Abstract
Background
The impact of antibiotic timing on sepsis outcomes remains controversial due to conflicting results from previous studies.
Objectives
This study investigated the association of door-to-antibiotic time with long-term mortality in ED patients with sepsis.
Methods
This retrospective cohort study included nontrauma adult ED patients with clinical sepsis admitted to four hospitals from 2013 to 2017. Only patients’ first eligible encounter was included. Multivariable logistic regression was used to measure the adjusted association between door-to-antibiotic time and 1-year mortality. Secondary analyses used alternative antibiotic timing measures (antibiotic initiation within 1 or 3 h and separate comparison of antibiotic exposure at each hour up to hour 6), alternative outcomes (hospital, 30-day, and 90-day mortality), and alternative statistical methods to mitigate indication bias.
Results
Among 10,811 eligible patients, median door-to-antibiotic time was 166 min (interquartile range, 115-230 min), and 1-year mortality was 19%. After adjustment, each additional hour from ED arrival to antibiotic initiation was associated with a 10% (95% CI, 5-14; P < .001) increased odds of 1-year mortality. The association remained linear when each 1-h interval of door-to-antibiotic time was independently compared with door-to-antibiotic time ≤ 1 h and was similar for hospital, 30-day, and 90-day mortality. Mortality at 1 year was higher when door-to-antibiotic times were > 3 h vs ≤ 3 h (adjusted OR, 1.27; 95% CI, 1.13-1.43) but not > 1 h vs ≤ 1 h (adjusted OR, 1.26; 95% CI, 0.98-1.62).
Conclusions
Delays in ED antibiotic initiation time are associated with clinically important increases in long-term, risk-adjusted sepsis mortality.
Key Words: antibiotic therapy, emergency medicine, epidemiology, mortality, sepsis
Abbreviations: aOR, adjusted OR; GCS, Glasgow Coma Scale; MEDS, Mortality in Emergency Department Sepsis
Sepsis is a common, costly, and lethal syndrome affecting nearly 1% of adults (at least 850,000 patients) treated in US EDs each year.1 Given the failure thus far of targeted treatments,2, 3 sepsis therapy currently focuses on controlling the underlying infection and individualized hemodynamic resuscitation and organ support.4
International guidelines now recommend that patients with sepsis receive antibiotics within 1 h of ED arrival,5 but the prioritization of early antibiotic administration in sepsis remains controversial.6, 7 Although some studies reported worse outcomes for each hour antibiotic administration is delayed,8, 9, 10, 11, 12 other studies and a widely cited meta-analysis did not find significant hour-upon-hour increases in sepsis mortality.13, 14, 15 Most studies, moreover, have focused on hospital mortality and used analytic methods that may have masked nonlinear associations between mortality and antibiotic initiation time. Because expediting antibiotic initiation has risks that include diagnostic and treatment delays for patients misdiagnosed with sepsis and complications due to unnecessary antibiotic therapy with potential long-term consequences, a better understanding of the strength and shape of the relationship between antibiotic timing and outcomes is important for clinicians and policy makers.
To address this issue, we applied robust risk-adjustment methods to a large, multicenter sepsis cohort to evaluate the association between both long-term and shorter term sepsis mortality and hourly delays in antibiotic initiation.
Patients and Methods
Study Design and Setting
This retrospective cohort study included adult patients presenting to the EDs of two community hospitals and two tertiary-care hospitals within an integrated hospital network in Utah. We included network hospitals for which detailed ED medication administration data were available. ED sepsis care was not protocolized, but adherence to a sepsis treatment bundle was monitored for patients admitted to ICUs. The Intermountain Healthcare Institutional Review Board approved the study and waived requirements for informed consent (IRB #1050172).
Subjects
Adult patients (age ≥ 18 years) admitted to the hospital after presenting to a study ED from July 2013 to January 2017 were eligible for study inclusion if they exhibited clinical sepsis while in the ED. Criteria for clinical sepsis were based on the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) standard.16 Organ failure was defined as a Sequential Organ Failure Assessment score at least two points above the patient’s baseline using only data prior to hospital admission.17 Diagnosed or suspected infection while in the ED was identified by using the combination of blood culture collection and IV antimicrobial (or oral vancomycin or oseltamivir) administration prior to hospital admission. Only patients’ first eligible encounter was included in the analysis. Trauma patients and patients who died in the ED were excluded.
Data Abstraction
Data on clinical and demographic characteristics, interventions (including antibiotic initiation), ED disposition, and hospital outcomes were obtained from the Electronic Data Warehouse at Intermountain Healthcare.18 Trained abstractors reviewed the medical record using standardized methods and data collection instruments to verify and, as needed, correct outlying and missing data. Details regarding data abstraction and validation are described in e-Appendix 1. Data linkage to Utah State death records and the US National Death Index provided long-term mortality data.
Exposure and Outcome Measures
The study’s prespecified primary outcome was 1-year morality. Secondary outcomes were hospital, 30-day, and 90-day mortality. The primary exposure was the time in hours from ED arrival to first antibiotic initiation (“door-to-antibiotic time”).5 Secondary analyses compared mortality for door-to-antibiotic times as follows: (1) ≤ 1 h vs each 1-h door-to-antibiotic interval beyond the first hour (eg, antibiotics started between 3 and 4 h compared with ≤ 1 h); (2) > 1 h vs ≤ 1 h; or (3) > 3 h vs ≤ 3 h from ED arrival.
Reported vital signs represent first-recorded values following ED arrival and prior to hospital admission. We measured comorbidities using a weighted Elixhauser score derived by Quan et al19 and von Walraven et al20 and mortality risk using the Mortality in Emergency Department Sepsis (MEDS) score.21, 22 The Glasgow Coma Scale (GCS) was dichotomized as abnormal (≤ 13) vs normal (≥ 14) mentation. Additional details on covariate measurement are included in e-Appendix 1.
Statistical Analysis
Bivariable comparisons used χ 2 tests, unpaired Student t tests with unequal variance, or Mann-Whitney U tests. The primary analysis used robust multivariable logistic regression to test the association between door-to-antibiotic time and 1-year mortality following adjustment for a prespecified list of known and potential confounders.23, 24, 25 Results are reported as adjusted OR (aOR) for mortality and, based on the average marginal effects method,26, 27 the adjusted change in expected mortality. Adjustment variables selected a priori included: triage acuity score; receipt of prehospital medical care (ie, arrival to hospital via ambulance); MEDS score; ED Sequential Organ Failure Assessment score; initial vital signs (systolic blood pressure, GCS score ≤ 13, heart rate, temperature, respiratory rate, and oxygen saturation); ED disposition (ICU vs ward); comorbidity score; marital status; insurance type; age; sex; Hispanic ethnicity or non-white race; hospital; preferred language other than English; initial WBC count; and a dichotomous variable indicating that the initial lactate level was both measured in the ED and > 2 mmol/L. This adjustment variable set exhibited good discrimination for 1-year mortality (C statistic, 0.81). No data were missing for these variables. Similar multivariable models were constructed for each secondary outcome and secondary exposure.
To evaluate the robustness of the study findings, several sensitivity analyses were performed. First, the primary adjusted association was retested in the subset of patients who: (1) received antibiotics within 6 h from ED arrival; or (2) had hospital discharge diagnoses consistent with sepsis per the modified Angus criteria.28, 29 The latter analysis was restricted to patients who presented to the ED prior to October 1, 2015, because the International Classification of Diseases, Ninth Revision, Clinical Modification discharge diagnosis codes required for the Angus method for sepsis diagnosis identification were no longer in use following this date. Second, we repeated the primary analysis with a more limited but still prespecified set of adjustment variables. Finally, we used the following: (1) inverse probability of treatment weighting30, 31 or (2) matching based on gain-weighted Gower’s distance32, 33 as alternative approaches to address indication bias when analyzing the association of door-to-antibiotic time ≥ 3 h and 1-year mortality. These analyses are described in detail in e-Appendix 1 and e-Figures 1 and 2.
To evaluate whether the association between mortality and door-to-antibiotic time varied as a function of patient factors or illness severity, we performed a preplanned exploratory analysis by adding to the multivariable logistic regression model an interaction term between the characteristics of interest (patient sex, hypotension, altered mental status [GCS score ≤ 13], MEDS score, triage acuity score, and lactate level > 2 mmol/L) and door-to-antibiotic time. For this analysis, triage acuity scores were dichotomized as high (scores of 3-5) or low (scores of 1-2), and the MEDS score was categorized (per the original published description) as high (score ≥ 8), moderate (score, 5-7), or low (score, ≤ 4). Finally, for a post hoc investigation designed to assess whether the association of antibiotic delays with 1-year mortality was mediated by ongoing excess mortality risk or durable differences in early mortality, we repeated the primary analysis after excluding patients who died within 7 days of ED arrival, died within 30 days, or died in the hospital.
Analyses were performed in R version 3.5.1 (R Foundation) and Stata version 14.2 (StataCorp LP). A P value < .05 using two-tailed tests was considered significant.
Results
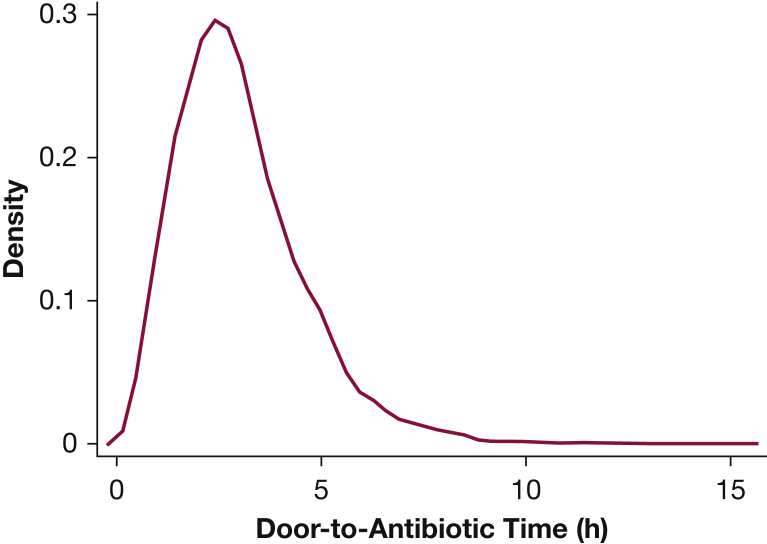
Among 10,811 adult ED patients with clinical sepsis included in this analysis (e-Fig 3), 887 (8%) died within 30 days, and 2,083 (19%) died within 1 year. Median door-to-antibiotic time was 166 min (interquartile range, 115-230 min) (Fig 1). Patients receiving antibiotics within 3 h of ED arrival were younger, less likely to be female, had more comorbidities, exhibited more organ failure, and had more physiologic derangement (Table 1). Consistent with more severely ill patients receiving antibiotics earlier, crude 30-day mortality was higher in patients whose door-to-antibiotic time was ≤ 3 h (9%) compared with those receiving antibiotics > 3 h from ED arrival (7%; P < .001). At 1 year, the difference in crude mortality between patients with door-to-antibiotic time ≤ 3 h vs > 3 h (20% vs 19%, respectively) was not statistically significant (P = .089).
Figure 1.
Density plot of observed door-to-antibiotic times.
Table 1.
Characteristics of ED Patients With Sepsis According to Timing of Antibiotic Initiation
| Characteristic | Antibiotic Initiation ≤ 3 h (n = 6,158) |
Antibiotic Initiation > 3 h (n = 4,653) | P Value |
|---|---|---|---|
| Age, y | 60.9 ± 19.3 | 62.1 ± 18.6 | .001 |
| Hispanic or non-white race | 985 (16.0%) | 756 (16.2%) | .72 |
| Female | 3,197 (51.9%) | 2,775 (59.6%) | < .001 |
| Preferred language other than English | 309 (5.0%) | 277 (6.0%) | .033 |
| Married | 3,155 (51.2%) | 2,368 (50.9%) | .72 |
| Primary insurance | .074 | ||
| Uninsured | 594 (9.6%) | 443 (9.5%) | |
| Private | 1,655 (26.9%) | 1,356 (29.1%) | |
| Medicaid | 587 (9.5%) | 435 (9.4%) | |
| Medicare | 3,322 (54.0%) | 2,419 (52.0%) | |
| Received prehospital medical care | 2,150 (34.9%) | 1,058 (22.7%) | < .001 |
| MEDS score | 5.1 ± 3.4 | 3.9 ± 3.1 | < .001 |
| SOFA score | 4.8 ± 2.9 | 4.0 ± 2.1 | < .001 |
| Weighted Elixhauser score | 6.7 ± 11.9 | 3.7 ± 9.6 | < .001 |
| Canadian triage acuity score | 2.4 ± 0.6 | 2.6 ± 0.5 | < .001 |
| Initial ED vital signs | |||
| Heart rate, beats/min | 105 ± 23 | 99 ± 21 | < .001 |
| Systolic blood pressure, mm Hg | 129 ± 28 | 131 ± 26 | .004 |
| Respiratory rate, breaths/min | 21.5 ± 6.2 | 19.7 ± 4.6 | < .001 |
| Glasgow Coma Scale score ≤ 13 | 431 (7.0%) | 130 (2.8%) | < .001 |
| Initial ED laboratory results | |||
| Lactate >2 mmol/L | 2,359 (41.2%) | 1,321 (28.4%) | < .001 |
| WBC count (1,000/dL) | 13.5 ± 14.5 | 12.7 ± 10.5 | < .001 |
| Vasopressor within 24 h | 639 (10.4%) | 153 (3.3%) | < .001 |
| Admit to ICU | 2,174 (35.3%) | 908 (19.5%) | < .001 |
| 30-Day mortality | 565 (9.2%) | 322 (6.9%) | < .001 |
| 90-Day mortality | 769 (12.5%) | 506 (10.9%) | .010 |
| 1-Year mortality | 1,221 (19.8%) | 862 (18.5%) | .089 |
Data are presented as mean ± SD or No. (%). MEDS = Mortality in Emergency Department Sepsis; SOFA = Sequential Organ Failure Assessment.
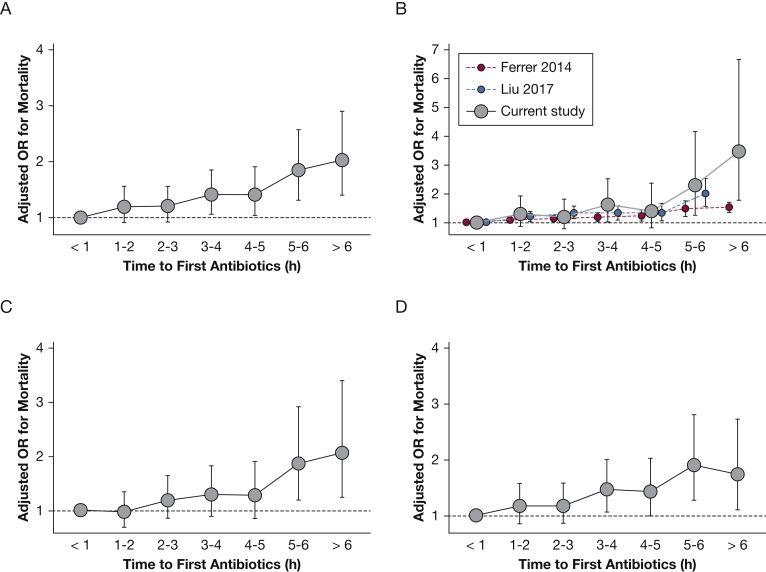
After adjustment, however, each 1-h increase in door-to-antibiotic time was associated with a 10% (95% CI, 5-14) increase in the odds of 1-year mortality (P < .001). This finding translated to a 1.1% (95% CI, 0.7-1.6) increase in expected mortality for each additional hour of door-to-antibiotic time (e-Table 1). A similar association was observed for hospital, 30-day, and 90-day mortality (Table 2). Delayed antibiotic initiation was also associated with increased 1-year mortality when comparing antibiotic initiation > 3 h vs ≤ 3 h (aOR, 1.27; 95% CI, 1.13-1.43; P < .001). However, the association of 1-year mortality with door-to-antibiotic time > 1 h vs ≤ 1 h (aOR, 1.26; 95% CI, 0.98-1.62) was not statistically significant (P = .070). When considering each extra hour of door-to-antibiotic time independently (relative to antibiotic initiation ≤ 1 h), the risk increase appeared linear for 1-year mortality and fairly linear for 30- or 90-day mortality (Fig 2).34 Similar to previous risk-adjusted reports,9, 10 the association with door-to-antibiotic time was less clearly linear for hospital mortality. Sensitivity analyses yielded similar results when restricted to patients with an ultimate International Classification of Diseases, Ninth Revision, Clinical Modification hospital discharge diagnosis consistent with sepsis, patients receiving antibiotics ≤ 6 h from ED arrival, and using an alternative set of adjustment variables (e-Table 2).
Table 2.
Adjusted Association of Door-to-Antibiotic Time and Mortality in ED Patients With Sepsis
| Variable | 1-Year Mortality |
In-Hospital Mortality |
30-Day Mortality |
90-Day Mortality |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted ORa (95% CI) | P Value | Adjusted ORa (95% CI) | P Value | Adjusted ORa (95% CI) | P Value | Adjusted ORa (95% CI) | P Value | |||||
| Door-to-antibiotic time, h | 1.10 | (1.05-1.14) | < .001 | 1.16 | (1.07-1.26) | < .001 | 1.12 | (1.06-1.18) | < .001 | 1.09 | (1.04-1.15) | < .001 |
| Door-to-antibiotic time > 1 h | 1.26 | (0.98-1.62) | .070 | 1.32 | (0.91-1.92) | .14 | 1.12 | (0.83-1.52) | .46 | 1.24 | (0.94-1.65) | .13 |
| Door-to-antibiotic time > 3 h | 1.27 | (1.13-1.43) | < .001 | 1.42 | (1.13-1.80) | .003 | 1.28 | (1.08-1.52) | .005 | 1.32 | (1.14-1.52) | < .001 |
| Door-to-antibiotic time interval | ||||||||||||
| ≤ 1 h | Reference | Reference | Reference | Reference | ||||||||
| > 1 to ≤ 2 h | 1.19 | (0.91-1.56) | .20 | 1.29 | (0.87-1.93) | .21 | 0.97 | (0.70-1.35) | .85 | 1.17 | (0.86-1.58) | .31 |
| > 2 to ≤ 3 h | 1.20 | (0.92-1.56) | .18 | 1.20 | (0.79-1.82) | .39 | 1.19 | (0.86-1.65) | .30 | 1.17 | (0.87-1.59) | .30 |
| > 3 to ≤ 4 h | 1.40 | (1.06-1.85) | .018 | 1.61 | (1.03-2.53) | .036 | 1.29 | (0.90-1.83) | .16 | 1.47 | (1.07-2.01) | .019 |
| > 4 to ≤ 5 h | 1.41 | (1.04-1.91) | .025 | 1.39 | (0.82-2.37) | .22 | 1.28 | (0.86-1.91) | .23 | 1.43 | (1.00-2.03) | .049 |
| > 5 to ≤ 6 h | 1.84 | (1.31-2.57) | < .001 | 2.28 | (1.26-4.16) | .007 | 1.87 | (1.20-2.92) | .006 | 1.90 | (1.28-2.81) | .001 |
| > 6 h | 2.02 | (1.40-2.90) | < .001 | 3.45 | (1.78-6.67) | < .001 | 2.06 | (1.25-3.40) | .004 | 1.74 | (1.11-2.73) | .015 |
Adjusted for pooled triage acuity score; receipt of prehospital medical care; MEDS score; SOFA score; initial vital signs (systolic blood pressure, abnormal Glasgow Coma Scale, heart rate, temperature, respiratory rate, and oxygen saturation); ED disposition (ICU vs ward); comorbidity score; marital status; insurance type; age; sex; Hispanic ethnicity or non-white race; hospital; non-English preferred language; initial WBC count; and initial lactate level tested and > 2 mmol/L. See Table 1 legend for expansion of abbreviations.
Figure 2.
Adjusted association of mortality with door-to-antibiotic time, comparing each hourly interval following the first hour to door-to-antibiotic time ≤ 1 h for (A) 1-year mortality, (B) hospital mortality, (C) 30-day mortality, and (D) 90-day mortality. For hospital mortality, results from the current analysis are compared with risk-adjusted associations with hospital mortality reported by Ferrer et al9 and Liu et al.10 Figure adapted with permission of the American Thoracic Society from Liu et al10 and with permission from Elsevier from Peltan and Liu.34 The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Both inverse probability of treatment weighting using patients’ propensity for receipt of antibiotics within 3 h of ED arrival (N = 10,811) and matching based on the gain-weighted Gower’s distance (2,255 matched pairs including 3,760 unique patients) yielded well-balanced groups (e-Fig 1). The sensitivity analyses both identified increased risks of 1-year mortality associated with door-to-antibiotic time > 3 h that were similar to the results derived from primary analysis using multivariable logistic regression (e-Table 3).
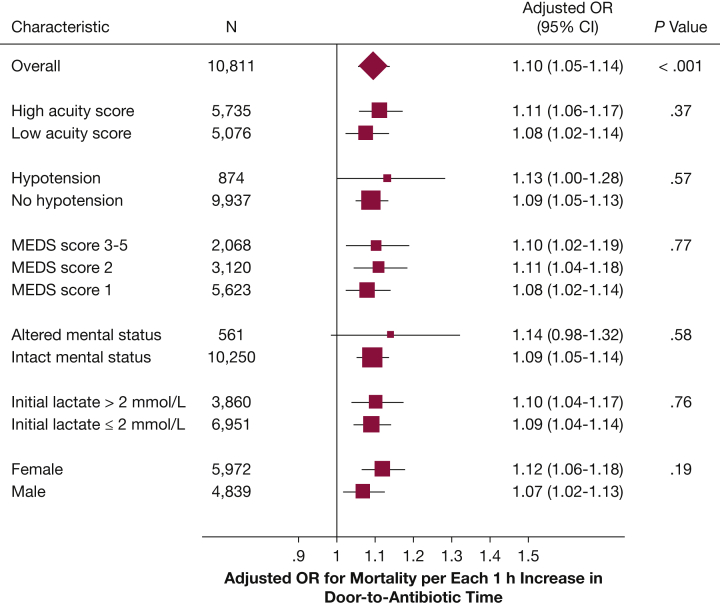
We observed no statistical evidence that the relative risk of 1-year mortality associated with door-to-antibiotic time varied according to illness severity or patient sex (Fig 3). Hourly increases in door-to-antibiotic time remained associated with risk-adjusted 1-year mortality in exploratory analyses that excluded patients who died within 7 days of ED arrival (aOR, 1.08; 95% CI, 1.04-1.12; P < .001), died within 30 days (aOR, 1.08; 95% CI, 1.03-1.12; P = .002), or died in the hospital (aOR, 1.09; 95% CI, 1.04-1.12; P < .001).22
Figure 3.
Variation in the adjusted association of door-to-antibiotic time and 1-year mortality according to patient and clinical factors. MEDS = Mortality in Emergency Department Sepsis.
Discussion
In this large, multicenter sepsis cohort, each 1-h increase in door-to-antibiotic time was associated with a 10% increase in the adjusted odds of death by 1 year. This finding translated into a 1.1% per hour increase in risk-adjusted absolute mortality and suggests that decreasing average door-to-antibiotic time to 1.5 h could prevent one death per 61 ED patients with sepsis, or more than four deaths per month just in the EDs included in this study. The association remained generally linear even when the analysis was structured to allow for a nonlinear relationship between antibiotic time and 1-year mortality. Door-to-antibiotic time cutoffs at 3 h were similarly associated with 1-year mortality, but the association between 1-year mortality and door-to-antibiotic time > 1 h did not reach statistical significance. In exploratory analyses, increased mortality risk persisted among initial survivors.
To our knowledge, this study is the first to examine the potential impact of antibiotic timing on mortality beyond 1 month in adults with sepsis, the largest study (by a factor of 10) to examine mortality beyond hospital discharge, and the first to find a significant association of antibiotic timing and long-term mortality in patients with general sepsis (e-Table 4).15, 35, 36, 37, 38 Confirmatory findings using different analytic strategies, related but not identical outcomes and measures of exposure, and a dose-response effect indicate that our findings are robust. Beyond buttressing findings from studies using early mortality end points, our results also address the controversy over whether sepsis outcomes only begin to worsen after door-to-antibiotic times exceed a specified threshold. We found that the association between increasing antibiotic delay and 1-year mortality was linear, suggesting that sepsis outcomes may improve continuously with earlier initiation of appropriate antibiotics rather than remaining equivalent as long as antibiotics are initiated within some early time window.
We observed that antibiotic delay was associated with increased risk of 1-year mortality even among patients who survived at least 7 days, 30 days, or through hospital discharge. This finding suggests that the association between long-term mortality and antibiotic timing does not depend solely on early but durable mortality differences. Potential mechanisms (which we were not able to investigate in the present study) by which antibiotic delay might result in excess mortality risk persisting long after the initial sepsis presentation include more severe or enduring sepsis-associated organ failure, increased persistent inflammation, more recurrent infection, or worse deconditioning.39 Importantly, these findings from exploratory analyses must be viewed as hypothesis generating and require both confirmation and mechanistic investigation in future studies.
Our study also could not address two reasons that the emphasis on early antibiotic initiation in sepsis remains controversial. First, quantitative data are lacking on whether and how much efforts to accelerate antibiotic initiation increase the frequency of inadequate antibiotics or antibiotic overtreatment (both excessively broad-spectrum and entirely unnecessary antibiotics) and the consequences of such mistreatment. As such, clinicians and policy makers cannot currently make fully informed decisions about the risk/benefit ratio of such efforts.40
Second, our findings build upon observational data regarding antibiotic timing in sepsis that meet multiple criteria for causal inference, including biologic plausibility, supportive evidence from preclinical studies, variation in the magnitude of effect consistent with theoretical predictions, and robust, replicable results across populations, variant exposures, and related outcomes.41, 42 However, our study is not a randomized trial and is not free from confounding by indication. In unadjusted analyses, such confounding will tend to abrogate or, as seen here and elsewhere,10, 13 produce apparently reversed association in crude analyses. Despite adjusting for a broad array of known and plausible confounders in the primary analysis and confirming results using alternative statistical methods, we cannot exclude the possibility of residual confounding in this observational study. Innovative trials are needed to confirm our findings, including adequately powered trials randomizing patients to receiving prehospital antibiotics or randomizing EDs to interventions designed to accelerate sepsis care.
Our study has several additional limitations. Although use of first antibiotic initiation is common in other studies in sepsis, using this marker as a surrogate for appropriate antibiotic initiation may have led us to underestimate the magnitude of the association between mortality and antibiotic delays. Mortality in the study cohort was comparable to a contemporary study enrolling similar patients with community-acquired clinical sepsis43 but was lower than in some historic and mostly higher risk cohorts.44 Inclusion of patients with clinical rather than confirmed sepsis could also lead to underestimated effect sizes, a concern potentially supported by the increased magnitude of the association when we restricted the analysis to patients with both clinical sepsis in the ED and a hospital discharge diagnosis consistent with sepsis. We were not able to assess cause of death in these patients, nor evaluate potential mechanisms (eg, increased sepsis relapse rates, persistent organ failure, or more frequent readmissions) of the observed association between late mortality and door-to-antibiotic time.
Conclusions
Increasing door-to-antibiotic time for ED patients with clinical sepsis was associated with a linear, hour-by-hour increase in 1-year mortality and possibly with persistent increases in mortality among survivors of the initial illness. Innovative trial designs are needed to test methods to accelerate appropriate antibiotic initiation and determine whether these interventions improve patient-centered outcomes.
Acknowledgments
Author contributions: I. D. P. is the guarantor of the paper and takes responsibility for the integrity of the work as a whole. I. D. P., S. M. B., J. R. B., T. L. L., and C. L. H. conceived the study. I. D. P. acquired the data and authored the manuscript. All authors participated in study design, data analysis, and interpretation of the results. All authors also contributed substantially to manuscript revision.
Financial/nonfinancial disclosures: None declared.
Role of the sponsors: The funding sources had no role in the study’s design, conduct, analysis, or reporting.
Other contributions: Thomas Oniki, PhD, Al Jephson, BA, Douglas Wirthlin, BA, Sierra McLean, BA, Emily Murnin, BA, Naresh Kumar, MPH, and Carolyn Klippel, BA, assisted with data abstraction and cleaning.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported by a grant from the Intermountain Research and Medical Foundation and by the University of Utah Study Design and Biostatistics Center, which is funded in part by the National Institutes of Health (UL1TR001067).
Supplementary Data
References
- 1.Wang H.E., Jones A.R., Donnelly J.P. Revised national estimates of emergency department visits for sepsis in the United States. Crit Care Med. 2017;45(9):1443–1449. doi: 10.1097/CCM.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent J.L. Individual gene expression and personalised medicine in sepsis. Lancet Respir Med. 2016;4(4):242–243. doi: 10.1016/S2213-2600(16)00068-0. [DOI] [PubMed] [Google Scholar]
- 3.Angus D.C., Barnato A.E., Bell D. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015;41(9):1549–1560. doi: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes A., Evans L.E., Alhazzani W. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 5.Levy M.M., Evans L.E., Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44(6):925–928. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 6.Singer M. Antibiotics for sepsis: does each hour really count, or is it incestuous amplification? Am J Respir Crit Care Med. 2017;196(7):800–802. doi: 10.1164/rccm.201703-0621ED. [DOI] [PubMed] [Google Scholar]
- 7.Klompas M., Calandra T., Singer M. Antibiotics for sepsis—finding the equilibrium. JAMA. 2018;320(14):1433–1434. doi: 10.1001/jama.2018.12179. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A., Roberts D., Wood K.E. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 9.Ferrer R., Martín-Loeches I., Phillips G. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 10.Liu V.X., Fielding-Singh V., Greene J.D. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seymour C.W., Kahn J.M., Martin-Gill C. Delays from first medical contact to antibiotic administration for sepsis. Crit Care Med. 2017;45(5):759–765. doi: 10.1097/CCM.0000000000002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seymour C.W. New York State Office of Quality and Patient Safety, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. New Engl J Med. 2017;376(23):2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterling S.A., Miller W.R., Pryor J. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care Med. 2015;43(9):1907–1915. doi: 10.1097/CCM.0000000000001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puskarich M.A., Trzeciak S., Shapiro N.I. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39(9):2066–2071. doi: 10.1097/CCM.0b013e31821e87ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloos F., Thomas-Rüddel D., Rüddel H. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care. 2014;18(2):R42. doi: 10.1186/cc13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer M., Deutschman C.S., Seymour C.W. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Clayton P.D., Narus S.P., Huff S.M. Building a comprehensive clinical information system from components: the approach at Intermountain Health Care. Methods Inf Med. 2003;42(1):1–7. [PubMed] [Google Scholar]
- 19.Quan H., Sundararajan V., Halfon P. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 20.van Walraven C., Austin P.C., Jennings A. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro N.I., Wolfe R.E., Moore R.B. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31(3):670–675. doi: 10.1097/01.CCM.0000054867.01688.D1. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro N.I., Howell M.D., Talmor D. Mortality in Emergency Department Sepsis (MEDS) score predicts 1-year mortality. Crit Care Med. 2007;35(1):192–198. doi: 10.1097/01.CCM.0000251508.12555.3E. [DOI] [PubMed] [Google Scholar]
- 23.Austin P.C., Steyerberg E.W. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat Methods Med Res. 2017;26(2):796–808. doi: 10.1177/0962280214558972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peduzzi P., Concato J., Kemper E. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 25.Lederer D.J., Bell S.C., Branson R.D. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 26.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308–331. [Google Scholar]
- 27.Muller C.J., MacLehose R.F. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962–970. doi: 10.1093/ije/dyu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angus D.C., Linde-Zwirble W.T., Lidicker J. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Iwashyna T.J., Odden A., Rohde J. Identifying patients with severe sepsis using administrative claims: patient-level validation of the Angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52(6):e39–e43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robins J.M., Hernán M.Á., Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Gower J.C. A general coefficient of similarity and some of its properties. Biometrics. 1971;27(4):857–871. [Google Scholar]
- 33.Podani J. Extending Gower's general coefficient of similarity to ordinal characters. Taxon. 1999;48(2):331–340. [Google Scholar]
- 34.Peltan ID, Liu VX. Does the timing of antibiotic administration matter in sepsis? In: Deutschman CS, Neligan PJ, eds. Evidence-Based Practice of Critical Care, 3rd ed. Philadelphia, PA: Elsevier. In press.
- 35.Larché J., Azoulay É., Fieux F. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29(10):1688–1695. doi: 10.1007/s00134-003-1957-y. [DOI] [PubMed] [Google Scholar]
- 36.de Groot B., Ansems A., Gerling D.H. The association between time to antibiotics and relevant clinical outcomes in emergency department patients with various stages of sepsis: a prospective multi-center study. Crit Care. 2015;19(1):194. doi: 10.1186/s13054-015-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryoo S.M., Kim W.Y., Sohn C.H. Prognostic value of timing of antibiotic administration in patients with septic shock treated with early quantitative resuscitation. Am J Med Sci. 2015;349(4):328–333. doi: 10.1097/MAJ.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 38.Han M., Fitzgerald J.C., Balamuth F. Association of delayed antimicrobial therapy with one-year mortality in pediatric sepsis. Shock. 2017;48(1):29–35. doi: 10.1097/SHK.0000000000000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prescott H.C., Angus D.C. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu V.X., Fielding-Singh V., Iwashyna T.J. Reply: the timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):935–936. doi: 10.1164/rccm.201704-0774LE. [DOI] [PubMed] [Google Scholar]
- 41.Gershon A.S., Jafarzadeh S.R., Wilson K.C. Clinical knowledge from observational studies: everything you wanted to know but were afraid to ask. Am J Respir Crit Care Med. 2018 doi: 10.1164/rccm.201801-0118PP. [DOI] [PubMed] [Google Scholar]
- 42.Hill A.B. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 43.Donnelly J.P., Safford M.M., Shapiro N.I. Application of the Third International Consensus Definitions for Sepsis (Sepsis-3) classification: a retrospective population-based cohort study. Lancet Infect Dis. 2017;17(6):661–670. doi: 10.1016/S1473-3099(17)30117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winters B.D., Eberlein M., Leung J. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.