Abstract
Proteolytic activation of the IL-33 precursor, full-length interleukin-33 (FLIL33), at multiple sites within the sensor domain (aa 95 – 109) yields several functionally mature (MIL33) forms. Unlike nuclear FLIL33, intracellular MIL33 occurs in the cytoplasm, is secreted from source cells, and exerts biological effects by activating the ST2 receptor on target cells. Previous studies and our findings in this report indicated that IL-33 forms that are substantially longer than those produced by cleavage with in the sensor domain are biologically indistinguishable from classical MIL33. We utilized a series of human and mouse N-terminal FLIL33 mutants to narrow down the boundaries of the nuclear localization sequence to aa 46 – 67, a segment known to include a portion of the chromatin-binding motif as well as another site controlling intracellular stability of FLIL33 in an importin-5-dependent fashion. The N-terminal FLIL33 deletion mutants starting prior to this region were intranuclear, non-secreted in cell culture, and manifested modest functional activity in vivo, similar to FLIL33. By contrast, the mutants starting after this region were cytoplasmic, secreted from cells in culture, and overtly biologically active in vivo, similar to MIL33. The deletion mutants starting within this region manifested an intermediate phenotype between FLIL33 and MIL33. Thus, this segment of IL-33 molecule controls multiple aspects of its biology, including subcellular localization, extracellular secretion, and functional maturation into the longest possible form of mature IL-33 cytokine. Future anti-IL-33 therapies may be based on interfering with this segment, thus restraining extracellular release and maturation of IL-33 into the active cytokine.
Keywords: interleukin-33, nuclear localization sequence, cytokine, cytokine precursor, cytokine maturation
INTRODUCTION
Unlike the majority of cytokines that are produced and secreted by source cells “on demand” in response to microenvironmental stimuli, IL-33 is constitutively and inducibly expressed as an intracellular, mostly intranuclear, precursor, full-length IL-33 (FLIL33) [1]. FLIL33 is released from necrotic cells and becomes proteolytically activated extracellularly into mature IL-33 (MIL33) [2, 3], binds to its cell-surface receptor ST2 on target cells, activates intracellular signaling, and elicits functional effects, thus acting as an alarmin and a powerful inducer of the Th2 phenotype, including recruitment of eosinophils [1–11]. Additionally, IL-33 may be actively secreted from live cells, including in its activated, MIL33 form [8, 12–18], although the mechanisms of such secretion are unknown. The intracellular precursor FLIL33 is also independently active and contributes to injury responses and to the pathophysiology of diseases [1, 4–8, 19–25].
Overall, IL-33 biology involves a complex interplay between its transcriptional regulation, intracellular protein stability, regulation of subcellular distribution, intracellular signaling from FLIL33, active secretion or necrotic release, and proteolytic activation to form the MIL33 cytokine. The precise regulation of each of these aspects and their interplay is poorly understood. Early studies roughly identified the region aa 60 – 80 as responsible for the nuclear localization of FLIL33 [22] and aa 40 – 58 as responsible for chromatin binding [26], but it remains unknown whether these regions simultaneously control functional maturation of IL-33. The same group later identified the region aa 95 – 109 as the “sensor domain” responsible for the proteolytic activation of FLIL33 into MIL33 variants of various lengths [2, 3, 10]. However, others have shown that a much longer form of mouse (m) IL-33, starting at aa 68, is localized cytoplasmically and acts in vivo as MIL33 [18], but they did not test even longer N-terminal deletion mutants. Here we describe a finding of a long-form human (h) MIL33, which spurred a more detailed analysis of the protein’s N-terminal sequence, leading to a more precise identification of the segment that controls such key aspects of IL-33 biology as its nuclear localization, secretion, and maturation into MIL33.
MATERIALS AND METHODS
Recombinant constructs
The plasmid and replication-deficient adenoviral (AdV) constructs, all driven by the cytomegalovirus promoter, were designed, prepared (plasmids by Genscript, Piscataway, NJ and AdVs by ViraQuest, North Liberty, IA), validated, and used as previously described in detail [8, 23–25]. The constructs that included the C-terminal portion of hIL-33 [hFLIL33, hFLIL33(71–79A9), hFLIL33(71–79DEL), and hMIL33] also contained a C-terminal HA tag (YPYDVPDYA) connected by a flexible linker peptide (G3S)3.
Primary human and mouse cell cultures
Deidentified primary human lung fibroblasts from adult healthy donors were purchased from Lonza (Walkersville, MD) and used at passages 4 – 6. To obtain primary mouse lung cell cultures, wild-type C57BL/6 mice were euthanized by CO2 asphyxiation followed by cervical dislocation. After opening the ribcage, lungs were perfused through the right ventricle with 10 ml of ice-cold PBS. Lungs were isolated, minced, and digested with collagenase and DNase I (both from Sigma-Aldrich, St. Louis, MO). Cells were strained through a 70 μm filter and plastic-adherent cells were propagated by two cycles of passaging in DMEM medium supplemented with 10% bovine calf serum. The cultures were maintained in a humidified atmosphere of 5% CO2 at a temperature of 37 °C in DMEM supplemented with 10% serum as well as glucose, L-glutamine, sodium pyruvate, nonessential amino acids, and antibiotic-antimycotic mixture as previously described [8, 23–25]. For experiments, the cells were harvested by trypsinization, washed, counted, and seeded on 6-well culture plates at a density of 3×105 cells/well in 0.5% bovine calf serum-containing DMEM. Gene delivery was achieved using electroporation of cells with recombinant plasmids utilizing the Amaxa Nucleofector (Lonza). In each reaction, 1×105 – 1×106 cells and 0.5 – 2.0 μg of plasmid were used, based on preliminary experiments to optimize expression of each delivered recombinant protein. Alternatively, infections of cultured cells with replication-deficient recombinant AdV constructs were used to overexpress proteins or peptides of interest. For these infections, 1×105 – 5×106 plaque-forming units/ml of AdVs were used per 3×105 cells in culture. Western blotting, ELISA, and fluorescent microscopy were performed as previously described [8, 23–25].
In vivo studies
All experiments were performed in 10 – 12 week-old mice in accordance with a research protocol approved by the University of Maryland Institutional Care and Use Committee. Wild-type C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). ST2-deficient mice on C57BL/6 background were a king gift from Dr. Andrew N. J. McKenzie, Medical Research Council Laboratory of Molecular Biology, Cambridge, UK [27]. The intratracheal instillation of AdVs, euthanasia, broncholaveolar lavage procedures, preparation of lung homogenates, and ELISAs of homogenates for selected cytokines were performed as previously described in detail [8, 23–25].
RESULTS
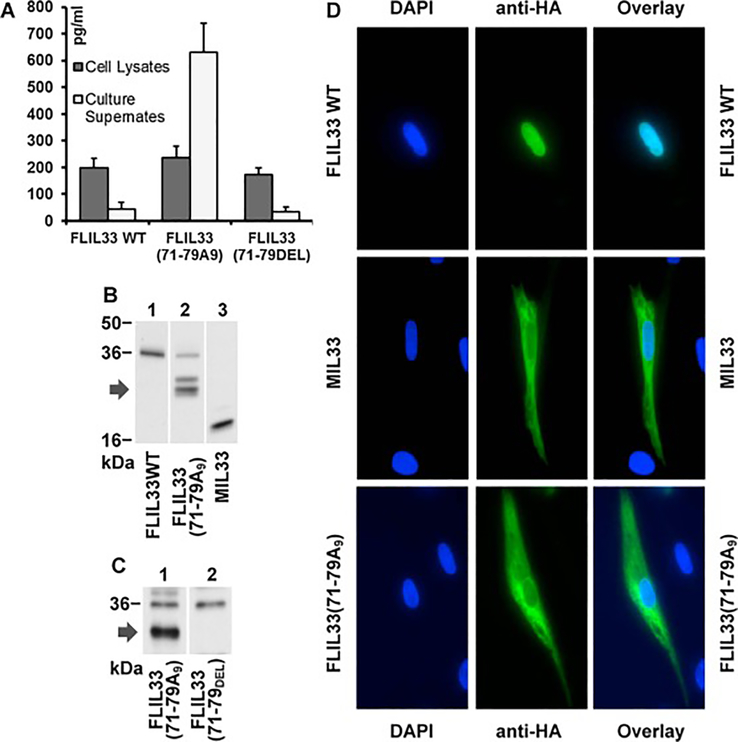
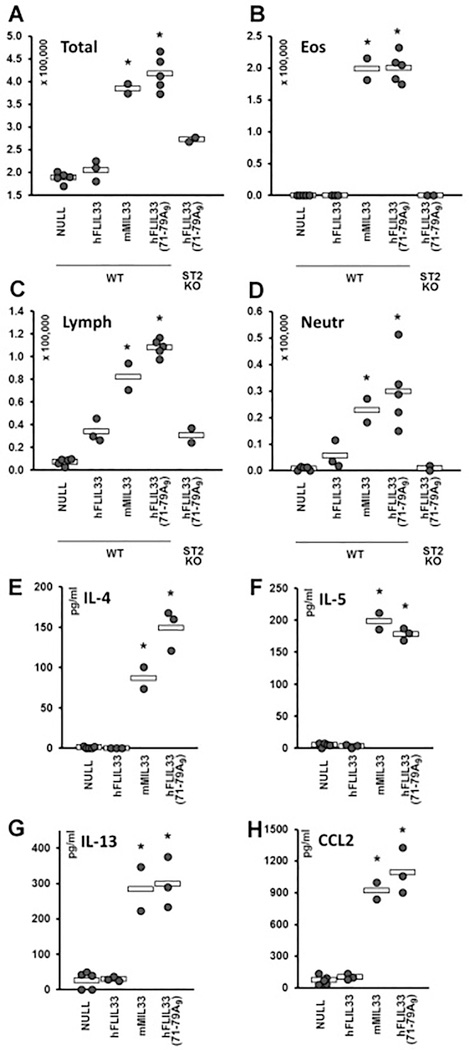
In our recent studies [24, 25], we examined intracellular stability of human (h) FLIL33, including by mutating segments of the N-terminal portion of the molecule. As a continuation of this systematic effort, we mutated a charged amino acid-rich region of hFLIL33 at aa 71 – 79, by either replacing this entire cluster (KTGRKHKRH) with poly-alanine [hFLIL33(71–79A9)] or by deleting it [hFLIL33(71–79DEL)]. Similar to the FLIL33, the hFLIL33(71–79DEL) mutant remained predominantly intracellular, whereas similar to MIL33, the hFLIL33(71–79A9) mutant was released in large amounts into the supernates of cells electroporated with the encoding plasmids (Fig. 1A, repeated in five primary cell cultures from separate donors, in duplicate cultures for each FLIL33 form). Western blots of these cell lysates revealed that hFLIL33(71–79A9)-expressing but not hFLIL33(71–79DEL)-expressing cells contained IL-33 bands that were intermediate in size between hFLIL33 and hMIL33; double or single intermediate-size bands were observed variably among the tested fibroblast cultures (Fig. 1B, C). Immunocytochemical staining for the HA tag, which was fused to the C-terminus of all these constructs through a flexible linker, revealed that unlike the predominantly intranuclear wild-type hFLIL33 and similar to the predominantly cytoplasmic MIL33 [25], the hFLIL33(71–79A9) mutant was abundant in the cytoplasm (Fig. 1D, repeated in five primary cell cultures from separate donors with similar results). These combined observations suggested that the hFLIL33(71–79A9) mutant undergoes partial degradation, perhaps proteolytic cleavage by a currently unknown enzyme, resulting in a protein product that behaves similarly to MIL33 in terms of subcellular localization and extracellular release, but is substantially larger than the known MIL33 variants [2, 3, 10]. Replication-deficient, recombinant adenoviral (AdV) constructs encoding wild-type hFLIL33, mMIL33, and hFLIL33(71–79A9), as well as a non-coding AdV-NULL vehicle control were prepared and administered intratracheally to mice (Fig. 2). Gene delivery of both mMIL33 and hFLIL33(71–79A9), but not wild-type hFLIL33, induced massive accumulation of inflammatory cells in the lungs based on bronchoalveolar lavage (BAL) cell counts (Fig. 2A), mostly due to pronounced pulmonary eosinophilia (Fig. 2B). Germline deficiency of ST2 abrogated mMIL33-induced [8] and hFLIL33(71–79A9)-induced eosinophilia (Fig. 2B), indicating that both molecules acted through this cell-surface receptor. Lymphocytes and neutrophils were also elevated by hFLIL33(71–79A9) gene delivery (Fig. 2C, D, respectively) similar to the effect of mMIL33 overexpression [8]. Both mMIL33 and hFLIL33(71–79A9) potently induced the levels of Th2 cytokines IL-4, IL-5, IL-13, and CCL2 (Fig. 2E – H).Thus, the product of hFLIL33(71–79A9) degradation, despite being substantially larger in size, behaves similarly to the classical MIL33 both in cell culture and in vivo.
Figure 1.
Properties of hFLIL33(71–79A9) and hFLIL33(71–79DEL) mutants in cultured cells. A. ELISA of cell lysates and cell culture supernates of primary human fibroblasts 72 h after electroporation with plasmids encoding the wild-type or mutant variants of hFLIL33, mean pg/ml ± SD. B, C. Western blots of primary human fibroblast lysates 72 h after transfection with plasmids encoding the indicated hFLIL33 variants. The arrows on the left indicate the intermediate-size bands observed in hFLIL33(71–79A9)-expressing cells. D. Immunofluorescence of cultured primary human fibroblasts using an anti-HA antibody. Cells were transfected with plasmids encoding the indicated hFLIL33 variants and tested 72 h later.
Figure 2.
Properties of hFLIL33(71–79A9) and hFLIL33(71–79DEL) mutants in vivo. A – D. Total and differential (eosinophils, lymphocytes, neutrophils) cell counts of BAL samples from wild type and ST2 knockout mice infected intratracheally with AdV constructs encoding the indicated variants of IL-33; day 14 after infection. E – H. ELISAs of lung homogenates for IL-4, IL-5, IL-13, and CCL2 in WT mice infected intratracheally with AdV constructs encoding the indicated variants of IL-33; day 14 after infection. In panels E – J, dots represent individual mice and horizontal bars show mean values for each group. Statistically significant differences (p < 0.05) from AdV-NULL-infected mice are indicated with asterisks.
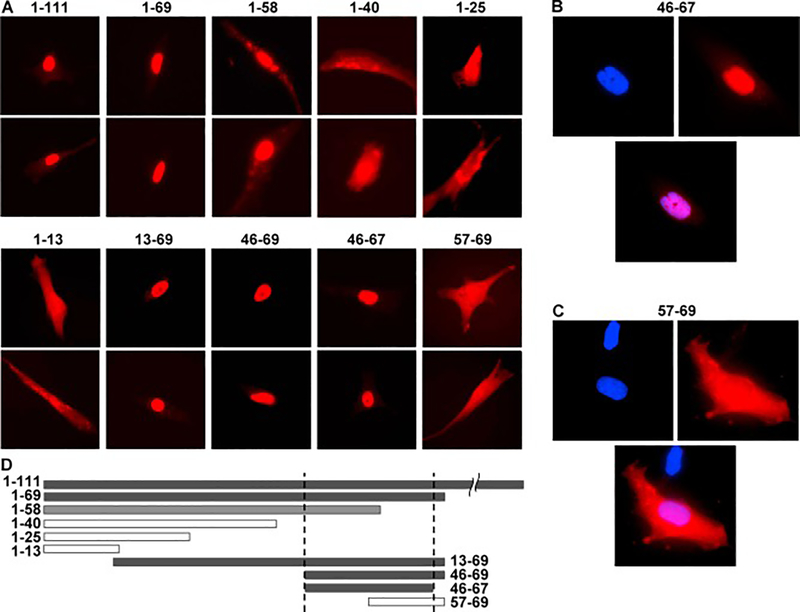
This observation of hFLIL33(71–79A9) activity, combined with a previous report of a long form of mMIL33, which begins at aa 68 [18], suggested that functional maturation of FLIL33 into larger MIL33 forms may occur in a substantially more N-terminal region than the known proteolytic activation domain at aa 95 – 109 [2, 3, 10]. To more precisely define this region, a series of plasmids were constructed and used to overexpress fusion proteins of various segments of the N-terminal region of FLIL33 with monomeric Scarlet-I (mScI) fluorescent protein [28] connected by a flexible linker (G4S)3 (Fig. 3). Selection of the segments was guided by the boundaries of charged amino acid clusters in the N-terminal part of the FLIL33 molecules. We reasoned that cytoplasmic rather than nuclear localization of an IL-33 form would be more conducive to its secretion and subsequent functional cytokine activity. As expected, the complete N-terminal segment of FLIL33 (aa 1 – 111) mediated nuclear localization of the fluorescent fusion construct 1–111mScI. Consistent with the notion that the region below aa 71 is necessary for nuclear localization of FLIL33, the aa 1 – 69 fragment of FLIL33 similarly mediated nuclear localization of the fusion construct 1–69mScI. However, a shorter peptide, aa 1 – 58 of FLIL33, while translocating the fused mScI (1–58mScI) to the nucleus, also remained present in the form of small granules in the cytoplasm. Thus, the aa 59 – 69 region is important for ensuring complete nuclear localization of the protein. The fusion constructs of shorter lengths of the N-terminal portion of FLIL33 with mScI (1–40mScI, 1–25mScI, and 1–13mScI) were either extranuclear or distributed through both the nucleus and cytoplasm. The 13–69mScI and 46–69mScI fusion constructs were intranuclear, suggesting that the area below aa 46 is not needed for the nuclear translocation. Amino acids 68 and 69 (P and S) are not charged, suggesting that they may not be required for the nuclear trafficking. Indeed, the 46–67mScI is localized entirely intranuclearly. As mentioned above, the region below aa 58 contributes to but is not by itself sufficient for the exclusively nuclear localization of the protein. Furthermore, the 57–69mScI fusion protein was distributed through both the nucleus and cytoplasm, suggesting that the integrity of the aa 46 – 67 segment is important for complete nuclear localization. We have thus narrowed down aa 46 – 67 (KLRSGLMIKKEACYFRRETTKR) as the nuclear localization sequence (NLS) of FLIL33. The combined observations described above suggested that any form of IL-33 starting C-terminally of aa 67 is an extranuclear, secreted, functionally mature IL-33 cytokine.
Figure 3.
Subcellular localization of the fusion constructs between the indicated segment peptides of FLIL33 and mScI. A. Fluorescence microscopy, red channel, of two representative cells for each FLIL33 segment is shown; repeated in primary NHLFs from three separate donors, with at least 50 overexpressing cells analyzed for each donor, with consistent results. B, C. Fluorescent images of cells with DAPI-stained nuclei (blue) expressing the indicated IL-33 segment peptide – mScI fusion proteins (red), and the overlays of the two channels. D. The horizontal bars represent the corresponding FLIL33 N-terminal peptide sequences to scale; closed bars represent nuclear and open bars extranuclear or combined localization.
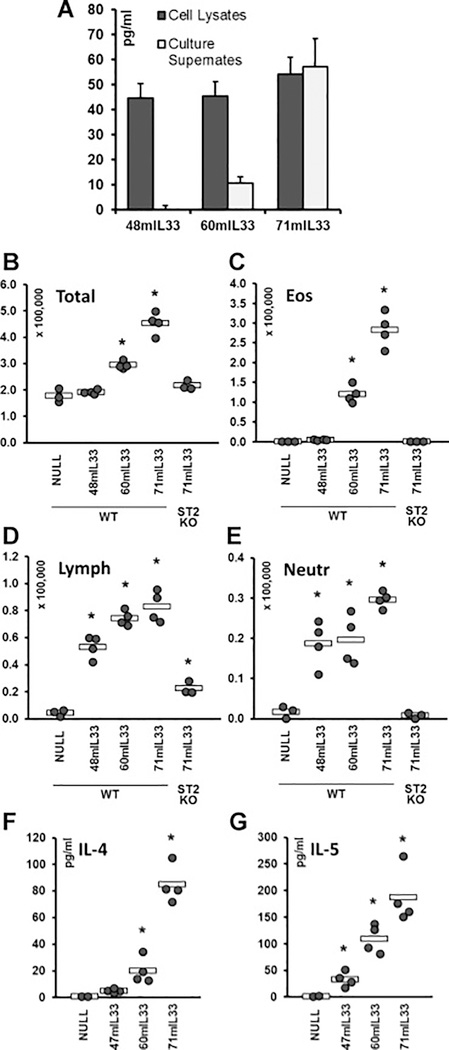
To ensure that the observations in Figs. 1 obtained with human IL-33 forms administered to mice were not artifactual cross-species effects, to generalize the observations presented in Figs. 1 – 3 to both human and mouse forms of IL-33, and to explore the functional activity of a partial NLS, N-terminal deletion constructs of mFLIL33 were prepared. The segment of mFLIL33 that is orthologous to aa 46 – 69 in hFLIL33 spans aa 48 – 71 (RLRSGLTIRKETSYFRKEPTKRYS). Plasmid and AdV constructs were used that encode N-terminal deletion mutants of mIL-33 starting at aa 48 (48mIL33), aa 60 (60mIL33), and aa 71 (71mIL33). Primary mouse lung cells were electroporated with these constructs, and cell lysates and culture supernates were tested 72 h later for IL-33 levels by ELISA. Consistent with the findings made with hFLIL33 mutants, 48mIL33 was not secreted, 71mIL33 was abundantly secreted, and 60mIL33 was partially secreted (Fig. 4A, repeated on three separate occasions, in duplicate cultures for each IL-33 form). To determine whether these differences in secretion are paralleled by functional activity, corresponding AdV constructs encoding these mutants were prepared and instilled intratracheally in mice. Similar to mFLIL33 [8], such gene delivery of 48mIL33 induced mild lymphocytosis and neutrophilia, but did not induce accumulation of eosinophils (Fig. 4B – E). By contrast, similar to mMIL33 [8], gene delivery of 71mIL33 induced overt eosinophilia (Fig. 4C), whereas overexpression of 60mIL33 resulted in an intermediate phenotype (Fig. 4B – E). Similar to mMIL33 [8], deficiency of the ST2 receptor abrogated the effects of 60mIL33 and 71mIL33 gene delivery (Fig. 4B – E). Moreover, gene delivery of 71mIL33 induced the levels of Th2 cytokines IL-4 and IL-5 similar to the effect of mMIL33 [8], with 60mIL33 having a similar but less pronounced effect (Fig. 4F, G).
Figure 4.
Properties of N-terminal deletion mutants of mFLIL33. A. ELISA of cell lysates and cell culture supernates of primary mouse cells 72 h after electroporation with plasmids encoding the indicated mFLIL33 mutants, mean pg/ml ± SD. B – E. Total and differential (eosinophils, lymphocytes, neutrophils) cell counts of BAL samples from wild type and ST2 knockout mice infected intratracheally with AdV constructs encoding the indicated variants of mIL-33; day 10 after infection. F, G. ELISAs of lung homogenates for IL-4 (F) and IL-5 (G). In panels B – G, dots represent individual mice and horizontal bars show mean values for each group. Statistically significant differences (p < 0.05) from AdV-NULL-infected mice are indicated with asterisks.
DISCUSSION
We made a serendipitous observation of spontaneous intracellular degradation of the human FLIL33(71–79A9) mutant. The product manifested biological properties of MIL33, such as cytoplasmic expression, secretion from expressing cells, and ST2-dependent pro-Th2 activity in vivo (Fig. 1, 2). However, its gel mobility suggested a substantially larger molecular size than that of a classical MIL33, which is generated through proteolysis of FLIL33 in the protease sensor domain spanning aa 95 – 109 [2, 3, 10]. These findings echoed the report of a similar long form of mouse MIL33 [18]. These combined findings indicated that IL-33 maturation may occur N-terminally of the protease sensor domain and prompted the need to definitively define the boundaries of the N-terminal region(s) that control(s) subcellular localization, secretion, and cytokine activity of IL-33 forms. We tested a series of FLIL33 N-terminal peptides for their ability to mediate intranuclear trafficking of a fluorescent fusion protein (Fig. 3). We reasoned that nuclear localization would not be conducive to IL-33 secretion from intact cells, whereas cytoplasmic localization could be permissive of secretion, with possible receptor-mediated functional activity. The results indicated that the integrity of the aa 46 – 67 segment of IL-33 precursor is important for complete nuclear localization, and its shortening permits cytoplasmic presence. Subsequently, N-terminal deletion mutants of mouse IL-33 precursor were also tested to determine whether the orthologous region controls nuclear localization, and whether its absence permits secretion and functional cytokine activity in both species. The combined observations presented in Figs. 1 – 3 suggested that the 48mIL33 mutant, which had the entire NLS preserved, would remain intracellular and non-secreted from cells. The 71mIL33 mutant, in which the entire NLS was removed, was expected to localize cytoplasmically, undergo secretion, and exert the cytokine effects of mMIL33. The 60mIL33 mutant, in which only a portion of the NLS was preserved, was expected to manifest an intermediate phenotype between mFLIL33 and mMIL33. Indeed, 71mIL33 was secreted from cells in culture, 48mIL33 was not, and 60mIL33 was partially secreted. Gene delivery in vivo revealed that 71mIL33 induces eosinophil recruitment and elevations in the levels of Th2 cytokines (Fig. 4) similarly to the Th2 phenotype-inducing effect of mMIL33 [8]. By contrast, similar to mFLIL33 [8], 48mIL33 had no effect on eosinophils and only induced mild lymphocytosis and neutrophilia, similar to FLIL33 [8]. The phenotype induced by 60mIL33 was intermediate, consistent with the notion that the entire NLS is needed to ensure complete nuclear localization of IL-33, and to prevent its secretion and cytokine activity.
Our findings narrow down the previous characterization of the NLS of FLIL33 [18, 22, 26], specifically to the aa 46 – 67 segment. We further demonstrate that the integrity of this NLS is important for the exclusive nuclear localization of IL-33, whereas its cleavage leads to partially nuclear and also cytoplasmic localization. Moreover, our data indicate that three key features of IL-33, including its subcellular localization, secretion from source cells, and cytokine activity through ST2 receptor, are all controlled by this region in the N-terminal portion of FLIL33. We have validated these observations not only in primary cell culture but also in mice in vivo, thus making the findings relevant to the complexity of the in vivo environment. It should be also noted that human IL-33 forms are biologically active in mice in vivo (Fig. 1). Although novel, our findings are consistent with previous observations. The aa 46 – 67 region of FLIL33 is encoded within exon 3 of the IL-33 gene, suggesting that IL-33 splice variants omitting this region would be present cytoplasmically in the producing cells, be secreted, and act as mature pro-Th2 cytokines. Indeed, such splice variants have been recently discovered [29, 30]. Further supporting the functional importance of this protein segment, a part of its sequence (aa 46 – 56) controls intracellular degradation of FLIL33 in an importin-5-dependent fashion [25].
In summary, we have precisely outlined the NLS of FLIL33, which simultaneously represents the most N-terminal region of the FLIL33 molecule that controls subcellular localization, intracellular stability, extracellular secretion, and functional activity of IL-33, and deletion of which produces the longest functional MIL33 cytokine. It remains to be determined which specific proteases natively cleave FLIL33 protein N-terminal to, within, or C-terminal to this region, and under which conditions. Nevertheless, the new information presented in this report may offer opportunities to therapeutically target IL-33 processing and function.
Highlights.
Nuclear localization sequence of full-length IL-33 was precisely identified
The same segment controls intracellular stability and extracellular secretion
N-terminal deletions within this segment produce long forms of mature IL-33
Acknowledgements
This work was supported by VA I01CX000101 (to I. G. L.) and I01BX002499 (to S. P. A.), NIH R01HL126897 (to S. P. A.), and by a Scleroderma Foundation award (to S. P. A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Veterans Administration, or Scleroderma Foundation.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Cayrol C, Girard JP, Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family, Immunol Rev, 281 (2018) 154–168. [DOI] [PubMed] [Google Scholar]
- [2].Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, Burlet-Schiltz O, Gonzalez-de-Peredo A, Girard JP, Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33, Nat Immunol, 19 (2018) 375–385. [DOI] [PubMed] [Google Scholar]
- [3].Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C, IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G, Proc Natl Acad Sci U S A, 109 (2012) 1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].De la Fuente M, MacDonald TT, Hermoso MA, The IL-33/ST2 axis: Role in health and disease, Cytokine Growth Factor Rev, 26 (2015) 615–623. [DOI] [PubMed] [Google Scholar]
- [5].Liew FY, Girard JP, Turnquist HR, Interleukin-33 in health and disease, Nat Rev Immunol, 16 (2016) 676–689. [DOI] [PubMed] [Google Scholar]
- [6].Martin NT, Martin MU, Interleukin 33 is a guardian of barriers and a local alarmin, Nat Immunol, 17 (2016) 122–131. [DOI] [PubMed] [Google Scholar]
- [7].Molofsky AB, Savage AK, Locksley RM, Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation, Immunity, 42 (2015) 1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Luzina IG, Pickering EM, Kopach P, Kang PH, Lockatell V, Todd NW, Papadimitriou JC, McKenzie AN, Atamas SP, Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion, J Immunol, 189 (2012) 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Travers J, Rochman M, Miracle CE, Habel JE, Brusilovsky M, Caldwell JM, Rymer JK, Rothenberg ME, Chromatin regulates IL-33 release and extracellular cytokine activity, Nat Commun, 9 (2018) 3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP, Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells, Proc Natl Acad Sci U S A, 111 (2014) 15502–15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Licona-Limon P, Kim LK, Palm NW, Flavell RA, TH2, allergy and group 2 innate lymphoid cells, Nat Immunol, 14 (2013) 536–542. [DOI] [PubMed] [Google Scholar]
- [12].Kakkar R, Hei H, Dobner S, Lee RT, Interleukin 33 as a mechanically responsive cytokine secreted by living cells, J Biol Chem, 287 (2012) 6941–6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hristova M, Habibovic A, Veith C, Janssen-Heininger YM, Dixon AE, Geiszt M, van der Vliet A, Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses, J Allergy Clin Immunol, 137 (2016) 1545–1556 e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee EJ, Kim JW, Yoo H, Kwak W, Choi WH, Cho S, Choi YJ, Lee YJ, Cho J, Single highdose irradiation aggravates eosinophil-mediated fibrosis through IL-33 secreted from impaired vessels in the skin compared to fractionated irradiation, Biochem Biophys Res Commun, 464 (2015) 20–26. [DOI] [PubMed] [Google Scholar]
- [15].Tung HY, Plunkett B, Huang SK, Zhou Y, Murine mast cells secrete and respond to interleukin-33, J Interferon Cytokine Res, 34 (2014) 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lefrancais E, Cayrol C, Mechanisms of IL-33 processing and secretion: differences and similarities between IL-1 family members, Eur Cytokine Netw, 23 (2012) 120–127. [DOI] [PubMed] [Google Scholar]
- [17].Balato A, Lembo S, Mattii M, Schiattarella M, Marino R, De Paulis A, Balato N, Ayala F, IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation, Exp Dermatol, 21 (2012) 892–894. [DOI] [PubMed] [Google Scholar]
- [18].Bessa J, Meyer CA, de Vera Mudry MC, Schlicht S, Smith SH, Iglesias A, Cote-Sierra J, Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation, J Autoimmun, 55 (2014) 33–41. [DOI] [PubMed] [Google Scholar]
- [19].Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard JP, Stappenbeck TS, Patterson GA, Pierce RA, Brody SL, Holtzman MJ, Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease, J Clin Invest, 123 (2013) 3967–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP, Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain, J Immunol, 188 (2012) 3488–3495. [DOI] [PubMed] [Google Scholar]
- [21].Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML, Martin MU, The dual function cytokine IL-33 interacts with the transcription factor NF-kappaB to dampen NF-kappaB-stimulated gene transcription, J Immunol, 187 (2011) 1609–1616. [DOI] [PubMed] [Google Scholar]
- [22].Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP, IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo, Proc Natl Acad Sci U S A, 104 (2007) 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luzina IG, Kopach P, Lockatell V, Kang PH, Nagarsekar A, Burke AP, Hasday JD, Todd NW, Atamas SP, Interleukin-33 potentiates bleomycin-induced lung injury, Am J Respir Cell Mol Biol, 49 (2013) 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kopach P, Lockatell V, Pickering EM, Haskell RE, Anderson RD, Hasday JD, Todd NW, Luzina IG, Atamas SP, IFN-gamma directly controls IL-33 protein level through a STAT1- and LMP2-dependent mechanism, J Biol Chem, 289 (2014) 11829–11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clerman A, Noor Z, Fishelevich R, Lockatell V, Hampton BS, Shah NG, Salcedo MV, Todd NW, Atamas SP, Luzina IG, The full-length interleukin-33 (FLIL33)-importin-5 interaction does not regulate nuclear localization of FLIL33 but controls its intracellular degradation, J Biol Chem, 292 (2017) 21653–21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Roussel L, Erard M, Cayrol C, Girard JP, Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket, EMBO Rep, 9 (2008) 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN, T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses, J Exp Med, 191 (2000) 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bindels DS, Haarbosch L, van Weeren L, Postma M, Wiese KE, Mastop M, Aumonier S, Gotthard G, Royant A, Hink MA, Gadella TW Jr., mScarlet: a bright monomeric red fluorescent protein for cellular imaging, Nat Methods, 14 (2017) 53–56. [DOI] [PubMed] [Google Scholar]
- [29].Tsuda H, Komine M, Karakawa M, Etoh T, Tominaga S, Ohtsuki M, Novel splice variants of IL-33: differential expression in normal and transformed cells, J Invest Dermatol, 132 (2012) 2661–2664. [DOI] [PubMed] [Google Scholar]
- [30].Gordon ED, Simpson LJ, Rios CL, Ringel L, Lachowicz-Scroggins ME, Peters MC, Wesolowska-Andersen A, Gonzalez JR, MacLeod HJ, Christian LS, Yuan S, Barry L, Woodruff PG, Ansel KM, Nocka K, Seibold MA, Fahy JV, Alternative splicing of interleukin-33 and type 2 inflammation in asthma, Proc Natl Acad Sci U S A, 113 (2016) 8765–8770. [DOI] [PMC free article] [PubMed] [Google Scholar]