Abstract
Intranasal esketamine for treatment-resistant depression
Keywords: ketamine, esketamine, treatment-resistant depression, suicide, suicidal ideation
INTRODUCTION
Major depressive disorder (MDD) affects more than 16 million adults each year in the United States.1 The incomplete response that many persons experience with current antidepressant (AD) therapies is one factor contributing to the difficulty of successfully treating MDD.2 Approximately 30% of patients meet the criteria for treatment-resistant depression (TRD), having no positive therapeutic response even after trying two or more AD medications. 2 According to the World Health Organization (WHO), depression is the leading cause of disability worldwide.3 Untreated depression is a major risk factor for suicide: Each year, approximately 800,000 people–– one person every 40 seconds––successfully complete suicide.4,5 Many patients with MDD are at risk for poor outcomes because of the limitations of currently approved treatments.6 These incomplete or poor responses to traditional ADs have led to the off-label use of many novel substances for the treatment of MDD.
Ketamine is one agent that has received attention as a potential fast-acting and effective treatment for patients with MDD with suicidal ideation (MDSI) and for those with TRD. 7,8 Traditional ADs generally require at least four to six weeks to produce an antidepressant effect.9 In contrast, ketamine has been found to produce that effect within hours of administration, with one study finding a two-hour post-dose effect for intravenous (IV) esketamine in adult TRD.7,10
INDICATIONS
Intranasal (IN) esketamine is approved by the Food and Drug Administration (FDA) for TRD when used in conjunction with a traditional oral AD. 8,10,34,44 The racemic mixture of ketamine is currently being used off-label for the treatment of depressive disorders.
The FDA granted fast track and breakthrough therapy designations to Janssen Pharmaceuticals, Inc., a subsidiary of Johnson & Johnson, Inc., for IN esketamine to treat patients with MDD and TRD with an imminent risk of suicide. The agent has been available in the U.S. since March 5, 2019, with a risk evaluation and mitigation strategy (REMS), due to the potential for sedation and dissociation. There are boxed warnings for sedation, dissociation, abuse, and misuse, along with the default warnings for suicidal thoughts and behaviors in pediatric and young adult populations.11,34 Diagnostic criteria for MDD are defined in Table 1.12 Suicidal ideation may range from passive to active thoughts of suicide, with or without an actionable plan.12
Table 1.
DSM-5 Diagnostic Criteria for Major Depressive Disorder12
For a diagnosis of MDD, ≥ 5 of the following symptoms must occur nearly every day for ≥ 2 weeks:
|
DSM-5 = Diagnostic and Statistical Manual, 5th edition; MDD = major depressive disorder
PHARMACOLOGY
Mechanism of Action
Ketamine, a non-competitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, was introduced to the U.S. market in the early 1970s as a replacement anesthetic and analgesic agent for phencyclidine (PCP).13,14 Ketamine is generally administered intravenously because of its poor oral bioavailability.13,14 The S-ketamine enantiomer has a higher affinity for the NMDA receptor, and is thought to produce fewer significant psychotomimetic effects, drowsiness, lethargy, or cognitive impairment than the R-ketamine enantiomer.8 For these reasons, S-ketamine (or esketamine) was selected as the active agent for IN-esketamine’s formulation.8 Intranasal esketamine has multiple benefits compared with other routes of administration. It is less invasive and less painful than injection, and has greater bioavailability than oral administration.13–15
Although ketamine’s mechanism of action (MOA) as an anesthetic has been extensively studied, the MOA of esketamine’s antidepressant effect is poorly understood. Suggested mechanisms include improved brain plasticity via the stimulation of brain-derived neurotropic factor (BDNF) production and activation of the mammalian target of rapamycin (mTOR).16–19 Ketamine’s action on NMDA receptors is believed to produce effects that activate amino-hydroxy-methyl-isoxazolepropionic acid (AMPA) receptors, which may modulate signaling pathways downstream that appear to reduce phosphorylation of eukaryotic elongation factor 2 kinase, and result in increased BDNF production.17
Downstream modulation of mTOR is thought to stimulate additional BDNF production, resulting in increased brain plasticity through dendritic growth and improved synaptic transmission.18,19 Current evidence suggests that ketamine has a more direct stimulation effect on BDNF and mTOR than the present oral ADs.20 This may explain the rapid onset of esketamine’s effect, as well as the mechanism by which it continues to exhibit effects even after complete drug elimination.20
PHARMACOKINETICS21–22
The Ki value of S-ketamine for the NMDA (PCP) receptor is 0.3–0.69 μM.
Absorption and Distribution22,44
The mean bioavailability is approximately 48% following nasal spray administration. The expected time to reach peak plasma concentration is 20 to 40 minutes after the last spray. The mean volume of distribution at steady state via the IV route is 709 L. Protein binding is approximately 43% to 45%. The enantiomer S-ketamine has an affinity approximately fourfold higher for the NMDA receptor in vitro compared with R-ketamine.
Metabolism and Elimination23–25,44
Intranasal esketamine will avoid first-pass metabolism. Esketamine is primarily metabolized to noresketamine via the CYP450 enzymes of CYP2B6 and CYP3A4, with a lesser contribution from CYP2C9 and CYP2C19. Esketamine’s half-life is biphasic, with an initial rapid decline in concentration within two to four hours and a terminal half-life of seven to 12 hours. Noresketamine is further metabolized via CYP-dependent pathways with hydroxylation to hydroxynoresketamine. Subsequent metabolites will then undergo glucuronidation.
Though esketamine is a substrate of the above CYP-enzyme pathways, it does not appear to be an inhibitor or inducer of said pathways. Noresketamine demonstrated weak inhibition of CYP3A4. Patients with moderate hepatic impairment may require increased monitoring for adverse drug reactions for a longer period of time as a result of the increased plasma levels and half-life of esketamine in that population. There is no clinical experience in patients receiving renal dialysis or in those with severe hepatic impairment.
CLINICAL TRIALS
A search for “intranasal esketamine” in the ClinicalTrials.gov database yields a total of 32 registered clinical trials (27 completed and five recruiting).26 Of these, 29 studies have been funded by Janssen Research & Development, LLC.25 Limited data are available from third-party off-label studies. Most studies set improvement in depressive symptoms as measured by a change in baseline Montgomery–Åsberg Depression Rating Scale (MADRS) score as their primary outcome measure.27–30 (It should be noted that all studies included in this section have been funded by Janssen Research & Development, LLC.)
NCT0209437827
This phase 1 randomized, double-blind, placebo-controlled, 2-period crossover study evaluated the cognitive effects associated with IN-esketamine administration in 24 healthy adults (aged 19–49 years old) using a previously validated computerized battery assessment (Cogstate®). Subjects were randomized 1:1 to either 84 mg IN esketamine or IN placebo. After a seven-day washout period, subjects were crossed over to the opposite group and the cognitive battery assessment was repeated. At 40 minutes post-dose, there was a significant difference in cognition between the IN-placebo and IN-esketamine groups compared with baseline. At two hours post-dose, no significant difference in cognition was found between the groups, demonstrating that the decrease in cognition with IN esketamine was limited to two hours post-dose. The most commonly reported adverse events included dizziness (67%), nausea (37.5%), disturbance in attention (29.2%), and fatigue (29.2%).
SYNAPSE (NCT01998958)10
This phase 2 randomized, double-blind, multicenter, placebo-controlled study evaluated the efficacy and dose-response of IN esketamine and standard of care compared with IN placebo and standard of care for the reduction of MDD symptoms in patients with TRD (n = 67). The trial consisted of four parts: screening, double-blind treatment (two 1-week periods), optional open-label treatment, and post-treatment follow-up. For week 1 of the treatment phase, patients were randomized 3:1:1:1 into four study arms: placebo (n = 33), and esketamine 28 mg (n = 11), 56 mg (n = 11), or 84 mg (n = 12) administered twice weekly. During week 2 of treatment, subjects were re-randomized 1:1:1:1. During the double-blind phase, subjects’ depressive symptoms decreased at hour 2, hour 24, day 8, and day 15 from baseline when compared with placebo. The change (least squares mean [standard error (SE)] difference vs. placebo) in MADRS total score for both periods combined in all three esketamine groups was superior to the change in the placebo group (esketamine 28 mg: −4.2 [2.09], P = 0.02; esketamine 56 mg: −6.3 [2.07], P = 0.001; esketamine 84 mg: −9.0 [2.13], P < 0.001). A correlation between higher doses of IN esketamine and a decrease in depressive symptoms was demonstrated (P < 0.001). During the open-label phase, administration of IN esketamine or placebo took place twice weekly for the first two weeks, weekly for the following three weeks, then once every two weeks. Response rates were evaluated on day 74 and showed that 65% of participants achieved a 50% or greater decrease in MADRS score. Remission rates were also evaluated, showing that 32% of participants achieved a MADRS total score of 10 or less on day 74, which suggests no-to-mild depression. During the follow-up phase, response rates indicated that 56% of participants were still demonstrating a 50% or greater decrease in MADRS total score.
Adverse events were also evaluated as a secondary outcome. Dissociative symptoms peaked at ~30 to 40 minutes post-dose and generally resolved by two hours post-dose. An increase in blood pressure (BP) recorded at 10 to 40 minutes post-dose usually resolved by two hours post-dose (no increase was observed for heart rate). Other common symptoms reported during both the double-blind and open-label phases included dizziness, headache, dysgeusia, sedation, and nausea. Only dizziness and nausea were dose-dependent. During the course of the study, four subjects discontinued participation due to adverse events (one event each of syncope, headache, dissociative syndrome, and ectopic pregnancy).
NCT021330018
This phase 2 randomized, double-blind, multicenter, placebo-controlled study evaluated IN-esketamine’s effect on depressive symptoms and suicidal ideation in patients with MDD at imminent risk of suicide (n = 68). Subjects were randomized 1:1 to standard of care and either IN esketamine 84 mg or IN placebo, administered twice weekly through day 25, once weekly through day 52, and once every other week through day 81. Changes in MADRS score from baseline to four hours, 24 hours, and day 25 post-dose were assessed, with four-hour post-dose response being the primary outcome measure. A follow-up phase was also completed at day 81.
The MADRS score at four hours post-dose improved significantly in IN-esketamine patients compared with placebo patients (least squares mean [SE] difference vs. placebo; −5.3 [2.10]). Also, improvements in MADRS suicidal-thought items were observed at four hours post-dose. Depressive-symptom improvement was continually greater in IN-esketamine patients than in IN-placebo patients over the four-week period. Significant differences were observed at 24-hours post-dose, on days 3 and 11.
TRANSFORM-2 (NCT02418585)28 and TRANSFORM-3 (NCT02422186)29
TRANSFORM-2 and TRANSFORM-3, phase 3 randomized, double-blind, multicenter, active-controlled studies, evaluated IN esketamine for efficacy and safety in patients with TRD. TRANSFORM-3 evaluated elderly participants (≥ 65 years old) and TRANSFORM-2 evaluated adults. It was necessary that participants had a previous, documented non-response to at least one oral AD. Subjects were switched from a prior oral AD to which they had not responded to a new oral AD, plus either 28-mg, 56-mg, or 84-mg flexible-dosing IN esketamine or IN placebo (1:1 randomization). Both the IN esketamine and IN placebo were administered twice weekly. Depressive symptoms using MADRS were collected on day 2 (in TRANSFORM-2 only), day 8, day 15, day 22, and day 28. The change in MADRS total score from baseline to day 28 served as the primary endpoint. Results from TRANSFORM-2 indicated a significant reduction in depressive symptoms, showing a least squares mean difference (LSMD) from placebo of −4.0 (P = 0.010). TRANSFORM-3 results, however, showed no significant difference from baseline for IN esketamine plus oral AD compared with placebo plus oral AD, with decreases in MADRS scores of −10 and −6.3, respectively (P 0.029 > P 0.025; P = 0.02 was considered statistically significant). However, the U.S. patient population demonstrated a significant improvement in MADRS total scores in the IN-esketamine group. The IN-esketamine group’s MADRS score decreased by −6.3 compared with the placebo group’s decrease in score of −4.7 (P = 0.016). Participants who were considered responders could then be entered into either the SUSTAIN-1 or SUSTAIN-2 trial.
Adverse events were collected during the study to evaluate safety. The main ones were mild to moderate in nature and included nausea, vertigo, dysgeusia, dissociation, dizziness, and headache. At 1.5 hours post-dose, 93% of subjects were ready for discharge. It is notable that no symptoms of psychosis were observed during this study. Day 28 (end of induction phase) response rates showed that patients receiving IN esketamine plus oral AD had fivefold higher remission rates than patients receiving placebo plus oral AD (16.7% vs. 2.9%, respectively). Also, IN-esketamine patients were 5.3 times more likely than placebo patients to show improved Clinical Global Impression-Severity scale (CGI–S) scores. Significant differences in Patient Health Questionnaire (PHQ-9) scores also were observed between the groups, favoring IN esketamine with an LSMD of −4.4 (P = 0.006).
SUSTAIN-1 (NCT02493868)30 and SUSTAIN-2 (NCT02497287) 31
The phase 3 multicenter, double-blind, randomized withdrawal trials SUSTAIN-1 and SUSTAIN-2 compared IN esketamine plus oral AD with IN placebo plus oral AD for long-term efficacy in TRD. SUSTAIN-2 also studied safety and tolerability. Participants had to be documented as non-responsive to at least two prior trials of traditional AD treatment. In SUSTAIN-1, 705 patients transferred from previous studies were enrolled. The primary endpoint of stable remission after 16 weeks of IN esketamine plus oral AD showed a significant delay to relapse compared with placebo, with the risk of relapse decreasing in favor of IN esketamine by 51%. Adverse events included dysgeusia, vertigo, dissociation, somnolence, dizziness, headache, nausea, blurred vision, and oral hypoesthesia. Symptoms resolved around 1.5 hours post-dose. In SUSTAIN-2, subjects received 28-mg, 56-mg, or 84-mg IN esketamine. The primary outcome was to determine treatment-emergent adverse events, most of which were mild to moderate and included dizziness, dissociation, nausea, headache, somnolence, dysgeusia, hypoesthesia, vertigo, vomiting, and viral upper respiratory tract infections.
Overview
Studies appear to support the use of IN esketamine in the settings of TRD and MDSI. In the long-term studies, a majority of patients responded to treatment doses of 84 mg and approximately one-third of patients responded to 56 mg, with doses administered either weekly or every other week. Findings suggest that IN esketamine decreases suicidal risk and symptoms of depression, as reflected by the decrease in MADRS scores.27–32 Furthermore, adverse effects such as increased blood pressure and dissociation were transient. Cognition was also demonstrated to return to baseline at two hours post-dose.25 However, long-term efficacy beyond one year has yet to be established.
WARNINGS AND PRECAUTIONS
Adverse Effects
Esketamine has boxed warnings for risk of sedation and dissociation; potential for abuse and misuse; and increased risk for suicidal thoughts and behaviors in pediatric and young adult populations. The medication will only be available through a restricted REMS program.
The dose of ketamine and esketamine required for an antidepressant response is lower than the necessary anesthetic dose, and reported side effects are typically mild and transient.8,28,44 Dissociation, measured using the Clinician-Administered Dissociative States Scale (CADSS), has been shown to begin shortly after the start of dosing, peaking at approximately 40 minutes post-dose and resolving at approximately two hours post-dose.8,28 However, the effect appears to decrease with repeated dosing.31 In one placebo-controlled study, dissociation occurred in 31.4% of patients in the esketamine arm, compared with 12.9% of patients in the placebo arm.8 Other reported side effects include unpleasant taste, hallucinations, euphoria, nausea, vomiting, dizziness, headache, and parasthesias.29 Transient increases in BP and heart rate have also been reported, and will be a caution or contraindication in some special populations at risk for cardiovascular and cerebrovascular conditions who are sensitive to increases in BP.10,32 Dizziness and nausea tended to be dose-related, while most other adverse effects did not appear to be related to dosage.10 Similar side-effect profiles were found throughout the completed studies regarding the adverse effects of IN esketamine.8,10,28–33,44
Contraindications
Esketamine is contraindicated in patients with a known hypersensitivity to esketamine, ketamine, or any component or excipient of the formulation, and in patients for whom an increase in BP would be hazardous.34,35,44 Specific contraindications for IN esketamine are aneurysmal vascular disease or arteriovenous malformation and intracerebral hemorrhage. Table 2 describes the use of esketamine in special populations.
Table 2.
Use in Special Populations
| Underlying Psychosis8 | Clinical trials have excluded patients with underlying psychosis; thus, there is little data for the administration of intranasal (IN) esketamine in these populations. Use caution in patients with current or prior MDD with psychosis, psychotic disorder, bipolar disorder, or other related disorders. Side effects such as dysgeusia and dissociation may exacerbate underlying psychosis. |
| Comorbid Substance Use Disorder31 | Use caution in patients with a history of substance use disorder(s). |
| Severe Hypertension or Cardiac Compensation10,31 | Monitor BP pre-dose and post-dose in all patients. Caution is warranted in patients with cardiac compensation or severe hypertension. Because the dose of IN esketamine for the treatment of TRD is much lower than the analgesic dose, respiratory depression is less likely to occur. IN esketamine increases BP, predominantly systolic, and increases cardiac output; therefore, medical conditions such as aneurysmal vascular diseases–including thoracic or abdominal aorta, intracranial, and peripheral arterial vessel disease and arteriovenous malformation–are contraindications to IN esketamine use. |
| Pulmonary Conditions20 | The impact of IN esketamine on patients with asthma, COPD, or other pulmonary conditions is unknown. One study on the administration of esketamine via nebulization showed no pulmonary or respiratory adverse events during or after inhalation. It cannot be assumed that IN esketamine will show the same results on pulmonary response. Nasal discomfort, oropharyngeal pain, and throat irritation were reported in the clinical trials. |
| Pediatric | IN esketamine has not been studied in pediatric populations. |
| Geriatric27–29 | Clinical trials demonstrate that the use of IN esketamine in the geriatric population is safe and effective. Studies in elderly patients ≥ 65 years old with TRD have found significant reduction of MADRS score with only mild-to-moderate side effects, similar to those found in the general population of adults aged 18–64 years. Clinical trials utilized similar dosing regimens, including frequency and dosing, across age groups. |
| Hepatic and Renal Impairment24 | Currently, the hepatic and renal adjustments for IN esketamine are unknown. According to package inserts, no dosage adjustments are needed for intravenous ketamine in hepatic or renal impairment. Individuals with moderate hepatic impairment receiving IN esketamine may show higher area-under-the-curve and half-life values. IN esketamine is not recommended in patients with severe hepatic impairment. Increased frequency of urination was observed in IN-ketamine trials. No recommendations are made regarding impaired renal impairment and there is no clinical experience in patients receiving dialysis treatment. |
| Pregnancy and Lactation32,33 | Use of IN esketamine has not been studied in pregnancy in humans. As animal studies suggest embryofetal toxicity, fetal harm may occur. Both clinician and patient should consider pregnancy planning and prevention if the patient is of childbearing age and has the potential for reproduction. Ketamine may cross the placental barrier and cause maternal complications. Increased uterine tone and uterine contractions/ pressure, ototoxic effects, excessive neonatal muscle tone, apnea, and other complications have been seen with higher dosages of ketamine. Many of these effects may be avoided by using lower dosages of ketamine. Little is known about the use of ketamine with breastfeeding. Refer to the package insert/product labeling for detailed information regarding animal studies with esketamine for more data and possible guidance. |
Drug Interactions with Esketamine
Esketamine is metabolized via the cytochrome P450 (CYP) system, suggesting a predisposition to potential drug–drug interactions of that system; however, no significant interactions have been reported thus far.35,36,44 The concomitant use of esketamine with central nervous system depressants can increase the risk of sedation. As concomitant use of esketamine with psychostimulants may result in increased BP, patients receiving amphetamines, methylphenidate, various weight-loss medications, modafanil, and armodafinil should have their BP closely monitored.44 In addition, the concomitant use of esketamine with monoamine oxidase inhibitors may also increase BP, warranting the close monitoring of BP in patients receiving this combination.
There are known interactions involving rifampin/rifampicin and ticlopidine. Rifampin/ rifampicin reduces the plasma concentration of esketamine in the area-under-the-curve (AUC) and the maximum serum concentration (Cmax) by 10% to 25%.37,44 Ticlopidine increases the plasma AUC of esketamine through the inhibition of the CYP2B6 liver enzyme.38,39,44
DOSAGE AND ADMINISTRATION28–33,44
Patients must be enrolled in the Spravato REMS program prior to administration. They should self-administer IN esketamine under the direct supervision of a health care provider. Patient BP should be assessed before and after administration; if it is already above a systolic reading of 140 mmHg and a diastolic reading of 90 mmHg, providers should reconsider the risks of increased BP versus the benefit of IN esketamine for that patient. Monitoring patients for at least two hours post-dose is required to ensure the resolution of possible transient adverse effects, such as increases in BP, cognitive impairment, sedation, and dissociation. Esketamine can impair the ability to drive or operate machinery and appropriate warnings are made regarding such activities. Because of potential nausea and vomiting caused by the treatment, patients are advised to avoid food at least two hours prior to administration and to avoid drinking liquids at least 30 minutes prior to administration. Other nasally administered medications such as corticosteroids or decongestants should be administered at least one hour before IN esketamine is given. The most-studied doses of IN esketamine are 56 mg and 84 mg; doses as low as 28 mg have also been studied but are not recommended for treatment.10,44 Each nasal-spray device contains a total of 28 mg, delivered via two sprays (one spray in each nostril). For the 56-mg dose, two devices are required, with five minutes of rest between each device. Three devices are required for the 84-mg dose, with another five minutes of rest between the second and third device. Detailed instructions for administration can be found in the package insert, including differences in frequency between the induction and maintenance phases of IN-esketamine treatment.44
COST
The exact cost of IN esketamine for the indication of TRD is currently unknown; it may depend on insurance coverage, large health-care system formulary contracts, and wholesale prices. IN esketamine will likely cost more in the initial phase of treatment and less during long-term management. Costs could range from $5,664 to $8,142 in the first month of induction-phase treatment, $2,832 to $4,248 in the second month, and possibly $1,416 to $4,248 each month thereafter, depending on whether dosing is weekly or bi-weekly.42
At the time of writing, the administration cost for off-label IV-ketamine infusions for depression ranged from $500 to $1,000 per infusion at a private clinic, not including consultation or lab fees.40,41 Off-label use is typically paid out-of-pocket by the patient, as it is not generally covered by major medical or prescription insurance policies.40,41 The average wholesale unit price of ketamine solution for injection is far lower, ranging from $0.42 to $2.30 per mL, depending on concentration and formulation.42
P&T COMMITTEE CONSIDERATIONS
Health care facilities considering the use of IN esketamine will need to factor in the many secondary costs that would be associated with implementing an IN-esketamine clinic. Based on current knowledge, patients will require post-dose monitoring of vital signs and mental status by health care professionals, and a quiet and calm environment in which they can recover from possible adverse effects such as sedation and dissociation. Facilities must be able to accommodate the scheduling of multiple appointments for each patient receiving IN esketamine, which could be as frequent as twice weekly at the outset, depending on the patient-specific duration of AD response. Also, facilities must have the capability to order and store a specialty controlled substance. Intranasal esketamine is a C-III controlled substance, similar to IV ketamine.33,44 To further ensure its proper use, the medication is distributed through limited specialty pharmacy distributors and administered via the REMS program. This is partly due to the abuse potential associated with ketamine, although the potential abuse of IN esketamine is not well documented.43 The limited indication of TRD in conjunction with an oral AD and the potential for abuse will likely result in the need for verification or prior authorization to utilize insurance benefits. The failure of prior oral AD therapy will need to be established before administration. However, the benefits of IN esketamine––a rapid-acting AD that can improve symptoms for many patients within hours or days instead of the weeks or months common with traditional ADs––make it a unique option that has long been needed by many treatment-resistant patients.
CONCLUSION
Depression is associated with high rates of disability and an increased risk of mortality. Traditional treatment options have a slow onset of action, limiting their utility in the most severely affected patients who also may be at a high risk for suicide. As few options exist for patients with TRD, a fast-acting therapy is attractive. In clinical trials, IN esketamine appears to provide significant short-term symptom improvement in TRD, with only transient and mild-to-moderate side effects commonly being reported. Some health care payers and providers may have concerns about access, treatment costs, storage requirements, staffing, and administrative logistics regarding the implementation and use of IN esketamine. Thus, it is difficult to make a definitive recommendation that pharmacy and therapeutics (P&T) committees adopt the drug for their particular facilities or patients. However, with the paucity of effective treatments for patients with TRD, IN esketamine may be a clinically useful option with a favorable risk-to-benefit profile for some of those patients. The committees will have to consider the needs and capabilities of their health care systems to determine whether IN esketamine is a viable therapeutic choice.
Figure 1.
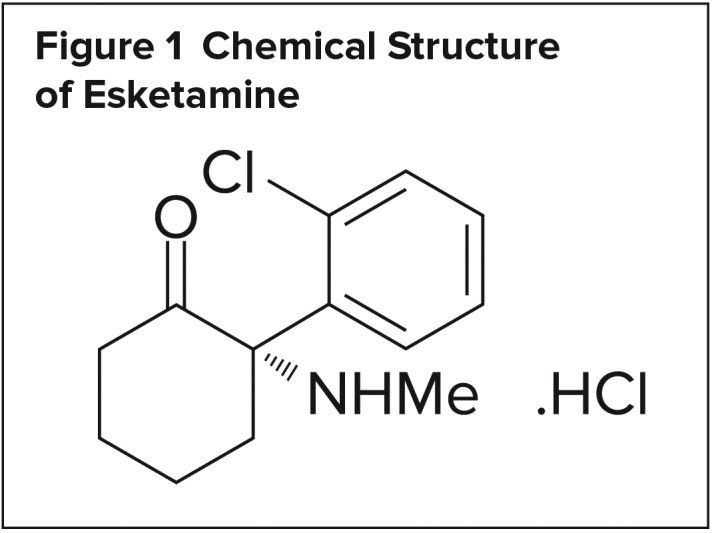
Chemical Structure of Esketamine
Footnotes
Disclosure: The authors report no commercial or financial interest in regard to this article.
REFERENCES
- 1.National Institute of Mental Health. Major depression. Feb, 2019. [Accessed April 15, 2019]. Available at: https://www.nimh.nih.gov/health/statistics/major-depression.shtml.
- 2.Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report [published online May 22, 2009] Psychological Medicine. 2010;40(1):41–50. doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Depression 2018. Mar 22, 2018. [Accessed April 15, 2019]. Available at: http://www.who.int/en/news-room/fact-sheets/detail/depression.
- 4.WHO. National suicide prevention strategies: progress examples and indicators. Jan 19, 2019. [Accessed May 7, 2019]. https://www.who.int/mental_health/suicide-prevention/national_strategies_2019/en/
- 5.Bobo WV, Vande Voort JL, Croarkin PE, et al. Ketamine for treatment-resistant unipolar and bipolar major depression: critical review and implications for clinical practice [published online April 6, 2016] Depress Anxiety. 2016;33(8):698–710. doi: 10.1002/da.22505. [DOI] [PubMed] [Google Scholar]
- 6.Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt [published online October 21, 2008]? J Clin Psychiatry. 2009;70(1):19–24. [PubMed] [Google Scholar]
- 7.Singh J, Fedgchin M, Daly E, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study [published online November 3, 2015] Biol Psychiatry. 2016;80(6):424–431. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620–630. doi: 10.1176/appi.ajp.2018.17060720. [DOI] [PubMed] [Google Scholar]
- 9.Fava M, Mischoulon D, Iosifescu D, et al. A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed out-patients with inadequate response to prior antidepressant therapy (ADAPT-A Study) [published online January 25, 2012] Psychother Psychosom. 2012;81(2):87–97. doi: 10.1159/000332050. [DOI] [PubMed] [Google Scholar]
- 10.Daly E, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139–148. doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esketamine receives breakthrough therapy designation from U.S. Food and Drug Administration for major depressive disorder with imminent risk for suicide [press release] Titusville, New Jersey: Johnson & Johnson Media Center; Aug 16, 2016. [Accessed April 16, 2019]. Available at: https://www.jnj.com/media-center/press-releases/esketamine-recieves-breakthrough-therapy-designation-from-us-food-and-drug-administration-for-major-depressive-disorder-with-imminent-risk-of-suicide. [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- 13.Covvey JR, Crawford AN, Lowe DK. Intravenous ketamine for treatment-resistant major depressive disorder [published online December 20, 2011] Ann Pharmacother. 2012;46(1):117–123. doi: 10.1345/aph.1Q371. [DOI] [PubMed] [Google Scholar]
- 14.Malhi GS, Byrow Y, Cassidy F, et al. Ketamine: stimulating antidepressant treatment? BJPsych Open. 2016;2(3):e5–e9. doi: 10.1192/bjpo.bp.116.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas R, Cetin M, Baker GB, Dursun SM. Comment on FDA’s breakthrough therapy designation of intranasal esketamine for the treatment of major depressive disorder with imminent risk of suicide. Klinik Psikofarmakol Bulteni. 2016;26(4):329–331. doi: 10.5455/bcp.20161027122045. [DOI] [Google Scholar]
- 16.Sattar Y, Wilson J, Khan AM, et al. A review of the mechanism of antagonism of N-methyl-D-aspartate receptor by ketamine in treatment-resistant depression. Cureus. 2018;10(5):e2652. doi: 10.7759/cureus.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ignácio ZM, Réus GZ, Arent CO, et al. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs [published online January 8, 2016] Br J Clin Pharmacol. 2016;82(5):1280–1290. doi: 10.1111/bcp.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsden WN. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog in Neuropsychopharmacol Biol Psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Moylan S, Maes M, Wray N, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications [published online April 24, 2012] Mol Psychiatry. 2013;18(5):595–606. doi: 10.1038/mp.2012.33. [DOI] [PubMed] [Google Scholar]
- 21.Jonkman K, Duma A, Olofsen E, et al. Pharmacokinetics and bioavailability of inhaled esketamine in healthy volunteers. Anesthesiology. 2017;127(4):675–683. doi: 10.1097/ALN.0000000000001798. [DOI] [PubMed] [Google Scholar]
- 22.Vollenweider FX, Leenders KL, Øye I, et al. Differential psychopathology and patterns of cerebral glucose utilization produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7(1):25–38. doi: 10.1016/S0924-977X(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 23.Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–660. doi: 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mössner LD, Schmitz A, Theurillat R, et al. Inhibition of cytochrome P450 enzymes involved in ketamine metabolism by use of liver microsomes and specific cytochrome P450 enzymes from horses, dogs, and humans. Am J Vet Res. 2011;72(11):1505–1513. doi: 10.2460/ajvr.72.11.1505. [DOI] [PubMed] [Google Scholar]
- 25.Adams JD, Jr, Baillie TA, Trevor AJ, Castagnoli N., Jr Studies on the biotransformation of ketamine 1: identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spectrom. 1981;8(11):527–538. doi: 10.1002/bms.1200081103. [DOI] [PubMed] [Google Scholar]
- 26.National Library of Medicine. Clinical Trials.gov. [Accessed April 22, 2019]. Available at: https://clinicaltrials.gov/ct2/results?cond=&term=intranasal+esketamine&cntry=&state=&city=&dist=
- 27.Morrison RL, Fedgchin M, Singh J, et al. Effect of intranasal esketamine on cognitive functioning in healthy participants: a randomized, double-blind, placebo-controlled study [published online February 1, 2018] Psychopharmacology (Berl) 2018;235(4):1107–1119. doi: 10.1007/s00213-018-4828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popova V, Daly E, Trivedi M, et al. Randomized, double-blind study of flexibly-dosed intranasal esketamine plus oral antidepressant vs. active control in treatment-resistant depression. Poster presented at American Psychiatric Association Annual Meeting; New York, New York. May 5–9, 2018; [Accessed April 16, 2019]. Abstract published in Biol Psychiatry 2018;83(suppl 9):S390. Available at: [DOI] [Google Scholar]
- 29.Ochs-Ross R, Daly EJ, Trivedi M, et al. Efficacy and safety of intranasal esketamine plus an oral antidepressant in elderly patients with treatment-resistant depression. Poster presented at American Psychiatric Association Annual Meeting; New York, New York. May 5–9, 2018; [Accessed April 16, 2019]. Abstract published in Biol Psychiatry 2018;83(suppl 9):S391. Available at: [DOI] [Google Scholar]
- 30.Daly E, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at American Society of Clinical Psychopharmacology Annual Meeting; Miami, Florida. May 29–June 1, 2018. [Google Scholar]
- 31.Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: phase 3, open-label, safety and efficacy study (SUSTAIN-2). Poster presented at American Society of Clinical Psychopharmacology Annual Meeting; Miami, Florida. May 29–June 1, 2018. [Google Scholar]
- 32.Starr L, Ochs-Ross R, Zhang Y, et al. Clinical response, remission, and safety of esketamine nasal spray in a US population of geriatric patients with treatment-resistant depression. Poster presented at American Psychiatric Association Annual Meeting; New York, New York. May 5–9, 2018. [Google Scholar]
- 33.Starr L, Ochs-Ross R, Zhang Y, et al. Clinical efficacy and safety of esketamine nasal spray in a US population of geriatric patients with treatment-resistant depression. Poster presented at American Psychiatric Association Annual Meeting; New York, New York. May 5–9, 2018. [Google Scholar]
- 34.FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic [news release] Silver Spring, Maryland: FDA; Mar 5, 2019. [Accessed April 16, 2019]. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm632761.htm. [Google Scholar]
- 35.DailyMed. Ketamine hydrochloride–ketamine hydrochloride injection. Jan 30, 2019. [Accessed April 16, 2019]. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58487c78-a641-4278-acc0-343596ee8683#LINK_fef97ab7-4e9e-4bef-85c8-267b54215625.
- 36.Briggs GG, Freeman RK, Towers CV, Forinash AB. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 11th ed. Philadelphia, Pennsylvania: Wolters Kluwer; 2017. p. 789. [Google Scholar]
- 37.Noppers I, Olofsen E, Niesters M, et al. Effect of rifampicin on S-ketamine and S-norketamine plasma concentrations in healthy volunteers after intravenous S-ketamine administration. Anesthesiology. 2011;114(6):1435–1445. doi: 10.1097/ALN.0b013e318218a881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashraf MW, Peltoniemi MA, Olkkola KT, et al. Semimechanistic population pharmacokinetic model to predict the drug–drug interaction between S-ketamine and ticlopidine in healthy human volunteers [published online September 10, 2018] CPT Pharmacometric Syst Pharmacol. 2018;7(10):687–697. doi: 10.1002/psp4.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peltoniemi MA, Saari TI, Hagelberg NM, et al. Exposure to oral S-ketamine is unaffected by itraconazole but greatly increased by ticlopidine [published online June 29, 2011] Clin Pharmacol Ther. 2011;90(2):296–302. doi: 10.1038/clpt.2011.140. [DOI] [PubMed] [Google Scholar]
- 40.Ketamine Wellness Centers. Cost of ketamine infusion treatments. 2019. [Accessed April 17, 2019]. Available at: https://www.ketaminewellnesscenters.com/treatment-cost/
- 41.Portland Ketamine Clinic. Medical forms. 2019. [Accessed April 17, 2019]. Available at: http://portlandketamineclinic.com/PKC/forms-cost-ketaminetreatment/
- 42.Ketamine HCL. Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; [Accessed April 17, 2019]. [Google Scholar]
- 43.Morgan CJ, Curran HV Independent Scientific Committee on Drugs. Ketamine use: a review [published online July 22, 2011] Addiction. 2012;107(1):27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 44.SpravatoTM (esketamine) prescribing information. Titusville, New Jersey, 08560: Janssen Pharmaceuticals, Inc; 2019. [Google Scholar]


