Abstract
Tendon disorders represent the most common musculoskeletal complaint for which patients seek medical attention; inflammation drives tendon degeneration before tearing and impairs healing after repair. Clinical evidence has implicated the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway as a correlate of pain-free return to function after surgical repair. However, it is currently unknown whether this response is a reaction to or a driver of pathology. Therefore, we aimed to understand the clinically relevant involvement of the NF-κB pathway in tendinopathy, to determine its potential causative roles in tendon degeneration, and to test its potential as a therapeutic candidate. Transcriptional profiling of early rotator cuff tendinopathy identified increases in NF-κB signaling, including increased expression of the regulatory serine kinase subunit IKKβ, which plays an essential role in inflammation. Using cre-mediated overexpression of IKKβ in tendon fibroblasts, we observed degeneration of mouse rotator cuff tendons and the adjacent humeral head. These changes were associated with increases in proinflammatory cytokines and innate immune cells within the joint. Conversely, genetic deletion of IKKβ in tendon fibroblasts partially protected mice from chronic overuse-induced tendinopathy. Furthermore, conditional knockout of IKKβ improved outcomes after surgical repair, whereas overexpression impaired tendon healing. Accordingly, targeting of the IKKβ/NF-κB pathway in tendon stromal cells may offer previously unidentified therapeutic approaches in the management of human tendon disorders.
INTRODUCTION
Twenty percent of all consultations made to primary care physicians are related to musculoskeletal diseases; 30% of these are associated with tendon injuries, namely tendinopathies (1, 2), representing a highly prevalent problem in musculoskeletal medicine. In the shoulder, rotator cuff tendinopathy is a frequent source of pain and disability for more than 17 million individuals in the United States alone, leading to lost days from work, occupational challenges, recreational limitations, and increased likelihood of tendon tears (3). Unfortunately, treatment outcomes are variable, operative management is equivalent to nonoperative care in some cases (4–6), and surgical repair failure rates range from 20 to 94% (7–10). Factors associated with failure include tear size, chronicity, patient age, and other environmental factors (9, 11, 12). Therefore, dissecting the cellular and molecular processes of tendon degeneration and healing after surgical repair will allow clinicians to implement preventative interventions and prescribe therapeutics to improve outcomes.
The role of inflammation in tendon degeneration and healing has been debated over the past three decades. Growing evidence supports its fundamental role in disease progression (2, 13–15). It is hypothesized that chronic inflammation may drive degeneration before tearing and may lead to fibrovascular scarring during healing and therefore is an attractive target for therapeutic intervention (15–17). Targeted blockade of inflammatory mechanisms after tendon repair can improve pre-clinical outcomes, but the mechanism of action remains unknown (18–20). Recent clinical investigations have revealed that early stages of tendinopathy involve nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (13, 15), a protein complex that controls cytokine production and apoptosis. Pathologic tendon stromal cells exhibit phenotypic plasticity with higher NF-κB target gene expression even without proinflammatory stimulus (21). Dissecting the pivotal role that the NF-κB signaling pathway plays in mediating tendinopathy and tendon healing is crucial to developing therapeutic strategies.
The canonical NF-κB pathway constitutes a family of “rapid-acting” protein complexes that are bound to dimer-specific inhibitor of NF-κB proteins (IκB) and held in latency within the cytoplasm (22). Inflammatory stimuli, acting through receptor-specific mechanisms, induce the recruitment of the IκB kinase (IKK) complex, which phosphorylates IκB, leading to its degradation. NF-κB dimers are then free to translocate to the nucleus and induce transcription. Thus, IKK is an indispensable fulcrum for NF-κB signaling. The IKK complex is composed of the scaffolding protein NF-κB essential modifier (NEMO) and catalytic subunits IKKα and IKKβ, the latter of which regulates inflammation and fibrosis by targeting the IκBα:p50:p65 NF-κB complex. Therefore, specifically restraining IKKβ expression or activity may slow or arrest tendinopathy progression and may improve healing outcomes without altering other essential physiologic activities of the NF-κB pathway. Here, we (i) confirm increased canonical NF-κB signaling in human rotator cuff tendinopathy, (ii) recapitulate tendon degeneration through persistent IKKβ activation in tendon stromal cells in vivo, (iii) identify IKKβ as a necessary component of tendinopathy in animal models, and (iv) demonstrate the therapeutic potential of blocking IKKβ activity.
RESULTS
NF-κB signaling is increased in clinical tendinopathy
Clinical samples ofearly-stage tendinopathy exhibited dysregulation of more than 65% of the NF-κB-associated genes assayed (Fig. 1, A and B). Transcripts of the NF-κB complex subunits [Nfkb1: mean, 6.95-fold increase (P = 0.019); Rel: mean, 8.41-fold increase (P = 0.007); Relb: mean, 13.53-fold increase (P = 0.019); Fig. 1C] and IKK complex proteins [Chuk: mean, 11.47-fold increase (P < 0.001); Ikbkb: mean, 6.71-fold increase (P = 0.045); Fig. 1D] were up-regulated in patients with rotator cuff disease. Because NF-κB signaling is present in the pathogenesis of rotator cuff disease, and IKKβ is essential for p65 phosphorylation (23) and nuclear translocation (Fig. 2A), we next asked whether IKKβ activation in tendon stromal cells was sufficient to drive tendon degeneration.
Fig. 1. NF-κB signaling in clinical tendinopathy.
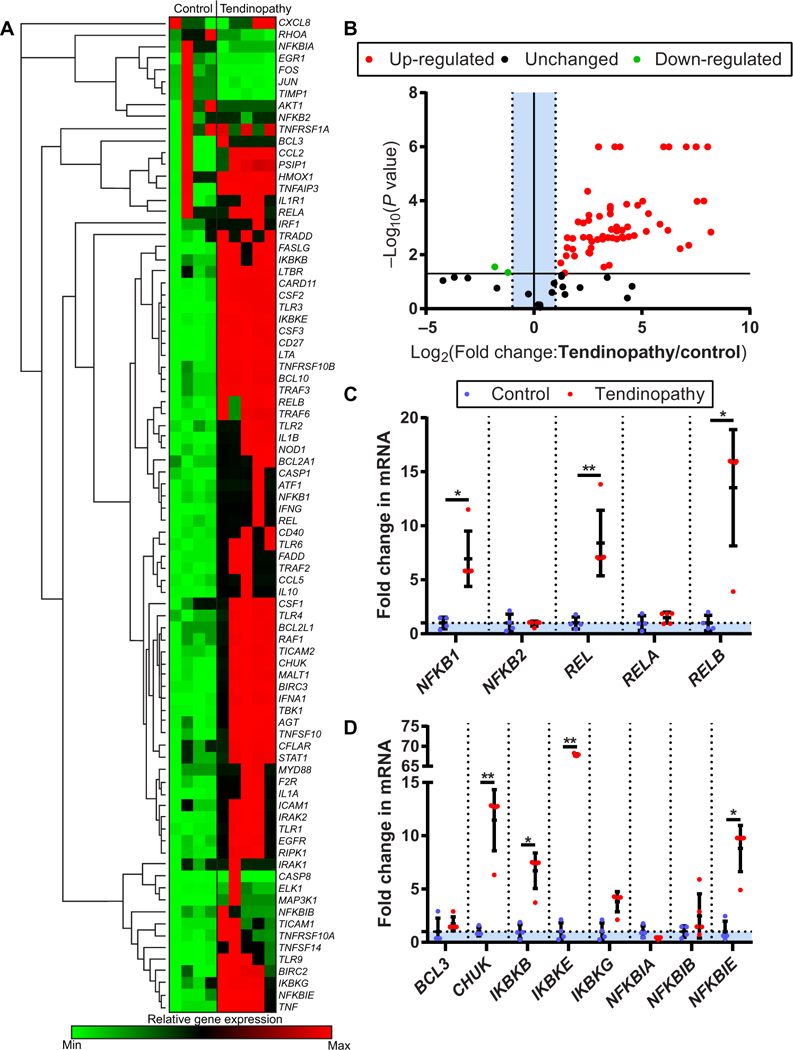
(A) Heat map of NF-κB profiling gene expression array from healthy human hamstrings tendons (control, n = 4) and early-stage diseased tendons (tendinopathy, n = 5). (B) Volcano plot of NF-κB profiling gene expression array. P < 10−6 are scaled for visualization purposes. (C) NF-κB complex protein coding genes NFKB1, REL, and RELB in control and tendinopathy tendon samples. (D) Regulatory NF-κB protein coding genes CHUK, IKBKB, IKBKE, and NFKBIE in control and tendinopathy tendon samples. Data are shown as means ± SD with individual points representing biologically independent samples. Statistically significant differences were calculated using multiple t tests with Holm-Šídák correction, **P < 0.01 and *P < 0.05.
Fig. 2. Modulation of IKKβ expression in murine tendon fibroblasts.
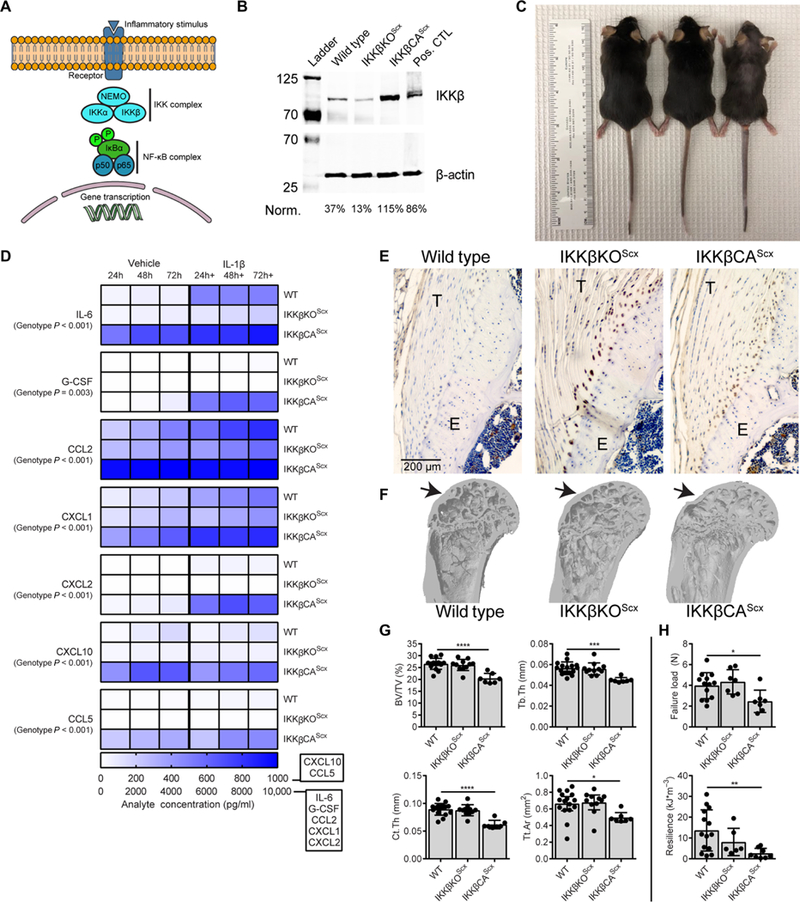
(A) Schematic of NF-κB signaling and gene transcription. NF-κB signaling was controlled by targeting inhibitor of NF-κB kinase subunit p (IKKβ), which acts upstream of the NF-κB complex. Tendon fibroblast IKKβ modulation was achieved by deletion of IKKβ (IKKβKOScx) and activation of IKKβ (IKKβCAScx) using Cre-loxP-mediated recombination under the Scx promoter. (B) Expression of IKKβ in tendon fibroblasts from WT, IKKβKOScx, and IKKβCAScx mice. Cultured mouse osteoclasts were used as a positive control (pos. CTL) (51). (C) Photograph of 16-week-old mice to demonstrate hair loss. (D) Secreted cytokines and growth factors in vehicle and IL-1β-treated tendon fibroblasts from WT, IKKβKOScx, and IKKβCAScx mice (n = 5 per group). (E) Immunolabeling for CD68 (brown) in the supraspinatus tendon from WT, IKKβKOScx,and IKKβCAScx mice. T, tendon; E, enthesis. (F) Microcomputed tomography (μCT) three-dimensional reconstruction of coronal section from proximal humerus. Arrows denote the supraspinatus tendon attachment site. (G) Quantification of bone morphometry: Bone volume normalized to total volume (BV/TV), trabecular thickness (Tb.Th), cortical thickness (Ct.Th), and total cortical area (Tt.Ar) (n = 8 to 9 per genotype). (H) Quantification of mechanical properties of the supraspinatus tendon-to-bone attachment (n = 8 to 9 per genotype). Data are shown as means ± SD with individual points representing biologically independent samples. Statistically significant differences were calculated using one-way analysis of variance (ANOVA) (genotype) with Fisher’s least significant difference (LSD) post hoc test. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
Chronic IKKβ overexpression in murine tendon fibroblasts mimics human rotator cuff disease
Conditional modulation of IKKβ was achieved using the tendon-specific scleraxis (Scx) promoter (24) driving cre-mediated recombination of LoxP sites flanking either exon 7 of the Ikbkb gene (IKKβKOScx) or a stop codon preceding a constitutively active form of IKKβ within an inserted Rosa26 locus (IKKβCAScx)(Fig. 2B) (25,26). IKKβCAScx mice exhibited noticeable hair loss by 8 weeks old, which became progressively worse throughout adulthood, whereas IKKβKOScx mice had no overt phenotype (Fig. 2C). Cultured tendon fibroblasts from IKKβCAScx mice responded to interleukin-1β (IL-1β) stimulus with increased expression of the downstream transcriptional targets tumor necrosis factor-α (Tnfα) and prostaglandin-endoperoxide synthase 2 (Ptgs2) of the NF-κB pathway in a dose-dependent manner (Tnfα, P = 0.0024; Ptgs2, P < 0.0001; fig. S1). Expression of matrix metalloproteinase (MMP)-1a, MMP-3, and MMP-13 was significantly decreased in IKKβKOScx compared to wild-type (WT) and IKKβCAScx tendon fibroblasts in vitro (MMP-1a: P < 0.0001, MMP-3: P < 0.0001, and MMP-13: P < 0.0001; fig. S1). Expression of Scx, a marker of tendon fibroblast differentiation, was not affected by IKKβ modulation, whereas expression of tenomodulin (Tnmd) was significantly increased in IKKβCAScx compared to WT and IKKβKOScx tendon fibroblasts (Tnmd, P < 0.0001; fig. S1). Expression of collagen (Col)–1a1 was significantly decreased in IKKβKOScx compared to WT tendon fibroblasts (Col1a1, P = 0.0004; fig. S1). At the translational level, IKKβCAScx tendon fibroblasts continually secreted high amounts of IL-6, C-C motif chemokine ligand (CCL)–2, CCL-5, and C-X-C motif chemokine ligand (CXCL)–10 in vitro (Fig. 2D and fig. S2). IKKβCAScx cells were also more sensitive to IL-1β stimulus, showing significant increases in granulocyte colony-stimulating factor (G-CSF) and CXCL-2 production (G-CSF: P=0.036 andCXCL-2: P=0.042; Fig.2D). In vitro studies of human tendon fibroblasts exposed to cytokines (IL-1β, IL-17A, and IL-33) exhibited similar inflammatory secretomes and were sensitized to repeated cytokine exposure (14, 15, 27, 28). Knockdown of IKKβ effectively desensitized tendon fibroblasts to proinflammatory stimulus (Fig. 2D and fig. S2).
Tendons from IKKβCAScx mice exhibited increases in cellularity and inflammatory cells (including CD68+ macrophages within the tendon and epitenon) and loss of metachromasia at the enthesis (implying a loss of proteoglycans at the fibrocartilaginous tendon-to-bone attachment) (Fig. 2E, fig. S3, and table S1). Furthermore, IKKβCAScx mice exhibited structural and functional losses of the rotator cuff, including less cortical and trabecular bone of the humeral head (Fig. 2G) and reduced tendon mechanical properties (Fig. 2H). These outcomes are similar to patients with rotator cuff disease, who also present with increased CD68+ tissue-resident macrophages (13, 15), loss of cortical and cancellous bone near the tendon enthesis (29), and increased compliance of the tendons (30). On the basis of these data, we next investigated the therapeutic potential ofmodulating the IKKβ/NF-κB signaling pathway for mitigating tendinopathy.
Chronic overuse degrades tissue function
Downhill treadmill running is a rodent model ofoveruse tendinopathy (31, 32); however, how closely this model aligns with clinical cases of rotator cuff disease and NF-κB signaling is unknown. Four weeks of overuse did not induce chronic dysregulation of NF-κB signaling (Fig. 3, A and B). Histologically, the tendons and tendon entheses of IKKβKOScx mice appeared similar to those in WT mice (Fig. 3C). The tendons were populated with spindle-shaped fibroblasts aligned with the direction of collagen fibers. The tendon entheses were populated by chondrocytes aligned in columns from tendon to bone. There were increased cell numbers and a loss of cell organization in the tendons and tendon entheses of IKKβCAScx mice.
Fig. 3. Modulation of IKK|β/NF-kB signaling with chronic overuse.
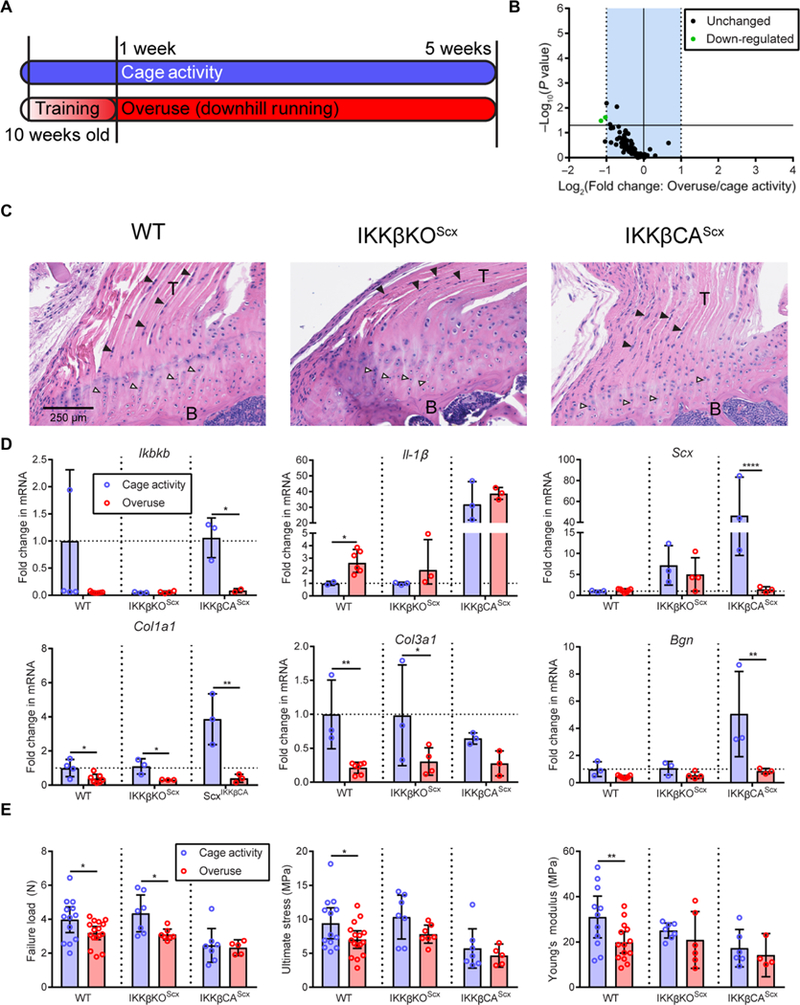
(A) Ten-week-old mice were subjected to a chronic overuse protocol with 1 week of progressive training, followed by 4 weeks of downhill running. Control mice were permitted normal cage activity. (B) NF-κB pathway-related gene regulation due to overuse. (C) Hematoxylin and eosin (H&E) images of WT, IKKβKOScx, and IKKβCAScx mice. B, bone; black arrowhead, spindle-shaped tendon fibroblast; white arrowhead, enthesis chondrocyte. (D) mRNA expression of Ikbkb, IL-1β, Scx, Col1a1, Col3a1, and Bgn in tendon from control cage-active or treadmill overuse-subjected WT (n = 4 to 6 per group), IKKβKOScx (n = 3 to 4 per group), and IKKβCAScx (n = 3 per group) mice. (E) Failure load, ultimate stress, and Young’s modulus of the supraspinatus tendon-to-bone attachment in cage-active and treadmill overuse-subjected mice (n = 5 to 13 per group). Data are shown as means ± SD with individual points representing biologically independent samples. Statistically significant differences were calculated using two-way ANOVA (genotype, overuse) with Fisher’s LSD post hoc test. **P < 0.01 and *P < 0.05.
Treadmill running down-regulated Ikbkb and tendon-associated (Scx, Col1a1, Col3a1, and Bgn) transcripts (Fig. 3D) across genotypes when compared to cage activity controls. WT mice exhibited degeneration of the tendon-to-bone attachment mechanical properties due to overuse (Fig. 3E). Abolishing IKKβ signaling in IKKβKOScx mice protected from losses of ultimate stress and Young’s modulus (Fig. 3E). Activation of IKKβ in IKKβCAScx mice, coupled with treadmill running, did not worsen the mechanical properties compared to cage activity. Overuse did not have an apparent effect on the bone microstructure (fig. S4). These results suggest that changes in tendon function due to chronic overuse are not solely dependent on the IKKβ/NF-κB signaling axis. To examine the role of NF-κB signaling after a tendon tear, we next investigated murine acute rotator cuff injury with immediate repair.
Blocking IKKβ improves surgical repair outcomes after acute injury
To examine the role of IKKb during tendon healing, mice underwent surgical transection and immediate repair of the supraspinatus tendon (Fig. 4, A and B). Injury and repair led to increased NF-κB signaling within the tendon 2 weeks after surgery (Fig. 4C), including up-regulation of protein coding genes for the IKK and NF-κB complexes (Fig. 4D). Injury and repair resulted in significant hypertrophy of the supraspinatus tendons from WT and IKKβKOScx mice (WT: P = 0.0051 and IKKβKOScx: P = 0.0024; Fig. 4E). The structural and material properties of the tendon-to-bone attachment were reduced in WT mice. Deletion of IKKβ resulted in no significant differences in stiffness (P = 0.21), resilience (P = 0.29), or Young’s modulus (P = 0.13) when compared to contralateral sham operation limbs (Fig. 4E). Constitutive activation of IKKβ suppressed healing, resulting in significantly lower tendon failure strength (P = 0.033). The acute injury and repair procedure did not have any apparent effect on the bone microstructure (fig. S5). On the basis of these results, we tested the potential therapeutic efficacy of pharmacological inhibition of IKKβ using a small-molecule inhibitor in an in vitro human inflammatory tendinopathy model.
Fig. 4. Modulation of IKKβ/NF-κB signaling with acute supraspinatus injury and repair.
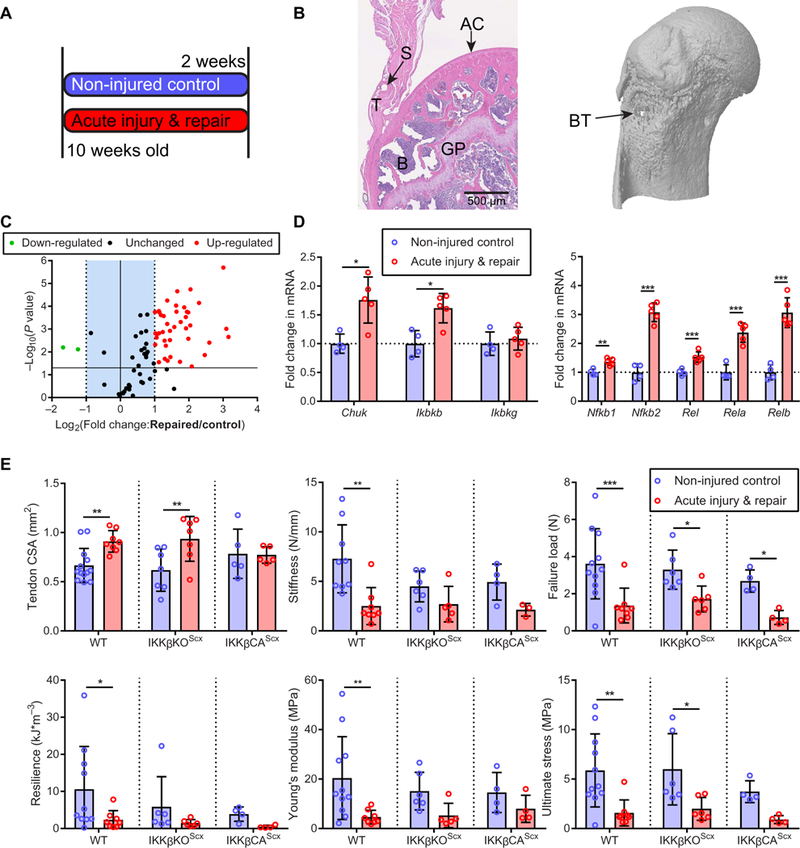
(A) Experimental protocol. Ten-week-old mice were subjected to a unilateral acute injury of the supraspinatus tendon and immediate repair, followed by 2 weeks of recovery. Sham operations were performed on contralateral limbs. (B) H&E image ofthe repaired tendon and new bone formation around the suture tunnel after 2 weeks of recovery. GP, growth plate; S, suture hole; AC, articular cartilage. The μCT image (right) shows the bone tunnel (BT) below the epiphysis. (C) NF-κB signaling gene expression 2 weeks after acute injury and repair (n = 4 to 5 per group). (D) mRNA expression of IKK complex-related genes and NF-κB complex-related genes in tendon 2 weeks after recovery (n = 4 to 5 per group). (E) Quantification of murine tendon cross-sectional area (CSA), stiffness, failure load, resilience, Young’s modulus, and ultimate stress 2 weeks after injury and repair (n = 4 to 5 per group). Data are shown as means ± SD with individual points representing biologically independent samples. Statistically significant differences were calculated using two-way ANOVA (genotype, injury) with Fisher’s LSD post hoc test. ***P < 0.01, **P <0.01,*P <0.05.
In vitro inhibition of IKKβ in human tendon stromal cells blocks NF-κB signaling and cytokine production
Tendon fibroblasts were isolated from healthy human hamstring tendons and cultured with IL-1β to model inflammatory tendinopathy in vitro. IKKβ inhibitor VIII successfully repressed transcription of most NF-κB signaling genes in the IL-1β-treated fibroblasts (Fig. 5A). Production of IL-6 and CCL-2 was reduced to control concentrations by the inhibitor treatment (Fig. 5B). These two cytokines were the most abundant in cultured tendon fibroblasts from IKKβCAScx mice and are present in human tendinopathy (17). Treatment did not significantly change the expression of extracellular matrix transcripts Col1a1 or Col3a1 (Col1a1: P = 0.21 and Col3a1: P = 0.61; fig. S6). Together, pharmacological inhibition of IKKβ desensitized human tendon fibroblasts to IL-1β stimulation in vitro while maintaining their transcriptional identity.
Fig. 5. Small-molecule inhibition of IKKβ in an in vitro model of inflammation.
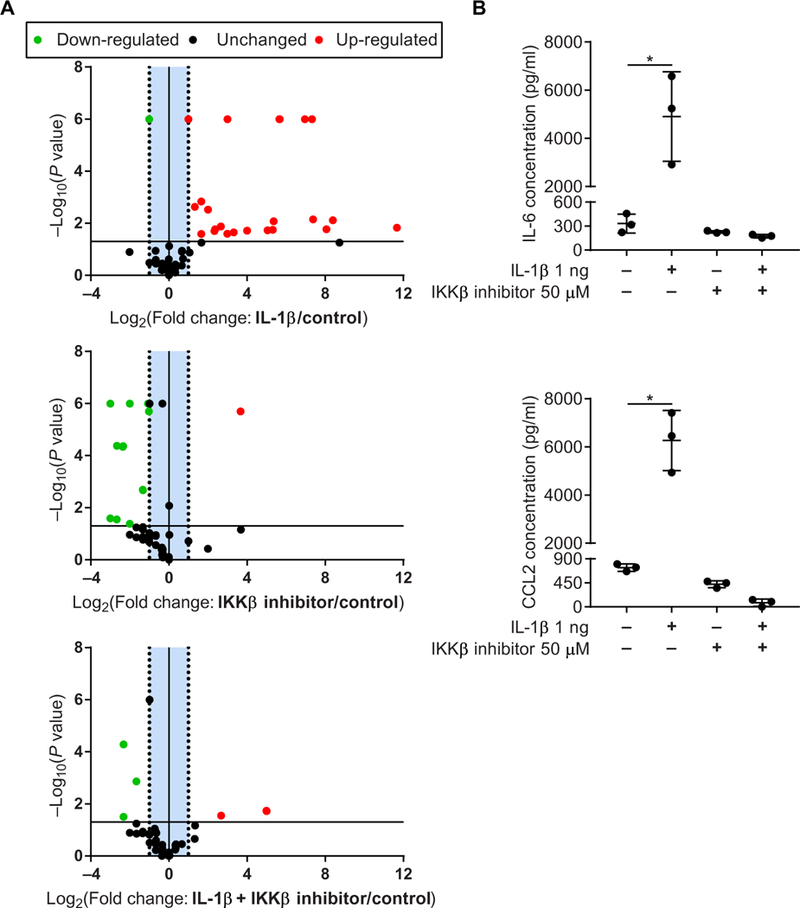
(A) Volcano plots of NF-κB signaling array in healthy human tendon fibroblasts treated with IL-1β with or without IKKβ inhibitor. (B) Proinflammatory cytokines IL-6 and CCL-2 produced by healthy human tendon fibroblasts treated with IL-1β with or without IKKβ inhibitor. Data are shown as means ± SD. Statistically significant differences were calculated using one-way ANOVA (treatment) with Fisher’s LSD post hoc test. *P < 0.05.
DISCUSSION
The NF-κB signaling pathway plays a central role in inflammation, stress response, and cell survival (22). In this study, we observed increases in NF-κB signaling in the subscapularis tendons of patients undergoing surgical repair of the rotator cuff. Intact subscapularis tendons in rotator cuff disease served as a model for the early stages of tendinopathy due to mechanical overuse (33). Studies of early-stage tendinopathic tissues, including the subscapularis, have previously revealed increases in NF-κB complex protein expression, downstream proinflammatory cytokine transcripts, and immune cell recruitment and activation (13,15). This inflammatory phenotype persists in patients with chronic pain after subacromial decompression treatment (17, 21). Specifically, fibroblasts within the diseased tendon microenvironment continued to exhibit phenotypic “memory” 4 years after treatment, were primed to be more responsive to IL-1β stimulation in vitro, and did not resolve mRNA expression of IL-6 and IL-8 after cessation of stimulus (21). Dakin et al. (21) proposed that this memory function is a crucial mechanism in the development of chronic inflammation. Therefore, resolution of NF-κB-mediated inflammation may be critical for successful outcomes in human tendon disease.
NF-κB may drive degeneration before tearing and impair healing after surgical repair. To better understand the intrinsic degenerative mechanisms of rotator cuff tendinopathy, NF-κB was selectively activated or inhibited in tendon fibroblasts of genetically modified mice (fig. S7). Analysis of mice with constitutively active IKKβ demonstrated that activation of the canonical NF-κB pathway was sufficient to induce degeneration in vivo, paralleling hallmarks of rotator cuff disease. Tendon stromal cells in these mice perpetually secreted various cytokines that are present in exercise adaptation and clinical tendinopathy (27, 34, 35). Cytokines of the interleukin family can exhibit various effects on tendon extracellular matrix remodeling. For example, IL-6 increases total collagen synthesis (36); however, IL-17 and IL-33 increase the ratio of type III to type I collagen and up-regulate MMP production (17). In addition to interleukins, we found that the tendon stromal compartment from IKKβCAScx mice produced many chemotactic (CCL-2, CCL-5, CXCL1, CXCL2, and CXCL10) and G-CSF proteins with and without IL-1β stimulation. These factors may drive the observed increases in CD68+ cells in tendons from IKKβCAScx mice. Clinical samples of early- and advanced-stage disease tendons exhibit active recruitment of CD14+ monocytes that mature into CD68+ macrophages (13, 15). Changes in tendinopathy gene and protein signatures are believed to follow macrophage activation. However, recent studies of fibroblast-macrophage signaling circuits in vitro demonstrated that dysregulation of the stromal compartment results in untethered macrophage expansion and activation, potentially overloading organ-carrying capacity and leading to degeneration (37). After a tendon tears, animal models have shown that macrophages are the primary immune cell at the site of injury and promote scar formation, cell death, and matrix degradation (38). Modulating macrophage behavior by promoting M2 polarization using adipose-derived stem cells and growth factors has a protective effect on tendon fibroblasts in this context (39, 40). Yet, tendon healing may also be improved by physical activity that drives a robust inflammatory response characterized by an increased cytokine transcription and a delayed switch to M2 polarization (41, 42). However, detensioning of engineered human tendon tissue also increases the expression of proinflammatory mediators and alters the response to growth factors, indicating that properly balanced mechanical loading is required to develop, maintain, and heal tendons (43). Thus, further studies examining the cross-talk among resident stromal, immune-sensing, and infiltrating compartments, as well as mechanical loading in tendinopathy and healing, are required.
At the tissue level, the constitutive activation of IKKβ resulted in mechanically weaker attachment of the supraspinatus tendon to the humeral head compared to WT controls. Recent advances in ultrasound elastography have revealed that clinically diagnosed tendinopathy correlates with increased compliance of the tendon (44). Similar results have been observed in many animal injury models (38), including the treadmill overuse protocol. We also found a loss of bone microstructure within the adjacent humeral head. Similarly, patients with partial- and full-thickness rotator cuff tears lose bone mass near the tendon insertion site, which might be attributed to decreased mechanical loading after a tear (29). However, we found that the constitutive activation of the canonical NF-κB pathway in tendons can also drive bone loss, suggesting a cellular signaling network beyond the local microenvironment.
To determine the role of the canonical NF-κB pathway in clinically relevant pathologic scenarios, we genetically ablated IKKβ-mediated signaling in the tendon fibroblasts of mice and subjected them to treadmill overuse (to model tendinopathy before a tendon tear) or acute supraspinatus tendon injury and repair (to model tendon healing after surgical repair). In both cases, IKKβKOScx mice were partially protected from biomechanical degeneration compared to WT controls or IKKβCAScx mice. In vitro, IKKβKOScx tendon fibroblasts were protected from IL-1β stimulus: They showed decreases in transcription of matrix turnover enzymes (Mmp-1a, Mmp-3, and Mmp-13) and in translation of proinflammatory cytokines and chemokines, while maintaining tenogenic markers (Scx) and matrix production (Col1a1). These data support the idea that NF-κB signaling is a key checkpoint for sustaining inflammatory signaling networks and directing tendon remodeling (17, 27). Many downstream effectors, such as cytokines, have been found at the tissue level in human and animal models of tendinopathy; however, there is limited evidence for increases in NF-κB signaling specifically in nonmyeloid cells of diseased tendons (14, 15, 21). Our findings mechanistically demonstrate the capacity of the tendon stromal compartment to shape the healing niche and provide a molecular target for improving the treatment of clinical tendinopathy. A potential crux of NF-κB-directed therapy is specifically targeting tendon stromal cells while allowing resident and infiltrating immune cells to initiate tissue repair. Leveraging technology such as nanoparticle-based delivery of NF-κB-targeted small interfering RNA in a cell-specific manner could provide a platform for advancing tendon therapeutics (45).
A limitation of the human biopsy portion of the study was the use of hamstrings tendon as healthy control samples. A second limitation is that murine models of tendinopathy cannot fully recreate the human condition (46). We chose to examine two different models of clinically relevant tendinopathy that address various aspects of tendon remodeling and surgically repaired healing. Although we did not observe the chronic dysregulation of NF-κB signaling due to treadmill overuse, IKKβKOScx mice were partly protected from degeneration. These results highlight a possible novel role of this pathway in tendon fibroblasts. Last, the in vitro model demonstrated therapeutic potential for suppressing the NF-κB pathway but only provides a limited model of inflammatory tendinopathy (i.e., inflammation was modeled with a single cytokine). Future in vivo studies are necessary to determine the efficacy of IKKβ inhibition for treating tendinopathy and/or improving tendon healing.
In summary, we observed increases in the canonical NF-κB signaling in patients with rotator cuff tendinopathy. Overexpression of IKKβ in murine tendon fibroblasts recreated rotator cuff disease in vivo. Inhibiting IKKβ in tendon fibroblasts limited biomechanical degeneration of the supraspinatus tendon due to overuse injury and partially improved healing after surgical repair.
MATERIALS AND METHODS
Study design
The goal of this study was to investigate the role of the NF-κB pathway in rotator cuff degeneration and healing. We compared gene signatures between patients with early-stage rotator cuff tendinopathy and control hamstrings samples from patients undergoing anterior cruciate ligament reconstruction. We further explored the mechanistic role of this signaling pathway in vivo using genetically modified mice by introducing gain- and loss-of-function IKKβ mutations in tendon fibroblasts. Last, we hypothesized that ablating IKKβ/NF-κB signaling could protect tendon fibroblasts from inflammation in the context of chronic overuse and acute injury and repair. Statistical analysis and sample sizes were determined from previous studies (13, 16, 39, 47) that were sufficiently powered to detect meaningful differences in clinical tendinopathy samples, murine rotator cuff microstructure, and biomechanical function and using an in vitro model of tendinopathy. Samples for biomechanical testing, μCT, and histological scoring were randomized, and analysis was performed by a blinded investigator. Sample size and replication are provided in the figure legends.
Study approval
Human study procedures and protocols were approved by the National Health Service West of Scotland Ethics Committee (REC 11/S0704/7). Full informed consent was obtained from all patients. Animal studies were approved by Washington University and Columbia University Institutional Animal Care and Use Committees.
Clinical samples
Samples of “early-stage” tendinopathy were obtained using a previously established protocol (13). The subscapularis tendon (n = 5; age, 33 to 56 years) was obtained from patients with rotator cuff tears undergoing reparative surgery using a standard three-portal technique. The tendon was arthroscopically biopsied from the superior border of the tendon 1 cm lateral to the glenoid labrum. Control hamstring tendons (confirmed by H&E staining; Bonar score 1) were taken from patients undergoing anterior cruciate ligament autograft and used as healthy controls for gene expression (n = 4; age, 22 to 44 years) or primary cell extraction (n = 3).
Generation of tendon-specific IKKβ mice
Genetic manipulation of the canonical NF-κB signaling in tendon fibroblasts was achieved using cre-loxP cross-breeding. Tendon specificity was determined using an Scx promoter driving a cre-recombinase (ScxCre) sequence in a bacterial artificial chromosome transgene (48). IKKβ expression was ablated in tendon fibroblasts by crossing ScxCre mice with Ikbkbtm2Cgn (IKKβ-floxed) mice to yield IKKβKOScx (25). IKKβ overexpression was accomplished using Gt(Rosa26)tm4(Ikbkb)Rsky (IKKβCA-floxed) mice harboring an internal ribosomal entry site-enhanced green fluorescent protein and complementary DNA (cDNA) sequence encoding Ikbkb preceded by a floxed stop codon to yield IKKβCAScx (26). All initial breeding pairs were set up with 8-week-old mice to produce the 31 female and 83 male mice used in this study [WT, n = 57 (19 females and 38 males); IKKβKOScx, n = 29 (6 females and 23 males); and IKKβCAScx, n = 28 (6 females and 22 males)].
Tendon fibroblast isolation and culture
After euthanasia, tail tendons were dissected from mice, minced, and placed in alpha-modified Eagle’s medium (alpha-MEM; Gibco). Tissue was digested in 0.2% collagenase type A (Sigma-Aldrich) in phosphate-buffered saline for 3 hours. The digested tissue was passed through a 100-μm cell strainer, pelleted by centrifugation at 460g for 5 min; the supernatant was discarded; and the cells were resuspended and plated in supplemented culture medium [alpha-MEM with 10% fetal bovine serum (Sigma-Aldrich), 1% penicillin/streptomycin (Gibco), and 1% amphotericin B (Invitrogen)] at 37°C, 5% CO2, and 95% humidity. The cells were used on passage 2. Human tendon-derived cells were extracted from biopsied hamstring tendon explants (age, 22 to 44 years). Cultures were maintained at 37°C in a humidified atmosphere of5% CO2 for 28 days. Cells were subcultured and trypsinized at subconfluency and used at passage 3.
In vitro model of tendinopathy
Mouse tendon stromal cells were plated in six-well plates at a density of 2 × 106 cells per well with 1.5 ml of supplemented culture medium. After 24 hours, cells were treated with IL-1β (10 ng/ml; R&D Systems). The culture medium was collected after 24, 48, and 72 hours and stored at −80°C. Human tendon cells were plated in 12-well culture plates at a density of 5 × 104 cells per well with 1 ml of supplemented culture medium, expanded for 48 hours, treated with 1 ng of IL-1β, 50 μM IKKβ inhibitor VIII (MilliporeSigma), or both for 4 hours, and compared to vehicle-treated controls. The supernatant was sampled to determine inflammatory cytokine concentration, and RNA was isolated from cell lysates.
Treadmill overuse model
Chronic overuse tendinopathy was established by subjecting 10-week-old mice (WT, n = 8 females and n = 22 males; IKKβKOScx, n = 3 females and n = 12 males; and IKKβCAScx, n = 2 females and n = 11 males) to 4 weeks of treadmill running at speeds of 20 m/min for 30 min/day, five times a week at a decline of 10°. Before protocol initiation, mice were subjected to a training week consisting of 5 min of running on the first day, followed by increasing durations by 5 min/day until 25 min of running was achieved on the fifth day. Cage activity mice served as controls (WT, n = 16; IKKβKOScx, n = 8; and IKKβCAScx, n = 8).
Acute injury and repair model
Shoulder injury and surgical repair methods were adapted from Bell et al. (49). Ten-week-old mice (WT, n = 7 females and n = 6 males; IKKβKOScx, n = 3 females and n = 4 males; and IKKβCAScx, n = 2 females and n = 5 males) were anesthetized using isoflurane and placed in a left lateral decubitus position. An incision in the skin was made to expose the deltoid. The deltoid was released to visualize the humerus, which was grasped with microforceps for stability. An 8–0 Ethilon suture was used to place a modified Mason-Allen stitch in the supraspinatus tendon. After the tendon grasping suture, the supraspinatus tendon was sharply detached from the humeral head. A 27-gauge needle was used to create a bone tunnel in the humeral head below the growth plate. The suture was then ligated through the bone tunnel, repairing the supraspinatus tendon to its original attachment site. The deltoid was reflected back over the humerus, and the skin was closed with a 5–0 PROLENE suture. After acute injury and repair, mice were allowed free cage activity and euthanized after 2 weeks. The uninjured contra-lateral shoulder was used as uninjured control.
Gene expression
Tissues were snap-frozen in liquid nitrogen and physically disrupted using a ball mill homogenizer (Mikro-Dismembrator U, Sartorius). RNA extraction was performed using guanidinium thiocyanate-phenol-chloroform (TRIzol, Thermo Fisher Scientific) and interphase separation (Phase Lock Gel, QuantaBio). RNA cleanup was performed using spin columns (RNeasy Mini Kit, Qiagen) with on-column deoxyribonuclease I treatment (Qiagen). Cells were lysed in lysis buffer (RLT Buffer, Qiagen), and RNA isolation was performed using spin columns (RNeasy Mini Kit, Qiagen). RNA quantity and quality were determined using a spectrophotometer (NanoDrop 1000, Thermo Fisher Scientific). For mouse studies, RNA was reverse-transcribed into cDNA using the SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific). Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was performed with TaqMan primers (Invitrogen) using a BioMark HD System. Gene expression changes were measured for Ikbkb (IKKβ, Mm01222247_m1), RelA (p65, Mm00501346_m1), RelB (p50, Mm01268877_m1), Chuk (IKKα, Mm00432529_m1), Ikbkg (NEMO, Mm00494927_m1), and Gapdh (Mm99999915_g1). NF-κB pathway profiling was performed using species-specific prepared RT2 Profiler arrays (Qiagen). RNA was reverse-transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Invitrogen). qRT-PCR was performed using PowerUp SYBR green (Invitrogen) and QuantStudio 6 Flex (Applied Biosystems). All gene expression data were analyzed using the ΔΔCt method, with the results first normalized to a housekeeping gene, as indicated in the figures, and then again to WT expression.
Western blot
Cells were plated in 60-mm Petri dishes at a density of 3 × 106 in 1ml of supplemented culture medium. After 24 hours, supplemented culture medium was replaced with 2 ml of alpha-MEM for 2 hours. Cells were lysed with radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific) supplemented with protease inhibitors (cOmplete, Sigma-Aldrich) and phosphatase inhibitors (PhosSTOP, Sigma-Aldrich). Total protein was quantified with the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of total cell lysate (100 μg) were subjected to SDS-electrophoresis on 8% acrylamide gels. Gels were run at a constant voltage of 100 mV (PowerPac 3000, Bio-Rad) and transferred onto a nitrocellulose membrane with the Trans-Blot Turbo Transfer System (Bio-Rad). Immunoblotting was completed for IKKβ (Cell Signaling Technology) and β-actin (Cell Signaling Technology). A Li-Cor Odyssey scanner was used to visualize immunoblots, and ImageJ software (NIH) was used for quantification.
Histology
Mouse shoulders were dissected, fixed in 4% paraformaldehyde, decalcified in 0.5 M EDTA, and embedded in paraffin using standard techniques. Sections (5 μm) were obtained and stained with H&E.
Immunohistochemistry
Histological sections were deparaffinized in xylene and rehydrated through graded ethanol. Endogenous peroxidase activity was quenched using 0.5% H2O2. Antigen retrieval was performed using Uni-Trieve (Innovex Biosciences). Blocking of nonspecific binding was performed using 2.5% horse blocking serum (Vector Laboratories). Sections were incubated overnight at 4°C with anti-CD68 (KP1, ab955, Abcam) or isotype control antibody. Staining of antigens was performed using the ImmPRESS Polymer Detection Kit (Vector Laboratories). Sections were counterstained using Gill’s hematoxylin (Vector Laboratories), differentiated in 2% glacial acetic acid, blued in 30% NH4OH, dehydrated, and mounted using Cytoseal XYL (Thermo Fisher Scientific).
Cytokine/chemokine quantification
Cytokine concentration from culture supernatant was determined using either single-antibody enzyme-linked immunosorbent assay (ELISA) kits (Invitrogen) or Milliplex Mouse Cytokine/Chemokine Magnetic Bead Panel (EMD Millipore) following the manufacturer’s protocol. Expression of cytokines and chemokines with three biological replicates and detectable concentrations was included for analysis.
Microcomputed tomography
The humerus with the supraspinatus tendon attached was dissected for bone morphometry analysis. Samples were scanned at an energy of 55 kilovolt peaks (kVp), an intensity of 145 μA, and a standard resolution of 12.3 μm (μCT 40, Scanco). Reconstructed images were evaluated using a segmentation algorithm to separate cortical and trabecular bone of the humeral head proximal to the growth plate (CTAn, Bruker).
Tendon-to-bone biomechanics
After μCT, the supraspinatus muscle was removed from the tendon in preparation for biomechanics (50). The humerus was potted in epoxy (Parbond 101, McMaster-Carr), and samples were tested in a saline bath at 37°C (30 N load cell; ElectroPuls 1000, Instron Corp.). Uniaxial load-to-failure tensile tests consisted of five cycles of preconditioning (5% strain and 0.2%/s), 300-s rest, and then extension to failure at 0.2%/s. Structural properties were determined from load-deformation data. Material properties were determined from normalized load deformation using tendon cross-sectional area measured from μCT and strain determined as grip-to-grip displacement relative to initial gauge length.
Statistical analysis
All data are shown as means ± SD. All statistical analyses, including Shapiro-Wilk normality test, ANOVA, Fisher’s least significant difference (LSD) with Bonferonni correction for multiple comparisons, and Student’s t test, as indicated in the figure legends, were performed using GraphPad Prism 7 software. A P value of <0.05 was considered significant.
Supplementary Material
Acknowledgments
Funding: This study was funded by the NIH (R01AR055580 and R01AR057836 to S.T.; 5F31AR066452–03 to S.A.S.; and R01AR049192, R01AR054326, and R01AR072623, to Y.A.-A.), Shriners Hospitals for Children (Biomedical grant 86200 to Y.A.-A.), Medical Research Council, UK (MR/R020515/1 to M.A. and N.L.M.), and Arthritis Research UK (21346 to N.L.M.)
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or in the Supplementary Materials.
REFERENCES AND NOTES
- 1.Jordan KP, Jöud A, Bergknut C, Croft P, Edwards JJ, Peat G, Petersson IF, Turkiewicz A, Wilkie R, Englund M, International comparisons of the consultation prevalence of musculoskeletal conditions using population-based healthcare data from England and Sweden. Ann. Rheum. Dis. 73, 212–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riley G, Tendinopathy—From basic science to treatment. Nat. Clin. Pract. Rheumatol. 4, 82–89 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Mather RC III, Koenig L, Acevedo D, Dall TM, Gallo P, Romeo A, Tongue J, Williams G Jr., The societal and economic value of rotator cuff repair. J. Bone Joint Surg. Am. 95, 1993–2000 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beard DJ, Rees JL, Cook JA, Rombach I, Cooper C, Merritt N, Shirkey BA, Donovan JL, Gwilym S, Savulescu J, Moser J, Gray A, Jepson M, Tracey I, Judge A, Wartolowska K, Carr AJ, Ahrens P, Baldwick C, Brinsden M, Brownlow H, Burton D, Butt MS, Carr A, Charalambous CP, Conboy V, Dennell L, Donaldson O, Drew S, Dwyer A, Gidden D, Hallam P, Kalogrianitis S, Kelly C, Kulkarni R, Matthews T, McBirnie J, Patel V, Peach C, Roberts C, Robinson D, Rosell P, Rossouw D, Senior C, Singh B, Sjolin S, Taylor G, Venkateswaran B, Woods D, Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): A multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet 391, 329–338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ketola S, Lehtinen JT, Arnala I, Arthroscopic decompression not recommended in the treatment of rotator cuff tendinopathy: A final review of a randomised controlled trial at a minimum follow-up often years. Bone Joint J. 99-B, 799–805 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Abrams GD, Gupta AK, Hussey KE, Tetteh ES, Karas V, Bach BR Jr., Cole BJ, Romeo AA, Verma NN, Arthroscopic repair of full-thickness rotator cuff tears with and without acromioplasty: Randomized prospective trial with 2-year follow-up. Am. J. Sports Med. 42, 1296–1303 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Harryman DT II, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA III, Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J. Bone Joint Surg. Am. 73, 982–989 (1991). [PubMed] [Google Scholar]
- 8.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K, The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Joint Surg. Am. 86-A, 219–224 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Clement ND, Nie YX, McBirnie JM, Management of degenerative rotator cuff tears: A review and treatment strategy. Sports Med. Arthrosc. Rehabil. Ther. Technol. 4, 48 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee YG, Cho NS, Yoo JH, Clinical outcome and repair integrity after rotator cuff repair in patients older than 70 years versus patients younger than 70 years. Arthroscopy 30, 546–554 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Lehman C, Cuomo F, Kummer FJ, Zuckerman JD, The incidence of full thickness rotator cuff tears in a large cadaveric population. Bull. Hosp. Jt. Dis. 54, 30–31 (1995). [PubMed] [Google Scholar]
- 12.Mall NA, Kim HM, Keener JD, Steger-May K, Teefey SA, Middleton WD, Stobbs G, Yamaguchi K, Symptomatic progression of asymptomatic rotator cuff tears: A prospective study of clinical and sonographic variables. J. Bone Joint Surg. Am. 92, 2623–2633 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millar NL, Hueber AJ, Reilly JH, Xu Y, Fazzi UG, Murrell GAC, McInnes IB, Inflammation is present in early human tendinopathy. Am. J. Sports Med. 38, 2085–2091 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA, Cytokines and apoptosis in supraspinatus tendinopathy. J. Bone Joint Surg. Br. 91, 417–424 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJF, Smith RDJ, Wheway K, Watkins B, Roche L, Carr AJ, Inflammation activation and resolution in human tendon disease. Sci. Transl. Med. 7, 311ra173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning CN, Havlioglu N, Knutsen E, Sakiyama-Elbert SE, Silva MJ, Thomopoulos S, The early inflammatory response after flexor tendon healing: A gene expression and histological analysis. J. Orthop. Res. 32, 645–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millar NL, Murrell GAC, McInnes IB, Inflammatory mechanisms in tendinopathy—Towards translation. Nat. Rev. Rheumatol. 13, 110–122 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Gulotta LV, Kovacevic D, Cordasco F, Rodeo SA, Evaluation of tumor necrosis factor α blockade on early tendon-to-bone healing in a rat rotator cuff repair model. Arthroscopy 27, 1351–1357 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Ko J-Y, Wang F-S, Huang H-Y, Wang C-J, Tseng S-L, Hsu C, Increased IL-1β expression and myofibroblast recruitment in subacromial bursa is associated with rotator cuff lesions with shoulder stiffness. J. Orthop. Res. 26, 1090–1097 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Blomgran P, Hammerman M, Aspenberg P, Systemic corticosteroids improve tendon healing when given after the early inflammatory phase. Sci. Rep. 7, 12468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dakin SG, Buckley CD, Hussein Al-Mossawi M, Hedley R, Martinez FO, Wheway K, Watkins B, Carr AJ, Persistent stromal fibroblast activation is present in chronic tendinopathy. Arthritis Res. Ther. 19, 16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayden MS, Ghosh S, NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 26, 203–234 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F, Tang E, Guan K, Wang C-Y, IKKβ plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 170, 5630–5635 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ, Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, Krampert M, Goebeler M, Gillitzer R, Israel A, Krieg T, Rajewsky K, Haase I, TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417, 861–866 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M, Canonical NF-κB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity 24, 729–739 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, Murrell GAC, Liew FY, Kurowska-Stolarska M, McInnes IB, MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat. Commun. 6, 6774 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millar NL, Akbar M, Campbell AL, Reilly JH, Kerr SC, McLean M, Frleta-Gilchrist M, Fazzi UG, Leach WJ, Rooney BP, Crowe LAN, Murrell GAC, McInnes IB, IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep. 6, 27149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waldorff EI, Lindner J, Kijek TG, Downie BK, Hughes RE, Carpenter JE, Miller BS, Bone density of the greater tuberosity is decreased in rotator cuff disease with and without full-thickness tears. J. Shoulder Elbow Surg. 20, 904–908 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Hou SW, Merkle AN, Babb JS, McCabe R, Gyftopoulos S, Adler RS, Shear wave ultrasound elastographic evaluation of the rotator cuff tendon. J. Ultrasound Med. 36, 95–106 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE, Neer award 1999: Overuse activity injures the supraspinatus tendon in an animal model: A histologic and biomechanical study. J. Shoulder Elbow. Surg. 9, 79–84 (2000). [PubMed] [Google Scholar]
- 32.Bell R, Li J, Gorski DJ, Bartels AK, Shewman EF, Wysocki RW, Cole BJ, Bach BR Jr., Mikecz K, Sandy JD, Plaas AH, Wang VM, Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model. J. Biomech. 46, 498–505 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Perry SM, Getz CL, Soslowsky LJ, After rotator cuff tears, the remaining (intact) tendons are mechanically altered. J. Shoulder Elbow Surg. 18, 52–57 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legerlotz K, Jones ER, Screen HRC, Riley GP, Increased expression of IL-6 family members in tendon pathology. Rheumatology (Oxford) 51, 1161–1165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langberg H, Olesen JL, Gemmer C, Kjœr M, Substantial elevation of interleukin-6 concentration in peritendinous tissue, in contrast to muscle, following prolonged exercise in humans. J. Physiol. 542, 985–990 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen MB, Pingel J, Kjœr M, Langberg H, Interleukin-6: A growth factor stimulating collagen synthesis in human tendon. J. Appl. Physiol. 110, 1549–1554 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, Franklin RA, Adler M, Jacox JB, Bailis W, Shyer JA, Flavell RA, Mayo A, Alon U, Medzhitov R, Circuit design features of a stable two-cell system. Cell 172, 744–757.e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA, Mechanisms of tendon injury and repair. J. Orthop. Res. 33, 832–839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manning CN, Martel C, Sakiyama-Elbert SE, Silva MJ, Shah S, Gelberman RH, Thomopoulos S, Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res. Ther. 6, 74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelberman RH, Linderman SW, Jayaram R, Dikina AD, Sakiyama-Elbert S, Alsberg E, Thomopoulos S, Shen H, Combined administration of ASCs and BMP-12 promotes an M2 macrophage phenotype and enhances tendon healing. Clin. Orthop. Relat. Res. 475, 2318–2331 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blomgran P, Blomgran R, Ernerudh J, Aspenberg P, A possible link between loading, inflammation and healing: Immune cell populations during tendon healing in the rat. Sci. Rep. 6, 29824 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammerman M, Dietrich-Zagonel F, Blomgran P, Eliasson P, Aspenberg P, Different mechanisms activated by mild versus strong loading in rat Achilles tendon healing. PLOS ONE 13, e0201211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayer ML, Schjerling P, Herchenhan A, Zeltz C, Heinemeier KM, Christensen L, Krogsgaard M, Gullberg D, Kjaer M, Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype. PLOS ONE 9, e86078 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aubry S, Nueffer J-P, Tanter M, Becce F, Vidal C, Michel F, Viscoelasticity in Achilles tendonopathy: Quantitative assessment by using real-time shear-wave elastography. Radiology 274, 821–829 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Yan H, Duan X, Pan H, Holguin N, Rai MF, Akk A, Springer LE, Wickline SA, Sandell LJ, Pham CTN, Suppression of NF-κB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc. Natl. Acad. Sci. U.S.A. 113, E6199–E6208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lake SP, Ansorge HL, Soslowsky LJ, Animal models of tendinopathy. Disabil. Rehabil. 30, 1530–1541 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Schwartz AG, Long F, Thomopoulos S, Enthesis fibrocartilage cells originate from a population of hedgehog-responsive cells modulated by the loading environment. Development 142, 196–206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, Johnson RL, Tabin CJ, Schweitzer R, Zelzer E, Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell 17, 861–873 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bell R, Taub P, Cagle P, Flatow EL, Andarawis-Puri N, Development of a mouse model of supraspinatus tendon insertion site healing. J. Orthop. Res. 33, 25–32 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Killian ML, Cavinatto LM, Ward SR, Havlioglu N, Thomopoulos S, Galatz LM, Chronic degeneration leads to poor healing of repaired massive rotator cuff tears in rats. Am. J. Sports Med. 43, 2401–2410 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otero JE, Dai S, Foglia D, Alhawagri M, Vacher J, Pasparakis M, Abu-Amer Y, Defective osteoclastogenesis by IKKβ-null precursors is a result of receptor activator of NF-κB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation. J. Biol. Chem. 283, 24546–24553 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


