Antimicrobial resistance (AMR) varies regionally. This study longitudinally maps Escherichia coli susceptibility leveraging Wisconsin antibiograms (n = 202) collected from 2009, 2013, and 2015 to inform the development of a novel clinical decision support tool.
KEYWORDS: antibiogram, antimicrobial resistance, antimicrobial stewardship, clinical decision support, geospatial analysis, surveillance studies
ABSTRACT
Antimicrobial resistance (AMR) varies regionally. This study longitudinally maps Escherichia coli susceptibility leveraging Wisconsin antibiograms (n = 202) collected from 2009, 2013, and 2015 to inform the development of a novel clinical decision support tool. Spatial interpolation methods were tested with E. coli susceptibilities to create geographic AMR visualizations and to estimate susceptibility in areas without AMR data. These visualizations and an interactive mapping tool, the AMR Tracker, provide a proof of concept for empirical antibiotic treatment decisions.
INTRODUCTION
Antimicrobial resistance (AMR) is a serious global health threat with local implications. The Centers for Disease Control and Prevention considers antibiotic-resistant infections to be one of the greatest health challenges of our time and advocates for targeted antibiotic prescribing practices to help address the threat. Antimicrobial stewardship guidelines also endorse this strategy, and stewardship program interventions are associated with improved clinical and economic outcomes (1–3). One strategy for ensuring antibiotic prescription practices that limit AMR development is to factor local AMR trends in empirical treatment decisions (1). These trends are currently captured in a tabulated antibiogram, which, while providing accurate data, is generally limited to one institution or unit. In addition, the use and function of antibiogram data have not changed in several decades, and many practitioners find them difficult to use and thus may not use them at all.
We engaged clinician partners from local health systems to assess current AMR data resources and to determine the desired format and content of an improved resource. Our survey found that only 13% of antibiotic-prescribing physicians used an antibiogram weekly. Fifty-seven percent of respondents indicated that visualization of regional antibiotic resistance would improve the translation of AMR data into practice “a great deal” (4). Although surveillance reports and geographic maps of AMR have been produced across Europe and the United States, including Wisconsin, they have had limited application to the clinical setting and can be refined for patient treatment decisions (5–12).
The objective of this study was to map regional Escherichia coli antibiotic susceptibility in Wisconsin and to create a useful tool for prescribing health care providers. Based on Wisconsin antibiogram data from 2009, 2013, and 2015, we created novel visualizations and an interactive mapping tool, AMR Tracker, as a proof-of-concept functional aid for practitioners to use during empirical treatment decisions.
Antibiograms were collected from health systems, hospitals, and clinics in Wisconsin, totaling 202 antibiograms (2009, n = 60; 2013, n = 74; 2015, n = 68), representing 201,091 Gram-negative isolates. We excluded tertiary care facilities because patients there are typically referred or transferred from distant locations. E. coli susceptibilities to amoxicillin-clavulanate, ampicillin, ampicillin-sulbactam, ciprofloxacin, levofloxacin, nitrofurantoin, and trimethoprim-sulfamethoxazole were included. If an antibiogram reported extended-spectrum β-lactamase susceptibility results separately, the number of isolates was used to calculate the average susceptibility, including all E. coli isolates at the same location.
For geographic analysis, each antibiogram was assigned geographic coordinates to a discrete location or geocoded. Specific addresses were used when identified, but when only general information was available, the facility was placed at the centroid of the county or city. This initial data preparation stage produced a series of spatial data sets containing geographic point features, with one point per health care facility, for each of the three sample years and E. coli susceptibility for each antibiotic of interest.
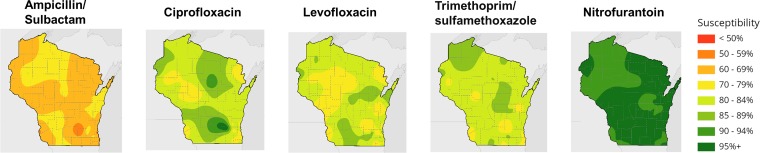
We explored various interpolation methods to estimate susceptibility values between geographic points. We tested the inverse distance-weighted and kriging methods (13–15). Ultimately, we found that a spline-with-barriers approach produced the most rational and visually appealing estimates of statewide conditions (16). After optimization, the mapping visualized regional differences in antibiotic susceptibilities (Fig. 1). The mapping demonstrated wide variability in susceptibility statewide and among antibiotics. Lower susceptibilities were observed near urban centers and valley areas.
FIG 1.
Interpolated 2015 Wisconsin E. coli antibiotic susceptibilities.
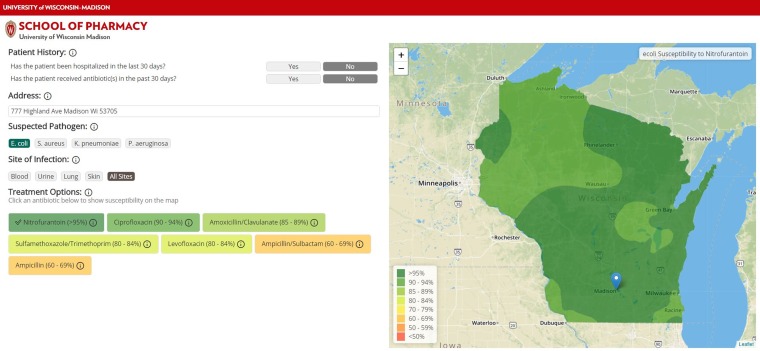
The AMR Tracker allows users to view estimated antibiotic resistance at a specific location for each antibiotic included (Fig. 2). When the user specifies an address by typing or clicking on the map, the tool displays a list of the included antibiotics ordered by predicted susceptibility for the geographic point specified. The colors of the antibiotics on the list indicate their predicted susceptibility, as do the colors used for the map layers. The map initially displays the regional pattern for the antibiotic with highest susceptibility. The user can then select any antibiotic from the list, loading that antibiotic’s resistance pattern into the map as a new visualization layer.
FIG 2.
AMR Tracker: prototype development of antimicrobial resistance visualization and clinical decision support tool.
Although we focused exclusively on E. coli data in this report, AMR Tracker was designed to include additional pathogens. Similarly, the application can support blood, urine, lung, and skin infection sites on data collection. We developed a highly interactive visual tool for making antibiotic treatment decisions that considers local antibiotic susceptibilities. The antibiogram data reveal geographic variations and suggestions of higher resistance near urban settings, similar to those in a U.S. hospital study (17).
Higher-precision data are needed for more accurate predictions before the tool can be broadly applied in the clinical practice setting. As data quality is expected to improve integration of health informatics, the application was designed with the capacity to display higher-precision maps when available. Thus, it may display susceptibility trends in real time in the future. The application can also accept and be updated with data from revised interpolation methods and data sources. Furthermore, mathematical predicative susceptibility modeling derived from each antibiotic and pathogen pair is likely to increase accuracy. Modeling can also identify clinically and statistically significant differences in susceptibility, as shown by Tlachac and colleagues (18, 19).
For community-onset infections, the assumption of the current interpolation method is that patients visit health care facilities nearest their homes. Data are anchored at one point without acknowledging the true geographic imprint of their origination, but rather they are approximated by the health system, county, or city as available. Patient-level data will allow for geographic distribution within the model. The current method creates susceptibility predictions by interpolating the space around each antibiogram by incorporating its values and those of surrounding antibiograms to create a three-dimensional surface. This interpolation method does not include the number of isolates. Therefore, areas with fewer antibiograms are at greater risk of distortion from outliers. Advances in the tool will consider data from overlapping health systems with higher-resolution geographic mapping and the number of isolates.
Ongoing research is investigating the associations among available geographic variables, such as socioeconomic measures, demographics, and population density. Additional types of interpolations and geospatial analyses, including spatial regression, will be used to test the relationship of these variables and AMR patterns. Future research includes validation of the predictions and engagement and refinement of the tool with health systems, followed by widespread dissemination, implementation, and evaluation. The maps and tool created will be utilized in continued efforts to improve the functionality of AMR data in clinical practice to optimize antimicrobial selection.
ACKNOWLEDGMENTS
We thank Erik Munson for providing the Wisconsin antibiograms.
This study was funded by a Fall Research Competition Award from the University of Wisconsin-Madison.
W.E.R. has received consulting fees from Visante, Inc.
REFERENCES
- 1.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. 2016. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karanika S, Paudel S, Grigoras C, Kalbasi A, Mylonakis E. 2016. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother 60:4840–4852. doi: 10.1128/AAC.00825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasan A. 2018. Antibiotic stewardship grows up. Jt Comm J Qual Patient Saf 44:65–67. doi: 10.1016/j.jcjq.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legenza LM, Barnett SG, Rose WE. 2018. A physician survey of antimicrobial stewardship culture and use of antimicrobial resistance data and resources. J Pharm Soc Wis 21:49–53. [Google Scholar]
- 5.Hay SI, Rao PC, Dolecek C, Day NPJ, Stergachis A, Lopez AD, Murray C. 2018. Measuring and mapping the global burden of antimicrobial resistance. BMC Med 16:78. doi: 10.1186/s12916-018-1073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. 2018. Surveillance of antimicrobial resistance in Europe – annual report of the European Antimicrobial Resistance Surveillance Network 2017 (EARS-Net). European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2015. Antibiotic resistance patient safety atlas executive summary. https://gis.cdc.gov/grasp/PSA/Downloads/AR-Summary.pdf. Accessed 9 January 2019.
- 8.Munson E, Block TK, Bowles EJ, Costello M, Dern R, Fritsche TR, Helgesen MA, Kropp JL, Podzorski RP, Siebers K, Simmons B, Smith MA, Spray F, Van TT, Warshauer DM. 2016. Surveillance of Wisconsin antibacterial susceptibility patterns. WMJ 115:29–36. [PubMed] [Google Scholar]
- 9.Center for Disease Dynamics, Economics & Policy. 2015. State of the world’s antibiotics. Center for Disease Dynamics, Economics & Policy, Washington, DC. [Google Scholar]
- 10.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrill HJ, Morton JB, Caffrey AR, Jiang L, Dosa D, Mermel LA, LaPlante KL. 2017. Antimicrobial resistance of Escherichia coli urinary isolates in the Veterans Affairs Health Care System. Antimicrob Agents Chemother 61:e02236-16. doi: 10.1128/AAC.02236-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez GV, Babiker A, Master RN, Luu T, Mathur A, Bordon J. 2016. Antibiotic resistance among urinary isolates from female outpatients in the united states in 2003 and 2012. Antimicrob Agents Chemother 60:2680–2683. doi: 10.1128/AAC.02897-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philip G, Watson D. 1985. A refinement of inverse distance weighted interpolation. Geoprocessing 2:315–327. [Google Scholar]
- 14.Oliver M. 1990. Kriging: a method of interpolation for geographical information systems. Int J Geogr Inf Syst 37:313–332. doi: 10.1080/02693799008941549. [DOI] [Google Scholar]
- 15.Environmental Systems Research Institute. 2018. ArcGIS Pro. Environmental Systems Research Institute, Redlands, CA. [Google Scholar]
- 16.Terzopoulos D. 1988. The computation of visible-surface representations. IEEE Trans Pattern Anal Machine Intell 10:417–438. doi: 10.1109/34.3908. [DOI] [Google Scholar]
- 17.Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, Palmore TN, Rhee C, Klompas M, Dekker JP, Powers JH, Suffredini AF, Hooper DC, Fridkin S, Danner RL, National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI). 2018. Difficult-to-treat resistance in Gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 67:1803–1814. doi: 10.1093/cid/ciy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tlachac ML, Rundensteiner E, Barton K, Troppy S, Beaulac K, Doron S. 2018. Predicting future antibiotic susceptibility using regression-based methods on longitudinal Massachusetts antibiogram data, p 103–114. In Proc 11th Int Joint Conf Biomed Eng Syst Technol (BIOSTEC), vol. 5. doi: 10.5220/0006567401030114. [DOI] [Google Scholar]
- 19.Tlachac ML, Rundensteiner E, Barton K, Troppy S, Beaulac K, Doron S, Zou J. 2018. CASSIA: an assistant for identifying clinically and statistically significant decreases in antimicrobial susceptibility. In IEEE EMBS Int Conf Biomed Health Informatics (BHI). doi: 10.1109/BHI.2018.8333450. [DOI] [Google Scholar]