osteoclast activity and survival 9. These agents are chemically similar to inorganic pyrophosphate in the mineral component of bone, allowing them to bind avidly to divalent metal ions such as Ca2+ and accumulate in bone 9. In contrast to the nitrogen‐containing bisphosphonates, bisphosphonates that do not contain nitrogen are taken up by osteoclasts and incorporated into adenosine triphosphate (ATP), making it nonhydrolyzable and ultimately leading to osteoclast apoptosis 10.
Denosumab, a fully humanized monoclonal antibody, impairs the development, activation, and survival of osteoclasts 11 by blocking the receptor activator of nuclear factor kappa‐B ligand (RANKL) with high affinity and specificity. In stark contrast to bisphosphonates, denosumab does not accumulate in bone over time.
Pharmacokinetics
Oral bisphosphonates are poorly absorbed throughout the gastrointestinal tract, with a bioavailability varying from 0.7–2.5%, and are better absorbed on an empty stomach 12, 13, 14. The majority of absorbed bisphosphonates are cleared by the kidney. Use in the setting of renal dysfunction must be undertaken with caution due to: uncertainties as to whether there is a greater proportion of bone retention as glomerular filtration rate (GFR) declines, the presence of adynamic bone disease in some patients with stage IV and V chronic kidney disease, and due to the potential renal toxicity of intravenous bisphosphonates. In addition to kidney excretion, bisphosphonates are released into the circulation and embedded in bone during bone formation. They dissociate from bone during subsequent resorption, explaining their long and slow elimination from the skeleton 15.
In contrast, denosumab does not bind to bone mineral and therefore is not recycled during bone turnover. The bioavailability of denosumab (60–120 mg, administered subcutaneously) was reported to be 61–64% 16. After administration of denosumab (60 mg), the mean maximum denosumab concentration was 6.75 μg ml−1 with a median time to maximum concentration of 10 days and a mean half‐life of 25.4 days. Unlike bisphosphonates, denosumab is not excreted from the kidney and therefore dosage adjustment is not required in patients with renal impairment 17. There is no risk of renal toxicity with denosumab. However, in some patients with renal impairment, denosumab has been reported to cause severe hypocalcaemia.
Pharmacodynamics: persistent vs. rebound effects on discontinuation
The optimal duration of antiresorptive drug treatment, both for osteoporosis and for cancer treatment‐induced bone loss, remains controversial. Rare adverse effects common to long‐term exposure to bisphosphonates and denosumab are osteonecrosis of the jaw and atypical femoral fracture 18. The United States Food and Drug Administration (FDA) has suggested individualizing treatment duration for bisphosphonate treatment of osteoporosis and, in low risk patients, to consider stopping the drug after 3–5 years 19. Per FDA label: ‘The optimal duration of use has not been determined. All patients on bisphosphonate therapy should have the need for continued therapy re‐evaluated on a periodic basis’ 20. In contrast, no such advice exists with denosumab. Given their differing pharmacokinetics, the impacts of stopping the two drugs classes are very distinct 11. In the context of breast cancer adjuvant therapy with aromatase inhibitors, a common clinical scenario would be to discontinue bone protective therapy (provided that residual fracture risk is not high) once the aromatase inhibitor is stopped.
Bisphosphonates
Because bisphosphonates can remain bound to bone for years, they continue to exert an antiresorptive (bone protective) effect even after discontinuation. Thus, after reviewing long‐term studies with fracture data subsequent to drug discontinuation, the FDA has suggested a potential ‘drug holiday’ after chronic bisphosphonate use 19. Two landmark trials in patients with osteoporosis have demonstrated maintenance of bone mineral density (BMD), or continued increases therein, for 3 or more years after stopping bisphosphonates. For example, in a randomized extension of the HORIZON Pivotal Fracture Trial (PFT), the BMD of patients who received zoledronate (5 mg annually) for 3 years was compared to that of patients receiving the drug for 6 years 21. At 2 years after discontinuation, BMD remained stable – at similar levels to those of patients still receiving the drug, and only began to decline slightly at 6 years (Figure 1). Similarly, the Fracture Intervention Long‐term Extension (FLEX) trial found that stopping alendronate after 5 years led to moderate declines in BMD 5 years later. This trial observed a statistically significant increase in clinical vertebral fractures in those subjects who discontinued alendronate compared with those who continued alendronate for 10 years, which adds complexity to the decision of whether or not to start a ‘bisphosphonate holiday’ 22.
Figure 1.
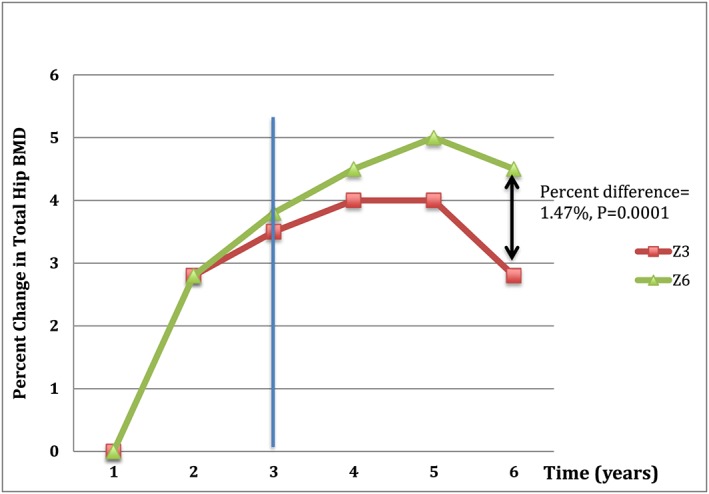
Residual effect of bisphosphonates after 3 years of treatment. Z3 is three yearly doses of zoledronic acid followed by no further treatment over the next three years. Z6 is six yearly doses of zoledronic acid. At the 6‐year mark, there is a small but significant 1.47% difference in bonemineral density between Z3 and Z6 (0.80–2.14; P = 0.0001)
Denosumab
Discontinuation of denosumab, in stark contrast to bisphosphonates, is associated with a rebound loss of BMD and increase in bone turnover markers (Figure 2). Recently, cases of spine fractures temporally linked to denosumab discontinuation have been reported. The rebound effects after drug discontinuation are not prevented or slowed by extended treatment and the accompanying usual large gains in BMD that follow it. Two recent studies demonstrated rapid bone loss at all clinically important sites within about a year of stopping denosumab in patients receiving at least 7 years of denosumab treatment 23, 24. Importantly, Popp et al. found that BMD fell below the pre‐treatment baseline at the hip: by 5.5% and 3.8% at total hip (TH) and femoral neck (FN), respectively 23. Similarly, bone turnover markers have also been observed to increase above pre‐treatment baseline levels within 6 months after discontinuing denosumab 25.
Figure 2.
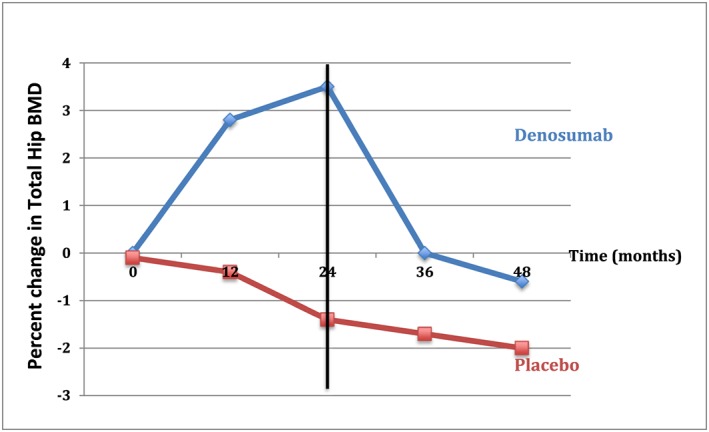
Effects of stopping denosumab after 24 months on total hip bone mineral density. There is an immediate drop in bone mineral density reaching a value below baseline (pre‐treatment) levels 24 months after denosumab cessation
A recent systematic review identified 24 patients with vertebral fractures 8–16 months after denosumab discontinuation, the majority (92%) of whom had multiple vertebral compression fractures 25. Five patients were on concurrent aromatase inhibitor treatment and the reason for denosumab discontinuation in four out of five was the end of aromatase inhibitor therapy. Thus, the fractures in these four patients could not have been caused by ongoing aromatase inhibitor therapy. A recent post‐hoc analysis of the FREEDOM trial also demonstrated that the vertebral fracture rate quickly increased upon denosumab discontinuation to the level observed in untreated participants; those with a history of vertebral fractures were at highest risk 26. Although most cases of ‘rebound’ vertebral fractures post‐denosumab occurred in patients naïve to other osteoporosis therapies, there are anecdotal reports of patients who sustained vertebral fractures despite previous teriparatide or bisphosphonate treatment 25. The underlying mechanism of rebound fractures post‐denosumab remains unclear, but one plausible explanation is that the bone remodelling rate increases markedly after denosumab discontinuation.
Bone loss after denosumab cessation may be partially preventable by alendronate or by a single post‐treatment dose of zoledronic acid 27, 28. A clinical trial (NCT02499237) investigating whether the latter strategy prevents the decrease in BMD and increase in bone turnover markers after discontinuation of denosumab is currently ongoing.
Use in breast cancer: impact on bone mineral density and fracture risk
Bisphosphonates
Both oral and intravenous bisphosphonates preserve BMD in postmenopausal breast cancer patients receiving endocrine adjuvant therapy (Table 1). The largest increases were reported in a study in which 25 osteoporotic patients and 22 osteopenic patients treated with anastrozole also received alendronate. At 3‐year follow‐up, lumbar spine BMD increased by 15.6% in the osteoporotic group and 6.3% in the osteopenic group with alendronate treatment, whereas patients without alendronate (n = 250) sustained a 5.4% loss 29. Oral bisphosphonates also preserve BMD in osteopenic patients; double‐blind, randomized, placebo‐controlled trials of ibandronate and risedronate found increases in lumbar spine (LS) and total hip (TH) BMD at 2‐year follow‐up (~2–3% and ~1–2%, respectively, vs. ~2–3% and ~1–4% losses with placebo) at 2‐year follow‐up 30. A third clinical trial investigated the effectiveness of oral risedronate in postmenopausal women with early stage breast cancer receiving anastrozole. Patients were further stratified according to their fracture risk. Patients with highest risk were all given risedronate while patients with moderate risk were randomly assigned to either risedronate or placebo. At 24 months, the moderate‐risk group treated with risedronate experienced a significant increase in LS (2.2% vs. −1.8%, P < 0.0001) and TH BMD (1.8% vs. −1.1%, P < 0.0001) compared with placebo 31. A similar BMD increase was also found in the high‐risk group (3.0% at LS, P = 0.006 and 2.0% at FN, P = 0.01) 32.
Table 1.
Clinical trials of bisphosphonates and denosumab use in postmenopausal women with early stage breast cancer that assessed change in bone mineral density
| Trials | Drug | N | Menopause status | Breast cancer stage | Application | Dose | Aromatase inhibitors | Duration (months) | BMD increase at lumbar spine (from baseline) | BMD increase at hip (from baseline) | Bone turnover marker change (from baseline) | Fracture risk a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BATMAN | Alendronate | 303 | Postmenopausal | Early stage | Oral | 70 mg weekly | Anastrozole | 36 | 15.6% in osteoporotic group | 5.60% | NTX −38% at 6 months | No fragility fracture in alendronate group |
| ARIBON | Ibandronate | 131 | Postmenopausal | Early stage | Oral | 150 mg monthly | Anastrozole | 24 | 2.98% | 0.60% | NTX‐30.9%, sCTX −26.3%, and B‐SAP −22.8% | No fragility fracture reported in either group |
| SABRE | Risedronate | 154 | Postmenopausal | Early stage | Oral | 35 mg weekly | Anastrozole | 24 | 2.2% in moderate risk, 3.0% in high risk | 1.8% in moderate risk, 2.0% in high risk | sCTX −43.8% in moderate risk, −46.0% in high risk | 4 fractures in placebo group; 1 in risedronate group |
| ZO‐FAST | Zoledronic acid | 1065 | Postmenopausal | Early stage | Intravenous | 4 mg every 6 months | Letrozole | 60 | 4.30% | 1.60% | N/A | N/A |
| Z‐FAST | Zoledronic acid | 602 | Postmenopausal | Early stage | Intravenous | 4 mg every 6 months | Letrozole | 60 | 6.20% | 2.60% | sNTX −20.1% at 12 months, B‐SAP −5.4% at 5 years | 9.3% in upfront group, 11% in delayed group, no significant difference |
| E‐ZO‐FAST | Zoledronic acid | 527 | Postmenopausal | Early stage | Intravenous | 4 mg every 6 months | Letrozole | 12 | 2.72% | 1.72% | N/A | 2.8% in upfront group, 3.3% in delayed group |
| HALT | Denosumab | 252 | Postmenopausal | Early stage | Subcutaneous | 60 mg every 6 months | Anastrozole, Letrozole, Exemestane | 24 | 4.8% (at 12 months) | 3.1% (at 12 months) | sCTX −80%; P1NP −73% | 2% in treatment group, 4% in placebo group |
| ABCSG‐18 a | Denosumab | 3420 | Postmenopausal | Early stage | Subcutaneous | 60 mg every 6 months | Anastrozole, Letrozole, Exemestane | 36 | 7.27% | 4.60% | N/A | Lower number of fracture vs placebo (92 vs. 176), delayed time to fracture HR 0.5 [95% CI 0.39–0.65], P < 0.0001 |
The only study sufficiently powered to evaluate fracture risk was the ABCSG‐18
Whether early administration of zoledronic acid is beneficial was tested in the Z‐FAST and ZO‐FAST trials 33, 34. In both trials, patients were randomized to receive either early treatment with zoledronate 4 mg every 6 months for 5 years or delayed administration when spine or hip T‐score decreased to <−2.0, or a clinical fracture occurred. In the Z‐FAST trial, at 61 months follow‐up, both LS and TH BMD were significantly better in the upfront treatment group than the delayed treatment group (adjusted mean difference 8.9% for LS and 6.7% for TH, respectively). After 5 years, 25% of women in the delayed treatment group received zoledronate 33. Similarly, in the ZO‐FAST trial, the mean change in LS BMD was +4.3% with immediate zoledronate and −5.4% with delayed intervention (P < 0.0001) 34.
Despite the beneficial effects of bisphosphonates in preserving BMD, none of the trials were powered to assess fracture incidence as the primary outcome. Though one meta‐analysis of six randomized placebo‐controlled clinical trials investigating adjuvant oral and/or intravenous bisphosphonates found no significant reduction in fracture incidence in women receiving adjuvant aromatase inhibitors for breast cancer, another more recent and larger meta‐analysis did show a fracture risk reduction (RR 0.85, 95% CI 0.75–0.97, P = 0.02) 8, 35. Given that these were adjuvant studies, fractures were not the primary endpoint, and may not have been adequately captured as adverse events.
Denosumab
Though few studies have examined the ability of denosumab to preserve BMD and reduce fracture risk in the context of early‐stage postmenopausal breast cancer, the one trial yet reported strongly supported its efficacy. The Austrian Breast and Colorectal Cancer Study Group‐18 (ABCSG‐18), a double blind, placebo‐controlled phase III trial, assigned 3420 postmenopausal women with early hormone receptor positive breast cancer treated with aromatase inhibitors to either denosumab 60 mg or placebo every 6 months. Subjects were randomized regardless of baseline BMD and/or fracture risk. The primary endpoint was time to first clinical fracture. At 36 months, patients in the denosumab group had a significant increase in BMD at all sites as compared with those in the placebo group (LS 10.02%, TH 7.92% and FN 6.51%, all adjusted P < 0.0001). Furthermore, time to clinical fracture was significantly delayed in patients receiving denosumab (HR 0.5 [95% CI 0.39–0.65], P < 0.0001). Denosumab also significantly decreased the vertebral fracture rate (OR 0.53 [95% CI 0.33–0.85], P = 0.009) 3. The fact that denosumab prevented fractures in a population unselected for traditional risk factors for fracture such as low BMD and advanced age implies that aromatase inhibitors may increase fracture risk largely independent of BMD and age. As ethnicity influences fracture risk, it is important to note that all the patients in this study were of Austrian or Swedish ethnicity.
Adjuvant effect of antiresorptive therapy in postmenopausal breast cancer
Bisphosphonates
Bisphosphonates have been shown to be effective in preventing loss of BMD in breast cancer patients, while their impact on breast cancer mortality has not been consistent. However, over many years and several randomized controlled trials of different bisphosphonates, clear evidence has emerged for adjuvant benefit in postmenopausal but not premenopausal patients.
The largest adjuvant bisphosphonate trial, Adjuvant Zoledronate to Reduce Recurrence (AZURE), included 3360 patients (both premenopausal and postmenopausal) randomized to receive standard adjuvant systemic therapy either with or without zoledronate. At long‐term follow‐up (84 months), neither disease‐free survival (DFS) nor overall survival (OS) differed between groups (DFS: HR 0.93, 95% CI 0.82–1.05, P = 0.22; OS: HR 0.93, 95% CI 0.81–1.07, P = 0.29). However, zoledronate reduced the development of bone metastases, both as a first event (HR 0.78, 95% CI 0.63–0.96, P = 0.020) and at any time during follow‐up (HR 0.81, 95% CI 0.68–0.97, P = 0.022). The effects of zoledronate on DFS were not affected by oestrogen‐receptor status. A preplanned analysis by menopausal status demonstrated that zoledronate improved DFS in those who were over 5 years since menopause at trial entry (n = 1041; HR 0.77, 95% CI 0.63–0.96) but not in other groups (premenopause, perimenopause and unknown status) (n = 2318; HR 1.03, 95% CI 0.89–1.20) 36.
Several meta‐analyses have evaluated the effect of bisphosphonates on survival and recurrence in early breast cancer and found various positive effects (Table 2). A 2010 analysis of 13 eligible trials and 6886 patients found that bisphosphonates did not reduce the overall number of deaths (OR 0.708, P = 0.079), bone metastases (OR 0.925, P = 0.413), overall disease recurrence (OR 0.843, P = 0.321) or distal relapse (OR 0.896, P = 0.453), but a subgroup analysis (n = 4142) of six zoledronate studies found a statistically significant lower risk of disease recurrence (OR 0.68, P = 0.025). A slightly smaller subgroup analysis (n = 2925) of three evaluable studies with zoledronate did not demonstrate a significant reduction in overall mortality or bone recurrence. 35. In contrast to the 2010 analysis, Valachis et al. showed that zoledronate decreased the risk of fractures and improved overall survival by reducing the risk of death by 19% (pooled HR 0.81, CI 0.70–0.94) in an analysis of 15 studies of zoledronate (seven studies of only postmenopausal women, five of perimenopausal women and three studies including both). There were no differences in DFS, disease recurrence rate or bone recurrence. A subgroup analysis to evaluate the impact of menopausal status was not performed 37. O'Carrigan et al. found that bisphosphonates improved OS and were associated with a reduced risk of bone metastasis compared with placebo (RR 0.86, P = 0.03). A subgroup analysis showed that this survival benefit was restricted to postmenopausal women (HR 0.77, P = 0.001); similarly, though bisphosphonates did not improve DFS in the overall analysis, they did in postmenopausal women (HR 0.82, P < 0.001). There was no effect on fracture rates (RR 0.77, P = 0.13) 38.
Table 2.
Meta‐analysis of studies on adjuvant effects of bisphosphonates in breast cancer patients
| Meta‐Analysis (by author) | Menopause status | Bisphosphonate | N of trials included | N | Breast cancer stage | Overall Recurrence | Distant Recurrence | Bone Recurrence | Mortality | Overall Survival Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Valachis et al. 2010 36 | Premenopausal, perimenopausal and postmenopausal | Pamidronate, Zoledronate, Clodronate, Risedronate | 13 | 6886 | Early | OR 0.84, 95% CI 0.60–1.18; P = 0.321 | OR 0.90; 95% CI, 0.67–1.19; P = 0.453 | OR 0.93, 95% CI 0.77–1.11; P = 0.413 | 0.71, 95% CI 0.48–1.04; P = 0.079 | |
| Zoledronate | 3–6 | 2925–4142 | OR 0.68, 95% CI 0.48–0.95; P = 0.025 | OR 0.66, 95% CI 0.38–1.15; P = 0.144 | OR 0.64, 95% CI 0.39–1.06; P = 0.085 | |||||
| Valachis et al. 2013 38 | Premenopausal, perimenopausal and postmenopausal | Zoledronate | 15 | 6414 | Early | HR 0.86, 95% CI 0.70–1.06; P = 0.15 | HR 0.88, 95% CI 0.70–1.010; P = 0.27 | HR 0.94, 95% CI 0.64–1.37; P = 0.74 | HR 0.81, 95% CI 0.70–0.94 | |
| EBCTCG (Meta‐analysis) 8 | Premenopausal, perimenopausal and postmenopausal |
Pamidronate, Zoledronate, Clodronate, Risedronate Ibandronate |
26 | 18 766 | Early | RR 0.94, 95% CI 0.87–1.01; P = 0.08 | RR 0.92, 95% CI 0.85–0.99; P = 0.03 | RR 0.83, 95% CI 0.73–0.94; P = 0.004 | RR 0.91, 95% CI 0.83–0.99; P = 0.04 | |
| Postmenopausal | 11 767 | Early | RR 0.86, 95% CI 0.78–0.94; P = 0.002 | RR 0.82, 95% CI 0.74–0.92; P = 0.0003 | RR 0.72, 95% CI 0.60–0.86; P = 0.0002 | RR 0.82, 95% CI 0.73–0.93; P = 0.002 | ||||
| O'Carrigan et al. 2017 39 | Premenopausal, perimenopausal and postmenopausal | Zoledronate, Clodronate, Ibandronate, Pamidronate | 11–7 | 15 005–12 578 | Early | RR 0.94 (95% CI 0.87–1.02; P = 0.13) | RR 0.86, 95% CI 0.75–0.99; P = 0.03 | |||
| 9 | 13 949 | Early | HR 0.91, 95% CI 0.83–0.99; P = 0.04 | |||||||
| Postmenopausal | 4–7 | 6048–8314 | Early | HR 0.82, 95% CI 0.74–0.91; P = 0.001 | HR 0.77, 95% CI 0.66–0.90; P = 0.001 | |||||
| Premenopausal | 2 | 3501 | Early | HR 1.03, 95% CI 0.86–1.22; P = 0.78 |
In the Early Breast Cancer Trialists' Collaborative Group study (EBCTCG), which analysed data on 18 766 women in 26 trials, including the AZURE study, the use of bisphosphonates was associated with a significant reduction in bone recurrence (RR 0.83, P = 0.004) and bone fractures (RR 0.85, P = 0.02). Reductions in overall recurrence (RR 0.94, 95% CI 0.87–1.01; P = 0.08), distant recurrence (0.92, 0.85–0.99; 2P = 0.03), and breast cancer mortality (0.91, 0.83–0.99; P = 0.04) were of borderline significance. Subgroup analysis found that these benefits were highly significant in women with documented menopause (if menopausal status was unknown, all women ≥ age 55 were considered postmenopausal), or who underwent medical suppression of ovarian function. Among such postmenopausal women (but not pre‐ or perimenopausal women), bisphosphonate use was strongly associated with a lower rate of recurrence (RR 0.86, 95% CI 0.78–0.94, P = 0.002), distant recurrence (RR 0.82, 95% CI 0.74–0.92, P = 0.0003), bone recurrence (RR 0.72, 95% CI 0.60–0.86, P = 0.0002), and breast cancer mortality (RR 0.82, 95% CI 0.73–0.93, P = 0.002). Importantly, the overall survival benefit appeared to be driven by lower rates of bone recurrence over the first 4 years of follow‐up, and, there was no difference in non‐breast cancer related mortality 8.
Based on these data, the Cancer Care Ontario and American Society of Clinical Oncology clinical practice guidelines recommend considering bisphosphonates as adjuvant therapy for postmenopausal women with breast cancer who are candidates for systemic therapy. Both zoledronate and clodronate are recommended over ibandronate given that the 50 mg daily dosing schedule of ibandronate in not available in the US and Canada 39. Insufficient data is available regarding the adjuvant activity of the oral bisphosphonates alendronate and risedronate.
Preclinical studies in breast cancer models point to various possible mechanisms by which bisphosphonates improve survival, such as preventing tumour cell adhesion to bone, inducing tumour cell apoptosis, inhibiting angiogenesis, and by activating gamma delta T cells 40, 41, 42. Many of these mechanisms, notably the effect on gamma delta T cells, are unique to bisphosphonates and are not shared by denosumab.
Biochemical markers of bone turnover reflect acute changes in bone resorption and dynamically respond to antiresorptive therapy. Hence, if antiresorptive activity is a driver of adjuvant effect, bone turnover markers would be a useful indicator of adjuvant effect. Higher pretreatment C‐terminal telopeptides (B‐CTx) (a serologic bone resorption marker) was associated with significantly shorter bone‐only recurrence‐free survival (RFS) in patients with postmenopausal breast cancer in a post hoc analysis of a randomized study (n = 667) 43. A retrospective analysis of a limited number of postmenopausal patients in the AZURE trial was suggestive of an association between baseline elevation in the bone formation marker Procollagen Type I N‐terminal propeptides (PINP) and an increased risk of bone metastases when compared with low baseline PINP (HR 1.58, 95% CI 1.00–2.50, P = 0.06) but not for distant recurrence overall. However, PINP levels did not predict benefit from zoledronic acid on bone or other recurrence. Baseline levels of serum CTx failed to be prognostic for bone metastases or distant recurrence and also were not prognostic of benefit from zoledronate on any recurrence 36. The patients analysed from the AZURE study may have been pre‐, peri‐ or postmenopausal at study entry, whereas the analysis by Lipton et al. was in postmenopausal patients, the population in which adjuvant benefit has been demonstrated.
Denosumab
In the ABCSG‐18 trial of denosumab, the initial report demonstrated a trend towards improved DFS (167 vs. 2013 recurrences or deaths), HR 0.82, P = 0.051. Subgroup analysis showed that denosumab significantly improved DFS in patients with tumours larger than 2 cm (HR 0.66, P = 0.016), that expressed both progesterone receptor (PR) and oestrogen receptor (ER) (HR 0.75, P = 0.013) and that had ductal histology (HR 0.79, P = 0.048) 3. However, subgroup analysis of relatively small numbers of patients may be misleading. The updated results from ABCSG‐18 demonstrated a clear improvement in DFS (HR 0.82, 95% CI 0.69–0.98, Cox P = 0.026*) 44.
Another large randomized trial, the D‐CARE trial, used a more aggressive dosing schedule of denosumab: 120 mg subcutaneously or matching placebo monthly for six months and then every three months for up to 5 years. Less than half (2149) of the 4509 subjects were postmenopausal, raising questions about adequate statistical power. In an initial report the trial failed to show an improvement in DFS or OS with denosumab; analysis of the postmenopausal subgroup showed similar results 45.
As with bisphosphonates, the mechanism of denosumab's adjuvant effect has yet to be fully elucidated. Preclinical studies have suggested that denosumab may have anti‐tumour effects via interaction with the immune system 46. However, the fact that both bisphosphonates and denosumab appear to improve DFS in postmenopausal breast cancer suggests that antiresorptive activity is important and that the antiresorptive effect on the bone microenvironment could be the key driver (Figure 3). In the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta‐analysis, mean scheduled treatment duration was 3.4 years. A total of 18 206 (97%) of 18 766 participants were in trials of 2–5 years of treatment. Since median follow‐up was 5.6 woman‐years (IQR 3.7–8.0), it was possible for 10‐year outcomes to be analysed 8. This ‘long‐term’ follow‐up supports (but does not prove) a persistent adjuvant effect of bisphosphonates after drug discontinuation, in keeping with their bone‐binding affinity and with the microenvironment hypothesis.
Figure 3.
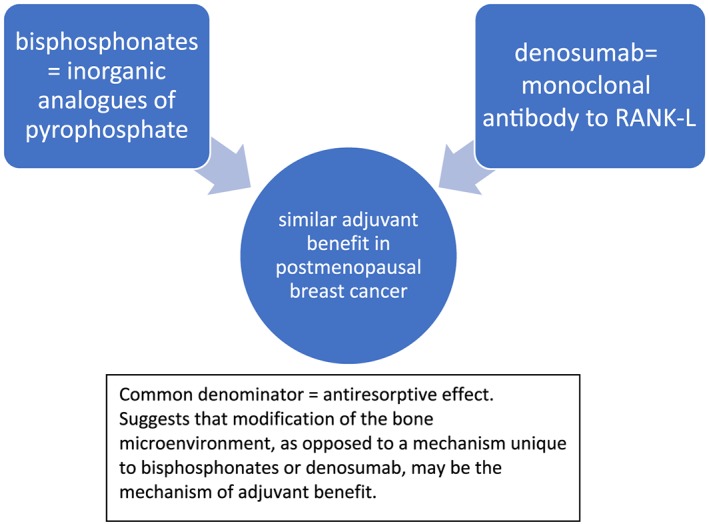
Many potential mechanisms to explain the adjuvant activity of bisphosphonates in postmenopausal breast cancer have been proposed. In light of the ABCSG‐18 denosumab adjuvant data, a unifying explanation is that the antiresorptive effect in the bone microenvironment (inhibiting release of matrix derived growth factors) is the mechanism
Following this logic, the marked increase in bone resorption after denosumab withdrawal would hypothetically be expected to be temporally associated with loss of adjuvant benefits; off‐treatment data from studies such as ABCSG‐18 could yield valuable insight into this potential issue (Table 3).
Table 3.
Effect of bisphosphonate and/or denosumab discontinuation on antifracture and adjuvant activity
| Antifracture activity | Adjuvant activity | |
|---|---|---|
| Bisphosphonate | Persists (not indefinitely) | Likely persists |
| Denosumab | Rapidly diminishes | ? |
Adjuvant benefit in postmenopausal patients only: implications for mechanism
It is not completely understood why the adjuvant benefit was only observed in the postmenopausal state for adjuvant bisphosphonate trials. Theoretically, this selective adjuvant benefit based on menopausal status could be related to the fact that postmenopausal oestrogen deficiency increases bone turnover. This liberates bone‐derived growth factors, theoretically creating a ‘fertile soil’ in which micrometastatic disease (‘seeds’) may propagate 47. Lowering bone resorption with powerful antiresorptive drugs (bisphosphonates and denosumab) could thus make the bone microenvironment less hospitable for metastatic bone disease to take root. In a mouse model, surgically induced menopause (which causes an abrupt increase in bone resorption) increased the number of bone tumours following inoculation of MDA‐MB‐231 breast tumour cells, and promoted their progression; these effects were blocked by zoledronate 48. With sham ovariectomy, zoledronate showed no anti‐tumour activity. Recombinant parathyroid hormone, which also increases bone resorption, similarly increased tumour development in bone. Overall, this preclinical study demonstrated a correlation between increased bone resorption and tumour growth in bone.
In the case of denosumab, the ABCSG‐18 data and the data from the postmenopausal cohort from the D‐CARE study conflict, with the former showing adjuvant DFS benefit while the latter did not. The two studies had very different study populations, with ABCSG‐18 having the vast majority of subjects being node negative (71%) and not treated with chemotherapy (75%), whereas the D‐CARE study did not (95% node positive and only 4% did not receive chemotherapy). Unlike the ABCSG‐18, the D‐CARE study did not require patients to have recently initiated aromatase inhibitor therapy. Aromatase inhibitors are known to increase bone resorption and therefore, according to the bone microenvironment hypothesis, this inclusion criterion may have facilitated the denosumab DFS benefit in ABCSG‐18 by enriching the study population. On the other hand, it does not appear likely that the ABCSG‐18 DFS benefit was driven by a reduction in metastatic bone disease. Final publication of ABCSG‐18 is awaited to resolve this issue.
Conclusion
Promising new data on the adjuvant use of the antiresorptive agents bisphosphonates and denosumab have demonstrated a positive impact on BMD and fractures in postmenopausal breast cancer patients. Bisphosphonates have been extensively studied in randomized adjuvant trials and the most definitive meta‐analysis shows that they improve survival due to reduction in metastatic bone disease. The two randomized trials of denosumab to date reflect different patient populations and have shown conflicting preliminary results (peer reviewed publication pending). Upon discontinuation, bisphosphonates have a persistent effect on BMD and bone turnover due to their high affinity to hydroxyapatite. In contrast, stopping denosumab leads to a rapid rebound in bone resorption and decline in BMD. In osteoporosis patients, cases of vertebral fragility have been attributed to the rebound effect, and strategies to block the rebound by following denosumab therapy with oral or intravenous bisphosphonates are being investigated. Following, or ‘chasing’, denosumab with a bisphosphonate appears particularly important in patients at high risk for osteoporotic fractures. Whether the ‘rebound effect’ has clinical implications for the potential adjuvant activity of denosumab in early breast cancer remains to be seen and likely depends on the mechanism of adjuvant effect, which itself has yet to be fully elucidated. However, the fact that two different classes of potent antiresorptives have yielded remarkably similar disease‐free survival benefits in postmenopausal breast cancer suggests that antiresorptive activity may be an important common denominator yielding adjuvant benefit (Figure 3). An unresolved question is the discrepancy in adjuvant outcomes between the two adjuvant studies of denosumab thus far. Finally, further research should investigate whether the loss of antiresorptive activity following denosumab discontinuation could have negative clinical implications for both the demonstrated fracture benefits as well as for the possible adjuvant activity of this agent.
Nomenclature of targets and ligands
Key ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 49.
Competing Interests
There are no competing interests to declare.
This research was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Chukir T., Liu Y., and Farooki A. (2019) Antiresorptive agents' bone‐protective and adjuvant effects in postmenopausal women with early breast cancer, Br J Clin Pharmacol, 85, 1125–1135. 10.1111/bcp.13834.
References
- 1. Chien AJ, Goss PE. Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol 2006; 24: 5305–5312. [DOI] [PubMed] [Google Scholar]
- 2. Cheung AM, Tile L, Cardew S, Pruthi S, Robbins J, Tomlinson G, et al Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol 2012; 13: 275–284. [DOI] [PubMed] [Google Scholar]
- 3. Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, et al Adjuvant denosumab in breast cancer (ABCSG‐18): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2015; 386: 433–443. [DOI] [PubMed] [Google Scholar]
- 4. Schousboe JT. Mortality after osteoporotic fractures: what proportion is caused by fracture and is preventable? J Bone Miner Res 2017; 32: 1783–1788. [DOI] [PubMed] [Google Scholar]
- 5. Rachner TD, Coleman R, Hadji P, Hofbauer LC. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol 2018; 6: 901–910. [DOI] [PubMed] [Google Scholar]
- 6. Wilson C, Holen I, Coleman RE. Seed, soil and secreted hormones: potential interactions of breast cancer cells with their endocrine/paracrine microenvironment and implications for treatment with bisphosphonates. Cancer Treat Rev 2012; 38: 877–889. [DOI] [PubMed] [Google Scholar]
- 7. Kanis JA, Powles T, Paterson AH, McCloskey EV, Ashley S. Clodronate decreases the frequency of skeletal metastases in women with breast cancer. Bone 1996; 19: 663–667. [DOI] [PubMed] [Google Scholar]
- 8. Early Breast Cancer Triallists’ Collaborative Group (EBCTCG) . Adjuvant bisphosphonate treatment in early breast cancer: meta‐analyses of individual patient data from randomised trials. Lancet 2015; 386: 1353–1361. [DOI] [PubMed] [Google Scholar]
- 9. Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 2008; 19: 733–759. [DOI] [PubMed] [Google Scholar]
- 10. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008; 83: 1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48: 677–692. [DOI] [PubMed] [Google Scholar]
- 12. Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 2005; 44: 551–570. [DOI] [PubMed] [Google Scholar]
- 13. Dunn CJ, Goa KL. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs 2001; 61: 685–712. [DOI] [PubMed] [Google Scholar]
- 14. Porras AG, Holland SD, Gertz BJ. Pharmacokinetics of alendronate. Clin Pharmacokinet 1999; 36: 315–328. [DOI] [PubMed] [Google Scholar]
- 15. Drake MT, Cremers SC. Bisphosphonate therapeutics in bone disease: the hard and soft data on osteoclast inhibition. Mol Interv 2010; 10: 141–152. [DOI] [PubMed] [Google Scholar]
- 16. Sutjandra L, Rodriguez RD, Doshi S, Ma M, Peterson MC, Jang GR, et al Population pharmacokinetic meta‐analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin Pharmacokinet 2011; 50: 793–807. [DOI] [PubMed] [Google Scholar]
- 17. Block GA, Bone HG, Fang L, Lee E, Padhi D. A single‐dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012; 27: 1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adler RA, El‐Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al Managing osteoporosis in patients on long‐term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31: 16–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis – where do we go from here? N Engl J Med 2012; 366: 2048–2051. [DOI] [PubMed] [Google Scholar]
- 20. Modi A, Sajjan S, Insinga R, Weaver J, Lewiecki EM, Harris ST. Frequency of discontinuation of injectable osteoporosis therapies in US patients over 2 years. Osteoporos Int 2017; 28: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 21. Black DM, Reid IR, Boonen S, Bucci‐Rechtweg C, Cauley JA, Cosman F, et al The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON‐Pivotal Fracture Trial (PFT). J Bone Miner Res 2012; 27: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long‐term Extension (FLEX): a randomized trial. JAMA 2006; 296: 2927–2938. [DOI] [PubMed] [Google Scholar]
- 23. Popp AW, Varathan N, Buffat H, Senn C, Perrelet R, Lippuner K. Bone mineral density changes after 1 year of denosumab discontinuation in postmenopausal women with long‐term denosumab treatment for osteoporosis. Calcif Tissue Int 2018; 103: 50–54. [DOI] [PubMed] [Google Scholar]
- 24. Zanchetta MB, Boailchuk J, Massari F, Silveira F, Bogado C, Zanchetta JR. Significant bone loss after stopping long‐term denosumab treatment: a post FREEDOM study. Osteoporos Int 2018; 29: 41–47. [DOI] [PubMed] [Google Scholar]
- 25. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011; 96: 972–980. [DOI] [PubMed] [Google Scholar]
- 26. Anastasilakis AD, Polyzos SA, Makras P, Aubry‐Rozier B, Kaouri S, Lamy O. Clinical features of 24 patients with rebound‐associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res 2017; 32: 1291–1296. [DOI] [PubMed] [Google Scholar]
- 27. Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JEB, McClung M, et al Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo‐controlled FREEDOM trial and its extension. J Bone Miner Res 2018; 33: 190–198. [DOI] [PubMed] [Google Scholar]
- 28. Freemantle N, Satram‐Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, et al Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24‐month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reid IR, Horne AM, Mihov B, Gamble GD. Bone loss after denosumab: only partial protection with zoledronate. Calcif Tissue Int 2017; 101: 371–374. [DOI] [PubMed] [Google Scholar]
- 30. Lomax AJ, Yee Yap S, White K, Beith J, Abdi E, Broad A, et al Prevention of aromatase inhibitor‐induced bone loss with alendronate in postmenopausal women: the BATMAN trial. J Bone Oncol 2013; 2: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lester JE, Dodwell D, Purohit OP, Gutcher SA, Ellis SP, Thorpe R, et al Prevention of anastrozole‐induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res 2008; 14: 6336–6342. [DOI] [PubMed] [Google Scholar]
- 32. Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, et al Prevention of aromatase inhibitor‐induced bone loss using risedronate: the SABRE trial. J Clin Oncol 2010; 28: 967–975. [DOI] [PubMed] [Google Scholar]
- 33. Llombart A, Frassoldati A, Paija O, Sleeboom HP, Jerusalem G, Mebis J, et al Immediate administration of zoledronic acid reduces aromatase inhibitor‐associated bone loss in postmenopausal women with early breast cancer: 12‐month analysis of the E‐ZO‐FAST trial. Clin Breast Cancer 2012; 12: 40–48. [DOI] [PubMed] [Google Scholar]
- 34. Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, et al Final 5‐year results of Z‐FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer 2012; 118: 1192–1201. [DOI] [PubMed] [Google Scholar]
- 35. Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, et al Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO‐FAST study): final 60‐month results. Ann Oncol 2013; 24: 398–405. [DOI] [PubMed] [Google Scholar]
- 36. Valachis A, Polyzos NP, Georgoulias V, Mavroudis D, Mauri D. Lack of evidence for fracture prevention in early breast cancer bisphosphonate trials: a meta‐analysis. Gynecol Oncol 2010; 117: 139–145. [DOI] [PubMed] [Google Scholar]
- 37. Coleman R, Cameron D, Dodwell D, Bell R, Wilson C, Rathbone E, et al Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open‐label phase 3 trial. Lancet Oncol 2014; 15: 997–1006. [DOI] [PubMed] [Google Scholar]
- 38. Valachis A, Polyzos NP, Coleman RE, Gnant M, Eidtmann H, Brufsky AM, et al Adjuvant therapy with zoledronic acid in patients with breast cancer: a systematic review and meta‐analysis. Oncologist 2013; 18: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Carrigan B, Wong MH, Willson ML, Stockler MR, Pavlakis N, Goodwin A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev 2017; 10: CD003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dhesy‐Thind S, Fletcher GG, Blanchette PS, Clemons MJ, Dillmon MS, Frank ES, et al Use of adjuvant bisphosphonates and other bone‐modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017; 35: 2062–2081. [DOI] [PubMed] [Google Scholar]
- 41. Daubiné F, Le Gall C, Gasser J, Green J, Clézardin P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst 2007; 99: 322–330. [DOI] [PubMed] [Google Scholar]
- 42. Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, et al Bisphosphonates inhibit angiogenesis in vitro and testosterone‐stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 2002; 62: 6538–6544. [PubMed] [Google Scholar]
- 43. Van der Pluijm G, Vloedgraven H, Van Beek E, Van der Wee‐Pals L, Löwik C, Papapoulos S. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro . J Clin Invest 1996; 98: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lipton A, Chapman JA, Demers L, Shepherd LE, Han L, Wilson CF, et al Elevated bone turnover predicts for bone metastasis in postmenopausal breast cancer: results of NCIC CTG MA.14. J Clin Oncol 2011; 29: 3605–3610. [DOI] [PubMed] [Google Scholar]
- 45. Coleman RE, Finkelstein D, Barrios CH, Martin M, Iwata H, Glaspy JA, et al Adjuvant denosumab in early breast cancer: first results from the international multicenter randomized phase III placebo controlled D‐CARE study. J Clin Oncol 2018; 36: 501. [Google Scholar]
- 46. De Groot AF, Appelman‐Dijkstra NM, Van der Burg SH, Kroep JR. The anti‐tumor effect of RANKL inhibition in malignant solid tumors – a systematic review. Cancer Treat Rev 2018; 62: 18–28. [DOI] [PubMed] [Google Scholar]
- 47. Wright LE, Guise TA. The microenvironment matters: estrogen deficiency fuels cancer bone metastases. Clin Cancer Res 2014; 20: 2817–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI, et al Zoledronic acid has differential antitumor activity in the pre‐ and postmenopausal bone microenvironment in vivo . Clin Cancer Res 2014; 20: 2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]


