Abstract
Introduction:
Altered striatal development contributes to core deficits in motor and inhibitory control, impulsivity, and inattention associated with attention-deficit/hyperactivity disorder (ADHD) and may likewise play a role in deficient reward processing and emotion regulation in psychosis and depression. The maturation of striatal connectivity has not been well-characterized, particularly as it relates to clinical symptomotology.
Methods:
Resting-state functional connectivity with striatal subdivisions was examined for 926 participants (ages 8-22 years, 44% male) from the general population who had participated in two large cross-sectional studies. Developing circuits were identified and growth-charting of age-related connections (ARCs) was performed to obtain individual scores reflecting relative neurodevelopmental attainment. Associations of clinical symptom scales (ADHD, psychosis, depression, and general psychopathology) with the resulting striatal connectivity age-deviation scores were then tested using Elastic Net regression.
Results:
Linear and non-linear developmental patterns occurred across 231 striatal ARCs. Both unique and overlapping striatal ARCs were associated with the four symptom domains. ADHD severity was related to age-advanced connectivity across several insula subregions, but age-delayed connectivity with nearby inferior frontal gyrus. Psychosis was associated with advanced connectivity with medial prefrontal cortex and superior temporal gyrus; while aberrant limbic connectivity predicted depression. The dorsal posterior insula, a region involved in pain processing, emerged as a strong contributor to general psychopathology as well as to each individual symptom domain.
Conclusions:
Developmental striatal pathophysiology in the general population is consistent with dysfunctional circuitry commonly found in clinical populations. Atypical age-normative connectivity may thereby reflect aberrant neurodevelopmental processes that contribute to clinical risk.
Keywords: striatum, development, ADHD, depression, psychosis, general psychopathology
Introduction
Cortico-striatal and cortico-striatal-cerebellar circuits underlie diverse cognitive functions such as cognitive control, motor control and inhibition, reward prediction and processing, and emotion regulation (1–7). It is unsurprising then that striatal maturation plays a critical role in several neurodevelopmental and psychiatric disorders (8). A number of risk factors influence striatal development and increase susceptibility to mental health disorders (9–12). The understanding of healthy striatal maturation is therefore important for identifying developmental biomarkers of psychopathology and for distinguishing such biomarkers of individual clinical domains.
Recent resting-state fMRI studies examining the development of striatal connectivity in the general population have either used small samples, with limited representation across developmental stages, or used categorical samples (e.g. childhood, adolescence, young adulthood), rather than looking continuously across the age range (13–15). Prefrontal maturation lags behind that of the striatum, a discrepancy that contributes to increased behavioral dysregulation during adolescence and thus may increase risk for several neuropsychiatric disorders (16, 17). Therefore, there is need for improved characterization of nonlinear changes in normative development of cortico-striatal connectivity.
Striatal dysfunction is implicated in core deficits in Attention-Deficit/Hyperactivity Disorder (ADHD) including inattention, hyperactivity, and impulsivity (12, 18, 19). This neurodevelopmental disorder is characterized by developmental delays across the brain in both structure and function (20, 21). Structural MRI studies find that children with ADHD have developmental trajectories lagging behind that of typically-developing children by 1-3 years (20, 22–24). Moreover, task and resting-state fMRI studies have found delayed functional development in several cortical networks (21, 25, 26). While striatal dysfunction in ADHD has been widely-reported (27–29), there is a dearth of research examining the development of striatal connectivity associated with ADHD.
Striatal development likewise plays a critical role in the neurodevelopment of psychosis and depression. For psychosis, the age of onset is later than in ADHD (i.e. adolescence or young adulthood); however, there is much evidence that genetic and environmental factors influencing striatal maturation alter dopamine levels and predispose toward psychosis (10, 30). Importantly, aberrant striatal connectivity has been observed during the psychosis prodrome (31, 32), as well as the first episode of psychotic illness (33, 34), suggesting that these abnormalities reflect disease etiology and are not merely the result of treatment or long-term illness processes. Understanding the development of altered striatal connectivity may also provide insight into treatment of psychotic disorders, insofar as normalization of cortico-striatal connectivity has been shown to predict response to antipsychotic treatment (35–37).
The incidence of depressive episodes increases in adolescence, which is likely the outcome of neurodevelopmental events increasing susceptibility (11, 38). As with psychosis, studies of patients in the earliest phases of illness, prior to exposure to medication, suggest a role for altered striatal connectivity in the etiopathogenesis of depression (39, 40). Reduced pregenual cingulate-striatal connectivity predicts greater subclinical depression severity (41). Further, general reductions in left caudate connectivity has been associated with subclinical depression (42). Thus, striatal circuits may be an early biomarker and key treatment target for depression. While atypical striatal maturation is a likely contributor to the pathophysiology of multiple disorders, it is not clear whether this reflects unique disorder-specific neurodevelopmental pathways or a common pathway indicative of general psychopathology. Recent interest in the “p” factor, a single dimension that explains symptom severity across disorders, stems from findings that there is high comorbidity between disorders and that familial and individual lifetime psychiatric histories commonly cross clinical domains (43, 44). Severity of general psychopathology is related to striatal dysfunction during reward processing in adults (45) as well as to resting-state striatal connectivity with the anterior cingulate cortex (ACC) in a developmental population (46). Therefore, it is unclear whether the maturation of particular striatal connections are related to particular symptom domains or to severity of psychopathology, more generally.
Several recent studies have used growth-charting to characterize age-normative brain development and to determine whether clinical or cognitive traits are related to delayed or precocious connectivity (47–49). The current study followed a series of steps to identify the neurodevelopment of striatal connectivity in the general population and to examine the role of age-inappropriate connectivity in clinical symptom domains. First, we examined the development of resting-state functional connectivity with striatal subdivisions (1) in the general population using large, cross-sectional developmental datasets. Next, growth-charting of age-related connections (ARCs) was performed to obtain individual scores reflecting age-normative connectivity. Associations of subclinical symptom scales (ADHD, psychosis, depression, and general psychopathology) with the resulting striatal connectivity age-deviation scores were then tested using Elastic Net (EN) regression. Given the strong contribution of neurodevelopmental factors in susceptibility to neurodevelopmental and psychiatric disorders, it was hypothesized that developing striatal connections would be predictive of clinical symptom domains in the general population. We further expected that those connections predictive of general psychopathology would overlap with those of other symptom domains, but that uniquely-predictive connections for each symptom domain would also be identified.
Methods and Materials
Participants
Participants were 926 children, 8-22 years of age, from a community sample drawn from two publicly-available databases. Resting-state fMRI and T1-weighted scans were collected from eight sites for the Pediatric, Imaging, Neurocognitive and Genetics (PING) study (http://ping.chd.ucsd.edu/) (50) and one site for the Philadelphia Neuroimaging Cohort (PNC) (51). Informed consent was acquired by each site according to each study’s protocol. Participants were excluded from analyses for the following reasons: incomplete covariate information, Frame-wise Displacement (FD) ≥ 0.5 mm, or post-scrubbed scan length ≤ 4 minutes. Table 1 summarizes each site’s resting-state scan acquisition and participant information for the final study sample.
Table 1.
Demographic and scanner information for the one Philadelphia Neuroimaging Cohort (PNC) site and the eight Pediatric, Neurocognition, Imaging, and Genetics (PING) sites.
| demographics | scanner | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| study | site | N | % male | age | age range | manufacturer | model | MR strength | TR | volumes | scan length |
| PNC | 1 | 601 | 44 | 15.74 (3.08) | 8 - 23 | Siemens | TrioTim | 3T | 3.0 | 126 | 6:18 |
| PING | 2 | 50 | 46 | 16.24 (3.16) | 9 - 21 | Siemens | TrioTim | 3T | 3.0 | 128 | 6:24 |
| PING | 3 | 41 | 44 | 18.45 (2.68) | 9 - 21 | Siemens | TrioTim | 3T | 2.0 | 300 | 10:00 |
| PING | 4 | 13 | 69 | 14.99 (3.48) | 10 - 21 | Siemens | TrioTim | 3T | 3.0 | 128 | 6:24 |
| PING | 5 | 59 | 46 | 15.68 (3.38) | 8 - 21 | Siemens | TrioTim | 3T | 3.0 | 128 | 6:24 |
| PING | 6 | 62 | 37 | 16.07 (3.73) | 8 - 21 | Philips | Achieva | 3T | 2.5 | 156 | 6:30 |
| PING | 7 | 17 | 35 | 14.21 (2.97) | 8 - 18 | Siemens | TrioTim | 3T | 2.0 | 180 | 6:00 |
| PING | 8 | 31 | 45 | 15.76 (3.22) | 8 - 21 | Philips | Achieva | 3T | 2.5 | 156 | 6:30 |
| PING | 9 | 52 | 46 | 14.46 (4.56) | 8 - 21 | Siemens | TrioTim | 3T | 3.0 | 128 | 6:24 |
| TOTAL | 926 | 44 | 15.79 (3.30) | 8 - 23 | |||||||
Neuroimaging Data Analysis
Details of the neuroimaging data preprocessing and nuisance regression is described in the Supplemental Information. Time-courses were extracted for three caudate and three putamen 4-millimeter seeds, which represented functionally-distinct anatomical sub-regions of the striatum (1). Full-brain connectivity maps for each seed were the Fisher’s Z-transform of the Pearson’s R-correlation at each voxel. General Linear Models assessed the linear and nonlinear effects of age, while co-varying for sex, FD-DVARS correlations, FD-DVARS2, scrubbed scan length, and acquisition site. Site was accounted for with one regressor per PING site, which consisted of ones for those participants scanned at that acquisition site and zeros for all other participants. Connections showing a significant relationship with age were identified as those with a voxel-level and cluster-level threshold of p<0.001 (52, 53). The cluster-level threshold was then multiple-comparisons corrected for the six seeds (i.e. p<0.001 / 6 = p<1.67×10−4). Follow-up analyses examined sex and age-by-sex interaction effects to determine whether striatal connections were significantly different between males and females.
Growth Charting of Developing Connections
Peak coordinates in the age statistical maps were defined as local maxima separated by >15 mm. 4-mm seeds were placed around each of these peak ARCs, Fisher’s Z-transformed connectivity values were extracted, and growth-charting was performed. This involved first regressing out the effects of sex, FD-DVARS correlations, FD-DVARS2, scrubbed scan length, and acquisition site (see the Supplementary Information for detailed assessment of site effects). Age was then fit for the full sample of 926 participants. A polynomial model of age, y = β0 + β1*age + β2*age2, was fit to the connectivity measures using the R-package nls2. For each of the ARCs, age trajectories corresponding to the 2.5th, 5th, 10th, 25th, 50th, 75th, 90th, 95th and 97.5th percentiles were created. This was done by first binning the age variable with a bin size of 1-year, obtaining the quantiles for a given age-bin, and then applying the quadratic model of age to each quantile.
Identifying Age-Normative Striatal Patterns
Each participant’s age-deviation score was their distance from the 50th percentile fit, with positive age-deviation scores falling above the age-normative fit line and negative age-deviation scores falling below the age-normative fit line. Age-deviation scores for each ARC were then entered into EN regression models to identify those connections in which distance from the age-normative fit was associated with symptom severity in the four symptom domains: ADHD, psychosis, depression, and general psychopathology. While age-normative fit was determined in the full-sample, associations with clinical symptom severity were examined exclusively in the PNC sample.
Assessing Symptom Severity in the PNC Sample
As part of the PNC protocol (51), participants underwent a computerized battery assessing almost 600 demographic, medical, and psychopathology items based on the Kiddie-Schedule for Affective Disorders and Schizophrenia (54). For the current study, ADHD, psychosis, depression, and general psychopathology domains were examined to determine their relationship with age-normative striatal connectivity. The particular items used for each of the symptom scales were previously described by Kaufmann and colleagues (55). Scores reflected the sum across all items within each scale.
Elastic Net (EN) Models Assessing Age-Normative Striatal Connectivity Related to Clinical Symptoms in the PNC Sample
EN is a form of regularized regression (56), which minimizes the following function:
Where y is the dependent variable (i.e. clinical symptom scores), xi are the predictor variables (i.e. age-deviation scores for each ARC), βi are the regression coefficients, λ is the regularization penalty, and α is the elastic net mixing parameter, which varies between 0 and 1, with lower values favoring the L2 norm penalty (i.e. ridge regression) and higher values favoring the L1 norm penalty (i.e. lasso regression). Negative binomial EN models were implemented using R.
EN models were fit at five values of α (i.e. 0.001, 0.25, 0.5, 0.75, and 1) and 100 values of λ. 10-fold cross validation was performed in which folds were kept constant across all models. The optimal model fit was assessed using the cross-validated log-likelihood across the 10-folds (i.e. the mean log-likelihood across the folds) and the optimal model was identified as the one with the maximum cross-validated log-likelihood. Additional assessment of the optimal EN model was done by examining stability of the EN model selection across different folds and by examining concordance of the EN variable selection with a second machine-learning method, Connectome-based Predictive Modeling (CPM) (57). These additional assessments are described in detail in the Supplemental Information.
Results
From the regression analyses examining striatal development across the full sample, a set of 231 ARCs were identified. Figure 1 displays the significant linear and nonlinear age effects for each of the striatal subdivisions. Analyses performed both with and without global signal regression yielded similar results both for developmental trends and symptom associations. Global signal included was used for the remainder of the paper. No significant sex or age-by-sex interaction effects were found.
Figure 1.

Significant age effects (p<1.67×10−4) for the six striatal seeds: Dorsal Caudate Right (DCR), Ventral Striatum superior Right (VSsR), Ventral Striatum inferior Right (VSiR), Dorsal Rostral Putamen Right (DRPR), Dorsal Caudal Putamen Right (DCPR), Ventral Rostral Putamen Right (VRPR). Positive linear age effects are in red. Negative linear age effects are in blue. Nonlinear age2 effects are in green. The striatal seed is in yellow. The top row displays the three caudate seeds and the bottom row displays the three putamen seeds.
Growth-Charting
The 231 peak coordinates in which significant age effects occurred are listed in Table S1. Growth charting was performed on the connectivity values of each of these 231 ARCs. Figure 2 displays developmental effects of two example striatal seeds and the growth-charting normative age fits for several of the peak coordinates. Various developmental trends were identified including linear increases and linear decreases with age, as well as nonlinear effects in which connectivity peaked during early or late adolescence. Importantly, the growth-charting approach permitted the calculation of each individual’s age-deviation score for each ARC, which was then used in subsequent analyses. Assessment of site effects revealed that there was no significant impact of acquisition site on connectivity values after confound regression (see the Supplementary Information for more discussion of site effects).
Figure 2.

Significant age effects (p<1.67×10−4) and growth charts for two example striatal seeds: Ventral Striatum inferior Right (VSiR) and Dorsal Rostral Putamen Right (DRPR). The top row shows significant age effects for VSiR and DRPR. Positive linear age effects are in red. Negative linear age effects are in blue. Nonlinear age2 effects are in green. The striatal seed is in yellow. The bottom row shows the growth charts for several of the Age-Related Connections (ARCs): the three on the left display VSiR ARCs and the three on the right display DRPR ARCs. Each green dot represents one participant; open, dark green dots are females and closed, light green dots are males. The sold blue line is the age 50th percentile, age-normative fit line and the dashed lines represent the 2.5th, 5th, 10th, 25th, 75th, 90th, 95th, and 97.5th percentile fits.
Optimal EN Model Selection
Table 2 lists the α and λ values for the optimal model for each of the four clinical symptom scales. The mean and standard deviation of the cross-validated log-likelihood is reported across the 10-times that the optimal model was re-run with varying folds. The log-likelihood was very stable over the 10 runs for all symptom scales. Model fit for depression was the most variable over runs, but the variation in log-likelihood values was still very low for this model (i.e. coefficient of variation 0.24/−90.61 = −0.0027). Therefore, the optimal model fit for all clinical symptom scales was very stable over varying cross-validation folds.
Table 2.
Elastic Net Parameters and Log-Likelihood for the Optimal Model Predicting Each of the Four Symptom Domains.
| alpha | lambda | mean log-likelihood | sd log-likelihood | |
|---|---|---|---|---|
| adhd | 0.0001 | 414.41 | −128.20 | 0.04 |
| psychosis | 0.25 | 0.25 | −193.38 | 0.08 |
| depression | 0.25 | 0.13 | −90.61 | 0.24 |
| general psychopathology | 0.0001 | 766.58 | −258.36 | 0.05 |
A second method, CPM (57), was applied to assess the validity of the variable selection for the optimal EN models. For this method, a threshold of p<0.10 on 95% of iterations was used to select predictive connections. Associations between striatal ARCs and clinical symptoms were also required to be in the same direction (i.e. positive or negative associations) on all suprathreshold iterations. CPM selected fewer connections than EN regression for ADHD (23 for CPM/35 for EN), depression (10 for CPM/42 for EN), and general psychopathology (20 for CPM/21 for EN). For each of these symptom sets, the connections resulting from CPM were 100% concordant with those emerging from EN. However, for psychosis, many more connections were selected using CPM (32 for CPM/8 for EN). Nonetheless, all 8 EN connections were also selected using the CPM method. Table S2 lists the particular connections that were selected for each method.
Clinical Symptoms Relate to Striatal Maturity
Figure 3 displays the selected connections (i.e. those connections with non-zero β values) for the optimal models and their penalized β values for each of the four symptom scales. Each β value was age-corrected (i.e. multiplied by the direction of the linear age effect) so that negative values reflected developmentally-delayed and positive values reflected developmentally-accelerated associations.
Figure 3.
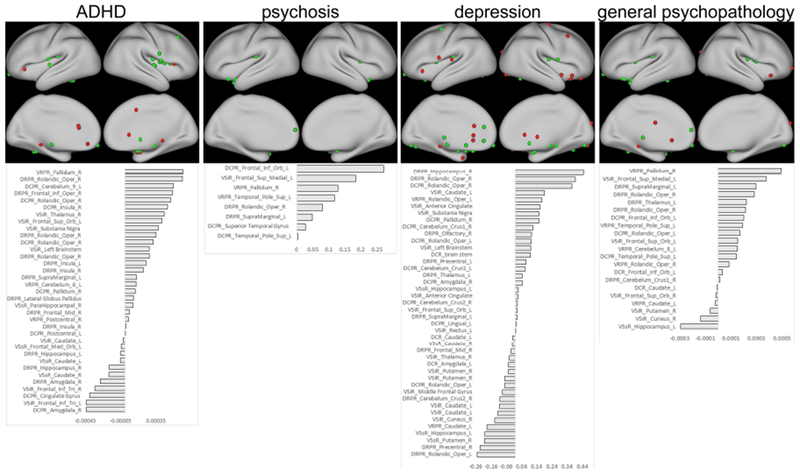
Connections with symptom associations. The peak ARCs with non-zero βs in the Elastic Net (EN) models are shown on the top row. Those ARCs in which greater symptom severity is associated with age-accelerated connectivity are displayed in green, while those ARCs in which greater symptom severity is associated with age-delayed connectivity are displayed in red. The ARC labels and the age-adjusted βs from the optimal EN model are shown in the bottom row.
For ADHD symptoms, associated connections included several dorsal putamen-insula connections, all of which were developmentally-accelerated with ADHD severity. VSiR-bilateral inferior frontal trigeminal cortex (BA 45), on the other hand, tended to be developmentally-delayed; along with a DCPR-anterior cingulate gyrus connection, and several connections within the ventral striatum. Connections between VSiR and subcortical regions, including the globus pallidus, substantia nigra, thalamus, and brain stem tended to be developmentally-accelerated in youth with more ADHD symptoms.
For psychosis symptoms, associated connections were limited to several putamen-anterior temporal pole connections, a putamen-OFC connection (BA 25), a ventral striatum-medial prefrontal cortex (mPFC) connection, and a couple of dorsal putamen-posterior insula connections. All predictive connections reflected accelerated development in those children with psychosis symptoms. Depression, on the other hand, was characterized by accelerated development of subgenual cingulate (SGC) connectivity as well as several subcortical limbic regions, including the hippocampus, substantia nigra, globus pallidus, brain stem, thalamus, and amygdala. Several intra-striatal connections, on the other hand, tended to be delayed with more depressive symptoms.
For general psychopathology, putamen-dorsal posterior insula connections tended to be developmentally-accelerated. Putamen connections to subcortical regions, such as globus pallidus and thalamus were also accelerated; whereas intra-striatal connections were delayed. Caudate-mPFC/OFC and putamen-anterior temporal pole connections, which were developmentally-accelerated with psychosis, were likewise accelerated with general psychopathology.
EN Model Fit and CPM Prediction
The optimal models emerging from the EN analysis accounted for 6-13% of the variance in symptom scores (ADHD: R2=0.10, psychosis: R2=0.060, depression: R2=0.13, general psychopathology: R2=0.083). For ADHD and depression scores, the majority of connections selected were unique to those clinical scores; while the majority of connections selected for the psychosis and general psychopathology scores were common to other symptom subscales. Figure 4 displays the association between the fitted values for the optimal EN models and the actual clinical symptom scores. To assess the reliability of these results using a different approach, the CPM method was used to generate prediction for the left-out participant during each iteration of the leave-one-out-cross-validation. Consistent with the EN results, the CPM test data resulted in significant prediction for all 4 clinical domains: ADHD R2=0.1025, p=8.88×10−16, psychosis R2=0.0070, p=0.040, depression R2=0.0119, p=0.0074, and general psychopathology R2=0.0192, p=6.61×10−4.
Figure 4.

Association between the actual dimensional symptom scores and the Elastic Net (EN) fitted values. The EN fit accounts for 10.0%, 6.0%, 13.0% and 8.3 % of the variance for ADHD, psychosis, depression, and general psychopathology, respectively.
Discussion
The current study examined the maturation of striatal functional subdivisions in 926 youth ranging from 8 to 22 years of age. The large sample size and broad developmental range allowed for improved characterization of linear and nonlinear age effects on striatal connectivity. While general decreases in intra-striatal and striatal-limbic connectivity were observed across all seeds, a number of previously-unreported cortical and cerebellar ARCs showed specific maturational patterns for different striatal subdivisions. EN models identified age-inappropriate striatal connectivity that was associated with symptom dimensions in the general population. Consistent with well-established pathophysiology of ADHD (58, 59), inferior frontal, insula, and anterior cingulate connections were among the most predictive of ADHD symptoms. Likewise, connections associated with psychosis and depression were consistent with known pathophysiology for each of these disorders. Medial prefrontal and superior temporal pole connections were predictive of psychosis, while limbic connections were predictive of depression. Connections associated with general psychopathology mostly overlapped with those of other symptom dimensions, notably for dorsal posterior insula as well as subcortical, and medial prefrontal connections.
Maturation of Striatal Connections
Several developmental trends in striatal connectivity across the brain were identified. The majority of striatal ARCs decreased connectivity with development particularly for subcortical, motor, visual, and limbic structures; suggesting that the striatum plays a more integral role in establishing motor and limbic circuits early in development, but plays a lesser role later on. Future research is needed to establish why this developmental shift occurs. Potential mechanisms include greater neural recruitment early in development necessary for learning, synaptic pruning of striatal connections, and/or changing dopamine concentrations.
Several developmentally-decreasing connections, such as intra-striatal and striatal-limbic, were non-specific, occurring across all striatal functional subdivisions. Other changes were, however, functionally-specific. For example, all putamen seeds increased connectivity with the cerebellar lobules Crus I and II, which are involved in higher-level cognition and are interconnected with the default mode and fronto-parietal networks (60–62). All putamen seeds also decreased connectivity with a spatially-distributed set of regions sub-serving basic motor control. This developmental reorganization suggests that the putamen is involved in establishing basic motor control early in development, but becomes more integral to circuits involved in higher-level cognition later.
Functionally-distinct developmental trends were found for striatal-ACC connections. Caudate seeds decreased connectivity with the rostral/pregenual ACC, which is commonly implicated in social and emotional aspects of cognition (63–65). Putamen seeds, on the other hand, decreased connectivity with the dorsal ACC, which is recruited for a variety of motor and executive function tasks and is in circuit with sensorimotor and cognitive control networks (66–68). Regional specificity likewise occurred in the reorganization of striatal-OFC connections, including developmental decreases with posterior OFC across all striatal subdivisions and developmental increases specifically between the ventral striatum and anterior OFC. While a number of studies have documented developmental changes in striatal-OFC connectivity between childhood and early adulthood (13–15), the finding that ventral striatum-anterior OFC connectivity strengthens is novel. Maturation of striatal-OFC circuits is consistent with previous literature documenting the development of reward and emotion-regulation circuitry through adolescence and young adulthood (69, 70). The current findings reveal previously undiscovered specificity in the development of distinct striatal circuitry.
Developing Striatal Connections Are Significantly Associated With Clinical Domains Striatal maturation plays a critical role in the emergence of neurodevelopmental and psychiatric disorders (8, 18, 28, 39). The current study is the first to distinguish striatal developmental patterns associated with specific clinical dimensions. This study is one of a growing number linking large-scale connectivity patterns with clinical symptom domains in developmental cohorts (46, 55, 71). Unlike previous research, the current study focused on the striatum and employed a novel approach in which the search for indicative connections was restricted to those showing robust developmental effects. This approach was based on a body of research identifying developmental factors that impact striatal function and potentially lead to the emergence of neurodevelopmental, psychotic, and mood disorders (8–10). As expected, the current study identified a set of ARCs associated to each symptom domain.
A critical question in linking brain function to clinical domains is whether meaningful distinctions between different domains exist. The current findings implicate both unique and overlapping connections for each clinical domain. Previous factor analyses across diagnostic items suggest that a single “p” factor indicating severity of general psychopathology explains common variance across different clinical domains (43, 44). Indeed, recent studies examining associations between cortical networks and clinical domains often find that those connections that are predictive of individual domains are likewise predictive of psychopathology, more generally (46, 55). However, distinct cortical connections have recently been associated with individual symptom domains (71). Most of the connections that were associated with general psychopathology overlapped with those identified for other domains (i.e. 16/21 general psychopathology connections overlapped with other domains). Further, the overlapping connections were those most strongly predictive of general psychopathology, and in all cases, they showed the same direction of effect (i.e. developmentally-delayed or developmentally-advanced) for each of predicted domains. Notably, the dorsal posterior insula (i.e. rolandic operculum) emerged as a particular locus associated with psychopathology across all domains. This granular region is commonly implicated in sensorimotor interoception (72–75) particularly to cardiac information (76), which is disrupted during stress (77, 78). It is integral to the processing of painful stimuli (79–81), encodes representations for social rejection (82), and may thereby be a general indicator of negative visceral and/or affective internal states (83). Putamen-dorsal posterior insula connections were associated with psychopathology across all four domains and included both overlapping and unique connections for each of the domains. Furthermore, the nearby left supramarginal gyrus was the only single connection associated with all four symptom dimensions. The results suggest that putamen-dorsal posterior insula connectivity is a biomarker of general psychopathology, which reflects elevated social and/or somatic distress associated with greater symptom severity.
Uniquely predictive connections were found for ADHD and depression symptom domains. Consistent with findings of altered function in the anterior insula (59, 84, 85) and abnormally-enhanced connectivity within cingulo-opercular and/or salience networks in individuals with ADHD (86–88), developmentally-accelerated connectivity in several middle and anterior dorsal insula regions was associated with greater ADHD symptom severity. Developmentally-delayed connectivity with nearby bilateral inferior frontal gyrus (BA 45), on the other hand, was associated with greater ADHD symptom severity, consistent with the well-established role of this region in inhibitory control dysfunction in ADHD (29, 59), and its upregulation with stimulant medication (89).
Limbic structures that are integral to the pathophysiology of depression were associated with depression symptomology. Accelerated maturation with posterior OFC (i.e. SGC) was uniquely predictive of depression, which is in line with the role of the SGC in affective processing and emotion regulation, as well as with its altered function in major depressive disorder (12, 90, 91). Other subcortical limbic structures that were predictive of depression, such as the hippocampus and amygdala, likewise play a critical role in affective processing in depression (92).
Unlike the other symptom domains, most of the connections associated with psychosis were also related to general psychopathology (i.e. 7 out of 8 connections). This included several medial prefrontal and superior temporal gyrus connections, which, while predictive of general psychopathology, were not predictive of ADHD or depressive symptoms. This overlap in the neural substrates of psychosis and general psychopathology is in line with previous characterizations suggesting that psychosis is indicative of more severe psychopathology than other symptom domains (44). Dysfunction in the medial prefrontal cortex, a core part of the default mode network, is commonly found in psychotic individuals (93–95) as is dysfunction in the superior temporal gyrus (31, 96–98).
The current study examined striatal maturation in a cross-sectional sample, spanning 8-22 years, taken from the general population. This is the largest such developmental study of striatal connectivity to-date and the growth-charting approach allowed for the identification of age-inappropriate striatal connectivity associated with ADHD, psychosis, depression, and general psychopathology. Future studies may improve characterization of striatal ARCs by employing a longitudinal study design, which could detect individual differences in developmental trajectories. Nonetheless, the present approach of examining age-deviation in striatal ARCs was successful for selecting predictive ARCs and accounted for a relatively high proportion of the variance. Thus, the EN models provided a powerful method for detecting atypical age-related connectivity associated with several clinical symptom domains.
Identification of such clinical biomarkers in the general population may be disadvantageous compared to case-control studies due to reduced symptom variability and smaller effect sizes, however, it is also advantageous in that it is robust to clinical confounds such as medication and it may identify early indicators of clinical risk. In summary, the current study found novel developmental trends in striatal connectivity, which lend insight into the maturation of reward-related, emotional, and cognitive functions. Novel findings of distinct striatal ARCs associated with ADHD, psychosis, and depression symptoms reveals distinct neurodevelopmental pathways for these domains. Identification of such pathways may indicate early clinical risk and provide clues for disease prevention.
Supplementary Material
Acknowledgments
Supported by NIMH grant K23MH110661 to Dr. Sarpal, R01MH108654 to Dr. Malhotra, R01MH085328 to Dr. Mostofsky, R01MH101506 to Dr. Karlsgodt, and a NARSAD young investigator award to Dr. Sarpal.
Data collection and sharing for this project was funded by the Pediatric Imaging, Neurocognition and Genetics Study (PING) (National Institutes of Health Grant RC2DA029475). PING is funded by the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health & Human Development. PING data are disseminated by the PING Coordinating Center at the Center for Human Development, University of California, San Diego. The names of the PING leadership, key senior contributors to the PING project design and/or organization, as well as collaborators from PING recruitment sites are listed at the following website: https://ping-dataportal.ucsd.edu/sharing/Authors10222012.pdf.
The full PING author list is:
Terry L. Jernigan, Ph.D.1, Connor McCabe, B.S. 1, Linda Chang, M.D.2, Natacha Akshoomoff, Ph.D.1, Erik Newman, Ph.D.1, Anders M. Dale, Ph.D.1, Thomas Ernst, Ph.D.2, Peter Van Zijl, Ph.D.3, Joshua Kuperman, Ph.D.1, Sarah Murray, Ph.D.4, Cinnamon Bloss, Ph.D.4, Nicholas J. Schork, Ph.D.4, Mark Appelbaum, Ph.D.1, Anthony Gamst, Ph.D. 1, Wesley Thompson, Ph.D. 1, Hauke Bartsch, Ph.D. 1, Brian Keating, Ph.D.2, David Amaral, Ph.D.5, Elizabeth Sowell, Ph.D.6, Walter Kaufmann, M.D. 3, Stewart Mostofsky, M.D.3, B.J. Casey, Ph.D.7, Erika J. Ruberry, B.A. 7, Alisa Powers, B.A.7, Bruce Rosen, M.D., Ph.D.8, Tal Kenet, Ph.D. 8, Jean Frazier, M.D. 9, David Kennedy, Ph.D. 9, Jeffrey Gruen, M.D.10.
1 University of California, San Diego, 2University of Hawaii, 3Kennedy Krieger Institute, 4Scripps Translational Science Institute, 5University of California, Davis, 6University of California, Los Angeles, 7Sackler Institute, Weill Cornell Medical College, Massachusetts General Hospital, Harvard University, 9University of Massachusetts: Yale University10.
Data access for the Philadelphia Neurodevelopmental Cohort (PNC) was provided by the National Center for Biotechnology Information (NCBI) database of Genotypes and Phenotypes (dbGaP), accession number phs000607.v1.p1. Support for the collection of the PNC dataset was provided by grant RC2MH089983 awarded to Raquel Gur and RC2MH089924 awarded to Hakon Hakonarson. All subjects were recruited through the Center for Applied Genomics at The Children’s Hospital in Philadelphia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Malhotra has served as a consultant for Forum Pharmaceuticals and has served on a scientific advisory board for Genomind, Inc. Dr. Lencz has served as a consultant to Genomind, Inc. The other authors report no biomedical financial interests or potential conflicts of interest.
References:
- 1.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. (2008): Functional connectivity of human striatum: a resting state FMRI study. Cerebral cortex. 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- 2.Maia TV, Frank MJ (2017): An Integrative Perspective on the Role of Dopamine in Schizophrenia. Biological psychiatry. 81:52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postuma RB, Dagher A (2006): Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral cortex. 16:1508–1521. [DOI] [PubMed] [Google Scholar]
- 4.Haber SN (2003): The primate basal ganglia: parallel and integrative networks. Journal of chemical neuroanatomy. 26:317–330. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 9:357–381. [DOI] [PubMed] [Google Scholar]
- 6.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL (2005): The cerebellum communicates with the basal ganglia. Nature neuroscience. 8:1491–1493. [DOI] [PubMed] [Google Scholar]
- 7.Kelly RM, Strick PL (2004): Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 143:449–459. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd GM (2013): Corticostriatal connectivity and its role in disease. Nature reviews Neuroscience. 14:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goff B, Tottenham N (2015): Early-life adversity and adolescent depression: mechanisms involving the ventral striatum. CNS spectrums. 20:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howes OD, Murray RM (2014): Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagan CC, Graham JM, Wilkinson PO, Midgley N, Suckling J, Sahakian BJ, et al. (2015): Neurodevelopment and ages of onset in depressive disorders. The lancet Psychiatry. 2:1112–1116. [DOI] [PubMed] [Google Scholar]
- 12.Arnsten AF, Rubia K (2012): Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 51:356–367. [DOI] [PubMed] [Google Scholar]
- 13.Fareri DS, Gabard-Durnam L, Goff B, Flannery J, Gee DG, Lumian DS, et al. (2015): Normative development of ventral striatal resting state connectivity in humans. Neuroimage. 118:422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter JN, Roy AK, Benson B, Carlisi C, Collins PF, Leibenluft E, et al. (2015): Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Developmental cognitive neuroscience. 11:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bo J, Lee CM, Kwak Y, Peltier SJ, Bernard JA, Buschkuehl M, et al. (2014): Lifespan differences in corticostriatal resting state connectivity. Brain connectivity. 4:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey BJ, Jones RM, Hare TA (2008): The adolescent brain. Annals of the New York Academy of Sciences. 1124:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crone EA, van Duijvenvoorde AC, Peper JS (2016): Annual Research Review: Neural contributions to risk-taking in adolescence--developmental changes and individual differences. Journal of child psychology and psychiatry, and allied disciplines. 57:353–368. [DOI] [PubMed] [Google Scholar]
- 18.Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U (2012): Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. The American journal of psychiatry. 169:264–272. [DOI] [PubMed] [Google Scholar]
- 19.Rubia K, Alegria AA, Brinson H (2014): Brain abnormalities in attention-deficit hyperactivity disorder: a review. Revista de neurologia. 58 Suppl 1:S3–16. [PubMed] [Google Scholar]
- 20.Shaw P, Gogtay N, Rapoport J (2010): Childhood psychiatric disorders as anomalies in neurodevelopmental trajectories. Human brain mapping. 31:917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sripada CS, Kessler D, Angstadt M (2014): Lag in maturation of the brain’s intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proceedings of the National Academy of Sciences of the United States of America. 111:14259–14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. (2007): Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 104:19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W (2013): Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biological psychiatry. 74:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, et al. (2017): Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. The lancet Psychiatry. 4:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, et al. (2010): Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biological psychiatry. 68:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein JN, Delbello MP, Adler CM, Altaye M, Kramer M, Mills NP, et al. (2009): Differential patterns of brain activation over time in adolescents with and without attention deficit hyperactivity disorder (ADHD) during performance of a sustained attention task. Neuropediatrics. 40:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oldehinkel M, Beckmann CF, Pruim RH, van Oort ES, Franke B, Hartman CA, et al. (2016): Attention-Deficit/Hyperactivity Disorder symptoms coincide with altered striatal connectivity. Biological psychiatry Cognitive neuroscience and neuroimaging. 1:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Rhein D, Oldehinkel M, Beckmann CF, Oosterlaan J, Heslenfeld D, Hartman CA, et al. (2016): Aberrant local striatal functional connectivity in attention-deficit/hyperactivity disorder. Journal of child psychology and psychiatry, and allied disciplines. 57:697–705. [DOI] [PubMed] [Google Scholar]
- 29.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (2013): Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA psychiatry. 70:185–198. [DOI] [PubMed] [Google Scholar]
- 30.Weinberger DR (1987): Implications of normal brain development for the pathogenesis of schizophrenia. Archives of general psychiatry. 44:660–669. [DOI] [PubMed] [Google Scholar]
- 31.Dandash O, Fornito A, Lee J, Keefe RS, Chee MW, Adcock RA, et al. (2014): Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 40:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubl D, Schultze-Lutter F, Hauf M, Dierks T, Federspiel A, Kaess M, et al. (2018): Striatal cerebral blood flow, executive functioning, and fronto-striatal functional connectivity in clinical high risk for psychosis. Schizophrenia research. [DOI] [PubMed] [Google Scholar]
- 33.Sarpal DK, Robinson DG, Fales C, Lencz T, Argyelan M, Karlsgodt KH, et al. (2017): Relationship between Duration of Untreated Psychosis and Intrinsic Corticostriatal Connectivity in Patients with Early Phase Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 42:2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin P, Wang X, Zhang B, Kirkpatrick B, Ongur D, Levitt JJ, et al. (2018): Functional dysconnectivity of the limbic loop of frontostriatal circuits in first-episode, treatment-naive schizophrenia. Human brain mapping. 39:747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarpal DK, Argyelan M, Robinson DG, Szeszko PR, Karlsgodt KH, John M, et al. (2016): Baseline Striatal Functional Connectivity as a Predictor of Response to Antipsychotic Drug Treatment. The American journal of psychiatry. 173:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, et al. (2015): Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA psychiatry. 72:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cadena EJ, White DM, Kraguljac NV, Reid MA, Lahti AC (2018): Evaluation of fronto-striatal networks during cognitive control in unmedicated patients with schizophrenia and the effect of antipsychotic medication. NPJ schizophrenia. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dean J, Keshavan M (2017): The neurobiology of depression: An integrated view. Asian J Psychiatr. 27:101–111. [DOI] [PubMed] [Google Scholar]
- 39.Pan PM, Sato JR, Salum GA, Rohde LA, Gadelha A, Zugman A, et al. (2017): Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disorder in a Longitudinal Community-Based Sample. The American journal of psychiatry. 174:1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong L, Yin Y, He C, Ye Q, Bai F, Yuan Y, et al. (2017): Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J Psychiatr Res. 84:9–17. [DOI] [PubMed] [Google Scholar]
- 41.Philippi CL, Motzkin JC, Pujara MS, Koenigs M (2015): Subclinical depression severity is associated with distinct patterns of functional connectivity for subregions of anterior cingulate cortex. J Psychiatr Res. 71:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao C, Wenhua L, Liu Y, Ruan X, Chen X, Liu L, et al. (2016): Decreased Subcortical and Increased Cortical Degree Centrality in a Nonclinical College Student Sample with Subclinical Depressive Symptoms: A Resting-State fMRI Study. Frontiers in human neuroscience. 10:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. (2014): The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clin PsycholSci. 2:119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caspi A, Moffitt TE (2018): All for One and One for All: Mental Disorders in One Dimension. The American journal of psychiatry.appiajp201817121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon JJ, Cordeiro SA, Weber MA, Friederich HC, Wolf RC, Weisbrod M, et al. (2015): Reward System Dysfunction as a Neural Substrate of Symptom Expression Across the General Population and Patients With Schizophrenia. Schizophr Bull. 41:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaczkurkin AN, Moore TM, Calkins ME, Ciric R, Detre JA, Elliott MA, et al. (2017): Common and dissociable regional cerebral blood flow differences associate with dimensions of psychopathology across categorical diagnoses. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erus G, Battapady H, Satterthwaite TD, Hakonarson H, Gur RE, Davatzikos C, et al. (2015): Imaging patterns of brain development and their relationship to cognition. Cerebral cortex. 25:1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, et al. (2009): Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain : a journal of neurology. 132:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kessler D, Angstadt M, Sripada C (2016): Growth Charting of Brain Connectivity Networks and the Identification of Attention Impairment in Youth. JAMA psychiatry. 73:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jernigan TL, Brown TT, Hagler DJ Jr., Akshoomoff N, Bartsch H, Newman E, et al. (2016): The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. NeuroImage. 124:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. (2014): Neuroimaging of the Philadelphia neurodevelopmental cohort. NeuroImage. 86:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J (2004): Unified univariate and multivariate random field theory. NeuroImage. 23 Suppl 1:S189–195. [DOI] [PubMed] [Google Scholar]
- 53.Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America. 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- 55. Kaufmann T, Alnaes D, Doan NT, Brandt CL, Andreassen OA, Westlye LT (2017): Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nature neuroscience. 20:513–515. [DOI] [PubMed] [Google Scholar]
- 56.Zou H, Hastie T (2005): Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B. 67 Part. 2:301–320. [Google Scholar]
- 57.Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, et al. (2017): Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nature protocols. 12:506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubia K, Alegria A, Brinson H (2014): Imaging the ADHD brain: disorder-specificity, medication effects and clinical translation. Expert review of neurotherapeutics. 14:519–538. [DOI] [PubMed] [Google Scholar]
- 59.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. (2016): Structural and Functional Brain Abnormalities in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder: A Comparative Meta-analysis. JAMA psychiatry. 73:815–825. [DOI] [PubMed] [Google Scholar]
- 60.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011): The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoodley CJ, Schmahmann JD (2009): Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 44:489–501. [DOI] [PubMed] [Google Scholar]
- 62.Stoodley CJ, Schmahmann JD (2010): Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex; a journal devoted to the study of the nervous system and behavior. 46:831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP (2009): Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation metaanalysis. Biological psychiatry. 65:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, et al. (2009): Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. The American journal of psychiatry. 166:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palomero-Gallagher N, Hoffstaedter F, Mohlberg H, Eickhoff SB, Amunts K, Zilles K (2018): Human Pregenual Anterior Cingulate Cortex: Structural, Functional, and Connectional Heterogeneity. Cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, et al. (2014): The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Human brain mapping. 35:2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2007): Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 37:579–588. [DOI] [PubMed] [Google Scholar]
- 68.Yu C, Zhou Y, Liu Y, Jiang T, Dong H, Zhang Y, et al. (2011): Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. NeuroImage. 54:2571–2581. [DOI] [PubMed] [Google Scholar]
- 69.Romer D, Reyna VF, Satterthwaite TD (2017): Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Developmental cognitive neuroscience. 27:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wahlstrom D, White T, Luciana M (2010): Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and biobehavioral reviews. 34:631–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, et al. (2018): Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 9:3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uddin LQ, Kinnison J, Pessoa L, Anderson ML (2014): Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. Journal of cognitive neuroscience. 26:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O (2017): Structure and Function of the Human Insula. J Clin Neurophysiol. 34:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deen B, Pitskel NB, Pelphrey KA (2011): Three systems of insular functional connectivity identified with cluster analysis. Cerebral cortex. 21:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K (2010): Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cerebral cortex. 20:1448–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schulz SM (2016): Neural correlates of heart-focused interoception: a functional magnetic resonance imaging meta-analysis. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gianaros PJ, Sheu LK (2009): A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. NeuroImage. 47:922–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD (2012): Brain systems for baroreflex suppression during stress in humans. Human brain mapping. 33:1700–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bowsher D, Brooks J, Enevoldson P (2004): Central representation of somatic sensations in the parietal operculum (SII) and insula. Eur Neurol. 52:211–225. [DOI] [PubMed] [Google Scholar]
- 80.Mazzola L, Faillenot I, Barral FG, Mauguiere F, Peyron R (2012): Spatial segregation of somato-sensory and pain activations in the human operculo-insular cortex. NeuroImage. 60:409–418. [DOI] [PubMed] [Google Scholar]
- 81.Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I (2015): The dorsal posterior insula subserves a fundamental role in human pain. Nature neuroscience. 18:499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, et al. (2014): Separate neural representations for physical pain and social rejection. Nat Commun. 5:5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Critchley HD, Garfinkel SN (2015): Interactions between visceral afferent signaling and stimulus processing. Frontiers in neuroscience. 9:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hawkey EJ, Tillman R, Luby JL, Barch DM (2018): Preschool Executive Function Predicts Childhood Resting-State Functional Connectivity and Attention-Deficit/Hyperactivity Disorder and Depression. Biological psychiatry Cognitive neuroscience and neuroimaging. 3:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vetter NC, Buse J, Backhausen LL, Rubia K, Smolka MN, Roessner V (2018): Anterior insula hyperactivation in ADHD when faced with distracting negative stimuli. Human brain mapping. 39:2972–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zepf FD, Bubenzer-Busch S, Runions KC, Rao P, Wong JWY, Mahfouda S, et al. (2017): Functional connectivity of the vigilant-attention network in children and adolescents with attention-deficit/hyperactivity disorder. Brain Cogn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barber AD, Carter CS (2005): Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cerebral cortex. 15:899–912. [DOI] [PubMed] [Google Scholar]
- 88.Janes AC, Gilman JM, Frederick BB, Radoman M, Pachas G, Fava M, et al. (2018): Salience network coupling is linked to both tobacco smoking and symptoms of attention deficit hyperactivity disorder (ADHD). Drug Alcohol Depend. 182:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, Radua J (2014): Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biological psychiatry. 76:616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. (2017): Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature medicine. 23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giacobbe P, Mayberg HS, Lozano AM (2009): Treatment resistant depression as a failure of brain homeostatic mechanisms: implications for deep brain stimulation. Exp Neurol. 219:44–52. [DOI] [PubMed] [Google Scholar]
- 92.Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG (2013): Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neuroscience and biobehavioral reviews. 37:152–163. [DOI] [PubMed] [Google Scholar]
- 93.Landin-Romero R, McKenna PJ, Salgado-Pineda P, Sarro S, Aguirre C, Sarri C, et al. (2015): Failure of deactivation in the default mode network: a trait marker for schizophrenia? Psychol Med. 45:1315–1325. [DOI] [PubMed] [Google Scholar]
- 94.Pomarol-Clotet E, Canales-Rodriguez EJ, Salvador R, Sarro S, Gomar JJ, Vila F, et al. (2010): Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Molecular psychiatry. 15:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. (2009): Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dutt A, Tseng HH, Fonville L, Drakesmith M, Su L, Evans J, et al. (2015): Exploring neural dysfunction in ‘clinical high risk’ for psychosis: a quantitative review of fMRI studies. J Psychiatr Res. 61:122–134. [DOI] [PubMed] [Google Scholar]
- 97.Crossley NA, Mechelli A, Fusar-Poli P, Broome MR, Matthiasson P, Johns LC, et al. (2009): Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Human brain mapping. 30:4129–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wojtalik JA, Smith MJ, Keshavan MS, Eack SM (2017): A Systematic and Meta-analytic Review of Neural Correlates of Functional Outcome in Schizophrenia. Schizophr Bull. 43:1329–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


